Abstract
In this present study, an amperometric immunosensor was developed based on disposable screen-printed carbon electrode (SPCE) for specific and sensitive detection of SARS-CoV-2 S1 protein. Anti-SARS-CoV-2 S1 monoclonal antibody was firstly immobilized onto the electrode surface. Then, the sandwich complex was formed by addition of S1 protein, secondary antibody and HRP-IgG, respectively. Chronoamperometry measurements were done in the presence of TMB mediator and the detection of SARS-CoV-2 S1 protein was performed by using 10 μL sample. The limit of detection (LOD) was found to be 0.19 ng/mL (equals to 24.7 amol in 10 μL sample) in the linear range of 0.5–10 ng/mL obtained in buffer medium. The applicability of this assay was investigated in the linear range of 0.5–3 ng/mL S1 protein in artificial saliva medium with the LOD as 0.13 ng/mL (equals to 16.9 amol in 10 μL sample). The selectivity study was examined in the presence of Hemagglutinin antigen (HA) in both mediums; buffer and artificial saliva while resulting with the successful discrimination between S1 protein and HA. The one of ultimate goals of our study is to present the possible implementation of this assay to point of care (POC) analysis. Under this aim, this assay was performed in combination with a portable device that is the commercial electrochemical analyzer. Amperometric detection of S1 protein in the range of 0.5–5 ng/mL was also successfully performed in artificial saliva medium with a resulting LOD as 0.15 ng/mL (equals to 19.5 amol in 10 μL sample). In addition, a selectivity study was similarly carried out by portable device.
Keywords: SARS-CoV-2 S1 protein, Electrochemical immunosensors, Chronoamperometry, COVID-19
Graphical abstract

1. Introduction
Biosensors are small devices that specifically detect the target analyte, enabling selective analysis. While performing the analysis with a biosensor, biomolecular recognition takes place in the biosensing surface, and the conversion of the sensing process into a measurable signal occurs in the transducer. The transducer can be electrochemical, optical, piezoelectric, magnetic or calorimetric. Many different biological molecules such as enzymes, peptides, nucleic acids, aptamers, antibodies can be used as receptor molecules. In electrochemical biosensors, biomolecular recognition occurs on an electrode surface [1,2].
Electrochemical techniques are frequently preferred in biosensor designs due to their fast and accurate response, low detection limit, performing analyzes with a small amount of analyte, being practical, not requiring the use of complex chemical substances [3].
SARS-CoV-2, a new and highly pathogenic coronavirus (severe acute respiratory syndrome coronavirus-2) has turned into a pandemic in the world. Coronavirus (COVID-19) disease started at the end of 2019 and spread rapidly out to worldwide. Since then, there has been a great progress to develop point-of-care systems on the diagnosis of COVID-19 disease.
The surface glycoproteins of coronaviruses that binds to the host cell receptor and allows the virus to enter the cell [4]. Spike protein, the one of surface glycoproteins, has a portion called the receptor-binding domain (RBD) and RBD interacts with the host cell receptor. After receptor binding, the relevant protease enzyme of host cleaves the spike protein and spike fusion proteins are released while facilitating the virus entry [[5], [6], [7]]. It has been reported that the surface glycoprotein S protein (spike protein) is substantially responsible for receptor binding as well as entry of the virus into the cell. Thus, the virus invades the host cell by binding to the host cell receptor human angiotensin converting enzyme 2 (hACE2) with the S protein on the surface of virus. S protein has two subunits known as S1 and S2 and the RBD site in the S1 subunit binds to hACE2 along with the S2 subunit directs the entry of the virus into the cell [8].
There are many studies in the literature using different techniques [[9], [10], [11], [12]] for the detection of COVID-19. Some of these techniques have presented disadvantages such as being relatively expensive, requiring multifaceted devices by requiring trained personnel. Conversely, electrochemical techniques have provided many advantages such as enabling fast and cost-effective analysis by resulting with low detection limit, being practical and enabling on-site analysis [1,2,13,14]. For these reason, the number of electrochemical biosensors developed for diagnosis of inherited diseases including COVID-19 is increasing day by day in the literature.
In this present study, amperometric immunosensing protocol specific to SARS-CoV-2 S1 protein was developed and applied for specific and sensitive detection of S1 protein in both mediums; buffer and artificial saliva. The selectivity of SARS-CoV-2 S1 protein specific immunosensor was tested against to a target marker of Influenza; Hemagglutinin antigen (HA) in both mediums. Under the optimum experimental conditions, the LOD value was calculated as well as the reproducibility and selectivity of this assay was investigated. The implementation of amperometric immunosensing protocol developed for detection of S1 protein in combination with a portable device was also demonstrated in the medium of artificial saliva with its results on the reproducibility with LOD value and also the selectivity.
2. Material and methods
2.1. Chemicals and apparatus
The chemicals; SARS-CoV-2 spike (subunit S1) protein (His tag), anti-SARS-CoV-2 S1 antibody (rabbit monoclonal, rabbit polyclonal) from ProSci; anti-SARS-CoV-2 S1 antibody (human monoclonal) from MyBioSource; HRP-IgG (Goat anti-Rabbit IgG, (H + L) HRP conjugate) from Merck; TMB (3,3′,5,5′-Tetramethylbenzidine) from abcam; Bovine serum albumin (BSA) and Hemagglutinin antigen (HA) from Sigma-Aldrich and Artificial Saliva from Pickering Laboratories were purchased and used in this present study. Phosphate saline buffer (PBS, 0.05 M, pH 7.00) was used as buffer solution in the experiments. Acetate buffer solution (ABS, 0.5 M, pH 4.80) was used for the pretreatment of each electrode. All chemicals were of analytical reagent grade. Millipore ultrapure water was used for preparation of all solutions.
Electrochemical measurements were carried out by using μAUTOLAB electrochemical analysis system with NOVA 1.11.1 software package (Eco Chemie, The Netherland). The Ref. DSC connector (Metrohm DropSens, Spain) was used for the connection between the screen-printed carbon electrodes (SPCEs) and the device during all electrochemical measurements.
