Abstract
The development of innovative antifungal agents is essential. Some fungicidal agents are no longer effective due to resistance development, various side effects, and high toxicity. Therefore, the synthesis and development of some new antifungal agents are necessary. 1,2,4-Triazole is one of the most essential pharmacophore systems between five-membered heterocycles. The structure-activity relationship (SAR) of this nitrogen-containing heterocyclic compound showed potential antifungal activity. The 1,2,4-triazole core is present as the nucleus in a variety of antifungal drug categories. The most potent and broad activity of triazoles have confirmed them as pharmacologically significant moieties. The goal of this review is to highlight recent developments in the synthesis and SAR study of 1,2,4-triazole as a potential fungicidal compound. In this study, we provide the results of a biological activity evaluation using various structures and figures. Literature investigation showed that 1, 2, 4-triazole derivatives reveal the extensive span of antifungal activity. This review will assist researchers in the development of new potential antifungal drug candidates with high effectiveness and selectivity.
1. Introduction
Heterocyclic organic chemistry is a main field in organic and medicinal chemistry [1–4]. Azoles are nitrogen-containing five-membered heterocyclic compounds [5, 6]. The presence of nitrogen in heterocycles has a major effect on biological activity. Recently, azole compounds have become hot topics around the world [2, 7]. Among the azoles fused as heterocyclic compounds, 1,2,4-triazole derivatives with molecular formula (C2H3N3) are the most stable compounds [8]. 1,2,4-Triazoles showed broad ranges of biological activities, such as antimalarial [9], antiurease [10], antiviral [11], anticonvulsant [12], antioxidant [13], and antifungal [14]. Some of the medicinal plants containing triazole scaffolds were demonstrated to be antifungal agents, including cyproconazole, triadimefon, metconazole, tebuconazole, propiconazole, epoxiconazole, and prothioconazole [15]. Annually, invasive fungal infections cause 1.7 million deaths in the world, which is a major public health issue [16]. One of the most serious issues is the rise of synthetic drug resistance to various fungal pathogens; thus, the synthesis and development of new 1,2,4-triazoles with low toxicity are essential worldwide [17]. This group of bioactive compounds acts by inhibiting the activity of cytochrome P450-dependent enzyme, the lanosterol 14α-demethylase (CYP51), which is an important enzyme in fungi ergosterol biosynthesis [18]. Azoles link to the iron in porphyrins, causing a blockade of the fungal ergosterol biosynthesis pathway resulting in the agglomeration of 14-demethylated sterols [19]. Recently, novel derivatives of 1,2,4-triazoles were prepared and evaluated for fungicidal activity and some of them showed potential activity against certain fungi. In previous years, many research articles have emphasized the importance of 1,2,4-triazoles as potent antifungal and antibacterial properties [20–31]. However, this review is focused on the latest papers (2015–2021) in the synthesis of new 1,2,4-triazole as an antifungal agent and evaluating structure-activity relationship (SAR) to provide an insight for the logical synthesis of more effective 1,2,4-triazole antifungal candidates. The diagram of choosing publications and the content of this review are illustrated in Figure 1.
Figure 1.
Chart related to the content of provided in this review.
2. Synthesis of 1,2,4-Triazoles
1,2,4-Triazoles are five-membered sp2 hybridization compounds containing three nitrogen atoms at the 1-, 2-, and 4-positions of the ring. There are two tautomeric forms: 4H-1,2,4-triazole and 1H-1,2,4-triazole (Scheme 1) [32].
Scheme 1.
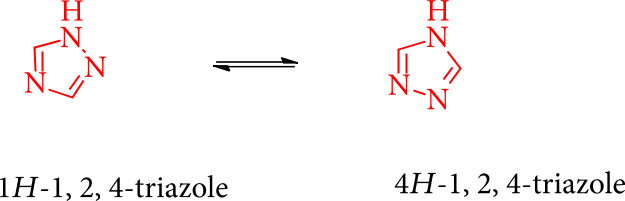
Tautomeric forms of the 1,2,4-triazole structure.
2.1. General Pathways
A simple method for preparing 1,2,4-triazoles has been introduced from the reaction of formamide and hydrazines without a catalyst using microwave irradiation (Scheme 2) [33].
Scheme 2.
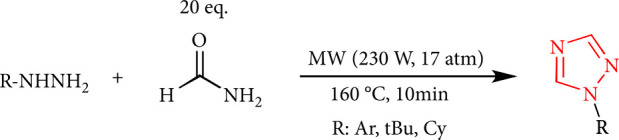
The preparation of 1,2,4-triazoles from hydrazines.
A series of 1,3-disubstituted-1,2,4-triazoles were synthesized by reacting amidine and trialkyl amines with K3PO4 as a base in the presence of a copper (II) catalyst (Scheme 3) [34].
Scheme 3.
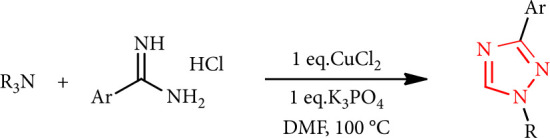
Synthesis of 1,2,4-triazole from amidine.
I2-mediated oxidative N-S and C-N bond formations are an ecologically friendly and effective approach for synthesizing novel 1,2,4-triazoles from isothiocyanates (Scheme 4) [35].
Scheme 4.
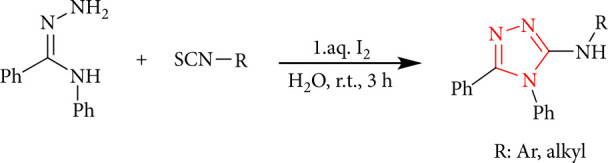
I2-mediated oxidative C-N and N-S bond formations.
1,5-Disubstituted-1,2,4-triazoles were prepared using copper (II) as the catalyst. This regioselective method makes it simple to produce 1,2,4-triazole moiety with wide substrate amplitude, high yield, and significant functional group compatibility (Scheme 5) [36].
Scheme 5.
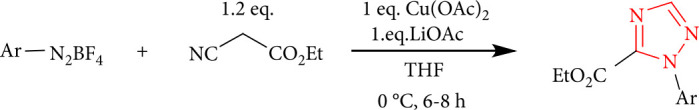
Synthesis of 1,2,4-triazoles in the presence of Cu (II) catalysis.
The synthesis of 1,2,4-triazoles from aliphatic amines and hydrazones has been developed using a cascade C-H functionalization, oxidative aromatization sequence, and double C-N bond formation under iodine as the catalyst (Scheme 6) [37].
Scheme 6.
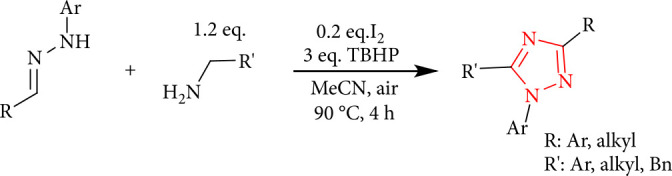
The synthesis of 1,2,4-triazoles of hydrazones and aliphatic amines.
2.2. Synthesis by Substitution of 1,2,4-Triazole
2.2.1. Arylation Reactions
Under reflux circumstances in pyridine, 1-aryl-1,2,4-1H-triazole is arylated by an aryl halide with electron-withdrawing groups using CuO as the catalyst (Scheme 7) [38, 39].
Scheme 7.
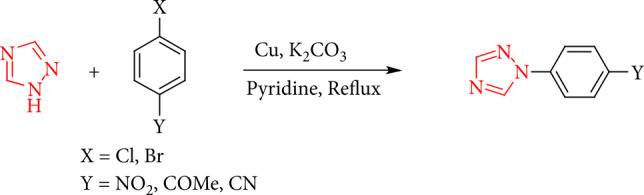
Arylation reactions of 1,2,4-triazoles.
2.2.2. Alkylation
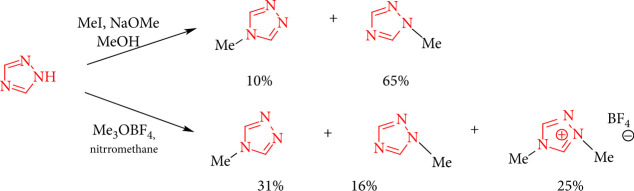
When sodium methoxide in methanol is used as the base, 1,2,4-triazole is alkylated at the N1 position, yielding in a mixture of 1-methyl- and 4-methyl-1,2,4-triazole with methyl sulfate alkylation in NaOH (Scheme 8) [40].
Scheme 8.
Alkylation reactions of 1,2,4-triazoles.
2.3. Direct the Synthesis of N4-Substituted Triazoles
A group of 4-Arylsubstituted-1,2,4-4H-triazoles yields via the reaction of N, N-diformylhydrazine with primary amine at high temperatures (Scheme 9) [41]. Pellizzari and Soldi pioneered this system by reacting simple arylamines such as naphthylamine, aniline, or toluidine with N, N′-diformylhydrazine [42, 43], as well as downloading via slight changes in amino heterocycles containing 3-amino-1,2,4-4H-triazole to generate 3,4-bitriazoles [44–46].
Scheme 9.
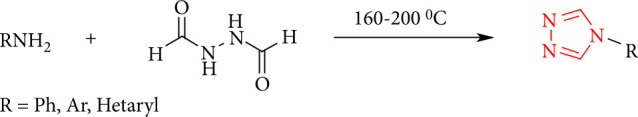
Direct synthesis of N4-substituted triazoles.
3. Pharmaceutical Drugs
3.1. Chemical Structures of 1, 2, 4-Triazole-Based Marketed Drugs
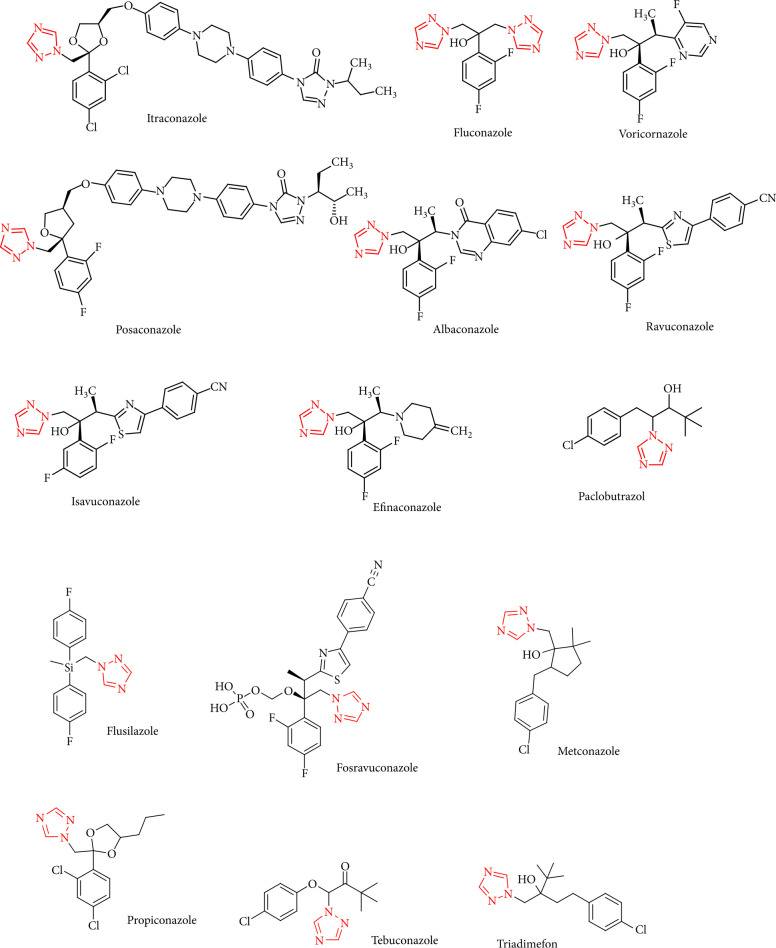
Bladin were the first to synthesize 1,2,4-triazoles in 1885 [31]. The primary procedures, such as the reaction of formamide with formylhydrazine, produced low yields of 1,2,4-triazole [47, 48]. Later, it was found that condensation of formamide with hydrazine sulfate yielded 1,2,4-triazole in average yield. Various pharmacological activities of 1,2,4-triazoles as antifungal [42, 49, 50] have been observed which included fluconazole, isavuconazole, itraconazole, voriconazole, ravuconazole, and posaconazole (Figure 2). Several 1,2,4-triazole-based drugs are under clinical use for the treatment of different diseases. Some of the most effective drugs available in the market are described in the following Table 1.
Figure 2.
Chemical structures of some bioactive 1,2,4-triazole-based marketed drugs.
Table 1.
Pharmacological properties and clinical implications of all marketing antifungal drugs.
| Drug/PubChem ID | Chemical name | Action of antifungal | Ref. |
|---|---|---|---|
| Itraconazole, CID: 55283 | 2-Butan-2-yl-4-[4-[4-[4-[[(2R,4S)-2-(2,4-dichlorophenyl)-2-(1,2,4-triazol-1-ylmethyl)-1,3-dioxolan-4-yl]methoxy]phenyl]piperazin-1-yl]phenyl]-1,2,4-triazol-3-on | Treatment of onychomycosis and seborrheic dermatitis | [51–53] |
| Fluconazole, CID: 3365 | 2-(2,4-Difluorophenyl)-1,3-bis(1H-1,2,4-triazol-1-yl)propan-2-ol | Effect on blastomycosis, cryptococcosis, candidiasis, coccidioidomycosis, histoplasmosis, dermatophytosis, and pityriasis versicolor | [54, 55] |
| Isavuconazole, CID: 6918485 | 4-[2-[(2R,3R)-3-(2,5-Difluorophenyl)-3-hydroxy-4-(1,2,4-triazol-1-yl) butan-2-yl]-1,3-thiazol-4-yl] benzonitrile | To treat invasive aspergillosis and invasive mucormycosis | [56, 57] |
| Efinaconazole, CID: 489181 | (2R, 3R)-2-(2, 4-Difluorophenyl)-3-(4-methylidenepiperidin-1-yl)-1-(1, 2, 4-triazol-1-yl) butan-2-ol | The treatment of onychomycosis (nail fungal infection) | [58–60] |
| Posaconazole, CID: 468595 | 4-[4-[4-[4-[[(3R,5R)-5-(2,4-Difluorophenyl)-5-(1,2,4-triazol-1-ylmethyl)oxolan-3-yl]methoxy]phenyl]piperazin-1-yl]phenyl]-2-[(2S,3S)-2-hydroxypentan-3-yl]-1,2,4-triazol-3-one | For the treatment of aspergillus and Candida and invasive fungal infections caused by the treatment of Scedosporium and fusarium species of pharyngeal candidiasis (OPC), including OPC retrofitting in the treatment of itraconazole and/or fluconazole | [61–66] |
| Voriconazole, CID: 71616 | (2R, 3S)-2-(2, 4-Difluorophenyl)-3-(5-fluoropyrimidin-4-yl)-1-(1, 2, 4-triazol-1-yl) butan-2-ol | Treatment includes candidiasis, coccidioidomycosis, histoplasmosis, penicilliosis, aspergillosis, and infections by Scedosporium or fusarium | [67–70] |
| Albaconazole, CID: 208952 | 7-Chloro-3-[(2R, 3R)-3-(2, 4-difluorophenyl)-3-hydroxy-4-(1, 2, 4-triazol-1-yl) butan-2-yl] quinazolin-4-one | Treatment of antiprotozoal agent | [71] |
| Ravuconazole, CID: 467825 | 4-[2-[(2R, 3R)-3-(2, 4-Difluorophenyl)-3-hydroxy-4-(1, 2, 4-triazol-1-yl) butan-2-yl]-1, 3-thiazol-4-yl] benzonitrile | Limited activity against species of Scedosporium, fusarium, and zygomycetes | [72–74] |
| Propiconazole, CID: 43234 | 1-[[2-(2,4-Dichlorophenyl)-4-propyl-1,3-dioxolan-2-yl]methyl]-1,2,4-triazole | In terms of agriculture as a systemic fungicide grown in meadow plants for seeds and aesthetic value, sports, wheat, mushrooms, corn, wild rice, peanuts, almonds, sorghum, oats, pecans, apricots, peaches, nectarines, plums, and prunes are used | [75, 76] |
| Fosravuconazole, CID: 9807507 | [(2R,3R)-3-[4-(4-Cyanophenyl)-1,3-thiazol-2-yl]-2-(2,4-difluorophenyl)-1-(1,2,4-triazol-1-yl)butan-2-yl]oxymethyl dihydrogen phosphate | Treatment of onychomycosis, fungal nail infections, and treatment of eumycetoma | [31, 77] |
| Fosfluconazole, CID: 214356 | {[2-(2,4-Difluorophenyl)-1,3-bis(1H-1,2,4triazole-1-yl)propan-2-yl]oxy}cphosphonic acid | Treatment and prevention of superficial and systemic fungal infections | [78] |
| Flusilazole, CID: 73675 | 1-{[Bis(4-fluorophenyl)methylsilyl]methyl}-1H-1,2,4-triazole | Used to control fungal infections in a variety of fruit and vegetable products | [79–81] |
| Tebuconazole, CID: 86102 | (RS)-1-(4-Chlorophenyl)-4,4-dimethyl-3-(1H, 1,2,4-triazol-1-ylmethyl)pentan-3-ol | Used in agriculture to treat pathogenic fungi of plants | [82] |
| Triadimefon, CID: 39385 | 1-(4-Chlorophenoxy)-3,3-dimethyl-1-(1H-1,2,4-triazol-1-yl)butan-2-one | Used in agriculture to control various fungal diseases | [83] |
| Metconazole, CID: 86210 | 5-[(4-Chlorophenyl)methyl]-2,2-dimethyl-1-(1,2,4-triazol-1-ylmethyl)cyclopentan-1-ol | To control a wide range of fungal infections including Alternaria, rust, fusarium, and Septoria | [84] |
| Paclobutrazol, CID: 158076 | (2RS,3RS)-1-(4-Chlorophenyl)-4,4-dimethyl-2-(1,2,4-triazol-1-yl)pentan-3-ol | Plant growth inhibitor, triazole fungicide, root growth, and drought stress resistance can be used as a chemical method to reduce the risk of habitat in cereal crops | [85] |
| Myclobutanil, CID: 6336 | 2-(4-Chlorophenyl)-2-(1,2,4-triazol-1-ylmethyl)hexanenitrile | Used as a fungicide which is a steroid demethylation inhibitor, specially inhibiting ergosterol biosynthesis | [86, 87] |
3.2. Structure-Activity Relationship of Fluconazole
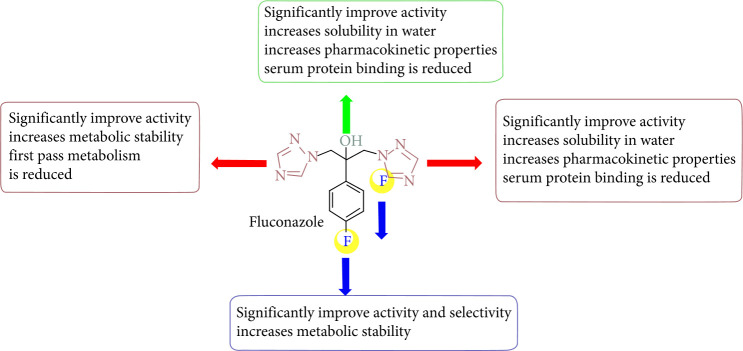
Fluconazole is well known as one of the most potent antifungal drugs with remarkable interest in medicinal chemistry. Due to the importance of fluconazole as a reference drug, the structure-activity relationship is shown in Figure 3.
Figure 3.
Structure-activity relationship of fluconazole.
4. 1,2,4 Triazole Scaffold for the Development of Antifungal Agents
This part of the review is classified into two parts based on the structural similarities of 1,2,4-triazole derivatives. First, we explained novel analogues of commercial 1,2,4-triazole drugs and then discussed 1,2,4-triazole-based scaffolds with various functional groups such as indole, benzimidazole, quinolone, quinazoline, amine, hydrazone, amide, sulfur, and oxime ether that showed remarkable antifungal properties, as well as SARs of all synthesized compounds.
