Abstract
The coronavirus disease 2019 (COVID-19) pandemic is responsible for 267 million infections and over 5 million deaths globally. COVID-19 is caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), a single-stranded RNA beta-coronavirus, which causes a systemic inflammatory response, multi-organ damage, and respiratory failure requiring intubation in serious cases. SARS-CoV-2 can also trigger neurological conditions and syndromes, which can be long-lasting and potentially irreversible. Since COVID-19 infections continue to mount, the burden of SARS-CoV-2-induced neurologic sequalae will rise in parallel. Therefore, understanding the spectrum of neurological clinical presentations in SARS-CoV-2 is needed to manage COVID-19 patients, facilitate diagnosis, and expedite earlier treatment to improve outcomes. Furthermore, a deeper knowledge of the neurological SARS-CoV-2 pathomechanisms could uncover potential therapeutic targets to prevent or mitigate neurologic damage secondary to COVID-19 infection. Evidence indicates a multifaceted pathology involving viral neurotropism and direct neuroinvasion along with cytokine storm and neuroinflammation leading to nerve injury. Importantly, pathological processes in neural tissue are non-cell autonomous and occur through a concerted breakdown in neuron-glia homeostasis, spanning neuron axonal damage, astrogliosis, microgliosis, and impaired neuron-glia communication. A clearer mechanistic and molecular picture of neurological pathology in SARS-CoV-2 may lead to effective therapies that prevent or mitigate neural damage in patients contracting and developing severe COVID-19 infection.
Keywords: Axon, Astrocyte, COVID-19, Cytokine storm, Extracellular vesicles, Immune system, Microglia, Neuron, Oligodendrocyte
1. Introduction
Late in 2019, a new coronavirus emerged, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), triggering the coronavirus disease 2019 (COVID-19) pandemic. Globally, 267 million individuals have contracted SARS-CoV-2, which has killed over 5 million people, as of December 2021 (https://coronavirus.jhu.edu/map.html, n.d). SARS-CoV-2 is a single-stranded RNA beta-coronavirus, which causes a systemic inflammatory response, multi-organ damage, and respiratory failure requiring intubation in serious cases. The virus is especially dangerous to older populations and patients with co-morbidities, such as obesity and diabetes (Feldman et al., 2020). The pathophysiology remains incompletely understood; however, neurological involvement is increasingly evident. This has important health implications for SARS-CoV-2 survivors, due to the frequent long-lasting and potentially irreversible nature of neurologic sequalae. Moreover, as infections continue to rise, so will the burden of SARS-CoV-2-induced neurologic complications.
Therefore, understanding the spectrum of neurological disorders in response to SARS-CoV-2 is needed to manage COVID-19 patients (Ellul et al., 2020). This will facilitate recognition of nervous system injury secondary to SARS-CoV-2, which could expedite earlier treatment to improve outcomes. Furthermore, a clearer understanding of the neurological SARS-CoV-2 pathomechanisms could uncover potential therapeutic targets. To date, the evidence suggests SARS-CoV-2-triggered neurological damage occurs through several avenues. First, the virus exhibits neurotropism, enabling direct invasion of neural tissue (Song et al., 2021). Second, systemic cytokine storm and a hyperactive inflammatory immune response secondary to viral infection, can cause nerve injury (Thepmankorn et al., 2021). Additionally, local induction of neuroinflammation within the central nervous system (CNS) also contributes to neuronal damage. Importantly, pathological processes in neural tissue are non-cell autonomous and occur through a concerted breakdown in neuron-glia homeostasis. These processes span neuron axonal damage, astrogliosis, microgliosis, and impaired neuron-glia communication.
This review will cover the topic of SARS-CoV-2-triggered loss of neuron-glial homeostasis. We will describe the breadth of neurological presentation in COVID-19 patients and describe evidence from clinical and autopsy studies of virally induced neuronal loss, endothelial astrogliosis, and neuroinflammation. We will also outline in vitro and animal research demonstrating putative viral entry routes and neurotropism, followed by a focus on neuron-glial interactions. Lastly, we will round up the discussion with potential therapeutic avenues based on currently known SARS-CoV-2 pathophysiology.
2. Neurological manifestation in COVID-19 patients
The SARS-CoV-2 pandemic precipitated a now recognized global increase in the prevalence of well-characterized and rare neurologic sequelae of the CNS and peripheral nervous systems (PNS) in response to viral illness (Mao et al., 2020). One United Kingdom (UK) study systematically documented the various neurologic syndromes experienced by COVID-19 survivors. Neurologic complications included encephalopathy, encephalitis with documented CNS inflammatory changes, ischemic stroke, and Guillain-Barré Syndrome (GBS), among other miscellaneous syndromes (Paterson et al., 2020). The postulated SARS-CoV-2-mediated pathomechanisms for CNS and PNS injury comprise direct neurotropic invasion and parainfectious endothelial dysfunction, coagulopathy, hyperinflammation, and autoimmunity (Fig. 1A) (Mehta et al., 2020; Zubair et al., 2020).
Fig. 1.
SARS-CoV-2-induced neurological manifestations.
(A) SARS-CoV-2-induced neurologic complications can affect the CNS and PNS. Damage to the brain stem and autonomic nervous systems can lead to respiratory and cardiac dysfunction. Severe COVID-19 patients exhibit systemic inflammation, marked by elevated white blood cells and pro-inflammatory cytokines. Antibodies specific to PNS injury can be detected in blood, including IgG, anti-GM1, anti-GD1a, and anti-GD1b. (B) SARS-CoV-2 binds to ACE2 (shown in figure) or NRP1 receptors, and a serine protease, e.g., TMPRSS2, cleaves the complex, leading to viral internalization. (C) The virus infects sustentacular cells of the nasal epithelium. ACE2, angiotensin-converting enzyme 2; BBB, blood-brain barrier; CNS, central nervous system; GBS, Guillain-Barré Syndrome; NRP1, neuropilin-1; PNS, peripheral nervous system; TMPRSS2, transmembrane serine protease 2. Created, in part, with BioRender.com.
Regarding endothelial dysfunction and coagulopathy, a prospective Belgian study of post-mortem brain magnetic resonance (n = 62) revealed subcortical micro- and macrobleeds, likely due to blood-brain barrier (BBB) breakdown (Coolen et al., 2020). This endothelial dysfunction, with or without direct viral infection, may underlie SARS-CoV-2 mediated CNS injury. SARS-CoV-2 related stroke syndromes tend to occur in the setting of markedly elevated D-dimer levels, frequently with concurrent large vessel and systemic venous thromboembolic events (Beyrouti et al., 2020). Notably, young patients suffer higher-than-expected stroke incidences, typically large vessel, which appears to implicate coagulopathy and endothelial dysfunction (Oxley et al., 2020). Antiphospholipid antibody syndrome from hypercoagulability has occurred in some cases, likely linked to pro-inflammatory cytokine states (Zhang et al., 2020).
In terms of immune response, post-mortem examination of six patients in Germany showed encephalitic and meningitic changes, with evidence of brainstem perivascular and inflammatory changes associated with neuronal loss (von Weyhern et al., 2020). Encephalitis, in particular, has figured prominently in SARS-CoV-2 mediated CNS injury. Acute demyelinating encephalomyelitis is a rare disorder lacking clear evidence of direct causality in SARS-CoV-2 injury. One autopsy study, however, illustrates some of the CNS inflammatory patterns seen in COVID-19-induced CNS injury, and sheds valuable insight into putative pathomechanisms. This post-mortem evaluation revealed hemorrhagic white matter lesions in the bilateral hemispheres, mostly subcortically, with macrophagic foci surrounding small vessels along with myelin breakdown and axonal damage (Reichard et al., 2020). Additionally, there was generalized widespread glial fibrillary acidic protein (GFAP) positive staining, indicative of astrogliosis, in white matter, but not in the hemorrhagic lesions. Furthermore, separate radiologic case reports of SARS-CoV-2-induced encephalitis document symmetric focal involvement of the bilateral thalami (Poyiadji et al., 2020) and the brainstem (Dixon et al., 2020). The neurologic UK study did not detect antibodies in COVID-19 patients with autoimmune encephalitis (Paterson et al., 2020). However, a number of studies have reported myelin oligodendrocyte glycoprotein (MOG)-associated demyelinating syndrome (Woodhall et al., 2020; Sinha et al., 2021), encephalitis (Peters et al., 2021), and optic neuritis (Sawalha et al., 2020) in the SARS-CoV-2 setting.
In the PNS, most neurological SARS-CoV-2-related clinical experience has focused on GBS, and numerous cases have been described (Padroni et al., 2020; Sedaghat and Karimi, 2020; Tiet and AlShaikh, 2020). As with most pathogen-mediated GBS, autoimmune cross-reactivity or molecular mimicry is thought to underlie SARS-CoV-2-related GBS. Antibodies specific to PNS disease are present in GBS cases post SARS-CoV-2, including IgG, anti-GM1, anti-GD1a, and anti-GD1b (Civardi et al., 2020; Dufour et al., 2021). One case of Miller Fisher syndrome with increased pro-inflammatory cytokine markers and positive anti-GD1b-IgG levels has been reported, supporting immune-mediated pathomechanisms (Gutiérrez-Ortiz et al., 2020).
Although it was initially thought that the pandemic would trigger a spike in GBS cases, data has shown otherwise. Instead, three studies found either stable incidence or a decline in GBS cases during the SARS-CoV-2 era (Keddie et al., 2021; Luijten et al., 2021; Umapathi et al., 2021). As with other novel viral pathogens, like Zika, there is an incontrovertible and emerging, albeit small, body of evidence that suggests a temporal link between COVID-19 infection and GBS (Aladawi et al., 2022). This seemingly contradictory finding lacks a definitive explanation, although putative reasons have been put forth. One suggestion for this discrepancy is the near-universal mask-wearing and stay-at-home orders of the pandemic (Foschi et al., 2021). It is postulated that this increased mask-wearing and limited social interaction reduced the incidence of non-SARS-CoV-2-related GBS, which would still constitute the bulk of total cases. Furthermore, it is possible that patients with milder GBS may not have sought medical attention to avoid a hospital setting. Limited hospital bed availability may have been another reason. Other explanations have also been explored (Foschi et al., 2021).
