Abstract
Dengue has become a worldwide affliction despite incessant efforts to search for a cure for this long-lived disease. Optimistic consequences for dengue vaccine are implausible as the efficiency is tied to previous dengue virus (DENV) exposure and a very high cost is required for large-scale production of vaccine. Medicinal plants are idyllic substitutes to fight DENV infection since they constitute important components of traditional medicine and show antiviral properties, although the mechanism behind the action of bioactive compounds to obstruct viral replication is less explored and yet to be discovered. This review includes the existing traditional knowledge on how DENV infects and multiplies in the host cells, conscripting different medicinal plants that obtained bioactive compounds with anti-dengue properties, and the probable mechanism on how bioactive compounds modulate the host immune system during DENV infection. Moreover, different plant species having such bioactive compounds reported for anti-DENV efficiency should be validated scientifically via different in vitro and in vivo studies.
Keywords: dengue, traditional medicine, plant medicaments, bioactive compound, immunomodulatory response
1 Introduction
Mosquito-borne diseases cause illness in approximately 700 million people per year globally (Genoud et al., 2018). Dengue is one of the mosquito-borne emerging viral disease endemics prominent in many urban areas of tropical countries. A considerable rise in the dengue infection incidences is noticed all over the world in recent decades. Due to its asymptomatic to mild infectious nature, actual numbers of dengue cases remain unregistered or wrongly diagnosed as other fever infections (Waggoner et al., 2016). According to the World Health Organization report, about 390 million cases of dengue virus (DENV) infections occur annually, of which nearly 96 million find medical treatment and about 20,000 individuals (mostly children and aged individuals) fail to survive the dengue infection (Bhatt et al., 2013; Saxena et al., 2017; World Health Organization 2021a). Remarkably, among 129 countries having a risk of dengue infection, Asia has almost 70% of the total infection load (Brady et al., 2012; Bhatt et al., 2013). Dengue, an arthropod-carried viral infection, is transmitted into humans by female Aedes mosquitoes (Ae. aegypti or, to a lesser extent, by Ae. albopictus and Ae. polynesiensis) (Cao-Lormeau 2009). Common symptoms in dengue infection are mild dengue fever to severe dengue hemorrhagic fever (i.e., DF and DHF), rashes, headache, vomiting, severe headache, low white blood cell count, joint and muscle pain, swollen glands, nausea, pain behind the eyes, and fever with dengue shock syndrome (DSS) (Murphy and Whitehead 2011; World Health Organization 2021a).
The natural reserve has offered various resources to mankind to combat various infectious diseases. Plants are the most frequently utilized natural sources among the local biodiversity and are linked to folk’s day-to-day needs (Robinson and Zhang 2011). Additionally, plant-based medicaments are in considerable demand because of their safer use and non-toxic nature, and because they are less harmful in comparison to synthetic treatments (Abd Kadir et al., 2013). Multiple uses, such as consumption as food, ethnomedicinal applications, cultural aspects, and sacred faith, are associated with their utilization (Svanberg and Berggren 2019; Fongnzossie et al., 2020). Traditional knowledge of plants that was commonly practiced among tribal or ethnic populations for their healthcare has now gained considerable attention from the modern populace. Nowadays, people in developing (60%–90%) as well as developed countries (23%–80%) are using ethno-medicines as the primary healthcare regimen (Borah and Prasad 2017; Sharma and Pareek 2021). Plant biodiversity used as medicaments is of utmost importance throughout human history, which has led to the accumulation of significant information in the form of scientific research and its validation (Pasa 2011). These conventional plant-based healthcare remedies led the Ayurvedic, Unani, Chinese, and Egyptian medical systems to classify various medicinal herbs (based on color, aroma, shape, flavor, and astronomic and magical attributes) (Larocca et al., 2021). Traditional use and ethnobotanical knowledge about many plant species in treating various ailments along their chemical validation through scientific research can be promising aspects in the development of contemporary medication to treat arboviral infections like dengue. Also, a plant-based antiviral product assures a more possible choice in combating dengue infection, which may be replacement of inadequate drugs with side effects (Bahuguna et al., 2019). WHO in the 1970s has effectively executed the spread of traditional knowledge via implementing Traditional Medicine Program, and consequently, following such initiative (taking into consideration the traditional knowledge and scientific research), the associated nations have been redeveloping and improving their public health systems (Larocca et al., 2021). Development of an efficient and safer anti-dengue vaccine is a challenging task, and because of its secondary infection (second time DENV serotype infection), it is linked to the serious clinical manifestations (Toman 2018; Redoni et al., 2020). Based on recent research, various plants and their derivatives have shown anti-dengue properties; among those, flavonoids are the most popular candidates, having the ability to supplement encouraging scope in the existing struggle of drug discovery (Carneiro et al., 2016). Some of the important flavonoids recently reported are discussed with various activities, such as antioxidative, anti-inflammatory, antitumoral, antiviral, and antibacterial effects (Raekiansyah et al., 2018; Deng et al., 2020b; Daneshzadeh et al., 2020; Macedo et al., 2020; Wang et al., 2020). In the present review, a brief idea of the role of these phytoconstituents has been described against dengue infections (Jayadevappa et al., 2020). In addition, the mechanism of action with future possibilities as contemporary medicaments of plant metabolites that endorses traditional knowledge is also included.
2 Epidemiology of Dengue
Dengue is a mosquito-borne viral infection, found in tropical and sub-tropical climates worldwide, mostly in urban and semi-urban areas, transmitted by Aedes aegypti and Aedes albopictus, known as the primary and secondary dengue vectors. A range of diseases has been transmitted via Ae. aegypti including yellow fever, chikungunya, zika, and, most importantly, DENV (Silva et al., 2020). DENV was first reported in the 1950s, during dengue epidemics in Thailand and Philippines. Since then, the spread of dengue has extensively widened from South Asian countries to African and South American countries. Over several decades, different countries of continental America conducted a program that aimed to eradicate Ae. aegypti. The program was proposed and commenced via the Pan American Health Organization (PAHO) in 1946 (Pinheiro and Rodrigues 1999). Venezuela, a country on the northern coast of South America, reported dengue fever outbreaks during the 1960s, when almost all South American countries had eradicated Ae. aegypti (Pinheiro and Corber 1997). Furthermore, during the 1970s, DENV-2 and DENV-3 were the main causative serotypes for dengue fever epidemics in Colombia, a country that had attained eradication of the vector during the PAHO program (Pinheiro 1997). In the 1980s, enhanced rate of dengue cases escorted the spreading of Ae. aegypti vector, and during this period, another efficient vector, the Asian mosquito, Ae. albopictus, was introduced in the region (Gubler 1989). In the last 5 decades, the American continent has been massively affected by dengue and an approximately 30-fold rise in disease level was recorded, where almost 390 million cases of dengue with 96 million medical manifestations were reported annually (Bhatt et al., 2013). In 2013, America has declared approximately 2.3 million new severe dengue cases, which was an alarming situation in viral infections (Salles et al., 2018).
Ae. albopictus was mostly found in Asia and further expanded over different countries of European and North American regions, having highly adaptive properties to survive extreme environments and therefore able to survive in cooler regions as well. The probability of an upsurge of arthropod-borne viruses is based on the abundance of vectors involved in transmission as well as the susceptibility of immune-deficient people. Hence, there is a necessity to develop an efficient way to control its population and the spread of the disease. Besides, several other causes such as increased urbanization, ecological changes, migration of people, exchange of goods, and biological contests (e.g., development of resistance for virus) stimulate the spread of disease to new regions (Chaudhry 2019).
3 Replicative Cycle and Pathogenesis of Virus
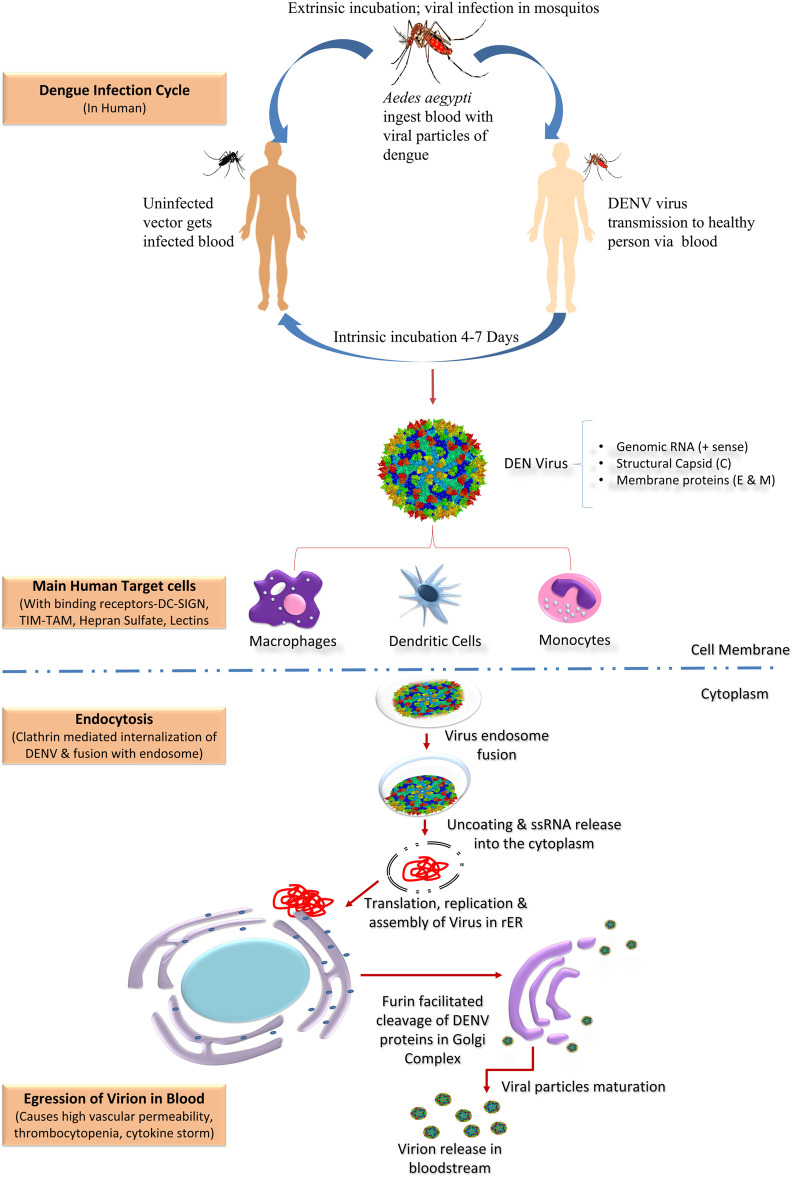
The genomic structure of DENV, approximately 10.7 kb in size, consists of ssRNA with +ve polarity (Chambers et al., 1990). Besides, the translation of viral genome was completed by three different structural proteins, i.e., Envelop (E), Capsid (C), and Pre-membrane (PrM), and seven non-structural (NS) proteins, NS1, NS2a, NS2b, NS3, NS4a, NS4b, and NS5. These proteins contribute to viral replication (Bartenschlager and Miller 2008; Xie et al., 2017). The infective form of dengue virion possesses a core of glycoprotein and ssRNA genome enclosed by icosahedral nucleocapsid (Akhtar 2019). Firstly, infected mosquito feed on host and DENV gets transmitted via injecting its saliva into the host. In the meantime, when half-length proboscis is inside the dermis, the vector releases DENV plaque-forming units (nearly 50,000) and causes infection in contiguous skin cells, i.e., Langerhans and keratinocytes (Marcial-Juárez et al., 2017; Schneider et al., 2021). Dendritic cells enter lymphatics after capturing DENV virions or antigens and transport them to local draining lymph nodes. DENV is also suggested to reach draining lymph nodes via the lymphatic flow in a cell-free manner (Yam-Puc et al., 2016). The E protein of DENV assists the binding between DENV and the cell membrane receptor. Receptor-mediated endocytosis is the principal way via which the virus is able to enter the host; a resultant sac-like structure is formed known as an endosome, depending on pH. Virus acutely penetrates and fuse with the membrane of the endosome due to the various irretrievable morphological reorganization as the endocytic vesicles become more acidic, followed by nucleocapsid opening and genetic material (RNA) release into the cytoplasm. RNA succeeds in translating into ribosomes allied to the ER (endoplasmic reticulum) by using the infected cell’s machinery; subsequently, the viral polyprotein is cleaved by cellular and viral proteases (Uno and Ross 2018). The newly synthesized RNA is wrapped in the capsid proteins (nucleocapsid), enters the host rER, and ultimately encloses the ER membrane. Further structure proteins (M and E) surround the ER-enveloped nucleocapsid forming immature virus. Subsequent processing in the Golgi apparatus results in the formation of the infectious form of the virus (Aruna 2014). Afterwards, it reaches the lymph node and further disseminates to other body organs. The virus incubation period may vary from 3 to 14 days based on the literature available (Chan and Johansson 2012). A general overview of the DENV infection cycle in humans is shown in Figure 1. After completion of the virus incubation period, the mosquito is capable of spreading the virus in the course of its life. Ae. aegypti feeds mostly on human blood and is a daytime feeder, efficiently causing infection in the early morning, and by evening, it infects multiple people during each suckling period (Weissenböck et al., 2010). The infecting efficiency of DENV for different cells including liver, blood vessels (endothelium), immune system, and retinal cells has been reported in the past (Carr et al., 2017; Begum et al., 2019).
FIGURE 1.
Dengue infection cycle in human body.
3.1 Dengue Infection
The occurrence of dengue has intensely spread worldwide in recent decades with a majority of asymptomatic cases (World Health Organization, 2021a). The infection efficiency can vary from subclinical disease to acute flu-like symptoms. Three different categories of symptomatic dengue, namely, dengue fever (DF), dengue hemorrhagic fever (DHF), and undifferentiated fever, were classified by WHO in 1997. Due to the changing epidemiology of infection, it was difficult to fulfill the suggested WHO guidelines (for dengue), and thus, classification was re-evaluated (World Health Organization 2009). In the new classification system, DHF was further categorized up to four levels based on severity of infection, with grades III and IV as DSS. Dengue asymptomatic cases were divided into two different categories: with warning and without warning signs of severe dengue (World Health Organization 2009). Dengue is associated with several complications, which are characterized by headache, appetite loss, high fever, retro-orbital pain, vomiting, abdominal pain, diarrhea, minor bleeding, rash, fatigue, etc. Severe dengue is classified by the presence of severe plasma bleeding, organ impairment, or dengue shock conditions (Khan and Bhutta 2016). It is also designated as “break-bone fever” (Rigau-Pérez 2006; Esler 2009). The severity of infection can be strongly provoked by numerous factors, such as virus serotypes, immunity power, and genomic background of host, among others (Rico-Hesse 2010; Dussart et al., 2012). Moreover, four different serotypes of viruses (DENV-1, DENV-2, DENV-3, and DENV-4) of the Flaviviridae family were deliberated as causal forms of dengue (Guzman and Istúriz 2010). Although every serotype has an equal potential to cause infection among the four serotypes of dengue, serotype variances that only affect pathogenesis such as DENV-2 have been associated with severe infection (Halstead 1988; Vaughn et al., 2000).
Recovery from infection provides enduring immunity against that serotype, although immunity for other serotypes known as cross-immunity is temporary and partial. Consequent contagions (secondary infection) by different serotypes increase the threat of emerging severe dengue. Dengue has discrete epidemiological forms, allied with the four serotypes of the virus (Halstead 2002; Rico-Hesse 2010; Dussart et al., 2012; Kim and Hwang 2020). Different studies based on the data analysis of infection status among the Indian population have been reported (Table 1). Despite this, numerous cases have been found in different regions of India, which is still unexplored in terms of proper data illustrations.
TABLE 1.
Dengue seroprevalence by age, reported in some studies from India.
| Padbidri et al. (2002) (n = 717) | (Garg et al., 2011) (n = 2,558) | (Rodríguez-Barraquer et al., 2015) (n = 800) | (Murhekar et al., 2019) (n = 12,300) | (Shah et al., 2017) (n = 700) | |||||
|---|---|---|---|---|---|---|---|---|---|
| Age (years) | Seroprevalence (%) | Age (years) | Seroprevalence (%) | Age (years) | Seroprevalence (%) | Age (years) | Seroprevalence (%) | Age (years) | Seroprevalence (%) |
| 0–9 | 47.60% | 5 | 40.70% | 9-May | 77% | 8-May | 33.00% | 0–10 | 13.70% |
| 19-Oct | 24.00% | 6 | 50.90% | 14-Oct | 90.30% | 17-Sep | 34.70% | 20-Nov | 30.10% |
| 20–29 | 26.80% | 7 | 58.60% | 15–19 | 91.70% | 18–45 | 32.30% | 21–30 | 45.10% |
| 30–39 | 25.00% | 8 | 67.40% | 20–29 | 96.30% | — | — | 31–40 | 59.70% |
| ≥40 | 23.30% | 9 | 70.80% | 30–40 | 98.80% | — | — | ≥40 | 64.10% |
| — | — | 10 | 73.40% | — | — | — | — | — | — |
| Total | 25.4 (95% CI = 22.3–28.7) | — | 59.6 (95% CI = 57.7–61.5) | — | 93 (95% CI = 91.1–94.6) | — | -— | — | 42.8 (CI = 40.9–44.7) |
4 Mode of Transmission
Aedes spp. mosquitoes (Ae. aegypti and Ae. albopictus) are the main source of dengue transmission. Recently, infection dispersed in almost all parts of tropical and subtropical regions and adapted to urban environments, mainly Ae. aegypti (Lambrechts et al., 2010). Among two different species, Ae. aegypti has been reported to have superior efficiency as a transmission vector; meanwhile, in urban environments, DENV is capable to endure its life cycle between humans and transmitter organisms (Lambrechts et al., 2010; Weissenböck et al., 2010). Human blood is the primary feeding source of Ae. aegypti, while Ae. albopictus (sylvatic strain) feeds on avian species as well as a variety of mammals (European Centre for Disease Prevention and Control 2018). Sylvatic strains are not that risky compared to “standard” dengue infection (Diallo et al., 2003; Vasilakis et al., 2008). In the initial stage of transmission, uninfected mosquitoes attack (susceptible to dengue) while feeding on host blood. In this process, viral particles are transferred into the mosquito midgut and start replicating and thus infecting the body cavity (hemocoel). From the hemocoel, the virus eventually makes its way to the salivary glands. Next, after completing the incubation period (approximately 2 weeks), the infected mosquito can disseminate the virus via salivary glands (Coudeville et al., 2009; Guzman et al., 2016).
