Abstract
Purpose
The sciatic nerve innervates the hamstring muscles. Occasionally, the sciatic nerve is injured along with a hamstring muscle. Detailed biomechanical and sensory thresholds of these structures are not well-characterized. Therefore, we designed a prospective study that explored high-resolution ultrasound (US) at multiple sites to evaluate properties of the sciatic nerve, including cross-sectional area (CSA) and shear-wave elastography (SWE). We also assessed SWE of each hamstring muscle at multiple sites. Mechanical algometry was obtained from the sciatic nerve and hamstring muscles to assess multi-site pressure pain threshold (PPT).
Methods
Seventy-nine asymptomatic sciatic nerves and 147 hamstring muscles (25 males, 24 females) aged 18–50 years were evaluated. One chiropractic radiologist with 4.5 years of US experience performed the evaluations. Sciatic nerves were sampled along the posterior thigh at four sites obtaining CSA, SWE, and algometry. All three hamstring muscles were sampled at two sites utilizing SWE and algometry. Descriptive statistics, two-way ANOVA, and rater reliability were assessed for data analysis with p ≤ 0.05.
Results
A significant decrease in sciatic CSA from proximal to distal was correlated with increasing BMI (p < 0.001). Intra-rater and inter-rater reliability for CSA was moderate and poor, respectively. Elastographic values significantly increased from proximal to distal with significant differences in gender and BMI (p = 0.002). Sciatic PPT significantly decreased between sites 1 and 2, 1 and 3, and 1 and 4. Significant correlation between gender and PPT was noted as well as BMI (p < 0.001). Hamstring muscle elastographic values significantly differed between biceps femoris and semitendinosus (p < 0.001) and biceps femoris and semimembranosus (p < 0.001). All three hamstring muscles demonstrated increased PPT in males compared to females (p < 0.001). In addition, PPT of the biceps femoris correlated with BMI (p = 0.02).
Conclusion
High-resolution US provided useful metrics of sciatic nerve size and biomechanical properties. PPT for the normal sciatic nerve and hamstring muscles was obtained for future clinical application.
Keywords: Sciatic nerve, Hamstring muscles, Ultrasonography, Elastography, Algometry
Introduction
Magnetic resonance imaging (MRI) and diagnostic ultrasound (US) are imaging modalities used in the assessment of sciatic nerve and hamstring muscles disorders [1–4]. Altered sciatic nerve function has been reported in association with hamstring injuries [5], however, sonographic and sensory characterization of this relationship requires clarification.
B-mode US has been increasingly utilized in sciatic nerve evaluation. The normal US appearance of the sciatic nerve is iso to hyperechoic epineurium, perineurium, and endoneurium surrounding multiple hypoechoic fascicles. Studies have defined upper limits of the sciatic nerve cross-sectional area (CSA) utilizing US [6].
Elastography is another US modality utilized to assess peripheral nerves, including the sciatic nerve. Shear-wave elastography (SWE) quantifies mechanical and elastic tissue properties. Shear wave uses a perpendicular sound wave that propagates along the tissue measured while the tissue is undergoing a shear force. This is defined as a change in shape of a tissue without volume change. SWE has been emerging in musculoskeletal (MSK) applications, including muscles, tendons, nerves, and ligaments [7]. Elastographic measures have been shown to increase as strain results from reduced fluid diffusion across the cell membrane [8]. This finding is exemplified in peripheral neuropathies [9–11].
There is limited sonoelastographic characterization of the sciatic nerve in an asymptomatic population. SWE has been described within the sciatic nerve during passive and active lower extremity motions [2, 12–14], as well as lumbar spine active ranges of motion [15]. Sonoelastography of the sciatic nerve-hamstring muscle interface has been assessed during active and passive flexion and extension of the knee in a healthy population cohort [16]. To our knowledge, there have been no studies utilizing sonoelastography in the assessment of the relationship of the sciatic nerve and hamstring muscles.
Hamstring injuries are the most common acute muscle injury, seen in 46.2%, of high-level athletes who participate in track and field and soccer, recurrence is common [17, 18]. Due to this close anatomical relationship, an eccentric neuropraxic sciatic injury [1, 19, 20], as well as entrapments [21–24] and iatrogenic causes [25], may accompany hamstring muscle injuries. Changes in the electrodiagnostic sciatic conduction velocity have even been noted in those with hamstring injuries [5]. US has proven a useful imaging modality in the assessment of the hamstring muscles.
Diagnostic B-mode ultrasound (US) has shown good reliability in assessing the hamstring muscle size and quality in young healthy subjects [3, 26]. Hamstring muscle dynamics have been studied extensively utilizing sonoelastography. Hamstring muscles between a resting state and passive stretching have shown higher stiffness qualities [14, 27]. Elastographic differences have also been demonstrated in muscle injury and atrophy [28, 29]. Similarly, a systematic review concluded an increase in elasticity has been observed in paretic and damaged muscles [30]. Discordant results have been reported. A decrease in SWE of muscles in the lower extremity was demonstrated in runners and was correlated with the distance completed [31].
Another quantitative means of assessing muscle and nerve sensory profile is pain pressure threshold (PPT). An algometer is a device employed to measure a patient’s PPT [32]. There are different types, such as mechanical algometry, cuff algometry, and thermal (heat and cold) induction algometry [32]. PPTs have been extensively assessed in muscles [33–36], whereas, little research has been performed in the investigation PPT in peripheral nerves, in particular the sciatic [37, 38]. Good to excellent inter- and intra-examiner reliability for pressure algometry of the sciatic nerve has been reported in asymptomatic subjects [39].
The primary aim of this study was to obtain at multiple sites SWE and CSA values of the entire sciatic nerve and SWE values of the hamstring musculature. We also obtained PPT values in normal sciatic nerves and hamstring muscles. The characterization of these findings in healthy volunteers will inform the pathophysiological relationship in the injured sciatic nerve and hamstring muscles.
