Abstract
Introduction:
Chemotherapy-induced peripheral neurotoxicity (CIPN) remains a significant toxicity in cancer survivors without preventative strategies or rehabilitation. Exercise and physical activity-based interventions have demonstrated promise in reducing existing CIPN symptoms and potentially preventing toxicity, however there is a significant gap in evidence due to the lack of quality clinical trials and appropriate outcome measures.
Areas Covered:
The authors systematically reviewed outcome measures in CIPN exercise and physical rehabilitation studies with expert panel consensus via the Peripheral Nerve Society Toxic Neuropathy Consortium to provide recommendations for future trials. Across 26 studies, 75 outcome measures were identified and grouped into 16 domains within three core areas - measures of manifestations of CIPN (e.g. symptoms/signs), measures of the impact of CIPN and other outcome measures.
Expert Opinion:
This article provides a conceptual framework for CIPN outcome measures and highlights the need for definition of a core outcome measures set. The authors provide recommendations for CIPN exercise and physical rehabilitation trial design and outcome measure selection. The development of a core outcome measure set will be critical in the search for neuroprotective and treatment approaches to support cancer survivors and to address the significant gap in the identification of effective rehabilitation and treatment options for CIPN.
Keywords: Chemotherapy induced peripheral neurotoxicity, exercise, rehabilitation, outcome measures, peripheral neuropathy, neurotoxicity, cancer survivorship, treatment
1. Introduction
Chemotherapy-induced peripheral neurotoxicity (CIPN) is a major side effect of the treatment of cancer, leading to early cessation of treatment and long-lasting disability [1]. CIPN develops after treatment with several conventional chemotherapy types including platinum compounds, taxanes, vinca alkaloids, thalidomide and bortezomib. CIPN produces mainly sensory or sensorimotor nerve damage in a stocking and glove distribution, whereas patients severely affected usually manifest sensory ataxia, gait disruption, increased falls risk, upper limb impairment and functional disability. Accurate diagnosis of CIPN is important, including differentiation from other syndromes including paraneoplastic neuropathies [1, 2]. Despite successful treatment and long-term survival prospects, up to 40% of cancer survivors may be left with long-term functional disability and reduced quality of life due to CIPN [2]. The growing population of cancer survivors with persistent CIPN has prompted research into potential preventative strategies as well as rehabilitation approaches to reduce the burden of CIPN.
Exercise and physical activity-based interventions have demonstrated promise for CIPN in both reducing the burden of existing symptoms and potentially for prevention of toxicity [3,4]. Although there is growing evidence that exercise rehabilitation strategies may be effective in patients with CIPN, there are significant limitations in existing studies, in part due to small sample sizes and variable outcome measures selection [5]. Recent reviews on the efficacy of exercise interventions in CIPN have identified heterogeneity in study design and inconsistent outcome measures, two factors that limit the ability to combine data across studies to provide evidence and form consensus [3,4,6]. Accordingly, although exercise is considered generally helpful, there remains insufficient evidence to recommend widespread implementation of exercise-based interventions for CIPN on the basis of current data [4,7,8].
Identification of optimal outcome measures in CIPN intervention trials represents a necessary step towards better trial design and ultimately finding successful strategies for CIPN prevention and treatment. Best practice in the design of randomized clinical trials (RCTs) aimed at examining efficacy of preventative approaches for CIPN have been highlighted [5,9]. However, in the context of exercise and physical rehabilitation trials, there are additional considerations in terms of endpoint selection and tailoring the study to intervention type. While consensus is growing that combination of patient and clinician reported outcome measures are likely to be best practice for clinical trial outcome measures in CIPN assessment [10], there remains no agreed framework on how best to measure sensory ataxia, upper-limb function, physical function, or balance in the context of CIPN. This review systematically examines existing outcome measures in CIPN exercise and physical rehabilitation studies to provide recommendations for future exercise and physical rehabilitation trials in CIPN to address the significant gap in the identification of effective rehabilitation and treatment options for CIPN through future high quality large-scale clinical trials.
2. Methods
2.1. Systematic review on rehabilitation in CIPN
We systematically reviewed papers exploring exercise-based rehabilitation strategies in CIPN to examine current outcome measures, conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) recommendations [11]. Detailed review methods and search strategy are presented in supplementary methods. Studies were included if they encompassed an exercise or physical activity-based intervention in people with CIPN or people being treated with neurotoxic chemotherapies at-risk of CIPN. Studies were excluded if they included a majority of patients who were not treated with neurotoxic chemotherapy or if chemotherapy types were unspecified. Patients treated with immune-checkpoint inhibitors were not included in the review. Extracted data included study design, chemotherapy and cancer types, sample size, symptom(s) targeted by the intervention and primary and secondary outcome measures: peripheral neuropathy, pain, gait and balance, falls, upper-limb function, fatigue, physical functioning, disability, quality of life, psychological status, and body composition measures.
2.2. Consensus meeting.
An expert panel discussion forum was held under the auspices of the Peripheral Nerve Society Toxic Neuropathy Consortium (TNC) [12]. The TNC Working Group on Chemotherapy-induced Peripheral Neurotoxicity and Physical Rehabilitation convened a meeting in April 2021 to review existing data and provide recommendations for outcome measure domains in CIPN exercise rehabilitation research. The Working Group comprised an international multidisciplinary group of oncologists, neurologists, and scientists with expertise in CIPN. Initial work via systematic review to examine currently available outcome measure domains was presented and a facilitated discussion was undertaken. Outcome measures were grouped into domains including manifestations and impacts as per the OMERACT Filter 2.1 framework [13], which was utilized to provide a structure for the core domain list.
3. Results
From a total of 2709 records, 26 studies were identified, comprising 17 randomized intervention studies and 9 non-randomized or pilot studies (Supplementary Figure 1; Supplementary Tables 1 and 2). There were a range of sensorimotor exercise, balance training, aerobic, and strength and resistance interventions trialled both during and after neurotoxic chemotherapy administration, which are reviewed elsewhere in terms of content and efficacy [3,4,6]. A total of 13 studies examined CIPN prevention and 13 studies examined CIPN treatment. 18 (69%) were published in the last 5 years. A total of 75 individual outcome measures were used across these studies, with substantial variation in outcome measure selection between studies (Supplementary Tables 1 and 2). These outcome measures were grouped into 16 domains within three core areas - measures of manifestations of CIPN (e.g. symptoms/signs), measures of the impact of CIPN and other outcome measures which are detailed below (Table 1).
Table 1.
