Abstract
Introduction
The aim of this work was to assess the impact of prolonged low immunoglobulin (IgG or IgM) serum concentrations on the potential cumulative serious infection (SI) risk in pediatric patients following rituximab treatment for granulomatosis with polyangiitis or microscopic polyangiitis (GPA/MPA) in PePRS.
Methods
Patients aged ≥ 2 to < 18 years received four weekly intravenous rituximab infusions of 375 mg/m2 and concomitant glucocorticoid taper. After 6 months, patients could receive further rituximab and/or other immunosuppressants per investigator discretion. Immunoglobulin levels and SIs were assessed throughout the 4.5-year observation period. Prolonged low IgG or IgM was defined as below the lower limit of normal age-specific reference range for ≥ 4 months.
Results
A total of 25 patients were included, of whom 19 (76%) had GPA and six (24%) had MPA; 18 (72%) had newly diagnosed disease and seven (28%) had relapsing disease. All 25 patients completed the rituximab induction regimen; 24 completed ≥ 18 months of follow-up. At month 18, eighteen patients (72%) had prolonged low IgG; 19 (76%), prolonged low IgM; and 15 (60%), both. Seven patients (28%) had nine SIs; one occurred during or after prolonged low IgG only, two during or after prolonged low IgM only, and six during or after concurrent prolonged low IgG and IgM. No patients died or discontinued the study due to SI. All patients had complete and sustained peripheral B-cell depletion for ≥ 6 months.
Conclusions
The majority of pediatric patients who received rituximab for GPA/MPA with prolonged low immunoglobulin levels did not experience SIs. In patients with SIs, these events were manageable, and the number of SIs did not increase over time or with multiple rituximab treatments. These observations are consistent with the rituximab safety profile in adults with GPA/MPA.
Trial registration
ClinicalTrials.gov identifier, NCT01750697.
Supplementary Information
The online version contains supplementary material available at 10.1007/s40744-022-00433-0.
Keywords: Granulomatosis with polyangiitis, Immunoglobulins, Infections, Microscopic polyangiitis, Pediatrics, Rituximab
Key Summary Points
| Why carry out this study? |
| Granulomatosis with polyangiitis (GPA) and microscopic polyangiitis (MPA) are rare autoimmune diseases that have high morbidity, involve multiple systems, and are prone to relapse. |
| The use of B-cell-depleting therapies, such as the anti-CD20 monoclonal antibody rituximab can lead to hypogammaglobulinemia (HGG); however, the clinical significance of HGG is unclear, as some patients remain relatively unaffected, while others experience severe or recurrent infections. |
| The objective of this study was to assess the occurrence of serious infection (SI) in pediatric patients with GPA or MPA who experienced prolonged low IgG or IgM serum concentrations following treatment with the rituximab in the PePRS clinical study. |
| What was learned from the study? |
| The majority of pediatric patients with GPA or MPA who experienced prolonged IgG or IgM levels after treatment with rituximab in PePRS did not experience SIs. |
| These observations are consistent with the rituximab safety profile in adults with GPA or MPA. |
Introduction
Granulomatosis with polyangiitis (GPA) and microscopic polyangiitis (MPA) are antineutrophil cytoplasmic antibody (ANCA)-associated vasculitides (AAVs) [1]. These rare autoimmune small-vessel vasculitides have high morbidity, involve multiple systems, and are prone to relapse [2, 3]. In pediatric patients, severe renal or respiratory involvement is common and delayed treatment can lead to irreversible organ damage [3, 4]. Due to the rarity of the disease, no treatment guidelines exist for pediatric GPA/MPA; current treatment strategies are adapted from adult regimens and expert consensus [3].
The anti-CD20 chimeric monoclonal antibody rituximab (MabThera/Rituxan) is a B-cell-targeting therapy approved for induction and maintenance of remission in adult patients with GPA/MPA [5, 6]. In 2019 and 2020, rituximab was approved by the US Food and Drug Administration and the European Medicines Agency, respectively, for the treatment of GPA/MPA in pediatric patients aged ≥ 2 years [5, 6]. This approval was based on the Phase 2a, international, open-label PePRS trial, which assessed safety, tolerability, pharmacokinetics, and exploratory efficacy outcomes in pediatric patients with GPA/MPA treated with rituximab [7].
The use of immunosuppressant therapies, including B-cell–depleting therapies, can lead to hypogammaglobulinemia (HGG), a laboratory abnormality (mostly immunoglobulin G [IgG] or immunoglobulin M [IgM] below the lower limit of normal [LLN] for the patient population) with varied causes and manifestations related to deficiencies of humoral immunity [8]. Reductions in immunoglobulin levels have been observed following rituximab treatment in adult and pediatric patients with autoimmune diseases, in adult patients with B-cell lymphoma, and in pediatric patients with steroid-dependent nephrotic syndrome (SDNS) [9–12]; however, the clinical significance of such decreases is not completely understood. The clinical phenotypes of patients with HGG are variable; some remain relatively unaffected, while others—particularly patients with a history of recurrent serious infection (SI) and preexisting severely low immunoglobulin serum levels—experience severe or recurrent infections [13–15]. Among pediatric patients who received rituximab for unapproved use in SDNS, 13 developed HGG and experienced concomitant infection while 33 developed HGG without experiencing infection [10]. In a long-term global clinical trial program of 3595 adult patients with rheumatoid arthritis who received rituximab over 11 years, SI rates were similar before and after low immunoglobulin [16].
Assessment of immunologic status and infection risk following rituximab treatment is often complicated by multiple disease- and therapeutic-associated risk factors for recurrent infections and HGG, including preexisting low immunoglobulin levels, prior use of cytotoxic or biologic therapies, and other disease- and age-related factors [13, 14, 17]. The objective of this evaluation was to assess the SI risk in pediatric GPA/MPA patients with prolonged low IgG or IgM serum concentrations following rituximab treatment in the PePRS clinical study [7]. Peripheral B-cell depletion was also assessed.
