INTRODUCTION:
Observational studies have suggested an increased risk of pancreatic ductal adenocarcinoma (PDAC) in patients with acute and chronic pancreatitis. We conducted a systematic review and meta-analysis to evaluate the magnitude of this association and summarize the published epidemiological evidence.
METHODS:
We searched electronic databases (MEDLINE, Embase, Web of Science, Cochrane, and Scopus) and reference lists until January 18, 2021. Studies reporting quantitative association between pancreatitis and PDAC were included and assessed for eligibility, data abstraction, and risk of bias. Standardized incidence ratios (SIRs) were pooled using the random-effects model.
RESULTS:
Twenty-five cohort and case-control studies met inclusion criteria. Meta-analysis of 12 chronic pancreatitis (CP) studies demonstrated an increased risk of PDAC in patients with CP (SIR: 22.61, 95% confidence interval [CI]: 14.42–35.44). This elevated risk persisted in subgroup analysis of studies that excluded patients diagnosed with PDAC within 2 years of CP diagnosis (SIR: 21.77, 95% CI: 14.43–32.720). The risk was higher in hereditary pancreatitis (SIR: 63.36, 95% CI: 45.39–88.46). The cumulative incidence rates of PDAC in CP increased with follow-up duration. Limited evidence in acute pancreatitis indicates higher PDAC risk in the subset of patients eventually diagnosed with CP. PDAC seems to be uncommon in patients with autoimmune pancreatitis, with 8 reported cases in 358 patients with autoimmune pancreatitis across 4 studies.
DISCUSSION:
There is an increased risk of PDAC in patients with CP, and incidence rates increase with CP disease duration. Our results indicate that PDAC surveillance may be considered in individuals with long-standing CP.
INTRODUCTION
Pancreatic ductal adenocarcinoma (PDAC) is a lethal malignancy with rising global incidence. Most patients present with incurable metastatic disease at diagnosis, and the 5-year survival in United States is approximately 10% (1–3). However, survival is substantially higher in the subgroup of patients diagnosed at an early stage. There is currently no recommended population screening tool for PDAC (4), but risk factor–based identification of individuals who are at a greater than average lifetime risk of PDAC has been proposed as a strategy to identify a cohort that may benefit from screening.
Chronic pancreatitis (CP) is a fibroinflammatory disease of the exocrine pancreas with varied etiology and a broad spectrum of clinical manifestations that range from asymptomatic disease to debilitating chronic pain and exocrine and endocrine insufficiency (5). CP is an established risk factor for PDAC (6–8). Long-standing inflammation increases cell turnover and stellate cell proliferation, and in CP, this creates a pancreatic tissue microenvironment that promotes carcinogenesis (9). The lifetime risk of PDAC is further elevated in the forms of CP characterized by an early onset of pancreatic inflammation, such as hereditary and tropical pancreatitis (5). Autoimmune pancreatitis (AIP) is a steroid-responsive form of CP associated with a marked inflammatory phase, potentially increasing the risk of malignancy (10). Although both pancreatic and extrapancreatic malignancies have been reported in patients with AIP, the lifetime risk of developing PDAC does not seem to be elevated in patients with AIP compared with the general population, and long-term follow-up data are limited (11). The relationship between acute pancreatitis (AP) and PDAC risk also remains unclear. Although AP can be the first clinical manifestation of PDAC, believed to be due to tumor-related ductal obstruction, the long-term risk of PDAC in individuals with AP has not been defined (12). Considering the clinical relevance of understanding the association between different forms of pancreatitis and pancreatic cancer, we conducted this systematic review and meta-analysis to evaluate the magnitude of association and the strength of the supporting evidence.
METHODS
This report adheres to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses Statement to complete of the systematic review (13).
Eligibility criteria
Studies were considered eligible for inclusion if participants were men and women older than 18 years. Also, diagnosis of 1 of the following:
AP diagnosed using 2 of 3: abdominal pain typical for AP; serum amylase and/or lipase greater than or equal to 3 times the upper normal limit; and evidence of AP on imaging (14).
CP confirmed diagnosis using imaging demonstrating pancreatic calcification with or without main pancreatic duct dilation, functional studies, or surgical pathology.
AIP with known radiological or histological findings diagnostic for AIP (International Consensus Diagnostic Criteria or histology, imaging, serology, other organ involvement, and response to therapy criteria) and/or clinical response to steroids (15,16).
Hereditary pancreatitis (HP) with confirmed gene mutation in either PRSS1, SPINK1, CFTR, or CTRC genes or with a family history consistent with HP.
Study design
The outcome was a quantitative association between exposure and histologically confirmed PDAC. Case-control, retrospective, or prospective cohort or single-arm cohort studies of any duration or setting studying >10 patients, reporting standardized incidence ratio (SIR), odds ratio (OR), relative risk (RR), and corresponding 95% confidence intervals (CIs) were included.
Exclusion criteria
Conference abstracts, case reports, letters, review articles, editorials, commentaries, qualitative articles, studies with less than 10 subjects, and studies with either patient-reported or International Classification of Diseases (ICD) code- based diagnosis of pancreatitis and/or PDAC.
Literature search
A comprehensive search of several databases from each database's inception was conducted, including Ovid MEDLINE In-Process and Other Non-Indexed Citations, Ovid MEDLINE, Ovid Embase, Ovid Cochrane Central Register of Controlled Trials, Ovid Cochrane Database of Systematic Reviews, and Scopus. The search strategy was designed and conducted by an experienced librarian with input from the study's investigators. Controlled vocabulary supplemented with keywords was used to search for pancreatitis and pancreatic cancer risk in human studies through January 18, 2021, with no language filter. Bibliographies of selected articles were reviewed for additional relevant studies. The detailed search strategy is available in the Appendix (see Supplementary Digital Content 1, http://links.lww.com/CTG/A764).