Of all post SARS-CoV-2 neurologic consequences, post-acute sequelae SARS-CoV-2 (PASC), also called long-COVID syndrome, remains the topic of greatest public interest, although the exact underlying pathomechanisms remain elusive. Patients report fatigue, cognitive slowing, and exertional intolerance, among many other symptoms. Some patients meet the criteria for orthostatic intolerance and postural tachycardia syndrome on formal autonomic testing (Shouman et al., 2021). It is suspected that SARS-CoV-2 binding to angiotensin-converting enzyme 2 (ACE2) receptors may disrupt the renin-angiotensin-aldosterone system, which regulates sympathetic outflow (Goldstein, 2021).
Therapeutic experience remains limited, although the neurologic UK study revealed that some encephalopathies improved without specific treatment (Paterson et al., 2020), while patients with inflammatory CNS syndromes improved with corticosteroids and/or immunoglobulin therapy.
3. SARS-CoV-2 neurotropism
Early in the pandemic, it was shown SARS-CoV-2 leverages the ACE2 receptor to facilitate entry into host cells, like its predecessors, SARS-CoV and Middle East respiratory syndrome coronavirus (Yan et al., 2020). Once the SARS-CoV-2 spike protein receptor-binding domain latches onto ACE2, proximal serine proteases, e.g., transmembrane serine protease 2 (TMPRSS2), cleave the spike-ACE2 complex, internalizing the virus (Fig. 1B) (Hoffmann et al., 2020). Tissue receptor expression dictates tropism; thus, widespread ACE2 expression across multiple tissues results in extensive SARS-CoV-2 tropism. ACE2 expression level in the CNS is moderate (Li et al., 2020a, Li et al., 2020b); however, since the discovery that SARS-CoV-2 binds ACE2, additional receptors have been identified, including neuropilin-1 (NRP1), a glycoprotein involved in neurogenesis (Zhang et al., 2021a, Zhang et al., 2021b). ACE2 and NRP1 are widely expressed in the brain; however, ACE2 is most highly expressed in endothelial vasculature and circumventricular organs (Hernández et al., 2021), whereas NRP1 expression is especially enhanced in the hippocampus, endothelial cells, mural cells, perivascular macrophages, and microglia (Davies et al., 2020).
Several mechanisms have been proposed for viral penetration into the brain. One suggested viral entry route is by disrupting the choroid plexus, thereby compromising the BBB and providing SARS-CoV-2 access to the CNS. The virus also triggers a pro-inflammatory response, which can render the BBB susceptible to damage, leading to immune cell infiltration into the brain along with viral penetration (Tremblay et al., 2020; Solomon, 2021). A possible avenue for dissemination throughout the CNS, once the virus has gained passage into the brain, is through the microvasculature. Autopsy examination of frontal cortex tissue from COVID-19 patients (n = 17) reveals an increase of so-called string vessels versus control tissue (n = 23) (Wenzel et al., 2021). String vessels are empty basement membrane tubes lacking endothelial cells, which are signs of capillary loss. The study found that Mpro, the primary SARS-CoV-2 protease, cleaves host endothelial NEMO (nuclear factor (NF)-κB essential modulator), leading to endothelial cell apoptosis, local hypoxia, and microglial and astrocytic reactivity (Wenzel et al., 2021). Therefore, microvascular pathology and endothelial dysfunction (Varga et al., 2020) in the brain may constitute a significant mode of viral dissemination and neuropathology in the brain.
Anosmia is a prominent symptom of COVID-19 infection, even in relatively mild cases. This promoted a putative mechanism of viral entry into the CNS intranasally through the olfactory epithelium, which expresses ACE2 (Fig. 1C) (Brann et al., 2020) and NRP1 (Cantuti-Castelvetri et al., 2020). In this proposed mechanism, the olfactory bulb would then serve as a conduit to the CNS, including ACE2-expressing circumventricular organs, such as the subfornical organ (de Melo et al., 2021), which are particularly vulnerable because they are not protected by the BBB. A recent study by Khan et al. of olfactory mucosa and whole olfactory bulb tissue from recently deceased COVID-19 patients (n = 85) detected SARS-CoV-2 primarily in the sustentacular cells of the mucosa (Khan et al., 2021). The virus was not detected in olfactory sensory neurons nor the olfactory bulb parenchyma (Khan et al., 2021), like another study, which only found sparse pathology of olfactory bulbs (Thakur et al., 2021). Thus, overall, an intranasal route into the CNS may be unlikely.
Far fewer studies have investigated SARS-CoV-2 penetration into the PNS, although case reports of peripheral neuropathies are reported within the same timeframe of COVID-19 infection (Padroni et al., 2020; Sedaghat and Karimi, 2020; Tiet and AlShaikh, 2020). PNS damage could occur secondary to SARS-CoV-2-induced inflammation, as occurs in the CNS. Alternatively, drawing parallels to other viruses, SARS-CoV-2 could be internalized into neurons through the endocytic pathway and hijack axonal trafficking to spread along the PNS (Fenrich et al., 2020).
Our understanding of SARS-CoV-2 neurotropism derives from both human, i.e., autopsy, and in vitro and mouse studies. Autopsy reveals invasion of the olfactory epithelium (Khan et al., 2021) and CNS (Mukerji and Solomon, 2021). Analysis of autopsy tissue from three COVID-19 patients demonstrated immunoreactivity against the viral spike protein in cortical neurons and endothelial cells, albeit to variable extents (Song et al., 2021). Cellular staining was perinuclear along with both intense puncta and diffuse cytoplasmic reactivity; subcortical microscopic ischemic infarcts were also present, which stained for viral proteins in the hyperacute stage. However, although neuroinflammation is a putative characteristic of severe COVID-19 infection, the areas that stained for viral protein were not infiltrated by lymphocytes or leukocytes (Song et al., 2021), though another autopsy study noted some CD3+, CD4+, and CD8+ T cells in the parenchyma (Schurink et al., 2020).
Analysis of brain autopsy tissue (n = 21) for RNA detected the virus in eight samples, but only at low SARS-CoV-2 copies per cell (Puelles et al., 2020). Another study (n = 18 patients) similarly detected viral RNA in various brain regions in most samples (Solomon et al., 2020). Interestingly, viral protein was not detected in neurons, glia, endothelium, or immune cells. A larger analysis (n = 40) found both viral RNA and protein in 8 (20%) of brain autopsy samples and either RNA or protein in 21 (53%) specimens (Matschke et al., 2020). One study detected viral RNA, but no protein, in most brain autopsy samples (n = 25), but levels were far lower than in mucosal samples (Thakur et al., 2021). The authors concluded that direct viral neuroinvasion in the brain was unlikely due to the significantly lower viral RNA levels in the brain relative to the nasal epithelium, and that most CNS neuropathology results from systemic inflammation, possibly compounded by local hypoxia and ischemia. It is worth noting that peripheral immune cell infiltration into the CNS has been detected in the brain, but also to variable extents (Schurink et al., 2020; Song et al., 2021), and primarily in the brain stem (Matschke et al., 2020; Thakur et al., 2021).
Another point of consideration, which has limited our understanding of SARS-CoV-2 neurotropism in the brain from autopsy samples, is that sensitivity for detecting viral RNA may be higher than for viral proteins (Solomon et al., 2020; Thakur et al., 2021). However, RNA analysis from bulk brain tissue does not allow localization of the RNA to specific cell types, and the virus detected in the brain may be more prevalent in endothelial cells versus neurons or glia (Nuovo et al., 2021; Wenzel et al., 2021). However, this possibility requires further investigation and single-cell sequencing of brain autopsy tissue may be one path forward (Fullard et al., 2021).
Regarding the PNS, consecutive autopsies (n = 35) from femoral nerve tissue revealed neuritis in nine COVID-19 patients without evidence of direct SARS-CoV-2 invasion, likely indicating inflammatory- or immune-mediated PNS damage rather than tissue tropism (Suh et al., 2021), though further studies are necessary.
Beyond autopsy studies, brain organoids have also expanded our understanding of viral neurotropism (Ng et al., 2021). The first obstacles to viral penetration into the CNS are the BBB and blood-cerebrospinal fluid (CSF) barriers, which regulate entry of material into the brain. The blood-CSF barrier lines the choroid plexus, which produces CSF. Infection rate was significantly higher in choroid plexus (10–20%) versus cortical (<1.5%), hippocampal (<1.0%), hypothalamic (<1.5%), and midbrain (<1.5%) organoids generated from human induced pluripotent stem cells (hiPSCs) (Jacob et al., 2020). Similarly, analysis of cell cultures found choroid plexus epithelial cells were robustly infected with SARS-CoV-2, whereas neurons and astrocytes were sparsely infected. Another study of hiPSC-derived choroid plexus organoids observed higher ACE2 expression by mature choroid plexus cells but not by neurons or other cell types, which mirrored greater SARS-CoV-2 infectivity of choroid cells (13%), but not of neurons or glia (Pellegrini et al., 2020). Additionally, the live virus also structurally and functionally compromised the choroid plexus epithelial barrier, and preference for choroid plexus cells and glia by SARS-CoV-2 over neurons was also reported by McMahon and colleagues (McMahon et al., 2021). Although human studies suggest that viral entry may proceed primarily from systemic inflammation (Solomon, 2021; Thakur et al., 2021), brain organoid studies suggests= that direct viral infection and/or destruction of choroid plexus may be feasible. However, if this pathway does occur in vivo, it may be to an insignificant extent.
Some brain organoid studies have observed higher infection of neurons by SARS-CoV-2 (Song et al., 2021; Wang et al., 2021), especially mature, MAP2-positive neurons, leading to neuronal death and loss (Song et al., 2021). A single-cell RNA-seq study identified multiple clusters of neuronal, neuronal progenitor, and radial glial cell populations (Song et al., 2021). Overlap of SARS-CoV-2 transcripts occurred with all cell-types, but to variable extents among the multiple clusters. Viral infection also induced a transcriptomic shift to a hypermetabolic and hypoxic cellular state, indicating that SARS-CoV-2 may be hijacking cellular metabolism to replicate. Differences among brain organoid studies may derive from maturation state of the organoid, since differentiated neurons are more susceptible than neural progenitor cells (Song et al., 2021) and differentiation boosts infection of astrocytes (Wang et al., 2021).
