Abstract
Introduction
To generalize safety and efficacy findings, it is essential that diverse populations are well represented in Alzheimer's disease (AD) drug trials. In this review, we aimed to investigate participant diversity in disease‐modifying AD trials over time, and the frequencies of participant eligibility criteria.
Methods
A systematic review was performed using Medline, Embase, the Cochrane Library, and Clinicaltrials.gov, identifying 2247 records.
Results
In the 101 included AD trials, participants were predominantly White (median percentage: 94.7%, interquartile range: 81.0–96.7%); and this percentage showed no significant increase or decrease over time (2001–2019). Eligibility criteria such as exclusion of persons with psychiatric illness (78.2%), cardiovascular disease (71.3%) and cerebrovascular disease (68.3%), obligated caregiver attendance (80.2%), and specific Mini‐Mental State Examination scores (90.1%; no significant increase/decrease over time) may have led to a disproportionate exclusion of ethnoracially diverse individuals.
Discussion
Ethnoracially diverse participants continue to be underrepresented in AD clinical trials. Several recommendations are provided to broaden eligibility criteria.
Keywords: clinical trial, clinical trial protocols, cultural diversity, ethnic groups, phase II, phase III, randomized controlled trials
1. INTRODUCTION
Although ethnoracially diverse individuals are at an increased risk of developing Alzheimer's disease (AD) dementia, 1 , 2 , 3 , 4 these populations are systematically underrepresented in AD clinical trials. 5 , 6 , 7 To generalize safety and efficacy findings from drug trials to the general population, it is essential to include a diverse population, as differences in pharmacokinetics and pharmacodynamics across diverse populations may impact treatment effect and safety; 8 , 9 for instance, drug metabolism rates may differ. 6 The lack of diversity among clinical trial participants is often attributed to enrolling and retaining practices, such as recruitment strategies that do not account for factors that play a role in diverse populations, including mistrust and worry because of historical racism in medical research or the possibility of injury or complications. 10
Although recruitment factors should be taken into consideration, other explanations need to be considered as well, especially because a number of studies have indicated that people from underrepresented populations may be equally willing to participate in health research. 11 , 12 One important potential cause is that there are inherent features of AD‐clinical trial eligibility criteria that lead to a disproportionate and systemic exclusion of underrepresented populations. 13 , 14 In 1997, Schneider et al. 14 demonstrated that applying the eligibility criteria of typical AD clinical trials to a Californian memory clinic population led to a systematic underrepresentation of people who are older, female, ethnoracially diverse, lower educated, and less wealthy; they provided several suggestions to improve provisional eligibility, such as a wider range of allowed scores on the Mini‐Mental State Examination (MMSE 15 ) or by allowing more patients with (mild) behavioral and psychological symptoms to participate.
This systematic review aims to take a closer look at diversity in clinical trials and eligibility criteria. The first goal was to investigate the level of participant diversity in AD clinical trials in the decades after the publication of Schneider et al. 14 The second goal was to identify which eligibility criteria have been used and how these eligibility criteria were defined. Third, we aimed to assess whether the use of criteria related to cognitive and neuropsychiatric instruments such as the MMSE have changed over time, as these were highlighted by Schneider et al. 14 as particularly problematic. Last, we will discuss how some eligibility criteria may have affected diversity levels in AD clinical trials.
2. METHODS
2.1. Search strategy
We performed a systematic review using Medline (which includes PubMed), Embase, the Cochrane Library, and ClinicalTrials.gov, without restrictions on the year of publication or location of the trial. Search terms included different terms for AD and mild cognitive impairment (MCI), terms referring to disease‐modifying drugs, terms related to amyloid beta (Aβ) and tau, and different terms for phase II and phase III trials (for the complete lists of the search terms used, see Text S1 in supporting information). Studies were included up to December 2019. Two independent authors screened all collected study data (JS and SF). Disagreement was resolved by a consensus agreement together with JMP. The PRISMA (Preferred Reporting Items for Systematic Reviews and Meta‐Analyses) guidelines 16 were followed, except for an assessment of the risk of bias—this step was omitted, as the aim was not to review or summarize the treatment effect reported in the included clinical trials.
2.2. Eligibility criteria
To be included in the review:
The study needed to be a planned, ongoing, completed, or early terminated phase II or phase III drug trial for patients with AD dementia, prodromal AD (early AD stage 3 17 ), or amnestic MCI (aMCI).
The experimental drug was a disease‐modifying treatment. Disease‐modifying was defined as targeting the pathogenic steps in the Aβ or tau pathways. This includes passive vaccination, monoclonal antibodies, agents disrupting accumulation or aggregation, and agents increasing clearance. As no agreed‐upon standards are currently available that definitively delineate which drugs are considered disease‐modifying, drug mechanisms were confirmed by consulting relevant literature (e.g., Galimberti and Scarpini 18 ) and examining trial features (e.g., outcomes measuring amyloid clearance).
To adequately capture recent developments; collate study results; and provide a clearly delineated, concise set of recommendations, we focused on a homogeneous set of trials and excluded several other types of trials and study populations from this review. First, we excluded studies focusing on other forms of dementia. Second, we excluded AD prevention trials (e.g., lifestyle intervention trials) and studies in preclinical AD (early AD stages 1–2 17 ) as these types of trials present with unique challenges and eligibility criteria. Third, we excluded studies focused on symptomatic treatment of AD, including studies of acetylcholinesterase inhibitors—tacrine, donepezil, rivastigmine, and galantamine—and memantine. Fourth, we excluded trials investigating herbal and dietary treatments (e.g., vitamin supplements, olive oil, huperzine). Conference abstracts, dissertations, comments, editorials, book chapters, white papers, and reviews were also excluded.
2.3. Data extraction
For each included study, all available study protocol sources—that is, published papers or National Clinical Trial (NCT) database, European Union Drug Regulating Authorities Clinical Trial Database (EudraCT), and Australian New Zealand Clinical Trial Registry (ANZCTR) clinical trial registrations—identified in the search were used for data extraction. When available, the year that the study was first posted, the study phase, the investigational drug, the inclusion and exclusion criteria, the number of recruited participants, and participant demographics were recorded. Information was compiled from all available sources to create the most complete account of each study's design and study sample.
2.4. Data analysis
Participant eligibility criteria were divided into three main categories: (1) criteria related to medical conditions; (2) criteria related to undergoing specific study procedures, such as neuropsychological tests and brain scans; and (3) criteria based on diagnostic tests and questionnaire outcomes. Analyses were mostly descriptive. We used Cochran‐Armitage trend tests (using the CATT package in R) to assess trends over time for binary variables, that is, whether a criterion was used in the trial or not. Spearman correlations were used to analyze associations between the study start year and continuous variables.
3. RESULTS
We identified 2247 records. The review process is summarized in the PRISMA flowchart in Figure 1. After deduplication, 1777 records remained; these records were screened on title and abstract. If the topic of the abstract fell within the criteria, but there was insufficient information on drug mechanism and/or trial phase, we reviewed the full text. A total of 506 records (clinical trial registrations or papers) were assessed in full for eligibility. A total of 17 NCT registrations, 35 EudraCT registrations, and one ANZCTR registration linked to published papers were retrieved manually. For three studies for which a published paper was available, we could not identify a clinical trial registration.
FIGURE 1.
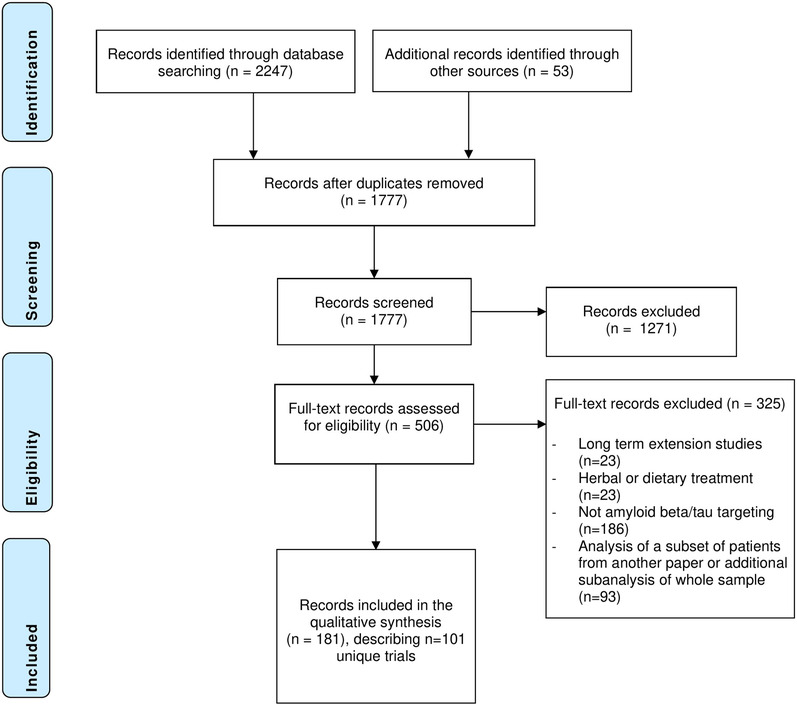
Results of database searches and selection process
RESEARCH IN CONTEXT
Systematic review: We reviewed the published literature and clinical trial registries to examine participant diversity in Alzheimer's disease drug trials, as well as developments in eligibility criteria for these trials. The trials covered a time period from 2001–2019.
