Abstract
A model of ascending unobstructed urinary tract infection (UTI) in mice was developed to study the significance of the antibiotic concentration in urine, serum, and kidney tissue for efficacy of treatment of UTI in general and pyelonephritis in particular. Outbred Ssc-CF1 female mice were used throughout the study, and Escherichia coli was used as the pathogen. The virulence of 11 uropathogenic E. coli isolates and 1 nonpathogenic laboratory E. coli strain was examined. Strain C175-94 achieved the highest counts in the kidneys, and this strain was subsequently used as the infecting organism. The model gave reproducible bladder infections, i.e., bacteria were recovered from 22 of 23 control mice after 3 days, and histological examination of kidney tissue showed that of 14 infected kidneys, 7 (50%) showed major histological changes, whereas 3 of 36 uninfected kidneys showed major histological changes (P = 0.018). Once the model was established, the efficacies of different doses of cefuroxime and gentamicin, corresponding to active concentrations in urine only or in urine, serum, and kidney tissue simultaneously, were examined. All cefuroxime doses resulted in significantly lower counts in urine than control treatments, but the dose which produced concentrations of cefuroxime only in urine and not in serum or kidney tissue had no effect on kidney infection. Even low doses of gentamicin (0.05 mg/mouse) resulted in concentrations in renal tissue for prolonged times due to accumulation. All gentamicin doses had a significant effect (compared to the effect of the control treatment) on bacterial counts in urine and kidneys. The antibiotic effect on bacterial counts in bladders was negligible for unknown reasons. Use of the mouse UTI model is feasible for study of the effect of an antibiotic in the urinary system, although the missing antibacterial effect in the bladder needs further evaluation.
Urinary tract infections (UTIs) are among the most commonly observed infections in clinical practice, and more than 25% of all women experience an UTI at least once during their lifetimes (6). The majority of UTIs are far from severe; i.e., they are not life-threatening and do not produce any irreversible damage. However, when the kidneys are involved in the infection, there is a risk of irreversible kidney tissue damage as well as an increased risk of bacteremia (6).
Escherichia coli is the causative agent in about 85% of community-acquired UTIs, 50% of nosocomial UTIs, and more than 80% of cases of uncomplicated pyelonephritis (6, 35). Several virulence factors among uropathogenic strains of E. coli have been recognized, including different types of adhesins, serum resistance, iron sequestration, and hemolysin production (17, 22, 30, 31).
Treatments for cystitis are well documented, especially different strategies concerning short-term treatment with a number of antibiotics (15, 21, 28), while the documentation concerning the duration of treatment of pyelonephritis is poor (1, 6, 27), with the failure rate for the treatment of pyelonephritis being approximately 30% (5). The importance of a sufficient antibiotic concentration in the kidneys, especially in the medulla, has been emphasized by several investigators (6, 8, 25). It is characteristic of antibiotics used for the treatment of UTIs that they have up to a 100-fold higher concentration in urine than the simultaneous concentrations in serum, but the significance of antibiotic levels in urine, serum, and kidney tissue for the efficacy of treatment of UTI has not been thoroughly evaluated. For several years, models of ascending unobstructed UTIs in mice have been used to study the factors that enable bacteria to colonize the urinary tract (12–14, 18–20, 33). Mice are chosen for these infection models because they seem to be sensitive to many of the same bacterial properties which cause UTI in humans, and like humans, they do not have a natural vesicoureteral reflux like other rodents do (16). In the literature, the development of animal models for investigation of the virulence factors possessed by bacteria known to be uropathogenic for humans is commonly described; however, aspects of treatment are less frequently dealt with. Here we describe the use of a murine model of infection that resembles the natural course of ascending UTI that causes pyelonephritis for the study of aspects of antibiotic treatment, i.e., antibiotic efficacy and course of disease.
MATERIALS AND METHODS
Bacteria.
The E. coli strains used in the study are described in Table 1. The 11 strains denoted C were isolated from patients with UTIs. Strain D2103-4 is a nonvirulent laboratory strain.
TABLE 1.
Twelve E. coli strains tested for pathogenicity for kidneys
| SSI no.a | Serotype and virulence factors | Origin | No. of infected kidneys/total no. | Range of no. of CFU/kidney | Median no. of CFU/kidney |
|---|---|---|---|---|---|
| C175-94 | O8:K48:H9, type 1 fimbriae | Fecesb | 5/6 | 0–3.4 × 107 | 1.8 × 105 |
| C165-94 | O2:K13:H1, type 1 fimbriae, hly+d | Fecesc | 4/6 | 0–3.2 × 106 | 3.0 × 104 |
| C760-85 | O1:K1:H7, type 1 fimbriae | Unknown | 4/6 | 0–6.6 × 106 | 2.2 × 104 |
| C711-92 | O18ac:K5:H− | Bloode | 5/6 | 0–3.6 × 105 | 7.8 × 103 |
| C190-92 | O15:K52:H1, P fimbriae | Blood | 6/10 | 0–2.2 × 105 | 3.5 × 103 |
| C153-94 | O1:K1:H−, P fimbriae, hly+ | Cervixf | 4/6 | 0–6.2 × 103 | 5.1 × 102 |
| C263-93 | O2:K2:H1, hly+ | Urine | 3/6 | 0–1.1 × 105 | 40 |
| C178-94 | O2:K1:H6, P fimbriae, hly+ | Urine | 3/6 | 0–1.2 × 106 | 0 |
| C561-93 | O2:K1:H7 | Urine | 2/6 | 0–3.8 × 104 | 0 |
| C309-94 | O6:K2:H1, hly+ | Urineg | 3/10 | 0–7.4 × 104 | 0 |
| C236-94 | O12:K1:H7 | Unknown | 1/6 | 0–1.5 × 106 | 0 |
| D2103-4 | Orough:H− | Laboratory | 0/10 | 0 | 0 |
| Control | PBS | 0/6 | 0 | 0 |
The strains denoted C were isolated from patients with UTIs. Strain D2103-4 is a nonvirulent laboratory strain.
