INTRODUCTION:
Extracellular vesicles (EVs) and their cargo may provide promising biomarkers for the early detection of pancreatic ductal adenocarcinoma (PDAC). Although blood-borne EVs are most frequently studied as cancer biomarkers, pancreatic juice (PJ) may represent a better biomarker source because it is in close contact with the ductal cells from which PDAC arises. It is, as yet, unknown whether PDAC results in a distinct type or increased number of particles in PJ and whether this has diagnostic value.
METHODS:
Secretin-stimulated PJ was collected from the duodenum of 54 cases and 117 nonmalignant controls under surveillance for PDAC. Serum was available for a subset of these individuals. The vesicular composition of these biofluids was analyzed with nanoparticle tracking analysis.
RESULTS:
The concentration of EVs did not differ between controls and PDAC cases. However, a higher number of large vesicles were found in PJ (but not serum) for patients with PDAC compared with controls.
DISCUSSION:
The composition of isolated EVs from PJ, but not serum, is altered in patients with PDAC. This suggests that PJ may carry disease-specific markers not present in serum and provides a valuable biomarker source for PDAC diagnosis. The nature of the larger particles in EV isolates from PJ of PDAC cases requires further investigation.
INTRODUCTION
Detection of pancreatic ductal adenocarcinoma (PDAC) at a curable stage is challenging because of the late presentation of symptoms and limited visibility of subcentimeter lesions on imaging. Therefore, accurate biomarkers for early detection are urgently needed. In recent years, extracellular vesicles (EVs) have gained interest as potential disease biomarkers. EVs carry a unique molecular cargo to communicate between cells and are expected to contain a cell-specific signature (1). Cancer cells release EVs to form a premetastatic niche (1). Thus, detection of cancer-derived EVs based on their content may predict the presence of disease. Although blood-borne EVs are most frequently studied in this context, for PDAC, pancreatic juice (PJ) may be a promising biomarker source because it is in close contact with the ductal cells from which PDAC arises. Indeed, detection of microRNA molecules from EVs obtained from PJ through ultracentrifugation was able to distinguish PDAC from controls (2).
Interestingly, the concentration of EVs was determined by nanoparticle tracking analysis in bile-discriminated patients with malignant (including PDAC) from nonmalignant common bile duct stenosis with 100% accuracy (3). These data suggest that cancer cells emit elevated numbers of EVs in their surrounding extracellular space (3), which may be exploited to detect the presence of cancer without studying specific EV content. Such analysis would greatly simplify testing in a clinical setting. We characterize the size and concentration of EVs in PJ and serum of patients with PDAC and the controls to establish whether this may present a promising biomarker for the early detection of PDAC.
METHODS
Selection of subjects
This study was executed at the Erasmus University Medical Center. Sequential inclusion occurred between August 2018 and May 2020 for patients who participate in one of the following prospective study cohorts: (1) patients with suspected (sporadic) PDAC (KRAS Panc study, MEC-2018-038), (2) high-risk individuals under surveillance for a hereditary predisposition or familial history of PDAC (N-cyclohexyl-3-aminopropanesulfonic acid study, MEC-2012-448, www.caps-registry.com) (4), and (3) individuals under surveillance for neoplastic pancreatic cysts (PACYFIC study, MEC-2014-021, www.pacyfic.net). The Erasmus Medical Center ethical review board approved the study, and the included individuals gave written consent before enrolment. This study was performed according to the ethical principles for medical research involving human subjects from the World Medical Association Declaration of Helsinki.
PJ collection
PJ was collected as described before (5). In short, during endoscopic ultrasound, PJ collection was performed after visualization of the ampullary orifice. To reduce duodenal contamination, duodenal fluid was aspirated before juice collection. Next, a washout of PJ was stimulated by intravenous administration of human synthetic secretin (ChriRhoStim, Burtonsville, MD; 16 µg/patient). PJ was collected for up to 8 minutes starting immediately after injection with the endoscope (Pentax Medical, Tokyo, Japan) and assembled in a mucus extractor (Pennine Healthcare, Derby, UK; 15 mL) attached to the proximal end of the endoscopic channel. PJ was aliquoted, snap-frozen within 10 minutes after collection, and stored at −80 °C until further use. In total, during the study period, sufficient PJ to perform the planned analyses was collected from 54 individuals with sporadic PDAC (cases) and 117 nonmalignant controls undergoing pancreatic surveillance. Of the included patients, serum was available for 46 cases and 58 controls.
