Abstract
The anti-human immunodeficiency virus (HIV) activity of aryloxyphosphoramidate protides of a number of anti-HIV nucleoside analogues was assessed in resting primary monocyte-macrophages (M/M). While 2′,3′-dideoxythymidine (d4T), 2′,3′-dideoxyadenosine (ddA), and 2′,3′-dideoxy-2′,3′-didehydroadenosine (d4A) protides showed an anti-HIV activity that was 25- to 625-fold greater than the parent nucleotides d4T, ddA, and d4A, respectively, other aryloxyphosphoramidate protides showed similar or even lower anti-HIV activities than their parent compounds. This variable anti-HIV effect is most likely related to the different dynamics of intracellular nucleoside monophosphate release from the protides. Our results indicate the potential advantage of therapeutic use of this approach for some nucleotide analogues to affect HIV replication in M/M, one of the major reservoirs of HIV in vivo.
In all body compartments, resting monocyte-macrophages (M/M) can be infected by human immunodeficiency virus (HIV) (1, 12–14, 27, 34, 37). Because of their resistance to the cytopathic effect of the virus, M/M are considered the most relevant reservoir of HIV in the body (10, 18, 26, 31) and a crucial target for a successful therapeutic approach (2, 3, 35).
M/M are characterized by some cellular peculiarities that affect not only virus replication (6, 28; S. Aquaro, P. Bagnarelli, M. Clementi, T. Guenci, R. Calio', and C. F. Perno, Program Abstr. 6th Conf. Retrovir. Opportunistic Infect., abstr. 607, 1999.) but also the activity of antiviral drugs (31, 33). Indeed, their resting status, and thus their limited DNA synthesis, does not require, for physiological functions, high intracellular levels of 2′-deoxynucleotide pools. As a consequence, and despite the limited phosphorylation of anti-HIV nucleoside analogues typically found in M/M, the ratio of the triphosphate forms of nucleoside reverse transcriptase inhibitors (NRTIs) to their natural triphosphorylated 2′-deoxynucleotide counterparts is higher than that found in lymphocytes (4). Thus, not surprisingly, the currently approved NRTIs for clinical use, i.e., azidothymidine (AZT), 2′,3′-dideoxyinosine, 2′,3′-dideoxycytidine (ddC), (−)(l)-3′-thia-2′,3′-dideoxycytidine (3TC), 2′,3′-didehydro-2′,3′-dideoxythymidine (d4T), show anti-HIV efficacies in M/M greater than those found in lymphocytes (4).
Despite this increased anti-HIV efficacy in M/M, the in vivo antiviral activity of most NRTIs in sequestered compartments is still suboptimal for a number of reasons, including the limited penetration of most NRTIs in sanctuaries (11, 16) and the high expression of p170 glycoprotein in M/M (17), that may affect the overall intracellular drug concentrations in M/M. Therefore, attempts should be made to increase the intracellular concentration of the triphosphate forms of the NRTIs in M/M.
To conveniently bypass the first phosphorylation step of NRTIs, masked nucleoside 5′-monophosphate (MP) prodrugs have been synthesized. These compounds were designed to act as monophosphorylated membrane-soluble prodrugs of the bioactive free nucleosides, which otherwise would be too polar to efficiently cross the membrane lipid bilayer of the target cells (22).
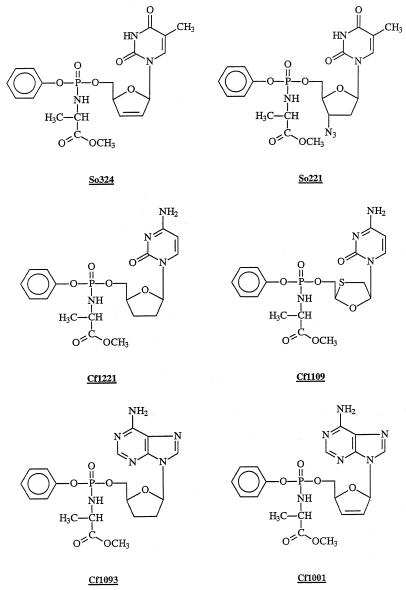
Several efforts have been made in this field (15, 23–25, 30, 36), and some significant results have been achieved, the aryl-phosphoramidate prodrugs of nucleoside analogues being among the most relevant (7, 21, 22). These prodrugs are characterized by the presence of a nucleoside analogue MP, containing typically (i) an aryl group linked to the phosphorus through an ester bond and (ii) the methyl ester of l-alanine linked to the phosphorus through a phosphoramidate bond with the primary amino moiety (Fig. 1).