Alternatively, variation in the hiPSC genetic background in genes linked to SARS-CoV-2 susceptibility could give rise to differential infectivity of neurons and various cell-types observed across organoid studies. For instance, the ApoE4 allele is a risk for severe COVID-19 infection (Kuo et al., 2020). Indeed, isogenic hiPSCs expressing ApoE3 versus ApoE4 demonstrates that ApoE4 renders differentiated neurons more prone to SARS-CoV-2 infection (2.1% ApoE4 versus 1.4% ApoE3) and viral-mediated neurite degeneration (Wang et al., 2021). ApoE4 in differentiated astrocytes similarly predisposes them to viral infection and a decrease in soma and process length. Another possibility for discordance in studies of cellular infectivity preference of SARS-CoV-2 is the viral strain. A German study concluded that a Düsseldorf isolate of SARS-CoV-2 preferentially targets neurons versus neuronal progenitor in human derived brain organoids (Ramani et al., 2020).
The striking differences between autopsy findings and in vitro brain organoids merits discussion. By and large, viral RNA or protein can be detected in brain tissue from COVID-19 patients, but only to low levels and their localization to specific cell types remains unclear, although endothelial cells may be a favored compartment versus neurons and glia (Nuovo et al., 2021; Wenzel et al., 2021). On the other hand, SARS-CoV-2 infection of neurons, glia, and choroid plexus cells is evident from brain organoid analysis. Several reasons may explain these disparate findings. If the virus does preferentially infect endothelial cells versus neurons and glia, microvasculature may be the preferred target in vivo. However, brain organoids are devoid of a comprehensive vasculature (Jacob et al., 2020; Song et al., 2021), and SARS-CoV-2 may invade neurons and glia in the absence of other targets, such as endothelial cells. Another possibility is differences in the viral load in vitro and in vivo. Brain organoids can be experimentally infected to a specified multiplicity of infection, at a burden that targets neurons and glia. However, the viral burden may be lower in vivo due to the BBB, despite damage from systemic inflammation, occurring at levels that do not infect neurons. Finally, viral attack is dynamic in vivo, and the temporal element is critical. COVID-19 patients may pass away before significant viral replication has occurred into the brain.
Cumulatively, however, human, in vitro, and animal data support invasion into the CNS. Possibly in humans, the dominant pathway is from systemic inflammation, though this does not fully exclude other less significant entry modes. Moreover, endothelial dysfunction in the brain is an important aspect of neuropathology secondary to SARS-CoV-2 infection. Additionally, the determinants of tropism among specific CNS cell-type still requires clarification. Understanding the determinants of infectivity could help identify patients at risk of developing COVID-19-mediated neurological disorders. If genetic background is a potential determinant of infection, it could explain variable susceptibly to SARS-CoV-2-induced neurological complications in COVID-19 patients. Finally, few studies have investigated neuroinvasion into the PNS, though SARS-CoV-2 may induce peripheral nerve damage.
4. SARS-CoV-2-induced disturbance in neuron-glial interactions
4.1. SARS-CoV-2 in the central nervous system
4.1.1. Astrocyte-neuron interactions
The brain is an incredibly complex organ; neurons are aided in their function by supportive glia, comprised primarily of oligodendrocytes, astrocytes, and microglia. Under homeostatic conditions, astrocytes contribute structurally to BBB maintenance (Linnerbauer and Rothhammer, 2020) and are thus part of the brain's first line of defense from invading pathogens, including SARS-CoV-2 (Fig. 2 ). Astrocytes also form tripartite synapses with neurons, providing support through neurotransmitter regulation and metabolic coupling. Virally induced disruption of astrocyte function could thus impair both neuronal transmission and metabolism (Cotto et al., 2019; Sher et al., 2019).
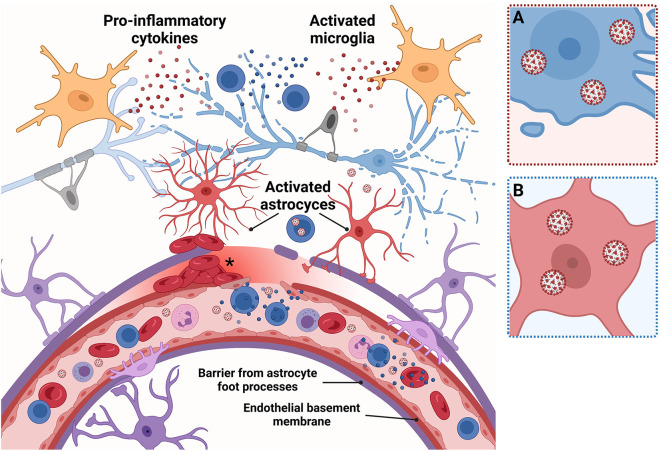
Fig. 2.
Neuron-glia homeostasis in the healthy brain.
Overview figure of the central nervous system (CNS) under homeostatic conditions. Brain neurons are supported by glia, astrocytes, microglia, and oligodendrocytes. Neurons (light blue): Functioning astrocytes and microglia support signal transmission in healthy neurons, which are wrapped with oligodendrocytes (grey). Blood vessels: Circulating species in blood vessels are separated from the brain by the blood-brain barrier (BBB; pink inset, A). The BBB is comprised of tight junctions between endothelial cells of the vasculature, along with structural reinforcement from the extracellular matrix from the basement membrane and astrocytic foot processes. The BBB regulates what species are permitted to traverse into the central nervous system (CNS). Astrocytes (purple): Contribute structurally to the BBB through astrocyte foot processes, which form a layer against the endothelial basement membrane, defending the brain from invading pathogens. Inset, blue, B: Astrocytes (purple) form tripartite synapses with neurons (light blue) to provide neurotransmitter and metabolic support. Microglia (dark blue): CNS resident immune cells, surveil for potentially harmful agents and clear the CNS of debris by phagocytosis. Inset, green, C: Microglia remodel neuronal circuits by pruning dendritic spines and synapses and promote myelin maintenance and remyelination. Created with BioRender.com.
Pathologic conditions, such as the presence of danger signals from viral invasion, called damage-associated molecular pattern molecules (DAMPs), induce astrogliosis, a neuroprotective phenotype characterized by morphological, transcriptomic, and biochemical reprogramming and glial fibrillary acidic protein (GFAP) upregulation (Fig. 3 ). However, astrogliosis can serve as a double-edged sword, promoting the disease process through a neurotoxic phenotype (Ding et al., 2021). In a longitudinal study of COVID-19 patients (n = 47) with varying disease severity, moderate and serious COVID-19 infection correlated with elevated plasma GFAP, and severe disease correlated with increased plasma neurofilament light chain (NfL), a marker of intra-axonal neuronal injury (Kanberg et al., 2021). In severe COVID-19 cases, plasma GFAP peaked earlier in the infection course, indicating initial astrogliosis, whereas NfL was persistently elevated, possibly from longer-lasting neuronal damage. Another study of hospitalized COVID-19 patients with (n = 34) and without (n = 94) neurological symptoms found elevated serum NfL levels, independent of neurologic presentation and uncorrelated to CSF NfL levels, possibly reflecting peripheral nerve damage in severe infection (Paterson et al., 2021). Serum NfL levels were not increased in COVID-19 community cases, suggesting mild disease may not produce nerve injury. Unlike some studies, however, GFAP was not prominent in CSF of patients with serious COVID-19, implying astrogliosis-induced neuronal damage may not always be part of SARS-CoV-2 pathophysiology. It is possible, however, that discrepancies between studies also arise from the time course of GFAP release, which is higher earlier in infection, leading to different results depending on when plasma/serum GFAP is sampled. Possibly, astrogliosis may be easier to detect in brain autopsy tissue. Indeed, investigation of brain samples from deceased COVID-19 patients (n = 43) documented astrogliosis through GFAP staining in 37 (86%) of cases (Matschke et al., 2020).
Fig. 3.
Putative SARS-CoV-2-induced disruption of neuron-glia homeostasis.
Overview figure of the CNS under SARS-induced pathologic conditions. Neurons (light blue): Excessive inflammation may cause neurons to degenerate. Inset, pink, A: SARS-CoV-2 may also potentially directly invade neurons, triggering apoptosis. Blood vessels: High levels of circulating pro-inflammatory cytokines (blue spheres) damage the BBB; peripheral cytotoxic T cells infiltrate the CNS, further augmenting pro-inflammatory cytokine levels. Circulating SARS-CoV-2 virions can also penetrate the CNS or hijack immune cells to enter. Microbleeds from SARS-CoV-2-induced endothelial dysfunction (asterisks) can also occur, causing CNS damage. Astrocytes (purple and red, activated): SARS-CoV-2 triggers astrogliosis, which raises production of damage-associated molecular pattern molecules, e.g., GFAP. Astrogliosis may also contribute to the breakdown in BBB integrity, e.g., through astrocytic foot processes detachment. Inset, blue, B: SARS-CoV-2 may infect astrocytes, possibly depending on the genotype. Microglia (orange): SARS-CoV-2 induces microgliosis, which elevates microglia-derived pro-inflammatory cytokines (red spheres). Both astrogliosis and microgliosis occur concomitant with neuronal loss. Created with BioRender.com.
Another primarily astroglial protein, S100B, also has a putative role as a DAMP and correlates with various neural CNS injuries, from traumatic acute brain damage to neurodegenerative diseases (Michetti et al., 2019). In a small longitudinal study of COVID-19 patients with neurological manifestations involving the CNS and cytokine storm (n = 5), elevated serum S100B was coincident with cytokine release in three patients with acute leukoencephalitis. Though small, the study does suggest that cytokine storm correlates with leukoencephalitis, which injures astrocytes and induces BBB leakage (Perrin et al., 2021).
In addition to a shift in astroglial phenotypes, COVID-19 may lead to astrocytic and microglial proliferation, concomitant with a decrease in neuronal density, as noted in a small neurohistopathological study of brain autopsy material (n = 3 COVID-19, n = 3 control) (Boroujeni et al., 2021). The astrocytes and microglia also adopted a pro-inflammatory phenotype, a scenario suggesting inflammation-induced neuronal loss.
