INTRODUCTION:
The aim of the study was to compare the effectiveness of a low fermentable oligosaccharide, disaccharide, monosaccharide, and polyol diet (LFD) vs psyllium on the frequency and severity of fecal incontinence (FI) episodes in patients with loose stools.
METHODS:
This was a single-center, randomized pilot trial of adult patients with FI (Rome III) with at least 1 weekly FI episode associated with loose stool. Eligible patients were randomized to 4 weeks of either a dietitian-led LFD or 6 g/d psyllium treatment.
RESULTS:
Forty-three subjects were randomized from October 2014 to May 2019. Thirty-seven patients completed the study (19 LFD and 18 psyllium). There was no statistically significant difference in the proportion of treatment responders (>50% reduction in FI episodes compared with baseline) for treatment weeks 1–4 (LFD 38.9%, psyllium 50%, P = .33). Compared with baseline, mean fecal incontinence severity index score significantly improved with LFD (39.4 vs 32.6, P = .02) but not with psyllium (35.4 vs 32.1, P = .29). Compared with baseline values, the LFD group reported improvements in fecal incontinence quality of life coping/behavior, depression/self-perception, and embarrassment subscales. The psyllium group reported improvement in incontinence quality of life coping/behavior.
DISCUSSION:
In this pilot study, there was no difference in the proportion of patients who reported a 50% reduction of FI episodes with the LFD or psyllium. Subjects in the psyllium group reported a greater reduction in overall FI episodes, whereas the LFD group reported consistent improvements in FI severity and quality of life. Further work to understand these apparently discrepant results are warranted but the LFD and psyllium seem to provide viable treatment options for patients with FI and loose stools.
INTRODUCTION
Fecal incontinence (FI) is a common, debilitating complaint that affects 5% of the population weekly (1). The most important risk factors of FI are urgency and loose or watery stools (1–4). Dietary management is the first important step in helping reduce episodes of FI. On a cross-sectional survey, Joh et al. noted that elderly women who consumed the lowest amount of weekly dietary fiber had a 2.66 higher likelihood of FI (5). Psyllium has also been shown to reduce the frequency and severity of FI episodes. Bliss et al. has demonstrated a 60% reduction in the number of episodes of FI per week in a pilot study of 39 patients (6) and 50% reduction in weekly FI episodes in a single blind, randomized controlled trial of 189 patients (7).
There may be other dietary interventions that might be of benefit to FI. Foods that are high in fermentable oligosaccharides, disaccharides, monosaccharides, and polyols (FODMAP) can cause symptoms of diarrhea and urgency (8). These highly fermentable carbohydrates can affect stool consistency by 2 mechanisms; the first is a pure osmotic effect by the poorly absorbed carbohydrates themselves drawing water into the stool and the second is bacterial fermentation leading to the production of short-chain fatty acids, which increase the osmotic load and accelerate intestinal transit.
Restricting dietary FODMAP intake reduces symptoms in patients with irritable bowel syndrome (IBS) (9–13). Therefore, it is reasonable to hypothesize that a low FODMAP diet (LFD) might offer clinical benefits to patients with FI, in which up to two-thirds of patients experience loose, frequent stools and urgency (2,4). We performed a retrospective case series of 65 patients with FI due to loose stool, in which 64% of subjects reported a reduction in their FI symptoms with an LFD (14). Therefore, the aim of this study was to conduct a randomized, controlled trial to compare the effectiveness of an LFD vs psyllium on clinical outcomes in patients with FI and loose stools.
