Abstract
Introduction
Despite recent advances in treatment for psoriatic arthritis (PsA), many patients experience inadequate response or intolerance to therapy, indicating that unmet treatment-related needs remain. To further characterize these unmet needs, we evaluated patients’ experiences regarding the burden of PsA symptoms and disease impacts, and patients’ preferences for treatment.
Methods
Patients from ArthritisPower, a rheumatology research registry, completed a web-based survey. Object case best–worst scaling (BWS) was used to evaluate the relative burden of 11 PsA-related symptoms and the relative importance of improvement in nine PsA-related disease impacts. BWS data were analyzed using a random-parameters logit model. Patient demographics, preferences for mode and frequency of therapy, and preferences for methotrexate were analyzed descriptively.
Results
Among the 332 participants, most were White (94%), female (80%), with mean age of 54 years (SD 11.4). In the BWS, joint pain was the most bothersome symptom, followed by other musculoskeletal pain and fatigue. The BWS for disease impacts found that improvements in the ability to perform physical activities were most important, followed by improvements in the ability to function independently, sleep quality, and the ability to perform daily activities. The most burdensome symptoms and desired disease impact improvements were similar in patients regardless of their experience with biologic disease-modifying antirheumatic drugs. The most preferred mode and frequency of treatment administration was oral, once-daily medication (preferred by 38% of respondents), and 74% prioritized therapies that significantly improved joint-related symptoms versus psoriasis-related symptoms. The majority of respondents (65%) preferred PsA treatment regimens that did not include methotrexate.
Conclusions
Patients with PsA from a rheumatology registry found musculoskeletal pain symptoms to be the most bothersome and prioritized improvements to functional impacts of their disease. These findings can better inform development of new therapies and guide shared patient-provider treatment decision-making.
Supplementary Information
The online version contains supplementary material available at 10.1007/s40744-022-00436-x.
Keywords: Best–worst scaling, Disease impacts, Joint pain, Patient preferences, Psoriatic arthritis
Key Summary Points
| Why carry out this study? |
| Despite recent advances in the treatment of psoriatic arthritis (PsA), many patients experience inadequate response or intolerance to therapy, indicating that unmet treatment-related needs remain. In this study, we sought to better understand the relative burden of common symptoms and disease impacts and measure patient treatment preferences. |
| What was learned from the study? |
| Study respondents from a rheumatology registry reported that musculoskeletal pain symptoms (joint, back/spine, and tendon or ligament pain) were the most bothersome and that the most important impact of PsA to improve was the ability to perform physical activities. |
| Participants strongly desired a treatment that improves musculoskeletal symptoms over psoriasis-related symptoms, with a preference toward oral therapy once daily and regimens that do not include methotrexate. |
| This study can inform drug development and encourage shared decision-making by elucidating patient priorities. |
Introduction
Psoriatic arthritis (PsA) is a chronic inflammatory disease that arises in 20–30% of patients with psoriasis, affecting men and women equally [1, 2]. PsA can result in a variety of symptoms including peripheral joint pain, swelling and stiffness, enthesitis (swollen tendons and ligaments), dactylitis (sausage fingers), spinal pain and stiffness, skin pain and itching, nail dystrophy/pitting, and fatigue [3].
PsA is typically treated with disease-modifying antirheumatic drugs (DMARDs), with the aim of reducing signs and symptoms, slowing disease progression, and improving quality of life (QoL). Conventional synthetic DMARDs (csDMARDs), which include medications such as methotrexate, were historically the cornerstone of therapy for both PsA and rheumatoid arthritis and are a major component of many combination therapies. More recently, several biologic DMARDs (bDMARDs) and targeted synthetic DMARDs (tsDMARDs) have been developed to treat PsA by targeting molecules that mediate inflammation. Patients with PsA are frequently treated with bDMARDs in combination with methotrexate to improve or prolong treatment effectiveness.
While the treatment landscape has continued to evolve, unmet needs remain for patients with PsA. Under half of patients in clinical trials for new PsA therapies report reaching minimal disease activity [4]. The PsA patient population is particularly heterogeneous, which poses challenges for effectively treating patients with widely varying disease presentations [4]. Patients with PsA may struggle with mental and emotional impacts of their disease as well, further decreasing their QoL [5, 6]. To adequately address the unique challenges of PsA, incorporating the patient perspective when developing new therapeutics is critical. Incorporating patient perspectives will not only help better tailor therapies in this heterogeneous population but also identify treatment targets that are relevant to the individual patients. Patient preferences are currently recommended for inclusion in clinical trials across major international PsA organizations [7–9].
In this study, we sought to better understand the preferences of patients with PsA by evaluating the relative burden of common PsA symptoms and the importance of improving common impacts of the disease, including those related to physical, social, and emotional functioning. We also evaluated treatment preferences, including preferences for mode and frequency of administration. Previous research has indicated that mode of administration is an important factor in patient treatment preferences with respect to the ease and convenience of treatment and may improve patient outcomes [10–12]. We also sought to better understand patient preferences with regards to methotrexate, because it is so widely used in PsA treatment regimens [13–15]. We also explored heterogeneity in preferences among patients with versus without bDMARD experience and among those patients who experienced all 11 of the assessed PsA symptoms versus those who did not.
Methods
Survey Design
This was a cross-sectional, web-based survey of adults (19 + years of age) with a self-reported physician diagnosis of PsA. Respondents were recruited through the Global Health Living Foundation’s (GHLF’s) ArthritisPower, a US-based rheumatology research registry. Patients who choose to participate in the ArthritisPower registry are encouraged to self-report a variety of details related to their diagnosis such as their current medications and the name of their rheumatologist. This study was performed in accordance with the Helsinki Declaration of 1964 and its later amendments. Participants in the survey pretest provided verbal consent, and respondents to the final survey provided electronic consent in the survey platform for their responses to be used for research purposes. The RTI International Institutional Review Board reviewed the study protocol and determined that the research met the criteria for exemption from IRB review.