Isolated from feces after a febrile UTI in a male.
Isolated from feces during a febrile UTI in a male.
hly+, hemolysin positive.
Isolated from a patient with bacteremia caused by a UTI.
Isolated from the asymptomatic female partner at the time of febrile UTI in a male.
Isolated from a boy with relapse of cystitis.
Preparation of inoculum.
The bacteria used to infect the mice were grown in filter-sterilized human urine and were passaged three times as described by Sharma et al. (33). The bacteria were incubated at 37°C, shaken at 200 rounds/min overnight, and centrifuged at 6,500 × g for 10 min. The pellet was then suspended in phosphate-buffered saline (PBS) to a concentration of approximately 1010 CFU/ml.
Animals.
Outbred female albino mice (Ssc-CF1 mice; weight, 30 ± 2 g) were used. The mice were housed six to a cage, were allowed a minimum of 24 h of acclimatization prior to inoculation, and at all times were allowed free access to chow and water.
Inoculation procedure.
The mice were anesthetized by intraperitoneal administration of 0.08 ml of a mixture of Hypnorm (fentanyl citrate, 0.315 mg/ml; fluanisone, 10 mg/ml) and Stesolid (diazepam, 5 mg/ml) at a ratio of 5:1.5.
Anesthetized mice were inoculated transurethrally with the bacterial suspension by use of plastic catheters. The catheters were made from tubing (autoclavable; inner diameter, 0.50 mm; outer diameter, 0.63 mm; reference no. 800-200-100-100; Portex Limited Non Sterile Tubing). Two-centimeter pieces of tubing were melted carefully over a gas flame so that the ends closed and became smooth. With a heated needle a hole was made just below the melted end. The tip had to be perfectly smooth so that it did not cause tissue injury during inoculation, and if necessary, the tip was reheated to melt off any irregularities. The pieces of tubing were placed on hypodermic needles (25 gauge by 5/8 in.) and autoclaved.
Through a microscope with ×10 enlargement the urethral orifice was localized, the catheter was carefully pushed in horizontally until it reached the top of the bladder, and 0.05 ml of bacterial suspension was injected in the bladder over 5 s in order to avoid vesicoureteral reflux (12, 18). The catheter was removed immediately after inoculation.
Specimens sampled for bacterial count determinations.
Before the mice were killed, urine from each mouse was collected in Eppendorf tubes by gentle compression of the abdomen, and the mice were killed by cervical dislocation. The organs were removed aseptically, the bladders were cut off near the urethra, and the kidneys were removed by blunt dissection to avoid bleeding. The organs were placed in cryotubes (Nunc 363452) containing a 750-μl suspension of collagenase (500 U/ml; Sigma C9891) and were stored at −80°C. Prior to homogenization, the infected organs were incubated for 1.5 h at room temperature and were then homogenized manually with inoculating loops and a whirl mixer. The numbers of bacteria recovered manually were found to be similar to those recovered by automatic homogenization (Kinematic Polytron PT3000 tissue homogenizer with a PTDA3007/2 knife) (data not shown). The manual procedure was preferred, since it was less time-consuming than the automatic method. Viable counts were performed on lactose bromthymol blue agar plates (Statens Serum Institut [SSI] 694).
Bacterial tests.
Bacteria from the inoculum, bacteria that were recovered from the urine samples and that had a phenotypic resemblance to the inoculated bacteria, and bacteria from either the bladder or one of the kidneys were characterized for specific O and K antigens, P and type 1 fimbriae, and hemolysin production.
The O and K antigens were detected by agglutination as described by Ørskov and Ørskov (29). The presence of P and type 1 fimbriae was shown by hemagglutination with either guinea pig or human erythrocytes with and without mannose as described previously (11).
The presence of hemolysin was illustrated by subculturing the strains on 10% horse blood agar plates (SSI 686). Positive reactions were interpreted as a transparent zone around the colonies.
Specimens sampled for correlation of kidney infection and histological changes.
Kidneys were collected, divided sagitally, and weighed. One half was placed in 0.5 ml of 10% formaldehyde solution (pH 7.4) and stained with hematoxylin and eosin. Histological examination was performed by the same person (N.C.) without knowledge of the culture results. Noninfected kidneys were blindly included as controls. Criteria for positive histology included accumulation of leukocytes or other inflammatory cells in the stroma of the cortex or the medulla and the presence of increased amounts of leukocytes in the tubules or the collecting ducts. The histological changes, when present, were always patchy in distribution. Viable counts were determined with the other half.
Antibiotics, dosage, and sampling.
The antibiotics used were cefuroxime (Zinacef; Glaxo), which was dissolved in PBS, and gentamicin (gentamicin sulfate; Sigma), which was dissolved in distilled water. The largest doses corresponded to the doses used for humans as related to surface area. The surface area of a mouse is considered to be 65 cm2 (7), and that of a human is considered to be 1.73 m2 (standard) (9). This resulted in the largest cefuroxime dosage being 5 mg/mouse given twice 8 h apart and the largest gentamicin dosage being 1 mg/mouse given once. The gentamicin dosage corresponds to approximately twice the human dosage, and it was chosen in order to achieve a high peak concentration, which has been found to be more effective (10) and less toxic than the same total amount of drug given repetitively (38). The same total amounts of both drugs were given by repetitive administration of smaller dosages, i.e., 2 mg of cefuroxime five times at 2-h intervals and 0.2 mg of gentamicin five times at 2-h intervals. Also, dosages that were intended to produce suboptimal concentrations in the kidney and urine were administered, i.e., 0.1 mg of cefuroxime given five times at 2-h intervals and 0.05 mg of gentamicin given five times at 2-h intervals. PBS was administered as a control five times at 2-h intervals. Mice were given injections of 0.25 ml of antibiotic solution subcutaneously in the neck with hypodermic needles (22 gauge by .25 in.), and subsequent injections were given farther down the back.
