Abstract
Wild poliovirus (WPV) is nearing eradication and only three countries have never interrupted wild poliovirus transmission (Pakistan, Afghanistan, Nigeria). WPV2 was last detected in 1999 and it was declared eradicated in 2015. WPV3 has not been detected since 2012. Since 2016, WPV1 has been detected in only two countries (Afghanistan and Pakistan), with only 22 cases reported in 2017 and 12 cases reported in 2018 (as of July 10). Because of WPV2 eradication and the risk of emergence of type 2 vaccine-derived polioviruses from continued use of trivalent oral polio vaccine (tOPV), tOPV was replaced by bivalent OPV (types 1 and 3) in a globally coordinated effort in 2016. WPV2 eradication and tOPV cessation also mean that breach of containment in a facility working with type 2 poliovirus is now a major risk to reseed type 2 circulation in the community. As a result, the World Health Organization has developed a “Global Action Plan to minimize poliovirus facility-associated risk after type-specific eradication of wild polioviruses and sequential cessation of oral polio vaccine use.” Because poliovirus has long been used as a standard for qualification of intravenous immunoglobulin, disinfectant products, and sanitation methods, poliovirus containment has implications far beyond poliovirus laboratories.
Background
Poliovirus (PV) occurs as three antigenically distinct serotypes, PV1, PV2, and PV3; humans are the only natural host. In susceptible children, approximately 70% of infections are asymptomatic and most of the remainder present with only mild, nonspecific illness with low-grade fever and sore throat1. Viral meningitis occurs in 1% to 5% of infections and less than 1% of infections result in acute flaccid paralysis (paralytic poliomyelitis; AFP) affecting one or more limbs. Paralysis is often permanent due to destruction of motor neurons in the anterior horn of the spinal cord, though some patients may regain at least some function in one or more affected limbs2. The virus is excreted in stool for 3–4 weeks post-infection3. Transmission is primarily by the fecal-oral route in areas with poor sanitation and by the oral-oral route in countries with improved sanitation2. Historically, poliovirus was a major cause of AFP in children. Therefore, the global polio eradication program has used AFP surveillance as the primary tool to detect poliovirus circulation. Stool samples from AFP cases are submitted to WHO-accredited laboratories for testing and the results are used to guide immunization efforts4. Environmental surveillance—testing of concentrated sewage for the presence of poliovirus—is often used to supplement AFP surveillance in countries considered to be at increased risk for poliovirus introduction or transmission4.
The number of paralytic poliomyelitis cases due to wild poliovirus infection is at an all-time low worldwide. When the Global Polio Eradication Initiative (GPEI) was initiated in 1988, there were approximately 350,000 paralytic poliomyelitis cases annually, in 125 countries. A total of 22 cases caused by wild poliovirus were reported In 2017, in only two countries, a reduction of >99.99%5,6. As of July 10, only 12 cases have been reported in 2018 (Figure 1A). Wild poliovirus (WPV) type 2 was last detected in 1999 and it was declared eradicated by the World Health Assembly in 20157. The last detection of WPV3 was in November 2012, leaving WPV1 as the only remaining wild poliovirus in circulation.
Figure 1.