The sets of symptoms and impacts measured in this study (Table 1) were developed based on the Outcome Measures in Rheumatology (OMERACT) core outcome set and input from clinical experts, a patient living with PsA, and patient advocates [16–18]. Object case best–worst scaling (BWS) was used to rank the relative burden of a set of 11 PsA symptoms and the relative importance of improvements in nine PsA-related impacts of disease. Object case BWS [19] has been previously applied in healthcare settings to understand the relative importance or burden of symptoms, outcomes, and treatment features [20–24]. Respondents were presented with a series of questions from two separate BWS exercises, each presenting a subset of the list of items, and asked to identify the most preferred (best) item and the least preferred (worst) item (Figure S-1). Each BWS exercise (symptom and impact) presented the respondent with a subset of items from the full list of PsA symptoms and disease-related impacts. The final set of BWS questions was created using an experimental design with a balanced, incomplete block design [17, 18, 25]. In both exercises, respondents were asked to make a choice regardless of whether they had ever experienced the symptom or impact.
Table 1.
Patient-friendly list of items and descriptions of symptoms and impacts for best–worst scaling exercise
| Item | Description |
|---|---|
| Symptoms | |
| Joint pain | Pain, tenderness, or discomfort in one or more joints |
| Joint swelling | Inflammation or swelling in one or more joints |
| Lower back or spine pain | Discomfort or pain in the lower back or spine |
| Sausage fingers or toes | Swelling in one or more fingers or toes including areas between and around joints |
| Morning stiffness | Stiffness after resting that makes it difficult to move your joints |
| Tender or painful tendons or ligaments | Inflammation at the places where tendons and ligaments connect to bone, such as the Achilles’ tendons, which stretch from your heel to your calf muscles and the soles of your feet |
| Fatigue or tiredness | Tiredness and lack of energy that doesn’t go away with sleep |
| Psoriasis patches on skin and scalp | Red, raised patches of skin with whitish-silver scales or plaques on the red patches that may flake |
| Nail pitting | Discoloration or pitting of the fingernails or toenails |
| Itching because of psoriasis patches | Physically irritated skin resulting in the urge to scratch |
| Skin pain and discomfort related to psoriasis patches | Painful, inflamed, or broken skin, burning, stinging, and skin tenderness |
| Impacts | |
| Sleep quality | Being able to have a restful sleep |
| Ability to perform physical activities (exercising, walking, climbing stairs) | Being able to perform physical activities such as exercising, walking, and climbing stairs |
| Emotional well-being | Feeling good about yourself |
| Ability to do daily activities (housework, chores, etc.) | Being able to do everyday tasks |
| Ability to live/function independently | Being able to maintain your independence, not being dependent on others for help |
| Ability to do work or school activities | Being able to perform activities related to your work/employment or school |
| Ability to participate in social activities | Being able to participate in social activities |
| Ability to participate in leisure activities | Being able to engage in leisure activities, such as reading, cooking, or other hobbies |
| Unpredictability of disease flare-up | Not knowing in the short term if you will have symptoms or be able to engage in activities |
In addition to the BWS questions, the survey instrument contained questions regarding the respondent’s current and past experience with the PsA symptoms and disease impacts assessed, treatment history, preferences for mode and frequency of administration (oral tablet once daily, oral tablet twice daily, biweekly injections, or monthly injections), and treatment regimens including methotrexate (including reasons for those reporting a preference), reasons behind their treatment preferences, and demographic questions.
The survey instrument was pretested using cognitive debriefing interviews on participants with PsA (n = 15). Based on interview feedback, the survey was adjusted to add symptom descriptions; expand the “physical functioning” label in the BWS questions to read, “ability to perform physical activities (exercising, walking, climbing stairs)”; and expand psoriasis symptom and diagnosis questions.
Statistical Analysis
Data were managed, described, and analyzed using STATA software, version 16.0 (StataCorp, LLC). Responses to the BWS scaling questions in each BWS exercise were analyzed by using two separate main effects models using a random-parameters logit (RPL) regression to estimate sample-level importance of each outcome and the intensity of feeling for each outcome (how much more bothersome/important patients find one outcome compared with another). An RPL model avoids potential estimation bias from unobserved preference heterogeneity among respondents by estimating a distribution of importance weights across the respondents in the sample and accounting for within-sample correlation when respondents answer multiple questions [26, 27]. To rescale the relative importance weights, the probability score of each item was divided by the probability score of one fixed reference item and multiplied by 10. Thus, the reference item has a scaled relative importance of 10, and the result for any other given item has a scaled relative importance with respect to the reference item. Differences in preferences across subgroups in the sample were investigated for two subgroups identified in post hoc exploratory analyses (patients with and without bDMARD experience and patients with experience with all joint- and skin-related symptoms assessed). A Chi-square test of the joint significance of the interaction terms indicated whether preferences between the groups were statistically significantly systematically different.
Patient demographics and treatment attribute preferences (methotrexate experience, joint or psoriasis symptom improvement, and treatment mode and frequency) were analyzed descriptively.
Results
A total of 332 patients completed the survey. Of these respondents, 94% were White, 80% were female, and 90% had attended at least some college (Table 2). Almost half of respondents were retired or unable to work (45%). Respondents were 22–79 years of age, with an average age of 54 years. Most participants had current or prior bDMARD experience (n = 258). The majority of respondents were currently taking a prescription medication to manage their PsA (95%); 58% of the total cohort were taking injectable biologics, 56% of respondents were taking prescription nonsteroidal anti-inflammatory drugs (NSAIDs), and 22.6% were taking csDMARDs. The majority of respondents in this study reported being under the care of a rheumatologist; 60.2% of respondents were under the care of a rheumatologist alone, and another 25.9% were under the care of both a rheumatologist and a dermatologist. Full respondent characteristics can be found in Table 2.