FIG. 1.
Chemical structures of So324, So221, Cf-1221, Cf-1109, Cf-1093, and Cf-1001.
The potent antiviral effect of the d4T-MP phosphoramidate (i.e., So324, previously described as the prototype compound of this class of prodrugs) (7) is particularly promising from a clinical standpoint, yet the results cannot be extended in principle to phosphoramidate analogues of nucleosides other than d4T, because of differences in the phosphorylation pathways. We thus focused our attention on the antiviral activity in lymphocytes and M/M of aryloxyphosphoramidates of the nucleoside analogues currently approved for the therapy of HIV infection and, additionally, the aryloxyphosphoramidates of 2′,3′-dideoxyadenosine (ddA) and of 2′,3′-dideoxy-2′,3′-didehydroadenosine (d4A; a compound devoid of marked antiviral activity in lymphocytes).
Human primary M/M were obtained from the blood of healthy seronegative donors as previously described (32). Adherent cells purified with this technique consist of >95% of differentiated M/M. CEM is a CD4+ lymphocytoid cell line highly susceptible to the cytopathic effect of HIV-1. Two different isolates of HIV were used in this study. The monocytotropic strain HIV-1Ba-L was used in all experiments involving primary M/M. In the experiments with lymphocytic cells, the lymphocytotropic strain HIV-1IIIB was used. Details about the synthesis of the protides of AZT (So221), d4T (So324), ddA (Cf-1093), d4A (Cf-1001), ddC (Cf-1221), and 3TC (Cf-1109) were previously described (19, 20, 22). Their structures are depicted in Fig. 1. Each compound was dissolved in 100% dimethyl sulfoxide (DMSO) and stored at −20°C until use. At the beginning of the experiment, and at each medium change, serial dilutions of each compound were prepared, and the appropriate amount was added to each well. Human primary M/M were exposed to various concentrations of aryloxyphosphoramidate prodrugs or their parent compounds for 20 min and then challenged with 500 50% cell culture infective doses per ml of HIV-1Ba-L. After 2 h of incubation, M/M were extensively and carefully washed with warm medium to remove the excess of virus and then cultured in the presence of drugs at the same conditions as before. M/M were washed and fed every 5 days with fresh medium and replenished with compounds. Unless differently stated, supernatants were collected at day 12 after virus challenge, and virus production was determined by the antigen-capture assay by using a commercially available kit. In an additional set of two experiments, drugs were added to M/M up to 1 h after virus challenge. Results, in terms of both antiviral activity and cytotoxicity, were superimposable to those obtained by adding the compounds before infection (data not shown). CEM cells were infected with the HIV-1IIIB strain at 100 50% cell culture infective doses/ml, then 100 μl of the infected cell suspensions was added to 200-μl microtiter plate wells containing 100 μl of an appropriate dilution of the test compounds. After 4 days incubation at 37°C, the cell cultures were examined for syncytium formation. The 50% effective concentration (EC50) was determined as the compound concentration required to inhibit syncytium formation by 50%. For the assessment of cytotoxicity, mock-infected M/M were treated with various concentrations of test compounds. Assessment of the cytotoxic effect was performed twice weekly by visual inspection and then by counting cells and trypan blue dye exclusion at day 12 after the beginning of treatment.
DMSO alone, used at the same final concentrations present in lymphocytes or M/M cultures (i.e., up to 0.05%), was devoid of any antiviral or cytotoxic effect (data not shown).
CEM cell toxicity was measured by counting the number of viable cells in CEM cultures exposed to different concentrations of the compounds.
Unless differently stated, all results presented are the averages of four independent experiments, each run in triplicate.
