Abstract
Background
Liver retransplantation remains as the only treatment for graft failure. This investigation aims to assess the incidence, post‐transplant outcomes, and risk factors in liver retransplantation recipients in Canada.
Materials and Methods
The Canadian Organ Replacement Register was used to obtain and analyse data on all adult liver retransplant recipients, matched donors, transplant-specific variables, and post‐transplant outcomes from January 2000 to December 2018.
Results
377 (6.5%) patients underwent liver retransplantation. Autoimmune liver disease and hepatitis C virus (HCV) were the most common underlying diagnoses. Graft failure was 7.9% and 12.5%, and overall survival was 77.1% and 65.6% at 1 year and 5 years, respectively. In contrast to recipients receiving their first graft transplant, the retransplantation group had a significantly higher incidence of graft failure (p < 0.001) and lower overall survival (p < 0.001). The graft failure and patient survival rates were comparable between second transplant and repeat retransplant recipients. Furthermore, there were no differences in graft failure and patient survival when stratified according to time to retransplantation. Recipient and donor age (HR = 1.12, p=0.011; HR = 1.09, p=0.008), recipient HCV status (HR = 1.81, p=0.014), and donor cytomegalovirus status (HR = 4.10, p=0.006) were predictors of patient mortality.
Conclusion
This analysis of liver retransplantation demonstrates that this is a safe treatment for early and late graft failure. Furthermore, even in patients requiring more than two grafts, similar outcomes to initial retransplantation can be achieved with careful selection.
1. Introduction
Liver retransplantation was first performed by Starzl et al. in 1968, with efforts being described to “have borne bitter fruit,” as only 6 of 27 (22%) patients survived past six months [1]. As retransplantation is the only treatment for irreversible graft failure after primary liver transplantation, further attempts demonstrated progress but with persistently increased mortality compared with primary transplantation [2–6]. Recent studies, however, have found no difference in survival between primary liver transplantation (LT) and liver retransplantation in appropriately selected recipients, partly due to the increase in sustained viral response with direct-acting antiviral agents (DAA) and the decrease in the number of patients undergoing repeat transplant for recurrent hepatitis C virus (HCV) [7].
Despite more contemporary reports, the allocation of donor livers continues to be an area of debate not only for primary liver transplantation but also for orthotopic liver retransplantation, which has inherently different medical, ethical, and economic considerations. Specifically, the opportunity cost of liver retransplantation is that it denies an already scarce resource to first-time liver transplant candidates. More broadly, the economic costs of liver retransplantation may have substantial downstream effects in a constrained healthcare system as it costs over twice that of primary transplantation [8, 9]. Given this, it is critical to re-evaluate the role of liver retransplantation over time as indications and outcomes have likely changed with advances in our understanding of donor selection and recipient management.
To date, liver retransplantation has yet to be studied broadly in Canada. Thus, this investigation aims to assess the incidence, post‐transplant outcomes, and risk factors in recipients of liver retransplantation in Canada. Furthermore, with this nationwide study group, we aim to conduct a subgroup analysis of the characteristics and outcomes of those undergoing more than two liver transplants (i.e., repeat retransplantation (RRT)).
2. Materials and Methods
Our study population included all adult (≥18 years) liver retransplant recipients and their matched donors registered on the Canadian Organ Replacement Register (CORR) from 1st January 2000 to 31st December 2018. CORR is a national registry maintained by the Canadian Institute for Health Information (CIHI) that collects information on the majority of Canadian liver transplants but does not include those performed in Quebec. Distinction between different centers are not available in this registry. For this study, simultaneous multiorgan transplants were excluded. The study was approved by CIHI and the research ethics board of University Health Network, Toronto, Canada (REB#19-5835).
Patient-level transplant data included information on liver recipients at the time of LT (age, gender, weight, height, blood type, medical status, model for end-stage liver disease (MELD) score, creatinine, serum bilirubin, international normalized ratio (INR), HCV status, liver disease diagnosis, and cytomegalovirus (CMV)), matched donors (age, gender, weight, height, ethnicity, blood type, cause of death, donor type, HCV status, and CMV status), transplant-specific variables (cold ischemic times), and distance from the donor procurement facility to the transplant facility. Furthermore, post‐transplant outcome data including graft failure, death, reason for death and graft failure, and date of last follow-up were collected. Medical status of a patient was defined as Status 1, patients at home; Status 1T, patients with tumors; Status 2, hospitalized patients; Status 3, patients hospitalized in intensive care unit (ICU); Status 3F, patients with fulminant failure in ICU; Status 4, patients in ICU with intubation/ventilation; and Status 4F, patients with fulminant failure in ICU with intubation/ventilation.
Trends in the number and proportion of yearly retransplantations were assessed with a linear regression least-squared model. A descriptive analysis was performed of all patients receiving retransplantation and stratified by those receiving second, third, and fourth grafts. Descriptive data for continuous variables were expressed as means with standard deviation if the distribution was normal and median with interquartile range (IQR) for non‐normal distribution. Categorical variables were expressed as numbers and percentages.
Given that a patient may die with a functioning graft, the cumulative incidence of graft failure was assessed with a competing risk analysis and compared with the Gray test with death as a competing event [10]. Patient survival was estimated using Kaplan–Meier analysis and compared using the log-rank test. Graft failure and patient survival were assessed at 1-, 3-, 5-, and 10-year time points. Patient survival and graft failure both were calculated from their most recent LT. Retransplant outcome according to acuity of the graft/liver failure was assessed by dividing the cohort by the time from previous LT to retransplantation (hyperacute: 0–7 days, acute: 7–30 days, and chronic: >30 days). A sensitivity analysis was performed in patients who survived at least 30 days post‐transplantation to consider the perioperative period.