Screen-printed carbon electrodes (SPCEs) (Metrohm DropSens, Spain) consist of carbon working and auxiliary electrode, silver reference electrode. The further details about SPCEs can be accessed from the supplier's website at https://www.dropsens.com.
2.2. Procedure
The procedure based on amperometric immunosensor was developed by following steps:
-
i.
Surface activation of SPCE: The electrochemical activation of SPCE surface was achieved by appling the potential +0.9 V for 60 s in acetate buffer (ABS, pH 4.80) using DPV technique. Chemical activation of the electrode surface was then performed using N-(3-Dimethylaminopropyl)-N′-ethylcarbodiimide hydrochloride (EDC) and N-Hydroxysuccinimide (NHS). A solution of 50 mM EDC and 50 mM NHS (1:1) prepared in PBS (0.05 M, pH 7.00) was used herein as reported in the study of Metzner et al., [15]. 10 μL of the prepared solution was dropped onto the electrode surface and kept for 1 h. All steps were performed at room temperature unless otherwise stated.
-
ii.
Immobilization of the capture (primary) antibody (CAb) onto the electrode surface: 6 μg/mL anti-SARS-CoV-2 S1 antibody was prepared in PBS (0.05 M, pH 7.00). 10 μL of antibody solution was dropped onto the electrode surface and kept at the electrode surface for 1 h.
-
iii.
Blocking of electrode surface: Blocking step was done by using BSA in order to cover the free sites at the electrode surface. 1% BSA solution was prepared in PBS (0.05 M, pH 7.00) and then 10 μL of BSA solution was added onto the electrode surface and left for 30 min.
-
iv.
Incubation of the target analyte (S1 protein; antigen) onto the electrode surface: Different concentrations of SARS-CoV-2 S1 protein were prepared in PBS (0.05 M, pH 7.00). 10 μL of S1 protein solution was dropped onto the electrode surface and incubated at 37 °C in drying-oven for 1 h.
-
v.
Incubation of the seconder antibody (dAb) onto the electrode surface: 0.5 μg/mL anti-SARS-CoV-2 S1 antibody was prepared in PBS (0.05 M, pH 7.00) and added onto the electrode surface for incubation step during 1 h.
-
vi.
Incubation of the HRP-labeled IgG antibody onto the electrode surface: 0.5 μg/mL Goat anti-Rabbit HRP-IgG antibody was prepared in PBS (0.05 M, pH 7.00) and added onto the electrode surface for incubation step during 30 min.
After each incubation step (iv,v and vi), the electrode surface was washed by using 30 μL of PBS (0.05 M, pH 7.00).
-
vii.
Chronoamperometry measurements: 40 μL of TMB solution was dropped onto the electrode surface prepared by following the steps listed above, and then it was kept for 3 min in order to let the enzymatic reaction be occurred properly. Accordingly, the chronoamperometry measurement was performed at −0.1 V for 150 s according to data presented in earlier literatures [[16], [17], [18]].
2.3. Selectivity studies
In order to present the selectivity of enzymatic based immunosensor specific to the SARS-CoV-2 S1 protein, a batch of experiment was performed in the presence of hemagglutinin antigen (HA). Due to the similarity of the COVID-19 and Influenza symptoms, the selectivity studies were performed herein by using the hemagglutinin antigen (HA). After the preparation of required concentration of HA, the experiment on selectivity was performed by following same procedure above.
2.4. Application of immunosensor on detection of SARS-CoV-2 S1 protein in artificial saliva samples
In order to present the applicability of the immunosensor to real samples, a batch of experiment was performed using artificial saliva samples. Artificial saliva was diluted in different ratios by using PBS (0.05 M, pH 7.00). The solutions of S1 protein in different concentrations were then prepared in diluted artificial saliva. Accordingly, the electrochemical measurements were carried out as above.
2.5. SARS-CoV-2 S1 protein detection by using a portable device
The application of amperometric immunosensing assay is furtherly examined for SARS-CoV-2 S1 protein detection in combination with a portable electrochemical analyzer. Hereafter, electrochemical measurements were carried out using Galvanoplot analyzer system with Galvanoplot Suite software package (Solar Biotechnology, Turkey) by following the optimum conditions of this procedure. The effect of changes at the concentration of S1 protein upon to response as well as the selectivity of this assay was examined in the artificial saliva medium.
3. Result & discussion
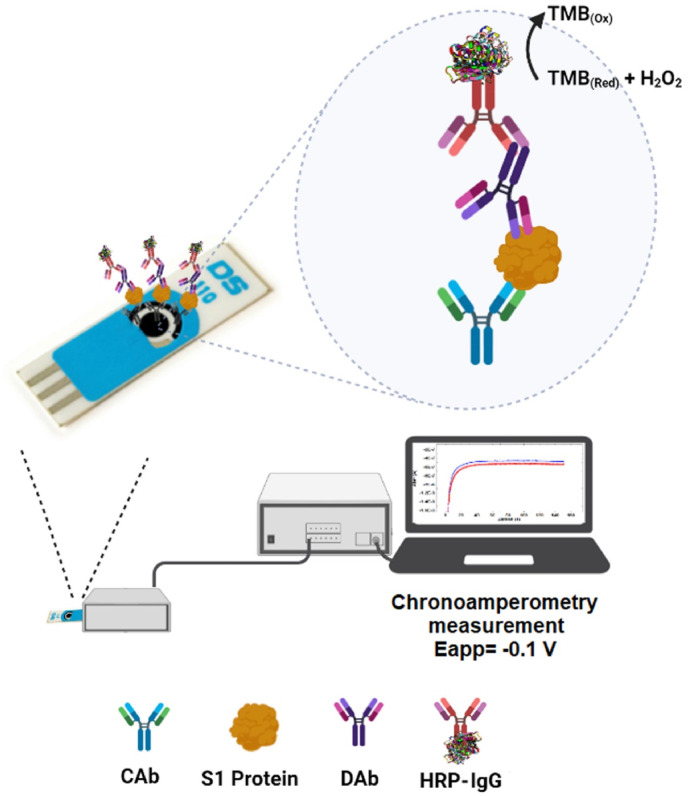
In this study, an amperometric immunosensor was developed for the rapid and sensitive detection of SARS-CoV-2 S1 protein. The schematic representation of this assay based on amperometric immunosensor is illustrated in Fig. 1 . At the end of whole procedure based on amperometric immunosensor, the chronoamperometric measurement was performed at −0.1 V for 150 s. Since the response is proportional to the concentration of SARS-CoV-2 S1 protein, the quantitative analysis of the S1 protein is achieved successfully in our study.
