INTRODUCTION:
Microscopic colitis, a common cause of diarrhea, is characterized by a largely normal appearance of the mucosa but increased numbers of lymphocytes in the epithelium and lamina propria on microscopy. We sought to determine whether T-cell percentage was associated with exposures or symptoms.
METHODS:
We conducted a case-control study that enrolled patients referred for colonoscopy for diarrhea. Patients were classified as microscopic colitis cases or controls by an experienced pathologist. Participants provided information on symptoms and exposures during a telephone or internet survey. Research biopsies from the ascending colon and descending colon were examined using immunofluorescence stains for CD3, CD8, and FOXP3 to determine percent T cells per total epithelial or lamina propria cells. Digital images were analyzed by regions of interest using Tissue Studio.
RESULTS:
There were 97 microscopic colitis cases and 165 diarrhea controls. There was no association between demographic factors and percentage of intraepithelial or lamina propria T cells. In cases, the mean percent T cells were similar in the right colon and left colon. There was no association between mean percent T cells and stool frequency or consistency. There was no association with irritable bowel syndrome, abdominal pain, or medications purported to cause microscopic colitis.
DISCUSSION:
The lack of association between the density of T cells and medications raises further doubts about their role in disease etiology. Loose and frequent stools in patients with microscopic colitis are not correlated with T-cell density.
INTRODUCTION
First described in 1976, microscopic colitis has become a common cause for diarrhea (1). The incidence has been increasing over the past few decades in Europe and Scandinavia (2–4) but has seemed to stabilize in the United States (5). Although previously thought to be uncommon, the incidence of microscopic colitis has surpassed Crohn's disease and ulcerative colitis in Denmark (3). Microscopic colitis has received far less study than the inflammatory bowel disease.
The condition is termed microscopic colitis because the colon appears normal or nearly normal at endoscopy. The diagnosis is established by biopsy. Based on the number of intraepithelial lymphocytes and the thickness of the subepithelial collagen band, patients are classified as having lymphocytic or collagenous colitis. Both conditions have more intraepithelial lymphocytes, more than 20 for lymphocytic colitis and 10–20 for collagenous colitis (6). There are more lymphocytes in the lamina propria in both subtypes. Collagenous colitis is characterized by a subepithelial collagen band >10 μm. Based on parallel trends in incidence and similarities in age, sex, and risk factors, lymphocytic and collagenous colitis are considered histologic subtypes of the same disease that is termed microscopic colitis (7).
Previous studies have found an increase in CD3+ cells (pan T-cell marker), CD8+ cells (cytotoxic T-cell marker), and forkhead box protein 3 (FOXP3)+ cells (regulatory T-cell marker) (8). Manual counting of lymphocytes is tedious and error-prone (9). We used immunofluorescence stains and automated counting of T cells to provide a more robust and quantitative measure of T-cell infiltration. The purpose of this study was to determine whether intraepithelial or lamina propria T cells were associated with demographic factors, symptoms, and exposures in patients with microscopic colitis compared with patients referred for colonoscopy to evaluate diarrhea. We also examined biopsies from the left colon and right colon to determine objective differences in T-cell counts by location.
MATERIALS AND METHODS
The methods have been previously described (10). Between April 1, 2015, and December 22, 2020, we conducted a case-control study at the University of North Carolina Hospitals. We enrolled patients who were referred for outpatient colonoscopy for diarrhea. Patients with a history of inflammatory bowel disease were excluded. To be eligible for this study, patients had to report a Bristol Stool Form Scale type of 5, 6, or 7 (mushy, loose, or watery) during the week before their colonoscopy, regardless of stool number (11). We also queried the hospital pathology database every month and contacted patients with biopsy-proven microscopic colitis who were not previously identified.
Colonoscopy and pathology
Patients were excluded if they had gross evidence of Crohn's disease or ulcerative colitis on colonoscopy. In addition to clinical biopsies, 2 single-pass biopsies were taken from the ascending colon and descending colon. The specimens were flattened on a strip of bibulous paper and placed in a vial containing 10% formalin. Biopsies were embedded in paraffin and sections for histology stained by hematoxylin and eosin (H&E).
An experienced gastroenterology pathologist (J.T.W.) reviewed the biopsies from every patient. Patients with an expanded number of lymphocytes in the epithelium and lamina propria or a thickened subepithelial collagen band were classified as having microscopic colitis. Patients without these pathologic findings were classified as controls. Patients with Crohn's disease or ulcerative colitis on biopsy were excluded. Patients with early microscopic colitis (lower numbers of lymphocytes) were excluded to avoid misclassification.
Interview
All participants completed a 30- to 40-minute structured interview by telephone or Internet. Participants self-reported demographics, medical history (including over-the-counter and prescription medications), reproductive history, bowel habits, and gastrointestinal symptoms. We asked patients if they had been told by a doctor that they had irritable bowel syndrome (IBS).
Immunofluorescent staining
We used immunofluorescence staining because of the ability to multiplex 2 markers and therefore reduce the cost and number of slides. Mouse monoclonal antibody for CD8 (108M-95, lot # 9925) was purchased from Cell Marque (Rocklin, CA), mouse monoclonal antibodies for FOXP3 (ab20034, lot # GR3266379-1) were purchased from Abcam (Cambridge, MA), and CD3 (NCL-L-CD3-565, lot # 6055982) was purchased from Leica Biosystems (Norwell, MA).
