Abstract
Purpose
Many patients with chronic pulmonary diseases, such as interstitial lung disease, cystic fibrosis, and non-cystic fibrosis bronchiectasis, suffer from dyspnea and exercise intolerance. Reduced lung compliance is the main cause of the patients’ dyspnea, but weak respiratory muscles could be an additional factor. The diaphragm is considered the major respiratory muscle. Our study aimed to detect diaphragmatic thickness and excursion by ultrasound in pediatric patients with chronic pulmonary diseases to assess respiratory muscle weakness in these patients.
Methods
A case–control study was conducted on 130 patients with pediatric chronic pulmonary diseases (childhood interstitial lung diseases, cystic fibrosis, and non-cystic fibrosis bronchiectasis) and 100 control subjects. Ultrasound was used to detect diaphragmatic excursion and thickness, which were correlated with the severity of the disease, both clinically and functionally.
Results
The right and left diaphragmatic excursions were significantly lower in the patients (19.469 ± 9.984 and 18.5 ± 10.131, respectively) than in the control subjects (29.6 ± 14.131 and 25.6 ± 12.827, respectively) (p values of 0.002 and 0.019). In contrast, the difference in the right and left diaphragmatic thicknesses between the patients and the controls was statistically insignificant (p values of 0.884 and 0.344). The left diaphragmatic excursion was positively correlated with the patients’ age and weight, while both the right and the left diaphragmatic excursion significantly correlated with the patients’ height, FEV1/FVC ratio, and heart rate.
Conclusion
The diaphragmatic excursion is lower in children and adolescents with chronic pulmonary diseases than in healthy control subjects. The diaphragmatic excursion is positively correlated with patients’ age, weight, height, FEV1/FVC ratio, and heart rate.
Keywords: Diaphragmatic ultrasound, Bronchiectasis, Cystic fibrosis, Interstitial lung diseases, Diaphragmatic excursion
Introduction
Pediatric patients with chronic pulmonary diseases suffer from dyspnea and exercise intolerance, which are mostly attributed to poor lung compliance and weak respiratory muscles. Patients are followed up for poor lung functions by spirometry, but they are usually not followed up for weak respiratory muscles. The diaphragm is responsible for about 75% of respiratory movements [1].
There are many possible causes of decreased diaphragm strength in chronic pulmonary disease patients, such as malnutrition, hypoxia, respiratory myopathy due to the chronic use of corticosteroids, systemic inflammation, or disuse [2].
Lung ultrasound (LUS) is a rapid, safe, and non-invasive tool to diagnose the lungs and pleura [3]. Both B-mode and M-mode ultrasound can be used to assess diaphragmatic movement or excursion (DE), which is the distance that the diaphragm can move during a respiratory cycle. This ultrasound parameter can be used as a predictor of decreased lung volumes due to weak respiratory muscles.
Ultrasound allows the visualization of the posterior and lateral parts of the diaphragm, which are the muscular crural parts that are innervated by the phrenic nerve. In contrast, fluoroscopy helps the visualization of the anterior central tendon, which moves 40% less than the muscular crural parts during respiration [4].
Our study aimed to evaluate chest ultrasound as a simple, non-invasive method to assess diaphragmatic thickness and excursion in pediatric patients with different chronic pulmonary diseases.
Materials and methods
Patients
A case–control study was performed on 130 patients with chronic pulmonary diseases (cystic fibrosis, non-cystic fibrosis bronchiectasis, and childhood interstitial lung diseases) and 100 control subjects (without any respiratory, abdominal, or neuromuscular diseases), age- and sex-matched with the patients.
Exclusion criteria
Any disease that increases intra-abdominal pressure and can affect diaphragmatic motility, any recent thoracic or abdominal surgery, any neuromuscular disease, or any active infection.
Study design
The study was carried out in the Chest Clinic, Children’s Hospital, and radiology department, Ain Shams University, Cairo, Egypt, from February 2019 to March 2020. It was approved by the local ethics committee, Children’s Hospital, Ain Shams University. Verbal and written consents were obtained from all the caregivers of our patients.
Data collected from the patients include age, sex, anthropometric measurements, and frequency of hospital admission in the past year.