Interpretation: Study samples in AD trials were predominantly white, and participant diversity did not change over time. Several characteristics of eligibility criteria may lead to systemic exclusion of diverse populations, such as the use of specific cognitive tests, requirements regarding language fluency, education, or caregiver attendance, and the exclusion of patients with comorbid conditions.
Future directions: This review highlights several eligibility criteria that require more research/consensus, such as how to best operationalize medical conditions and which cognitive and clinical tests may best be used in diverse populations. In addition, it highlights the need for revision of race/ethnicity categorization and for better reporting of diversity‐related data.
A total of 101 trials were included in this review. We extracted information about these trials from 181 unique papers and clinical trial registrations, as well as from 21 full protocols that were attached to the included papers or clinical trial registrations. The full protocols were not publicly available for the remaining trials. The sample consisted of 67 phase II trials and 34 phase III trials, investigating 47 different drugs. The studies covered 2001 to 2019, during which 79 studies had finished recruitment, and 22 studies had not yet commenced or were registered as active/recruiting. A listing of the included papers and clinical trial registration numbers is provided in Table S1 in supporting information. Several of the eligibility criteria were more prevalent in studies for which a full protocol was available as opposed to studies for which a full protocol was not available (see Text S2 in supporting information).
3.1. Diversity in clinical trial participants
Of the 101 trials, most had one or more study site(s) in North America (79.2%) or Europe (60.4%), and less frequently Asia (36.6%), or Oceania (32.7%); even fewer trials included study sites in South America (14.9%) or Africa (6.9%). Race/ethnicity data of the enrolled participants was available for less than half of the clinical trials (46 studies, 45.5%). Of these trials, 10 (9.9%) reported only the percentage of White participants without specifying percentages for any other ethnoracial groups, and four (4.0%) included White participants only. Race/ethnicity data was available for 58.2% (46/79) of the studies that were registered as completed or early terminated. When looking specifically at trials for which a published paper was available, 75.5% reported any race/ethnicity data (40/53). Different race/ethnicity categorizations were used across studies. Trials in Clinicaltrials.gov often reported race and/or ethnicity according to the National Institutes of Health/Office of Management and Budget (NIH/OMB) categories. Although few papers explicitly reported using the NIH/OMB categorization, a selection of these categories was often used in papers as well, whereas other categorizations were used very infrequently—one trial conducted across Asia, Europe, North America, and South America reported numbers for “Caucasian,” “African,” “Hispanic,” “East Asian,” and “West Asian” participants, and a paper about a trial conducted in the UK and Singapore reported the numbers of “Afro‐Caribbean,” “Asian,” and “Caucasian” participants.
The median reported percentage of White participants in all studies was 94.7% (interquartile range [IQR]: 81.0–96.7%). This percentage of White participants was invariably high across both trials that did and those that did not use specific eligibility criteria (see Table S2 in supporting information). Only seven studies reported the number of participants with a Latinx (Latina/o) ethnic background (median: 5.6%, IQR: 4.2–11.4%); specifically, 20.0% of the trials that included a North American site for which race/ethnicity data was available (7/35) reported the number of participants with a Latinx ethnic background. Data regarding (non‐)Latinx background was often presented separate from the number of participants in each racial group; it was therefore unclear how many participants with a Latinx background were included across racial groups (e.g., Latinx–White). The median percentage of Black/African American participants was 1.2% (IQR: 0.4–1.7%), and the median percentage of Asian participants was 4.4% (IQR: 0.3–17.3%; NB: three studies from Asia had samples consisting of 100% Asian participants). The median percent of other or multiracial participants was 0.9% (IQR: 0.0–1.9%).
We found no statistically significant relationship between the percentage of White participants and the study start year (ρ = –.26, P = .09). Of the studies for which a published paper was available 47.2% (25/53) reported the number of people who did not meet the eligibility criteria. Only 17.0% (9/53) specified which criteria most frequently were the cause of participant exclusion. Although one study (NCT00105547) reported whether the excluded and included patients differed on age and sex, none of the studies reported whether included and excluded participants differed on race/ethnicity.
Of the studies reporting race/ethnicity, none explicitly referred to socioeconomic status (SES), while 41.3% (19/46) reported on the participants’ education level. We extracted the mean education level of the total sample for each of these studies and calculated the average of the reported means across placebo and intervention groups for studies that did not report the total sample mean. The average mean number of years of education across these studies was 13.3 years, and a higher mean level of education was significantly correlated with a higher percentage of White participants included in the trial (ρ = .61, P = .02).
3.2. Eligibility criteria
3.2.1. Criteria related to medical conditions
The frequency of exclusion criteria related to medical conditions is displayed in the first columns of Table 1, ranked from most prevalent (top) to least prevalent (bottom). In the remaining columns to the right, we present the prevalence of these medical conditions in several ethnoracial groups to provide context for the potential impact on ethnoracial diversity of participants. In addition to ethnoracial groups within the United States 19 (non‐Latinx White, Latinx, non‐Latinx Black, American Indian/Alaska Native), we have included prevalence estimates from the Indigenous Australian population 20 as an example to illustrate the potential impact of eligibility criteria on an international scale (see note to Table 1 for additional sources used to compile this table).
TABLE 1.
Frequencies of eligibility criteria related to medical conditions and prevalence of medical conditions in American and Australian ethnoracial groups*
| Criterion frequency in all trials (N = 101) | % in n‐L White Americans | % in Latinx Americans | % in n‐L Black Americans | % in American Indian and Alaska Native | % in Indigenous Australians † | ||
|---|---|---|---|---|---|---|---|
| Other neurological disease | 81 | 80.2% | — | — | — | — | — |
| Psychiatric disorder | 79 | 78.2% | 6.9% | 9.4% | 9.7% | — | 12% (9.6%) |
| Cardiovascular disease | 72 | 71.3% | 11.5% | 8.2% | 10.0% | 14.6% | 13% (1.2x) |
| Cerebrovascular disease | 69 | 68.3% | 2.6% | 2.5% | 3.9% | 3.0% | — |
| ‐ Hachinski ischemia scale score >4 | 53 | 52.5% | — | — | — | — | — |
| ‐ Cerebrovascular evidence on MRI | 48 | 47.5% | — | — | — | — | — |
| Childbearing/conception | 62 | 61.4% | — | — | — | — | — |
| Unspecified systemic illness | 62 | 61.4% | — | — | — | — | — |
| Alcohol or drug abuse | 59 | 58.4% | 8.4% | 8.6% | 7.4% | 14.9% | 18% (19%) |
| Vitals or lab abnormalities | 53 | 52.5% | — | — | — | — | — |
| Infections/infectious diseases | 50 | 49.5% | — | — | — | — | — |
| ‐ HIV status ‡ | 26 | 25.7% | 4.8 ‡ | 16.4 ‡ | 39.2 ‡ | 7.7 ‡ | 5.5 ‡ (4.5 ‡ ) |
| Liver disease | 48 | 47.5% | 1.7% | 2.7% | 1.1% | 2.5% |
15%‐23% (1.4x‐2.1x) |
| Autoimmune disease | 47 | 46.5% | 22.0% | 16.8% | 21.0% | 30.6% | 10.0% (1.1x) |
| Renal disease | 46 | 45.5% | 2.0% | 2.2% | 3.1% | — | 3.0% (≈3.7x) |
| Seizure disorder | 44 | 43.6% | — | — | — | — | — |
| Cancer | 41 | 40.6% | 9.1% | 4.2% | 5.1% | 7.1% | 1.7% (1.5%) |
| Respiratory illness § | 26 | 25.7% |
7.5% § ; 3.6% |
6.0% § ; 2.7% |
9.1% § ; 3.4% |
9.5%; — |
18% (1.9x) — |
| Endocrine dysfunction | 25 | 24.8% | — | — | — | — | — |
| Brain/head trauma | 25 | 24.8% | — | — | — | — | — |
| Diabetes ¶ | 23 | 22.8% |
8.6% ¶ ; 13.0% |
13.2% ¶ ; 21.5% |
13.1% ¶ ; 19.6% |
23.5% — |
11% (3.3x) — |
| Weight or BMI cut‐off | 21 | 20.8% | 31.0% | 34.9% | 38.0% | 48.1% | 37% (1.6x) |
| Gastrointestinal disease | 18 | 17.8% | 5.7% | 4.3% | 4.9% | 8.3% | — |
| Excessive smoking (≥20 cigarettes per day) | 9 | 8.9% | — | — | — | — | — |
| CNS inflammation | 8 | 7.9% | — | — | — | — | — |
| Systemic inflammation | 6 | 5.9% | — | — | — | — | — |
Abbreviations: BMI, body mass index; CNS, central nervous system; HIV, human immunodeficiency virus; MR, magnetic resonance imaging; n‐L, non‐Latinx.
