Abstract
Objective:
Acute synovial inflammation following joint trauma is associated with posttraumatic arthritis. Synovial macrophages have been implicated in degenerative changes. In this study, we sought to elucidate the role of intra-articular macrophages in the acute inflammatory response to fracture in the mouse knee.
Method:
A closed articular fracture was induced in two models of synovial macrophage depletion: genetically-modified MaFIA mice administered AP20187 to induce programmed macrophage apoptosis, and wild-type C57BL/6 mice administered clodronate liposomes, both via intra-articular injection. Synovial inflammation, bone morphology, and levels of F4/80+ macrophages, NOS2+ M1 macrophages, and CD206+ M2 macrophages were quantified 7 days after fracture using histology and micro-computed tomography.
Results:
Intra-articular macrophage depletion with joint injury did not reduce acute synovitis or the number of synovial macrophages 7 days after fracture in either macrophage-depleted MaFIA mice or in clodronate-treated C57BL/6 mice. In macrophage-depleted MaFIA mice, macrophage polarity shifted to a dominance of M1 macrophages and a reduction of M2 macrophages in the synovial stroma, indicating a shift in M1/M2 macrophage ratio in the joint following injury. Interestingly, MaFIA mice depleted 2 days prior to fracture demonstrated increased synovitis (P = 0.003), reduced bone mineral density (P = 0.0004), higher levels of M1 macrophages (P = 0.013), and lower levels of M2 macrophages (not statistically significant, P=0.084) compared to control-treated MaFIA mice.
Conclusion:
Our findings indicate that macrophages play a critical immunomodulatory role in the acute inflammatory response surrounding joint injury and suggest that inhibition of macrophage function can have prominent effects on joint inflammation and bone homeostasis after joint trauma.
Keywords: Post traumatic, Inflammation, Synovium, Fracture, Trauma
Introduction
Posttraumatic arthritis (PTA) is a degenerative form of osteoarthritis (OA) that occurs following joint injury and is a major cause of disability nationwide. Of the nearly 27 million Americans living with OA, 12% are estimated to have a posttraumatic etiology1–3. Despite efforts to understand the mechanisms of PTA development, there are no currently available pharmacologic agents in clinical practice that have been shown to alter its progression.
PTA develops most commonly following fracture of the articular surface of the joint, resulting in acute joint inflammation4. The process of inflammation encompasses a complex array of cellular changes that are components of an integrated response5. Although mild inflammation after injury can support healing, mounting evidence suggests that inflammation at the time of joint injury may exacerbate the degenerative changes in PTA6–9. At 7 days after an articular fracture, significant synovitis of the joint capsule is evident histologically both in the mouse10 and in human patient biopsy samples11, suggesting that trauma to the articular surface may lead to acute pathology in surrounding joint tissues through various mechanisms. C57BL/6 mice, a strain that predictably develops PTA after closed articular fracture, demonstrate increased synovitis, higher levels of inflammatory cytokines such as interleukin-1 (IL-1) and tumor necrosis factor-alpha (TNF-α) within the synovial fluid, and an influx of macrophages to the joint capsule 7 days after fracture10,12. Conversely, MRL/MpJ mice, a “superhealer” strain that is protected from developing severe PTA, have a reduced inflammatory response following joint injury, suggesting that inflammation at the time of injury may contribute to arthritic changes13–15.
Macrophages exhibit a spectrum of phenotypes, but can be classified generally into pro-inflammatory M1 macrophages and regulatory M2 macrophages. The pro-inflammatory response of M1 macrophages is a component of host defense, producing inflammatory cytokines and nitric oxide synthase 2 (NOS2)16,17, whereas the tissue remodeling regulatory M2 macrophages express CD206 and other scavenger receptors, producing interleukin-10 (IL-10) and transforming growth factor beta (TGF-β)18,19. Specifically for arthritis, inflammation, activated macrophages, and a high M1 to M2 ratio were strongly associated with joint symptoms and radiographic severity of human knee OA20,21. Synovial macrophages have been implicated in the production of pro-inflammatory cytokines and matrix metalloproteinases (MMPs)16,22,23, which are highly upregulated following intraarticular fracture13. Intra-articular inhibition of IL-1 through the use of IL-1 receptor antagonist (IL-1Ra), delivered either transiently or in a sustained manner at the time of articular fracture in mice, has been shown to attenuate the development of PTA24,25. Furthermore, systemic depletion of macrophages has been shown to decrease joint swelling in a collagen-induced arthritis model26 and reduce systemic levels of inflammatory cytokines in a hyperlipidemia model of OA27; however, two separate studies of macrophage depletion in high-fat diet mouse models demonstrated an increased systemic inflammatory response28,29. We initially sought to systemically deplete macrophages with joint fracture using clodronate liposomes; however, the immune response to the trauma of the fracture coupled with the systemic macrophage depletion resulted in a high fatality rate (data not shown). These previous studies indicate a complex role for macrophages in systemic immunoregulation. Understanding the role of acute synovial inflammation and macrophages following articular fracture could help identify novel therapeutic approaches.
In this study, we investigated the effect of a transient synovial depletion of macrophages on the acute inflammatory response following joint injury using two different methods of macrophage depletion. Macrophage Fas-induced Apoptosis (MaFIA) mice are a transgenic mouse strain with Fas-suicide gene on a macrophage promoter that allows for the specific depletion of macrophages using the small molecule AP2018730. By injecting AP20187 directly into the joint space, we locally depleted macrophages in the knee joint surrounding a closed articular fracture. Alternatively, we locally depleted macrophages in C57BL/6 mice with joint injury using an intra-articular injection of clodronate encapsulated in liposomes31,32. We hypothesized that acute transient intra-articular depletion of macrophages following joint injury would reduce synovitis and alter the inflammatory response. On the contrary, however, we found that macrophage depletion induced intensive synovitis 7 days after fracture, indicating macrophages play a critical role in modulating the acute inflammatory response to joint injury.
Methods
Animals
All animal procedures were performed in accordance with an IACUC-approved protocol at Duke University. Male MaFIA transgenic mice generated on the C57BL/6 background (n = 27) and male C57BL/6 mice (n = 27) were purchased from The Jackson Laboratories. MaFIA mice have mutant human FK-506 binding protein 1A, 12 kDa (FKBP12)-Fas suicide construct and enhanced green fluorescent protein (eGFP) placed under the mouse colony stimulating factor 1 receptor promoter (Csf1r), which allows for inducible and reversible apoptosis of macrophages and dendritic cells in the presence of the dimerization drug AP20187 and fluorescent visualization of Csf1r cells30. The AP20187 molecule preferentially binds to mutant human FKBP on Csf1r cells and initiates apoptosis through the Fas-suicide pathway.
Articular fracture and macrophage depletion
MaFIA mice and C57BL/6 mice received a closed articular fracture of the left tibial plateau at 16 weeks of age, as previously described13,14,33. Briefly, animals were anesthetized (iso-flurane, concentration 2%) and placed in a custom cradle with the left hindlimb in neutral position (90° flexion). Using a precision material testing system (ELF 3200, TA Instruments, Newcastle, DE), a custom indenter applied a 10N compressive pre-load to the anterior aspect of the left proximal tibial plateau, followed by compression applied at a rate of 20N/s until fracture. The maximum displacement of the indenter was limited to 2.7 mm to create moderate fractures of the tibial plateau. The right hind limb of each mouse served as an uninjured contralateral control.
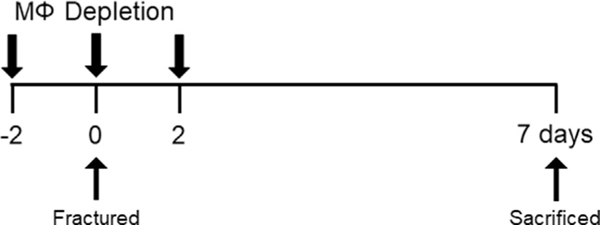
Under sterile conditions, MaFIA mice received an intra-articular injection of either carrier control solution (n = 3 per time point) or 60 μg of AP20187 (6 μL injection of 10 mg/ml concentration), consisting of 4% ethanol,10% polyethylene glycol 400, and 1.7% Tween 20 in water34 to locally deplete macrophages (n = 6 per time point). In another set of experiments, C57BL/6 mice received an intra-articular injection of either liposomes containing PBS (n = 3 per time point) or 30 μg of clodronate encapsulated in liposomes (6 μL injection of 5 mg/ml concentration)35 to locally deplete macrophages (n = 6 per time point). Mice received intraarticular injections either 2 days prior to fracture (Day −2, n = 9 per genotype), immediately following fracture (Day 0, n = 9 per genotype), or 2 days following fracture (Day −2, n = 9 per geno-type). Mice were sacrificed 7 days post-fracture at 17 weeks of age; no fixation or surgical intervention were implemented (Fig. 1).
Fig 1. Experimental timeline.
Mice received a closed articular fracture of the left knee and a single intra-articular injection of AP20187, clodronate, or carrier control administered either 2 days prior to fracture (Day −2, n = 9), immediately following fracture (Day 0, n = 9), or 2 days following fracture (Day 2, n = 9). Mice were sacrificed 7 days following fracture.