CIPN exercise and physical rehabilitation study outcome measure domains
| Manifestations of CIPN (Signs/symptoms) | Domain | Outcome measures | Patients examined | References |
| Patient reported outcome measures | ||||
| CIPN Symptoms | EORTC-CIPN20* FACT/GOG-NTx* NRS CIPNAT PNQ |
1246 | [14–31,36] | |
| Pain symptoms | S-LANSS PainDETECT NRS Pain Brief Pain Index |
351 | [21,23,24,26,30–33] | |
| Clinical neuropathy outcome measures | ||||
| Clinician Global Impression | NCI CTCAE | 173 | [15,25] | |
| Neurological Examination | Clinical neurological examination (including pinprick, MRC strength) Vibratory threshold Total Neuropathy Score* |
629 | [14,15,17–19,23,25,26,31,34,36,38,39] | |
| Neurophysiology | Nerve conduction studies | 263 | [18,19,26,30] | |
| Quantitative Sensory Testing | Quantitative sensory testing Pain pressure threshold |
179 | [25,31] | |
| Impact | Overall Disability | CIPN R-ODS | 29 | [18] |
| Impact of CIPN | CIPNAT PNQ |
145 | [23,28,30] | |
| Impact on QoL | EORTC QLQC30* FACT-G SF-36 McGill QoL |
820 | [15–20,22,25–27,30,32,33,38,43] | |
| Impact on physical function | ||||
| Upper-limb function | 6-hole buttoning test Coin test Hand function (DASH) |
127 | [24,31] | |
| Strength and Physical Fitness | Barthel Index Short physical performance battery Chair rise test* Sit to stand* Partial curl up Standing vertical jump test Timed up and go Sit and reach Functional reach Walking tests* (50 step walk; Stair walking with modified Borg scale; 6MWT) Strength (1-repetition maximum; dynamometry; handgrip) Activity level Cardiorespiratory fitness (V02 max; individual anaerobic threshold) |
817 | [15,17–21,23–25,27,31,32,36,38,39,43] | |
| Balance and gait | Postural sway* Tandem stance Limit of stability test Unipedal stance time Berg Balance Scale Fullerton Advanced Balance Scale GGT-reha balance scale Modified clinical test for sensory interaction in balance Gait performance, dynamic gait index Falls efficacy scale Number of falls |
561 | [15,17–20,26,27,33,34,36,38,39,44] | |
| Other outcome measures | Other symptoms, signs, and outcome measures | Fatigue (Questionnaire, MFI20) Pittsburgh Sleep Quality Index Perceived stress Psychological distress (Brief symptom inventory) FACIT-spiritual Mindful attention awareness scale HADS Cognitive impairment Cancer type specific symptoms (BR23, LC13) Salivary cortisol level |
152 | [16,20,22,23,38] |
| Body composition and diet | Body composition (bioelectrical impedance analysis, skinfold test) Diet (Subjective Global Assessment, Mini Nutritional Assessment) |
66 | [32,39] | |
| Chemotherapy dose | Relative dose intensity | 310 | [14,19,24,30] | |
| Exercise adherence | Exercise adherence | 965 | [14–19,21–23,26,27,29,30,38,39,44] | |
Number of patients examined indicates the total number of patients assessed with each domain of outcome measure in the included CIPN exercise and physical rehabilitation studies. Outcome measure domains are grouped and presented in the same order as the text. Outcome measures are starred where provisional recommendations for inclusion can be made.
Abbreviations: 6MWT Six minute walking test; BR23 Breast cancer specific module; CIPN Chemotherapy induced peripheral neurotoxicity; CIPNAT CIPN Assessment Tool; CIPN-R-ODS Rasch-built Overall Disability Scale for patients with CIPN; CIPN DASH Disability of the Arm, Shoulder and Hand Scale; EORTC CIPN20 European Organization for Research and Treatment of Cancer Chemotherapy Induced Peripheral Neuropathy Questionnaire; EORTC QLQ-C30 European Organization for Research and Treatment of Cancer Quality of Life questionnaire; FACIT Functional Assessment of Chronic Illness Therapy; FACT-G Functional Assessment of Cancer Therapy – General; FACT/GOG-Ntx Functional Assessment of Cancer Therapy/Gynecologic Cancer Group Neurotoxicity Subscale; GGT-Reha Gleichgewichtstest-Reha; HADS Hospital Anxiety and Depression Scale; LC13 Lung cancer specific module; MFI20 Multidimensional Fatigue Inventory; MRC Medical Research Council; NCI-CTCAE National Cancer Institute Common Terminology for Adverse Events; NRS numerical rating scale; PNQ Patient Neurotoxicity Questionnaire; QoL Quality of Life; SF-36 36-item Short Form Health Survey; S-LANSS Self- administered Leeds Assessment of Neuropathic Symptoms and Signs; VO2 max Maximal oxygen consumption
3.1. Measures of manifestations of CIPN
A range of measures have been used to quantify the manifestations of CIPN, including those assessing symptoms of neuropathy and pain, neurological examination, and instrumental measures (Table 1).
3.1.1. CIPN symptoms - patient reported outcome measures
Patient reported outcome measures (PROMs) of CIPN symptoms were the most utilized outcome measure across studies, with use of six different PROMs in 19 studies (73%). The most used were the EORTC-CIPN20 or its variants (n= 7 studies [14–20]) and the FACT/GOG-Ntx [17,21–27] (n=8 studies). CIPN PROMs were used as primary outcome measures in four studies [14,21,27,28].
In some studies examining prevention of CIPN during chemotherapy administration, CIPN PROMs FACT/GOG-Ntx and EORTC-CIPN20 failed to demonstrate CIPN prevention with intervention but instead identified an increase in CIPN symptoms with chemotherapy administration (4 of 6 studies) [14–16,20]. A small RCT (n=30) of endurance, resistance and balance training in metastatic colorectal cancer patients demonstrated CIPN symptom stability (via FACT/GOG-Ntx) in the intervention group while the control group experienced significant symptom worsening [27]. A larger RCT (n=159) of sensorimotor training or resistance exercise vs usual care found reduced sensory symptoms in the feet using a modified version of the EORTC-CIPN20 scale but no effects in the entire scale or in intention-to-treat analyses [19]. However, interestingly, in a small RCT (n=36) of sensorimotor training vs control group, while the EORTC-CIPN20 did not detect any difference between groups, measures of balance and strength demonstrated worse performance in the control group [20], suggesting that the CIPN PROM did not detect functional differences between groups. In another RCT, a numerical rating scale (NRS; 0–10 scale) of CIPN symptoms numbness and tingling and hot/coldness in hands/feet was used to measure benefits from a walking and resistance exercise intervention during chemotherapy (n=170 vs 185 controls) [29]. Symptoms of hot/coldness were reduced following intervention compared to the control group [29], with a trend towards reduced numbness and tingling in the intervention group.
In contrast, 8 of 10 exercise studies in patients with established CIPN demonstrated significant benefits post-intervention using CIPN PROMs (EORTC-CIPN20 [17,18], FACT/GOG-Ntx [17,21,24,25], patient neurotoxicity questionnaire PNQ [23], chemotherapy-induced peripheral neuropathy assessment tool CIPNAT [28,30]). However, the FACT/GOG-NTx did not detect significant benefits in two CIPN treatment studies: one RCT that evaluated 8-week sensorimotor training and whole-body vibration (n=20 vs 20 controls)[26] and another that evaluated an 8-week yoga intervention (n=10) [23]. Interestingly, while there was no significant improvement in the FACT/GOG-Ntx in the sensorimotor training and whole-body vibration study, 95% of patients reported symptom improvement on a global perception of change scale [26]. In addition, significant improvement was detected by the PNQ but not the FACT/GOG-Ntx in the yoga study, suggesting that PROM content may be important [23]. Conversely, an 8-week yoga intervention in patients with established CIPN (n=21 vs 20 controls) found improvement in the intervention group in the FACT/GOG-Ntx but not a NRS for numbness or tingling [21]. Further, another study identified significant changes only in FACT/GOG-Ntx items relating to lower limbs [25]. Taken in total, this may suggest that CIPN PROM content should be reviewed before implementation in exercise trials to ensure that these outcome measures are able to appropriately capture exercise-related improvement.
3.1.2. Pain symptoms - patient reported outcome measures
Pain specific PROMs were utilized in eight studies (32% of studies) and specified as a primary outcome measure in three studies. Pain specific PROMs included the self-report version of the Leeds Assessment of Neuropathic Symptoms and Signs pain scale (S-LANSS [30–32]), PainDETECT questionnaire [26,33], brief pain inventory (BPI [23,24]) and NRSs for pain [21] or fingertip pain [31]. All studies demonstrated a positive effect of exercise interventions on pain, but it should be noted that the small number of studies and diversity of interventions preclude any conclusions on the suitability of these PROMs as outcome measures. Moreover, these pain scales are heterogeneous, in that S-LANSS and PainDETECT are designed to separate neuropathic from nociceptive pain and not to measure its intensity, while the BPI explores pain and related disability. Further, neuropathic pain is not always a component of CIPN, so the use of pain PROMs should be limited to trials in which the intervention is intended to modulate pain and in patients who had received chemotherapies that produce neuropathic pain.