Methods
Subjects
This study was approved by the university’s Institutional Review Board. Participants were recruited internally through the university by class announcements. All subjects were provided a written informed consent. Criteria for inclusion were either gender, age 18–65 with a BMI less than 29 lbs/in2, and the provision of informed consent. Exclusion criteria included a history of hip, knee, or ankle pain, surgery, or fracture/internal derangement; inability to tolerate mild and transient pressure or bruising of the posterior thigh; collagen disorders, such as rheumatoid arthritis or systemic lupus; lumbar central canal or foraminal stenosis; lumbar radiculopathy; spondylotic myelopathy; ischiofemoral impingement or piriformis syndrome; and diabetes mellitus. Body mass index (BMI) was calculated for all subjects. Additionally, demographic information regarding gender, height, and weight were recorded. Subjects’ thighs that met the inclusion criteria were examined.
Sonography
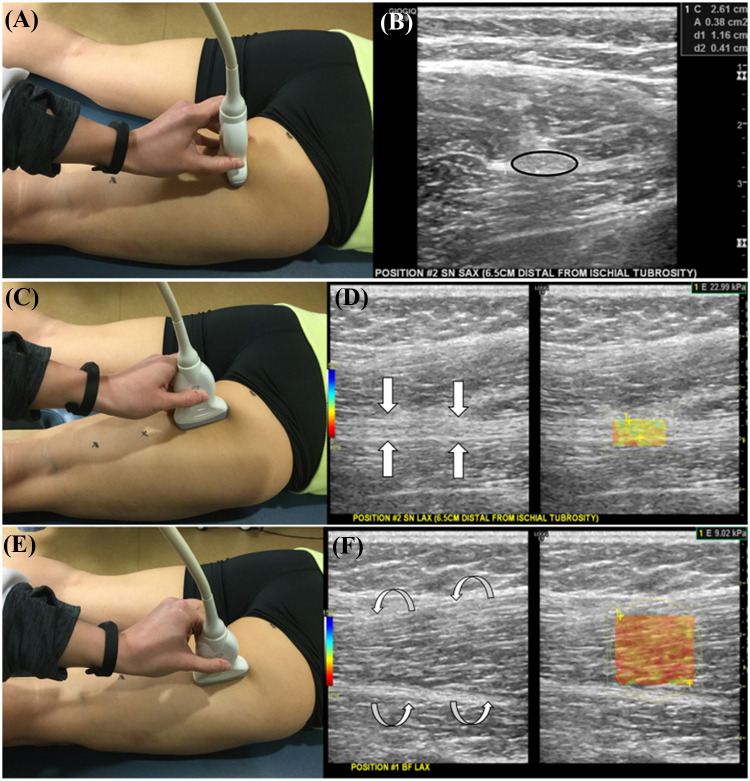
All US examinations were performed in the sonography suite of the radiology department. US examination of the sciatic nerve and each hamstring muscle was performed with subjects in a prone position with the knee extended and hip in a neutral position. All images were acquired by a chiropractic radiologist with 4.5 years of experience in musculoskeletal ultrasound. A Logiq E9 (General Electric Healthcare, Wauwatosa, WI) US system operating at a 9 MHz high-frequency linear array transducer and coupling agent was employed. The sonographic parameters of the sciatic nerve were measured at four sites along the posterior thigh. First, the sciatic nerve was imaged at a site (site 1) adjacent to the ischial tuberosity and then a short axis linear slide to site 4 just proximal to the bifurcation of the tibial and common fibular nerves. Waterproof marker was used to identify both locations. These marks identified sites 1 and 4. A measurement was made between the two marked points which established two other central locations on the thigh (sites 2 and 3). At each of the four sites, the sonographic probe was applied in the axial (short axis) and sagittal (long axis) planes (Fig. 1a–d). All three hamstring muscles (biceps femoris, semimembranosus, and semitendinosus) were evaluated in long axis for SWE (Fig. 1e, f).
Fig. 1.
Probe position (a) and B-mode image (b) of the sciatic nerve in short axis (black circle) at site #2. Probe position (c) and B-mode and elastographic image (d) of the sciatic nerve in long axis (white arrows) at site #2. Probe position (e) and B-mode and elastographic image (f) of the biceps femoris muscle in long axis (white curved arrows) at site #1
Sciatic nerve CSA (cm2) and SWE (kPa) was evaluated at all four sites. CSA of the sciatic nerve was measured at its maximum diameter using the digital ellipse function tool (Fig. 1b). The SWE region of interest (ROI) was established from epineurium to epineurium in the anterior to posterior dimension and set at the widest width in the superior to inferior dimension (Fig. 1d). All three hamstring muscle SWE measures were evaluated at the two central locations as described above. The ROI for the SWE measurements of the hamstring muscles was established using the epimysium as the anterior to posterior boundary and the maximum width (Fig. 1f). Sequential SWE evaluation started with biceps femoris and ended with semimembranosus. The SWE range was set from 0 to 250 kiloPascals (kPa) utilizing a musculoskeletal setting. All US images were assessed for technical quality prior to analysis. Images were analyzed and stored in the Logiq E9 hard drive.
Pressure pain threshold
Pressure pain threshold (PPT) was obtained utilizing an algometer. This measure was obtained overlying the sciatic nerve along the posterior thigh at all four sites, as well as each hamstring muscle at the two central sites. The algometer measurements, expressed in units of pounds, were recorded three times at each site and averaged. The algometer (CommanderTM Algometer; JTECH Medical Industries, Midvale, UT) utilized a 1.0 cm circular probe with a rubber tip connected to the pressure transducer. Algometer pressure was gradually increased over the sciatic nerve until the participant indicated pain (PPT) by verbal response. Each reading required a 30 s rest period between measurements. All PPT measurements were performed and recorded by the same examiner.
Statistical analysis
Descriptive statistics were presented as means, standard deviations (SD), and ranges for continuous data or numbers (n) and percentages (%). The threshold of statistical significance was established for all measurements at p < 0.05.
The outcome measures (CSA, PPT, SWE) were compared using mixed-effects random-intercept, random-slope models using Matlab (The MathWorks Inc., Natick, MA, USA). Site, Gender, and BMI were included as fixed effects in the model. Factors significance was tested using ANOVA, followed by post hoc comparisons when appropriate to determine the significance of the findings.
