Abstract
Cyclosporine (CsA) is an immunosuppressive and antimicrobial drug which, in complex with cyclophilin A, inhibits the protein phosphatase calcineurin. We recently found that Cryptococcus neoformans growth is resistant to CsA at 24°C but sensitive at 37°C and that calcineurin is required for growth at 37°C and pathogenicity. Here CsA analogs were screened for toxicity against C. neoformans in vitro. In most cases, antifungal activity was correlated with cyclophilin A binding in vitro and inhibition of the mixed-lymphocyte reaction and interleukin 2 production in cell culture. Two unusual nonimmunosuppressive CsA derivatives, (γ-OH) MeLeu4-Cs (211-810) and D-Sar (α-SMe)3 Val2-DH-Cs (209-825), which are also toxic to C. neoformans were identified. These CsA analogs inhibit C. neoformans via fungal cyclophilin A and calcineurin homologs. Our findings identify calcineurin as a novel antifungal drug target and suggest nonimmunosuppressive CsA analogs warrant investigation as antifungal agents.
Cryptococcus neoformans is a basidiomycetous opportunistic fungal pathogen that causes systemic mycosis in patients with immunosuppression as a result of chemotherapy, immune system dysfunction, solid-organ transplantation, or infection with the human immunodeficiency virus. Primary infections begin in the lung following inhalation of the infectious propagule and then spread hematogenously to the brain where severe meningoencephalitis develops (10, 12, 35, 40). Two primary antifungal therapies are available, amphotericin B and fluconazole, which target the fungal membrane sterol ergosterol. However, amphotericin B has a number of adverse serious side effects, fluconazole is fungistatic, and drug-resistant mutants are arising in Candida species and in C. neoformans (3, 49, 61, 62). Therefore, there is a need to find new antifungal agents that are more fungicidal and less toxic for the treatment of cryptococcal meningitis and that have different mechanisms of action for use in combination drug therapies.
Cyclosporine (CsA) is an immunosuppressant that inhibits signal transduction events required for T-cell activation following antigen presentation (reviewed in references 8, 9, 23, and 55). CsA enters the cell by diffusion and associates with an intracellular receptor, cyclophilin A, which belongs to a family of proteins that catalyze cis-trans peptidyl-prolyl isomerization, a rate-limiting step in protein folding (for reviews, see references 17, 23, and 53). CsA binds to the active site of cyclophilin and potently inhibits prolyl isomerase activity. However, immunosuppression is not related to the inhibition of this enzyme activity. The target of the cyclophilin A-CsA complex is a Ca2+-calmodulin-dependent serine-threonine-specific protein phosphatase, calcineurin (27, 36, 37). In T cells responding to antigen presentation, an increase in intracellular Ca2+ activates calcineurin, which subsequently dephosphorylates a transcription factor, NF-AT, allowing nuclear import and expression of T-cell activation genes (11, 18, 30, 45, 46, 56).
CsA is a natural product of a soil fungus and exhibits potent antimicrobial activities (reviewed in reference 6). Previous studies in the yeast Saccharomyces cerevisiae reveal that the mechanisms of CsA immunosuppressive and antifungal action are essentially identical (2, 7, 19, 24–26, 43). CsA binds to S. cerevisiae cyclophilin A (21), which shares 65% identity with the human homolog, to form a protein-drug complex that inhibits the S. cerevisiae calcineurin homolog (2, 5, 7, 13–15, 20, 26, 43, 58).
It has been suggested that the antimicrobial activities of CsA might have clinical applicability (28). For instance, CsA is toxic to the pathogenic fungus Coccidioides immitis (34), to Neurospora crassa (60), and to Aspergillus niger (34). However, in contrast to S. cerevisiae, little is known about the mechanism(s) of drug action in these other fungi. We have previously reported that CsA is markedly toxic to the opportunistic fungal pathogen C. neoformans at 37°C but not at 24°C in vitro (47). By gene disruption, we demonstrated that calcineurin, the target of the cyclophilin A-CsA complex, is required for growth at 37°C and virulence of C. neoformans (47).
These observations suggest that drugs which inhibit calcineurin should similarly prevent C. neoformans infections in vivo. Indeed, CsA can protect mice from cryptococcal pneumonia (41). However, because in previous studies CsA also exacerbated cryptococcal meningitis in mice and rabbits because of potent immunosuppressive activity (42, 50), we sought to identify nonimmunosuppressive CsA analogs that retain antifungal activity. We report here an analysis of a collection of CsA analogs with alterations in the effector domain of the drug, which interacts with calcineurin. Antifungal activity is generally correlated with binding to human cyclophilin A in vitro and to immunosuppressive activity in vivo, supporting a model in which the cyclophilin A-CsA complex is toxic to C. neoformans by inhibiting calcineurin. More importantly, we identify two nonimmunosuppressive CsA analogs that retain antifungal activity in vitro. Our studies reveal that these analogs inhibit C. neoformans growth via cyclophilin A-dependent inhibition of calcineurin. We suggest that these drugs take advantage of structural differences between host and fungal cyclophilin A and calcineurin to inhibit fungal growth but spare immune system function. Our studies suggest further examination of CsA analogs as potential novel antifungal agents is warranted.
MATERIALS AND METHODS
Strains and compounds.
The pathogenic serotype A strain H99 has been previously described (59). C. neoformans T1 and 89-610 are azole-resistant strains and were kindly provided by Mahmoud Ghannoum (University of California at Los Angeles-Harbor) and John Graybill (University of Texas Health Science Center at San Antonio), respectively. CsA and analogs were prepared at Novartis (Basel, Switzerland). Compounds were dissolved in dimethyl sulfoxide at a concentration of 10 mg/ml and stored frozen at −20°C.
In vitro susceptibility testing.
Experiments for determination of MICs were performed by the broth macrodilution method following the recommendations of the National Committee for Clinical Laboratory Standards (NCCLS) (44). The only difference from the standardized method was the choice of drug dilutions, which ranged from 100 to 0.09 μg/ml. The fungal growth inhibition assay was performed with drug concentrations of 0.01, 0.1, 1, and 10 μg/ml. Drug dilutions and inoculum preparation were done, using the NCCLS criteria (44). Optical density at 600 nm (OD600) was measured with a Beckman spectrophotometer following incubation for 72 h at 24, 30, and 37°C. The MIC was defined as the lowest drug concentration in which a visual turbidity less than or equal to 80% inhibition compared to that produced by the growth control tube was observed. The minimum fungicidal concentration (MFC) was determined as previously described (16). Briefly, 10-μl aliquots from tubes with growth inhibition were plated onto Sabouraud agar plates. The lowest drug concentration that yielded three or fewer C. neoformans colonies was recorded as the MFC.
Preparation of protein extracts and [3H]CsA LH-20 drug binding assays.
Protein extracts were prepared from 100 OD600 units of cell pellet by glass bead homogenization in 1 ml of lysis buffer (150 mM Tris [pH 7.5], 10 mM MgCl2, 1 mM dithiothreitol, 10% [vol/vol] glycerol, 1 mM phenylmethylsulfonyl fluoride, 3 μg of benzamide per ml, 1 μg of aprotinin per ml, 1 μg of leupeptin per ml) with eight 1-min bursts with cooling on ice. Extracts were centrifuged at 12,000 rpm for 10 min at 4°C in a Sorvall Microfuge MC12, and the supernatants were transferred to fresh tubes. Protein content was quantified by the Bradford assay, and extracts were stored frozen at −80°C.