The severity of the chest disease was assessed by the following tests:
The six-minute walk test (6MWT) [5].
Oxygen saturation as measured by a pulse oximeter.
Pulmonary function tests: spirometry for forced vital capacity (FVC) and forced expiratory volume in the first second (FEV1), using VIASYS Healthcare GmbH.
The modified Medical Research Council (mMRC) dyspnea score [6].
Ultrasound was used to assess the diaphragmatic thickness and excursion
The ultrasound examinations were carried out by a single radiologist with 20 years of experience in pediatric ultrasound using the Logiq p7 ultrasound machine (GE Healthcare, Waukesha, Wisconsin, USA).
The patients were examined while breathing to aid the random detection of diaphragmatic thickness and motion. Because of less uncertainty and greater reproducibility, the test was performed in the supine position.
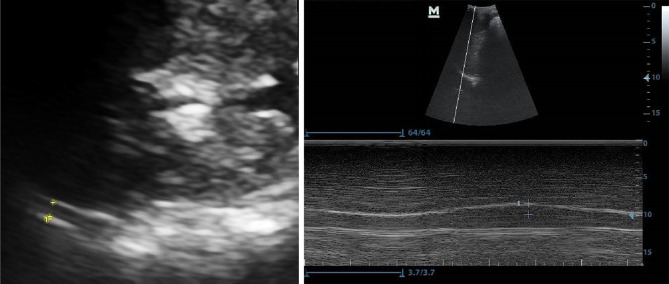
Diaphragmatic thickness was measured in B-mode with a 2–5-MHz curvilinear transducer placed over the diaphragm’s opposition zone, near the costophrenic angle, between the anterior and posterior axillary lines. Diaphragmatic thickness was measured from the most superficial hyperechoic line (pleural line) to the deepest hyperechoic line (peritoneal line). We measured the thickness of the diaphragm during a breath-holding manoeuvre after maximal inspiration (corresponding to the total lung capacity, TLC), and the average value of three consecutive measurements was recorded [7] (Fig. 1a).
For the viewing of the DE, the anterior subcostal method was needed. The transducer was positioned between the midclavicular and anterior axillary lines, using the M-mode to achieve the optimum excursion [8] (Fig. 1b).
Fig. 1.
Method of measuring the diaphragmatic thickness (a) and excursion (b)
Statistical analysis
Statistical analysis was done using the Statistical Package for the Social Sciences (SPSS, version 18.0, Toronto, Canada). Qualitative variables were expressed as a number and percentage, while quantitative variables were expressed as the mean ± SD. Student’s t test, the one-way ANOVA test, and Pearson’s correlation coefficient were used to test for significance; p values less than 0.05 were considered significant.
Results
Our study included 50 patients with cystic fibrosis, 50 patients with non-cystic fibrosis bronchiectasis (post-infectious, genetic, and idiopathic bronchiectasis), and 30 patients with childhood interstitial lung disease (idiopathic interstitial pneumonia, hypersensitivity pneumonitis, and bronchiolitis obliterans). The patients consisted of 69 males (53%) and 61 females (47%). Their age ranged from 2 to 17 years, with a mean of 7.82 ± 3.9 years. In total, 100 control subjects were assessed for diaphragmatic thickness and excursion, and they were age- and sex-matched to our patients. Clinical parameters and the severity of the disease are presented in Table 1.
Table 1.