Data abstraction
Two independent reviewers (S.G. and J.d.l.F.) screened the titles and abstracts of studies based on inclusion and exclusion criteria, followed by a full-text analysis of relevant articles. A third reviewer (S.M.) adjudicated disagreements between the 2 reviewers. Study data were abstracted in duplicate to verify the accuracy of the studies. The following information was abstracted from each study: study type, author, year of publication, population and setting (study site), sample size, number of patients who developed PDAC, method of verification of pancreatitis and PDAC, matched variables, risk estimates, and their corresponding 95% CI. Disagreements were settled by consensus. Data on the cumulative incidence rates (CIRs) of PDAC after CP diagnosis were extracted when available.
Assessment of risk of bias
Risk of bias assessment was analyzed independently for each article by 2 reviewers (S.G. and J.d.l.F.) using the Newcastle-Ottawa Scale for case-control and cohort studies (17) and a modification of the scale for single-arm cohort studies (18). We considered that the follow-up was adequate when patients were followed for more than 2 years after pancreatitis diagnosis and when less than 20% patients were lost to follow-up. The most important factors in assessing the risk of bias in this specific research question were ascertainment of exposure, demonstration that the outcome was not present at the start of the study, assessment of outcome, and adequate length of follow-up in cohort studies. Studies that fulfilled all these criteria were considered to be at low risk of bias. Studies that only fulfilled 1 or none were considered to be at high risk of bias.
Statistical analyses
We meta-analyzed cohort studies (1 case-control study is reported narratively). All the studies reported SIRs except 1 that reported RR. The reported SIR was calculated as the ratio of the observed to expected number of patients with PDAC in the population studied. We conducted meta-analysis with and without the study that reported RR to determine whether the conclusions of the meta-analysis would change by excluding this study.
We pooled SIRs that compared the association between pancreatitis and PDAC risk at a 95% CI, using the random-effects model (19) because of anticipated heterogeneity across study settings and populations. Heterogeneity was assessed using the I2 statistic, and a value exceeding 50% implied substantial heterogeneity (20). We also conducted a sensitivity analysis on studies that excluded patients who developed PDAC within 2 years of follow-up. SIRs of PDAC in CP in smokers and nonsmokers were pooled when available to evaluate the effect of smoking on PDAC. Publication bias was assessed using the funnel plot.
RESULTS
Study selection
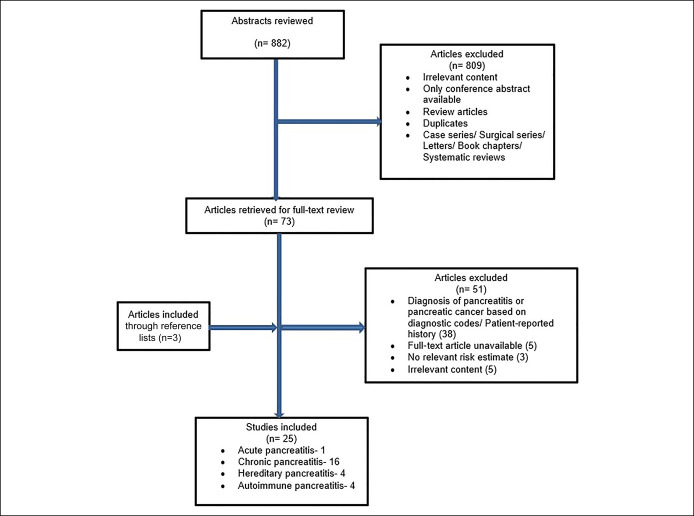
The initial database search identified 882 studies. Eight hundred nine studies were excluded based on title and abstract in the initial review. A full-text review was performed of the remaining 73 studies, and 51 studies were further excluded (Figure 1). Three additional studies were identified by searching bibliographies of relevant articles.
Figure 1.
Study flowchart for selection criteria.
Study characteristics
We found 25 studies that met our inclusion criteria: 1 for AP and CP, 1 for AIP and CP, 3 for AIP, 4 for HP, and 16 for CP only. Table 1 summarizes the descriptive characteristics of the 17 cohort studies, 7 single-arm cohort studies, and 2 case-control studies (21–45). Midha et al. (23) conducted both case-control and cohort studies and reported their findings in the same article (Midha a: Cohort study; Midha b: Case-control study). Rijkers et al. (38) described the outcomes separately for patients with AP who developed CP (Rijkers a: Outcomes for AP patients and Rijkers b: Outcomes for AP patients who developed CP). Ikeura et al. (42) examined the development of PDAC in both patients with AIP and CP (Ikeura a: Outcomes for AIP patients and Ikeura b: Outcomes for CP patients) (Table 1).
Table 1.