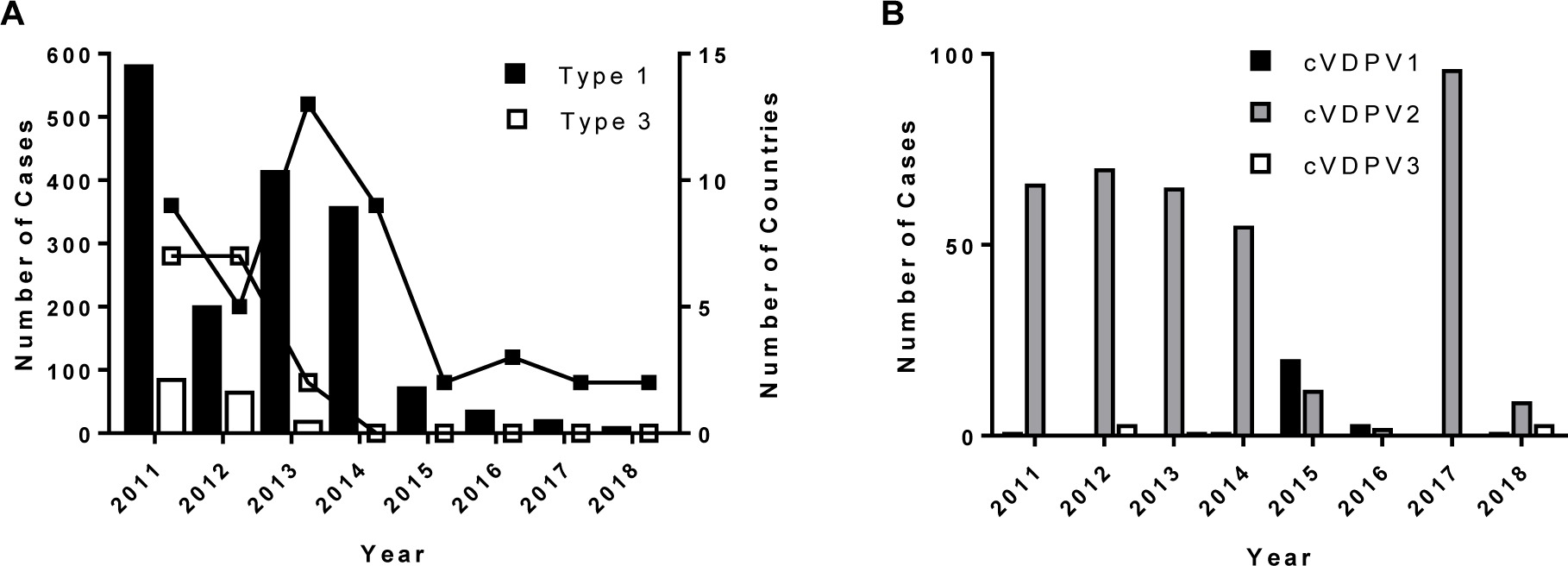
Paralytic poliomyelitis cases, 2011–2018. A. WPV cases and number of affected countries. B. Circulating vaccine-derived poliovirus cases. Data are from www.polioeradication.org 6 and Burns et al.13
Two vaccines are available to protect against paralytic poliomyelitis. The inactivated polio vaccine (IPV), introduced by Salk and colleagues in 1955 is produced from wild poliovirus strains that were isolated from paralytic cases in the 1930s and 1940s8; until the formalin inactivation step, the IPV strains are highly pathogenic and neurovirulent, requiring particular care during the production process9. IPV is highly immunogenic, inducing humoral antibodies that protect against disease, probably by inhibiting systemic infection and preventing virus from entering the central nervous system. Its disadvantages are the need for trained medical staff to administer injections by needle and syringe and its relatively high cost, both of which have limited its use in developing country settings. The live, attenuated oral polio vaccine (OPV) was developed by Sabin and colleagues just a few years after the introduction of IPV2. OPV is cheaper to produce than IPV and can be delivered by minimally trained volunteers, but it is somewhat less immunogenic than IPV on a per-dose basis. The attenuated virus replicates in the intestinal tract of immunized individuals, stimulating both mucosal and humoral antibodies. Like wild virus, OPV viruses are excreted in stool, though the duration of excretion is generally shorter than for WPV3. In an immune population, OPV excretion can result in secondary transmission to close contacts of the primary vaccinee, providing additional protection in the community. With both vaccines, immune individuals can still be reinfected by wild or vaccine viruses, but they are fully protected from disease. Mucosal immunity induced by OPV has the added advantage of limiting the replication of a subsequent wild virus infection, resulting in reduced virus titers in stool and thus reduced transmission of the vaccine virus. IPV, on the other hand, can induce mucosal immunity only after previous exposure to live virus (either from OPV or natural exposure to wild virus). Because of its low cost and ease of administration, OPV has been GPEI’s primary tool to induce polio immunity worldwide.
