INTRODUCTION:
Overt hepatic encephalopathy (HE) is a major complication of transjugular intrahepatic portosystemic shunt (TIPS). This study aimed to develop and validate prognostic models to identify patients at different risks of overt HE within 3 months after TIPS.
METHODS:
Two cohorts of patients with cirrhosis undergoing TIPS insertion were retrospectively included. In the derivation cohort of 276 patients, 3 models were established in increasing order of complexity: core model (age + Child-Pugh class), sarcopenia model (core model + sarcopenia), and full model (sarcopenia model + post-TIPS portal pressure gradient). All models were internally validated for discrimination and calibration and externally validated in an independent cohort of 182 patients.
RESULTS:
During a 3-month follow-up period, 61 (22.1%) and 33 patients (18.1%) developed overt HE in the derivation and validation cohort, and sarcopenia was associated with increased risk of the outcome. In the derivation cohort, the core model showed a c-statistic of 0.68 (95% confidence interval [CI] 0.61–0.75), and discrimination improved in the sarcopenia model (c-statistic 0.73; 95% CI 0.66–0.80). The full model that extended the core model with inclusion of sarcopenia and post-TIPS portal pressure gradient showed a significant improvement in discriminative ability (0.77; 95% CI 0.71–0.83; P = 0.001). Both sarcopenia and full model yielded comparable performances in the validation cohort.
DISCUSSION:
We developed and externally validated 2 prediction models applied before (sarcopenia model) and after TIPS (full model) to estimate the risk of post-TIPS overt HE. These tools could aid to select appropriate candidates for TIPS and guide postoperative management.
INTRODUCTION
Overt hepatic encephalopathy (HE) is one of the major complications of the transjugular intrahepatic portosystemic shunt (TIPS) procedure (1). As previously reported, the 1-year cumulative incidence of post-TIPS overt HE ranges from 10% to 50% (2) and reaches to the highest level within 3 months (3). Because only 2 available randomized controlled trials achieved controversial results regarding the efficacy of drug prophylaxis (4,5), identification of patients at different risks and appropriate case selection for TIPS insertion remain the most effective approach in the prevention of this complication (2,4).
The pathogenesis of HE is multifactorial, and hyperammonemia plays a central role, which is related to abnormal ammonia metabolism in various organs (6,7). On the one hand, urea synthesis in the liver is the main route of ammonia catabolism (8). Thus, declined liver detoxification function is one of the major mechanisms responsible for hyperammonemia. On the other hand, for patients receiving TIPS, the shunt of portal blood into the systemic circulation further reduces hepatic clearance of gut-derived neurotoxins and contributes to ammonia accumulation. More recently, sarcopenia was suggested as a strong predictor of post-TIPS overt HE independent of the severity of cirrhosis (9,10), but the evidence was mainly derived from studies with small sample size and requires further validation (11,12).
Several risk factors for postprocedural overt HE have been identified (13–16), and a few risk prediction models were proposed (17–19), but their use in clinical practice was limited. Contributory reasons may be that they were not specifically developed for post-TIPS overt HE (17), included only demographic and laboratory data without taking the effect of nutritional status and degree of diversion into account, and lack of external validation (18,19). Therefore, the first aim of this study was to validate the correlation between sarcopenia and post-TIPS overt HE in a relatively large cohort of patients with cirrhosis, and the second aim was to develop and validate easy-to-perform tools to accurately estimate the risk of the outcome within 3 months after TIPS implantation.
MATERIALS AND METHODS
Study design and data source
The derivation cohort retrospectively included patients from a prospective database of consecutive patients admitted to Wuhan Union Hospital to receive TIPS treatment from January 2016 to November 2020. Baseline data regarding demographic characteristics, laboratory tests, and radiological findings were collected during hospitalization. Details of treatment were retrieved from electronic medical records. The follow-up at outpatient was scheduled for patients at 1, 3, 6, and 12 months and then annually after the TIPS procedure, supplemented with telephone interviews every 3 months. Patients were followed until death, liver transplantation, or the end of the study (July 2021), and data were censored at the end of follow-up. The validation cohort retrospectively included patients admitted to the First Affiliated Hospital of Soochow University to undergo TIPS from November 2015 to July 2020, and the data collection and follow-up protocol were in accordance with the derivation cohort.
Patients with a confirmed diagnosis of cirrhosis (clinical, radiological, and/or histologic) were considered eligible for this study. We excluded patients with previous TIPS insertion, previous episode of overt HE, advanced hepatocellular carcinoma outside Milan criterial or other extrahepatic malignancy, and who lost to follow-up within 3 months. Besides, patients with alcohol consumption within 1 month before the procedure, psychoactive drug that may cause cognitive dysfunction and unrelated neurological diseases were also excluded.
Variable selection and definition
We searched for candidate predictors of post-TIPS HE that were repeatedly highlighted in published studies, and variables were considered if they (i) can be easily ascertained in different clinical settings and (ii) are part of routine evaluations for the TIPS procedure. Finally, 12 candidate variables were identified: age, etiology of cirrhosis, premorbid diabetes mellitus, Child-Pugh score/class, model for end-stage liver disease (MELD) score, MELD-sodium score, serum creatinine level, serum albumin level, natremia, sarcopenia, and post-TIPS portal pressure gradient (PPG).
Enhanced computed tomography (CT) was performed as a standard of care within 3 days before TIPS to assess the portal system and to facilitate anatomical orientation. Meanwhile, CT is regarded as the optimal tool for the detection of muscle depletion (20). A transverse CT image at the level of the third lumbar vertebra (L3) was selected to measure the cross-sectional skeletal muscle area (see Supplementary Figure 1, Supplementary Digital Content 1, http://links.lww.com/CTG/A762). Then, the skeletal muscle area was normalized for stature to obtain the skeletal muscle index (SMI) in square centimeters per square meter. Sarcopenia was defined according to previously validated cutoffs: L3-SMI <39 cm2/m2 for female patients and <50 cm2/m2 for male patients (21).