Methods
Study Design and Patient Population
The study design and patient population of the Phase 2a, international, multicenter, open-label, uncontrolled PePRS study (WA25615; NCT01750697; EudraCT 2012-002062-13) has previously been described [7]. Patients aged ≥ 2 to < 18 years with newly diagnosed or relapsing GPA/MPA were enrolled. Exclusion criteria included IgG < 5.65 g/l, or IgM levels < LLN for age-specific reference ranges (Supplemental Table S1). Written informed consent from all patients or parent(s)/legal guardians, with patient assent as appropriate, depending on age and level of understanding, was obtained. The study was conducted in accordance with the principles of the Declaration of Helsinki; ethics approvals were obtained from institutional review boards/ethics committees (Supplemental Methods).
The study consisted of a 6-month remission induction phase and a minimum 12-month follow-up phase. During remission induction, patients received four weekly intravenous (IV) rituximab infusions of 375 mg/m2 and concomitant oral glucocorticoid taper. After 6 months, patients could receive further rituximab and/or other immunosuppressants at the investigator’s discretion. At the common closeout (CCO) date, patients whose peripheral B cells remained depleted (< LLN of 100 cells/µl) entered the extended safety follow-up (SFU) with study visits every 3 months until peripheral B-cell counts returned to pre-rituximab baseline levels or to within the normal range for the population, whichever was lower. No further administration of rituximab or any other B-cell–depleting agent was permitted in the extended SFU. Serum samples were provided for determining circulating CD19+ B-cell populations via fluorescence-activated cell sorter analysis.
Laboratory and Safety Assessments
Total immunoglobulin, IgG, and IgM serum levels were measured every 4–12 weeks throughout remission induction and follow-up. Laboratory abnormalities of prolonged low IgG or IgM levels were defined as IgG or IgM levels < LLN (age-specific reference range; Supplemental Table S1) for ≥ 4 months (1 month = 28 days). SI occurrence was assessed throughout the study (up to 4.5 years of observation), including during and after occurrence of low IgG or IgM levels. Adverse event (AE) severity was graded by the National Cancer Institute Common Terminology Criteria for Adverse Events. SIs were reported as serious AEs with the MedDRA System Organ Class designation of “infections and infestations.” During the extended SFU, safety, laboratory, and other immunological parameters were assessed.
Statistical Methods
In this post hoc analysis, odds ratios (ORs) and CIs for ORs were calculated from contingency tables, along with P values from χ2 tests of independence.
Results
Patient Disposition and Baseline Characteristics
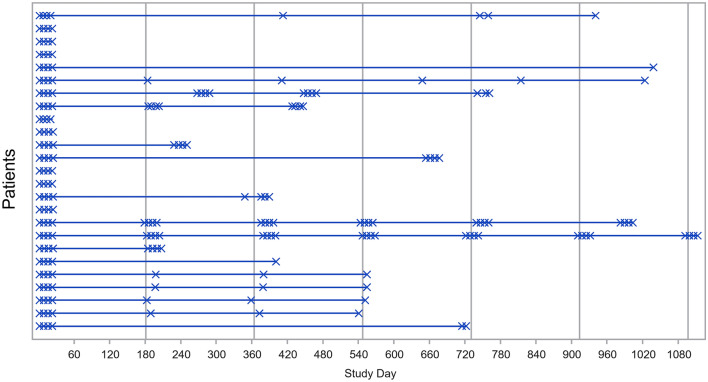
Overall, 25 patients were enrolled from six countries; 80% were female, 68% White, and 76% between 12 and < 18 years of age (Table 1). Nineteen patients (76%) had GPA and six (24%) had MPA; 18 (72%) had newly diagnosed disease and seven (28%) had relapsing disease. All patients completed four weekly rituximab infusions and the 6-month remission induction phase; 24 completed ≥ 18 months of follow-up. Seventeen patients (68%) received additional rituximab treatment on or after month 6 until the CCO, up to a maximum of 4.5 years of observation (minimum, 15.7 months), as shown in Fig. 1, the dosing regimen of which was variable. During the overall study period, patients received a mean of eight infusions of rituximab (range, 4–28). Fourteen patients received concomitant immunosuppressants (e.g., cyclophosphamide [CYC], azathioprine, mycophenolate mofetil [MMF]) at any time during the overall study period (remission induction phase + follow-up phase).
Table 1.
Patient demographic and baseline characteristics
| Rituximab (N = 25) | |
|---|---|
| Female, n (%) | 20 (80.0) |
| Age, median (range), years | 14.0 (6–17) |
| Age group, n (%) | |
| < 12 years | 6 (24.0) |
| ≥ 12 years | 19 (76.0) |
| Race, n (%) | |
| Asian | 4 (16.0) |
| Black or African American | 1 (4.0) |
| White | 17 (68.0) |
| Other or multiple | 3 (12.0) |
| Height, median (range), cm | 154.9 (120.0–175.2) |
| Weight, median (range), kg | 50.9 (23.0–80.8) |
| Diagnosis, n (%) | |
| GPA, newly diagnosed | 13 (52.0) |
| MPA, newly diagnosed | 5 (20.0) |
| GPA, relapsed | 6 (24.0) |
| MPA, relapsed | 1 (4.0) |
| Disease duration, median (range), months | 0.5 (0.2–72.1) |
| ANCA positive, n (%)a | 22 (88.0) |
| MPO-ANCA positive | 8 (32.0) |
| PR3-ANCA positive | 14 (56.0) |
| eGFR, ml/min/1.73 m2, median (IQR) | 138 (120–157) |
| PVAS, median (IQR), 0–63 | 8.0 (5.0–15.0) |
| PGADA, median (IQR) | 46.0 (29.0–71.0) |
| PVDI, median (IQR), 0–72 | 0.0 (0.0–3.0) |
| Previous cyclophosphamide therapy, n (%) | 2 (8.0) |
ANCA antineutrophil cytoplasmic antibody, eGFR estimated glomerular filtration rate, GPA granulomatosis with polyangiitis, IQR interquartile range, MPA microscopic polyangiitis, MPO myeloperoxidase, PGADA Physician’s Global Assessment of Disease Activity, PR3 proteinase 3, PVAS Pediatric Vasculitis Activity Score, PVDI Pediatric Vasculitis Damage Index
aThree patients with relapsed disease were ANCA negative at screening but were ANCA positive at the time of diagnosis
Fig. 1.