Study characteristics of the included studies
| Cohort studies | |||||||||
| Study author | Type of pancreatitis | Setting and period | Source population | Population size/control | PDAC cases | Pancreatitis verification | PDAC verification | Adjusted variables | EE value and 95% CI |
| Jeon | CP | United States 2006–2015 | Single-center | 1,766 | 46 | Imaging | Pathology/radiology | Age and sex | 12 (8.8–16)a |
| Hao | CP | China 2000–2013 | Single-center | 1,656 | 21 | Imaging or invasive functional testing | Pathology or multidisciplinary evaluation | Age and sex | 20.22 (12.53–30.89)a |
| Midha a | CP | India 2004–2009 | Single-center | 402 | 5 | Imaging and clinical characteristics | Pathology | Age and sex | 121 (39.7–295.9)a |
| Ueda | CP | Japan 2009–2010 | Muticenter | 506 | 19 | Histology/imaging/invasive functional testing | Pathology/radiology | Age and sex | 11.8 (7.1–18.4)a |
| Wang | CP | China 1997–2007 | Single-center | 420 | 4 | Pathology/imaging | Pathology | Age and sex | 27.2 (7.4–69.6)a |
| Zheng | CP | China 2009–2017 | Single-center | 650 | 12 | Pathology | Pathology | Age | 68.12 (35.2–118.99)a |
| Talamini | CP | Italy 1971–1995 | Single-center | 715 | 14 | Imaging/clinical characteristics | Pathology | Age and sex | 18.5 (10–30)a |
| Chari | CP | India 1987–1991 | Single-center | 185 | 6 | Imaging/clinical characteristics | Pathology, operative examination, and clinical characteristics | Age and sex | 100 (37–218)b |
| Malka | CP | France 1973–1997 | Single-center | 373 | 4 | Imaging/pathology | Pathology | Age and sex | 26.7 (7.3–68.3)a |
| Lowenfels | CP | Multicountry 1946–1989 | Multicenter | 1,552 | 29 | Imaging/biochemical testing/clinical evaluation | Pathology/imaging | Age, sex, and center | 16.5 (11.1–23.7)a |
| Pedrazzoli | CP | Italy 1970–1999 | Single-center | 170 | 2 | Pathology | Pathology | Age, sex, and calendar period | 2.93 (0.36–10.6)a |
| Rocca | CP | Italy 1970–1984 | Single-center | 172 | 2 | Pathology/Imaging | Pathology | Age and sex | NA |
| Hirano | AIP | Japan 1997–2012 | Multicenter | 95 | 2 | ICDC criteria | Pathology | Age and sex | 3.65 (0.42–12.5)a |
| Hamoir | HP | Belgium 1999–2012 | Single-center | 61 | 5 | AP-Atlanta criteria; CP-imaging; HP-genetic testing/family history | Pathology | Age | 26.5 (8.6–61.9)a |
| Rebours | HP | France 2005 | Multicenter | 200 | 10 | AP-Atlanta criteria; CP-imaging/pathology; HP-genetic testing or family history | Pathology | Age and sex | 87 (42–114)a |
| Howes | HP | Multicountry 1997 | Multicenter | 418 | 26 | AP-Atlanta criteria; CP-imaging/pathology/biochemical studies; HP-or family history | Pathology/imaging | Age, sex, nationality, and surgical intervention | 67 (50–82)a |
| Lowenfels | HP | Multicountry 1995–1996 | Multicenter | 246 | 8 | AP-Atlanta criteria/HP-family history | Pathology | Age, sex, and country | 53 (23–105)a |
| Single-arm cohort studies | ||||||||
| Study author | Type of pancreatitis | Setting and period | Source population | Population size/control | PDAC cases | Pancreatitis verification | PDAC verification | EE value and 95% CI |
| Rijkers a | AP | Netherlands 2004–2011 | Multicenter | 680 | 3 | Atlanta criteria | Pathology | 1.1 (0.3–3.3)c |
| Rijkers b | CP | Netherlands 2004–2011 | Multicenter | 51 | 2 | Imaging/pathology/functional testing | Pathology | 9.0 (2.3–35.7)c |
| Sakorafas | CP | United States 1976–1997 | Single-center | 484 | 14 | Pathology | Pathology | NA |
| Agarwal | CP | India 1998–2019 | Single-center | 1,415 | 29 | Pathology/imaging | Pathology | NA |
| Dite | CP | Czech Republic 1992–2003 | Unclear | 223 | 13 | Imaging/functional test | Pathology | NA |
| Ikeura | AIP | Japan 2002–2011 | Single-center | 63 | 3 | ICDC criteria | Pathology | 0.92%d |
| Ikeura | CP | Japan 2002–2011 | Single-center | 41 | 1 | Imaging/pathology/functional testing | Pathology | 0.59%d |
| Gupta | AIP | United States | Single-center | 84 | 2 | Pathology | Pathology | |
| Vujasinovic | CP | Sweden 2003–2019 | Single-center | 581 | 6 | Imaging/Pathology | Pathology | 0.2%d |
| Case-control studies | ||||||||||
| Study author | Type of pancreatitis | Setting and period | Source population | Control recruitment | Cases/control (n) | Exposed cases/control | Pancreatitis verification | PDAC verification | Matching/Adjusted variables | OR (95% CI) |
| Midha b | CP | India 2004–2009 | Single-center | “Healthy” relative of the cases | 249/1,000 | 24/1 | Imaging | Pathology/imaging | Age, sex, and socioeconomic status | 97.67 (12.69–751.36) |
| Hart | AIP | United States 1985–2011 | Single-center | 344 primary care clinic patients | 116/344 | 1/unknown | Pathology, HISORt criteria, and response to steroids | Pathology | Age, sex, and registration date | NA |
AP, acute pancreatitis; AIP, autoimmune pancreatitis; CI, confidence interval; CP, chronic pancreatitis; EE, effect estimate; HISORt, histology, imaging, serology, other organ involvement, and response to therapy (criteria for autoimmune pancreatitis); HP, hereditary pancreatitis; ICDC, International Consensus Diagnostic Criteria (for autoimmune pancreatitis); NA, not available; OR, odds ratio; PDAC, pancreatic ductal adenocarcinoma.
Standardized incidence ratio.
Relative risk.
Incidence rate per 1,000 person-years.
Annual incidence rate.
The number of study participants ranged from 61 to 1,766 in cohort studies, 41 to 1,415 in single-arm cohort studies and 116 to 249 cases in case-control studies. The studies differed in populations studied, settings, study periods, and the data sources ranged from single centers to multiple centers. In both case-control studies, cases and controls were matched for age and sex. All cohort studies were standardized by age; all but 5 studies were standardized for sex. Table 1 summarizes the risk estimates of the studies. The risk estimates reported were SIRs in 15 studies, incidence rate in 2 studies, RR in 1 study, and OR in 1 study.
The domains of the Newcastle-Ottawa Scale are listed for each study in Table 2 (21–45). The risk of bias in the included studies was low to moderate because of limitations in demonstration of outcome at the start of the study and adequate length of follow-up.
Table 2.