3.1.3. Clinical neuropathy outcome measures - neurological examination and clinician grading
Clinical and neurological examination outcome measures were utilized in 13 studies (50% of studies) and specified as a primary outcome measure in three studies [19,26,34].
The National Cancer Institute Common Terminology for Adverse Events (NCI-CTCAE) clinician grading scale was used as an outcome measure in two studies [15,25]. However, previous studies have highlighted the caveats to its utility as a robust outcome measure for CIPN clinical trials [9,35] and it did not detect improvement following exercise intervention in either study [15,25].
Composite clinical neurological examination was an outcome measure in seven studies [15,18,19,25,26,34,36], mainly comprising assessment of deep tendon reflexes, proprioception, vibration, light touch, pinprick sensibility or combination of these modalities. In four of these studies, neurological examination was combined with patient symptoms report in the validated and comprehensive Total Neuropathy Score (©Johns Hopkins University, TNSc [18,19]; TNSr [19,36]) or modified TNS [19,34]. Three of these studies were undertaken to treat established CIPN and all three identified significant improvement in TNS scores post-exercise intervention [18,34,36]. Multiple variants of the TNS were utilised in a RCT of sensorimotor or resistance exercise to prevent CIPN development (n=112 vs 58 usual care) but failed to demonstrate any differences following exercise intervention compared to usual care [19]. Accordingly, further studies are required to demonstrate the utility of the TNS as an outcome measure in CIPN treatment and prevention trials.
The most commonly assessed examination modality was vibration sense, which was assessed as an indicator of large sensory fiber damage [37] using a semi-quantitative tuning fork [14,15,17,18,25,26,38,39] or biothesiometer [23,31] in nine studies. In exercise interventions designed to prevent CIPN, vibration sensibility was demonstrated to improve in only one study (out of five studies), which was a RCT (n=61) of sensorimotor, endurance and strength training [38]. In this study, a significantly lower proportion of patients from the intervention group demonstrated abnormal vibration sense at follow-up compared to the control group [38]. In other studies of exercise interventions administered during neurotoxic chemotherapy, vibration sense did not demonstrate significant improvement [15,31,39] or only at a single timepoint [14]. In patients with established CIPN, improved vibration sense post-exercise intervention relative to controls was also identified in a single study of sensorimotor training or whole-body vibration training (n=20 vs 20 controls) [26], with improved vibration sense at medial malleolus following intervention compared to controls. Other studies either did not report vibration results or found improvement at only selective sites [23,31].
Another examination modality involved testing of deep tendon reflexes. There was partial [26] or no significant improvement [25] of reflex function following exercise intervention to treat established CIPN. This may be due to the often-irreversible nature of reflex loss in established CIPN.
3.1.4. Instrumental measures: nerve conduction and quantitative sensory measures
Both nerve conduction studies (NCS) and quantitative sensory testing (QST) provide information regarding CIPN but require specialized equipment and technical expertise. NCS provide mechanistic information relevant to CIPN pathophysiology which correlate with clinical examination [37,40]. Only four studies (15%) have utilized NCS as an outcome measure in exercise trials [18,19,26,30]. Two studies in patients with established CIPN failed to demonstrate significant changes with intervention [18,26]. Two studies in patients receiving chemotherapy treatment also failed identify any inter-group differences [19,30]. Because the hallmark of CIPN on NCS is reduction in sensory amplitudes [41,42], a major limitation of one study was testing of nerve conduction velocity, rather than amplitude [30]. Because patients may present symptomatic recovery in the context of limited neurophysiological recovery, the utility of NCS as an outcome measure in studies of established CIPN is unclear. Examination of NCS during treatment as a marker of CIPN development may provide more utility, but this has not been demonstrated in the single study to date [19].
Quantitative Sensory Testing (QST) is a non-invasive outcome measure quantifying sensory fiber functionality [40]. QST measures have been utilized in only two exercise trials (one CIPN treatment study [25,31]) with conflicting results. While improved warm detection threshold [25] or improved pain pressure threshold [31] were identified following exercise-based interventions, all other QST parameters were unchanged.
3.2. Measures of CIPN impact
A range of measures have been used to quantify the impact of CIPN, both on overall quality of life and on physical function (Table 1), which are outlined below.
3.2.1. Overall disability and impact of CIPN: PROMs
While CIPN PROMs often focus on symptom expression and severity, the CIPNAT tool incorporates symptom experience and interference subscales, encompassing symptom interference and physical activities participation limitations. CIPNAT demonstrated significant improvement following intervention in two studies [28,30]. The PNQ also incorporates elements addressing the functional impact of CIPN and identified improvement following intervention in a single study [23]. Similarly, the Rasch-built Overall Disability Scale (CIPN-R-ODS) was designed to capture changes in daily activities associated with CIPN but still requires further validation. The CIPN-R-ODS detected significant improvement in 29 patients who completed an 8-week multimodal exercise training intervention [18], with no change in the preceding control period.
3.2.2. Impact on quality of life
Quality of life (QoL) was assessed in 16 studies (62%), with four different instruments used (most commonly the EORTC-QLQC30, n=12 studies). QoL was the primary outcome in three studies. Half of studies investigating exercise as a preventative strategy for CIPN did not report significant differences in global QoL following intervention (5 of 10 studies) [15,16,20,27,43]. However, some randomized studies of exercise interventions showed significant improvement in overall global health status in EORTC-QLQ-C30 following exercise intervention during taxane chemotherapy [14], lymphoma treatment [38] or neurotoxic chemotherapy of multiple types [19,33]. In addition, a single arm study demonstrated improved QoL using the McGill scale [32], but this warrants study in randomized trials. In exercise interventions designed to treat CIPN, only two of six studies found improved QoL following intervention using the SF-36 [18] or the QLQ30 [30], while other studies did not demonstrate change in QoL following intervention [17,22,25,26]. Overall, the variation between patient populations, sample size and intervention type make it difficult to determine the optimal QoL assessment tool in this setting.
3.2.3. Impact on physical function – upper limb function
The impact of CIPN on physical function was measured using measures of upper-limb function, overall strength and physical fitness, and balance and gait.
A single study utilized simple tests of patient upper limb function [24]. These tests measured the time taken to button up a six-button shirt and pick up four coins from a table (timed coin test), and improved following a 2-week gymnastics and walking intervention. Another study included the patient reported Disabilities of the Arm, Shoulder and Hand questionnaire to probe upper-limb function but did not yield significant improvement [31]. While such measures may provide insight into upper limb functional disability in patients with CIPN, they have not been validated or investigated for measurement property suitability for inclusion as CIPN clinical trial outcome measures.
3.2.4. Impact on physical function – strength and physical fitness
A total of 16 studies assessed strength and physical fitness (62%; Table 1). Measures of strength and physical fitness spanned multiple domains of fitness, including global physical fitness, strength, aerobic capacity, endurance, and flexibility. Measures of strength and physical fitness were the primary outcome in two studies [39,43].
Global physical function was assessed using the Short Physical Performance Battery (incorporating 10-meter gait speed, 10-second stance balance, and chair rise test) [39] and the Barthel Index assessing independence in activities of daily living [43]. Although the Barthel Index captured significantly improved physical function following aerobic and strength training intervention in platinum-treated patients [43], it lacks content validity and specificity in measuring CIPN-related functional deficits. The Short Physical Performance Battery showed no changes in physical function following aerobic exercise intervention in 44 gastrointestinal cancer patients undergoing chemotherapy treatment [39]. A single study reported exercise-logged physical activity [38] which demonstrated improvements in the intervention group during chemotherapy.