Some of OPV’s strengths (live virus, secondary transmission) are also its biggest weaknesses. Due to their error-prone RNA-dependent RNA polymerase, polioviruses (and picornaviruses, in general) evolve extremely rapidly at the nucleotide level, on the order of 1% per year along a chain of transmission10. However, ~90% of these are synonymous substitutions (i.e. there is no change in the encoded amino acid), so that the same vaccines developed over 60 years ago still provide immunity to the genetically distinct wild viruses that circulate today. OPV strains were derived by serial passage of wild polioviruses in cell culture and/or non-human primates, resulting in attenuation of replication efficiency as well as neurovirulence2. The genomic sites that confer the attenuated phenotype are known for all three serotypes2. Due to the high error rate of the viral polymerase and strong selection for reversion of the attenuating phenotype during replication in the human gut, the vaccine viruses can accumulate nucleotide substitutions at the attenuation sites, recovering their replication and neurovirulence properties.
Despite its well-established safety record, OPV can rarely cause vaccine-associated paralytic poliomyelitis (VAPP), at a rate of about one case per 2.7 million OPV doses11. OPV use can also be associated with rare emergence of genetically divergent vaccine-derived polioviruses (VDPVs) whose genetic drift from the parental OPV strains indicates prolonged replication or circulation12,13. Circulating vaccine-derived polioviruses (cVDPVs) can emerge very rarely in areas with inadequate OPV coverage. A number of cVDPV outbreaks have been documented since the identification of a type 1 cVDPV outbreak in Hispaniola in 200013 and 14 cVDPV cases, the majority of which are type 2, have been reported to date in 20186, as of July 10 (Figure 1B). VDPVs can also emerge among persons with primary immunodeficiencies (PIDs) if they are immunized with OPV (usually at a very young age, before diagnosis of their immunodeficiency, since OPV is contraindicated in immunocompromised individuals) or if they are exposed to a recent vaccinee (often a sibling or other close contact). Because immunity to poliovirus is primarily antibody-mediated, persons with deficits in humoral immunity (e.g. agammaglobulinemia, hypogammaglobulinemia, combined variable immunodeficiency, severe combined immunodeficiency) are particularly at risk for prolonged excretion of vaccine virus and possible paralytic disease14. In OPV-using developed countries where prophylactic immunoglobulin therapy is also available, PID patients are generally protected from paralytic disease even if they become infected with a poliovirus vaccine strain. However, immunoglobulin preparations must have serotype-specific antibodies to the infecting poliovirus to be effective. Immunodeficiency-associated VDPVs (iVDPVs) can replicate for years in some persons with PIDs15. Antiviral drugs are being developed to treat chronically infected PID patients16.
OPV cessation and poliovirus containment
As a result of WPV2 eradication and risk of emergence of type 2 VDPVs from continued use of trivalent oral polio vaccine (tOPV), tOPV was replaced by bivalent OPV (bOPV; types 1 and 3) in a globally coordinated effort in April 201617. Concomitant with OPV2 withdrawal, WHO recommended inclusion of at least one dose of IPV in routine immunization schedules worldwide. Monovalent type 2 OPV (mOPV2) is maintained in a global stockpile for emergency use and has been used to respond to a number of VDPV2 outbreaks since the cessation of trivalent OPV use. Because type 2 poliovirus is an eradicated agent (except for cVDPV2 outbreaks and mOPV2 emergency use), release of the virus from a laboratory or vaccine facility now pose the greatest risk to maintaining its eradication. The World Health Organization has developed a Global Action Plan to minimize poliovirus facility-associated risk after type-specific eradication of wild polioviruses and sequential cessation of oral polio vaccine use (“GAPIII”) that describes poliovirus containment requirements18. GAPIII serves as the primary guide to facilities that must retain live poliovirus materials for essential functions that support eradication, such as vaccine production, polio diagnostics, or certain critical research. Poliovirus has been used for decades as a model system in basic, applied, and clinical research, as well as in quality control for such diverse products as chemical disinfectants and intravenous immune globulin. As a result, poliovirus containment has implications for industries and facilities beyond those directly linked to polio eradication.