Fig. 1.
Schematic representation of the amperometric immunosensor developed for detection of SARS-CoV-2 S1 Protein.
3.1. Optimization of experimental parameters
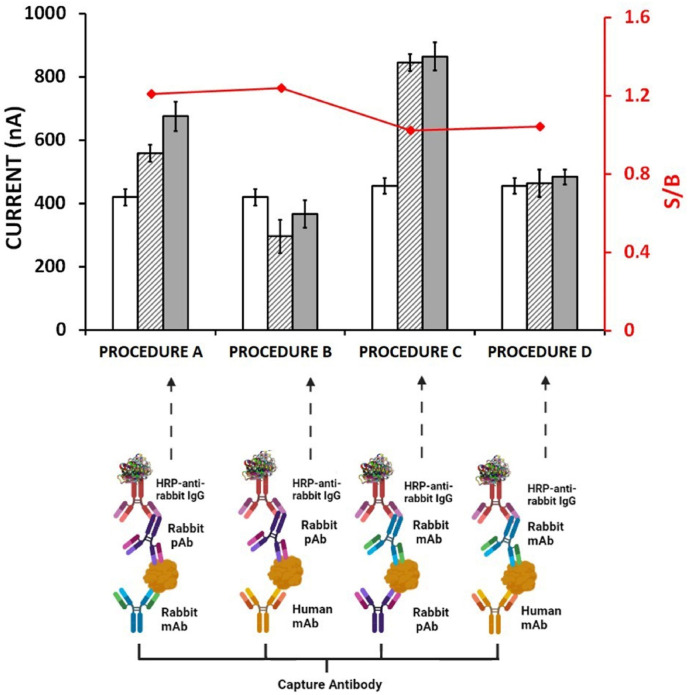
Firstly, the selection of antibody source used for development of sandwich immunosensor is very important [[19], [20], [21], [22]]. In order to perform more sensitive analysis and also to prevent cross-reactions, immunosensors based on four different configurations (procedure A, B, C and D) were designed and prepared in our study. Under this aim, human monoclonal, rabbit monoclonal and rabbit polyclonal anti-SARS-CoV-2 antibodies were used and hence, the effect of the changes at antibodies upon to the response was examined. The schematic representation with the results was shown in Fig. 2 .
Fig. 2.
Histograms and schematic representations obtained from immunosensors prepared using different antibody pairs. Empty columns represent the control group without CAb, striped columns represent the control group without antigen and gray columns represent the full procedure. The S/B (signal/blank) ratio was obtained by dividing the full procedure signal with the control group without antigen signal. Assayed configurations: Procedure A: Rabbit mAb (CAb), Rabbit pAb. Procedure B: Human mAb (CAb), Rabbit pAb. Procedure C: Rabbit pAb (CAb), Rabbit mAb. Procedure D: Human mAb (CAb), Rabbit mAb.
According to the results presented in Fig. 2, the highest differentiation between full procedure and negative control (in the absence of S1 protein) was obtained by following the Procedure A based on rabbit monoclonal antibody as CAb and rabbit polyclonal antibody as dAb of all. In the presence of human monoclonal antibody (in Procedure D) or rabbit polyclonal antibody (in Procedure C) used as CAb herein, it was found that a negligible differentiation between full procedure and negative control was recorded. However there was a differentiation between full procedure and negative control in Procedure B, it was not preferred since the higher control signal was measured in the absence of CAb in contrast to the one of full procedure as well as negative control.
In our study, a high affinity and specificity on detection of S1 protein was achieved in the presence of CAb immobilization onto the electrode surface by using rabbit monoclonal antibody similarly to the results of earlier studies confirming the higher affinity and specificity of rabbit monoclonal antibodies [23,24]. By the immobilization of rabbit monoclonal antibody onto the electrode surface, the stability of immuno-complex between CAb and its target antigen occurred on the electrode surface is ensured. While monoclonal CAb is binding to the antigen from a single epitope, dAb can recognize the antigen from multiple epitopes [24].
Hence, the rabbit polyclonal detector antibody (dAb) could recognize S1 protein by binding from its different epitopes in our study, it could also facilitate the binding of HRP-labeled IgG to dAb. Resembling our study, Čadková et al. [25] developed a sandwich immunosensor by using rabbit-derived CAb and dAb antibody pair. Cross-linking was prevented by blocking the electrode surface using BSA after CAb incubation. In addition, the use of rabbit polyclonal detector antibody enabled specific binding to HRP-labeled Goat Anti-Rabbit as reported in earlier studies [[26], [27], [28]]. Therefore, our assay was optimized by following the Procedure A, and applied for detection of SARS-CoV-2 in this present study.
The experimental parameters, such as CAb concentration, BSA concentration, incubation time of antigen, seconder antibody concentration with its incubation time, HRP-IgG concentration with its incubation time were optimized for development of amperometric immunosensor specific to S1 protein. The optimum experimental conditions were summarized in Table 1 and the results were also shown in Fig. S1.
Table 1.
The experimental parameters investigated in the optimization of the amperometric immunosensor for the detection of S1 protein and optimum experimental conditions.
| Parameters | Tested Range | Selected Value |
|---|---|---|
| CAb concentration (μg/mL) | 0.25-2-4-6-8-10 | 6 |
| BSA concentration (%) | 0.2-0.6-1-2 | 1 |
| Antigen incubation time (min) | 30-60-90 | 60 |
| dAb concentration (μg/mL) | 0.25-0.5-1-1.5-2 | 0.5 |
| dAb incubation time (min) | 30-45-60 | 60 |
| HRP-IgG concentration (μg/mL) | 0.2-0.5-1-2-4 | 0.5 |
| HRP-IgG incubation time (min) | 15-30 | 30 |
| Antigen incubation temperature | Room temperature-37 °C | 37 °C |
3.2. Analytical features of amperometric immunosensor for S1 protein detection
The analytical performance of the amperometric immunosensor was examined under the optimized conditions while increasing concentrations of S1 protein in buffer medium (0.05 M PBS, pH 7.00) and the chronoamperometric measurements were performed (shown in Fig. S2). The resulted amperograms based on the response of immunosensor in different concentrations of S1 protein from 0 to 10 ng/mL were given in Fig. 3 A. In the concentration range of S1 protein from 0.5 ng/mL to 10 ng/mL, there was an increase observed at current value proportionally to S1 protein concentration. Based on the average current values (n = 6), the resulting calibration curve was established in the range of S1 protein from 0.5 ng/mL to 10 ng/mL with regression equation: (Fig. 3B). According to the Miller and Miller method [29], the limit of detection (LOD) was calculated and found to be 0.19 ng/mL.