Table 2.
Respondent demographics, disease characteristics, and treatment experience
| Characteristic | Respondents (n = 332) |
|---|---|
| Age | |
| Mean (SD, range) | 53.9 (11.4, 22–79) |
| Gender | |
| Female | 266 (80.1%) |
| Race/ethnicity | |
| White | 312 (94.0%) |
| Employment status | |
| Employed (full-time, part-time, self-employed) | 162 (48.8%) |
| Retired | 52 (15.7%) |
| Disabled/unable to work | 96 (28.9%) |
| Other | 18 (5.4%) |
| Missing | 4 (1.2%) |
| Education | |
| Less than high school degree | 6 (1.8%) |
| High school or equivalent (e.g., GED) | 23 (6.9%) |
| More than high school but less than 4-year college degree | 139 (41.9%) |
| 4-year college degree or higher | 160 (48.2%) |
| Missing | 4 (1.2%) |
| Physician who diagnosed PsAa | |
| Primary care provider | 76 (22.9%) |
| Dermatologist | 74 (22.3%) |
| Rheumatologist | 282 (84.9%) |
| Other | 12 (3.6%) |
| Don’t know or not sure | 3 (0.9%) |
| Specialist currently treating PsA | |
| Dermatologist only | 16 (4.8%) |
| Rheumatologist only | 200 (60.2%) |
| Both a dermatologist and a rheumatologist | 86 (25.9%) |
| None of the above | 30 (9.0%) |
| Past treatment experiencea | |
| Steroids | 270 (81.3%) |
| Prescription NSAIDs | 296 (89.2%) |
| Injectable biologics | 250 (75.3%) |
| csDMARDs | 144 (43.3%) |
| Current treatmenta | |
| Steroids | 82 (24.8%) |
| Prescription NSAIDs | 185 (55.9%) |
| Injectable biologics | 193 (58.3%) |
| csDMARDs | 75 (22.6%) |
| Patient global assessment of pain (0–10 NRS) | |
| Mean (SD) | 6.1 (2.1) |
| Current joint symptom severity during past week | |
| Mild | 56 (16.9%) |
| Moderate | 155 (46.7%) |
| Severe or very severe | 115 (34.6%) |
| None or missing | 6 (1.8%) |
| Approximate body surface area affected by psoriasisb | |
| ≤ 2% | 101 (52.9%) |
| 3–10% | 61 (31.9%) |
| > 10% | 17 (8.9%) |
| Don’t know or missing | 12 (6.3%) |
csDMARD conventional synthetic disease-modifying antirheumatic drug, NRS numeric rating scale, NSAID nonsteroidal anti-inflammatory drug, PsA psoriatic arthritis, SD standard deviation
aRespondents could select more than response option; therefore, totals may not add up to 100%
bAmong respondents experiencing psoriasis in the past week (n = 191). Respondents were asked to estimate the extent of their body surface area affected by psoriasis, where the area of 1 palm is approximately equivalent to 1% body surface area
Joint pain (98%), fatigue (94%), and morning stiffness (94%) were the most common symptoms ever experienced among respondents. Similarly, the most common symptoms patients reported experiencing in the past week were joint pain (89%), morning stiffness (85%), and fatigue (84%). Approximately 37% of respondents had ever experienced all 11 included symptoms over the course of their disease. The most reported disease impacts of PsA ever experienced were related to the ability to perform physical activities (93%) and sleep quality (92%); these were also the two most reported impacts experienced by respondents in the preceding week (76 and 79% of respondents, respectively). The majority of patients (60%) also experienced impacts to their emotional well-being stemming from their PsA over the past week. Approximately 42% of the cohort had experienced all nine functional, social/emotional, and QoL impacts.
Joint pain was the most burdensome symptom relative to all other symptoms included in the BWS exercise, followed by lower back or spine pain, tender or painful tendons and ligaments, and fatigue or tiredness (Fig. 1). For example, based on the analysis of the responses, joint pain was 2.5 times more burdensome than joint swelling (4.0) and almost equally (1.1 times) as burdensome as lower back or spine pain (9.0) (Fig. 1) (P < 0.05). The least burdensome symptoms were skin-related symptoms such as nail pitting (0.02), psoriasis patches on skin and scalp (0.8), and itching because of psoriasis patches (0.8); however, due to the overlapping confidence intervals, respondents did not generally differentiate between these three symptoms.
Fig. 1.
Relative burden of PsA disease symptoms. PsA psoriatic arthritis. The relative burden estimates for the full sample from the symptom best–worst scaling exercise, where the most burdensome symptom, joint pain, is set to 10.0. For example, joint pain is 2.5 times more burdensome than joint swelling (10/4 = 2.5) and is almost as burdensome as lower back or spine pain (10.0/9.0 = 1.1)
Subgroup analysis based on respondents’ experience with bDMARDs or having experienced all joint and skin symptoms was performed to determine whether these patient populations weighed symptom burdens differently. In general, preferences were relatively similar, with both bDMARD-experienced and bDMARD-naive respondents ranking musculoskeletal pain-related symptoms as most bothersome, while the least bothersome symptoms were psoriasis related (Figure S-2). The same trend was observed whether patients had experienced all joint- and skin-related symptoms or not (Figure S-3). However, bDMARD-naive respondents found morning stiffness significantly more burdensome (P < 0.05) than bDMARD-experienced respondents (Figure S-2). Respondents who had ever experienced all joint and skin symptoms ranked lower back or spine pain as the most burdensome symptom, while patients who did not experience all joint and skin symptoms ranked joint pain as the most burdensome symptom. However, this difference was not statistically significant (Figure S-3).