4.1. Analogues of Commercial 1,2,4-Triazole Drugs
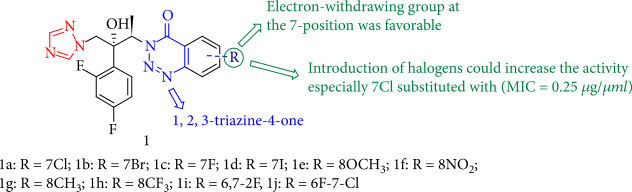
Most triazole compounds containing 1,2,3-benzotriazine-4-one demonstrated more antifungal activity against Candida albicans and Cryptococcus neoformans than reference drug with MIC values ranging from 0.0156 to 2.0 μg/mL. Furthermore, a strong SAR investigation revealed that derivatives with groups −NO2 and CF3 at the 7-position exhibited more effective antifungal activity than derivatives with groups at the 5-, 6-, and 8-positions. In addition, products containing halogens such as Cl and F demonstrated more excellent antifungal activity than those containing electron-withdrawing groups. Meanwhile, compound 1a (R = 7Cl) demonstrated remarkable antifungal activity specifically against Aspergillus fumigatus (MIC = 0.25 μg/mL) and moderate activity against fluconazole-resistant Candida albicans strains (Figure 4) [88].
Figure 4.
The structure of 1, 2, 3-benzotriazin-4-one.
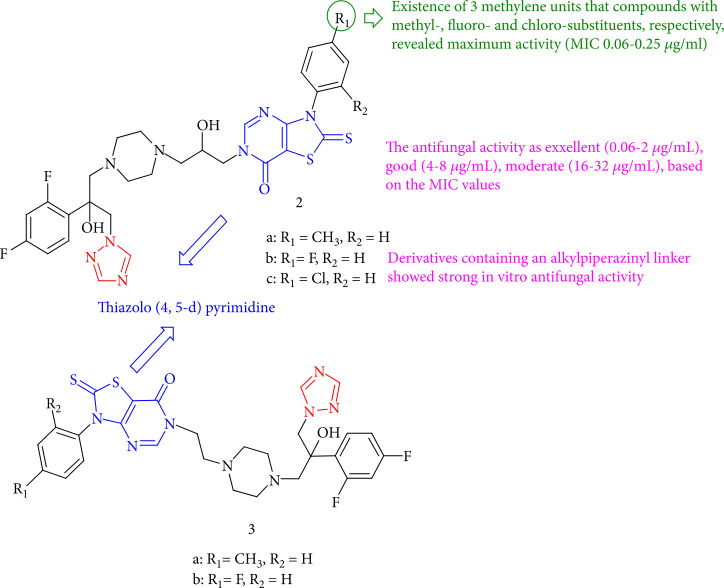
Blokhina et al. [29] investigated the fungicidal activity of thiazolo[4,5-d] pyrimidine hybrids with (1H-1,2,4) triazole. All derivatives include the methyl-(2a), fluoro-(2b), and chloro-(2c) substituents at the para position. In vitro evaluation of various compounds with a potent alkylpiperazinyl linker demonstrated antifungal activity similar to the standard drug. The most active compounds are methyl-(2a), fluoro-(2b), chloro-(2c) methyl-(3a), and fluoro-(3b). Based on MIC values, antifungal activity is classified as poor (≥32 μg/mL), modest (16–32 μg/mL), good (4–8 μg/mL), excellent (0.06–2 μg/mL), or outstanding based on MIC values (Figure 5).
Figure 5.
Antifungal screening of novel hybrids of triazole derivatives.
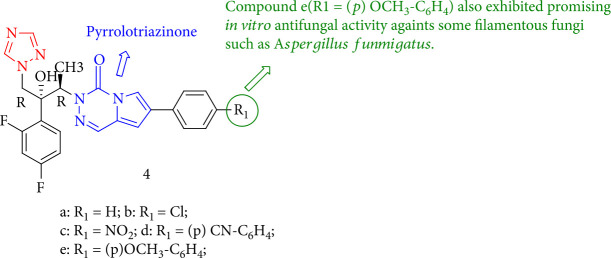
Montoir et al. [89] reported a novel class of azole antifungal compounds based on a pyrrolotriazinone scaffold. As a result, these compounds demonstrated fungicidal activity against pathogenic Candida species in vitro (fluconazole susceptible and fluconazole resistant) and were more active than voriconazole against two Candida albicans candidates. Compound 4e also showed promising in vitro activity against several filamentous fungi, including Aspergillus fumigatus (Figure 6).
Figure 6.
Chemical structure of novel azole antifungals containing a fused triazinone scaffold.
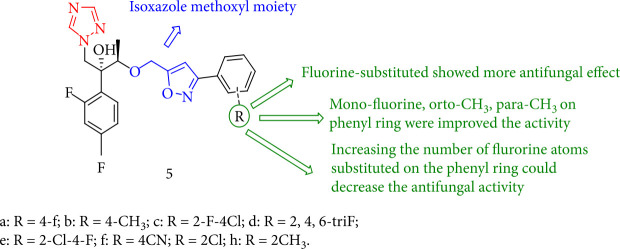
Xie et al. [90] demonstrated that the entire series of triazole containing isoxazole compounds (5a-f) were antifungal against eight human pathogenic fungi. Compound 5a showed a strong inhibitory activity toward Candida parasilosis and Candida albicans with MIC80 values of 0.0313 μg/mL. According to the SARs study, mono-fluorine on the phenyl ring possesses antifungal activity. On the other hand, enhancing the number of fluorine atoms (5c-d) may result in a reduction in antifungal activity (Figure 7).
Figure 7.
Chemical structure of novel triazole analogues featuring isoxazole moieties.
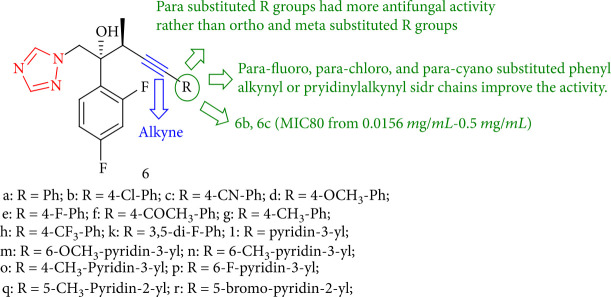
In comparison to the reference drugs, voriconazole, fluconazole, and ravuconazole, an alkyne linked in the side chain of the triazole derivatives demonstrated good fungicidal activity against eight human pathogenic fungi, with particularly noticeable activity against Cryptococcus species and Candida. Compounds 6b and 6c shown in vitro antifungal activity against all the investigated fungi with (MIC80 = 0.0156–0.5 mg/mL), which is greater than fluconazole and ravuconazole. SAR study shows that para fluoro (6e, 6h), para chloro (6b), and para cyano (6c) substituted phenylalkynyl, or pyridinyl alkynyl (6m, 6n) side chains improve activity. Compounds with R groups in the para position are shown to be more active than meta or ortho compounds (Figure 8) [91].
Figure 8.
Novel triazole derivatives bearing alkynyl side chains.
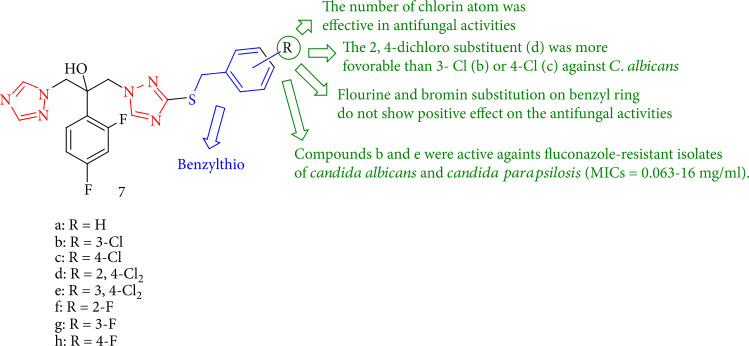
The antifungal efficacy of triazole alcohol derivatives toward 16 Candida isolates from five different species, including fluconazole-susceptible and fluconazole-resistant isolates, was investigated. All of these derivatives with MIC values of 0.063–1 mg/mL showed higher activity than fluconazole (MICs = 0.5–4 mg/mL) against fluconazole-susceptible isolates; significantly, compounds 7b and 7e were also active against fluconazole-resistant species. However, the effect of chloro substitution depends on the type of species. For example, the 2,4-dichloro substituent 7d was shown to be more effective against C. albicans than 3-Cl (7b) or 4-Cl (7c). In the case of C. krusei, however, the 3-chloro group was better than the 4-chloro or 2,4-dichloro substituents. The addition of fluoro or bromo groups to the benzyl residue, however, had no beneficial impact. Among the fluorobenzyl regioisomers (7f-h), the 3-fluoro 7g analog was more active against Candida species (Figure 9) [92].
Figure 9.
Benzylthio analogues of fluconazole.
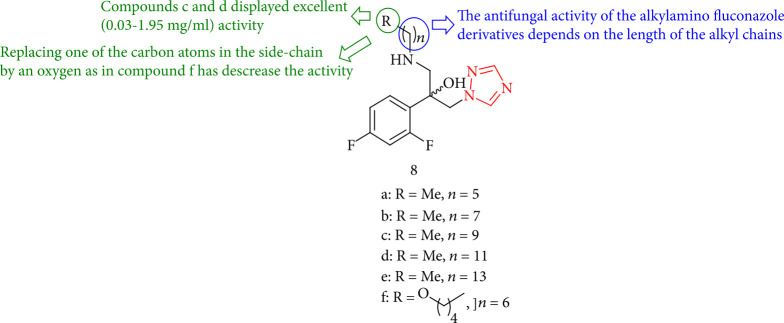
Chandrika et al. [93] investigated novel fluconazole (FLC) compounds for antifungal activity against the clinical strains of C. parapsilosis, C. glabrata, and Candida with aryl, alkyl, cycloalkyl, and dialkyl-amino substituents for neoformans using MIC determination. The activity of the alkylamino FLC derivatives was shown to be directionally related to the length of the alkyl chains (Figure 10).
Figure 10.
The study of antifungal properties in new fluconazole derivatives.
Tekale et al. [94] investigated the antifungal activities of triazole compounds, including imidazole. The impact of the imidazole side chain on the in vitro fungicidal activity of novel synthesized compounds toward various microorganisms such as aspergillus, niger, aspergillus fumigates, and Candida albicans was demonstrated. Compound 9e had the lowest activity against C. albicans, and compounds 9b & 9d had higher activity against A. niger than the other compounds (Figure 11).
Figure 11.
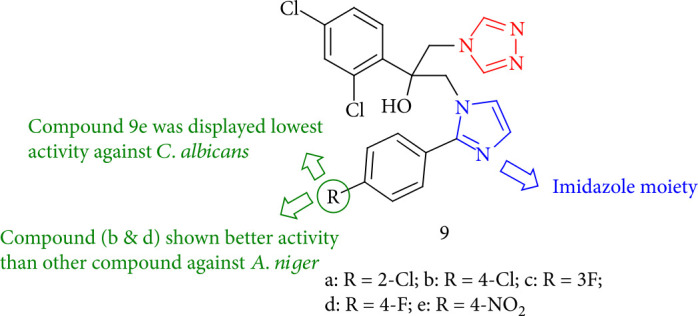
Structure of azoles containing imidazole derivatives.
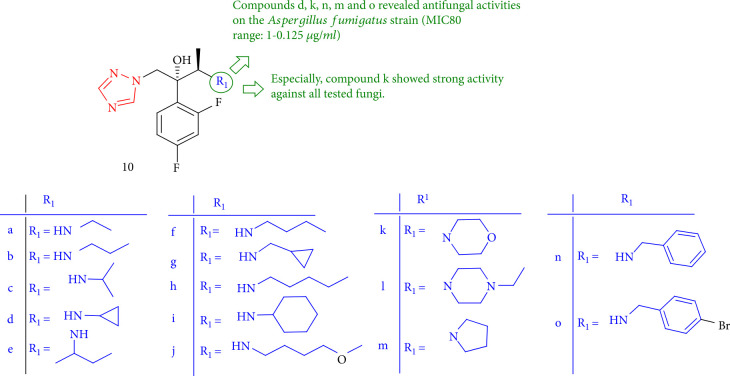
The MIC80 values of new triazole compounds containing 1,2,3-triazoles or substituted amines as side chain 10a-o derivatives demonstrated better antifungal properties than those of fluconazole on three significant fungal infections except for 10i. Furthermore, the considerable compounds 10d, 10 k, 10n, 10 m, and 10o were reported on the Aspergillus fumigatus strain (MIC80 range: 0.125–1 μg/mL). In addition, 10k can be applied to almost all fungi tested, especially Aspergillus spp. In vitro biological assessments of the compounds 10d and 10k showed potent antifungal properties (Figure 12) [95].
Figure 12.
Antifungal assessment of new triazole derivatives.
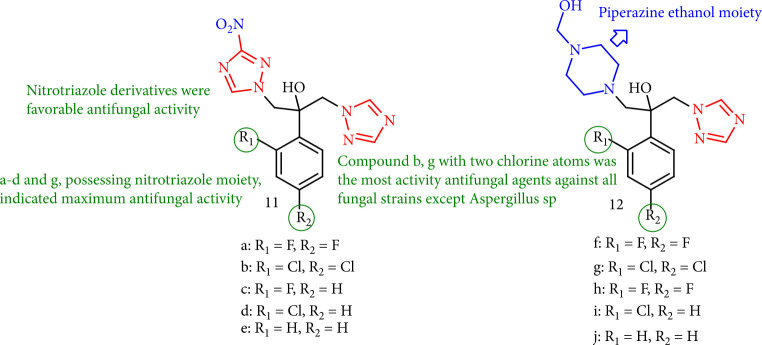
Sadeghpour et al. [96] reported two classes of novel fluconazole-derivatives containing nitrotriazole or 2-(piperazin-1-yl) ethanol moieties, which were evaluated for antifungal activity against standards and clinically isolated yeasts, and their MIC structures were compared with those of fluconazole. Nitrotriazole derivatives 11a-d and compounds 12g and 11b containing two chlorine atoms exhibited good activity against the tested fungus, notably some fluconazole-resistant species. Compounds 11a, 11b, and 12g with 2,4-difluorophenyl or 2,4-dichlorophenyl groups had more excellent antifungal activity (Figure 13).
Figure 13.
Fluconazole-derivatives bearing nitrotriazol or 2-(piperazine-1-ylFig) ethanol moieties.
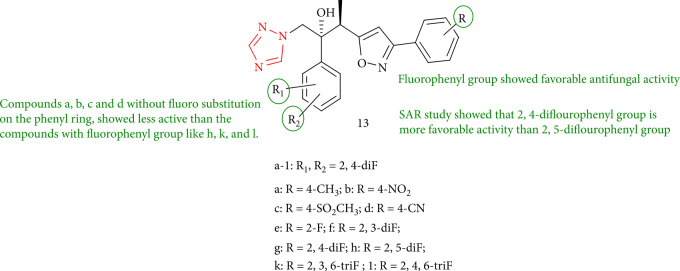
In vitro antifungal activities of new triazole derivatives of ravuconazole and isavuconazole were demonstrated against eight fungal isolates. Compounds 13e (2-F), 13f (2, 3-diF), and 13g (2, 4-diF) in particular displayed activity to ravuconazole, demonstrating that the 2,4-diflourophenyl group is more active than the 2,5-diflourophenyl group. Compounds without a fluoro substitution on the phenyl ring, such as 13a (4-CH3), 13c (4-SO2-CH3), 13b (4-NO2), and 13d (4-CN), were less active than those with fluorophenyl groups, such as 13k (2, 6-diF),13h (2, 5-diF), and 13l (2, 4, 6-triF) (Figure 14) [97].
Figure 14.
New triazole derivatives of ravuconazole and isavuconazole.
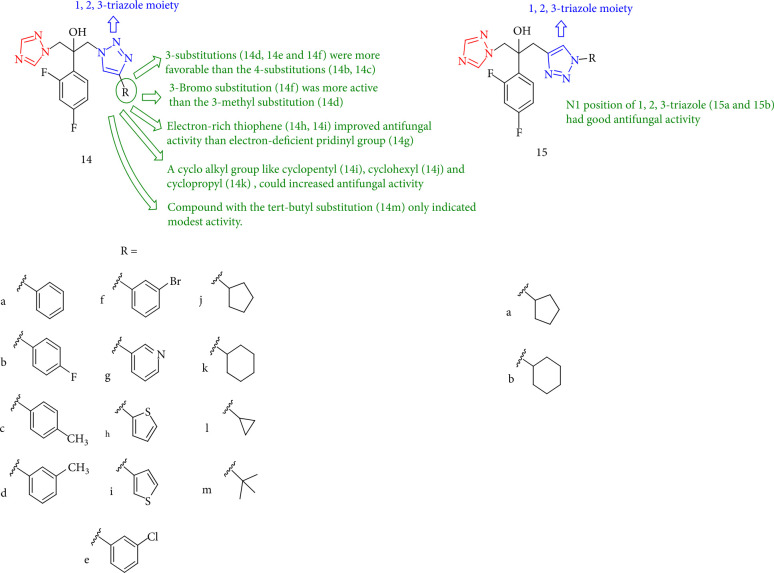
Chen et al. [98] investigated a class of novel antifungal triazoles, and compound 14l (MIC = 0.125 μg/mL) showed greater antifungal activity versus Candida glabrata and Candida albicans. Furthermore, compounds 14j, 14k, 14l, 15a, and 15b (MIC = 0.125–0.5 μg/mL) were shown to be more effective than fluconazole (MIC = 0.25 μg/mL) against C. glabrata. SARs revealed that 3-substitutions (14d, 14e, and 14f) were more favorable than the 4-substitutions (14b, 14c), while electron-rich thiophene (14h, 14i) significantly outperformed the electron-deficient pridinyl group in antifungal activity (14g). Antifungal activity may be enhanced by replacement of the phenyl group of compound 14a by a cycloalkyl group, namely, cyclopentyl (14j), cyclohexyl (14k), and cyclopropyl (7l). In contrast, tert-butyl substitution (14m) only showed modest activity (Figure 15).
Figure 15.
Structure of triazole-piperdine-heterocycle.
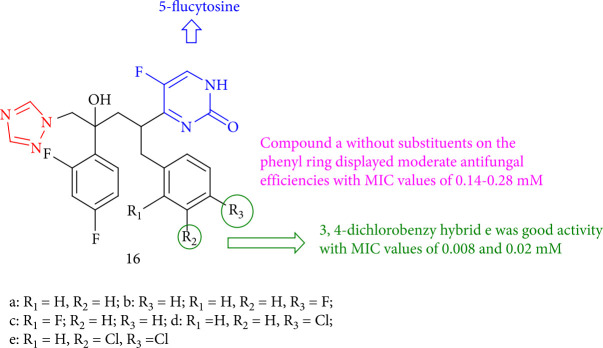
Compared to fluconazole and 5-flucytosine, the new fungicidal hybrids of 5-flucytosine and fluconazole showed modest antifungal activity. Surprisingly, a hybrid of 3,4-dichlorobenzene can inhibit clinical-resistant strain C. albicans and the growth of C. albicans ATCC 90023 with MIC values of 0.02 and 0.008 mM, respectively. Compound 16e inhibited C. albicans rapidly, whereas compound 16a lacked fungicidal activity due to the lack of substituents on the phenyl ring (ure 16) [99].
Figure 16.
Structure of novel t hybrids of 5-flucytosine and fluconazole.
Xu et al. [100] described a series of novel triazole derivatives having γ-lactam that were screened for antifungal activity against six pathogenic fungi in vitro. Furthermore, the pyridyl- and phenyl-substituted compounds 17d and 17e showed moderate antimicrobial activity against Cryptococcus neoformans and Candida spp. (Figure 17).
Figure 17.
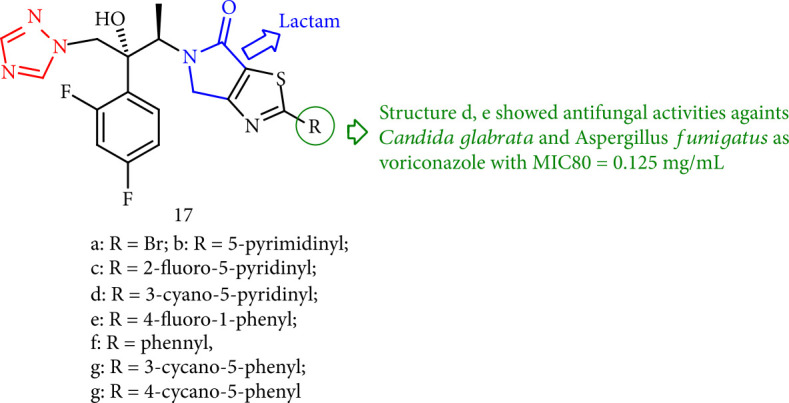
Novel triazole derivatives bearing γ-lactam.