LH-20 drug binding assays were performed as previously described (22). His6-tagged S. cerevisiae cyclophilin A protein was overexpressed and affinity purified on Ni2+-nitriloacetic acid-resin (Qiagen) as previously described (4). [3H]CsA was obtained from Amersham (specific activity, 7.0 Ci/mmol). LH-20 assays were performed with ∼5 μg of purified S. cerevisiae cyclophilin A protein and with 180 μg of C. neoformans total protein.
Enzyme immunoassays.
Human recombinant cyclophilin A protein (CYP) was expressed in Escherichia coli, purified, and biotinylated as previously described (54). The solid-phase enzyme immunoassays (enzyme-linked immunosorbent assay) were performed as previously described (52, 54). Briefly, a d-Lys-CsA derivative was coupled to bovine serum albumin (BSA) and used to coat the wells of polyvinyl microtiter plates (2 μg/ml in phosphate-buffered saline [PBS] [pH 7.4] for 2 h at 37°C). After saturation of the plate with PBS containing 2% BSA (1 h at 37°C) and washing once with PBS containing 0.05% Tween 20 and three times with PBS, CYP-biotin was incubated overnight at 4°C (in PBS containing 1% BSA). Bound CYP-biotin was detected with streptavidin coupled to alkaline phosphatase (Jackson Immunoresearch Labs, Inc.) (1:6,000 in PBS containing 1% BSA for 2 h at 37°C). The absorbance at 405 nm was measured after hydrolysis of p-nitrophenyl phosphate (1 mg/ml in diethanolamine buffer [pH 9.6] for 1 to 2 h at 37°C). In the competitive assays, the CsA derivatives (1 mg/ml in ethanol) were immediately added to the CYP solutions (at 1:100 dilution, 0.83 × 10−5 M) and further 10-fold dilutions were made directly in the microtiter plate. After incubation overnight at 4°C, unbound CYP was removed and the assay was calculated as the percent inhibition of the control reaction between CYP and coated CsA in the absence of inhibitor (10 replicates per plate). The 50% inhibitory concentrations for the CsA derivatives were compared with the 50% inhibitory concentration for CsA run in triplicate on each microtiter plate.
Mouse MLR.
The mixed-lymphocyte reaction (MLR) was performed essentially as previously described (38, 57). Equal amounts of spleen cells from CBA and BALB/c mice were mixed and incubated (2 × 105 cells per well) with appropriate serial dilutions of compounds in 200 μl of serum-free CG medium (Bioreba, Basel, Switzerland) in flat-bottom tissue culture microtiter plates at 37°C in 5% CO2. After 4 days, 1 μCi of [3H]thymidine (2 Ci/mmol) was added to each well and the plates were subsequently incubated for an additional 16 h. Cells were harvested on filter paper and counted in a β-counter. Background values (low control, proliferation of BALB/c cells alone) were subtracted from all values. Proliferation of mixed cells without any compound was taken as 100% proliferation.
IL-2 reporter gene assay.
The interleukin 2 (IL-2) reporter gene assay was performed as previously described (1). Briefly, the E. coli gene lacZ (reporter gene), which encodes the enzyme β-galactosidase, was placed under the transcriptional control of the human IL-2 promoter and stably transfected into the human T-cell line Jurkat. Assay plates (96-well microtiter plates) were prepared containing the compounds at appropriate dilutions. Transfected Jurkat cells (5 × 104 per well) which were stimulated with phorbol myristate acetate and phytohemagglutinin were added. The plates were incubated for 16 h at 37°C in 5% CO2. The IL-2 promoter-driven β-galactosidase expression, which was quantified by measuring the fluorescence of its cleaved substrate, correlates with IL-2 expression and is a direct readout of IL-2 gene transcription.
Cloning, expression, purification, and binding of intein-cryptococal cyclophilin A to chitin beads.
The cloned cryptococcal CPA1 gene encoding the cyclophilin A homolog (P. Wang and J. Heitman, unpublished data) was PCR amplified with two flanking oligonucleotides, 5′-GGGAATTCCATATGTCCCAAGTTTACTTTGACATTGCCATTAAC (1189) and 5′-CAGTCAGCTCTTCCGCAGACAGTGCCGGAGGCAGTGATGGTGATCTTGGC, cleaved with NdeI and SapI, cloned into the IMPACT I (for intein-mediated purification with affinity chitin-binding tag) system expression vector pCYB1 (New England Biolabs) and confirmed by DNA sequence analysis. Cells expressing the intein-tagged cryptococcal cyclophilin A protein were grown at 37°C overnight in a 3-ml culture of FB medium (63) supplemented with 200 μg of ampicillin per ml. A portion (1 ml) of this culture was pelleted and resuspended in 700 ml FB medium with ampicillin and grown at 37°C to an OD600 of 0.6. At this point, 2 mM isopropyl-β-d-thiogalactopyranoside (IPTG) was added to the culture and incubation continued for 4 h at 37°C. Cells were collected by centrifugation at 10,000 rpm for 10 min in a Sorvall GS-3 rotor and resuspended in 20 ml of ice-cold lysis buffer (20 mM Tris [pH 7.5], 0.1 mM EDTA, 0.1% Triton X-100, 0.5 mM phenylmethylsulfonyl fluoride) and 500 mM KCl. Cells were lysed by sonicating eight times for 1 min each time, with periods of cooling between. The cell lysate was clarified by centrifugation at 40,000 rpm for 45 min in a Beckman Ti-70 rotor. Chitin beads (1 ml) (preequilibrated in lysis buffer) were added to the cell lysates, and the suspension was stirred gently for 1 h at 4°C. The bead-cell extract mix was loaded onto an econocolumn (Bio-Rad) and washed three times, first with 150 ml of lysis buffer containing 500 mM NaCl, second with 50 ml of lysis buffer containing 1 M NaCl, and third with 50 ml of lysis buffer (without Triton X-100) with 100 mM KCl. Intein-cyclophilin A-chitin beads were resuspended in 1 ml of lysis buffer (without Triton X-100) and stored at 4°C in 0.2% NaN3.
Cryptococcal cyclophilin A-calcineurin interactions in vitro.
Cyclophilin A bound to chitin beads described above was used for cyclophilin-calcineurin binding assays. Incubation mixtures contained 800 μl of cryptococcal cell extract (4 mg of protein) and 40 μl of cyclophilin A-chitin beads (50% [vol/vol] suspension). CsA and CsA analogs D-Sar (α-SMe)3 Val2-DH-Cs (209-825) and (γ-OH) MeLeu4-Cs (211-810) were added where indicated to a final concentration of 100 μM. The binding mixtures were incubated at 4°C on a nutator shaker for 2 h. The chitin beads were collected by centrifugation for 10 s and washed four times with lysis buffer (described above). Bound proteins were eluted from the beads by boiling for 4 min in 30 μl of sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis sample buffer, fractionated on SDS–12.5% polyacrylamide gels, and transferred to a nitrocellulose membrane. The membrane was blocked overnight in block-wash buffer (10 mM imidazole [pH 7.3], 100 mM KCl, 5 mM CaCl2, 5% BSA, 0.05% Tween 20, 0.02% NaN3), transferred to fresh buffer containing 106 cpm of 125I-calmodulin, and incubated at room temperature for 2 h with gentle agitation. The membrane was washed twice in block-wash buffer, air dried, and exposed to film overnight at −80°C.
Site-directed mutagenesis of C. neoformans calcineurin A.