2018 US National Health Interview study data 19 and 2015 Australian Institute of Health and Welfare data are presented 20 (unless otherwise specified), providing prevalence rates for the following specific conditions within the broader categories specified in the first column: psychiatric disorders = moderate to severe depressive symptoms (US 78 ) versus feeling depressed (AUS); cardiovascular disease = any; cerebrovascular disease = stroke; alcohol or drug abuse = substance dependence or abuse (US 79 ) versus lifetime risky alcohol consumption (AUS); infections – HIV status (US 80 ); autoimmune disease = arthritis diagnosis; renal disease = weak or failing kidneys (USA) versus chronic kidney disease stages 3‐5 (AUS); liver disease = any (US) versus abnormal ALT/GGT (AUS); cancer = any; weight or BMI = obesity; gastrointestinal disease = ulcers (duodenal, stomach, peptic).
In parentheses: times increased risk compared to non‐Indigenous Australians or prevalence rate in non‐Indigenous Australians.
Diagnosis rate per 100,000.
Respiratory illness = current asthma (top) and chronic bronchitis (bottom).
Diabetes = diagnosed (top) versus diagnosed and undiagnosed combined (bottom 81 ).
Non‐AD neurological diseases and (major) psychiatric disorders were used as an exclusion criterion in more than three quarters of the included AD trials (Table 1, column 3), followed by cardiovascular disease (71.3%) and a history of cerebrovascular disease (68.3%). The last five columns of Table 1 demonstrate that the prevalence of some medical conditions is higher in either non‐Latinx Black US residents, Latinx US residents, American Indian/Native Alaskan US residents, or Indigenous Australians than in non‐Latinx White US residents or non‐Indigenous Australians: diabetes, major psychiatric disease, cerebrovascular disease, renal disease, alcohol/substance use disorder, liver disease, higher weight/body mass index (BMI), and human immunodeficiencey virus (HIV) diagnosis rates. For diabetes, studies sometimes referred to specific HbA1c levels, but these levels varied substantially from <6.0% to <9.0%; other studies included “insulin dependent” diabetes, “poorly controlled” diabetes, or merely “diabetes.” Studies with a BMI criterion mostly required participants to have a minimum BMI of 18 or higher, but the upper cut‐off value varied considerably from 28 to 40. Weight criteria specified a minimum weight of between 35 and 45 kg (≈77–99 pounds), mostly with a maximum of 120 kg (≈265 pounds). For hepatic disease, specific alanine transaminase (ALT; 1.5–3 times upper limit of normal, or ULN), aspartate transaminase (AST; 1.5–3 times ULN), and/or bilirubin (1.5–2.5 times ULN) cut‐off levels were generally defined. For renal conditions, some studies referred to specific levels of creatinine clearance, whereas others only described “severe” renal disease, “impaired renal function,” or specified dialysis requirement as the exclusion criterion.
3.2.2. Criteria related to study procedures
Caregiver attendance was the most prevalent criterion related to study procedures (80.2%, see Table 2), which often specified that the same caregiver had to attend all study visits and sometimes that the caregiver either had to live at the patient's home or had to visit a minimum number of times (range: <1–5 times/week) or hours per week (range: 4–24 hours/week). Some studies were more flexible, for example, by requiring the caregiver to accompany the patient only on key follow‐up visits and allowing the patient to be accompanied by a “delegate” on the other visits. Written informed consent (52.5%) and a contraindication to undergoing positron emission tomography (PET)/magnetic resonance imaging (MRI; 51.5%) were used as a criterion in the majority of the included AD clinical trials.
TABLE 2.
Frequencies of criteria related to undergoing study procedures
| Criterion frequency in all trials (N = 101) | ||
|---|---|---|
| Caregiver attendance | 81 | 80.2% |
| Written informed consent | 53 | 52.5% |
| Contraindication to MRI/PET | 52 | 51.5% |
| Adequate sensory abilities | 42 | 41.6% |
| Language ability | 35 | 34.7% |
| Residence in the community | 35 | 34.7% |
| Caregiver consent | 28 | 27.7% |
| Education requirement | 19 | 18.8% |
| Reading or writing ability | 19 | 18.8% |
| Determined likely to complete | 15 | 14.9% |
| Recent hospitalization | 4 | 4.0% |
Abbreviations: MRI, magnetic resonance imaging; PET, positron emission tomography.
Of the 19 studies using an education criterion, eight studies also allowed a work history consistent with no intellectual disabilities. For language fluency, most studies required fluency in the test language (n = 11), in the “local” language (n = 11), or in English (n = 8), while four studies allowed fluency in one of a number of languages. One study allowed fluency in any language with sponsor approval, as long as (1) staff were also fluent in that language, and (2) required study documents were available in that language. A subset of studies (14.9%) included a criterion whether patients or patient–caregiver dyads were likely to complete the study in the opinion of the investigator; an operationalization of this criterion was not provided.
3.2.3. Criteria related to diagnostic tests and questionnaires
Cognitive tests, batteries, or screeners were used as an inclusion criterion in nearly all studies, with little variety in the tests that were used; the MMSE score was a criterion in over 90% of the studies (Table 3). Aside from the MMSE, a handful of other screening tests/short batteries were used, such as the Repeatable Battery for the Assessment of Neuropsychological Status (RBANS 21 ), the Alzheimer's Disease Assessment Scale‐Cognitive Subscale (ADAS‐Cog 22 ), and the Montreal Cognitive Assessment (MoCA 23 ). Additionally, some studies used memory‐specific tests: the Free and Cued Selective Reminding Test (FCSRT 24 ), tests from the Wechsler Memory Scale–Revised (WMS‐R 25 ), and the International Shopping List Test (ISLT 26 ). One study used different cut‐off scores for the test they used (WMS‐R) to correct for education (0–7, 8–15, and ≥16 years); none of the other studies described different cut‐offs based on demographic or sociocultural characteristics known to impact cognitive test performance (e.g., age, sex, ethnicity, quality of education, acculturation, etc.).
TABLE 3.
Frequencies of neurocognitive and neuropsychiatric screening tests and measures
| Criterion frequency in all trials (N = 101) | ||
|---|---|---|
| COGNITIVE TESTS | ||
| MMSE | 91 | 90.1% |
| Memory‐specific test* | 7 | 6.9% |
| RBANS | 4 | 4.0% |
| ADAS‐Cog | 3 | 3.0% |
| MoCA | 1 | 1.0% |
| GLOBAL & FUNCTIONAL MEASURES | ||
| CDR | 36 | 35.6% |
| Eastern Cooperative Oncology Group status | 1 | 1.0% |
| FAQ | 1 | 1.0% |
| PSYCHIATRIC ASSESSMENTS | ||
| Geriatric Depression Scale | 25 | 24.8% |
| Hamilton Depression Rating Scale | 6 | 5.9% |
| Other depression instrument | 1 | 1.0% |
| C‐SSRS | 5 | 5.0% |
| Other/unspecified suicide or self‐harm risk scale | 14 | 13.9% |
Abbreviations: ADAS‐Cog, Alzheimer's Disease Assessment Scale‐Cognitive Subscale; C‐SSRS, Columbia Suicide Severity Rating Scale; CDR, Clinical Dementia Rating; FAQ, Functional Activities Questionnaire; MMSE, Mini‐Mental State Examination; MoCA, Montreal Cognitive Assessment; RBANS, Repeatable Battery for the Assessment of Neuropsychological Status.
Includes Free and Cued Selective Reminding Test (FCSRT), Wechsler Memory Scale‐Revised (WMS‐R), and International Shopping List Test (ISLT).