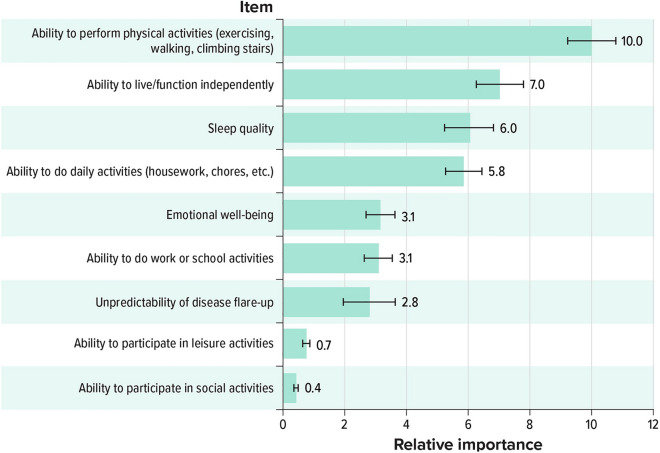
When considering the importance of improving certain disease impacts, respondents ranked improving the ability to perform physical activities (exercising, walking, and climbing stairs) highest (10.0), while the ability to participate in social activities (0.4) was ranked as the least important impact to improve (Fig. 2). Subgroup analysis revealed that an improvement in sleep quality was of greater importance to bDMARD-naive respondents than to bDMARD-experienced respondents (Figure S-2). This difference was statistically significant at the P < 0.05 level of significance.
Fig. 2.
Relative importance of improving PsA disease impacts. PsA psoriatic arthritis. The scaled relative importance of improving PsA disease impacts, where the most important impact to improve is set to 10.0. For example, the ability to perform physical activities is approximately three times more important to improve than emotional well-being (10.0/3.1 = 3.2)
Over half of respondents (57%) reported being somewhat or very satisfied with their current PsA treatment. When asked to choose between a series of hypothetical treatment options, ranging from no improvement in joint symptoms and complete improvement in skin symptoms to complete improvement in joint symptoms and no improvement in skin symptoms, most respondents (74%) preferred a medicine that would provide significant or complete improvements in joint symptoms with mild to no improvements in skin symptoms (Fig. 3). Only 5% of respondents prioritized skin symptom improvement over joint symptom improvement.
Fig. 3.
Respondents’ preferences for mode and frequency of treatment administration
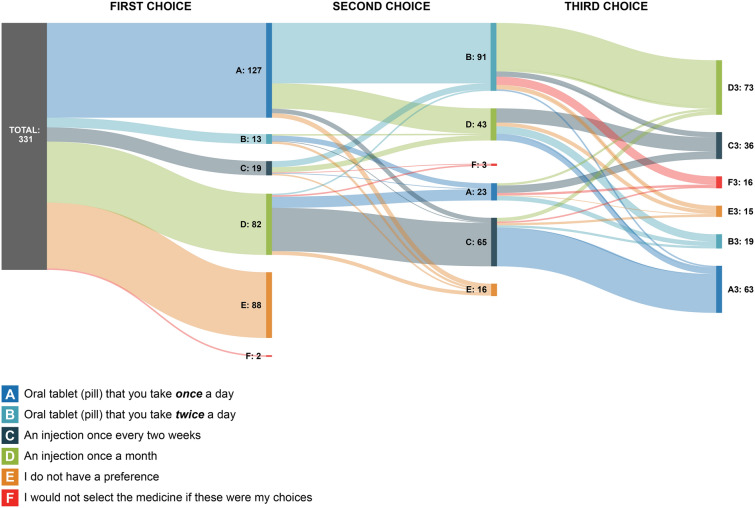
When considering the mode and frequency of PsA treatment administration, nearly half of respondents (46%) felt that mode was an important factor when making treatment decisions. When asked to select their first choice for mode of administration, 38% of respondents chose once-daily oral medications, followed by injection once a month (25%) (Fig. 4). Approximately one in four respondents (27%) reported no preference among the four options. The flow of the subsequent mode and frequency of administration choices is shown in Fig. 4. For instance, among patients who chose an oral tablet (pill) once a day (n = 127) as their preferred mode and frequency of administration, approximately 72% (n = 91) stayed with an oral mode of administration (i.e., selected oral tablet [pill] twice a day) as their second choice. For those who preferred oral dosing, the top reasons cited included convenience (fast and easy to take [68%], easier to travel with [49%], and easier to remember [34%]) and dislike of needles/injections (27%).
Fig. 4.
Respondents’ preferences for a treatment improving joint versus skin symptoms
Ninety-two respondents (28%) had experience taking methotrexate. Nearly half of these respondents (47%) stated they would strongly prefer a treatment that does not include methotrexate; 35% of respondents with methotrexate experience reported feeling satisfied with it. Top reasons for dissatisfaction with methotrexate included dislike of short-term side effects (58%), lack of efficacy (53%), and fear of long-term side effects (44%) (Table 3).
Table 3.