Two C. neoformans calcineurin mutant strains were created by transformation of the wild-type serotype A strain H99 with mutant alleles of the calcineurin A CNA1 gene. The C. neoformans CNA1-1 (Val344Arg) and CNA1-2 (Val344Lys) alleles were created by PCR overlap mutagenesis (29), using genomic DNA as the template. The first round of PCR was performed, using primer 5′-GTTAGAGTATCGATCAAGGTAGTTAGGTG (1006) with flanking primer 5′-GTGATTTCACTATTATCCTCCATC (1003) and 5′-CTACCTTGATCGATACTCTAACAAGGCCGCTG (1005) with flanking primer 5′-GAGTTAGCGACCAATGGAGTGTGACG (1004) for the CNA1-1 (Val344Arg) allele (mutations in boldface). To construct the CNA1-2 (Val344Lys) allele, PCR was performed, using primer 5′-GTTAGAGTACTTATCAAGGTAGTTAGGTG (1008) with flanking primer 1003 and 5′-CTACCTTGATAAGTACTCTAACAAGGCCGCTG (1007) with flanking primer 1004. First-round PCR overlap products were gel purified and used as template for the second-round PCR with flanking primers 1003 and 1004. The PCR protocol was as follows: an initial step of 10 min at 96°C; 35 cycles of PCR, with 1 cycle consisting of 30 s at 95°C, 30 s at 55°C, and 2 min at 72°C; and a final step of 5 min at 72°C. The resulting PCR product was cloned into the TA vector (Invitrogen) and confirmed by sequencing. The second-round-confirmed PCR fragments were used to transform wild-type strain H99 by the biolistic method (59). Transformants were first grown on plates containing 1 M sorbitol for 8 h to allow phenotypic expression, and CsA-resistant colonies were selected on YPD medium containing CsA (100 μg/ml) at 37°C. Colonies that were CsA resistant and FK506 sensitive contained the CNA1-1 (MCC11 strain) or CNA1-2 (MCC12 strain) allele and could be readily distinguished from spontaneous CsA-resistant mutants, which were all cross-resistant to FK506 (1 μg/ml) at 37°C.
RESULTS
CsA is toxic to C. neoformans at 37°C but not at 24°C.
We previously reported that the immunosuppressive drugs CsA and FK506 are toxic to C. neoformans when grown at 37°C but not at 24°C in vitro (47). In these experiments, CsA and FK506 toxicity was determined by growth on solid YPD medium (1% yeast extract, 2% peptone, 2% glucose, 2% agar) containing these compounds. We extended these observations, using the NCCLS standardized growth inhibition criteria (44) for assessing antifungal activity in C. neoformans. In this assay, cells of C. neoformans were cultured in RPMI 1640 medium for 72 h with increasing concentrations of CsA and growth was assessed by measuring OD600. Representative CsA growth inhibition curves for the pathogenic C. neoformans serotype A strain H99 are presented in Fig. 1 for cultures incubated at 24, 30, and 37°C. CsA toxicity was also readily detectable in the standardized in vitro assay, with MICs of 0.39 μg/ml at 37°C and 12.5 μg/ml at 30°C and MFCs of 0.78 μg/ml at 37°C and >100 μg/ml at 30°C. In contrast, no growth inhibition was observed at 24°C (data not shown), even at drug concentrations up to 1,000-fold greater than the MIC at 37°C. These observations are in accord with our previous findings and confirm that CsA is toxic to C. neoformans in vitro at elevated growth temperatures.
FIG. 1.
CsA is toxic to C. neoformans at 37°C but not at 24°C. C. neoformans H99 was grown in RPMI 1640 medium with the indicated concentrations of CsA for 72 h at 24, 30, and 37°C. Growth was assayed by determining the OD600, and three independent experiments were done.
C. neoformans expresses CsA binding activity.
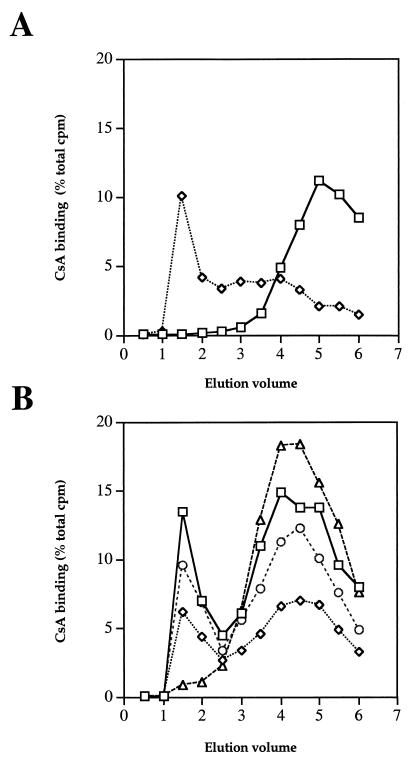
Previous studies with the yeast S. cerevisiae and with T lymphocytes have established that CsA binds to the CsA-binding protein cyclophilin A to form an active drug-protein complex. To determine whether CsA could exert its toxic effects in C. neoformans via a similar mechanism, protein extracts were prepared from C. neoformans H99 and assayed for CsA-binding protein. We employed the standard LH-20 drug binding assay, in which [3H]CsA is mixed with protein extract and then chromatographed through Sephadex LH-20, a hydrophobic matrix that retards the mobility of free CsA compared to that of the cyclophilin-CsA complexes (22). As shown in Fig. 2A, whereas free [3H]CsA elutes as a late peak in the LH-20 drug binding assay, [3H]CsA incubated with purified S. cerevisiae cyclophilin A protein eluted primarily as an early peak attributable to a cyclophilin A-CsA complex, with some free [3H]CsA eluting as a less-pronounced later peak. Similarly, [3H]CsA incubated with C. neoformans protein extract eluted as both an early cyclophilin-[3H]CsA complex and a late peak of free [3H]CsA in the LH-20 drug binding assay (Fig. 2B). Extracts were also analyzed for isogenic mutant strains lacking the cyclophilin A protein CPA1 (cpa1), CPA2 (cpa2), or both (cpa1 cpa2). CsA binding was reduced to ∼50% of the wild-type level in extracts containing CPA2 and lacking CPA1, to ∼80% of the wild-type level in extracts containing CPA1 and lacking CPA2, and to ∼9% in extracts lacking both CPA1 and CPA2 (Fig. 2B). These observations provide evidence that C. neoformans expresses two CsA-binding forms of the cyclophilin A protein and that the CPA1 protein contributes more of the total CsA binding activity than the CPA2 form of cyclophilin A. CsA toxicity in C. neoformans could be mediated by these cyclophilin-CsA complexes, as is the case in other organisms.
FIG. 2.
C. neoformans expresses [3H]CsA binding activities. CsA-binding protein activity was measured by the LH-20 drug binding assay. (A) Tritiated CsA alone (squares) or incubated with purified S. cerevisiae cyclophilin A protein (diamonds) was subjected to Sephadex LH-20 chromatography. Fractions were analyzed by scintillation counting, and the percentages of total counts per minute were plotted. (B) Protein extracts from the C. neoformans wild-type CPA1 CPA2 strain H99 (squares), cpa1 mutant strain PW67 (diamonds), cpa2 mutant strain PW71 (circles), and cpa1 cpa2 mutant strain PW62 (triangles) were subjected to Sephadex LH-20 chromatography. Fractions were analyzed by scintillation counting, and the percentages of total counts per minute were plotted. Elution volume is given in milliliters.
Nonimmunosuppressive CsA analogs have altered binding to human cyclophilin A and reduced ability to inhibit calcineurin in T cells.