Both intra- and inter-rater reliability of the CSA of the sciatic nerve was performed using intraclass correlation coefficient (ICC). Images of the sciatic nerve were randomly selected for rater evaluations and performed independently. For intra-rater reliability, rater #1 re-measured 20 sciatic nerve CSAs 6 weeks after the original data collection. For inter-rater reliability, a second rater measured 20 sciatic nerves at the same time frame as the intra-rater reliability. Bland-Altmann plots were utilized for ICC analysis.
Results
Sciatic nerve
Seventy-nine sciatic nerves (25 males, 24 females) aged 18–50 years (mean age 27 years) were enrolled. The average BMI of our participants was 24.3 lbs/in2 (range 17.7–29.0 lbs/in2). The descriptive statistics (means and standard deviations) of the sciatic nerve CSA, SWE, and PPT are in Table 1. There was a significant decrease in CSA between sites 1 and 4 (p < 0.001), sites 1 and 3 (p = 0.012), sites 2 and 4 (p < 0.001), and sites 3 and 4 (p = 0.002) (Fig. 2a). There was a significant direct correlation between increasing CSA and BMI (p < 0.001, r = 0.62) (Fig. 2b). Intra-rater and inter-rater reliability were moderate (0.70) and poor (0.40), respectively (Fig. 3).
Table 1.
Descriptive statistics for cross-sectional area (CSA), shear-wave elastography (SWE), and pressure pain threshold (PPT) of the sciatic nerve by testing site
| Means and standard deviations of the CSA, SWE, and PPT of the sciatic nerve by site | ||||
|---|---|---|---|---|
| Site 1 | Site 2 | Site 3 | Site 4 | |
| CSA (cm2) | 0.46 ± 0.085 | 0.45 ± 0.08 | 0.44 ± 0.075 | 0.42 ± 0.07 |
| SWE (kPa) | 28.0 ± 12.44 | 33.9 ± 16.46 | 48.6 ± 20.46 | 63.9 ± 27.37 |
| PPT (lbs) | 19.5 ± 4.28 | 17.7 ± 4.52 | 17.0 ± 4.54 | 17.9 ± 4.62 |
Fig. 2.
Sciatic nerve CSA mean and standard deviation at each acquisition site. Note the significant decrease in CSA from Site 1 to Site 4 (a). Linear regression analysis demonstrated a significant correlation between increasing BMI and increasing CSA (b). Sciatic nerve SWE mean and standard deviation at each acquisition site. Note the significant increase in SWE from Site 1 to Site 4 (c). Mean and standard deviation of SWE between males and females. Note the significant increase of SWE in males compared to females (d). Sciatic nerve PPT mean and standard deviation at each acquisition site. Note the significant decrease in PPT from Site 1 to Site 3 (e). Linear regression analysis demonstrated a significant correlation between increasing BMI and Increasing PPT (f). Significance was measured at p < 0.05
Fig. 3.
Bland–Altman plots for inter- and intra-reliability analysis of the sciatic nerve CSA. Intra-rater and inter-rater reliability for CSA was moderate (0.70) and poor (0.40), respectively
Elastography of the sciatic nerve revealed significant increasing values from sites 1 and 4 (p < 0.001), sites 1 and 2 (p 0.013), sites 1 and 3 (p < 0.001), sites 2 and 3 (p < 0.001), sites 2 and 4 (p < 0.001), and sites 3 and 4 (p < 0.001) (Fig. 2c). There was a significant difference between male and female sciatic nerve elastography with males retaining higher values (p < 0.001) (Fig. 2d). There was also a positive direct correlation between sciatic nerve elastography and BMI (p = 0.002).
Sciatic nerve PPT demonstrated a significant decrease between sites 1 and 2 (p < 0.001), sites 1 and 3 (p < 0.001), and sites 1 and 4 (p = 0.014). There were no significant decreases between sites 2 and 3 and sites 3 and 4 (Fig. 2e). There was a significant positive correlation between gender and PPT with males having a higher threshold (p = 0.05). There was also a positive direct correlation between PPT and BMI (p < 0.001) (Fig. 2f).
Hamstring muscles
The descriptive statistics (means and standard deviations) of all three hamstring muscle SWE and PPT are in Table 2. Elastography of the biceps femoris muscle demonstrated no significant effect between sites, gender, or BMI. Although, semitendinosus and semimembranosus elastography revealed a significant inverse relationship with BMI (p = 0.05 and p = 0.002, respectively) (Figs. 4a and 5a). Semitendinosus and semimembranosus muscle elastography demonstrated no relationship between sites or gender. Elastography between muscles revealed a significant increase in values between biceps femoris and semitendinosus muscles (p < 0.001) and biceps femoris and semimembranosus muscles (p < 0.001) (Fig. 6a). There was also a significance difference between site 1 and site 2 across all three muscles with site 2 having higher values (p < 0.001) (Fig. 6b).
Table 2.
Descriptive statistics for shear-wave elastography (SWE) and pressure pain threshold (PPT) of the hamstring muscles by testing site
| Means and standard deviations of the SWE and PPT of the Hamstring muscles by site | ||
|---|---|---|
| Hamstring muscle | Site 1 | Site 2 |
| Biceps femoris | ||
| SWE (kPa) | 14.43 ± 5.29 | 16.27 ± 6.62 |
| PPT (lbs) | 15.57 ± 4.37 | 15.68 ± 4.69 |
| Semitendinosus | ||
| SWE (kPa) | 22.23 ± 9.75 | 24.70 ± 10.02 |
| PPT (lbs) | 15.61 ± 4.46 | 16.61 ± 4.58 |
| Semimembranosus | ||
| SWE (kPa) | 21.15 ± 9.44 | 23.43 ± 9.41 |
| PPT (lbs) | 12.72 ± 4.79 | 14.37 ± 4.99 |
Fig. 4.
Linear regression analysis of the semitendinosus muscle demonstrated a significant correlation between increasing BMI and decreasing SWE (a). Semitendinosus muscle PPT for each gender. A significant increased mean PPT in males compared to females was noted (b). Semitendinosus muscle PPT for each site. A significant increased mean PPT between site #1 and site #2 noted (c). Significance was measured at p < 0.05
Fig. 5.