5 Impact of Dengue Infection Globally and in India
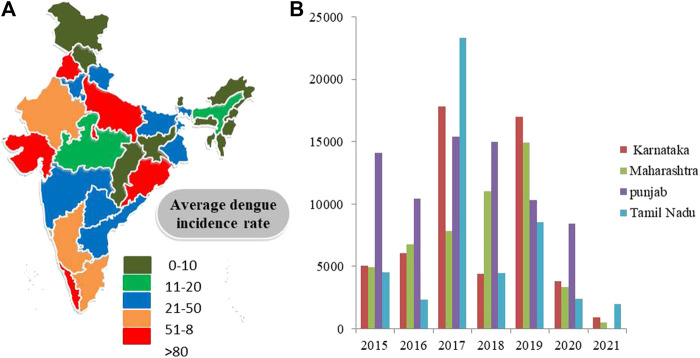
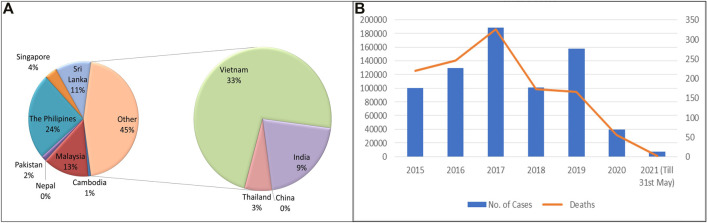
Dengue has emerged as one of the most important mosquito-borne, flaviviruses diseases, deceptively intensifying as a global health issue (Zeng et al., 2021). Dengue has been designated as the topmost worldwide health risk by WHO (Kumar et al., 2017; Biswal et al., 2019). A comparison of prior approximations of entire global dengue contagions has been illustrated. Average incidences in India with maximum infected states (Karnataka, Punjab, Tamil Nadu, and Maharashtra) are shown (Alagarasu et al., 2021) (Figure 2). Moreover, dengue infection frequency of different Asian countries and their percentage contribution have been determined where India was found to comprise 9% of total dengue incidences, higher than Pakistan, Singapore, China, Thailand, Nepal, and Cambodia, while Vietnam and Philippines are the leading countries and recorded the highest number of dengue cases in 2021 (till May) (Figure 3A) (European Centre for Disease Prevention and Control 2018; World Health Organization 2021b). Also, dengue infection status recorded in the past 7 years in India can be seen where the average infection was recorded to be the maximum in 2017 (Figure 3B) (NVBDCP, 2021).
FIGURE 2.
(A) Dengue cases in different regions of India 2021. (B) State-wise distribution of dengue cases (y-axis) in four states of India from 2015 to 2021 (x-axis) (Source: National Vector Borne disease Control Programme).
FIGURE 3.
(A) Comparative analysis of dengue incidences in different Asian countries (Source: WHO and European Center for disease Prevention and Control). (B) Number of dengue cases and deaths in India (2015–2021 May). (Source: National Vector Borne disease Control Programme).
6 Plant Medicaments Used Based on Traditional Knowledge
Plants have been the main remedial source to cure a variety of ailments since ancient times. Ancestral folks empirically isolate medicinal plants for medical home remedies and pass this knowledge from generation to generation till the present day. Ethnobotany has confirmed the necessity for conserving such information in societies that utilize plants in the treatment of different types of diseases (Gu et al., 2014; Mussin and Giusiano 2020). However, it is critical to note that these practices of exploiting plant medicaments, without scientific understanding, have led to placing various plants in the IUCN Red List consisting of threatened species (Trujillo-Correa et al., 2019). The dengue treatment period is suggested to be 10–15 days, which may vary according to the infection level of severity, the regimen carried out during treatment, the conditions, and the response of the organism (Yogarajalakshmi et al., 2020). Different plant-based formulations are reported to be used in various affected parts of India against dengue and have also been approved through scientific research, although limited studies in this reference have been recorded in India (Deep et al., 2018). There are many plant species such as Curcuma longa L., Lonicera japonica Thunb., Acorus calamus L., Carica papaya L., Euphorbia hirta L., Tinospora sinensis (Lour.) Merr., and Sambucus canadensis L. used globally as ethnomedicine against dengue fever (Ichsyani et al., 2017; Lee et al., 2017; Deep et al., 2018; Yao et al., 2018; Larocca et al., 2021) (Table 2). In another report, plants from a diverse group of plant families (31 in total with 54 species) are reported to exhibit anti-dengue activity based on traditional knowledge, where major representatives reported were Lamiaceae (10.5%), Asteraceae (9.9%), Aristolochiaceae, and Loganiaceae (each 7.2%) families (Saleh and Kamisah 2021). Plants revealed by the local population to exhibit anti-dengue activity included Baccharis trimera (Less.) DC., Aristolochia surinamensis Willd., Momordica charantia L., Dysphania ambrosioides (L.) Mosyakin and Clemants, Ocimum gratissimum L., Strychnos pseudoquina A. St.-Hil., and Stachytarpheta cayennensis (Rich.) Vahl (Pongthanapisith et al., 2013; Sharma et al., 2020; Larocca et al., 2021). Although many of these plant species do not have any scientific evidence as suggested in the available literature, their ethnomedicinal potential may lead to the real success in treating arboviral diseases like dengue (Pilla et al., 2006; Mendoza et al., 2020). However, limited literature presenting scientific validation of these plants showing anti-dengue activity is available. Traditional use of C. papaya leaf juice has revealed very promising outcomes in treating dengue disease (Prakash Kala, 2012; Sarala and Paknikar 2014), which is further authorized through scientific evidence demonstrating lower cytotoxic and efficient anti-DENV effects (Saptawati et al., 2017; Sharma et al., 2019; Sarker et al., 2021). Similarly, the use of Psidium guajava L. as folkloric medication has also been cited in literature, which enhances thrombocyte count in dengue infection (Berlian et al., 2017). Traditional medicinal values being the foundation for advanced scientific research lead to the revelations of possible mechanisms behind the anti-dengue activities that suggest a promising role of phytoconstituent-associated antiviral activities via inhibition of viral cell adhesion, replication, etc. (Saptawati et al., 2017; Dewi et al., 2020). Other traditionally used plants such as Ocimum tenuiflorum L. (Tulsi), Tinospora sinensis (Lour.) Merr. (Giloy leaves and stems), Trigonella gladiata M. Bieb. (fenugreek leaves), Azadirachta indica A. Juss. (Neem leaves), Cymbopogon citratus (DC.) Stapf (Lemon grass), and Psiloxylon mauritianum (Bouton ex Hook. f) Baill. with potential ethnopharmacological uses in treating dengue still need clarification about their action mechanism technically (Parida et al., 2002; Abd Kadir et al., 2013; Ling et al., 2014; Singh et al., 2016; Deep et al., 2018; Arunabha 2019; Clain et al., 2019; Rosmalena et al., 2019; Paul et al., 2021). There are very few reports validating the antiviral activities of A. indica. According to these reports, aqueous A indica leaf extracts and Gedunin and Pongamol (neem constituents) are capable of repressing DENV-2 serotype by targeting viral replication (Parida et al., 2002) and targeting human as well as viral proteins (responsible for viral attachment) (Rao and Yeturu 2020). Quercus lusitanica Lam. and Gastrodia elata Blume have also been approved to have an antiviral response against DENV-2 replication and DENV multiplication cycle, respectively (Rahman et al., 2006; McDowell et al., 2010; Ahmad et al., 2011). Also, the polyphenol-rich extract of Aphloia theiformis (Vahl) Benn. has recently been reported against ZIKV and DENV infections, which prohibits viral entry (Clain et al., 2018).
TABLE 2.
List of plants studied for anti-dengue response based on ethnopharmacological use.
| Sr. No | Plant/Common name/Family | Geographical location | Part Used | Studied Model, DENV Serotype | Optimum dosage | Mode of action | Used cell line/animal/human model | Citation | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Section1 : In vitro studies | |||||||||||||
| 1 | Acacia catechu. (L.f.) Willd./Khair/Fabaceae | Maharashtra, Madhya Pradesh, Gujarat, Tamil Nadu, Uttar Pradesh, Karnataka, Andhra Pradesh, and Rajasthan | Dried powder of fruits | DENV 1–4 | IC50 values 1.54 μg/ml and 0.18 μg/ml | Extract contains peptides, subdued DENV infection in the initial phase of infection via decrease foci formation and level of intracellular envelope proteins were also decreased in all serotypes of DENV. | Vero cells (kidney epithelial cells isolated from African green monkeys) and Huh7 cells (hepatocellular carcinoma cell line) | Panya et al. (2019); Panya et al. (2020) | |||||
| Most effective peptide (designated Pep-RTYM) interacted with DENV particles and inhibited cellular entry | |||||||||||||
| 2 | Acorus calamus L./sweet flag, sway or calamus/Acoraceae | Europe, China, northern Asia Minor, Indonesia, southern Siberia, in southern Russia, Japan, Sri Lanka, Australia, Burma, as well as southern Canada and Northern United States | Roots | DENV2 | Tatanan A, EC50 = 3.9 μM | Potentially affect DENV2, treatment constrained the initial steps of RNA synthesis as well as post translation modifications | Mosquito larva C6/36 cells were for DENV2 replication and Mouse kidney fibroblast cells (BHK-21) | Yao et al. (2018) | |||||
| 3 | Andrographis paniculata (Burm.f.) Nees/Green chiretta/Acanthaceae | Widely cultivated in Southern and South eastern Asia, Malaysia | Methanolic extract | DENV 2,4 | Andrographolide, the maximum non-toxic dose 15.62 μg/ml | — | C6/36 cell line for DENV replication | Kaushik et al. (2021) | |||||
| 4 | Basilicum polystachyon L. Monecch/Musk Basil/Lamiaceae | Asia, Asterids, Africa, Borneo, Indochina Australia, India, China, Indian Ocean | Whole plant | DENV serotype not mentioned | IC50 = 1.4 ± 2/1 μM | — | Vero cells (African green monkey kidney) | Tan et al. (2019) | |||||
| 5 | Cissampelos pareira L./Velvet leaf/Menispermaceae | Western and Eastern Cape Provinces Sandy slopes and scrub of the Northern, northwards into Namibia | Aerial parts | DENV-1–4 | IC50 values 100, 125, 78, and 100 μg/ml, respectively | It obsessed the efficiency to downregulate the synthesis of TNF-α, a type of cytokine allied with acute dengue disease | Mosquito cell line C6/36 and monkey kidney cell lines LLCMK2 | Sood et al. (2015) | |||||
| 6 | Curcuma longa L./Turmeric/Zingiberaceae | Originate from South or Southeast Asia, most probably from Vietnam, western India, or China | Not mentioned | DENV-2 | IC50 = 17,91 μg/ml CC50 = 85,4 μg/ml | - | Huh7it-1 cells | Ichsyani et al. (2017) | |||||
| 7 | Cymbopogon citratus (DC.) Stapf/Lemongrass/Gramineae | Indigenous to Sri Lanka and South India, recently cultivated in the tropical areas of Asia and America | Not mentioned | DENV-2 | CC50 = 183.74 and EC50 = 29.37 μg/ml | After treatment viral replication inhibition increased | Antiviral activity in Huh7it-1 cell lines infected by DENV | Rosmalena et al. (2019) | |||||
| 8 | Doratoxylon apetalum (Poir.) Radlk./Sapindaceae | Native to Mascarene Islands | Aerial parts | DENV1–4 | IC50 = 96.35, 16.75, 25.90, and 23.30 μg/ml for DENV1–4 individually | Extract-mediated DENV inhibition is allied to an erosion of infectivity | Human lung epithelial A549 cells, Vero cells, and human-derived Huh-7 hepatoma cells | Haddad et al. (2019) | |||||
| 9 | Ficus septica Burm.f./Hauli tree/Moraceae | Japan, Indonesia, Malaysia, Philippines, Solomon Islands, Papua New Guinea, and Taiwan | Stem fruit, heartwood, and leaves | DENV-1 and DENV-2 | IC50 = 3.05–>100 μg/ml | — | DENV-1 766733A and DENV-2 PL046 (GenBank accession no. AJ968413.1) | Huang et al. (2017) | |||||
| 10 | Oldenlandia uniflora L./Geta Kola/Rubiaceae | Mostly found in Sri Lanka | Leaves, stems, and roots | DENV-2 NS2B-NS3pro | IC50 ≤ 100 μg/ml | — | Dengue NS2B-NS3pro | Rothan et al. (2014) | |||||
| 11 | Kaempferia parviflora Wall. ex Baker/krachai Dam/Zingiberaceae | — | Leaves and stems | DENV-2 | — | -— | — | Umesh Kanna and Krishnakumar (2019) | |||||
| 12 | Nephelium lappaceum L./Rambutan/Sapindaceae | Southeast Asian native to the Malaysian–Indonesian region | Rind | DENV-2 | IC50 = 1.75 μM | Restricts early phases of cell and virus interaction via inhibiting the attachment of virus with the binding of E-DIII protein | African Green Monkey kidney cells (Vero) | Abdul Ahmad et al. (2017) | |||||
| 13 | Ocimum tenuiflorum L./Tulsi, basil/Lamiaceae | Indigenous to Iran and India and currently cultivated in France, Egypt, Italy, Hungary, Morocco, and United States. | Whole aerial body | DENV-1 | Maximum non-toxic dose: 23.44 μg/ml | O. sanctum unveiled antiviral efficacy for DENV-1 via inhibiting CPE formation and multiplication of virus | HepG2 cells | Ling et al. (2014) | |||||
| 14 | Pavetta canescens DC./Papari/Rubiaceae | E. Asia—India, Sri Lanka, and Philippines | Leaves | DENV-2 | Least LC50 and LC90 values (5.968 and 7.493 μg/ml) | — | Monolayer culture of C6/C36 mosquito cell line | Pratheeba et al. (2019) | |||||
| 15 | Psidium guajava L./Common guava/Myrtaceae | Native to the Caribbean, Central America, and South America | Bark | DENV-2 | Catechin, CC50 = 1,000.0; μg/ml EC50 = 7.8 | — | Epithelial VERO cells and C6/36HT cells (from Aedes albopictus mosquito larvae) | Trujillo-Correa et al. (2019) | |||||
| 16 | Schisandra chinensis (Turcz.) Baill./Magnolia-vine, Chinese magnolia-vine/Schisandraceae | Indigenous to forests of Northern China and the Russian Far East and Korea | — | DENV-1, 2, 3, and 4 | Schisandrin A, EC50 = 28.1 ± 0.42 μM | Isolated bioactive compound, restricted RNA replication and translation as well as ominously raised DENV-reduced IFN-α gene expression | DENV-infected Huh-7 cells | Yu et al. (2017) | |||||
| 17 | Tarenna asiatica (L.) Kuntze ex K. Schum./Bingi Papadi/Rubiaceae | Southern part of India, Sri Lanka, and Malaysia | Leaves | DENV serotype not mentioned | Tetracontane, LC50 and LC90 values (1.288 and 1.992 μg/ml) | — | Monolayer culture of C6/C36 mosquito cell line | Pratheeba et al. (2019) | |||||
| 18 | Zostera marina L./Eagrass or eelgrass/Zosteraceae | Northern Hemisphere as well as Australia, New Zealand, Southeast Asia, and southern Africa | Designed and provided by CernoFina, LLC (Portland, ME) | DENV-1–4 | ZA, IC50 = 2.3 mM | — | Monkey kidney cell line LLCMK-2 | Rees et al. (2008) | |||||
| Section 2: In vitro gene or protein expression studies | |||||||||||||
| 19 | Allium sativum L./Garlic/Liliaceae | United States and Canada | Purchased organosulfur garlic compounds | DENV-2 | — | Addition of Allium sativum compounds abridged the level of different pro-inflammatory cytokines including IL-8, TNF-α, IL-10 as well as iNOS (nitric oxide synthase) | Cell lines Huh-7 and U937 | Hall et al. (2017) | |||||
| 20 | Garcinia × mangostana L./Purple mangosteen/Clusiaceae | Native to island nations of Southeast Asia and Thailand | Pericarp | DENV-1–4 | α-Mangostin, 20 μM | Remarkably reduced transcription of IL-6, TNF-α (cytokine), IP-10, RANTES, and MIP-1β (chemokine) | Human hepatocellular carcinoma (HepG2), (Huh-7) and African green monkey kidney (Vero) cells | Tarasuk et al. (2017) | |||||