In addition to cognitive tests, roughly one‐third of the trials used the Clinical Dementia Rating (CDR 27 ) global score as a criterion. A similar proportion of studies used a measure of psychiatric symptoms as part of the eligibility criteria. For depression, the 15‐item version of the Geriatric Depression Scale (GDS 28 ) was used most often, as well as the Hamilton Depression Rating Scale. 29 The allowed range of scores for the GDS was relatively homogeneous across studies: the majority of studies (n = 22, 88% of studies with GDS) included patients with a score below 6 or 7, one study used the original 30‐item version and used a cut‐off score of ≤10, and two studies using a cut‐off of <8 did not specify whether the long or short version of the GDS was used. The Columbia Suicide Severity Rating Scale 30 was used a few times, but the majority of studies with a suicide risk criterion left the interpretation of this criterion to the opinion of the investigator (in contrast with depressive and cognitive symptoms).
Diagnostic tests and screeners: the use of the MMSE, CDR, and GDS over time
Additional Cochran‐Armitage trend analyses of the use of the MMSE revealed that the study start year did not differ between studies with or without an MMSE‐eligibility criterion (Z = 0.14, P = .89); that is, the MMSE cut‐off scores were not used significantly less (or more) often with time. As displayed in Figure 2, the cut‐off score for the MMSE increased over time (MMSE lower limit ρ = 0.53, P < .001; MMSE upper limit ρ = 0.48, P < .001). Furthermore, the range of allowed MMSE scores narrowed over time (ρ = –0.44, P < .001). Similar to the MMSE, the Cochran‐Armitage trend test showed that there was no statistically significant increase or decrease in the use of the GDS by study year (Z = 0.0, P = .99); the CDR, however, was used significantly more frequently in later years (Z = –2.48, P = .01).
FIGURE 2.
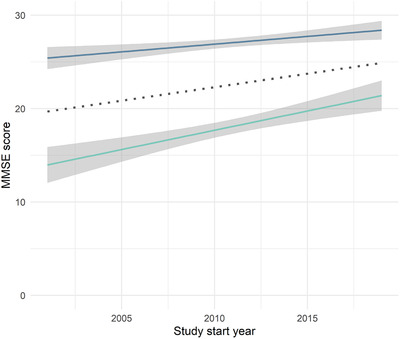
Changes in Mini‐Mental State Examination (MMSE) upper and lower cut‐off scores (midpoint in dotted line)
4. DISCUSSION
In this systematic review, we aimed to (1) investigate the level of participant diversity in AD clinical trials targeting Aβ and tau; (2) identify which eligibility criteria have been used and how these criteria were defined; and (3) discover whether the use of criteria related to cognitive and neuropsychiatric instruments changed over time. The results showed that study samples were predominantly composed of White individuals, and ethnoracial diversity levels did not show a significant increase (or decrease) over time. Some of the most frequently reported criteria were the exclusion of participants with non‐AD neurological disease, psychiatric illness, cardiovascular and cerebrovascular disease, obligated caregiver attendance, and cognitive impairment as defined by a specific score on the MMSE. The MMSE was used in an overwhelming majority of cases as the main cognitive eligibility criterion and was used consistently over time, with cut‐off scores increasing over the years, but with the range of allowed scores decreasing over the years. The criteria related to medical conditions and study procedures often were not well operationalized and cut‐off scores were often wide ranging. In addition to these main aims, our goal was to discuss how these eligibility criteria may have affected diversity levels. In the following paragraphs, we will discuss the main outcomes of this review and provide recommendations for future clinical trials, an overview of which can be found in Table 4.
TABLE 4.
Issues with eligibility criteria of clinical trials and recommendations
| Issue/criterion | Recommendations |
|---|---|
| Overarching issues | |
|
|
| Criteria related to medical conditions | |
|
|
| Criteria related to study procedures | |
|
|
| Criteria related to neurocognitive and neuropsychiatric measures | |
|
|
Abbreviations: AD, Alzheimer's disease; CDR, Clinical Dementia Rating; MMSE, Mini‐Mental State Examination.
We could not retrieve race/ethnicity data for more than half of the studies included in this review; for those studies for which a paper was published, a little over three quarters reported race/ethnicity data. This is somewhat higher than in a review of cholinesterase inhibitors and memantine randomized controlled trials (59.2% 5 ). The studies that reported race/ethnicity data included an overwhelming majority of White participants (≈95%), and no significant increase or decrease in this ratio was observed over time. For most trials, data regarding Latinx ethnicity was not reported, and in the handful of cases in which it was described, it was presented separately from the numbers by racial group. It was therefore not possible to determine how many Latinx versus non‐Latinx participants were included, and whether these proportions may have changed over time. However, based on the studies that did report the number of Latinx participants, as well as the data from Black, Asian, and other racial groups, it seems unlikely that Latinx participants were well represented. This lack of diversity, as well as the underreporting of Latinx background are particularly notable for studies with a North American site (79.2%), given the rapidly increasing diversification of the United States during this review period. Whitfield et al. 31 describe how, as the ratio of White participants to other ethnoracial groups increases, the statistical power to detect group differences decreases drastically, and samples will typically have to include a larger proportion of diverse ethnoracial participants than a representative sample of the general population (e.g., more than 15% Black participants in the sample). As it stands, the limited percentage of ethnoracially diverse individuals precludes sufficiently powered analyses of safety and efficacy across ethnoracial groups. In addition, currently used racial/ethnic categories themselves may need to be revised to fully represent global diversity—for example, categorizing all individuals from Europe, North Africa, and the Middle East as “White” does not do justice to the diversity within and between persons originating from these regions.
Our results showed that trials targeting Aβ or tau in AD often provide unclear definitions of their eligibility criteria; these imprecise definitions, such as “diabetes” or “impaired renal function” (not further specified), likely result in the exclusion of all or most patients with a specific medical condition. When specific ranges on indices of certain medical conditions were provided, such as BMI or ALT/AST levels, the allowed ranges differed substantially between studies. There thus seems to be a lack of consensus on how these conditions are best defined in the context of Aβ and tau trials. These ill‐defined eligibility criteria may particularly affect the inclusion of underrepresented populations that are characterized by health disparities. Kim et al. 32 made several suggestions to broaden inclusion criteria in oncology trials that may provide inspiration for AD trials. One of these recommendations is to include persons living with HIV (PWH) based on current and past CD4+ and T‐cell counts instead of excluding all PWH—unless antiretroviral therapy is expected to interact with the investigational product. Additionally, one might take into consideration whether PWH are medically stable and whether they have a (non‐)detectable viral load. Furthermore, Kim et al. 32 provided examples of how to improve the clarity of the definitions used in clinical trials eligibility criteria, such as the use of validated clinical classifications (of disease staging) as opposed to more generic definitions.
With regard to the impact of criteria related to medical conditions on the inclusion of ethnoracially diverse groups specifically, it is still uncertain if, how, and when race corrections should be used to evaluate various clinical laboratory results as indicators of specific medical conditions, such as indicators of kidney functioning 33 and several other common laboratory values. 34 Although such race corrections could potentially make the process of inclusion in clinical trials more inclusive, they may also inadvertently perpetuate or amplify existing disparities. 35 The field is in need of expert guidance to reach a consensus on whether and when to apply these race corrections.
Criteria related to undergoing study procedures were commonly part of the eligibility criteria. In the following paragraphs, the eligibility criteria related to language, education, caregiver attendance, written informed consent/reading and writing abilities, and whether patients are considered likely to complete the study, are discussed in more detail, specifically in the context of the inclusion of diverse individuals.
First, language requirements, such as fluency in the English language, were included in more than one third of the clinical trials. Depending on their definition, specific language requirements may lead to disproportionate exclusion of individuals from underrepresented populations. The lack of guidance on how to handle language barriers in clinical trials was acknowledged as a problem by multicenter research ethics committees in the UK. 36 A more inclusive solution may be to allow fluency in any preferred language, as long as the required test materials are available in that language and there is a staff member available who speaks the language to the degree necessary for cognitive testing—as was allowed in one trial (NCT00676143). This would, however, require the development/adaptation and validation of test materials across a number of languages. In addition, it may be worthwhile to investigate if assessment with experienced formal interpreters could be a viable option at study sites where the population is exceptionally diverse.
Regarding education, a minimum of 6 years of formal education was often used as a criterion—sometimes stating this was to ensure that patients with intellectual disabilities were not included. This criterion is problematic for several reasons; first, many diverse elderly patients across the world did not receive any formal education during childhood due to reasons other than intellectual disabilities—such as a lack of financial means or a large geographic distance to educational facilities (e.g., in first generation immigrants in Europe). Second, mandatory primary education across the world has historically been variable—although some countries required 6 years of primary education, others may have required only 4 or 5. Therefore, years or level of education cannot serve as a suitable proxy for intellectual disabilities in diverse patients. Some studies acknowledged these barriers by allowing people with a work history consistent with no intellectual disabilities to participate in the study. Future studies should focus on developing ways to screen for intellectual disabilities that do not result in the exclusion of patients without intellectual disabilities who had limited access to formal education.