Methodological quality assessment of studies using the Newcastle-Ottawa Scale
| Cohort studies | ||||||||
| Study | Representativeness of the exposed cohort | Selection of the nonexposed cohort | Ascertainment of the exposure | Demonstration that outcome of interest was not present at start of study | Comparability | Assessment of outcome | Follow-up long enough | Follow-up adequacy |
| Jeon | *Truly representative | *Same community as exposed cohort | *Secure record | No | **Age and sex | *Independent assessment | *Yes | No statement |
| Hao | *Truly representative | *Same community as exposed cohort | *Secure record | *Yes | **Age and sex | *Independent assessment | *Yes | No statement |
| Midha a | *Somewhat representative | *Same community as exposed cohort | *Secure record | *Yes | **Age and sex | *Independent assessment | *Yes | *Yes |
| Ueda | *Truly representative | *Same community as exposed cohort | *Secure record | *Yes | *Age | *Independent assessment | *Yes | No statement |
| Wang | *Truly representative | *Same community as exposed cohort | *Secure record | *Yes | **Age and sex | *Independent assessment | *Yes | *Yes |
| Zheng | *Truly representative | *Same community as exposed cohort | *Secure record | *Yes | *Age | *Independent assessment | *Yes | *Yes |
| Talamini | *Truly representative | *Same community as exposed cohort | *Secure record | *Yes | **Age and sex | *Independent assessment | *Yes | *Yes |
| Chari | *Truly representative | *Same community as exposed cohort | *Secure record | No | **Age and sex | *Independent assessment | *Yes | *Yes |
| Malka | *Truly representative | *Same community as exposed cohort | *Secure record | *Yes | **Age and sex | *Independent assessment | *Yes | No |
| Lowenfels (CP) | *Truly representative | *Same community as exposed cohort | *Secure record | *Yes | **Age and sex | *Independent assessment | *Yes | No |
| Pedrazzoli | *Somewhat representative | *Same community as exposed cohort | *Secure record | *Yes | *Age | *Independent assessment | *Yes | *Yes |
| Rocca | *Truly representative | *Same community as exposed cohort | *Secure record | No | **Age and sex | *Independent assessment | *Yes | No statement |
| Hirano | *Truly representative | *Same community as exposed cohort | *Secure record | *Yes | **Age and sex | *Independent assessment | *Yes | No statement |
| Hamoir | *Truly representative | *Same community as exposed cohort | *Secure record | No | *Age | *Independent assessment | *Yes | *Yes |
| Rebours | *Truly representative | *Same community as exposed cohort | *Secure record | No | **Age and sex | *Independent assessment | *Yes | No statement |
| Howes | *Truly representative | *Same community as exposed cohort | *Secure record | No | **Age and sex | *Independent assessment | *Yes | No statement |
| Lowenfels (HP) | *Truly representative | *Same community as exposed cohort | *Secure record | *Yes | **Age and sex | *Independent assessment | *Yes | No statement |
| Single-arm cohort studies | |||||
| Study | Selection | Adequate ascertainment of exposure | Adequate ascertainment of outcome | Follow-up long enough? | Replication/Inferences |
| Rijkers a | *Truly representative | *Yes | No | No | *Yes |
| Rijkers b | *Truly representative | *Yes | *Yes | *Yes | *Yes |
| Sakorafas | *Somewhat representative | *Yes | *Yes | No | *Yes |
| Agarwal | *Truly representative | *Yes | No | *Yes | *Yes |
| Dite | Unclear | No description | No | *Yes | No |
| Ikeura | *Truly representative | *Yes | *Yes | *Yes | *Yes |
| Gupta | *Truly representative | *Yes | *Yes | *Yes | *Yes |
| Vujasinovic | *Truly representative | *Yes | *Yes | *Yes | *Yes |
| Case-control studies | |||||||
| Study | Is case definition adequate? | Representativeness of the cases | Selection of controls | Definition of controls | Comparability | Ascertainment of exposure | Same method for ascertainment for cases and controls |
| Midha b | *Yes, with independent validation | *Consecutive or obviously representative series of cases | *Community controls | No mention | **Age, sex, and socioeconomic status | *Secure record | No |
| Hart | *Yes, with independent validation | *Consecutive or obviously representative series of cases | *Community controls | No mention | **Age, sex, and registration date | *Secure record | No |
CP, chronic pancreatitis; HP, hereditary pancreatitis. *denotes 1 point, and ** denotes 2 points.
Meta-analysis
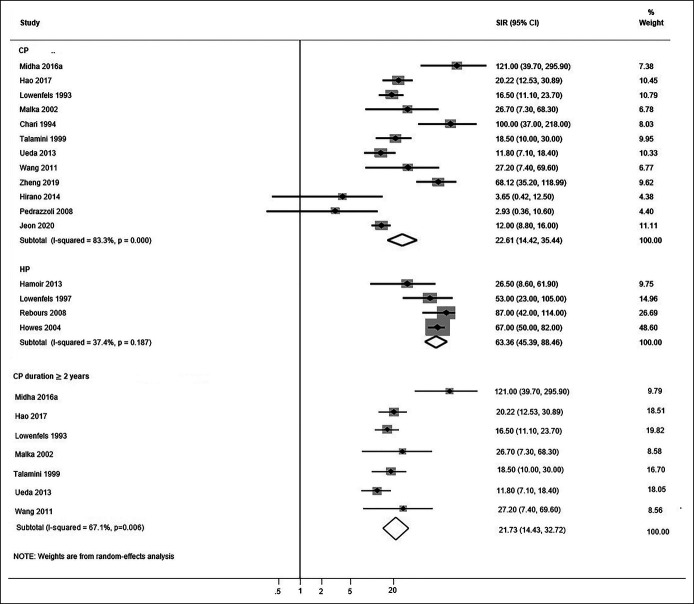
Sixteen studies were included in the meta-analysis (12 studies on CP and 4 studies on HP). Figure 2 depicts forest plots of the association of CP and HP with PDAC.
Figure 2.
Forest plots of the observational studies examining the association between CP, HP, and PDAC risk. CI, confidence interval; CP, chronic pancreatitis; HP, hereditary pancreatitis; PDAC, pancreatic ductal adenocarcinoma; SIR, standardized incidence ratio.
Meta-analysis of CP studies demonstrated that CP was associated with a statistically significant increased risk of PDAC (SIR: 22.61, 95% CI: 14.42–35.44). Heterogeneity among these studies was high (I2 = 83.3%, P < 0.001). Pooling the risk estimates of 4 HP studies yielded an increased risk of PDAC (SIR: 63.36, 95% CI: 45.39–88.46) compared with the non-HP CP studies. Heterogeneity among the HP studies was low (I2 = 37.4%, P = 0.187). Figure 2 also depicts sensitivity analysis on 7 CP studies that excluded patients diagnosed with PDAC within 2 years of CP diagnosis. The pooled risk ratio did not substantially change (SIR: 21.77, 95% CI: 14.43–32.720), and the heterogeneity among these studies was high (I2 = 67.1%, P = 0.006).