Enzyme-linked immunosorbent assay
The total protein concentration in PJ was assessed by Lowry protein assay (Bio-Rad, Hercules, CA) (6). To disrupt vesicles before measurement of the total protein in EVs, 0.2% sodium dodecyl sulfate (SDS) was added. As a measure of pancreatic specific content, we measured the levels of phosphatidylcholine 2-acyl hydrolase 1B (PLA2G1B), which is primarily expressed and secreted by the acinar cells of the pancreas and plays a central role in phospholipid digestion and metabolism (7). PLA2G1B concentration was determined by enzyme-linked immunosorbent assay using precoated, preblocked plates as per the manufacturer's instructions (MyBiosource, San Diego, CA).
EV analysis
PJ was centrifuged for 10 minutes at 4,000 revolutions per minute at 4 °C to remove debris. Then, 100 μL of Total Exosome Isolation Reagent (Thermo Fisher Scientific, Waltham, MA; #4478359) was added to 200 μL of PJ supernatant and kept on a rollerbank overnight at 4 °C. After this, samples were centrifuged for 1 hour at 14,000 RPM and the pellet was resuspended in 400 μL of phosphate buffered saline (PBS) (prefiltered with 0.2 μM filter). Serum was centrifuged for 30 minutes at 2,000g at 4 °C. Forty microliters of Total Exosome Isolation Reagent (Thermo Fisher Scientific, Waltham, MA; #4478360) were added to 200 μL of serum supernatant and incubated 30 minutes at 4 °C. Then, samples were centrifuged 10 minutes at 10,000g, and the pellet was resuspended in 200 μL of filtered PBS. EVs were stored at −80 °C until further analysis.
EV characterization
For nanoparticle tracking analysis (NTA), samples were diluted 1:1,000 in filtered PBS. The size and concentration were detected using NanoSight NS300 (NTA 3.4 Build 3.4.003 software). Two measurements of each sample were performed. EVs were visualized using transmission electron microscopy (TEM). For this, 10 μL droplets were deposited on formvar/carbon-coated 400 mesh Cu grids and incubated for 10 minutes. After that, the remaining liquid was drained with a filter paper and samples were stained with a drop of UranyLess stain for 1 minute. The remaining liquid was drained, and grids were allowed to air dry. Grids were observed under the electron microscope Talos L120C TEM from Thermo Fisher Scientific at 120 kV. For Western blot analysis, total proteins were extracted in 300 μL Laemmli buffer [SDS 4%, glycerol 20%, Tris-Cl (pH 6.8) 120 mM, bromophenol blue 0.02% (w/v), and dithiothreitol 0.1 M]. Proteins were resolved by SDS-PAGE and blotted onto Immobilon-FL polyvinylidene fluoride membranes (Millipore, Bedford, MA). Membranes were blocked in Odyssey blocking buffer (Thermo Fisher Scientific) and incubated overnight at 4 °C with primary antibody (caveolin-1 [Cell Signaling Technology, Danvers, MA; #3238], CD81 [GeneTex, Irvine, CA; #GTX43505], and GAPDH [Santa Cruz Biotechnology, Dallas, TX; #sc-51906]), followed by the appropriate Alexa-linked secondary antibodies, at 1:5,000 dilution, in Odyssey blocking buffer for 1 hour. The fluorescent bands were detected using fluorescent Odyssey Imaging.
Statistical analysis
Graphpad Prism 9 (GraphPad Software Inc.) and US IBM SPSS statistic 25 (SAS Institute, Cary, NC) software were used for the generation of graphs and statistical analyses. The Shapiro-Wilk test was used to determine data distribution; the Mann-Whitney U test was performed to compare the 2 groups.