Outcome measure
The primary outcome of the study was the development of overt HE within 3 months after TIPS creation. The spectrum of cognitive impairment occurred in a continuum and was subdivided into 5 grades according to the West Haven criteria, and only a grade Ⅱ or higher was regarded as an episode of overt HE (2).
Model development
The association between the potential variables and the outcome was analyzed by fitting logistic regression models, with calculation of odds ratios and 95% confidence intervals (CIs) to quantify prognostic strength for each variable. To further explore the linearity in the effect of sarcopenia, restricted cubic splines were applied by entering L3-SMI as a continuous variable into the logistic analysis.
We categorized the following continuous variables with clinically validated cutoffs because they showed a nonlinear relation with the outcome: creatinine (1.5 mg/dL), albumin (25 g/L), and natremia (130 mmol/L). Afterward, all candidate variables were included to fit the full logistic regression model, with least absolute shrinkage and selection operator to exclude weaker covariates. After simplification, the most predictive variables were age, Child-Pugh class, sarcopenia, and post-TIPS PPG. We thereby established 3 models in increasing order of complexity for the application in a different clinical context. The first model (core model) included only clinical data (age and Child-Pugh class). The second model (sarcopenia model) was built based on the core model supplemented with a nutritional parameter (sarcopenia), and the third model (full model) was developed by extending the sarcopenia model to include post-TIPS PPG.
Model performance and validation
The performance of prediction models was assessed for discrimination and calibration. Discrimination was evaluated with the Harrell c-statistic. To further investigate the added predictive ability of additional predictors to a pre-existing model, we conducted reclassification analyses between 2 models and calculated the net reclassification index (NRI) (22). Calibration was evaluated graphically with a calibration plot and statistically by computing the Hosmer-Lemeshow test and Brier score. Calibration slopes and calibration-in-large were measured and added to the calibration plot. Moreover, we evaluated the overall performance of the models with the R2 statistic and Akaike information criterion (AIC). A higher R2 value and lower AIC indicate better performance. Clinical usefulness and net benefit of these tools were estimated with decision curve analysis (23).
To assess the stability of the developed tools to random changes in sample composition, internal validation using a bootstrap resampling method with 1,000 replicates was performed. In addition, these tools were further applied to the validation cohort to assess model performance in various populations.
Sensitivity analysis
Three sets of sensitivity analyses were conducted to verify the robustness of our results. First, subgroup analysis according to sex, TIPS indication, etiology, pre-existing portal vein thrombosis, and balloon size was conducted to examine whether there was heterogeneity in the predictive ability of the tools. Second, we performed a case-wise deletion analysis by omitting cases with missing values and compared the results with that of our main analysis, which used multiple imputations to replace missing data. Finally, considering the possible influence of competing events on the occurrence of overt HE when applying a logistic regression model, the Fine and Gray subdistributional hazards model was used to refit the 3 models with the same parameters determined in the main analysis and the coefficient of each variable was compared with that of the original model.
Data processing
For our main analysis, multiple imputations with chained equations were performed to replace missing values for body height, laboratory results, and pre-TIPS PPG/post-TIPS PPG, with 10 imputations to correct for bias. The significance level was set at 5%. All analyses were performed using IBM SPSS (version 25.0; IBM SPSS, Armonk, NY) and R software (version 4.0.3) with the add-on packages MICE, rcs, cmprsk, and nricens. This study adhered to the Transparent Reporting of a Multivariable Prediction Model for Individual Prognosis Or Diagnosis, TRIPOD statement for reporting (24).
RESULTS
Study population and main outcome
Three hundred eighty-three consecutive patients undergoing TIPS implantation were retrospectively screened, and 276 (72%) patients were included to constitute the derivation cohort (Figure 1), with a mean (SD) age of 54.6 years (11.5) and a sex distribution of 65.6% males. The etiology of cirrhosis was related to virus infection in 67.8% of the population (187/276) and to alcohol in 8.7% (24/276). Descriptive clinical characteristics are presented in Table 1.
Figure 1.
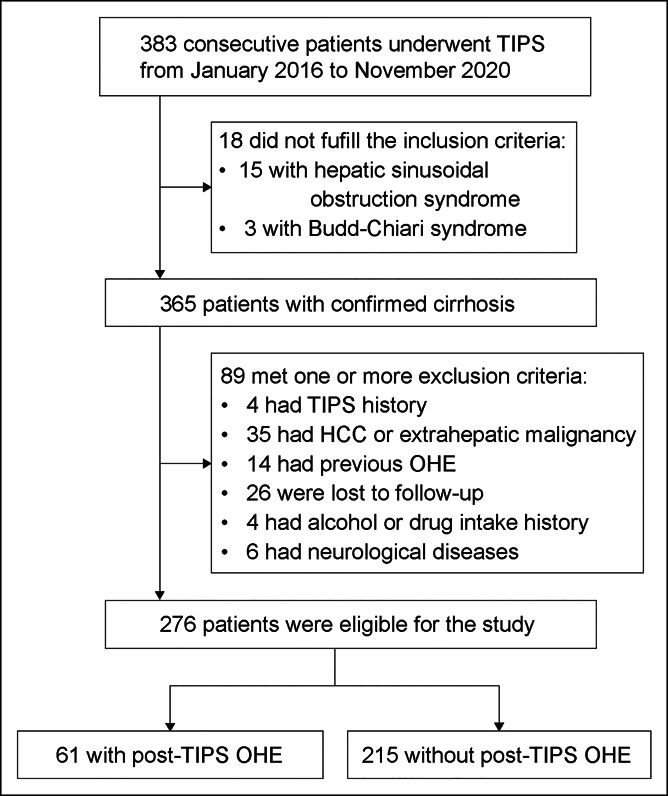
Flow chart of the patient selection protocol. HCC, hepatocellular carcinoma; OHE, overt hepatic encephalopathy; TIPS, transjugular intrahepatic portosystemic shunt.