Rituximab administration over the course of the study. Vertical lines represent month 6, month 12, month 18, and every 6 months thereafter. X one rituximab infusion. Five patients received rituximab infusions (375 mg/m2) approximately every 6 months administered once weekly for 4 weeks, and five received one infusion of rituximab (375 mg/m2) every 6 months; a further seven patients received other varied doses of rituximab within individualized regimens
Six patients entered the extended SFU, as peripheral B cells remained < LLN following their last rituximab dose. Two of these patients continued concomitant oral steroids as treatment for GPA/MPA; one received hydrocortisone 10 mg and MMF 1 g; the other received prednisone 5 mg. All six patients completed the extended SFU when peripheral B-cells returned to within normal range or when they were transferred to adult care to receive treatment for GPA/MPA. Patient participation in the extended SFU ranged from 3 months to > 2 years.
All Infections
Seventeen patients (68%) experienced 31 infections (serious and nonserious AEs) during the remission induction phase; 28 (90%) were nonserious and resolved without sequelae. During the overall study period (up to 4.5 years observation), 23 patients reported 105 infections; 91% were nonserious AEs. Details of infections in PePRS have been reported previously [7].
During remission induction, three patients (12%) experienced three SIs. During the overall study period, seven patients (28%) had nine SIs (Table 2). Most SIs resolved without sequelae (eight of nine events). There were no deaths or study discontinuations due to SI. No SIs were reported during the extended SFU. The most frequent SIs were influenza and lower respiratory tract infection.
Table 2.
Serious infections in patients with prolonged low IgG and/or prolonged low IgM levelsa
| Patient no. | Serious infection (SI) | Study day of SI onset | Normal IgG and IgM valueb at baseline (day 1) | Prolonged low IgG | Prolonged low IgM | Caused by study drugc | Previous steroid and IST medications before baseline | Concomitant steroid and IST medications | Outcome | Occurrence during remission induction vs. follow-up |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Lower respiratory tract infection | 215 | Yes | Yes | Yes | No | MMF; prednisolone; methylprednisolone; CYC | Prednisolone; dexamethasone; CYC | Recovered/resolved | Follow-up |
| 2 | Lower respiratory tract infection | 75 | Yes | Yes | Yes | No | Dexamethasone; prednisolone | Prednisone; methylprednisolone; MMF; triamcinolone acetonide | Recovered/resolved | Remission induction |
| 3 | Eye infection bacteriald | 811 | Yes | Yes | No | No | MMF; prednisolone | Prednisolone; methylprednisolone; MMF | Recovered/resolved with sequelae | Follow-up |
| 4 | Device-related sepsis | 456 | No (IgM < LLN) | No | Yes | No | – | Prednisone | Recovered/resolved | Follow-up |
| 5 | Gastroenteritis viral | 129 | Yes | No | Yes | No | Prednisolone; methylprednisolone | Prednisolone; MMF | Recovered/resolved | Remission induction |
| Influenza | 1396 | Yes | Yes | Yes | No | Recovered/resolved | Follow-up | |||
| Sinusitis | 1404 | Yes | Yes | Yes | No | Recovered/resolved | Follow-up | |||
| 6 | Gastroenteritis norovirus | 205 | No (IgM < LLN) | Yes | Yes | No | Prednisone | Prednisone | Recovered/resolved | Follow-up |
| 7 | Influenza | 161 | Yes | Yes | Yes | Yes | Prednisone; methylprednisolone; azathioprine; CYC | Prednisone | Recovered/resolved | Remission induction |
CYC cyclophosphamide, IgG immunoglobulin G, IgM immunoglobulin M, IST immunosuppressant, MMF mycophenolate mofetil
aAll SIs occurred during or after the first occurrence of prolonged low IgG or IgM
bNormal levels at baseline: IgG > 5.65 g/l; IgM levels > LLN age-specific reference range
cAs reported by the study investigator
dA serious bacterial eye infection due to Ureaplasma urealyticum occurred 51 days after the last rituximab infusion. The patient had surgical history, previous immunosuppressant treatment, previous grade 2 (National Cancer Institute Common Terminology Criteria for Adverse Events) conjunctivitis, and concomitant steroid use
Overview of Serum Immunoglobulin Levels
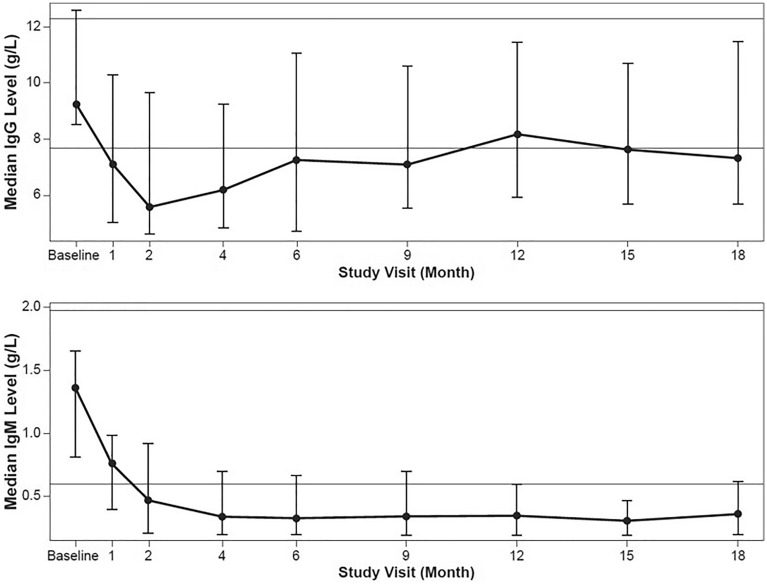
At day 1 (baseline), a total of two patients had IgM levels < LLN (highest LLN, 0.6 g/l; Supplemental Table S1). Median IgG and IgM levels decreased from baseline over the course of the study. Median IgG levels were < LLN by month 1, with the lowest level (5.59 [2.4–15.4] g/l) at month 2. At month 6, median IgG levels were 7.26 (2.5–15.4) g/l and returned to within normal limits between months 9 and 12. At month 18, the median IgG levels were 7.33 (2.1–16.8) g/l (Fig. 2, Table 3). Median IgM levels were < LLN by month 2 and remained < LLN until month 18. At month 6, the median IgM level was 0.33 (0.2–1.4) g/l (Fig. 2, Table 3). After the remission induction phase (month 6), no consistent trend was observed across individual patients in the reconstitution of IgG and IgM levels over time or by the number of rituximab follow-up treatments.