Cefuroxime concentrations in serum and kidney tissue versus time were determined for groups of three mice each that were given injections of 5, 2, and 0.1 mg of cefuroxime and that were killed 15, 30, 45, 60, 90, and 120 min after the injection. The concentrations in urine were determined for groups of five mice each that were given injections of 5, 1, and 0.1 mg of cefuroxime and that were killed 0.5, 1, 2, 3, 4, 5, 6, and 8 h after the injection.
Gentamicin concentrations in serum versus time were determined for groups of five mice each that were given injections of 1, 0.2, and 0.05 mg of gentamicin and that were killed 15, 30, 45, 60, 90, and 150 min after the injection. Concentrations in urine and kidney tissue were determined for groups of five mice each that were given injections of the same gentamicin doses and that were killed 0.25, 0.5, 1.5, 2.5, 4, 6, 12, and 24 h after the injection. Urine collected at the time of death was used to estimate the amount of drug eliminated in urine since fractional collection of urine proved to be difficult. Antibiotic concentrations in kidney tissue were derived from homogenates of whole organs.
Antibiotic concentration determinations.
Drug concentrations in urine, serum, and kidney tissue were determined by the agar disc diffusion method. The coefficients of variation for the bioassay controls for both antibiotics ranged from 4 to 8%. Blood was sampled by ocular cut-down after anesthetizing the mice with CO2. The blood was centrifuged at 2,000 × g for 15 min, and the supernatant (serum) was transferred to Eppendorf tubes. Kidneys sampled for estimation of antibiotic concentrations were homogenized with the Kinematic homogenizer.
The bacteria used in the assay were the cefuroxime-sensitive organism Sarcina lutea ATCC 9441 and the gentamicin-sensitive organism Staphylococcus haemolyticus P903 grown on Mueller-Hinton agar (SSI 24243). Standard curves of five threefold dilutions starting at 100 μg/ml were prepared in mouse serum for determination of the concentrations in serum and kidney tissue and in PBS for determination of the concentrations in urine. For cefuroxime the coefficients of variation for the highest standard concentration (100 μg/ml) were 6.6% in serum and 7.2% in PBS. The coefficients of variation for the highest standard concentration of gentamicin (100 μg/ml) in serum and PBS were 5.8 and 8.5%, respectively.
MICs.
The MICs were determined by the E-test (Biodisk, Stockholm, Sweden), whereas the minimum bactericidal concentration was not determined.
Statistical methods.
Two-by-two tables were used for statistical evaluation of the correlation of infected kidneys and histological changes. Fischer's exact test was used for values <50, and the χ2 test was used for values ≥50.
The number of mice needed in each group for treatment was calculated by using the binomial scale. The type 1 error was set to 5%, the type 2 error was set to 20%, the expected effect rate in control groups was set to 50%, and the minimal difference between rates not to be overlooked was set to 40%. With these conditions 24 mice in each group would be necessary. The Mann-Whitney U test was used for statistical evaluation, and parameters for antibiotic efficacy were chosen as the numbers of bacteria (CFU) recovered from the specimens. The efficacy of each regimen was compared to those of the other regimens with the same drug, to that of the control treatment, and to the corresponding regimen with the other drug. P values less than 0.05 were considered significant.
RESULTS
Testing different E. coli clinical isolates in the UTI model.
In the search for an infecting organism with a high degree of uropathogenicity, the infective abilities of 12 E. coli strains were examined in the mouse model (Table 1). Each strain was inoculated into three mice. After 3 days the mice were killed and their kidneys were collected.
As seen in Table 1, strain C175-94, serotype O8:K48:H9, which expresses type 1 fimbriae, produced the largest numbers of CFU per kidney, and the strain was subsequently used as the infecting organism.
The MICs for strain C175-94 were 3.0 μg/ml for cefuroxime and 0.75 μg/ml for gentamicin.
Course of infection.
The uropathogenicity of strain C175-94 in the mouse model was compared with that of the nonpathogenic laboratory strain D2103-4 by monitoring the course of infection with regard to the number of CFU at different times after inoculation and examining the histological changes in kidneys from inoculated animals.
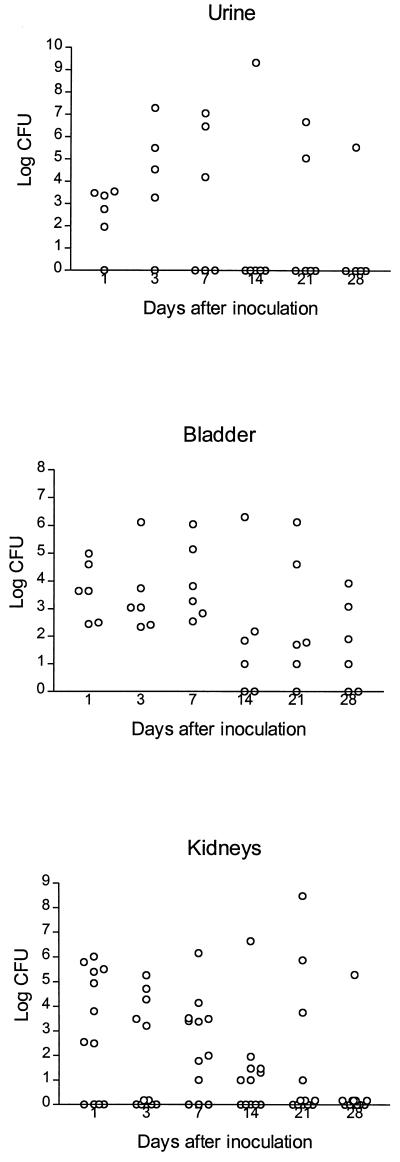
The number of bacteria recovered from mice killed at 1, 3, 7, 14, 21, and 28 days after inoculation with strain C175-94 are shown in Fig. 1. Seven days after inoculation, all mice had positive bladder cultures, and after 28 days, bacteria were recovered from bladders from four of six animals. The number of bacteria recovered from the kidneys decreased throughout the period, but after 14 days more than half the kidney cultures were still positive. In contrast, strain D2103-4 was eliminated rapidly from the urinary tract, with no kidney cultures being positive 7 days after inoculation (data not shown). In approximately half the mice with pyelonephritis both kidneys were found to be infected during the period. When both kidneys were infected, the numbers of bacteria recovered from the two kidneys were generally similar, and in the majority of mice less than a 10-fold difference in the numbers of CFU was observed.