Table 1 reports the results obtained with the aryloxyphosphoramidate derivatives of d4T- and AZT-MP. The antiviral activity of d4T and its phosphoramidate derivative So324 in CEM lymphocytes was only fivefold greater than that of d4T (EC50s, 0.07 and 0.35 μM, respectively). Conversely, So324 showed an antiviral activity in M/M that was about 25-fold greater than that of its parent compound, the EC50s being 0.008 and 0.2 μM, respectively (Table 1). It is worth noting that So324 at 0.03 μM afforded 90% inhibition of HIV replication in M/M (Table 1), while the same concentration of d4T decreased virus production in these cells less than 28% (data not shown). Interestingly, So324 was about ninefold more active in M/M than in CEM cells (EC50s, 0.008 and 0.07 μM, respectively), while the parent d4T was almost equiactive in the two cellular systems. Thus, the chemical modification of d4T provided an increase of its antiviral potency in M/M that was much more pronounced than that achieved in lymphocytes. This is also in substantial agreement with our metabolism studies where a fourfold enhancement of the intracellular levels of d4T-triphosphate in M/M was observed following treatment with So324, as compared to d4T; in lymphocytes, the increase proved to be markedly less pronounced (7).
TABLE 1.
Anti-HIV activity of So221 and So324, the aryloxyphosphoramidate prodrug derivatives of AZT- and d4T-MP, respectivelya
| Compound | EC90 (μM) for M/M | EC50 (μM) for:
|
CC50b (μM) for:
|
SIc of:
|
|||
|---|---|---|---|---|---|---|---|
| M/M | CEM | M/M | CEM | M/M | CEM | ||
| d4T | 0.78 ± 0.02 | 0.20 ± 0.05 | 0.35 ± 0.07 | >100 | 143 ± 1.4 | >500 | 497 |
| So324 | 0.03 ± 0.001 | 0.008 ± 0.001 | 0.07 ± 0.007 | >100 | ≥100 | >12,500 | ≥769 |
| AZT | 0.02 ± 0.003 | 0.005 ± 0.001 | 0.002 ± 0.002 | >100 | >100 | >20,000 | >33,300 |
| So221 | 0.1 ± 0.02 | 0.012 ± 0.005 | 0.06 ± 0.02 | >100 | >100 | >8,300 | >12,500 |
Results are presented as means ± standard deviations of tree values obtained in four different experiments.
CC50, 50% cytotoxic concentration.
SI = CC50/EC50.
The next step was the assessment of the antiviral effect of So221, the aryloxyphosphoramidate prodrug of AZT (Table 1). In agreement with previously published results, AZT was much more active in M/M than d4T (EC50s, 0.005 and 0.2 μM, respectively), yet the delivery of AZT–AZT-MP via treatment with its aryloxyphosphoramidate prodrug So221 caused an antiviral effect in M/M (EC50, 0.012 μM) that was comparable, or even lower, than that obtained with AZT alone (Table 1). A similar result was obtained with So221 in CEM lymphocytes, with a two- to threefold reduction of antiviral activity of So221, compared to that of the parent AZT, both in M/M and CEM cells. This discrepancy in the antiviral activity of aryl-phosphoramidate derivatives of d4T and AZT should be interpreted in view of the different metabolic properties of AZT, as compared to d4T. For AZT, the bottleneck in the phosphorylation pathway is at the level of the dTMP kinase-catalyzed AZT-MP–to–AZT-diphosphate conversion, whereas for d4T, the bottleneck is at the first phosphorylation (thymidine kinase-catalyzed) step, bypassed by the intracellular delivery of d4T-MP through its protide (5).
The differential antiviral effects of the AZT-MP and d4T-MP prodrug derivatives prompted us to assess the antiviral effects of other ddNMP aryloxyphosphoramidate prodrugs. The antiviral effect of ddA in M/M was dramatically enhanced by treatment with Cf-1093, the phosphoramidate prodrug ddA-MP (Table 2). Indeed, the EC50s of ddA and its phosphoramidate prodrug Cf-1093 were 1 and 0.004 μM, respectively, with an increase of efficacy of about 250-fold. Interestingly, the markedly enhanced activity of Cf-1093 was not counteracted by a similar increase of toxicity (only sixfold). Thus, the selectivity index (SI) of the Cf-1093 aryloxyphosphoramidate prodrug derivative of ddA in M/M was more than 40-fold greater than that of ddA. The increased antiviral activity of Cf-1093 found in M/M was similar to that found in lymphocytes, where the EC50s of Cf-1093 and ddA were 0.016 and 3.7 μM, respectively.
TABLE 2.