Zhang et al. [101] reported the triazole sequence as a miconazole analogue with antifungal against five fungi. Among these compounds, 18b, 3,4-dichlorobenzyl had the highest activity. Furthermore, the antifungal activity of 3,4-dichlorobenzyl compound 18b (MIC = 0.5 μg/mL), 2,4-difluorobenzyl derivative 18c (MIC = 4 μg/mL), and 2-fluorobenzyl miconazole analogue 18e (MIC = 16 μg/mL) due to F or Cl may be significantly improved over nitro group compound 18d (nitrobenzyl). Surprisingly, substituted benzyl triazoles (MIC = 0.5–16 μg/mL) showed more potency than monosubstituted benzyl compounds (MIC = 0.5–32 μg/mL) (Figure 18).
Figure 18.
Structure-activity relationship between triazoles as miconazole analogues.
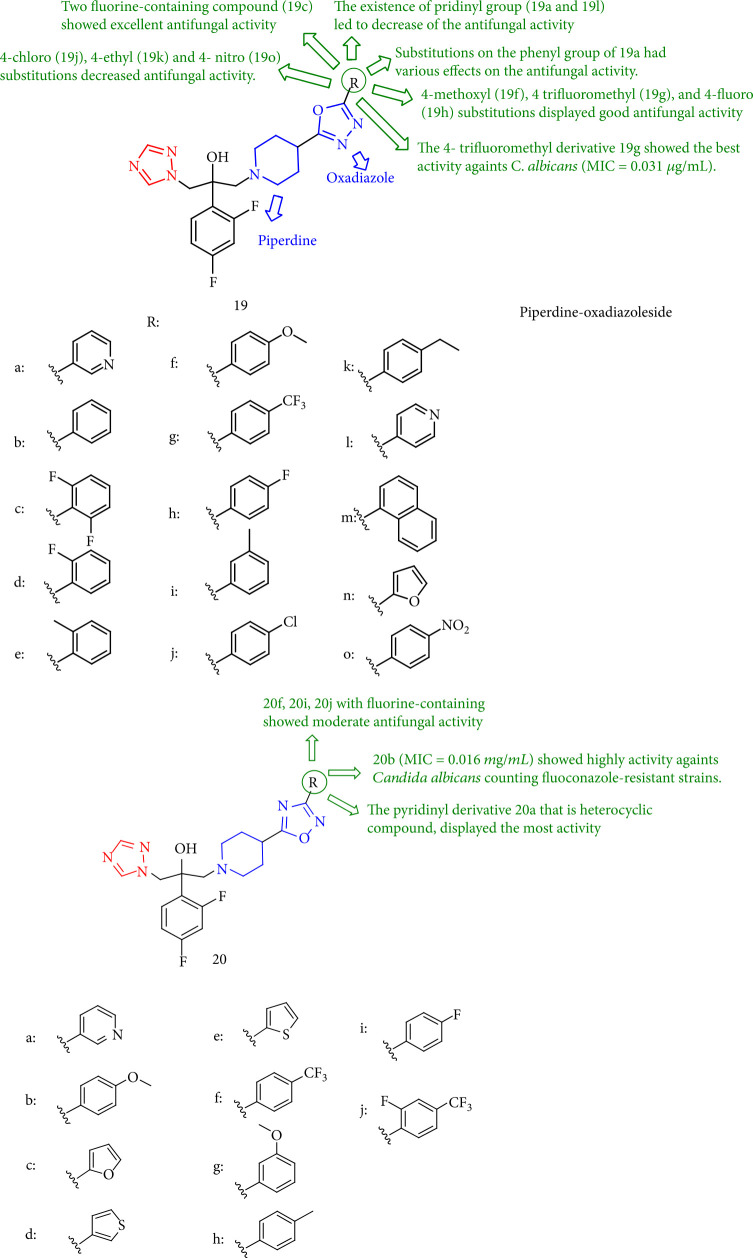
The antifungal activity of a novel triazole-piperdine-oxadiazoleside group against clinically important fungal pathogens was investigated. Particularly, 19g (MIC = 0.031 μg/mL) and 20b (MIC = 0.016 μg/mL) showed high activity versus Candida albicans including fluconazole-resistant strains. Compounds 19c, 19d, 19h, 19n, and 20a had greater activity (MIC ≤ 0.125 μg/mL) than fluconazole (MIC = 0.25 μg/mL). Additionally, the presence of pridinyl group (19a and 19l) 1,2,4-oxadiazole derivatives reduces antifungal activity. On the contrary, compound 19n with furan demonstrated better activity than compound 19b with substitutions on the phenyl ring that revealed distinct effects on antifungal activity. Furthermore, 4-methoxyl (19f), 4-fluoro (19h), and 4-trifluoromethyl (19g) alterations demonstrated promising antifungal activity, with 19g showing the most activity against C. albicans (MIC = 0.031 μg/mL). Surprisingly, compound 19c exhibited significant fungicidal activity. In contrast, the antifungal activities of 4-ethyl (19k), 4-chloro (19j), and 4-nitro (19o) derivatives were decreased. On the other hand, fluorine-containing 1,2,4-oxadiazole derivatives such as 20f, 20i, and 20j demonstrated modest antifungal activity. Finally, the heterocyclic pyridinyl derivative 20a demonstrated the greatest activity (Figure 19) [102].
Figure 19.
Structure-activity relationship between triazoles bearing piperdine-oxadiazoleside chains.
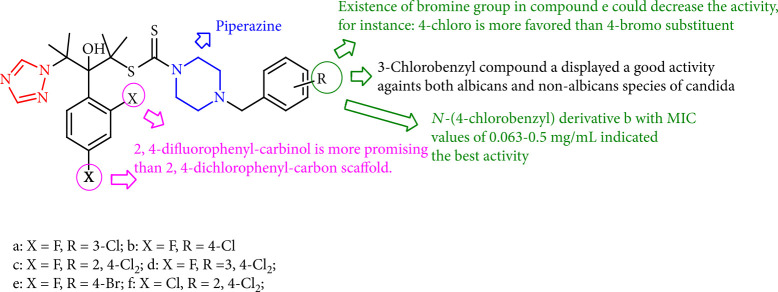
Mahmoudi et al. [103] evaluated some 1,2,4-triazole alcohols bearing N-(halobenzyl) piperazine carbodithioate scaffold as effective antifungal agent in vitro bioassays versus C. albicans, C. glabrata, C. parapsilosis, C. krusei, and C. tropicalis in which the best activity indicated N-(4-chlorobenzyl) derivative 21b with MIC values of 0.063–0.5 mg/mL, being several times more effective than fluconazole. Furthermore, the 3-chlorobenzyl compound 21a displayed a good activity toward both albicans and non-albicans species of Candida. Generally, according to MICs, 2, 4-difluorophenyl derivatives were more active than their dichlorophenyl compounds. In addition, SAR studies revealed that 2,4-difluorophenyl-carbinol was higher than the 2, 4-dichlorophenyl-carbinol scaffold. Moreover, assessment against fluconazole-resistant isolates showed that compound 21b was active against C. albicans, C. krusei, and C. parapsilosis isolates, with MIC values of 2 to 16 mg/mL (Figure 20).
Figure 20.
Triazole alcohols holding N-benzylpiperazine carbodithioate moiety.
Ciprofloxacin and itraconazole were employed to screen 1,2,4-triazole derivatives fused with novel benzene-ethanol which were assessed at concentrations ranging from 0.125 to 64 mg/mL. Furthermore, compounds 22a, 22g, and 22i showed much better growth inhibitory activity on C. albicans with MIC of 32 mg/mL (itraconazole was introduced as the standard drug MIC 1 mg/mL). Electronegativity, like substituent groups on the para and ortho positions of a benzene ring, can be effective in antifungal activity (Figure 21) [104].
Figure 21.
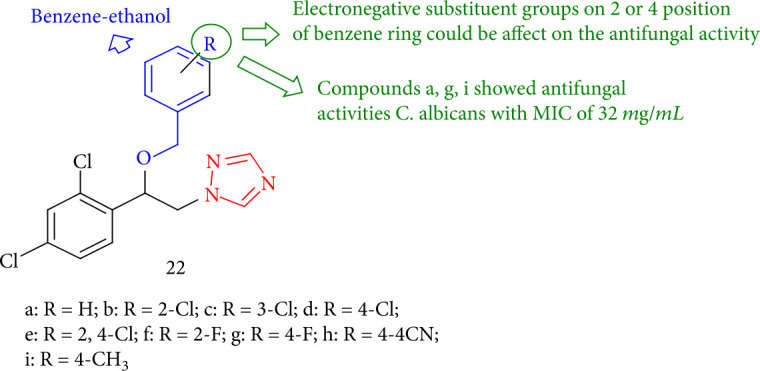
Novel benzene-ethanol bearing 1,2,4-triazole derivatives.
A class of 1,2,4-triazole derivatives has been tested toward Magnaporthe oryzae. Aromatic ring structures revealed that the methyl group at position 1,4 of the phenyl ring 23b and the phenyl moiety at the para position of phenyl 23c reduced antifungal activity. When an electron-withdrawing fluorine atom entered this position (23e), the antifungal activity increased slightly. An electron-withdrawing group (trifluoromethyl group) had a positive efficacy on increasing the antifungal activity of this synthetic series (comparison of antifungal activity 23b with 23f). The introduction of two chlorine atoms to the phenyl moiety had a distinct effect on increasing antifungal activity. Compound 23g with the 2,4-dichlorophenyl analogue slightly increased the antifungal activity, while compound 23h with the 3,4-dichlorophenyl analogue notably reduced the antifungal activity. The mono chlorine substitution at position 4 of the phenyl ring (23i) reduced the antifungal activity of these synthesized derivatives. According to the preceding considerations, 23e demonstrated remarkable fungicidal activity in this synthetic series. The effect of the chlorine atom on the various positions of the phenoxy moiety (ring B) such as 24a, 24b, and 24c can lead to an increase in antifungal activity. As a result, the fungicidal activity of the analogue without a chlorine substituent at ring B (24j) was the most effective against M. oryzae among these compounds (Figure 22) [105].
Figure 22.
1-(4-Phenoxymethyl-2-phenyl-[1, 3] dioxolan-2-ylmethyl)-1H-1,2,4-triazole derivatives.
4.2. 1, 2, 4-Triazole Hybrids
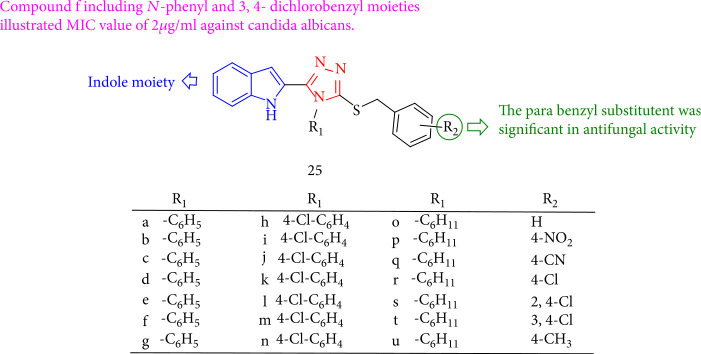
Al-Wabli et al. [106] estimated the antifungal characteristics of a variety of novel indole-triazole compounds. The MIC value of compound 25f, which included N-phenyl and 3,4-dichlorobenzyl moieties, was 2 mg/mL against Candida albicans. In addition, the para benzyl substituent exhibits antifungal activity. Also, MIC values for compounds 25b, 25c, 25d, 25e, 25f, and 25g having a phenyl moiety on the triazole ring are 250–500 μg/mL against Bacillus subtilis. Meanwhile, compounds 25o, 25p, and 25q with an N-cyclohexyl substituent showed moderate to good activity toward the tested Candida albicans strain (Figure 23).
Figure 23.
Evaluation of the antifungal activity of new indole-1,2,4-triazole conjugates.
The nortopsentin analogues containing 1,2,4-triazole demonstrated good antifungal activity. Compounds 26a, 26d, and 26f were more fungicidal toward Cercospora arachidicola Hori than chlorothalonil and carbendazim (commercial fungicides). Compounds 26d and 26f indicated better actions against most of the fourteen plant pathogens (Figure 24) [107].
Figure 24.

1,2,4-Triazole derivatives containing nortopsentin.
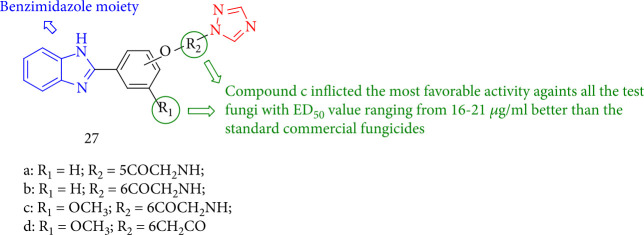
According to the study by Ahuja et al. [108], compound 27c has a lower ED50 value than the triazole fungicide propiconazole. Significantly, compound 27c showed the highest activity compared with other experimental fungi, with an ED50 value of 16 to 21 μg/mL, which is higher than the ED50 values of the standard commercial fungicides used (tilt: 20–25 μg/mL and carbendazim: 150–230 μg/mL (Figure 25).
Figure 25.
Benzimidazolyl-1,2,4-triazole.
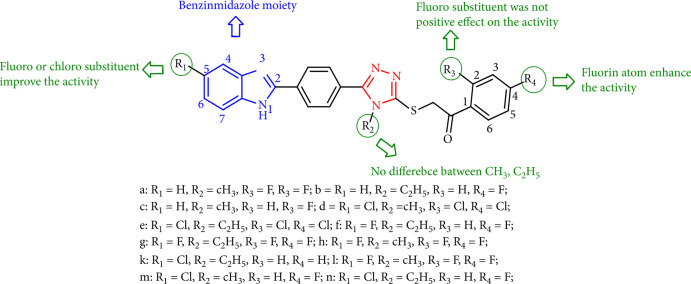
Microbiological studies revealed that benzimidazole-1,2,4-triazole hybrid compounds 28m, 28n, 28f, and 28g had good fungicidal activity (MIC50 values of 0.78 to1.56 μg/mL) because of the presence of a fluoro or chloro substituent at the C-para position of phenyl, whereas compounds 28c, 28a, and 28b did not. Compounds 28d and 28e demonstrated adequate fungicidal activity (MIC50 values = 1.56–3.12 μg/mL). Compound 28l exhibited comparable antifungal activity with reference drugs fluconazole and ketoconazole. As a result, chloro or fluoro substitution at the C-5 position of benzimidazole is vital and could have had a significant influence on antifungal activity (Figure 26) [109].
Figure 26.
SAR outline of the benzimidazole-1,2,4-triazole hybrid compounds.
The benzimidazole-triazole compounds showed moderate antifungal activity toward Candida krusei (ATCC 6258), Candida glabrata (ATCC 90030), Candida albicans (ATCC 24433), and Candida parapsilosis (ATCC 22019), with MIC50 values ranging from 12.5 to 0.78 mg/mL. The findings revealed that compound 3,4-dihydroxy has an influence on the activity (Figure 27) [110].
Figure 27.
Structure of new benzimidazole-triazoles.
Novel tri-substituted 1,2,4-triazoles containing benzimidazole were tested for antifungal efficacy against three plant pathogenic fungus, and compounds 30e and 30g showed potent activity against Venturia nashicola. However, 30d and 30f indicated sufficient activity against Fusarium graminearum (Figure 28) [111].
Figure 28.
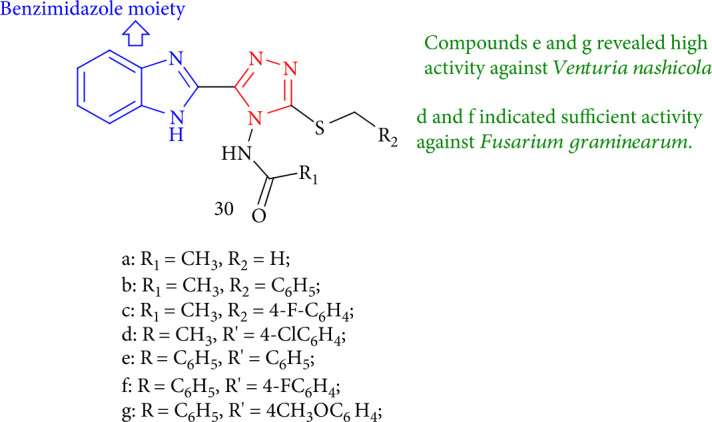
Tri-substituted 1,2,4-triazoles containing benzimidazole moiety.
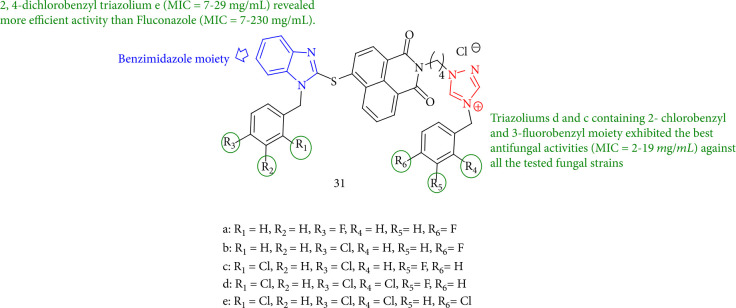
Luo et al. [112] reported a new group of benzimidazole-derived triazoliums and naphthalimide triazoles that have been thoroughly tested for antifungal activities. Triazoliums 31g and 31f with 3-fluorobenzyl and 2-chlorobenzyl moiety demonstrated the highest antifungal activity (MIC = 2–19 mg/mL) against all tested fungal strains. However, 2,4-di-chlorobenzene triazolium 31h (MIC = 7–29 mg/mL) showed more efficacy than fluconazole (MIC = 7–230 mg/mL). Furthermore, bis (4-fluorobenzyl) triazolium 31b (MIC = 4–19 mg/mL) displayed high activity against all of the microorganisms tested except S. cerevisiae (Figure 29).
Figure 29.
Structure-activity relationships between benzimidazole-derived naphthalimide triazoles.
The antifungal efficacy of 1,2,4-triazoles having quinoline moiety against A. fumigatus and Candida albicans was highest owing to methoxy and chloro substituents. As a result, 32e, 32g, and 32m derivatives with methoxy and chloro substituents had the highest enhanced activity (Figure 30) [113].
Figure 30.
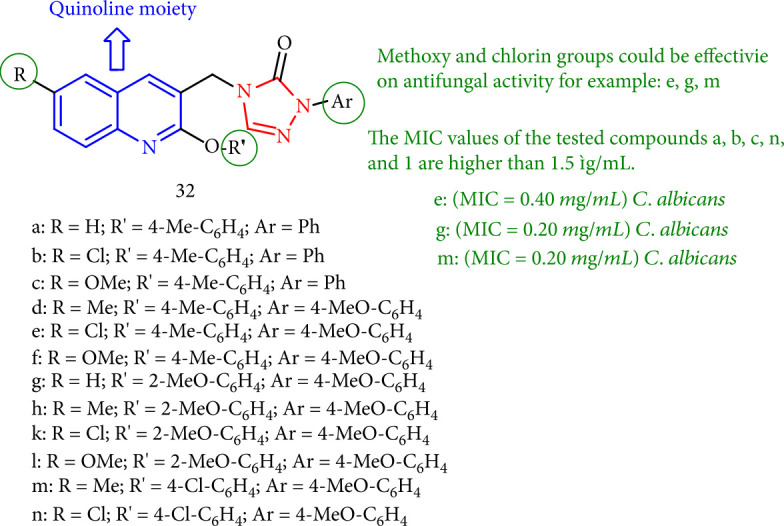
1,2,4-Triazole hybrids of 2-(aryloxy) quinolones.
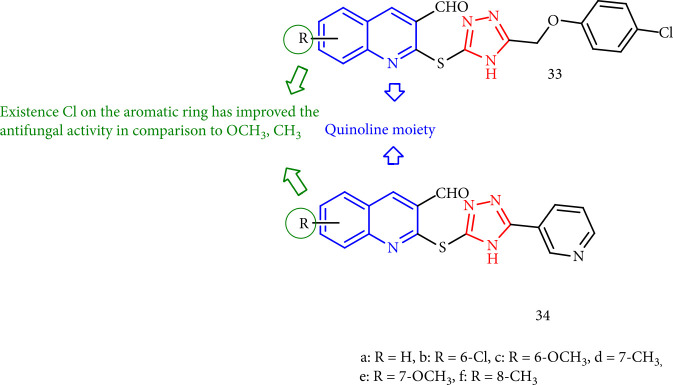
D'Souza et al. [114] investigated the fungicidal of new quinoline-triazoles. Compounds 33b and 34b with chlorine substituents on the aromatic ring demonstrated more antifungal activity than (33e, 33f, 34e, and 34f) that included OCH3 and CH3 (Figure 31).
