Abstract
Point-of-care ultrasound (POCUS) refers to the use of portable ultrasound (US) applications at the bedside, performed directly by the treating physician, for either diagnostic or procedure guidance purposes. It is being rapidly adopted by traditionally non-imaging medical specialties across the globe. Recent international evidence-based guidelines on POCUS for critically ill neonates and children were issued by the POCUS Working Group of the European Society of Pediatric and Neonatal Intensive Care (ESPNIC). Currently there are no standardized national or international guidelines for its implementation into clinical practice or even the training curriculum to monitor quality assurance. Further, there are no definitions or methods of POCUS competency measurement across its varied clinical applications.
Conclusion: The Hippocratic Oath suggests medical providers do no harm to their patients. In our continued quest to uphold this value, providers seeking solutions to clinical problems must often weigh the benefit of an intervention with the risk of harm to the patient. Technologies to guide diagnosis and medical management present unique considerations when assessing possible risk to the patient. Frequently risk extends beyond the patient and impacts providers and the institutions in which they practice. POCUS is an emerging technology increasingly incorporated in the care of children across varied clinical specialties. Concerns have been raised by clinical colleagues and regulatory agencies regarding appropriate POCUS use and oversight. We present a framework for assessing the risk of POCUS use in pediatrics and suggest methods of mitigating risk to optimize safety and outcomes for patients, providers, and institutions.
| What is Known: |
|---|
| • The use POCUS by traditionally non-imaging pediatric specialty physicians for both diagnostic and procedural guidance is rapidly increasing. |
| • Although there are international guidelines for its indications, currently there is no standardized guidance on its implementation in clinical practice. |
| What is New: |
| • Although standards for pediatric specialty-specific POCUS curriculum and training to competency have not been defined, POCUS is likely to be most successfully incorporated in clinical care when programmatic infrastructural elements are present. |
| • Risk assessment is a forward-thinking process and requires an imprecise calculus that integrates considerations of the technology, the provider, and the context in which medical care is delivered. Medicolegal considerations vary across countries and frequently change, requiring providers and institutions to understand local regulatory requirements and legal frameworks to mitigate the potential risks of POCUS. |
Keywords: Point-of-care ultrasound (POCUS), Risk assessment, Framework, Neonates, Children
Introduction
The 2020 publication of international point-of-care (POCUS) guidelines for neonatal and critical care providers identified numerous clinical POCUS applications supported by both graded level of evidence and expert opinion [1]. These guidelines build upon increasing data suggesting that, across pediatric disciplines, ultrasound imaging improves patient safety and provider procedural performance [2–9], introduces new clinical data [10, 11], expedites and changes management [12–15], and may improve outcomes [16–18]. Alongside robust adult data supporting POCUS use by clinicians in non-traditional imaging specialties, the question of why do we use POCUS continues to be asked and answered across clinical applications.
Although POCUS has experienced significant growth in clinical use by traditionally non-imaging based specialties, little time has been spent asking (or answering) the question “how” do we integrate POCUS in our practice? Quality healthcare ensures the delivery of safe and effective care, the value of which is assessed in the context of cost. Risk potentially impacts both the delivery of quality care and cost to providers, patients, and institutions. Frameworks for risk assessment exist across varied professional endeavors. A leading group within the National Health Service (NHS) in England recently published a risk assessment framework (RAF) to standardize methods of prospectively evaluating risks associated with clinical practice [19]. Importantly, the authors suggest that the RAF “should be tailored to the specific needs of the assessment” and is “not intended to be exhaustive.” Thus, the RAF provides a flexible and wholistic approach to identify, analyze, evaluate, and manage risk in the clinical setting (Table 1). This manuscript seeks to integrate concerns, experiences, and opinions from the literature as well as from our diverse international co-authorship within the RAF framework to assess pediatric POCUS risk.
Table 1.
Risk assessment framework for point-of-care ultrasound
| Risk assessment step | Considerations |
|---|---|
| Identify |
• Describe the system including elements and interactions • Define undesired outcomes including patient, provider and institution • Identify potential contributory factors to undesired outcomes • Describe potential consequences |
| Analyze |
• What controls are in place to identify and prevent undesired outcomes • Assess the severity and likelihood of undesired outcomes • Identify risk level |
| Evaluate |
• Describe the risk tolerability • Identify ineffective or non-existent controls to mitigate risk • Define required actions and plan on methods of communicating results |
| Manage |
• Develop a multidisciplinary group of experts to address risk and manage activities • Review data and develop analytic techniques for prospective risk assessment |
Kaya GK, Ward JR, Clarkson PJ. A framework to support risk assessment in hospitals. Int J Qual Health Care. 2019;31:393–401
POCUS risk: current concerns
In 2020, the European Society of Paediatric Radiology (ESPR) published a position paper on the use of POCUS by non-radiology performers. In this position paper, multiple concerns were raised regarding translation of this technology to new practice environments. Current training platforms were described as a “gimmick,” and the practice itself is suggested to result in missed diagnosis, delayed therapeutics and increased costs for families and institutions [20]. Similarly, in 2020 the Emergency Care Research Institute (ECRI), a nonprofit organization designated as an Evidence-based Practice Center in the USA providing guidance for US healthcare regulatory agencies such the Agency for Healthcare Research and Quality (AHRQ) and the Joint Commission, identified POCUS as number 2 among the top 10 greatest technology hazards within healthcare. The ECRI stated that “safeguards for ensuring that POCUS users have the requisite training, experience, and skill have not kept pace with the speed of adoption. The lack of sufficient oversight increases the potential that patients will be adversely affected by problems associated with use, or lack of use, of the technology.”[21] Thus safety commissions, regulatory agencies, as well as our very own colleagues suggest that POCUS places providers, patients, and institutions at varied risks of adverse outcomes.