Clinical data and investigations of the studied patients
| Mean ± SD (Range) | |
|---|---|
| Weight (kg) | 23.48 ± 11.21 (9–50) |
| Height (cm) | 119.09 ± 24.15 (71–165) |
| BMI (kg/m2) | 16.51 ± 2.48 (13.02–23.31) |
| Heart rate (beats/min) | 99.69 ± 11.55 (80–112) |
| Respiratory rate (breath/min) | 29 ± 5.93 (24–44) |
| FEV1 (% of predicted) | 84.36 ± 18.21 (59.9–112) |
| FVC (% of predicted) | 96.26 ± 15.02 (71.3–116.6) |
| FEV1/FVC | 79.23 ± 12.5 (53.47–95) |
| MMEF (% of predicted) | 51.26 ± 23.9 (18.5–72.9) |
| MRC dyspnea score | 1.87 ± 1.18 (0–4) |
| Frequency of hospital admission (times/year) | 6.73 ± 6.49 (0–20) |
| Oxygen saturation (%) | 97.35 ± 1.6 (94–100) |
| 6MWT (m) | 286.23 ± 112.52 (80–495) |
| Right diaphragmatic thickness (mm) | 4.03 ± 1.52 (1.3–6.4) |
| Right diaphragmatic excursion (mm) | 19.47 ± 9.98 (1–38) |
| Left diaphragmatic thickness (mm) | 4.14 ± 1.6 (1.8–8.2) |
| Left diaphragmatic excursion (mm) | 18.5 ± 10.13 (2–43) |
BMI body mass index, FEV1 forced expiratory volume in one second, FVC forced vital capacity, MRC modified Medical Research Center, 6MWT six-minute walk test
Regarding diaphragmatic thickness and excursion, there was no statistically significant difference between patients with cystic fibrosis, bronchiectasis, or childhood interstitial lung diseases (Table 2).
Table 2.
Comparison between patients with cystic fibrosis, bronchiectasis, and childhood interstitial lung diseases as regards diaphragmatic thickness and excursion
| Patients | Mean | SD | F* | p value** | |
|---|---|---|---|---|---|
| Right diaphragmatic thickness | C.F | 3.646 | 1.717 | 1.518 | 0.236 |
| Bronchiectasis | 3.950 | 1.257 | |||
| chILD | 4.857 | 1.414 | |||
| Right diaphragmatic excursion | C.F | 20.308 | 12.278 | 0.222 | 0.802 |
| Bronchiectasis | 17.917 | 9.765 | |||
| chILD | 20.571 | 5.635 | |||
| Left diaphragmatic thickness | C.F | 3.638 | 1.601 | 2.453 | 0.104 |
| Bronchiectasis | 4.058 | 1.190 | |||
| chILD | 5.214 | 1.881 | |||
| Left diaphragmatic excursion | C.F | 18.077 | 12.783 | 0.024 | 0.976 |
| Bronchiectasis | 18.583 | 9.700 | |||
| chILD | 19.143 | 5.490 |
C.F cystic fibrosis, chILD: childhood interstitial lung diseases
*F: one-way ANOVA test; **Statistically significant p value less than 0.05
A statistically significant difference between patients and controls was obtained regarding the right and left DEs, but no significant difference was found between them concerning diaphragmatic thickness (Table 3).
Table 3.
Comparison between patients and controls as regards diaphragmatic thickness and excursion
| Mean ± SD (mm) | t* | p value | ||
|---|---|---|---|---|
| Right diaphragmatic thickness | Patients (n = 130) | 4.025 ± 1.519 | 0.147 | 0.884 |
| Controls (n = 100) | 4.08 ± 1.429 | |||
| Right diaphragmatic excursion | Patients (n = 130) | 19.469 ± 9.984 | 3.241 | 0.002** |
| Controls (n = 100) | 29.6 ± 14.131 | |||
| Left diaphragmatic thickness | Patients (n = 130) | 4.141 ± 1.596 | 0.955 | 0.344 |
| Controls (n = 100) | 3.8 ± 1.195 | |||
| Left diaphragmatic excursion | Patients (n = 130) | 18.5 ± 10.131 | 2.408 | 0.019** |
| Controls (n = 100) | 25.6 ± 12.827 | |||
*t Students’ t test; **Statistically significant p value less than 0.05
There was a significant positive correlation between the left DE and the patients’ age and weight. Also, a significant positive correlation was found between the right and left DEs and the height, FEV1/FVC ratio, and heart rate of the patients (Table 4). However, the diaphragmatic thickness was not correlated with age, anthropometric measurements, mMRC dyspnea scale, or any other variables (Table 4).
Table 4.