Respondents’ experience and satisfaction with methotrexate
| Question | Participants (n = 332) |
|---|---|
| Among those who have ever taken methotrexate Trexall (methotrexate) (n = 92) | |
| On a scale ranging from very satisfied to very dissatisfied, please rate your satisfaction with taking methotrexate | |
| Very satisfied | 5 (5.4%) |
| Somewhat satisfied | 27 (29.3%) |
| Neither satisfied nor dissatisfied | 16 (17.4%) |
| Somewhat dissatisfied | 14 (15.2%) |
| Very dissatisfied | 29 (31.5%) |
| Missing | 1 (1.1%) |
| Among those who are dissatisfied or who are neither satisfied nor dissatisfied with taking methotrexate (n = 59) | |
| From the list below, please select up to three reasons that you are, or were, not satisfied with methotrexatea | |
| I don’t like to have the short-term side effects of methotrexate after my dose each week (fatigue, upset stomach, headache) | 34 (57.6%) |
| Methotrexate does not work well to treat my symptoms | 31 (52.5%) |
| I fear the long-term side effects related to methotrexate | 26 (44.1%) |
| I would like to take less medicine in general | 12 (20.3%) |
| I must limit my alcohol intake | 7 (11.9%) |
| I do not like going to the doctor’s office for regular lab tests | 5 (8.5%) |
| I had abnormal lab test results | 5 (8.5%) |
| I often forget to take it | 2 (3.4%) |
| Cost | 0 (0.0%) |
| It is not safe to use during pregnancy | 0 (0.0%) |
| Other | 8 (13.6%) |
| Among those who are satisfied with taking Trexall (methotrexate) (n = 32) | |
| From the list below, please select the main reason that you are, or were, satisfied with methotrexate | |
| Methotrexate works well to treat my symptoms | 14 (43.8%) |
| I can afford it | 1 (3.1%) |
| It is easy to take | 3 (9.4%) |
| It is easy to get the prescription filled | 0 (0.0%) |
| My doctor recommended that I take it and keep taking it | 12 (37.5%) |
| Other | 2 (6.3%) |
| All participants | |
| How would you rate your interest in a treatment for psoriatic arthritis that either includes methotrexate or does not include methotrexate? | |
| I would strongly prefer a treatment that did not include methotrexate | 155 (46.7%) |
| I would somewhat prefer a treatment that did not include methotrexate | 61 (18.4%) |
| I have no preference | 63 (19.0%) |
| I somewhat prefer a treatment that includes methotrexate | 18 (5.4%) |
| I strongly prefer a treatment that includes methotrexate | 7 (2.1%) |
| Don’t know or not sure | 27 (8.1%) |
| Missing | 1 (0.3%) |
aRespondents could provide multiple responses to these questions. For this reason, the totals may exceed the number of respondents
Discussion
This study found that symptoms related to musculoskeletal pain were the most bothersome symptoms of PsA, compared with psoriasis-related symptoms, in rheumatology-focused patients with overall low skin disease severity. This finding mirrors other patient impact studies in PsA [3, 16, 28, 29], and patients and rheumatologists are generally in agreement that joint pain is the most burdensome symptom of PsA [30]. However, our findings also highlight other interesting trends. While the top three most bothersome symptoms are related to pain (joint pain, lower back or spine pain, and tender or painful tendons and ligaments), the next most burdensome symptom was fatigue. Pain and fatigue are commonly cited as highly burdensome PsA symptoms [5, 31]. Sleep quality was also ranked highly as an impact to improve, behind improvements in physical activity and independent function. Sleep disturbances and fatigue have been found to be significant burdens to patients with PsA [28, 32], which may arise as a result of other PsA symptoms and impacts [33]. Multiple studies have found discrepancies between physicians’ understanding of disease burdens and the actual burdens felt by patients [30, 34]. Some of this disconnect may stem from the use of patient-reported outcome measures that fail to account for all symptoms and impacts [29]. Ensuring that clinicians and researchers fully understand the burden of symptoms on patients is key to better disease management and the development of appropriate treatments.
When probing patient treatment preferences, respondents in this study most strongly desired an improvement in function and joint symptoms, which is reflected in the findings of other studies [5, 16]. Additionally, many respondents preferred a treatment regimen that did not include methotrexate, citing their dissatisfaction with the drug’s side effects and its poor efficacy. Reducing side effects is a high priority to PsA patients [31]. However, around one-third (35%) of respondents who reported having experience with methotrexate reported satisfaction with methotrexate because it worked well for their symptoms. These mixed opinions on methotrexate efficacy are common in patients with PsA and clinicians [13, 14, 35, 36]. These findings indicate that, despite the beneficial affordability and accessibility of methotrexate, there is an unmet need for many patients for whom methotrexate does not work well, and that clinicians need early access to tools to properly tailor treatment plans to respondents if their current therapy is not sufficiently managing their disease.
When expanding our toolbox for treating PsA, clinicians and providers should also consider patient preferences for treatment modality. Studies have shown that shared decision-making, in which patient perspectives are considered in treatment plans, can improve outcomes, especially affective-cognitive outcomes [37]. As of 2016, the US Food and Drug Administration (FDA) has encouraged the use of patient preferences on acceptable risk–benefit profiles and disease impacts in new drug applications to ensure patients find new treatments acceptable. As new therapies to treat PsA become available, and in light of research indicating respondents’ unmet needs, clinicians may need to adopt more personalized approaches to patient care to ensure that respondents are achieving the best possible outcomes [38]. We found that most respondents in this study preferred once-daily oral dosing, although mode of administration was not a high priority to many respondents when considering treatment options. The convenience of daily oral dosing often makes it a highly desired mode of administration among patients, according to respondents in this study and previous literature [12, 39]. This preference was consistent both in patients with bDMARD experience and in patients without bDMARD experience, indicating that familiarity with parenteral administration used in bDMARDs did not change preferences for oral administration.
There are some limitations associated with our study. The BWS methodology provides a measure of the relative importance of various improvements to PsA disease impacts (i.e., how important it was to improve one disease impact over another) as well as the relative burden of PsA symptoms (i.e., how burdensome one symptom was relative to another); however, it does not provide an estimate of each symptom or impact’s absolute level of importance. For instance, improving the ability to function physically was ranked three times as important as improving emotional well-being, but this does not necessarily indicate that improving emotional well-being is not important to patients with PsA, only that it is less important relative to improving physical function. Therefore, it is important for physicians to discuss with patients all the impacts PsA has on their lives and strategies to help patients reduce the impact of their disease. In addition, the survey included questions about select comorbidities, some of which may have similar symptoms to PsA symptoms, and each respondent’s set of comorbidities might impact their ranking of the burden of symptoms.