RESULTS
Patients
In total, PJ was available from 54 PDAC cases and 117 controls. A summary of clinical characteristics is provided in Table 1. Cases were slightly older than controls (age 67.5 vs 62 years, P = 0.001), included more men (63% vs 34%, P < 0.001), and more often suffered from diabetes (38.9% vs 13.7%, P = 0.001). By contrast, controls showed a higher BMI (25.7 vs 23.7, P = 0.001).
Table 1.
Clinical characteristics at time of pancreatic juice collection
| Cases (N = 54) | Controls (N = 117) | P value | |
| Age in yr, median (IQR) | 67.5 (10.3) | 62.0 (6.0) | 0.001 |
| Male sex, n (%) | 34 (63.0) | 40 (34.2) | <0.001 |
| BMI in kg/m2, median (IQR) | 23.7 (3.7) | 25.7 (5.0) | 0.001 |
| Familial/genetic predisposition, n (%) | 0 (0.0) | 66 (56.4) | |
| Member of FPC familya | . | 31 (26.5) | |
| CDKN2A p16 | . | 24 (20.5) | |
| BRCA2 + ≥2 blood relatives with PDAC | . | 5 (4.3) | |
| BRCA1 + ≥2 blood relatives with PDAC | . | 1 (0.9) | |
| PALB2 + ≥2 blood relatives with PDAC | . | 1 (0.9) | |
| BRCA2 + CDKN2A p16 | . | 1 (0.9) | |
| STK11/LKB1 | . | 2 (1.7) | |
| Diabetes mellitus, n (%) | 21 (38.9) | 16 (13.7) | 0.001 |
| Indication EUS, n (%) | |||
| Suspected PDAC | 35 (64.8) | 4 (3.4) | |
| Fiducial placementb | 18 (33.3) | 0 (0.0) | |
| Surveillance | 1 (1.9) | 114 (96.6) | |
| CBD stent in situ, n (%) | <0.001 | ||
| CBD stent in situ | 9 (16.7) | 0 (0.0) | . |
| No CBD stent and CBD dilation | 14 (25.9) | 3 (2.5) | . |
| No CBD stent and no CBD dilation | 31 (57.4) | 115 (97.5) | . |
| Relative or absolute indications for surgeryc [8], n (%) | 54 (100.0) | 26 (22.2) | <0.001 |
| Enhancing mural nodule or hypodense lesion | 54 (100.0) | 4 (3.4) | |
| Caliber change | 41 (75.9) | 0 (0.0) | |
| Diffuse PD dilation > 5 mm | 0 (0.0) | 14 (12.0) | |
| CA19.9 ≥37 kU/L | 34 (63.0) | 7 (6.0) | |
| Cyst size >40 mm | 0 (0.0) | 2 (1.7) | |
| New-onset diabetesd | 9 (16.6) | 2 (1.7) | |
| Recent acute pancreatitise,f | 2 (3.7) | 6 (5.1) | |
| Lymphadenopathy | 23 (42.6) | 0 (0.0) | |
| Working diagnosis, n (%) | |||
| No abnormalities | . | 41 (35.0) | |
| Unspecified cyst | . | 9 (7.7) | |
| SB-IPMN | 1 (1.9) | 50 (41.9) | |
| MD-IPMN or MT-IPMN | . | 14 (12.0) | |
| MCN | . | 1 (0.9) | |
| NET | . | 1 (0.9) | |
| Indeterminate lesion, not suspect for malignancy | . | 2 (1.7) | |
| Resectable PDAC | 10 (18.5) | 0 (0.0) | |
| Locally advanced PDAC | 43 (79.6) | 0 (0.0) | |
| Distal metastases (on imaging), n (%) | 8 (14.8) | 0 (0.0) | <0.001 |
BMI, body mass index; CBD, common bile duct; ERCP, endoscopic retrograde cholangiopancreatography; EUS, endoscopic ultrasound; FPC, familial pancreatic cancer; IPMN, intraductal papillary mucinous neoplasm; IQR, interquartile range; MCN, mucinous cystic neoplasms; MD, main duct; MT, mixed-type; NET, pancreatic neuroendocrine tumors; PD = pancreatic duct; PDAC, pancreatic ductal adenocarcinoma; SB, side branch.