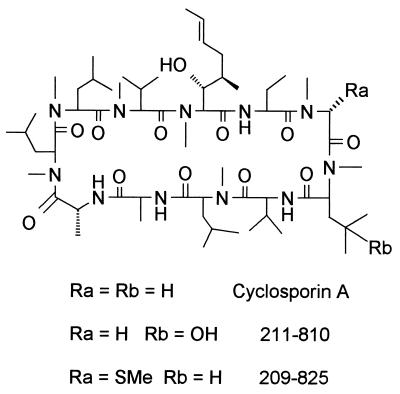
In previous studies, CsA was found to exacerbate C. neoformans infections in the immunosuppressed rabbit model of cryptococcal meningitis (50). Thus, the potent immunosuppressive effects of CsA outweigh any antifungal effect in this animal model of central nervous system infection. We therefore assessed the activity of a series of CsA analogs that are modified on the cyclophilin binding and calcineurin effector surfaces of CsA. The activities of these CsA analogs were compared to that of CsA in binding to human cyclophilin A and in two bioassays: inhibition of IL-2 gene expression (1) and inhibition of the MLR (38, 57). These bioassays measure cyclophilin-dependent inhibition of calcineurin function by CsA. Several CsA analogs have increased or reduced binding to cyclophilin A and reduced immunosuppressive activity as a result of these structural modifications (Table 1). Interestingly, one derivative [(γ-OH) MeLeu4-Cs (211-810)] exhibited markedly reduced (∼100-fold) immunosuppressive activity. The structure of CsA is depicted in Fig. 3. The upper portion of the CsA ring interacts with cyclophilin A, whereas the lower portion of the ring is responsible for calcineurin binding. In the 211-810 analog, a hydroxyl group has been introduced onto the surface of CsA that interacts with calcineurin.
TABLE 1.
Cyclophilin A binding and inhibition of MLR and IL-2 production by CsA analogsa
| CSA derivativeb | MLR | IL-2 | CYP A binding |
|---|---|---|---|
| CsA | 100 | 100 | 100 |
| (γ-OH) MeLeu4-Cs (211–810) | 0.8 | 0.9 | 100 |
| D-Sar (α-SMe)3 Val2-DH-Cs (209–825) | 10 | 55 | 200 |
| Allo-Thr-2-Cs | 9 | 11 | 20 |
| Norvaline-2-Cs | 48 | 37 | 20 |
| d-Ala(3-acetylamino)-8-Cs | 55 | 33 | 125 |
| Thr-2-Cs | 59 | 100 | 71 |
| d-MeSer-3-Cs | 77 | 42 | 333 |
| d-Ser(O-CH2CH2-OH)-8-Cs | 100 | 100 | 200 |
| d-Ser-8-Cs | 139 | 167 | 167 |
The ability of a series of CsA analogs to inhibit an MLR assay, IL-2 production, or cyclophilin A (CYP A) binding was compared to that of CsA and is expressed here as percentage of activity relative to CsA activity.
In CsA derivatives, CsA is shown as Cs.
FIG. 3.
Structures of CsA and CsA analogs 211-810 and 209-825. The structures of CsA and two nonimmunosuppressive CsA analogs are depicted. The upper half of the CsA ring binds cyclophilin A, and the effector surface of CsA that is exposed to the solvent in the cyclophilin A-CsA complex and which interacts with calcineurin is the lower half of the ring.
Nonimmunosuppressive CsA analogs are toxic to C. neoformans.
We next assessed the antifungal activity of these CsA analogs in the serotype A C. neoformans H99 strain, using the NCCLS standardized criteria. A number of immunosuppressive CsA analogs potently inhibited the growth of strain H99 at 37°C (Table 2) but not at 30°C (data not shown). In general, the immunosuppressive activity correlated well with antifungal activity, indicating that the ability to interact with cyclophilin A and effectively inhibit calcineurin likely accounts for the antifungal activity of these CsA analogs. Importantly, two CsA analogs whose immunosuppressive activity is reduced by 10- to 100-fold compared to that of CsA in the MLR assay retained potent antifungal activity against C. neoformans. These two nonimmunosuppressive CsA analogs were toxic to C. neoformans at 37°C but not at 30°C (Tables 2 and 3), suggesting that calcineurin is their common target. These analogs, (γ-OH) MeLeu4-Cs (211-810) and D-Sar (α-SMe)3 Val2-DH-Cs (209-825), have substitutions on the effector surface of CsA (Fig. 3), which is exposed to the solvent in the cyclophilin A-CsA complex and interacts with calcineurin (31–33, 39, 51). Alterations of this effector surface are known to reduce the affinity of the cyclophilin A-CsA complex for mammalian calcineurin, resulting in reduced or abolished immunosuppressive activity (36).
TABLE 2.
CsA and CsA analogs are toxic to fluconazole-sensitive and resistant C. neoformans isolatesa
| CsA derivativeb or drug |
C. neoformans H99
|
C. neoformans T1
|
C. neoformans 89-610
|
|||
|---|---|---|---|---|---|---|
| MIC | MFC | MIC | MFC | MIC | MFC | |
| CsA | 0.39 | 3.12 | 0.78 | 25 | 0.78 | 12.5 |
| (γ-OH) MeLeu4-Cs (211–810) | 0.78 | 1.56 | 0.39 | 6.25 | 0.78 | 6.25 |
| D-Sar (α-SMe)3 Val2- DH-Cs (209–825) | 0.78 | 0.78 | 0.19 | 1.56 | 0.39 | 0.78 |
| aThr-2-Cs | 1.56 | 25 | ≤0.09 | 25 | 0.78 | 12.5 |
| Nva-2-Cs | 0.39 | 25 | 1.56 | >100 | 0.39 | >12.5 |
| d-Ala(3-acetylamino)- 8-Cs | 0.78 | 6.25 | ≤0.09 | 12.5 | 0.39 | 6.25 |
| Thr-2-Cs | 0.78 | 1.56 | ≤0.09 | >12.5 | 0.39 | 1.56 |
| d-MeSer-3-Cs | 0.78 | 1.56 | 0.78 | 3.12 | 0.39 | 3.12 |
| d-Ser(O-CH2CH2-OH)- 8-Cs | 0.78 | 3.12 | <0.09 | 25 | 0.39 | 6.25 |
| d-Ser-8-Cs | 0.78 | 1.56 | 0.39 | >12.5 | 0.78 | 6.25 |
| Fluconazole | 1 | 8 | 4 | 64 | 32 | >64 |
All MICs and MFCs are given in micrograms per milliliter.
In CsA derivatives, CsA is shown as Cs.
TABLE 3.
Calcineurin and cyclophilin A mutations confer resistance to CsA and CsA analogsa
| Strain | Genotype | CsA
|
209–825
|
211–810
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 30°C
|
37°C
|
30°C
|
37°C
|
30°C
|
37°C
|
||||||||
| MIC | MFC | MIC | MFC | MIC | MFC | MIC | MFC | MIC | MFC | MIC | MFC | ||
| H99 | CNA1 CPA1 CPA2 | 12.5 | >100 | 0.39 | 0.78 | >100 | – | 0.78 | 1.56 | >100 | – | 1.56 | 25 |
| PW26 | CPA1 cpa1::ADE2 | >100 | – | 50 | >100 | >100 | – | 50 | >100 | >100 | – | 1.56 | >50 |
| PW47 | cpa1 cpa2 | >100 | – | >100 | – | >100 | – | >100 | – | >100 | – | >100 | – |
| PW48 | cpa1 cpa2 | >100 | – | >100 | – | >100 | – | >100 | – | >100 | – | >100 | – |
| MCC11 | CNA1-1 | >100 | – | >100 | – | >100 | – | >100 | – | >100 | – | >100 | – |
| MCC12 | CNA1-2 | >100 | – | >100 | – | >100 | – | >100 | – | >100 | – | >100 | – |
All MICs and MFCs given in micrograms per milliliter. –, not determined.