Fig. 3.
(A) Amperograms showing the response of immunosensor at increasing concentration of S1 Protein in the range of 0–10 ng/mL in buffer medium. (B) Calibration curve presenting the data for amperometric determination of SARS-CoV-2 S1 Protein (S1P) in buffer medium.
In the literature presenting the immunosensors developed for electrochemical detection of SARS-CoV-2, the microfluidic magneto immunosensor was developed in the same concentration range (0–10 ng/mL) of SARS-CoV-2 N Protein by Li and Lillehoj [30]. In the study of Mojsoska et al. [31], the analysis was performed in the higher concentration values of SARS-CoV-2 S1 Protein ranging of 20–80 μg/mL.
The immunosensor sensitivity in buffer was estimated from the slope of the calibration curve (shown in Fig. 3B) divided by the surface area of SPCE and found to be 45.39 μA mL ng−1 cm−2.
Concerning to the repeatability of immunosensor, two measurements were performed for each group in three days to detect S1 protein in its concentration range from 0.5 to 10 ng/mL. Therefore, the RSD values were calculated and shown in Table S1. A lower RSD value than 8% was recorded in our study by indicating the electrochemical detection of S1 protein with a good repeatability.
In this study, the developed immunosensor has a lot of advantages such as using disposable electrodes with a low sample volume (10 μL) and resulting analysis in a very short time. In addition there is no need to use of time-consuming surface modification by nanomaterials, polymers, etc.
Besides, a low detection limit (0.19 ng/mL, equals to 24.7 amol in 10 μL sample) was achieved herein by following the sandwich-immunoassay similarly to earlier reports [26,28,32]. The detection limit in the linear concentration range of S1 protein obtained by amperometric immunosensor was found lower than the LODs reported in earlier literatures [31,33].
3.3. Selectivity studies performed in buffer medium by amperometric immunosensor
Since COVID-19 and Influenza have similar symptoms, including cough, runny nose, sore throat, fever, headache and fatigue [34,35], the selectivity study was investigated in our study by using Influenza hemagglutinin antigen (HA) that is target marker of Influenza. Due to the similarity in symptoms of COVID-19 and Influenza in patients, it is aimed to test the selectivity of immunosensors specific to SARS-CoV-2 S1 protein contrast to HA.
When the studies reported for the detection of SARS-CoV-2 in the literature are examined, it was also noticed that the selectivity of immunosensors was examined by using different proteins that can cross-react with different target analyte. In the study reported by Fabiani et al. [33], Influenza virus A (H1N1) and 2009 Influenza virus pH1N1 were used as off-target species in the selectivity studies for detection of SARS-CoV-2 S and N Protein. Similarly, Influenza A and B antigens were used as off-target species in the selectivity studies in another work presented by Rahmati et al. [36].
In order to explore the selectivity of SARS-CoV-2 S1 specific immunosensor, a batch of experiment was performed in our study by using 1 ng/mL and 10 ng/mL of S1 protein or HA prepared in buffer medium (Fig. 4 ). Even if there was 10 times increase at HA concentration, the average current values remained almost constant without significant change. This result indicates that the developed immunosensor is very selective to its target S1 protein.
Fig. 4.
Histograms presenting the data obtained in the selectivity study performed by using 1 ng/mL and 10 ng/mL S1 protein or HA prepared in buffer medium. Blue striped columns represent control group without antigen as negative control, gray columns and green striped columns respectively represent full procedure in the presence of S1 protein and HA. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
3.4. Application of immunosensor on detection of SARS-CoV-2 S1 protein in artificial saliva samples
The application of amperometric immunosensor was carried out in the medium of artificial saliva. Since COVID-19 is transmitted through saliva and it is easier and non-invasive way to analyze COVID-19 in saliva, the applicability of our assay is investigated in the medium of artificial saliva.
In the literature, there are earlier studies [[37], [38], [39]] presenting the application of immunosensors to real sample by following the spiking method of target analyte. For instance, in the study of Aydin et al. [37], the detection of SARS-CoV-2 RBD was demonstrated in artificial nasal secretion samples. In the study of Liv [39], the detection of COVID-19 was performed using saliva and oropharyngeal swab samples while monitoring of SARS-CoV-2 spike antibody that was spiked into the samples. Similar to our work, in these studies, COVID-19 determination was explored via antigen or antibody spiked into the samples.
In our study, S1 protein determination was performed in artificial saliva samples. In the literature, there are studies using undiluted or diluted artificial saliva with dilution ratio varying from 1:5 to 1:100 [33,40]. Accordingly, undiluted artificial saliva (raw sample) and the diluted ones -with the ratio of 1:5 and 1:20 were tested. As the most reproducible results were obtained in the samples with 1:5 and 1:20 dilution ratios, 1 ng/mL S1 protein was prepared in 1:5 and 1:20 diluted artificial saliva in order to examine its effect on the immunosensor response (shown in Table S2).
Ever since a higher differentiation with more reproducible results was observed with 1 ng/mL S1 protein prepared in 1:20 artificial saliva medium in contrast to the result obtained by the negative group (Table S2), the detection of S1 protein in its varying concentrations was performed in 1:20 artificial saliva medium. The samples containing S1 protein were prepared in 1:20 artificial saliva medium in its various concentrations from 0.5 to 5 ng/mL, and then the electrochemical detection protocol was followed. According to the results shown in Fig. S3, an increase at current proportional to concentration was obtained in the concentration range of S1 protein between 0.5 and 3 ng/mL (Table S3). Therefore, a calibration curve was obtained based on the average current values (n = 3) and shown in Fig. 5 with the regression equation: According to the Miller and Miller method [29], the limit of detection was calculated and found to be 0.13 ng/mL (equals to 16.9 amol in 10 μL sample).