MicroCT analysis
All hind limbs from MaFIA mice and C57BL/6 mice were placed in 10% neutral-buffered formalin for 48 h and then scanned by microCT (Skyscan 1176, Bruker, Madison, Wisconsin). Morphometric bone parameters were determined in the distal femoral condyles, proximal tibial plateau immediately distal to subchondral bone, and metaphyseal region of tibia beginning at the fibular attachment, as previously described12. Parameters reported in the femoral condyles were trabecular bone fraction (bone volume/total volume) and bone density (mg/cm3). Parameters reported in the tibial plateau and metaphyseal regions include bone volume (mm3) and bone density (mg/cm3).
Histology
Following micro-CT analysis, limbs were decalcified in 1.35N hydrochloric acid solution (Cal-Ex Decalcification Solution, Fisher Scientific) for 5 days. The limbs were then dehydrated in ethanol, infiltrated with xylene, and paraffin-embedded in the coronal orientation. Serial sections (8 μm) in the mid-joint region of both hind limbs from MaFIA mice and clodronate-treated C57BL/6 mice were stained with standard hematoxylin and eosin (H&E) to visualize inflammatory changes in the synovial structure and cellularity. Digital images of the synovial insertion of the lateral femur, medial femur, lateral tibia, and medial tibia were obtained and evaluated separately using a modified form of an established synovitis score for changes in synovial lining thickness and cellular density in the synovial stroma (maximum site score 6) by three blinded graders10,36. The lining and stroma scores were summed for all four regions to generate a total synovitis score for each knee joint37.
Consecutive joint sections (8 mm) were also used for immune-histochemistry (IHC) to identify macrophages (F4/80, Serotec MCA497G), M1 macrophages (iNOS, Abcam ab3523, reported as NOS2+ in text) and M2 macrophages (CD206, Abcam ab64693) present within the lining and stroma of the synovial capsule of the fractured limb (n = 3 from carrier control groups; n = 3 from macrophage depleted groups; subset of joints selected with synovitis scores closest to the mean and demonstrate cellularity), as previously reported28,38–40. The F4/80+, NOS2+, and CD206+ cells were quantified for the lining and stroma separately by graders from digital images.
For immunohistochemistry of F4/80 macrophages, sections from MaFIA mice and clodronate-treated C57BL/6 mice were pretreated with 0.01% proteinase K (Sigmae–Aldrich) for 5 min at 37°C for antigen retrieval. Endogenous peroxidase was quenched with 3% H2O2 in methanol for 30 min. Sections were incubated with a rat anti-mouse monoclonal antibody against a surface marker of activated macrophages (F4/80, Serotec MCA497G) at a 1:150 dilution at room temperature for 1 h.
For immunohistochemistry of NOS2 M1 macrophages, sections from MaFIA mice and clodronate-treated C57BL/6 mice were pretreated with 0.005% proteinase K (Sigmae–Aldrich) for 5 min at 37°C for antigen retrieval. Endogenous peroxidase was quenched with 3% H2O2 in methanol for 1 h. Sections were incubated with a rabbit anti-mouse polyclonal antibody against NOS2, an enzymatic marker of activated classical M1 macrophages (iNOS, Abcam ab3523) at a 1:300 dilution at 4°C overnight.
For immunohistochemistry of CD206 M2 macrophages, sections from MaFIA mice and clodronate-treated C57BL/6 mice were pretreated with Citrate Buffer, pH 6.0 (H-3300, Vector laboratories, Burlingame, CA) for 15 min at 90°C for antigen retrieval. Endogenous peroxidase was quenched with 3% H2O2 in methanol for 30 min. Sections were incubated with a rabbit anti-mouse polyclonal antibody against the mouse Mannose Receptor surface marker of activated M2 macrophages (CD206 Mouse anti-Human, Abcam ab64693) at a 1:500 dilution at room temperature for 1 h.
For all immunohistochemistry, negative controls received blocking serum instead of the primary antibody. Following incubation, appropriate species-specific polymer detection kits were utilized (ImmPRESS anti-rat kit MP-7440; ImmPRESS anti-rabbit kit MP-7401, Vector laboratories), followed by DAB substrate (SK-4100, Vector laboratories) for chromogenic detection and hematoxylin counter stain (H-3494, Vector laboratories). Digital images of the joint tissue were obtained (BX53 with DP73 camera, cellSens software, Olympus, Center Valley, PA) for stained and negative control sections.
Statistical analysis
A priori sample size calculations using previous data13,28 determined that a sample size of n = 3 calculated for 2-tailed independent samples t-test was needed to detect a 25% difference in IHC assessment of macrophages (Supplemental S1). As our prior experience with systemic macrophage depletion (data not shown) resulted in adverse events and death in some animals, the sample size of the macrophage depleted groups were increased to n = 6. We report no adverse events or loss of animals with local intraarticular depletion. To examine statistical difference in synovitis and bone morphology, a multifactorial analysis of variance (ANOVA) was used with depletion or carrier treated as categorical variable and fractured or contralateral control limb treated as a repeated measure with treatment time points analyzed independently. To examine statistical differences in immunohistochemical assessment of macrophages a Student’s t-test was used which compared treatment group of depleted or carrier in fractured limbs with treatment time points and region (lining or synovial stroma) within the joint analyzed independently. In all cases, statistical significant is reported at the 95% confidence level and residual diagnostics presented in Supplemental S2 (STATISTICA v.7, StatSoft, Inc.).
Results
Macrophage depletion with joint injury does not reduce synovitis
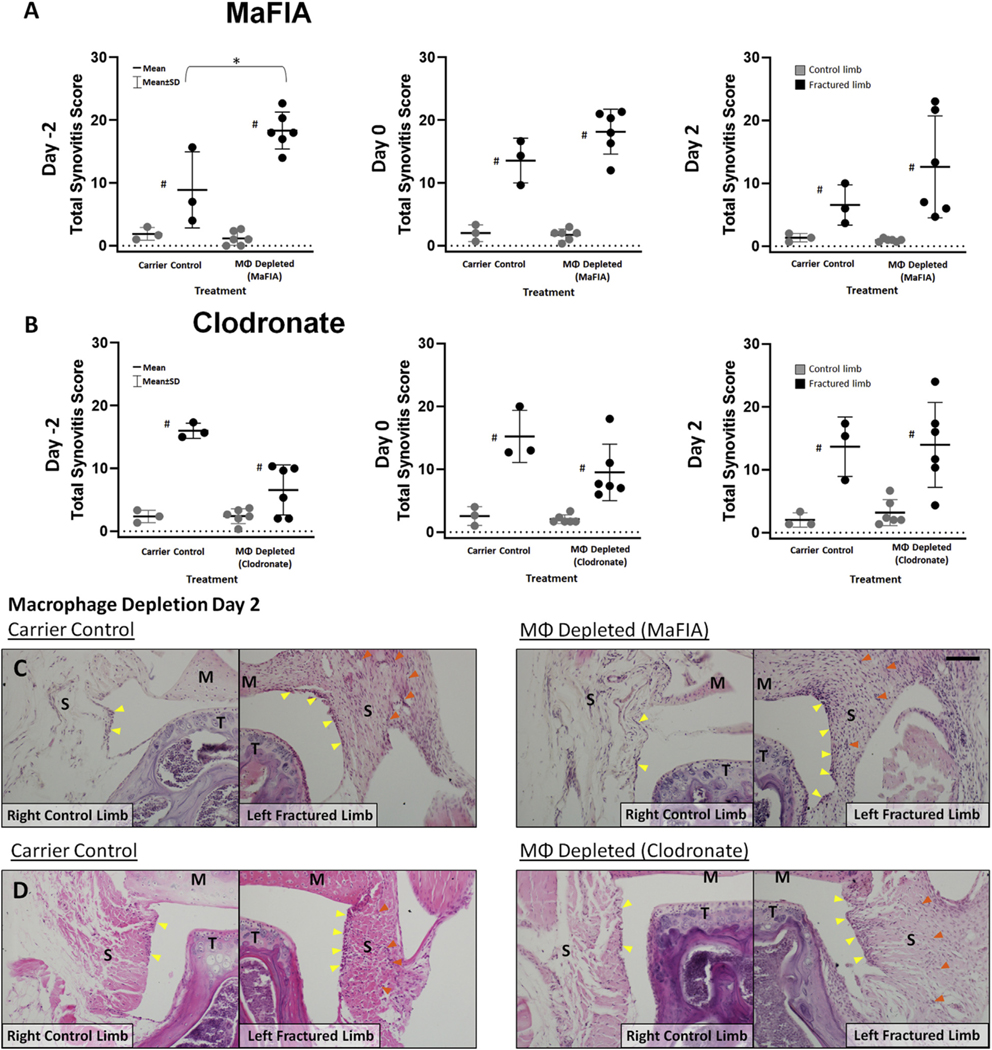
Intra-articular depletion of macrophages did not reduce joint synovitis at any time point for either MaFIA mice or clodronate-treated C57BL/6 mice [Fig. 2(A) and (B)]. Surprisingly, pre-depletion of macrophages 2 days prior to injury (Day–2) in MaFIA mice significantly increased synovitis in the injured limb when compared to a carrier control-treated injured limb (Fig. 2(A), P = 0.003; estimate of between-group difference 9.44 with 95% CI 1.77, 17.11), and with the clodronate pre-depletion, synovitis scores in the fractured limbs were lower but not statistically significant in comparison to fractured limbs with carrier control (Fig. 2(B), P = 0.071; estimate of between-group difference −4.17 with 95% CI − 9.14, 0.81). H&E staining of synovial tissue from MaFIA and clodronate-treated C57BL/6 mice treated 2 days after fracture (Day 2) shows increased cellularity and thickness of the synovial lining layer in fractured joints when compared to contralateral control joints [Fig. 2(C) and (D)].