METHODS
Study participants
The study was approved by the University of Michigan Institutional Review board and registered on Clinicaltrials.gov (NCT02828384). Subjects 18 years and older who met Rome III criteria for functional fecal incontinence were recruited from the Gastroenterology Clinics and Michigan Bowel Control Program Clinic at Michigan Medicine Health System. Eligible criteria included adult FI patients who experienced at least 1 FI episode associated with loose stool per week as defined by Bristol Stool Form Scale (BSFS, Type 5, 6, or 7) and occurring for at least 3 months. A mean weekly BSFS of a 5, 6, or 7 was not required for inclusion. Exclusion criteria included the following: cognitive dysfunction or inability to provide or understand written informed consent, abnormal innervation caused by lesion(s) within the brain (e.g., dementia), spinal cord, or sacral nerve roots, or mixed lesions (e.g., multiple sclerosis), or as part of a generalized peripheral or autonomic neuropathy (e.g., due to diabetes), anal sphincter abnormalities associated with a multisystem disease (e.g., scleroderma), structural or neurogenic abnormalities believed to be the major or primary cause of fecal incontinence, overflow diarrhea due to fecal loading, pregnancy, solid stool FI only, comorbid medical problems that may affect gastrointestinal transit or motility such as inflammatory bowel disease, extraintestinal diseases known to affect the gastrointestinal system (i.e., scleroderma and unstable thyroid disease), severe renal or hepatic disease, previous abdominal surgery other than appendectomy, cholecystectomy, and gynecologic/urologic surgery if performed less than 6 months before enrollment, previous treatment with an LFD, concurrent medications not permitted including probiotics, antibiotics, or narcotics, and active participation in another form of dietary therapy.
Randomization and interventions
After a 2-week screening period, eligible patients were randomized by computer generation in a 1:1 ratio to a dietitian-led LFD vs 6 g/d psyllium for 4 weeks. Participants who were randomized to the LFD group met with an experienced, specially trained registered dietician (RD) at the Michigan Clinical Research Unit (MCRU). For practical reasons, we were unable to blind participants to the study arm to which they were randomized. However, the physicians analyzing the data were blinded as to which group the patients were randomized. The MCRU dieticians administered instructions in a standardized manner using teaching materials published from Monash University and created at Michigan Medicine. Standard dietary compliance measures used in the counseling environment included prospectively recorded 3-day food diaries and 24-hour dietary recall at a 2-week and 4-week visit with the MCRU dietician. Vital signs, body mass index, and adverse events were collected at each visit. Participants randomized to psyllium arm were given standardized instructions regarding the use of the daily supplement dosing. Using the Nutrition Data System for Research (NDSR) computer program, food diaries were analyzed for fermentable carbohydrate measurement.
Statistical analysis
The primary outcome was the proportion of treatment responders in each group, defined as a reduction in the number of FI episodes by ≥50% compared with baseline. Key secondary outcomes included fecal incontinence severity index (FISI) and fecal incontinence quality of life (FIQL) scores in each treatment group before and after the dietary intervention. Subjects also reported daily stool consistency with a responder defined as a decrease in mean daily BSFS value of 1 or more compared with baseline for 3 or greater of 4 treatment weeks. Stool frequency will be compared for the 2 groups by the change from baseline in mean daily stool frequency for each treatment week. The presence of urgency was assessed by the inability “to hold my bowel movement long enough to get to the bathroom” into the following 4 categories: most of the time, some of the time, a little of the time, or none of the time. Patients with a response of “most of the time” or “some of the time” were designated as having urgency. A responder was defined by a response of either “none of the time” or “a little of the time” at weeks 3 and 4 with the proportion of responders between the 2 groups compared. Abdominal pain, discomfort, and fatigue were measured using a daily numerical rating scale (NRS; 0, no pain; 10, intolerable pain), with the change from baseline in daily NRS scores averaged over each treatment week were compared between the 2 groups. Differences in proportions were assessed using Pearson χ2 and differences in continuous variables used Student t test. Because this was a pilot trial, we were assuming a response rate of a 50% in the psyllium and LFD groups. A two-sample t test for mean difference will have 80% power to detect the difference between a mean of 2 at baseline and a mean of 1 at follow-up for both the control and psyllium groups when the sample size in each group is 17. Anticipating a dropout rate of 15%, this equates to a sample size of 20 for each of the 2 treatment arms. We included 14 subjects in the LFD group and 9 subjects in the psyllium group for the continuous outcome variable. A post hoc power analysis for the difference in each group from baseline to week 4 (using a test of paired means) showed power for the LFD and psyllium groups at 0.64 and 0.83. The analysis also showed that each group would need 75 subjects to achieve 80% power with an average FI episodes at week 4 of 1 (standard deviation [SD] = 2) in the LFD group and .4 (SD = 0.5) in the psyllium group. Although powered for a continuous variable, we used the aforementioned dichotomous outcome as our primary outcome because this is the common FDA end point used in other FI studies.