For comparative purposes, post‐transplant survival and graft failure in those with single (i.e., primary only) liver transplantations, which met the same inclusion criteria as the retransplantation group, were described. Additionally, to investigate the temporal effects on post‐transplant survival and graft failure in LT, especially for patients who received LT for HCV, the transplant patients were divided into two groups (pre-DAA era: 2000–2010 and post-DAA era: 2011–2018) and were compared.
The association between various predictors and graft failure and patient survival was evaluated using the Fine–Gray competing risk model and Cox regression analyses, respectively. The Fine–Gray model fits a proportional subdistribution hazards' regression model and assesses the effects of covariates on the subdistribution hazard ratio of a particular type of failure in a competing risk setting, in this case, graft failure [11]. The proportional hazard assumption was assessed, and if the assumption was not met, a time-dependent analysis was conducted using the appropriate time period. Multivariable analyses were performed using backward stepwise regression and using all variables with p < 0.15 on univariate analysis. Statistical significance was defined as a value of p < 0.05. All statistical analyses were performed using IBM SPSS Statistics for Windows, version 26.0 (IBM Corp., Armonk, NY, USA), R (R Core Team (2019) R: A language and environment for statistical computing), and Statistical Analysis System (SAS) version 9.4 (SAS Institute, Cary, NC).
3. Results
Over the 18-year study period, 5,805 patients underwent liver transplantation in Canada, with 377 (6.5%) having retransplantations. Of these, 340 (90.2%) received a second graft, 34 (9.0%) a third graft, and 3 (0.8%) a fourth graft. Retransplantation numbers significantly increased over the study period, in which the yearly increase in retransplantations was 1.1 (95% CI: 0.8–1.4, p < 0.001), as shown in Figure 1(a). There were nine retransplantations in 2000; meanwhile, 31 retransplantations were performed in 2018. The proportion of retransplantations also increased over the study period with a yearly increase of 0.2% (95% CI: 0.1–0.3, p=0.002) (Figure 1(b)). The median follow-up was 3.0 years (IQR: 0.5–8.4). Retransplantation recipient and donor characteristics are summarized in Tables 1 and 2. Notably, autoimmune liver diseases and HCV were the most common underlying liver diagnoses (102 (27.1%) and 64 (17.0%), respectively). Almost a third of the recipients were in the ICU and intubated (Status 4 and 4F) preoperatively. The majority of donors were after declaration of brain death (336, 89.1%). The median distance to the donor procurement facility was 172.7 (IQR: 2.1–21.6) kilometers, and the median cold ischemia time was 443 (IQR: 327–572) minutes.
Figure 1.
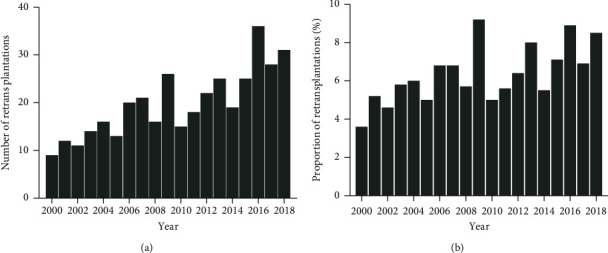
Number and proportion of retransplantations by year from 2000 to 2018.
Table 1.
Liver retransplant recipient characteristics.
| Overall | 2 transplants | 3 transplants | 4 transplants | |
|---|---|---|---|---|
| N = 377 | N = 340 (90.2%) | N = 34 (9.0%) | N = 3 (0.8%) | |
| Age at LT, years, median (IQR) | 52 (42–59) | 52 (44–59) | 43 (33–52) | 34 |
| Male, number (%) | 225 (59.7) | 203 (59.7) | 19 (55.9) | 3 (100.0) |
| Time from previous transplant, days, median (IQR) | 5 (10–809) | 47 (10–642) | 546 (43–1998) | 805 |
| Unknown (n) = 76 (20.2%) | ||||
| Creatinine at LT, μmol/L, median (IQR) | 104 (82–183) | 103 (80–186) | 113 (97–179) | 117 |
| Unknown (n) = 150 (39.8%) | ||||
| Serum bilirubin at LT, μmol/L, median (IQR) | 135.5 (52.8–409.5) | 135.5 (53.8–414.5) | 117.0 (32.0–370.0) | 277.0 |
| Unknown (n) = 151 (40.1%) | ||||
| INR at LT, median (IQR) | 1.6 (1.3–2.3) | 1.6 (1.3–2.4) | 1.6 (1.2–1.8) | 1.4 |
| Unknown (n) = 153 (40.6%) | ||||
| Blood type, number (%) | ||||