Fig. 2. Effect of intra-articular macrophage (MΦ) depletion on synovitis in the mouse knee at 7 days after fracture.
(A) Total joint synovitis score of the knee in contralateral control and fractured limbs for MaFIA treatment groups. *Significantly increased synovitis of the knee in macrophage depleted fractured limbs with AP20187 compared to fractured limbs receiving carrier control (*P < 0.05) with treatment at Day −2. #Significantly increased synovitis of the knee in fractured limbs compared to respective contralateral control limbs (#P < 0.05). (B) Total joint synovitis score of the knee in contralateral control and fractured limbs for Clodronate treatment groups. #Significantly increased synovitis of the knee in fractured limbs compared to respective contralateral control limbs (#P < 0.05). (C–D) Representative histological sections stained with H&E demonstrating increased cellularity in the synovial joint capsule with fracture in both carrier control and macrophage depleted groups treated at Day 2 for (C) MaFIA and (D) Clodronate groups with yellow arrows indicating cellularity in synovial lining with increase in fractured limbs, and S indicating synovial stroma with orange arrows indicating increased cellularity, T for tibia and M for meniscus in contralateral control and fractured limbs, scale bar = 50 μm.
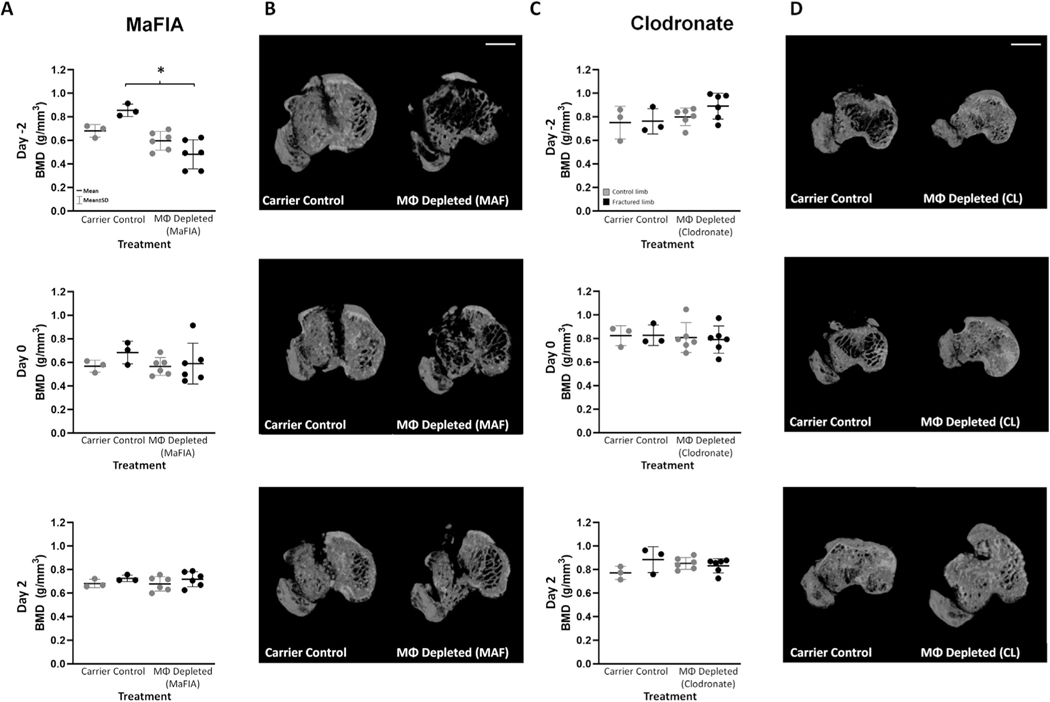
Pre-injury macrophage depletion in MaFIA mice decreases bone mineral density
Compared with carrier control-treated injured limbs, macrophage depletion in MaFIA mice 2 days prior to fracture (Day −2, P = 0.002; estimate of between-group difference −0.37 with 95% CI 0.61, −0.14) significantly decreased Bone Mineral Density (BMD) in the metaphyseal region of the tibia [Fig. 3(A) and (B)]. This reduction in BMD, illustrated in microCT images [Fig. 3(B)], coincided with the increased synovitis observed in Day–2 macrophage-depleted MaFIA mice. BMD was not altered in MaFIA mice depleted on Day 0 or Day 2 [Fig. 3(A) and (B)]. Similarly, there were no significant differences in BMD in the metaphyseal region between control and macrophage-depleted C57BL/6 mice with clodronate liposome treatment [Fig. 3(C) and (D)]. Other statistically significant changes in bone morphology are detailed in Table I with the majority of changes being a reduction in bone volume or fraction and BMD in fractured limbs compared to contralateral control limbs, and in select experimental conditions, a reduction in bone volume or fraction with macrophage depletion in both fractured and control limbs.
Fig. 3. Bone Morphology in tibial metaphysis of macrophage (MΦ) depleted and carrier control mice.
A) Bone Mineral Density (BMD) of control and fractured limbs for all MaFIA treatment groups with *P < 0.05. (B) MicroCT axial cross-sectional images in MaFIA fractured experimental joints. (C) Bone Mineral Density (BMD) of control and fractured limbs for all clodronate treatment groups. (D) MicroCT axial cross-sectional images in clodronate fractured experimental joints. Scale bar is 1 mm.
Table I. Bone morphology.
Measures of experimental groups at 7 days post-fracture for contralateral control and fractured experimental limbs as assessed by microCT for the femoral condyles, tibial plateau, and tibial metaphysis (mean ± 95% confidence intervals)
| Macrophage Depletion at Day −2 | |||||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Carrier Control | MaFIA MO Depleted | ANOVA(P value) | |||||
|
|
|
|
|||||
| Location and Measurement | nonfx limb | fx limb | nonfx limb | fx limb | Group | Limb | Group*Limb |
| Femoral Condyles | |||||||
| Cancellous Bone Fraction (BV/TV) | 77.4 (72.2–82.7) | 70.7 (62.2–79.3) | 70.5 (66.7–74.2) | 66.9 (62.3–71.5) | 0.034 | 0.045 | 0.519 |
| Bone Mineral Density (mg/mm3) | 1.54(1.43–1.64) | 1.40 (1.22–1.57) | 1.39(1.31–1.64) | 1.32 (1.22–1.41) | 0.032 | 0.046 | 0.525 |
| Tibiai Plateau | |||||||
| Bone Volume (mm3) | 70.4 (66.1–74.8) | 65.1 (53.4–76.8) | 65.4 (63.3–67.5) | 64.4 (61.1–67.8) | 0.158 | 0.019 | 0.076 |
| Bone Mineral Density (mg/mm3) | 1.38 (1.28–1.47) | 1.26(1.02–1.51) | 1.27 (1.23–1.32) | 1.25 (1.18–1.32) | 0.159 | 0.018 | 0.074 |
| Tibial Metaphysis | |||||||
| Bone Volume (mm3) | 197(138–256) | 267 (176–357) | 175 (142–209)* | 154(113–195)* | 0.004 | 0.226 | 0.040 |
| Bone Mineral Density (mg/mm3) | 0.681 (0.548–0.814) | 0.855 (0.725 – 0.986)+ | 0.597 (0.513–0.680) | 0.482 (0.353 – 0.611)+ | 0.002 | 0.520 | 0.014 |
| Carrier Control | Coldronate MO Depleted | ANOVA(P value) | |||||
|
|
|
|
|||||
| Location and Measurement | nonfx limb | fx limb | nonfx limb | fx limb | Group | Limb | Group*Limb |
|
| |||||||
| Femoral Condyles | |||||||
| Cancellous Bone Fraction (BV/TV) | 76.6 (73.0–80.3) | 73.4 (67.8–79.0) | 75.6 (73.2–77.9) | 73.3 (70.1–76.4) | 0.728 | 0.068 | 0.745 |
| Bone Mineral Density (mg/mm3) | 1.54 (1.47–1.62) | 1.47 (1.35–1.60) | 1.52 (1.47–1.57) | 1.47 (1.40–1.54) | 0.722 | 0.068 | 0.749 |
| Tibial Plateau | |||||||