Anti-HIV activity of Cf-1093 and Cf-1001, the aryloxyphosphoramidate prodrug derivatives of ddA- and d4A-MP, respectivelya
| Compound | EC90 (μM) for M/M | EC50 (μM) for:
|
CC50b (μM) for:
|
SIc of M/M | ||
|---|---|---|---|---|---|---|
| M/M | CEM | M/M | CEM | |||
| ddA | NAd | 1 ± 0.5 | 3.7 ± 2.1 | 60 | >250 | 60 |
| Cf-1093 | 0.02 ± 0.003 | 0.004 ± 0.001 | 0.016 ± 0.0 | 10 | 2.6 ± 0.64 | 2500 |
| d4A | NA | 5 ± 1 | 29 ± 19 | 50 | 95 ± 7.1 | 10 |
| Cf-1001 | 0.03 ± 0.005 | 0.008 ± 0.003 | 0.006 ± 0.0007 | >5e | 3.75 ± 0.92 | 500 |
Results are presented as means ± standard deviations of tree values obtained in four different experiments.
CC50, 50% cytotoxic concentration.
SI = CC50/EC50.
NA, not achieved.
A higher concentration could not be tested due to DMSO toxicity. The stock solution of Cf-1001 was made in DMSO at a lower Cf-1001 concentration than those for the other compounds.
Since the prodrugs of two nucleosides with the same base, such as AZT and d4T, gave results markedly different from each other, we studied the antiviral effect of another ddA derivative, i.e., d4A, which was characterized by a very limited anti-HIV activity and a substantial toxicity in lymphocytes when administered as free nucleoside analogue. The antiviral effect of d4A was also limited in M/M (EC50, 5 μM). However, the Cf-1001 prodrug derivative showed a markedly greater efficacy in both M/M (Table 2) and the lymphocytic CEM cell line, with EC50s of 0.008 and 0.006 μM, respectively. Also in this case, as for ddA, a 625-fold increase in antiviral activity of Cf-1001, compared to d4A, was not accompanied by a marked enhancement of toxicity. Indeed, since the toxicity increased only 6- to 10-fold, the net-to-SI of Cf-1001 became 50-fold greater than that of d4A in M/M (Table 2). Thus, in the case of both adenosine prodrug analogues, a substantially greater antiviral activity and selectivity was noted, in comparison with the corresponding parent compounds. It's not coincidental, as it is for d4T, that also in the cases of ddA and d4A, the bottleneck in their phosphorylation pathway is at the level of the first phosphorylation, bypassed by the treatment with their phosphorylated protides (9). Thus, Cf-1001 and Cf-1093 display high SI values in primary M/M, which considerably improve the therapeutic window of these new nucleotide prodrugs. Overall, this suggests that the increased antiviral activity of these compounds (and perhaps other purine analogues currently in development) can be obtained, in vitro and perhaps in vivo, without increasing the toxicity for the host.
Different results again were obtained in the cases of Cf-1221 and Cf-1109, the phosphoramidate derivatives of ddC and 3TC, respectively. The antiviral activity of Cf-1109 (the prodrug of 3TC) was slightly lower than that of the parent 3TC in M/M, with EC50s of 0.032 and 0.016 μM, respectively (Table 3). In the case of the ddC prodrug Cf-1221, the difference of the antiviral activity, compared to ddC, was even more striking, with a net loss of about 30-fold in terms of EC50s (0.09 and 0.003 μM, respectively). Thus, in the case of derivatives of 2′-deoxycytidine analogues, a loss of antiviral activity in M/M, but also in lymphocytes, was obtained if ddC and 3TC were administered to the cells as their aryloxyphosphoramidate prodrugs.
TABLE 3.
Anti-HIV activity of Cf1221 and Cf-1109, the aryloxyphosphoramidate prodrug derivatives of ddC- and 3TC-MP, respectivelya
| Compound | EC90 (μM) for M/M | EC50 (μM) for:
|
CC50b (μM) for:
|
SIc of:
|
|||
|---|---|---|---|---|---|---|---|
| M/M | CEM | M/M | CEM | M/M | CEM | ||
| 3TC | 0.13 ± 0.01 | 0.016 ± 0.004 | 0.01 ± 0.003 | >100 | >100 | >6,250 | >10,000 |
| Cf-1109 | 0.18 ± 0.02 | 0.032 ± 0.003 | 2.5 ± 0.7 | >100 | >100 | >3,100 | >40 |
| ddC | 0.01 ± 0.004 | 0.003 ± 0.001 | 0.04 ± 0.01 | >10 | 3.5 ± 0.53 | >3,300 | 80 |
| Cf-1221 | 0.48 ± 0.02 | 0.09 ± 0.01 | 0.60 ± 0.0 | >20 | 114 ± 23 | >200 | 190 |
Results are presented as means ± standard deviations of tree values obtained in four different experiments.