| A | 172 (45.6) | 153 (45.0) | 17 (50.0) | 2 (66.7) |
| AB | 17 (4.5) | 15 (4.4) | 1 (2.9) | 1 (33.3) |
| B | 58 (15.4) | 56 (16.5) | 2 (5.9) | 0 (0.0) |
| O | 129 (34.2) | 116 (34.1) | 13 (38.2) | 0 (0.0) |
| Unknown | 1 (0.3) | 0 (0.0) | 1 (2.9) | 0 (0.0) |
| Liver diagnosis, number (%) | ||||
| AIH/PSC/PBC | 102 (27.1) | 91 (26.8) | 10 (29.4) | 1 (33.3) |
| Alcoholic cirrhosis | 20 (5.3) | 20 (5.9) | 0 (0.0) | 0 (0.0) |
| Hepatocellular carcinoma | 21 (5.6) | 18 (5.3) | 3 (8.8) | 0 (0.0) |
| HBV | 21 (5.6) | 20 (5.9) | 1 (2.9) | 0 (0.0) |
| HCV | 64 (17.0) | 61 (17.9) | 3 (8.8) | 0 (0.0) |
| NASH | 10 (2.7) | 10 (2.9) | 0 (0.0) | 0 (0.0) |
| Cryptogenic | 62 (16.4) | 51 (15.0) | 10 (29.4) | 1 (33.3) |
| Other | 77 (20.4) | 69 (20.3) | 7 (20.6) | 1 (33.3) |
| Previous organ failure cause, number (%) | ||||
| Primary nonfunction | 31 (8.2) | 29 (8.5) | 2 (5.9) | 0 (0.0) |
| Portal vein thrombosis | 4 (1.1) | 4 (1.2) | 0 (0.0) | 0 (0.0) |
| Hepatic vein thrombosis | 18 (4.8) | 16 (4.7) | 2 (5.9) | 0 (0.0) |
| Hepatic artery thrombosis | 38 (10.1) | 36 (10.6) | 1 (2.9) | 1 (33.3) |
| Acute rejection | 4 (1.1) | 4 (1.2) | 0 (0.0) | 0 (0.0) |
| Chronic rejection | 11 (2.9) | 11 (3.2) | 0 (0.0) | 0 (0.0) |
| Recurrence of original disease | 23 (6.1) | 21 (6.2) | 2 (5.9) | 0 (0.0) |
| Other | 19 (5.0) | 18 (5.3) | 1 (2.9) | 0 (0.0) |
| Unknown/uncertain | 229 (60.8) | 201 (59.1) | 26 (76.5) | 2 (66.7) |
| BMI, kg/m2, median (IQR) | 24.9 (21.9–28.7) | 25.2 (22.2–29.0) | 23.9 (20.1–25.9) | 24.2 |
| Unknown (n) = 27 (7.2%) | ||||
| MELD score at LT, median (IQR) | 24 (17–31) | 24 (17–32) | 23 (16–27) | 23 |
| Unknown (n) = 154 (40.8%) | ||||
| Medical status, number (%) | ||||
| At home (1, 1T) | 74 (19.6) | 65 (19.1) | 8 (23.5) | 1 (33.3) |
| Hospitalized (2) | 75 (19.9) | 65 (19.1) | 10 (29.4) | 0 (0.0) |
| Hospitalized/ICU (3, 3F) | 44 (11.7) | 40 (11.8) | 3 (8.8) | 1 (33.3) |
| ICU/ventilated (4, 4F) | 124 (32.9) | 117 (34.4) | 6 (17.7) | 1 (33.3) |
| Unknown | 60 (16.0) | 53 (15.6) | 7 (20.6) | 0 (0.0) |
| HCV positive, number (%) | 54 (14.3) | 53 (15.6) | 1 (2.9) | 0 (0.0) |
| Unknown (n) = 139 (36.9%) | ||||
| CMV positive, number (%) | 182 (48.3) | 165 (48.5) | 17 (50.0) | 0 (0.0) |
| Unknown (n) = 116 (30.8%) | ||||
| CIT, minutes, median (IQR) | 443 (327–572) | 443 (329–572) | 456 (289–590) | 474 |
| Unknown (n) = 87 (23.1%) | ||||
LT, liver transplantation; IQR, interquartile range; INR, international normalized ratio; AIH, autoimmune hepatitis; PSC, primary sclerosing cholangitis; PBC, primary biliary cholangitis; HBV, hepatitis B virus; HCV, hepatitis C virus; NASH, nonalcoholic steatohepatitis; BMI, body mass index; MELD, model for end-stage liver disease; ICU, intensive care unit; CMV, cytomegalovirus; CIT, cold ischemia time.
Table 2.
Liver retransplant donor characteristics.
| Overall | 2 transplants | 3 transplants | 4 transplants | |
|---|---|---|---|---|
| N = 377 | N = 340 | N = 34 | N = 3 | |
| Age at death, years, median (IQR) | 41 (24–54) | 41 (23–54) | 43 (29–51) | 44 |
| Unknown (n) = 33 (8.8%) | ||||
| BMI, kg/m2, median (IQR) | 24.8 (22.0–27.7) | 25.0 (22.0–27.5) | 23.7 (22.0–28.2) | 30.1 |
| Unknown (n) = 52 (13.8%) | ||||
| Male sex, number (%) | 187 (49.6) | 171 (50.3) | 15 (44.1) | 1 (33.3) |
| Cause of death, number (%) | ||||
| Anoxia | 63 (16.7) | 57 (16.8) | 6 (17.6) | 0 (0.0) |
| Cerebrovascular accident | 138 (33.6) | 121 (35.6) | 15 (44.1) | 2 (66.7) |
| Trauma | 101 (26.9) | 94 (27.7) | 6 (17.6) | 1 (33.3) |
| Overdose | 9 (2.4) | 7 (2.1) | 2 (5.9) | 0 (0.0) |
| Unknown | 49 (13.0) | 47 (13.8) | 2 (5.9) | 0 (0.0) |
| Other | 17 (4.6) | 14 (4.1) | 3 (8.8) | 0 (0.0) |
| Ethnicity, number (%) | ||||
| Caucasian | 219 (58.1) | 194 (57.1) | 24 (70.6) | 1 (33.3) |
| Asian | 13 (3.4) | 11 (3.2) | 1 (2.9) | 1 (33.3) |
| Black | 7 (1.9) | 6 (1.8) | 1 (2.9) | 0 (0.0) |
| Other/unknown | 138 (36.6) | 129 (37.9) | 8 (23.6) | 1 (33.3) |