| Bone Volume (mm3) | 63.4 (58.1–68.8) | 65.0 (59.8–70.2) | 61.0 (57.3–64.7) | 61.3 (57.6–65.1) | 0.044 | 0.637 | 0.762 |
| Bone Mineral Density (mg/mm3) | 1.22 (1.11–1.33) | 1.25 (1.17–1.33) | 1.17 (1.10–1.24) | 1.18 (1.10–1.26) | 0.041 | 0.556 | 0.808 |
| Tibial Metaphysis | |||||||
| Bone Volume (mm3) | 182 (−201 –565) | 245 (157–333) | 259 (229–289) | 286 (238–334) | 0.068 | 0.287 | 0.658 |
| Bone Mineral Density (mg/mm3) | 0.750 (0.405–1.10) | 0.762 (0.495–1.03) | 0.800 (0.722–0.878) | 0.891 (0.775–1.01) | 0.057 | 0.434 | 0.542 |
| Macrophage Depletion at Day 0 | |||||||
|
| |||||||
| Carrier Control | MaFIA MO Depleted | ANOVA(P value) | |||||
|
|
|
|
|||||
| Location and Measurement | nonfx limb | fx limb | nonfx limb | fx limb | Group | Limb | Group*Limb |
|
| |||||||
| Femoral Condyles | |||||||
| Cancellous Bone Fraction (BV/TV) | 74.8 (69.0–80.7) | 66.3 (61.9–70.8) | 67.9 (63.3–72.6) | 62.4 (57.0–67.8) | 0.092 | 0.002 | 0.453 |
| Bone Mineral Density (mg/mm3) | 1.48 (1.36–1.60) | 1.31 (1.21–1.40) | 1.34(1.24–1.44) | 1.22 (1.11–1.34) | 0.092 | 0.002 | 0.443 |
| Tibial Plateau | |||||||
| Bone Volume (mm3) | 67.4 (57.6–77.1) | 62.5 (54.9–70.0) | 63.3 (62.3–64.3) | 62.7 (59.7–65.7) | 0.269 | 0.022 | 0.053 |
| Bone Mineral Density (mg/mm3) | 1.31 (1.10–1.52) | 1.21 (1.05–1.37) | 1.23 (1.20–1.25) | 1.21 (1.15–1.28) | 0.266 | 0.022 | 0.053 |
| Tibial Metaphysis | |||||||
| Bone Volume (mm3) | 152 (117–187) | 214 (108–320) | 158 (133–182) | 172 (115–228) | 0.408 | 0.073 | 0.229 |
| Bone Mineral Density (mg/mm3) | 0.568 (0.442–0.693) | 0.684 (0.446–0.921) | 0.566 (0.487–0.645) | 0.591 (0.409–0.772) | 0.523 | 0.181 | 0.366 |
| Carrier Control | Coldronate MO Depleted | ANOVA(P value) | |||||
|
|
|
|
|||||
| Location and Measurement | nonfx limb | fx limb | nonfx limb | fx limb | Group | Limb | Group*Limb |
|
| |||||||
| Femoral Condyles | |||||||
| Cancellous Bone Fraction (BV/TV) | 77.2 (73.5–80.8) | 70.5 (64.4–76.6) | 78.8 (75.1–82.5) | 74.8 (70.4–79.3) | 0.288 | 0.001 | 0.287 |
| Bone Mineral Density (mg/mm3) | 1.53 (1.45–1.61) | 1.39(1.26–1.52) | 1.56(1.49–1.64) | 1.48 (1.39–1.58) | 0.288 | 0.001 | 0.288 |
| Tibial Plateau | |||||||
| Bone Volume (mm3) | 71.9 (67.8–76.0) | 68.9 (54.0–83.8) | 71.8 (68.2–75.4) | 70.2 (67.5–72.8) | 0.807 | 0.059 | 0.531 |
| Bone Mineral Density (mg/mm3) | 1.41 (1.32–1.50) | 1.35 (1.03–1.67) | 1.41 (1.33–1.48) | 1.37 (1.31–1.43) | 0.826 | 0.064 | 0.568 |
| Tibial Metaphysis | |||||||
| Bone Volume (mm3) | 257 (165–348) | 261 (111–411) | 241 (208–273) | 244 (206–282) | 0.508 | 0.798 | 0.978 |
| Bone Mineral Density (mg/mm3) | 0.825 (0.615–1.04) | 0.828 (0.612–1.04) | 0.809 (0.676–0.941) | 0.791 (0.670–0.912) | 0.686 | 0.882 | 0.828 |
| Macrophage Depletion at Day 2 | |||||||
|
| |||||||
| Carrier Control | MaFIA MO Depleted | ANOVA(P value) | |||||
|
|
|
|
|||||
| Location and Measurement | nonfx limb | fx limb | nonfx limb | fx limb | Group | Limb | Group*Limb |
|
| |||||||
| Femoral Condyles | |||||||
| Cancellous Bone Fraction (BV/TV) | 72.1 (66.6–77.5) | 68.2 (62.1–74.2) | 73.0 (70.1–76.0) | 67.0 (62.5–71.5) | 0.965 | 0.014 | 0.566 |
| Bone Mineral Density (mg/mm3) | 1.43 (1.31–1.54) | 1.34 (1.22–1.47) | 1.44 (1.38–1.51) | 1.32 (1.22–1.41) | 0.941 | 0.014 | 0.555 |
| Tibiai Plateau | |||||||
| Bone Volume (mm3) | 67.2 (63.6–70.7) | 64.4 (58.2–70.6) | 67.3 (64.4–70.3) | 64.3 (59.0–69.5) | 0.989 | 0.137 | 0.929 |
| Bone Mineral Density (mg/mm3) | 1.31 (1.23–1.38) | 1.25 (1.12–1.38) | 1.31 (1.25–1.37) | 1.25 (1.13–1.36) | 0.984 | 0.139 | 0.924 |
| Tibiai Metaphysis | |||||||
| Bone Volume (mm3) | 186 (121–251) | 218 (148–288) | 197 (170–223) | 223 (202–243) | 0.602 | 0.020 | 0.770 |
| Bone Mineral Density (mg/mm3) | 0.682 (0.594–0.770) | 0.726 (0.658–0.793) | 0.679 (0.614–0.744) | 0.719 (0.651–0.786) | 0.825 | 0.248 | 0.948 |
| Carrier Control | Coldronate MO Depleted | ANOVA(P value) | |||||
|
|
|
|
|||||
| Location and Measurement | nonfx limb | fx limb | nonfx limb | fx limb | Group | Limb | Group*Limb |
|
| |||||||
| Femoral Condyles | |||||||
| Cancellous Bone Fraction (BV/TV) | 76.4 (70.0–82.9) | 73.1 (67.4–78.8) | 80.0 (76.2–83.7) | 73.5 (68.9–78.1) | 0.470 | 0.013 | 0.384 |
| Bone Mineral Density (mg/mm3) | 1.52 (1.38–1.65) | 1.44 (1.32–1.56) | 1.59(1.51–1.67) | 1.46(1.36–1.55) | 0.445 | 0.013 | 0.405 |
| Tibial Plateau | |||||||
| Bone Volume (mm3) | 72.6 (68.2–77.0) | 70.9 (61.0–80.8) | 72.3 (69.8–74.8) | 71.1 (67.7–74.4) | 0.971 | 0.361 | 0.881 |
| Bone Mineral Density (mg/mm3) | 1.42 (1.33–1.52) | 1.39 (1.18–1.60) | 1.42 (1.36–1.47) | 1.39(1.32–1.46) | 0.954 | 0.369 | 0.891 |
| Tibial Metaphysis | |||||||
| Bone Volume (mm3) | 246 (218–274)* | 305 (276–334)*+ | 254 (237–271) | 256 (240–273)+ | 0.031 | 0.004 | 0.006 |
| Bone Mineral Density (mg/mm3) | 0.771 (0.632–0.911) | 0.884 (0.612–1.16) | 0.852 (0.799–0.905) | 0.832 (0.769–0.895) | 0.732 | 0.092 | 0.026 |
MQ = macrophage, nonfx = non-fractured, fx = fractured. Values represent Mean ± SD. Tukey post-hoc testing for Group*limb effect: P < 0.05 for
fx vs nonfx limbs;
depleted fx vs control fx limbs.