FIG. 1.
Bacterial counts in urine, bladder, and kidneys of mice killed at different times after inoculation with C175-94. At each time of killing, specimens were obtained from six mice. The number of urine samples at some time points was less than six due to unsuccessful urine sample collection.
For the uropathogenic strain E. coli C175-94 there was a significant correlation between the presence of bacteria in cultures of renal tissue and histological changes (Table 2).
TABLE 2.
Results of histological examination and bacterial culture of kidneysa
| Bacterial recovery from renal tissue | No. of kidneys with major histological changes
|
||
|---|---|---|---|
| Yes | No | Total | |
| Yes | 7b | 7 | 14 |
| No | 3 | 23 | 26 |
| Total | 10 | 30 | 40 |
Transected kidneys, with one half used for histologic comparison and the other half processed for quantitative bacteriology. Kidneys from noninfected mice were included as controls.
Number of mice; 7 of 7 versus 3 of 23 (P = 0.018).
Concentrations of cefuroxime and gentamicin.
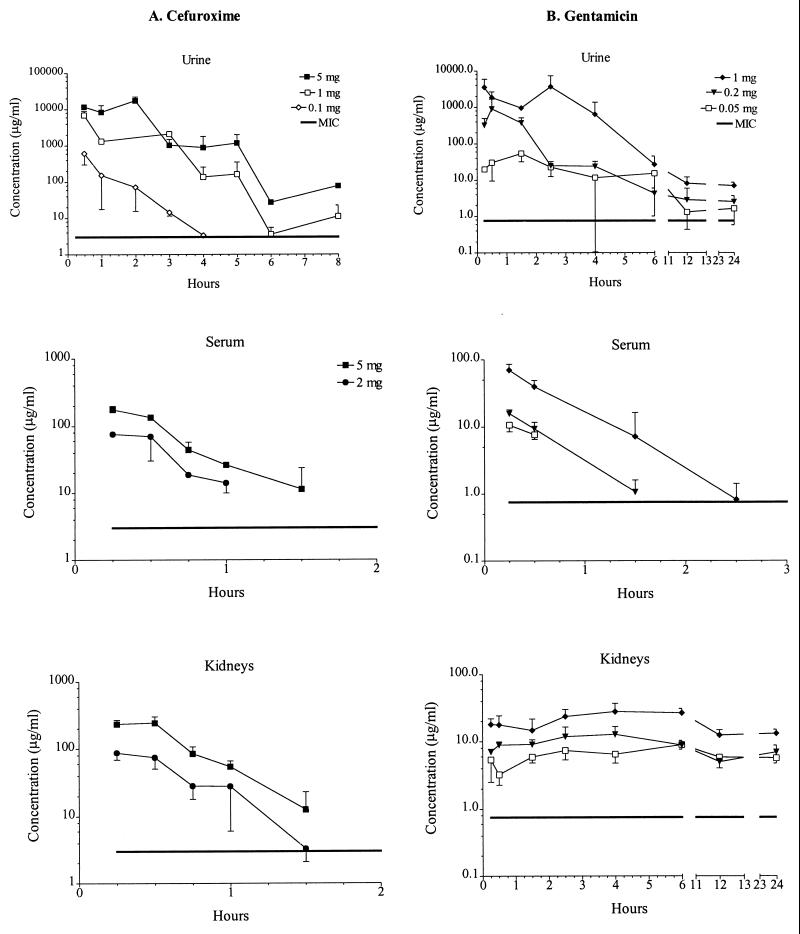
The curves of the log concentration versus time are shown in Fig. 2. Cefuroxime was present at similar concentrations in the serum and kidneys, and peak concentrations in urine were approximately 100-fold larger than those in the serum and kidneys (Fig. 2A). The 5- and the 1-mg doses were detectable in urine for at least 8 h, the 0.1-mg dose was detectable in urine for 4 h, and the 5- and 2-mg doses were detectable in serum and kidneys for 1.5 h, whereas the 0.1-mg dose was undetectable in either type of sample. Peak concentrations in serum and kidneys were found in the first samples drawn, i.e., those obtained 15 min after administration, for the 5- and 2-mg doses. Peak concentrations in urine occurred 2 h after administration of the 5-mg dose and 30 min after administration of the 1- and 0.1-mg doses.
FIG. 2.
Concentrations of cefuroxime (A) and gentamicin (B) in urine, serum, and kidneys following subcutaneous administration of the stated doses. At each time of killing, specimens were obtained from three or five mice, and the average drug concentrations in the specimens are plotted against times of sampling.