Linear regression analysis of the semimembranosus muscle demonstrated a significant correlation between increasing BMI and decreasing SWE (a). Semimembranosus muscle PPT for each gender. A significant increased mean PPT in males compared to females was noted (b). Semimembranosus muscle PPT for each site. A significant increased mean PPT between site #1 and site #2 noted (c). Significance was measured at p < 0.05
Fig. 6.
SWE values between the biceps femoris muscle and semimembranosus muscle revealed a significant increase as well as significantly increased SWE values between the biceps femoris muscle and semitendinosus muscle (a). Overall data for the three hamstring muscles demonstrated a significantly increased SWE from site 1 to site 2 (b). Significance was measured at p < .05
Some significant changes in PPT were noted within each hamstring muscle. Semitendinosus, semimembranosus, and biceps femoris muscles demonstrated a significant increase in PPT of males compared to females (p < 0.001) (Figs. 4b, 5b, and 7). There was also a significance correlation between PPT of the biceps femoris muscle and BMI (p = 0.02), although the semitendinosus and semimembranosus muscles did not demonstrate this correlation. There was a significance increase in PPT between site 1 and site 2 within the semitendinosus and semimembranosus muscles (p = 0.012 and p < 0.001, respectively) (Fig. 4c and 5c). The biceps femoris demonstrated no significant difference in PPT between sites.
Fig. 7.
Biceps femoris muscle PPT for each gender. A significant increased mean PPT in males compared to females was noted. Significance was measured at p < 0.05
Discussion
The sciatic nerve is the longest nerve in the body and is easily accessible with diagnostic US. The B-mode US appearance of the sciatic nerve is similar to other peripheral nerves in the body with a “cluster of grapes” appearance in short axis and a loose fascicular appearance in long axis [6]. The sciatic nerve is hypoechoic with an echogenic epineural membrane. In B-mode evaluation of the sciatic nerve, a CSA is often measured. Chen et al. demonstrated in 400 sciatic nerves a mean range CSA of 0.47–0.59 cm2 at the level of the gluteal sulcus and 0.39–0.50 cm2 at the midpoint of the thigh in a normal population [6]. Our mean CSA measurements were 0.46 cm2 at the ischial tuberosity, 0.45 cm2 at site 2 just proximal to mid-thigh, 0.44 cm2 at site 3 just distal to mid-thigh, and 0.42 cm2 just proximal to the bifurcation. Even though our mean CSAs were slightly smaller in comparison to Chen et al., we similarly observed a decrease in CSA of the sciatic nerve as we moved more distal through the four sites of data acquisition from ischial level to the bifurcation.
Unlike Chen et al., we did not show a significant difference between gender CSAs, although our sample age range was limited to middle age. Cartwright et al., revealed a gradient of sciatic CSA as a function of age. They compared pediatric and geriatric populations and concluded that the CSA of the sciatic nerve increased with age [40]. This relationship may explain the CSA discrepancy between our study and Chen et al. [6]. Concordant with Chen et al. [6] and Bedewi et al. [41], we also showed a strong linear correlation between nerve CSA and BMI. Although, this relationship varied depending on the peripheral nerve evaluated. Werner et al. [42] noted no difference between obese and thin groups in the evaluation of the median nerve CSA.
Sonoelastography is another technology utilized to assess the sciatic nerve as it evaluates the biomechanical properties of musculoskeletal tissues [7]. Andrade et al. studied biomechanical characteristics of the sciatic nerve noting the sciatic nerve had the highest stiffness (10.4 ± 2.4 m/s) at 180˚ of knee flexion with full ankle dorsiflexion [2]. When the m/s units are converted to kPa, this translated to a much higher stiffness compared with our data. One explanation for this discrepancy is we evaluated our participants in 180˚ of knee extension with a neutral ankle position, which may have reduced strain on the sciatic nerve. Topp et al. found compressive and tensile forces on the nerve could reduce the capacity to conduct action potentials and reduce axonal transportation and intraneural blood flow. If stress exceeds this capacity, neural injury occurs. The nerve is exposed to increased tensile stress when it crosses a joint. Due to viscoelastic properties of healthy nerves, the nerve has a greater capacity to stretch for a modest increase in length. Excursion of the nerve was drawn toward the moving joint when the limb is lengthened and inversely when the limb is shortened. Shear forces along nonneural tissues, e.g., muscles, during nerve sliding imposes pressure on the nerve. Increased pressure on the nerve exceeding > 30 mm Hg decreases perfusion leading to efferent conduction dysfunction [43]. Greening et al., noted similar postural changes demonstrated increased stiffness in the tibial and median nerves [12]. Coppieters et al. reported that cadavers displayed the greatest strain on the sciatic nerve in hip flexion with ankle dorsiflexion while performing a modified SLR test [13]. Neto et al. noted no significant difference in stiffness of the sciatic nerve during the slump test, and attributed this lack of significance to the compliance of the nerve to these tensile loads [15], although this may change with age [44]. Interestingly, a more recent study by Neto et al. demonstrated decreasing SWE values with the slump test in patients with sciatica, suggesting tissues changes with pathology versus normal tissues[45]. Unlike Andrade et al., Greening et al., and Coppieters et al., we did not evaluate for dynamic changes of the nerve stiffness.
Interestingly, we found that the SWE stiffness of the sciatic nerve progressively increased moving from proximal to distal (site 1 to site 4), although CSA was reduced. A possible explanation for this gradual increase may be the proximity of the sciatic nerve to the distal femur. Bortolotto et al. found a stiffness increase of the median nerve as it approached the carpal tunnel attributing this finding to “bone-proximity” hardening artifact [46]. We also noted a significant increase in SWE in males compared to females as well as a positive correlation between SWE and BMI within the sciatic nerve. A few studies had similar differences in SWE between genders [12, 46], although these studies only noted a moderate correlation between height and weight and SWE.