≥2 first-degree relatives or 3 relatives (either first or second degree) or ≥2 second-degree relatives of which ≥1 with age <50 yr at time of diagnosis.
Received previous chemotherapy.
One can have developed multiple worrisome features.
Development of diabetes mellitus in 2 yr before biomaterial collection.
Acute pancreatitis in 2 yr before biomaterial collection (not related to performed ERCP).
Three extra post-ERCP pancreatitis.
Characterization of PJ
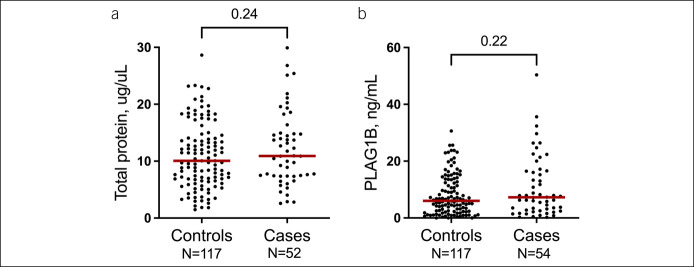
Pancreas-specific PLA2G1B was detected in PJ of all patients with PDAC and 115 of 117 controls. The concentrations of PLA2G1B (P = 0.22) and total protein content (P = 0.24) did not differ between cases and controls, indicating similar PJ quality (Figure 1).
Figure 1.
No difference was seen between controls and cases in pancreatic juice concentration of total protein (a) and phospholipase A2 group IB (PLA2G1B) (b) as a measure of pancreatic origin, indicating similar composition of pancreatic juice.
Characterization of EVs
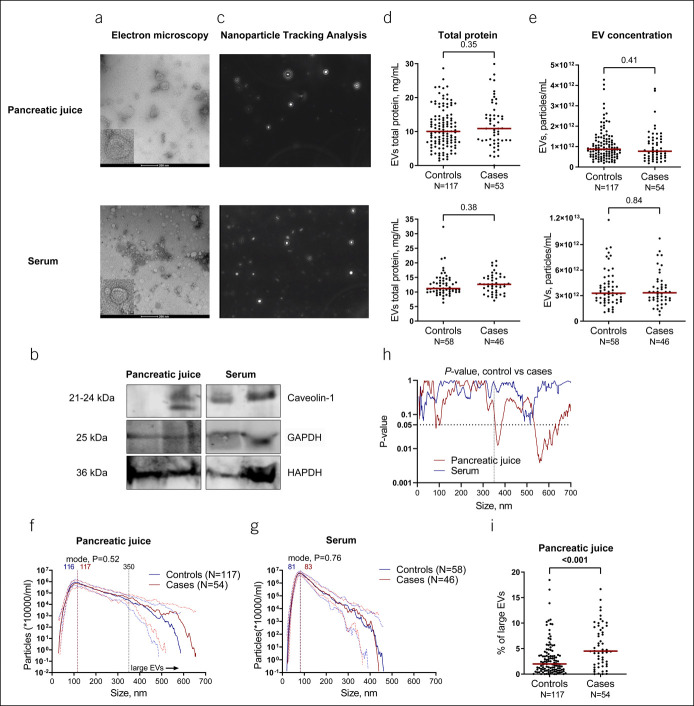
For both PJ and serum, isolated EV fractions showed round double-membrane vesicle-like structures, typical of EVs (Figure 2a), which express membrane and cytoplasmic EV markers, such as CD81, caveolin-1, and GAPDH (8–10) (Figure 2b). NTA analysis allowed the visualization of heterogeneous populations of spherical nanoparticles moving under Brownian motion (Figure 2c). The concentration of EVs was significantly higher in serum (median 3.28E12 particles/mL; 95% CI 2.8512–3.6812) than in PJ (median 8.42E11 particles/mL; 95% CI 7.5311–9.4911, P < 0.001). When comparing the concentration of EVs between cases and controls, no difference was found for either biofluid as seen from NTA analysis and total protein concentration in EV isolates (in PJ median, 8.7111 particles/mL [95% CI 7.6711–9.8611] for controls vs 7.7311 [95% CI 5.9211–1.14512] for cases, P = 0.41; in serum median, 3.2812 [95% CI 2.8212–3.9612] for controls vs 3.3412 [95% CI 2.7112–3.9412; P = 0.84] for cases) (Figure 2d–e).