RESULTS
Demographics and primary end point
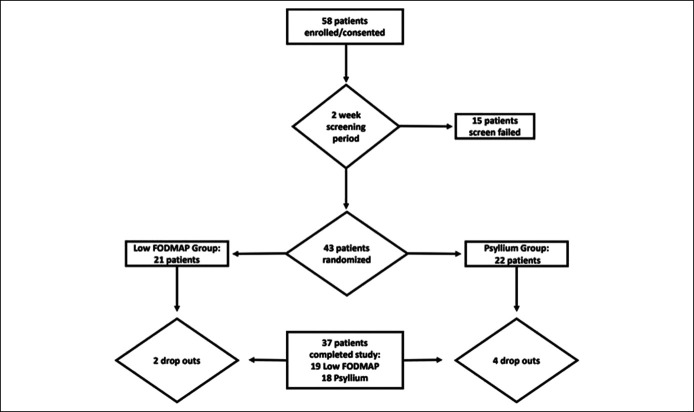
After screening, 58 patients consented for enrollment in the trial (Figure 1). Forty-three subjects (86% women, 83% Caucasian, and mean age 61.4 years) were randomized from October 2014 through May 2019, of which 37 patients completed the study (18 LFD and 19 psyllium) (Table 1). For both groups at baseline, 36.1%, 33.3%, and 25% reported FI with loose stool daily, weekly, and monthly by FISI, respectively. For the primary outcome, there was no statistically significant difference in the proportion of treatment responders (>50% reduction in FI episodes [average daily per specified study week] compared with baseline) for treatment weeks 1–4 (Table 2). At the end of 4 weeks, 38.9% of the LFD group and 50% of the psyllium group reported a 50% reduction in FI episodes when compared with baseline (P = .33). Compared with baseline, mean FISI scores significantly improved from baseline to week 4 with LFD (39.2 vs 32.6, P = .02) but not with psyllium (35.2 vs 32.5, P = .22) treatment. Compared with baseline, the LFD group reported improvements in the FIQL coping/behavior, depression/self-perception, and embarrassment subscales (Table 3). The psyllium group reported improvement in FIQL coping/behavior subscale.
Figure 1.
Flow chart of participants.
Table 1.
Demographics and baseline characteristics of patients
| Characteristic | Low FODMAP (n = 21) | Psyllium (n = 22) | P value |
| Average age, yr | 63.8 ± 17 | 64.8 ± 11 | .82 |
| Average age, yr, n of patients (%) | .7 | ||
| 32–58 | 8 (38) | 7 (32) | |
| 61–70 | 7 (33) | 6 (27) | |
| 71–98 | 6 (29) | 9 (41) | |
| Sex, n of patients (%) | .41 | ||
| Female | 18 (82) | 19 (90) | |
| Race, n of patients (%) | .38 | ||
| White | 19 (90) | 17 (77) | |
| Black | 2 (10) | 2 (9) | |
| Asian | 0 (0) | 1 (5) | |
| Unknown | 0 (0) | 2 (9) | |
| Ever smoker, n of patients (%) | .39 | ||
| Yes | 6 (32) | 3 (19) | |
| Average daily measures | |||
| Bristol Stool Form Scale | 5.1 ± 1 (3.4–7.0) | 5.0 ± .9 (3.7–6.4) | .77 |
| Stool frequency | 3.1 ± 1 | 3.6 ± 1 | .33 |
| FI frequency | 2.0 ± 2 | 1.5 ± 1 | .32 |
| Solid FI frequency | 0.6 ± 1 | 0.7 ± 7 | .93 |
| Liquid FI frequency | 1.3 ± 1 | 0.8 ± 1 | .31 |
FI, fecal incontinence; FODMAP, fermentable oligosaccharides, disaccharides, monosaccharides, and polyols.
Table 2.
Proportion (%) of subjects with ≥50% reduction in FI episodes compared with baseline by treatment weeks
| Variable | Low FODMAP | Psyllium | P value |
| Treatment week | |||
| 1 | 15.8 | 18.7 | .81 |
| 2 | 31.6 | 37.5 | .71 |
| 3 | 26.3 | 43.8 | .27 |
| 4 | 38.9 | 50.0 | .33 |
FI, fecal incontinence; FODMAP, fermentable oligosaccharides, disaccharides, monosaccharides, and polyols.
Table 3.