Algometry has not been studied extensively within the peripheral nerves. It is measured at the point when pressure stimulus transitions into a painful stimulus [32]. Sterling et al. found that the mean PPTs of the median, radial, and ulnar nerves ranged from 240 to 380 kPa and was overall higher for males compared to females [37]. Cornelson et al. demonstrated a mean PPT of 12.2 lb for the ulnar nerve [47]. We found a mean PPT of the sciatic nerve proximally to distally (site 1 to site 4) decreased significantly. There were also significant decreases between each site, except sites 3 and 4 where there was an insignificant increase in PPT. There also was a positive correlation between PPT and BMI. Concordant to Sterling et al. [37], our data showed a significant difference in PPT between males and females with males having a higher mean PPT than females. Even though, there are not very many PPT studies on peripheral nerves, Fingleton et al. demonstrated excellent (0.9) intrarater and good (0.75) interrater reliability for the sciatic nerve [39].
There are not many studies assessing the direct physiologic relationship between the sciatic nerve and the hamstring muscles. One study evaluated the sciatic nerve-hamstring muscle interface with SWE. Ellis et al. found no significant differences in SWE at the sciatic nerve-hamstring muscle interface with passive (63.26 ± 40.29%) and active (59.84 ± 32.24%) ranges of knee motion [16]. This study may explain the relationship between hamstring tears and the neuropraxic injury of the sciatic nerve. The sciatic nerve courses in the fascial plane between the adductor magnus, semitendinosus, and biceps femoris muscles [48]. All the hamstring muscles and occasionally the adductor magnus [49], are innervated by the sciatic nerve [48]. Perez-Bellmunt et al. found fibrotic bands of connective tissue around the sciatic nerve at the level of the proximal hamstring complex may compress the nerve, producing a compressive neuropathy. Epimysial expansions from the gluteus maximus form around the sciatic nerve creating a tunnel. Fibrous attachments from the gluteus maximus epimysium also connect to the biceps femoris origin on the ischial tuberosity [50]. This close anatomic relationship of the sciatic and hamstring muscles has its effects in injury. For example, Kouzaki et al. found sciatic nerve conductivity was decreased in hamstring injuries as compared to the unaffected limb and healthy controls [5]. Recurrence of hamstring injuries are reported at 79%. Injury often recurred at the original site [18], and the rate of recurrence increased with intratendinous extension of the injury [51]. Although it is a rare occurrence, sciatic nerve entrapment by a hamstring tear or scar tissue from a previous tear has been described [22]. The hamstring muscles have been shown to be easily accessible and evaluated by US for normal muscle architecture and injury.
Blackburn et al. measured normal noncontracted hamstring muscle architecture utilizing US. They utilized US to measure the CSA and fascicle length of the biceps femoris. Other parameters were also assessed, such as tendon stiffness and strength utilizing a damped oscillatory technique, all of which were greater in males as compared to females [26]. SWE is another way to evaluate the stiffness of the muscles and has been studied extensively during passive and active motions and stretching [30, 52]. Lacourpaille et al. showed an increase in SWE 30 min after muscle damaging eccentric exercise within the knee extensors [28]. Dynamic hamstring muscle mechanics have also been assessed utilizing SWE. Le Sant et al. demonstrated a significant increase in SWE of all the hamstring muscles with increasing knee extension and hip flexion. Higher SWE for long head of the biceps femoris muscle compared to semimembranosus muscle and the semimembranosus was higher than the semitendinosus muscle [14]. Even though our participants were evaluated in a neutral hip posture, we found that the biceps femoris muscle SWE was significantly lower than the semimembranosus and semitendinosus muscles. We hypothesize this difference in SWE of the biceps femoris muscle may lead to the increased rates of injury in this muscle [53]. Sadeghi et al. evaluated the SWE in thigh and leg muscles, including the biceps femoris and semitendinosus, which showed a significant decrease in SWE of the biceps femoris muscle in short and long-distance runners. There was also a significant decrease in SWE of the semitendinosus muscle between short and medium distance runners [31]. Nakamura et al. showed semimembranosus muscle had a significantly higher SWE than semitendinosus and biceps femoris muscles in all pelvic positions. The semimembranosus muscle also had a higher increase in SWE with passive elongation. All hamstrings showed increased SWE with anterior pelvic tilt [27]. Umegaki et al. demonstrated an overall significant decrease in SWE of the hamstring muscles after 5 min of static stretching. Even though they evaluated their participants in 90˚ knee flexion and hip flexion, their pre-stretching SWE values were similar to ours [54].
Pain pressure thresholds (PPT) have been well studied in most muscle groups and has been shown to decrease in myofascial pain syndromes [55]. A significant effect of BMI and PPT was identified within the biceps femoris muscle, but not in the semimembranosus and semitendinosus. Tashani et al. found that in obese individuals BMI and body fat distribution decreased PPT compared to normal weight individuals [33]. Dissimilarly, Torensma et al. demonstrated that the obese population had hypoalgesia to electrical and thermal stimuli compared to those within a normal BMI [35]. Our data showed PPT differences in males and females with males exhibiting a higher threshold. This was like other studies assessing muscle PPT. Cheatham et al. evaluated males and females PPT pre and post foam rolling. They found foam rolling overall increased PPT in males and females, although there were no differences in PPT between males and females [34]. Similarly, Jay et al. demonstrated massage with roller device increased PPTs in participants with delayed onset muscle soreness [56]. Kim et al. showed similar effects from instrument-assisted soft tissue mobilization [36]. Our data could not be directly compared to these studies as our measurements were in differing units. Uniquely, our data showed a significant increase in PPT between the first site compared to the second site. To our knowledge, intramuscular differences in PPT have not been demonstrated.
We are reporting the most extensive evaluation of SWE and CSA of the sciatic nerve by US to date. The SWE increase of the sciatic nerve from proximal to distal is novel. Some limitations of this study require discussion. First, this was a cross-sectional study of asymptomatic participants aged 18–50 years, limiting generalizability of the population. Second, our asymptomatic volunteers were not subject to clinical evaluation, only self-report. Lastly, our sciatic nerve CSA intra-rater and inter-rater reliability was moderate and poor, respectively. This limits reproducibility of the CSA measurements, especially amongst those with less US experience.