Table 1.
Demographics and baseline characteristics of the derivation cohort
| Total (n = 276) | Without overt HE (n = 215) | With overt HE (n = 61) | P | |
| Demographics and clinical characteristics | ||||
| Age (per year) | 54.6 (11.5) | 53.4 (11.2) | 58.6 (11.9) | 0.003 |
| Sex (male) | 181 (65.6) | 138 (64.2) | 43 (70.5) | 0.446 |
| Etiology of cirrhosis | 0.769 | |||
| Virus-related | 187 (67.8) | 148 (68.8) | 39 (63.9) | |
| Alcohol-related | 24 (8.7) | 18 (8.37) | 6 (9.84) | |
| Others | 65 (23.6) | 49 (22.8) | 16 (26.2) | |
| TIPS indication | 0.529 | |||
| Variceal bleeding | 248 (89.9) | 195 (90.7) | 53 (86.9) | |
| Refractory ascites | 28 (10.1) | 20 (9.30) | 8 (13.1) | |
| Diabetes mellitus | 47 (17.0) | 35 (16.3) | 12 (19.7) | 0.668 |
| L3 SMI (cm2/m2) | ||||
| Male | 45.7 (7.7) | 46.6 (7.8) | 43.0 (6.6) | 0.004 |
| Female | 38.8 (7.1) | 40.3 (7.1) | 37.5 (7.1) | 0.342 |
| Sarcopenia (yes) | 170 (61.6) | 123 (57.2) | 47 (77.0) | 0.008 |
| Child-Pugh score | 7.68 (1.67) | 7.53 (1.69) | 8.22 (1.51) | 0.003 |
| Child-Pugh class | 0.006 | |||
| A | 63 (22.8) | 58 (27.0) | 5 (8.2) | |
| B | 177 (64.1) | 131 (60.9) | 46 (75.4) | |
| C | 32 (11.6) | 22 (10.2) | 10 (16.4) | |
| MELD | 11.8 (3.86) | 11.4 (3.48) | 13.0 (4.82) | 0.022 |
| MELD-Na | 12.6 (4.77) | 12.2 (4.58) | 13.8 (5.23) | 0.038 |
| Laboratory parameters | ||||
| Bilirubin (μmol/L) | 28.0 (29.3) | 27.2 (28.7) | 30.5 (31.6) | 0.468 |
| Albumin (g/L) | 30.7 (5.69) | 31.2 (5.78) | 28.9 (4.98) | 0.002 |
| ALT (U/L) | 37.5 (76.5) | 38.6 (85.3) | 33.6 (29.5) | 0.477 |
| AST (U/L) | 49.4 (76.5) | 48.5 (81.7) | 52.4 (54.8) | 0.672 |
| Creatinine (μmol/L) | 75.8 (62.4) | 70.5 (30.7) | 99.3 (59) | 0.029 |
| Sodium (mmol/L) | 139 (4.80) | 138 (4.81) | 139 (4.74) | 0.378 |
| Prothrombin time (s) | 16.8 (2.65) | 16.6 (2.63) | 17.4 (2.67) | 0.038 |
| INR | 1.39 (0.29) | 1.37 (0.28) | 1.45 (0.28) | 0.040 |
| Hemoglobin (g/L) | 80.8 (22.2) | 80.9 (22.0) | 80.7 (23.1) | 0.949 |
| Platelet count (×109/L) | 102 (83.6) | 104 (87.1) | 95.3 (69.5) | 0.428 |
| Radiological findings | ||||
| Ascites | 0.249 | |||
| Mild | 101 (36.6) | 80 (37.2) | 21 (34.4) | |
| Moderate | 47 (17.0) | 37 (17.2) | 10 (16.4) | |
| Severe | 79 (28.6) | 56 (26.1) | 23 (37.7) | |
| Portal vein thrombosis | 90 (32.6) | 72 (33.5) | 18 (29.5) | 0.667 |
| TIPS procedure | ||||
| Embolization | 168 (60.9) | 138 (64.2) | 30 (49.2) | 0.049 |
| Pre-PPG | 27.6 (5.47) | 28.1 (5.45) | 25.9 (5.26) | 0.013 |
| Post-PPG | 11.7 (3.76) | 12.0 (3.79) | 10.3 (3.30) | 0.002 |
| PPG change | 15.9 (4.28) | 16.0 (4.23) | 15.6 (4.51) | 0.540 |
Data are presented as mean (SD) or n (%) unless otherwise specified.
ALT, alanine aminotransferase; AST, aspartate aminotransferase; HE, hepatic encephalopathy; INR, international normalized ratio; MELD, model for end-stage liver disease; MELD-Na, model for end-stage liver disease-sodium; PPG, portal pressure gradient; SMI, skeletal muscle index; TIPS, transjugular intrahepatic portosystemic shunt.
During a follow-up period of 3 months, 61 patients (22.1%) in the derivation cohort experienced at least 1 episode of overt HE after TIPS creation. Compared with patients who did not develop post-TIPS overt HE, patients with the outcome were older, had worse liver function (higher Child-Pugh score and MELD score), and had a lower PPG before and after the procedure (Table 1).