Immunofluorescent (IF) staining was performed in the Leica BOND Rx fully automated staining platform (Leica Biosystems). Slides were dewaxed in BOND Dewax solution (AR9222) and hydrated in BOND Wash solution (AR9590). Epitope retrieval for CD3 was performed for 10 minutes in BOND Epitope Retrieval Solution 2 pH 9.0 (AR9640). All remaining targets were exposed for 20 minutes in BOND Epitope Retrieval Solution 1 pH6.0 (AR9661). The epitope retrieval was followed with 5-minute endogenous peroxidase blocking using BOND peroxide blocking solution (DS9800) and 10-minute protein blocking for CD8 and FOXP3. For the FOXP3, the IF stain application order and incubation times of the primary and secondary antibodies and the tyramide signal amplification systems were as follows: FOXP3 (1:75)—1 hour, BOND post primary—8 minutes (DS9800), BOND polymer —8 minutes (DS9800), and tyramide signal amplification-Cy5 (1:50)—15 minutes (#SAT705A001EA, Perkin Elmer). IF-stained slides were counterstained with Hoechst 33258 (#H3569, Life Technologies) and mounted with ProLong Diamond Antifade Mountant (P36961; Life Technologies/Thermal Fisher Scientific, Waltham, MA). A control tissue microarray was used to evaluate staining reproducibility between staining batches.
High-resolution acquisition of IF slides was performed with the Aperio Versa 200 scanner (Leica Biosystems, Buffalo Grove, IL) at an apparent magnification of 20×. Images were uploaded to the eSlideManager database (Aperio; eSlideManager version 12.3.3.7075) at the University of North Carolina Translational Pathology Laboratory.
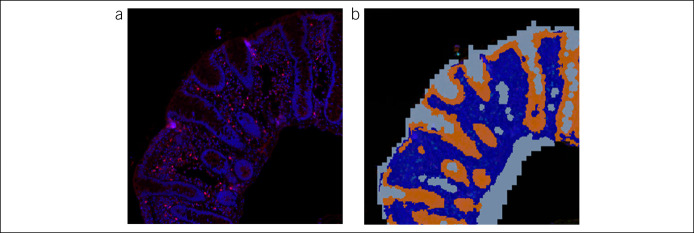
Digital images were analyzed using Tissue Studio version 2.7 with Tissue Studio Library version 4.4.2 (Definiens, Carlsbad, CA). Briefly, images from stained colon tissue sections from samples and control specimens were digitally separated into epithelial and lamina propria regions of interest using Tissue Studio Composer software (Definiens) (Figure 1). Then, cells were analyzed separately in each region of interest for signal from each of the T-cell markers of interest. Percent positively stained cells were calculated as the number of stained cells per 100 epithelial or lamina propria cells as identified by the Hoechst 33258 nuclear stain (Figure 2).
Figure 1.
Representative images from stained colon tissue sections showing identification of the ROIs for scoring. The tissue was digitally separated into epithelial and lamina propria ROIs using Tissue Studio Composer software (Definiens). The cells within each region were analyzed separately for signal from each of the markers of interest. Percent positively stained cells were calculated as the number of stained cells per 100 epithelial or lamina propria cells. (a) Immunofluorescent-stained section (b) Mask demarcating ROIs—epithelium (orange), lamina propria (dark blue), and background (light blue). ROI, regions of interest.
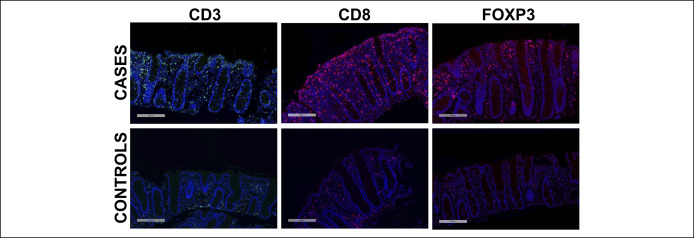
Figure 2.
Representative images showing CD3+, CD8+, and FOXP3+ cells by immunofluorescent in the colon specimens of MC case subjects (top panel) and non-MC controls (bottom panel). 10× magnification. MC, microscopic colitis.
Statistical analysis
Data analysis was conducted using Stata 17.0 (Stata, College Station, TX). The analysis was limited to patients with both exposure data and biological samples. Variables were examined one-by-one in bivariate analyses using χ2 tests for categorical variables and Student t tests for continuous variables. T-cell percentages were compared using t tests and analysis of variance (ANOVA). The association between T-cell percentages and age and body mass index was evaluated using linear regression. Missing data were not imputed.
This study was approved by the University of North Carolina Office of Human Research Ethics. All patients gave informed consent. All authors had access to the study data and reviewed and approved the final manuscript.
RESULTS
Between April 1, 2015, and December 22, 2020, 1,008 patients were nominally eligible. Of those, 176 cancelled their colonoscopy, 161 were ineligible, 99 were missed because the research assistant was not available, and 196 refused. We excluded 14 with indeterminate disease. After excluding patients without research biopsies, there were 97 cases and 165 controls.
Characteristics of the study population are provided in Table 1. Cases were older than controls, mean age 63.0 (12.8 SD) vs 54.4 years (12.1). Study participants were predominantly married and White. The cases were more likely to have a college education and beyond compared with controls, 66% for cases and 44% for controls. Among the cases, 10.6% were current smokers compared with 23.4% of the controls. The mean body mass index was 25.6 (6.4 SD) for cases and 29.1 (7.0 SD) for controls.
Table 1.