| Donor type, number (%) | ||||
| DCD | 8 (2.1) | 7 (2.1) | 1 (2.9) | 0 (0.0) |
| NDD | 336 (89.1) | 301 (88.5) | 32 (94.1) | 0 (0.0) |
| Unknown | 33 (8.8) | 32 (9.4) | 1 (2.9) | 3 (100.0) |
| CMV, number (%) | 153 (40.6) | 137 (40.3) | 14 (41.2) | 2 (66.7) |
| Unknown (n) = 75 (19.9%) | ||||
| Hepatitis C, number (%) | 4 (1.1) | 3 (0.9) | 1 (2.9) | 0 (0.0) |
| Unknown (n) = 66 (17.5%) | ||||
| Blood type, number (%) | ||||
| A | 124 (32.9) | 109 (32.1) | 14 (41.2) | 1 (33.3) |
| AB | 11 (2.9) | 9 (2.6) | 1 (2.9) | 1 (33.3) |
| B | 41 (10.9) | 39 (11.5) | 2 (5.9) | 0 (0.0) |
| O | 167 (44.3) | 150 (44.1) | 16 (47.1) | 1 (33.3) |
| Unknown | 34 (9.0) | 33 (9.7) | 1 (2.9) | 0 (0.0) |
| Distance from donor harvest to the facility of LT, km, median (IQR) | 172.7 (2.1–621.6) | 169.6 (2.0–672.3) | 273.8 (2.2–520.4) | 1,398.4 |
| Unknown (n) = 84 (22.3%) | ||||
IQR, interquartile range; BMI, body mass index; DCD, donor after cardiac death; NDD, neurological determination of death; CMV, cytomegalovirus; LT, liver transplantation.
With regard to the timing of transplantation, the median time between the initial and the second transplants was 47 (IQR: 10–642) days, the second and the third transplants was 546 (IQR: 43–1,998) days, and the third and the fourth transplants was 805 (IQR: NA) days. Retransplantation occurred within 30 days in 123/273 (45.1%) patients in the second transplant group and 5/28 (17.9%) patients in the RRT group.
In all patients with a retransplantation, the rate of graft failure was 7.9%, 12.5%, and 13.0% and patient survival was 77.1%, 65.6%, and 60.0% at 1, 5, and 10 years, respectively (Table 3 and Figure S1). Causes of graft failure and death in the retransplantation group are presented in Table S1. In contrast to recipients receiving their first graft transplant, the retransplantation group had a significantly higher incidence of graft failure (p < 0.001) and lower overall survival (p < 0.001) at 1, 5, and 10 years, respectively (Table 3 and Figure S1). For patients who survived beyond 30 days post‐transplantation, the patient survival differences remained between the primary transplantation group and the retransplantation group (Figure S1). Furthermore, there were no significant differences in graft failure and overall survival between the second transplant group and the RRT group (i.e., those receiving a third or fourth graft) (Figure 2). When the time from previous LT to retransplantation was assessed (0–7 days (n = 63, 20.9%), 7–30 days (n = 65, 21.6%), and >30 days (n = 173, 57.4%)), there were no significant differences in graft survival and overall survival.
Table 3.
Graft failure incidence and patient survival at various time points according to the number of transplantations.
| Graft failure incidence (%) (95%CI) | ||||
| 1 year | 3 year | 5 year | 10 year | |
| Primary LT | 2.9 (2.5–3.4) | 5.0 (4.4–5.6) | 6.4 (5.8–7.2) | 8.6 (7.8–9.5) |
| Retransplantation | 8.4 (5.7–11.7) | 11.0 (7.8–14.8) | 12.3 (8.9–16.3) | 12.8 (9.3–17.0) |
| RRT | 2.7 (0.2–12.3) | 10.2 (2.4–24.7) | 14.3 (4.3–30.2) | 14.3 (4.3–30.2) |
| All retransplantations | 7.9 (5.4–10.9) | 10.9 (7.9–14.5) | 12.5 (9.2–16.4) | 13.0 (9.6–16.9) |
|
| ||||
| Patient survival (%) (95%CI) | ||||
| 1 year | 3 year | 5 year | 10 year | |
| Primary LT | 91.4 (90.6–92.1) | 86.0 (85.0–86.9) | 81.8 (80.7–82.9) | 72.9 (71.4–74.3) |
| Retransplantation | 76.8 (71.8–81.0) | 70.5 (65.0–75.3) | 65.6 (59.8–70.8) | 60.1 (53.5–66.1) |
| RRT | 80.6 (63.6–90.3) | 69.5 (50.1–82.5) | 65.4 (45.4–79.5) | 59.9 (39.0–75.7) |
| All retransplantations | 77.1 (72.4–81.1) | 70.4 (65.2–74.9) | 65.6 (60.1–70.5) | 60.0 (53.7–65.7) |
LT, liver transplantation; RRT, repeat retransplantation.
Figure 2.
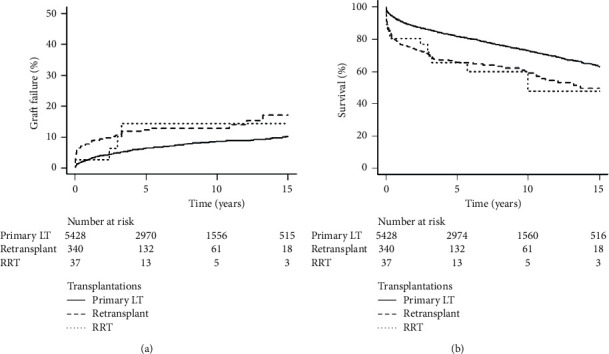
Incidence of graft failure (a) and overall survival (b) by the number of transplantations (LT: liver transplantation; RRT: repeat retransplantation).