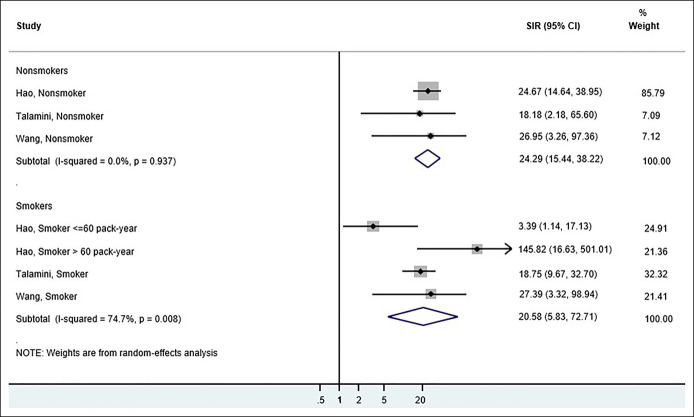
Risk in smokers
Three CP studies compared the risk estimates for PDAC in CP in smokers vs nonsmokers (22,25,26). On pooling the risk estimates, we found no significant difference in SIRs between the 2 groups (Figure 3).
Figure 3.
Forest plots of the observational studies comparing the SIR of pancreatic ductal adenocarcinoma in chronic pancreatitis for smokers vs nonsmokers. CI, confidence interval; SIR, standardized incidence ratio.
Systematic review of single-arm cohort studies and case-control studies
Acute pancreatitis
Although our eligibility criteria included AP, we identified only 1 study that met our a priori inclusion criteria of strict diagnostic ascertainment (38). In this study, the authors concluded that during a median follow-up of 55 months, the risk of future PDAC was significantly higher in patients with AP who eventually developed CP compared with those who did not (incidence rate per 1,000 person-years 9.0 vs 1.1, P = 0.049), indicating a possible association with CP and not standalone AP.
Chronic pancreatitis
We identified 4 single-arm cohort studies on CP that could not be included in the meta-analysis. In the study by Agarwal et al. (40), 2% of patients with CP developed PDAC over a median follow-up of 3.5 years, and smoking was a significant risk factor predicting the development of malignancy (hazard ratio 6.48; CI: 2.2–19.0; P < 0.001). Vujasinovic et al. (44) reported an annual incidence PDAC rate of 0.2% among patients with CP. About 83.3% of patients with CP who developed PDAC were smokers. In the study by Dite et al. (41), all 13 of 223 patients who developed PDAC over 2–11 years of follow-up were smokers. The interval between CP diagnosis and PDAC was <2 years in 1 patient, 2–5 years in 4 patients, 5–10 years in 5 patients, and >10 years in 3 patients. In the study by Sakorafas et al. (39), PDAC was diagnosed in 2.9% of patients undergoing surgery after a mean duration of follow-up of 3.4 years (2 months–11 years). In 1 case-control study (Midha b), 24 CP cases vs 1 control developed PDAC (OR: 97.67, 95% CI: 12.69–751.36) (23).
Autoimmune pancreatitis
In the case-control study by Hart et al. (45), 1 of 116 AIP cases developed PDAC. The single-arm cohort study by Gupta et al. (43) reported 2 cases of PDAC in 84 patients with AIP after a follow-up period of 6 and 10 years. In another single-arm cohort study by Ikeura et al. (42), 3 of 63 patients with AIP developed PDAC.
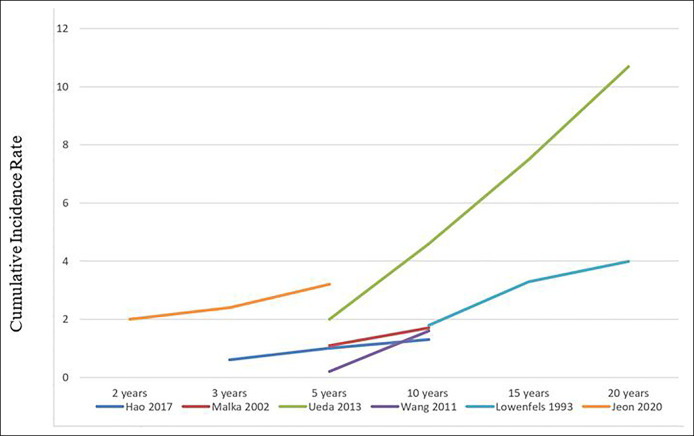
Incidence rate of PDAC during CP follow-up
Six studies reported the CIRs of PDAC over time after the diagnosis of CP (21,22,24,25,29,30). Data were insufficient to conduct meta-regression because of the small number of studies, and data for covariates of interest were not consistently available. Figure 4 demonstrates a trend of increasing CIR with increased duration of CP within each individual study. Zheng et al. reported a rising cumulative incidence of PDAC after surgery for CP: 1.48% at 3 years, 2.63% at 6 years, and 3.71% 9 years after the surgery for CP complications. Talamini et al. also described a rising cumulative probability of PDAC occurrence after the onset of CP.
Figure 4.
Cumulative incidence rate of pancreatic ductal adenocarcinoma after diagnosis of chronic pancreatitis.
Publication bias
Figure 5 depicts the funnel plot to visualize the effect of publication bias in our study. Presence of asymmetry was considered potentially indicative of bias.
Figure 5.
Funnel plot assessing publication bias.
DISCUSSION
Main findings
In this systematic review and meta-analysis, available data confirm higher than average risk of PDAC in patients diagnosed with CP and HP.