4.1.2. Microglia-neuron interactions
Microglia are the resident immune cells of the CNS (Prinz et al., 2019). During homeostatic conditions in the adult brain, they surveil the CNS milieu for potentially harmful agents, e.g., infectious pathogens, tissue injury (Fig. 2). They also perform numerous “housekeeping” roles, such as clearing the CNS of debris by phagocytosis. Microglia also remodel neuronal circuits by pruning dendritic spines and synapses during learning and memory and participate in axonal myelin maintenance and remyelination. Therefore, microglia have a direct impact on neuronal health. Under pathologic conditions during infection, microglia launch an immune response in order to clear the invading pathogen from the CNS (Fig. 3) (Hatton and Duncan, 2019). This response is complex and involves variably activated microglia with anti- and pro-inflammatory phenotypes (Cherry et al., 2014). Unfortunately, in certain instances, an overly pro-inflammatory state can develop, which is toxic to neurons.
A comparable situation may occur during SARS-CoV-2, with a microglial-mediated local inflammatory response within the CNS, which is compounded by the presence of systemic hyperinflammation that can also spread throughout the brain (Solomon, 2021). Post-mortem evaluation of COVID-19 patients (n = 43) sheds light on this dual inflammatory process in the brain, which demonstrates both microglial activation and cytotoxic T lymphocyte infiltration, mostly in the brainstem and cerebellum (in up to 79% of cases) (Matschke et al., 2020). Analysis of cerebral autopsy samples (n = 3) also demonstrates activation of the NLRP3 inflammasome (Cama et al., 2021), a sensor of pathogen-associated molecular patterns (PAMPs) involved in the antiviral response (Zhao and Zhao, 2020). NLRP3 colocalizes with CD68+ macrophages in the brain and the periphery (lung) of the deceased, implicating a role for NLRP3 in SARS-CoV-2 pathology (Cama et al., 2021).
Single-nucleus RNA sequencing of brain samples for COVID-19 patients that died from severe infection confirm immunohistological microglia analyses. The brain transcriptome in COVID-19 (n = 8 COVID-19; n = 13 controls) reveals choroid plexus barrier disruption along with signaling cues into the CNS (Yang et al., 2021). Peripheral T cell infiltration is present along with resident microglia, which have adopted a phenotype reminiscent of neurodegenerative disease. Another transcriptomic analysis of three distinct brain areas (n = 5 COVID-19; n = 4 controls) found an influx of monocytes and macrophages in the choroid plexus along with a signature in cortical microglia linked to cellular activation, mobility, and phagocytosis (Fullard et al., 2021). In both instances, molecular traces of SARS-CoV-2 were not detected (Fullard et al., 2021; Yang et al., 2021), possibly suggestive of persistent inflammation and longer-lasting neural injury, even after the virus has cleared. Using a paradigm of spatial profiling by imaging mass cytometry at single-cell resolution, an evaluation was performed of autopsy brain stem and olfactory bulb (Schwabenland et al., 2021). Significant neuropathology was seen, involving axonal damage, astrocytosis, and BBB leakage, concomitant with viral antigens in ACE2+ vascular cells. Perivascular microglial nodules enriched with activated CD8+ T cells were also noted, which correlated with clinical measures of systemic inflammation.
Research of in vitro and in vivo models paints a similar picture. Exposure of rodent BV-2 microglia to SARS-CoV-2 spike protein increases tumor necrosis factor alpha (TNF-α), interleukin-6 (IL-6), IL-1β, and inducible nitric oxide synthase (iNOS)/ nitric oxide (NO) production (Olajide et al., 2021). Microglial contact with spike protein also enhanced NLRP3 inflammasome and caspase-1 activity. Similar observations were made following exposure of human HMC3 microglia to SARS-CoV-2 spike protein, leading to heightened TNF-α, IL-8, IL-1β, and reactive oxygen species generation and increased NOS and caspase-3/7 expression (Clough et al., 2021). This pro-inflammatory response was accompanied by microglial morphological changes along with mitochondrial fragmentation. In vivo, in SARS-CoV-2-infected K18-hACE2 transgenic mice (cytokeratin-18 gene promoter driven human ACE2 expression) and Syrian hamsters, microglia proliferated and increased TNF-α and IL-6 expression (Zhang et al., 2021b).
CNS microglia may also be activated by exosomes carrying viral material, rather than the virus itself. Exosomes are extracellular vesicles that bud off from cells and carry cargo to recipient cells. This intercellular communication mode can occur as part of normal physiologic function, or as a pathological process during disease states, including a viral infection. Circulating exosomes have been detected in patients in the context of SARS-CoV-2 infection, carrying cargo harboring SARS-CoV-2 RNA and a distinct proteomic signature strongly involved in host response to infection, immune processes, inflammation, and coagulation (Barberis et al., 2021). It is conceivable that exosomes can penetrate the CNS and activate microglia; indeed, an in vitro study of exosomes derived from HEK293T cells transfected with SARS-CoV-2 spike plasmid were found to transfect human microglial CHME3 cells (Mishra and Banerjea, 2021). Exosome cargo was enriched in microRNAs (miR) 148a and miR-590-3p. microRNAs are small non-coding RNAs that negatively regulate expression of target genes. The exosomes suppressed ubiquitin specific peptidase 33 (USP33) and interferon regulatory factor 9 (IRF9) expression in recipient microglia, the latter being involved in immune regulation.
5. Summary
Overall, human, in vitro, and animal evidence suggests a recurrent theme of astrogliosis and pro-inflammatory microglial activation in SARS-CoV-2 infection in the brain (Fig. 3). Some studies suggest these processes occur in tandem with neuron axonal damage and loss, possibly indicating neuronal injury arising secondary to a breakdown in neuron-glia homeostasis. Future studies are needed to better understand the molecular steps or mediators of neuron-glia or axo-glia communications during SARS-CoV-2 infection leading to brain damage, with the goal of developing mechanism-based therapies. Furthermore, understanding of potential neuron-oligodendrocyte interactions is lacking in the context of SARS-CoV-2. However, a study of another coronavirus, mouse hepatitis virus (MHV), suggests surviving oligodendrocytes post viral infection may prolong the inflammatory phase (Pan et al., 2020).
5.1. SARS-CoV-2 in the peripheral nervous system
In contrast to CNS studies, there is a paucity of reports in the PNS. However, PNS neurotropism is a facet of SARS-CoV-2 pathophysiology as evidenced by the emerging literature linking autoimmune peripheral neuropathies to COVID-19 infection (Padroni et al., 2020; Sedaghat and Karimi, 2020; Tiet and AlShaikh, 2020) and the presence of IgG, anti-GM1, anti-GD1a, and anti-GD1b antibodies (Civardi et al., 2020; Dufour et al., 2021). Elevated serum NfL hints at PNS damage during severe COVID-19 infection (Paterson et al., 2021). However, mechanistic studies in SARS-CoV-2 are lacking, although clinical findings suggest an immune-mediated component, at least in part.
Drawing from other neurotropic viruses, such as MHV, demyelinating strains are transported in a retrograde fashion from the brain via axons, inducing optic neuritis with macrophage infiltration and axonal demyelination and loss (Shindler et al., 2011). Human coronavirus OC43 can also spread via neuron axonal transport machinery (Dubé et al., 2018). Thus, similar modes of spread like MHV and OC43 have been proposed for SARS-CoV-2 (Li et al., 2020b); however, this is speculative, and experimental evidence for SARS-CoV-2 is presently missing. One study reported direct nociceptor infection on dorsal root ganglia by SARS-CoV-2, which suggests potential PNS neurotropism (McFarland et al., 2021). SARS-CoV-2 attack of sensory dorsal root ganglia nociceptors implicates potential pathways for pain during severe COVID-19, and possibly into the PASC post-infection phase.
Overall, more studies are needed and will likely evolve over time as we increase our understanding of PNS disorders secondary to SARS-CoV-2.
6. Potential therapeutic avenues of SARS-CoV-2-mediated neurologic injury
To our knowledge, there is no specific treatment protocol for preventing neurological complications in COVID-19 patients. Some neurological conditions that develop from SARS-CoV-2 infection improve without specific treatment or can otherwise be treated per standard of care, e.g., corticosteroids or immunoglobulins for autoimmune encephalitis (Paterson et al., 2020). Overall, therapeutic experience remains limited. Fortunately, current understanding of SARS-CoV-2 pathomechanisms suggests potential therapeutic avenues. First, lowering systemic and neural inflammation through broad-acting anti-inflammatory corticosteroid therapy, for example, could mitigate brain vascular and neural damage. Dexamethasone is part of the present repertoire of therapies being used to treat COVID-19 patients. In an open-label trial, dexamethasone reduced 28-day mortality in hospitalized COVID-19 patients on respiratory support, e.g., invasive mechanical ventilation, oxygen administration, but not in those off of respiratory support (Horby et al., 2021). Whether dexamethasone specifically prevents neurological injury from SARS-CoV-2 remains to be seen. Other approaches to lowering systemic inflammation include immunomodulating antibodies (Izda et al., 2021), e.g., tocilizumab, sarilumab.
The second potential therapeutic target, that of SARS-CoV-2 invasion and/or neuroinvasion, may be mitigated by blocking viral replication and penetration into cells (Izda et al., 2021). Replication-inhibitors include antivirals, such as remdesivir. Preventing cellular entry can be achieved by binding to the virus spike protein or by blocking the ACE2-protease machinery, which grants the virus entry into cells. Casirivimab and imdevimab are monoclonal antibodies that bind to SARS-CoV-2 spike protein and obstruct ACE-2 binding (Izda et al., 2021). The combined casirivimab and imdevimab cocktail is effective in preventive and therapeutic paradigms and lowers the incidence and severity of COVID-19 infection (O'Brien et al., 2021; Weinreich et al., 2021). However, the efficacy of this casirivimab/imdevimab cocktail at preventing neurological complications has not been studied. Other approaches of preventing SARS-CoV-2 entry into cells rely on pharmacological inhibition of ACE2-protease machinery (Zhang et al., 2021a). Such approaches have only had mixed success thus far, as with hydroxychloroquine.
Preclinical and clinical research is ongoing, and the need is great for more definitive COVID-19 therapeutics, specifically those aimed at neural protection.