There were temporal effects on post‐transplant overall survival in primary LT from the first era (2000–2010) to the second era (2011–2018) (HCV: p < 0.001, other etiologies: p=0.03) (Figure 3). For those retransplanted for HCV, post‐transplant overall survival was higher in those from the second era, yet no significant difference was detected, likely due to the small number of patients (p=0.15). For other etiologies, there were no statistically significant improvements in post‐transplant survival over time (p=0.38).
Figure 3.
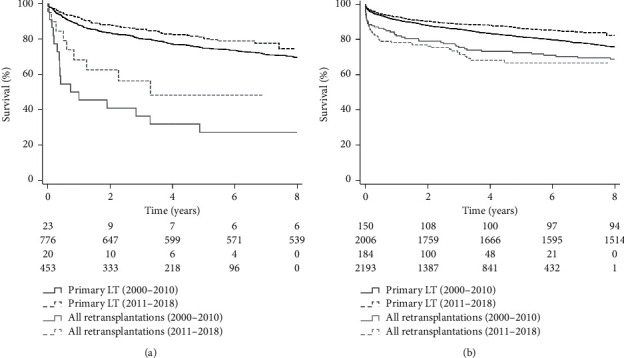
Temporal effects on post‐transplant overall survival (pre-DAA era: 2000–2010 vs. post-DAA era: 2011–2018) (DAA: direct-acting antiviral agents; LT: liver transplantation).
In the univariate analysis for graft failure, the following parameters were included in the backward stepwise model selection (p < 0.15): blood group, weight, donor CMV status, and recipient CMV status. In the overall survival model, the following parameters were included: recipient and donor age, recipient HCV and CMV status, medical status (ventilation), donor CMV status, DCD donor, and facility volume.
In multivariable analysis, donor CMV status was the only factor significantly associated with graft failure (HR = 3.03, 95%CI: 1.28–7.17, p=0.01, Table 4). In contrast, recipient age (5-year increase), donor age (5-year increase), and donor CMV status were associated with overall survival (Table 5). Older recipient and donor ages were associated with increased risk for death (recipient: HR = 1.12, 95%CI: 1.03–1.22, p=0.011; donor: HR = 1.09, 95%CI: 1.03–1.16, p=0.008). The association of donor CMV status with survival was not constant over time and therefore was evaluated as a time-dependent covariate. Donor positivity for CMV was only associated with an increased risk for death following three years after retransplantation (HR = 4.10, 95%CI: 1.50–11.25, p=0.006). Whether donor CMV and recipient CMV status were matched had no impact on post‐transplant outcomes. In univariate analysis of post‐transplant survival, the HR for CMV match was 1.13 (95%CI: 0.71–1.80, p=0.61) and 1.06 (95%CI: 0.66–1.68, p=0.83) in multivariable analysis. For graft survival, CMV match had an HR of 1.13 (95%CI: 0.49–2.57, p=0.78) and 0.91 (95%CI: 0.38–2.19, p=0.83) in univariate and multivariable analysis, respectively. Furthermore, there were no significant interactions between donor CMV status and recipient CMV status, suggesting that the effect of donor CMV on outcomes is consistent irrespective of recipient CMV status.
Table 4.
Multivariable analysis after backward stepwise selection of predictors for graft failure in retransplantation.
| Graft failure (n = 283) | |||
|---|---|---|---|
| HR | 95% CI | p value | |
| Recipient weight | 0.98 | 0.96–1.00 | 0.073 |
| Donor CMV-positive | 3.03 | 1.28–7.17 | 0.01 |
Table 5.
Multivariable analysis after backward stepwise selection of predictors for patient survival in retransplantation.
| Patient survival (n = 196) | Patient survival (n = 302) | |||||
|---|---|---|---|---|---|---|
| Including HCV status | Excluding HCV status | |||||
| HR | 95% CI | p value | HR | 95% CI | p value | |
| Recipient age (5-year increase) | — | — | — | 1.12 | 1.03–1.22 | 0.011 |
| Recipient HCV-positive | 1.81 | 1.06–3.08 | 0.014 | |||
| Donor age (5-year increase) | — | — | — | 1.09 | 1.03–1.16 | 0.008 |
| Donor CMV-positive (≤3 years) | 1.29 | 0.73–2.28 | 0.37 | 1.26 | 0.80–1.97 | 0.32 |
| Donor CMV-positive (>3 years) | 6.59 | 1.85–23.54 | 0.004 | 4.10 | 1.50–11.25 | 0.006 |
Given that the HCV status of recipients was available in a limited number of patients, a secondary model was created, which also included HCV status in the backward stepwise selection, in which it was demonstrated to be associated with overall survival (HR = 1.81, 95%CI: 1.06–3.08, p=0.014).
4. Discussion
Retransplantation remains the only salvage for patients experiencing graft failure, which would otherwise result in mortality. Yet, scrutiny surrounding the indications and debate on the utility and futility of liver retransplantation persists. This is the largest Canadian study of liver retransplantation, which was noted to be performed in 6.5% of all recipients. Our findings suggest that retransplantation is a valid treatment, with a 5-year graft failure and patient survival of 12.5% and 65.6%, respectively. This is true not only for early graft failure from vascular complications and acute rejection but also for late graft failure from disease recurrence and chronic rejection. RRT, although performed uncommonly, resulted in similar results compared to initial retransplantation in the appropriately selected recipient and should remain an option in the setting of recurrent graft failure.