Figure 31.
Antifungal activity evaluation of quinolone-triazole derivatives.
Fan et al. [115] investigated the antifungal activity of 1,2,4-triazolo [4,3-a]pyridine-containing quinazoline thioether derivatives at 50 mg/mL. Except for compound 35c against the fungi Verticillium dahlias and Fusarium oxysporum (inhibition rates of 65.4 and 52.5%, respectively), all of these compounds failed to demonstrate apparent fungicidal activity (≥45%) against the case fungi, with compound 35h against the pathogen V. dahliae (46.8%) (Figure 32).
Figure 32.
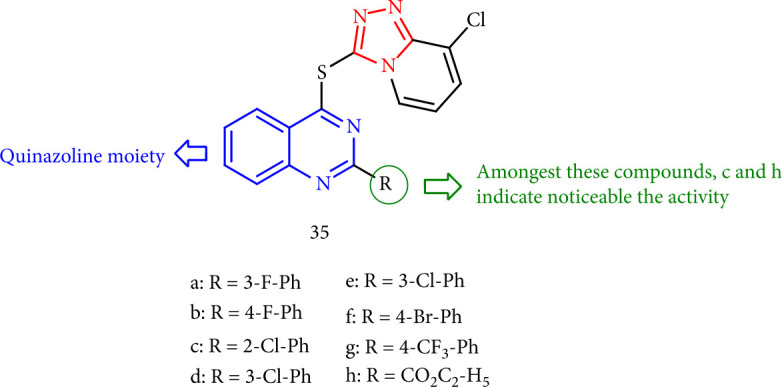
Quinazoline thioether-1,2,4-triazolo [4,3-a] pyridine derivatives.
Fan et al. [116] investigated the antifungal activity of quinazolin-containing 1,2,4-triazoles against six significant phytopathogenic fungi in agriculture. Furthermore, compounds 36h and 36g were showed a remarkable fungicidal activity toward Gloeosporium fructigenum at 50 mg/mL, comparable to the commercial antifungal hymexazol (Figure 33).
Figure 33.
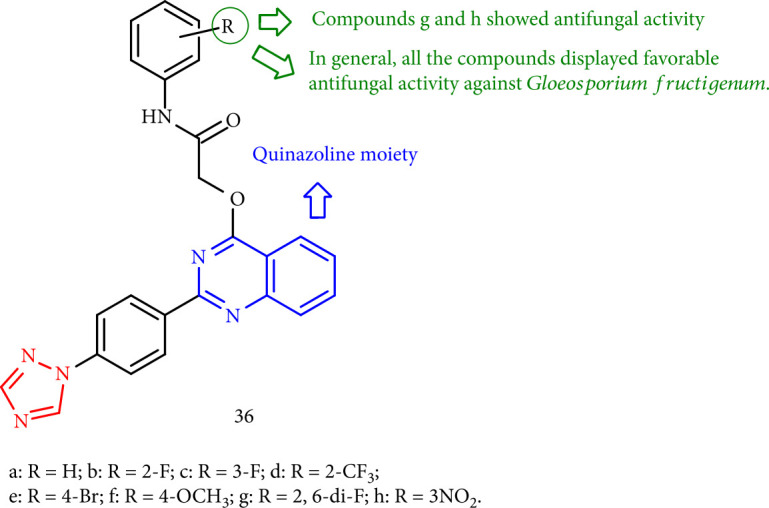
1,2,4-Triazole containing quinazolin derivatives.
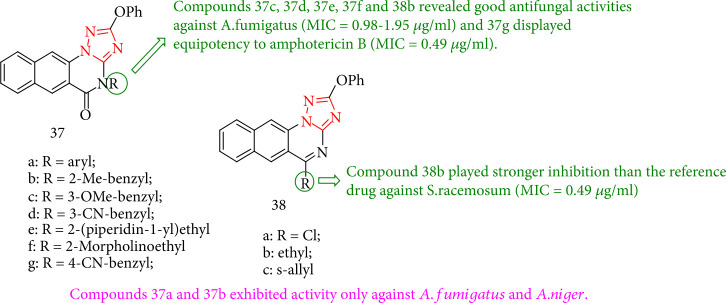
Most of the 2-phenoxy-benzo [g] [1, 2, 4] triazolo [1,5-a] quinazoline derivatives indicated in vitro antifungal activity against ten fungal strains except C. neoformans. Nevertheless, 37a and 37b exhibited activity only against A. niger and A. fumigatus. Compounds 37c, 37d, 37e, 37f, and 38b revealed excellent fungicidal activities against A. fumigatus (MIC = 0.98–1.95 μg/mL), and 37g showed the ability to produce amphotericin B (MIC = 0.49 μg/mL). In contrast, 38b had stronger inhibition with respect to the reference drug toward S. racemosum (MIC = 0.49 μg/mL) and 37e confirmed similar activities to amphotericin B (MIC = 0.98 μg/mL). In addition, compounds 37c-37e, 38a, and 38b showed the highest activity versus G. candidum (MIC = 0.49–0.98 μg/mL) as compared with amphotericin B (MIC = 1.95 μg/mL) (Figure 34) [117].
Figure 34.
Chemical structure of 2-phenoxy-benzo-triazole quinazoline derivatives.
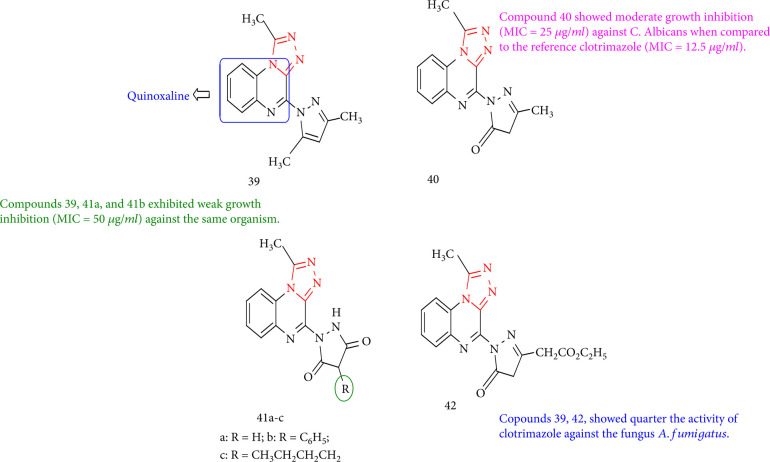
El-Attar et al. [118] investigated the antifungal activities of 1,2,4-triazolos [4,3-a]-quinoxaline derivatives with various substituted pyrazole moieties at position 4. When compared with the reference clotrimazole (MIC = 12.5 μg/mL), compound 39 demonstrated reasonable growth inhibition (MIC = 25 μg/mL) against C. Albicans. Compounds 39, 41a, and 41b inhibited only weakly (MIC = 50 μg/mL) growth against the same organism. Clotrimazole activity against the fungus A. fumigatus was reduced by one-quarter in studies 39 and 42. Regarding the activity against R. oryzae, compounds 39, 40, 41a-c, and 42 showed weak growth inhibition (MIC = 25 μg/mL) when compared with the reference clotrimazole (MIC = 6.25 μg/mL) (Figure 35).
Figure 35.
Antifungal study on pyrazol-1,2,4-triazol-quinoxalines.
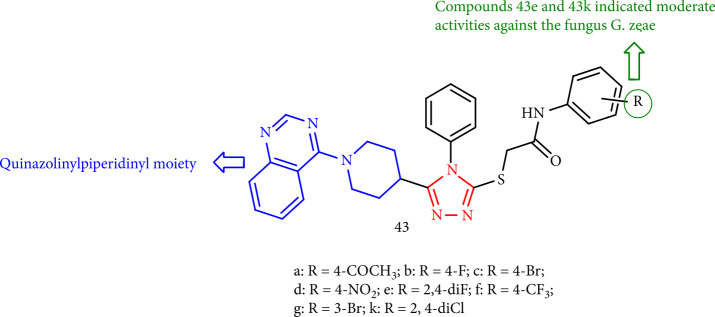
Yang and Bao [119] demonstrated that 1,2,4-triazole derivatives (43a-43k) containing N-(substituted phenyl) acetamide and the quinazolinylpiperidinyl moiety group did not exhibit remarkable inhibition activity against phytopathogenic fungi such as Phytophthora infestans, Verticillium dahliae, and Gibberella zeae) at 50 mg/mL save compounds 43e and 43k that showed modest inhibitory activity against the fungus G. zeae (Figure 36).
Figure 36.
1,2,4-Triazole derivatives containing the quinazolinylpiperidinyl moiety.
Sompalle et al. [120] investigated the antifungal activity of a class of 1,2,4-triazole-quinazolinethiones (44a-l) against Aspergillus niger (A. niger) and Aspergillus flavus (A. flavus) in combination with the commonly used antifungal drug fluconazole (Figure 37).
Figure 37.
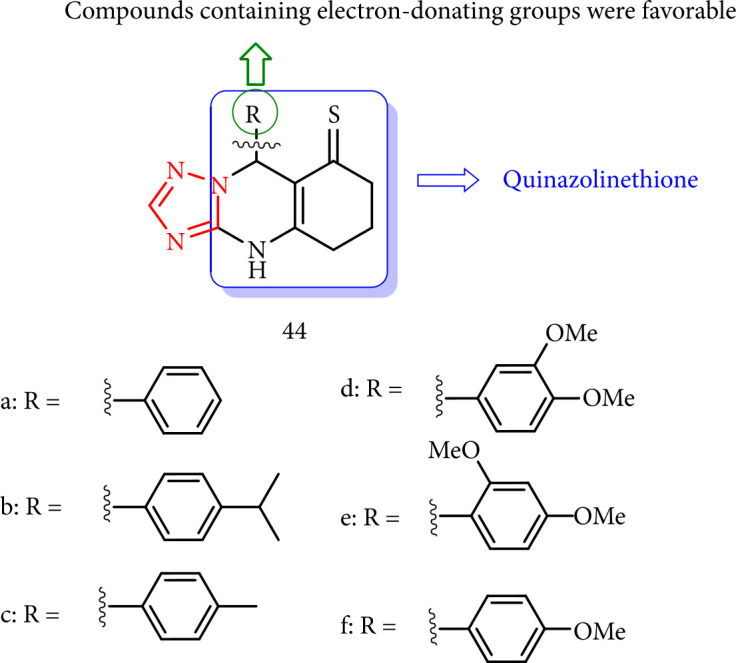
SAR study of triazolo-quinazolinethiones.
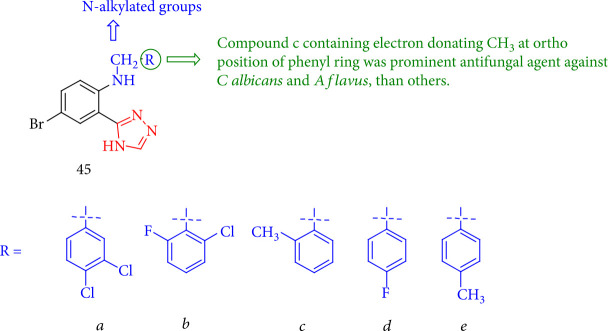
All triazole derivatives with N-alkylated groups were tested for fungicidal activity toward Candida albicans and Aspergillus flavus and anthelmintic activity against Pheretima posthuma, and the compound containing group CH3 at the ortho position of the phenyl ring showed good inhibition with the inhibition zone 24.17 ± 0.32 and 15.02 ± 0.41 mm against A flavus and C albicans in comparison with a standard antifungal drug, Nystatin, while the antifungal activity of the other structures was lower (Figure 38) [121].
Figure 38.
Structure of triazole derivatives containing N-alkylation.
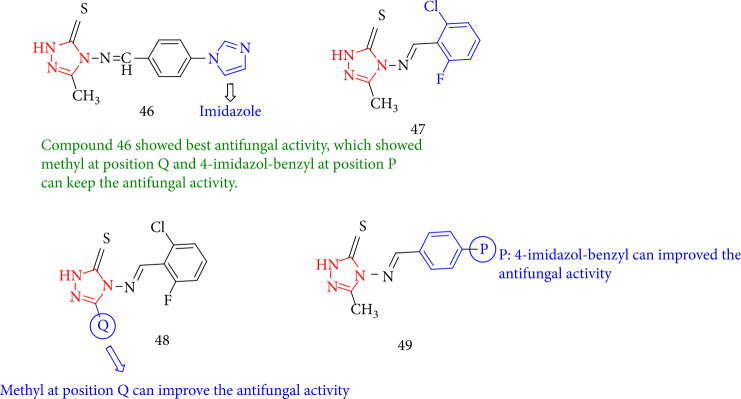
Jin et al. [122] investigated the fungicidal activity of novel compounds containing 1,2,4-triazole with different substituted groups toward Gibberlla nicotiancola, Pythium solani, Gibberlla saubinetii, and Fusarium oxysporum f.sp. niveum in vitro. Compound 46 had good activity against the case fungus, indicating that 1,2,4-triazole-imidazole can contribute to antifungal properties. Methyl at position Q increased the activity, the activity order is 47>46, and compound 47 demonstrated a remarkable antifungal activity. As a result, positions P and Q may have an impact on the activity at the same time (Figure 39).
Figure 39.
Chemical structure of novel 1,2,4-triazole derivatives.
1,2,4-Triazole derivatives with a pyrimidine moiety were evaluated for fungicidal activity, with compounds 50c and 50d showing the best antifungal activity against Phompsis sp. that was even better than pyrimethanil (32.1 mg/mL). Compound 50d, on the other hand, had higher activity against B. cinerea and B. dothidea with 55.1 and 40.1 mg/mL, respectively, when compared with Pyrimethanil (57.6 and 62.8 mg/mL) (Figure 40) [123].
Figure 40.
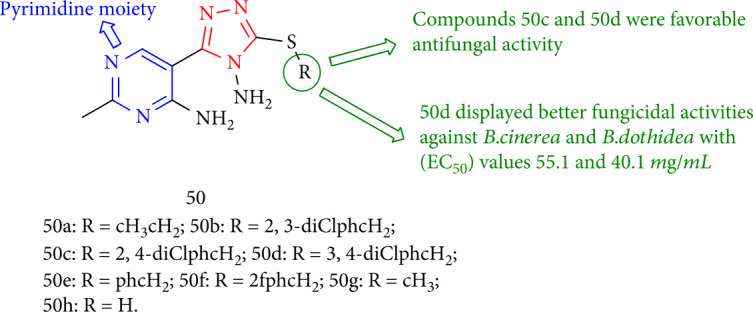
The structure of triazole derivatives with a pyrimidine moiety.
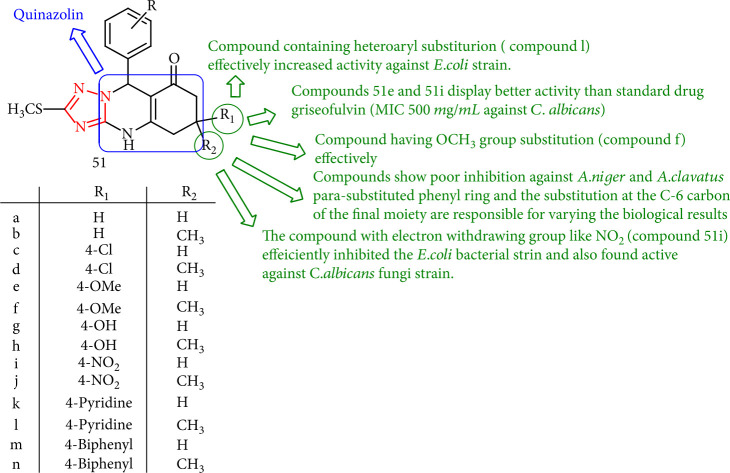
Antifungal evaluation [1, 2, 4] of triazolo [5,1-b] quinazolin-8(4H) one scaffolds (51a-n) in vitro exhibited that compounds 51e and 51i display higher activity than standard drug griseofulvin (MIC 500 mg/mL) against C. albicans. Surprisingly, the substitution at the C-6 carbon of the final moiety and para-substituted phenyl ring was responsible for variable biological results, while the triazole with nonsubstituted or diversely para-substituted (Cl, OCH3, and NO2) phenyl core or heterocyclic nucleus showed the best properties. In addition, the compounds having OCH3 group substitution (compound 51f) effectively showed poor inhibition toward A. clavatus and A. niger inhibited the S. aeruginosa, P. aeruginosa, and S. pneumonia strains, although the derivative with the electron-withdrawing group such as NO2 (compound 51i) efficiently inhibited the E. coli bacterial strain as well as was found potent toward the C. albicans strain. Finally, compound bearing heteroaryl substitution (compound 51l) led to the improvement in the activity against the E. coli strain (Figure 41) [124].
Figure 41.
[1, 2, 4] Triazolo [5,1-b] quinazolin-8(4H) derivatives.
All 1,2,4-triazole having amine derivatives were evaluated and shown to be effective in inhibiting fungal pathogens with MIC values ranging from 1 to 256 μg/mL. They were proposed as the potential antifungal agents that synthesized under optimized conditions as 3(5)-substituted 1,2,4-triazol-5(3)-amine 52. As starting materials, however, several heteroaryl hydrazides and aryls were used as starting materials (Figure 42) [125].
Figure 42.
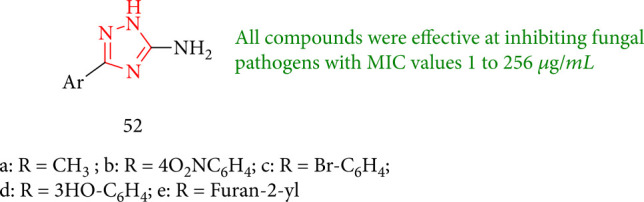
Study of 1,2,4-triazole derivatives.
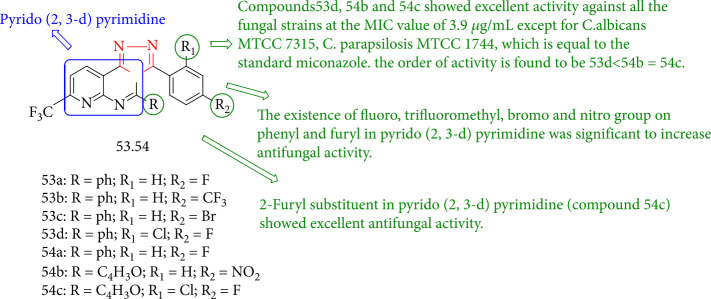
Appna et al. [126] described the fungicidal activity of novel 1,2,4-triazole fused pyrido [2,3-d] pyrimidine derivatives (53a-d and 54a-c) against different Candida strains. The antifungal activities of the synthesized compounds 53d, 54b, and 54c were shown. SAR investigations revealed that trifluoromethyl, fluoro, bromo, and nitro groups on the furyl and phenyl rings of pyrido [2,3-d] pyrimidine could increase antifungal activity. Compounds (53d, 54b, and 54c) 4-fluoro-2-chlorophenyl triazole and 2-furyl substituent in pyrido [2,3-d] pyrimidine exhibited the best activity. As well, 4-nitrophenyl triazole in combination with 2-furyl pyrido [2,3-d] pyrimidine (54b) exhibited the same activity. The antifungal effects of 2-chloro-4-fluoro phenyl triazole with 2-phenyl pyrido [2,3-d] pyrimidine (53d) were favorable (Figure 43).
Figure 43.
Chemical structure of new 4-hydrazone functionalized/1,2,4-triazole fused pyrido [2,3-d] pyrimidine derivatives.
A new series of 1,2,4-triazole derivatives were synthesized by Singh et al. The antifungal characteristics of the compounds showed that most of them could effectively inhibit the growth of the tested fungal strains. However, none of them were superior to the reference drug fluconazole. Compound 55l had the most potent antifungal activity against both fungi. 55l revealed comparable activity (A. niger: MIC = 11.7 μM; C. albicans: MIC = 10.9 μM) with the reference fluconazole (A. niger: MIC = 9.4 μM; Candida albicans: MIC = 10.2 μM). Apart from that, antifungals 55f (A. niger: MIC = 15.6 μM; C. albicans: MIC = 14.1 μM) and 55a (A. niger: MIC = 28.1 μM; C. albicans: MIC = 18.8 μM) were found to be potent (Figure 44) [127].
Figure 44.
Antifungal evaluation of novel bioactive 1,2,4-triazoles.