The POCUS community response to these statements has been swift and pointed. Representatives from the European Society of Emergency Pediatrics (ESEP), the Ultrasound Section of the European Society for Emergency Medicine (EUSEM), and the Pediatric Emergency Medicine Point-of Care Ultrasound (P2) “respectfully disagree with the conclusions [of the ESPR statement], especially the need for further oversight from our radiology colleagues.”[22] While the ESPR position statement recommends a well thought-out curriculum for each clinical specialty wishing to perform POCUS and lists European credentialing/certification methods for undergraduates, general radiology training, and radiology subspecialization, it should be noted that these are not requirements for licensing and performing pediatric ultrasound as a radiologist in Europe [20]. The “need for credentialing non-radiologists who want to become involved in non-radiologist point-of-care [ultrasound]” should be balanced by what is expected of radiologists themselves [23]. Similarly, a perspective published by adult and pediatric POCUS experts suggest that “if these statements [by the ECRI] are used to guide the governance of POCUS use in pediatric intensive care units (PICUs), the resulting policies may be overly restrictive of a practice that actually has several potential benefits” and that, within the ECRI report, “no objective data were presented” as a basis for concerns [24].
Three publications review POCUS litigation, none of which found medicolegal cases from the use of POCUS [25–27]. In fact, the only cases related to POCUS arose from its lack of use when the technology was available. Assessing medicolegal risk, though, is a forward-thinking process to prevent harm, whether to patient, provider, or institution. Therefore, components within a conceptual POCUS RAF may not be evidence-based and specific contributors to risk (and the weight of their contribution) will be heavily influenced by the local practice setting.
POCUS risk: identify
The identify phase defines the process being evaluated and the system in which the process is being performed. In this phase we must assess not only the safety of ultrasound technology itself but also how it is used by varied clinicians. In research terms, this might be referred to as assessing the efficacy of the technology and the effectiveness of its use when employed by providers. The Curie brothers discovered piezoelectricity over 140 years ago, and for over 80 years, the medical field has utilized ultrasound in clinical practice [28]. Ultrasound is also conceptually familiar to people outside of the medical profession, whether through knowledge of music, understanding methods of echolocation used by animals, or exposure to fetal assessment in pregnancy. Among technologies physicians incorporate at the bedside, there is likely a comfort with ultrasound that is shared between providers and patients.
Ultrasound is frequently cited as possessing a desirable safety profile since it does not utilize ionizing radiation for image acquisition. Risk assessment requires us to assess aspects of the technology that may result in harm. There are, indeed, safety concerns associated with the use of ultrasound including thermal bioeffects and inertial and non-inertial cavitation [29–32]. Neonates and pediatric patients, especially extremely premature infants, theoretically may be at greater risk of this non-thermal injury phenomena compared to adults, but no studies have been published demonstrating actual risk in human beings. The physical machine itself may also expose patients to unnecessary harm if not maintained appropriately. Ultrasound probes may be exposed to contaminated body fluids and present a risk of cross-transmission of pathogenic organisms [33, 34]. Finally, like any device, age and maintenance may impact machine capabilities. Aging transducer crystals and reduced processing speeds may result in diminished quality images [35]. Other electrical devices within clinical care may create artifacts within images on an ultrasound machine [36]. Thus, even the influence of the environment on the machine could result in suboptimal images for procedures and diagnostics.
The greatest risk regarding ultrasound use is from the users themselves. While literature does not identify prior litigation resulting from POCUS studies, we must acknowledge the potential for error and harm. From a procedural standpoint, ultrasound use has robust data supporting improved pediatric provider performance and patient safety and for almost 20 years has been promoted as standard of care for central vascular access by the National Institute for Health and Care Excellence [37]. For pediatric specialists performing invasive procedures, the greatest risk is likely not learning and employing ultrasound during these procedures.
Diagnostic POCUS carries the risk of misdiagnosis or missed diagnosis within the scope of clinical care. However, such a risk cannot be viewed solely specific to ultrasound. In fact, almost no clinical exam findings, serologic studies, or imaging modalities have 100% etiologic and/or pathophysiologic sensitivity and specificity. Our physical exam is fraught with inaccuracies, inconsistencies, and misleading data driving therapeutics and outcomes. The stethoscope, having over 200 years of integration within the practice of medicine, cannot always be trusted regardless of experience [38]. How we define risk requires contextualization within current practice and an appreciation for its benefit within pediatric and neonatal clinical care.