7. Conclusion
It is now clear that SARS-CoV-2 induces a host of acute neurological complications of varying clinical severity. In some instances, these neurologic sequelae evolve into long-term and irreversible complications. As the virus becomes endemic (Emanuel et al., 2022), this could have ramifications for many recovering from COVID-19 infection, although emerging variants my not trigger as much neurological damage as the original and delta variants. Fortunately, the putative mechanism of COVID-19 induced acute neurologic injury is becoming more apparent, thus opening the window for therapeutic interventions. These pathologic mechanisms include systemically and locally induced inflammatory responses and/or endothelial dysfunction, potential direct viral neuroinvasion, astrogliosis, and pro-inflammatory microglial activation, all leading to neuronal injury. Thus, anti-inflammatories and monoclonal antibodies may help prevent or minimize neurologic damage in the context of acute infection, although formal studies are needed. Moreover, a clearer mechanistic and molecular picture of neurological pathology in acute SARS-CoV-2 infection may lead to more effective therapies for mitigating neural damage in patients developing PASC (or long covid syndrome) post infection. For these patients, the landscape remains poorly defined, and therapies to date are primarily supportive. In summary, as COVID-19 infections continue to rise, the burden of SARS-CoV-2-induced acute neurologic disorders and PASC will also increase, necessitating a greater understanding of disease pathophysiology to enable the development of mechanism-based therapies targeting both the CNS and PNS.
Search terms
“COVID-19”, “SARS-CoV-2”, “astrocyte”, “autopsy”, “axon-glia”, “brain”, “cell”, “central nervous system”, “clinical”, “exosome”, “glia”, “microglia”, “neurotropism”, “oligodendrocyte”, “PASC”, “peripheral”, “peripheral nerve”, “peripheral nervous system”, “Schwann cell”, “syndromes”. Conducted initially during September and then again in December to update.
Declaration of Competing Interest
MGS, ELF, and AMS declare no conflicts of interest.
Acknowledgments
ELF acknowledges funding from the NIH NIDDK (U01DK119083), the Michigan Diabetes Research Center Elizabeth Weiser Caswell Diabetes Institute COVID-19 and Metabolic Disease Grant Program, the Robert E. Nederlander Sr. Program for Alzheimer's Research, the Andrea and Lawrence A. Wolfe Brain Health Initiative Fund, the Sinai Medical Staff Foundation, and the NeuroNetwork for Emerging Therapies. AMS acknowledges funding from Bristol Myers Squibb, the GBS-CIDP Foundation, NeuroNEXT, and the Foundation for Peripheral Neuropathy.
References
- Aladawi M., Elfil M., Abu-Esheh B., Abu Jazar D., Armouti A., Bayoumi A., Piccione E. Guillain Barre syndrome as a complication of COVID-19: a systematic review. Can J Neurol Sci. 2022;49(1):38–48. doi: 10.1017/cjn.2021.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barberis E., Vanella V.V., Falasca M., Caneapero V., Cappellano G., Raineri D., Ghirimoldi M., De Giorgis V., Puricelli C., Vaschetto R., Sainaghi P.P., Bruno S., Sica A., Dianzani U., Rolla R., Chiocchetti A., Cantaluppi V., Baldanzi G., Marengo E., Manfredi M. Circulating exosomes are strongly involved in SARS-CoV-2 infection. Front. Mol. Biosci. 2021;8 doi: 10.3389/fmolb.2021.632290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beyrouti R., Adams M.E., Benjamin L., Cohen H., Farmer S.F., Goh Y.Y., Humphries F., Jäger H.R., Losseff N.A., Perry R.J., Shah S., Simister R.J., Turner D., Chandratheva A., Werring D.J. Characteristics of ischaemic stroke associated with COVID-19. J. Neurol. Neurosurg. Psychiatry. 2020;91:889–891. doi: 10.1136/jnnp-2020-323586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boroujeni M.E., Simani L., Bluyssen H.A.R., Samadikhah H.R., Zamanlui Benisi S., Hassani S., Akbari Dilmaghani N., Fathi M., Vakili K., Mahmoudiasl G.R., Abbaszadeh H.A., Hassani Moghaddam M., Abdollahifar M.A., Aliaghaei A. Inflammatory response leads to neuronal death in human post-mortem cerebral cortex in patients with COVID-19. ACS Chem. Neurosci. 2021;12(12):2143–2150. doi: 10.1021/acschemneuro.1c00111. [DOI] [PubMed] [Google Scholar]
- Brann D.H., Tsukahara T., Weinreb C., Lipovsek M., Van den Berge K., Gong B., Chance R., Macaulay I.C., Chou H.J., Fletcher R.B., Das D., Street K., de Bezieux H.R., Choi Y.G., Risso D., Dudoit S., Purdom E., Mill J., Hachem R.A., Matsunami H., Logan D.W., Goldstein B.J., Grubb M.S., Ngai J., Datta S.R. Non-neuronal expression of SARS-CoV-2 entry genes in the olfactory system suggests mechanisms underlying COVID-19-associated anosmia. Sci. Adv. 2020;6(31) doi: 10.1126/sciadv.abc5801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cama V.F., Marín-Prida J., Acosta-Rivero N., Acosta E.F., Díaz L.O., Casadesús A.V., Fernández-Marrero B., Gilva-Rodríguez N., Cremata-García D., Cervantes-Llanos M., Piniella-Matamoros B., Sánchez D., Del Rosario-Cruz L., Borrajero I., Díaz A., González Y., Pentón-Arias E., Montero-González T., Guillen-Nieto G., Pentón-Rol G. The microglial NLRP3 inflammasome is involved in human SARS-CoV-2 cerebral pathogenicity: a report of three post-mortem cases. J. Neuroimmunol. 2021;361 doi: 10.1016/j.jneuroim.2021.577728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantuti-Castelvetri L., Ojha R., Pedro L.D., Djannatian M., Franz J., Kuivanen S., van der Meer F., Kallio K., Kaya T., Anastasina M., Smura T., Levanov L., Szirovicza L., Tobi A., Kallio-Kokko H., Österlund P., Joensuu M., Meunier F.A., Butcher S.J., Winkler M.S., Mollenhauer B., Helenius A., Gokce O., Teesalu T., Hepojoki J., Vapalahti O., Stadelmann C., Balistreri G., Simons M. Neuropilin-1 facilitates SARS-CoV-2 cell entry and infectivity. Science. 2020;370(6518):856–860. doi: 10.1126/science.abd2985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherry J.D., Olschowka J.A., O’Banion M.K. Neuroinflammation and M2 microglia: the good, the bad, and the inflamed. J. Neuroinflammation. 2014;11:98. doi: 10.1186/1742-2094-11-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Civardi C., Collini A., Geda D.J., Geda C. Antiganglioside antibodies in Guillain-Barré syndrome associated with SARS-CoV-2 infection. J Neurol Neurosurg Psychiatry. England. 2020;91:1361–1362. doi: 10.1136/jnnp-2020-324279. [DOI] [PubMed] [Google Scholar]
- Clough E., Chean K.T., Inigo J., Tubbesing K.E., Chandra D., Chaves L., Reynolds J.L., Aalinkeel R., Schwartz S.A., Khmaladze A., Mahajan S.D. Mitochondrial dynamics in SARS-COV2 spike protein treated human microglia: implications for neuro-COVID. J. NeuroImmune Pharmacol. 2021:1–15. doi: 10.1007/s11481-021-10015-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coolen T., Lolli V., Sadeghi N., Rovai A., Trotta N., Taccone F.S., Creteur J., Henrard S., Goffard J.C., Dewitte O., Naeije G., Goldman S., De Tiège X. Early postmortem brain MRI findings in COVID-19 non-survivors. Neurology. 2020;95(14):e2016–e2027. doi: 10.1212/WNL.0000000000010116. [DOI] [PubMed] [Google Scholar]
- Cotto B., Natarajaseenivasan K., Langford D. Astrocyte activation and altered metabolism in normal aging, age-related CNS diseases, and HAND. J. Neuro-Oncol. 2019;25(5):722–733. doi: 10.1007/s13365-019-00721-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies J., Randeva H.S., Chatha K., Hall M., Spandidos D.A., Karteris E., Kyrou I. Neuropilin-1 as a new potential SARS-CoV-2 infection mediator implicated in the neurologic features and central nervous system involvement of COVID-19. Mol. Med. Rep. 2020;22(5):4221–4226. doi: 10.3892/mmr.2020.11510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Melo I.S., Sabino-Silva R., Cunha T.M., Goulart L.R., Reis W.L., Jardim A.C.G., Shetty A.K., de Castro O.W. Hydroelectrolytic disorder in COVID-19 patients: evidence supporting the involvement of Subfornical organ and paraventricular nucleus of the hypothalamus. Neurosci. Biobehav. Rev. 2021;124:216–223. doi: 10.1016/j.neubiorev.2021.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding Z.B., Song L.J., Wang Q., Kumar G., Yan Y.Q., Ma C.G. Astrocytes: a double-edged sword in neurodegenerative diseases. Neural Regen. Res. 2021;16(9):1702–1710. doi: 10.4103/1673-5374.306064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon L., Varley J., Gontsarova A., Mallon D., Tona F., Muir D., Luqmani A., Jenkins I.H., Nicholas R., Jones B., Everitt A. COVID-19-related acute necrotizing encephalopathy with brain stem involvement in a patient with aplastic anemia. Neurol Neuroimmunol Neuroinflamm. 2020;7(5) doi: 10.1212/NXI.0000000000000789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubé M., Le Coupanec A., Wong A.H.M., Rini J.M., Desforges M., Talbot P.J. Axonal transport enables neuron-to-neuron propagation of human coronavirus OC43. J. Virol. 2018;92(17) doi: 10.1128/JVI.00404-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dufour C., Co T.K., Liu A. GM1 ganglioside antibody and COVID-19 related Guillain Barre syndrome - a case report, systemic review and implication for vaccine development. Brain Behav Immun Health, © 2021 The Author(s). 2021;12 doi: 10.1016/j.bbih.2021.100203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellul M.A., Benjamin L., Singh B., Lant S., Michael B.D., Easton A., Kneen R., Defres S., Sejvar J., Solomon T. Neurological associations of COVID-19. Lancet Neurol. 2020;19(9):767–783. doi: 10.1016/S1474-4422(20)30221-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emanuel E.J., Osterholm M., Gounder C.R. A National Strategy for the “new Normal” of life with COVID. Jama. 2022;327(3):211–212. doi: 10.1001/jama.2021.24282. [DOI] [PubMed] [Google Scholar]