Once complete eradication of all three serotypes is achieved, live poliovirus will exist only in settings such as laboratories and vaccine manufacturing facilities. This is the case for PV2 now, in 2018, with the exception of ongoing cVDPV2 circulation in Nigeria, Democratic Republic of the Congo, and the Horn of Africa6. In recent years, there have been several known breaches of containment in vaccine manufacturing facilities, underlining the risks associated with retaining live poliovirus materials post-eradication19,20.
Recent containment breaches/incidents.
In September 2014, 45 liters of infectious WPV3 (the type 3 Saukett strain used for manufacture of IPV; ~1013 infectious particles) was released by an IPV vaccine production facility in Belgium into the sewage system and discharged to a wastewater treatment plant whose treated water enters a river network and ultimately ends up in the North Sea off the coast of Belgium and the Netherlands20. No virus was detected in the wastewater treatment plant, surface waters, sewage in the affected areas in the Netherlands, in mussels in the contaminated waters, but warnings were issued related to shellfish consumption and swimming; no human cases were reported. Production was halted at the facility during the investigation.
In April 2017, two workers were exposed to partly aerosolized WPV2 in an IPV vaccine production facility in the Netherlands19. Both workers were properly immunized against polio and were considered to have low risk to develop disease, but even fully immunized persons can become infected and excrete virus in their feces. The worker who was nearest to the spill became infected and excreted infectious virus for 28 days post-exposure. No virus was detected in the worker who was further from the actual spill, in samples taken 4, 7, and 11 days after exposure. Samples from household contacts of the infected worker were poliovirus-negative in all samples tested. However, WPV2 was detected in sewage immediately downstream from the infected worker’s home up to 30 days post-exposure. Remediation of the spill required closure of the IPV production process and premises, disinfection of the site and inventory of the persons with risk of exposure.
It is likely there have been other, smaller spills of poliovirus materials that were not widely reported, both in vaccine production facilities and in laboratories. At the very least, these spills and the response should be documented by the institutional biosafety office, both to assess the risk of infection of staff in the particular incident and to develop mitigation strategies to prevent future spills and exposures. The risks of exposure can be different in different types of poliovirus facilities. For example, the total culture volume in a vaccine production lot is approximately 1000-fold greater than the largest volumes used in a typical laboratory setting. Nevertheless, the consequences of a spill/exposure can be the same—infection of a worker and possible transmission to the community. Even in a highly immunized population, there may be susceptible individuals or groups of individuals, as well as immunodeficient individuals who are at high risk of developing serious disease should they become infected.
Implementation of polio containment
Basic principles.
GAPIII specifies a biorisk management standard composed of 16 elements that are key to proper containment of polioviruses in poliovirus-essential facilities (Table 1). Certification as a poliovirus-essential facility (PEF) is a formal process that requires full compliance with the elements of GAPIII, which must be verified by onsite inspection by WHO-trained polio containment auditors. The containment requirements are slightly different depending on whether the facility plans to work with wild poliovirus strains or only with vaccine strains. In the near future, highly attenuated poliovirus strains, specifically engineered to resist reversion, may be approved for use outside stringent containment facilities, but none are yet available as of September 2018. Most of the elements should already be part of the biosafety and quality management systems of vaccine production facilities and laboratories with quality control, public health, or research functions, and many should also be part of standard practice in any academic laboratory that handles human or animal pathogens. However, several elements, including Biorisk Management System, Emergency Response and Contingency Planning, and Facility Physical Requirements may present a relatively high bar for compliance at some facilities.
Table 1.