Maximum strength or maximum power was assessed using dynamometry [19,20,31,32,36] or 1-repetition maximum (1RM) estimations (using upper or lower body resistance exercise machines or isometric force production) [15,27,39]. Strength improved with exercise and physical rehabilitation intervention as assessed via handgrip dynamometry in two of three studies [20,31,32]. Limited data regarding expected changes in hand dynamometry in CIPN is available which may reduce the utility of this outcome measure. Lower limb strength assessed via 1RM or dynamometry improved following interventions in four out of five studies [15,19,27,36,39].
Physical fitness or endurance were assessed using the chair rising or sit-to-stand test [18,20,21,25,33], timed up and go [23,36], curl-up test [32], and a standing vertical jump test [17]. Sit-to-stand, chair rising or timed up and go tests were utilized in multiple studies, and improved following intervention in all studies in patients with established CIPN [18,21,23,25,36]. However, improvement was not seen following interventions delivered during chemotherapy [20,33]. Other measures including standing vertical jump height [17] and partial curl up test [32] were assessed in single studies and demonstrated some improvement with intervention but require further study.
Physical fitness and aerobic capacity was also assessed using walking, stair-climbing or treadmill tests. The 6-minute walk test (6MWT) demonstrated improvement with intervention in three studies [15,18,43] out of four [27]. Other tests of walking ability included the 50-step walk [24] and stair climbing test [43], which both demonstrated improvement following intervention. Aerobic capacity was also examined using maximal oxygen consumption (VO2max) tests [17,32] or lactic acid threshold test [17,38] using treadmill or cycle ergometer and identified benefits of exercise intervention in three of the four studies. Flexibility tests (modified sit and reach, functional reach) were utilized in three studies and identified improvement following yoga interventions [21,23] but not a strength and endurance exercise program [32].
3.2.5. Balance and gait
Thirteen studies assessed balance or gait (50%), either using objective assessment, clinician-rated performance measures or patient report. Balance was the primary outcome in five studies [17,20,33,34,44].
The clinician-rated performance measures, such as the Berg Balance Scale [33,34], Fullerton Advanced Balance Scale [20], GGT-Reha [27] and modified clinical test for sensory interaction in balance [33,36], quantified static and dynamic balance based on individuals’ performance in various standing and sitting tasks into clinical scores. These improved following intervention in three of five studies, including in a single group study of patients with established CIPN [34] and between groups among participants in active chemotherapy treatment balance training intervention [20,33].
Balance was also assessed as postural sway using a variety of methods including force plates [17,19,20,27,38,39], horizontal waist level posturography measured via electronic tablet [18], and wearable sensor-based functional assessment [44]. The majority of studies identified improvement in postural sway following intervention (7 of 9 studies), both conducted during chemotherapy [20,27,38,39] and for treatment of established CIPN [17,18,44]. Other tests of balance did not demonstrate significant improvement following intervention including timed unilateral and tandem stance tests [15], force plates with visual surroundings [33], or gait speed [21,39]. Unipedal standing time decreased in the usual care group but not in exercise intervention groups in a RCT of sensorimotor or resistance training for CIPN prevention but there was no effect on postural sway between groups [19]. Fear of falling was assessed via patient report and captured improvements with intervention in patients during chemotherapy [19] but not in CIPN treatment trials [23,44]. There was no difference in the number of falls reported following exercise intervention [19].
3.2.6. Other outcome measures
A number of other symptom scales (none of which were primary outcome measures) have been utilized in CIPN exercise studies and are listed in Table 1. These include scales addressing fatigue [16,20], psychological status [22,23,38], cognitive impairment [38] and sleep quality [23] as well as inclusion of specific PROM subscales developed for breast cancer [20] and lung cancer [43]. Body composition and diet outcome measures have also been used to evaluate the efficacy of exercise interventions in CIPN [32,38,39]. Exercise adherence was collected or included as an outcome measure in 17 studies (Table 1), to illustrate the feasibility of conducting exercise intervention, particularly in patients undergoing chemotherapy treatment. Finally, relative chemotherapy dose intensity was compared between groups in four studies of CIPN prevention. Exercise intervention patients were more likely to reach 85% relative dose intensity than those in the usual care or delayed intervention groups in two studies [14,19] and received a higher chemotherapy dose in another study [30]. However, there was no difference between groups in another study [24].
4. Conclusions
The search for effective exercise and physical rehabilitation-based interventions for CIPN is accelerating. This paper systematically reviewed current evidence to make suggestions for the best outcome measures for such studies in CIPN. Although 26 studies examining exercise or physical rehabilitation interventions in patients with CIPN were identified, there was overall a lack of high-quality evidence to support specific measures, largely due to study design limitations [3,4]. We identified 75 different individual outcome measures across 16 domains (Table 1), highlighting the range and heterogeneity of outcome measures, and need for definition of a core outcome set to optimize outcome measures and study design for future use.
4.1. Conceptual framework for CIPN outcome measures
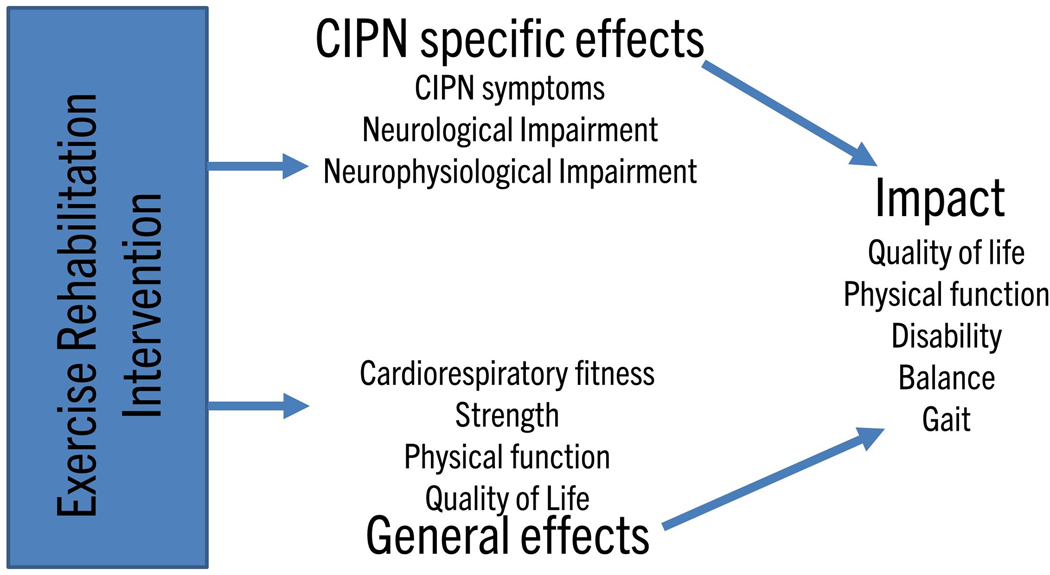
A critical feature of optimal outcome measure selection is identification of the key constructs that the outcome measures aim to capture. In the setting of CIPN exercise studies, this may be directly related to neuropathy (CIPN specific effects) or more generally (e.g. physical performance; Fig 1). Accordingly, a combination of outcome measures may be necessary to identify relevant change across these multiple areas. Due to the broad spectrum of effects of physical rehabilitation, the effects of any intervention may be general, specific to CIPN or a combination and require different outcome measures to be fully captured. As per the OMERACT 2.1 Framework [13], a core set of outcome measure domains can ensure content validity across manifestations of CIPN (incorporating symptoms, signs, biomarkers) and impact on daily life, lifespan considerations and societal/resource use. Definition of the outcomes of interest is essential and this framework is applied to CIPN exercise studies below (Fig 2).
Figure 1. Conceptual framework for chemotherapy-induced peripheral neurotoxicity outcome measures.