Association between sarcopenia and post-TIPS overt HE
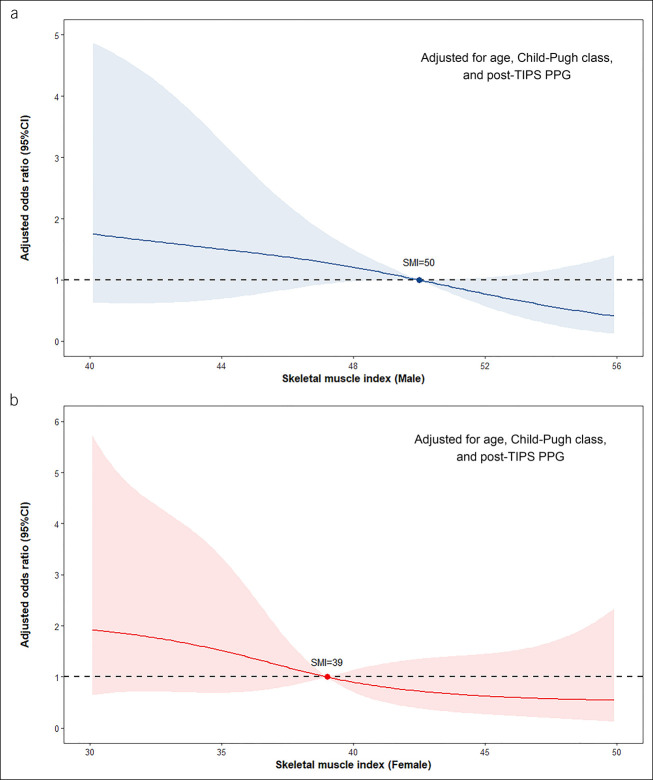
In the derivation cohort, basal sarcopenia according to L3-SMI cutoffs was detected in 170 patients (61.6%). Compared with those without sarcopenia, patients with sarcopenia were more frequently male, with a higher Child-Pugh score and with a higher percentage of premorbid ascites. In addition, the presence of sarcopenia was associated with increased risk of post-TIPS overt HE (odds ratio 3.66; 95% CI 1.87–7.77; P < 0.001). Figure 2 shows the continuous correlation between L3-SMI and risk of the outcome stratified by sex. For male patients, the effect of SMI could be fitted almost as a linear relation because the risk of overt HE increased parallel to the decrease in SMI, and the risk was approximately twice as the reference when SMI decreased from 50 to 35 cm2/m2. A similar pattern was presented in the female subgroup, but this relation was not apparent when SMI improved to normal.
Figure 2.
Continuous association between SMI and risk of post-TIPS overt HE stratified by sex. (a) Male subgroup and (b) female subgroup. ORs were calculated with reference (OR = 1) to SMI = 50 in male patients and 39 in female patients. Restricted cubic splines were fitted using logistic regression models adjusted for age, Child-Pugh class, and post-TIPS PPG. CI, confidence interval; HE, hepatic encephalopathy; OR, odds ratio; PPG portal pressure gradient; SMI, skeletal muscle index; TIPS, transjugular intrahepatic portosystemic shunt.
Model performance and internal validation
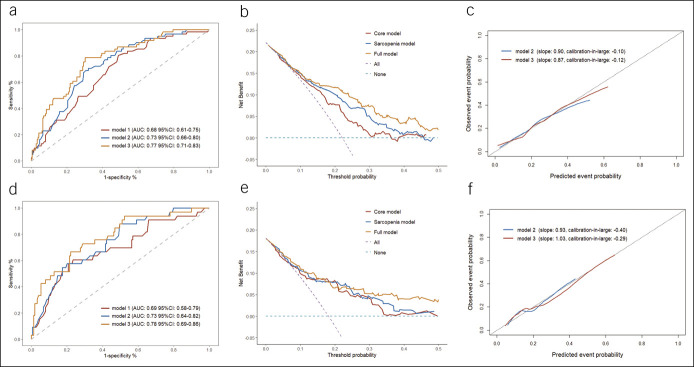
In the derivation cohort, the core model fitted with age and Child-Pugh class showed a c-statistic of 0.68 (95% CI 0.61–0.75), an AIC of 278, and an R2 of 11.2%. The sarcopenia model was built based on the core model supplemented with sarcopenia, which showed an improvement in model performance (c-statistic 0.73 [95% CI 0.66–0.80]; AIC 268; R2 17.4%). When including post-TIPS PPG to establish the full model, all predictors showed statistical significance in multivariable analysis, and they collectively explained 23.6% of the variation in the outcome. Compared with the core model, inclusion of sarcopenia and post-TIPS PPG achieved a substantial improvement in discrimination (c-statistic 0.77; 95% CI 0.71–0.83; P = 0.001) (Table 2 and Figure 3a).
Table 2.
Performance of 3 prediction models in the derivation cohort
| Indices | Model 1 | Model 2 | Model 3 |
| Discrimination | |||
| c-statistic | 0.68 (0.61 to 0.75) | 0.73 (0.66 to 0.80) | 0.77 (0.71 to 0.83) |
| NRIa | |||
| NRI for events | Reference | 3.3 (−14.6 to 29.3) | 16.4 (−7.7 to 41.6) |
| NRI for nonevents | Reference | 9.7 (−6.4 to 28.8) | 12.6 (−3.5 to 31.7) |
| Overall NRI | Reference | 13.0 (−4.5 to 33.4) | 29.0 (6.8 to 56.6) |
| P | 0.079 | 0.002 | |
| Calibration | |||
| Brier score | 0.16 (0.13 to 0.18) | 0.15 (0.13 to 0.18) | 0.14 (0.12 to 0.17) |
| Pb | 0.83 | 0.66 | 0.52 |
| Overall performance | |||
| R2 statistic (%) | 11.2 | 17.4 | 23.6 |
| AIC | 278 | 268 | 257 |
Model 1/2/3 indicates the core/sarcopenia/full model.
AIC, Akaike information criterion; NRI, net reclassification improvement.
NRI was calculated with reference to model 1 (model 2/model 3 vs model 1).
P value was calculated by the Hosmer-Lemeshow goodness-of-fit test.
Figure 3.