Biorisk management standard for poliovirus-essential facilities holding wild poliovirus materials18
| Element | Notes/Key Challenges |
|---|---|
| Biorisk Management System | Oversight by Biorisk Management Committee, with expertise in biosafety, biosecurity, physical security, facility engineering, and occupational health |
| Risk Assessment | Written risk assessments must be developed for each laboratory protocol/method in the containment laboratory; risk mitigation plans |
| Poliovirus Inventory and Information | Accurate inventory of poliovirus infectious materials; periodic review of inventory |
| General Safety | |
| Personnel and Competency | Recruitment, training, competence, continuity and succession planning |
| Good Microbiological Technique | |
| Clothing and Personal Protective Equipment (PPE) | |
| Human Factors | Institutional policies to address risk associated with human behavior |
| Health Care | Occupational health program, proof of poliovirus immunization and proof of immunity (requires neutralization assay) |
| Emergency Response and Contingency Planning | Anticipate all credible emergency scenarios; logistics for specimen collection and testing in the event of a release or laboratory exposure; coordination with local, state/provincial, and national emergency and public health authorities |
| Accident/Incident Investigation | |
| Facility Physical Requirements | Facility design and verification; infrastructure and operations: emergency shower, effluent decontamination |
| Equipment and Maintenance | |
| Decontamination, Disinfection, and Sterilization | Management of biological waste, inactivation of poliovirus (e.g. RNA extraction for downstream processes) by validated methods |
| Transport Procedures | Material transport within the facility and to/from other facilities |
| Security | Physical security, information security, personnel control, personal security, contractors/visitors/suppliers |
Biorisk Management System.
Implementation of a GAPIII-compliant biorisk management system requires that a facility take a broad approach to managing containment. A biorisk management committee, whose members have expertise in biosafety, biosecurity, physical security, facility engineering, and occupational health should provide oversight for the containment laboratory. Written policies and procedures must be developed to provide a foundation for all aspects of the containment facility and the activities that are carried out within the containment perimeter. Though most of these components should be present in larger research and production facilities, maintaining a poliovirus-specific biorisk management committee with broad expertise is generally beyond the scope of most institutional biosafety offices.
Emergency Response and Contingency Planning.
Any workplace should have a response plan to deal with emergencies such as fire, flooding, severe weather, power outages, etc., and facilities working with human pathogens must have an exposure response plan to prevent and respond to potential laboratory-acquired infections, but work with eradicated polioviruses poses additional challenges. The usual response to potential exposure of a laboratory worker is to monitor the worker(s) for signs of infection and disease, to provide post-exposure prophylaxis if available, and to refer the individual for treatment if they are confirmed to be infected. The response may also include isolation of the infected person if there is risk of transmission to close contacts or the community. A worker in a polio-essential facility should have demonstrated immunity to poliovirus and is therefore protected from disease; however, immunity does not fully protect from infection and shedding of virus in stool2. Therefore, the greatest risk is to unimmunized or underimmunized household and community contacts, with the possibility of further spread as population immunity levels drop in the years post-eradication and post-OPV cessation. The exposure response plan must encompass every step in the response, from the site of the exposure, to the facility occupational health practitioner, and reporting to local, state/provincial, and national health authorities. Each of these outside entities should be consulted as the exposure response plan is developed. The plan must include collection of stool specimens from exposed persons, testing of specimens in a competent laboratory, and criteria for release from isolation (often two or three consecutive negative stool specimens). Laboratory testing to identify poliovirus infection is not widely available in clinical or public health laboratories, other than those in the Global Polio Laboratory Network.
Facility Physical Requirements.
Facility requirements are the most costly requirements of poliovirus containment, and the most difficult to implement. The facility must be located in an area with high polio vaccine coverage and the coverage estimate must be supported by data. The latest guidance requires coverage data for the area within a 100 km commuting distance of the polio-essential facility21. Such a distance can encompass multiple jurisdictions, and even multiple countries, making it difficult to collect and validate the data. The most challenging aspect of this requirement, however, is the actual physical design of the facility. Entry to the laboratory should be controlled, with access limited to those with a legitimate need to work in the containment space. Two doors should separate the laboratory area from public areas, preferably with interlocking doors. Materials must be decontaminated by a method that has been validated to inactivate poliovirus before they are removed from containment; this can be accomplished by using a pass-through autoclave, airlock/decontamination chamber, or dunk tank. GAPIII currently calls for mandatory showering upon exiting the laboratory and decontamination of the effluent water; it would be very expensive to retrofit an existing facility with a shower system. Showering out is not required if all poliovirus work is conducted in a class III biosafety cabinet. There are additional requirements for facilities where work with poliovirus-infected animals will be conducted. Once eradication of all wild polioviruses has been certified, additional requirements will apply, including HEPA filtration of air exhausted from the containment laboratory and disinfection of liquid effluent.