Fig. 5.
Calibration curve presenting the data obtained by amperometric determination of SARS-CoV-2 S1 Protein (S1P) in 1:20 artificial saliva medium.
The immunosensor sensitivity in artificial saliva medium was estimated from the slope of the calibration curve (shown in Fig. 5) divided by the surface area of SPCE and found to be 115.88 μA mL ng−1 cm−2.
Likewise to earlier works, the application of the amperometric immunosensor was demonstrated herein in artificial saliva medium containing SARS-CoV-2 S1 protein in the concentration range of 0.5–3 ng/mL. Basso et al. [41], performed the detection of SARS-CoV-2 antigen in saliva samples obtained from COVID-19 patients by using gold standard rRT-PCR technique. In the presence of COVID-19 positive patients, the concentration range of antigen obtained by PCR is consistent with the concentration range of SARS-CoV-2 S1 protein in our study. Based on the results reported in the study of Basso et al. [41], a higher virus density is observed in the early stages of COVID-19. As a result, the antigen detection is extremely important in early diagnosis and prognosis of COVID-19 pandemic.
The selectivity study was also carried out using 0.5 and 1 ng/mL of S1 protein, or HA prepared in artificial saliva medium and the results were shown in Fig. S4. While increasing the concentration of protein, an increase at current was recorded in the presence of S1 protein whereas no significant increase at current was observed in the presence of HA. According to these results, it was concluded that the amperometric immunosensor is specific to the S1 protein even it is in a complex sample matrix as saliva.
3.5. SARS-CoV-2 S1 protein detection by portable device
The one of ultimate goals in our study is to present the possible implementation of our assay to point of care (POC) analysis. The development of portable device towards to the point-of-care analysis has an essential prominence for the ability of patients to perform their own test for diagnosis of inherited diseases. In the case of COVID-19 pandemic, an effort to perform widespread testing has been facilitated and positive cases have been isolated to prevent the spread of the COVID-19. Under this intention, our assay based on amperometric immunosensor was tested by using a portable device, i.e, the commercial electrochemical analyzer.
First, the samples containing S1 protein varying from 0.5 ng/mL to 7 ng/mL were prepared in 1:20 artificial saliva medium and then, the electrochemical detection of S1 protein was investigated (shown in Fig. S5). An increase at current proportional to the concentration of S1 protein was observed in the range between 0.5 and 5 ng/mL. Therefore, a calibration curve was obtained based on the average current values (n = 3) and shown in Fig. 6 with the regression equation: According to the Miller and Miller method [29], the limit of detection was achieved 0.15 ng/mL (equals to 19.5 amol in 10 μL sample).
Fig. 6.
Calibration presenting the data for the amperometric determination of SARS-CoV-2 S1 protein (S1P) in 1:20 artificial saliva medium by using a portable device.
The sensitivity of immunosensor in combination with portable device was estimated from the slope of the calibration curve (shown in Fig. 6) divided by the surface area of SPCE and found to be 94.17 μA mL ng−1 cm−2.
Next, the selectivity study was also carried out in 1:20 artificial saliva medium by using immunosensor integrated portable device. In the presence of 0.5 ng/mL S1 protein or HA, the immunosensor response was examined and accordingly the results were shown in Fig. S6. Reminiscent of the results in buffer medium, a high current value was obtained with S1 protein whereas almost same current value to control group was recorded in the presence of HA. Hereafter, it can be concluded that S1 protein was detected specifically over to HA by immunosensor integrated portable device.
The analytical parameters for detection of SARS-CoV-2 S1 protein in artificial saliva medium using a potentiostat and a hand-held potentiostat was summarized in Table S4.
4. Conclusions
The rapid and reliable diagnosis of COVID-19, and accordingly the isolation of positive cases are extremely important to prevent the transmission of COVID-19 disease.
Since S1 protein plays a major role in the entry of SARS-CoV-2 virus into the cell, the present study aims to determine the COVID-19 through by the detection of the virus surface S1 protein. Due to the need for development of rapid, sensitive and selective antigen test kits, an amperometric immunosensor was developed herein for the detection of SARS-CoV-2 S1 protein with its application in artificial saliva was demonstrated. In buffer medium, the limit of detection was found to be 0.19 ng/mL (equals to 24.7 amol in 10 μL sample) and the immunosensor was found to be specific for S1 protein over to HA. Then, application of the immunosensor was carried out in 1:20 diluted artificial saliva medium while exploring the LOD as 0.13 ng/mL (equals to 16.9 amol in 10 μL sample) by presenting a very selective detection of S1 protein in the presence of HA. The implementation of amperometric immunosensor in combination with a portable device was also demonstrated with the LOD of S1 protein as 0.15 ng/mL (equals to 19.5 amol in 10 μL sample) in the medium of 1:20 diluted artificial saliva by resulting a good selectivity to target S1 protein over to HA.
Our assay based on amperometric immunosensor presented many advantages over to earlier studies; such as using single-use electrodes without any time-consuming modification steps, as well as using low sample volume (10 μL) while resulting the analysis in a short time. The comparison of earlier studies on electrochemical detection of COVID-19 over to present work was given in Table S5. As it can be seen, the target analyte, i.e, SARS-CoV-2 S1 protein could be analyzed herein in a shorter time in aspects of the preparation time of electrode and immunosensor contrast to many studies [30,31,33,36,37,39,40,[42], [43], [44], [45], [46], [47], [48], [49]] mentioned in Table S5. The detection of SARS-CoV-2 virus based on different bioreceptors such as spike protein (S) and nucleocapsid protein (N) [30,42,44] has been reported by following different procedures. In addition to the studies targeting spike protein [33,36,37,39,47,48], other studies targeting S1 subunits are also reported [[31], [45]]. S1 protein was determined using different techniques such as voltammetric [31] and amperometric [45] techniques. Even though, the LOD of S1 protein as 0.15 ng/mL reported in the study of Li et al. [45], was found a little competitive with the one found in the present work, their immunoassay was performed by using different antibody labeled with HRP as well as following very time-consuming procedure due to overnight immobilization procedure [45].