Characteristics of the study population
| Cases n = 97 |
Controls N = 165 |
P value | |||
| N or mean | % or SD | N or mean | % or SD | ||
| Age (mean, SD) | 63.0 | 12.8 SD | 54.4 | 12.1 | 0.0001 |
| Marital status | |||||
| Married | 66 | 70.2% | 93 | 66.0% | 0.49 |
| Not married | 28 | 29.8 | 48 | 34.0 | |
| Race | |||||
| White | 90 | 95.7% | 123 | 88.5% | 0.05 |
| Non-White | 4 | 4.3 | 16 | 11.5 | |
| Sex | |||||
| Female | 84 | 86.6% | 120 | 72.7% | 0.01 |
| Male | 13 | 13.4 | 45 | 27.3 | |
| Education | |||||
| College and beyond | 62 | 66.0 | 62 | 44.0 | 0.001 |
| Less than college | 32 | 34.0 | 79 | 56.0 | |
| Cigarette smoking | |||||
| Current smoker | 10 | 10.6% | 33 | 23.4% | 0.01 |
| Nonsmoker | 84 | 89.4 | 108 | 76.6 | |
| BMI (mean, SD) | 25.6 | 6.4 SD | 29.1 | 7.0 SD | 0.0001 |
BMI, body mass index.
Celiac disease has been associated with microscopic colitis (12,13). We asked cases and controls if they had a history of celiac disease and confirmed results by review of the medical record. There were 2 confirmed celiac cases among the microscopic colitis cases and 1 control. The numbers were too small (3 patients) to support separate analyses. To assess the validity of the pathologic classification, we compared the mean percent T cells in the cases of microscopic colitis with the nonmicroscopic colitis controls. As expected, the mean percentages of total T cells (CD3+) and CD8+ and FOXP3+ T-cell subsets were greater in the cases than the controls, and the differences were highly statistically significant for both the epithelium and the lamina propria for all markers (P < 0.0001) (Table 2).
Table 2.
Mean percent T cellsa by case-control status and location
|
T cell type |
Cases n = 97 |
Controls* n = 165 |
||||||||
| Ascending | Descending | P value | Ascending | Descending | P value | |||||
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | |||
| CD3+ epithelium | 11.9 | 5.6 | 10.3 | 5.4 | 0.05 | 5.6 | 3.4 | 5.1 | 3.2 | 0.12 |
| CD3+ lamina propria | 17.0 | 7.7 | 17.6 | 7.1 | 0.56 | 11.4 | 4.8 | 10.7 | 4.60 | 0.19 |
| CD8+ epithelium | 6.9 | 5.0 | 6.2 | 4.6 | 0.32 | 2.4 | 1.6 | 1.9 | 1.4 | 0.001 |
| CD8+ lamina propria | 7.6 | 4.1 | 7.2 | 3.8 | 0.39 | 3.7 | 2.0 | 3.0 | 1.8 | 0.0009 |
| FOXP3+ epithelium | 1.7 | 2.2 | 1.5 | 1.8 | 0.50 | 1.1 | 1.5 | 0.7 | 1.0 | 0.08 |
| FOXP3+ lamina propria | 5.9 | 5.3 | 5.7 | 4.2 | 0.78 | 3.1 | 2.5 | 2.1 | 1.8 | 0.0001 |
Percent T cells calculated as the no. of stained cells per 100 epithelial or lamina propria cells.
P- < 0.0001 for cases compared with controls for all makers and locations.
We next examined whether there were differences in lymphocyte percentages for biopsies from the ascending colon compared with the descending colon. The results are given in Table 2. For the cases, the means in the ascending colon and the descending colon were similar for CD8+ and FOXP3+ T-cell subsets from the epithelium and the lamina propria. The P value was 0.05 for total CD3+ cells from the epithelium, but the mean number of cells was not meaningfully different, 11.9% ascending and 10.3% descending. For controls, on the other hand, T-lymphocyte counts were higher in the ascending colon than in the descending colon for each lymphocyte marker. The differences were significant for CD8+ T cells in both the epithelium and lamina propria and FOXP3+ T cells in the lamina propria. All subsequent analyses for cases are based on mean values from the ascending colon and the descending colon combined.
In an exploratory analysis, we combined the biopsies from the ascending colon and descending colon and compared the number of lymphocytes for each location and each T-cell marker in collagenous colitis vs lymphocytic colitis. The results are provided in Supplementary Table 1 (see Supplementary Digital Content, http://links.lww.com/CTG/A768). The percent lymphocytes were slightly higher in the lymphocytic colitis than the collagenous colitis patients, but the differences were not statistically significant. Patients can have characteristics of both lymphocytic and collagenous colitis. As expected, the mean percent lymphocytes for the “both” category were intermediate between the lymphocytic and collagenous colitis means. The controls were significantly lower.