Change in FIQL subscales from baseline to 4 weeks by intervention group
| FIQL Category | Low FODMAP | Psyllium | ||||
| Variable | Baseline | Week 4 | P value within group | Baseline | Week 4 | P value within group |
| Baseline vs week 4 | ||||||
| Lifestyle | 2.82 | 3.15 | .06 | 2.79 | 2.91 | .13 |
| Coping/behavior | 1.97 | 2.42 | .03 | 1.89 | 2.06 | .04 |
| Depression/self-perception | 2.76 | 3.15 | .01 | 2.65 | 2.81 | .11 |
| Embarrassment | 2.07 | 2.57 | .01 | 2.02 | 2.16 | .38 |
FIQL, fecal incontinence quality of life; FODMAP, fermentable oligosaccharides, disaccharides, monosaccharides, and polyols.
Episodes of FI in the LFD group by FISI subscales
At baseline, 36% of patients in the LFD group had daily episodes, 33% weekly episodes, and 25% monthly episodes, with 5% reporting no episodes of FI with loose stool (Table 4). At the end of 4 weeks, 17% experienced daily episodes, 36% weekly episodes, 31% monthly episodes, and 17% reported no episodes of FI with loose stool. The LFD group had significant reduction in episodes of FI with loose stool (P = .01). There was no statistical difference in the change in FI with gas (P = .50) or FI with solid stool (P = .53), but significant reduction was noted in the amount of FI with mucus after an LFD, with a P value of .02 with a largest proportion (27%) shifting from daily or weekly episodes of mucus to experiencing monthly or no episodes.
Table 4.
Change in FISI subscale, liquid stool incontinence, from baseline to 4 weeks by intervention group
| FIQL Category | Low FODMAP | Psyllium | ||||
| Variable | Baseline | Week 4 | P value within group | Baseline | Week 4 | P value within group |
| Liquid stool incontinence Baseline vs week 4 |
||||||
| Daily FI | 36% | 17% | 17% | 11% | ||
| Weekly FI | 33% | 36% | 56% | 39% | ||
| Monthly FI | 25% | 31% | .02 | 22% | 28% | .11 |
| No FI | 5% | 17% | 6% | 22% | ||
FI, fecal incontinence; FIQL, fecal incontinence quality of life; FISI, fecal incontinence severity index; FODMAP, fermentable oligosaccharides, disaccharides, monosaccharides, and polyols.
Episodes of FI in the psyllium group by FISI subscales
At baseline, 17% of patients in the psyllium group had daily episodes, 56% weekly episodes, and 22% monthly episodes, with 6% reporting no episodes of FI with loose stool (Table 4). At the end of 4 weeks, 11% had daily episodes, 37% weekly episodes, 32% monthly episodes, and 21% no episodes of FI with loose stool. There was a reduction in daily and weekly episodes of liquid stool FI with an increase in those experiencing no episodes of FI with psyllium supplement, but this was not statistically significant (P = .19). There was no statistical difference in the change of FI with gas (P = .52) and FI with mucus (P = .91) stool but significant reduction in FI with solid stool with a P value of .002.
Response of LFD and psyllium by FIQL
At baseline, both LFD and psyllium groups reported quality of life issues some of the time in the lifestyle, depression/self-perception, and embarrassment FIQL categories (Table 3). Both participant arms reported most of the time quality of life issues with the coping/behavior category. At 4 weeks, in the LFD group, there was significant improvement in coping/behavior, depression/self-perception, and embarrassment domains. Although all FIQL domains improved with psyllium treatment, only the coping/behavior domain was statistically significant.
Average daily measures
There were no statistically significant differences in stool variables, except in FI frequency, among those participants in whom we had daily data (Table 5). The LFD led to a statistically significantly change in BSFS from types 5 to type 4. Both groups had reduction in FI episodes, but it was statistically significant in the psyllium arm for a within-group change from baseline to 4 weeks (P = .0003) and between intervention arms (P < .0001). In the LFD group, there was improvement in urgency, abdominal pain, bloating, and fatigue, but these changes were not statistically significant. Among the psyllium group, there was an increase in urgency and bloating, with a slight reduction in abdominal pain and fatigue. For urgency responder, both groups experienced an improvement from baseline to week 4 with the LFD arm seeing a 14% improvement (P = .21) and the psyllium arm seeing a 25% improvement (P = .07). The magnitude of improvement with the LFD vs psyllium at 4 weeks was not statistically significant.