Figure 2.
Analysis of EVs in PJ of controls and individuals with PDAC (cases) shows a different size distribution between these groups. (a) Representative images of EVs extracted from PJ and serum by transmission electron microscopy showing the presence of double-membrane vesicles. Note a larger size EVs in PJ compared with serum. (b) Western blot analysis of typical EV markers commonly found in exosome subpopulations. (c) Representative NTA images for PJ and serum. (d,e) Total protein content in EVs isolated from PJ (upper panel) or serum (lower panel) is equal between controls and cases, and NTA showed no difference in particle concentrations between controls and cases in PJ (E, median concentration of 8.7111 particles/mL [95% CI 7.6711–9.8611] for controls vs 7.7311 [95% CI 5.9211–1.14512] for cases, P = 0.41) or serum (E, median 3.2812 [95% CI 2.8212–3.9612] and 3.3412 [95% CI 2.7112–3.9412; P = 0.84], respectively. (f,g) Median concentration of EVs of different sizes (from 0 to 750 with stepwise increments of 0.5 nm) in PJ (f) and serum (g). For PJ, vertical lines indicate mode size of 116 and 117 nm for controls and cases, respectively (P = 0.52). A threshold line of 350 nm indicating large size particles is indicated, with cases having more EVs with diameter >350 nm than controls. For serum, vertical lines indicate mode diameter of 81 and 83 nm for controls and cases, respectively (P = 0.76). Scattered lines indicate interquartile range. (h) Comparison of the concentration of EVs of different sizes (from 0 to 750 with stepwise increments of 0.5 nm) between controls and cases. P values for PJ are indicated in red and serum in blue. Although the number of EVs in serum is similar between cases and controls across the size ranges, cases present significantly more EVs in the larger range as compared with controls in PJ. The dotted line indicates a significance threshold level of P = 0.05. (i) Percentage of large EVs (>350 nm) in PJ is higher in cases vs controls. CI, confidence interval; EV, extracellular vesicles; NTA, nanoparticle tracking analysis; PDAC, pancreatic ductal adenocarcinoma; PJ, pancreatic juice.
PJ-derived EVs appeared larger than serum EVs in TEM analysis, which was confirmed by NTA analysis (mode diameter of 116 nm [95% CI 114.2–120.2] for PJ and 82 nm [95% CI 80.2–84.1] for serum, P < 0.0001). No difference in mode diameter was observed between controls and cases for either PJ or serum (Figure 2f,g). We then compared the concentration of EVs between cases and controls for all measured EV sizes, ranging from 0.5 nm to 750 nm, with a stepwise increment of 0.5 nm. This analysis showed a significant difference in EV size distribution between cases and controls for PJ, but not serum. In PJ, the difference between cases and controls was significant mostly for larger particles with diameters starting from 355.5 (P < 0.05) and a trend starting from 350 nm (P < 0.1). When choosing a threshold of 350 nm, cases had a higher concentration of large EVs in PJ as compared with controls (P < 0.001, Figure 2i). Specifically, EVs ranging in size from 355.5 to 385.5 nm, 534.5–629.5 nm, 631.5–642.5 nm, and 645.5–647.5 nm reached significantly higher concentrations (P < 0.05) in cases, as compared with controls (Figure 2h). Particles with a size of 102.5 nm were more prevalent in cases compared with controls. After correction for age, sex, body mass index, and diabetes mellitus, the percentage of larger EVs (size >350 nm) remained significantly higher in cases vs controls in PJ (see Supplementary Table 1, Supplementary Digital Content, http://links.lww.com/CTG/A769), thus showing an independent association with PDAC.