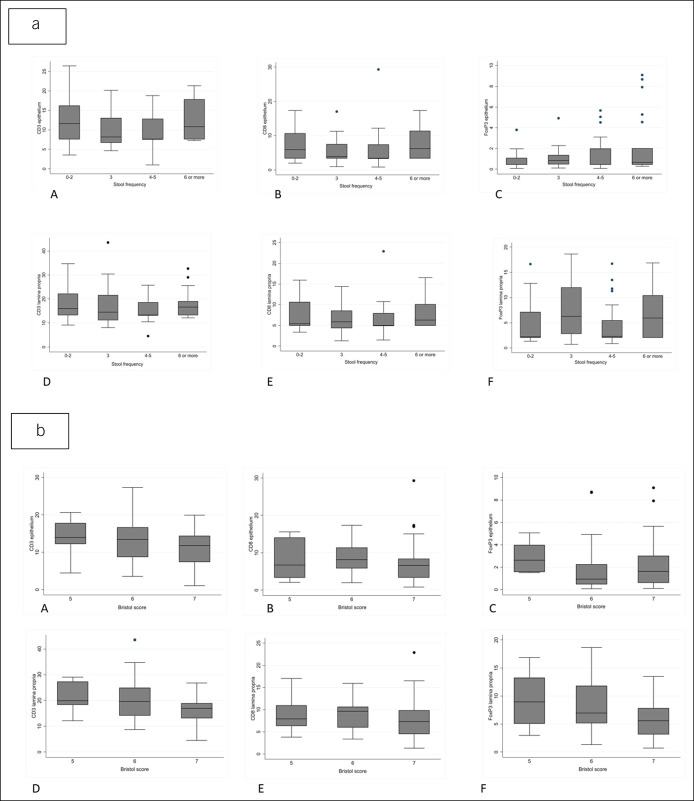
We next examined whether the percentage of T cells correlated with stool frequency in the microscopic colitis cases. There were 86 patients who provided information on stool frequency and had biopsies examined. As provided in Table 3 and Figure 3a, we categorized the number of loose stools per day into 4 categories and compared the means in each stool frequency category for each T-cell marker using ANOVA. There was no correlation between the mean percent T cells and number of loose stools per day. We similarly examined the mean percent T cells by marker according to stool consistency as measured by the Bristol Stool Form Scale again using ANOVA in 68 patients (Table 3 and Figure 3b). There was no association between stool form and any of the T-cell markers in the epithelium or the lamina propria. We further examined whether there was an association with abdominal pain or physician-diagnosed IBS (Table 4).
Table 3.
Mean (SD) percent T cellsa by location in microscopic colitis cases by category of liquid stools per day (n = 86) and Bristol stool score (n = 68)
| No. of loose stools per day | P value | Bristol stool typeb | P value | ||||||
| Lymphocyte type and location | 0–2 (n = 13) | 3 (n = 16) | 4–5 (n = 35) | 6 or more (n = 22) | Type 5 (n = 6) | Type 6 (n = 30) | Type 7 (n = 32) | ||
| CD3+ epithelium | 12.1 (6.2) | 10.0 (4.7) | 9.7 (3.9) | 12.3 (5.0) | 0.15 | 14.9 (5.5) | 13.6 (5.9) | 11.4 (4.6) | 0.21 |
| CD3+ lamina propria | 17.7 (6.8) | 17.6 (9.5) | 15.6 (4.7) | 17.7 (5.7) | 0.54 | 21.1 (6.3) | 21.2 (9.2) | 16.7 (5.2) | 0.05 |
| CD8+ epithelium | 7.3 (5.1) | 5.8 (4.1) | 5.6 (4.9) | 7.4 (4.3) | 0.42 | 8.1 (5.6) | 8.4 (3.8) | 7.2 (5.8) | 0.73 |
| CD8+ lamina propria | 7.9 (4.2) | 6.8 (3.5) | 6.7 (3.6) | 7.7 (3.2) | 0.60 | 9.0 (4.6) | 8.9 (3.2) | 8.0 (4.6) | 0.82 |
| FOXP3+ epithelium | 0.89 (1.0) | 1.2 (1.2) | 1.4 (1.5) | 2.2 (2.9) | 0.15 | 2.9 (1.5) | 1.8 (2.2) | 2.3 (2.2) | 0.47 |
| FOXP3+ lamina propria | 5.6 (5.20) | 7.2 (5.2) | 4.3 (3.8) | 6.6 (4.6) | 0.13 | 9.3 (5.4) | 8.4 (5.0) | 6.0 (3.7) | 0.06 |
Percent T cells calculated as the number of stained cells per 100 epithelial or lamina propria cells.
Type 5—soft blobs with clear-cut edges passed easily; type 6—fluffy pieces with ragged edges, a mushy stool; type 7—watery, no solid pieces, and entirely liquid.
Figure 3.
(a) Mean percent T cells by region and stool frequency. (b) Mean percent T cells by region and Bristol stool scale (type 5—soft blobs with clear-cut edges passed easily; type 6—fluffy pieces with ragged edges, a mushy stool; and type 7—watery, no solid pieces). Top row epithelium: (A) CD3+, (B) CD8+, and (C) FOXP3+. Bottom row lamina propria: (D) CD3+, (E) CD8+, and (F) FOXP3+.
Table 4.
Mean percent (and SD) T cellsa by location in microscopic colitis cases by abdominal pain and physician-diagnosed IBS
| Abdominal pain | Physician-diagnosed IBS | |||||||||
| Yes | No | P value | Yes | No | ||||||
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | P value | ||
| CD3+ epithelium | 10.3 | 5.3 | 11.2 | 4.2 | 0.36 | 11.9 | 4.7.2 | 11.0 | 0.3.5 | |
| CD3+ lamina propria | 16.3 | 6.4 | 17.3 | 6.4 | 0.47 | 16.3 | 4.3 | 17.2 | 6.8 | 0.61 |
| CD8+ epithelium | 6.2 | 4.0 | 6.5 | 5.2 | 0.74 | 6.3 | 2.3 | 6.7 | 5.0 | 0.72 |
| CD8+ lamina propria | 7.0 | 3.1 | 7.31 | 4.0 | 0.73 | 7.2 | 2.1 | 7.5 | 3.9 | 0.81 |
| FOXP3+ epithelium | 1.54 | 2.1 | 1.43 | 1.6 | 0.79 | 2.2 | 2.3 | 1.6 | 1.9 | 0.28 |
| FOXP3+ lamina propria | 5.3 | 4.7 | 5.9 | 4.4 | 0.55 | 5.1 | 3.4 | 5.7 | 4.5 | 0..67 |
IBS, irritable bowel syndrome.