Because of the nature of this study, which solicited the opinions of patients with PsA through an online survey, the respondent pool may not be fully representative of the population of patients with PsA. The respondents were predominantly White, female, and well educated, whereas the characteristics of the PsA population are more balanced with respect to gender. This may be due to the demographics of the ArthritisPower registry; the majority of participants with PsA in ArthritisPower are female. Additionally, most respondents were under the care of a rheumatologist, with fewer seeking care primarily in a dermatology setting. Patients may choose to primarily seek the care of a rheumatologist when suffering most strongly from musculoskeletal symptoms rather than skin symptoms. This survey was conducted through ArthritisPower, a rheumatology-focused patient organization, which may also have led to recruiting bias toward patients experiencing joint pain symptoms over psoriasis-associated symptoms, resulting in overrepresentation of musculoskeletal symptoms. However, compared with other patient preference literature in the rheumatology-based PsA population, the relative symptom burden appears to be in line with other studies [3, 12, 30, 39].
Conclusions
In conclusion, this study found that patients with PsA, even those on advanced therapies, are most burdened by symptoms related to musculoskeletal pain. Patients with PsA strongly desire a treatment option that could improve their daily function and generally prefer a daily, oral option as well as a treatment regimen that does not include a methotrexate co-prescription. These findings can be used to facilitate future treatment decisions and treatment strategies.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
The authors thank Kip Burgess for his assistance and perspective in the development of the study protocol and survey. The authors thank Kip Burgess for his perspective as a person living with PsA in the development of this study.
Funding
AbbVie, Inc. provided the financial support for the study for the journal’s rapid service fee. RTI Health Solutions, an independent nonprofit research organization, received funding under a research contract with AbbVie, Inc. to conduct this study and provide publication support in the form of manuscript writing, styling, and submission.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Author Contributions
Alexis Ogdie, Kelley Myers, Carol Mansfield, Colton Leach, W. Benjamin Nowell, Kelly Gavigan, Patrick Zueger, and Jessica Walsh contributed to the study conception and design. Material preparation, data collection and analysis were performed by Colton Leach, Kelley Myers, and Carol Mansfield. Patient recruitment was performed by Kelly Gavigan and W. Benjamin Nowell. All authors commented on previous versions of the manuscript and read and approved the final manuscript.
Medical Writing, Editorial, and Other Assistance
Medical writing and editing were provided by Sara Musetti and John Forbes, respectively, of RTI Health Solutions and were funded by AbbVie Inc, North Chicago, IL. Project management was provided by Kimberly Moon of RTI Health Solutions and was funded by AbbVie Inc, North Chicago, IL.
Prior Presentation
This study was previously presented at the 2021 EULAR Conference and the 2021 American College of Rheumatology Conference. The 2021 EULAR conference was held June 2-5, 2021 and the 2021 American College of Rheumatology Conference was held November 3-10, 2021.
Disclosures
Kelley Myers, Carol Mansfield, and Colton Leach are full time employees of RTI Health Solutions, an independent nonprofit research organization, which was retained by AbbVie, Inc to conduct the research which is the subject of this manuscript. Their compensation is unconnected to the studies on which they work. Patrick Zueger and Erin McDearmon-Blondell are employees of AbbVie, Inc and may hold shares and/or stock options in the company. Alexis Ogdie has received consulting fees and/or honoraria from AbbVie, Amgen, Bristol Myers Squibb, Celgene, CorEvitas, Gilead, Janssen, Eli Lilly, Novartis, Pfizer, and UCB; and grants from AbbVie, Novartis, and Pfizer to the trustees of University of Pennsylvania, Amgen to Forward, and royalties to husband from Novartis. Jessica Walsh has received grants and/or consulting fees from AbbVie, Pfizer, Merck, Amgen, Eli Lilly, Novartis, Janssen, and UCB. W. Benjamin Nowell is a principal investigator on grants/contracts from AbbVie, Eli Lilly and Company, and PCORI. W. Benjamin Nowell and Kelly Gavigan are employees of the Global Healthy Living Foundation (GHLF). GHLF receives grants, sponsorships and contracts from pharmaceutical manufacturers and private foundations. A full list of GHLF funders is publicly available here: https://www.ghlf.org/our-partners/. William Tillett has received consulting fees from AbbVie, Amgen, Celgene, Eli Lilly, Janssen, MSD, Novartis, Pfizer, and UCB; grant/research support from AbbVie, Celgene, Eli Lilly, and Janssen; and is on the speakers bureau for AbbVie, Amgen, Celgene, Eli Lilly, Janssen, Novartis, Pfizer, and UCB. Peter Nash has received honoraria and/or research grants from AbbVie, Amgen, Janssen, Novartis, Pfizer, and UCB.