CC50, 50% cytotoxic concentration.
SI = CC50/EC50.
There are tree possibilities that could account for this decreased activity. One, the poorer uptake of 3TC-ddC prodrugs, compared to the parental compounds, is rather unlikely, since the prodrugs are thought to enter the cells by passive diffusion, thus independent from the nature of the nucleoside but governed by the high lipophilicity of all the protides. At the same time, it is rather difficult to account for this difference with a second hypothesis, that is, a different rate of di- and triphosphorylation of the MP derived from the parent compound and its aryloxyphosphoramidate protides. Thus, a low efficacy of intracellular release of ddC–ddC-MP and 3TC–3TC-MP from the prodrug molecules could more likely explain the limited efficacy, in lymphocytes and M/M, of the cytosine-containing prodrugs compared with the thymine- or adenine-containing prodrugs. Regarding this latter hypothesis, it has been observed that Cf-1109 has identical antiviral potency against hepatitis B virus in hepatocytes as 3TC (8). Thus, we may conclude that the enzymes responsible for the release of 3TC-MP (and ddC-MP) may be less active and operative in lymphocytes and M/M than in hepatoma cells.
Regarding the SIs, their precise evaluation has been hampered by the limited toxicity of some nucleoside analogues and their aryloxyphosphoramidate derivatives showing lack of signs of toxicity at concentrations of 100 μM. Nevertheless, where this calculation was possible (as in the case of the 2′-deoxyadenosine analogues) the SIs were greatly increased following administration of the aryloxyphosphoramidate derivatives. This suggests that the increased antiviral activity can be obtained, in vitro and perhaps in vivo, without necessarily increasing the toxicity for the host.
In conclusion, the success and efficiency of the aryloxyphosphoramidate prodrug approach to enhance the antiviral potency of nucleoside analogues depends to a large extent on the nature of the nucleoside analogues and the cell system evaluated. Most prodrugs exhibited superior anti-HIV activity in M/M, not only when compared to their parent compounds (i.e., d4T, ddA, and d4A) but also when compared to their antiviral activity in lymphocytes (i.e., d4T, 3TC, and ddC). Therefore, given the pivotal role of HIV-infected M/M in the HIV infection process and eventual pathogenicity, application of the aryloxyphosphoramidate technology to several (but not all) ddN derivatives currently in the clinic may further improve their specificity, antiviral potency, and/or therapeutic potential.
Acknowledgments
This work was supported by grants from CNR, ISS Ministry of Health, Italy, the European Commission, and the United Kingdom Medical Research Council. Stefano Aquaro was supported by a grant from ISS and Ministry of Health.
We thank Franca Serra, Tania Guenci, and Fabio Marcuccilli for their technical assistance.