Specific Challenges.
Current US Food and Drug Administration regulations require manufacturers of immune globulin products to test each lot of final product for neutralizing antibodies to at least one poliovirus serotype, among other quality control measures22. The neutralization test is a cell-based assay that uses live poliovirus to measure antibody-mediated protection of cells from virus cytopathic effect23. Similarly, the US Environmental Protection Agency recommends using a Sabin vaccine poliovirus as a standard in their method for the recovery and assay of total culturable viruses from sewage sludge24. Polioviruses are also often used as one of several standards for products used to disinfect environmental surfaces. Manufacturers who currently use type 2 poliovirus for the neutralization test will need to either perform the work in a GAPIII-compliant containment facility (and apply to become a PEF) or switch to a different serotype. However, while it is currently permissible to use poliovirus types 1 and 3, this will change after certification of eradication, so alternatives will need to be explored. There are currently no validated assays that can test for poliovirus neutralizing antibodies without the use of live virus.
Footnotes
The authors declare that they have no conflicts of interest relevant to the manuscript submitted to TRANSFUSION.
This material was presented in part at the FDA/IDF/PPTA/NIH public workshop, “Immune Globulin Potency in the 21st Century,” November 8–9, 2017.
The findings and conclusions in this report are those of the author and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
References
- 1.American Academy of Pediatrics. Poliovirus infections. In: Kimberlin DW, Brady MT, Jackson MA, Long SS, eds. Red Book: 2018 Report of the Committee on Infectious Diseases. Itaska, IL: American Academy of Pediatrics, 2018:657–64. [Google Scholar]
- 2.Sutter RW, Kew OM, Cochi SL, Aylward RB. Poliovirus vaccine--live. In: Plotkin SA, Orenstein WA, Offit PA, eds. Vaccines. Philadelphia: Elsevier Saunders, 2013:598–645. [Google Scholar]
- 3.Alexander JP Jr., Gary HE Jr., Pallansch MA. Duration of poliovirus excretion and its implications for acute flaccid paralysis surveillance: a review of the literature. Journal of Infectious Diseases 1997;175: S176–82. [DOI] [PubMed] [Google Scholar]
- 4.Gardner TJ, Diop OM, Jorba J, Chavan S, Ahmed J, Anand A. Surveillance to Track Progress Toward Polio Eradication - Worldwide, 2016–2017. MMWR Morb Mortal Wkly Rep 2018;67: 418–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Khan F, Datta SD, Quddus A, Vertefeuille JF, Burns CC, Jorba J, Wassilak SGF. Progress Toward Polio Eradication - Worldwide, January 2016-March 2018. MMWR Morb Mortal Wkly Rep 2018;67: 524–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Global Polio Eradication Initiative. Global Polio Eradication Initiative Home 2018. [Google Scholar]
- 7.Global Commission for the Certification of Poliomyelitis Eradication. 14th Meeting of the Global Commission for the Certification of Poliomyelitis Eradication (GCC) [monograph on the internet]. Geneva: World Health Organization; 2015. Available from: http://polioeradication.org/wp-content/uploads/2016/07/1Report.pdf [Google Scholar]
- 8.Vidor E, Plotkin SA. Polio vaccine--inactivated. In: Plotkin SA, Orenstein WA, Offit PA, eds. Vaccines. Philadelphia: Elsevier Saunders, 2013:573–97. [Google Scholar]
- 9.World Health Organization. Guidelines for the safe production and quality control of inactivated poliomyelitis vaccine manufactured from wild polioviruses (Addendum, 2003, to the Recommendations for the Production and Quality Control of Poliomyelitis Vaccine (Inactivated) WHO Technical Report Series No. 926. Geneva, 2004. [Google Scholar]