Correlation between diaphragm thickness and mobility and other variables for the studied patients
| Variables | Right diaphragmatic thickness | Right diaphragmatic excursion | Left diaphragmatic thickness | Left diaphragmatic excursion | ||||
|---|---|---|---|---|---|---|---|---|
| r* | p** | r* | p** | r* | p** | r* | p** | |
| Age | 0.031 | 0.87 | 0.309 | 0.084 | 0.12 | 0.512 | 0.456 | 0.009** |
| MRC dyspnea score | 0.245 | 0.178 | 0.117 | 0.524 | 0.156 | 0.394 | 0.176 | 0.335 |
| Weight | 0.076 | 0.68 | 0.316 | 0.078 | 0.127 | 0.49 | 0.395 | 0.025** |
| Height | 0.031 | 0.89 | 0.456 | 0.029** | 0.087 | 0.694 | 0.493 | 0.017** |
| BMI | 0.39 | 0.076 | 0.085 | 0.707 | 0.401 | 0.058 | 0.058 | 0.792 |
| FEV1 | 0.109 | 0.74 | 0.539 | 0.707 | 0.073 | 0.822 | 0.451 | 0.141 |
| FVC | 0.018 | 0.96 | 0.341 | 0.278 | 0.17 | 0.596 | 0.268 | 0.400 |
| FEV1/FVC | 0.49 | 0.104 | 0.686 | 0.014** | 0.308 | 0.331 | 0.596 | 0.041** |
| MMEF | 0.419 | 0.301 | 0.630 | 0.094 | 0.140 | 0.741 | 0.540 | 0.167 |
| Frequency of hospital admission | 0.24 | 0.189 | 0.018 | 0.92 | 0.196 | 0.291 | 0.039 | 0.832 |
| Respiratory rate | 0.11 | 0.67 | 0.044 | 0.863 | 0.317 | 0.2 | 0.089 | 0.726 |
| Heart rate | 0.37 | 0.14 | 0.553 | 0.017** | 0.236 | 0.345 | 0.47 | 0.047** |
| Oxygen saturation | 0.12 | 0.612 | 0.164 | 0.489 | 0.432 | 0.057 | 0.164 | 0.490 |
| 6MWT | 0.32 | 0.28 | 0.06 | 0.845 | 0.356 | 0.232 | 0.065 | 0.834 |
BMI body mass index, FEV1 forced expiratory volume in one second, FVC forced vital capacity, MRC modified Medical Research Center, 6MWT six-minute walk test
*r Pearson correlation coefficient; **Statistically significant p value less than 0.05
Discussion
The diaphragm is the main respiratory muscle during quiet breathing. Different diagnostic modalities are used to assess diaphragmatic function. Fluoroscopy is the simplest and easiest method to interpret the diaphragm’s function, but it has the risk of significant radiation exposure. Also, chest X-ray and computed axial tomography (CT) are used but with limited dynamic imaging; on the other hand, dynamic magnetic resonance imaging (MRI) is expensive [9]. Moreover, a drawback of these techniques is the limited availability, making ultrasound superior to them all as an easy, available, and safe diagnostic modality.
Our study revealed a statistically significant difference between patient and control groups regarding the DE, which means that ultrasound is a useful modality to assess changes in the DE in different pulmonary diseases. Similarly, Boccatonda et al.’s [10] study on idiopathic pulmonary fibrosis (IPF) patients showed a significant reduction in the DE in patients compared with control subjects, but this difference was detectable in deep breathing only, which is affected by the degree of fibrosis. This is against the results of He et al. [11] who found no statistically significant differences in diaphragmatic motility measured by M-mode LUS between IPF patients and control subjects.
In our study, the decreased FEV1/FVC ratio in patients with mixed obstructive and restrictive lung disease (n = 44, 33.85%) significantly correlated with the DE, meaning that as the obstructive element increases, the excursion decreases. This might indicate that air trapping and lung hyperinflation flatten the diaphragm, causing the contraction of the diaphragm muscle fibres to be less effective, resulting in less expansion of the chest cage and a lower transverse thoracic diameter during inspiration [12]. On the other hand, in their research on obese patients, Adel et al. [13] recorded a significant correlation between the DE and the percentage of predicted FEV1 and FVC, but they, too, reached the conclusion that there was no significant correlation between the degree of restrictive pattern in spirometry and the DE. Furthermore, in adults, many studies on chronic obstructive pulmonary disease (COPD) patients, such as Dos Santos et al. [14] and Baria et al. [15], revealed that the DE was strongly correlated with air trapping. However, these studies disagree with several other studies, such as Scott et al. [16] and Smargiassi et al. [17], which did not find a correlation between the DE and spirometric parameters.