Peak gentamicin concentrations in urine were approximately 100-fold larger than those in serum and kidney, but from 12 to 24 h after injection, concentrations in the urine and kidneys were alike (Fig. 2B). All gentamicin doses were detectable in the urine and kidneys for at least 24 h, whereas in serum the 1-mg dose was detectable for 2.5 h, the 0.2-mg dose was detectable for 1.5 h, and the 0.05-mg dose was detectable for 30 min. Peak concentrations in serum and kidneys were found in the first samples drawn, i.e., those obtained 15 min after administration, for all doses. Peak concentrations in urine were seen 2.5 h after administration of the 1-mg dose, 30 min after administration of the 0.2-mg dose, and 1.5 h after administration of the 0.05-mg dose. Table 3 shows the peak concentration above the MIC and the area under the curve (AUC) above the MIC for all doses, when it could be measured and calculated, for the two drugs in serum, kidney, and urine. There was a strong correlation between peak concentration and AUCs at the three doses for both drugs and sites. Therefore, it was impossible to differentiate between the different parameters and effect.
TABLE 3.
Peak concentration above the MIC and AUC above the MIC for the two drugs in serum, renal tissue, and urine
| Drug (dose [mg/mouse]) | Peak concn above MIC (mg/liter)
|
AUC above the MIC (mg/liter/h)
|
||||
|---|---|---|---|---|---|---|
| Serum | Kidney | Urine | Serum | Kidney | Urine | |
| Gentamicin | ||||||
| 1 | 69.1 | 27.0 | 3,493.1 | 38.6 | 396.2 | 8,477.5 |
| 0.2 | 15.0 | 12.0 | 898.6 | 7.0 | 168.7 | 1,099.5 |
| 0.05 | 8.1 | 8.1 | 52.5 | 1.9 | 145.6 | 200.5 |
| Cefuroxime | ||||||
| 5 | 172.0 | 238.9 | 11,588.0 | 126.1 | 126.1 | 29,912.0 |
| 1 | 72.2 | 172.0 | 6,050.5 | 39.5 | 39.5 | 9,277.0 |
| 0.1 | 607.6 | 326.8 | ||||
Significance of concentration in urine, serum, and kidney tissue for effect on treatment for UTI.
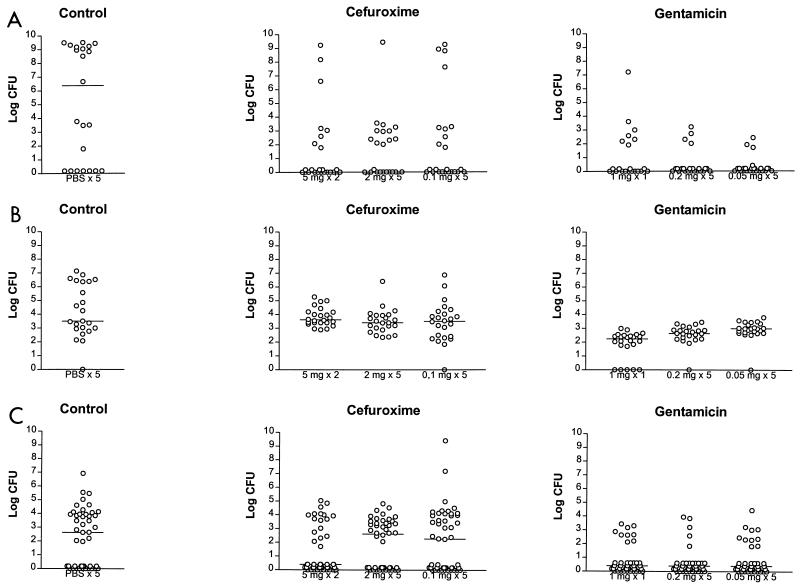
Antibiotic treatment was given 3 days after inoculation. Mice were killed in groups of 24 the day after treatment as well as 10 days later. Bacterial counts on the day after treatment for mice treated with cefuroxime or gentamicin are shown in Fig. 3.
FIG. 3.
Bacterial counts recovered from urine (A), bladder (B), and kidneys (C) of mice inoculated with C175-94 and treated with cefuroxime or gentamicin. Twenty-four mice were treated with one of the stated dosage regimens and were killed 1 day after treatment. The number of urine samples at some time points was less than 24 due to unsuccessful urine sample collection. The horizontal line represents the median bacterial count.
On the day after treatment with cefuroxime, significantly fewer bacteria were found in kidneys from mice from the 5-mg group, i.e., mice treated twice with 5 mg of cefuroxime, than in kidneys from mice in the control group, i.e., mice treated five times with PBS (P = 0.015) (Fig. 3C). Significantly fewer bacteria were found in the kidneys from the 5-mg group than in mice treated five times with 2 mg (P = 0.007) and five times with 0.1 mg (P = 0.003) (Fig. 3C). The numbers of bacteria found in the kidneys of the 2- and 0.1-mg groups were not different from those found in the kidneys of the control group (for the 2-mg group, P = 0.32; for the 0.1-mg group, P = 0.67) (Fig. 3C). The bladders of all groups had similar numbers of bacteria (Fig. 3B). Significantly lower bacterial counts were found in the urine of treated groups than in the urine of the control group (for the 5-mg group, P = 0.004; for the 2-mg group, P = 0.013; for the 0.1-mg group, P = 0.014) (Fig. 3A), but no differences in bacterial counts were found between the treated groups. Ten days after treatment no differences in the numbers of bacteria were found in either the kidneys, bladder, or urine between any of the groups.