REFERENCES
- 1.Amerongen H M, Weltzin R, Farnet C M, Michetti P, Haseltine W A, Neutra M R. Transepithelial transport of HIV-1 by intestinal M cells: a mechanism for transmission of AIDS. J Acquir Immune Defic Syndr. 1991;4:760–765. [PubMed] [Google Scholar]
- 2.Aquaro S, Balestra E, Cenci A, Francesconi M, Calio R, Perno C F. HIV infection in macrophage: role of long-lived cells and related therapeutical strategies. J Biol Regul Homeost Agents. 1997;11:69–73. [PubMed] [Google Scholar]
- 3.Aquaro S, Calio R, Balestra E, Bagnarelli P, Cenci A, Bertoli A, Tavazzi B, Di Pierro D, Francesconi M, Abdelahad D, Perno C F. Clinical implications of HIV dynamics and drug resistance in macrophages. J Biol Regul Homeost Agents. 1998;12:23–27. [PubMed] [Google Scholar]
- 4.Aquaro S, Perno C F, Balestra E, Balzarini J, Cenci A, Francesconi M, Panti S, Serra F, Villani N, Calio R. Inhibition of replication of HIV in primary monocyte/macrophages by different antiviral drugs and comparative efficacy in lymphocyte. J Leukoc Biol. 1997;62:138–143. doi: 10.1002/jlb.62.1.138. [DOI] [PubMed] [Google Scholar]
- 5.Avramis V I, Markson W, Jackson R L, Gomperts E. Biochemical pharmacology of zidovudine in human T-lymphoblastoid cells (CEM) AIDS. 1989;3:417–422. doi: 10.1097/00002030-198907000-00002. [DOI] [PubMed] [Google Scholar]
- 6.Bagnarelli P, Valenza A, Menzo S, Sampaolesi R, Varaldo P E, Butini L, Montroni M, Perno C F, Aquaro S, Mathez D, Leibowitch J, Balotta C, Clementi M. Dynamics and modulation of human immunodeficiency virus type 1 transcripts in vitro and in vivo. J Virol. 1996;70:7603–7613. doi: 10.1128/jvi.70.11.7603-7613.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Balzarini J, Karlsson A, Aquaro S, Perno C F, Cahard D, Naesens L, De Clercq E, McGuigan C. Mechanism of anti-HIV action of masked alaninyl d4T-MP derivatives. Proc Natl Acad Sci USA. 1996;93:7295–7299. doi: 10.1073/pnas.93.14.7295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Balzarini J, Wedgwood O, Kruining J, Pelemans H, Heijtink R, De Clercq E, McGuigan C. Anti-HIV and anti-HBV activity and resistance profile of 2′,3′-dideoxy-3′-thiacytidine (3TC) and its arylphosphoramidate derivative CF 1109. Biochem Biophys Res Commun. 1996;225:363–369. doi: 10.1006/bbrc.1996.1181. [DOI] [PubMed] [Google Scholar]
- 9.Cooney D A, Ahluwalia G, Mitsuya H, Fridland A, Johnson M, Hao Z, Dalal M, Balzarini J, Broder S, Johns D G. Initial studies on the cellular pharmacology of 2′,3′-dideoxyadenosine, an inhibitor of HTLV-III infectivity. Biochem Pharmacol. 1987;36:1765–1768. doi: 10.1016/0006-2952(87)90235-8. [DOI] [PubMed] [Google Scholar]
- 10.Gartner S, Markovits P, Markovitz D M, Kaplan M H, Gallo R C, Popovic M. The role of mononuclear phagocytes in HTLV-III/LAV infection. Science. 1986;233:215–219. doi: 10.1126/science.3014648. [DOI] [PubMed] [Google Scholar]
- 11.Haworth S J, Christofalo B, Anderson R D, Dunkle L M. A single-dose study to assess the penetration of stavudine into human cerebrospinal fluid in adults. J Acquir Immune Defic Syndr Hum Retrovirol. 1998;17:235–238. doi: 10.1097/00042560-199803010-00008. [DOI] [PubMed] [Google Scholar]
- 12.Kalter D C, Gendelman H E, Meltzer M S. Monocytes, dendritic cells, and Langerhans cells in human immunodeficiency virus infection. Dermatol Clin. 1991;9:415–428. [PubMed] [Google Scholar]
- 13.Koenig S, Gendelman H E, Orenstein J M, Dal Canto M C, Pezeshkpour G H, Yungbluth M, Janotta F, Aksamit A, Martin M A, Fauci A. Detection of AIDS virus in macrophages in brain tissue from AIDS patients with encephalopathy. Science. 1986;233:1089–1093. doi: 10.1126/science.3016903. [DOI] [PubMed] [Google Scholar]