Several studies included a criterion that patients should be likely to complete the study. However, the interpretation of this criterion often was not defined, requiring the investigator to make this judgment call. Although such a criterion may be necessary to prevent costly missed visits in clinical trials, especially for studies using PET‐ligands, likeliness to complete should be well defined at the outset. For example, a protocol may state that the patient and caregiver should complete a first run‐in period of a specific number of screening visits fully compliant with the specified study procedures and in line with a specified time schedule. If this criterion is left undefined, it may prove problematic, as studies have indicated that participant selection may be influenced by implicit bias of the clinicians, that is, compliance stereotyping. 37
More than three quarters of the studies required some form of caregiver participation, often explicitly stating caregivers had to engage in frequent contact with patients—one study required caregivers to spend at least 24 hours per week with the patient. In some diverse ethnoracial groups, the main caregiver is often an adult child, rather than a spouse, 38 , 39 , 40 and previous research has indicated that adult children are less likely than spouses to be eligible to participate alongside patients in dementia clinical trials. 41 Adult–child caregivers are more likely to still be active in the workforce, 39 potentially limiting their opportunities to engage in frequent study visits due to the practical and financial burden of missed work. Researchers may provide more flexibility by allowing others to accompany patients on a subset of visits; by having appointments taking place outside of weekday business hours; or by exploring options for remote administration of interviews, such as over the phone or via video calls. 39
More than half of the AD clinical trials in this review explicitly required written informed consent. Although this currently seems to be the standard, requiring written informed consent will lead to the exclusion of people with low literacy skills—either because these patients will not be asked, or because they will be hesitant to sign a document they have difficulty understanding. Globally, ≈781 million adults are illiterate, with a high prevalence in lower‐ and middle‐income countries, 42 although disparities in literacy are also prevalent in some underrepresented populations in high‐income countries. For example, so‐called “guest workers” in Europe often received little if any formal education, 43 , 44 and Latinx adults—and to a lesser degree Black and American Indian/Alaska Native adults—in the United States were overrepresented in the “below basic” level on the National Assessment of Adult Literacy. 45 To facilitate the enrollment of underrepresented populations, informed consent procedures will have to be tailored to patients and caregivers with low literacy skills. Over two decades ago, the US Food and Drug Administration (FDA) described the possibility of non‐written consent procedures in illiterate English‐speaking subjects, in which an impartial third party cosigns the consent document, preferably with a videotape recording. 46 A recent study in a different medical field (cardiology/endocrinology) has indicated that using a video informed consent procedure can increase the enrollment of patients from underrepresented populations. 47 As an additional example, in India, audiovisual recording of the informed consent procedure has been mandatory since 2013, and standard operating procedures have consequently been developed. 48 AD research would benefit from efforts to incorporate alternatives to written informed consent developed in other research areas that include diverse and vulnerable populations, as well as from initiatives examining the feasibility of integrating such approaches in AD research.
Regarding cognitive screening tests and questionnaires, we found that the MMSE was used almost invariably as an inclusion criterion, and its use remained stable over time, with cut‐off scores even increasing over the years. This is notable, given the fact that Schneider et al. 14 warned about the use of the MMSE in dementia trials in 1997. There is an abundant literature describing how MMSE‐scores are substantially influenced by literacy and education 49 , 50 , 51 and likely also by cultural background. 50 In particular the subtests of orientation to time and place, serial 7s, figure copy, writing, and reading will be substantially influenced by someone's educational and cultural background. 52 Developing alternatives to written informed consent will only solve half of the problem as long as the cognitive tests used for screening and to measure primary and secondary outcomes require reading and writing skills. Moving forward toward more valid and inclusive global clinical trials will entail using other cognitive tests that are more suitable for diverse populations. For instance, the Rowland Universal Dementia Assessment Scale (RUDAS 53 )—a test to assess the general level of cognitive impairment—or the International Shopping List Test 26 —for the inclusion of patients with memory impairment specifically—may be relevant options for further study. Before any instrument is selected for a clinical trial, it is imperative that a thorough review of the literature is carried out to determine whether the instrument is a valid and reliable measure of cognition in all groups that are to be included in the trial. As selection bias is often present in reliability/validity studies—for example, by excluding persons with low education levels or limited language fluency—it may be necessary to specifically check the demographic characteristics of these original study samples to ensure they reflect the intended trial sample. At a minimum, trials can be made more equitable by using different cut‐off scores for groups with different levels of education in cognitive screeners and memory tests, as was done by one trial in this review (NCT00890890).
In addition to the MMSE, this study showed a rise in the use of the CDR as an inclusion criterion. The CDR has considerable merits, but researchers and clinicians need to be aware of possible cultural differences that may bias the results, such as (1) downplaying of cognitive symptoms out of respect for older family members, (2) different perceptions of what “normal” daily functioning may entail, (3) the need for adaptations to questions relating to hobbies that may be uncommon in some groups—for example, crossword puzzles—and social or cultural practices, (4) the potential influence of traditional gender roles, and (5) the potential influence of limited literacy on some activities of daily life. 54 Aside from the extensive training that is already needed to administer the CDR in a reliable and valid way in the general population, it is likely that additional training and/or adaptations to the instrument itself are needed to make it more suitable for the assessment of diverse populations across the globe.
In addition to these specific recommendations pertaining to criteria related to medical conditions, undergoing study procedures, and cognitive screeners and questionnaires, some general recommendations may further improve inclusion of underserved populations in AD clinical trials. In the design phase, the FDA 55 specifically recommends revisiting and revising the criteria when moving from a restrictive phase II to a more inclusive phase III trial. 32 , 55 Furthermore, they encourage the inclusion of samples known as “expansion cohorts” in trials—consisting of patients with specific comorbidities that may not fit the inclusion criteria for the main study—to determine the safety of doses in these populations as well. 32 Aside from changes to the trial design, more insight can be gained into the mechanisms behind the underrepresentation of diverse patients in clinical trials, if studies were to report the ethnoracial characteristics of all patients that (1) were considered for eligibility, (2) were invited, (3) were screened, and (4) were excluded/screen failed. In addition, reports should provide specifications regarding the eligibility criteria that were most often the reason for exclusion.