Calcineurin A mutations confer resistance to CsA and to nonimmunosuppressive CsA analogs.
By site-directed PCR overlap mutagenesis, we introduced mutations into the CNA1 gene encoding the calcineurin A catalytic subunit in the region proposed to contain the cyclophilin A-CsA binding site. In previous studies, these mutations were found to confer CsA resistance in the yeast S. cerevisiae by preventing cyclophilin A-CsA binding to calcineurin (7). We found that C. neoformans MCC11 and MCC12, which express the Val344Arg and Val344Lys calcineurin A mutant proteins, respectively, were resistant to CsA (Table 3), but not to FK506 (data not shown). In addition, these calcineurin A mutant strains are also resistant to the nonimmunosuppressive CsA analogs D-Sar (α-SMe)3 Val2-DH-Cs (209-825) and (γ-OH) MeLeu4-Cs (211-810), demonstrating that these CsA analogs inhibit the fungal calcineurin homolog by a mechanism similar to that of CsA (Table 3).
Cyclophilin A mediates antifungal activity of CsA and nonimmunosuppressive CsA analogs.
In recent studies we have identified the genes encoding cyclophilin A homologs in C. neoformans and generated mutants with reduced or no cyclophilin A expression (P. Wang and J. Heitman, unpublished data). Because cyclophilin A mediates CsA action in S. cerevisiae and T lymphocytes, we tested whether cyclophilin A also mediates CsA antifungal effects in C. neoformans. The MIC of CsA was increased from 0.78 μg/ml at 37°C in the cyclophilin A-expressing wild-type strain H99 to >50 μg/ml at 37°C in strain PW26 in which cyclophilin A expression is reduced but not abolished as a consequence of transgene-induced gene repression (Wang and Heitman, unpublished) (Table 3). In addition, strain PW26 was resistant to the toxic effects of the CsA analog D-Sar (α-SMe)3 Val2-DH-Cs (209-825) but, most interestingly, was not resistant to the CsA analog (γ-OH) MeLeu4-Cs (211-810). These observations confirm that cyclophilin A mediates the action of both CsA and the CsA analog D-Sar (α-SMe)3 Val2-DH-Cs (209-825) in C. neoformans and suggest that the CsA analog (γ-OH) MeLeu4-Cs (211-810) has an unusual mechanism of action or can still inhibit calcineurin when the levels of cyclophilin A are reduced (see below).
Recently we have obtained mutant strains PW47 and PW48 in which two genes encoding cyclophilin A homologs (CPA1 and CPA2) have been disrupted by homologous recombination. Both of these mutants lack all cyclophilin A protein (Fig. 2 and data not shown) and are completely resistant to the toxic effects of CsA and both the 209-825 and 211-810 CsA analogs (Table 3). These findings demonstrate that cyclophilin A mediates the toxic effects of both CsA and the nonimmunosuppressive CsA analogs.
Nonimmunosuppressive CsA analogs promote cryptococcal cyclophilin-calcineurin complexes in vitro.
We used affinity chromatography to test whether CsA and the two CsA analogs with reduced immunosuppressive activity mediate cryptococcal cyclophilin A-calcineurin interactions in vitro. Purified cryptococcal cyclophilin A bound to chitin beads was incubated with cryptococcal cell extract in the presence or absence of CsA and the D-Sar (α-SMe)3 Val2-DH-Cs (209-825) and (γ-OH) MeLeu4-Cs (211-810) analogs. Proteins associated with the cyclophilin A-drug complex were eluted, fractionated on SDS-polyacrylamide gels, and transferred to nitrocellulose membranes. The calcineurin A catalytic subunit was detected by its ability to associate with 125I-calmodulin in an overlay blot. Because cell extracts used for the affinity chromatography contained equal amounts of calcineurin, we were able to compare the affinity of each cyclophilin A-drug complex for cryptococcal calcineurin.
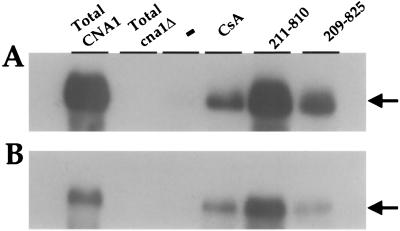
As expected, CsA promoted cyclophilin A binding to cryptococcal calcineurin (Fig. 4). In comparison, the cyclophilin A-calcineurin interaction was stimulated to an even greater extent by the CsA analog (γ-OH) MeLeu4-Cs (211-810) (Fig. 4). This finding provides a molecular explanation as to why the (γ-OH) MeLeu4-Cs (211-810) analog retains antifungal activity even in strains with reduced expression of cyclophilin A: namely, that the increased affinity of the cyclophilin A-analog complex for calcineurin mitigates the effects of reduced cyclophilin A levels in mutant strain PW26. The CsA analog D-Sar (α-SMe)3 Val2-DH-Cs (209-825) also promoted binding to calcineurin A (Fig. 4A). Similar results were obtained when serotype D strains were used as the source of protein extracts used for the affinity chromatography (Fig. 4B). Taken together, these findings indicate CsA and nonimmunosuppressive CsA analogs promote cyclophilin A binding to calcineurin.
FIG. 4.
CsA and CsA analogs promote cryptococcal cyclophilin A binding to calcineurin. Binding assays with purified cryptococcal cyclophilin A immobilized on chitin beads were performed with equal amounts of protein extract from serotype A strain H99 (A) and serotype D strain JEC21 (B) in the absence (−) or presence of 100 μM CsA and CsA analogs (γ-OH) MeLeu4-Cs (211-810) and D-Sar (α-SMe)3 Val2-DH-Cs (209-825). Bound proteins were eluted and fractionated by SDS-polyacrylamide gel electrophoresis, and the calcineurin A catalytic subunit was detected by an overlay blot with 125I-calmodulin. The migration position of the CNA1 protein is indicated by an arrow. Total protein extracts from serotype A and D CNA1 wild-type and cna1 mutant strains served as controls for the identity and electrophoretic mobility of the calcineurin A catalytic subunit CNA1.
Fluconazole-resistant C. neoformans clinical isolates are sensitive to immunosuppressive and nonimmunosuppressive CsA analogs.
We assessed the action of CsA and CsA analogs against two fluconazole-resistant isolates of C. neoformans obtained from AIDS patients. As shown in Table 2, both CsA and several CsA analogs exhibited antifungal activity against fluconazole-resistant clinical isolates T1 and 89-610. These observations confirm that CsA and its analogs retain activity against C. neoformans clinical isolates which have decreased susceptibility to azole compounds.
DISCUSSION
C. neoformans is a fungal pathogen of increasing importance, given its worldwide distribution, common occurrence in AIDS and other immunosuppressed patients, and the appearance of azole-resistant strains (10). Because current antifungal agents have a number of undesirable adverse side effects and poor fungicidal properties and because drug-resistant strains are emerging, the identification of novel antifungal drug targets and inhibitors is of significant clinical importance and may allow us to further exploit the use of combination antifungal therapy in the future.