Table 5.
The effect of dietary intervention on individual symptoms
| Variable | Low FODMAP (n = 14) | Psyllium (n = 9) | P value between groups | ||||||
| Baseline | Week 4 | Difference | P value within group | Baseline | Week 4 | Difference | P value within group | ||
| Baseline vs week 4 | |||||||||
| Bristol Stool Form Scale, mean ± SD | 5.3 ± 1 | 4.4 ± 1 | −0.9 | .02 | 4.6 ± 0.7 | 4.3 ± 1 | −0.3 | .43 | .27 |
| Stool frequency, mean ± SD | 3.2 ± 1 | 2.6 ± 9 | −0.6 | .14 | 3.3 ± 1 | 2.8 ± 0.6 | −0.5 | .17 | .83 |
| FI Frequency, mean ± SD | 2.2 ± 2 | 1 ± 2 | −1.2 | .13 | 1.1 ± 0.7 | 0.4 ± 0.5 | −0.7 | .0003 | < .0001 |
| Solid FI frequency, mean ± SD | .6 ± 2 | 0.4 ± 1 | −0.2 | .66 | 0.9 ± 0.8 | 0.3 ± 0.1 | −0.6 | .003 | .49 |
| Liquid FI frequency, mean ± SD | 1.4 ± 1.3 | 0.6 ± 0.8 | −0.8 | .08 | 0.3 ± 0.5 | 0.1 ± 0.2 | −0.2 | .15 | .20 |
| Variable | Low FODMAP (n = 14) | Psyllium (n = 10) | P Value between groups | ||||||
| Week 1 | Week 4 | Difference | P Value within group | Week 1 | Week 4 | Difference | P Value within group | ||
| Week 1 vs week 4 | |||||||||
| Urgency, mean ± SD | 4.3 ± 3 | 3.4 ± 2 | −0.9 | .15 | 3.5 ± 2 | 3.8 ± 3 | +0.3 | .7 | .20 |
| Abdominal pain, mean ± SD | 2.2 ± 2 | 1.5 ± 2 | −0.7 | .18 | 1.5 ± 1 | 1.3 ± 2 | −0.2 | .63 | .12 |
| Bloating, mean ± SD | 2.6 ± 2 | 2.3 ± 2 | −0.3 | .62 | 1.6 ± 1 | 2.2 ± 2 | +0.6 | .23 | .27 |
| Fatigue, mean ± SD | 4.2 ± 2 | 3.2 ± 3 | −0.9 | .11 | 3.5 ± 4 | 3.3 ± 2.6 | −0.2 | .8 | .50 |
Between-group P values refer to the change from baseline between groups at week 4 for low FODMAP and psyllium subjects.
FI, fecal incontinence; FODMAP, fermentable oligosaccharides, disaccharides, monosaccharides, and polyols.
Nutritional data
Baseline dietary nutritional intake was similar in the 2 study groups (Table 6). However, by the end of 4 weeks, nutritional intake varied significantly between the 2 groups. Participants in the LFD had a significant reduction in calories, carbohydrates, monosaccharides, fructose, and lactose when compared with the psyllium arm.
Table 6.