POCUS risk: analyze
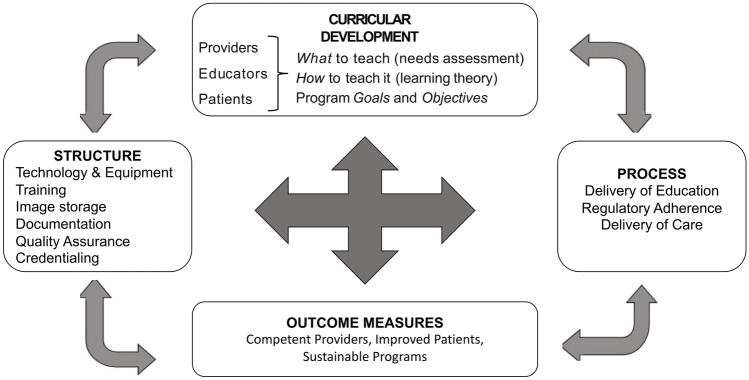
This leads us to the analyze phase of risk assessment. What is the severity and likelihood of risk that we assume in performing ultrasound? And are there controls or methods by which we may mitigate this risk? The severity of risk is dependent upon the POCUS application performed. It is unlikely that severe harm will occur in a failed ultrasound-guided peripheral intravenous catheter insertion attempt. But imagine a POCUS evaluation of cardiac contractility interpreted as normal qualitative systolic function in a child with a clinical respiratory viral illness. What if this child is later identified to have severe systolic dysfunction on a complete echocardiogram and is subsequently diagnosed with myocarditis? Maybe the provider was correct in the initial interpretation and the cardiac dysfunction evolved over time. Or maybe the provider mis-interpreted the images and the dysfunction was present at the time of POCUS assessment. The risk to the patient is obvious, as therapies may not align with underlying pathophysiology, thereby increasing the risk of morbidity. But the risk to the provider, the department, and the institution is potentiated by factors other than the POCUS study itself. Risk increases if the provider did not receive adequate training or adequate supervision. Risk increases if the provider did not document findings. Risk increases if the provider documented an interpretation, but images were not saved and incorporated into the medical record. Risk increases if there is not a method of longitudinal assessment of physician skills. Risk increases if there are no national or institutional standards, for example, a credentialing process, for POCUS clinical integration. Thus, controls for risk involve developing infrastructural programmatic elements to standardize practice and ensure the adequate translation of training to practice (Fig. 1). Such controls are variably developed in current POCUS practice settings.
Fig. 1.
Suggested elements for program development. Infrastructural elements allow for support of effective curricular development and, through implementation processes, translates to quality care. Structure and process outcomes can be measured to assure benefit to patient, providers and institutions
Quantifying the level of risk for a patient, a provider and an institution is an imprecise form of calculus that integrates the potential likelihood and severity of risks and associated consequences and must be balanced with potential benefit. Robust data exists in pediatrics supporting effectiveness of both procedural and diagnostic ultrasound in the hands of skilled providers, and this literature is only going to increase with time and experience [39, 40]. Strategies for mitigating risk should be viewed as opportunities to promote patient safety and optimize clinical outcomes while also serving to protect providers and their local environment. Hence, developing a robust clinical governance around use of ultrasound in clinical practice can help in minimizing such risks.
POCUS risk: evaluate
The evaluate phase of risk assessment inventories current processes within the scope of mitigation strategies and solutions. This phase also evaluates the environment of practice for greater precision in assessing the degree of risk. The presence and absence of strategies and solutions are tied to local, national, and international standards. For example, training in specialty-specific POCUS is not standardized in any pediatric disciplines and there is no definition or methods of assessing for competency. Courses sponsored by societies and institutions exist to expose learners to limited applications, and there exist few practice guidelines or consensus statements regarding the role of POCUS in pediatric clinical care [1, 41–43]. Although POCUS and Targeted Neonatal Echocardiography (TNE) are certified in Switzerland by the Swiss Federation of Physicians, this is one of the very few certification processes that exist within pediatrics. Without external certification processes, nations and their individual pediatric institutions must define for themselves what entails appropriate training.
Infrastructural elements including image storage, documentation and quality assurance processes are essential components of sustainable POCUS programs, and a robust outline of elements to develop successful programs has been published by the American College of Emergency Physicians (ACEP) [44]. Quality assurance processes, in particular, can be challenging to build and maintain and will often require multidisciplinary support for implementation. Institutions with cardiology and radiology specialists may require their involvement in reviewing POCUS studies to confirm appropriate image acquisition quality and interpretative accuracy. There is no formal definition of quality assurance in POCUS, but there should be an effort made to create a longitudinal educational environment for refinement of POCUS skill. Mechanisms ensuring the oversight of educational objectives should ideally involve multidisciplinary specialist collaboration.