Transient local macrophage depletion alters synovial macrophage polarity
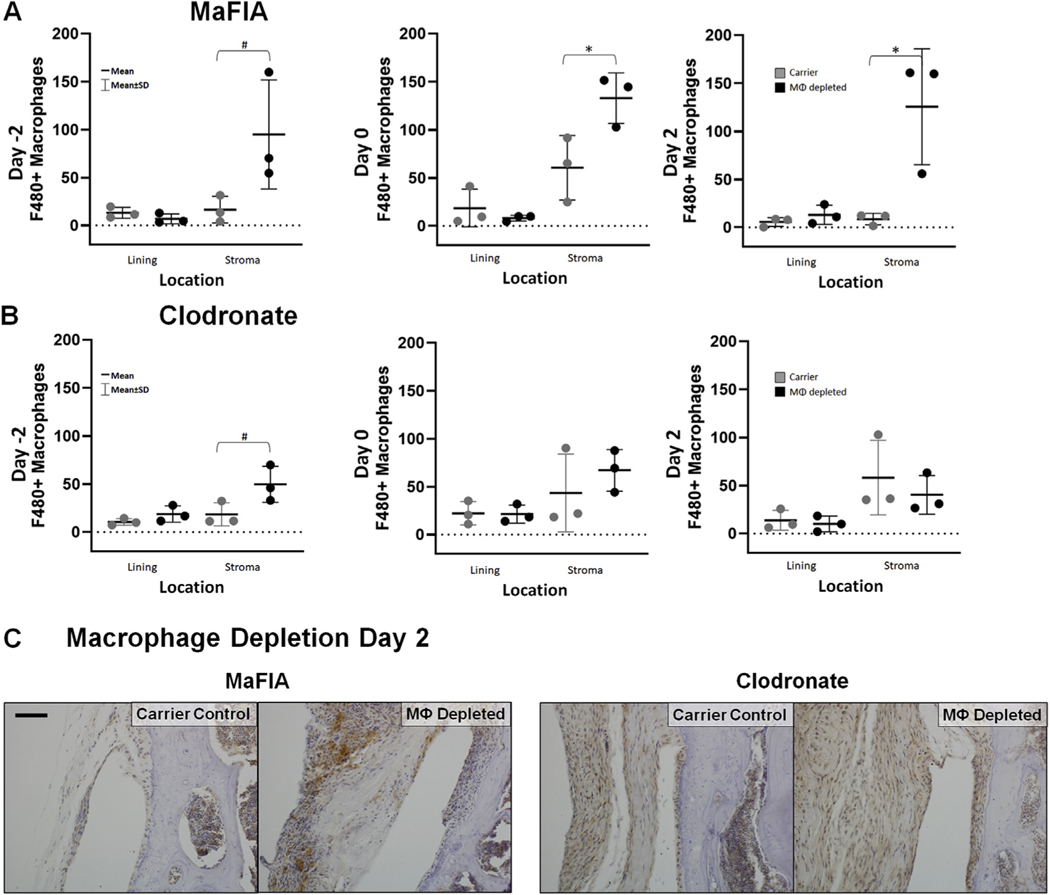
Surprisingly, transient local macrophage depletion within the acute window of joint injury did not reduce the number of synovial F4/80+ cells, a marker for activated murine macrophages, at 7 days post-fracture in either MaFIA mice or clodronate-treated C57BL/6 mice [Fig. 4(A) and (B)]. In fact, macrophage-depleted MaFIA mice depleted on Day 0 (P = 0.04; estimate of between-group difference 72.4 with 95% CI 4.1, 140.8) and Day 2 (P = 0.03; estimate of between-group difference 117.0 with 95% CI 19.8, 214.2) had significantly higher numbers of F4/80+ macrophages in the synovial stroma compared with the carrier control [Fig. 4(A)], with an increase of F4/80+ macrophages in the synovial stroma in MaFIA mice depleted on Day–2 that was not statistically significant (P = 0.08; estimate of between-group difference 78.4 with 95% CI - 15.3, 172.2). The synovial lining of macrophage-depleted MaFIA mice showed a small reduction was F4/80+ cells at Day –2 and Day 0, but this reduction was not significant at 7 days post-fracture (Day –2, P = 0.23; estimate of between-group difference −6.3 with 95% CI - 18.7, 6.1 and Day 0, P.= 0.42; estimate of between-group difference −10.4 with 95% CI - 42.5, 21.6). C57BL/6 mice pre-depleted with clodronate liposomes at Day–2 also demonstrated higher levels of F4/80+ cells in the synovial stroma 7 days post-fracture [Fig. 4(B)], but the results were also not statistically significant (P = 0.07; estimate of between-group difference 31.2 with 95% CI - 4.5, 67.0). Differences in F4/80+ cells within the synovial tissue in Day 2 MaFIA and clodronate-treated C57BL/6 mice can be visualized in digital immunohistochemistry images [Fig. 4(C)].
Fig. 4. Effect of intra-articular macrophage (MΦ) depletion on levels of F4/80+ macrophages.
(A) Total number of F4/80+ macrophages in lining and stroma of fractured limbs for carrier control and experimental macrophage depleted MaFIA treatment groups. *Significant increase of F4/80+ macrophages in experimental treatment groups compared to carrier control groups (*P < 0.05). Experimental depleted groups showed increased F4/80+ macrophages within the stroma compared to carrier control groups, but the differences were not statistically significant (#P < 0.08). (B) Total number of F4/80+ macrophages in lining and stroma of fractured limbs for carrier control and experimental macrophage depleted clodronate treatment groups. With depletion on Day −2, the experimental treatment group demonstrated increased F4/80+ macrophages in the stroma compared to the carrier control, but the differences were also not statistically significant (#P < 0.08). (C) Medial femur of histological sections stained with an antibody against the F4/80 surface marker of activated macrophages from the control and experimental treatment groups for MaFIA and clodronate mice receiving local injection 2 days after fracture (Day 2). Scale bar is 50 mm; brown = positive IHC stain, blue = hematoxylin counterstain.
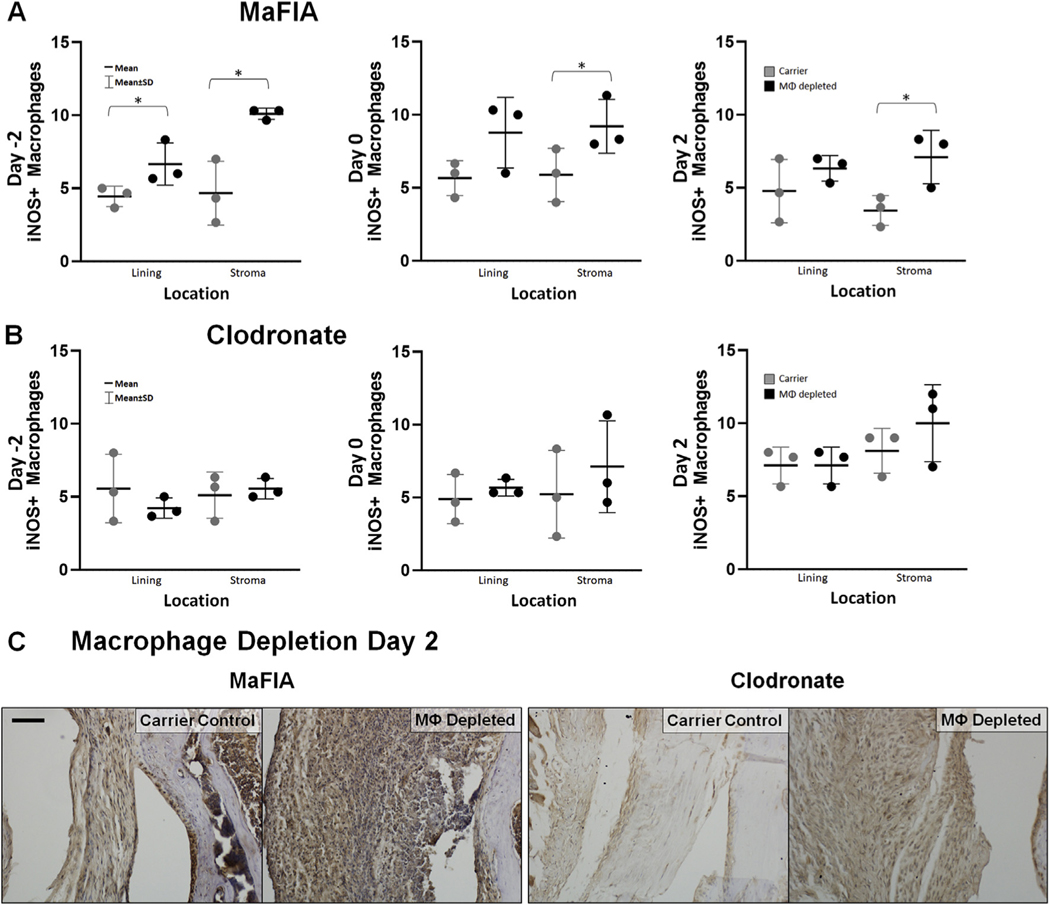
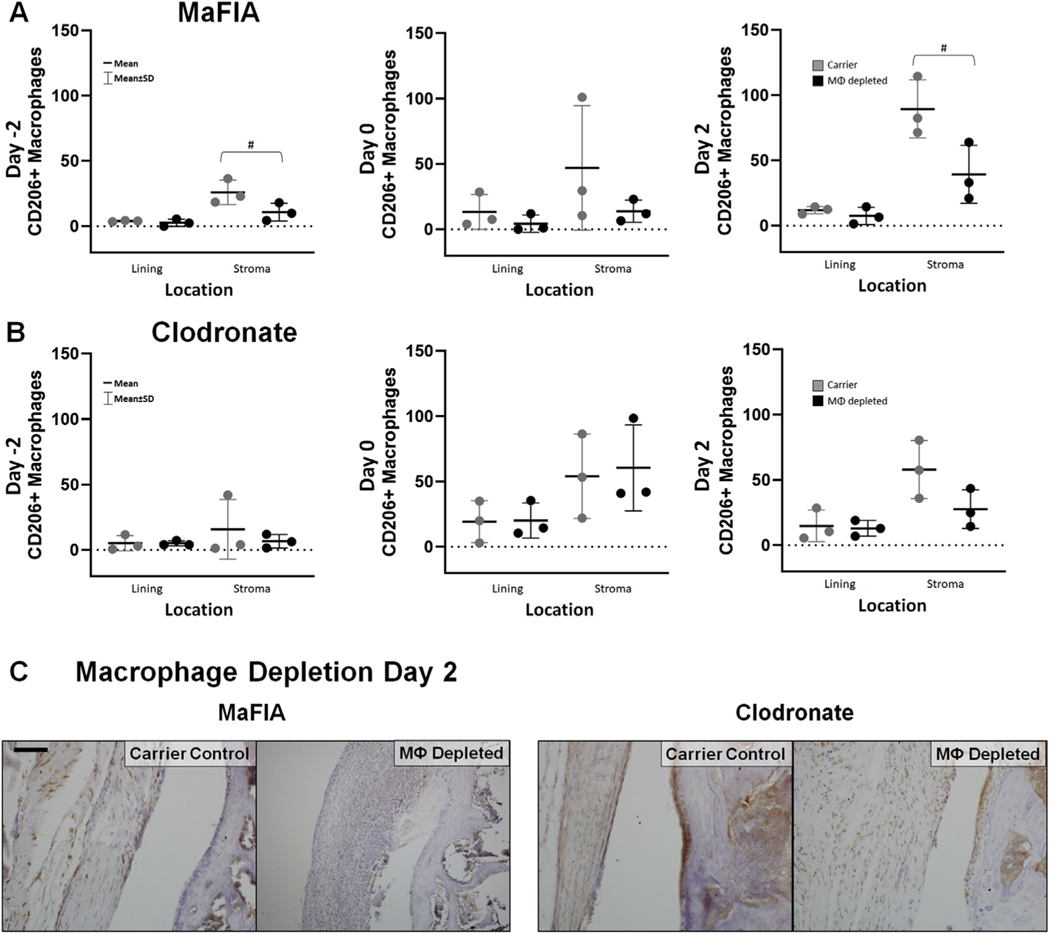
Interestingly, the observed increase in F4/80+ macrophage cells in the synovial stroma of macrophage-depleted MaFIA mice coincided with a significant increase in the number of inflammatory M1 macrophages, as indicated by NOS2+ cells on immunohistochemistry (Day –2, P = 0.013, estimate of between-group difference 5.44 with 95% CI 1.89, 9.00; Day 0, P = 0.09, estimate of between-group difference 3.33 with 95% CI - 0.83, 7.50; Day 2, P = 0.038, estimate of between-group difference 3.67 with 95% CI 0.30, 7.03) [Fig. 5(A) and (C)]. Additionally, this increased number of F4/80+ macrophages and NOS2+ M1 macrophages in macrophage-depleted MaFIA mice corresponded to lower, although not statistically different levels of CD206+ cells, a marker for regulatory M2 macrophages, in mice depleted on Day 2 (P = 0.086, estimate of between-group difference −15.2 with 95% CI - 33.7, 3.4) and Day –2 (P – 0.05, estimate of between-group difference −50.17 with 95% CI - 100.64, 0.30) [Fig. 6(A) and (C)]. These findings indicate a dominance of M1 macrophages relative to M2 macrophages following macrophage depletion and joint injury in MaFIA mice. Macrophage pre-depletion using clodronate in C57BL/6 mice (Day –2) resulted in an increase of F4/80+ cells in the synovial stroma that was not statistically significant (P = 0.07; estimate of between-group difference 31.2 with 95% CI - 4.5, 67.0), but clodronate depletion showed no statistically significant differences in NOS2+ cells or CD206+ cells in macrophage-depleted C57BL/6 mice in the lining or stroma at any time point [Figs. 5(B) and 6(B)]. Differences in Day 2 depleted MaFIA and clodronate-treated C57BL/6 mice of NOS2+ cells [Fig. 5(C)] and CD206+ cells [Fig. 6(C)] within the synovial tissue can be visualized in digital images of immunohistochemistry. In reviewing the overall changes as quantified from all regions in the joint, intra-articular macrophage depletion regardless of method preferentially depleted CD206+ M2 macrophages in the articular fracture injury model at 7 days post-fracture (P = 0.03; estimate of between-group difference −24.5 with 95% CI - 46.5, −2.6) with the pre-depletion time at Day –2 having the lowest level of CD206+ M2 macrophages (P = 0.003; estimate of between-group difference 46.4 with 95% CI 13.0, 79.8), as demonstrated in Table II.
Fig. 5. Effect of intra-articular macrophage (MΦ) depletion on levels of NOS2+ inflammatory macrophages.
(A) Total scores for the number of NOS2+ macrophages in lining and stroma of fractured limbs for carrier control and experimental macrophage depleted MaFIA treatment groups. *Significant increase of NOS2+ macrophages in experimental treatment groups compared to carrier control groups (*P < 0.05). (B) Total scores for number of NOS2+ macrophages in lining and stroma of fractured limbs for carrier control and experimental macrophage depleted clodronate treatment groups. (C) Medial femur of histological sections stained with an antibody against the NOS2 marker of activated macrophages from the fractured knee of carrier control and experimental treatment groups for MaFIA and clodronate mice receiving local injection 2 days after fracture (Day 2). Scale bar is 50 mm; brown = positive IHC stain, blue = hematoxylin counterstain.
Fig. 6. Effect of intra-articular macrophage (MΦ) depletion on levels of CD206+ regulatory macrophages.