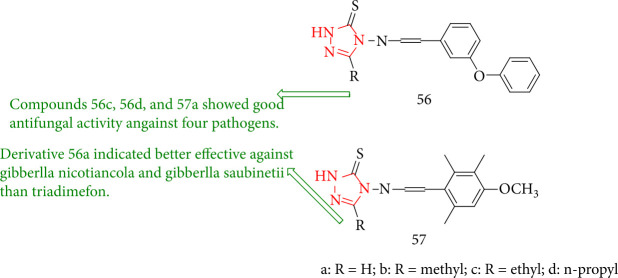
Jin et al. [128] investigated the antifungal effects of a variety of 4-amino-5-substituent-1,2,4-triazole-3-thione Schiff bases toward Pythium solani, Gibberlla nicotiancola, Gibberlla saubinetii, and Fusarium oxysporum f.sp. niveum. Compounds 56a and 56b showed considerable activity against the majority of the test fungi, while derivative 56a was more potent toward Gibberlla saubinetii and Gibberlla nicotiancola than triadimefon. The antifungal activity of 56a-d and 57a-d analogues was tested against four plant pathogenic fungi, including Pythium solani, Fusarium oxysporium f.sp. niveum, Gibberlla nicotiancola, and Gibberlla saubinetii (Figure 45).
Figure 45.
Chemical structure of new 1,2,4-triazole Schiff base derivatives.
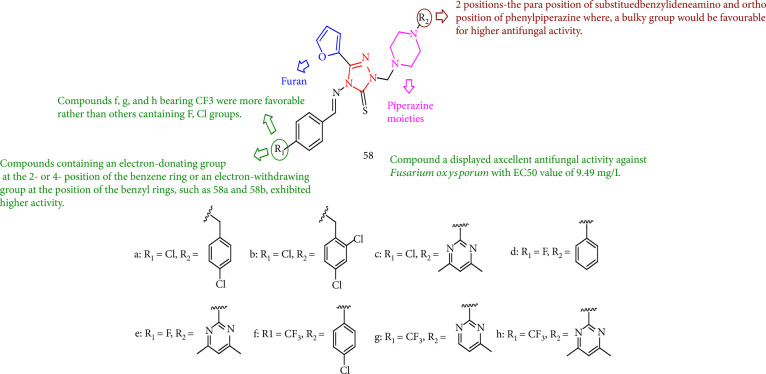
Zhang et al. [129] displayed a new class of piperazine-containing 3-(furan-2-yl)-1,2,4-triazole important in vitro fungicidal activity toward a variety of plant fungi. In particular, compounds 58a, 58b, 58c, 58d, 58e, 58f, 58g, and 58h showed triadimefon against a variety of test fungi. Compounds 58g, 58f, and 58h having R1 = CF3 performed better than others (R1 = F or Cl). In comparison, the SARs of the compounds revealed 2-positions of the para position of substituted benzylideneamino and ortho position of phenylpiperazine, where a large group would be favorable for higher fungicidal activity. Finally, compounds with an EWG at the benzyl ring position or an electron-donating group at the 2,4-position of the benzene ring, such as 58b and 58a, demonstrated higher activity (Figure 46).
Figure 46.
Study of the structure of new piperazine-bearing 3-(furan-2-yl)-1, 2, 4-triazole.
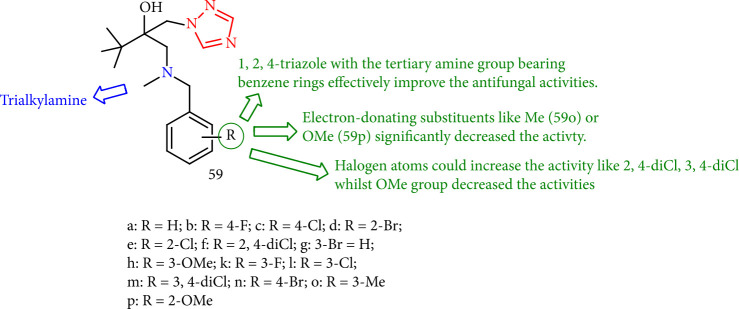
Trialkylamine compounds having a triazole moiety were evaluated in vitro for antifungal activity against six phytopathogenic fungi at 50 mg/mL (Magnaporthe grisea, Curvularia lunata, Alternaria solani, Fusarium solani, A. alternata, and F. graminearum). Compounds 59k (3-F), 59m (3,4-diCl), and 59n (4-Br) had good activity toward A. solani with EC50 values of 2.88, 8.20, and 1.92 mg/mL, respectively. Furthermore, compounds 59c (4-Cl), 59f (3,4-diCl), and 59d (2-Br) showed good antifungal activity against F. graminearum with EC50 values of 11.60, 5.14, and 16.24 mg/mL, respectively. Also, electron-donating groups 59o (Me) or 59p (OMe) considerably reduced the activity. In contrast, the presence of halogen atoms such as 59k (3-F), 59c (4-Cl), (3,4-diCl), and 59n (4-Br) might increase the activity (Figure 47) [130].
Figure 47.
Structure of trialkylamine derivatives bearing a triazole moiety.
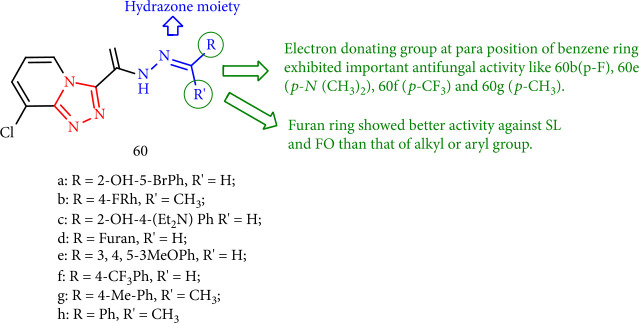
1,2,4-Triazole-pyridine products with hydrazone scaffold (compounds 60a-60h) were tested in vivo at 100 mg/mL against Stemphylium lycopersici (Enjoji) Yamamoto (SL) and Fusarium oxysporum sp. Cucumebrium (FO). Compound 60d as well as compounds having electron-donating groups at the 4-position of benzene such as 60e (p-N (CH3)2), 60b (p-F), 60f (p- CF3), and 60g (p-CH3) demonstrated strong antifungal activities. As a result, the furan ring-substitution exhibited more activity against SL and FO than the aryl or alkyl groups. Furthermore, both poly- and single-substituted benzene compounds showed excellent activity against FO (Figure 48) [131].
Figure 48.
(SAR) analysis of 1,2,4-triazolo-pyridine derivatives containing hydrazone moiety.
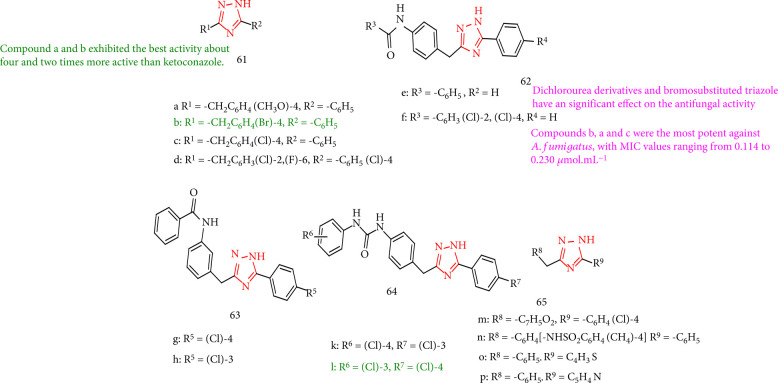
Remarkable antifungal activity of a number of new 1,2,4-triazole derivatives against different strains of Aspergillus fumigatus, Candida albicans, and Candida crocus has been reported in comparison with those of commercial fungicides ketoconazole and itraconazole. All of the derivatives investigated, the dichloro urea analogue and bromo substituted triazole, stand out as the most favorable compounds. The most potent compounds against A. fumigatus were 64l, 61b, 61a, and 61c, with MIC values ranging from 0.114 to 0.230 μmol/mL. Instead, amide analogues such as 62f can influence the activity, with the amide moiety 62f having higher activity than less bulky triazoles such as 65o and 65p. Furthermore, compound 63h with the sulfonamide substitution is responsible for the activity reduction. Compounds 64l and 61b have the highest activity, being several times more potent than ketoconazole. Conversely, these derivatives were less active than itraconazole (Figure 49) [132].
Figure 49.
The study of antifungal properties of 1,2,4-triazoles.
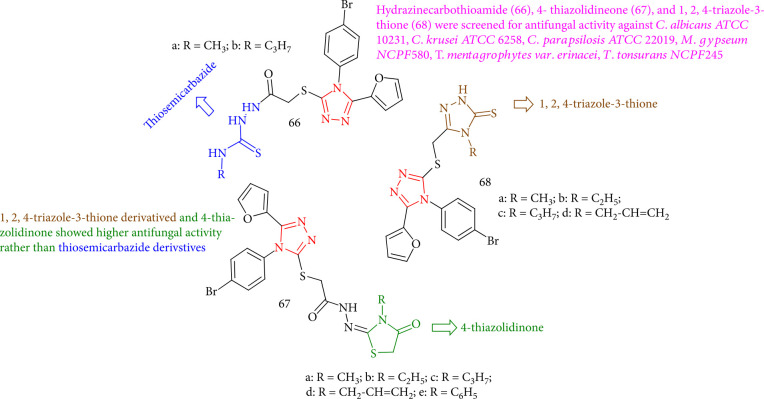
Dincel et al. [133] screened a group of novel hydrazinecarbothioamide (66), 4-thiazolidinone (67), and 1,2,4-triazole-3-thione (68) for the fungicidal properties against C. parapsilosis ATCC 22019, C. Albicans ATCC 10231, M. gypseum NCPF580, C. krusei ATCC 6258, T. tonsurans NCPF245, and T. mentagrophytes var. echinacea. Generally, 1,2,4-triazole-3-thiones and 4-thiazolidinones showed better fungicidal activity rather than thiosemicarbazide derivatives. As a result, the 3-allyl substitution of 4-thiazolidinones is critical for their antifungal activity. Compounds 67d (R = CH2-CH = CH2), 68c (R = C3H7), and 68d (R = CH2CH = CH2) had the highest fungicidal activity against S. aureus (MIC = 32 μg/mL). Also, 68c (R = C3H7) and 68d (R = CH2CH = CH2) showed the greatest activity against E. coli (MIC = 32 μg/mL). In addition, 68d (R = CH2CH = CH2) showed the greatest activity towards P. aeruginosa (MIC = 32 μg/mL) (Figure 50).
Figure 50.
Structure of hydrazinecarbothioamides, 4-thiazolidinones, and 1,2,4-triazole-3-thiones.
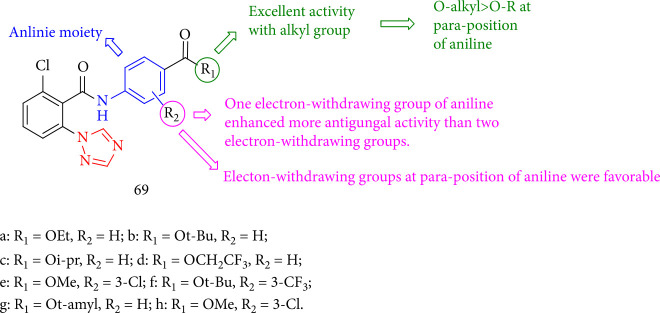
Cheng et al. [134] investigated the fungicidal activity of new groups of 1,2,4-triazole benzoyl aryl amines. The findings revealed a clear relationship between the structure and training in these compounds as well. The electron-withdrawing group oi-pr(isopropyl) at the para position has a favorable impact on high activity, and the preferred groups were alkoxy carbonyls. This compound indicated the most effective fungicidal activities with EC50 values of 0.12, 0.19, and 0.01 mg/mL against S. sclerotiorum, F. graminearum, and G. graminis var. tritici, respectively. Alkoxy carbonyl of these ester carbonyls revealed the highest activities (69a-b and 69c-g). In contrast, no significant increase in the activity was observed when more than one electron-withdrawing group was added to aniline. For instance, if the second electron-withdrawing groups such as CF3 or Cl were added to the meta situation of aniline, the activity against G. graminis var. tritici would be reduced (69e and 69f) (Figure 51).
Figure 51.
The structure of 1,2,4-triazole benzoyl arylamine compounds.
The evaluation indicated that all 1,2,4-triazole derivatives had fungicidal activity, with MIC values ranging from 0.02 to 0.52 mM, which was better than bifonazole (MIC values of 0.32–0.64 mM) and ketoconazole (MIC values of 0.28–1.88 mM). Compound 70c, having a MIC value of 0.02–0.04 mM, exhibited the best antifungal activity rather than compound 70a (Figure 52) [135].
Figure 52.
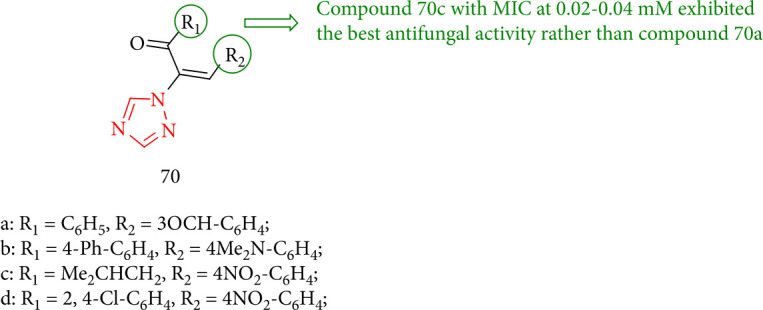
New vinyl-1, 2, 4-triazole analogues.
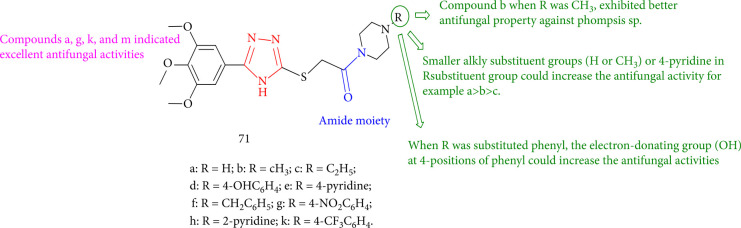
Wu et al. [136] evaluated the fungicidal activity of a novel series of 1,2,4-triazole derivatives containing an amide moiety. Compounds 71a, 71d, 71e, and 71f had the highest antifungal activity against Botrytis cinerea. Meanwhile, compound 71b, when R was CH3, exhibited better antifungal property against Phomopsis sp., compared with that of pyrimethanil. SAR studies revealed that 4-pyridine in the R substituent group and the smaller alkyl substituent groups (H or CH3) could have a favorable influence on the activity, such as 71a>71b>71c. Meanwhile, when R = OH is added to the 4-positions of phenyl and substituted phenyl, the action against Phomopsis sp., B. dothidea, and B. cinerea rises in the sequence 71d>71g>71k. Furthermore, when R = 4-pyridine, the antifungal activities of the corresponding compound 71h against Phomopsis sp., B. dothidea, and B. cinerea were higher than those of compound 71e (R = 2-pyridine) (Figure 53).
Figure 53.
1,2,4-Triazole derivatives bearing an amide moiety.
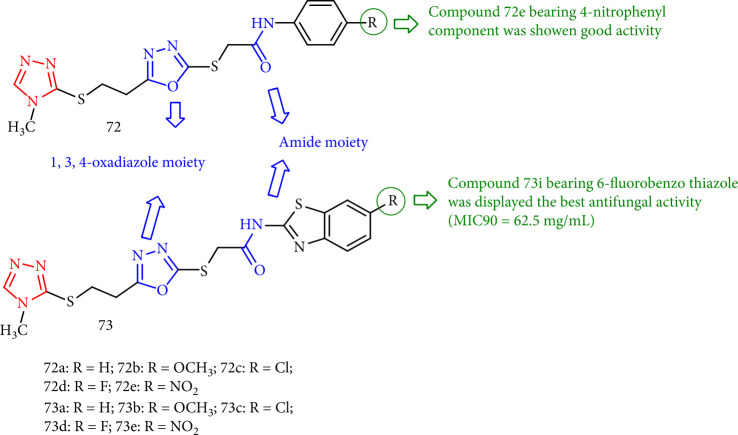
Yurttaş and CantŘrk [137] investigated triazole-oxadiazole compounds against C. krusei, C. glabrata, C. albicans, and C. parapsilosis and found that triazole-oxadiazole derivatives 72e and, particularly, 73i had the highest activity against C. glabrata and C. albicans (MIC90 = 62.5 mg/mL). The oxadiazole rings of these derivatives differ due to the benzothiazole and phenyl rings linked to the acetamide molecule. Meanwhile, compounds 72e (R = NO2) and compound 73e (R = F) were found to have the highest activity (Figure 54).
Figure 54.
The study of some triazole-oxadiazole compounds.
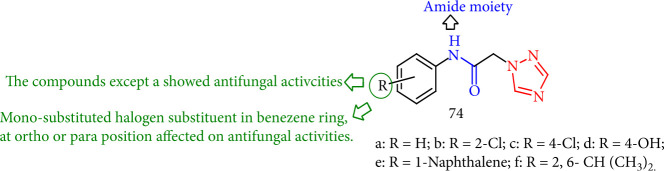
Li et al. [138] reported a group of N-phenylacetamide containing 1,2,4-triazole derivatives (74a-f) that were screened in vitro for antifungal assessment, and specific compounds, such as 74b-f derivatives, inhibited the growth of the tested fungus. Among all synthesized compounds, 74a exhibited no antifungal activity. Moreover, mono-substituted halogen substituents in the benzene ring, in either the ortho or the para position, displayed antifungal activity (Figure 55).
Figure 55.
The study of N-phenylacetamide containing 1,2,4-triazole derivatives.
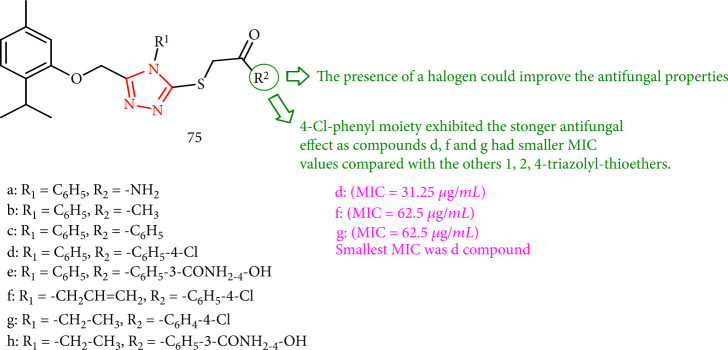
The antifungal properties of the synthesized 1,2,4-triazole-3-yl-mercapto derivatives toward two Candida albicans strains (C. albicans ATCC 10231 and C. albicans ATCC 18804) and one non-Candida albicans strain (C. krusei ATCC 6258) were evaluated. Its antifungal activity was shown by the presence of a halogenated aryl substituent linked to the 3-mercapto group. Compounds 75d, 75f, and 75g had smaller MIC values than the other 1,2,4-triazolyl-thioethers, indicating that the 1,2,4-triazole-3-yl-mercapto derivatives with a 4-Cl-phenyl component had more excellent antifungal activity against the Candida krusei ATCC 6258 strain. In this series, compound 75d in this series has the lowest MIC value (Figure 56) [139].
Figure 56.
The chemical structures of 1, 2, 4-triazole-3-yl-mercapto derivatives.
Antifungal activities of new myrtenal derivatives containing 1,2,4-triazole were tested against Physalospora piricola, Fusarium oxysporum f.sp. cucumerinum, Cercospora arachidicola, Alternaria solani, and Gibberella zeae at 50 mg/mL. Among these compounds, 76a (R = Et), 76c (R = i − Pr), and 76e (R = o − NO2 Bn) had the most significant antifungal activity against P. piricola. Among these derivatives, 76a (R = Et) 76c (R = i − Pr), and 76e (R = o − NO2 Bn) indicated the highest antifungal activity against P. piricola (Figure 57) [140].
Figure 57.
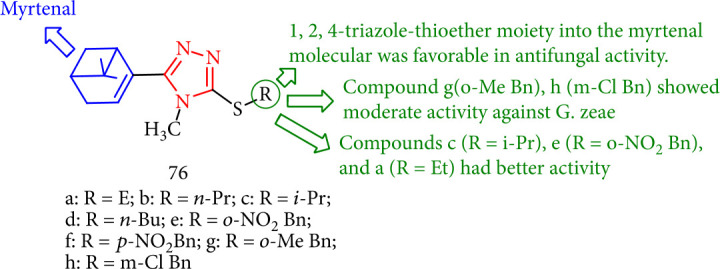
Novel myrtenal-based 4-methyl-1, 2, 4-triazole-thioethers.