We previously discovered that two immunosuppressive compounds, CsA and FK506, are toxic to C. neoformans at 37°C but not at 24°C in vitro (47). In addition, we found that C. neoformans mutants lacking the target of CsA and FK506, calcineurin, exhibited temperature-sensitive growth in vitro and were nonpathogenic in vivo. These observations identify calcineurin as a novel antifungal drug target.
In previous studies, Mody and coworkers found that CsA protected mice from extraneural cryptococcal infections and reported that CsA was toxic to C. neoformans under some in vitro conditions (41, 42). In contrast, Perfect and Durack found no evidence for CsA toxicity in vitro (50). Our findings that CsA toxicity is temperature dependent resolved the earlier discrepancies over CsA toxicity against C. neoformans in vitro, as the studies of Mody et al. were performed at 35°C and those of Perfect and Durack were conducted at 30°C. In both mice and rabbits, CsA exacerbated cryptococcal meningitis (42, 50), so the immunosuppressive activity of CsA outweighs any antifungal effect. In addition, CsA does not cross the blood-brain barrier, which likely also diminishes effectiveness against cryptococcal meningitis. Although complicated by steroid use, organ transplant recipients receiving CsA are similarly at risk for development of cryptococcal infection. These observations prompted us to identify and study nonimmunosuppressive CsA analogs to determine whether there was a difference in drug interactions between the mammalian and fungal target proteins which could be exploited to develop antifungal compounds.
Here we have assessed the antifungal activities of CsA analogs with diminished or absent immunosuppressive activities. In general, the immunosuppressive activity of this series of analogs correlated well with antifungal action, providing additional support for our model that CsA toxicity results from calcineurin inhibition by a cyclophilin A-CsA complex. In addition, we found two CsA analogs, (γ-OH) MeLeu4-Cs (211-810) and D-Sar (α-SMe)3 Val2-DH-Cs (209-825), that have dramatically decreased immunosuppressive activity but retain potent antifungal activity against C. neoformans. In related studies, we have also identified a nonimmunosuppressive FK506 analog, L-685,818, which similarly retains antifungal activity (48). Here we establish that CsA and the nonimmunosuppressive analogs are toxic to C. neoformans via cyclophilin A-dependent inhibition of calcineurin. First, we show that the compounds are toxic at 37°C and not 30°C, which correlates with the temperature-dependent growth of calcineurin mutant strains. Second, we show that mutations on the cyclophilin A-CsA binding surface of calcineurin (Val344Arg and Val344Lys) confer resistance to CsA and CsA analogs. Third, reduction in the level of the cyclophilin A protein confers resistance to CsA and the D-Sar (α-SMe)3 Val2-DH-Cs (209-825) analog. Fourth, two mutant strains completely lacking the cyclophilin A protein are resistant to CsA and both CsA analogs (Table 3). Finally, we present biochemical evidence that CsA and the CsA analogs D-Sar (α-SMe)3 Val2-DH-Cs (209-825) and (γ-OH) MeLeu4-Cs (211-810) promote cyclophilin A binding to fungal calcineurin. Most interestingly, the affinity of the cyclophilin A–(γ-OH) MeLeu4-Cs (211-810) analog complex for calcineurin is increased relative to that of cyclophilin A-CsA, and this analog is toxic to C. neoformans. These findings suggest that the γ-hydroxy substitution on the leucine 4 residue on the effector surface of CsA prevents binding to mammalian calcineurin but increases binding to the fungal calcineurin homolog.
We suggest that these CsA nonimmunosuppressive analogs take advantage of subtle inherent structural differences between host and fungal cyclophilin A and possibly also the calcineurin A catalytic and calcineurin B regulatory subunits, such that host immune function is spared but fungal growth is inhibited. Elucidation of the X-ray structure of the cyclophilin A-CsA-calcineurin complex and cloning and sequencing of the fungal calcineurin B subunit should allow further structural insights into these mechanisms of drug and drug analog action. It remains to be tested whether these nonimmunosuppressive CsA analogs will have therapeutic effects in animal models of cryptococcal meningitis, possibly in conjunction with agents that permeabilize the blood-brain barrier, but we suggest that these and other calcineurin inhibitors warrant consideration as novel antifungal agents in further studies.
ACKNOWLEDGMENTS
We thank Tamara Breuder, Dena Toffaletti, and Lora Cavallo for technical assistance. We are indebted to Tony Means and Elizabeth MacDougall for generously providing 125I-calmodulin.
This work was supported in part by RO1 grants AI39115, AI41937, and AI42159 from NIAID and PO1 grant AI44975 from NIAID to the Duke University Mycology Research Unit. Joseph Heitman is an associate investigator of the Howard Hughes Medical Institute and a Burroughs Wellcome Scholar in Molecular Pathogenic Mycology.
REFERENCES
- 1.Baumann G, Andersen E, Quesniaux V, Eberle M K. Cyclosporine and its analogue SDZ IMM 125 mediate very similar effects on T-cell activation—a comparative analysis in vitro. Transplant Proc. 1992;24:43–48. [PubMed] [Google Scholar]
- 2.Breuder T, Hemenway C S, Movva N R, Cardenas M E, Heitman J. Calcineurin is essential in cyclosporin A- and FK506-sensitive yeast strains. Proc Natl Acad Sci USA. 1994;91:5372–5376. doi: 10.1073/pnas.91.12.5372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cameron M L, Schell W A, Bruch S, Bartlett J, Waskin H A, Perfect J R. Correlation of in vitro fluconazole resistance of Candida isolates in relation to therapy and symptoms of individuals seropositive for human immunodeficiency virus type 1. Antimicrob Agents Chemother. 1993;37:2449–2453. doi: 10.1128/aac.37.11.2449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cardenas M E, Hemenway C, Muir R S, Ye R, Fiorentino D, Heitman J. Immunophilins interact with calcineurin in the absence of exogenous immunosuppressive ligands. EMBO J. 1994;13:5944–5957. doi: 10.1002/j.1460-2075.1994.tb06940.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cardenas M E, Lim E, Heitman J. Mutations that perturb cyclophilin A ligand binding pocket confer cyclosporin A resistance in Saccharomyces cerevisiae. J Biol Chem. 1995;270:20997–21002. doi: 10.1074/jbc.270.36.20997. [DOI] [PubMed] [Google Scholar]
- 6.Cardenas M E, Lorenz M, Hemenway C, Heitman J. Yeast as model-T cells. Perspect Drug Discov Des. 1994;2:103–126. [Google Scholar]