- 14.Kure K, Lyman W D, Weidenheim K M, Dickson D W. Cellular localization of an HIV-1 antigen in subacute AIDS encephalitis using an improved double-labeling immunohistochemical method. Am J Pathol. 1990;136:1085–1092. [PMC free article] [PubMed] [Google Scholar]
- 15.Lefebvre I, Perigaud C, Pompon A, Aubertin A M, Girardet J L, Kirn A, Gosselin G, Imbach J L. Mononucleoside phosphotriester derivatives with S-acyl-2-thioethyl bioreversible phosphate-protecting groups: intracellular delivery of 3′-azido-2′,3′-dideoxythymidine 5′-monophosphate. J Med Chem. 1995;38:3941–3950. doi: 10.1021/jm00020a007. [DOI] [PubMed] [Google Scholar]
- 16.Lewis L L, Venzon D, Church J, Farley M, Wheeler S, Keller A, Rubin M, Yuen G, Mueller B, Sloas M, Wood L, Balis F, Shearer G M, Brouwers P, Goldsmith J, Pizzo P A. Lamivudine in children with human immunodeficiency virus infection: a phase I/II study. The National Cancer Institute Pediatric Branch-Human Immunodeficiency Virus Working Group. J Infect Dis. 1996;174:16–25. doi: 10.1093/infdis/174.1.16. [DOI] [PubMed] [Google Scholar]
- 17.Malorni W, Lucia M B, Rainaldi G, Cauda R, Cianfriglia M, Donelli G, Ortona L. Intracellular expression of P-170 glycoprotein in peripheral blood mononuclear cell subsets from healthy donors and HIV-infected patients. Haematologica. 1998;83:13–20. [PubMed] [Google Scholar]
- 18.McElrath M J, Pruett J E, Cohn Z A. Mononuclear phagocytes of blood and bone marrow: comparative roles as viral reservoirs in human immunodeficiency virus type 1 infections. Proc Natl Acad Sci USA. 1989;86:675–679. doi: 10.1073/pnas.86.2.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McGuigan C, Cahard D, Sheeka H M, De Clercq E, Balzarini J. Aryl phosphoramidate derivatives of d4T have improved anti-HIV efficacy in tissue culture and may act by the generation of a novel intracellular metabolite. J Med Chem. 1996;39:1748–1753. doi: 10.1021/jm950605j. [DOI] [PubMed] [Google Scholar]
- 20.McGuigan C, Cahard D, Sheeka H M, De Clercq E, Balzarini J. Phosphoramidate derivatives of d4T with improved anti-HIV efficacy retain full activity in thymidine kinase-deficient cells. Bioorg Med Chem Lett. 1996;6:1183–1186. [Google Scholar]
- 21.McGuigan C, Kinchington D, Wang M F, Nicholls S R, Nickson C, Galpin S, Jeffries D J, O'Connor T J. Nucleoside analogues previously found to be inactive against HIV may be activated by simple chemical phosphorylation. FEBS Lett. 1993;322:249–252. doi: 10.1016/0014-5793(93)81580-s. [DOI] [PubMed] [Google Scholar]
- 22.McGuigan C, Pathirana R N, Balzarini J, De Clercq E. Intracellular delivery of bioactive AZT nucleotides by aryl phosphate derivatives of AZT. J Med Chem. 1993;36:1048–1052. doi: 10.1021/jm00060a013. [DOI] [PubMed] [Google Scholar]
- 23.McIntee E J, Remmel R P, Schinazi R F, Abraham T W, Wagner C R. Probing the mechanism of action and decomposition of amino acid phosphomonoester amidates of antiviral nucleoside prodrugs. J Med Chem. 1997;40:3323–3331. doi: 10.1021/jm960694f. [DOI] [PubMed] [Google Scholar]
- 24.Meier C, Aubertin A M, de Monte M, Faraj A, Sommadossi J P, Perigaud C, Imbach J L, Gosselin G. Synthesis and antiviral evaluation of SATE-foscarnet prodrugs and new foscarnet-AZT conjugates. Antivir Chem Chemother. 1998;9:41–52. doi: 10.1177/095632029800900105. [DOI] [PubMed] [Google Scholar]
- 25.Meier C, Lorey M, De Clercq E, Balzarini J. cycloSal-2′,3′-dideoxy-2′,3′-didehydrothymidine monophosphate (cycloSal-d4TMP): synthesis and antiviral evaluation of a new d4TMP delivery system. J Med Chem. 1998;41:1417–1427. doi: 10.1021/jm970664s. [DOI] [PubMed] [Google Scholar]