Model performance in the derivation and validation cohort. (a–c) Model performance in the derivation cohort. (d–f) Model performance in the validation cohort. (a and d) A receiver operating characteristic curve for the models to predict post-TIPS overt HE. (b and e) Calibration plots showed the predicted event probability against the observed probability. (c and f) Decision curve analysis for the outcome prediction. AUC, area under the receiver operating characteristic curve; CI, confidence interval; HE, hepatic encephalopathy; TIPS, transjugular intrahepatic portosystemic shunt.
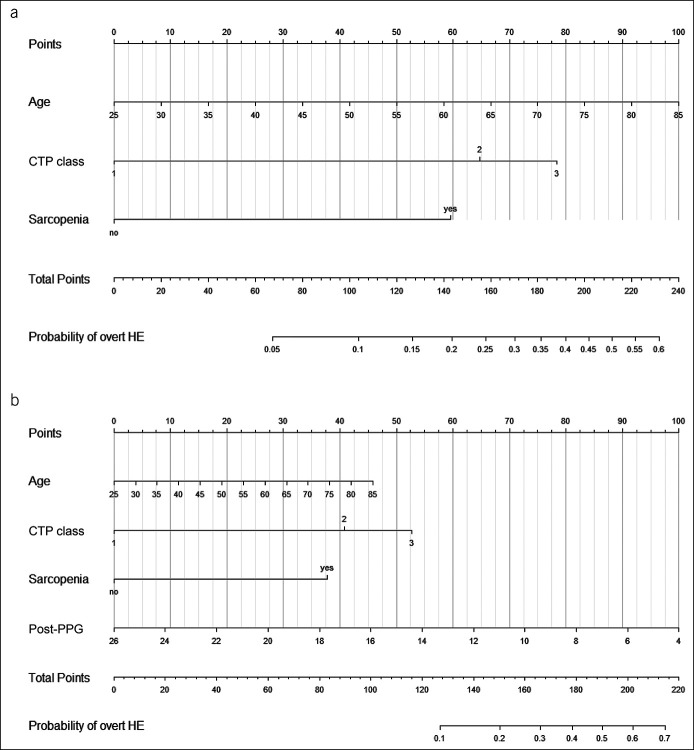
The added value of sarcopenia and post-TIPS PPG was confirmed in reclassification analyses. When the sarcopenia model was used as the reclassifying model to compare with the core model, the NRI for events (with post-TIPS overt HE) was 3.3% and for nonevents (without post-TIPS overt HE) was 9.7%, with an overall NRI of 13.0% (95% CI −4.5% to 33.4%; P = 0.079), suggesting that 13% of the patients misclassified by the core model were classified correctly by the sarcopenia model. Although the comparison was conducted between the core model and full model, the overall NRI improved markedly (29.0% [95% CI 6.8%–56.6%]; P = 0.002), confirming the additional ability gained from sarcopenia and post-TIPS PPG (Table 2). When decision curve analysis was performed, the full model provided the largest net benefit across the range of threshold probabilities for the outcome (Figure 3b). Subsequently, 2 nomograms based on the sarcopenia model and full model were created (Figure 4), and regression coefficients of corresponding tools were provided to support independent validation (Table 3).
Figure 4.
Nomograms to predict the risk of post-TIPS overt HE. (a) A nomogram based on the sarcopenia model. (b) A nomogram based on the full model. Each independent predictor associated with the outcome was assigned a weighted score using the regression coefficient from the multivariable analysis. When using the nomogram, first draw a vertical line to the Points row to assign corresponding points for each variable, then add the points from each variable, and drop a vertical line from the Total Points row to the bottom row to obtain the probability of developing overt HE. CTP, Child-Turcotte-Pugh; HE, hepatic encephalopathy; PPG, portal pressure gradient; TIPS, transjugular intrahepatic portosystemic shunt.
Table 3.
OR and regression coefficients of each variable in 3 models
| Variable | Core model | Sarcopenia modela | Full modelb | |||
| OR (95% CI) | Coefficient | OR (95% CI) | Coefficient | OR (95% CI) | Coefficient | |
| Intercept | — | −4.36 | — | −5.14 | — | −2.98 |
| Age | 1.03 (1.01–1.06) | 0.03 | 1.03 (1.01–1.06) | 0.03 | 1.03 (1.01–1.06) | 0.03 |
| Child-Pugh class | ||||||
| B | 4.21 (1.59–14.5) | 1.44 | 3.65 (1.36–12.7) | 1.30 | 3.45 (1.26–12.2) | 1.24 |
| C | 5.82 (1.67–23.8) | 1.76 | 4.85 (1.37–20.2) | 1.58 | 5.38 (1.46–23.1) | 1.68 |
| Sarcopenia | — | — | 3.40 (1.70–7.33) | 1.22 | 3.83 (1.87–8.43) | 1.34 |
| Post-TIPS PPG | — | — | — | — | 0.84 (0.76–0.92) | −0.17 |
CI, confidence interval; OR, odds ratio; PPG, portal pressure gradient; TIPS, transjugular intrahepatic portosystemic shunt.
The sarcopenia model was calculated with the formula: 0.03 × age + (0 × CP-A/1.30 × CP-B/1.58 × CP-C) + 1.22 × sarcopenia (yes) − 5.14.
The full model was calculated with the formula: 0.03 × age + (0 × CP-A/1.24 × CP-B/1.68 × CP-C) + 1.34 × sarcopenia (yes) −2.98.
Three prediction models demonstrated good performance in calibration, with a Brier score of 0.16, 0.15, and 0.14 and a Hosmer-Lemeshow P value of 0.83, 0.66, and 0.52, respectively (Table 2). Similarly, calibration plots showed fair agreement between predicted and observed probabilities, particularly in the low-risk subgroup (Figure 3c). Internal bootstrap validation indicated only minimal overfitting (optimism-corrected c-statistic: 0.66, 0.72, and 0.75; optimism-corrected Brier score: 0.16, 0.16, and 0.15).