Percent T cells calculated as the number of stained cells per 100 epithelial or lamina propria cells.
Microscopic colitis has been associated with a number of different drugs, particularly proton-pump inhibitors (PPIs), nonsteroidal anti-inflammatory drugs (NSAIDs), statins, and selective serotonin reuptake inhibitors (SSRIs). We examined the mean percent T cells for drug users and nonusers (both cases and controls; Table 5). If these drugs were associated with microscopic colitis, we would expect higher mean percent T cells in drug users than nonusers. There were no differences in the percentages of any of the T-cell populations in the lamina propria or epithelium for any of the medications.
Table 5.
Mean (SD) percent T cells by location in microscopic colitis cases by medication use
| PPI | NSAID | Statin | SSRI | |||||||||
| Yes N = 98 |
No N = 144 |
P value | Yes N = 120 |
No N = 121 |
P value | Yes N = 74 |
No N = 168 |
P value | Yes N = 99 |
No N = 142 |
P value | |
| CD3+ epithelium | 7.57 | 7.97 | 0.53 | 7.25 | 8.22 | 0.12 | 7.97 | 7.73 | 0.75 | 7.95 | 7.68 | 0.68 |
| CD3+ lamina propria | 12.95 | 14.06 | 0.15 | 13.17 | 13.91 | 0.33 | 13.62 | 13.61 | 0.98 | 13.6 | 13.54 | 0.88 |
| CD8+ epithelium | 3.47 | 4.36 | 0.08 | 3.63 | 4.27 | 0.19 | 1.04 | 3.98 | 0.90 | 4.05 | 3.96 | 0.84 |
| CD8+ lamina propria | 4.42 | 5.43 | 0.02 | 4.5 | 5.41 | 0.05 | 5.11 | 4.98 | 0.77 | 5.00 | 5.03 | 0.95 |
| FOXP3+ epithelium | 1.23 | 2.20 | 0.89 | 1.21 | 1.21 | 0.97 | 1.05 | 1.28 | 0.26 | 1.46 | 1.04 | 0.03 |
| FOXP3+ lamina propria | 3.41 | 4.18 | 0.10 | 3.83 | 3.84 | 0.99 | 1.08 | 3.77 | 0.53 | 3.82 | 3.90 | 0.86 |
NSAID, nonsteroidal anti-inflammatory drug; PPI, proton-pump inhibitors; SSRI, statins and selective serotonin reuptake inhibitor.
DISCUSSION
We used immunofluorescence staining and automated counting to characterize lymphocyte type and location in a large cohort of patients with microscopic colitis compared with controls with diarrhea. As expected, percentages of total T cells and CD8+ and FOXP3+ T-cell subsets were more frequent in cases than controls in both the epithelium and the lamina propria. We found that T-cell percentages were similar in the ascending colon and the descending colon for microscopic colitis cases, supporting the conclusion that a limited colonoscopy would generally be reliable for making the diagnosis (14). In controls, percentages of CD8+ and FOXP3+ T cells were higher in the right colon lamina propria as previously reported, which serves to validate our method of counting T cells (15). We found no association between T-cell percentages and the frequency of loose stools or stool consistency as measured by the Bristol Stool Form Scale. There was no association between T-cell percentages and the drugs that are most commonly linked with microscopic colitis—PPIs, NSAIDs, statins, and SSRIs.
The term microscopic colitis refers to the fact that the mucosa is normal grossly, although in many cases, there are subtle changes, such as edema, friability, alteration of the vasculature, or nodularity (15). Histologically, there are more intraepithelial lymphocytes (15). Crypt architectural irregularity can sometimes be seen (16). Lymphocytic colitis is conventionally defined by more intraepithelial lymphocytes, >20 per 100 surface epithelial cells along with an increased inflammatory infiltrate in lamina propria on H&E slides (17). Collagenous colitis is also characterized by both intraepithelial and lamina propria lymphocytes. Immunologically, most of the intraepithelial lymphocytes are characterized as CD8+ T cells (18). FOXP3+ T regulatory cells have also been reported to be increased in patients with microscopic colitis compared with controls (8,18–20). FOXP3 is a transcription factor that is specifically expressed in certain regulatory T cells that suppress inflammation (19).
To provide a quantitative analysis of T-cell percentages, we used immunofluorescence and automated analysis. Using immunohistochemistry, Goranzon et al. (8) found elevated numbers of CD8+ lymphocytes in the epithelium and the lamina propria in 23 patients with microscopic colitis They also found that FOXP3+ cells were more abundant. The increase in regulatory FOXP3+ T cells is likely a compensatory response that attenuates the severity of inflammation (8). Fiehn et al. (21) used immunohistochemistry to study 40 patients with lymphocytic colitis. The number of intraepithelial lymphocytes was higher using immunohistochemistry than H&E-stained slides and even higher when they used image analysis. In addition, because customary diagnostic criteria are based on H&E staining that does not distinguish between lymphocyte types (e.g., B cells and T cells), and because we only measured total T cells, CD8+ T cells, and FOXP3+ T cells, our percentages may underestimate the total lymphocyte count observed in H&E-stained tissues.