Worldwide, the incidence of retransplantation ranges from 5.5% to 7% [12–14]. At 6.5%, the overall retransplantation rate in our Canadian cohort was similar. Temporally, we noted that the frequency of retransplantation has steadily increased in Canada from 9 cases in 2000 to 31 cases in 2018, with approximately 0.2% yearly increase in retransplantation rates. Similarly, Australia and New Zealand have also noticed an increase in retransplantation since 2001 [13]. Conversely, this has not been the case in Europe, where an analysis of the European Liver Transplant Registry (ELTR) from 2000–2009 revealed a 5% decrease in the utilization of retransplantation [14].
In our study, compared to initial transplantation, retransplantation was associated with a significantly lower patient and graft survival. Despite this, the results of our multicenter Canadian cohort were consistent with those from other countries, demonstrating an acceptable graft failure rate of 7.9%, 12.5%, and 13% with an overall survival of 77.1%, 65.6%, and 60% at 1, 5, and 10 years, respectively [13–17]. Notably, the most significant drop in survival was observed in the first year post‐transplantation. A study of the ELTR also noted this trend in which half of the deaths and three-quarters of graft failure for European retransplantation occurred within a year [14].
Multiple earlier studies have addressed the interval from previous transplantation as an essential predictor for outcome [3, 15, 17, 18]. These studies state that early retransplantation (<30 days) often has worse outcomes than late retransplantation. In our study, early versus late retransplantation did not have a significant impact on graft failure and overall survival. However, the prevalence of early graft failure differed according to the number of grafts transplanted. Specifically, of recipients with a second graft, 45.1% (n = 123) had this within 30 days of their primary transplantation. In contrast, only 17.9% (n = 5) of the RRT group had their subsequent transplantation within 30 days.
Given its rarity, minimal data on RRT are available in the current literature. In a single-center review of RRT, the overall incidence was noted to be 2.1% and was performed most commonly for chronic rejection (33%), arterial thrombosis (28%), or primary nonfunction (17%) [19]. In these patients, 90-day mortality for RRT was 18% compared to 15% in those receiving a first retransplant [19]. Notably, of the eight patients receiving a fourth graft, 90-day mortality was 50% [19]. Clinically relevant independent risk factors for 90-day mortality in the RRT group were extrahepatic sepsis and the need for vasopressor support at the time of retransplantation [19]. By performing a nationwide analysis, we were able to perform a subgroup analysis on recipients of RRT. Our RRT rate was 0.6%, similar to the reported incidences of 0.5 to 2.1% from Australia, New Zealand, France, and the USA [13, 19, 20]. Concerning outcomes, Canadian patients with RRT had an acceptable 1-, 5-, and 10-year graft failure and patient survival of 2.7%, 14.3%, and 14.3% and 80.6%, 65.4%, and 59.9%, respectively. This was similar to those receiving a second graft. Thus, RRT remains an option for recurrent graft failure. However, careful selection of patients to achieve the best results remains necessary, given the lack of significant long-term survival benefit in this group of recipients [15, 16, 21].
Over the years, there has been an effort in the literature to identify independent predictors of patient and graft survival after liver retransplantation. Recipient age, creatinine, bilirubin, ventilatory status, MELD, United Network for Organ Sharing (UNOS) status, urgency of retransplantation, cold ischemia time, and causes of graft failure have been noted to be significant variables [16, 18, 22, 23]. Our Canadian cohort study adds to the heterogeneity in the literature as it demonstrated recipient age, donor age, and donor CMV status to be independent predictors of overall survival. Donor age has been confirmed as a predictor of prognosis for retransplantation [21]. Marudanayagam et al. showed that donor age <55 years (OR:1.02, 95%CI: 1.00–1.04, p=0.036) correlates with lower mortality along with MELD score <23 (OR:1.103, 95%CI: 1.00–1.06, p=0.029) in their single-center series [21]. This was confirmed in our study, in which older recipients and patients who received grafts from an older donor had worse outcomes. CMV is a common viral pathogen that can adversely affect liver transplant recipients and is associated with CMV syndrome, tissue-invasive diseases, an increased predisposition to rejection and mortality, accelerated HCV recurrence, and other opportunistic infections [24]. Although there is a lack of specific evidence regarding the correlation between CMV status and liver retransplantation outcome, its impact can be inferred from studies on primary liver transplants. Donor and recipient CMV-positive status have been shown to adversely affect recipient survival in primary liver transplantation (RR:4.6, 95%CI: 1.9–10.7, p < 0.001) [25]. CMV-positive patients who received a liver transplant are also more prone to have graft failure than CMV-negative patients [26]. Our Canadian series has demonstrated its negative impact on graft failure (HR: 3.03, 95%CI: 1.28–7.17, p=0.01) and patient survival following 3 years post‐retransplantation (HR: 4.10, 95%CI: 1.50–11.25, p=0.006). Contrastingly, CMV match status between the donor and the recipient had no impact on post‐transplantation outcomes in addition to donor CMV status, as shown in our multivariable analysis of post‐transplant outcomes.