Daily nutritional intake as measured by NSRD for each dietary intervention, comparisons made per protocol (mean ±SD)
| Variable | Low FODMAP | Psyllium | P value between groups: baseline | P value between groups: week 4 | ||||
| Baseline (n = 15) | Week 4 (n = 15) | P value within group | Baseline (n = 15) | Week 4 (n = 12) | P value within group | |||
| Kilocalories | 1,639 ± 543 | 1,398 ± 435 | .1 | 1,927 ± 666 | 2,020 ± 973 | .7 | .2 | .04 |
| Protein (g) | 64.8 ± 26 | 61.6 ± 19 | .7 | 73.2 ± 26 | 73.4 ± 29 | .7 | .4 | .2 |
| Fat (g) | 65.2 ± 27 | 64.6 ± 29 | .99 | 84.8 ± 33 | 88.8 ± 42 | .3 | .1 | .09 |
| Alcohol (g) | 7 ± 13 | 8.2 ± 18 | .8 | 5.5 ± 11 | 3.7 ± 8 | .8 | .7 | .4 |
| Carbohydrates (g) | 193 ± 58 | 136 ± 52 | < .0001 | 219 ± 88 | 237 ± 141 | .8 | .4 | .02 |
| Monosaccharides (g) | 40.1 ± 11 | 23.8 ± 11 | .003 | 42.2 ± 25 | 46.8 ± 38 | .9 | .8 | .03 |
| Fructose (g) | 20.3 ± 6 | 10.1 ± 5 | .0004 | 21.1 ± 13 | 23.1 ± 21 | .9 | .8 | .03 |
| Total dietary fiber (g) | 18.1 ± 11 | 15.7 ± 7 | .2 | 19.5 ± 7 | 18.5 ± 8 | .1 | .7 | .4 |
| Insoluble dietary fiber (g) | 12.8 ± 10 | 10.8 ± 6 | .2 | 13.4 ± 4 | 13.1 ± 7 | .5 | .8 | .3 |
| Soluble dietary fiber (g) | 5.3 ± 3 | 4.9 ± 2 | .3 | 5.9 ± 3 | 5.2 ± 3 | .02 | .6 | .7 |
| Lactose (g) | 6.1 ± 5 | 1.1 ± 1 | .0014 | 10.7 ± 8 | 12.9 ± 11 | .5 | .08 | .0003 |
| Polyols (g) | 1.1 ± 1 | 0.7 ± 0.7 | .3 | 1.5 ± 1 | 1.1 ± 0.9 | .1 | .4 | .3 |
FODMAP, fermentable oligosaccharides, disaccharides, monosaccharides, and polyols.
Side effects and study dropout
Both interventions were well tolerated by participants. For the LFD arm, 11.1% of participants reported constipation, bloating, and gas. For the psyllium arm, 15.7% reported gas, diarrhea, abdominal cramping, and abdominal bloating. Two participants dropped out from the LFD arm due to the highly restrictive nature of the diet and ineffectiveness. In the psyllium arm, 4 participants dropped out due to diarrhea (1), bloating and diarrhea (1), and ineffectiveness (2). There were no serious adverse events or deaths reported.
DISCUSSION
In the first pilot study comparing LFD vs psyllium, there was no difference in the proportion of patients who reported a 50% reduction of FI episodes. Subjects in the psyllium group reported a greater reduction in overall FI episodes when compared with the LFD. However, the LFD group reported consistent improvements in FI severity and disease-related quality of life than those who received psyllium. Using the FISI subscales, there was a statistically significant reduction in FI episodes due to liquid stool but not in solid stool for subjects in the LFD arm. However, for the psyllium arm, there was a statistically significant reduction in the FI episodes due to solid stool but not to liquid stool in subject in the psyllium arm. Our study provides further evidence for the use of an LFD in the management of FI associated with loose stool and confirms the utility of psyllium.
Psyllium has been shown to reduce FI episodes in 3 randomized controlled trials (6,7,15). In 2001, Bliss et al. published the first pilot trial comparing psyllium vs gum Arabic vs placebo, which demonstrated more than 50% reduction in FI with the use of both those agents (6). In the psyllium arm (7.1 g), 21.4% of subjects used loperamide. Bliss et al. then went on to publish in 2014, a single-blind, randomized controlled trial of 189 patients comparing psyllium (14.6 g) vs gum Arabic vs carboxymethylcellulose vs placebo on FI frequency (7). The authors demonstrated a 50% reduction with psyllium, with no change with gum Arabic or placebo and almost a one-third increase of FI events with carboxymethylcellulose (4% of the psyllium arm used loperamide). Finally, Markland et al. compared psyllium (7.4 mg/d) vs loperamide in a randomized, double-blind, placebo-controlled crossover trial (FIRM). The authors found no difference between both arms with approximately 33% reduction in FI episodes (15). Our study used the similar psyllium dosing similar to the Bliss 2001 pilot trial and the FIRM trial, and we demonstrated similar and greater efficacy of FI episode reductions with the use of low-dose psyllium and without the use of loperamide, respectively. It is interesting to point out that we found more evidence for reduction of FI with solid stool for psyllium and no significant improvement in the FI with loose stool using FISI subscores; however, psyllium did outperform a low FODMAP diet in reducing FI episodes.