| 21 | Schisandra chinensis (Turcz.) Baill/Magnolia-vine, Chinese magnolia-vine, schisandra/Schisandraceae | — | Purchased compounds | DENV-1–4 | Schisandrin A | Inductive efficacy of antiviral IFN-I exerts gene expression | DENV-infected Huh-7 cells | Yu et al. (2017) | |||||
| 22 | Lonicera japonica Thunb./Japanese honeysuckle/Caprifoliaceae | Native to eastern Asia | Flower buds | DENV-2 | — | Subdue DENV2 multiplication via luciferase-reporter activity and diminishes NS1 RNA prevention level and treatment occur via instigation of the distinctive miRNA let-7a | Human hepatoma, baby hamster kidney (BHK-21 and Aedes albopictus cells C6/36) | Lee et al. (2017) | |||||
| 23 | Carica papaya L./Pawpaw/Caricaceae | Native to the tropics of the Americas but now is widely cultivated in other tropical regions | Leaves | NM | — | Level of NS1 and envelope protein decreased in the THP-1 cells, erythrocyte damage also declines | DENV-infected THP-1 cells | Sharma et al. (2019) | |||||
| 24 | Schwartzia brasiliensis (Choisy) Bedell ex Gir.-Cañas/Norantea/Marcgraviaceae | Vine native to Brazil | Leaves | DENV-2 | — | Downregulated IL-6, TNF-α, IL-10, and IFN-α secretion, cellular antigenic viral load, and secreted NS1 protein reduction | Buffy coat cells and peripheral blood mononuclear leukocytes | Fialho et al. (2017) | |||||
| Section3: Clinical studies based on animal model | |||||||||||||
| 25 | Carica papaya L./pawpaw/Caricaceae | Native to the tropics of the Americas but now is widely cultivated in other tropical regions | Leaves | — | — | Upsurge in cytokines of plasma in DENV infested AG129 mice with the dosage of freeze-dried 500 and 1,000 mg/kg | AG129 mice infected with DEN-2 | Norahmad et al. (2019) | |||||
| 26 | Cissampelos pareira L./Abuta, ice vine/Menispermaceae | Tamilnadu, Himachal Pradesh, Bihar, west Bengal, Nagpur, Punjab, and Rajasthan | Aerial parts | DENV-1–4 | Doses as high as 2 g/kg body weight for up to 1 week | — | AG129 mouse model | Sood et al. (2015) | |||||
| 27 | Lonicera japonica Thunb./Japanese honeysuckle/Caprifoliaceae | Native to eastern Asia | Flower buds | DENV-2 | Honeysuckle (40 μl) | Up to 30% virus reduction was observed via suppression of DENV2 replication as well as viral titer | C57/B6 and ICR suckling mouse models | Lee et al. (2017) | |||||
| Section 4: Clinical studies based on human model | |||||||||||||
| 28 | Euphorbia hirta L./Asthma Weed, Cats hair/Euphorbiaceae | Warmer regions of India and Australia | Plant parts not specifically mentioned–herbal water | DENV serotype not mentioned | — | Leukocyte count ominously improved from 4,000 to 11,000 mm3 in both female and male patients | — | Mir et al. (2012) | |||||
| 29 | Lonicera japonica Thunb./Japanese honeysuckle/Caprifoliaceae | Native to eastern Asia | Flower buds | DENV-2 | — | Upregulated miRNAs–let-7a showed highest expression level | — | Lee et al. (2017) | |||||
| 30 | Boerhavia diffusa L./Tarvine/Nyctaginaceae | India, Australia, Sudan, Pakistan, Sri Lanka, South Africa, Brazil, and the southern United States | Stem | DENV serotype not mentioned | Stems of Boerhavia diffusa L. (10 g) | Lowers body temperature and increases platelet counts more than 85,000. Again, after 24 h, they have normal platelet count and no symptoms of dengue | — | Bharati and Sinha (2012) | |||||
| Section 5: In silico molecular docking studies | |||||||||||||
| 31 | Nephelium lappaceum L./Rambutan/Sapindaceae | Southeast Asian native to the Malaysian–Indonesian region | Rind | DENV-2 | — | Geraniin binds with DENV E, specifically at DIII region | — | Abdul Ahmad et al. (2017) | |||||
| 32 | Glycyrrhiza glabra L./Licorice/Fabaceae | England, Iran, Spain, Iraq, Sicily, and Russia | Not specifically mentioned – | — | — | Polyphenolic compounds, chalcones, flavonoids and some phenolics were strong docking ligands for target of dengue virus protein | — | Powers and Setzer (2016) | |||||
| 33 | Psidium guajava L./Common guava/Myrtaceae | Native to the Caribbean, Central America, and South America | Bark | DENV-2 | Catechin, CC50 = 1,000.0; μg/ml EC50 = 7.8 | Interactions of isolated compounds with the viral envelope protein in silico by docking, only naringin and hesperidin had better scores than the theoretical threshold of −7.0 kcal/mol (−8.0 kcal/mol and −8.2 kcal/mol, respectively) | — | Trujillo-Correa et al. (2019) | |||||
7 Plant Extract as Mosquito Repellent and Larvicide
Ae. aegypti and Ae. albopictus, epidemiologically related arboviruses in the community health context, including Zika, dengue, and chikungunya viruses. Different types of synthetic insecticides are easily accessible in the market, associated with numerous side effects such as the development of resistance to these insecticides, having toxic effects on other organs, and ecological health problems (Rodrigues et al., 2020). However, biological control as an alternative for these vectors could be very helpful due to its eco-friendly and cost-effective nature. Govindarajan et al. (2015) reported that methanol extracts of Delonix elata (L.) Gamble leaves and seed (highest concentrations used: 5.0 mg/cm2) offered protection for over 180 and 150 min, respectively, against Ae. aegypti. Herbal oil formulation of different plant species including Eucalyptus globulus Labill., A. indica, Mentha × piperita L., Ocimum basilicum L., and rhizome of Zingiber officinale Roscoe has also been reported as repellents and bite protectors against Ae. aegypti and Ae. albopictus (Nasir et al., 2015). Terpinen-4-ol, 1,8-cineole, and β-pinene (0.4 mg/cm2) isolated from Artemisia vulgaris L. showed up to 91% inhibition of Ae. aegypti (Hwang et al., 1985). Salvia elegans Vahl possess acetate (11.4%), β-caryophyllene (6.4%), and caryophyllene oxide (13.5%), which exhibited good larvicidal activity against Ae. aegypti (Ali et al., 2015). Essential oils (EOs) extracted from the Hazomalania voyronii (Jum.) Capuron dried bark, stem, and wood are being used to attain protection against the mosquito vector Ae. aegypti (Benelli et al., 2020). As per WHO protocol, extracts were prepared using leaves of Lantana camara L., Hyptis suaveolens (L.) Poit., Nerium oleander L., and Tecoma stans (L.) Juss. ex Kunth, revealing effective larvicidal efficiency in contrast to larvae of Ae. aegypti (Hari and Mathew 2018). The chloroform bark extract of Terminalia arjuna (Roxb. ex DC.) Wight and Arn. showed maximum mortality on Ae. aegypti larvae. The LC50 and LC90 values of T. arjuna on Ae. aegypti larvae were 4.61 and 24.17 µg/ml, respectively (Tamilventhan and Jayaprakash 2019).
8 Some Phytoconstituents With Anti-Dengue Activity and Their Action Mechanism
Numerous plants and plant-based products exhibit enormous biological properties (like an antibiotic, antitumor, and antiviral), which are responsible for treating millions of individuals with serious diseases. Among those flavonoids, polysaccharides, hemicelluloses, and hydrophilic colloids are involved in the antiviral properties of plants (Table 3). Some of these biologically active compounds are based on recent research, and their promising role as contemporary medication in the near future has been discussed briefly.
TABLE 3.
Effective bioactive compounds from plant sources against dengue infection.
| Sr. no | Phytochemical | Chemical structure | Class | Plant studied for anti-dengue activity | Plant Part used | Other plants containing this phytoconstituent | Citation |
|---|---|---|---|---|---|---|---|
| 1 | Castanospermine |
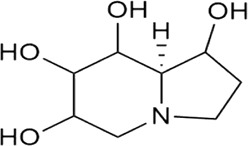
|
Tetrahydroxyindolizidine alkaloid | Castanospermum australe A. Cunn. and C. Fraser (Fabaceae) | — | 1. Swainsona canescens (Lindl.) F. Muell. (Fabaceae) | Häusler et al. (2000); Whitby et al. (2005) |
| 2 | Baicalin |
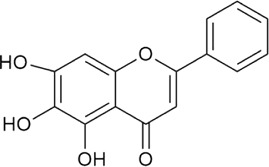
|
Glycosyloxyflavone | Scutellaria baicalensis Georgi (Lamiaceae) | Roots | 1. Scutellaria baicalensis Georgi (Lamiaceae) inhibit human T-cell leukemia virus type 1(HTLV-I) | Baylor et al. (1992); Zhao et al. (2016); Zhou et al. (2016); Rojsanga et al. (2020) |
| 2. Oroxylum indicum (L.) Kurz (Bignoniaceae) | |||||||
| 3 | Gallic acid |
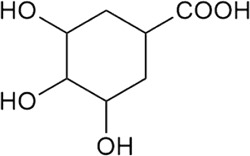
|
Phenolic acid | Psidium guajava L. (Myrtaceae) | Bark | 1. Camellia sinensis (L.) Kuntze (Theaceae) | Trujillo-Correa et al. (2019); Zhou et al. (2020); Baite et al. (2021) |
| 2. Ficus auriculata Lour. (Moraceae) | |||||||
| 4 | Quercetin |
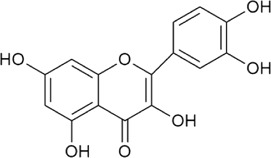
|
Flavonoid glycosides | Momordica charantia L. (Cucurbitaceae) | — | 1. Allium roseum L. (Amaryllidaceae) | Han et al. (2005); Eid et al. (2010); Agrawal and Pal (2013); Nile et al. (2017) |
| 2. Vaccinium vitis-idaea L. (Ericaceae) | |||||||
| 3. Nyctanthes arbor-tristis L. (Oleaceae) | |||||||
| 5 | Epigallocatechin gallate |
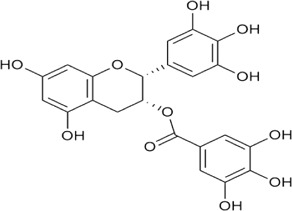
|
Polyphenols | Camellia sinensis (L.) Kuntze (Theaceae) | — | — | Vázquez-Calvo et al. (2017) |
| 6 | Galactomannan |
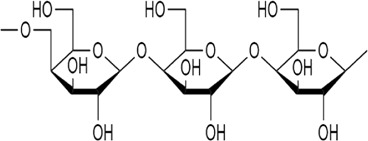
|
Polysaccharides | Leucaena leucocephala (Lam.) de. Wit (Fabaceae), Mimosa scabrella Benth. (Fabaceae), Lippia alba (Mill.) N.E.Br. ex Britton and P. Wilson (Verbenaceae) | Seed | 1. Trigonella foenum-graecum L. (Fabaceae) | Latgé et al. (1991); Chaubey and Kapoor (2001); Ono et al. (2003); Mathur and Mathur (2005); Ocazionez et al. (2010); Prajapati et al. (2013) |
| 2. Ceratonia siliqua L. (Fabaceae) | |||||||
| 3. Cyamopsis tetragonoloba (L.) Taub. (Fabaceae) | |||||||
| 4. Caesalpinia spinosa (Molina) Kuntze (Fabaceae) | |||||||
| 5. Senna alexandrina Mill. (Fabaceae) | |||||||
| 7 | Glabranine, 7-O-methylglabranine |
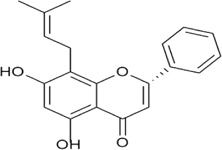
|
Flavonoid | Tephrosia species | Leaf, Flower | 1. Linum usitatissimum L. (Linaceae) | Sánchez et al. (2000) |
| 8 | Galactan |
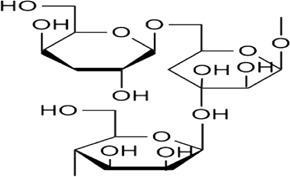
|
Hemicelluloses | Cryptonemia crenulata J. Agardh (Halymeniaceae), Gymnogongrus torulosus (J.D. Hooker and Harvey) F. Schmitz (Phyllophoraceae) | Whole Plant | 1.Chenopodium quinoa Willd (Amaranthaceae) | Talarico et al. (2005); Wefers et al. (2014) |
| 9 | Kappa carrageenan |
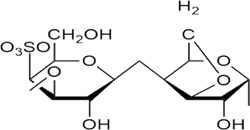
|
Hydrophilic colloids | Meristiella gelidium (J. Agardh) (Solieriaceae), Gymnogongrus griffithsiae (Turner) C. Martius (Phyllophoraceae) | — | 1. Hypnea musciformis (Wulfen) J.V. Lamouroux (Cystocloniaceae) | Talarico et al. (2005); Arman and Qader (2012); Nur Fatin Nazurah and Nur Hanani (2017) |
| 2. Eucheuma cottoni Weber-van Bosse (Solieriaceae) | |||||||
| 10 | 4-Hydroxypanduratin A, panduratin A | — | — | Boesenbergia rotunda (L.) Mansf. (Zingiberaceae) | — | 1. Boesenbergia rotunda (L.) Mansf. (Zingiberaceae) | Kiat et al. (2006); Pham et al. (2020) |
| 11 | Zosteric acid |
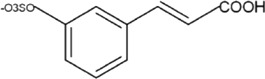
|
Flavonoid | Zostera marina L. (Zosteraceae) | — | — | Rees et al. (2008) |
| 12 | Morin |
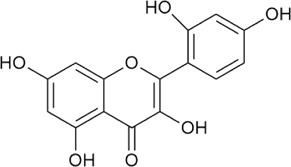
|
Flavonoid | Zingiber officinale Roscoe (Zingiberaceae) | — | 1. Tinospora crispa (L.) Hook. f. and Thomson (Menispermaceae) | Yang and Lee (2012); Hussain et al. (2014); Mbadiko et al. (2020); Taguchi et al. (2020); Warsinah et al. (2020) |
| 2. Morus alba (L.) (Moraceae) | |||||||
| 3. Acridocarpus orientalis A. Juss. (Malpighiaceae) | |||||||
| 13 | Hyperoside |
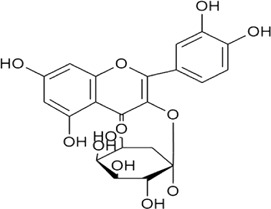
|
Flavonoid | Houttuynia cordata Thunb. (Saururaceae) | Whole plants, aerial stem and leaves | 1. Camptotheca acuminata Decne. (Cornaceae) | Li et al. (2005); Kim et al. (2011); Leardkamolkarn et al. (2012); Ahn and Lee (2017); Zhang et al. (2021) |
| 2. Hypericum perforatum L. (Hypericaceae) | |||||||
| 3. Oenanthe javanica (Blume) DC. (Apiaceae) | |||||||
| 4. Zanthoxylum bungeanum Maxim. (Rutaceae) | |||||||
| 14 | Fucoidan |
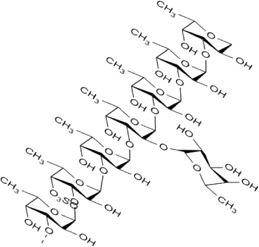
|
Polysaccharides | Cladosiphon okamuranus Tokida (Chordariaceae) | Whole plants | 1.Utricularia aurea Lour. (Lentibulariaceae) | Jiang et al. (2010); Skriptsova et al. (2010); Marudhupandi and Kumar (2013); Minh Ly et al. (2017); Palanisamy et al. (2017); Lim et al. (2019) |
| 2. Undaria pinnatifida (Alariaceae) | |||||||
| 3. Sargassum wightii Greville ex J. Agardh (Sargassaceae) | |||||||
| 4. Sargassum polycystum C. Agardh (Sargassaceae) | |||||||
| 5. Ascophyllum nodosum | |||||||
| (L.) Le Jolis (Fucaceae) | |||||||
| 6. Vietnam Sargassum species | |||||||
| 15 | Daidzein |
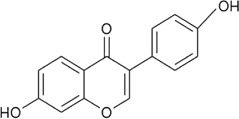
|
Bioflavonoid | — | — | 1. Glycine max (L.) Merr. (antioxidant) (Fabaceae) | Pongkitwitoon et al. (2011); Juliana et al. (2020) |
| 2. Pluchea lanceolata (DC.) C.B. Clarke (Asteraceae) | |||||||
| 3. Pueraria candollei Benth. (Fabaceae) |
8.1 Castanospermine
Castanospermine (a natural alkaloid) is a derivative of Castanospermum australae A. Cunn. and C. Fraser (black bean) (Whitby et al., 2005) that can easily be isolated through simple purification methods (Pan et al., 1993). One of the possible mechanisms through which it prevents infection is misfolding of viral proteins via obstructing the removal of glucose residues from N-linked glycans (Whitby et al., 2005). This may further lead to inhibit molecular interaction of viral and host proteins (Zhang et al., 1997; Molinari and Helenius 1999). Castanospermine has shown not only inhibitory effects on all the dengue serotypes by preventing the production of DENV but also evidence to prevent mice death when infected with DENV-2 through the intracranial route (Rathore et al., 2011). Evidence of celgosivir (6-O-butanoyl castanospermine) being an antiviral agent against DENV virus both in vitro and in vivo has also been concluded. Castanospermine exhibits glucosidase inhibitor properties that result in early-stage inhibition of glycoprotein processing. As a result, the formation of unstable complexes produces non-productive viral protein (prM and E) folding pathways and ultimately the antiviral response of Castanospermine (Courageot et al., 2000). Celgosivir is the butylated prodrug cleaved in cells to produce castanospermine, a bicyclic iminosugar (Miller et al., 2018). It causes misfolding and accumulation of NS1 (DENV non-structural protein) in the endoplasmic reticulum and also alters host protein response leading to the antiviral activity. Based on the previous research and data availability, castanospermine use in anti-dengue medication can be an important contribution in fighting dengue infection (Rathore et al., 2011).