The elevated risk of PDAC in patients with CP and HP in our meta-analysis is consistent with results reported in previous studies. In a systematic review on CP and pancreatic cancer by Kirkegard et al. (46), the risk estimates at 2, 5, and 9 years after CP diagnosis were 16.16, 95% CI: 12.59–20.73; 7.90, 95% CI: 4.26–14.66; and 3.53, 95% CI: 1.69–7.38, respectively. In a meta-analysis by Raimondi et al. (7), the pooled estimate of 6 CP studies was 13.3, 95% CI: 6.1–28.9 and the pooled estimate of 3 HP studies was 69, 95% CI: 56.4–84.4. In meta-analysis of 6 CP studies by Tong et al. (47), the pooled estimate was 10.35, 95% CI: 9.13–11.75. Using stringent case definitions as is often necessary in clinical practice, we were able to demonstrate and quantify the risk association between CP and PDAC in this meta-analysis. The risk estimates in our study are higher than previously reported estimates. A possible explanation is the inclusion in previously published reports of studies with unverified exposure and outcome (based on self-reported history or ICD codes), increasing the risk of misclassification bias. A recent meta-analysis of 11 studies on AP by Liu et al. (48) reported a higher risk of PDAC in patients with AP (RR: 2.07, 95% CI: 1.36–2.78). We were unable to perform a meta-analysis assessing the PDAC risk in AP because only 1 study met our stringent inclusion criteria. It remains unclear whether PDAC is associated with standalone AP or limited to the subset where AP is the first manifestation of underlying CP. Studies that use strict diagnostic criteria for AP and limit inclusion to biopsy-proven PDAC are needed to improve our understanding of accurate risk estimates. Similarly, only 1 AIP study (Hirano) reports SIRs; thus, a separate sensitivity analysis could not be performed. Multicenter prospective cohort studies with long-term follow-up are needed to effectively understand PDAC risk in patients with AIP.
Practice implications
PDAC is a lethal malignancy with an advanced-stage clinical presentation at diagnosis; early detection using surveillance provides a chance for improving prognosis. The goal of surveillance is to detect and manage precursor lesions of cancer, although the ability to do so is limited in current clinical practice. Because of a substantially increased risk of PDAC in patients with HP, expert consensus supports initiating pancreas surveillance in these patients at age 40 or 20 years after the first pancreatitis attack, irrespective of gene mutation status (49). However, no clear guidelines for screening exist for the patients with CP without an underlying genetic mutation. Our study indicates that PDAC risk increases with duration of CP likely because of chronicity of inflammation-driven carcinogenesis (50). One could envision a pancreas cancer surveillance strategy in patients with CP similar to inflammatory bowel disease, where colon cancer surveillance starts 8–10 years after diagnosis. For a possible surveillance program for these patients, more data are needed on the timing of development of pancreatic cancer after CP diagnosis. Furthermore, screening patients with CP for PDAC requires enrichment strategies by building multivariable models to identify patients with CP at a higher risk of PDAC and concomitant development of novel biomarkers that can accurately detect PDAC in patients with CP with high accuracy (51).
Strengths and limitations
This systematic review has several strengths. It includes an up-to-date quantitative meta-analysis assessing the association of pancreatitis and PDAC using specific diagnostic criteria for both the disease and the outcome. It avoids the use of patient-reported diagnosis and billing codes and its associated pitfalls. This allows for a more reliable risk assessment and minimizes the risk of misclassification of pancreatitis and PDAC, but some degree of misclassification is unavoidable because the clinical diagnosis of CP and other forms of pancreatitis can be challenging. In addition, PDAC diagnosis in CP is challenging and can be delayed because imaging can miss lesions, given the abnormal nature of the pancreatic parenchyma in CP (52). Excluding patients diagnosed within a short interval after CP diagnosis as was performed in the sensitivity analysis in our study is critical for accurate risk estimation. We also specifically report on CIRs of PDAC during CP follow-up, and our data indicate the potential role of pancreatic cancer screening in long-standing CP. Finally, we conducted a comprehensive search of multiple databases; studies were reviewed by independent reviewers, and the study team included experts in pancreatic diseases and research methodology.
This study has some limitations. A high heterogeneity was seen in the overall pooled SIR in CP studies, limiting the interpretation of the summary estimate. Differences in the study population, covariates assessed, and ability to control for confounding by variables such as smoking, alcohol consumption, and diabetes mellitus might account for this heterogeneity. In addition, CP is a heterogeneous disease with no uniform diagnostic criteria which makes pooling studies challenging. Furthermore, there is likely an overlap between HP and CP because a substantial number of studies report idiopathic CP (before routine genetic testing), and some patients might have an undiagnosed genetic mutation. Most HP studies primarily focused on PRSS1 mutation (3 of the 4 studies), so these data may not represent HP caused by CTRC, SPINK1 and CFTR mutations. There is a risk of selection bias in studies because of variable follow-up periods and loss of patients to follow-up. The risk estimates may be inflated and have low precision in studies with very few patients with exposure and outcome and in studies including patients with complicated CP undergoing surgery. Only 3 studies reported risk estimates in smokers vs nonsmokers; thus, we could not evaluate the effect of smoking on PDAC in patients with CP. Moreover, we do not have a good way to statistically assess publication bias because the funnel plot is not reliable when the number of studies is small or when heterogeneity is present.
In summary, CP is associated with an increased risk of PDAC, and the increased risk persists after excluding potentially incident cases in whom PDAC was diagnosed in close temporal proximity to CP diagnosis. Moreover, the risk of PDAC in CP seems to increase with the duration of disease. The risk association between PDAC and AP and AIP remains poorly defined and warrants further study. Future management guidelines should consider incorporating pancreas cancer surveillance in individuals with long-standing CP.
CONFLICTS OF INTEREST
Guarantor of the article: Shounak Majumder, MD.
Specific author contributions: S.G.: study concept and design, acquisition of data, analysis and interpretation of data, and drafting of manuscript. J.d.l.F: study concept and design, acquisition of data, analysis and interpretation of data, drafting of manuscript, and critical revision of manuscript for important intellectual content. M.H.M.: study concept and design, analysis and interpretation of data, statistical analysis, and critical revision of manuscript for important intellectual content. S.M.: study concept and design, analysis and interpretation of data, drafting of manuscript, critical revision of manuscript for important intellectual content, and study supervision.
Financial support: None to report.
Potential competing interests: Mayo Clinic and Exact Sciences have an intellectual property development agreement. S.M. is listed as an inventor under this agreement and could share potential future royalties paid to Mayo Clinic. The other authors declare no conflict of interest.
Data, analytic methods, and study materials pertinent to this manuscript may be made available to other researchers on reasonable request.
Study Highlights.