In assessing risk, providers and institutions must also understand local, national, and international laws and regulations related to medical care. There are two main categories of law: civil law and common law. Differences exist regarding assessment and assignment of negligence within these systems [45]. Medical malpractice in a common law system falls under the negligence tort which is summarized as a duty and failure to provide an accepted standard of care resulting in harm [46, 47]. In common law, negligence is assigned to an individual or individuals and results in compensation. A no-fault legal system requires identification of a causal relationship between treatment and injury but does not assign responsibility at the level of the individual [48]. Medical malpractice law in the USA is regulated by individual states, so there are subtle differences in tort negligence laws between states. Similarly, European countries may integrate tort law and no-fault systems in varied ways to optimize system efficiency and may also cap compensation [49–51]. Many countries, including Canada and most of those within Europe, have tort liability assessed by a judge, thereby shortening the time in which a decision is rendered. No-fault systems are practiced outside of the courtroom, and claims in New Zealand are adjudicated by a Medical Board review [52]. Practicing pediatrics, or a subspecialty within pediatrics, also reduces the risk of litigation as a recent survey demonstrated pediatrics as the 24th of 25 specialties in proportion of physicians facing malpractice claims. Yet the mean indemnity payments of malpractice suits were highest in pediatrics [53, 54]. In the USA, 34% of all physicians have been involved in a lawsuit [55]. When we integrate literature regarding malpractice claims with geographic and specialty-specific considerations, pediatric POCUS likely represents a low risk (but not “no riskˮ) practice in any current clinical setting, and risk tolerability is dependent upon local contexts of practice.
POCUS risk: manage
The approach to manage POCUS risk requires developing previously discussed infrastructural elements including technology and equipment, standardized training, image storage solutions, documentation processes, quality assurance methods and pathways to ensure provider competency in POCUS applications (Fig. 1, Table 2). A recent survey of academic pediatric critical care programs in the USA found that over 60% of divisions were performing diagnostic ultrasound in the clinical setting. Yet, despite frequent use of ultrasound, less than 25% of institutions were represented within core infrastructural elements suggested by ACEP [56]. At the time of publication, no division had over 25% of pediatric critical care clinicians credentialed in POCUS applications. Attention to each of these aspects of program development is another step towards protecting patients, providers, and institutions from risk, especially when POCUS will be widely performed by physicians and emerges as a standard of care.
Table 2.
Point-of-care ultrasound risk and mitigation strategies by programmatic infrastructural element
| Infrastructural element | Risk | Mitigation strategy | Challenges to mitigation |
|---|---|---|---|
|
Technology and equipment |
Direct harm to patient from ultrasound and related equipment | Embed as knowledge objectives within educational processes | Limited knowledge of current standards and human data on actual risk |
| Training | Incompetent in knowledge, psychomotor and/or interpretative educational domains | Development of initial and longitudinal specialty-specific training |
Lack of POCUS educational experts No standardized educational curriculum No definition or method of measuring competency |
| Documentation | Absent or insufficient documentation resulting in a loss of important information from POCUS | Development of POCUS application-specific documentation templates for inclusion in the medical record |
Current differences in documentation practice between and within institutions (e.g., paper versus electronic) Identification of appropriate person to interpret and document results |
| Image storage | Absent or insufficient image storage capabilities resulting in a loss of review capabilities for initial interpretation or longitudinal assessment of changing physiologies | Development of a local POCUS image storage solution |
Solutions may not be technologically available in a local clinical environment Storage solutions external to a hospital system (e.g., “cloud-basedˮ) may not be linked to a medical record and may not be viewable to other clinicians |
| Quality assurance | A lack of a review processes and/or a review process led by unqualified individuals results in the clinical translation of inadequate POCUS skills integrated in patient care | Development of a quality assurance process providing timely feedback to providers across educational domains led by appropriate specialists |
Specialists for oversight likely found in other specialties, particularly in the early phases of POCUS program development No definition of “specialistˮ in many POCUS applications Significant time and effort to build multidisciplinary team for the review of images and to create feedback mechanisms |
|
Processes to define and confirm competency (e.g., credentialing) |
Absent institutional or national processes for clinical provider integration of POCUS in patient care | Institutional or national POCUS credentialing or certification processes resulting in clinical privileges for providers completing POCUS training |
Requires many of the above elements to be in place or actively in development Resistance from administrators with little knowledge of POCUS |
The development of programmatic infrastructural elements embedded with risk mitigation strategies likely results in a symbiotic reduction of overall risk given their obvious interdependence with one another
This leads us to the most important element in risk mitigation: collaboration. Collaboration requires listening to the concerns expressed by others, whether physicians, administrators, or regulatory agencies. It involves working together to develop shared solutions. Curriculum build and training requires not only ideas and skill development by those within a specialty but also guidance from consultants outside the specialty. The American Society of Echocardiography recently published a statement recognizing and supporting the use of POCUS by adult clinicians and suggested methods by which echocardiography laboratories can assist in program development [57]. Departments and institutions may be unable to support robust quality assurance processes. External support systems may be modeled after telemedicine solutions used by groups such as Médecins Sans Frontières for POCUS quality assurance platforms in remote settings, and information technology growth has resulted in faster and cheaper storage platforms to integrate images and documents in the medical record [58]. Electronic health record solutions will undoubtedly be available to all practice settings if not already present. Finally, development of institutional processes to confirm competency requires collaboration with multi-specialty clinical and administrative leaders and represents institutional investment in safe and high quality pediatric care.