Although not technically part of the CONSORT (Consolidated Standards of Reporting Trials) guidelines, 56 a short summary of the main reasons for exclusion may provide valuable insights to researchers on the eligibility criteria that have the strongest effect on eligibility. This information was only provided in a handful of studies in this review, and none of the studies specified whether there was a disproportionate exclusion of patients from underrepresented populations. It therefore remains unclear whether there was a disproportionate exclusion of patients from these groups based on overly strict eligibility criteria, or whether these patients were not invited in the first place or did not consent to study participation after invitation. For example, patients from underrepresented populations may experience geographical, financial, or logistical barriers that prevent them from participating in research. 55 , 57 Additionally, recruitment strategies need to be tailored to suit the needs of underrepresented populations, such as by investing in community‐outreach programs, trust‐building initiatives, and cultural‐sensitivity training. 10 , 36 , 58 , 59 Financial support from funding agencies and/or the trial sponsor to facilitate such initiatives may be needed. In addition, more general financial or regulatory incentives from funding organizations or governmental bodies to actively enroll patients from underrepresented populations may further improve inclusion, for example, similar to the changes in the field of pediatrics, in which the Pediatric Research Equity Act (PREA) now requires manufacturers to complete studies in children if a substantial number of children is expected to use the drug. 60
Although this review specifically examined race/ethnicity, we acknowledge that race is a social construct and that health disparities are often driven by social determinants of health, such as education, literacy, socioeconomic status, racially patterned social stress, and access to care. 61 , 62 , 63 Although some trials in this review with race/ethnicity data reported the education level of the included participants, none mentioned SES. This limited reporting of social determinants of health is in line with a previous review in symptomatic treatment of AD, in which no studies reported on variables such as lifetime occupation, individual/household income, or wealth, and few studies on education. 64 It remains unclear how these variables may have affected enrollment of diverse participants in the trials included in this review; however, participants are often recruited in memory clinics, and these facilities may not be accessible to some underrepresented groups, for example because of limited health literacy, 65 or because medical care is expensive and insufficiently covered by insurance. 66
Several limitations to this review should be mentioned. Although we did not exclude studies based on the language in which the record was written, our study did not identify any articles that were not written in English. Therefore, some local trials may have been missed. Second, race/ethnicity data was not available for a substantial number of studies, and the full protocols describing all eligibility criteria were only available for about one fifth of the included trials. As can be seen in the supporting information, the frequencies of the eligibility criteria may differ between studies with and without a full protocol available, and the rates we presented in this review may be an underestimation of the actual frequencies. For example, it seems unlikely that only slightly more than half of the clinical trials required written informed consent, particularly as the studies without such a criterion did not describe any alternative consent requirements. Likewise, trials that did not report race/ethnicity data may have included even fewer diverse participants—or, less likely, more—than the studies that did report race/ethnicity data. Third, in this review, we presented data from diverse ethnoracial populations in Australia and the United States alongside the frequencies of the eligibility criteria related to medical conditions to provide the reader with a better sense of the potential impact on diversity in clinical trials. These populations cannot be seen as directly representative of all underrepresented populations across the world, and given that these data were obtained in the general population, health disparities may actually be even more systemic and striking when zooming in on elderly populations specifically. For example, the prevalence of overweight and obesity in indigenous populations in Australia is 35% in those aged 15 to 17, but rises to 80% in those 55 and over. 20 Although we only showed data from the United States and Australia, similar health disparities are observed in populations outside those two countries, such as across different ethnoracial groups in Europe—particularly in the prevalence of diabetes, stroke, hypertension, and cardiovascular disease, 67 , 68 , 69 but also in kidney disease. 70 , 71 Fourth, it is important to note that the data based on Latinx American samples is based on a pan‐Latinx construction of this population. These studies did not account for the significant within‐group variance that has important implications for health disparities and cognitive test performance (e.g., origin/nativity [Mexican, Puerto Rican, etc.], acculturation). Fifth, we only focused on Aβ and tau trials in this review. Although many of these recommendations can likely also be applied to other types of trials across neurodegenerative diseases, such as lifestyle trials like World‐Wide FINGERS, 72 some of these trials will come with their own unique challenges—such as a lack of suitable cross‐cultural instruments measuring social cognition, language, and behavioral changes in frontotemporal dementia trials 73 as well as issues regarding the applicability of the diagnostic criteria for primary progressive aphasia subtypes across global languages, such as Chinese. 74 Last, we were unable to determine the direct effect of each criterion on the representation of diverse individuals using inferential statistics. Several factors precluded such analyses, such as the fact that some criteria were used either very infrequently or invariably (e.g., the MMSE, Table S2), as well as the fact that race/ethnicity data was not reported for each global region/country specifically, precluding any comparisons of the makeup of the study samples with a priori disease estimates in the general populations in these countries/regions. The contribution of each individual eligibility criterion to the underrepresentation of diverse individuals across trials therefore remains unclear—even more so given the underreporting of the main reasons for exclusion.
Both federal law (Public Health Service Act §492B 75 ) and NIH policy 76 require studies involving human subjects to address the inclusion of “minorities,” and Alzheimer Europe 77 similarly calls upon researchers, ethics committees, and funders to address inequity in research. This review illustrates that there is a continuous, systemic underrepresentation of ethnoracially diverse groups in AD clinical trials. To generalize safety and efficacy data of AD clinical trials to the general population, more diverse individuals need to be enrolled, and modifying or changing the eligibility criteria in AD clinical trials may play a key role in reaching this goal.
FUNDING INFORMATION
SF and JMP report a grant from The Netherlands Organisation for Health Research and Development/Alzheimer Nederland (ZonMw Memorabel; grant number 733050834). JES reports a personal James B. Reynolds scholarship for foreign study. LSS reports a grant from NIH (P30 AG066530). GMB reports grants from NIH/NIA (R01AG068183, R01AG067428, A2021142S) and the BrightFocus Foundation (A2021142S). MRM is supported by grants from the NIA/NIH (R13 AG071313‐01, R01AG065110‐01A1, NIH/NIA 5U19AG024904‐14, NIH/NIA R01AG066471‐01A1), NIH/NIMH (U24MH100931‐03), NIH/NIA (5R24AG065163), National Science Foundation, the Genentech Health Equity 2020 Fund (G‐89294), and the Alzheimer's Association (AARGD‐16‐446038).
CONFLICTS OF INTEREST
RLvB‐V, JES, ELA, GMB, JMP, EvdB have nothing to disclose (aside from the funding reported in the “Funding Information” section). SF received support to attend meetings/conferences from Alzheimer Nederland and the Erasmus Trustfonds. She served as the executive committee member of the Cultural Diversity & Psychology commission of the Dutch Association of Psychologists and serves as executive committee member to the Diversity and Disparities PIA of ISTAART. NDP is consultant to Boehringer Ingelheim, Amylyx, and Aribio (payments made to his institution). He is co‐PI of studies with EIP Pharma and Fuji Film Toyama Chemical. He serves on the DSMB of Abbvie's M15‐566 trial (payment to his institution). He is CEO and co‐owner of the Brain Research Center, the Netherlands. He is also on the scientific program committee of the Alzheimer's Association. LSS reports grants/contracts by Eisai, Eli Lilly, Roche/Genentech, Biogen, Biohaven, Novartis, and Washington University/NIA‐DIAN‐TU paid to the institution. In addition, he reports consulting fees from Abbott, AC Immune, Avraham Ltd, Boehringer Ingelheim, Cognition Therapeutics, Cortexyme, Eisai, FujiFilm, Immunobrain Checkpoint Ltd, Neurally Inc, Neurim Ltd, Neuronix Ltd, Samus, Takeda, vTv. MRM is the past president of the Hispanic Neuropsychological Society and standing member of the NIH NIA‐T Study Section. MRM also has a relationship with the Alzheimer's Association Harem Community and Academic partnership. MRM received support from NIH/NIA to attend meetings. MRM received payment/honoraria for being panelist/chair/speaker/plenary speaker at the following events: AAIC Neuroscience Next 2020; mid‐year conference of the International Neuropsychological Society 2019; Latinos and Alzheimer's Disease Symposium 2019; International Association of Forensic Mental Health Services Conference 2017; International Neuropsychological Society Annual Conference 2012; International Neuropsychological Society Annual Conference, 2012; 37th Annual Conference of the International Neuropsychological Society Annual Conference, 2010 American Academy of Clinical Neuropsychology annual meeting [2021 delayed due to COVID]; Harvard MGH Psychology Assessment Center Seminar 2021; University of Washington Department of Neurology Grand Rounds [delayed due to COVID 2020]; Annual Conference of the Pacific Northwest Neuropsychological Society, 2020; Annual Conference of the Council of University Directors of Clinical Psychology, 2020; National Academy of Sciences/Simons Foundation: The Science & Entertainment Exchange, 2019; Emory University HIV & Aging Conference; Brown University Alpert Medical School, Department of Psychiatry and Human Behavior Grand Rounds, 2019; Wisconsin Alzheimer's Institute/University of Wisconsin School of Medicine & Public Health 16th Annual Alzheimer's Disease Update Conference, 2018; 38th annual meeting of the National Academy of Neuropsychology, 2018; Council of Science Editors, Technica Editorial Services Webinar. The Peer Review Ecosystem: Where Does Diversity & Inclusion Fit In? 2018; 12) Colloquium Presentation, Dept. of Psychology, Ohio University, Athens, OH, 2018.
Supporting information
Supporting information
ACKNOWLEDGMENTS
The authors would like to thank Wichor Bramer from the Erasmus MC University Medical Center Rotterdam for his help in developing the search strategy and Jolien Franzen for her contribution to the results section. We also acknowledge the ABOARD consortium for supporting the work on diversity in primary and secondary prevention of Alzheimer’s disease in the Netherlands.