In the current study, HCV was the second most common underlying liver diagnosis (64, 16.9%) and associated with increased patient mortality (HR: 1.81, 95%CI: 1.06–3.08, p=0.014). Historically, HCV positivity as an indicator for worse outcomes in retransplantation has been controversial. Rosen et al. identified HCV infection as an independent risk factor for death after retransplantation (RR: 1.36, p=0.0038) [27]. Watt et al. showed through analysis of UNOS data on 2,129 retransplantation patients that HCV patients had no different survival compared to retransplantation for HBV and autoimmune hepatitis [28]. This was further supported by this group's subsequent study, where 1- and 3-year survival rates after retransplantation were not significantly different for HCV and non‐HCV groups (69 vs. 73% and 49 vs. 55%, respectively) [29]. These data are changing with the increased use of DAA (after 2010) to manage this underlying etiology of end-stage liver disease. This temporal effect was observed in our post‐transplant outcomes in primary LT from the first era (2000–2010) to the second era (2011–2018). Primary LT patients for HCV in the second era had superior overall survival than patients in the first era (p < 0.001). However, for those retransplanted for HCV, the difference in post‐transplant survival was only observed without statistical significance likely due to the small number of patients (p=0.15).
This study is limited in that we do not address death on the waitlist in patients listed for retransplantation. Additionally, selection bias cannot be accounted for given the multicenter design of this study and the lack of insight into donor and recipient matching considerations. Furthermore, inherent to the dataset, the analysis was limited by each center's accuracy and completeness of data entered into the national registry.
5. Conclusion
This report represents the largest analysis of liver retransplantation in Canada. It demonstrates that this is a feasible and safe treatment for early and late liver graft failure. Furthermore, even in patients requiring RRT, with careful selection, similar outcomes to initial retransplantation can be achieved.
Abbreviations
- CI:
Confidence interval
- CIHI:
Canadian Institute for Health Information
- CORR:
Canadian Organ Replacement Register
- CMV:
Cytomegalovirus
- ELTR:
European Liver Transplant Registry
- HCV:
Hepatitis C virus
- HR:
Hazard ratio
- ICU:
Intensive care unit
- INR:
International normalized ratio
- IQR:
Interquartile range
- LT:
Liver transplantation
- MELD:
Model for end-stage liver disease
- NA:
Not applicable
- OR:
Odds ratio
- RR:
Relative ratio
- RRT:
Repeat retransplantation
- UNOS:
United Network for Organ Sharing.
Data Availability
Our study population included all adult (≥18 years) liver retransplant recipients and their matched donors registered on the Canadian Organ Replacement Register (CORR) from 1st January 2000 to 31st December 2018. CORR and the centers participating in the CORR are the source of the data used herein; they have not verified and are not responsible for the statistical validity of the data analysis of the conclusions derived by the authors.
Disclosure
The research was performed in association with Toronto General Hospital, Toronto, Canada, and University of Toronto, Toronto, Canada.
Conflicts of Interest
The authors declare that there are no conflicts of interest regarding the publication of this article.
Supplementary Materials
Table S1. Cause of graft failure and death in patients with retransplantation. Supplementary Figure 1. Incidence of graft failure and patient survival from the time of transplantation (A) and from 30 days after transplantation (B), according to retransplantation status (LT: liver transplantation).
References
- 1.Starzl T. E., Iwatsuki S., Van Thiel D. H., et al. Evolution of liver transplantation. Hepatology . 1982;2(5):614–636. doi: 10.1002/hep.1840020516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ghobrial R. M., Farmer D. G., Baquerizo A., et al. Orthotopic liver transplantation for hepatitis C. Annals of Surgery . 1999;229(6):824–831. doi: 10.1097/00000658-199906000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Markmann J. F., Markowitz J. S., Yersiz H., et al. Long-term survival after retransplantation of the liver. Annals of Surgery . 1997;226(4):408–420. doi: 10.1097/00000658-199710000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Magee J. C., Barr M. L., Basadonna G. P., et al. Repeat organ transplantation in the United States, 1996-2005. American Journal of Transplantation: Official Journal of the American Society of Transplantation and the American Society of Transplant Surgeons . 2007;7(5 Pt 2):1424–1433. doi: 10.1111/j.1600-6143.2007.01786.x. [DOI] [PubMed] [Google Scholar]
- 5.Lang H., Sotiropoulos G. C., Beckebaum S., et al. Incidence of liver retransplantation and its effect on patient survival. Transplantation Proceedings . 2008;40(9):3201–3203. doi: 10.1016/j.transproceed.2008.09.039. [DOI] [PubMed] [Google Scholar]
- 6.Pfitzmann R., Benscheidt B., Langrehr J. M., Schumacher G., Neuhaus R., Neuhaus P. Trends and experiences in liver retransplantation over 15 years. Liver Transplantation . 2007;13(2):248–257. doi: 10.1002/lt.20904. [DOI] [PubMed] [Google Scholar]
- 7.Croome K. P., Mathur A. K., Pungpapong S., et al. Equivalent outcomes with retransplantation and primary liver transplantation in the direct-acting antiviral era. Transplantation . 2019;103(6):1168–1174. doi: 10.1097/tp.0000000000002460. [DOI] [PubMed] [Google Scholar]
- 8.D’Alessandro A. M., Ploeg R. J., Knechtle S. J., et al. Retransplantation of the liver—a seven-year experience. Transplantation . 1993;55(5):1083–1087. doi: 10.1097/00007890-199305000-00028. [DOI] [PubMed] [Google Scholar]