Conclusion
Risk analysis of any practice in medicine is a complex calculus incorporating the delivery of care by providers and institutions, the outcomes of patients, and the setting of clinical practice. POCUS use is likely to increase across all pediatric specialties and its translation to clinical care likely represents a low risk, but not no-risk practice. Though a clinician may currently work in an environment with limited medicolegal risk, legal frameworks are likely to evolve with an emerging emphasis on quality and safety across the medical field. We suggest that listening to concerns and partnering with experienced providers, administrators, and regulatory agencies will not only help to develop strategies towards risk reduction, but also result in a practice structure that improves provider performance and patient outcome.
Abbreviations
- POCUS
Point-of-care ultrasound
- ESPR
European Society of Paediatric Radiology
- ESEP
European Society of Emergency Pediatrics
- ESEM
European Society for Emergency Medicine
- BMUS
British Medical Ultrasound Society
- ACGME
Accreditation Council for Graduate Medical Education
- TNE
Targeted Neonatal Echocardiography
- ACEP
American College of Emergency Physicians
- AHRQ
Agency for Healthcare Research and Quality
- ECRI
Emergency Care Research Institute
Authors’ contribution
YS and TC conceptualized the idea and prepared the initial draft. All the co-authors (YS, TC, NY, JMC, CT, MVF, SB, PS, AMV, and BS) critically reviewed the manuscript for important intellectual content and edited and approved its final version.
Funding
This study received no specific grant from any funding agency in the public, commercial or not-for-profit sectors.
Availability of data and material
None.
Code availability
Not applicable.
Declarations
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Conflict of interest
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Thomas W. Conlon, Email: ConlonT@chop.edu
Nadya Yousef, Email: nadya.yousef@aphp.fr.
Maria V. Fraga, Email: FRAGAM@chop.edu
Shazia Bhombal, Email: sbhombal@stanford.edu.
Bijan Siassi, Email: bijans@msn.com.
References
- 1.Singh Y, Tissot C, Fraga MV, Yousef N, Cortes RG, Lopez J, et al. International evidence-based guidelines on Point of Care Ultrasound (POCUS) for critically ill neonates and children issued by the POCUS Working Group of the European Society of Paediatric and Neonatal Intensive Care (ESPNIC) Crit Care. 2020;24(1):65. doi: 10.1186/s13054-020-2787-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.de Souza TH, Brandão MB, Nadal JAH et al (2018) Ultrasound Guidance for Pediatric Central Venous Catheterization: A Meta-analysis. Pediatrics 142: e20181719 [DOI] [PubMed]
- 3.Hanada S, Van Winkle MT, Subramani S et al (2017) Dynamic ultrasound-guided short-axis needle tip navigation technique vs. landmark technique for difficult saphenous vein access in children: a randomised study. Anaesthesia 72:1508–1515 [DOI] [PubMed]
- 4.Takeshita J, Yoshida T, Nakajima Y, et al. Superiority of Dynamic Needle Tip Positioning for Ultrasound-Guided Peripheral Venous Catheterization in Patients Younger Than 2 Years Old: A Randomized Controlled Trial. Pediatr Crit Care Med. 2019;20:e410–e414. doi: 10.1097/PCC.0000000000002034. [DOI] [PubMed] [Google Scholar]
- 5.Kantor DB, Su E, Milliren CE, et al. Ultrasound Guidance and Other Determinants of Successful Peripheral Artery Catheterization in Critically Ill Children. Pediatr Crit Care Med. 2016;17:1124–1130. doi: 10.1097/PCC.0000000000000936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Katheria AC, Fleming SE, Kim JH. A randomized controlled trial of ultrasound-guided peripherally inserted central catheters compared with standard radiograph in neonates. J Perinatol. 2013;33:791–794. doi: 10.1038/jp.2013.58. [DOI] [PubMed] [Google Scholar]
- 7.Helgeson SA, Fritz AV, Tatari MM, et al. Reducing Iatrogenic Pneumothoraces: Using Real-Time Ultrasound Guidance for Pleural Procedures. Crit Care Med. 2019;47:903–909. doi: 10.1097/CCM.0000000000003761. [DOI] [PubMed] [Google Scholar]
- 8.Hayes J, Borges B, Armstrong D, et al. Accuracy of manual palpation vs ultrasound for identifying the L3–L4 intervertebral space level in children. Paediatr Anaesth. 2014;24:510–515. doi: 10.1111/pan.12355. [DOI] [PubMed] [Google Scholar]