Malnutrition in patients with chronic pulmonary disease is an additional factor for weak respiratory muscles, leading to limited exercise capacity in these patients, which may be wrongly interpreted as parenchymal or airway disease [18]. In our study, the patients’ weight correlated positively with the left DE. Also, Hida et al. [19] and El-Halaby et al. [7] mentioned a positive correlation between patients’ weight and the DE. On the other hand, Adel et al. [13] reported a negative correlation between the DE and the body mass index (BMI), which can be explained by the fact that most patients in our sample were underweight children, with a BMI of 16.51 ± 2.48 kg/m2, as opposed to the adult obese patients with a low DE involved in Adel et al.’s study.
We also found a significant positive correlation between patients’ age and DE. This agrees with the study by El-Halaby et al. [7], but disagrees with the studies by Boussuges et al. [20], Hida et al. [19], and Hayat et al. [21], as they did not find any correlation between diaphragmatic mobility and age. On the other hand, Scarlata et al. [22] found a negative correlation between the DE and age in deep breathing. This discrepancy is attributable to the different age ranges of the patients studied, as our research and that by El-Halaby et al. [7] were conducted on children as opposed to adults.
In our research, only the left DE was positively correlated with the age and weight of the patients, indicating that the left diaphragm is more accurate in evaluating the DE, possibly because the DE is limited by the presence of the liver on the right side [4]. Also, Hida et al. [19] showed that the left DE is greater than the right DE, and that in both the inspiratory and the expiratory stage, the left diaphragm reaches its peak motion faster than the right one.
In our study, there was a significant correlation between the DE and body height. A similar finding was reported by Boussuges et al. [20], who found a weak positive correlation between diaphragmatic mobility and height and a strong positive correlation between diaphragmatic mobility and weight.
We also found a significant positive correlation between the DE and the heart rate. This can be explained by the physiological hemodynamics of the heart, as patients with better diaphragmatic mobility have increased venous return to the heart, leading to an increased heart rate [23, 24].
In cystic fibrosis patients, the strength of the diaphragm is affected by the nutritional state of the patient, which is the result of impaired energy intake, decreased absorption, defective intestinal motility, and increased energy requirements. Hart et al. [25] found a positive correlation between diaphragm strength and nutritional status in children but not in adults.
In our study, although we expected to find a difference in the diaphragmatic thickness and excursion between patients with cystic fibrosis and patients with non-cystic fibrosis bronchiectasis because of the different pathophysiology and greater nutritional depletion in cystic fibrosis patients, there was no statistically significant difference. Also, Rosenthal et al. [26] did not find any specific skeletal muscle affection in patients with cystic fibrosis compared with patients with non-cystic fibrosis bronchiectasis.
In adult COPD patients, an improved DE after physical rehabilitation was reported by Crimi et al. [27]. Similar research on the impact of physical therapy on the DE in pediatric patients with chronic pulmonary diseases needs to be performed.
Conclusion
The DE measured by ultrasound is lower in pediatric patients with chronic pulmonary diseases than in healthy subjects. The DE is positively correlated with patients’ age, weight, height, FEV1/FVC ratio, and heart rate.
Abbreviations
- CT
Computed tomography.
- DE
Diaphragm excursion
- FRC
Functional residual capacity
- FVC
Forced vital capacity
- ILD
Interstitial lung diseases
- IPF
Idiopathic pulmonary fibrosis
- LUS
Lung ultrasound
- mMRC
Modified Medical Research Council
- MRI
Magnetic resonance imaging
- TF
Thickness fraction
- TLC
Total lung capacity
Funding
This research did not receive any funding.
Availability of data and materials
The data that support the results of this research are available upon request from the corresponding author. The data are not publicly available for the privacy of research participants.
Compliance with ethical standards
Conflict of interest
The authors have no conflict of interest to disclose.
Ethical approval
The study was approved by the local ethics committee, Children’s Hospital, Ain Shams University.