Fig. 2.
Median immunoglobulins by study visit. a IgG. b IgMa. Error bars represent the interquartile range. The horizontal lines on the graphs show the ULN and the LLN for IgG (12.29 g/l and 7.68 g/l) and IgM (1.97 g/l and 0.6 g/l). IgG immunoglobulin G, IgM immunoglobulin M, LLN lower limit of normal, ULN upper limit of normal. aFigure originally published in (7)
Table 3.
Number and proportiona of patients with low (< LLN, for age-specific reference ranges) IgG and IgM at key study visits
| Baseline (study day 1)b n = 25 |
Month 6 n = 25 |
Month 12 n = 24 |
Month 18 n = 23 |
|
|---|---|---|---|---|
| IgG | 0 (0%)c | 12 (48%) | 10 (42%) | 11 (48%) |
| IgM | 2 (16%) | 17 (68%) | 18 (75%) | 16 (70%) |
IgG immunoglobulin G, IgM immunoglobulin M, LLN lower limit of normal, n number of patients with a valid measurement at that visit
aPercentages are based on n
bBefore rituximab treatment
cBased on study exclusion criteria: IgG < 5.65 mg/ml at baseline
At the CCO, six patients whose peripheral B cells remained depleted entered the extended SFU. Among them, four experienced an increase in total immunoglobulin concentration by the end of the extended SFU, compared with the last value measured before entering the extended SFU, and two had a slight decrease (2.59–2.13 g/l and 12.66–11.08 g/l).
Prolonged Low IgG Levels and Serious Infections
Overall, 18 patients (72%) had prolonged low IgG levels for ≥ 4 months during the study (of whom 15 also had prolonged low IgM; Table 2 and Fig. 3), and all had past and/or concomitant treatment with steroids and/or immunosuppressants. Of these 18 patients, the IgG levels returned to within normal range by study end in seven patients. Six patients (33%) experienced seven SIs during or after prolonged low IgG, of which one occurred during or after prolonged low IgG only and six occurred during or after concurrent prolonged low IgG and prolonged low IgM (Table 2). Three of these patients received treatment with IV immunoglobulin after experiencing an AE of HGG. Six of the SIs resolved with no reported sequelae and one resolved with sequelae (a serious bacterial eye infection due to Ureaplasma urealyticum occurred 51 days after the last rituximab infusion, Table 2).
Fig. 3.
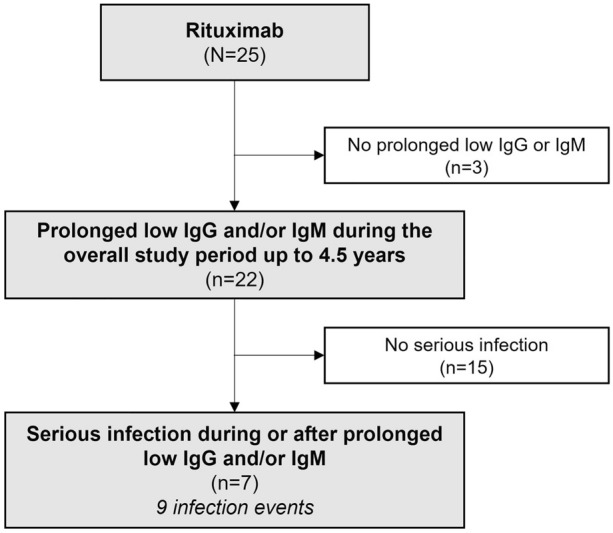
Patient flow chart: distribution of patients with prolonged low IgG and/or IgM levels and SI. IgG immunoglobulin G, IgM immunoglobulin M
In this post hoc analysis, the calculation of ORs and P values using χ2 tests of independence based on the contingency table (Supplemental Table S2; OR, 3.0; 95% CI, 0.29–30.92; P = 0.3507) suggests that there is no clear statistical association between prolonged low IgG level and probability of experiencing an SI in relation to rituximab treatment.
In the extended SFU, three patients had IgG levels < LLN during the overall study period and afterwards had IgG levels within the normal range until the CCO and throughout the extended SFU, and three patients had IgG levels < LLN during the overall study period and through the extended SFU. No infections occurred during the extended SFU in the patients with IgG < LLN.
Prolonged Low IgM Levels and Serious Infections
Overall, 19 patients (76%) had prolonged low IgM levels (of whom 15 also had prolonged low IgG; Table 2 and Fig. 3), two of these patients had IgM levels < LLN at study day 1 (before starting rituximab). All had past and/or concurrent treatment with steroids and/or immunosuppressants. Six of 19 patients (32%) experienced eight SIs during or after prolonged low IgM, of which two occurred during or after prolonged low IgM only and six occurred during or after concurrent prolonged low IgG and prolonged low IgM (Table 2). All SIs resolved without sequelae.