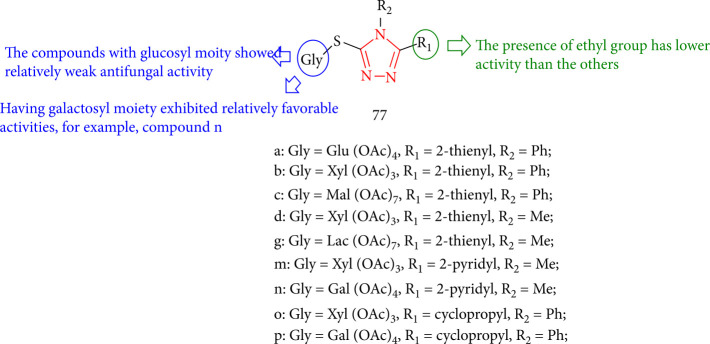
Cheng et al. [141] evaluated a series of 4,5-disubstituted-3-S-(β-D-acetyl glycosyl)-1,2,4-triazoles for their antifungal activities in which compounds revealed reasonable activities at the concentration of 50 μg/mL. Particularly, compounds 77c, 77g, 77n, and 77p displayed 60–68.6% inhibitory rates against B. cinerea and 77c, 77d, 77g, 77m, 77n, and 77p derivatives exhibited 63.6%–78.8% inhibitory rates against S. sclerotiorum, with the antifungal activity of R2 = ethyl being lower than those of the other compounds against S. sclerotiorum. In other words, compounds containing a galactosyl moiety, such as compound 77n, demonstrated highly favorable antifungal action, whereas compounds with a glucosyl moiety showed comparably weak antifungal activity (Figure 58).
Figure 58.
1,2,4-Triazole with glucosyl moiety compounds.
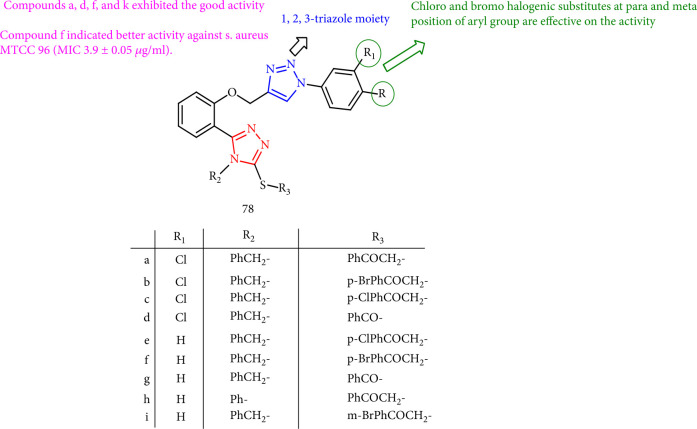
Bitla et al. [142] synthesized and screened bis(1,2,3 and 1,2,4)-triazole derivatives for antifungal activity, and compounds 78a, 78d, 78f, and 78i had the highest activity. It is remarked that bromo and chloro substitutes at meta and para positions of the aryl ring were highly important. Compound 78f indicated superior activity against S. aureus MTCC 96 (MIC 3.9 ± 0.05 μg/ml) (Figure 59).
Figure 59.
Assessment of bis-1,2,4-triazole derivatives as antifungal agents.
Beyzaei et al. [143] synthesized and tested a new class of 1,2,4-triazole-3-thiones in glycerol/potassium carbonate and assessed them for antifungal activity. Significant inhibitory special effects were detected notably against fungal infections. Fusarium oxysporum and Aspergillus fumigatus were inhibited with all of them. The most excellent antifungal activities indicated triazole 79c that contains R = 4-nitrophenyl has the highest antifungal activity as well as the high-affinity binding to the receptor. Hydrogen bonds between the N-1 azole ring and some amino acid residues in the target enzyme interact predominantly. These findings might aid in the development of antifungal drugs (Figure 60).
Figure 60.
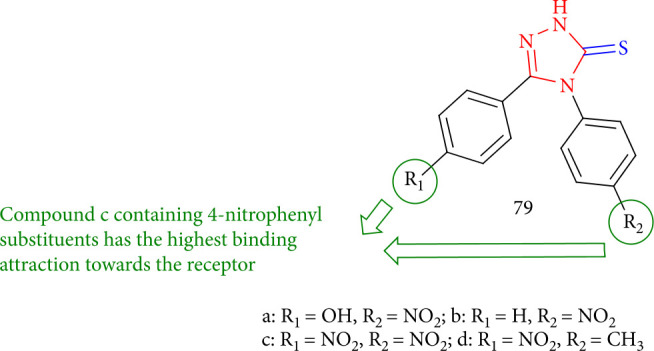
Antifungal study on 4,5-substituted 1,2,4-triazole-3-thiones.
Some studies have been conducted on the fungicidal activities of the novel 1,2,4-triazole derivatives. Compounds 80a-d (Figure 61) in particular showed high antifungal activity. The relationship between biological activity and structure revealed that compounds with the sulfur atom exclusively in the thiol form exhibited activity. Furthermore, compounds 80a and 80c, at a concentration of 1000 mm, inhibit the growth of C. albicans by 35–40%, respectively [144].
Figure 61.
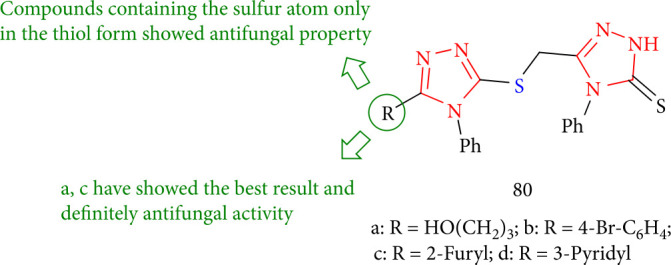
Derivatives of 1,2,4-triazole with a thiomethyl bridge.
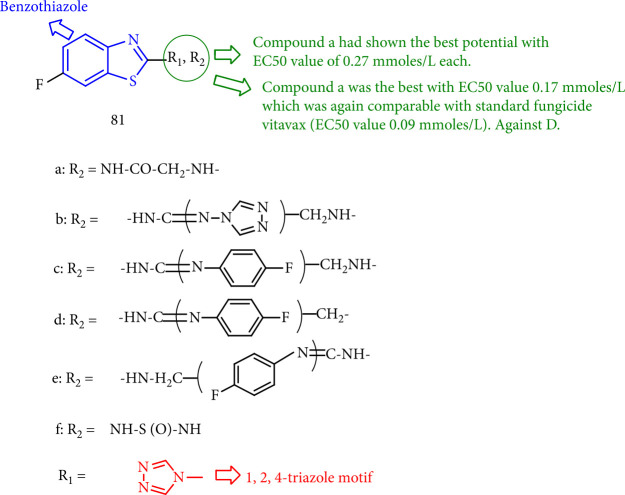
Sidhu and Kukreja [145] reported new compounds based on lead hybridization of 1,2,4-triazoles with fluorinated benzothiazol-2-yl that were tested for fungicidal activity against P. striiformis, D. oryzae, and U. hordei in contrast with conventional fungicides. Furthermore, derivatives 81b and 81c are active against most of the experimental fungi. Compounds 81a and 81e caused the antifungal potential of EC50 0.23 and 0.19 mmoles/L, respectively, against P. striiformis that was compared to the standard fungicide (EC50 value 0.10 of mmoles/L). Compound 81a has the greatest EC50 value (0.17 mmoles/L) against U. hordei when compared to Vitavax (EC50 value of 0.09 mmoles/L) (Figure 62).
Figure 62.
Series of new fluorinated benzothiazol-2-yl-1,2,4-triazoles.
Shingare et al. [146] presented a new series of pyrazole bearing triazolo-thiadiazole derivatives (82a-l) which were evaluated to have antifungal activity versus A. Niger, C. albicans, and A. clavatus along with nystatin and griseofulvin as standard drugs. Amongst them, compounds 82b and 82j revealed good antifungal activity. Compound 82j has shown the most activity (Figure 63).
Figure 63.
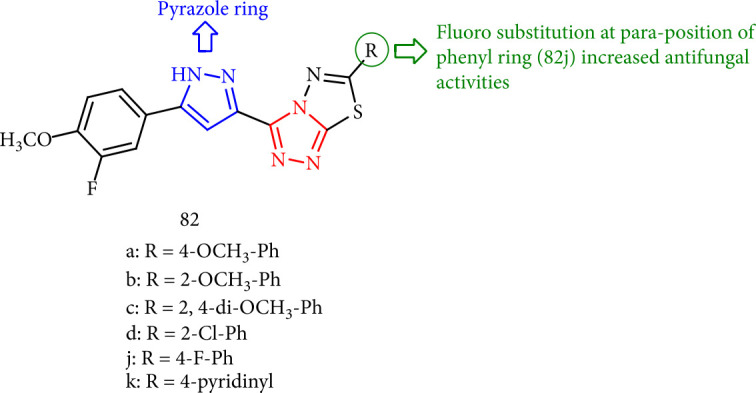
Chemical structures of pyrazole bearing triazolo-thiadiazole derivatives.
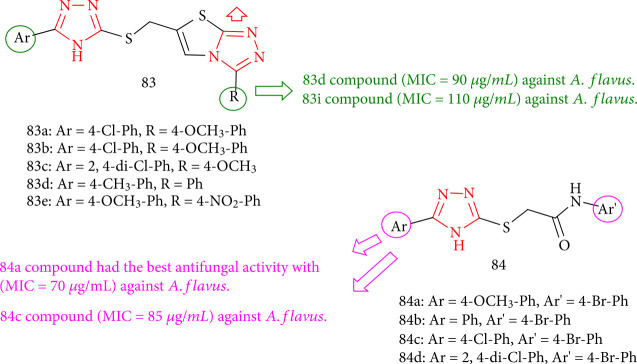
The antifungal activity of 1,2,4-triazolo containing thiadiazoles (83a-e) and 1,2,4-triazol-3-ylthio-N-4-aryl) acetamides (84a-d) was evaluated. Compound 84a had good activity toward A. flavus with a MIC value of 70 μg/mL compared with standard fluconazole; derivatives 83d, 83a, 84c, and 84a demonstrated moderate activity (Figure 64) [147].
Figure 64.
The study of the chemical structure of triazole based heterocycles.
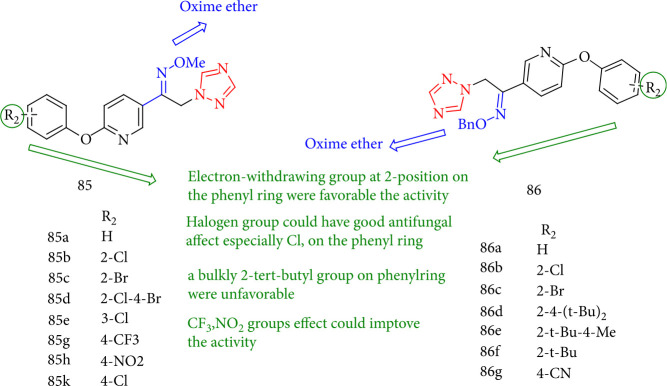
Bai et al. [148] assessed several novel 1,2,4-triazole analogues for antifungal activity against eight phytopathogens and found that the majority of them exhibited acceptable to outstanding fungicidal characteristics. Almost all of the compounds demonstrated moderate to excellent fungicidal activity toward the tested phytopathogens. In general, the fungicidal activity of methyl oxime ether group 85 (R1 = methyl) was significantly greater than benzyl oxime ether group 86 (R1 = benzyl). It is clear that electron-drawing groups at the 2-position of the phenyl ring, such as 85b, 85d, 86b were more helpful for fungicidal activity. Because of the halogen substituent effect, compounds 85b, 85d, 85g, 85k, and 86b demonstrated significantly higher inhibitory activities against fungal pathogens than other compounds, with the chlorine atom playing a more significant role in improving fungicidal activity in each position of the benzene ring.
Furthermore, the prevention rates of compounds 85d, 86e, and 85f against all of the fungi examined were meager. It is shown that for benzyl oxime ether series 85, a bulky 2-tert-butyl group on the benzene substituent was not best for the activity. Compound 85d (2-Cl-4-Br) containing two mixed halogen atoms showed broad-spectrum fungicidal activity, with EC50 values of 1.59, 0.46, 0.27, and 11.39 mg/L against four fungal pathogens (Figure 65).
Figure 65.
1,2,4-Triazole analogues having oxime ether and phenoxyl pyridinyl moiety.
5. Conclusion
A privileged structure in medicinal and organic chemistry is 1,2,4-triazole-hybrids having a broad spectrum of antifungal activity. The 1,2,4-triazole nucleus and its derivatives are essential scaffolds in the discovery and development of drugs that have a multitude of biological activities. An acceptable reason for its broad biological profile is a small and stable cyclic ring structure wherein the nitrogen atoms can act both as hydrogen bond donor and as acceptors at the active site of the receptor. The pentacyclic triazole ring processes plasticity for the synthesis of a number of derivatives due to of its multifold binding sites. This potent scaffold will act as a lead molecule in drug synthesis in the future. The various methods for the regioselective synthesis of 1,2,4-triazole-scaffold will be a great tool in medicinal chemistry in the future. The most challenging problem in fungal therapy is antifungal resistance, which may be progressed by drug target overexpression. This review is focused to summarizing recent research on 1,2,4-triazole-hybrids as fungicidal agents over the last decade. It will aid researchers and medicinal chemists in the discovery and the synthesis of new antifungal compounds with 1,2,4-triazole-moiety.
Acknowledgments
The authors wish to thank the Noncommunicable Diseases Research Center, Fasa University of Medical Sciences.
Abbreviations
- A. flavus:
Aspergillus flavus
- A. niger:
Aspergillus niger
- C. albicans:
Candida albicans
- C2H3N3:
Molecular formula of 1,2,4-triazole derivatives
- CuO:
Copper (II) oxide or cupric oxide
- CYP51:
Lanosterol 14α-demethylase
- ED50:
Effective dose for 50% of the population
- EWG:
Electron withdrawing group
- FLC:
Fluconazole
- FO:
Fusarium oxysporum
- K3PO4:
Tripotassium phosphate
- MIC:
Minimum inhibitory concentration
- NaOH:
Sodium hydroxide
- SAR:
Structure-activity relationship
- SL:
Stemphylium lycopersici.
Data Availability
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request. We have presented all data in the form of figures.
Ethical Approval
This research has been ethically approved (no. 99310) IR.FUMS.REC.1400.013.
Conflicts of Interest
The authors declare that they have no competing interests.
Authors' Contributions
The conception, design, writing, and revision of the study were done by E. Z. The first draft of the manuscript was written by E. Z., Z. K., M. M., A. S., M. F., and A.K. Also, E. Z. and M. M. played the role of the first author in this manuscript; all authors approved the final manuscript.