(A) Total number of CD206+ macrophages in lining and stroma of fractured limbs for carrier control and experimental macrophage depleted MaFIA treatment groups. With depletion on Day −2 and Day 2, experimental treatment limbs showed a decrease in CD206+ macrophages in the stroma compared to carrier control, but the differences were not statistically significant (#P < 0.09). (B) Total number of CD206+ macrophages in lining and stroma of fractured limbs for carrier control and experimental macrophage depleted clodronate treatment groups. (C) Medial femur of histological sections stained with an antibody against the CD206 surface marker of activated macrophages from the control and experimental treatment groups for MaFIA and clodronate mice receiving local injection 2 days after fracture (Day 2). Scale bar is 50 mm; brown = positive IHC stain, blue = hematoxylin counterstain.
Table II.
Quantification of CD206+ M2 macrophages from immunohistochemistry.
| Depletion Days Since Depletion | Control* | Depleted | |
|---|---|---|---|
| Day.−2** | 9 | 25.4 (4.83–46.1) | 12.6(5.77–19.5) |
| Day 0 | 7 | 66.8 (14.7–119) | 49.4 (1.16–97.7) |
| Day 2 | 5 | 87.1 (55.5–119) | 43.8(24.8–62.7) |
| ANOVA: | depletion time P = 0.003 | depletion group P = 0.03 | time • group P = 0.47 |
Mean (95% confidence intervals).
ANOVA:
P=0.03
P=0.003 depletion time: Day−2<Day 0 (P = 0.004) and Day 2 (P = 0.02) Tukey’s post-hoc
Discussion
Transient, resolving inflammation plays a critical role in wound repair; however, severe, non-resolving inflammation associated with joint injury has been shown to contribute to joint degeneration and arthritic changes6–9. Particularly, infiltration of synovial macrophages, the major cell sources responsible for elevating levels of inflammatory cytokines, including IL-1 and TNF-α, within the synovial fluid, are observed in C57BL/6 mice following joint fracture10,12. The inflammation-associated joint tissue catabolism, however, is diminished in MRL/MpJ “superhealer” mice following articular fracture, and subsequently prevents PTA development13,14. Furthermore, targeted inhibition of IL-1 using intra-articular delivery of interleukin 1 receptor antagonist (IL-1Ra) reduces joint inflammation and prevents the onset of PTA24,25. Thus, we hypothesized that a transient, intra-articular depletion of macrophages would reduce joint synovitis during the acute phase of joint injury. However, we found that acute local macrophage depletion with articular fracture, using both the transgenic MaFIA mouse model or using clodronate liposomes in C57BL/6 mice, did not reduce synovitis or levels of macrophages in the synovium 7 days after fracture. In macrophage-depleted MaFIA mice, intraarticular macrophage depletion paradoxically altered the polarity of macrophages to a dominance of M1 inflammatory macrophages within the joint synovium. Our data suggest that the synovial macrophage population plays a pivotal immunomodulatory role in the acute inflammatory response to joint injury.
Macrophages are key modulators of bone homeostasis by regulating osteoblasts through osteal macrophages (osteomacs), cells critical for bone remodeling and mineralization34,41. Synovial macrophages, specifically, have been shown to mediate osteophyte formation42. In a study of parathyroid hormone (PTH)-induced bone anabolism, systemic macrophage depletion in MaFIA mice resulted in a reduction of cortical and trabecular bone mass that was not responsive to PTH43. However, systemic depletion of macrophages in the same model using clodronate liposomes had the opposite effect43, suggesting that these methods may target different macrophage subpopulations. While we did not observe a significant increase in BMD when synovial macrophages were depleted locally using clodronate, we did observe a significant decrease in BMD in pre-depleted MaFIA mice (Day–2). This decrease in bone density accompanied by a significant increase in synovitis at 7 days following fracture is similar to early rheumatoid arthritis pathology of periarticular bone loss in association with active synovitis and inflammation44,45. Findings from this study further strengthen the evidence that macrophages play a critical role in bone homeostasis, particularly within the region of a fracture.
Surprisingly, local macrophage depletion using either method did not reduce acute joint synovitis following articular fracture at any time point evaluated. A limitation of the study was that macrophage depletion was not evaluated at 1–2 days after administration. Assessment of the synovium at 7 days post-fracture was selected based on previously reported results that significant synovitis of the joint capsule with infiltration of macrophages is evident histologically both in the mouse10,13 and in human patient biopsy samples11 at this time point. The finding that neither depletion method reduced synovitis with articular fracture may be due to a preferential depletion of anti-inflammatory cells46. Furthermore, when MaFIA mice were depleted 2 days prior to fracture (Day –2), joint synovitis significantly increased with joint injury. Consistent with these findings, macrophage depletion with joint injury also did not reduce the number of F4/80+ macrophages present within the synovium 7 days post-fracture. While others have successfully reduced the synovial macrophage population using systemic administration of AP20187 to MaFIA mice28 or intraarticular injection of clodronate liposomes22,42, our model involving joint trauma via articular fracture prevented this effect. In macrophage-depleted MaFIA mice on Day 0 and Day 2, we observed a significant increase of F4/80+ macrophages within the deep layers of the synovial stroma, suggesting either a compensatory influx or proliferation of macrophages. In diet-induced obese MaFIA mice, systemic macrophage depletion similarly induced systemic inflammation and did not attenuate the severity of OA28. Systemic macrophage depletion using clodronate liposomes in diet-induced obese C57BL/6 mice likewise resulted in increased systemic inflammation in the form of neutrophilia and altered circulating cytokines29. Also a limitation of this study is that additional cell types within the synovium were not identified. However, we can speculate that similar to previous findings28, neutrophils may increase as a compensatory mechanism. Future investigations may look at immune cell phenotyping via polychromatic flow cytometry to better characterize the cell subsets involved following articular fracture.