In this post hoc analysis, the calculation of the ORs and P values using χ2 tests of independence based on the contingency table (Supplemental Table S3; OR, 2.3; 95% CI, 0.22–24.32; P = 0.4871) suggests that there is no association between prolonged low IgM level and probability of experiencing an SI in relation to rituximab treatment.
In the extended SFU, all six patients had IgM levels < LLN during the study before entering the extended SFU and had IgM levels < LLN during the extended SFU. IgM levels returned to within normal ranges during the extended SFU in one patient; in five patients they remained < LLN throughout the extended SFU. No infections occurred during the extended SFU in patients with IgM < LLN.
Representative Cases of Immunoglobulin Levels Over Time
Examples of four representative patient profiles showing variability of rituximab dosing regimens, subsequent immunoglobulin levels, and clinical consequence (with or without occurrence of SI) are shown in Fig. 4a–d, respectively. Patient A received multiple rituximab courses, had prolonged low IgG and IgM levels, and experienced SI. IgG levels decreased to < LLN after remission induction (lowest level, 4.52 g/l), and afterward returned to within normal range. The patient received IV immunoglobulins for the AE of HGG (study days 233, 268, and 330). IgM levels were < LLN from study day 33 throughout the study (lowest level, < 0.2 g/l). The patient had previous use of, and additional concomitant treatments with, immunosuppressants and steroids, and experienced a serious lower respiratory tract infection that was reported as unrelated to study medication and related to concurrent illness. Ig levels at onset were not available; however, the previous IgG level was within normal range, while the previous IgM level was < LLN. Patient B received multiple rituximab treatments and had prolonged low IgG (lowest level, 4.9 g/l) and IgM (lowest level, < 0.2 g/l) levels throughout the study and no SI. The patient received additional concomitant treatments with prednisolone and azathioprine.
Fig. 4.
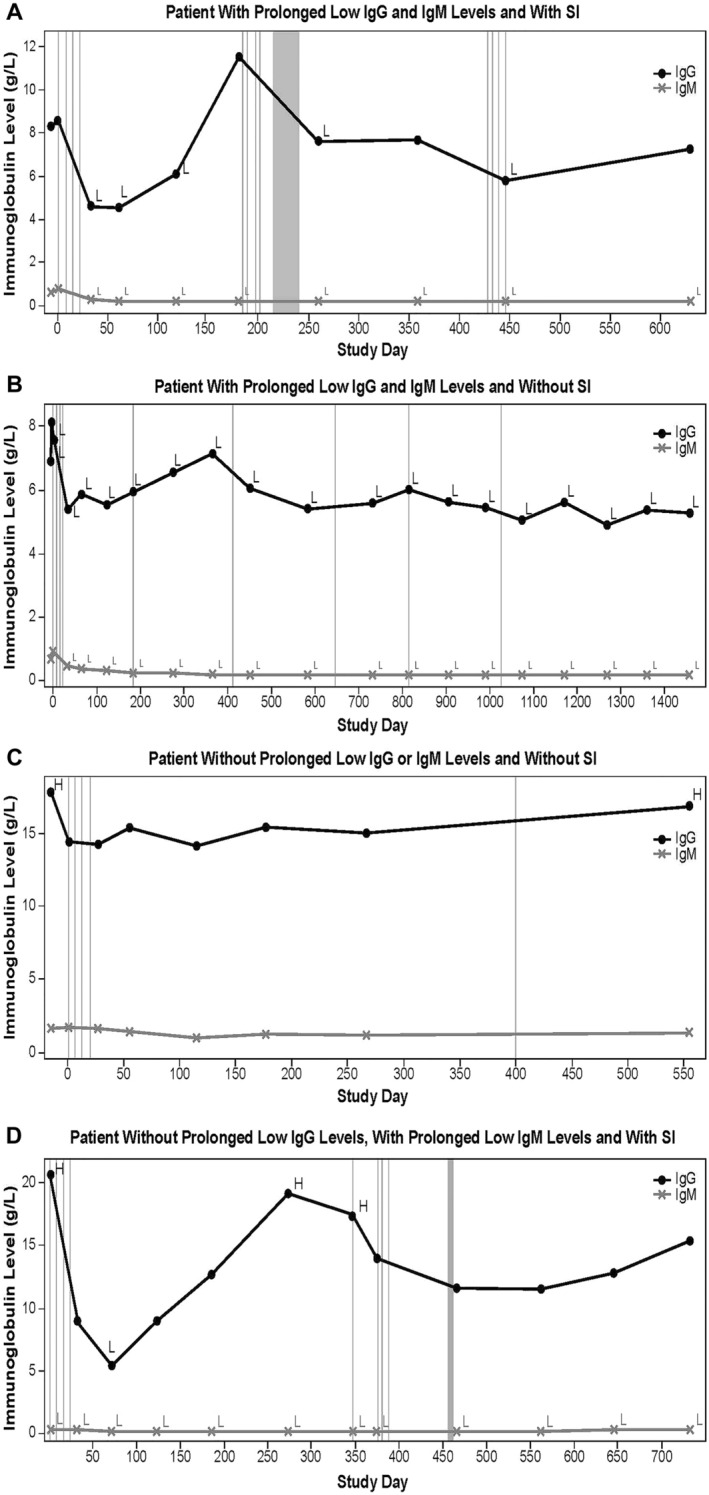
Representative individual patient plots of IgG and IgM values over time. a A patient with prolonged low IgG and IgM levels and with SI. b A patient with prolonged low IgG and IgM levels and without SI. c A patient without prolonged low IgG or IgM levels and without IS. d A patient without prolonged low IgG levels, with prolonged low IgM levels and with SI. Vertical lines represent rituximab infusion. Vertical gray block indicates timing of serious infection. H indicates immunoglobulin levels > ULN; L indicates immunoglobulin levels < LLN. IgG immunoglobulin G, IgM immunoglobulin M, LLN lower limit of normal, SI serious infection, ULN upper limit of normal
Patient C received multiple rituximab treatments and did not experience prolonged low IgG or IgM levels or SI; IgG and IgM levels were within or above normal range throughout the study. Patient D received two rituximab treatments and did not have prolonged low IgG and experienced SI. IgG levels decreased to < LLN at study day 71 and afterward were within or above normal range until study end. At baseline and until study end, IgM levels were < LLN (lowest, < 0.20 g/l). Concomitant treatment with prednisone was received throughout the study. The patient experienced an SI (device-related sepsis) while hospitalized for sickle cell crisis, which resolved and was reported as not caused by study drug. IgG levels at infection onset were within normal range. During the study, the patient experienced three nonserious infections; all resolved and were reported as unrelated to study drug.