External validation
The validation cohort included 182 patients from 218 consecutive patients admitted for TIPS treatment, in which the inclusion and exclusion criteria were in accordance with the derivation cohort. Compared with the derivation cohort, the validation cohort included patients with older age, higher incidence of malnutrition, and a lower Child-Pugh score and MELD score. In addition, the proportion of patients who reached the outcome was lower (18.1%). Baseline data of the validation cohort are presented in Supplementary Table 1 (Supplementary Digital Content 2, http://links.lww.com/CTG/A763).
Three prediction tools performed well in the validation cohort. The sarcopenia model yielded a comparable discrimination (c-statistic 0.73, 95% CI 0.64–0.82), while the core model and full model achieved slightly higher c-statistic (0.69 [95% CI 0.58–0.79] in the core model and 0.78 [95% CI 0.68–0.86] in full model) (Figure 3d and Supplementary Table 2, Supplementary Digital Content 2, http://links.lww.com/CTG/A763). The added value of sarcopenia and post-TIPS PPG was confirmed in the reclassification analysis and decision curve analysis (Figure 3e). Besides, all models showed satisfactory calibration in the validation cohort (Figure 3f).
Sensitivity analyses
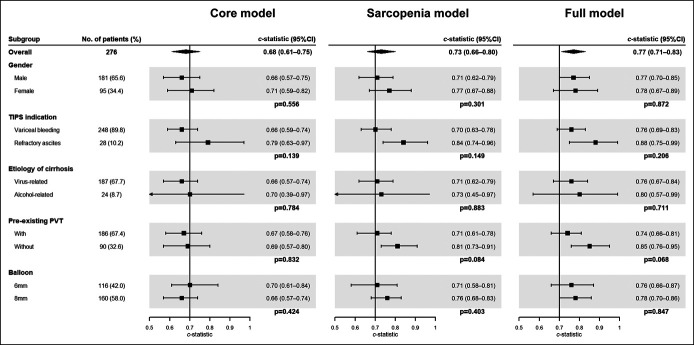
In the subgroup analysis, 3 models showed higher discrimination in the subgroup of female patients with alcoholic cirrhosis, with pre-existing portal vein thrombosis and treated for refractory ascites, although the difference between the subgroups was not significant (Figure 5). The complete case analysis achieved similar results to that of our main analysis (see Supplementary Table 3, Supplementary Digital Content 2, http://links.lww.com/CTG/A763). Furthermore, similar model coefficients obtained in the competing risk analysis confirmed that death and liver transplantation did not remarkably affect the models (see Supplementary Table 4, Supplementary Digital Content 2, http://links.lww.com/CTG/A763).
Figure 5.
Forest plot of model discrimination in different subgroups. CI, confidence interval; PVT, portal vein thrombosis; TIPS, transjugular intrahepatic portosystemic shunt.
DISCUSSION
In this multicenter cohort study of patients with cirrhosis receiving TIPS treatment, we confirmed the negative effect of sarcopenia on the development of post-TIPS overt HE and that inclusion of sarcopenia and post-TIPS PPG could significantly improve the predictive accuracy of a model comprising only clinical data. Two prediction models (the sarcopenia model and full model) were developed to be applied before and after the TIPS procedure to determine the risk of postprocedural overt HE, which presented good discrimination and calibration both in the derivation and validation cohort. Moreover, the predictive ability of the 2 models was consistent across prespecified subgroups.
Variables showed that a strong prognostic effect included age, Child-Pugh class, sarcopenia, and post-TIPS PPG. Among them, age and Child-Pugh class are the most frequently reported determinants (14). Sarcopenia is a common comorbidity in patients with cirrhosis, with a prevalence that has been reported to be as high as 30%–70% (25). As previously suggested, the skeletal muscle disposes more ammonia than the liver in the presence of cirrhosis (6,26). Thus, depletion of the muscle quantity results in an elevation in circulating ammonia. Meanwhile, ammonia accumulated in the skeletal muscle activates molecular alternations, which inhibit protein synthesis and consequently aggravate sarcopenia (27,28). This vicious cycle increases the risk of post-TIPS overt HE in patients with baseline sarcopenia.
For patients with cirrhosis undergoing TIPS, another major mechanism of post-TIPS overt HE is related to the portal-systemic shunt of blood containing neurotoxins (29). Because the optimal PPG threshold to achieve a balance between preventing rebleeding and reducing the risk of overt HE has been undetermined (30), we inputted post-TIPS PPG as a continuous variable. Two prediction models were developed to be applied before and after TIPS to estimate the risk of postprocedural overt HE. Items in the sarcopenia model are routinely assessed and readily available before the procedure. For patients classified as high-risk, TIPS placement may be considered as a contraindication and the net benefit should be carefully weighed. For patients with medium risk, an underdilated strategy to achieve a higher post-TIPS PPG may be an optimal choice (31). After the procedure, the full model incorporating post-TIPS PPG should be applied to refine the risk estimation, and patients with high risk of overt HE may benefit from personalized dietary management to improve nutritional status (11) and pharmacological prophylaxis (5). Furthermore, the full model could be implemented before the procedure to calculate an optimal post-TIPS PPG, which could decrease the risk of the outcome below a specific level.
To establish easy-to-perform tools for clinical practice, the final set of variables were selected at discretion with exclusion of several potential predictors. Myosteatosis was reported as an independent predictor of post-TIPS overt HE, but the routine examination for the TIPS procedure is enhanced computed tomography, in which muscle attenuation may not be measured accurately. Although the final 4 variables showed the strongest prognostic effect, they collectively explained a small proportion of the variation in the outcome (23.6%), which might be related to the multifactorial pathogenesis of overt HE. Therefore, future studies may refine these models with inclusion of other potential risk factors that were not fully captured by this study.