Chemotherapy-induced peripheral neurotoxicity (CIPN) exercise studies may produce CIPN-specific effects (improvement of CIPN symptoms, neurological or neurophysiological impairment) and/or general effects (improved cardiorespiratory fitness, strength, physical function or quality of life). Both of these effects will collectively impact on overall patient quality of life, physical function, disability, balance and gait. Outcome measure selection requires identification of the primary outcome of interest and may be directly related to CIPN or to physical performance.
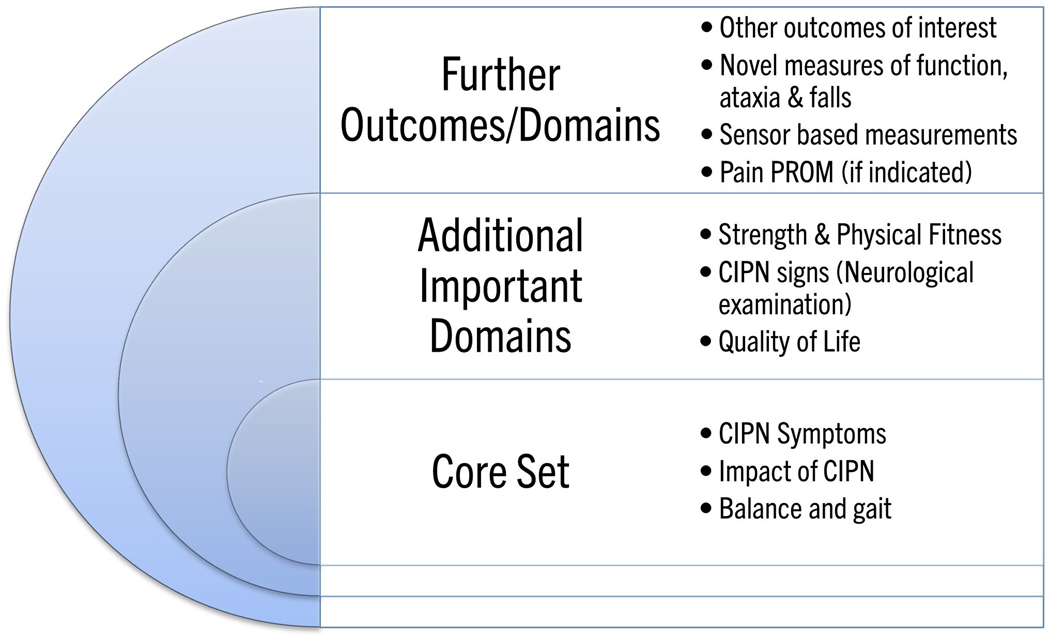
Figure 2. Proposal for outcome measure core set for chemotherapy-induced peripheral neurotoxicity exercise and rehabilitation studies.
Core outcomes suggested for inclusion in all chemotherapy-induced peripheral neurotoxicity (CIPN) exercise and physical rehabilitation trials, additional important domains suggested for inclusion as appropriate and further domains/outcomes suggested for future research.
4.2. Considerations for outcome measures selection in CIPN exercise studies
4.2.1. Assessment of manifestations of CIPN
Outcome measures addressing manifestations of CIPN include neurological and neurophysiological examination and symptoms-based measures. CIPN PROMs are crucial to examine CIPN symptoms and are necessary for inclusion in a core outcome measures set but may not be sufficient alone, particularly in CIPN prevention trials. CIPN PROMs are patient relevant and fulfil the increasing requirement by regulatory authorities and other bodies for clinical trials to incorporate patient-relevant endpoints [45]. Given the limitations of existing studies, it is difficult to identify the best PROM to quantify amelioration of CIPN symptoms following exercise. Further, the ideal PROM must be precise; for example, the use of neuropathic pain PROMs is appropriate solely for studies recruiting patients specifically selected to have neuropathic pain symptoms, which are typically only experienced by 25%−40% of patients with CIPN [46]. Additionally, some evidence suggests that CIPN PROMs may not capture relevant changes in patient function and development of sensory ataxia in exercise trials. Therefore, the development of other measures (including PROMs focused on sensory ataxia and patient function or objective and/or semi-objective clinical assessments) should be considered.
Although neurological examination-based outcome measures are valid measures of CIPN pathophysiology [47], responsiveness in the clinical trial setting needs to be demonstrated, particularly when assessing recovery post-treatment. The majority of studies utilizing clinical examination focused on a single modality such as vibration sensation, typically assessed at one or a few discrete sites. However, a battery of clinical tests (such as the TNS) may be more appropriate to capture CIPN and its severity changes. NCS lacked efficacy in identifying exercise effects, which may reflect the low sample sizes assessed or suggest that routine NCS lack sensitivity to change within the timeframe under study. NCS assessment of the dorsal branch of sural nerve was reported to be more sensitive and may be beneficial [41,42]. Further, examination of NCS during treatment as a marker of CIPN development may provide more utility, but this remains to be demonstrated. While the need for specialised equipment, time and patient cooperation must be considered when incorporating instrumental measures, there are some outcome measures which have never been implemented in CIPN exercise studies, such as skin biopsy [40]. Such techniques may provide complementary information into CIPN pathophysiology (particularly in prevention studies) with the advantage of centralized processing to reduce local variability. Taken in total, neurological examination-based outcome measures represent an important additional domain to assess in CIPN exercise trials but may not be practicable to include in every trial.
4.2.2. Assessment of CIPN impact
Assessment of CIPN impact has been measured via a number of domains including patient function, disability, quality of life, physical function, balance and gait. While many of these measures incorporate both the impact of CIPN and other constructs, which may be affected by exercise (Fig 1), these elements are relevant to patients and their wellbeing and still warrant inclusion in CIPN exercise studies. Accordingly, assessment of CIPN impact, balance and gait are recommended as core outcome measure domains and quality of life and strength and physical fitness are recommended as important additional domains (Fig 2).
Assessing the impact of CIPN on patient function is incompletely addressed through current outcome measures. This is particularly evident as widely used CIPN PROMs do not specifically probe symptoms of sensory ataxia or address physical performance. Sensory ataxia in CIPN patients has been demonstrated to associate with increased risk of falls [48], highlighting its functional significance. However, while functional scales of sensory ataxia have been developed [49], these have not been validated in CIPN patient populations. Accordingly, it may be necessary to develop additional tools which encompass CIPN symptoms as well as impact of symptoms related to sensory ataxia and functional disturbance. While some current CIPN PROMs (e.g. CIPNAT, PNQ) contain more content addressing the impact of CIPN on function, there remains insufficient studies to date incorporating these measures to recommend their use.
Physical function outcome measures are identified as important additional domains as they assess the practical, physical benefits of exercise interventions for the patient. However, simple measures of physical function such as timed up and go, six-minute walk and chair rising tests may capture CIPN-specific benefits of exercise but also additional constructs [50] and it may be difficult to dissociate an effect as being due to reduction in sensory ataxia or other non-specific effects such as improvement in fatigue or strength [51]. Further, other comorbidities may affect physical function such as cancer-related cachexia, sarcopenia or obesity [52]. Accordingly, physical function outcome measures should always be considered in combination with CIPN specific outcome measures. In addition, upper limb physical function was not frequently collected (e.g., pegboard test), despite patient reports of distress from CIPN-related difficulty with performing tasks such as buttoning, opening a jar, and typing on a laptop.
Quantification of balance and gait provides an index of CIPN-relevant physical function. There is not sufficient evidence to highlight specific measures of balance as preferable, but there are a range of objective and semi-objective measures that have been utilized. Simple tests of balance, such as eyes open and closed stance tests on static and dynamic surfaces, are clinically feasible and sensitive to differences between groups. A further factor which has been ill-addressed in currently available CIPN exercise studies is the risk of falling, which is an important outcome of physical improvement programs. Although assessing rate of falls within the limited timeframe of a clinical trial may not be feasible, there are other methods developed to address this construct [53].