Our randomized controlled trial is the first of its kind to assess the effect of a specific dietary modification, a low FODMAP diet, on FI incidence. We previously published a 5-year retrospective chart review in patients with FI and loose stools without alarming features who were recommended a low FODMAP diet and underwent formal dietary instruction with a GI dietician. Sixty-five patients were included and with the use of a low FODMAP diet, 64.6% reported a reduction in FI episodes with 35% having complete continence on the low FODMAP diet. This recorded response was higher than the primary outcome results of this study. However, despite randomization, participants in the LFD arm did have more severe symptoms with a higher percentage having daily FI when compared to those in the psyllium arm. We observed significant reduction in the FISI score for liquid stool, which may be a better reflection of what the low FODMAP diet is meant to accomplish. From the IBS literature, a diet low in fermentable carbohydrates has demonstrated efficacy in IBS patients (9,12,16). Fermentable carbohydrates can be powerful osmotic agents, drawing water into the stool and overwhelming colonic absorption leading to loose stool (8). Therefore, we found evidence to support the use of a low FODMAP diet in FI with loose stool if trained dieticians are available to lead patients through the 3 phases of the low FODMAP diet.
Our study has a number of limitations. This study is a pilot trial, and as such, the sample size is modest. Furthermore, we had incomplete data due to a lack of daily symptom scores for a number of study participants (4 LFD arm and 9 psyllium arm), which affected our primary outcome and final data analysis. Our study is underpowered for a dichotomous outcome. Despite our efforts to recruit participants with FI and loose stool, some of the enrolled participants at baseline did not have FI nor loose stools by the FISI. These participants did qualify for the study in a 2-week screening period, which also highlights the variability of FI and stool consistency. This could have affected the effect of the LFD. Our findings also have implications on future clinical trial design. For future FI dietary studies, it would be judicious to consider a longer run-in period to ensure participant eligibility. Finally, we included type 5 on the BSFS, which is defined as soft blobs with clear-cut edges in our inclusion criteria for the FI episodes. In clinical practice, we have found that anything less than a formed stool can precipitate FI.
In conclusion, through a randomized controlled trial, we found that both psyllium and low FODMAP diet reduced FI episodes in patients with FI and loose stool. We add further evidence to the literature for the use of psyllium as a first-line treatment and new data for the use for a low FODMAP diet led by trained dieticians. The low FODMAP diet reduced FI severity and improved quality of life in 3 of the 4 subscales of FIQOL whereas psyllium did not significantly reduce FI severity but improved quality of life in the coping/behavior subscale only. Our preliminary study suggested both an LFD and psyllium are viable treatment options for patients with FI and loose stools.
CONFLICTS OF INTEREST
Guarantor of the article: Stacy B. Menees, MD, MSCRDSA.
Specific author contributions: S.B.M.: study concept and design; analysis and interpretation of data; drafting of the manuscript; critical revision of the manuscript for important intellectual content; statistical analysis; administrative, technical, or material support; and study supervision. J.R.B., S.E., B.N., R.S., and A.A.L.: critical revision of the manuscript for important intellectual content; statistical analysis; and administrative, technical, or material support. K.J.: statistical support and critical revision of the manuscript for important intellectual content. D.E.F.: study concept and design; analysis and interpretation of data; critical revision of the manuscript for important intellectual content; and statistical analysis. W.D.C.: study concept and design; analysis and interpretation of data; critical revision of the manuscript for important intellectual content; statistical analysis; and study supervision.
Financial support: Rome Foundation Grant.
Potential competing interests: W.D.C. is a consultant for Abbvie, Allakos, Alnylam, Ardelyx, Arena, Bayer, Biomerica, Comvita, Ironwood, Nestle, QOL Medical, Salix/Valeant, Takeda, Urovant Sciences, and Vibrant; has received grant and/or research study funding from Biomerica, Commonwealth Diagnostics International, QOL Medical, and Salix; has stock options in GI on Demand, Modify Health; serves on the Rome Board of Directors; and is a member of the Board of Trustees of the American College of Gastroenterology and Board of Directors of the International Foundation for Gastrointestinal Disorders. D.E.F. is a consultant for Takeda Phamece. All other authors have no related conflicts.
Study Highlights.