On the day after treatment with gentamicin the numbers of bacteria in kidneys from mice from all three dosing groups, i.e., mice treated with 1 mg once, 0.2 mg five times, or 0.05 mg five times, were significantly lower than the numbers found in the kidneys from mice from the PBS-treated control group (P = 0.0001, P = 0.00005, and P = 0.0003, respectively) (Fig. 3C). No differences in the numbers of bacteria in kidneys between any of the gentamicin-treated groups were found. The bladders of the 1-mg group had significantly fewer bacteria than the bladders of the control group (P < 0.000005) and those of the 0.2-mg (P = 0.002) and 0.05-mg (P = 0.000001) groups. The number of bacteria per bladder in the 0.2-mg group was significantly lower than those for the control group (P = 0.0002) and the 0.05-mg group (P = 0.014), and the bladders of the 0.05-mg group also had significantly fewer bacteria than those of the control group (P = 0.0003) (Fig. 3B). Significant differences in the number of bacteria per milliliter of urine were found for all three treatment groups compared to those for the control group (P = 0.0013, P = 0.0002, and P = 0.00002, respectively) (Fig. 3A). However, there were no differences in counts in urine between the three gentamicin dosing groups. Ten days after treatment, no differences in the number of bacteria in urine and kidneys were found between any of the groups. The numbers of bacteria in bladders were significantly lower for the 0.2- and 0.05-mg groups than for the control group (for the 0.2-mg group, P = 0.007; for the 0.05-mg group, P = 0.0002) and for the 0.2-mg group than for the 0.05-mg group (P = 0.021).
The efficacies of the largest doses (5 mg of cefuroxime twice and 1 mg of gentamicin once) of the two antibiotics were compared, as were the efficacies of the medium (2 mg of cefuroxime five times and 0.2 mg of gentamicin five times) and the smallest (0.1 mg of cefuroxime five times and 0.05 mg of gentamicin once) doses. On the day after treatment, mice that received any of the gentamicin regimens, with two exceptions, had significantly fewer bacteria in all specimens than mice that received the cefuroxime regimens. The exceptions were the colony counts in urine in mice treated with the largest doses and the colony counts in kidneys in mice treated with the medium doses. Comparisons 10 days after treatment showed a similar pattern, but the exceptions were colony counts in urine and colony counts in kidneys in mice treated with the largest doses.
DISCUSSION
The significant correlation between positive kidney cultures and histopathological findings indicated that the model produced a true infection rather than a mere colonization of the kidney tissue. The histological changes were patchy in distribution, which may explain why bacteria were cultured from some kidneys in which no changes were detected. The course of the untreated infection with the virulent strain (strain C175-94) showed increases in bacterial numbers in the urine, bladder, and kidneys until day 7 after inoculation, after which the infection tended to clear by itself; counts in the bladders were still high, however, even after 28 days postinoculation. This course of UTI follows closely that shown by Hagberg et al. (11–14). The fact that the infection subsides after 3 to 4 weeks sets the limits for antibiotic treatment to within the first 2 weeks.
Cefuroxime had high concentrations in urine compared to those in serum; i.e., concentrations in urine were approximately 100-fold higher than those in serum. This result correlates to the findings for humans (34). It has previously been reported that cephalosporins produced higher or similar concentration levels in renal tissue compared to the simultaneous levels in serum, corresponding to our findings of slightly higher concentrations in renal tissue compared to those in serum (4). The antibiotic concentrations found in kidney tissue were based on whole organs; i.e., the concentration consists of a blend of the concentrations in serum, urine, lymph tissue, and interstitial liquid immersed in 500 μl of PBS. Maigaard et al. (23) showed that the trimethoprim and sulfadizine concentrations in renal lymph tissue and interstitial fluid were lower than those in plasma, which is presumably also the case for cefuroxime and gentamicin. Furthermore, it was reported that the antibiotic concentration in urine reflects the concentration in the collecting ducts but not the concentration in the interstitial fluid of the medulla (4). Since the urine that is produced is led from the kidneys, the concentrations in kidney tissue obtained in our model presumably reflect the concentrations in serum rather than the concentrations in urine.
Gentamicin accumulated in the kidneys and was detectable in urine for a prolonged period. This finding is due to the accumulation of gentamicin in kidney tubular cells, and since the accumulation causes lysis of the cells, the drug will be present in urine for a period corresponding to the turnover rate of lysis (32). This accumulation is also found in humans (34). In healthy dogs it was found that aminoglycosides reached higher concentrations in renal tissue than the simultaneous levels in serum (39); however, our findings showed higher initial levels in serum than in kidneys.
The duration of treatment in this study was chosen to be short for two reasons. First, short-duration treatment (1 to 3 days) is commonly used for the treatment of UTI in humans in order to improve compliance and reduce side effects, and second, shorter-duration regimens were considered necessary for studying the differences in the effects of the individual dosing regimens.
None of the antibiotic dosage regimens given were able to sterilize the urinary tracts of the infected mice. However, 1 day after treatment, specimens from mice treated with 5 mg of cefuroxime twice and 1 mg of gentamicin once generally had lower bacterial counts than specimens from mice treated with the other regimens. Specifically, 5 mg of cefuroxime given twice was significantly better than the control treatment and the two other dosing regimens at eradicating bacteria from the kidneys; i.e., the high concentrations of antibiotic in urine alone without measurable concentrations in renal tissue were unable to clear the infection from the kidneys. The effect on the number of bacteria in the kidneys following administration of the largest dosage regimen (5 mg given twice) indicated that the concentration in the kidney tissue is of importance. This finding correlates with the findings of others (3, 6, 8, 36, 37). The effect of gentamicin on eradicating bacteria from the kidneys compared to the effect of the control treatment was similar for the three dosing regimens, also indicating that concentrations in tissue are important for achieving an effect. The similarities of the effects of all gentamicin dosage regimens, including 0.05 mg given five times, could be due to accumulation in kidney tubular cells, followed by excretion through lysis of the cells (32), thereby prolonging the antibacterial effect.