Franzen S, Smith JE, van den Berg E, et al. Diversity in Alzheimer's disease drug trials: The importance of eligibility criteria. Alzheimer's Dement. 2022;18:810–823. 10.1002/alz.12433
REFERENCES
- 1. Alzheimer's Association . 2015 Alzheimer's disease facts and figures. Alzheimers Dement. 2015;11:324‐384. [DOI] [PubMed] [Google Scholar]
- 2. Barnes LL, Bennett DA. Alzheimer's disease in African Americans: Risk factors and challenges for the future. Health Aff. 2014;33(4):580‐586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Prince M, Bryce R, Albanese E, Wimo A, Ribeiro W, Ferri CP. The global prevalence of dementia: A systematic review and metaanalysis. Alzheimers Dement. 2013;9(1):63‐75 e2. [DOI] [PubMed] [Google Scholar]
- 4. Selten J‐P, Termorshuizen F, van Sonsbeek M, Bogers J, Schmand B. Migration and dementia: A meta‐analysis of epidemiological studies in Europe. Psychol Med. 2020:1‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Canevelli M, Bruno G, Grande G, et al. Race reporting and disparities in clinical trials on Alzheimer's disease: A systematic review. Neurosci Biobehav Rev. 2019;101:122‐128. [DOI] [PubMed] [Google Scholar]
- 6. Faison WE, Schultz SK, Aerssens J, et al. Potential ethnic modifiers in the assessment and treatment of Alzheimer's disease: challenges for the future. Int Psychogeriatr. 2007;19(3):539‐558. [DOI] [PubMed] [Google Scholar]
- 7. Shin J, Doraiswamy PM. Underrepresentation of African‐Americans in Alzheimer's trials: A call for affirmative action. Frontiers in Aging Neuroscience. 2016;8:123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bjornsson TD, Wagner JA, Donahue SR, et al. A review and assessment of potential sources of ethnic differences in drug responsiveness. J Clin Pharmacol. 2003;43:943‐967. [DOI] [PubMed] [Google Scholar]
- 9. Goldstein DB, Tate SK, Sisodiya SM. Pharmacogenetics goes genomic. Nat Rev Genet. 2003;4(12):937‐947. [DOI] [PubMed] [Google Scholar]
- 10. Gilmore‐Bykovskyi AL, Jin Y, Gleason C, et al. Recruitment and retention of underrepresented populations in Alzheimer's disease research: A systematic review. Alzheimers Dement (N Y). 2019;5:751‐770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Garza MA, Quinn SC, Li Y, et al. The Influence of Race and Ethnicity on Becoming a Human Subject: Factors Associated with Participation in Research. Contemp Clin Trials Commun. 2017;7:57‐63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wendler D, Kington R, Madans J, et al. Are racial and ethnic minorities less willing to participate in health research? PLoS Med. 2006;3(2):e19. https://doi.org10.1371/journal.pmed.0030019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cooper C, Ketley D, Livingston G. Systematic review and meta‐analysis to estimate potential recruitment to dementia intervention studies. Int J Geriatr Psychiatry. 2014;29(5):515‐525. [DOI] [PubMed] [Google Scholar]
- 14. Schneider LS, Olin JT, Lyness SA, Chui HC. Eligibility of Alzheimer's disease clinic patients for clinical trials. J Am Geriatr Soc. 1997;45(8):923‐928. [DOI] [PubMed] [Google Scholar]
- 15. Folstein MF, Folstein SE, McHugh PR. “Mini‐mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189‐198. [DOI] [PubMed] [Google Scholar]
- 16. Moher D, Liberati A, Tetzlaff J, Altman DG, The Prisma Group. Preferred reporting items for systematic reviews and meta‐analyses: The PRISMA statement. BMJ. 2009;339:b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Food and Drug Administration . Early Alzheimer's Disease: Developing Drugs for Treatment Guidance for Industry. Rockville, MD. 2020. https://www.fda.gov/regulatory‐information/search‐fda‐guidance‐documents/alzheimers‐disease‐developing‐drugs‐treatment‐guidance‐industy. Accessed March 6, 2021 [Google Scholar]
- 18. Galimberti D, Scarpini E. Disease‐modifying treatments for Alzheimer's disease. Ther Adv Neurol Disord. 2011;4(4):203‐216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. National Center for Health Statistics. Tables of Summary Health Statistics for U.S. Adults: 2018 National Health Interview Survey. 2019.
- 20. Australian Institute of Health and Welfare The health and welfare of Australia's Aboriginal and Torres Strait Islander peoples. Cat. no. IHW 147. Canberra: AIHW; 2015. https://www.aihw.gov.au/getmedia/584073f7‐041e‐4818‐9419‐39f5a060b1aa/18175.pdf.aspx?inline=true. Accessed April 8, 2021
- 21. Randolph C. RBANS update: Repeatable Battery for the Assessment of Neuropsychological Status: Manual. Bloomington, MN: Pearson; 2009. [Google Scholar]
- 22. Rosen WG, Mohs RC, Davis KL. A new rating scale for Alzheimer's disease. Am J Psychiatry. 1984;141(11): 1356‐1364. [DOI] [PubMed] [Google Scholar]
- 23. Nasreddine ZS, Phillips NA, Bedirian V, et al. The Montreal Cognitive Assessment, MoCA: A brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53(4):695‐699. [DOI] [PubMed] [Google Scholar]
- 24. Buschke H. Free and Cued Selective Reminding Test N Engl J Med. 2002;347(22):1761‐1768. [DOI] [PubMed] [Google Scholar]
- 25. Wechsler D. Manual for the Wechsler Memory Scale‐Revised. San Antonio, TX: The Psychological Corporation; 1987. [Google Scholar]
- 26. Thompson TAC, Wilson PH, Snyder PJ, et al. Sensitivity and test‐retest reliability of the International Shopping List Test in assessing verbal learning and memory in mild Alzheimer's disease. Arch Clin Neuropsych. 2011;26(5):412‐424. [DOI] [PubMed] [Google Scholar]
- 27. Hughes CP, Berg L, Danziger WL, Coben LA, Martin RL. A new clinical scale for the staging of dementia. Br J Psychiatry 1982;140:566‐572. [DOI] [PubMed] [Google Scholar]
- 28. Yesavage JA, Brink TL, Rose TL, et al. Development and validation of a geriatric depression screening scale: A preliminary report. J Psychiatr Res. 1982;17(1):37‐49. [DOI] [PubMed] [Google Scholar]
- 29. Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56‐62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Posner K, Brown GK, Stanley B, et al. The Columbia‐Suicide Severity Rating Scale: initial validity and internal consistency findings from three multisite studies with adolescents and adults. Am J Psychiatry. 2011;168(12):1266‐1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Whitfield KE, Allaire JC, Belue R, Edwards CL. Are comparisons the answer to understanding behavioral aspects of aging in racial and ethnic groups? J Gerontol B Psychol Sci Soc Sci. 2008;63(5):P301‐P308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kim ES, Bruinooge SS, Roberts S, et al. Broadening eligibility criteria to make clinical trials more representative: American Society of Clinical Oncology and Friends of Cancer Research joint research statement. J Clin Oncol. 2017;35(33):3737‐3744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130(6):461‐470. [DOI] [PubMed] [Google Scholar]
- 34. Lim E, Miyamura J, Chen JJ. Racial/Ethnic‐Specific Reference Intervals for Common Laboratory Tests: A Comparison among Asians, Blacks, Hispanics, and White. Hawaii J Med Public Health. 2015;74(9):302‐310. [PMC free article] [PubMed] [Google Scholar]
- 35. Vyas DA, Eisenstein LG, Jones DS. Hidden in Plain Sight ‐ Reconsidering the Use of Race Correction in Clinical Algorithms. N Engl J Med. 2020;383(9):874‐882. [DOI] [PubMed] [Google Scholar]
- 36. Hussain‐Gambles M, Atkin K, Leese B. Why ethnic minority groups are under‐represented in clinical trials: A review of the literature. Health Soc Care Community. 2004;12(5):382‐388. [DOI] [PubMed] [Google Scholar]
- 37. Cooper LA, Roter DL, Carson KA, et al. The associations of clinicians' implicit attitudes about race with medical visit communication and patient ratings of interpersonal care. Am J Public Health. 2012;102(5):979‐987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Llanque SM, Enriquez M. Interventions for Hispanic caregivers of patients with dementia: a review of the literature. Am J Alzheimers Dis Other Demen. 2012;27(1):23‐32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Largent EA, Karlawish J, Grill JD. Study partners: essential collaborators in discovering treatments for Alzheimer's disease. Alzheimers Res Ther. 2018;10(1):101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. van Wezel N, Francke AL, Kayan‐Acun E, Ljm Deville W, van Grondelle NJ, Blom MM. Family care for immigrants with dementia: The perspectives of female family carers living in The Netherlands. Dementia (London). 2016;15(1):69‐84. [DOI] [PubMed] [Google Scholar]