8.2 Baicalin
Baicalin (a flavonoid) demonstrates considerable antiviral potential against DENV-2 by targeting the replication stages post-viral infection (Zandi et al., 2012). Baicalein is reported to be isolated naturally from the roots of Scutellaria baicalensis Georgi, a medicinal plant of China, and can be further converted into baicalin after intake by animals or humans (Xu et al., 2010). Based on the successful results obtained from Baicalin against dengue infection, other plant species having baicalin as a biomolecule can be explored for their anti-dengue properties. The mechanistic approach behind the anti-dengue properties has been demonstrated by researchers where possible ways of antiviral activity (96–99%) are direct inactivation of free DENV-2 particles, inhibited intracellular viral replication, and binding of DENV-2 cells to the host cell (Moghaddam et al., 2014). In molecular docking analysis, both baicalin and baicalein are reported to inhibit the DENV replication by pursuing key DENV genes and exhibit anti-DENV protease (NS2B/NS3) activity. The results of docking studies show that both baicalein and baicalin may interact with NS3–NS2B, E, and NS5 proteins, which can be responsible for their antiviral impact (Hassandarvish et al., 2016; Rasool et al., 2019). Further investigation using these phytoconstituents is still needed to obtain promising results in the form of contemporary medications and future implementation in clinical use.
8.3 Gallic Acid
Gallic acid (main phenolic plant component) exhibits various pharmacological activities (antioxidant, antiviral, antifungal, etc.) and therapeutic uses against neural disorders, dengue, cancer, asthma, and allergic rhinitis, without any cytotoxic effects (Suganthi and Ravi 2019). In a study, inhibitory response of gallic acid with no cytotoxic effect has been elucidated in in vitro conditions on DENV-2 virus along with another compound, emodin, and some plant extracts, i.e., Antennaria microphylla Rydb., Rubus scaber Weihe, Ziziphus jujuba Mill., and Commelina benghalensis L. Gallic acid was found to be active against dengue disease through prophylactic treatment rather than post-infection medication (Batool et al., 2018), while another report has suggested successful viral inhibition in both prophylactic (52.6%) and post-treatment (67.3%) approaches (Trujillo-Correa et al., 2019). More complete investigations are warranted on these plants for isolation, purification, and characterization of bioactive principles responsible for the anti-dengue activity, and to elucidate their underlying mechanisms of DENV inhibition (Batool et al., 2018). Moreover, isobutyl gallate (a derivative of gallic acid) due to low cellular toxicity and antiviral response at low concentration has been suggested as a good alternative for anti-DENV activity (by inhibiting viral replication) (Dewi et al., 2019). Considering all mentioned findings and inconsistencies about cellular toxicity and anti-dengue effects, more clarification is still needed, which can lead to formulating a successful treatment against the DENV.
8.4 Quercetin
Quercetin (flavonoid) exhibits various pharmacological responses against viruses, tumors, inflammation, etc. In accordance with previous studies available, quercetin is one of the main components of different plant extracts (E. hirta and P. guajava) exerting consistent antiviral effect against dengue infection (DENV-2) (Zandi et al., 2011; Saptawati et al., 2017; Suganthi and Ravi 2019). A possible mechanism is similar to the previously discussed metabolites that inhibit viral replication by targeting RNA polymerase enzyme. Quercetin is reported to exhibit antiviral properties via impeding viral attachment and viral replication against DENV serotypes (1–4) (Zandi et al., 2011; Sharma et al., 2018). In addition, quercetin is one of the main components present in P. guajava leaf extract demonstrating the inhibitory effect on DENV infection with no involvement in the inhibition of viral surface proteins and receptors. These findings also suggest the presence of other bioactive compounds in P. guajava leaf formulations that may exhibit anti-dengue behavior (Dewi et al., 2020). Houttuynia cordata Thunb. leaves ethyl acetate fraction (including quercetin, quercitrin, and rutin) has also been reported, which reveals the efficient anti-DENV-2 activity of bioactive components quercetin (IC50 of 176.76 μg/ml) and quercitrin (IC50 of 467.27 μg/ml). The synergistic effect of both components is also depicted in the past presenting antiviral properties with an IC50 of 176.76 μg/ml (Chiow et al., 2016). These results conclude that plant formulations and their metabolites signify a promising preventative substance in P. guajava L. leaf extract, which might prevent DENV attachment by inhibition of DENV surface protein along with the receptor inhibition (Dewi et al., 2020).
8.5 Epigallocatechin Gallate
Epigallocatechin gallate (flavonoid) and delphinidin (structurally related polyphenol) from plants and their products (wine, green tea, curcumin, and delphinidin) have proved their antiviral qualities regardless of the DENV serotypes. These compounds are suggested to exhibit virucidal effects or inhibit viral production by acting upon the viral life cycle at its attachment and entry points (Vázquez-Calvo et al., 2017; Raekiansyah et al., 2018; Clain et al., 2019). In another study, epigallocatechin gallate, a biologically active component of green tea, curcumin, and delphinidin, is reported to have the least cytotoxic effects. It is believed to prevent viral infection at early stages by direct interaction with the virion (when the virus is preincubated with epigallocatechin gallate), which leads to an approximate drop of 90% in DENV antigen present in intracellular fluid. Similarly, epigallocatechin, another bioactive compound, also has a strong repressive impact on viral growth (Raekiansyah et al., 2018). The inhibition mechanism as suggested by Raekiansyah et al. is to develop a virucidal effect through direct binding with molecules present on the DENV surface (Raekiansyah et al., 2018).
8.6 Other Bioactive Compounds
Apiofuranoside (flavanone glycosides), obtained from Faramea bahiensis Mull. Arg., is efficiently involved in the treatment of dengue via controlling viral replication as well as reducing the number of infected cells (12%) and RNA copies of DENV-2 (67%) in HepG2 cells (Nascimento et al., 2017). Flacourtoside A, a phenolic glycoside exhibited in Flacourtia indica (Burm.f.) Merr., effectively inhibited DENV replication with the 9.3 µM IC50 values (Bourjot et al., 2012). Trigocherrin A and Trigocherriolide B and C (diterpenoids) found in Trigonostemon cherrieri Veillon are also able to impede DENV replication with 12.7 µM and 3.1 and 16.0 µM IC50 values, respectively (Allard et al., 2012). Arrabidaea pulchra (Cham.) L.G. Lohmann synthesizes another important bioactive compound, verbascoside (phenyl glycoside), which is reported to display good anti-DENV-2 (IC50 = 3.4 μg/ml) properties (Brandão et al., 2013). Also, pectolinarin, a type of flavone found in Amphilophium elongatum (Vahl) L.G. Lohmann exhibited anti-DENV-2 effects with an EC50 value of 86.4 μg/ml (Simões et al., 2011).
Note that the action mechanism and the minimal cytotoxicity levels of natural compounds can contribute to the development of anti-dengue medication, which will be safer as well as more effective in use, although limited research has been done in this aspect and there are limited publications. Thus, further investigations are necessary to develop a fruitful antiviral drug (to cure DENV).
9 Immunomodulatory Response of Plant Extracts
With the onset of dengue infection, the host can experience a complex interplay of host immune factors and viral particles, which may include amplified immune cell infection due to the presence of non-deactivating antibodies and stimulation of cross-reactive autoimmunity responses (i.e., activation of T cells and autoantibodies, cytokine deregulation, complement, and coagulation systems) (Jasso-Miranda et al., 2019). Being an acute febrile infection (thrombycytopenia, arthralgia, and hemorrhagic symptoms), it may lead to the host deterioration due to the hemorrhagic attack, plasma leak, intense shock, organ collapse, and ultimately, death (Pan American Health Organization (PAHO) 2016; Jasso-Miranda et al., 2019). Dengue infection is hypothesized to be more fatal due to the antibody-dependent enhancement of infection, which is associated with the transformation of the disease. Two DENV infections in a specific sequence, the interval of two infections, and how much the human host is causative in terms of age, health, ethnicity, and genetic background (Guzman et al., 2013). Generation of cross-reactive antibodies, post-first-time dengue infection in association with the second infecting virus, results in the formation of more harmful immune compounds like non-deactivating antibodies (critical players of the pathogenic response in severe dengue conditions) (Kuczera et al., 2018). This consequently increases the infected cell count and thus increased viral output in the form of high cytokine production, which further causes vascular permeability, intense shock, and, ultimately, death (Puerta-Guardo et al., 2013). Various other immune factors like interferons (IFN-α and -γ), interleukin (IL-6, -8, and -10), and tumor necrosis factors (TNF-α), and elevated expression of cytokine signaling suppressors are linked to severe dengue infections (Bozza et al., 2008; Chen et al., 2014; Flores-Mendoza et al., 2017). Another study revealed a potential immunomodulatory response (against DENV virus) by Schwartzia brasiliensis (Choisy) Bedell ex Gir.-Cañas Choisy extract, which helps in antigen load reduction (NS1 protein, a viral load indicator) in the cells. Crude S. brasiliensis extract was most efficient against dengue activity while the dichloromethane fraction resulted in strong immunoregulatory activities. Downregulation of various immune factors like TNF-α, IFN-α, IL-6, and IL-10 was evidenced as the key reason behind the antiviral effects (Fialho et al., 2017). Significant immunoregulation and antiviral response with Uncaria tomentosa (Willd ex Schult.) DC. fractions have also been demonstrated, in which DENV-infected monocytes (human) were tested. The anti-DENV activity was associated with decreased cytokine levels such as TNF-α and modulation of IL-10 (Reis et al., 2008). Successful molecular assessment and manipulations of various immune responses and associated factors can be useful research to develop anti-dengue drugs. Traditionally, C. papaya leaf extract has been proven for its immunomodulatory activities (Pandey et al., 2016; Anjum et al., 2017; Razak et al., 2021). In dengue patients (suffering from thrombocytopenia), oral administration of papaya leaf extract has resulted in increased platelet count activity (40–48 h) (Subenthiran et al., 2013). In other similar findings with the use of C. papaya leaf extract, beneficial impacts are reported where a significant increase in WBC and platelet count is evidenced with negligible side effects (Hettige 2008; Gowda et al., 2015). Rhodiola imbricata Edgew. (a flowering plant) is also demonstrated to induce pharmacological modifications in response to the DENV infection. It induces NK cells and cytokines like interferon b (IFN), IL-8, IL-1b, IL-6, and TNF-a and upregulates phosphorylated NF-kB, eIF-2a, and PKR in DENV-infected cells (Diwaker et al., 2014). In addition, the immunomodulatory potential against DENV is stated by its ability to regulate cytokine production, phagocytic activity, and white blood cell proliferation (Razak et al., 2021).
10 Complementary and Alternative Dengue Therapeutics
Dengue vaccines have been classified into five main categories, i.e., live attenuated vaccines (CYD-TDV, TV003/TV005), DENVaxin activated virus vaccine (PIV), recombinant subunit vaccine (V180), DNA vaccine (D1ME100, TVDV), and viral vectored vaccine (TLAV Prime/PIV boost and reverse order) (Deng et al., 2020a). Dengvaxia (CYD-TDV) by Sanofi Pasteur (the first dengue vaccine) was registered in several endemic countries for individuals of the age group 9–45 years (Deep et al., 2018; Deng et al., 2020a). It consists of DENV (1–4) serotype structural genes (encoding PrM and E proteins) introduced in the attenuated yellow fever vaccine strain genome. According to the reports, it exerts 56%–61% virucidal efficacy against dengue in Asia and Latin America (Capeding et al., 2014; Biswal et al., 2019). This anti-dengue vaccine is only recommended for patients having evidence of past DENV infection due to infection severity in seronegative candidates via stimulating non-neutralizing antibody generation (Sridhar et al., 2018). This drawback is considerable in the development of other alternatives of anti-dengue drug discovery. Another tetravalent dengue medicine, TAK-003 (Takeda), consisting of live attenuated DENV-2 genetic backbone for all DENV serotypes, has been designed by experts at the Division of Vector-Borne Diseases, Centers for Disease Control and Prevention (CDC) (Huang et al., 2013). Overall vaccine efficiency is documented to be 80.9%, and the seronegative populations showed 74.9% vaccine efficacy for the new dengue vaccine (TAK-003) (Biswal et al., 2019). The vaccination priming with drugs used in combination has been reported to provide more effective immunogenic protection in animals. In addition, analysis of the immune response and the mechanism of viral elements can be a revolutionary success in vaccine development (Liu et al., 2016). Anti-dengue drugs, such as chloroquine, celgosivir, balapiravir, prednisolone, and lovastatin, have been stated to undergone medical examinations against DENV infection (Kaptein and Neyts 2016; Low et al., 2017), although no effective dengue treatment has been developed from any of these composites. Therefore, the search for more effective antiviral compounds (plant-derived or second-use medicines) is still a vital prerequisite in overcoming dengue’s lethal effects (Trujillo-Correa et al., 2019). Besides, the specific anti-dengue vaccine is not yet developed, as few to none of the potential anti-dengue candidates have been tested clinically (Razak et al., 2021).
11 Conclusion
An update about various plants with pharmacological and ethnobotanical uses against dengue treatment was presented. Besides the global status of the dengue epidemic, existing vaccination measures have been conferred in the current review. Solely, the folkloric knowledge and uses of natural resources have been promising due to the exhaustive investigation carried out on the ethnopharmacologically important plant species. Many plant species and their respective extracts and pure compounds have been shown to exhibit potential as anti-DENV medicaments. The plant bioactive metabolites exhibit antiviral response directly or through the stimulation of immunomodulatory response cascades against DENV at different infection stages, i.e., viral adsorption, intracellular replication, and proliferation. Plant metabolites also help to reduce the antigen load and manipulate various immune factors in the host. Plant-mediated immunomodulation leads to the regulated cytokine production, enhanced platelet count, and phagocytic pathway activation. However, there are several plants still used for their anti-dengue properties via traditional methods and are yet to be investigated for scientific approval. Whether the antiviral properties are a result of a single phytoconstituent or the interaction of multiple phytoconstituents present in the plant extract(s) is critical to justify. Thus, extensive research is required to assess the immunologic potentials along with the phytochemical richness of plants and further clinical probes to develop an effective medicine. Another significant aspect of medicinal plants is their insecticidal properties, which make them an eco-friendly and sustainable substitute for Aedes mosquito control. Since vaccine development is a time-consuming process, finding an alternate treatment is crucial to overcome the lethal impacts of the DENV virus. Plant natural compounds are valuable sources to accomplish the speedy discovery of anti-DENV drugs because of their safer use and positive immunomodulatory responses. Nevertheless, the most crucial task is to reveal the molecular machinery of viral components and their capability to mutate rapidly during replication. Moreover, there are some vaccines available to treat dengue infection, but with some limitations. In this scenario, research is being carried out on some medicinal plant species, i.e., Nyctanthes arbor-tristis L., Firmiana simplex (L.) W. Wight, T. sinensis, and Moringa oleifera Lam., to investigate their metabolic profiles supporting their anti-dengue properties (antilarval or anti-DENV, in the authors’ laboratory). Besides, N. arbor-tristis, M. oleifera, and T. sinensis are also reported for their larvicidal activities. Furthermore, the GC-MS profiling of F. simplex also reveals the presence of natural compounds having insecticidal properties against dengue vectors.
Besides, a lot of scientific research is dedicated towards anti-DENV drug development and further research is needed to identify phytochemicals for their antiviral efficacy, mode of action, and successful clinical implementation.
Acknowledgments
The authors are grateful to Manipal University Jaipur, Rajasthan, India, for supporting the present work. The entire team is thankful to Manipal University Jaipur for vital support and amenities.