Conclusion
US, SWE, and PPT are different qualitative and quantitative imaging modalities used in the assessment of nerves and muscles. The sciatic nerve has a gradually decreasing CSA and increasing SWE from the ischial tuberosity to the bifurcation and is easily accessible by US. SWE measurements of the sciatic nerve may differ between genders and classes of BMI. Also, those with a higher BMI had higher PPT on the sciatic nerve. PPT also differs at different sites along the sciatic nerve. Males generally had higher PPT compared to females within the sciatic nerve and hamstring muscles. Elastographic measurements vary across all three of the hamstring muscles, as well as intramuscular. This US, SWE, and PPT data of the sciatic nerve and the hamstring muscles can be used as a foundation for future research a pathophysiologic relationship between sciatic nerve and hamstring muscle injury.
Compliance of ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. This article does not contain any studies with animals performed by any of the authors.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Agnollitto PM, Chu MWK, Simao MN, Nogueira-Barbosa MH. Sciatic neuropathy: findings on magnetic resonance neurography. Radiol Bras. 2017;50:190–196. doi: 10.1590/0100-3984.2015.0205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andrade RJ, Nordez A, Hug F, Ates F, Coppieters MW, Pezarat-Correia P, et al. Non-invasive assessment of sciatic nerve stiffness during human ankle motion using ultrasound shear wave elastography. J Biomech. 2016;49:326–331. doi: 10.1016/j.jbiomech.2015.12.017. [DOI] [PubMed] [Google Scholar]
- 3.Palmer TB, Akehi K, Thiele RM, Smith DB, Thompson BJ. Reliability of panoramic ultrasound imaging in simultaneously examining muscle size and quality of the hamstring muscles in young, healthy males and females. Ultrasound Med Biol. 2015;41:675–684. doi: 10.1016/j.ultrasmedbio.2014.10.011. [DOI] [PubMed] [Google Scholar]
- 4.Bucknor MD, Steinbach LS, Saloner D, Chin CT. Magnetic resonance neurography evaluation of chronic extraspinal sciatica after remote proximal hamstring injury: a preliminary retrospective analysis. J Neurosurg. 2014;121:408–414. doi: 10.3171/2014.4.JNS13940. [DOI] [PubMed] [Google Scholar]
- 5.Kouzaki K, Nakazato K, Mizuno M, Yonechi T, Higo Y, Kubo Y, et al. Sciatic nerve conductivity is impaired by hamstring strain injuries. Int J Sports Med. 2017;38:803–808. doi: 10.1055/s-0043-115735. [DOI] [PubMed] [Google Scholar]
- 6.Chen J, Liu J, Zeng J, Wu S, Ren J. Ultrasonographic reference values for assessing normal sciatic nerve ultrasonography in the normal population. J Med Ultrasound. 2018;26:85–89. doi: 10.4103/JMU.JMU_6_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Taljanovic MS, Gimber LH, Becker GW, Latt LD, Klauser AS, Melville DM, et al. Shear-wave elastography: basic physics and musculoskeletal applications. Radiographics. 2017;37:855–870. doi: 10.1148/rg.2017160116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cingoz M, Kandemirli SG, Alis DC, Samanci C, Kandemirli GC, Adatepe NU. Evaluation of median nerve by shear wave elastography and diffusion tensor imaging in carpal tunnel syndrome. Eur J Radiol. 2018;101:59–64. doi: 10.1016/j.ejrad.2018.02.005. [DOI] [PubMed] [Google Scholar]
- 9.Arslan H, Yavuz A, Ilgen F, Aycan A, Ozgokce M, Akdeniz H, et al. The efficiency of acoustic radiation force impulse (ARFI) elastography in the diagnosis and staging of carpal tunnel syndrome. J Med Ultrason. 2001;2018(45):453–459. doi: 10.1007/s10396-017-0857-7. [DOI] [PubMed] [Google Scholar]
- 10.Dikici AS, Ustabasioglu FE, Delil S, Nalbantoglu M, Korkmaz B, Bakan S, et al. Evaluation of the tibial nerve with shear-wave elastography: a potential sonographic method for the diagnosis of diabetic peripheral neuropathy. Radiology. 2017;282:494–501. doi: 10.1148/radiol.2016160135. [DOI] [PubMed] [Google Scholar]
- 11.Neto T, Freitas SR, Andrade RJ, Vaz JR, Mendes B, Firmino T, et al. Noninvasive measurement of sciatic nerve stiffness in patients with chronic low back related leg pain using shear wave elastography. J Ultrasound Med. 2018 doi: 10.1002/jum.14679. [DOI] [PubMed] [Google Scholar]
- 12.Greening J, Dilley A. Posture-induced changes in peripheral nerve stiffness measured by ultrasound shear-wave elastography. Muscle Nerve. 2017;55:213–222. doi: 10.1002/mus.25245. [DOI] [PubMed] [Google Scholar]
- 13.Coppieters MW, Alshami AM, Babri AS, Souvlis T, Kippers V, Hodges PW. Strain and excursion of the sciatic, tibial, and plantar nerves during a modified straight leg raising test. J Orthop Res. 2006;24:1883–1889. doi: 10.1002/jor.20210. [DOI] [PubMed] [Google Scholar]
- 14.Le Sant G, Ates F, Brasseur JL, Nordez A. Elastography study of hamstring behaviors during passive stretching. PLoS ONE. 2015;10:e0139272. doi: 10.1371/journal.pone.0139272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Neto T, Freitas SR, Andrade RJ, Gomes J, Vaz J, Mendes B, et al. Sciatic nerve stiffness is not changed immediately after a slump neurodynamics technique. Muscles Ligaments Tendons J. 2017;7:583–589. doi: 10.11138/mltj/2017.7.3.583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ellis R, Rohan M, Fox J, Hitt J, Langevin H, Henry S. Ultrasound elastographic measurement of sciatic nerve displacement and shear strain during active and passive knee extension. J Ultrasound Med. 2018;37:2091–2103. doi: 10.1002/jum.14560. [DOI] [PubMed] [Google Scholar]