This study has several strengths. To the best of our knowledge, this is the first study relating the presence of malnutrition (sarcopenia) and degree of diversion (post-TIPS PPG) to the risk of postprocedural overt HE and synthesizing these variables to build prognostic models. Besides, all candidate variables were selected based on clinical relevance according to the latest relevant studies, and final predictors are objective, readily available at admission or after the procedure, and routinely measured for each patient scheduled for TIPS, supporting their applicability and generalizability in clinical practice. Furthermore, we only assessed the occurrence of post-TIPS overt HE over the first 3 months. In this restricted period, the incidence was high and patients were minimally affected by confounding factors, such as high-protein diet.
Potential limitations exist in this study. First, we excluded patients with previous overt HE, and the tools might not be applicable to this subset of cases. However, previous overt HE was identified as the strongest predictor of post-TIPS overt HE by most studies (14), and these patients should be regarded as high-risk whether they acquire the above risk factors. Second, patients with baseline covert HE were not identified and excluded, which was reported as another independent predictor (32). Third, the reliability of the tools might be confounded by different methods of L3-SMI measurement, and more convenient and accurate instruments are warranted to identify sarcopenia automatically. Fourth, both the derivation and validation cohort included a relatively small number of patients. Thus, the established tools should be validated in diverse clinical settings and populations before clinical application.
In conclusion, we developed and externally validated 2 risk prediction models for different clinical settings, which have the potential to reliably identify patients at different risks of post-TIPS overt HE within 3 months. These tools could provide evidence for further studies to establish risk-based selection criteria for the TIPS procedure and guide premature strategies after the procedure to reduce the risk of the outcome, such as active drug prophylaxis and personalized dietary management.
CONFLICTS OF INTEREST
Guarantor of the article: Bin Xiong, PhD.
Specific author contributions: C.S.: statistical assistant. Conceptualization: B.X.; Methodology: C.Y. and J.L.; Data-collection: X.Z., Q.S. and H.D.; Data-analysis and interpretation: Y.C., Y.B. and W.Y.; Software: Y.W. and J.L.; Writing-original draft: C.Y. and C.Z.; Writing-review and editing: T.L., C.W. and S.J.
Financial support: National Natural Science Foundation of China (81873917).
Potential competing interests: None to report.
Study Highlights.
WHAT IS KNOWN
✓ Overt hepatic encephalopathy (HE) is a major complication of transjugular intrahepatic portosystemic shunt (TIPS) implantation, which limits its clinical use.
✓ Sarcopenia is an independent predictor of post-TIPS overt HE.
WHAT IS NEW HERE
✓ Inclusion of parameters indicating nutritional status (sarcopenia) could significantly improve the predictive ability of a model comprising only clinical data.
✓ We developed and externally validated 2 prognostic models before and after TIPS which could accurately predict the risk of overt HE.
Supplementary Material
ACKNOWLEDGEMENT
We want to express our gratitude to Chuang Sun for statistical analysis.
Footnotes
SUPPLEMENTARY MATERIAL accompanies this paper at http://links.lww.com/CTG/A762, http://links.lww.com/CTG/A763
Chongtu Yang and Xiaoli Zhu contributed equally to this work.
Contributor Information
Chongtu Yang, Email: henrys1011@163.com.
Xiaoli Zhu, Email: zhuxiaoli@163.com.
Jiacheng Liu, Email: jiacheng6jc@163.com.
Qin Shi, Email: shiq1010@163.com.
Hang Du, Email: 18435148567@163.com.
Yang Chen, Email: yang_chen_@hust.edu.cn.
Songjiang Huang, Email: hsjhzkjdx@163.com.
Chen Zhou, Email: zhouchenjr@163.com.
Yingliang Wang, Email: 18345197920@163.com.
Tongqiang Li, Email: 17728096221@163.com.
Yaowei Bai, Email: baiyaowei918@163.com.
REFERENCES
- 1.Sanyal AJ, Freedman AM, Shiffman ML, et al. Portosystemic encephalopathy after transjugular intrahepatic portosystemic shunt: Results of a prospective controlled study. Hepatology 1994;20:46–55. [DOI] [PubMed] [Google Scholar]
- 2.Vilstrup H, Amodio P, Bajaj J, et al. Hepatic encephalopathy in chronic liver disease: 2014 Practice Guideline by the American Association for the Study of Liver Diseases and the European Association for the Study of the Liver. Hepatology 2014;60:715–35. [DOI] [PubMed] [Google Scholar]
- 3.Nolte W, Wiltfang J, Schindler C, et al. Portosystemic hepatic encephalopathy after transjugular intrahepatic portosystemic shunt in patients with cirrhosis: Clinical, laboratory, psychometric, and electroencephalographic investigations. Hepatology 1998;28:1215–25. [DOI] [PubMed] [Google Scholar]
- 4.Riggio O, Masini A, Efrati C, et al. Pharmacological prophylaxis of hepatic encephalopathy after transjugular intrahepatic portosystemic shunt: A randomized controlled study. J Hepatol 2005;42:674–9. [DOI] [PubMed] [Google Scholar]
- 5.Bureau C, Thabut D, Jezequel C, et al. The use of rifaximin in the prevention of overt hepatic encephalopathy after transjugular intrahepatic portosystemic shunt: A randomized controlled trial. Ann Intern Med 2021;174(5):633–40. [DOI] [PubMed] [Google Scholar]
- 6.Olde Damink SW, Jalan R, Redhead DN, et al. Interorgan ammonia and amino acid metabolism in metabolically stable patients with cirrhosis and a TIPSS. Hepatology 2002;36:1163–71. [DOI] [PubMed] [Google Scholar]