Author Contributions
MMS: Conceptualization, Validation, Supervision. MD: Writing—original draft preparation, Formal analysis, Validation, Writing—review and editing. LS: Writing—original draft preparation, Formal analysis, Validation, Writing—review and editing. AD: Data collection, Formal analysis. PD: Chemical structure.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
- Abd Kadir S. L., Yaakob H., Mohamed Zulkifli R. (2013). Potential Anti-dengue Medicinal Plants: A Review. J. Nat. Med. 67, 677–689. 10.1007/s11418-013-0767-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdul Ahmad S. A., Palanisamy U. D., Tejo B. A., Chew M. F., Tham H. W., Syed Hassan S. (2017). Geraniin Extracted from the Rind of Nephelium Lappaceum Binds to Dengue Virus Type-2 Envelope Protein and Inhibits Early Stage of Virus Replication. Virol. J. 14, 229. 10.1186/s12985-017-0895-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agrawal J., Pal A. (2013). Nyctanthes Arbor-Tristis Linn--a Critical Ethnopharmacological Review. J. Ethnopharmacol. 146, 645–658. 10.1016/j.jep.2013.01.024 [DOI] [PubMed] [Google Scholar]
- Ahmad N., Fazal H., Ayaz M., Abbasi B. H., Mohammad I., Fazal L. (2011). Dengue Fever Treatment with Carica Papaya Leaves Extracts. Asian Pac. J. Trop. Biomed. 1, 330–333. 10.1016/S2221-1691(11)60055-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahn H., Lee G. S. (2017). Isorhamnetin and Hyperoside Derived from Water Dropwort Inhibits Inflammasome Activation. Phytomedicine 24, 77–86. 10.1016/j.phymed.2016.11.019 [DOI] [PubMed] [Google Scholar]
- Akhtar I. N. (2020). “Viral Genetics and Structure,” in Dengue Virus Disease: From Origin to Outbreak (Academic Press; ), 85–113. 10.1016/B978-0-12-818270-3.00006-0 [DOI] [Google Scholar]
- Alagarasu K., Patil J. A., Kakade M. B., More A. M., Yogesh B., Newase P., et al. (2021). Serotype and Genotype Diversity of Dengue Viruses Circulating in India: a Multi-centre Retrospective Study Involving the Virus Research Diagnostic Laboratory Network in 2018. Int. J. Infect. Dis. 111, 242–252. 10.1016/j.ijid.2021.08.045 [DOI] [PubMed] [Google Scholar]
- Ali A., Tabanca N., Demirci B., Blythe E. K., Ali Z., Baser K. H., et al. (2015). Chemical Composition and Biological Activity of Four Salvia Essential Oils and Individual Compounds against Two Species of Mosquitoes. J. Agric. Food Chem. 63, 447–456. 10.1021/jf504976f [DOI] [PubMed] [Google Scholar]
- Allard P. M., Leyssen P., Martin M. T., Bourjot M., Dumontet V., Eydoux C., et al. (2012). Antiviral Chlorinated Daphnane Diterpenoid Orthoesters from the Bark and wood of Trigonostemon Cherrieri. Phytochemistry 84, 160–168. 10.1016/j.phytochem.2012.07.023 [DOI] [PubMed] [Google Scholar]
- Anjum V., Arora P., Ansari S. H., Najmi A. K., Ahmad S. (2017). Antithrombocytopenic and Immunomodulatory Potential of Metabolically Characterized Aqueous Extract of Carica Papaya Leaves. Pharm. Biol. 55, 2043–2056. 10.1080/13880209.2017.1346690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arman M., Qader S. A. U. (2012). Structural Analysis of Kappa-Carrageenan Isolated from Hypnea Musciformis (Red Algae) and Evaluation as an Elicitor of Plant Defense Mechanism. Carbohydr. Polym. 88, 1264–1271. 10.1016/j.carbpol.2012.02.003 [DOI] [Google Scholar]
- Aruna R. (2014). Review on Dengue Viral Replication, Assembly and Entry into the Host Cells. Int. J. Microb. Appl. Sci. 8, 1. [Google Scholar]
- Arunabha M. (2019). Trigonella Foenum-Graecum: A Review on its Traditional Uses, Phytochemistry and Pharmacology. Int. J. Adv. Scientific Res. 5 (5), e5217. 10.7439/ijasr [DOI] [Google Scholar]
- Bahuguna V., Matura R., Sharma N. (2019). Potential Use of Medicinal Plants for Dengue: a Systematic Mini Review of Scientific Evidence. J. Appl. Life Sci. 2, 10–16. [Google Scholar]
- Baite T. N., Mandal B., Purkait M. K. (2021). Ultrasound Assisted Extraction of Gallic Acid from Ficus Auriculata Leaves Using green Solvent. Food Bioproducts Process. 128, 1–11. 10.1016/j.fbp.2021.04.008 [DOI] [Google Scholar]
- Bartenschlager R., Miller S. (2008). Molecular Aspects of Dengue Virus Replication. Future Microbiol. 3, 155–165. 10.2217/17460913.3.2.155 [DOI] [PubMed] [Google Scholar]
- Baylor N. W., Fu T., Yan Y. D., Ruscetti F. W. (1992). Inhibition of Human T Cell Leukemia Virus by the Plant Flavonoid Baicalin (7-glucuronic Acid, 5,6-dihydroxyflavone). J. Infect. Dis. 165, 433–437. 10.1093/infdis/165.3.433 [DOI] [PubMed] [Google Scholar]
- Begum F., Das S., Mukherjee D., Mal S., Ray U. (2019). Insight into the Tropism of Dengue Virus in Humans. Viruses 11, 1. 10.3390/v11121136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benelli G., Pavela R., Rakotosaona R., Nzekoue F. K., Canale A., Nicoletti M., et al. (2020). Insecticidal and Mosquito Repellent Efficacy of the Essential Oils from Stem Bark and wood of Hazomalania Voyronii. J. Ethnopharmacol. 248, 112333. 10.1016/j.jep.2019.112333 [DOI] [PubMed] [Google Scholar]
- Berlian G., Tandrasasmita O. M., Tjandrawinata R. R. (2017). Trombinol, a Bioactive Fraction of Psidium Guajava , Stimulates Thrombopoietin Expression in HepG2 Cells. Asian Pac. J. Trop. Biomed. 7, 437–442. 10.1016/j.apjtb.2016.09.010 [DOI] [Google Scholar]
- Bharati P., Sinha R. (2012). Study the Effect of Tinospora Cardifolia (wild) Miers and Boerhaavia diffusia Linn on dengue. Int. J. Ayurvedic Herb. Med.. [Google Scholar]
- Bhatt S., Gething P. W., Brady O. J., Messina J. P., Farlow A. W., Moyes C. L., et al. (2013). The Global Distribution and burden of Dengue. Nature 496, 504–507. 10.1038/nature12060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswal S., Reynales H., Saez-Llorens X., Lopez P., Borja-Tabora C., Kosalaraksa P., et al. (2019). Efficacy of a Tetravalent Dengue Vaccine in Healthy Children and Adolescents. N. Engl. J. Med. 381, 2009–2019. 10.1056/nejmoa1903869 [DOI] [PubMed] [Google Scholar]
- Borah M. P., Prasad S. B. (2017). Ethnozoological Study of Animals Based Medicine Used by Traditional Healers and Indigenous Inhabitants in the Adjoining Areas of Gibbon Wildlife Sanctuary, Assam, India. J. Ethnobiol. Ethnomed. 13, 39. 10.1186/s13002-017-0167-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourjot M., Leyssen P., Eydoux C., Guillemot J. C., Canard B., Rasoanaivo P., et al. (2012). Flacourtosides A-F, Phenolic Glycosides Isolated from Flacourtia Ramontchi. J. Nat. Prod. 75, 752–758. 10.1021/np300059n [DOI] [PubMed] [Google Scholar]
- Bozza F. A., Cruz O. G., Zagne S. M., Azeredo E. L., Nogueira R. M., Assis E. F., et al. (2008). Multiplex Cytokine Profile from Dengue Patients: MIP-1beta and IFN-Gamma as Predictive Factors for Severity. BMC Infect. Dis. 8, 86. 10.1186/1471-2334-8-86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brady O. J., Gething P. W., Bhatt S., Messina J. P., Brownstein J. S., Hoen A. G., et al. (2012). Refining the Global Spatial Limits of Dengue Virus Transmission by Evidence-Based Consensus. Plos Negl. Trop. Dis. 6, e1760. 10.1371/journal.pntd.0001760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandão G., Kroon E., Souza D., Filho J., Oliveira A. (2013). Chemistry and Antiviral Activity of Arrabidaea Pulchra (Bignoniaceae). Molecules 18, 9919–9932. 10.3390/molecules18089919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao-Lormeau V. M. (2009). Dengue Viruses Binding Proteins from Aedes aegypti and Aedes polynesiensis Salivary Glands. Virol. J. 6, 35. 10.1186/1743-422X-6-35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capeding M. R., Tran N. H., Hadinegoro S. R., Ismail H. I., Chotpitayasunondh T., Chua M. N., et al. (2014). Clinical Efficacy and Safety of a Novel Tetravalent Dengue Vaccine in Healthy Children in Asia: a Phase 3, Randomised, Observer-Masked, Placebo-Controlled Trial. Lancet 384, 1358–1365. 10.1016/S0140-6736(14)61060-6 [DOI] [PubMed] [Google Scholar]
- Carneiro B. M., Batista M. N., Braga A. C. S., Nogueira M. L., Rahal P. (2016). The green tea Molecule EGCG Inhibits Zika Virus Entry. Virology 496, 215–218. 10.1016/j.virol.2016.06.012 [DOI] [PubMed] [Google Scholar]
- Carr J. M., Ashander L. M., Calvert J. K., Ma Y., Aloia A., Bracho G. G., et al. (2017). Molecular Responses of Human Retinal Cells to Infection with Dengue Virus. Mediators Inflamm. 2017, 3164375. 10.1155/2017/3164375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers T. J., Hahn C. S., Galler R., Rice C. M. (1990). Flavivirus Genome Organization, Expression, and Replication. Annu. Rev. Microbiol. 44, 649–688. 10.1146/annurev.mi.44.100190.003245 [DOI] [PubMed] [Google Scholar]
- Chan M., Johansson M. A. (2012). The Incubation Periods of Dengue Viruses. PLoS One 7, e50972. 10.1371/journal.pone.0050972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaubey M., Kapoor V. P. (2001). Structure of a Galactomannan from the Seeds of Cassia Angustifolia Vahl. Carbohydr. Res. 332, 439–444. 10.1016/S0008-6215(01)00104-5 [DOI] [PubMed] [Google Scholar]
- Chaudhry M. R. A. (2020). “Dengue Virus Infection Outbreak: Comparison with Other Viral Infection Outbreak,” in Dengue Virus Disease: From Origin to Outbreak (IEEE; ), 17–35. 10.1016/B978-0-12-818270-3.00003-5 [DOI] [Google Scholar]
- Chen R. F., Yang K. D., Lee I. K., Liu J. W., Huang C. H., Lin C. Y., et al. (2014). Augmented miR-150 Expression Associated with Depressed SOCS1 Expression Involved in Dengue Haemorrhagic Fever. J. Infect. 69, 366–374. 10.1016/j.jinf.2014.05.013 [DOI] [PubMed] [Google Scholar]
- Chiow K. H., Phoon M. C., Putti T., Tan B. K., Chow V. T. (2016). Evaluation of Antiviral Activities of Houttuynia Cordata Thunb. Extract, Quercetin, Quercetrin and Cinanserin on Murine Coronavirus and Dengue Virus Infection. Asian Pac. J. Trop. Med. 9, 1–7. 10.1016/j.apjtm.2015.12.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow V., Batool R., Aziz E., Mahmood T., Tan B. (2018). Inhibitory Activities of Extracts of Rumex Dentatus, Commelina Benghalensis, Ajuga Bracteosa, Ziziphus Mauritiana as Well as Their Compounds of Gallic Acid and Emodin against Dengue Virus. Asian Pac. J. Trop. Med. 11, 265. 10.4103/1995-7645.231466 [DOI] [Google Scholar]
- Clain E., Haddad J. G., Koishi A. C., Sinigaglia L., Rachidi W., Desprès P., et al. (2019). The Polyphenol-Rich Extract from Psiloxylon Mauritianum, an Endemic Medicinal Plant from Reunion Island, Inhibits the Early Stages of Dengue and Zika Virus Infection. Int. J. Mol. Sci. 20, 1. 10.3390/ijms20081860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clain E., Sinigaglia L., Koishi A. C., Gorgette O., Gadea G., Viranaicken W., et al. (2018). Extract from Aphloia Theiformis, an Edible Indigenous Plant from Reunion Island, Impairs Zika Virus Attachment to the Host Cell Surface. Sci. Rep. 8, 1–12. 10.1038/s41598-018-29183-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coudeville L., Shepard D. S., Zambrano B., Dayan G. (2009). Dengue Economic burden in the Americas: Estimates from Dengue Illness. Am. J. Trop. Med. Hyg. 8, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courageot M. P., Frenkiel M. P., Dos Santos C. D., Deubel V., Desprès P. (2000). Alpha-glucosidase Inhibitors Reduce Dengue Virus Production by Affecting the Initial Steps of Virion Morphogenesis in the Endoplasmic Reticulum. J. Virol. 74, 564–572. 10.1128/jvi.74.1.564-572.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daneshzadeh M. S., Abbaspour H., Amjad L., Nafchi A. M. (2020). An Investigation on Phytochemical, Antioxidant and Antibacterial Properties of Extract from Eryngium Billardieri F. Delaroche. Food Measure 14, 708–715. 10.1007/s11694-019-00317-y [DOI] [Google Scholar]
- Deep P., Srivastava V., Verma S. (2018). Current Perspectives of Medicinal Plants Having Anti Dengue Potential. Int. J. Pharm. Sci. Rev. Res. 8, 1. [Google Scholar]
- Deng S.-Q., Yang X., Wei Y., Chen J.-T., Wang X.-J., Peng H.-J. (2020a). A Review on Dengue Vaccine Development. Vaccines 8, 63. 10.3390/vaccines8010063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng Y., Li S., Wang M., Chen X., Tian L., Wang L., et al. (2020b). Flavonoid-rich Extracts from Okra Flowers Exert Antitumor Activity in Colorectal Cancer through Induction of Mitochondrial Dysfunction-Associated Apoptosis, Senescence and Autophagy. Food Funct. 8, 1. 10.1039/d0fo02081h [DOI] [PubMed] [Google Scholar]
- Dewi B. E., Angelina M., Nuwwaaridya F., Desti H., Sudiro T. M. (2019). Antiviral Activity of Isobutyl Gallate to Dengue Virus Serotype 2 In Vitro . IOP Conf. Ser. Earth Environ. Sci. 251, 012018. 10.1088/1755-1315/251/1/012018 [DOI] [Google Scholar]
- Dewi B. E., Taufiqqurrachman I., Desti H., Sudiro M., Fithriyah, Angelina M. (2020). Inhibition Mechanism of Psidium Guajava Leaf to Dengue Virus Replication In Vitro . IOP Conf. Ser. Earth Environ. Sci. 462, 012034. 10.1088/1755-1315/462/1/012034 [DOI] [Google Scholar]
- Diallo M., Ba Y., Sall A. A., Diop O. M., Ndione J. A., Mondo M., et al. (2003). Amplification of the Sylvatic Cycle of Dengue Virus Type 2, Senegal, 1999-2000: Entomologic Findings and Epidemiologic Considerations. Emerg. Infect. Dis. 9, 362–367. 10.3201/eid0903.020219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diwaker D., Mishra K. P., Ganju L., Singh S. B. (2014). Rhodiola Inhibits Dengue Virus Multiplication by Inducing Innate Immune Response Genes RIG-I, MDA5 and ISG in Human Monocytes. Arch. Virol. 159, 1975–1986. 10.1007/s00705-014-2028-0 [DOI] [PubMed] [Google Scholar]
- Dussart P., Baril L., Petit L., Beniguel L., Quang L. C., Ly S., et al. (2012). Clinical and Virological Study of Dengue Cases and the Members of Their Households: The Multinational Denframe Project. Plos Negl. Trop. Dis. 6, e1482. 10.1371/journal.pntd.0001482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eid H. M., Martineau L. C., Saleem A., Muhammad A., Vallerand D., Benhaddou-Andaloussi A., et al. (2010). Stimulation of AMP-Activated Protein Kinase and Enhancement of Basal Glucose Uptake in Muscle Cells by Quercetin and Quercetin Glycosides, Active Principles of the Antidiabetic Medicinal Plant Vaccinium Vitis-Idaea. Mol. Nutr. Food Res. 54, 991–1003. 10.1002/mnfr.200900218 [DOI] [PubMed] [Google Scholar]
- Esler D. (2009). Dengue - Clinical and Public Health Ramifications. Aust. Fam. Physician 38, 876–879. [PubMed] [Google Scholar]
- European Centre for Disease Prevention and Control (2018). Aedes albopictus - {Factsheet} for Experts. Eur. Cent. Dis. Prev. Control. 1, 1. [Google Scholar]
- Fialho L. G., Da Silva V. P., Reis S. R. N. I., Azeredo E. L., Kaplan M. A. C., Figueiredo M. R., et al. (2017). Antiviral and Immunomodulatory Effects of Norantea Brasiliensis Choisy on Dengue Virus-2. Intervirology 59, 217–227. 10.1159/000455855 [DOI] [PubMed] [Google Scholar]
- Flores-Mendoza L. K., Estrada-Jiménez T., Sedeño-Monge V., Moreno M., Manjarrez M. D. C., González-Ochoa G., et al. (2017). IL-10 and Socs3 Are Predictive Biomarkers of Dengue Hemorrhagic Fever. Mediators Inflamm. 2017, 5197592. 10.1155/2017/5197592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fongnzossie E. F., Nyangono C. F. B., Biwole A. B., Ebai P. N. B., Ndifongwa N. B., Motove J., et al. (2020). Wild Edible Plants and Mushrooms of the Bamenda Highlands in Cameroon: Ethnobotanical Assessment and Potentials for Enhancing Food Security. J. Ethnobiol. Ethnomed. 16, 12. 10.1186/s13002-020-00362-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genoud A. P., Basistyy R., Williams G. M., Thomas B. P. (2018). Optical Remote Sensing for Monitoring Flying Mosquitoes, Gender Identification and Discussion on Species Identification. Appl. Phys. B 124, 1. 10.1007/s00340-018-6917-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govindarajan M., Rajeswary M., Hoti S., Benelli G., Benelli G., Amsath A. (2015). Ovicidal Activity of Pithecellobium dulce (Family: Fabaceae) Leaf and Seed Extracts against Filariasis Vector Mosquito Culex quinquefasciatus (Diptera: Culicidae). J. Med. Herbs Ethnomed 1, 1. 10.5455/jmhe.2015.10.024 [DOI] [Google Scholar]
- Gowda A., Kumar N., Kasture P., Nagabhushan K. (2015). A Pilot Study to Evaluate the Effectiveness of Carica Papaya Leaf Extract in Increasing the Platelet Count in Cases of Dengue with Thrombocytopenia. Available at: http://medind.nic.in/ice/t15/i3/icet15i3p109.pdf.