A further important consideration in outcome measure development is the need for simple measures that can be easily implemented in resource poor environments or non-specialized centers. Simple tests include the chair-rising test, the 6MWT, the stair-walking test, and the handgrip dynamometry test (requires equipment but not expensive or hard to use). The 6MWT also translates well into real-world activities that patients with CIPN report difficulty with, including walking (e.g., balance issues and loss of confidence). It is critical that these tests be done safely and that researchers are prepared in case of a medical emergency (e.g., falling, cardiac event). Additionally, there was limited use of the Dynamic Gait Index, Functional Reach Test, and Timed Up and Go, which have been suggested as valid measures for use among individuals with diabetic peripheral neuropathy and may be relevant to CIPN [54].
5. Expert opinion
5.1. Considerations for study design and future research agenda
Overall, outcome measures were less likely to demonstrate efficacy in demonstrating prevention of CIPN during chemotherapy treatment compared to trials of rehabilitation in patients with established CIPN. While this may be due to the lack of effectiveness of exercise and physical activity-based interventions in preventing CIPN onset, it may also be due to the substantial difficulties inherent in demonstrating reduced development of toxicity during chemotherapy administration. Identification of intervention effectiveness is more difficult due challenges in identifying clinical improvement on the background of overall progressive worsening of CIPN symptoms with increasing dose. In prevention trials, confounds related to active chemotherapy administration including greater dropouts, lack of intervention adherence as well as other treatment related side effects and fatigue are likely. Further, intergroup differences in CIPN severity may only be evident at later timepoints during chemotherapy given the progressive nature of CIPN development. Accordingly, the requirements for a CIPN prevention trial are different from a treatment trial, including larger sample size to account for participants that do not develop CIPN, methods to deal with missing data and consideration of composite endpoints incorporating chemotherapy dose/completion [9].
In addition, there may be specific considerations regarding composition of the control group for CIPN exercise trials. Usual care or waitlist control groups insufficiently account for expectancy effects inherent to a behavioral intervention such as exercise, which may over-estimate effectiveness [55]. A nutritional intervention or a generic survivorship health program or stretching may be a more appropriate control condition to reduce patient expectancy of benefit and match the time and attention inherent to an exercise intervention. In contrast, there is the potential for a waitlist group to pre-emptively exercise prior to trial which may also contaminate the findings. A delayed-start design (i.e., the active group starts treatment earlier than the control group) may overcome some issues related to a waitlist group, as long as there is not a strong effect of time (i.e., this design is more appropriate to treat existing CIPN which is stable over time, as opposed to preventing CIPN during chemotherapy).
The development of technology-based approaches to monitor home activity via inexpensive sensor systems linked to a smartwatch or phone may provide relevant and readily implementable outcome measures for CIPN exercise studies. However, such devices and related software would need to be cost-effective and accessible for widespread use as well as validated as an outcome measure in future studies. As part of future research, it might be beneficial to examine biological or psychosocial factors underlying exercise effects to assist in identifying patients most likely to respond [4], which may also assist in patient stratification for future trials.
5.2. Recommendations
Inconsistency in outcome measures limits the ability to pool results and ultimately reduces ability to achieve evidence and consensus on the utility of exercise rehabilitation interventions in CIPN. This review represents a step towards development of a core set of outcome measures for clinical trials on CIPN exercise and physical rehabilitation interventions, with appropriate consensus-based methods and patient and stakeholder involvement required for confirmation of a core set. We provide recommendations for CIPN exercise and physical rehabilitation trial design and outcome measure selection (Box 1). Given the lack of available interventions to support patients with CIPN, it is critical to facilitate research to definitively recommend rehabilitation interventions to patients. Development of a core outcome measure set and examination of its sensibility, specificity, and responsiveness in the setting of CIPN exercise trials is warranted. Further, the development of a core outcome measure set will likely apply to CIPN intervention studies more broadly and be helpful in the search for neuroprotective and treatment approaches to support cancer survivors.
Box 1.
Recommendations for CIPN exercise and physical rehabilitation study design
| Patient selection and CIPN diagnosis |
| – Criteria for classification of CIPN should be clear |
| – Patient characteristics (cancer and chemotherapy types) should be reported |
| – Consideration of CIPN phenotype (in relation to pain and functional disturbance) |
| – Additional complication of assessing intervention outcomes during neurotoxic chemotherapy |
| Outcome measure selection |
| – A core outcome set is needed to enable comparison and meta-analysis |
| – Outcome measures should be tailored to prevention vs treatment trials |
| – CIPN symptoms, impact of CIPN and balance measures should be included as core outcome measures |
| – Global physical function, strength and physical fitness, quality of life and neurological examination are recommended additional outcome measures |
| – Neuropathic pain PROMs may warrant inclusion but only in CIPN types where pain is expected to represent a relevant symptom |
| Specificity of outcome measures |
| – A battery of clinical tests (such as the TNS) may be more appropriate to capture CIPN than a single site and modality assessment tool |
| – Consideration of outcome measures assessing both upper and lower limb function may be appropriate |
| – Routine NCS may lack sensitivity as an outcome measure |
| – Physical function measures reflect performance not specific to CIPN but are relevant and warrant inclusion |
| – Outcome measures addressing other symptoms, body composition and diet do not assess CIPN but may be warranted depending on study aims |
| Practical considerations |
| – Simple outcome measures that can be implemented in resource-poor settings |
| – Adherence and drop-out rate are important factors and must be reported |
| – Intention to treat analysis should be undertaken using the last observation carried forward method to avoid bias |
| – Control group should be carefully considered to match time, attention, and expectancy effects present in the exercise condition |
| – Effect sizes should be reported to facilitate future meta-analyses (e.g., table with mean and standard deviation and sample size at each time point for each study arm) |
| – Interventions delivered during chemotherapy require flexibility and simplicity due to the burden of chemotherapy and may be affected by confounding factors |
| Future research agenda |
| – Develop better tools of patient-reported impact of CIPN, QoL, functional deficits, sensory ataxia and falls risk |
| – Additional functional outcome measures used in clinical trials in other peripheral neuropathies (e.g., diabetic neuropathy) should be examined for utility |
| – Examination of information and communication technology-based approaches and sensor systems as outcome measures |
| – Study of biological and psychosocial factors underlying the response to exercise (which patients respond best, how does exercise affect CIPN mechanistically) |
| – Longitudinal quantification of blood biomarkers, such as serum NfL levels during and post chemotherapy, might be of use |
Supplementary Material
Article highlights.
Chemotherapy-induced peripheral neurotoxicity (CIPN) is a significant toxicity in cancer survivors.
Exercise and physical activity-based interventions have demonstrated promise in reducing the burden of symptoms and potentially preventing toxicity.
There is a lack of definitive studies and outcome measure consistency.
We undertook a systematic review and consensus expert opinion approach to provide a conceptual framework for CIPN outcome measures.
A total of 75 outcome measures were identified from 26 studies, grouped into measures of manifestations of CIPN (e.g. symptoms/signs), measures of the impact of CIPN and other outcome measures.
This article highlights the need for definition of a core outcome measures set, providing recommendations for CIPN exercise and physical rehabilitation trial design and outcome measure selection.
The development of a core outcome measure set will be critical in the search for neuroprotective and treatment approaches to support cancer survivors and to address the significant gap in the identification of effective rehabilitation and treatment options for CIPN.
Funding
This work was supported by the National Health and Medical Research Council of Australia under Grants (#1148595, #1080521 to SBP), Cancer Institute NSW under Grant (#14/TPG/1–05 to SB Park), National Cancer Institute under Grant (K07CA221931 to IR Kleckner), Instituto de Salud Carlos III under Grant (project PI20/00283 co-funded by European Regional Development Fund. ERDF, a way to build Europe, to R Velasco), CERCA Programme /Generalitat de Catalunya for institutional support to R Velasco and University of Milano-Bicocca Under Grant (“Bicocca Starting Grant” to P Alberti). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Declaration of interests
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or conflict with the subject matter or materials discussed in this manuscript apart from those disclosed.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
References
Papers of special note have been highlighted as either of interest (*) or of considerable interest (**) to readers.