In our study, no time-consuming modification process was applied for the surface of SPCE while resulting fast, sensitive and selective SARS-CoV-2 S1 protein determination based on single-use immunosensor. Moreover, a lower detection limit was obtained in the present work in comparison to the study of Fabiani et al. [33] presenting the double surface technique based on magnetic nanoparticles and carbon black modified SPCE, and also the study of Mojsoska et al. [31] using graphene electrodes.
Since the implementation of our immunosensor developed for detection of SARS-CoV-2 S1 protein has been presented successfully by using portable device in a good selectivity and sensitivity, it can be concluded that this assay can be implemented furtherly into point-of-care analysis. Furthermore, our assay based on amperometric immunosensor in combination with portable device could be applied not only detection of SARS-CoV-2, but also the detection of many types of inherited diseases in future.
Credit author statement
Arzum Erdem: Investigation, Conceptualization, Methodology, Data acquisition, Supervision, Project administration, Funding acquisition, Writing – review & editing. Huseyin Senturk: Investigation, Conceptualization, Data acquisition, Writing – original draft. Esma Yildiz: Investigation, Data acquisition, Writing – original draft. Meltem Maral: Data acquisition.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
A.E acknowledges the financial support from Turkish Scientific and Technological Research Council (TÜBİTAK, Turkey) (Project No. 120S419) as the Project Investigator, and she also would like to express her gratitude to the Turkish Academy of Sciences (TÜBA, Turkey) as an Principal member for its partial support. H.S, E.Y and M.M acknowledge project scholarship by TÜBİTAK (Project No. 120S419). The authors acknowledge BioRender.com for aid in creating partially some graphical elements in graphical abstract, Fig. 1, Fig. 2.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.talanta.2022.123422.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Wang J. Electrochemical biosensors: towards point-of-care cancer diagnostics. Biosens. Bioelectron. 2006;21:1887–1892. doi: 10.1016/j.bios.2005.10.027. [DOI] [PubMed] [Google Scholar]
- 2.Paleček E., Fojta M., Tomschik M., Wang J. Electrochemical biosensors for DNA hybridization and DNA damage. Biosens. Bioelectron. 1998;13:621–628. doi: 10.1016/S0956-5663(98)00017-7. [DOI] [PubMed] [Google Scholar]
- 3.Erdem A. Nanomaterial-based electrochemical DNA sensing strategies. Talanta. 2007;74:318–325. doi: 10.1016/j.talanta.2007.10.012. [DOI] [PubMed] [Google Scholar]
- 4.Li F. Structure, function, and evolution of coronavirus spike proteins. Annu. Rev. Virol. 2016;3:237–261. doi: 10.1146/annurev-virology-110615-042301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Simmons G., Zmora P., Gierer S., Heurich A., Pöhlmann S. Proteolytic activation of the SARS-coronavirus spike protein: cutting enzymes at the cutting edge of antiviral research. Antivir. Res. 2013;100:605–614. doi: 10.1016/j.antiviral.2013.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gierer S., Bertram S., Kaup F., Wrensch F., Heurich A., Kramer-Kuhl A., Welsch K., Winkler M., Meyer B., Drosten C., Dittmer U., von Hahn T., Simmons G., Hofmann H., Pohlmann S. The spike protein of the emerging betacoronavirus EMC uses a novel coronavirus receptor for entry, can Be activated by TMPRSS2, and is targeted by neutralizing antibodies. J. Virol. 2013;87:5502–5511. doi: 10.1128/jvi.00128-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Belouzard S., Chu V.C., Whittaker G.R. Activation of the SARS coronavirus spike protein via sequential proteolytic cleavage at two distinct sites. Proc. Natl. Acad. Sci. U. S. A. 2009;106:5871–5876. doi: 10.1073/pnas.0809524106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hoffmann M., Kleine-Weber H., Schroeder S., Krüger N., Herrler T., Erichsen S., Schiergens T.S., Herrler G., Wu N.H., Nitsche A., Müller M.A., Drosten C., Pöhlmann S. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181:271–280. doi: 10.1016/j.cell.2020.02.052. e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Seo G., Lee G., Kim M.J., Baek S.H., Choi M., Ku K.B., Lee C.S., Jun S., Park D., Kim H.G., Kim S.J., Lee J.O., Kim B.T., Park E.C., Il Kim S. Rapid detection of COVID-19 causative virus (SARS-CoV-2) in human nasopharyngeal swab specimens using field-effect transistor-based biosensor. ACS Nano. 2020;14:5135–5142. doi: 10.1021/acsnano.0c02823. [DOI] [PubMed] [Google Scholar]
- 10.Zhou L., Hao P., Li H., Zhang Z. Electrochemical resonance of molecular motion enabling label-, antibody-, and enzyme-free detection of SARS-CoV-2. ACS Sens. 2021;6:1613–1620. doi: 10.1021/acssensors.1c00022. [DOI] [PubMed] [Google Scholar]