- Feldman E.L., Savelieff M.G., Hayek S.S., Pennathur S., Kretzler M., Pop-Busui R. COVID-19 and diabetes: a collision and collusion of two diseases. Diabetes. 2020;69(12):2549–2565. doi: 10.2337/dbi20-0032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenrich M., Mrdenovic S., Balog M., Tomic S., Zjalic M., Roncevic A., Mandic D., Debeljak Z., Heffer M. SARS-CoV-2 dissemination through peripheral nerves explains multiple organ injury. Front. Cell. Neurosci. 2020;14:229. doi: 10.3389/fncel.2020.00229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foschi M., D'Anna L., Abdelhak A., Mayer B., Tumani H., Otto M., Abu-Rumeileh S. Ongoing challenges in unravelling the association between COVID-19 and Guillain-Barré syndrome. Brain. 2021;144 doi: 10.1093/brain/awab069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fullard J.F., Lee H.C., Voloudakis G., Suo S., Javidfar B., Shao Z., Peter C., Zhang W., Jiang S., Corvelo A., Wargnier H., Woodoff-Leith E., Purohit D.P., Ahuja S., Tsankova N.M., Jette N., Hoffman G.E., Akbarian S., Fowkes M., Crary J.F., Yuan G.C., Roussos P. Single-nucleus transcriptome analysis of human brain immune response in patients with severe COVID-19. Genome Med. 2021;13(1):118. doi: 10.1186/s13073-021-00933-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein D.S. The possible association between COVID-19 and postural tachycardia syndrome. Heart Rhythm. 2021;18(4):508–509. doi: 10.1016/j.hrthm.2020.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutiérrez-Ortiz C., Méndez-Guerrero A., Rodrigo-Rey S., San Pedro-Murillo E., Bermejo-Guerrero L., Gordo-Mañas R., de Aragón-Gómez F., Benito-León J. Miller fisher syndrome and polyneuritis cranialis in COVID-19. Neurology. 2020;95(5):e601–e605. doi: 10.1212/WNL.0000000000009619. [DOI] [PubMed] [Google Scholar]
- Hatton C.F., Duncan C.J.A. Microglia are essential to protective antiviral immunity: lessons from mouse models of viral encephalitis. Front. Immunol. 2019;10:2656. doi: 10.3389/fimmu.2019.02656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernández V.S., Zetter M.A., Guerra E.C., Hernández-Araiza I., Karuzin N., Hernández-Pérez O.R., Eiden L.E., Zhang L. ACE2 expression in rat brain: implications for COVID-19 associated neurological manifestations. Exp. Neurol. 2021;345 doi: 10.1016/j.expneurol.2021.113837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann M., Kleine-Weber H., Schroeder S., Krüger N., Herrler T., Erichsen S., Schiergens T.S., Herrler G., Wu N.H., Nitsche A., Müller M.A., Drosten C., Pöhlmann S. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181(2):271–280.e278. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horby P., Lim W.S., Emberson J.R., Mafham M., Bell J.L., Linsell L., Staplin N., Brightling C., Ustianowski A., Elmahi E., Prudon B., Green C., Felton T., Chadwick D., Rege K., Fegan C., Chappell L.C., Faust S.N., Jaki T., Jeffery K., Montgomery A., Rowan K., Juszczak E., Baillie J.K., Haynes R., Landray M.J. Dexamethasone in hospitalized patients with Covid-19. N. Engl. J. Med. 2021;384(8):693–704. doi: 10.1056/NEJMoa2021436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- https://coronavirus.jhu.edu/map.html
- Izda V., Jeffries M.A., Sawalha A.H. COVID-19: a review of therapeutic strategies and vaccine candidates. Clin. Immunol. 2021;222 doi: 10.1016/j.clim.2020.108634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob F., Pather S.R., Huang W.K., Zhang F., Wong S.Z.H., Zhou H., Cubitt B., Fan W., Chen C.Z., Xu M., Pradhan M., Zhang D.Y., Zheng W., Bang A.G., Song H., Carlos de la Torre J., Ming G.L. Human pluripotent stem cell-derived neural cells and brain organoids reveal SARS-CoV-2 Neurotropism predominates in choroid plexus epithelium. Cell Stem Cell. 2020;27(6):937–950.e939. doi: 10.1016/j.stem.2020.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanberg N., Simrén J., Edén A., Andersson L.M., Nilsson S., Ashton N.J., Sundvall P.D., Nellgård B., Blennow K., Zetterberg H., Gisslén M. Neurochemical signs of astrocytic and neuronal injury in acute COVID-19 normalizes during long-term follow-up. EBioMedicine. 2021;70 doi: 10.1016/j.ebiom.2021.103512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keddie S., Pakpoor J., Mousele C., Pipis M., Machado P.M., Foster M., Record C.J., Keh R.Y.S., Fehmi J., Paterson R.W., Bharambe V., Clayton L.M., Allen C., Price O., Wall J., Kiss-Csenki A., Rathnasabapathi D.P., Geraldes R., Yermakova T., King-Robson J., Zosmer M., Rajakulendran S., Sumaria S., Farmer S.F., Nortley R., Marshall C.R., Newman E.J., Nirmalananthan N., Kumar G., Pinto A.A., Holt J., Lavin T.M., Brennan K.M., Zandi M.S., Jayaseelan D.L., Pritchard J., Hadden R.D.M., Manji H., Willison H.J., Rinaldi S., Carr A.S., Lunn M.P. Epidemiological and cohort study finds no association between COVID-19 and Guillain-Barré syndrome. Brain. 2021;144(2):682–693. doi: 10.1093/brain/awaa433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan M., Yoo S.J., Clijsters M., Backaert W., Vanstapel A., Speleman K., Lietaer C., Choi S., Hether T.D., Marcelis L., Nam A., Pan L., Reeves J.W., Van Bulck P., Zhou H., Bourgeois M., Debaveye Y., De Munter P., Gunst J., Jorissen M., Lagrou K., Lorent N., Neyrinck A., Peetermans M., Thal D.R., Vandenbriele C., Wauters J., Mombaerts P., Van Gerven L. Visualizing in deceased COVID-19 patients how SARS-CoV-2 attacks the respiratory and olfactory mucosae but spares the olfactory bulb. Cell. 2021;184(24):5932–5949.e5915. doi: 10.1016/j.cell.2021.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo C.L., Pilling L.C., Atkins J.L., Masoli J.A.H., Delgado J., Kuchel G.A., Melzer D. APOE e4 genotype predicts severe COVID-19 in the UK biobank community cohort. J. Gerontol. A Biol. Sci. Med. Sci. 2020;75(11):2231–2232. doi: 10.1093/gerona/glaa131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M.Y., Li L., Zhang Y., Wang X.S. Expression of the SARS-CoV-2 cell receptor gene ACE2 in a wide variety of human tissues. Infect Dis Poverty. 2020;9(1):45. doi: 10.1186/s40249-020-00662-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z., Liu T., Yang N., Han D., Mi X., Li Y., Liu K., Vuylsteke A., Xiang H., Guo X. Neurological manifestations of patients with COVID-19: potential routes of SARS-CoV-2 neuroinvasion from the periphery to the brain. Front Med. 2020;14(5):533–541. doi: 10.1007/s11684-020-0786-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linnerbauer M., Rothhammer V. Protective functions of reactive astrocytes following central nervous system insult. Front. Immunol. 2020;11 doi: 10.3389/fimmu.2020.573256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luijten L.W.G., Leonhard S.E., van der Eijk A.A., Doets A.Y., Appeltshauser L., Arends S., Attarian S., Benedetti L., Briani C., Casasnovas C., Castellani F., Dardiotis E., Echaniz-Laguna A., Garssen M.P.J., Harbo T., Huizinga R., Humm A.M., Jellema K., van der Kooi A.J., Kuitwaard K., Kuntzer T., Kusunoki S., Lascano A.M., Martinez-Hernandez E., Rinaldi S., Samijn J.P.A., Scheidegger O., Tsouni P., Vicino A., Visser L.H., Walgaard C., Wang Y., Wirtz P.W., Ripellino P., Jacobs B.C. Guillain-Barré syndrome after SARS-CoV-2 infection in an international prospective cohort study. Brain. 2021;144(11):3392–3404. doi: 10.1093/brain/awab279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao L., Jin H., Wang M., Hu Y., Chen S., He Q., Chang J., Hong C., Zhou Y., Wang D., Miao X., Li Y., Hu B. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol. 2020;77(6):683–690. doi: 10.1001/jamaneurol.2020.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matschke J., Lütgehetmann M., Hagel C., Sperhake J.P., Schröder A.S., Edler C., Mushumba H., Fitzek A., Allweiss L., Dandri M., Dottermusch M., Heinemann A., Pfefferle S., Schwabenland M., Sumner Magruder D., Bonn S., Prinz M., Gerloff C., Püschel K., Krasemann S., Aepfelbacher M., Glatzel M. Neuropathology of patients with COVID-19 in Germany: a post-mortem case series. Lancet Neurol. 2020;19(11):919–929. doi: 10.1016/S1474-4422(20)30308-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFarland A.J., Yousuf M.S., Shiers S., Price T.J. Neurobiology of SARS-CoV-2 interactions with the peripheral nervous system: implications for COVID-19 and pain. Pain Rep. 2021;6(1) doi: 10.1097/PR9.0000000000000885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahon C.L., Staples H., Gazi M., Carrion R., Hsieh J. SARS-CoV-2 targets glial cells in human cortical organoids. Stem Cell Reports. 2021;16(5):1156–1164. doi: 10.1016/j.stemcr.2021.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta P., McAuley D.F., Brown M., Sanchez E., Tattersall R.S., Manson J.J. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395(10229):1033–1034. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michetti F., D’Ambrosi N., Toesca A., Puglisi M.A., Serrano A., Marchese E., Corvino V., Geloso M.C. The S100B story: from biomarker to active factor in neural injury. J. Neurochem. 2019;148(2):168–187. doi: 10.1111/jnc.14574. [DOI] [PubMed] [Google Scholar]