- 1.Park SB, Goldstein D, Krishnan AV, et al. Chemotherapy-induced peripheral neurotoxicity: a critical analysis. CA Cancer J Clin. 2013; 63: 419–37 [DOI] [PubMed] [Google Scholar]
- 2.Staff NP, Grisold A, Grisold W, et al. Chemotherapy-induced peripheral neuropathy: A current review. Ann Neurol. 2017; 81: 772–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kanzawa-Lee GA, Larson JL, Resnicow K, et al. Exercise Effects on Chemotherapy-Induced Peripheral Neuropathy: A Comprehensive Integrative Review. Cancer Nurs. 2020; 43: E172–85 * Excellent review of exercise and physical rehabilitation in CIPN, outlining current evidence on efficacy.
- 4.Kleckner IR, Park SB, Streckmann F, et al. Systematic review of exercise for prevention and management of chemotherapy-induced peripheral neuropathy. In: Lustberg MB and Loprinzi CL, eds. Diagnosis, Management and Emerging Strategies for Chemotherapy Induced Neuropathy. Springer; 2021 [Google Scholar]
- 5.Dorsey SG, Kleckner IR, Barton D, et al. The National Cancer Institute Clinical Trials Planning Meeting for Prevention and Treatment of Chemotherapy-Induced Peripheral Neuropathy. J Natl Cancer Inst. 2019; 111: 531–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tanay MAL, Armes J, Moss-Morris R, et al. A systematic review of behavioural and exercise interventions for the prevention and management of chemotherapy-induced peripheral neuropathy symptoms. J Cancer Surviv. 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Jordan B, Margulies A, Cardoso F, et al. Systemic anticancer therapy-induced peripheral and central neurotoxicity: ESMO-EONS-EANO Clinical Practice Guidelines for diagnosis, prevention, treatment and follow-up. Ann Oncol. 2020; 31: 1306–19 ** Clinical practice guidelines for CIPN, incorporating assessment tools and management strategies.
- 8. Loprinzi CL, Lacchetti C, Bleeker J, et al. Prevention and Management of Chemotherapy-Induced Peripheral Neuropathy in Survivors of Adult Cancers: ASCO Guideline Update. J Clin Oncol. 2020; 38: 3325–48 ** Updated guidelines for prevention and management of CIPN, outlining evidence gaps for treatment strategies.
- 9. Gewandter JS, Brell J, Cavaletti G, et al. Trial designs for chemotherapy-induced peripheral neuropathy prevention: ACTTION recommendations. Neurology. 2018; 91: 403–13 ** Excellent overview of imporant considerations for trial design in CIPN prevention trials.
- 10.Alberti P, Rossi E, Cornblath DR, et al. Physician-assessed and patient-reported outcome measures in chemotherapy-induced sensory peripheral neurotoxicity: two sides of the same coin. Ann Oncol. 2014; 25: 257–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021; 372: n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Argyriou AA, Cavaletti G, and Park SB. The Toxic Neuropathy Consortium of the Peripheral Nerve Society. J Peripher Nerv Syst. 2019; 24 Suppl 2: S4–5 [DOI] [PubMed] [Google Scholar]
- 13.Boers M, Beaton DE, Shea BJ, et al. OMERACT Filter 2.1: Elaboration of the Conceptual Framework for Outcome Measurement in Health Intervention Studies. J Rheumatol. 2019; 46: 1021–27 [DOI] [PubMed] [Google Scholar]
- 14.Bland KA, Kirkham AA, Bovard J, et al. Effect of Exercise on Taxane Chemotherapy-Induced Peripheral Neuropathy in Women With Breast Cancer: A Randomized Controlled Trial. Clin Breast Cancer. 2019; 19: 411–22 [DOI] [PubMed] [Google Scholar]
- 15.Chen SC, Huang HP, Huang WS, et al. Non-randomized preliminary study of an education and elastic-band resistance exercise program on severity of neuropathy, physical function, muscle strength and endurance & quality of life in colorectal cancer patients experiencing oxaliplatin-induced peripheral neuropathy. Eur J Oncol Nurs. 2020; 49: 101834 [DOI] [PubMed] [Google Scholar]
- 16.Hatlevoll I, Oldervoll LM, Wibe A, et al. Physical exercise during adjuvant chemotherapy for colorectal cancer-a non-randomized feasibility study. Support Care Cancer. 2021; 29: 2993–3008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kneis S, Wehrle A, Müller J, et al. It’s never too late - balance and endurance training improves functional performance, quality of life, and alleviates neuropathic symptoms in cancer survivors suffering from chemotherapy-induced peripheral neuropathy: results of a randomized controlled trial. BMC Cancer. 2019; 19: 414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McCrary JM, Goldstein D, Sandler CX, et al. Exercise-based rehabilitation for cancer survivors with chemotherapy-induced peripheral neuropathy. Support Care Cancer. 2019; 27: 3849–57 [DOI] [PubMed] [Google Scholar]
- 19. Müller J, Weiler M, Schneeweiss A, et al. Preventive effect of sensorimotor exercise and resistance training on chemotherapy-induced peripheral neuropathy: a randomised-controlled trial. Br J Cancer. 2021 * Second largest study of exercise to prevent CIPN, incorporating a range of CIPN assessment tools across multiple domains.
- 20.Vollmers PL, Mundhenke C, Maass N, et al. Evaluation of the effects of sensorimotor exercise on physical and psychological parameters in breast cancer patients undergoing neurotoxic chemotherapy. J Cancer Res Clin Oncol. 2018; 144: 1785–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bao T, Zhi I, Baser R, et al. Yoga for Chemotherapy-Induced Peripheral Neuropathy and Fall Risk: A Randomized Controlled Trial. JNCI Cancer Spectr. 2020; 4: pkaa048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Clark PG, Cortese-Jimenez G, and Cohen E. Effects of Reiki, Yoga, or Meditation on the Physical and Psychological Symptoms of Chemotherapy-induced Peripheral Neuropathy: A Randomized Pilot Study. J Evid Based Complement Alternat Med. 2012; 17: 161–71 [Google Scholar]
- 23.Galantino ML, Tiger R, Brooks J, et al. Impact of Somatic Yoga and Meditation on Fall Risk, Function, and Quality of Life for Chemotherapy-Induced Peripheral Neuropathy Syndrome in Cancer Survivors. Integr Cancer Ther. 2019; 18: 1534735419850627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gui Q, Li D, Zhuge Y, et al. Efficacy of Exercise Rehabilitation Program in Relieving Oxaliplatin Induced Peripheral Neurotoxicity. Asian Pac J Cancer Prev. 2021; 22: 705–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schönsteiner SS, Bauder Mißbach H, Benner A, et al. A randomized exploratory phase 2 study in patients with chemotherapy-related peripheral neuropathy evaluating whole-body vibration training as adjunct to an integrated program including massage, passive mobilization and physical exercises. Exp Hematol Oncol. 2017; 6: 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Streckmann F, Lehmann HC, Balke M, et al. Sensorimotor training and whole-body vibration training have the potential to reduce motor and sensory symptoms of chemotherapy-induced peripheral neuropathy-a randomized controlled pilot trial. Support Care Cancer. 2019; 27: 2471–8 [DOI] [PubMed] [Google Scholar]
- 27.Zimmer P, Trebing S, Timmers-Trebing U, et al. Eight-week, multimodal exercise counteracts a progress of chemotherapy-induced peripheral neuropathy and improves balance and strength in metastasized colorectal cancer patients: a randomized controlled trial. Support Care Cancer. 2018; 26: 615–24 [DOI] [PubMed] [Google Scholar]
- 28.Şimşek NY and Demir A. Cold Application and Exercise on Development of Peripheral Neuropathy during Taxane Chemotherapy in Breast Cancer Patients: A Randomized Controlled Trial. Asia Pac J Oncol Nurs. 2021; 8: 255–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kleckner IR, Kamen C, Gewandter JS, et al. Effects of exercise during chemotherapy on chemotherapy-induced peripheral neuropathy: a multicenter, randomized controlled trial. Support Care Cancer. 2018; 26: 1019–28 * Largest trial to date of exercise to prevent CIPN in patients undergoing chemotherapy.