- 9.Lamperti M, Bodenham AR, Pittiruti M, Blaivas M, Augoustides JG, Elbarbary M, Pirotte T, Karakitsos D, Ledonne J, Doniger S, Scoppettuolo G, Feller-Kopman D, Schummer W, Biffi R, Desruennes E, Melniker LA, Verghese ST. International evidence-based recommendations on ultrasound-guided vascular access. Intensive Care Med. 2012;38(7):1105–1117. doi: 10.1007/s00134-012-2597-x. [DOI] [PubMed] [Google Scholar]
- 10.Kutty S, Attebery JE, Yeager EM, et al. Transthoracic echocardiography in pediatric intensive care: Impact on medical and surgical management. Pediatr Crit Care Med. 2014;15:329–335. doi: 10.1097/PCC.0000000000000099. [DOI] [PubMed] [Google Scholar]
- 11.Harabor A, Soraisham AS. Utility of targeted neonatal echocardiography in the management of neonatal illness. J Ultrasound Med. 2015;34:1259–1263. doi: 10.7863/ultra.34.7.1259. [DOI] [PubMed] [Google Scholar]
- 12.Papadhima I, Louis D, Purna J, Deshpande P, Diambomba Y, Lee S, Shah P, Weisz D, El-Khuffash A, McNamara PJ, Mertens L, Jain A. Targeted neonatal echocardiography (TNE) consult service in a large tertiary perinatal center in Canada. J Perinatol. 2018;38(8):1039–1045. doi: 10.1038/s41372-018-0130-y. [DOI] [PubMed] [Google Scholar]
- 13.Jain A, Sahni M, El-Khuffash A, et al. Use of targeted neonatal echocardiography to prevent postoperative cardiorespiratory instability after patent ductus arteriosus ligation. J Pediatr. 2012;160:584–589. doi: 10.1016/j.jpeds.2011.09.027. [DOI] [PubMed] [Google Scholar]
- 14.Teixeira L, Shivananda S, Stephens D, et al. Postoperative cardiorespiratory instability following ligation of the preterm ductus arteriosus is related to early need for intervention. J Perinatol. 2008;28:803–810. doi: 10.1038/jp.2008.101. [DOI] [PubMed] [Google Scholar]
- 15.De Martino L, Yousef N, Ben-Ammar R, Raimondi F, Shankar-Aguilera S, De Luca D (2018) Lung Ultrasound Score Predicts Surfactant Need in Extremely Preterm Neonates. Pediatrics 142(3):e20180463 [DOI] [PubMed]
- 16.Becker DM, Tafoya CA, Becker SL, et al. The use of portable ultrasound devices in low- and middle-income countries: a systematic review of the literature. Trop Med Int Health. 2016;21:294–311. doi: 10.1111/tmi.12657. [DOI] [PubMed] [Google Scholar]
- 17.Arnoldi S, Glau CL, Walker SB, Himebauch AS, Parikh DS, Udeh SC, Weiss SL, Fitzgerald JC, Nishisaki A, Conlon TW. Integrating Focused Cardiac Ultrasound Into Pediatric Septic Shock Assessment. Pediatr Crit Care Med. 2021;22(3):262–274. doi: 10.1097/PCC.0000000000002658. [DOI] [PubMed] [Google Scholar]
- 18.Elsayed YN, Amer R, Seshia MM. The impact of integrated evaluation of hemodynamics using targeted neonatal echocardiography with indices of tissue oxygenation: a new approach. J Perinatol. 2017;37(5):527–535. doi: 10.1038/jp.2016.257. [DOI] [PubMed] [Google Scholar]
- 19.Kaya GK, Ward JR, Clarkson PJ. A framework to support risk assessment in hospitals. Int J Qual Health Care. 2019;31(5):393–401. doi: 10.1093/intqhc/mzy194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.van Rijn RR, Stafrace S, Arthurs OJ, Rosendahl K, European Society of Paediatric Radiology (2021) Non-radiologist-performed point-of-care ultrasonography in paediatrics - European Society of Paediatric Radiology position paper. Pediatr Radiol 51(1):161–167 [DOI] [PMC free article] [PubMed]
- 21.ECRI (2020) Top 10 Health Technology Hazards Executive Brief. Available at. https://www.ecri.org/landing-2020-top-ten-health-technology-hazards/. Accessed 20 October 2020
- 22.Parri N, Berant R, Giacalone M, Corsini I, Titomanlio L, Connolly J, Kwan C, Teng D. European Society of Emergency Pediatrics (EUSEP), the Ultrasound Section of the European Society for Emergency Medicine (EUSEM), and the Pediatric Emergency Medicine Point-of-Care Ultrasound (P2) Network. Point-of-care ultrasonography in pediatrics Pediatr Radiol. 2021;51(7):1271–1272. doi: 10.1007/s00247-021-05077-w. [DOI] [PubMed] [Google Scholar]
- 23.Andronikou S, Otero HJ, Belard S, Heuvelings CC, Ruby LC, Grobusch MP. Radiologists should support non-radiologist point-of-care ultrasonography in children: a case for involvement and collaboration. Pediatr Radiol. 2021;24:1–4. doi: 10.1007/s00247-021-05185-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Su E, Soni NJ, Blaivas M, Bhargava V, Steffen K, Haileselassie B. Regulating Critical Care Ultrasound, It Is All in the Interpretation. Pediatr Crit Care Med. 2021;22(4):e253–e258. doi: 10.1097/PCC.0000000000002600. [DOI] [PubMed] [Google Scholar]