- 9.Reed A., Howard R. J., Fujita S., et al. Liver retransplantation: a single-center outcome and financial analysis. Transplantation Proceedings . 2005;37(2):1161–1163. doi: 10.1016/j.transproceed.2004.11.046. [DOI] [PubMed] [Google Scholar]
- 10.Scrucca L., Santucci A., Aversa F. Competing risk analysis using R: an easy guide for clinicians. Bone Marrow Transplantation . 2007;40(4):381–387. doi: 10.1038/sj.bmt.1705727. [DOI] [PubMed] [Google Scholar]
- 11.Fine J. P., Gray R. J. A proportional hazards model for the subdistribution of a competing risk. Journal of the American Statistical Association . 1999;94(446):496–509. doi: 10.1080/01621459.1999.10474144. [DOI] [Google Scholar]
- 12.Rana A., Petrowsky H., Kaplan B., et al. Early liver retransplantation in adults. Transplant International . 2014;27(2):141–151. doi: 10.1111/tri.12201. [DOI] [PubMed] [Google Scholar]
- 13.Jeffrey A. W., Delriviere L., McCaughan G., et al. Excellent contemporary graft survival for adult liver retransplantation: an Australian and New Zealand registry analysis from 1986 to 2017. Transplantation Direct . 2019;5(8):p. e472. doi: 10.1097/txd.0000000000000920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Adam R., Karam V., Delvart V., et al. Evolution of indications and results of liver transplantation in Europe. A report from the European Liver Transplant Registry (ELTR) Journal of Hepatology . 2012;57(3):675–688. doi: 10.1016/j.jhep.2012.04.015. [DOI] [PubMed] [Google Scholar]
- 15.Busuttil R. W., Farmer D. G., Yersiz H., et al. Analysis of long-term outcomes of 3200 liver transplantations over two decades. Annals of Surgery . 2005;241(6):905–918. doi: 10.1097/01.sla.0000164077.77912.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Azoulay D., Linhares M. M., Huguet E., et al. Decision for retransplantation of the liver. Annals of Surgery . 2002;236(6):713–721. doi: 10.1097/00000658-200212000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Maggi U., Andorno E., Rossi G., et al. Liver retransplantation in adults: the largest multicenter Italian study. PLoS One . 2012;7(10) doi: 10.1371/journal.pone.0046643.e46643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Doyle H. R., Morelli F., McMichael J., et al. Hepatic retransplantation-an analysis of risk factors associated with Outcome1. Transplantation . 1996;61(10):1499–1505. doi: 10.1097/00007890-199605270-00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Memeo R., Laurenzi A., Pittau G., et al. Repeat liver retransplantation: rationale and outcomes. Clinical Transplantation . 2016;30(3):312–319. doi: 10.1111/ctr.12691. [DOI] [PubMed] [Google Scholar]
- 20.Akpinar E., Selvaggi G., Levi D., et al. Liver retransplantation of more than two grafts for recurrent failure. Transplantation . 2009;88(7):884–890. doi: 10.1097/tp.0b013e3181b6f20e. [DOI] [PubMed] [Google Scholar]
- 21.Marudanayagam R., Shanmugam V., Sandhu B., et al. Liver retransplantation in adults: a single-centre, 25-year experience. International Hepato-Pancreato-Biliary Association . 2010;12(3):217–224. doi: 10.1111/j.1477-2574.2010.00162.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rosen H. R., Madden J. P., Martin P. A model to predict survival following liver retransplantation. Hepatology . 1999;29(2):365–370. doi: 10.1002/hep.510290221. [DOI] [PubMed] [Google Scholar]
- 23.Rosen H., Prieto M., Casanovas-Taltavull T., Cuervas-Mons V., Guckelberger O., Muiesan P. Validation and refinement of survival models for liver retransplantation. Hepatology . 2003;38(2):460–469. doi: 10.1053/jhep.2003.50328. [DOI] [PubMed] [Google Scholar]
- 24.Razonable R.-R. Cytomegalovirus infection after liver transplantation: current concepts and challenges. World Journal of Gastroenterology . 2008;14(31):4849–4860. doi: 10.3748/wjg.14.4849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Falagas M. E., Snydman D. R., Griffith J., Ruthazer R., Werner B. G. Effect of cytomegalovirus infection status on first-year mortality rates among orthotopic liver transplant recipients. Annals of Internal Medicine . 1997;126(4):275–279. doi: 10.7326/0003-4819-126-4-199702150-00003. [DOI] [PubMed] [Google Scholar]
- 26.Burak K., Kremers W. K., Batts K. P., Wiesner R. H., Rosen C. B., Razonable R. R. Impact of cytomegalovirus infection, year of transplantation, and donor age on outcomes after liver transplantation for hepatitis C. Liver Transplantation . 2002;8(4):362–369. doi: 10.1053/jlts.2002.32282. [DOI] [PubMed] [Google Scholar]
- 27.Rosen H. R., Martin P. Hepatitis C infection in patients undergoing liver Retransplantation1,2. Transplantation . 1998;66(12):1612–1616. doi: 10.1097/00007890-199812270-00007. [DOI] [PubMed] [Google Scholar]
- 28.Watt K., Lyden E. R., McCashland T. M. Poor survival after liver retransplantation: is hepatitis C to blame? Liver Transplantation . 2003;9(10):1019–1024. doi: 10.1053/jlts.2003.50206. [DOI] [PubMed] [Google Scholar]
- 29.McCashland T., Watt K., Lyden E., et al. Retransplantation for hepatitis C: results of a U.S. multicenter retransplant study. Liver Transplantation . 2007;13(9):1246–1253. doi: 10.1002/lt.21322. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Cause of graft failure and death in patients with retransplantation. Supplementary Figure 1. Incidence of graft failure and patient survival from the time of transplantation (A) and from 30 days after transplantation (B), according to retransplantation status (LT: liver transplantation).
Data Availability Statement
Our study population included all adult (≥18 years) liver retransplant recipients and their matched donors registered on the Canadian Organ Replacement Register (CORR) from 1st January 2000 to 31st December 2018. CORR and the centers participating in the CORR are the source of the data used herein; they have not verified and are not responsible for the statistical validity of the data analysis of the conclusions derived by the authors.