WHAT IS KNOWN
✓ There is an increased risk of pancreatic ductal adenocarcinoma (PDAC) in patients with pancreatitis, but the magnitude of association, the risk in different forms of pancreatitis, and association with duration of disease is unclear.
WHAT IS NEW HERE
✓ This up-to-date systematic review and quantitative meta-analysis including studies with verified diagnosis of pancreatitis and pancreatic cancer suggests an increased risk of PDAC in patients with chronic and hereditary forms of pancreatitis.
✓ There are insufficient data to definitively study the risk of PDAC in acute pancreatitis and autoimmune pancreatitis.
✓ In chronic pancreatitis, the PDAC incidence rates increase with duration of disease, indicating possible role of pancreatic cancer screening in patients with long-standing chronic pancreatitis.
Footnotes
SUPPLEMENTARY MATERIAL accompanies this paper at http://links.lww.com/CTG/A764
Contributor Information
Sonal Gandhi, Email: sonalgandhi1894@gmail.com.
Jaime de la Fuente, Email: delafuente.jaime@mayo.edu.
Mohammad Hassan Murad, Email: Murad.Mohammad@mayo.edu.
REFERENCES
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin 2020;70:7–30. [DOI] [PubMed] [Google Scholar]
- 2.Society AC. Cancer Facts & Figures. American Cancer Society: Atlanta, GA, 2016. [Google Scholar]
- 3.Huang J, Lok V, Ngai CH, et al. Worldwide burden of, risk factors for, and trends in pancreatic cancer. Gastroenterology 2021;160:744–54. [DOI] [PubMed] [Google Scholar]
- 4.Owens DK, Davidson KW, Krist AH, et al. Screening for pancreatic cancer: US Preventive Services Task Force reaffirmation recommendation statement. JAMA 2019;322:438–44. [DOI] [PubMed] [Google Scholar]
- 5.Majumder S, Chari ST. Chronic pancreatitis. Lancet 2016;387:1957–66. [DOI] [PubMed] [Google Scholar]
- 6.Ekbom A, McLaughlin JK, Karlsson BM, et al. Pancreatitis and pancreatic cancer: A population-based study. J Natl Cancer Inst 1994;86:625–7. [DOI] [PubMed] [Google Scholar]
- 7.Raimondi S, Lowenfels AB, Morselli-Labate AM, et al. Pancreatic cancer in chronic pancreatitis; aetiology, incidence, and early detection. Best Prac Res Cllin Gastroenterol 2010;24:349–58. [DOI] [PubMed] [Google Scholar]
- 8.Duell E, Lucenteforte E, Olson S, et al. Pancreatitis and pancreatic cancer risk: A pooled analysis in the International Pancreatic Cancer Case-Control Consortium (PanC4). Ann Oncol 2012;23:2964–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Algül H, Treiber M, Lesina M, et al. Mechanisms of disease: Chronic inflammation and cancer in the pancreas—A potential role for pancreatic stellate cells? Nat Clin Pract Gastroenterol Hepatol 2007;4:454–62. [DOI] [PubMed] [Google Scholar]
- 10.Hart PA, Zen Y, Chari ST. Recent advances in autoimmune pancreatitis. Gastroenterology 2015;149:39–51. [DOI] [PubMed] [Google Scholar]
- 11.Majumder S, Takahashi N, Chari ST. Autoimmune pancreatitis. Dig Dis Sci 2017;62:1762–9. [DOI] [PubMed] [Google Scholar]
- 12.Li S, Tian B. Acute pancreatitis in patients with pancreatic cancer: Timing of surgery and survival duration. Medicine (Baltimore) 2017;96:e5908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shamseer L, Moher D, Clarke M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: Elaboration and explanation. BMJ 2015;350:g7647. [DOI] [PubMed] [Google Scholar]
- 14.Banks PA, Bollen TL, Dervenis C, et al. Classification of acute pancreatitis—2012: Revision of the Atlanta classification and definitions by international consensus. Gut 2013;62:102–11. [DOI] [PubMed] [Google Scholar]
- 15.Shimosegawa T, Chari ST, Frulloni L, et al. International consensus diagnostic criteria for autoimmune pancreatitis: Guidelines of the International Association of Pancreatology. Pancreas 2011;40:352–8. [DOI] [PubMed] [Google Scholar]
- 16.Chari ST. Diagnosis of autoimmune pancreatitis using its five cardinal features: Introducing the Mayo Clinic's HISORt criteria. J Gastroenterol 2007;42(Suppl 18):39–41. [DOI] [PubMed] [Google Scholar]
- 17.Wells G, Shea B, O'Connell D, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. 2014. (http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp). Accessed January 30, 2021.