The efficacy of gentamicin was found to be superior to that of cefuroxime, which is likely to be explained partly by its more rapid bactericidal activity as well as its continuing excretion from the kidneys. From clinical trials on the treatment of UTI with antibiotics which yield high concentrations in urine but concentrations in tissue insufficient to be active, it was deduced that cure was dependent on the drug concentration in urine and was independent of the drug concentration in serum (24, 36, 37). Our data seem to contradict those observations, at least when renal infection is concerned.
The in vivo activities of antibiotics can be influenced by the patient's state of hydration and the pH of the patient's urine, and the mere presence of infection may jeopardize the penetration of antibiotics into tissue as well as modify the intrarenal pharmacokinetics of the drugs (2, 4, 6, 39).
The bacterial counts in the bladder were surprisingly little affected by the high concentrations of either drug in urine. Perhaps a longer duration of treatment would be more effective, which we pursued in the present investigation.
Our findings suggest that the ascending UTI model in mice is useful for study of the effect of antibiotics in vivo against UTIs involving the kidneys, since it is possible to evaluate the antibiotic concentrations in various tissue or fluid compartments. Furthermore, the results of our study indicate that the use of antibiotics that achieve adequate concentrations in the kidney tissue is necessary for the effective treatment of pyelonephritis. Furthermore, we show that despite high concentrations in urine, bacteria were able to survive in bladder tissue. Very recently it was showed by scanning electron microscopy that the bacteria are engulfed in the bladder wall and are not killed by treatment with gentamicin ex vivo (26). Further experiments will be designed to reveal the mechanism of bacterial survival in the bladder during antibiotic treatment.
ACKNOWLEDGMENTS
We thank Jytte Mark Andersen and Jakob Vang, SSI, for excellent technical assistance.
C.S. was partly supported by a fellowship from Plasmidfondet.
REFERENCES
- 1.Andriole V T. Urinary tract infections in the 90s: pathogenesis and management. Infection. 1992;20:251–256. doi: 10.1007/BF01710009. [DOI] [PubMed] [Google Scholar]
- 2.Auclair P, Lessard C, Bergeron M G. Renal pharmacokinetic changes of gentamicin during enterococcal pyelonephritis. Antimicrob Agents Chemother. 1988;32:736–739. doi: 10.1128/aac.32.5.736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bergeron G, Marois Y. Benefit from high intrarenal levels of gentamicin in the treatment of Escherichia coli pyelonephritis. Kidney Int. 1986;30:481–487. doi: 10.1038/ki.1986.211. [DOI] [PubMed] [Google Scholar]
- 4.Bergeron M G. A review of models for the therapy of experimental infections. Scand J Infect Dis. 1978;14(Suppl.):189–206. [PubMed] [Google Scholar]
- 5.Bergeron M G. Current concepts in treatment of pyelonephritis. Intern Med. 1989;10:65–84. [Google Scholar]
- 6.Bergeron M G. Treatment of pyelonephritis in adults. Med Clin N Am. 1995;75:619–649. doi: 10.1016/s0025-7125(16)30060-8. [DOI] [PubMed] [Google Scholar]
- 7.Diack S L. The determination of the surface area of the white rat. J Nutr. 1930;3:289–296. [Google Scholar]
- 8.Francois P. Traitement des infections de l'appareil urinaire. Ann Pediatr. 1991;38:557–562. [PubMed] [Google Scholar]
- 9.Frimodt-Møller N, Sebbesen O, Thomsen V F. The pneumococcus and the mouse protection test: importance of the lag phase in vivo. Chemotherapy (Basel) 1983;29:128–134. doi: 10.1159/000238186. [DOI] [PubMed] [Google Scholar]
- 10.Gerber A U. Comparison of once-daily versus thrice-daily human equivalent dosing of aminoglycosides: basic considerations and experimental approach. J Drug Dev. 1988;1:17–24. [Google Scholar]
- 11.Hagberg L, Jodal U, Korhonen T K, Lidin-Janson G, Lindberg U, Svandborg-Edén C. Adhesion, hemagglutination and virulence of Escherichia coli causing urinary tract infections. Infect Immun. 1981;31:564–570. doi: 10.1128/iai.31.2.564-570.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hagberg L, Engberg I, Freter R, Lam J, Olling S, Svanborg-Edén C. Ascending, unobstructed urinary tract infection in mice caused by pyelonephritogenic Escherichia coli of human origin. Infect Immun. 1983;40:273–283. doi: 10.1128/iai.40.1.273-283.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hagberg L, Hull R, Hull S, Falkow S, Freter R, Svanborg Edén C. Contribution of adhesion to bacterial persistence in the mouse urinary tract. Infect Immun. 1983;40:265–272. doi: 10.1128/iai.40.1.265-272.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hagberg L, Hull R, Hull S, McGhee R, Michalek M, Svanborg Edén C. Difference in susceptibility to gram-negative urinary tract infection between C3H/HeJ and C3H/HeN mice. Infect Immun. 1984;46:839–844. doi: 10.1128/iai.46.3.839-844.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hooton T M, Stamm W E. Management of acute uncomplicated urinary tract infection in adults. Med Clin N Am. 1991;75:339–357. doi: 10.1016/s0025-7125(16)30458-8. [DOI] [PubMed] [Google Scholar]