DISCUSSION
This study shows that, as compared with serum, EVs from PJ are larger, whereas their absolute concentration is lower, indicating a distinct proportional composition of vesicular subtypes in PJ. We and others (3), did not find differences in EV concentrations between PDAC cases and controls in serum where most of the EVs may be of nontumor origin. In contrast to previous reports for bile (3), where elevated numbers of EVs were seen for patients with cholangiocarcinoma and PDAC with biliary stenosis, we did not find differences in the absolute concentration of PJ-derived EVs between cases and controls. Interestingly, bile-derived EVs appeared to be larger in patients with cancer compared with patients with chronic pancreatitis, although size distribution was not quantified in that study (3). In our study, the number of large EVs (>350 nm) in PJ of PDAC cases was significantly increased, suggesting a different prevalence of particular subtypes of EVs in these groups. The number of large EVs correlated with pancreas-specific PLA2G1B levels (see Supplementary Figure 1, Supplementary Digital Content, http://links.lww.com/CTG/A769), implying that these larger-sized EVs are of pancreatic origin and that EV size may be a promising tool to discriminate patients with PDAC from controls.
EVs are classified based on size and their biogenesis: Exosomes (<150 nm) are released through multivesicular bodies (MVBs) in the endosomal pathway, microvesicles (200–500 nm) are formed by budding from the plasma membrane, and apoptotic bodies of various sizes derive from programmed cell death. In addition, many other specialized EV subtypes have been described (11). Lipoproteins can also be coisolated to varying degrees using diverse EV isolation methods (12). These lipid and apolipoprotein assemblies resemble EVs in size, with the largest chylomicrons ranging in size from 75 to 600 nm, while having a lipid core rather than a aqueous pore (13). Owing to a significant overlap in size, similarities in composition, and lack of specific markers, it is difficult to assign individual EVs to one of the biogenesis pathways, but the nature of the large particles found in EV isolates in PJ of patients with PDAC represents an interesting research question.
We show that EV extraction from PJ with isolation kits requiring microcentrifuges yields similar concentrations as reported for extraction with ultracentrifugation (2,14). Because microcentrifuges are commonly available in laboratories, this finding facilitates the application of EVs as a clinical biomarker. We also acknowledge several limitations to our study. First, it is conceivable that our findings consist of chance findings. Despite the fact samples were isolated and measured in batches with results within each batch similar to our overall conclusion, an independent confirmation of our findings would be our recommended next step. Another limitation is the difference between groups in age, sex body mass index, and diabetes. Although for some characteristics, this is unavoidable (e.g., pancreatic cancer is associated with diabetes), and our results demonstrate that EV size associates with PDAC independent from these clinical characteristics, confirmation in a better-matched cohort is important. Finally, to be of clinical use, investigation of the presence of larger EVs at precancerous stages would be required.
In summary, we characterized the vesicular composition of PJ in PDAC and controls undergoing surveillance and found that PJ from individuals with PDAC harbors increased amounts of large EVs, which may be useful for future biomarker development.
CONFLICTS OF INTEREST
Guarantor of the article: Gwenny M. Fuhler, PhD.
Specific author contributions: K.N.: conceptualization, project coordination, writing—original draft, data analysis and visualization, EVs, and total protein analysis. I.J.M.L.: subjects inclusion, pancreatic juice and serum collection, writing—review and editing, PLAG1B, and total protein analysis. N.F.J.D.: electron microscopy. D.L.C.: pancreatic juice collection, writing—review and editing. M.P.P.: writing—review and editing. M.J.B.: pancreatic juice collection, writing—review and editing, and supervisor. G.M.F.: conceptualization, writing—original draft, writing—review and editing, and supervisor.
Financial support: None to report.
Potential competing interests: M.J.B. is a consultant to Boston Scientific (consultant, support for industry and investigator initiated studies), Cook Medical (consultant, support for industry and investigator initiated studies), Pentax Medical (consultant, support for investigator initiated studies), Mylan (support for investigator initiated studies), ChiRoStim (support for investigator initiated studies), 3M (support for investigator initiated studies), and InterScope (support for investigator initiated studies). None of the other authors have any conflicts of interest to report.
Study Highlights.
WHAT IS KNOWN
✓ Extracellular vesicles (EVs) carry cancer-specific information.
✓ Serum EVs have been widely studied for diagnostic purposes in cancer, including pancreatic ductal adenocarcinoma (PDAC).
✓ Pancreatic juice may provide an alternative biomarker source, being in close contact with the cancerous ductal cells.