- Gu R., Wang Y., Long B., Kennelly E., Wu S., Liu B., et al. (2014). Prospecting for Bioactive Constituents from Traditional Medicinal Plants through Ethnobotanical Approaches. Biol. Pharm. Bull. 37, 903–915. 10.1248/bpb.b14-00084 [DOI] [PubMed] [Google Scholar]
- Gubler D. J. (1989). Aedes aegypti and Aedes aegypti-borne Disease Control in the 1990s: Top Down or Bottom up. Charles Franklin Craig Lecture. Am. J. Trop. Med. Hyg. 40, 571–578. 10.4269/ajtmh.1989.40.571 [DOI] [PubMed] [Google Scholar]
- Guzman A., Istúriz R. E. (2010). Update on the Global Spread of Dengue. Int. J. Antimicrob. Agents 36, S40–S42. 10.1016/j.ijantimicag.2010.06.018 [DOI] [PubMed] [Google Scholar]
- Guzman M. G., Alvarez M., Halstead S. B. (2013). Secondary Infection as a Risk Factor for Dengue Hemorrhagic Fever/dengue Shock Syndrome: An Historical Perspective and Role of Antibody-dependent Enhancement of Infection. Arch. Virol. 158, 1445–1459. 10.1007/s00705-013-1645-3 [DOI] [PubMed] [Google Scholar]
- Guzman M. G., Gubler D. J., Izquierdo A., Martinez E., Halstead S. B. (2016). Dengue Infection. Nat. Rev. Dis. Primers 2, 16055. 10.1038/nrdp.2016.55 [DOI] [PubMed] [Google Scholar]
- Haddad J. G., Koishi A. C., Gaudry A., Nunes Duarte Dos Santos C., Viranaicken W., Desprès P., et al. (2019). Doratoxylon Apetalum, an Indigenous Medicinal Plant from Mascarene Islands, Is a Potent Inhibitor of Zika and Dengue Virus Infection in Human Cells. Ijms 20, 2382. 10.3390/ijms20102382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall A., Troupin A., Londono-Renteria B., Colpitts T. M. (2017). Garlic Organosulfur Compounds Reduce Inflammation and Oxidative Stress during Dengue Virus Infection. Viruses 9, 159. 10.3390/v9070159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halstead S. B. (2002). Dengue Hemorrhagic Fever: Two Infections and Antibody Dependent Enhancement, a Brief History and Personal Memoir. Rev. Cubana Med. Trop. 54, 171–179. [PubMed] [Google Scholar]
- Halstead S. B. (1988). Pathogenesis of Dengue: Challenges to Molecular Biology. Science 239, 476–481. 10.1126/science.3277268 [DOI] [PubMed] [Google Scholar]
- Han Y., Bu L. M., Ji X., Liu C. Y., Wang Z. H. (2005). Modulation of Multidrug Resistance by Andrographolid in a HCT-8/5-FU Multidrug-Resistant Colorectal Cancer Cell Line. Chin. J. Dig. Dis. 6, 82–86. 10.1111/j.1443-9573.2005.00197.x [DOI] [PubMed] [Google Scholar]
- Hari I., Mathew N. (2018). Larvicidal Activity of Selected Plant Extracts and Their Combination against the Mosquito Vectors Culex quinquefasciatus and Aedes aegypti . Environ. Sci. Pollut. Res. Int. 25, 9176–9185. 10.1007/s11356-018-1515-3 [DOI] [PubMed] [Google Scholar]
- Hassandarvish P., Rothan H. A., Rezaei S., Yusof R., Abubakar S., Zandi K. (2016). In Silico study on Baicalein and Baicalin as Inhibitors of Dengue Virus Replication. RSC Adv. 6, 31235–31247. 10.1039/c6ra00817h [DOI] [Google Scholar]
- Häusler H., Kawakami R. P., Mlaker E., Severn W. B., Wrodnigg T. M., Stütz A. E. (2000). Sugar Analogues with Basic Nitrogen in the Ring as Anti-infectives. J. Carbohydr. Chem. 19, 435–449. 10.1080/07328300008544092 [DOI] [Google Scholar]
- Huang C. Y., Kinney R. M., Livengood J. A., Bolling B., Arguello J. J., Luy B. E., et al. (2013). Genetic and Phenotypic Characterization of Manufacturing Seeds for a Tetravalent Dengue Vaccine (DENVax). Plos Negl. Trop. Dis. 7, e2243. 10.1371/journal.pntd.0002243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang N. C., Hung W. T., Tsai W. L., Lai F. Y., Lin Y. S., Huang M. S., et al. (2017). Ficus Septica Plant Extracts for Treating Dengue Virus In Vitro . PeerJ 5, e3448. 10.7717/peerj.3448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussain J., Ali L., Khan A. L., Rehman N. U., Jabeen F., Kim J. S., et al. (2014). Isolation and Bioactivities of the Flavonoids Morin and Morin-3-O-β-D-Glucopyranoside from Acridocarpus Orientalis-A Wild Arabian Medicinal Plant. Molecules 19, 17763–17772. 10.3390/molecules191117763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang Y. S., Wu K. H., Kumamoto J., Axelrod H., Mulla M. S. (1985). Isolation and Identification of Mosquito Repellents inArtemisia Vulgaris. J. Chem. Ecol. 11, 1297–1306. 10.1007/BF01024117 [DOI] [PubMed] [Google Scholar]
- Ichsyani M., Ridhanya A., Risanti M., Desti H., Ceria R., Putri D. H., et al. (2017). Antiviral Effects of Curcuma Longa L. Against Dengue Virus In Vitro and In Vivo . IOP Conf. Ser. Earth Environ. Sci. 101, 012005. 10.1088/1755-1315/101/1/012005 [DOI] [Google Scholar]
- Jasso-Miranda C., Herrera-Camacho I., Flores-Mendoza L. K., Dominguez F., Vallejo-Ruiz V., Sanchez-Burgos G. G., et al. (2019). Antiviral and Immunomodulatory Effects of Polyphenols on Macrophages Infected with Dengue Virus Serotypes 2 and 3 Enhanced or Not with Antibodies. Infect. Drug Resist. 12, 1833–1852. 10.2147/IDR.S210890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayadevappa M. K., Karkera P. R., Siddappa R. Y., Telkar S., Karunakara P. (2020). Investigation of Plant Flavonoids as Potential Dengue Protease Inhibitors. J. Herbmed Pharmacol. 9, 366–373. 10.34172/jhp.2020.46 [DOI] [Google Scholar]
- Jiang Z., Okimura T., Yokose T., Yamasaki Y., Yamaguchi K., Oda T. (2010). Effects of Sulfated Fucan, Ascophyllan, from the Brown Alga Ascophyllum Nodosum on Various Cell Lines: A Comparative Study on Ascophyllan and Fucoidan. J. Biosci. Bioeng. 110, 113–117. 10.1016/j.jbiosc.2010.01.007 [DOI] [PubMed] [Google Scholar]
- Juliana C., Lister I. N. E., Girsang E., Nasution A. N., Widowati W. (2020). Antioxidant and Elastase Inhibitor from Black Soybean (Glycine max L.) and its Compound (Daidzein). J. Biomed. Transl. Res. 6, 11–14. 10.14710/jbtr.v6i1.5540 [DOI] [Google Scholar]
- Kaptein S. J., Neyts J. (2016). Towards Antiviral Therapies for Treating Dengue Virus Infections. Curr. Opin. Pharmacol. 30, 1–7. 10.1016/j.coph.2016.06.002 [DOI] [PubMed] [Google Scholar]
- Kaushik S., Dar L., Kaushik S., Yadav J. P. (2021). Identification and Characterization of New Potent Inhibitors of Dengue Virus NS5 Proteinase from Andrographis Paniculata Supercritical Extracts on in Animal Cell Culture and In Silico Approaches. J. Ethnopharmacol. 267, 113541. 10.1016/j.jep.2020.113541 [DOI] [PubMed] [Google Scholar]
- Khan A. M., Bhutta Z. A. (2017). “Childhood Infectious Diseases: Overview,” in International Encyclopedia of Public Health (Elsevier; ), 517–538. 10.1016/B978-0-12-803678-5.00065-5 [DOI] [Google Scholar]
- Khanh Pham N., Tuan Nguyen H., Binh Nguyen Q. (2021). A Review on the Ethnomedicinal Uses, Phytochemistry and Pharmacology of Plant Species Belonging to Kaempferia Genus (Zingiberaceae). Pharm. Sci. Asia 48, 1–24. 10.29090/PSA.2021.01.19.070 [DOI] [Google Scholar]
- Kiat T. S., Pippen R., Yusof R., Ibrahim H., Khalid N., Rahman N. A. (2006). Inhibitory Activity of Cyclohexenyl Chalcone Derivatives and Flavonoids of Fingerroot, Boesenbergia Rotunda (L.), towards Dengue-2 Virus NS3 Protease. Bioorg. Med. Chem. Lett. 16, 3337–3340. 10.1016/j.bmcl.2005.12.075 [DOI] [PubMed] [Google Scholar]
- Kim J., Hwang E.-S. (2020). Multiplexed Diagnosis of Four Serotypes of Dengue Virus by Real-Time RT-PCR. Biochip J. 14, 421–428. 10.1007/s13206-020-4409-7 [DOI] [Google Scholar]
- Kim S. J., Um J. Y., Lee J. Y., Lee J. Y. (2011). Anti-inflammatory Activity of Hyperoside through the Suppression of Nuclear Factor-Κb Activation in Mouse Peritoneal Macrophages. Am. J. Chin. Med. 39, 171–181. 10.1142/S0192415X11008737 [DOI] [PubMed] [Google Scholar]
- Kuczera D., Assolini J. P., Tomiotto-Pellissier F., Pavanelli W. R., Silveira G. F. (2018). Highlights for Dengue Immunopathogenesis: Antibody-dependent Enhancement, Cytokine Storm, and beyond. J. Interferon Cytokine Res. 38, 69–80. 10.1089/jir.2017.0037 [DOI] [PubMed] [Google Scholar]
- Kularatne S. A. M. (2008). Dengue Fever. Sri Lankan Fam. Physician 1, h4661. 10.1136/bmj.h4661 [DOI] [Google Scholar]
- Kumar A., Rongpharpi S. R., Dewan Duggal S., Gur R., Choudhary S., Khare P. (2017). Clinical, Epidemiological and Microbiological Profile of Dengue Fever at a Tertiary Care Hospital in Delhi, India. J. Infect. Dis. Med. 02, 1. 10.4172/2576-1420.1000110 [DOI] [Google Scholar]
- Lambrechts L., Scott T. W., Gubler D. J. (2010). Consequences of the Expanding Global Distribution of Aedes albopictus for Dengue Virus Transmission. Plos Negl. Trop. Dis. 4, e646. 10.1371/journal.pntd.0000646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larocca D. G., Júnior N. G. R., Vicente R., Silva I. V. (2021). Ethnobotanical Treatment of Tropical Diseases, Malaria and Dengue, Prescribed by Bioenergetico Practitioners and Profile of the Involved Population in Meridional Amazon. Revista Etnobiología. 19, 114–128. [Google Scholar]
- Latgé J. P., Debeaupuis J. P., Moutaouakil M., Diaquin M., Sarfati J., Prévost M. C., et al. (1991). Galactomannan and the Circulating Antigens of Aspergillus fumigatus. Springer, 143–155. 10.1007/978-3-642-76074-7_11 [DOI] [Google Scholar]
- Leardkamolkarn V., Sirigulpanit W., Phurimsak C., Kumkate S., Himakoun L., Sripanidkulchai B. (2012). The Inhibitory Actions of Houttuynia Cordata Aqueous Extract on Dengue Virus and Dengue-Infected Cells. J. Food Biochem. 36, 86–92. 10.1111/j.1745-4514.2010.00514.x [DOI] [Google Scholar]
- Lee Y. R., Yeh S. F., Ruan X. M., Zhang H., Hsu S. D., Huang H. D., et al. (2017). Honeysuckle Aqueous Extract and Induced Let-7a Suppress Dengue Virus Type 2 Replication and Pathogenesis. J. Ethnopharmacol. 198, 109–121. 10.1016/j.jep.2016.12.049 [DOI] [PubMed] [Google Scholar]
- Li S., Zhang Z., Cain A., Wang B., Long M., Taylor J. (2005). Antifungal Activity of Camptothecin, Trifolin, and Hyperoside Isolated from Camptotheca Acuminata. J. Agric. Food Chem. 53, 32–37. 10.1021/jf0484780 [DOI] [PubMed] [Google Scholar]
- Lim S. J., Wan Aida W. M., Schiehser S., Rosenau T., Böhmdorfer S. (2019). Structural Elucidation of Fucoidan from Cladosiphon Okamuranus (Okinawa Mozuku). Food Chem. 272, 222–226. 10.1016/j.foodchem.2018.08.034 [DOI] [PubMed] [Google Scholar]
- Ling A. P. K., Khoo B. F., Seah C. H., Foo K. Y., Cheah R. K., Chye S. M., et al. (2014). “Inhibitory Activities of Methanol Extracts of Andrographis Paniculata and Ocimum Sanctum against Dengue-1 Virus,” in International Conference on Biological Environmental and Food Engineering (Bali, Indonesia: IEEE; ). 10.15242/iicbe.c814013 [DOI] [Google Scholar]
- Liu Y. X., Li F. X., Liu Z. Z., Jia Z. R., Zhou Y. H., Zhang H., et al. (2016). Integrated Analysis of miRNAs and Transcriptomes in Aedes albopictus Midgut Reveals the Differential Expression Profiles of Immune-Related Genes during Dengue Virus Serotype-2 Infection. Insect Sci. 23, 377–385. 10.1111/1744-7917.12339 [DOI] [PubMed] [Google Scholar]
- Low J. G., Ooi E. E., Vasudevan S. G. (2017). Current Status of Dengue Therapeutics Research and Development. J. Infect. Dis. 215, S96. 10.1093/infdis/jiw423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macedo T., Ribeiro V., Oliveira A. P., Pereira D. M., Fernandes F., Gomes N. G. M., et al. (2020). Anti-inflammatory Properties of Xylopia Aethiopica Leaves: Interference with Pro-inflammatory Cytokines in THP-1-Derived Macrophages and Flavonoid Profiling. J. Ethnopharmacol. 248, 112312. 10.1016/j.jep.2019.112312 [DOI] [PubMed] [Google Scholar]
- Marcial‐Juárez E., Yam‐Puc J. C., Cedillo‐Barrón L., García‐Cordero J., Calderón‐Amador J., Maqueda‐Alfaro R. A., et al. (2017). “Travelling with Dengue: from the Skin to the Nodes,” in Dengue - Immunopathology and Control Strategies (London, United Kingdom: WHO; ). 10.5772/intechopen.68338 [DOI] [Google Scholar]
- Marudhupandi T., Kumar T. T. A. (2013). Antibacterial Effect of Fucoidan from Sargassum Wightii against the Chosen Human Bacterial Pathogens. Int. Curr. Pharm. J. 2, 156–158. 10.3329/icpj.v2i10.16408 [DOI] [Google Scholar]
- Mathur V., Mathur N. K. (2005). Fenugreek and Other Lesser Known Legume Galactomannan-Polysaccharides: Scope for Developments. New Delhi, India: J. Sci. Ind. Res. (India). [Google Scholar]
- Mbadiko C. M., Inkoto C. L., Gbolo B. Z., Lengbiye E. M., Kilembe J. T., Matondo A., et al. (2020). A Mini Review on the Phytochemistry, Toxicology and Antiviral Activity of Some Medically Interesting Zingiberaceae Species. Jocamr 8, 44–56. 10.9734/jocamr/2020/v9i430150 [DOI] [Google Scholar]
- McDowell M., Gonzales S. R., Kumarapperuma S. C., Jeselnik M., Arterburn J. B., Hanley K. A. (2010). A Novel Nucleoside Analog, 1-Beta-D-Ribofuranosyl-3-Ethynyl-[1,2,4]triazole (ETAR), Exhibits Efficacy against a Broad Range of Flaviviruses In Vitro . Antivir. Res 87, 78–80. 10.1016/j.antiviral.2010.04.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendoza A., Silva M., Castro E. A. (2020). Etnobotánica medicinal de comunidades Ñuu Savi de la Montaña de Guerrero. México. Etnobiología 2, 1. [Google Scholar]
- Miller J. L., Tyrrell B. E., Zitzmann N. (2018). Mechanisms of Antiviral Activity of Iminosugars against Dengue Virus. Adv. Exp. Med. Biol. 18, 277–301. 10.1007/978-981-10-8727-1_20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minh Ly B., Quoc Buu N., Duy Nhut N., Duc Thinh P., Thi Thanh Van T. (2017). Studies on Fucoidan and its Production from Vietnamese Brown Seaweeds. Ajstd 22, 371. 10.29037/ajstd.173 [DOI] [Google Scholar]
- Mir M., Khurshid R., Aftab R. (2012). Management of Thrombocytopenia and Flu-like Symptoms in Dengue Patients with Herbal Water of Euphorbia Hirta. J. Ayub Med. Coll. Abbottabad. 24, 6–9. [PubMed] [Google Scholar]
- Moghaddam E., Teoh B. T., Sam S. S., Lani R., Hassandarvish P., Chik Z., et al. (2014). Baicalin, a Metabolite of Baicalein with Antiviral Activity against Dengue Virus. Sci. Rep. 4, 5452. 10.1038/srep05452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohd Abd Razak M. R., Norahmad N. A., Md Jelas N. H., Afzan A., Mohmad Misnan N., Mat Ripen A., et al. (2021). Immunomodulatory Activities of Carica Papaya L. Leaf Juice in a Non-lethal, Symptomatic Dengue Mouse Model. Pathogens 10, 501. 10.3390/pathogens10050501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molinari M., Helenius A. (1999). Glycoproteins Form Mixed Disulphides with Oxidoreductases during Folding in Living Cells. Nature 402, 90–93. 10.1038/47062 [DOI] [PubMed] [Google Scholar]