- 16.Johnson D E, Russell R G, Warren J W. Animal models of urinary tract infections. In: Mobley H T L, Warren J W, editors. Urinary tract infections: molecular pathogenesis and clinical management. Washington, D.C.: ASM Press; 1996. pp. 377–405. [Google Scholar]
- 17.Johnson J R. Virulence factors in Escherichia coli urinary tract infection. Clin Microbiol Rev. 1991;4:80–128. doi: 10.1128/cmr.4.1.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Johnson J R, Manivel J C. Vesicoureteral reflux induces renal trauma in a mouse model of ascending, unobstructed pyelonephritis. J Urol. 1991;145:1306–1311. doi: 10.1016/s0022-5347(17)38620-2. [DOI] [PubMed] [Google Scholar]
- 19.Johnson J R, Berggren T, Manivel J C. Histopathologic-microbiologic correlates of invasiveness in a mouse model of ascending unobstructed urinary tract infection. J Infect Dis. 1992;165:299–305. doi: 10.1093/infdis/165.2.299. [DOI] [PubMed] [Google Scholar]
- 20.Johnson J R, Berggren T, Newburg D S, McCluer R H, Manivel J C. Detailed histopathological examination contributes to the assessment of Escherichia coli urovirulence. J Urol. 1992;147:1160–1166. doi: 10.1016/s0022-5347(17)37507-9. [DOI] [PubMed] [Google Scholar]
- 21.Kunin C M. Duration of treatment of urinary tract infections. Am J Med. 1981;71:849–854. doi: 10.1016/0002-9343(81)90383-1. [DOI] [PubMed] [Google Scholar]
- 22.Mabeck C E, Ørskov F, Ørskov I. Escherichia coli and renal involvement in urinary tract infection. Lancet. 1971;i:1312–1314. doi: 10.1016/s0140-6736(71)91884-8. [DOI] [PubMed] [Google Scholar]
- 23.Maigaard S, Frimodt-Møller N, Naber K G, Madsen P O. Renal lymph and interstitial fluid concentration of co-trimazine: an experimental study in dogs. Infection. 1979;7:349–353. doi: 10.1007/BF01639012. [DOI] [PubMed] [Google Scholar]
- 24.McCabe W R, Jackson G G. Treatment of pyelonephritis. N Engl J Med. 1965;272:1037–1044. doi: 10.1056/NEJM196505202722002. [DOI] [PubMed] [Google Scholar]
- 25.Meyrier A, Guibert J. Diagnosis and drug treatment of acute pyelonephritis. Drugs. 1992;44:356–367. doi: 10.2165/00003495-199244030-00005. [DOI] [PubMed] [Google Scholar]
- 26.Mulvey M A, Lopez-Boado Y S, Wilson C L, Roth R, Parks W C, Heuser J, Hultgren S J. Induction and evasion of host defenses by type 1-piliated uropathogenic Escherichia coli. Science. 1998;282:1494–1497. doi: 10.1126/science.282.5393.1494. [DOI] [PubMed] [Google Scholar]
- 27.Naber K G. Optimal management of uncomplicated and complicated urinary tract infections. Med Microbiol Lett. 1996;5:372–380. [Google Scholar]
- 28.Norrby S R. Short-term treatment of uncomplicated lower urinary tract infections in women. Rev Infect Dis. 1990;12:458–467. doi: 10.1093/clinids/12.3.458. [DOI] [PubMed] [Google Scholar]
- 29.Ørskov F, Ørskov I. Serotyping of Escherichia coli. Methods Microbiol. 1984;14:43–112. [Google Scholar]
- 30.Ørskov I, Ørskov F. Escherichia coli in extra-intestinal infections. J Hyg Camb. 1985;95:551–575. doi: 10.1017/s0022172400060678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sandberg T, Kaijser B, Linid-Janson G, Lincoln K, Ørskov F, Ørskov I, Stokland E, Svanborg-Edén C. Virulence and Escherichia coli in relation to host factors in women with symptomatic urinary tract infection. J Clin Microbiol. 1988;26:1471–1476. doi: 10.1128/jcm.26.8.1471-1476.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schentag J J. Aminoglycoside pharmacokinetics as a guide to therapy and toxicology: renal tubular transport and intrarenal aminoglycoside distribution. 1982. pp. 143–168. and 191–222. In A. Whelton and C. N. Harols (ed.), The aminoglycosides. Microbiology, clinical use and toxicology. Marcel Dekker Inc., New York, N.Y. [Google Scholar]
- 33.Sharma S, Harjai K, Mittal R. Enhanced siderophore production and mouse kidney pathogenicity in Escherichia coli grown in urine. J Med Microbiol. 1991;35:325–329. doi: 10.1099/00222615-35-6-325. [DOI] [PubMed] [Google Scholar]
- 34.Slack R C B. Urinary tract infections. In: Lambert H P, O'Grady W, editors. Antibiotics and chemotherapy. 6th ed. London, United Kingdom: Churchill Livingstone; 1992. pp. 407–415. [Google Scholar]
- 35.Sobel J D. Bacterial etiologic agents in the pathogenesis of urinary tract infections. Med Clin N Am. 1991;75:253–273. doi: 10.1016/s0025-7125(16)30452-7. [DOI] [PubMed] [Google Scholar]
- 36.Stamey T A, Govan D E, Palmer J M. The localization and treatment of urinary tract infections: the role of bactericidal urine levels as opposed to serum levels. Medicine. 1965;44:1–36. doi: 10.1097/00005792-196501000-00001. [DOI] [PubMed] [Google Scholar]
- 37.Stamey T A, Fair W R, Timothy M M, Millar M A, Mihara G, Lowery Y G. Serum versus urinary antimicrobial concentrations in cure of urinary-tract infections. N Engl J Med. 1974;291:1159–1163. doi: 10.1056/NEJM197411282912204. [DOI] [PubMed] [Google Scholar]
- 38.Tulkens P M, Clercq-Braun F, Donnez J, Ibrahim J, Kállay Z, Delmee M, et al. Safety and efficacy of aminoglycosides once-a-day: experimental data and randomized, controlled evaluation in patients suffering from pelvic inflammatory disease. J Drug Dev. 1988;1:71–83. [Google Scholar]
- 39.Whelton A, Walker W G. Intrarenal antibiotic distribution in health and diseases. Kidney Int. 1974;6:131–137. doi: 10.1038/ki.1974.91. [DOI] [PubMed] [Google Scholar]