B-Cell Depletion
Consistent with rituximab’s mode of action, patients experienced sustained CD19+ peripheral B-cell depletion following rituximab treatment, which persisted until at least month 6 and in some cases throughout the duration of follow-up, with administration of repeat rituximab infusions (Supplemental Figure S1). The median B-cell level at baseline (pre-treatment) was 599 (range, 197–2173) cells/µl and deceased rapidly after the first infusion by week 1 (median, 1 cell/µl). At month 18, B-cell levels had increased slightly but remained low (median, 58 cells/µl).
Of the six patients who entered the extended SFU, all had peripheral B-cell counts within or above normal laboratory range (100–616 cells/µl) at baseline; upon entering the extended SFU, patients' peripheral B-cell counts were < 100 cells/µl following the patients’ last dose of rituximab within the treatment phase of the study. Four patients completed the extended SFU when their B-cell counts returned to within the normal laboratory range for the population. One patient with a baseline B-cell count of 197 cells/µl completed 18 months of extended SFU, at which time they were moved into adult care per the investigator’s clinical judgment with a B-cell count of 3 cells/µl, 819 days since their last rituximab dose. The investigator confirmed that the patient did not receive rituximab or other B-cell–depleting therapy or other agent that would affect the return of B-cells on or after the CCO. One patient with a baseline B-cell count of 341 cells/µl completed the study after approximately 3 months in extended SFU (last CD19+ B-cell count, 4 cells/µl). This patient received additional rituximab treatment approximately 6 months after CCO as part of the post-trial access program; therefore, per protocol the patient was no longer required to remain in extended SFU for B-cell monitoring.
Discussion
This post hoc analysis examined SI risk and prolonged low IgG or IgM levels following rituximab treatment among pediatric patients with GPA/MPA in the phase 2a global PePRS study. There was a decrease in IgG and/or IgM levels during and after remission induction; afterward, no consistent pattern in IgG and IgM reconstitution over time and/or by number of rituximab follow-up treatments was observed across patients. Overall, 72 and 76% of patients experienced prolonged low IgG or IgM levels, respectively, during the overall study period, and most of them did not experience SI. Although this study was not designed or powered to specifically study an association between prolonged low IgM levels and SIs, our analyses suggest that there is no association between prolonged low IgG or IgM levels and probability of experiencing an SI in relation to rituximab treatment.
Studies have reported that HGG does not always translate to impaired response to antigens (e.g., infections) and does not necessarily result in recurrent infections [13], whereas in other cases, low IgG levels can predispose patients to an increased risk of SI [15]. The clinical significance of low immunoglobulin levels in PePRS appeared to be variable (three of 25 patients received IV immunoglobulin for AEs of HGG, leading to resolutions of these AEs), and not all patients with low immunoglobulin experienced SI, although the patients who experienced SI (n = 7) had prolonged low immunoglobulin levels. This could be due to variability in previous and/or concomitant steroid and immunosuppressant use, number of rituximab follow-up treatments, and medical history and underlying disease severity. Prolonged low IgM levels, but not prolonged low IgG levels, occurred more frequently and generally did not recover during the study; however, as reported in previous studies, low IgM is less clinically significant with regards to a possible increase in SI risk [13]. Most of the observed laboratory abnormalities of prolonged low immunoglobulin were asymptomatic, and there was no increase in the number of SI over time, through the extended SFU, or with increasing rituximab treatments. No trend of recurrent SI, even during periods of prolonged low IgG and/or IgM levels, was observed, and most SIs could be treated, had a favorable outcome, and were consistent with the safety profile in adult patients with GPA/MPA receiving rituximab.
Overall, no firm conclusions can be made regarding a possible relationship between prolonged low IgG and/or IgM levels and risk of SI following rituximab treatment as this study was not adequately powered to detect such a relationship. The attribution of a direct role of rituximab for SI development in the presence of low immunoglobulin levels was limited by potential alternative etiologies, including concomitant immunosuppressive medications and/or clinical characteristics of the underlying disease; however, these observations are consistent with the rituximab safety profile in adult patients with GPA/MPA and in other approved autoimmune indications [5, 6, 18, 19]. In the 18-month follow-up of the RAVE trial, a multicenter non-inferiority trial of rituximab in 197 adults with GPA/MPA, there was no significant association between SI and low IgG levels [9]. However, in a retrospective study of adults with B-cell lymphoma receiving rituximab, the number of rituximab doses significantly correlated with development of symptomatic HGG [20]. A study of 28 pediatric patients with post-transplant lymphoproliferative disorder (PTLD) demonstrated that among patients treated with rituximab (n = 17), a significantly higher proportion developed HGG and there was a significantly higher cumulative rate of bacterial infection compared with those who did not receive rituximab; recurrent bacterial infection and/or related deaths within 1 year post-PTLD was significantly associated with HGG (P = 0.047) and near-significantly with rituximab (P = 0.180) [21]. A retrospective study of 2875 pediatric patients found that infection occurrence after rituximab may depend on an underlying condition, where patients with autoimmune conditions had lower rates of infections compared with patients with primary immunodeficiency, malignancy, or transplant [22]. In a chart review, among ten pediatric patients with ANCA-associated vasculitis treated with rituximab, 60% experienced HGG, with mean (SD) onset 4.2 (3.4) months after rituximab initiation; one patient experienced frequent or severe infection [12]. This study noted that the prevalence of HGG in patients with autoimmune diseases treated with rituximab appeared to be higher in children than adults. In a study of 107 pediatric patients with SDNS treated with rituximab, of 86 patients without HGG prior to rituximab, 25 (29%) developed HGG after rituximab treatment; of these 25 patients, nine (36%) had an infection during HGG [10]. Notably, patients were more likely to develop HGG if they were younger at first infusion; low baseline IgG levels increased the risk of aggravated or persistent HGG [10]. In our study, no specific pattern was observed with regards to the influence of the Ig levels at baseline (study day 1) on the results of the study. Overall, two out of the seven patients who experienced SI had IgM < LLN and normal IgG levels at baseline. These patients continued to have low IgM after rituximab administration and during the course of the study, while IgG levels remained within normal laboratory reference ranges. On the other hand, patients with normal IgG and/or IgM levels at baseline also showed decreased Ig levels or prolonged low Ig and/or had SI during the study.