- Mishra R., Banerjea A.C. SARS-CoV-2 spike targets USP33-IRF9 Axis via Exosomal miR-148a to activate human microglia. Front. Immunol. 2021;12 doi: 10.3389/fimmu.2021.656700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukerji S.S., Solomon I.H. What can we learn from brain autopsies in COVID-19? Neurosci. Lett. 2021;742 doi: 10.1016/j.neulet.2020.135528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng J.H., Sun A., Je H.S., Tan E.K. Unravelling pathophysiology of neurological and psychiatric complications of COVID-19 using brain organoids. Neuroscientist. 2021;10738584211015136 doi: 10.1177/10738584211015136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuovo G.J., Magro C., Shaffer T., Awad H., Suster D., Mikhail S., He B., Michaille J.J., Liechty B., Tili E. Endothelial cell damage is the central part of COVID-19 and a mouse model induced by injection of the S1 subunit of the spike protein. Ann. Diagn. Pathol. 2021;51 doi: 10.1016/j.anndiagpath.2020.151682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Brien M.P., Forleo-Neto E., Musser B.J., Isa F., Chan K.C., Sarkar N., Bar K.J., Barnabas R.V., Barouch D.H., Cohen M.S., Hurt C.B., Burwen D.R., Marovich M.A., Hou P., Heirman I., Davis J.D., Turner K.C., Ramesh D., Mahmood A., Hooper A.T., Hamilton J.D., Kim Y., Purcell L.A., Baum A., Kyratsous C.A., Krainson J., Perez-Perez R., Mohseni R., Kowal B., DiCioccio A.T., Stahl N., Lipsich L., Braunstein N., Herman G., Yancopoulos G.D., Weinreich D.M. Subcutaneous REGEN-COV antibody combination to prevent Covid-19. N. Engl. J. Med. 2021;385(13):1184–1195. doi: 10.1056/NEJMoa2109682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olajide O.A., Iwuanyanwu V.U., Adegbola O.D., Al-Hindawi A.A. SARS-CoV-2 Spike Glycoprotein S1 Induces Neuroinflammation in BV-2 Microglia. Mol Neurobiol. 2021:1–14. doi: 10.1007/s12035-021-02593-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oxley T.J., Mocco J., Majidi S., Kellner C.P., Shoirah H., Singh I.P., De Leacy R.A., Shigematsu T., Ladner T.R., Yaeger K.A., Skliut M., Weinberger J., Dangayach N.S., Bederson J.B., Tuhrim S., Fifi J.T. Large-vessel stroke as a presenting feature of Covid-19 in the young. N. Engl. J. Med. 2020;382 doi: 10.1056/NEJMc2009787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padroni M., Mastrangelo V., Asioli G.M., Pavolucci L., Abu-Rumeileh S., Piscaglia M.G., Querzani P., Callegarini C., Foschi M. Guillain-Barré syndrome following COVID-19: new infection, old complication? J. Neurol. 2020;267:1877–1879. doi: 10.1007/s00415-020-09849-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan R., Zhang Q., Anthony S.M., Zhou Y., Zou X., Cassell M., Perlman S. Oligodendrocytes that survive acute coronavirus infection induce prolonged inflammatory responses in the CNS. Proc. Natl. Acad. Sci. U. S. A. 2020;117(27):15902–15910. doi: 10.1073/pnas.2003432117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paterson R.W., Brown R.L., Benjamin L., Nortley R., Wiethoff S., Bharucha T., Jayaseelan D.L., Kumar G., Raftopoulos R.E., Zambreanu L., Vivekanandam V., Khoo A., Geraldes R., Chinthapalli K., Boyd E., Tuzlali H., Price G., Christofi G., Morrow J., McNamara P., McLoughlin B., Lim S.T., Mehta P.R., Levee V., Keddie S., Yong W., Trip S.A., Foulkes A.J.M., Hotton G., Miller T.D., Everitt A.D., Carswell C., Davies N.W.S., Yoong M., Attwell D., Sreedharan J., Silber E., Schott J.M., Chandratheva A., Perry R.J., Simister R., Checkley A., Longley N., Farmer S.F., Carletti F., Houlihan C., Thom M., Lunn M.P., Spillane J., Howard R., Vincent A., Werring D.J., Hoskote C., Jäger H.R., Manji H., Zandi M.S. The emerging spectrum of COVID-19 neurology: clinical, radiological and laboratory findings. Brain. 2020;143(10):3104–3120. doi: 10.1093/brain/awaa240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paterson R.W., Benjamin L.A., Mehta P.R., Brown R.L., Athauda D., Ashton N.J., Leckey C.A., Ziff O.J., Heaney J., Heslegrave A.J., Benedet A.L., Blennow K., Checkley A.M., Houlihan C.F., Mummery C.J., Lunn M.P., Manji H., Zandi M.S., Keddie S., Chou M., Vinayan Changaradil D., Solomon T., Keshavan A., Barker S., Jäger H.R., Carletti F., Simister R., Werring D.J., Spyer M.J., Nastouli E., Gauthier S., Rosa-Neto P., Zetterberg H., Schott J.M. Serum and cerebrospinal fluid biomarker profiles in acute SARS-CoV-2-associated neurological syndromes. Brain Commun. 2021;3(3):fcab099. doi: 10.1093/braincomms/fcab099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellegrini L., Albecka A., Mallery D.L., Kellner M.J., Paul D., Carter A.P., James L.C., Lancaster M.A. SARS-CoV-2 infects the brain choroid plexus and disrupts the blood-CSF barrier in human brain organoids. Cell Stem Cell. 2020;27(6):951–961.e955. doi: 10.1016/j.stem.2020.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrin P., Collongues N., Baloglu S., Bedo D., Bassand X., Lavaux T., Gautier-Vargas G., Keller N., Kremer S., Fafi-Kremer S., Moulin B., Benotmane I., Caillard S. Cytokine release syndrome-associated encephalopathy in patients with COVID-19. Eur. J. Neurol. 2021;28(1):248–258. doi: 10.1111/ene.14491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters J., Alhasan S., Vogels C.B.F., Grubaugh N.D., Farhadian S., Longbrake E.E. MOG-associated encephalitis following SARS-COV-2 infection. Mult Scler Relat Disord. 2021;50 doi: 10.1016/j.msard.2021.102857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poyiadji N., Shahin G., Noujaim D., Stone M., Patel S., Griffith B. COVID-19-associated acute hemorrhagic necrotizing encephalopathy: imaging features. Radiology. 2020;296(2):E119–e120. doi: 10.1148/radiol.2020201187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prinz M., Jung S., Priller J. Microglia biology: one century of evolving concepts. Cell. 2019;179(2):292–311. doi: 10.1016/j.cell.2019.08.053. [DOI] [PubMed] [Google Scholar]
- Puelles V.G., Lütgehetmann M., Lindenmeyer M.T., Sperhake J.P., Wong M.N., Allweiss L., Chilla S., Heinemann A., Wanner N., Liu S., Braun F., Lu S., Pfefferle S., Schröder A.S., Edler C., Gross O., Glatzel M., Wichmann D., Wiech T., Kluge S., Pueschel K., Aepfelbacher M., Huber T.B. Multiorgan and renal tropism of SARS-CoV-2. N. Engl. J. Med. 2020;383:590–592. doi: 10.1056/NEJMc2011400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramani A., Müller L., Ostermann P.N., Gabriel E., Abida-Islam P., Müller-Schiffmann A., Mariappan A., Goureau O., Gruell H., Walker A., Andrée M., Hauka S., Houwaart T., Dilthey A., Wohlgemuth K., Omran H., Klein F., Wieczorek D., Adams O., Timm J., Korth C., Schaal H., Gopalakrishnan J. SARS-CoV-2 targets neurons of 3D human brain organoids. EMBO J. 2020;39(20) doi: 10.15252/embj.2020106230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichard R.R., Kashani K.B., Boire N.A., Constantopoulos E., Guo Y., Lucchinetti C.F. Neuropathology of COVID-19: a spectrum of vascular and acute disseminated encephalomyelitis (ADEM)-like pathology. Acta Neuropathol. 2020;140(1):1–6. doi: 10.1007/s00401-020-02166-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawalha K., Adeodokun S., Kamoga G.R. COVID-19-induced acute bilateral optic neuritis. J Investig Med High Impact Case Rep. 2020;8 doi: 10.1177/2324709620976018. (2324709620976018) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schurink B., Roos E., Radonic T., Barbe E., Bouman C.S.C., de Boer H.H., de Bree G.J., Bulle E.B., Aronica E.M., Florquin S., Fronczek J., Heunks L.M.A., de Jong M.D., Guo L., du Long R., Lutter R., Molenaar P.C.G., Neefjes-Borst E.A., Niessen H.W.M., van Noesel C.J.M., Roelofs J., Snijder E.J., Soer E.C., Verheij J., Vlaar A.P.J., Vos W., van der Wel N.N., van der Wal A.C., van der Valk P., Bugiani M. Viral presence and immunopathology in patients with lethal COVID-19: a prospective autopsy cohort study. Lancet Microbe. 2020;1(7):e290–e299. doi: 10.1016/S2666-5247(20)30144-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwabenland M., Salié H., Tanevski J., Killmer S., Lago M.S., Schlaak A.E., Mayer L., Matschke J., Püschel K., Fitzek A., Ondruschka B., Mei H.E., Boettler T., Neumann-Haefelin C., Hofmann M., Breithaupt A., Genc N., Stadelmann C., Saez-Rodriguez J., Bronsert P., Knobeloch K.P., Blank T., Thimme R., Glatzel M., Prinz M., Bengsch B. Deep spatial profiling of human COVID-19 brains reveals neuroinflammation with distinct microanatomical microglia-T-cell interactions. Immunity. 2021;54(7):1594–1610.e1511. doi: 10.1016/j.immuni.2021.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sedaghat Z., Karimi N. Guillain Barre syndrome associated with COVID-19 infection: a case report. J. Clin. Neurosci. 