Compliance with Ethics Guidelines
This study was performed in accordance with the Helsinki Declaration of 1964 and its later amendments. Participants in the survey pretest provided verbal consent, and respondents to the final survey provided electronic consent in the survey platform for their responses to be used for research purposes. The RTI International Institutional Review Board reviewed the study protocol and determined that the research met the criteria for exemption from IRB review.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
- 1.Alinaghi F, Calov M, Kristensen LE, Gladman DD, Coates LC, Jullien D, et al. Prevalence of psoriatic arthritis in patients with psoriasis: a systematic review and meta-analysis of observational and clinical studies. J Am Acad Dermatol. 2019;80(1):251–65 e19. doi: 10.1016/j.jaad.2018.06.027. [DOI] [PubMed] [Google Scholar]
- 2.Chandran V, Raychaudhuri SP. Geoepidemiology and environmental factors of psoriasis and psoriatic arthritis. J Autoimmun. 2010;34(3):J314–J321. doi: 10.1016/j.jaut.2009.12.001. [DOI] [PubMed] [Google Scholar]
- 3.Lebwohl MG, Bachelez H, Barker J, Girolomoni G, Kavanaugh A, Langley RG, et al. Patient perspectives in the management of psoriasis: results from the population-based Multinational Assessment of Psoriasis and Psoriatic Arthritis Survey. J Am Acad Dermatol. 2014;70(5):871–881 e1–30. doi: 10.1016/j.jaad.2013.12.018. [DOI] [PubMed] [Google Scholar]
- 4.Ogdie A, Coates L. The changing face of clinical trials in psoriatic arthritis. Curr Rheumatol Rep. 2017;19(4):21. doi: 10.1007/s11926-017-0642-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dures E, Hewlett S, Lord J, Bowen C, McHugh N, PROMPT Study Group et al. Important treatment outcomes for patients with psoriatic arthritis: a multisite qualitative study. Patient. 2017;10(4):455–462. doi: 10.1007/s40271-017-0221-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tillett W, Merola JF, Thaci D, Holdsworth E, Booth N, Lobosco LS, et al. Disease characteristics and the burden of joint and skin involvement amongst people with psoriatic arthritis: a population survey. Rheumatol Ther. 2020;7(3):617–637. doi: 10.1007/s40744-020-00221-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Coates LC, Kavanaugh A, Mease PJ, Soriano ER, Laura Acosta-Felquer M, Armstrong AW, et al. Group for research and assessment of psoriasis and psoriatic arthritis 2015 treatment recommendations for psoriatic arthritis. Arthritis Rheumatol. 2016;68(5):1060–1071. doi: 10.1002/art.39573. [DOI] [PubMed] [Google Scholar]
- 8.Gossec L, Smolen JS, Ramiro S, de Wit M, Cutolo M, Dougados M, et al. European League Against Rheumatism (EULAR) recommendations for the management of psoriatic arthritis with pharmacological therapies: 2015 update. Ann Rheum Dis. 2016;75(3):499–510. doi: 10.1136/annrheumdis-2015-208337. [DOI] [PubMed] [Google Scholar]
- 9.Singh JA, Guyatt G, Ogdie A, Gladman DD, Deal C, Deodhar A, et al. Special article: 2018 American College of Rheumatology/National Psoriasis Foundation Guideline for the Treatment of Psoriatic Arthritis. Arthritis Rheumatol. 2019;71(1):5–32. doi: 10.1002/art.40726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kjeken I, Dagfinrud H, Mowinckel P, Uhlig T, Kvien TK, Finset A. Rheumatology care: involvement in medical decisions, received information, satisfaction with care, and unmet health care needs in patients with rheumatoid arthritis and ankylosing spondylitis. Arthritis Rheum. 2006;55(3):394–401. doi: 10.1002/art.21985. [DOI] [PubMed] [Google Scholar]
- 11.Leung YY, Tam LS, Lee KW, Leung MH, Kun EW, Li EK. Involvement, satisfaction and unmet health care needs in patients with psoriatic arthritis. Rheumatology (Oxford) 2009;48(1):53–56. doi: 10.1093/rheumatology/ken410. [DOI] [PubMed] [Google Scholar]
- 12.Aletaha D, Husni ME, Merola JF, Ranza R, Bertheussen H, Lippe R, et al. Treatment mode preferences in psoriatic arthritis: a qualitative multi-country study. Patient Prefer Adherence. 2020;14:949–961. doi: 10.2147/PPA.S242336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Coates LC, Helliwell PS. Methotrexate efficacy in the tight control in psoriatic arthritis study. J Rheumatol. 2016;43(2):356–361. doi: 10.3899/jrheum.150614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Coates LC, Merola JF, Grieb SM, Mease PJ, Callis DK. Methotrexate in psoriasis and psoriatic arthritis. J Rheumatol Suppl. 2020;96:31–35. doi: 10.3899/jrheum.200124. [DOI] [PubMed] [Google Scholar]
- 15.Kingsley GH, Kowalczyk A, Taylor H, Ibrahim F, Packham JC, McHugh NJ, et al. A randomized placebo-controlled trial of methotrexate in psoriatic arthritis. Rheumatology (Oxford) 2012;51(8):1368–1377. doi: 10.1093/rheumatology/kes001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Orbai AM, de Wit M, Mease P, Shea JA, Gossec L, Leung YY, et al. International patient and physician consensus on a psoriatic arthritis core outcome set for clinical trials. Ann Rheum Dis. 2017;76(4):673–680. doi: 10.1136/annrheumdis-2016-210242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cheung KL, Wijnen BF, Hollin IL, Janssen EM, Bridges JF, Evers SM, et al. Using best–worst scaling to investigate preferences in health care. Pharmacoeconomics. 2016;34(12):1195–1209. doi: 10.1007/s40273-016-0429-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Parvin S, Wang P, Uddin J, Wright LT. Using best–worst scaling method to examine consumers’ value preferences: a multidimensional perspective. Cogent Bus Manag. 2016;3(1):1199110. doi: 10.1080/23311975.2016.1199110. [DOI] [Google Scholar]