- Murhekar M. V., Kamaraj P., Kumar M. S., Khan S. A., Allam R., Barde P., et al. (2019). Burden of Dengue Infection in India, 2017: A Cross-Sectional Population Based Serosurvey. Lancet Glob. Heal. 7 (8), e1065–e1073. 10.1016/S2214-109X(19)30250-5 [DOI] [PubMed] [Google Scholar]
- Murphy B. R., Whitehead S. S. (2011). Immune Response to Dengue Virus and Prospects for a Vaccine. Annu. Rev. Immunol. 29, 587–619. 10.1146/annurev-immunol-031210-101315 [DOI] [PubMed] [Google Scholar]
- Mussin J., Giusiano G. (2020). Ethno-Phytopharmacology: Product Validation Process Based on Traditional Knowledge of Medicinal Plants. in Agric. For. Bioindustry Biotechnol. Biodiscovery 43, 331–353. 10.1007/978-3-030-51358-0_17 [DOI] [Google Scholar]
- Nascimento A. C., Valente L. M. M., Gomes M., Barboza R. S., Wolff T., Neris R. L. S., et al. (2017). Antiviral Activity of Faramea Bahiensis Leaves on Dengue Virus Type-2 and Characterization of a New Antiviral Flavanone Glycoside. Phytochemistry Lett. 19, 220–225. 10.1016/j.phytol.2017.01.013 [DOI] [Google Scholar]
- Nasir S., Batool M., Qureshi N. A., Debboun M., Qamer S., Nasir I., et al. (2015). Repellency of Medicinal Plant Extracts against Dengue Vector Mosquitoes, Aedes albopictus and Ae. Aegypti (Diptera: Culicidae). Pak. J. Zool. 2, 1. [Google Scholar]
- Nile S. H., Nile A. S., Keum Y. S., Sharma K. (2017). Utilization of Quercetin and Quercetin Glycosides from Onion (Allium cepa L.) Solid Waste as an Antioxidant, Urease and Xanthine Oxidase Inhibitors. Food Chem. 235, 119–126. 10.1016/j.foodchem.2017.05.043 [DOI] [PubMed] [Google Scholar]
- Norahmad N. A., Mohd Abd Razak M. R., Mohmad Misnan N., Md Jelas N. H., Sastu U. R., Muhammad A., et al. (2019). Effect of Freeze-Dried Carica Papaya Leaf Juice on Inflammatory Cytokines Production during Dengue Virus Infection in AG129 Mice. BMC Complement. Altern. Med. 19, 44. 10.1186/s12906-019-2438-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nur Fatin Nazurah R., Nur Hanani Z. A. (2017). Physicochemical Characterization of Kappa-Carrageenan (Euchema Cottoni) Based Films Incorporated with Various Plant Oils. Carbohydr. Polym. 157, 1479–1487. 10.1016/j.carbpol.2016.11.026 [DOI] [PubMed] [Google Scholar]
- NVBDCP (2021). Dengue/DHF Situation In INDIA. Available at: https://nvbdcp.gov.in/index4.php?lang=1&level=0&linkid=431&lid=3715 (Accessed June, 2021). [Google Scholar]
- Ocazionez R. E., Meneses R., Torres F. A., Stashenko E. (2010). Virucidal Activity of Colombian Lippia Essential Oils on Dengue Virus Replication In Vitro . Mem. Inst. Oswaldo Cruz 105, 304–309. 10.1590/S0074-02762010000300010 [DOI] [PubMed] [Google Scholar]
- Ono L., Wollinger W., Rocco I. M., Coimbra T. L., Gorin P. A., Sierakowski M. R. (2003). In Vitro and In Vivo Antiviral Properties of Sulfated Galactomannans against Yellow Fever Virus (BeH111 Strain) and Dengue 1 Virus (Hawaii Strain). Antivir. Res 60, 201–208. 10.1016/S0166-3542(03)00175-X [DOI] [PubMed] [Google Scholar]
- Padbidri V. S., Wairagkar N. S., Joshi G. D., Umarani U. B., Risbud A. R., Gaikwad D. L. (2002). A Serological Survey of Arboviral Diseases Among the Human Population of the Andaman and Nicobar Islands, India. Southeast Asian J. Trop. Med. Public Health 33, 794–800. [PubMed] [Google Scholar]
- Palanisamy S., Vinosha M., Marudhupandi T., Rajasekar P., Prabhu N. M. (2017). Isolation of Fucoidan from Sargassum Polycystum Brown Algae: Structural Characterization, In Vitro Antioxidant and Anticancer Activity. Int. J. Biol. Macromol. 102, 405–412. 10.1016/j.ijbiomac.2017.03.182 [DOI] [PubMed] [Google Scholar]
- Pan American Health Organization (PAHO) (2016). Dengue. United States: Guidelines for patient care in the region of the Americas. [Google Scholar]
- Pan Y. T., Ghidoni J., Elbein A. D. (1993). The Effects of Castanospermine and Swainsonine on the Activity and Synthesis of Intestinal Sucrase. Arch. Biochem. Biophys. 303, 134–144. 10.1006/abbi.1993.1264 [DOI] [PubMed] [Google Scholar]
- Pandey S., Cabot P. J., Shaw P. N., Hewavitharana A. K. (2016). Anti-inflammatory and Immunomodulatory Properties of Carica Papaya. J. Immunotoxicol 13, 590–602. 10.3109/1547691X.2016.1149528 [DOI] [PubMed] [Google Scholar]
- Panya A., Sawasdee N., Songprakhon P., Tragoolpua Y., Rotarayanont S., Choowongkomon K., et al. (2020). A Synthetic Bioactive Peptide Derived from the Asian Medicinal Plant Acacia Catechu Binds to Dengue Virus and Inhibits Cell Entry. Viruses 12, 1. 10.3390/v12111267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panya A., Yongpitakwattana P., Budchart P., Sawasdee N., Krobthong S., Paemanee A., et al. (2019). Novel Bioactive Peptides Demonstrating Anti-dengue Virus Activity Isolated from the Asian Medicinal Plant Acacia Catechu. Chem. Biol. Drug Des. 93, 100–109. 10.1111/cbdd.13400 [DOI] [PubMed] [Google Scholar]
- Parida M. M., Upadhyay C., Pandya G., Jana A. M. (2002). Inhibitory Potential of Neem (Azadirachta indica Juss) Leaves on Dengue Virus Type-2 Replication. J. Ethnopharmacol. 79, 273–278. 10.1016/S0378-8741(01)00395-6 [DOI] [PubMed] [Google Scholar]
- Pasa A. E. (2011). Biodiversity Study of a Smallholder-Protected forest Ecosystem in Leyte, Philippines. Biodiversity 12, 38–45. 10.1080/14888386.2011.573702 [DOI] [Google Scholar]
- Paul A., Vibhuti A., Raj V. S. (2021). Evaluation of Antiviral Activity of Andrographis Paniculata and Tinospora Cordifolia Using In Silico and In Vitro Assay against DENV-2. J. Pharmacogn. Phytochem. 10, 486–496. 10.22271/phyto.2021.v10.i2f.13847 [DOI] [Google Scholar]
- Pilla M. A. C., Amorozo M. C. d. M., Furlan A. (2006). Obtenção e uso das plantas medicinais no distrito de Martim Francisco, Município de Mogi-Mirim, SP, Brasil. Acta Bot. Bras. 20, 789–802. 10.1590/s0102-33062006000400005 [DOI] [Google Scholar]
- Pinheiro F. P., Corber S. J. (1997). Global Situation of Dengue and Dengue Haemorrhagic Fever, and its Emergence in the Americas. Geneva, Switzerland: World Heal. Stat. Q. [PubMed] [Google Scholar]
- Pinheiro F. (1997). Re-Emergence of Dengue and Emergence of Dengue Haemorrhagic Fever in the Americas. Geneva, Switzerland: WHO Regional Office for South-East Asia. [Google Scholar]
- Pinheiro F., Rodrigues S. (1999). Aedes aegypti, Dengue and Re-urbanization of Yellow Fever in Brazil and Other South American Countries Past and Present Situation and Future Presentative. Geneva, Switzerland: WHO. [Google Scholar]
- Pongkitwitoon B., Sakamoto S., Tanaka H., Tsuchihashi R., Kinjo J., Morimoto S., et al. (2011). Development of an Enzyme-Linked Immunosorbent Assay to Determine Puerarin and its Aglycone Daidzein. J. Nat. Med. 65, 31–36. 10.1007/s11418-010-0448-z [DOI] [PubMed] [Google Scholar]
- Pongthanapisith V., Ikuta K., Puthavathana P., Leelamanit W. (2013). Antiviral Protein of Momordica Charantia L. Inhibits Different Subtypes of Influenza A. Evid. Based Complement. Alternat Med. 2013, 729081. 10.1155/2013/729081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powers C. N., Setzer W. N. (2016). An In-Silico Investigation of Phytochemicals as Antiviral Agents against Dengue Fever. Comb. Chem. High Throughput Screen. 19, 516–536. 10.2174/1386207319666160506123715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prajapati V. D., Jani G. K., Moradiya N. G., Randeria N. P., Nagar B. J., Naikwadi N. N., et al. (2013). Galactomannan: A Versatile Biodegradable Seed Polysaccharide. Int. J. Biol. Macromol. 60, 83–92. 10.1016/j.ijbiomac.2013.05.017 [DOI] [PubMed] [Google Scholar]
- Prakash Kala C. (2012). Leaf Juice of Carica Papaya L.: A Remedy of Dengue Fever. Med. Aromatic Plants 01, 1. 10.4172/2167-0412.1000109 [DOI] [Google Scholar]
- Pratheeba T., Taranath V., Sai Gopal D., Natarajan D. (2019). Antidengue Potential of Leaf Extracts of Pavetta Tomentosa and Tarenna Asiatica (Rubiaceae) against Dengue Virus and its Vector Aedes aegypti (Diptera: Culicidae). Heliyon 5, e02732. 10.1016/j.heliyon.2019.e02732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puerta-Guardo H., Raya-Sandino A., González-Mariscal L., Rosales V. H., Ayala-Dávila J., Chávez-Mungía B., et al. (2013). The Cytokine Response of U937-Derived Macrophages Infected through Antibody-dependent Enhancement of Dengue Virus Disrupts Cell Apical-junction Complexes and Increases Vascular Permeability. J. Virol. 87, 7486–7501. 10.1128/jvi.00085-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raekiansyah M., Buerano C. C., Luz M. A. D., Morita K. (2018). Inhibitory Effect of the green tea Molecule EGCG against Dengue Virus Infection. Arch. Virol. 163, 1649–1655. 10.1007/s00705-018-3769-y [DOI] [PubMed] [Google Scholar]
- Rahman N. A., HadinurMuliawan S., Rashid N. N., Muhamad M., Yusof R. (2006). Studies on Quercus Iusitanica Extracts on DENV-2 Replication. Dengue Bull. 1, 1. [Google Scholar]
- Rao V. B., Yeturu K. (2020). Possible Anti-viral Effects of Neem (Azadirachta indica) on Dengue Virus. United States: bioRxiv. 10.1016/s0378-8741(01)00395-6 [DOI] [Google Scholar]
- Rasool N., Ashraf A., Waseem M., Hussain W., Mahmood S. (2019). Computational Exploration of Antiviral Activity of Phytochemicals against NS2B/NS3 Proteases from Dengue Virus. Turkish J. Biochem. 44, 261–277. 10.1515/tjb-2018-0002 [DOI] [Google Scholar]
- Rathore A. P., Paradkar P. N., Watanabe S., Tan K. H., Sung C., Connolly J. E., et al. (2011). Celgosivir Treatment Misfolds Dengue Virus NS1 Protein, Induces Cellular Pro-survival Genes and Protects against Lethal challenge Mouse Model. Antivir. Res 92, 453–460. 10.1016/j.antiviral.2011.10.002 [DOI] [PubMed] [Google Scholar]
- Redoni M., Yacoub S., Rivino L., Giacobbe D. R., Luzzati R., Di Bella S. (2020). Dengue: Status of Current and Under-development Vaccines. Rev. Med. Virol. 30, e2101. 10.1002/rmv.2101 [DOI] [PubMed] [Google Scholar]
- Rees C. R., Costin J. M., Fink R. C., McMichael M., Fontaine K. A., Isern S., et al. (2008). In Vitro inhibition of Dengue Virus Entry by P-Sulfoxy-Cinnamic Acid and Structurally Related Combinatorial Chemistries. Antivir. Res 80, 135–142. 10.1016/j.antiviral.2008.05.007 [DOI] [PubMed] [Google Scholar]
- Reis S. R., Valente L. M., Sampaio A. L., Siani A. C., Gandini M., Azeredo E. L., et al. (2008). Immunomodulating and Antiviral Activities of Uncaria Tomentosa on Human Monocytes Infected with Dengue Virus-2. Int. Immunopharmacol. 8, 468–476. 10.1016/j.intimp.2007.11.010 [DOI] [PubMed] [Google Scholar]
- Rico-Hesse R. (2010). Dengue Virus Virulence and Transmission Determinants. Curr. Top. Microbiol. Immunol. 338, 45–55. 10.1007/978-3-642-02215-9_4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigau-Pérez J. G. (2006). Severe Dengue: the Need for New Case Definitions. Lancet Infect. Dis. 3, 1. 10.1016/S1473-3099(06)70465-0 [DOI] [PubMed] [Google Scholar]
- Robinson M. M., Zhang X. (2011). Traditional Medicines. Geneva, Switzerland: global situation, issues and challenges. [Google Scholar]
- Rodrigues A., Morais S., Martins V. E. (2020). Larvicidal Efficacy of Plant Extracts and Isolated Compounds from Annonaceae and Piperaceae against Aedes aegypti and Aedes albopictus . Asian Pac. J. Trop. Med. 13, 384. 10.4103/1995-7645.290583 [DOI] [Google Scholar]
- Rodríguez-Barraquer I., Solomon S. S., Kuganantham P., Srikrishnan A. K., Vasudevan C. K., Qbal S. H., et al. (2015). The Hidden Burden of Dengue and Chikungunya in Chennai, India. PLoS Negl. Trop. Dis. 9, e0003906. 10.1371/journal.pntd.0003906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojsanga P., Bunsupa S., Sithisarn P. (2020). Flavones Contents in Extracts from Oroxylum Indicum Seeds and Plant Tissue Cultures. Molecules 25, 1. 10.3390/molecules25071545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosmalena R., Elya B., Dewi B. E., Fithriyah F., Desti H., Angelina M., et al. (2019). The Antiviral Effect of Indonesian Medicinal Plant Extracts against Dengue Virus In Vitro and In Silico. Pathogens 8, 1. 10.3390/pathogens8020085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothan H. A., Zulqarnain M., Ammar Y. A., Tan E. C., Rahman N. A., Yusof R. (2014). Screening of Antiviral Activities in Medicinal Plants Extracts against Dengue Virus Using Dengue NS2B-NS3 Protease Assay. Trop. Biomed. 31, 286–296. [PubMed] [Google Scholar]
- Saleh M. S. M., Kamisah Y. (2021). Potential Medicinal Plants for the Treatment of Dengue Fever and Severe Acute Respiratory Syndrome-Coronavirus. Biomolecules 11, 42. 10.3390/biom11010042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salles T. S., da Encarnação Sá-Guimarães T., De Alvarenga E. S. L., Guimarães-Ribeiro V., De Meneses M. D. F., De Castro-Salles P. F., et al. (2018). History, Epidemiology and Diagnostics of Dengue in the American and Brazilian Contexts: a Review. Parasites Vectors 11, 1. 10.1186/s13071-018-2830-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sánchez I., Gómez-Garibay F., Taboada J., Ruiz B. H. (2000). Antiviral Effect of Flavonoids on the Dengue Virus. Phyther. Res. 1, 1. [DOI] [PubMed] [Google Scholar]
- Saptawati L., Febrinasari R. P., Yudhani R. D., Yono H., Faza A. G., Luthfiani S., et al. (2017). In Vitro study of Eight Indonesian Plants Extracts as Anti Dengue Virus. Health Sci. J. Indonesia 8, 1. 10.22435/hsji.v8i1.6601.12-18 [DOI] [Google Scholar]