- 7.Jaffe A, Lim JK, Jakab SS. Pathophysiology of hepatic encephalopathy. Clin Liver Dis 2020;24:175–88. [DOI] [PubMed] [Google Scholar]
- 8.Meijer AJ, Lamers WH, Chamuleau RA. Nitrogen metabolism and ornithine cycle function. Physiol Rev 1990;70:701–48. [DOI] [PubMed] [Google Scholar]
- 9.Nardelli S, Lattanzi B, Merli M, et al. Muscle alterations are associated with minimal and overt hepatic encephalopathy in patients with liver cirrhosis. Hepatology 2019;70:1704–13. [DOI] [PubMed] [Google Scholar]
- 10.Bhanji RA, Moctezuma-Velazquez C, Duarte-Rojo A, et al. Myosteatosis and sarcopenia are associated with hepatic encephalopathy in patients with cirrhosis. Hepatol Int 2018;12:377–86. [DOI] [PubMed] [Google Scholar]
- 11.Gioia S, Merli M, Nardelli S, et al. The modification of quantity and quality of muscle mass improves the cognitive impairment after TIPS. Liver Int 2019;39:871–7. [DOI] [PubMed] [Google Scholar]
- 12.Nardelli S, Lattanzi B, Torrisi S, et al. Sarcopenia is risk factor for development of hepatic encephalopathy after transjugular intrahepatic portosystemic shunt placement. Clin Gastroenterol Hepatol 2017;15:934–6. [DOI] [PubMed] [Google Scholar]
- 13.Riggio O, Angeloni S, Salvatori FM, et al. Incidence, natural history, and risk factors of hepatic encephalopathy after transjugular intrahepatic portosystemic shunt with polytetrafluoroethylene-covered stent grafts. Am J Gastroenterol 2008;103:2738–46. [DOI] [PubMed] [Google Scholar]
- 14.Bai M, Qi X, Yang Z, et al. Predictors of hepatic encephalopathy after transjugular intrahepatic portosystemic shunt in cirrhotic patients: A systematic review. J Gastroenterol Hepatol 2011;26L:943–51. [DOI] [PubMed] [Google Scholar]
- 15.Butt Z, Jadoon NA, Salaria ON, et al. Diabetes mellitus and decompensated cirrhosis: Risk of hepatic encephalopathy in different age groups. J Diabetes 2013;5:449–55. [DOI] [PubMed] [Google Scholar]
- 16.Liu J, Zhou C, Wang Y, et al. The combination of Child-Pugh score and quantitative CT-based spleen volume could predict the risk of hepatic encephalopathy after transjugular intrahepatic portosystemic shunt creation. Abdom Radiol (NY) 2021;46:3464–70. [DOI] [PubMed] [Google Scholar]
- 17.Riggio O, Amodio P, Farcomeni A, et al. A model for predicting development of overt hepatic encephalopathy in patients with cirrhosis. Clin Gastroenterol Hepatol 2015;13:1346–52. [DOI] [PubMed] [Google Scholar]
- 18.Li Y, He X, Pang H. A model to predict early hepatic encephalopathy in patients undergoing transjugular intrahepatic portosystemic shunt. Turk J Gastroenterol 2019;30:702–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yin X, Zhang F, Guo H, et al. A nomogram to predict the risk of hepatic encephalopathy after transjugular intrahepatic portosystemic shunt in cirrhotic patients. Sci Rep 2020;10:9381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cruz-Jentoft AJ, Baeyens JP, Bauer JM, et al. Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Ageing 2010;39:412–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Carey EJ, Lai JC, Wang CW, et al. A multicenter study to define sarcopenia in patients with end-stage liver disease. Liver Transpl 2017;23:625–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pencina MJ, D'Agostino RB, D'Agostino RB, et al. Evaluating the added predictive ability of a new marker: From area under the ROC curve to reclassification and beyond. Stat Med 2008;27:157–72. [DOI] [PubMed] [Google Scholar]
- 23.Vickers AJ, Elkin EB. Decision curve analysis: A novel method for evaluating prediction models. Med Decis Making 2006;26:565–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Collins GS, Reitsma JB, Altman DG, et al. Transparent reporting of a multivariable prediction model for individual prognosis or diagnosis (TRIPOD): The TRIPOD statement. BMJ 2015;350:g7594. [DOI] [PubMed] [Google Scholar]
- 25.Dasarathy S, Merli M. Sarcopenia from mechanism to diagnosis and treatment in liver disease. J Hepatol 2016;65:1232–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ganda OP, Ruderman NB. Muscle nitrogen metabolism in chronic hepatic insufficiency. Metabolism 1976;25L:427–35. [DOI] [PubMed] [Google Scholar]
- 27.Dasarathy S, McCullough AJ, Muc S, et al. Sarcopenia associated with portosystemic shunting is reversed by follistatin. J Hepatol 2011;54:915–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Qiu J, Thapaliya S, Runkana A, et al. Hyperammonemia in cirrhosis induces transcriptional regulation of myostatin by an NF-κB-mediated mechanism. Proc Natl Acad Sci USA 2013;110:18162–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rossi P, Salvatori FM, Fanelli F, et al. Polytetrafluoroethylene-covered nitinol stent-graft for transjugular intrahepatic portosystemic shunt creation: 3-year experience. Radiology 2004;231:820–30. [DOI] [PubMed] [Google Scholar]
- 30.Pereira K, Carrion AF, Martin P, et al. Current diagnosis and management of post-transjugular intrahepatic portosystemic shunt refractory hepatic encephalopathy. Liver Int 2015;35:2487–94. [DOI] [PubMed] [Google Scholar]
- 31.Schepis F, Vizzutti F, Garcia-Tsao G, et al. Under-dilated TIPS associate with efficacy and reduced encephalopathy in a prospective, non-randomized study of patients with cirrhosis. Clin Gastroenterol Hepatol 2018;16:1153–62. [DOI] [PubMed] [Google Scholar]
- 32.Nardelli S, Gioia S, Pasquale C, et al. Cognitive impairment predicts the occurrence of hepatic encephalopathy after transjugular intrahepatic portosystemic shunt. Am J Gastroenterol 2016;111L:523–8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.