- 18.Murad MH, Sultan S, Haffar S, et al. Methodological quality and synthesis of case series and case reports. BMJ Evid Based Med 2018;23:60–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986;7:177–88. [DOI] [PubMed] [Google Scholar]
- 20.Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ 2003;327:557–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jeon CY, Chen Q, Yu W, et al. Identification of individuals at increased risk for pancreatic cancer in a community-based cohort of patients with suspected chronic pancreatitis. Clin Transl Gastroenterol 2020;11:e00147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hao L, Zeng XP, Xin L, et al. Incidence of and risk factors for pancreatic cancer in chronic pancreatitis: A cohort of 1656 patients. Dig Liver Dis 2017;49:1249–56. [DOI] [PubMed] [Google Scholar]
- 23.Midha S, Sreenivas V, Kabra M, et al. Genetically determined chronic pancreatitis but not alcoholic pancreatitis is a strong risk factor for pancreatic cancer. Pancreas 2016;45:1478–84. [DOI] [PubMed] [Google Scholar]
- 24.Ueda J, Tanaka M, Ohtsuka T, et al. Surgery for chronic pancreatitis decreases the risk for pancreatic cancer: A multicenter retrospective analysis. Surgery 2013;153:357–64. [DOI] [PubMed] [Google Scholar]
- 25.Wang W, Liao Z, Li G, et al. Incidence of pancreatic cancer in Chinese patients with chronic pancreatitis. Pancreatology 2011;11:16–23. [DOI] [PubMed] [Google Scholar]
- 26.Talamini G, Falconi M, Bassi C, et al. Incidence of cancer in the course of chronic pancreatitis. Am J Gastroenterol 1999;94:1253–60. [DOI] [PubMed] [Google Scholar]
- 27.Zheng Z, Chen Y, Tan C, et al. Risk of pancreatic cancer in patients undergoing surgery for chronic pancreatitis. BMC Surg 2019;19:83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chari ST, Mohan V, Pitchumoni CS, et al. Risk of pancreatic carcinoma in tropical calcifying pancreatitis: An epidemiologic study. Pancreas 1994;9:62–6. [DOI] [PubMed] [Google Scholar]
- 29.Malka D, Hammel P, Maire F, et al. Risk of pancreatic adenocarcinoma in chronic pancreatitis. Gut 2002;51:849–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lowenfels AB, Maisonneuve P, Cavallini G, et al. Pancreatitis and the risk of pancreatic cancer. International Pancreatitis Study Group. N Engl J Med 1993;328:1433–7. [DOI] [PubMed] [Google Scholar]
- 31.Pedrazzoli S, Pasquali C, Guzzinati S, et al. Survival rates and cause of death in 174 patients with chronic pancreatitis. J Gastrointest Surg 2008;12:1930–7. [DOI] [PubMed] [Google Scholar]
- 32.Rocca G, Gaia E, Iuliano R, et al. Increased incidence of cancer in chronic pancreatitis. J Clin Gastroenterol 1987;9:175–9. [DOI] [PubMed] [Google Scholar]
- 33.Hirano K, Tada M, Sasahira N, et al. Incidence of malignancies in patients with IgG4-related disease. Intern Med 2014;53:171–6. [DOI] [PubMed] [Google Scholar]
- 34.Hamoir C, Pepermans X, Piessevaux H, et al. Clinical and morphological characteristics of sporadic genetically determined pancreatitis as compared to idiopathic pancreatitis: Higher risk of pancreatic cancer in CFTR variants. Digestion 2013;87:229–39. [DOI] [PubMed] [Google Scholar]
- 35.Rebours V, Boutron-Ruault MC, Schnee M, et al. Risk of pancreatic adenocarcinoma in patients with hereditary pancreatitis: A national exhaustive series. Am J Gastroenterol 2008;103:111–9. [DOI] [PubMed] [Google Scholar]
- 36.Howes N, Lerch MM, Greenhalf W, et al. Clinical and genetic characteristics of hereditary pancreatitis in Europe. Clin Gastroenterol Hepatol 2004;2:252–61. [DOI] [PubMed] [Google Scholar]
- 37.Lowenfels AB, Maisonneuve P, DiMagno EP, et al. Hereditary pancreatitis and the risk of pancreatic cancer. International Hereditary Pancreatitis Study Group. JNCI 1997;89:442–6. [DOI] [PubMed] [Google Scholar]
- 38.Rijkers AP, Bakker OJ, Ahmed Ali U, et al. Risk of pancreatic cancer after a primary episode of acute pancreatitis. Pancreas 2017;46:1018–22. [DOI] [PubMed] [Google Scholar]
- 39.Sakorafas GH, Sarr MG. Pancreatic cancer after surgery for chronic pancreatitis. Dig Liver Dis 2003;35:482–5. [DOI] [PubMed] [Google Scholar]
- 40.Agarwal S, Sharma S, Gunjan D, et al. Natural course of chronic pancreatitis and predictors of its progression. Pancreatology 2020;14:14. [DOI] [PubMed] [Google Scholar]
- 41.Dite P, Novotny I, Precechtelova M, et al. Incidence of pancreatic carcinoma in persons with chronic pancreatitis [in Czech]. Vnitr Lek 2009;55:18–21. [PubMed] [Google Scholar]
- 42.Ikeura T, Miyoshi H, Uchida K, et al. Relationship between autoimmune pancreatitis and pancreatic cancer: A single-center experience. Pancreatology 2014;14:373–9. [DOI] [PubMed] [Google Scholar]
- 43.Gupta R, Khosroshahi A, Shinagare S, et al. Does autoimmune pancreatitis increase the risk of pancreatic carcinoma? A retrospective analysis of pancreatic resections. Pancreas 2013;42:506–10. [DOI] [PubMed] [Google Scholar]
- 44.Vujasinovic M, Dugic A, Maisonneuve P, et al. Risk of developing pancreatic cancer in patients with chronic pancreatitis. J Clin Med 2020;9:3720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hart PA, Law RJ, Dierkhising RA, et al. Risk of cancer in autoimmune pancreatitis: A case-control study and review of the literature. Pancreas 2014;43:417–21. [DOI] [PubMed] [Google Scholar]
- 46.Kirkegård J, Mortensen FV, Cronin-Fenton D. Chronic pancreatitis and pancreatic cancer risk: A systematic review and meta-analysis. Am J Gastroenterol 2017;112:1366–72. [DOI] [PubMed] [Google Scholar]
- 47.Tong GX, Geng QQ, Chai J, et al. Association between pancreatitis and subsequent risk of pancreatic cancer: A systematic review of epidemiological studies. Asian Pac J Cancer Prev 2014;15:5029–34. [DOI] [PubMed] [Google Scholar]
- 48.Liu J, Wang Y, Yu Y. Meta-analysis reveals an association between acute pancreatitis and the risk of pancreatic cancer. World J Clin Cases 2020;8:4416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Goggins M, Overbeek KA, Brand R, et al. Management of patients with increased risk for familial pancreatic cancer: Updated recommendations from the International Cancer of the Pancreas Screening (CAPS) Consortium. Gut 2020;69:7–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kolodecik T, Shugrue C, Ashat M, et al. Risk factors for pancreatic cancer: Underlying mechanisms and potential targets. Front Physiol 2013;4:415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Singhi AD, Koay EJ, Chari ST, et al. Early detection of pancreatic cancer: Opportunities and challenges. Gastroenterology 2019;156:2024–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Raman SP, Horton KM, Fishman EK. Multimodality imaging of pancreatic cancer—Computed tomography, magnetic resonance imaging, and positron emission tomography. Cancer J 2012;18:511–22. [DOI] [PubMed] [Google Scholar]