References
- 1.Ahmad S., Alam O., Naim M. J., Shaquiquzzaman M., Alam M. M., Iqbal M. Pyrrole: an insight into recent pharmacological advances with structure activity relationship. European Journal of Medicinal Chemistry . 2018;157:527–561. doi: 10.1016/j.ejmech.2018.08.002. [DOI] [PubMed] [Google Scholar]
- 2.Afsarian M. H., Farjam M., Zarenezhad E., Behrouz S., Rad M. N. S. Synthesis, antifungal evaluation and molecular docking studies of some tetrazole derivatives. Acta Chimica Slovenica . 2019;66(4):874–887. doi: 10.17344/acsi.2019.4992. [DOI] [PubMed] [Google Scholar]
- 3.Zarenezhad E., Farjam M., Iraji A. Synthesis and biological activity of pyrimidines-containing hybrids: focusing on pharmacological application. Journal of Molecular Structure . 2021;1230:p. 129833. doi: 10.1016/j.molstruc.2020.129833. [DOI] [Google Scholar]
- 4.Mosslemin M. H., Zarenezhad E., Shams N., Rad M. N. S., Anaraki-Ardakani H., Fayazipoor R. Green synthesis of 5-aryl-(1H,3H,5H,10H)-pyrimido[4,5-b]quinoline-2,4-diones catalysed by 1,4-diazabicyclo[2.2.2]octane in water. Journal of Chemical Research . 2014;38(3):169–171. doi: 10.3184/174751914X13917105358323. [DOI] [Google Scholar]
- 5.Dymińska L. Imidazopyridines as a source of biological activity and their pharmacological potentials--Infrared and Raman spectroscopic evidence of their content in pharmaceuticals and plant materials. Bioorganic & Medicinal Chemistry . 2015;23(18):6087–6099. doi: 10.1016/j.bmc.2015.07.045. [DOI] [PubMed] [Google Scholar]
- 6.Zarenezhad E., Soltani Rad M. N., Behrouz S., Esmaielzadeh S., Farjam M. Immobilized [Cu (cdsalMeen)] on silica gel: a highly efficient heterogeneous catalyst for ‘Click’[3+2] Huisgen cycloaddition. Journal of the Iranian Chemical Society . 2017;14(2):509–519. doi: 10.1007/s13738-016-0999-3. [DOI] [Google Scholar]
- 7.Martínez-Matías N., Rodríguez-Medina J. R. Fundamental concepts of azole compounds and triazole antifungals: a beginner’s review. Puerto Rico Health Sciences Journal . 2018;37(3):135–142. [PubMed] [Google Scholar]
- 8.Cox J. R., Woodcock S., Hillier I. H., Vincent M. A. Tautomerism of 1,2,3- and 1,2,4-triazole in the gas phase and in aqueous solution: a combined ab initio quantum mechanics and free energy perturbation study. Journal of Physical Chemistry . 1990;94(14):5499–5501. doi: 10.1021/j100377a016. [DOI] [Google Scholar]
- 9.Chu X.-M., Wang C., Wang W. L., et al. Triazole derivatives and their antiplasmodial and antimalarial activities. European Journal of Medicinal Chemistry . 2019;166:206–223. doi: 10.1016/j.ejmech.2019.01.047. [DOI] [PubMed] [Google Scholar]
- 10.Bekircan O., Menteşe E., Ülker S., Kucuk C. Synthesis of some new 1, 2, 4-triazole derivatives starting from 3-(4-chlorophenyl)-5-(4-methoxybenzyl)-4H-1, 2, 4-triazol with anti-lipase and anti-urease activities. Archiv der Pharmazie . 2014;347(6):387–397. doi: 10.1002/ardp.201300344. [DOI] [PubMed] [Google Scholar]
- 11.Cao X., Wang W., Wang S., Bao L. Asymmetric synthesis of novel triazole derivatives and their _in vitro_ antiviral activity and mechanism of action. European Journal of Medicinal Chemistry . 2017;139:718–725. doi: 10.1016/j.ejmech.2017.08.057. [DOI] [PubMed] [Google Scholar]
- 12.Kaproń B., Czarnomysy R., Wysokiński M., et al. 1, 2, 4-Triazole-based anticonvulsant agents with additional ROS scavenging activity are effective in a model of pharmacoresistant epilepsy. Journal of Enzyme Inhibition and Medicinal Chemistry . 2020;35(1):993–1002. doi: 10.1080/14756366.2020.1748026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pokuri S., Singla R., Bhat V., Shenoy G. Insights on the antioxidant potential of 1, 2, 4-triazoles: synthesis, screening & QSAR studies. Current Drug Metabolism . 2014;15(4):389–397. doi: 10.2174/1389200215666140908101958. [DOI] [PubMed] [Google Scholar]
- 14.Shafiei M., Peyton L., Hashemzadeh M., Foroumadi A. History of the development of antifungal azoles: A review on structures, SAR, and mechanism of action. Bioorganic Chemistry . 2020;104:p. 104240. doi: 10.1016/j.bioorg.2020.104240. [DOI] [PubMed] [Google Scholar]
- 15.Russell P. A century of fungicide evolution. The Journal of Agricultural Science . 2005;143(1):11–25. doi: 10.1017/S0021859605004971. [DOI] [Google Scholar]
- 16.Pianalto K. M., Alspaugh J. A. New horizons in antifungal therapy. Journal of Fungi . 2016;2(4):p. 26. doi: 10.3390/jof2040026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Roemer T., Krysan D. J. Antifungal drug development: challenges, unmet clinical needs, and new approaches. Cold Spring Harbor Perspectives in Medicine . 2014;4(5):p. a019703. doi: 10.1101/cshperspect.a019703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Campoy S., Adrio J. L. Antifungals. Biochemical Pharmacology . 2017;133:86–96. doi: 10.1016/j.bcp.2016.11.019. [DOI] [PubMed] [Google Scholar]
- 19.Dawson J. H., Sono M. Cytochrome P-450 and chloroperoxidase: thiolate-ligated heme enzymes. Spectroscopic determination of their active-site structures and mechanistic implications of thiolate ligation. Chemical Reviews . 1987;87(5):1255–1276. doi: 10.1021/cr00081a015. [DOI] [Google Scholar]
- 20.Kathiravan M. K., Salake A. B., Chothe A. S., et al. The biology and chemistry of antifungal agents: a review. Bioorganic & Medicinal Chemistry . 2012;20(19):5678–5698. doi: 10.1016/j.bmc.2012.04.045. [DOI] [PubMed] [Google Scholar]
- 21.Miyamoto Y., Yamazaki C. Synthesis of nitrogen-containing heterocycles. 3. Formation and structure of new 1, 2, 4-triazole derivatives. Journal of Heterocyclic Chemistry . 1989;26(2):327–332. doi: 10.1002/jhet.5570260211. [DOI] [Google Scholar]
- 22.Tratrat C. 1, 2, 4-triazole: a privileged scaffold for the development of potent antifungal agents-a brief review. Current Topics in Medicinal Chemistry . 2020;20(24):2235–2258. doi: 10.2174/1568026620666200704140107. [DOI] [PubMed] [Google Scholar]
- 23.Strzelecka M., Świątek P. 1, 2, 4-Triazoles as important antibacterial agents. Pharmaceuticals . 2021;14(3):p. 224. doi: 10.3390/ph14030224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Samura Т. Review of antibacterial and antifungal activity of 1, 2, 4-triazole derivatives. Farmatsevtychnyi zhurnal . 2018;5(5):63–68. doi: 10.32352/0367-3057.5.15.02. [DOI] [Google Scholar]
- 25.Sahoo S., Veliyath S. K., Mahendra Kumar C. B. Review on substituted 1, 2, 4-triazole as potent antifungal and antibacterial agents. International Journal of Pharmaceutical Sciences and Research . 2012;3(2012) [Google Scholar]
- 26.Garratt P. J. 1, 2, 4-Triazoles . Elsevier; 1996. [Google Scholar]
- 27.Huang T., Jiang H., Zhao Y., He J., Cheng H., Martyniuk C. J. A comprehensive review of 1,2,4-triazole fungicide toxicity in zebrafish (Danio rerio): A mitochondrial and metabolic perspective. Science of the Total Environment . 2022;809, article 151177 doi: 10.1016/j.scitotenv.2021.151177. [DOI] [PubMed] [Google Scholar]
- 28.Zafar W., Sumrra S. H., Chohan Z. H. A review: pharmacological aspects of metal based 1,2,4-triazole derived Schiff bases. European Journal of Medicinal Chemistry . 2021;222, article 113602 doi: 10.1016/j.ejmech.2021.113602. [DOI] [PubMed] [Google Scholar]
- 29.Blokhina S. V., Sharapova A. V., Ol'khovich M. V., Doroshenko I. A., Levshin I. B., Perlovich G. L. Synthesis and antifungal activity of new hybrids thiazolo[4,5-d]pyrimidines with (1 H-1,2,4)triazole. Bioorganic & Medicinal Chemistry Letters . 2021;40, article 127944 doi: 10.1016/j.bmcl.2021.127944. [DOI] [PubMed] [Google Scholar]
- 30.Aliste M., Pérez-Lucas G., Garrido I., Fenoll J., Navarro S. Risk assessment of 1, 2, 4-triazole-typed fungicides for groundwater pollution using leaching potential indices. Water, Air, & Soil Pollution . 2021;232(11):1–13. doi: 10.1007/s11270-021-05428-1. [DOI] [Google Scholar]
- 31.Bladin J. A. Ueber von dicyanphenylhydrazin abgeleitete verbindungen. Berichte der deutschen chemischen Gesellschaft . 1885;18(1):44–51. [Google Scholar]
- 32.Benson F. R., Savell W. L. The chemistry of the vicinal triazoles. Chemical Reviews . 1950;46(1):1–68. doi: 10.1021/cr60143a001. [DOI] [PubMed] [Google Scholar]
- 33.Shelke G. M., Rao V., Jha M., Cameron T., Kumar A. Microwave-assisted catalyst-free synthesis of substituted 1, 2, 4-triazoles. Synlett . 2015;26(3):404–407. doi: 10.1055/s-0034-1379734. [DOI] [Google Scholar]
- 34.Huang H., Guo W., Wu W., Li C. J., Jiang H. Copper-catalyzed oxidative C (sp 3)–H functionalization for facile synthesis of 1, 2, 4-triazoles and 1, 3, 5-triazines from amidines. Organic Letters . 2015;17(12):2894–2897. doi: 10.1021/acs.orglett.5b00995. [DOI] [PubMed] [Google Scholar]
- 35.Jatangi N., Tumula N., Palakodety R. K., Nakka M. I2-mediated oxidative C–N and N–S bond formation in water: a metal-free synthesis of 4, 5-disubstituted/n-fused 3-amino-1, 2, 4-triazoles and 3-substituted 5-amino-1, 2, 4-thiadiazoles. The Journal of Organic Chemistry . 2018;83(10):5715–5723. doi: 10.1021/acs.joc.8b00753. [DOI] [PubMed] [Google Scholar]
- 36.Liu J.-Q., Shen X., Wang Y., Wang X. S., Bi X. [3 + 2] cycloaddition of isocyanides with aryl diazonium salts: catalyst-dependent regioselective synthesis of 1,3- and 1,5-Disubstituted 1,2,4-Triazoles. Organic Letters . 2018;20(21):6930–6933. doi: 10.1021/acs.orglett.8b03069. [DOI] [PubMed] [Google Scholar]
- 37.Chen Z., Li H., Dong W., Miao M., Ren H. I2-catalyzed oxidative coupling reactions of hydrazones and amines and the application in the synthesis of 1, 3, 5-trisubstituted 1, 2, 4-triazoles. Organic Letters . 2016;18(6):1334–1337. doi: 10.1021/acs.orglett.6b00277. [DOI] [PubMed] [Google Scholar]
- 38.Khan M. A., Polya J. Syntheses of heterocyclic compounds. Part II. N-arylazoles by Ullmann condensation. Journal of the Chemical Society C: Organic . 1970;1(1):85–91. doi: 10.1039/j39700000085. [DOI] [Google Scholar]
- 39.Kauffmann T., Legler J., Ludorff E., Fischer H. Synthesis and properties of azole-pyridine combinations: problem of the hydrolytic cleavage of Hetarene combinations. Angewandte Chemie International Edition in English . 1972;11(9):846–847. doi: 10.1002/anie.197208461. [DOI] [Google Scholar]
- 40.Olofson R. A., Kendall R. Protection by acylation in the selective alkylation of heterocycles. The Journal of Organic Chemistry . 1970;35(7):2246–2248. doi: 10.1021/jo00832a031. [DOI] [Google Scholar]
- 41.Ainsworth C., Jones R. Isomeric and nuclear-substituted β-Aminoethyl-1, 2, 4-triazoles. Journal of the American Chemical Society . 1955;77(3):621–624. doi: 10.1021/ja01608a028. [DOI] [Google Scholar]
- 42.Pellizzari G., Soldi A. Via treatment of the 1-benzyl derivative with phenyllithium in THF ether the position of alkylation is assumed to follow previous examples. Gazzetta Chimica Italiana . 1905;35:p. 373. [Google Scholar]
- 43.Naik A. D., Marchand-Brynaert J., Garcia Y. A simplified approach to N-and N, N’-linked 1, 2, 4-triazoles by transamination. Synthesis . 2008;2008(1):149–154. doi: 10.1055/s-2007-983896. [DOI] [Google Scholar]
- 44.Wiley R. H., Hart A. J. Reaction of diformylhydrazine with aminoheterocycles. The Journal of Organic Chemistry . 1953;18(10):1368–1371. doi: 10.1021/jo50016a016. [DOI] [Google Scholar]
- 45.Liu B., Zhang X.-C., Wang Y.-F. The syntheses, structures and fluorescent properties of two 3-D hydrogen- bonded frameworks constructed from monomeric Zn(II) and Cd(II) assemblies containing bitriazole. Inorganic Chemistry Communications . 2007;10(2):199–203. doi: 10.1016/j.inoche.2006.10.028. [DOI] [Google Scholar]
- 46.Becker H., Hoffmann G., Gwan K. M., Knüpfer L. Azocoupling of quaternary 1, 2, 4-triazolium salts to form 5-p-N, N-dimethylaminophenylazo-1, 2, 4-triazolium salts. Journal für Praktische Chemie . 1988;330(3):325–337. doi: 10.1002/prac.19883300302. [DOI] [Google Scholar]
- 47.Pellizzari G. Nuova sintesi del triazolo e dei suoi derivati. Gazzetta Chimica Italiana . 1894;24:222–229. [Google Scholar]
- 48.Potts K. The chemistry of 1, 2, 4-triazoles. Chemical Reviews . 1961;61(2):87–127. doi: 10.1021/cr60210a001. [DOI] [Google Scholar]
- 49.von Meyer W. C., Greenfield S. A., Seidel M. C. Wheat leaf rust: control by 4-n-butyl-1, 2, 4-triazole, a systemic fungicide. Science . 1970;169(3949):997–998. doi: 10.1126/science.169.3949.997. [DOI] [PubMed] [Google Scholar]
- 50.Tombo G. M. R., Belluš D. Chirality and crop protection. Angewandte Chemie International Edition in English . 1991;30(10):1193–1215. doi: 10.1002/anie.199111933. [DOI] [Google Scholar]
- 51.De Beule K., Van Gestel J. Pharmacology of itraconazole. Drugs . 2001;61(Supplement 1):27–37. doi: 10.2165/00003495-200161001-00003. [DOI] [PubMed] [Google Scholar]
- 52.Westerberg D. P., Voyack M. J. Onychomycosis: current trends in diagnosis and treatment. American Family Physician . 2013;88(11):762–770. [PubMed] [Google Scholar]
- 53.Gupta A., Richardson M., Paquet M. Systematic review of oral treatments for seborrheic dermatitis. Journal of the European Academy of Dermatology and Venereology . 2014;28(1):16–26. doi: 10.1111/jdv.12197. [DOI] [PubMed] [Google Scholar]
- 54.Rossi S. Australian Medicines Handbook . Adelaide; 2006. [Google Scholar]
- 55.Dayer M. R. Old drugs for newly emerging viral disease, COVID-19: Bioinformatic Prospective. 2020, https://arxiv.org/abs/2003.04524.
- 56.Donnelley M. A., Zhu E. S., Thompson G. R., 3rd Isavuconazole in the treatment of invasive aspergillosis and mucormycosis infections. Infection and Drug Resistance . 2016;9:p. 79. doi: 10.2147/IDR.S81416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Miceli M., Kauffman C. Isavuconazole: a new broadspectrum triazole antifungal agent. Clinical Infectious Diseases . 2015;61(10):1558–1565. doi: 10.1093/cid/civ571. [DOI] [PubMed] [Google Scholar]
- 58.Patel T., Dhillon S. Efinaconazole: first global approval. Drugs . 2013;73(17):1977–1983. doi: 10.1007/s40265-013-0152-x. [DOI] [PubMed] [Google Scholar]
- 59.Tschen E. H., Bucko A. D., Oizumi N., Kawabata H., Olin J. T., Pillai R. Efinaconazole solution in the treatment of toenail onychomycosis: a phase 2, multicenter, randomized, double-blind study. Journal of Drugs in Dermatology . 2013;12(2):186–192. [PubMed] [Google Scholar]
- 60.Tatsumi Y., Nagashima M., Shibanushi T., et al. Mechanism of action of efinaconazole, a novel triazole antifungal agent. Antimicrobial Agents and Chemotherapy . 2013;57(5):2405–2409. doi: 10.1128/AAC.02063-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Schiller D. S., Fung H. B. Posaconazole: an extended-spectrum triazole antifungal agent. Clinical Therapeutics . 2007;29(9):1862–1886. doi: 10.1016/j.clinthera.2007.09.015. [DOI] [PubMed] [Google Scholar]
- 62.Rachwalski E. J., Wieczorkiewicz J. T., Scheetz M. H. Posaconazole: an oral triazole with an extended spectrum of activity. Annals of Pharmacotherapy . 2008;42(10):1429–1438. doi: 10.1345/aph.1L005. [DOI] [PubMed] [Google Scholar]
- 63.Li X., Brown N., Chau A. S., et al. Changes in susceptibility to posaconazole in clinical isolates of Candida albicans. Journal of Antimicrobial Chemotherapy . 2003;53(1):74–80. doi: 10.1093/jac/dkh027. [DOI] [PubMed] [Google Scholar]
- 64.Walsh T. J., Raad I., Patterson T. F., et al. Treatment of invasive aspergillosis with posaconazole in patients who are refractory to or intolerant of conventional therapy: an externally controlled trial. Clinical Infectious Diseases . 2007;44(1):2–12. doi: 10.1086/508774. [DOI] [PubMed] [Google Scholar]
- 65.Raad I. I., Hachem R. Y., Herbrecht R., et al. Posaconazole as salvage treatment for invasive fusariosis in patients with underlying hematologic malignancy and other conditions. Clinical Infectious Diseases . 2006;42(10):1398–1403. doi: 10.1086/503425. [DOI] [PubMed] [Google Scholar]
- 66.Colon B. L., Rice C. A., Guy R. K., Kyle D. E. Phenotypic screens reveal posaconazole as a rapidly acting amebicidal combination partner for treatment of primary amoebic meningoencephalitis. The Journal of Infectious Diseases . 2019;219(7):1095–1103. doi: 10.1093/infdis/jiy622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Vanham D., Spinewine A., Hantson P., Wittebole X., Wouters D., Sneyers B. Drug-drug interactions in the intensive care unit: do they really matter? Journal of Critical Care . 2017;38:97–103. doi: 10.1016/j.jcrc.2016.09.014. [DOI] [PubMed] [Google Scholar]
- 68.Patterson T. F., Thompson G. R., III, Denning D. W., et al. Practice guidelines for the diagnosis and management of aspergillosis: 2016 update by the Infectious Diseases Society of America. Clinical Infectious Diseases . 2016;63(4):e1–e60. doi: 10.1093/cid/ciw326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Omrani A. S., Almaghrabi R. S. Complications of hematopoietic stem transplantation: fungal infections. Hematology/Oncology and Stem Cell Therapy . 2017;10(4):239–244. doi: 10.1016/j.hemonc.2017.05.013. [DOI] [PubMed] [Google Scholar]
- 70.Herbrecht R., Denning D. W., Patterson T. F., et al. Voriconazole versus amphotericin B for primary therapy of invasive aspergillosis. New England Journal of Medicine . 2002;347(6):408–415. doi: 10.1056/NEJMoa020191. [DOI] [PubMed] [Google Scholar]
- 71.Salvador Velez F. M., Sánchez Montalvá A., Molina Romero I. Experimental and clinical treatment of Chagas disease: a review. The American Journal of Tropical Medicine and Hygiene . 2017;97(5):1289–1303. doi: 10.4269/ajtmh.16-0761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kumar S., Kimball A. B. New antifungal therapies for the treatment of onychomycosis. Expert Opinion on Investigational Drugs . 2009;18(6):727–734. doi: 10.1517/13543780902810352. [DOI] [PubMed] [Google Scholar]
- 73.Pasqualotto A. C., Thiele K. O., Goldani L. Z. Novel triazole antifungal drugs: focus on isavuconazole, ravuconazole and albaconazole. Current Opinion in Investigational Drugs . 2010;11(2):165–174. [PubMed] [Google Scholar]
- 74.Pfaller M., Messer S. A., Hollis R. J., Jones R. N. Antifungal activities of posaconazole, ravuconazole, and voriconazole compared to those of itraconazole and amphotericin B against 239 clinical isolates of Aspergillus spp. and other filamentous fungi: report from SENTRY antimicrobial surveillance program. Antimicrobial Agents and Chemotherapy . 2002;46(4):1032–1037. doi: 10.1128/AAC.46.4.1032-1037.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Toribio L., del Nozal M., Bernal J. L., Jiménez J. J., Alonso C. Chiral separation of some triazole pesticides by supercritical fluid chromatography. Journal of Chromatography A . 2004;1046(1-2):249–253. doi: 10.1016/S0021-9673(04)01064-7. [DOI] [PubMed] [Google Scholar]
- 76.Sunderland M. R., Cruickshank R. H., Leighs S. J. The efficacy of antifungal azole and antiprotozoal compounds in protection of wool from keratin-digesting insect larvae. Textile Research Journal . 2014;84(9):924–931. doi: 10.1177/0040517513515312. [DOI] [Google Scholar]
- 77.Yamaguchi H. Potential of ravuconazole and its prodrugs as the new OralTherapeutics for onychomycosis. Medical Mycology Journal . 2016;57(4):E93–E110. doi: 10.3314/mmj.16-00006. [DOI] [PubMed] [Google Scholar]
- 78.Tran N. T., Zivin A., Mozaffarian D., Karmy-Jones R. Right atrial perforation secondary to implantable cardioverter defibrillator insertion. Canadian Respiratory Journal . 2001;8(4) doi: 10.1155/2001/257641. [DOI] [PubMed] [Google Scholar]
- 79.Moberg W. K., Basarab G. S., Cuomo J., Liang P. H. Biologically Active Organosilicon Compounds: Fungicidal Silylmethyltriazoles . ACS; 1987. [DOI] [Google Scholar]
- 80.Bostanian N. J., Larocque N., Chouinard G., Coderre D. Baseline toxicity of several pesticides to Hyaliodes vitripennis (Say)(Hemiptera: Miridae) Pest Management Science: Formerly Pesticide Science . 2001;57(11):1007–1010. doi: 10.1002/ps.374. [DOI] [PubMed] [Google Scholar]