- 25.Reaume M, Farishta M, Costello JA, Gibb T, Melgar TA. Analysis of lawsuits related to diagnostic errors from point-of-care ultrasound in internal medicine, paediatrics, family medicine and critical care in the USA. Postgrad Med J. 2021;97(1143):55–58. doi: 10.1136/postgradmedj-2020-137832. [DOI] [PubMed] [Google Scholar]
- 26.Nguyen J, Cascione M, Noori S. Analysis of lawsuits related to point-of-care ultrasonography in neonatology and pediatric subspecialties. J Perinatol. 2016;36(9):784–786. doi: 10.1038/jp.2016.66. [DOI] [PubMed] [Google Scholar]
- 27.Blaivas M, Pawl R. Analysis of lawsuits filed against emergency physicians for point-of-care emergency ultrasound examination performance and interpretation over a 20-year period. Am J Emerg Med. 2012;30(2):338–341. doi: 10.1016/j.ajem.2010.12.016. [DOI] [PubMed] [Google Scholar]
- 28.Newman PG, Rozycki GS. The history of ultrasound. Surg Clin North Am. 1998;78(2):179–195. doi: 10.1016/s0039-6109(05)70308-x. [DOI] [PubMed] [Google Scholar]
- 29.Shankar H, Pagel PS. Potential adverse ultrasound-related biological effects: a critical review. Anesthesiology. 2011;115(5):1109–1124. doi: 10.1097/ALN.0b013e31822fd1f1. [DOI] [PubMed] [Google Scholar]
- 30.Miller MW, Ziskin MC. Biological consequences of hyperthermia. Ultrasound Med Biol. 1989;15(8):707–722. doi: 10.1016/0301-5629(89)90111-7. [DOI] [PubMed] [Google Scholar]
- 31.Palte HD, Gayer S, Arrieta E, Scot Shaw E, Nose I, Lee E, Arheart KL, Dubovy S, Birnbach DJ, Parel JM. Are ultrasound-guided ophthalmic blocks injurious to the eye? A comparative rabbit model study of two ultrasound devices evaluating intraorbital thermal and structural changes. Anesth Analg. 2012;115:194–201. doi: 10.1213/ANE.0b013e318253622e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dalecki D. Mechanical bioeffects of ultrasound. Annu Rev Biomed Eng. 2004;6:229–248. doi: 10.1146/annurev.bioeng.6.040803.140126. [DOI] [PubMed] [Google Scholar]
- 33.Chu K, Obaid H, Babyn P, Blondeau J. Bacterial contamination of ultrasound probes at a tertiary referral university medical center. AJR Am J Roentgenol. 2014;203(5):928–932. doi: 10.2214/AJR.13.12407. [DOI] [PubMed] [Google Scholar]
- 34.Nyhsen CM, Humphreys H, Koerner RJ, Grenier N, Brady A, Sidhu P, Nicolau C, Mostbeck G, D'Onofrio M, Gangi A, Claudon M. Infection prevention and control in ultrasound - best practice recommendations from the European Society of Radiology Ultrasound Working Group. Insights Imaging. 2017;8(6):523–535. doi: 10.1007/s13244-017-0580-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Doblhoff G, Satrapa J, Coulthard P. Recognising small image quality differences for ultrasound probes and the potential of misdiagnosis due to undetected side lobes. Ultrasound. 2017;25(1):35–44. doi: 10.1177/1742271X16689281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Quien MM, Saric M. Ultrasound imaging artifacts: How to recognize them and how to avoid them. Echocardiography. 2018;35(9):1388–1401. doi: 10.1111/echo.14116. [DOI] [PubMed] [Google Scholar]
- 37.National Institute for Clinical Excellence (2002) Guidance on the use of ultrasound locating devices for placing central venous catheters. Technology Appraisal Guidance No. 49. Available from. http://www.nice.org.uk
- 38.Arts L, Lim EHT, van de Ven PM, Heunks L, Tuinman PR. The diagnostic accuracy of lung auscultation in adult patients with acute pulmonary pathologies: a meta-analysis. Sci Rep. 2020;10(1):7347. doi: 10.1038/s41598-020-64405-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fraga MV, Stoller JZ, Glau CL, De Luca D, Rempell RG, Wenger JL, Yek Kee C, Muhly WT, Boretsky K, Conlon TW (2019) Seeing Is Believing: Ultrasound in Pediatric Procedural Performance. Pediatrics 144(5):e20191401 [DOI] [PubMed]
- 40.Conlon TW, Nishisaki A, Singh Y, Bhombal S, De Luca D, Kessler DO, Su ER, Chen AE, Fraga MV (2019) Moving Beyond the Stethoscope: Diagnostic Point-of-Care Ultrasound in Pediatric Practice. Pediatrics 144(4):e20191402 [DOI] [PubMed]
- 41.Frankel HL, Kirkpatrick AW, Elbarbary M, Blaivas M, Desai H, Evans D, Summerfield DT, Slonim A, Breitkreutz R, Price S, Marik PE, Talmor D, Levitov A. Guidelines for the Appropriate Use of Bedside General and Cardiac Ultrasonography in the Evaluation of Critically Ill Patients-Part I: General Ultrasonography. Crit Care Med. 2015;43(11):2479–2502. doi: 10.1097/CCM.0000000000001216. [DOI] [PubMed] [Google Scholar]