Consent to participate
Verbal and written consents were taken from all the caregivers of our patients.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Sally R. Ishak, Email: sally.raafat@med.asu.edu.eg
Hossam M. Sakr, Email: drhossamsakr@gmail.com
References
- 1.Nava S, Rubini F. Lung and chest wall mechanics in ventilated patients with end stage idiopathic pulmonary fibrosis. Thorax. 1999;54(5):390–395. doi: 10.1136/thx.54.5.390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Santana PV, Prina E, Albuquerque AL, Carvalho CR, Caruso P. Identifying decreased diaphragmatic mobility and diaphragm thickening in interstitial lung disease: the utility of ultrasound imaging. J Bras Pneumol. 2016;42(2):88–94. doi: 10.1590/S1806-37562015000000266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Davidsen JR, Bendstrup E, Henriksen DP, Graumann O, Laursen CB. Lung ultrasound has limited diagnostic value in rare cystic lung diseases: a cross-sectional study. Eur Clin Respir J. 2017;4(1):1330111. doi: 10.1080/20018525.2017.1330111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sarwal A, Walker FO, Cartwright MS. Neuromuscular ultrasound for evaluation of the diaphragm. Muscle Nerve. 2013;47(3):319–329. doi: 10.1002/mus.23671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.American Thoracic Society (ATS) statement Guidelines for the six-minute walk test. Am J Respir Crit Care Med. 2002;166:111–117. doi: 10.1164/ajrccm.166.1.at1102. [DOI] [PubMed] [Google Scholar]
- 6.Papiris SA, Daniil Z, Malagari K, et al. The Medical Research Council dyspnea scale in the estimation of disease severity in idiopathic pulmonary fibrosis. Respir Med. 2005;99:755–761. doi: 10.1016/j.rmed.2004.10.018. [DOI] [PubMed] [Google Scholar]
- 7.El-Halaby H, Abdel-Hady H, Alsawah G, et al. Sonographic evaluation of diaphragmatic excursion and thickness in healthy infants and children. J Ultrasound Med. 2016;35:167–175. doi: 10.7863/ultra.15.01082. [DOI] [PubMed] [Google Scholar]
- 8.Epelman M, Navarro OM, Daneman A, Miller SF. M-mode sonography of diaphragmatic motion: description of technique and experience in 278 pediatric patients. Pediatr Radiol. 2005;35(7):661–667. doi: 10.1007/s00247-005-1433-7. [DOI] [PubMed] [Google Scholar]
- 9.Nason LK, Walker CM, Mcneeley MF, Burivong W, Fligner CL, Godwin JD. Imaging of the diaphragm: anatomy and function. Radiographics. 2012;32(2):E51–E70. doi: 10.1148/rg.322115127. [DOI] [PubMed] [Google Scholar]
- 10.Boccatonda A, Decorato V, Cocco G, Marinari S, Schiavone C. Ultrasound evaluation of diaphragmatic mobility in patients with idiopathic lung fibrosis: a pilot study. Multidiscip Respir Med. 2019;14:1. doi: 10.1186/s40248-018-0159-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.He L, Zhang W, Zhang J, Cao L, Gong L, Ma J, et al. Diaphragmatic motion studied by M-mode ultrasonography in combined pulmonary fibrosis and emphysema. Lung. 2014;192(4):553–561. doi: 10.1007/s00408-014-9594-5. [DOI] [PubMed] [Google Scholar]
- 12.Yamaguti W, Paulin E, Shibao S, Chammas M, Salge JM, Ribeiro M, et al. Air trapping: the major factor limiting diaphragm mobility in chronic obstructive pulmonary disease patients. Respirology. 2008;13:138–144. doi: 10.1111/j.1440-1843.2007.01194.x. [DOI] [PubMed] [Google Scholar]
- 13.Adel SM, Hieba EG, Hossam SH. Assessment of diaphragmatic mobility by chest ultrasound in relation to BMI and spirometric parameters. Egypt J Bronchol. 2019;13:232–243. doi: 10.4103/ejb.ejb_73_18. [DOI] [Google Scholar]
- 14.Dos Santos Yamaguti WP, Paulin E, Shibao S. Air trapping: the major factor limiting diaphragm mobility in chronic obstructive pulmonary disease patients. Respirology. 2008;13:138–144. doi: 10.1111/j.1440-1843.2007.01194.x. [DOI] [PubMed] [Google Scholar]