Our analysis also demonstrated that pediatric patients with GPA/MPA treated with rituximab had peripheral B-cell depletion after induction therapy, which was maintained through at least month 18. Previous exploratory analyses of pediatric patients in PePRS and adult patients in the RAVE trial demonstrated that higher rituximab exposure was correlated with longer-lasting B-cell depletion [7].
This study has some limitations. The lack of a placebo arm and the small patient number limits the generalizability of the results; the small sample size is partly due to the rare nature of the disease. The lack of correlation between SI onset and recording of immunoglobulin levels was due to protocol-defined time points for laboratory assessments and limits the ability to draw strong conclusions regarding a possible relationship. Potential alternative etiologies (such as underlying diseases or exposure to immunosuppressive agents) pose another possible limitation.
Conclusions
In PePRS, laboratory abnormalities of prolonged low immunoglobulin after rituximab treatment in pediatric patients with GPA/MPA were generally asymptomatic without clinical consequence (most patients with prolonged low IgG or IgM levels did not experience SI). There was no increase in the number of SI over time, through the extended SFU, or with the number of follow-up rituximab treatments. In patients who experienced SI in the presence of low immunoglobulin, infections were manageable and consistent with the known rituximab safety profile in adult patients with GPA/MPA. These findings indicate that repeated peripheral B-cell depletion with rituximab did not increase SI risk over time, despite prolonged low Ig. Vigilance is recommended in monitoring for SI in pediatric patients with prolonged low immunoglobulin levels during or after rituximab treatment.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
Funding
This study and journal’s Rapid Service Fee was funded by F. Hoffmann-La Roche Ltd, Basel, Switzerland.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Authors’ Contributions
All authors were involved in drafting the article or revising it critically for important intellectual content, and all authors approved the final version to be published. S. Melega, P.B. Lehane, and P. Brogan were involved in the study conception and design. P. Brogan, G. Cleary, A.O. Hersh, O. Kasapcopur, S. Rangaraj, R.S.M. Yeung, A. Zeft, J. Cooper, and P.B. Lehane were involved in the acquisition of data. All authors contributed to the analysis and interpretation of data.
Medical Writing, Editorial, and Other Assistance
Support for medical writing assistance, furnished by Claire Stedden PhD, and Ellen Mercado, PhD, of Health Interactions, Inc, was provided by F. Hoffmann-La Roche, Ltd. P. Brogan acknowledges support from the Great Ormond Street Hospital (GOSH) NIHR Biomedical Research Centre and the GOSH Clinical Research Facility for this study.
Prior Presentation
This manuscript is based on work that has been previously presented at the International Vasculitis and ANCA Workshop, April 7–10, 2019, Philadelphia, PA, USA, and at the EULAR European E-Congress of Rheumatology, June, 3–6, 2020.
Disclosures
S. Melega and P. Kirchner are employees of F. Hoffmann La-Roche Ltd, Basel, Switzerland. P. Brogan has received institutional grants from ChemoCentryx, Novartis, Novimmune, Roche, and SOBI, and consultancy/speaker fees from Novartis, Roche, SOBI, and UCB. G. Cleary has received speaker fees from AbbVie. A.O. Hersh, O. Kasapcopur, and S. Rangaraj have nothing to disclose. R.S.M. Yeung received consultancy/speaker fees from AbbVie and Novartis. A. Zeft has stock in Merck Pharmaceuticals, Teva Pharmaceuticals, and OPKO Health. J. Cooper was a research fellow at Genentech, Inc., at the time of this study. P. Pordeli is an employee of Roche Products Ltd., Mississauga, Canada. P.B. Lehane is an employee of Roche Products Ltd, Welwyn Garden City, UK.
Compliance with Ethics Guidelines
Written informed consent from all patients or patients’ parent(s)/legal guardians, with assent as appropriate by the patient, depending on age and level of understanding, was obtained. The study was conducted in accordance with the principles of the Declaration of Helsinki; ethics approvals were obtained from institutional review boards/ethics committees (Supplemental Methods).
Data Availability
Qualified researchers may request access to individual patient level data through the clinical study data request platform (https://vivli.org/). Further details on Roche's criteria for eligible studies are available at https://vivli.org/members/ourmembers/. For further details on Roche's Global Policy on the Sharing of Clinical Information and how to request access to related clinical study documents, see https://www.roche.com/research_and_development/who_we_are_how_we_work/clinical_trials/our_commitment_to_data_sharing.htm.