- 7.Cardenas M E, Muir R S, Breuder T, Heitman J. Targets of immunophilin-immunosuppressant complexes are distinct highly conserved regions of calcineurin A. EMBO J. 1995;14:2772–2783. doi: 10.1002/j.1460-2075.1995.tb07277.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cardenas M E, Sanfridson A, Cutler N S, Heitman J. Signal-transduction cascades as targets for therapeutic intervention by natural products. Trends Biotechnol. 1998;16:427–433. doi: 10.1016/s0167-7799(98)01239-6. [DOI] [PubMed] [Google Scholar]
- 9.Cardenas M E, Zhu D, Heitman J. Molecular mechanisms of immunosuppression by cyclosporine, FK506, and rapamycin. Curr Opin Nephrol Hypertens. 1995;4:472–477. doi: 10.1097/00041552-199511000-00002. [DOI] [PubMed] [Google Scholar]
- 10.Casadevall A, Perfect J R. Cryptococcus neoformans. Washington, D.C.: ASM Press; 1998. [Google Scholar]
- 11.Clipstone N A, Crabtree G R. Identification of calcineurin as a key signalling enzyme in T-lymphocyte activation. Nature. 1992;357:695–697. doi: 10.1038/357695a0. [DOI] [PubMed] [Google Scholar]
- 12.Cox G M, Perfect J R. Fungal infections. Curr Opin Infect Dis. 1993;6:422–426. [Google Scholar]
- 13.Cunningham K W, Fink G R. Calcineurin-dependent growth control in Saccharomyces cerevisiae mutants lacking PMC1, a homolog of plasma membrane Ca2+ ATPases. J Cell Biol. 1994;124:351–363. doi: 10.1083/jcb.124.3.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cyert M S, Kunisawa R, Kaim D, Thorner J. Yeast has homologs (CNA1 and CNA2 gene products) of mammalian calcineurin, a calmodulin-regulated phosphoprotein phosphatase. Proc Natl Acad Sci USA. 1991;88:7376–7380. doi: 10.1073/pnas.88.16.7376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cyert M S, Thorner J. Regulatory subunit (CNB1 gene product) of yeast Ca2+/calmodulin-dependent phosphoprotein phosphatases is required for adaptation to pheromone. Mol Cell Biol. 1992;12:3460–3469. doi: 10.1128/mcb.12.8.3460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Del Poeta M, Schell W A, Dykstra C C, Jones S, Tidwell R R, Czarny A, Bajic M, Bajic M, Kumar A, Boykin D, Perfect J R. Structure-in vitro activity relationships of pentamidine analogues and dication-substituted bis-benzimidazoles as new antifungal agents. Antimicrob Agents Chemother. 1998;42:2495–2502. doi: 10.1128/aac.42.10.2495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dolinski K, Heitman J. Peptidyl-prolyl isomerases (PPIases) In: Tooze S, editor. Guidebook to molecular chaperones and protein folding catalysts. Oxford, United Kingdom: Oxford University Press; 1997. pp. 359–369. [Google Scholar]
- 18.Flanagan W M, Corthésy B, Bram R J, Crabtree G R. Nuclear association of a T-cell transcription factor blocked by FK-506 and cyclosporin A. Nature. 1991;352:803–807. doi: 10.1038/352803a0. [DOI] [PubMed] [Google Scholar]
- 19.Foor F, Parent S A, Morin N, Dahl A M, Ramadan N, Chrebet G, Bostian K A, Nielsen J B. Calcineurin mediates inhibition by FK506 and cyclosporin of recovery from α-factor arrest in yeast. Nature. 1992;360:682–684. doi: 10.1038/360682a0. [DOI] [PubMed] [Google Scholar]
- 20.Garrett-Engele P, Moilanen B, Cyert M S. Calcineurin, the Ca2+/calmodulin-dependent protein phosphatase, is essential in yeast mutants with cell integrity defects and in mutants that lack a functional vacuolar H+-ATPase. Mol Cell Biol. 1995;15:4103–4114. doi: 10.1128/mcb.15.8.4103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Haendler B, Keller R, Hiestand P C, Kocher H P, Wegmann G, Movva N R. Yeast cyclophilin: isolation and characterization of the protein, cDNA and gene. Gene. 1989;83:39–46. doi: 10.1016/0378-1119(89)90401-0. [DOI] [PubMed] [Google Scholar]
- 22.Heitman J, Koller A, Cardenas M E, Hall M N. Identification of immunosuppressive drug targets in yeast. Methods Companion Methods Enzymol. 1993;5:176–187. [Google Scholar]
- 23.Heitman J, Movva N R, Hall M N. Proline isomerases at the crossroads of protein folding, signal transduction, and immunosuppression. New Biol. 1992;4:448–460. [PubMed] [Google Scholar]
- 24.Heitman J, Movva N R, Hall M N. Targets for cell cycle arrest by the immunosuppressant rapamycin in yeast. Science. 1991;253:905–909. doi: 10.1126/science.1715094. [DOI] [PubMed] [Google Scholar]
- 25.Heitman J, Movva N R, Hiestand P C, Hall M N. FK506-binding protein proline rotamase is a target for the immunosuppressive agent FK506 in Saccharomyces cerevisiae. Proc Natl Acad Sci USA. 1991;88:1948–1952. doi: 10.1073/pnas.88.5.1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hemenway C S, Dolinski K, Cardenas M E, Hiller M A, Jones E W, Heitman J. vph6 mutants of Saccharomyces cerevisiae require calcineurin for growth and are defective in vacuolar H+-ATPase assembly. Genetics. 1995;141:833–844. doi: 10.1093/genetics/141.3.833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hemenway C S, Heitman J. Calcineurin structure, function, and inhibition. Cell Biochem Biophys. 1999;30:115–151. doi: 10.1007/BF02737887. [DOI] [PubMed] [Google Scholar]
- 28.High K P. The antimicrobial activities of cyclosporine, FK506, and rapamycin. Transplantation. 1994;57:1689–1700. [PubMed] [Google Scholar]
- 29.Ho S N, Hunt H D, Horton R M, Pullen J K, Pease L R. Site-directed mutagenesis by overlap extension using the polymerase chain reaction. Gene. 1989;77:51–59. doi: 10.1016/0378-1119(89)90358-2. [DOI] [PubMed] [Google Scholar]
- 30.Jain J, McCaffrey P G, Miner Z, Kerppola T K, Lambert J N, Verdine G L, Curran T, Rao A. The T-cell transcription factor NFATp is a substrate for calcineurin and interacts with Fos and Jun. Nature. 1993;365:352–355. doi: 10.1038/365352a0. [DOI] [PubMed] [Google Scholar]
- 31.Kallen J, Spitzfaden C, Zurini M G M, Wider G, Widmer H, Wuthrich K, Walkinshaw M D. Structure of human cyclophilin and its binding site for cyclosporin A determined by X-ray crystallography and NMR spectroscopy. Nature. 1991;353:276–279. doi: 10.1038/353276a0. [DOI] [PubMed] [Google Scholar]
- 32.Ke H, Mayrose D, Cao W. Crystal structure of cyclophilin A complexed with substrate ala-pro suggests a solvent-assisted mechanism of cis-trans isomerization. Proc Natl Acad Sci USA. 1993;90:3324–3328. doi: 10.1073/pnas.90.8.3324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ke H, Zydowsky L D, Liu J, Walsh C T. Crystal structure of recombinant human T-cell cyclophilin A at 2.5 A resolution. Proc Natl Acad Sci USA. 1991;88:9483–9487. doi: 10.1073/pnas.88.21.9483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kirkland T N, Fierer J. Cyclosporin A inhibits Coccidioides immitis in vitro and in vivo. Antimicrob Agents Chemother. 1983;24:921–924. doi: 10.1128/aac.24.6.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kwon-Chung K J, Bennett J E. Medical mycology. Malvern, Pa: Lea & Febiger; 1992. Cryptococcosis; pp. 397–446. [Google Scholar]