- 17.Crema MD, Jarraya M, Engebretsen L, Roemer FW, Hayashi D, Domingues R, et al. Imaging-detected acute muscle injuries in athletes participating in the Rio de Janeiro 2016 Summer Olympic Games. Br J Sports Med. 2018;52:460–464. doi: 10.1136/bjsports-2017-098247. [DOI] [PubMed] [Google Scholar]
- 18.Wangensteen A, Tol JL, Witvrouw E, Van Linschoten R, Almusa E, Hamilton B, et al. Hamstring reinjuries occur at the same location and early after return to sport: a descriptive study of MRI-confirmed reinjuries. Am J Sports Med. 2016;44:2112–2121. doi: 10.1177/0363546516646086. [DOI] [PubMed] [Google Scholar]
- 19.Aggen PD, Reuteman P. Conservative rehabilitation of sciatic nerve injury following hamstring tear. N Am J Sports Phys Ther. 2010;5:143–154. [PMC free article] [PubMed] [Google Scholar]
- 20.Shim HY, Lim OK, Bae KH, Park SM, Lee JK, Park KD. Sciatic nerve injury caused by a stretching exercise in a trained dancer. Ann Rehabil Med. 2013;37:886–890. doi: 10.5535/arm.2013.37.6.886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Martin HD, Khoury A, Schroder R, Palmer IJ. Ischiofemoral Impingement and hamstring syndrome as causes of posterior hip pain: where do we go next? Clin Sports Med. 2016;35:469–486. doi: 10.1016/j.csm.2016.02.010. [DOI] [PubMed] [Google Scholar]
- 22.Lohrer H, Nauck T, Konerding MA. Nerve entrapment after hamstring injury. Clin J Sport Med. 2012;22:443–445. doi: 10.1097/JSM.0b013e318257d76c. [DOI] [PubMed] [Google Scholar]
- 23.Haus BM, Arora D, Upton J, Micheli LJ. Nerve wrapping of the sciatic nerve with acellular dermal matrix in chronic complete proximal hamstring ruptures and ischial apophyseal avulsion fractures. Orthop J Sports Med. 2016;4:2325967116638484. doi: 10.1177/2325967116638484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mattiussi G, Moreno C. Treatment of proximal hamstring tendinopathy-related sciatic nerve entrapment: presentation of an ultrasound-guided "Intratissue Percutaneous Electrolysis" application. Muscles Ligaments Tendons J. 2016;6:248–252. doi: 10.11138/mltj/2016.6.2.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Iyer VG. Iatrogenic injury to the sciatic nerve during surgical repair of proximal hamstring avulsion. Muscle Nerve. 2015;52:465–466. doi: 10.1002/mus.24678. [DOI] [PubMed] [Google Scholar]
- 26.Blackburn JT, Pamukoff DN. Geometric and architectural contributions to hamstring musculotendinous stiffness. Clin Biomech (Bristol, Avon) 2014;29:105–110. doi: 10.1016/j.clinbiomech.2013.10.011. [DOI] [PubMed] [Google Scholar]
- 27.Nakamura M, Hasegawa S, Umegaki H, Nishishita S, Kobayashi T, Fujita K, et al. The difference in passive tension applied to the muscles composing the hamstrings—comparison among muscles using ultrasound shear wave elastography. Man Ther. 2016;24:1–6. doi: 10.1016/j.math.2016.03.012. [DOI] [PubMed] [Google Scholar]
- 28.Lacourpaille L, Nordez A, Hug F, Doguet V, Andrade R, Guilhem G. Early detection of exercise-induced muscle damage using elastography. Eur J Appl Physiol. 2017;117:2047–2056. doi: 10.1007/s00421-017-3695-9. [DOI] [PubMed] [Google Scholar]
- 29.Sun Y, Xiao Y, Li F, Wang C, Wu T, Zhou M, et al. Diagnosing muscle atrophy by use of a comprehensive method of assessing the elastic properties of muscle during passive stretching. AJR Am J Roentgenol. 2020;214:862–870. doi: 10.2214/AJR.19.21174. [DOI] [PubMed] [Google Scholar]
- 30.Lima K, Costa Junior JFS, Pereira WCA, Oliveira LF. Assessment of the mechanical properties of the muscle-tendon unit by supersonic shear wave imaging elastography: a review. Ultrasonography. 2018;37:3–15. doi: 10.14366/usg.17017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sadeghi S, Newman C, Cortes DH. Change in skeletal muscle stiffness after running competition is dependent on both running distance and recovery time: a pilot study. PeerJ. 2018;6:e4469. doi: 10.7717/peerj.4469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Parhizgar SEH. A review on experimental assessments of pain threshold in healthy human subjects. BCN. 2010;1:62–67. [Google Scholar]
- 33.Tashani OA, Astita R, Sharp D, Johnson MI. Body mass index and distribution of body fat can influence sensory detection and pain sensitivity. Eur J Pain. 2017;21:1186–1196. doi: 10.1002/ejp.1019. [DOI] [PubMed] [Google Scholar]
- 34.Cheatham SW, Baker R. Differences in pressure pain threshold among men and women after foam rolling. J Bodyw Mov Ther. 2017;21:978–982. doi: 10.1016/j.jbmt.2017.06.006. [DOI] [PubMed] [Google Scholar]
- 35.Torensma B, Oudejans L, van Velzen M, Swank D, Niesters M, Dahan A. Pain sensitivity and pain scoring in patients with morbid obesity. Surg Obes Relat Dis. 2017;13:788–795. doi: 10.1016/j.soard.2017.01.015. [DOI] [PubMed] [Google Scholar]
- 36.Kim DH, Lee JJ, Sung Hyun You J. Effects of instrument-assisted soft tissue mobilization technique on strength, knee joint passive stiffness, and pain threshold in hamstring shortness. J Back Musculoskelet Rehabil. 2018;31:1169–1176. doi: 10.3233/BMR-170854. [DOI] [PubMed] [Google Scholar]
- 37.Sterling M, Treleaven J, Edwards S, Jull G. Pressure pain thresholds of upper limb peripheral nerve trunks in asymptomatic subjects. Physiother Res Int. 2000;5:220–229. doi: 10.1002/pri.202. [DOI] [PubMed] [Google Scholar]