Additionally, in MaFIA mice, systemic depletion of macrophages in a skeletal muscle injury model has been shown to cause a compensatory increase, or rebound, of macrophages, while systemic clodronate-treated mice did not have the same effect47,48.We have previously shown that the acute 7-day period following articular fracture is associated with greater synovitis, which may contribute to long-term dysregulation of chondrocyte function and lead to degenerative changes in the articular cartilage characteristic of PTA10. Our goal in this study was to modulate the acute inflammatory response with a transient depletion of macrophages. However, we found no reduction in the number of macrophages and in some cases an increased number, suggesting a possible rebound effect in response to a transient depletion of macrophage in the setting of an intra-articular fracture. Fate-mapping approaches used in a recent study determined that monocyte-derived macrophages recruited to the synovium actively contribute to joint inflammation, but a local population of synovial epithelial-like macrophages provided a protective barrier48. This new insight suggests that intra-articular depletion may preferentially deplete this anti-inflammatory population of macrophages.
Interestingly, our macrophage depletion method resulted in a change in the polarity of macrophages to a dominance of the M1 classically-activated lineage in macrophage-depleted MaFIA mice with a trend toward lower levels of M2 regulatory macrophages within the synovial stroma. One possible explanation of this shift in macrophage polarity is that an influx of monocytes, from locations such as the bone marrow, may have further differentiated into M1 macrophages in response to fracture. Alternatively, native synovial M2 macrophages may have switched their phenotype to M1 macrophages following injury. Dissecting the precise mechanisms by which the transient intra-articular macrophage depletion significantly increased M1, but not M2, synovial macrophages in MaFIA mice warrants further investigation.
A similar shift in macrophage polarity in AP20187-treated MaFIA mice has been reported in a glioma model, resulting in both an influx of M1 macrophages and a reduction in the proliferation of M2 macrophages49. In a model of systemic macrophage depletion in MaFIA mice with a destabilized medial meniscal injury, synovial macrophages were successfully reduced at day 0; this depletion predominantly affected the M2 macrophage population at 9 weeks post-depletion28. The M1/M2 ratio is critical for healthy bone homeostasis; enhancing the M2 macrophage population can improve bone healing outcomes50. The dominant M1 macrophage population in our macrophage-depleted MaFIA mice with decreased BMD suggests that M1 macrophages may be reciprocally detrimental for bone homeostasis, and further reinforces the importance of the ratio of M1 to M2 macrophages.
Although we observed an increase in F4/80+ macrophages in the clodronate-treated C57BL/6 mice, a shift in macrophage polarity was not observed. Additionally, no differences in bone morphology were observed in the clodronate-treated C57BL/6 mice. These differences may be the result of inherent characteristics of the macrophage depletion methods. Mechanistically, administration of AP20187, a small molecule inhibitor, targets all CSF+ cells in MaFIA mice, while clodronate liposomes induce apoptosis in phagocytic cells. As a result, AP20187 depletes all monocytic cells in MaFIA mice, while clodronate primarily targets mature macrophage cells. This may explain the stable M1/M2 polarity in the clodronate-treated mice due to the lack of effect on the monocytic lineage itself. Clodronate also has anti-inflammatory effects independent of its effects on macrophages by inhibiting pro-inflammatory cytokines, making it useful in preventing synovial inflammation in models of rheumatoid arthritis and ankylosing spondylitis51. This may account for the attenuated inflammatory phenotype of the clodronate-treated mice when compared to the MaFIA depleted mice. In addition to its effect on inflammation, clodronate inhibits bone resorption by reducing osteoclastic activity52, providing a potential explanation for the variation in bone morphological alterations between MaFIA and C57BL/6 mice. Furthermore, clodronate liposomes are much larger than the AP20187 molecule, which leads to difficulty in penetrating the synovium and vasculature53, making their effect less potent.
Our findings suggest that while prolonged inflammation has degenerative effects on cartilage, macrophages are critical immunomodulators in the setting of joint injury28. This study provides new insight on the regulatory role of macrophages in joint synovitis and bone homeostasis after joint fracture.
Supplementary Material
Acknowledgments
We would like to acknowledge Steve Johnson, RVT for his technical support with the animal protocol and Carrie Williams for her technical support with immunohistochemistry. Tyler Vovos, MD and Daniel Cunningham, MD for assistance with intra-articular injections, and the following funding sources, Arthritis Foundation Grant 5244, NIH grants AG46927, AG15768, OD010707, and Synthes/DePuy Research grant 13126.
Footnotes
Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.joca.2020.01.015.
Conflict of interest
The authors have no financial or personal relationships that could potentially influence or bias this work and conclusions.
References
- 1.Brown TD, Johnston RC, Saltzman CL, Marsh JL, Buckwalter JA.Posttraumatic osteoarthritis: a first estimate of incidence, prevalence, and burden of disease. J Orthop Trauma 2006;20: 739–44. [DOI] [PubMed] [Google Scholar]
- 2.Anderson DD, Chubinskaya S, Guilak F, Martin JA, Oegema TR,Olson SA, et al. Post-traumatic osteoarthritis: improved understanding and opportunities for early intervention. J Orthop Res 2011;29:802e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Olson SA, Guilak F. Post-traumatic Arthritis. New York, NY: Springer US; 2015. [Google Scholar]
- 4.Guilak F, Fermor B, Keefe FJ, Kraus VB, Olson SA, Pisetsky DS,et al. The role of biomechanics and inflammation in cartilage injury and repair. Clin Orthop Relat Res 2004:17–26. [DOI] [PubMed]
- 5.Scott A, Khan KM, Roberts CR, Cook JL, Duronio V. What do wemean by the term “inflammation”? A contemporary basic science update for sports medicine. Br J Sports Med 2004;38: 372–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Furman BD, Kimmerling KA, Zura RD,Reilly RM, Zlowodzki MP, Huebner JL, et al. Articular ankle fracture results in increased synovitis, synovial macrophage infiltration, and synovial fluid concentrations of inflammatory cytokines and chemokines. Arthritis Rheum 2015;67:1234–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Elsaid KA, Fleming BC, Oksendahl HL, Machan JT, Fadale PD,Hulstyn MJ, et al. Decreased lubricin concentrations and markers of joint inflammation in the synovial fluid of patients with anterior cruciate ligament injury. Arthritis Rheum 2008;58:1707–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brophy RH, Rai MF, Zhang Z, Torgomyan A, Sandell LJ. Molecular analysis of age and sex-related gene expression in meniscal tears with and without a concomitant anterior cruciate ligament tear. J Bone Joint Surg Am 2012;94:385–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Scanzello CR, McKeon B, Swaim BH, DiCarlo E, Asomugha EU,Kanda V, et al. Synovial inflammation in patients undergoing arthroscopic meniscectomy: molecular characterization and relationship to symptoms. Arthritis Rheum 2011;63:391–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lewis JS, Hembree WC, Furman BD, Tippets L, Cattel D, Huebner JL, et al. Acute joint pathology and synovial inflammation is associated with increased intra-articular fracture severity in the mouse knee. Osteoarthritis Cartilage 2011;19: 864–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Furman BD, Kimmerling KA, Zura RD,Reilly RM,Zlowodzki MP, Huebner JL, et al. Articular ankle fracture results in increased synovitis, synovial macrophage infiltration, and synovial fluid inflammatory cytokines and chemokines. Arthritis Rheum 2015. [DOI] [PMC free article] [PubMed]
- 12.Furman BD, Strand J, Hembree WC, Ward BD, Guilak F, Olson SA. Joint degeneration following closed intraarticular fracture in the mouse knee: a model of posttraumatic arthritis. J Orthop Res 2007;25:578–92. [DOI] [PubMed] [Google Scholar]
- 13.Lewis JS Jr, Furman BD, Zeitler E, Huebner JL, Kraus VB,Guilak F, et al. Genetic and cellular evidence of decreased inflammation associated with reduced incidence of posttraumatic arthritis in MRL/MpJ mice. Arthritis Rheum 2013;65: 660–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ward BD, Furman BD, Huebner JL, Kraus VB, Guilak F, Olson SA.Absence of posttraumatic arthritis following intraarticular fracture in the MRL/MpJ mouse. Arthritis Rheum 2008;58: 744–53. [DOI] [PubMed] [Google Scholar]
- 15.Deng Z, Gao X, Sun X, Amra S, Lu A, Cui Y, et al. Characterization of articular cartilage homeostasis and the mechanism of superior cartilage regeneration of MRL/MpJ mice. Faseb J 2019;33:8809–21. [DOI] [PubMed] [Google Scholar]