- 30.Dhawan S, Andrews R, Kumar L, et al. A Randomized Controlled Trial to Assess the Effectiveness of Muscle Strengthening and Balancing Exercises on Chemotherapy-Induced Peripheral Neuropathic Pain and Quality of Life Among Cancer Patients. Cancer Nurs. 2020; 43: 269–80 [DOI] [PubMed] [Google Scholar]
- 31.Andersen Hammond E, Pitz M, Steinfeld K, et al. An Exploratory Randomized Trial of Physical Therapy for the Treatment of Chemotherapy-Induced Peripheral Neuropathy. Neurorehabil Neural Repair. 2020; 34: 235–46 [DOI] [PubMed] [Google Scholar]
- 32.Wonders KY. The Effect of Supervised Exercise Training on Symptoms of Chemotherapy-Induced Peripheral Neuropathy. Int J Phys Med Rehabil. 2014; 2: 4 [Google Scholar]
- 33.Bahar-Ozdemir Y, Akyuz G, Kalkandelen M, et al. The Effect of Therapeutic Exercises on Balance, Quality of Life, and Pain in Patients Who Were Receiving Neurotoxic Chemotherapy. Am J Phys Med Rehabil. 2020; 99: 291–9 [DOI] [PubMed] [Google Scholar]
- 34.Fernandes J and Kumar S. Effect of lower limb closed kinematic chain exercises on balance in patients with chemotherapy-induced peripheral neuropathy: a pilot study. Int J Rehabil Res. 2016; 39: 368–71 [DOI] [PubMed] [Google Scholar]
- 35.Frigeni B, Piatti M, Lanzani F, et al. Chemotherapy-induced peripheral neurotoxicity can be misdiagnosed by the National Cancer Institute Common Toxicity scale. J Peripher Nerv Syst. 2011; 16: 228–36 [DOI] [PubMed] [Google Scholar]
- 36.Tofthagen C, Visovsky C, Beckstead J, et al. Results of a Strength and Balance Training Pilot Study for Colorectal Cancer Survivors with Peripheral Neuropathy Caused by Oxaliplatin. Rehabil Oncol. 2014; 32:38–44 [Google Scholar]
- 37.Griffith KA, Dorsey SG, Renn CL, et al. Correspondence between neurophysiological and clinical measurements of chemotherapy-induced peripheral neuropathy: secondary analysis of data from the CI-PeriNomS study. J Peripher Nerv Syst. 2014; 19: 127–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Streckmann F, Kneis S, Leifert JA, et al. Exercise program improves therapy-related side-effects and quality of life in lymphoma patients undergoing therapy. Ann Oncol. 2014; 25: 493–9 [DOI] [PubMed] [Google Scholar]
- 39.Stuecher K, Bolling C, Vogt L, et al. Exercise improves functional capacity and lean body mass in patients with gastrointestinal cancer during chemotherapy: a single-blind RCT. Support Care Cancer. 2019; 27: 2159–69 [DOI] [PubMed] [Google Scholar]
- 40. Argyriou AA, Park SB, Islam B, et al. Neurophysiological, nerve imaging and other techniques to assess chemotherapy-induced peripheral neurotoxicity in the clinical and research settings. J Neurol Neurosurg Psychiatry. 2019; 90: 1361–9 * Important review outlining assessment tools in CIPN.
- 41.Alberti P, Rossi E, Argyriou AA, et al. Risk stratification of oxaliplatin induced peripheral neurotoxicity applying electrophysiological testing of dorsal sural nerve. Support Care Cancer. 2018; 26: 3143–51 [DOI] [PubMed] [Google Scholar]
- 42.Velasco R, Bruna J, Briani C, et al. Early predictors of oxaliplatin-induced cumulative neuropathy in colorectal cancer patients. J Neurol Neurosurg Psychiatry. 2014; 85: 392–8 [DOI] [PubMed] [Google Scholar]
- 43.Henke CC, Cabri J, Fricke L, et al. Strength and endurance training in the treatment of lung cancer patients in stages IIIA/IIIB/IV. Support Care Cancer. 2014; 22: 95–101 [DOI] [PubMed] [Google Scholar]
- 44.Schwenk M, Grewal GS, Holloway D, et al. Interactive Sensor-Based Balance Training in Older Cancer Patients with Chemotherapy-Induced Peripheral Neuropathy: A Randomized Controlled Trial. Gerontology. 2016; 62: 553–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Crossnohere NL, Brundage M, Calvert MJ, et al. International guidance on the selection of patient-reported outcome measures in clinical trials: a review. Qual Life Res. 2021; 30: 21–40 [DOI] [PubMed] [Google Scholar]
- 46.Timmins HC, Li T, Kiernan MC, et al. Quantification of Small Fiber Neuropathy in Chemotherapy-Treated Patients. J Pain. 2020; 21: 44–58 [DOI] [PubMed] [Google Scholar]
- 47. Cavaletti G, Frigeni B, Lanzani F, et al. The Total Neuropathy Score as an assessment tool for grading the course of chemotherapy-induced peripheral neurotoxicity: comparison with the National Cancer Institute-Common Toxicity Scale. J Peripher Nerv Syst. 2007; 12: 210–5 * Important clinical study comparing CIPN assessment methods - with neurological grading demonstrating greater sensitivity to change than clinician reported outcome tools.
- 48.Argyriou AA, Bruna J, Anastopoulou GG, et al. Assessing risk factors of falls in cancer patients with chemotherapy-induced peripheral neurotoxicity. Support Care Cancer. 2020; 28:1991–5 [DOI] [PubMed] [Google Scholar]
- 49.Martinez ARM, Martins MP, Martins CR Jr. et al. Sensory ataxia rating scale: Development and validation of a functional scale for patients with sensory neuronopathies. J Peripher Nerv Syst. 2019; 24: 242–6 [DOI] [PubMed] [Google Scholar]
- 50.Patrizio E, Calvani R, Marzetti E, et al. Physical Functional Assessment in Older Adults. J Frailty Aging. 2021; 10: 141–9 [DOI] [PubMed] [Google Scholar]
- 51.Agarwala P and Salzman SH. Six-Minute Walk Test: Clinical Role, Technique, Coding, and Reimbursement. Chest. 2020; 157: 603–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Aversa Z, Costelli P, and Muscaritoli M. Cancer-induced muscle wasting: latest findings in prevention and treatment. Ther Adv Med Oncol. 2017; 9: 369–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Park SH. Tools for assessing fall risk in the elderly: a systematic review and meta-analysis. Aging Clin Exp Res. 2018; 30: 1–16 [DOI] [PubMed] [Google Scholar]
- 54.Dixon CJ, Knight T, Binns E, et al. Clinical measures of balance in people with type two diabetes: A systematic literature review. Gait Posture. 2017; 58: 325–32 [DOI] [PubMed] [Google Scholar]
- 55.Sherman KJ. The Trials and Tribulations of Selecting Comparison Groups in Randomized Trials of Nonpharmacological Complementary and Integrative Health Interventions. J Altern Complement Med. 2020; 26: 449–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.