- 26.Meltzer M S, Nakamura M, Hansen B D, Turpin J A, Kalter D C, Gendelman H E. Macrophages as susceptible targets for HIV infection, persistent viral reservoir in tissue, and key immunoregulatory cells that control levels of virus replication and extent of disease. AIDS Res Hum Retrovir. 1990;6:967–971. doi: 10.1089/aid.1990.6.967. [DOI] [PubMed] [Google Scholar]
- 27.Nakata K, Weiden M, Harkin T, Ho D, Rom W N. Low copy number and limited variability of proviral DNA in alveolar macrophages from HIV-1-infected patients: evidence for genetic differences in HIV-1 between lung and blood macrophage populations. Mol Med. 1995;1:744–757. [PMC free article] [PubMed] [Google Scholar]
- 28.O'Brien W A, Namazi A, Kalhor H, Mao S H, Zack J A, Chen I S. Kinetics of human immunodeficiency virus type 1 reverse transcription in blood mononuclear phagocytes are slowed by limitations of nucleotide precursors. J Virol. 1994;68:1258–1263. doi: 10.1128/jvi.68.2.1258-1263.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Orenstein J M, Fox C, Wahl S M. Macrophages as a source of HIV during opportunistic infections. Science. 1997;276:1857–1861. doi: 10.1126/science.276.5320.1857. [DOI] [PubMed] [Google Scholar]
- 30.Perigaud C, Aubertin A M, Benzaria S, Pelicano H, Girardet J L, Maury G, Gosselin G, Kirn A, Imbach J L. Equal inhibition of the replication of human immunodeficiency virus in human T-cell culture by ddA bis(SATE)phosphotriester and 3′-azido-2′,3′-dideoxythymidine. Biochem Pharmacol. 1994;48:11–14. doi: 10.1016/0006-2952(94)90217-8. [DOI] [PubMed] [Google Scholar]
- 31.Perno C F, Newcomb F M, Davis D A, Aquaro S, Humphrey R W, Calio R, Yarchoan R. Relative potency of protease inhibitors in monocytes/macrophages acutely and chronically infected with human immunodeficiency virus. J Infect Dis. 1998;178:413–422. doi: 10.1086/515642. [DOI] [PubMed] [Google Scholar]
- 32.Perno C F, Yarchoan R. Culture of HIV in monocytes and macrophages. In: Coligan J E, Kruisbeek A M, Margulies D H, Shevach E M, Strober W, editors. Current protocols in immunology. New York, N.Y: John Wiley & Sons; 1993. pp. 12.4.1–12.4.11. [Google Scholar]
- 33.Perno C F, Yarchoan R, Cooney D A, Hartman N R, Gartner S, Popovic M, Hao Z, Gerrard T L, Wilson Y A, Johns D G, Broder S. Inhibition of human immunodeficiency virus (HIV-1/HTLV-IIIBa-L) replication in fresh and cultured human peripheral blood monocytes/macrophages by azidothymidine and related 2′,3′-dideoxynucleosides. J Exp Med. 1988;168:1111–1125. doi: 10.1084/jem.168.3.1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schmitt M P, Gendrault J L, Schweitzer C, Steffan A M, Beyer C, Royer C, Jaeck D, Pasquali J L, Kirn A, Aubertin A M. Permissivity of primary cultures of human Kupffer cells for HIV-1. AIDS Res Hum Retrovir. 1990;6:987–991. doi: 10.1089/aid.1990.6.987. [DOI] [PubMed] [Google Scholar]
- 35.Schrager L K, D'Souza M P. Cellular and anatomical reservoirs of HIV-1 in patients receiving potent antiretroviral combination therapy. JAMA. 1998;280:67–71. doi: 10.1001/jama.280.1.67. [DOI] [PubMed] [Google Scholar]
- 36.Thumann Schweitzer C, Gosselin G, Perigaud C, Benzaria S, Girardet J L, Lefebvre I, Imbach J L, Kirn A, Aubertin A M. Anti-human immunodeficiency virus type 1 activities of dideoxynucleoside phosphotriester derivatives in primary monocytes/macrophages. Res Virol. 1996;147:155–163. doi: 10.1016/0923-2516(96)80230-5. [DOI] [PubMed] [Google Scholar]
- 37.Zambruno G, Giannetti A, Bertazzoni U, Girolomoni G. Langerhans cells and HIV infection. Immunol Today. 1995;16:520–524. doi: 10.1016/0167-5699(95)80044-1. [DOI] [PubMed] [Google Scholar]