- 36.Liu J, Albers M W, Wandless T J, Luan S, Alberg D G, Belshaw P J, Cohen P, MacKintosh C, Klee C B, Schreiber S L. Inhibition of T cell signaling by immunophilin-ligand complexes correlates with loss of calcineurin phosphatase activity. Biochemistry. 1992;31:3896–3901. doi: 10.1021/bi00131a002. [DOI] [PubMed] [Google Scholar]
- 37.Liu J, Farmer J D, Lane W S, Friedman J, Weissman I, Schreiber S L. Calcineurin is a common target of cyclophilin-cyclosporin A and FKBP-FK506 complexes. Cell. 1991;66:807–815. doi: 10.1016/0092-8674(91)90124-h. [DOI] [PubMed] [Google Scholar]
- 38.Meo T. The MLR in the mouse. In: Lefkovits L, Pernis B, editors. Immunological methods. New York, N.Y: Academic Press; 1979. pp. 227–239. [Google Scholar]
- 39.Mikol V, Kallen J, Pflugl G, Walkinshaw M D. X-ray structure of a monomeric cyclophilin A-cyclosporin A crystal complex at 2.1 A resolution. J Mol Biol. 1993;234:1119–1130. doi: 10.1006/jmbi.1993.1664. [DOI] [PubMed] [Google Scholar]
- 40.Mitchell T G, Perfect J R. Cryptococcosis in the era of AIDS—100 years after the discovery of Cryptococcus neoformans. Clin Microbiol Rev. 1995;8:515–548. doi: 10.1128/cmr.8.4.515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mody C H, Toews G B, Lipscomb M F. Cyclosporin A inhibits the growth of Cryptococcus neoformans in a murine model. Infect Immun. 1988;56:7–12. doi: 10.1128/iai.56.1.7-12.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mody C H, Toews G B, Lipscomb M F. Treatment of murine cryptococcosis with cyclosporin-A in normal and athymic mice. Am Rev Respir Dis. 1989;139:8–13. doi: 10.1164/ajrccm/139.1.8. [DOI] [PubMed] [Google Scholar]
- 43.Nakamura T, Liu Y, Hirata D, Namba H, Harada S-I, Hirokawa T, Miyakawa T. Protein phosphatase type 2B (calcineurin)-mediated, FK506-sensitive regulation of intracellular ions in yeast is an important determinant for adaptation to high salt stress conditions. EMBO J. 1993;12:4063–4071. doi: 10.1002/j.1460-2075.1993.tb06090.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.National Committee for Clinical Laboratory Standards. Reference method for broth dilution susceptibility testing of yeasts. Approved standard M27-A. Wayne, Pa: National Committee for Clinical Laboratory Standards; 1997. [Google Scholar]
- 45.Northrop J P, Ho S N, Chen L, Thomas D J, Timmerman L A, Nolan G P, Admon A, Crabtree G R. NF-AT components define a family of transcription factors targeted in T-cell activation. Nature. 1994;369:497–502. doi: 10.1038/369497a0. [DOI] [PubMed] [Google Scholar]
- 46.O'Keefe S J, Tamura J I, Kincaid R L, Tocci M J, O'Neill E A. FK-506- and CsA-sensitive activation of the interleukin-2 promoter by calcineurin. Nature. 1992;357:692–694. doi: 10.1038/357692a0. [DOI] [PubMed] [Google Scholar]
- 47.Odom A, Muir S, Lim E, Toffaletti D L, Perfect J, Heitman J. Calcineurin is required for virulence of Cryptococcus neoformans. EMBO J. 1997;16:2576–2589. doi: 10.1093/emboj/16.10.2576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Odom A, Poeta M D, Perfect J, Heitman J. The immunosuppressant FK506 and its nonimmunosuppressive analog L-685,818 are toxic to Cryptococcus neoformans by inhibition of a common target protein. Antimicrob Agents Chemother. 1997;41:156–161. doi: 10.1128/aac.41.1.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Paugam A, Dupouy-Camet J, Blanche P, Gangneux J P, Tourte-Schaefer C, Sicard D. Increased fluconazole resistance of Cryptococcus neoformans isolated from a patient with AIDS and recurrent meningitis. Clin Infect Dis. 1994;19:975–976. doi: 10.1093/clinids/19.5.975-a. [DOI] [PubMed] [Google Scholar]
- 50.Perfect J R, Durack D T. Effects of cyclosporine in experimental cryptococcal meningitis. Infect Immun. 1985;50:22–26. doi: 10.1128/iai.50.1.22-26.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pflugl G, Kallen J, Schirmer T, Jansonius J N, Zurini M G M, Walkinshaw M D. X-ray structure of a decameric cyclophilin-cyclosporin crystal complex. Nature. 1993;361:91–94. doi: 10.1038/361091a0. [DOI] [PubMed] [Google Scholar]
- 52.Quesniaux V F, Schreier M H, Wenger R M, Hiestand P C, Harding M W, Regenmortel M H V. Cyclophilin binds to the region of cyclosporine involved in its immunosuppressive activity. Eur J Immunol. 1987;17:1359–1365. doi: 10.1002/eji.1830170921. [DOI] [PubMed] [Google Scholar]
- 53.Schmid F X. Prolyl isomerase—enzymatic catalysis of slow protein-folding reactions. Annu Rev Biophys Biomol Struct. 1993;22:123–143. doi: 10.1146/annurev.bb.22.060193.001011. [DOI] [PubMed] [Google Scholar]
- 54.Schneider H, Charara N, Schmitz R, Wehrli S, Mikol V, Zurini M G M, Quesniaux V F J, Movva N R. Human cyclophilin C: primary structure, tissue distribution, and determination of binding specificity for cyclosporins. Biochemistry. 1994;33:8218–8224. doi: 10.1021/bi00193a007. [DOI] [PubMed] [Google Scholar]
- 55.Schreiber S L, Crabtree G R. The mechanism of action of cyclosporin A and FK506. Immunol Today. 1992;13:136–142. doi: 10.1016/0167-5699(92)90111-J. [DOI] [PubMed] [Google Scholar]
- 56.Shaw K T-Y, Ho A M, Raghavan A, Kim J, Jain J, Park J, Sharma S, Rao A, Hogan P G. Immunosuppressive drugs prevent a rapid dephosphorylation of transcription factor NFAT1 in stimulated immune cells. Proc Natl Acad Sci USA. 1995;92:11205–11209. doi: 10.1073/pnas.92.24.11205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Strong D M, Ahmed A A, Thurman G B, Sell K W. In vitro stimulation of murine spleen cells using a microculture system and a multiple automated sample harvester. J Immunol Methods. 1973;2:279–291. doi: 10.1016/0022-1759(73)90054-9. [DOI] [PubMed] [Google Scholar]
- 58.Tanida I, Hasegawa A, Iida H, Ohya Y, Anraku Y. Cooperation of calcineurin and vacuolar H+-ATPase in intracellular Ca2+ homeostasis of yeast cells. J Biol Chem. 1995;270:10113–10119. doi: 10.1074/jbc.270.17.10113. [DOI] [PubMed] [Google Scholar]
- 59.Toffaletti D L, Rude T H, Johnston S A, Durack D T, Perfect J R. Gene transfer in Cryptococcus neoformans by use of biolistic delivery of DNA. J Bacteriol. 1993;175:1405–1411. doi: 10.1128/jb.175.5.1405-1411.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tropschug M, Barthelmess I B, Neupert W. Sensitivity to cyclosporin A is mediated by cyclophilin in Neurospora crassa and Saccharomyces cerevisiae. Nature. 1989;342:953–955. doi: 10.1038/342953a0. [DOI] [PubMed] [Google Scholar]
- 61.White T C. Antifungal drug resistance in Candida albicans. ASM News. 1998;63:427–433. [Google Scholar]
- 62.White T C, Marr K A, Bowden R A. Clinical, cellular, and molecular factors that contribute to antifungal drug resistance. Clin Microbiol Rev. 1998;11:382–402. doi: 10.1128/cmr.11.2.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zinder N D, Boeke J D. The filamentous phage (Ff) as vectors for recombinant DNA—a review. Gene. 1982;19:1–10. doi: 10.1016/0378-1119(82)90183-4. [DOI] [PubMed] [Google Scholar]