- Sarala N., Paknikar S. (2014). Papaya Extract to Treat Dengue: A Novel Therapeutic Option? Ann. Med. Health Sci. Res. 4, 320–324. 10.4103/2141-9248.133452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarker M. M. R., Khan F., Mohamed I. N. (2021). Dengue Fever: Therapeutic Potential of Carica Papaya L. Leaves. Front. Pharmacol. 12, 1. 10.3389/fphar.2021.610912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxena S. K., Haikerwal A., Gadugu S., Bhatt M. L. (2017). Complementary and Alternative Medicine in alliance with Conventional Medicine for Dengue Therapeutics and Prevention. Future Virol. 12, 399–402. 10.2217/fvl-2017-0047 [DOI] [Google Scholar]
- Schneider C. A., Calvo E., Peterson K. E. (2021). Arboviruses: How Saliva Impacts the Journey from Vector to Host. Int. J. Mol. Sci. 22, 9173. 10.3390/ijms22179173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah P. S., Deoshatwar A., Karad S., Mhaske S., Singh A., Bachal R. V., et al. (2017). Seroprevalence of Dengue in a Rural and an Urbanized Village: A Pilot Study From Rural Western India. J. Vector Borne Dis. 54 (2), 172. 176. [PubMed] [Google Scholar]
- Sharma A., Kashyap D., Sak K., Tuli H. S., Sharma A. K. (2018). Therapeutic Charm of Quercetin and its Derivatives: A Review of Research and Patents. Pharm. Pat. Anal. 7 (1), 15–32. 10.4155/ppa-2017-0030 [DOI] [PubMed] [Google Scholar]
- Sharma K., Guleria S., Razdan V. K. (2020). Green Synthesis of Silver Nanoparticles Using Ocimum Gratissimum Leaf Extract: Characterization, Antimicrobial Activity and Toxicity Analysis. J. Plant Biochem. Biotechnol. 29, 213–224. 10.1007/s13562-019-00522-2 [DOI] [Google Scholar]
- Sharma N., Mishra K. P., Chanda S., Bhardwaj V., Tanwar H., Ganju L., et al. (2019). Evaluation of Anti-dengue Activity of Carica Papaya Aqueous Leaf Extract and its Role in Platelet Augmentation. Arch. Virol. 164, 1095–1110. 10.1007/s00705-019-04179-z [DOI] [PubMed] [Google Scholar]
- Sharma N., Pareek A. (2021). Ethnobotanical Properties of Plants Used by the Rural Community of Dausa District of rajasthan, india. Ijbi 03, 179–185. 10.46505/ijbi.2021.3118 [DOI] [Google Scholar]
- Silva N. M., Santos N. C., Martins I. C. (2020). Dengue and Zika Viruses: Epidemiological History, Potential Therapies, and Promising Vaccines. TropicalMed 5, 150. 10.3390/tropicalmed5040150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simões L. R., Maciel G. M., Brandão G. C., Kroon E. G., Castilho R. O., Oliveira A. B. (2011). Antiviral Activity of Distictella Elongata (Vahl) Urb. (Bignoniaceae), a Potentially Useful Source of Anti-dengue Drugs from the State of Minas Gerais, Brazil. Brazil. Lett. Appl. Microbiol. 53, 602–607. 10.1111/j.1472-765X.2011.03146.x [DOI] [PubMed] [Google Scholar]
- Singh K., Zeeshan M., Ansari V. A., Ahmad Z., Bagga P., Shakya P. (2016). Prevention and Control of Dengue by Herbal Remedies. J. Chem. Pharm. Res. 1, 1. [Google Scholar]
- Skriptsova A. V., Shevchenko N. M., Zvyagintseva T. N., Imbs T. I. (2010). Monthly Changes in the Content and Monosaccharide Composition of Fucoidan from Undaria Pinnatifida (Laminariales, Phaeophyta). J. Appl. Phycol. 22, 79–86. 10.1007/s10811-009-9438-5 [DOI] [Google Scholar]
- Sood R., Raut R., Tyagi P., Pareek P. K., Barman T. K., Singhal S., et al. (2015). Cissampelos Pareira Linn: Natural Source of Potent Antiviral Activity against All Four Dengue Virus Serotypes. Plos Negl. Trop. Dis. 9, e0004255. 10.1371/journal.pntd.0004255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sridhar S., Luedtke A., Langevin E., Zhu M., Bonaparte M., Machabert T., et al. (2018). Effect of Dengue Serostatus on Dengue Vaccine Safety and Efficacy. N. Engl. J. Med. 379, 327–340. 10.1056/NEJMoa1800820 [DOI] [PubMed] [Google Scholar]
- Subenthiran S., Choon T. C., Cheong K. C., Thayan R., Teck M. B., Muniandy P. K., et al. (2013). Carica Papaya Leaves Juice Significantly Accelerates the Rate of Increase in Platelet Count Among Patients with Dengue Fever and Dengue Haemorrhagic Fever. Evid. Based Complement. Alternat Med. 2013, 616737. 10.1155/2013/616737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suganthi A., Ravi T. K. (2019). Chemical Methodologies Estimation of Anti-dengue Phytochemical Markers Gallic Acid, Rutin and Quercetin in Methanolic Extract of Euphorbia Hirta (L.) and Tawa-Tawa Capsule Formulation by Validated RP-HPLC Method. Chem. Methodol. 3, 43–54. 10.22034/CHEMM.2018.129381.1051 [DOI] [Google Scholar]
- Svanberg I., Berggren Å. (2019). Ant Schnapps for Health and Pleasure: The Use of Formica Rufa L. (Hymenoptera: Formicidae) to Flavour Aquavit. J. Ethnobiol. Ethnomed. 15, 68. 10.1186/s13002-019-0347-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taguchi K., Tano I., Kaneko N., Matsumoto T., Kobayashi T. (2020). Plant Polyphenols Morin and Quercetin rescue Nitric Oxide Production in Diabetic Mouse Aorta through Distinct Pathways. Biomed. Pharmacother. 129, 110463. 10.1016/j.biopha.2020.110463 [DOI] [PubMed] [Google Scholar]
- Talarico L. B., Pujol C. A., Zibetti R. G., Faría P. C., Noseda M. D., Duarte M. E., et al. (2005). The Antiviral Activity of Sulfated Polysaccharides against Dengue Virus Is Dependent on Virus Serotype and Host Cell. Antivir. Res 66, 103–110. 10.1016/j.antiviral.2005.02.001 [DOI] [PubMed] [Google Scholar]
- Tamilventhan A., Jayaprakash A. (2019). Larvicidal Activity of Terminalia Arjuna Bark Extracts on Dengue Fever Mosquito Ades Aegypti. Rese. Jour. Pharm. Technol. 12, 87. 10.5958/0974-360X.2019.00017.9 [DOI] [Google Scholar]
- Tan Y. P., Xue Y., Savchenko A. I., Houston S. D., Modhiran N., McMillan C. L. D., et al. (2019). Basimarols A, B, and C, Highly Oxygenated Pimarane Diterpenoids from Basilicum Polystachyon. J. Nat. Prod. 82, 2828–2834. 10.1021/acs.jnatprod.9b00522 [DOI] [PubMed] [Google Scholar]
- Tarasuk M., Songprakhon P., Chimma P., Sratongno P., Na-Bangchang K., Yenchitsomanus P. T. (2017). Alpha-mangostin Inhibits Both Dengue Virus Production and Cytokine/chemokine Expression. Virus. Res. 240, 180–189. 10.1016/j.virusres.2017.08.011 [DOI] [PubMed] [Google Scholar]
- Toman J. (2018). Anti-infective Properties of Epigallocatechin-3-Gallate (EGCG), a Component of green tea. J. Sci. Food Agric. 1, 1. 10.4137/MBI.S943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trujillo-Correa A. I., Quintero-Gil D. C., Diaz-Castillo F., Quiñones W., Robledo S. M., Martinez-Gutierrez M. (2019). In Vitro and In Silico Anti-dengue Activity of Compounds Obtained from Psidium Guajava through Bioprospecting. BMC Complement. Altern. Med. 19, 298. 10.1186/s12906-019-2695-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umesh Kanna S., Krishnakumar N. (2019). Anti-dengue Medicinal Plants: A Mini Review. J. Pharmacogn. Phytochem. 1, 1. 10.1007/s11418-013-0767-y [DOI] [Google Scholar]
- Uno N., Ross T. M. (2018). Dengue Virus and the Host Innate Immune Response. Emerg. Microbes Infect. 7, 167. 10.1038/s41426-018-0168-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasilakis N., Fokam E. B., Hanson C. T., Weinberg E., Sall A. A., Whitehead S. S., et al. (2008). Genetic and Phenotypic Characterization of Sylvatic Dengue Virus Type 2 Strains. Virology 377, 296–307. 10.1016/j.virol.2008.04.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughn D. W., Green S., Kalayanarooj S., Innis B. L., Nimmannitya S., Suntayakorn S., et al. (2000). Dengue Viremia Titer, Antibody Response Pattern, and Virus Serotype Correlate with Disease Severity. J. Infect. Dis. 181, 2–9. 10.1086/315215 [DOI] [PubMed] [Google Scholar]
- Vázquez-Calvo Á., Jiménez de Oya N., Martín-Acebes M. A., Garcia-Moruno E., Saiz J.-C. (2017). Antiviral Properties of the Natural Polyphenols Delphinidin and Epigallocatechin Gallate against the Flaviviruses West Nile Virus, Zika Virus, and Dengue Virus. Front. Microbiol. 8, 1. 10.3389/fmicb.2017.01314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waggoner J. J., Gresh L., Vargas M. J., Ballesteros G., Tellez Y., Soda K. J., et al. (2016). Viremia and Clinical Presentation in Nicaraguan Patients Infected with Zika Virus, Chikungunya Virus, and Dengue Virus. Clin. Infect. Dis. 63, 1584–1590. 10.1093/cid/ciw589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M. Y., Ma Z. L., He C. L., Yuan X. Y. (2020). The Antioxidant Activities of Flavonoids in Jerusalem Artichoke (Helianthus Tuberosus L.) Leaves and Their Quantitative Analysis. Nat. Prod. Res. , 1, 1, 5.doi: 10.1080/14786419.2020.1839464 [DOI] [PubMed] [Google Scholar]
- Warsinah W., Baroroh H. N., Harwoko H. (2020). Phytochemical Analysis and Antioxidant Activity of Brotowali (Tinospora Crispa L. Mier) Stem. Molekul 15, 73. 10.20884/1.jm.2020.15.2.533 [DOI] [Google Scholar]
- Wefers D., Tyl C. E., Bunzel M. (2014). Novel Arabinan and Galactan Oligosaccharides from Dicotyledonous Plants. Front. Chem. 2, 100. 10.3389/fchem.2014.00100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissenböck H., Hubálek Z., Bakonyi T., Nowotny N. (2010). Zoonotic Mosquito-Borne Flaviviruses: Worldwide Presence of Agents with Proven Pathogenicity and Potential Candidates of Future Emerging Diseases. Vet. Microbiol. 140, 271–280. 10.1016/j.vetmic.2009.08.025 [DOI] [PubMed] [Google Scholar]
- Whitby K., Pierson T. C., Geiss B., Lane K., Engle M., Zhou Y., et al. (2005). Castanospermine, a Potent Inhibitor of Dengue Virus Infection In Vitro and In Vivo . J. Virol. 79, 8698–8706. 10.1128/jvi.79.14.8698-8706.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization (2021a). Dengue and Severe Dengue. Available at: https://www.who.int/news-room/fact-sheets/detail/dengue-and-severe-dengueue (Accessed May 30, 2021). [Google Scholar]
- World Health Organization (2009). Dengue Guidelines for Diagnosis, Treatment, Prevention and Control. Geneva, Switzerland: WHO. [PubMed] [Google Scholar]
- World Health Organization (2021b). Dengue Situation Updates 2021. WHO. Available at: https://apps.who.int/iris/bitstream/handle/10665/341149/Dengue-20210520.pdf (Accessed May 30, 2021). [Google Scholar]
- Xie Q., Zhang B., Yu J., Wu Q., Yang F., Cao H., et al. (2017). Structure and Function of the Non-structural Protein of Dengue Virus and its Applications in Antiviral Therapy. Curr. Top. Med. Chem. 17, 371–380. 10.2174/1568026616666160829155327 [DOI] [PubMed] [Google Scholar]
- Xu G., Dou J., Zhang L., Guo Q., Zhou C. (2010). Inhibitory Effects of Baicalein on the Influenza Virus In Vivo Is Determined by Baicalin in the Serum. Biol. Pharm. Bull. 33, 238–243. 10.1248/bpb.33.238 [DOI] [PubMed] [Google Scholar]
- Yam-Puc J. C., Cedillo-Barrón L., Aguilar-Medina E. M., Ramos-Payán R., Escobar-Gutiérrez A., Flores-Romo L. (2016). The Cellular Bases of Antibody Responses during Dengue Virus Infection. Front. Immunol. 7, 1. 10.3389/fimmu.2016.00218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J.-Y., Lee H.-S. (2012). Evaluation of Antioxidant and Antibacterial Activities of Morin Isolated from mulberry Fruits (Morus alba L.). J. Korean Soc. Appl. Biol. Chem. 55, 485–489. 10.1007/s13765-012-2110-9 [DOI] [Google Scholar]
- Yao X., Ling Y., Guo S., Wu W., He S., Zhang Q., et al. (2018). Tatanan A from the Acorus calamus L. Root Inhibited Dengue Virus Proliferation and Infections. Phytomedicine 42, 258–267. 10.1016/j.phymed.2018.03.018 [DOI] [PubMed] [Google Scholar]
- Yogarajalakshmi P., Venugopal Poonguzhali T., Ganesan R., Karthi S., Senthil-Nathan S., Krutmuang P., et al. (2020). Toxicological Screening of marine Red Algae Champia Parvula (C. Agardh) against the Dengue Mosquito Vector Aedes aegypti (Linn.) and its Non-toxicity against Three Beneficial Aquatic Predators. Aquat. Toxicol. 222, 105474. 10.1016/j.aquatox.2020.105474 [DOI] [PubMed] [Google Scholar]
- Yu J. S., Wu Y. H., Tseng C. K., Lin C. K., Hsu Y. C., Chen Y. H., et al. (2017). Schisandrin A Inhibits Dengue Viral Replication via Upregulating Antiviral Interferon Responses through STAT Signaling Pathway. Sci. Rep. 7, 45171. 10.1038/srep45171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zandi K., Teoh B. T., Sam S. S., Wong P. F., Mustafa M. R., Abubakar S. (2011). Antiviral Activity of Four Types of Bioflavonoid against Dengue Virus Type-2. Virol. J. 8, 560. 10.1186/1743-422X-8-560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zandi K., Teoh B. T., Sam S. S., Wong P. F., Mustafa M. R., AbuBakar S. (2012). Novel Antiviral Activity of Baicalein against Dengue Virus. BMC Complement. Altern. Med. 12, 214. 10.1186/1472-6882-12-214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng Z., Zhan J., Chen L., Chen H., Cheng S. (2021). Global, Regional, and National Dengue burden from 1990 to 2017: A Systematic Analysis Based on the Global burden of Disease Study 2017. EClinicalMedicine 32, 100712. 10.1016/j.eclinm.2020.100712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J. X., Braakman I., Matlack K. E., Helenius A. (1997). Quality Control in the Secretory Pathway: the Role of Calreticulin, Calnexin and BiP in the Retention of Glycoproteins with C-Terminal Truncations. Mol. Biol. Cel 8, 1943–1954. 10.1091/mbc.8.10.1943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Yu X., Wang M., Ding Y., Guo H., Liu J., et al. (2021). Hyperoside from Z. Bungeanum Leaves Restores Insulin Secretion and Mitochondrial Function by Regulating Pancreatic Cellular Redox Status in Diabetic Mice. Free Radic. Biol. Med. 162, 412–422. 10.1016/j.freeradbiomed.2020.10.320 [DOI] [PubMed] [Google Scholar]
- Zhao Q., Chen X. Y., Martin C. (2016). Scutellaria Baicalensis, the golden Herb from the Garden of Chinese Medicinal Plants. Sci. Bull. (Beijing) 61, 1391–1398. 10.1007/s11434-016-1136-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X., Zeng L., Chen Y., Wang X., Liao Y., Xiao Y., et al. (2020). Metabolism of Gallic Acid and its Distributions in tea (Camellia Sinensis) Plants at the Tissue and Subcellular Levels. Int. J. Mol. Sci. 21, 1. 10.3390/ijms21165684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y., Yang Z. Y., Tang R. C. (2016). Bioactive and UV Protective Silk Materials Containing Baicalin - the Multifunctional Plant Extract from Scutellaria Baicalensis Georgi. Mater. Sci. Eng. C Mater. Biol. Appl. 67, 336–344. 10.1016/j.msec.2016.05.063 [DOI] [PubMed] [Google Scholar]