- 38.Walsh J, Hall T. Reliability, validity and diagnostic accuracy of palpation of the sciatic, tibial and common peroneal nerves in the examination of low back related leg pain. Man Ther. 2009;14:623–629. doi: 10.1016/j.math.2008.12.007. [DOI] [PubMed] [Google Scholar]
- 39.Fingleton CP, Dempsey L, Smart K, Doody CM. Intraexaminer and interexaminer reliability of manual palpation and pressure algometry of the lower limb nerves in asymptomatic subjects. J Manipulative Physiol Ther. 2014;37:97–104. doi: 10.1016/j.jmpt.2013.12.006. [DOI] [PubMed] [Google Scholar]
- 40.Cartwright MS, Mayans DR, Gillson NA, Griffin LP, Walker FO. Nerve cross-sectional area in extremes of age. Muscle Nerve. 2013;47:890–893. doi: 10.1002/mus.23718. [DOI] [PubMed] [Google Scholar]
- 41.Bedewi MA, Yousef AM, Abd-Elghany AA, El-Sharkawy MS, Awad EM. Estimation of ultrasound reference values for the ulnar nerve fascicular number and cross-sectional area in young males: a cross-sectional study. Medicine (Baltimore) 2017;96:e6204. doi: 10.1097/MD.0000000000006204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Werner RA, Jacobson JA, Jamadar DA. Influence of body mass index on median nerve function, carpal canal pressure, and cross-sectional area of the median nerve. Muscle Nerve. 2004;30:481–485. doi: 10.1002/mus.20125. [DOI] [PubMed] [Google Scholar]
- 43.Topp KS, Boyd BS. Peripheral nerve: from the microscopic functional unit of the axon to the biomechanically loaded macroscopic structure. J Hand Ther. 2012;25:142–51, quiz 52. doi: 10.1016/j.jht.2011.09.002. [DOI] [PubMed] [Google Scholar]
- 44.Hirata K, Yamadera R, Akagi R. Associations between range of motion and tissue stiffness in young and older people. Med Sci Sports Exerc. 2020 doi: 10.1249/MSS.0000000000002360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Neto T, Freitas SR, Andrade RJ, Vaz JR, Mendes B, Firmino T, et al. Shear wave elastographic investigation of the immediate effects of slump neurodynamics in people with sciatica. J Ultrasound Med. 2020;39:675–681. doi: 10.1002/jum.15144. [DOI] [PubMed] [Google Scholar]
- 46.Bortolotto C, Turpini E, Felisaz P, Fresilli D, Fiorina I, Raciti MV, et al. Median nerve evaluation by shear wave elastosonography: impact of "bone-proximity" hardening artifacts and inter-observer agreement. J Ultrasound. 2017;20:293–299. doi: 10.1007/s40477-017-0267-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cornelson SM, Sclocco R, Kettner NW. Ulnar nerve instability in the cubital tunnel of asymptomatic volunteers. J Ultrasound. 2019;22:337–344. doi: 10.1007/s40477-019-00370-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Woodley SJ, Mercer SR. Hamstring muscles: architecture and innervation. Cells Tissues Organs. 2005;179:125–141. doi: 10.1159/000085004. [DOI] [PubMed] [Google Scholar]
- 49.Takizawa M, Suzuki D, Ito H, Fujimiya M, Uchiyama E. The adductor part of the adductor magnus is innervated by both obturator and sciatic nerves. Clin Anat. 2014;27:778–782. doi: 10.1002/ca.22274. [DOI] [PubMed] [Google Scholar]
- 50.Perez-Bellmunt A, Miguel-Perez M, Brugue MB, Cabus JB, Casals M, Martinoli C, et al. An anatomical and histological study of the structures surrounding the proximal attachment of the hamstring muscles. Man Ther. 2015;20:445–450. doi: 10.1016/j.math.2014.11.005. [DOI] [PubMed] [Google Scholar]
- 51.Pollock N, Patel A, Chakraverty J, Suokas A, James SL, Chakraverty R. Time to return to full training is delayed and recurrence rate is higher in intratendinous ('c') acute hamstring injury in elite track and field athletes: clinical application of the British Athletics Muscle Injury Classification. Br J Sports Med. 2016;50:305–310. doi: 10.1136/bjsports-2015-094657. [DOI] [PubMed] [Google Scholar]
- 52.Shinohara M, Sabra K, Gennisson JL, Fink M, Tanter M. Real-time visualization of muscle stiffness distribution with ultrasound shear wave imaging during muscle contraction. Muscle Nerve. 2010;42:438–441. doi: 10.1002/mus.21723. [DOI] [PubMed] [Google Scholar]
- 53.Petersen J, Thorborg K, Nielsen MB, Skjodt T, Bolvig L, Bang N, et al. The diagnostic and prognostic value of ultrasonography in soccer players with acute hamstring injuries. Am J Sports Med. 2014;42:399–404. doi: 10.1177/0363546513512779. [DOI] [PubMed] [Google Scholar]
- 54.Umegaki H, Ikezoe T, Nakamura M, Nishishita S, Kobayashi T, Fujita K, et al. Acute effects of static stretching on the hamstrings using shear elastic modulus determined by ultrasound shear wave elastography: differences in flexibility between hamstring muscle components. Man Ther. 2015;20:610–613. doi: 10.1016/j.math.2015.02.006. [DOI] [PubMed] [Google Scholar]
- 55.Graven-Nielsen T, Vaegter HB, Finocchietti S, Handberg G, Arendt-Nielsen L. Assessment of musculoskeletal pain sensitivity and temporal summation by cuff pressure algometry: a reliability study. Pain. 2015;156:2193–2202. doi: 10.1097/j.pain.0000000000000294. [DOI] [PubMed] [Google Scholar]
- 56.Jay K, Sundstrup E, Sondergaard SD, Behm D, Brandt M, Saervoll CA, et al. Specific and cross over effects of massage for muscle soreness: randomized controlled trial. Int J Sports Phys Ther. 2014;9:82–91. [PMC free article] [PubMed] [Google Scholar]