- 19.Flynn TN, Louviere JJ, Peters TJ, Coast J. Best–worst scaling: what it can do for health care research and how to do it. J Health Econ. 2007;26(1):171–189. doi: 10.1016/j.jhealeco.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 20.Yuan Z, Levitan B, Burton P, Poulos C, Hauber AB, Berlin J. Relative importance of benefits and risks associated with antithrombotic therapies of acute coronary syndrome: patient and physician perspectives. Value Health. 2013;16(3):A292–A293. doi: 10.1016/j.jval.2013.03.1517. [DOI] [PubMed] [Google Scholar]
- 21.Hauber AB, Mohamed AF, Johnson FR, Cook M, Arrighi HM, Zhang J, et al. Understanding the relative importance of preserving functional abilities in Alzheimer's disease in the United States and Germany. Curr Med Res Opin. 2014;30(9):1733–1741. doi: 10.1007/s11136-013-0620-5. [DOI] [PubMed] [Google Scholar]
- 22.Peay HL, Hollin I, Fischer R, Bridges JF. A community-engaged approach to quantifying caregiver preferences for the benefits and risks of emerging therapies for Duchenne muscular dystrophy. Clin Ther. 2014;36(5):624–637. doi: 10.1016/j.clinthera.2014.04.011. [DOI] [PubMed] [Google Scholar]
- 23.Ross M, Bridges JF, Ng X, Wagner LD, Frosch E, Reeves G, et al. A best–worst scaling experiment to prioritize caregiver concerns about ADHD medication for children. Psychiatr Serv. 2015;66(2):208–211. doi: 10.1176/appi.ps.201300525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ungar WJ, Hadioonzadeh A, Najafzadeh M, Tsao NW, Dell S, Lynd LD. Quantifying preferences for asthma control in parents and adolescents using best–worst scaling. Respir Med. 2014;108(6):842–851. doi: 10.1016/j.rmed.2014.03.014. [DOI] [PubMed] [Google Scholar]
- 25.Khare M, Federer WT. A simple construction procedure for resolvable incomplete block designs for any number of treatments. Biom J. 1981;23(2):121–132. doi: 10.1002/bimj.4710230203. [DOI] [Google Scholar]
- 26.Train K. Discrete choice methods with simulation. 2. Cambridge: Cambridge University Press; 2009. [Google Scholar]
- 27.Train K, Sonnier G. Mixed logit with bounded distributions of correlated partworths. In: Applications of simulation methods in environmental and resource economics. Dordrecht: Springer; 2005.
- 28.Ogdie A, Michaud K, Nowak M, Bruce R, Cantor S, Hintzen C, et al. Patient's experience of psoriatic arthritis: a conceptual model based on qualitative interviews. Arthritis Care Res. 2020;6(3):e001321. doi: 10.1136/rmdopen-2020-001321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sunkureddi P, Doogan S, Heid J, Benosman S, Ogdie A, Martin L, et al. Evaluation of self-reported patient experiences: insights from digital patient communities in psoriatic arthritis. J Rheumatol. 2018;45(5):638–647. doi: 10.3899/jrheum.170500. [DOI] [PubMed] [Google Scholar]
- 30.Husni ME, Fernandez A, Hauber B, Singh R, Posner J, Sutphin J, et al. Comparison of US patient, rheumatologist, and dermatologist perceptions of psoriatic disease symptoms: results from the DISCONNECT study. Arthritis Res Ther. 2018;20(1):102. doi: 10.1186/s13075-018-1601-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tillett W, Dures E, Hewlett S, Helliwell PS, FitzGerald O, Brooke M, et al. A multicenter nominal group study to rank outcomes important to patients, and their representation in existing composite outcome measures for psoriatic arthritis. J Rheumatol. 2017;44(10):1445–1452. doi: 10.3899/jrheum.161459. [DOI] [PubMed] [Google Scholar]
- 32.Sandikci SC, Colak S, Aydogan Baykara R, Oktem A, Cure E, Omma A, et al. Evaluation of restless legs syndrome and sleep disorders in patients with psoriatic arthritis. Z Rheumatol. 2019;78(10):987–995. doi: 10.1007/s00393-018-0562-y. [DOI] [PubMed] [Google Scholar]
- 33.Skougaard M, Jorgensen TS, Rifbjerg-Madsen S, Coates LC, Egeberg A, Amris K, et al. Relationship between fatigue and inflammation, disease duration, and chronic pain in psoriatic arthritis: an observational DANBIO registry study. J Rheumatol. 2020;47(4):548–552. doi: 10.3899/jrheum.181412. [DOI] [PubMed] [Google Scholar]
- 34.Eder L, Thavaneswaran A, Chandran V, Cook R, Gladman DD. Factors explaining the discrepancy between physician and patient global assessment of joint and skin disease activity in psoriatic arthritis patients. Arthritis Care Res (Hoboken) 2015;67(2):264–272. doi: 10.1002/acr.22401. [DOI] [PubMed] [Google Scholar]
- 35.Katchamart W, Trudeau J, Phumethum V, Bombardier C. Efficacy and toxicity of methotrexate (MTX) monotherapy versus MTX combination therapy with non-biological disease-modifying antirheumatic drugs in rheumatoid arthritis: a systematic review and meta-analysis. Ann Rheum Dis. 2009;68(7):1105–1112. doi: 10.1136/ard.2008.099861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Van den Bosch F, Coates L. Clinical management of psoriatic arthritis. Lancet. 2018;391(10136):2285–2294. doi: 10.1016/s0140-6736(18)30949-8. [DOI] [PubMed] [Google Scholar]
- 37.Shay LA, Lafata JE. Where is the evidence? A systematic review of shared decision making and patient outcomes. Med Decis Making. 2015;35(1):114–131. doi: 10.1177/0272989X14551638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ogdie A, Coates LC, Mease P. Measuring outcomes in psoriatic arthritis. Arthritis Care Res (Hoboken) 2020;72(Suppl 10):82–109. doi: 10.1002/acr.24242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xu Y, Sudharshan L, Hsu M-A, Koenig AS, Cappelleri JC, Liu WF, et al. Patient preferences associated with therapies for psoriatic arthritis: a conjoint analysis. Am Health Drug Benefits. 2018;11(8):408–416. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.