WHAT IS NEW HERE
✓ EVs can be isolated from pancreatic juice using commercially available kits.
✓ The number of EVs in serum and pancreatic juice is similar between PDAC cases and controls.
✓ The size distribution of EVs isolated from pancreatic juice, but not serum, is altered in PDAC cases compared with controls.
Supplementary Material
ACKNOWLEDGEMENT
We acknowledge I.J. Visser and E. de Vries for their help with sample acquisition and analysis, respectively.
Footnotes
SUPPLEMENTARY MATERIAL accompanies this paper at http://links.lww.com/CTG/A769
Contributor Information
Kateryna Nesteruk, Email: kv.nesteruk@gmail.com.
Iris J.M. Levink, Email: i.levink@erasmusmc.nl.
Natasja F.J. Dits, Email: n.dits@erasmusmc.nl.
Djuna L. Cahen, Email: d.cahen@erasmusmc.nl.
Maikel P. Peppelenbosch, Email: m.peppelenbosch@erasmusmc.nl.
Marco J. Bruno, Email: m.bruno@erasmusmc.nl.
REFERENCES
- 1.Costa-Silva B, Aiello NM, Ocean AJ, et al. Pancreatic cancer exosomes initiate pre-metastatic niche formation in the liver. Nat Cell Biol 2015;17(6):816–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nakamura S, Sadakari Y, Ohtsuka T, et al. Pancreatic juice exosomal MicroRNAs as biomarkers for detection of pancreatic ductal adenocarcinoma. Ann Surg Oncol 2019;26(7):2104–11. [DOI] [PubMed] [Google Scholar]
- 3.Severino V, Dumonceau JM, Delhaye M, et al. Extracellular vesicles in bile as markers of malignant biliary stenoses. Gastroenterology 2017;153(2):495–504.e8. [DOI] [PubMed] [Google Scholar]
- 4.Overbeek KA, Levink IJM, Koopmann BDM, et al. Long-term yield of pancreatic cancer surveillance in high-risk individuals. Gut 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Levink IJM, Nesteruk K, Visser DI, et al. Optimization of pancreatic juice collection: A first step toward biomarker discovery and early detection of pancreatic cancer. Am J Gastroenterol 2020;115(12):2103–8. [DOI] [PubMed] [Google Scholar]
- 6.Lowry OH, Rosebrough NJ, Farr AL, et al. Protein measurement with the Folin phenol reagent. J Biol Chem 1951;193(1):265–75. [PubMed] [Google Scholar]
- 7.Dennis EA. Diversity of group types, regulation, and function of phospholipase A2. J Biol Chem 1994;269(18):13057–60. [PubMed] [Google Scholar]
- 8.Campos A, Salomon C, Bustos R, et al. Caveolin-1-containing extracellular vesicles transport adhesion proteins and promote malignancy in breast cancer cell lines. Nanomedicine (Lond) 2018;13(20):2597–609. [DOI] [PubMed] [Google Scholar]
- 9.Andreu Z, Yáñez-Mó M. Tetraspanins in extracellular vesicle formation and function. Front Immunol 2014;5:442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Logozzi M, De Milito A, Lugini L, et al. High levels of exosomes expressing CD63 and caveolin-1 in plasma of melanoma patients. PLoS One 2009;4(4):e5219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maas SLN, Breakefield XO, Weaver AM. Extracellular vesicles: Unique intercellular delivery vehicles. Trends Cell Biol 2017;27(3):172–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brennan K, Martin K, FitzGerald SP, et al. A comparison of methods for the isolation and separation of extracellular vesicles from protein and lipid particles in human serum. Sci Rep 2020;10(1):1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Simonsen JB. What are we looking at? Extracellular vesicles, lipoproteins, or both? Circ Res 2017;121(8):920–2. [DOI] [PubMed] [Google Scholar]
- 14.Zheng J, Hernandez JM, Doussot A, et al. Extracellular matrix proteins and carcinoembryonic antigen-related cell adhesion molecules characterize pancreatic duct fluid exosomes in patients with pancreatic cancer. HPB (Oxford) 2018;20(7):597–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.