- 15.Baria MR, Shahgholi L, Sorenson EJ, et al. B-mode ultrasound assessment of diaphragm structure and function in patients with COPD. Chest. 2014;146(3):680–685. doi: 10.1378/chest.13-2306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Scott S, Fuld JP, Carter R, McEntegart M, MacFarlane NG. Diaphragm ultrasonography as an alternative to whole-body plethysmography in pulmonary function testing. J Ultrasound Med. 2006;25(2):225–232. doi: 10.7863/jum.2006.25.2.225. [DOI] [PubMed] [Google Scholar]
- 17.Smargiassi A, Inchingolo R, Tagliaboschi L, Di Marco BA, Valente S, Corbo GM. Ultrasonographic assessment of the diaphragm in chronic obstructive pulmonary disease patients: relationships with pulmonary function and the influence of body composition—a pilot study. Respiration. 2014;87:364–371. doi: 10.1159/000358564. [DOI] [PubMed] [Google Scholar]
- 18.Singer J, Yelin EH, Katz PP, et al. Respiratory and skeletal muscle strength in chronic obstructive pulmonary disease: impact on exercise capacity and lower extremity function. J Cardiopulm Rehabil Prev. 2011;31(2):111–119. doi: 10.1097/HCR.0b013e3182033663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hida T, Yamada Y, Ueyama M, Araki T, Nishino M, Kurosaki A, Jinzaki M, Honda H, Hatabu H, Kudoh S. Time-resolved quantitative evaluation of diaphragmatic motion during forced breathing in a health screening cohort in a standing position: dynamic chest phrenicography. Eur J Radiol. 2019;113:59–65. doi: 10.1016/j.ejrad.2019.01.034. [DOI] [PubMed] [Google Scholar]
- 20.Boussuges A, Gole Y, Blanc P. Diaphragmatic motion studied by m-mode ultrasonography methods, reproducibility, and normal values. Chest. 2008;135(2):391–400. doi: 10.1378/chest.08-1541. [DOI] [PubMed] [Google Scholar]
- 21.Hayat A, Khan A, Khalil A, Asghar A. Diaphragmatic excursion: does it predict successful weaning from mechanical ventilation? J Coll Phys Surg Pak. 2017;27(12):743–746. [PubMed] [Google Scholar]
- 22.Scarlata S, Mancini D, Laudisio A, Benigni A, Antonelli Incalzi R. Reproducibility and clinical correlates of supine diaphragmatic motion measured by M-mode ultrasonography in healthy volunteers. Respiration. 2018;96:259–266. doi: 10.1159/000489229. [DOI] [PubMed] [Google Scholar]
- 23.Bordoni B, Zanier E. Anatomic connections of the diaphragm: influence of respiration on the body system. J Multidiscip Healthc. 2013;6:281–291. doi: 10.2147/JMDH.S45443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Russo MA, Santarelli DM, O'Rourke D. The physiological effects of slow breathing in the healthy human. Breathe (Sheff) 2017;13(4):298–309. doi: 10.1183/20734735.009817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hart N, Tounian P, Clément A, Boulé M, Polkey MI, Lofaso F, Fauroux B. Nutritional status is an important predictor of diaphragm strength in young patients with cystic fibrosis. Am J Clin Nutr. 2004;80(5):1201–1206. doi: 10.1093/ajcn/80.5.1201. [DOI] [PubMed] [Google Scholar]
- 26.Rosenthal M, Narang I, Edwards L, Bush A. Non-invasive assessment of exercise performance in children with cystic fibrosis (CF) and non-cystic fibrosis bronchiectasis: Is there a CF specific muscle defect? Pediatr Pulmonol. 2009;44(3):222–230. doi: 10.1002/ppul.20899. [DOI] [PubMed] [Google Scholar]
- 27.Crimi C, Heffler E, Augelletti T, Campisi R, Noto A, Vancheri C, Crimi N. Utility of ultrasound assessment of diaphragmatic function before and after pulmonary rehabilitation in COPD patients. Int J Chron Obstruct Pulmon Dis. 2018;13:3131–3139. doi: 10.2147/COPD.S171134. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the results of this research are available upon request from the corresponding author. The data are not publicly available for the privacy of research participants.