- 10.Peck KM, Lauring AS. Complexities of Viral Mutation Rates. J Virol 2018;92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.World Health Organization. Vaccine-associated paralytic polio (VAPP) and vaccine-derived poliovirus (VDPV) [monograph on the internet]. Geneva: World Health Organization; 2015. Available from: http://www.who.int/immunization/diseases/poliomyelitis/endgame_objective2/oral_polio_vaccine/VAPPandcVDPVFactSheet-Feb2015.pdf [Google Scholar]
- 12.Dowdle WR, De Gourville E, Kew OM, Pallansch MA, Wood DJ. Polio eradication: the OPV paradox. Rev Med Virol 2003;13: 277–91. [DOI] [PubMed] [Google Scholar]
- 13.Burns CC, Diop OM, Sutter RW, Kew OM. Vaccine-derived polioviruses. J Infect Dis 2014;210 Suppl 1: S283–93. [DOI] [PubMed] [Google Scholar]
- 14.Macklin G, Liao Y, Takane M, Dooling K, Gilmour S, Mach O, Kew OM, Sutter RW, i VWG. Prolonged Excretion of Poliovirus among Individuals with Primary Immunodeficiency Disorder: An Analysis of the World Health Organization Registry. Front Immunol 2017;8: 1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dunn G, Klapsa D, Wilton T, Stone L, Minor PD, Martin J. Twenty-Eight Years of Poliovirus Replication in an Immunodeficient Individual: Impact on the Global Polio Eradication Initiative. PLoS Pathog 2015;11: e1005114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McKinlay MA, Collett MS, Hincks JR, Oberste MS, Pallansch MA, Okayasu H, Sutter RW, Modlin JF, Dowdle WR. Progress in the Development of Poliovirus Antiviral Agents and their Essential Role in Reducing Risks that Threaten Eradication. Journal of Infectious Diseases 2014;210: S447–53. [DOI] [PubMed] [Google Scholar]
- 17.Hampton LM, Farrell M, Ramirez-Gonzalez A, Menning L, Shendale S, Lewis I, Rubin J, Garon J, Harris J, Hyde T, Wassilak S, Patel M, Nandy R, Chang-Blanc D, Immunization Systems Management Group of the Global Polio Eradication I. Cessation of Trivalent Oral Poliovirus Vaccine and Introduction of Inactivated Poliovirus Vaccine - Worldwide, 2016. MMWR Morb Mortal Wkly Rep 2016;65: 934–8. [DOI] [PubMed] [Google Scholar]
- 18.World Health Organization. WHO Global Action Plan to minimize poliovirus facility-associated risk after type-specific eradication of wild polioviruses and sequential cessation of oral polio vaccine use. Geneva, 2015. [Google Scholar]
- 19.Duizer E, Ruijs WL, van der Weijden CP, Timen A. Response to a wild poliovirus type 2 (WPV2)-shedding event following accidental exposure to WPV2, the Netherlands, April 2017. Euro Surveill 2017;22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Duizer E, Rutjes S, de Roda Husman AM, Schijven J. Risk assessment, risk management and risk-based monitoring following a reported accidental release of poliovirus in Belgium, September to November 2014. Euro Surveill 2016;21: 30169. [DOI] [PubMed] [Google Scholar]
- 21.World Health Organization. 15th Meeting of the SAGE Polio Working Group: Conclusions and Recommendations, Note for the Record [monograph on the internet]. Geneva: World Health Organization; 2018. Available from: http://www.who.int/immunization/sage/meetings/2018/april/2_WHO_Polio_SAGE_Apr2018.pdf?ua=1 [Google Scholar]
- 22.United States Food and Drug Administration. Code of Federal Regulations Title 21, Part 640, Subpart J, Section 640.104: Additional standards for human blood and blood products, immune globulin (human), potency, 2017. [Google Scholar]
- 23.Weldon WC, Oberste MS, Pallansch MA. Standardized Methods for Detection of Poliovirus Antibodies. Methods Mol Biol 2016;1387: 145–76. [DOI] [PubMed] [Google Scholar]
- 24.United States Environmental Protection Agency. Environmental Regulations and Technology: Control of Pathogens and Vector Attraction in Sewage Sludge, 2003. [Google Scholar]