We found that the percentages of T cells and T-cell subsets were similar in the right colon and the left colon in patients with microscopic colitis. Most previous studies report either no differences or modest differences based on the colon location. In a small study (8 patients with lymphocytic colitis), Chadwick et al.(22) found a gradient from the right colon to the rectum. A multicenter study from France reported that the diagnostic sensitivity was highest in the transverse colon and lowest in the rectum, but the median number of lymphocytes (median 30) was similar in the sigmoid and left colon compared with the right colon (14). Although our data support a limited colonoscopy to the descending colon to make a diagnosis of microscopic colitis, a full colonoscopy is likely to be necessary to exclude diseases in the right colon that might be causing diarrhea, for example, ileocolonic Crohn's disease. Biopsies from both the ascending colon and descending colon have been shown to make the diagnosis of microscopic colitis in 100% of cases (23). In a frail patient, a more limited examination could be considered.
We found no association between diarrhea symptoms and T-cell infiltration in either the epithelium or the lamina propria of microscopic colitis cases. Although one might expect that the extent of inflammation, as measured by the percentage of lymphocytes, might correlate with symptoms, that has generally not been the case. Olsen et al. (6) found that neither the total cell density in the lamina propria, proportion of CD3+ lymphocytes in the lamina propria, nor surface epithelium correlated with the number of daily stools or watery stools in 60 patients from a European prospective registry. Similarly, Shaz et al. (24) found no association between symptoms and histologic findings. There was no correlation between the intensity of histologic inflammation and the severity of clinical symptoms in a study of 129 patients with microscopic colitis (14). The mechanism for diarrhea in patients with microscopic colitis is unknown. Reduced sodium and chloride absorption is thought to be the predominant mechanism along with a secretory component of active electrogenic chloride secretion (25).
Animal studies have shown that T cells may exert visceral analgesic effects (26). We did not find an association between T-cell numbers and abdominal pain or between percentages of T cells or T-cell subsets and IBS.
Microscopic colitis has been linked with a number of drugs, most notably PPIs, NSAIDs, statins, and SSRIs. The associations are surprising because the drugs belong to different classes. Many studies were case series without a control group (27–34). Other studies have compared patients with microscopic colitis with community or population controls (35,36). Studies that have used diarrhea controls found no association (37,38). A recently published study compared patients with microscopic colitis with patients with diarrhea undergoing colonoscopy (39). They found an inverse association between microscopic colitis and PPIs, H2 blockers. If medications are responsible for the development of microscopic colitis, one might anticipate that the offending drugs would be associated with higher numbers of lymphocytes. We found no association between overall T cells, CD8+ T cells, or FOXP3+ T cells and PPI, NSAIDs, statins, or SSRIs in microscopic colitis cases. These results suggest that these drugs do not play a role in the etiology of microscopic colitis.
A notable strength of our study was the relatively large size compared with many published reports. All the patients enrolled in this study were referred to a single medical center for colonoscopy for diarrhea. Because we compared the patients with microscopic colitis with nonmicroscopic colitis diarrhea controls, the groups were comparable for symptoms, access to care, completion of a colonoscopy, and willingness to participate in a research study. We used objective methods to quantify T-cell percentages, and we examined intraepithelial and lamina propria T cells using stains for CD3, CD8, and FOXP3. The patient data included information on drug exposure and autoimmune diseases.
Microscopic colitis is the term used to describe lymphocytic colitis and collagenous colitis. We included both lymphocytic and collagenous colitis in this article. Both forms of microscopic colitis have had parallel increases in incidence and similar symptoms, age of onset, female predominance, and response to treatment (7,40). When lymphocytic and collagenous colitis are considered separately, the sample size is reduced in each group, making it difficult to demonstrate associations. In stratified analyses, we did not find differences between lymphocytic and collagenous colitis.
Despite the fact that this study was larger than most previous studies, the sample size was still relatively modest. We used very specific lymphocyte markers to avoid misclassification. This strategy may have led to the detection of lower numbers of lymphocytes and prevented direct comparison of our numbers with other reports. In addition, because customary diagnostic criteria are based on H&E staining that does not distinguish between lymphocyte types (e.g., B cells and T cells), and because we only measured total T cells, CD8+ T cells, and FOXP3+ T cells, our percentages may underestimate the total lymphocyte count observed in H&E-stained tissues.
We found similar numbers of T cells in the left colon and right colon in patients with microscopic colitis. We found no association between mean percentages of T cells and demographic factors, medications purportedly related to microscopic colitis, symptoms, and stool characteristics in patients with microscopic colitis. For future studies of microscopic colitis, we would advocate objective and quantitative measurement of lymphocyte infiltration, diarrhea controls to prevent selection bias, and detailed information on exposures. Now that microscopic colitis is as common as Crohn's disease and ulcerative colitis, it is time for more research.
CONFLICT OF INTEREST
Guarantor of the article: Robert S. Sandler, MD, MPH.
Specific author contributions: R.S.S.: study concept and design, acquisition of data, analysis and interpretation, drafting manuscript, critical revisions, statistical analysis, obtaining funding, and approved final draft. J.J.H.: critical revision. A.F.P.: analysis and interpretation, critical revisions, statistical analysis, and approved final draft. J.T.W.: study concept and design, acquisition of data, analysis and interpretation, critical revisions, and approved final draft. J.A.G.: analysis and interpretation, critical revisions, statistical analysis, and approved final draft. T.O.K.: study concept and design, acquisition of data, analysis and interpretation, critical revision, obtaining funding, and approved final draft.