2020;76:233–235. doi: 10.1016/j.jocn.2020.04.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sher A.A., Glover K.K.M., Coombs K.M. Zika virus infection disrupts astrocytic proteins involved in synapse control and axon guidance. Front. Microbiol. 2019;10:596. doi: 10.3389/fmicb.2019.00596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shindler K.S., Chatterjee D., Biswas K., Goyal A., Dutt M., Nassrallah M., Khan R.S., Das Sarma J. Macrophage-mediated optic neuritis induced by retrograde axonal transport of spike gene recombinant mouse hepatitis virus. J. Neuropathol. Exp. Neurol. 2011;70(6):470–480. doi: 10.1097/NEN.0b013e31821da499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shouman K., Vanichkachorn G., Cheshire W.P., Suarez M.D., Shelly S., Lamotte G.J., Sandroni P., Benarroch E.E., Berini S.E., Cutsforth-Gregory J.K., Coon E.A., Mauermann M.L., Low P.A., Singer W. Autonomic dysfunction following COVID-19 infection: an early experience. Clin. Auton. Res. 2021;31(3):385–394. doi: 10.1007/s10286-021-00803-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha R., Wander A., Kapoor A., Yadav R., Kumar A., Gulati S. Acute demyelinating syndrome (MOG antibody positive) associated with COVID-19 infection: a widening spectrum. Clin. Pediatr. (Phila) 2021;60(13):501–503. doi: 10.1177/00099228211037210. [DOI] [PubMed] [Google Scholar]
- Solomon T. Neurological infection with SARS-CoV-2 - the story so far. Nat. Rev. Neurol. 2021;17(2):65–66. doi: 10.1038/s41582-020-00453-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon I.H., Normandin E., Bhattacharyya S., Mukerji S.S., Keller K., Ali A.S., Adams G., Hornick J.L., Padera R.F., Jr., Sabeti P. Neuropathological features of Covid-19. N. Engl. J. Med. 2020;383:989–992. doi: 10.1056/NEJMc2019373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song E., Zhang C., Israelow B., Lu-Culligan A., Prado A.V., Skriabine S., Lu P., Weizman O.E., Liu F., Dai Y., Szigeti-Buck K., Yasumoto Y., Wang G., Castaldi C., Heltke J., Ng E., Wheeler J., Alfajaro M.M., Levavasseur E., Fontes B., Ravindra N.G., Van Dijk D., Mane S., Gunel M., Ring A., Kazmi S.A.J., Zhang K., Wilen C.B., Horvath T.L., Plu I., Haik S., Thomas J.L., Louvi A., Farhadian S.F., Huttner A., Seilhean D., Renier N., Bilguvar K., Iwasaki A. Neuroinvasion of SARS-CoV-2 in human and mouse brain. J. Exp. Med. 2021;218(3) doi: 10.1084/jem.20202135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suh J., Mukerji S.S., Collens S.I., Padera R.F., Jr., Pinkus G.S., Amato A.A., Solomon I.H. Skeletal muscle and peripheral nerve histopathology in COVID-19. Neurology. 2021;97(8):e849–e858. doi: 10.1212/WNL.0000000000012344. [DOI] [PubMed] [Google Scholar]
- Thakur K.T., Miller E.H., Glendinning M.D., Al-Dalahmah O., Banu M.A., Boehme A.K., Boubour A.L., Bruce S.S., Chong A.M., Claassen J., Faust P.L., Hargus G., Hickman R.A., Jambawalikar S., Khandji A.G., Kim C.Y., Klein R.S., Lignelli-Dipple A., Lin C.C., Liu Y., Miller M.L., Moonis G., Nordvig A.S., Overdevest J.B., Prust M.L., Przedborski S., Roth W.H., Soung A., Tanji K., Teich A.F., Agalliu D., Uhlemann A.C., Goldman J.E., Canoll P. COVID-19 neuropathology at Columbia University Irving medical center/New York Presbyterian hospital. Brain. 2021;144(9):2696–2708. doi: 10.1093/brain/awab148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thepmankorn P., Bach J., Lasfar A., Zhao X., Souayah S., Chong Z.Z., Souayah N. Cytokine storm induced by SARS-CoV-2 infection: the spectrum of its neurological manifestations. Cytokine. 2021;138 doi: 10.1016/j.cyto.2020.155404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiet M.Y., AlShaikh N. Guillain-Barré syndrome associated with COVID-19 infection: a case from the UK. BMJ Case Rep. 2020;13(7) doi: 10.1136/bcr-2020-236536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremblay M.E., Madore C., Bordeleau M., Tian L., Verkhratsky A. Neuropathobiology of COVID-19: the role for glia. Front. Cell. Neurosci. 2020;14 doi: 10.3389/fncel.2020.592214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umapathi T., Er B., Koh J.S., Goh Y.H., Chua L. Guillain-Barré syndrome decreases in Singapore during the COVID-19 pandemic. J. Peripher. Nerv. Syst. 2021;26:235–236. doi: 10.1111/jns.12439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varga Z., Flammer A.J., Steiger P., Haberecker M., Andermatt R., Zinkernagel A.S., Mehra M.R., Schuepbach R.A., Ruschitzka F., Moch H. Endothelial cell infection and endotheliitis in COVID-19. Lancet. 2020;395(10234):1417–1418. doi: 10.1016/S0140-6736(20)30937-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Weyhern C.H., Kaufmann I., Neff F., Kremer M. Early evidence of pronounced brain involvement in fatal COVID-19 outcomes. Lancet. 2020;395(10241) doi: 10.1016/S0140-6736(20)31282-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C., Zhang M., Garcia G., Jr., Tian E., Cui Q., Chen X., Sun G., Wang J., Arumugaswami V., Shi Y. ApoE-isoform-dependent SARS-CoV-2 Neurotropism and cellular response. Cell Stem Cell. 2021;28(2):331–342.e335. doi: 10.1016/j.stem.2020.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinreich D.M., Sivapalasingam S., Norton T., Ali S., Gao H., Bhore R., Musser B.J., Soo Y., Rofail D., Im J., Perry C., Pan C., Hosain R., Mahmood A., Davis J.D., Turner K.C., Hooper A.T., Hamilton J.D., Baum A., Kyratsous C.A., Kim Y., Cook A., Kampman W., Kohli A., Sachdeva Y., Graber X., Kowal B., DiCioccio T., Stahl N., Lipsich L., Braunstein N., Herman G., Yancopoulos G.D. REGN-COV2, a neutralizing antibody cocktail, in outpatients with Covid-19. N. Engl. J. Med. 2021;384(3):238–251. doi: 10.1056/NEJMoa2035002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenzel J., Lampe J., Müller-Fielitz H., Schuster R., Zille M., Müller K., Krohn M., Körbelin J., Zhang L., Özorhan Ü., Neve V., Wagner J.U.G., Bojkova D., Shumliakivska M., Jiang Y., Fähnrich A., Ott F., Sencio V., Robil C., Pfefferle S., Sauve F., Coêlho C.F.F., Franz J., Spiecker F., Lembrich B., Binder S., Feller N., König P., Busch H., Collin L., Villaseñor R., Jöhren O., Altmeppen H.C., Pasparakis M., Dimmeler S., Cinatl J., Püschel K., Zelic M., Ofengeim D., Stadelmann C., Trottein F., Nogueiras R., Hilgenfeld R., Glatzel M., Prevot V., Schwaninger M. The SARS-CoV-2 main protease M(pro) causes microvascular brain pathology by cleaving NEMO in brain endothelial cells. Nat. Neurosci. 2021;24(11):1522–1533. doi: 10.1038/s41593-021-00926-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodhall M., Mitchell J.W., Gibbons E., Healy S., Waters P., Huda S. Case report: myelin oligodendrocyte glycoprotein antibody-associated relapse with COVID-19. Front. Neurol. 2020;11 doi: 10.3389/fneur.2020.598531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan R., Zhang Y., Li Y., Xia L., Guo Y., Zhou Q. Structural basis for the recognition of SARS-CoV-2 by full-length human ACE2. Science. 2020;367(6485):1444–1448. doi: 10.1126/science.abb2762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang A.C., Kern F., Losada P.M., Agam M.R., Maat C.A., Schmartz G.P., Fehlmann T., Stein J.A., Schaum N., Lee D.P., Calcuttawala K., Vest R.T., Berdnik D., Lu N., Hahn O., Gate D., McNerney M.W., Channappa D., Cobos I., Ludwig N., Schulz-Schaeffer W.J., Keller A., Wyss-Coray T. Dysregulation of brain and choroid plexus cell types in severe COVID-19. Nature. 2021;595(7868):565–571. doi: 10.1038/s41586-021-03710-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Xiao M., Zhang S., Xia P., Cao W., Jiang W., Chen H., Ding X., Zhao H., Zhang H., Wang C., Zhao J., Sun X., Tian R., Wu W., Wu D., Ma J., Chen Y., Zhang D., Xie J., Yan X., Zhou X., Liu Z., Wang J., Du B., Qin Y., Gao P., Qin X., Xu Y., Zhang W., Li T., Zhang F., Zhao Y., Li Y. Coagulopathy and antiphospholipid antibodies in patients with Covid-19. N. Engl. J. Med. 2020;382 doi: 10.1056/NEJMc2007575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q., Xiang R., Huo S., Zhou Y., Jiang S., Wang Q., Yu F. Molecular mechanism of interaction between SARS-CoV-2 and host cells and interventional therapy. Signal Transduct Target Ther. 2021;6(1):233. doi: 10.1038/s41392-021-00653-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L., Zhou L., Bao L., Liu J., Zhu H., Lv Q., Liu R., Chen W., Tong W., Wei Q., Xu Y., Deng W., Gao H., Xue J., Song Z., Yu P., Han Y., Zhang Y., Sun X., Yu X., Qin C. SARS-CoV-2 crosses the blood-brain barrier accompanied with basement membrane disruption without tight junctions alteration. Signal Transduct Target Ther. 2021;6(1):337. doi: 10.1038/s41392-021-00719-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao C., Zhao W. NLRP3 Inflammasome-a key player in antiviral responses. Front. Immunol. 2020;11:211. doi: 10.3389/fimmu.2020.00211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zubair A.S., McAlpine L.S., Gardin T., Farhadian S., Kuruvilla D.E., Spudich S. Neuropathogenesis and neurologic manifestations of the coronaviruses in the age of coronavirus disease 2019: a review. JAMA Neurol. 2020;77(8):1018–1027. doi: 10.1001/jamaneurol.2020.2065. [DOI] [PMC free article] [PubMed] [Google Scholar]