- 42.Levitov A, Frankel HL, Blaivas M, Kirkpatrick AW, Su E, Evans D, Summerfield DT, Slonim A, Breitkreutz R, Price S, McLaughlin M, Marik PE, Elbarbary M. Guidelines for the Appropriate Use of Bedside General and Cardiac Ultrasonography in the Evaluation of Critically Ill Patients-Part II: Cardiac Ultrasonography. Crit Care Med. 2016;44(6):1206–1227. doi: 10.1097/CCM.0000000000001847. [DOI] [PubMed] [Google Scholar]
- 43.Marin JR, Lewiss RE (2015) American Academy of Pediatrics, Committee on Pediatric Emergency Medicine, 2013–2014; Society for Academic Emergency Medicine (Reviewers); American College of Emergency Physicians, Pediatric Emergency Medicine Committee, 2013–2014; World Interactive Network Focused on Critical Ultrasound Board of Directors (reviewers); Point-of-care ultrasonography by pediatric emergency physicians. Policy statement. Ann Emerg Med 65(4):472–8 [DOI] [PubMed]
- 44.Guidelines U. Emergency, Point-of-Care and Clinical Ultrasound Guidelines in Medicine. Ann Emerg Med. 2017;69(5):e27–e54. doi: 10.1016/j.annemergmed.2016.08.457. [DOI] [PubMed] [Google Scholar]
- 45.Traina F. Medical malpractice: the experience in Italy. Clin Orthop Relat Res. 2009;467(2):434–442. doi: 10.1007/s11999-008-0582-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bal BS. An introduction to medical malpractice in the United States. Clin Orthop Relat Res. 2009;467(2):339–347. doi: 10.1007/s11999-008-0636-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cheluvappa R, Selvendran S. Medical negligence - Key cases and application of legislation. Ann Med Surg (Lond) 2020;17(57):205–211. doi: 10.1016/j.amsu.2020.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Watson K, Kottenhagen R. Patients’ Rights, Medical Error and Harmonisation of Compensation Mechanisms in Europe, European. J Health Law. 2018;25(1):1–23. [Google Scholar]
- 49.Antoci A, Fiori Maccioni A, Russu P (2016) The Ecology of Defensive Medicine and Malpractice Litigation. PLoS One 11(3):e0150523 [DOI] [PMC free article] [PubMed]
- 50.Yan SC, Hulsbergen AFC, Muskens IS, van Dam M, Gormley WB, Broekman MLD, Smith TR. Defensive medicine among neurosurgeons in the Netherlands: a national survey. Acta Neurochir (Wien) 2017;159(12):2341–2350. doi: 10.1007/s00701-017-3323-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kessler DP, Summerton N, Graham JR. Effects of the medical liability system in Australia, the UK, and the USA. Lancet. 2006;368(9531):240–246. doi: 10.1016/S0140-6736(06)69045-4. [DOI] [PubMed] [Google Scholar]
- 52.Wallis KA. No-fault, no difference: no-fault compensation for medical injury and healthcare ethics and practice. Br J Gen Pract. 2017;67(654):38–39. doi: 10.3399/bjgp17X688777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jena AB, Seabury S, Lakdawalla D, Chandra A. Malpractice risk according to physician specialty. N Engl J Med. 2011;365(7):629–636. doi: 10.1056/NEJMsa1012370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jena AB, Chandra A, Seabury SA. Malpractice risk among US pediatricians. Pediatrics. 2013;131(6):1148–1154. doi: 10.1542/peds.2012-3443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Guardado JR (2017) Policy Research Perspectives: Medical Liability Claim Frequency Among U.S. Physicians. Chicago, IL: American Medical Association
- 56.Conlon TW, Kantor DB, Su ER, Basu S, Boyer DL, Haileselassie B, Petersen TL, Su F, Nishisaki A. Diagnostic Bedside Ultrasound Program Development in Pediatric Critical Care Medicine: Results of a National Survey. Pediatr Crit Care Med. 2018;19(11):e561–e568. doi: 10.1097/PCC.0000000000001692. [DOI] [PubMed] [Google Scholar]
- 57.Kirkpatrick JN, Grimm R, Johri AM, Kimura BJ, Kort S, Labovitz AJ, Lanspa M, Phillip S, Raza S, Thorson K, Turner J. Recommendations for Echocardiography Laboratories Participating in Cardiac Point of Care Cardiac Ultrasound (POCUS) and Critical Care Echocardiography Training: Report from the American Society of Echocardiography. J Am Soc Echocardiogr. 2020;33(4):409–422.e4. doi: 10.1016/j.echo.2020.01.008. [DOI] [PubMed] [Google Scholar]
- 58.Halton J, Kosack C, Spijker S, Joekes E, Andronikou S, Chetcuti K, Brant WE, Bonnardot L, Wootton R. Teleradiology usage and user satisfaction with the telemedicine system operated by médecins sans frontières. Front Public Health. 2014;28(2):202. doi: 10.3389/fpubh.2014.00202. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
None.
Not applicable.