Financial support: This research was supported, in part, by grants from the National Institutes of Health (P30 DK034987, R01 DK105114).
Potential competing interests: None to report.
Study Highlights.
WHAT IS KNOWN
✓ Microscopic colitis is a common cause of watery diarrhea.
✓ Information on risk factors is limited based on small numbers of cases and variable controls.
WHAT IS NEW HERE
✓ There was no association between T-cell percent and symptoms, stool characteristics, or medication exposure.
✓ Comparing microscopic colitis cases with controls with diarrhea may provide new insights.
Supplementary Material
ACKNOWLEDGEMENTS
We thank Stephanie Montgomery, Dr. Nana Nikolaishvili-Feinberg, and Bentley Midkiff in the UNC Translational Pathology Laboratory Services Core for expert technical assistance with immunofluorescence and digital image analysis. The PSC is supported in part by an NCI Center Core Support Grant (5P30CA016080-42). Histology was performed by Ms. Carolyn Suitt at the CGIBD Histology Core at the University of North Carolina at Chapel Hill, which is supported by an NIDDK Grant (P30 DK034987). We thank Ms. Amber McCoy for laboratory expertise and assistance with coordinating specimen handling and processing for histology and digital image analysis.
Footnotes
SUPPLEMENTARY MATERIAL accompanies this paper at http://links.lww.com/CTG/A768
Contributor Information
Jonathan J. Hansen, Email: jonathan_hansen@med.unc.edu.
Anne F. Peery, Email: anne_peery@med.unc.edu.
John T. Woosley, Email: john_woosley@med.unc.edu.
Joseph A. Galanko, Email: joe_galanko@hotmail.com.
Temitope O. Keku, Email: temitope_keku@med.unc.edu.
REFERENCES
- 1.Lindstrom CG. “Collagenous colitis” with watery diarrhoea–a new entity? Pathol Eur 1976;11:87–9. [PubMed] [Google Scholar]
- 2.Maye H, Safroneeva E, Godat S, et al. Increasing incidence of microscopic colitis in a population-based cohort study in Switzerland. Clin Gastroenterol Hepatol 2020;19:2205–6. [DOI] [PubMed] [Google Scholar]
- 3.Weimers P, Ankersen DV, Lophaven S, et al. Incidence and prevalence of microscopic colitis between 2001 and 2016: A Danish nationwide cohort study. J Crohns Colitis 2020;14:1717–23. [DOI] [PubMed] [Google Scholar]
- 4.Bergman D, Clements MS, Khalili H, et al. A nationwide cohort study of the incidence of microscopic colitis in Sweden. Aliment Pharmacol Ther 2019;49:1395–400. [DOI] [PubMed] [Google Scholar]
- 5.Tome J, Sehgal K, Kamboj AK, et al. The epidemiology of microscopic colitis in Olmsted County, Minnesota: Population-based study from 2011 to 2019. Clin Gastroenterol Hepatol 2021. doi: 10.1016/j.cgh.2021.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Olsen LM, Engel PJH, Goudkade D, et al. Histological disease activity in patients with microscopic colitis is not related to clinical disease activity or long-term prognosis. Aliment Pharmacol Ther 2021;54:43–52. [DOI] [PubMed] [Google Scholar]
- 7.Rasmussen MA, Munck LK. Systematic review: Are lymphocytic colitis and collagenous colitis two subtypes of the same disease - microscopic colitis? Aliment Pharmacol Ther 2012;36:79–90. [DOI] [PubMed] [Google Scholar]
- 8.Goranzon C, Kumawat AK, Hultgren-Hornqvist E, et al. Immunohistochemical characterization of lymphocytes in microscopic colitis. J Crohns Colitis 2013;7:e434–42. [DOI] [PubMed] [Google Scholar]
- 9.Diem K, Magaret A, Klock A, et al. Image analysis for accurately counting CD4+ and CD8+ T cells in human tissue. J Virol Methods 2015;222:117–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sandler RS, Keku TO, Woosley JT, et al. Medication use and microscopic colitis. Aliment Pharmacol Ther 2021;54:1193–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lewis SJ, Heaton KW. Stool form scale as a useful guide to intestinal transit time. Scand J Gastroenterol 1997;32:920–4. [DOI] [PubMed] [Google Scholar]
- 12.Vigren L, Tysk C, Strom M, et al. Celiac disease and other autoimmune diseases in patients with collagenous colitis. Scand J Gastroenterol 2013;48:944–50. [DOI] [PubMed] [Google Scholar]
- 13.Liu PH, Lebwohl B, Burke KE, et al. Dietary gluten intake and risk of microscopic colitis among US women without celiac disease: A prospective cohort study. Am J Gastroenterol 2019;114:127–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Macaigne G, Lahmek P, Locher C, et al. Over 90% of cases of microscopic colitis can be diagnosed by performing a short colonoscopy. Clin Res Hepatol Gastroenterol 2017;41:333–340. [DOI] [PubMed] [Google Scholar]
- 15.Langner C, Aust D, Ensari A, et al. Histology of microscopic colitis-review with a practical approach for pathologists. Histopathology 2015;66:613–26. [DOI] [PubMed] [Google Scholar]
- 16.Ayata G, Ithamukkala S, Sapp H, et al. Prevalence and significance of inflammatory bowel disease-like morphologic features in collagenous and lymphocytic colitis. Am J Surg Pathol 2002;26:1414–23. [DOI] [PubMed] [Google Scholar]