References
- 1.Yates M, Watts RA, Bajema IM, et al. EULAR/ERA-EDTA recommendations for the management of ANCA-associated vasculitis. Ann Rheum Dis. 2016;75(9):1583–1594. doi: 10.1136/annrheumdis-2016-209133. [DOI] [PubMed] [Google Scholar]
- 2.de Graeff N, Groot N, Brogan P, et al. European consensus-based recommendations for the diagnosis and treatment of rare paediatric vasculitides - the SHARE initiative. Rheumatology (Oxford) 2019;58(4):656–671. doi: 10.1093/rheumatology/key322. [DOI] [PubMed] [Google Scholar]
- 3.Jariwala MP, Laxer RM. Primary vasculitis in childhood: GPA and MPA in childhood. Front Pediatr. 2018;6:226. doi: 10.3389/fped.2018.00226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Finkielman JD, Lee AS, Hummel AM, et al. ANCA are detectable in nearly all patients with active severe Wegener's granulomatosis. Am J Med. 2007;120(7):643.e9–14. doi: 10.1016/j.amjmed.2006.08.016. [DOI] [PubMed] [Google Scholar]
- 5.(Rituximab) R. Prescribing information. South San Francisco, CA. Genentech, Inc. 2019.
- 6.MabThera. Summary of product characteristics. Grenzach-Wyhlen, Germany. Roche Registration GmbH. 2020.
- 7.Brogan P, Yeung RSM, Cleary G, et al. Phase IIa global study evaluating rituximab for the treatment of pediatric patients with granulomatosis with polyangiitis or microscopic polyangiitis. Arthritis Rheumatol. 2022;74(1):124–133. doi: 10.1002/art.41901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Patel SY, Carbone J, Jolles S. The expanding field of secondary antibody deficiency: causes, diagnosis, and management. Front Immunol. 2019;10:33. doi: 10.3389/fimmu.2019.00033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Specks U, Merkel PA, Seo P, et al. Efficacy of remission-induction regimens for ANCA-associated vasculitis. N Engl J Med. 2013;369(5):417–427. doi: 10.1056/NEJMoa1213277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Parmentier C, Delbet JD, Decramer S, Boyer O, Hogan J, Ulinski T. Immunoglobulin serum levels in rituximab-treated patients with steroid-dependent nephrotic syndrome. Pediatr Nephrol. 2020;35(3):455–462. doi: 10.1007/s00467-019-04398-1. [DOI] [PubMed] [Google Scholar]
- 11.Khan DA. Hypersensitivity and immunologic reactions to biologics: opportunities for the allergist. Ann Allergy Asthma Immunol. 2016;117(2):115–120. doi: 10.1016/j.anai.2016.05.013. [DOI] [PubMed] [Google Scholar]
- 12.Khojah AM, Miller ML, Klein-Gitelman MS, et al. Rituximab-associated hypogammaglobulinemia in pediatric patients with autoimmune diseases. Pediatr Rheumatol Online J. 2019;17:61. doi: 10.1186/s12969-019-0365-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kado R, Sanders G, McCune WJ. Diagnostic and therapeutic considerations in patients with hypogammaglobulinemia after rituximab therapy. Curr Opin Rheumatol. 2017;29(3):228–233. doi: 10.1097/BOR.0000000000000377. [DOI] [PubMed] [Google Scholar]
- 14.Sacco KA, Abraham RS. Consequences of B-cell-depleting therapy: hypogammaglobulinemia and impaired B-cell reconstitution. Immunotherapy. 2018;10(8):713–728. doi: 10.2217/imt-2017-0178. [DOI] [PubMed] [Google Scholar]
- 15.Barmettler S, Ong MS, Farmer JR, Choi H, Walter J. Association of immunoglobulin levels, infectious risk, and mortality with rituximab and hypogammaglobulinemia. JAMA Netw Open. 2018;1(7):e184169. doi: 10.1001/jamanetworkopen.2018.4169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.van Vollenhoven RF, Fleischmann RM, Furst DE, et al. Long-term safety of rituximab: final report of the rheumatoid arthritis global clinical trial program over 11 years. Rheumatology. 2015;42(10):1761–1766. doi: 10.3899/jrheum.150051. [DOI] [PubMed] [Google Scholar]
- 17.Worch J, Makarova O, Burkhardt B. Immunreconstitution and infectious complications after rituximab treatment in children and adolescents: what do we know and what can we learn from adults? Cancers (Basel) 2015;7(1):305–328. doi: 10.3390/cancers7010305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stone JH, Merkel PA, Spiera R, et al. Rituximab versus cyclophosphamide for ANCA-associated vasculitis. N Engl J Med. 2010;363(3):221–232. doi: 10.1056/NEJMoa0909905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jones RB, Tervaert JW, Hauser T, et al. Rituximab versus cyclophosphamide in ANCA-associated renal vasculitis. N Engl J Med. 2010;363(3):211–220. doi: 10.1056/NEJMoa0909169. [DOI] [PubMed] [Google Scholar]
- 20.Casulo C, Maragulia J, Zelenetz AD. Incidence of hypogammaglobulinemia in patients receiving rituximab and the use of intravenous immunoglobulin for recurrent infections. Clin Lymphoma Myeloma Leuk. 2013;13(2):106–111. doi: 10.1016/j.clml.2012.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chiou FK, Beath SV, Patel M, Gupte GL. Hypogammaglobulinemia and bacterial infections following pediatric post-transplant lymphoproliferative disorder in the rituximab era. Pediatr Transplant. 2019;23(6):e13519. doi: 10.1111/petr.13519. [DOI] [PubMed] [Google Scholar]
- 22.Kavcic M, Fisher BT, Seif AE, et al. Leveraging administrative data to monitor rituximab use in 2875 patients at 42 freestanding children's hospitals across the United States. J Pediatr. 2013;162(6):1252–1258. doi: 10.1016/j.jpeds.2012.11.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Qualified researchers may request access to individual patient level data through the clinical study data request platform (https://vivli.org/). Further details on Roche's criteria for eligible studies are available at https://vivli.org/members/ourmembers/. For further details on Roche's Global Policy on the Sharing of Clinical Information and how to request access to related clinical study documents, see https://www.roche.com/research_and_development/who_we_are_how_we_work/clinical_trials/our_commitment_to_data_sharing.htm.