- 41. Grill JD, Monsell S, Karlawish J. Are patients whose study partners are spouses more likely to be eligible for Alzheimer's disease clinical trials? Dement Geriatr Cogn Disord. 2012;33(5):334‐40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. UNESCO . Education for all 2000‐2015: achievements and challenges n.d. https://unesdoc.unesco.org/images/0023/002322/232205e.pdf. Accessed March 6, 2021
- 43. Schellingerhout R. Gezondheid en welzijn van allochtone ouderen. The Hague, the Netherlands: Sociaal en Cultureel Planbureau; 2004. [DOI] [PubMed] [Google Scholar]
- 44. Danmarks Statistik Indvandrere i Danmark . 2018. p. 51‐72. https://www.dst.dk/Site/Dst/Udgivelser/GetPubFile.aspx?id=29445&sid=indv2018. Accessed April 8, 2021
- 45. Kutner M, Greenberg E, Jin Y, Boyle B, Hsu Y, Dunleavy E. Literacy in Everyday Life: Results From the 2003 National Assessment of Adult Literacy (NCES 2007‐480). Washington, DC: National Center for Education Statistics; 2007. [Google Scholar]
- 46. Food and Drug Administration . A guide to informed consent: guidance for institutional review board and clinical investigators. Rockville, MD. 1998. https://www.fda.gov/regulatory‐information/search‐fda‐guidance‐documents/guide‐informed‐consent. Accessed April 8, 2021 [Google Scholar]
- 47. Fanaroff AC, Li S, Webb LE, et al. An Observational Study of the Association of Video‐ Versus Text‐Based Informed Consent With Multicenter Trial Enrollment Lessons From the PALM Study (Patient and Provider Assessment of Lipid Management). Circ‐Cardiovasc Qual. 2018;11(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Parikh PM, Prabhash K, Govind KB, et al. Standard operating procedure for audio visual recording of informed consent: An initiative to facilitate regulatory compliance. Indian J Cancer. 2014;51(2):113‐116. [DOI] [PubMed] [Google Scholar]
- 49. Steis MR, Schrauf RW. A review of translations and adaptations of the Mini‐Mental State Examination in languages other than English and Spanish. Res Gerontol Nurs. 2009;2(3):214‐224. [DOI] [PubMed] [Google Scholar]
- 50. Tombaugh TN, McIntyre NJ. The mini‐mental state examination: a comprehensive review. J Am Geriatr Soc. 1992;40(9):922‐935. [DOI] [PubMed] [Google Scholar]
- 51. Murden RA, McRae TD, Kaner S, Bucknam ME. Mini‐Mental State exam scores vary with education in blacks and whites. J Am Geriatr Soc. 1991;39(2):149‐155. [DOI] [PubMed] [Google Scholar]
- 52. Zwart LAR, Goudsmit M, van Campen JPCM, Rijkers CJM, Wind AW. Using the MMSE as a cognitive screener among Turkish and Moroccan migrants. Tijdschr Gerontol Geriatr. 2015;46(1):28‐36. [DOI] [PubMed] [Google Scholar]
- 53. Storey JE, Rowland JT, Basic D, Conforti DA, Dickson HG. The Rowland Universal Dementia Assessment Scale (RUDAS): a multicultural cognitive assessment scale. Int Psychogeriatr. 2004;16(1):13‐31. [DOI] [PubMed] [Google Scholar]
- 54. Lim WS, Chong MS, Sahadevan S. Utility of the clinical dementia rating in Asian populations. Clin Med Res. 2007;5(1):61‐70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Food and Drug Administration . Enhancing the diversity of clinical trial populations ‐ Eligibility criteria, enrollment practices, and trial designs guidance for industry. Rockville, MD; 2019. https://www.fda.gov/regulatory‐information/search‐fda‐guidancedocuments/enhancing‐diversity‐clinical‐trial‐populations‐eligibility‐criteria‐enrollment‐practices‐and‐trial. Accessed April 8, 2021 [Google Scholar]
- 56. Schulz KF, Altman DG, Moher D. CONSORT 2010 statement: Updated guidelines for reporting parallel group randomised trials. J Pharmacol Pharmacother. 2010;1(2):100‐107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Clark LT, Watkins L, Pina IL, et al. Increasing Diversity in Clinical Trials: Overcoming Critical Barriers. Curr Probl Cardiol. 2019;44(5):148‐712. [DOI] [PubMed] [Google Scholar]
- 58. Gauthier MA, Clarke WP. Gaining and sustaining minority participation in longitudinal research projects. Alzheimer Dis Assoc Disord. 1999;13:S29‐S33. [DOI] [PubMed] [Google Scholar]
- 59. Shavers VL, Lynch CF, Burmeister LF. Racial differences in factors that influence the willingness to participate in medical research studies. Ann Epidemiol. 2002;12(4):248‐256. [DOI] [PubMed] [Google Scholar]
- 60. Pediatric Research Equity Act, Pub. L. No. 108‐155, 117 Stat. 1936. 2003.
- 61. Zahodne LB, Sharifian N, Kraal AZ, et al. Socioeconomic and psychosocial mechanisms underlying racial/ethnic disparities in cognition among older adults. Neuropsychology. 2021;35(3):265‐275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Llibre‐Guerra JJ, Li Y, Allen IE, et al. Race, genetic admixture and cognitive performance in the Cuban population. J Gerontol A Biol Sci Med Sci. 2021. [DOI] [PubMed] [Google Scholar]
- 63. Resende EDF, Guerra JJL, Miller BL. Health and Socioeconomic Inequities as Contributors to Brain Health. Jama Neurol. 2019;76(6):633‐634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Canevelli M, Bruno G, Vico C, et al. Socioeconomic disparities in clinical trials on Alzheimer's disease: a systematic review. Eur J Neurol. 2018;25(4):626‐e43. [DOI] [PubMed] [Google Scholar]
- 65. Mukadam N, Cooper C, Livingston G. Improving access to dementia services for people from minority ethnic groups. Curr Opin Psychiatry. 2013;26(4):409‐14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. National Research Council Panel on Race, Ethnicity, and Health in Later Life . Understanding Racial and Ethnic Differences in Health in Late Life: A Research Agenda. Washington, DC: National Academies Press (US). 2004. [PubMed] [Google Scholar]
- 67. Khunti K, Kumar S, Brodie J. Diabetes UK and South Asian Health Foundation recommendations on diabetes research priorities for British South Asians. London, UK: Diabetes UK; 2009. [Google Scholar]
- 68. Kunst AE, Stronks K, Agyemang C. Non‐communicable diseases. In: Bernd R, Mladovsky P, Devillé W, Rijks B, Petrova‐Benedict R, McKee M, editors. Migration and Health in the European Union. Maidenhead, England: Open University Press; 2011. pp. 101‐20. [Google Scholar]
- 69. Chaturvedi N. Ethnic differences in cardiovascular disease. Heart. 2003;89(6):681‐686. https://doi.org10.1136/heart.89.6.681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Adjei DN, Stronks K, Adu D, et al. Relationship between educational and occupational levels, and Chronic Kidney Disease in a multi‐ethnic sample ‐ The HELIUS study. PLoS One. 2017;12(11):e0186460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Fischbacher CM, Bhopal R, Rutter MK, et al. Microalbuminuria is more frequent in South Asian than in European origin populations: a comparative study in Newcastle, UK. Diabet Med. 2003;20(1):31‐36. [DOI] [PubMed] [Google Scholar]
- 72. Kivipelto M, Mangialasche F, Snyder HM, et al. World‐Wide FINGERS Network: A global approach to risk reduction and prevention of dementia. Alzheimers Dement. 2020;16(7):1078‐1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Franzen S, Papma JM, van den Berg E, Nielsen TR. Cross‐cultural neuropsychological assessment in the European Union: a Delphi expert study. Arch Clin Neuropsych. 2021;36(5):815‐830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Ting SKS, Foo H, Chia PS, et al. Dyslexic Characteristics of Chinese‐Speaking Semantic Variant of Primary Progressive Aphasia. J Neuropsychiatry Clin Neurosci. 2018;30(1):31‐37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Public Health Service Act §492B 42 U.S.C. §289a‐2. Inclusion of women and minorities in clinical research.
- 76. National Institute of Health NIH policy and guidelines on the inclusion of women and minorities as subjects in clinical research. 2001. https://grants.nih.gov/grants/guide/notice‐files/NOT‐OD‐02‐001.html. Accessed April 8, 2021
- 77. Alzheimer Europe . Overcoming ethical challenges affecting the involvement of people with dementia in research: recognising diversity and promoting inclusive research. Luxembourg, Luxembourg: 2019. [Google Scholar]
- 78. Pratt LA, Brody DJ. Depression in the U.S. household population, 2009‐2012. NCHS data brief, no 172. Hyattsville, MD: National Center for Health Statistics; 2014. [PubMed] [Google Scholar]
- 79. Substance Abuse and Mental Health Services Administration . Results from the 2013 national survey on drug use and health: Summary of national findings, NSDUH series H‐48, HHS publication no. (SMA) 14‐4863. Rockville, MD. 2014. [Google Scholar]
- 80. Centers for Disease Control and Prevention HIV Surveillance Report, 2018 (Updated). 2020. https://www.cdc.gov/hiv/library/reports/hiv‐surveillance.html. Accessed April 8, 2021
- 81. Center for Disease Control and Prevention . Health, United States, 2018. Hyattsville, MD: National Center for Health Statistics; 2019. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting information