- 11.Li Z., Yi Y., Luo X., Xiong N., Liu Y., Li S., Sun R., Wang Y., Hu B., Chen W., Zhang Y., Wang J., Huang B., Lin Y., Yang J., Cai W., Wang X., Cheng J., Chen Z., Sun K., Pan W., Zhan Z., Chen L., Ye F. Development and clinical application of a rapid IgM-IgG combined antibody test for SARS-CoV-2 infection diagnosis. J. Med. Virol. 2020;92:1518–1524. doi: 10.1002/jmv.25727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Corman V.M., Landt O., Kaiser M., Molenkamp R., Meijer A., Chu D.K.W., Bleicker T., Brünink S., Schneider J., Schmidt M.L., Mulders D.G.J.C., Haagmans B.L., Van Der Veer B., Van Den Brink S., Wijsman L., Goderski G., Romette J.L., Ellis J., Zambon M., Peiris M., Goossens H., Reusken C., Koopmans M.P.G., Drosten C. Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR. Euro Surveill. 2020;25 doi: 10.2807/1560-7917.ES.2020.25.3.2000045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grieshaber D., MacKenzie R., Vörös J., Reimhult E. Electrochemical biosensors - sensor principles and architectures. Sensors. 2008;8:1400–1458. doi: 10.1016/j.talanta.2014.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Erdem A., Ozsoz M. Electrochemical DNA biosensors based on DNA-drug interactions. Electroanalysis. 2002;14:965–974. doi: 10.1002/1521-4109(200208)14:14<965::AID-ELAN965>3.0.CO;2-U. [DOI] [Google Scholar]
- 15.Metzner J., Luckert K., Lemuth K., Hämmerle M., Moos R. Towards an electrochemical immunosensor system with temperature control for cytokine detection. Sensors. 2018;18:1–18. doi: 10.3390/s18051309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Radi A.E., Muñoz-Berbel X., Cortina-Puig M., Marty J.L. An electrochemical immunosensor for ochratoxin A based on immobilization of antibodies on diazonium-functionalized gold electrode. Electrochim. Acta. 2009;54:2180–2184. doi: 10.1016/j.electacta.2008.10.013. [DOI] [Google Scholar]
- 17.Sarkar P., Ghosh D., Bhattacharyay D., Setford S.J., Turner A.P.F. Electrochemical immunoassay for free prostate specific antigen (f-PSA) using magnetic beads. Electroanalysis. 2008;20:1414–1420. doi: 10.1002/elan.200804194. [DOI] [Google Scholar]
- 18.Ricci F., Adornetto G., Palleschi G. A review of experimental aspects of electrochemical immunosensors. Electrochim. Acta. 2012;84:74–83. doi: 10.1016/j.electacta.2012.06.033. [DOI] [Google Scholar]
- 19.Gamella M., Bueno-Díaz C., Ruiz-Valdepeñas Montiel V., Povedano E., Reviejo A.J., Villalba M., Campuzano S., Pingarrón J.M. First electrochemical immunosensor for the rapid detection of mustard seeds in plant food extracts. Talanta. 2020;219 doi: 10.1016/j.talanta.2020.121247. [DOI] [PubMed] [Google Scholar]
- 20.Burry R.W. Controls for immunocytochemistry: an update. J. Histochem. Cytochem. 2011;59:6–12. doi: 10.1369/jhc.2010.956920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pan Y., Sonn G.A., Sin M.L.Y., Mach K.E., Shih M.C., Gau V., Wong P.K., Liao J.C. Electrochemical immunosensor detection of urinary lactoferrin in clinical samples for urinary tract infection diagnosis. Biosens. Bioelectron. 2010;26:649–654. doi: 10.1016/j.bios.2010.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Prieto-Simón B., Campàs M., Marty J.L., Noguer T. Novel highly-performing immunosensor-based strategy for ochratoxin A detection in wine samples. Biosens. Bioelectron. 2008;23:995–1002. doi: 10.1016/j.bios.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 23.Kumar R., Christensen N.D., Kaddis Maldonado R.J., Bewley M.C., Ostman A., Sudol M., Chen E.C., Buchkovich N.W., Gontu A., Surendran Nair M., Nissly R.H., Minns A.M., Kapur V., Rossi R., Kuchipudi S.V., Lindner S.E., Parent L.J., Flanagan J.M., Buchkovich N.J. Monoclonal antibodies to s and n sars-cov-2 proteins as probes to assess structural and antigenic properties of coronaviruses. Viruses. 2021;13:1–14. doi: 10.3390/v13101899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ramos-Vara J.A. Technical aspects of immunohistochemistry. Vet. Pathol. 2005;42:405–426. doi: 10.1354/vp.42-4-405. [DOI] [PubMed] [Google Scholar]
- 25.Čadková M., Metelka R., Holubová L., Horák D., Dvořáková V., Bílková Z., Korecká L. Magnetic beads-based electrochemical immunosensor for monitoring allergenic food proteins. Anal. Biochem. 2015;484:4–8. doi: 10.1016/j.ab.2015.04.037. [DOI] [PubMed] [Google Scholar]
- 26.Valverde A., Povedano E., Ruiz-Valdepeñas Montiel V., Yáñez-Sedeño P., Garranzo-Asensio M., Rodríguez N., Domínguez G., Barderas R., Campuzano S., Pingarrón J.M. Determination of cadherin-17 in tumor tissues of different metastatic grade using a single incubation-step amperometric immunosensor. Anal. Chem. 2018;90:11161–11167. doi: 10.1021/acs.analchem.8b03506. [DOI] [PubMed] [Google Scholar]
- 27.Angulo-Ibáñez A., Eletxigerra U., Lasheras X., Campuzano S., Merino S. Electrochemical tropomyosin allergen immunosensor for complex food matrix analysis. Anal. Chim. Acta. 2019;1079:94–102. doi: 10.1016/j.aca.2019.06.030. [DOI] [PubMed] [Google Scholar]
- 28.Serafín V., Razzino C.A., Gamella M., Pedrero M., Povedano E., Montero-Calle A., Barderas R., Calero M., Lobo A.O., Yáñez-Sedeño P., Campuzano S., Pingarrón J.M. Disposable immunoplatforms for the simultaneous determination of biomarkers for neurodegenerative disorders using poly(amidoamine) dendrimer/gold nanoparticle nanocomposite. Anal. Bioanal. Chem. 2021;413:799–811. doi: 10.1007/s00216-020-02724-3. [DOI] [PubMed] [Google Scholar]
- 29.Miller J.N., Miller J.C. sixth ed. Pearson education; Essex: 2010. Statistics and Chemometrics for Analytical Chemistry. [Google Scholar]
- 30.Li J., Lillehoj P.B. Microfluidic magneto immunosensor for rapid, high sensitivity measurements of SARS-CoV-2 nucleocapsid protein in serum. ACS Sens. 2021;6:1270–1278. doi: 10.1021/acssensors.0c02561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mojsoska B., Larsen S., Olsen D.A., Madsen J.S., Brandslund I., Alatraktchi F.A. Rapid SARS-CoV-2 detection using electrochemical immunosensor. Sensors. 2021;21:1–11. doi: 10.3390/s21020390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jamil A., Lim H.N., Yusof N.A., Tajudin A.A., Huang N.M., Pandikumar A., Golsheikh A.M., Lee Y.H., Andou Y. Preparation and characterization of silver nanoparticles-reduced graphene oxide on ITO for immunosensing platform. Sensor. Actuator. B Chem. 2015;221:1423–1432. doi: 10.1016/j.snb.2015.06.156. [DOI] [Google Scholar]