- 17.Miehlke S, Guagnozzi D, Zabana Y, et al. European guidelines on microscopic colitis: United European Gastroenterology and European Microscopic Colitis Group statements and recommendations. United Eur Gastroenterol J 2021;9:13–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Carrasco A, Esteve M, Salas A, et al. Immunological differences between lymphocytic and collagenous colitis. J Crohns Colitis 2016;10:1055–66. [DOI] [PubMed] [Google Scholar]
- 19.Fernandez-Banares F, Casalots J, Salas A, et al. Paucicellular lymphocytic colitis: Is it a minor form of lymphocytic colitis? A clinical pathological and immunological study. Am J Gastroenterol 2009;104:1189–98. [DOI] [PubMed] [Google Scholar]
- 20.Bai S, Siegal GP, Jhala NC. Foxp3 expression patterns in microscopic colitides: A clinicopathologic study of 69 patients. Am J Clin Pathol 2012;137:931–6. [DOI] [PubMed] [Google Scholar]
- 21.Fiehn AK, Miehlke S, Aust D, et al. Distribution of histopathological features along the colon in microscopic colitis. Int J Colorectal Dis 2021;36:151–9. [DOI] [PubMed] [Google Scholar]
- 22.Chadwick VS, Chen W, Shu D, et al. Activation of the mucosal immune system in irritable bowel syndrome. Gastroenterology 2002;122:1778–83. [DOI] [PubMed] [Google Scholar]
- 23.Virine B, Chande N, Driman DK. Biopsies from ascending and descending colon are sufficient for diagnosis of microscopic colitis. Clin Gastroenterol Hepatol 2020;18:2003–9. [DOI] [PubMed] [Google Scholar]
- 24.Shaz BH, Reddy SI, Ayata G, et al. Sequential clinical and histopathological changes in collagenous and lymphocytic colitis over time. Mod Pathol 2004;17:395–401. [DOI] [PubMed] [Google Scholar]
- 25.Burgel N, Bojarski C, Mankertz J, et al. Mechanisms of diarrhea in collagenous colitis. Gastroenterology 2002;123:433–43. [DOI] [PubMed] [Google Scholar]
- 26.Cremon C, Gargano L, Morselli-Labate AM, et al. Mucosal immune activation in irritable bowel syndrome: Gender-dependence and association with digestive symptoms. Am J Gastroenterol 2009;104:392–400. [DOI] [PubMed] [Google Scholar]
- 27.Bohr J, Tysk C, Eriksson S, et al. Collagenous colitis: A retrospective study of clinical presentation and treatment in 163 patients. Gut 1996;39:846–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fumery M, Kohut M, Gower-Rousseau C, et al. Incidence, clinical presentation, and associated factors of microscopic colitis in northern France: A population-based study. Dig Dis Sci 2017;62:1571–9. [DOI] [PubMed] [Google Scholar]
- 29.Goff JS, Barnett JL, Pelke T, et al. Collagenous colitis: Histopathology and clinical course. Am J Gastroenterol 1997;92:57–60. [PubMed] [Google Scholar]
- 30.Olesen M, Eriksson S, Bohr J, et al. Lymphocytic colitis: A retrospective clinical study of 199 Swedish patients. Gut 2004;53:536–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Veress B, Lofberg R, Bergman L. Microscopic colitis syndrome. Gut 1995;36:880–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Abdo A, Raboud J, Freeman HJ, et al. Clinical and histological predictors of response to medical therapy in collagenous colitis. Am J Gastroenterol 2002;97:1164–8. [DOI] [PubMed] [Google Scholar]
- 33.Simondi D, Pellicano R, Reggiani S, et al. A retrospective study on a cohort of patients with lymphocytic colitis. Rev Esp Enferm Dig 2010;102:381–4. [DOI] [PubMed] [Google Scholar]
- 34.Pardi DS, Ramnath VR, Loftus EV, Jr, et al. Lymphocytic colitis: Clinical features, treatment, and outcomes. Am J Gastroenterol 2002;97:2829–33. [DOI] [PubMed] [Google Scholar]
- 35.Bonderup OK, Fenger-Gron M, Wigh T, et al. Drug exposure and risk of microscopic colitis: A nationwide Danish case-control study with 5751 cases. Inflamm Bowel Dis 2014;20:1702–7. [DOI] [PubMed] [Google Scholar]
- 36.Masclee GM, Coloma PM, Kuipers EJ, et al. Increased risk of microscopic colitis with use of proton pump inhibitors and non-steroidal anti-inflammatory drugs. Am J Gastroenterol 2015;110:749–59. [DOI] [PubMed] [Google Scholar]
- 37.Pascua MF, Kedia P, Weiner MG, et al. Microscopic colitis and medication use. Clin Med Insights Gastroenterol 2010;2010:11–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Guagnozzi D, Lucendo AJ, Angueira T, et al. Drug consumption and additional risk factors associated with microscopic colitis: Case-control study. Rev Esp Enferm Dig 2015;107:347–53. [PubMed] [Google Scholar]
- 39.Zylberberg HM, Kamboj AK, De Cuir N, et al. Medication use and microscopic colitis: A multicentre retrospective cohort study. Aliment Pharmacol Ther 2021;53:1209–15. [DOI] [PubMed] [Google Scholar]
- 40.Bjornbak C, Engel PJ, Nielsen PL, et al. Microscopic colitis: Clinical findings, topography and persistence of histopathological subgroups. Aliment Pharmacol Ther 2011;34:1225–34. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.