WHAT IS KNOWN
✓ Foods high in fermentable oligosaccharides, disaccharides, monosaccharides, and polyols (FODMAP) can lead to diarrhea and fecal incontinence (FI).
✓ Psyllium reduces FI frequency.
WHAT IS NEW HERE
✓ Dietary measures of low FODMAP or psyllium reduced FI frequency.
✓ A low FODMAP diet reduced the FI severity.
✓ A low FODMAP diet improved the quality of life in patients with FI.
References
- 1.Menees SB, Almario CV, Spiegel BMR, et al. Prevalence of and factors associated with fecal incontinence: Results from a population-based survey. Gastroenterology 2018;154:1672–87.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Whitehead WE, Borrud L, Goode PS, et al. Fecal incontinence in US adults: Epidemiology and risk factors. Gastroenterology 2009;137:512–7, 517 e1–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bharucha AE, Seide BM, Zinsmeister AR, et al. Relation of bowel habits to fecal incontinence in women. Am J Gastroenterol 2008;103:1470–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Smith TM, Menees SB, Xu X, et al. Factors associated with quality of life among women with fecal incontinence. Int Urogynecol J 2013;24:493–9. [DOI] [PubMed] [Google Scholar]
- 5.Joh HK, Seong MK, Oh SW. Fecal incontinence in elderly Koreans. J Am Geriatr Soc 2010;58:116–21. [DOI] [PubMed] [Google Scholar]
- 6.Bliss DZ, Jung HJ, Savik K, et al. Supplementation with dietary fiber improves fecal incontinence. Nurs Res 2001;50:203–13. [DOI] [PubMed] [Google Scholar]
- 7.Bliss DZ, Savik K, Jung HJ, et al. Dietary fiber supplementation for fecal incontinence: A randomized clinical trial. Res Nurs Health 2014;37:367–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Spencer M, Chey WD, Eswaran S. Dietary renaissance in IBS: Has food replaced medications as a primary treatment strategy? Curr Treat Options Gastroenterol 2014;12:424–40. [DOI] [PubMed] [Google Scholar]
- 9.Eswaran SL, Chey WD, Han-Markey T, et al. A randomized controlled trial comparing the low FODMAP diet vs. modified NICE guidelines in US adults with IBS-D. Am J Gastroenterol 2016;111:1824–32. [DOI] [PubMed] [Google Scholar]
- 10.Halmos EP, Power VA, Shepherd SJ, et al. A diet low in FODMAPs reduces symptoms of irritable bowel syndrome. Gastroenterology 2014;146:67–75 e5. [DOI] [PubMed] [Google Scholar]
- 11.Austin GL, Dalton CB, Hu Y, et al. A very low-carbohydrate diet improves symptoms and quality of life in diarrhea-predominant irritable bowel syndrome. Clin Gastroenterol Hepatol 2009;7:706–8 e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shepherd SJ, Parker FC, Muir JG, et al. Dietary triggers of abdominal symptoms in patients with irritable bowel syndrome: Randomized placebo-controlled evidence. Clin Gastroenterol Hepatol 2008;6:765–71. [DOI] [PubMed] [Google Scholar]
- 13.Staudacher HM, Whelan K, Irving PM, et al. Comparison of symptom response following advice for a diet low in fermentable carbohydrates (FODMAPs) versus standard dietary advice in patients with irritable bowel syndrome. J Hum Nutr Diet 2011;24:487–95. [DOI] [PubMed] [Google Scholar]
- 14.Menees SB, Chandhrasekhar D, Liew EL, et al. A low FODMAP diet may reduce symptoms in patients with fecal incontinence. Clin Transl Gastroenterol 2019;10:e00060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Markland AD, Burgio KL, Whitehead WE, et al. Loperamide versus psyllium fiber for treatment of fecal incontinence: The fecal incontinence prescription (Rx) management (FIRM) randomized clinical trial. Dis Colon Rectum 2015;58:983–93. [DOI] [PubMed] [Google Scholar]
- 16.Bohn L, Storsrud S, Liljebo T, et al. Diet low in FODMAPs reduces symptoms of irritable bowel syndrome as well as traditional dietary advice: A randomized controlled trial. Gastroenterology 2015;149:1399–407 e2. [DOI] [PubMed] [Google Scholar]