- 33.Fabiani L., Saroglia M., Galatà G., De Santis R., Fillo S., Luca V., Faggioni G., D'Amore N., Regalbuto E., Salvatori P., Terova G., Moscone D., Lista F., Arduini F. Magnetic beads combined with carbon black-based screen-printed electrodes for COVID-19: a reliable and miniaturized electrochemical immunosensor for SARS-CoV-2 detection in saliva. Biosens. Bioelectron. 2021;171 doi: 10.1016/j.bios.2020.112686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.WHO . 2021. Coronavirus Disease COVID-19 Similarities and Differences with Influenza.https://www.who.int/emergencies/diseases/novel-coronavirus-2019/question-and-answers-hub/coronavirus-disease-covid-19-similarities-and-differences-with-influenza [Google Scholar]
- 35.CDC . 2022. The Symptoms of Flu and COVID-19.https://www.cdc.gov/flu/symptoms/flu-vs-covid19.htm [Google Scholar]
- 36.Rahmati Z., Roushani M., Hosseini H., Choobin H. Electrochemical immunosensor with Cu2O nanocube coating for detection of SARS-CoV-2 spike protein. Microchim. Acta. 2021;188 doi: 10.1007/s00604-021-04762-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Aydın E.B., Aydın M., Sezgintürk M.K. Highly selective and sensitive sandwich immunosensor platform modified with MUA-capped GNPs for detection of spike Receptor Binding Domain protein: a precious marker of COVID 19 infection. Sensor. Actuator. B Chem. 2021;345:130355. doi: 10.1016/j.snb.2021.130355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Aydın E.B., Aydın M., Sezgintürk M.K. New impedimetric sandwich immunosensor for ultrasensitive and highly specific detection of spike receptor binding domain protein of SARS-CoV-2. ACS Biomater. Sci. Eng. 2021;7:3874–3885. doi: 10.1021/acsbiomaterials.1c00580. [DOI] [PubMed] [Google Scholar]
- 39.Liv L. Electrochemical immunosensor platform based on gold-clusters, cysteamine and glutaraldehyde modified electrode for diagnosing COVID-19. Microchem. J. 2021;168:106445. doi: 10.1016/j.microc.2021.106445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Torrente-Rodríguez R.M., Lukas H., Tu J., Min J., Yang Y., Xu C., Rossiter H.B., Gao W., SARS-CoV-2 RapidPlex A graphene-based multiplexed telemedicine platform for rapid and low-cost COVID-19 diagnosis and monitoring. Matter. 2020;3:1981–1998. doi: 10.1016/j.matt.2020.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Basso D., Aita A., Padoan A., Cosma C., Navaglia F., Moz S., Contran N., Zambon C.F., Maria Cattelan A., Plebani M. Salivary SARS-CoV-2 antigen rapid detection: a prospective cohort study. Clin. Chim. Acta. 2021;517:54–59. doi: 10.1016/j.cca.2021.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Eissa S., Alhadrami H.A., Al-Mozaini M., Hassan A.M., Zourob M. Voltammetric-based immunosensor for the detection of SARS-CoV-2 nucleocapsid antigen. Microchim. Acta. 2021;188 doi: 10.1007/s00604-021-04867-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Raziq A., Kidakova A., Boroznjak R., Reut J., Öpik A., Syritski V. Development of a portable MIP-based electrochemical sensor for detection of SARS-CoV-2 antigen. Biosens. Bioelectron. 2021;178 doi: 10.1016/j.bios.2021.113029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tian J., Liang Z., Hu O., He Q., Sun D., Chen Z. An electrochemical dual-aptamer biosensor based on metal-organic frameworks MIL-53 decorated with Au@Pt nanoparticles and enzymes for detection of COVID-19 nucleocapsid protein. Electrochim. Acta. 2021;387 doi: 10.1016/j.electacta.2021.138553. [DOI] [Google Scholar]
- 45.Li J., Lin R., Yang Y., Zhao R., Song S., Zhou Y., Shi J., Wang L., Song H., Hao R. Multichannel immunosensor platform for the rapid detection of SARS-CoV-2 and influenza A(H1N1) virus. ACS Appl. Mater. Interfaces. 2021;13:22262–22270. doi: 10.1021/acsami.1c05770. [DOI] [PubMed] [Google Scholar]
- 46.Zhao H., Liu F., Xie W., Zhou T.C., OuYang J., Jin L., Li H., Zhao C.Y., Zhang L., Wei J., Zhang Y.P., Li C.P. Ultrasensitive supersandwich-type electrochemical sensor for SARS-CoV-2 from the infected COVID-19 patients using a smartphone. Sensor. Actuator. B Chem. 2021;327:128899. doi: 10.1016/j.snb.2020.128899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Torres M.D.T., de Araujo W.R., de Lima L.F., Ferreira A.L., de la Fuente-Nunez C. Low-cost biosensor for rapid detection of SARS-CoV-2 at the point of care. Matter. 2021;4:2403–2416. doi: 10.1016/j.matt.2021.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rashed M.Z., Kopechek J.A., Priddy M.C., Hamorsky K.T., Palmer K.E., Mittal N., Valdez J., Flynn J., Williams S.J. Rapid detection of SARS-CoV-2 antibodies using electrochemical impedance-based detector. Biosens. Bioelectron. 2021;171:112709. doi: 10.1016/j.bios.2020.112709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Liv L., Çoban G., Nakiboğlu N., Kocagöz T. A rapid, ultrasensitive voltammetric biosensor for determining SARS-CoV-2 spike protein in real samples. Biosens. Bioelectron. 2021;192 doi: 10.1016/j.bios.2021.113497. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.