- 81.Eckert M. R., Rossall S., Selley A., Fitt B. D. L. Effects of fungicides onin vitrospore germination and mycelial growth of the phytopathogensLeptosphaeria maculansandL. biglobosa(phoma stem canker of oilseed rape) Pest Management Science: Formerly Pesticide Science . 2010;66(4):396–405. doi: 10.1002/ps.1890. [DOI] [PubMed] [Google Scholar]
- 82.Rani M., Shanker U. Removal of chlorpyrifos, thiamethoxam, and tebuconazole from water using green synthesized metal hexacyanoferrate nanoparticles. Environmental Science and Pollution Research . 2018;25(11):10878–10893. doi: 10.1007/s11356-018-1346-2. [DOI] [PubMed] [Google Scholar]
- 83.Wang Z., Tian Z., Chen L., et al. Stereoselective metabolism and potential adverse effects of chiral fungicide triadimenol on Eremias argus. Environmental Science and Pollution Research . 2020;27(8):7823–7834. doi: 10.1007/s11356-019-07205-4. [DOI] [PubMed] [Google Scholar]
- 84.Krämer W., Schirmer U. Modern Crop Protection Compounds . Wiley-VCH; 2007. [Google Scholar]
- 85.Berova M., Zlatev Z. Physiological response and yield of paclobutrazol treated tomato plants (Lycopersicon esculentum Mill.) Plant Growth Regulation . 2000;30(2):117–123. doi: 10.1023/A:1006300326975. [DOI] [Google Scholar]
- 86.Schnabel G., Jones A. L. The 14α-demethylasse(CYP51A1) gene is overexpressed inVenturia inaequalisstrains resistant to myclobutanil. Phytopathology . 2001;91(1):102–110. doi: 10.1094/PHYTO.2001.91.1.102. [DOI] [PubMed] [Google Scholar]
- 87.Allen J. W., Wolf D. C. Toxicity profiles in mice treated with hepatotumorigenic and non-hepatotumorigenic triazole conazole fungicides: propiconazole, triadimefon, and myclobutanil. Toxicologic Pathology . 2006;34(7):853–862. doi: 10.1080/01926230601047816. [DOI] [PubMed] [Google Scholar]
- 88.Ding Z., Ni T., Xie F., et al. Design, synthesis, and structure-activity relationship studies of novel triazole agents with strong antifungal activity against Aspergillus fumigatus. Bioorganic & Medicinal Chemistry Letters . 2020;30(4):p. 126951. doi: 10.1016/j.bmcl.2020.126951. [DOI] [PubMed] [Google Scholar]
- 89.Montoir D., Guillon R., Gazzola S., et al. New azole antifungals with a fused triazinone scaffold. European Journal of Medicinal Chemistry . 2020;189, article 112082 doi: 10.1016/j.ejmech.2020.112082. [DOI] [PubMed] [Google Scholar]
- 90.Xie F., Ni T., Ding Z., et al. Design, synthesis, and _in vitro_ evaluation of novel triazole analogues featuring isoxazole moieties as antifungal agents. Bioorganic Chemistry . 2020;101:p. 103982. doi: 10.1016/j.bioorg.2020.103982. [DOI] [PubMed] [Google Scholar]
- 91.Khakzad S., Rahmani F., Hojjati M., Tabandeh M. R. Anti-carcinogenic effects of Satureja khuzistanica and Zataria multiflora essential oils on K562 cell line proliferation. Journal of Food and Bioprocess Engineering . 2019;2(2):127–132. [Google Scholar]
- 92.Motahari K., Badali H., Hashemi S. M., et al. Discovery of benzylthio analogs of fluconazole as potent antifungal agents. Future Medicinal Chemistry . 2018;10(9):987–1002. doi: 10.4155/fmc-2017-0295. [DOI] [PubMed] [Google Scholar]
- 93.Chandrika N. T., Shrestha S. K., Ngo H. X., Howard K. C., Garneau-Tsodikova S. Novel fluconazole derivatives with promising antifungal activity. Bioorganic & Medicinal Chemistry . 2018;26(3):573–580. doi: 10.1016/j.bmc.2017.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Tekale S., Diwan M. F., Farooqui M., Pardeshi R. K. Design and synthesis of azole containing Imidazole derivatives and evaluation of their antifungal activity. Chemistry & Biology Interface . 2018;8(6) [Google Scholar]
- 95.Wu J., Ni T., Chai X., et al. Molecular docking, design, synthesis and antifungal activity study of novel triazole derivatives. European Journal of Medicinal Chemistry . 2018;143:1840–1846. doi: 10.1016/j.ejmech.2017.10.081. [DOI] [PubMed] [Google Scholar]
- 96.Sadeghpour H., Khabnadideh S., Zomorodian K., et al. Design, synthesis, and biological activity of new triazole and nitro-triazole derivatives as antifungal agents. Molecules . 2017;22(7):p. 1150. doi: 10.3390/molecules22071150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Xie F., Ni T., Zhao J., et al. Design, synthesis, and in vitro evaluation of novel antifungal triazoles. Bioorganic & Medicinal Chemistry Letters . 2017;27(10):2171–2173. doi: 10.1016/j.bmcl.2017.03.062. [DOI] [PubMed] [Google Scholar]
- 98.Chen H.-J., Jiang Y. J., Zhang Y. Q., et al. New triazole derivatives containing substituted 1,2,3-triazole side chains: design, synthesis and antifungal activity. Chinese Chemical Letters . 2017;28(4):913–918. doi: 10.1016/j.cclet.2016.11.027. [DOI] [Google Scholar]
- 99.Fang X.-F., Li D., Tangadanchu V. K. R., Gopala L., Gao W. W., Zhou C. H. Novel potentially antifungal hybrids of 5-flucytosine and fluconazole: design, synthesis and bioactive evaluation. Bioorganic & Medicinal Chemistry Letters . 2017;27(22):4964–4969. doi: 10.1016/j.bmcl.2017.10.020. [DOI] [PubMed] [Google Scholar]
- 100.Xu Y.-Y., Qian A. R., Cao X. F., et al. Design and synthesis of novel triazole derivatives containing γ -lactam as potential antifungal agents. Chinese Chemical Letters . 2016;27(5):703–706. doi: 10.1016/j.cclet.2016.01.040. [DOI] [Google Scholar]
- 101.Zhang Y., Damu G. L. V., Cui S. F., Mi J. L., Tangadanchu V. K. R., Zhou C. H. Discovery of potential antifungal triazoles: design, synthesis, biological evaluation, and preliminary antifungal mechanism exploration. MedChemComm . 2017;8(8):1631–1639. doi: 10.1039/C7MD00112F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.He X., Jiang Y., Zhang Y., et al. Discovery of highly potent triazoleantifungal agents with piperidine-oxadiazole side chains. MedChemComm . 2015;6(4):653–664. doi: 10.1039/C4MD00505H. [DOI] [Google Scholar]
- 103.Mahmoudi Y., Badali H., Hashemi S. M., et al. New potent antifungal triazole alcohols containing N-benzylpiperazine carbodithioate moiety: Synthesis, in vitro evaluation and in silico study. Bioorganic Chemistry . 2019;90, article 103060 doi: 10.1016/j.bioorg.2019.103060. [DOI] [PubMed] [Google Scholar]
- 104.Li B., Zhang D., Zhang Y., et al. Synthesis and evaluation of novel benzene-ethanol bearing 1, 2, 4-triazole derivatives as potential antimicrobial agents. Medicinal Chemistry Research . 2017;26(1):44–51. doi: 10.1007/s00044-016-1724-6. [DOI] [Google Scholar]
- 105.Hoshi T., Yamada K., Yoshizawa Y., Oh K. Structure-activity relationship study for fungicidal activity of 1-(4-phenoxymethyl-2-phenyl-[1, 3] dioxolan-2-ylmethyl)-1H-1, 2, 4-triazole derivatives against rice blast. Journal of Plant Protection Research . 2015;55(4):383–388. doi: 10.1515/jppr-2015-0051. [DOI] [Google Scholar]
- 106.Al-Wabli R. I., Alsulami M. A., Bukhari S. I., Moubayed N., Al-Mutairi M. S., Attia M. I. Design, synthesis, and antimicrobial activity of certain new Indole-1, 2, 4 Triazole conjugates. Molecules . 2021;26(8):p. 2292. doi: 10.3390/molecules26082292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Zhao X., Liao A., Zhang F., et al. Design, synthesis, and bioactivity of nortopsentin analogues containing 1,2,4‐triazole moieties. Journal of Heterocyclic Chemistry . 2020;57(2):761–767. doi: 10.1002/jhet.3817. [DOI] [Google Scholar]
- 108.Ahuja R., Sidhu A., Bala A., Arora D., Sharma P. Structure based approach for twin-enzyme targeted benzimidazolyl-1,2,4-triazole molecular hybrids as antifungal agents. Arabian Journal of Chemistry . 2020;13(6):5832–5848. doi: 10.1016/j.arabjc.2020.04.020. [DOI] [Google Scholar]
- 109.Karaca Gençer H., Acar Çevik U., Levent S., et al. New benzimidazole-1, 2, 4-triazole hybrid compounds: synthesis, anticandidal activity and cytotoxicity evaluation. Molecules . 2017;22(4):p. 507. doi: 10.3390/molecules22040507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Can N. Ö., Acar Çevik U., Sağlık B. N., et al. Synthesis, molecular docking studies, and antifungal activity evaluation of new benzimidazole-triazoles as potential lanosterol 14α-demethylase inhibitors. Journal of Chemistry . 2017;2017:15. doi: 10.1155/2017/9387102. [DOI] [Google Scholar]
- 111.Jiang L., Wang M. Y., Wan F. X., Qu Z. Q. Synthesis and biological activity of tri-substituted 1, 2, 4-triazoles bearing benzimidazole moiety. Phosphorus, Sulfur, and Silicon and the Related Elements . 2015;190(10):1599–1605. [Google Scholar]
- 112.Luo Y.-L., Baathulaa K., Kannekanti V. K., Zhou C. H., Cai G. X. Novel benzimidazole derived naphthalimide triazoles: synthesis, antimicrobial activity and interactions with calf thymus DNA. Science China Chemistry . 2015;58(3):483–494. doi: 10.1007/s11426-014-5296-3. [DOI] [Google Scholar]
- 113.Somagond S. M., Kamble R. R., Kattimani P. P., Joshi S. D., Dixit S. R. Design, synthesis, docking and in vitro antifungal study of 1, 2, 4-triazole hybrids of 2-(aryloxy) quinolines. Heterocyclic Communications . 2017;23(4):317–324. [Google Scholar]
- 114.D’Souza V. T., Nayak J., D’Mello D. E., Dayananda P. Synthesis and characterization of biologically important quinoline incorporated triazole derivatives. Journal of Molecular Structure . 2021;1229:p. 129503. doi: 10.1016/j.molstruc.2020.129503. [DOI] [Google Scholar]
- 115.Fan Z., Shi J., Luo N., Ding M., Bao X. Synthesis, crystal structure, and agricultural antimicrobial evaluation of novel quinazoline thioether derivatives incorporating the 1, 2, 4-triazolo [4, 3-a] pyridine moiety. Journal of Agricultural and Food Chemistry . 2019;67(42):11598–11606. doi: 10.1021/acs.jafc.9b04733. [DOI] [PubMed] [Google Scholar]
- 116.Fan Z., Shi J., Luo N., Bao X. Synthesis, crystal structure and antimicrobial activity of 2-((2-(4-(1H-1,2,4-triazol-1-yl)phenyl)quinazolin-4-yl)oxy)-N-phenylacetamide derivatives against phytopathogens. Molecular Diversity . 2019;23(3):615–624. doi: 10.1007/s11030-018-9896-2. [DOI] [PubMed] [Google Scholar]
- 117.Abuelizz H. A., el-Dib R. A., Marzouk M., al-Salahi R. In vitro evaluation of new 2-phenoxy-benzo[g][1,2,4]triazolo[1,5- a]quinazoline derivatives as antimicrobial agents. Microbial Pathogenesis . 2018;117:60–67. doi: 10.1016/j.micpath.2018.02.018. [DOI] [PubMed] [Google Scholar]
- 118.El-Attar Z. Synthesis of pyrazolo-1, 2, 4-triazolo [4, 3-a] quinoxalines as antimicrobial agents with potential inhibition of DHPS enzyme. Future Medicinal Chemistry . 2018;10(18):2155–2175. doi: 10.4155/fmc-2018-0082. [DOI] [PubMed] [Google Scholar]
- 119.Yang L., Bao X.-P. Synthesis of novel 1, 2, 4-triazole derivatives containing the quinazolinylpiperidinyl moiety and N-(substituted phenyl) acetamide group as efficient bactericides against the phytopathogenic bacterium Xanthomonas oryzae pv. oryzae. RSC Advances . 2017;7(54):34005–34011. doi: 10.1039/C7RA04819J. [DOI] [Google Scholar]
- 120.Sompalle R., Roopan S. M., al-Dhabi N. A., Suthindhiran K., Sarkar G., Arasu M. V. 1,2,4-Triazolo-quinazoline-thiones: non-conventional synthetic approach, study of solvatochromism and antioxidant assessment. Journal of Photochemistry and Photobiology B: Biology . 2016;162:232–239. doi: 10.1016/j.jphotobiol.2016.06.051. [DOI] [PubMed] [Google Scholar]
- 121.Khan G., Sreenivasa S., Govindaiah S., Chandramohan V., Shetty P R. Synthesis, biological screening, in silico study and fingerprint applications of novel 1, 2, 4-triazole derivatives. Journal of Heterocyclic Chemistry . 2020;57(4):2010–2023. doi: 10.1002/jhet.3929. [DOI] [Google Scholar]
- 122.Jin R., Wang Y., Guo H., et al. Design, synthesis, biological activity, crystal structure and theoretical calculations of novel 1,2,4-triazole derivatives. Journal of Molecular Structure . 2020;1202, article 127234 doi: 10.1016/j.molstruc.2019.127234. [DOI] [Google Scholar]
- 123.Wu W.-N., Jiang Y. M., Fei Q., du H. T. Synthesis and fungicidal activity of novel 1, 2, 4-triazole derivatives containing a pyrimidine moiety. Phosphorus, Sulfur, and Silicon and the Related Elements . 2019;194(12):1171–1175. doi: 10.1080/10426507.2019.1633321. [DOI] [Google Scholar]
- 124.Patel D. M., Vala R. M., Sharma M. G., Rajani D. P., Patel H. M. A practical green visit to the functionalized [1, 2, 4] triazolo [5, 1-b] quinazolin-8 (4H) one scaffolds using the group-assisted purification (GAP) chemistry and their pharmacological testing. Chemistry Select . 2019;4(3):1031–1041. [Google Scholar]
- 125.Beyzaei H., Khosravi Z., Aryan R., Ghasemi B. A green one-pot synthesis of 3 (5)-substituted 1, 2, 4-triazol-5 (3)-amines as potential antimicrobial agents. Journal of the Iranian Chemical Society . 2019;16(12):2565–2573. doi: 10.1007/s13738-019-01714-2. [DOI] [Google Scholar]
- 126.Appna N. R., Nagiri R. K., Korupolu R. B., et al. Design and synthesis of novel 4-hydrazone functionalized/1, 2, 4-triazole fused pyrido [2, 3-d] pyrimidine derivatives, their evaluation for antifungal activity and docking studies. Medicinal Chemistry Research . 2019;28(9):1509–1528. doi: 10.1007/s00044-019-02390-w. [DOI] [Google Scholar]
- 127.Singh R., Kashaw S. K., Mishra V. K., Mishra M., Rajoriya V., Kashaw V. Design and synthesis of new bioactive 1, 2, 4-Triazoles, potential antitubercular and antimicrobial agents. Indian Journal of Pharmaceutical Sciences . 2018;80(1):36–45. doi: 10.4172/pharmaceutical-sciences.1000328. [DOI] [Google Scholar]
- 128.Jin R.-Y., Zeng C. Y., Liang X. H., et al. Design, synthesis, biological activities and DFT calculation of novel 1,2,4-triazole Schiff base derivatives. Bioorganic Chemistry . 2018;80:253–260. doi: 10.1016/j.bioorg.2018.06.030. [DOI] [PubMed] [Google Scholar]
- 129.Zhang Y., Zhan Y. Z., Ma Y., et al. Synthesis, crystal structure and 3D-QSAR studies of antifungal (bis-)1,2,4-triazole Mannich bases containing furyl and substituted piperazine moieties. Chinese Chemical Letters . 2018;29(3):441–446. doi: 10.1016/j.cclet.2017.08.035. [DOI] [Google Scholar]
- 130.Sui G., Zhang W., Zhou K., et al. Trialkylamine derivatives containing a triazole moiety as promising ergosterol biosynthesis inhibitor: design, synthesis, and antifungal activity. Chemical and Pharmaceutical Bulletin . 2017;65(1):82–89. doi: 10.1248/cpb.c16-00732. [DOI] [PubMed] [Google Scholar]
- 131.Mu J.-X., Shi Y. X., Wu H. K., et al. Microwave assisted synthesis, antifungal activity, DFT and SAR study of 1, 2, 4-triazolo [4, 3-a] pyridine derivatives containing hydrazone moieties. Chemistry Central Journal . 2016;10(1):1–9. doi: 10.1186/s13065-016-0196-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Zoidis G., Kritsi E., Lecinska P., et al. The triazole ring as a privileged scaffold for putative antifungals: synthesis and evaluation of a series of new analogues. ChemMedChem . 2021;16(1):134–144. doi: 10.1002/cmdc.202000312. [DOI] [PubMed] [Google Scholar]
- 133.Dincel E. D., Ulusoy-Güzeldemirci N., Şatana D., Küçükbasmacı Ö. Design, synthesis, characterization and antimicrobial evaluation of some novel hydrazinecarbothioamide, 4-thiazolidinone and 1,2,4‐triazole‐3‐thione derivatives. Journal of Heterocyclic Chemistry . 2021;58(1):195–205. doi: 10.1002/jhet.4159. [DOI] [Google Scholar]
- 134.Cheng Y.-N., Jiang Z. H., Sun L. S., Su Z. Y., Zhang M. M., Li H. L. Synthesis of 1, 2, 4-triazole benzoyl arylamine derivatives and their high antifungal activities. European Journal of Medicinal Chemistry . 2020;200:p. 112463. doi: 10.1016/j.ejmech.2020.112463. [DOI] [PubMed] [Google Scholar]
- 135.Stingaci E., Zveaghinteva M., Pogrebnoi S., et al. New vinyl-1,2,4-triazole derivatives as antimicrobial agents: Synthesis, biological evaluation and molecular docking studies. Bioorganic & Medicinal Chemistry Letters . 2020;30(17, article 127368) doi: 10.1016/j.bmcl.2020.127368. [DOI] [PubMed] [Google Scholar]
- 136.Wu W. N., Jiang Y. M., Fei Q., du H. T., Yang M. F. Synthesis and antifungal activity of novel 1,2,4‐triazole derivatives containing an amide moiety. Journal of Heterocyclic Chemistry . 2020;57(3):1379–1386. doi: 10.1002/jhet.3874. [DOI] [Google Scholar]
- 137.Yurttaş L., CantŘrk Z. The synthesis, antifungal and apoptotic effects of triazole-oxadiazoles against Candida species. European Journal of Medicinal Chemistry . 2018;144:255–261. doi: 10.1016/j.ejmech.2017.12.020. [DOI] [PubMed] [Google Scholar]
- 138.Li B., Lin X., Zhang Y., Zhang D., Xiao Y., Lin F. Synthesis and characterization of novel N-phenylacetamide bearing 1, 2, 4-triazole derivatives as potential antimicrobial agents. Chemical Research in Chinese Universities . 2017;33(1):70–73. doi: 10.1007/s40242-017-6327-3. [DOI] [Google Scholar]
- 139.Pricopie A.-I., Vlase L., Pîrnău A., Cristian D. Design and synthesis of some novel 1, 2, 4-triazole-3-yl-mercapto derivatives as potential anti-Candida agents. Enzyme . 2018;66(6):948–958. doi: 10.31925/farmacia.2018.6.4. [DOI] [Google Scholar]
- 140.Lin G.-S., Duan W. G., Yang L. X., Huang M., Lei F. H. Synthesis and antifungal activity of novel myrtenal-based 4-methyl-1, 2, 4-triazole-thioethers. Molecules . 2017;22(2):p. 193. doi: 10.3390/molecules22020193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Cheng D., Wei W., Li Y., et al. Design, synthesis and biological activities of novel 4, 5-disubstituted-3-S-(β-D-acetylglycosyl)-1, 2, 4-triazole derivatives. Chemical Research in Chinese Universities . 2017;33(5):758–764. doi: 10.1007/s40242-017-7053-6. [DOI] [Google Scholar]
- 142.Bitla S., Gayatri A. A., Puchakayala M. R., et al. Design and synthesis, biological evaluation of bis-(1,2,3- and 1,2,4)-triazole derivatives as potential antimicrobial and antifungal agents. Bioorganic & Medicinal Chemistry Letters . 2021;41, article 128004 doi: 10.1016/j.bmcl.2021.128004. [DOI] [PubMed] [Google Scholar]
- 143.Beyzaei H., Ghanbari Kudeyani M., Samareh Delarami H., Aryan R. Synthesis, antimicrobial and antioxidant evaluation, and molecular docking study of 4,5-disubstituted 1,2,4-triazole-3-thiones. Journal of Molecular Structure . 2020;1215, article 128273 doi: 10.1016/j.molstruc.2020.128273. [DOI] [Google Scholar]
- 144.Galstyan A. S., Ghochikyan T. V., Samvelyan M. A., Frangyan V. R., Sarfraz M. Synthesis, study of the biological activity of new 1,2,4‐Triazole derivatives and characteristics of the relationship of the structure and biological activity in a series of the latter. ChemistrySelect . 2019;4(42):12386–12390. doi: 10.1002/slct.201902761. [DOI] [Google Scholar]
- 145.Sidhu A., Kukreja S. Synthesis of novel fluorinated benzothiazol-2-yl-1,2,4-triazoles: Molecular docking, antifungal evaluation and in silico evaluation for SAR. Arabian Journal of Chemistry . 2019;12(8):2118–2127. doi: 10.1016/j.arabjc.2015.01.009. [DOI] [Google Scholar]
- 146.Shingare R. M., Patil Y. S., Sangshetti J. N., Damale M. G., Rajani D. P., Madje B. R. Synthesis, antimicrobial evaluation and docking study of some pyrazole bearing [1, 2, 4] triazolo [3, 4-b][1, 3, 4] thiadiazole derivatives. ChemistrySelect . 2018;3(14):3899–3903. doi: 10.1002/slct.201800373. [DOI] [Google Scholar]
- 147.Sahi S., Sodhi R. K., Jamwal B., Paul S. Synthesis andin vitrobiological evaluation of some novel triazole-based heterocycles as potential antimicrobial agents. Journal of Heterocyclic Chemistry . 2018;55(7):1596–1603. doi: 10.1002/jhet.3193. [DOI] [Google Scholar]
- 148.Bai H., Liu X., Chenzhang P., Xiao Y., Fu B., Qin Z. Design, synthesis and fungicidal activity of new 1, 2, 4-triazole derivatives containing oxime ether and phenoxyl pyridinyl moiety. Molecules . 2020;25(24):p. 5852. doi: 10.3390/molecules25245852. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request. We have presented all data in the form of figures.