- 16.Bondeson J, Wainwright SD, Lauder S, Amos N, Hughes CE. Therole of synovial macrophages and macrophage-produced cytokines in driving aggrecanases, matrix metalloproteinases, and other destructive and inflammatory responses in osteoarthritis. Arthritis Res Ther 2006;8:R187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jablonski KA, Amici SA, Webb LM, Ruiz-Rosado Jde D, Popovich PG, Partida-Sanchez S, et al. Novel markers to delineate murine M1 and M2 macrophages. PloS One 2015;10. e0145342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sudan B, Wacker MA, Wilson ME, Graff JW. A systematicapproach to identify markers of distinctly activated human macrophages. Front Immunol 2015;6:253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pajarinen J, Lin T, Gibon E, Kohno Y, Maruyama M, Nathan K,et al. Mesenchymal stem cell-macrophage crosstalk and bone healing. Biomaterials 2019;196:80–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kraus VB, McDaniel G, Huebner JL, Stabler TV, Pieper CF,Shipes SW, et al. Direct in vivo evidence of activated macrophages in human osteoarthritis. Osteoarthritis Cartilage 2016;24:1613–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu B, Zhang M, Zhao J, Zheng M, Yang H. Imbalance of M1/M2 macrophages is linked to severity level of knee osteoarthritis. Exp Ther Med 2018;16:5009–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Blom AB, van Lent PL, Libregts S, Holthuysen AE, van derKraan PM, van Rooijen N, et al. Crucial role of macrophages in matrix metalloproteinase-mediated cartilage destruction during experimental osteoarthritis: involvement of matrix metalloproteinase 3. Arthritis Rheum 2007;56:147–57. [DOI] [PubMed] [Google Scholar]
- 23.Van Lent PL, Van den Hoek AE, Van den Bersselaar LA, Spanjaards MF, Van Rooijen N, Dijkstra CD, et al. In vivo role of phagocytic synovial lining cells in onset of experimental arthritis. Am J Pathol 1993;143:1226–37. [PMC free article] [PubMed] [Google Scholar]
- 24.Furman BD, Mangiapani DS, Zeitler E, Bailey KN, Horne PH,Huebner JL, et al. Targeting pro-inflammatory cytokines following joint injury: acute intra-articular inhibition of interleukin-1 following knee injury prevents post-traumatic arthritis. Arthritis Res Ther 2014;16:R134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kimmerling KA, Furman BD, Mangiapani DS, Moverman MA,Sinclair SM, Huebner JL, et al. Sustained intra-articular delivery of IL-1RA from a thermally-responsive elastin-like polypeptide as a therapy for post-traumatic arthritis. Eur Cell Mater 2015;29:124–39. discussion 139–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li J, Hsu HC, Yang P, Wu Q, Li H, Edgington LE, et al. Treatment of arthritis by macrophage depletion and immunomodulation: testing an apoptosis-mediated therapy in a humanized death receptor mouse model. Arthritis Rheum 2012;64:1098–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Uchida K, Satoh M, Inoue G, Onuma K, Miyagi M, Iwabuchi K,et al. CD11c(+) macrophages and levels of TNF-alpha and MMP-3 are increased in synovial and adipose tissues of osteoarthritic mice with hyperlipidaemia. Clin Exp Immunol 2015;180:551–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wu CL, McNeill J, Goon K, Little D, Kimmerling K, Huebner J, et al. Conditional macrophage depletion increases inflammation and does not inhibit the development of osteoarthritis in obese macrophage Fas-induced apoptosis-transgenic mice. Arthritis Rheum 2017;69:1772–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bader JE, Enos RT, Velazquez KT, Carson MS, Sougiannis AT, McGuinness OP, et al. Repeated clodronate-liposome treatment results in neutrophilia and is not effective in limiting obesity-linked metabolic impairments. Am J Physiol Endocrinol Metab 2019;316:E358–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Burnett SH, Kershen EJ, Zhang J, Zeng L, Straley SC, Kaplan AM, et al. Conditional macrophage ablation in transgenic mice expressing a Fas-based suicide gene. J Leukoc Biol 2004;75: 612–23. [DOI] [PubMed] [Google Scholar]
- 31.Van Rooijen N, Sanders A. Liposome mediated depletion of macrophages: mechanism of action, preparation of liposomes and applications. J Immunol Methods 1994;174:83–93. [DOI] [PubMed] [Google Scholar]
- 32.van Lent PL, Holthuysen AE, van den Bersselaar LA, van Rooijen N, Joosten LA, van de Loo FA, et al. Phagocytic lining cells determine local expression of inflammation in type II collagen-induced arthritis. Arthritis Rheum 1996;39:1545–55. [DOI] [PubMed] [Google Scholar]
- 33.Furman BD, Olson SA, Guilak F. The development of posttraumatic arthritis after articular fracture. J Orthop Trauma 2006;20:719–25. [DOI] [PubMed] [Google Scholar]
- 34.Alexander KA, Chang MK, Maylin ER, Kohler T, Muller R, Wu AC, et al. Osteal macrophages promote in vivo intramembranous bone healing in a mouse tibial injury model. J Bone Miner Res 2011;26:1517–32. [DOI] [PubMed] [Google Scholar]
- 35.Van Lent PL, Holthuysen AE, Van Rooijen N, Van De Putte LB,Van Den Berg WB. Local removal of phagocytic synovial lining cells by clodronate-liposomes decreases cartilage destruction during collagen type II arthritis. Ann Rheum Dis 1998;57: 408–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Krenn V, Morawietz L, Burmester GR, Kinne RW, MuellerLadner U, Muller B, et al. Synovitis score: discrimination between chronic low-grade and high-grade synovitis. Histopathology 2006;49:358–64. [DOI] [PubMed] [Google Scholar]
- 37.Louer CR, Furman BD, Huebner JL, Kraus VB, Olson SA, Guilak F.Diet-induced obesity significantly increases the severity of posttraumatic arthritis in mice. Arthritis Rheum 2012;64: 3220–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shoulders H, Garner KH, Singla DK. Macrophage depletion byclodronate attenuates bone morphogenetic protein-7 induced M2 macrophage differentiation and improved systolic blood velocity in atherosclerosis. Transl Res 2019;203:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Utomo L, Fahy N, Kops N, van Tiel ST, Waarsing JH, Verhaar JA,et al. Peripheral blood monocyte and synovial macrophage subsets in the mouse model of osteoarthritis induced by destabilization of the medial meniscus. Osteoarthritis Cartilage 2019;27:S376. [Google Scholar]
- 40.Sun AR, Wu X, Liu B, Chen Y, Armitage CW, Kollipara A, et al. Pro-resolving lipid mediator ameliorates obesity induced osteoarthritis by regulating synovial macrophage polarisation. Sci Rep 2019;9:426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chang MK, Raggatt LJ, Alexander KA, Kuliwaba JS, Fazzalari NL,Schroder K, et al. Osteal tissue macrophages are intercalated throughout human and mouse bone lining tissues and regulate osteoblast function in vitro and in vivo. J Immunol 2008;181: 1232–44. [DOI] [PubMed] [Google Scholar]
- 42.Blom AB, van Lent PL, Holthuysen AE, van der Kraan PM,Roth J, van Rooijen N, et al. Synovial lining macrophages mediate osteophyte formation during experimental osteoarthritis. Osteoarthritis Cartilage 2004;12:627–35. [DOI] [PubMed] [Google Scholar]
- 43.Cho SW, Soki FN, Koh AJ, Eber MR, Entezami P, Park SI, et al. Osteal macrophages support physiologic skeletal remodeling and anabolic actions of parathyroid hormone in bone. Proc Natl Acad Sci U S A 2014;111:1545–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Green MJ, Deodhar AA. Bone changes in early rheumatoidarthritis. Best Pract Res Clin Rheumatol 2001;15:105–23. [DOI] [PubMed] [Google Scholar]
- 45.Shimizu S, Shiozawa S, Shiozawa K, Imura S, Fujita T. Quantitative histologic studies on the pathogenesis of periarticular osteoporosis in rheumatoid arthritis. Arthritis Rheum 1985;28:25–31. [DOI] [PubMed] [Google Scholar]
- 46.Scanzello CR. Role of low-grade inflammation in osteoarthritis. Curr Opin Rheumatol 2017;29:79–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cote CH, Bouchard P, van Rooijen N, Marsolais D, Duchesne E.Monocyte depletion increases local proliferation of macrophage subsets after skeletal muscle injury. BMC Muscoskel Disord 2013;14:359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Culemann S, Gruneboom A, Nicolas-Avila JA, Weidner D, Lammle KF, Rothe T, et al. Locally renewing resident synovial macrophages provide a protective barrier for the joint. Nature 2019;572:670–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gabrusiewicz K, Hossain MB, Cortes-Santiago N, Fan X, Kaminska B, Marini FC, et al. Macrophage ablation reduces M2like populations and jeopardizes tumor growth in a MAFIAbased glioma model. Neoplasia 2015;17:374–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schlundt C, El Khassawna T, Serra A, Dienelt A, Wendler S,Schell H, et al. Macrophages in bone fracture healing: their essential role in endochondral ossification. Bone 2018;106: 78–89. [DOI] [PubMed] [Google Scholar]
- 51.Toussirot E, Wendling D. Antiinflammatory treatment with bisphosphonates in ankylosing spondylitis. Curr Opin Rheumatol 2007;19:340–5. [DOI] [PubMed] [Google Scholar]
- 52.Lin HN, O’Connor JP. Osteoclast depletion with clodronate liposomes delays fracture healing in mice. J Orthop Res 2017;35:1699–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Van Lent PLEM, Van den Bersselaar LAM, Holthuyzen AEM,Van Rooijen N, Van de Putte LBA, Van den Berg WB. Phagocytic synovial lining cells in experimentally induced chronic arthritis: down-regulation of synovitis by CL2MDP-liposomes. Rheumatol Int 1994;13:221–8. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.