Abstract
Introduction
Baricitinib has been shown to improve patient-reported outcomes (PROs) in patients with rheumatoid arthritis (RA) who are inadequate responders (IR) to conventional synthetic and biologic disease-modifying antirheumatic drugs (csDMARDs and bDMARDs, respectively). We assessed the ability of baricitinib 2-mg to maintain minimal clinically important differences (MCIDs) in PROs until week 24 among week 4 and 12 responders.
Methods
Data were from two phase 3 trials, RA-BUILD (NCT01721057; csDMARD-IR patients) and RA-BEACON (NCT01721044; bDMARD-IR patients). PROs included Pain Visual Analogue Scale, Health Assessment Questionnaire-Disability Index, Functional Assessment of Chronic Illness Therapy-Fatigue, Short-Form 36 Physical Component Score, and Patient’s Global Assessment of Disease Activity. Outcomes were evaluated by proportions of patients achieving MCID improvements, number needed to treat (NNT) at weeks 4, 12, and 24, proportions of patients maintaining MCID responses at week 24 among week 4 or 12 responders, and median time to achieve substantial response with baricitinib 2-mg versus placebo.
Results
A higher proportion of baricitinib-treated patients achieved MCID improvements, with NNTs ranging from 5 to 8 for baricitinib 2-mg versus placebo at week 24. Generally, early MCID responses in PROs at weeks 4 or 12 were better maintained through week 24 in RA patients treated with baricitinib 2-mg versus placebo. Patients treated with baricitinib 2-mg also achieved substantial PRO responses or normative values more quickly than placebo.
Conclusions
These results suggest baricitinib-treated patients with RA achieving MCID improvement in PROs at weeks 4 and 12 maintained those improvements over time and that substantial PRO responses were achieved quickly.
Keywords: Baricitinib, Janus kinase inhibitors, Minimal clinically important differences, Patient-reported outcomes, Rheumatoid arthritis
Key Summary Points
| Why carry out this study? |
| Patient-reported outcomes (PROs), an important measure of symptoms and perceived disease severity, are included in rheumatoid arthritis (RA) clinical trials in order to assess treatment efficacy from the patient perspective. |
| This study used PRO data from two clinical trials of inadequate responders (IRs) to conventional synthetic and biologic disease-modifying antirheumatic drugs (csDMARDs and bDMARDs, respectively) to evaluate the maintenance of minimal clinically important differences (MCIDs) in PROs until week 24 and the time to substantial response for the same set of PROs in patients with RA treated with baricitinib 2-mg. |
| What was learned from the study? |
| Early MCIDs in PROs were maintained until week 24 in patients with active RA treated with baricitinib 2-mg, and the time to reach a substantial response in PROs was shorter in patients treated with baricitinib 2-mg vs. placebo. |
| These results support that treatment with baricitinib 2-mg allows patients to quickly achieve and maintain improvements in PROs. |
Introduction
Rheumatoid arthritis (RA) is a chronic, immune-mediated inflammatory arthritis that negatively affects patients’ health-related quality of life (HRQoL) [1–4]. HRQoL is defined by patients’ self-perception of well-being and encompasses pain, physical function, and fatigue, alongside psychosocial factors [4]. Patient-reported outcomes (PROs), which reflect different aspects of HRQoL, are evaluated by the patient directly; therefore, serving as an important measure of symptoms and overall perceived disease severity [5–8]. PROs are included in RA clinical trials as a way to evaluate efficacy from the patient perspective alongside endpoints measuring disease activity that are interpreted by physicians [9].
The 2021 American College of Rheumatology Guidelines for the Treatment of RA emphasize the importance of shared decision-making regarding treatment that considers patients’ experiences and preferences [10]. Treating RA with the goal of reducing objective markers of disease activity also improves PROs and HRQoL as perceived by patients [2].
Baricitinib is an oral selective Janus kinase (JAK)1/JAK2 inhibitor that is approved for the treatment of moderate-to-severe RA in adults who have had an inadequate response to csDMARDs or, in the United States, one or more TNFi [11]. In the phase 3 RA-BUILD (NCT01721057) and RA-BEACON (NCT01721044) trials, treatment with baricitinib was associated with significant improvements in PROs including pain, physical function, fatigue, quality of life, and patient global assessment compared to placebo in patients who were inadequate responders (IR) to csDMARDs and bDMARDs, respectively [2, 3, 7, 9].
Using PRO (pain, physical function, fatigue, health-related quality of life), and patient global assessment data from the RA-BUILD and RA-BEACON trials of inadequate responders (IR) to csDMARDs and bDMARDs, post hoc analyses were conducted to assess the ability of baricitinib 2-mg to quickly achieve and maintain minimal clinically important differences (MCIDs) until week 24 among those who achieved PRO MCIDs at weeks 4 or 12, separately. In addition, the time to substantial response in the same set of PROs was evaluated.
Methods
Study Design
Full study designs for RA-BUILD and RA-BEACON have been published previously [2, 3]. Both trials were phase 3, double-blind studies randomizing patients 1:1:1 to placebo, baricitinib 2-mg, or baricitinib 4-mg once daily. The present post hoc analyses focused on evaluating baricitinib 2-mg, the recommended dose in the United States and Canada. Patients enrolled in either study were adults with active RA (≥ 6/68 tender joint count, ≥ 6/66 swollen joint count). Patients enrolled in RA-BUILD had high sensitivity CRP levels greater than 3.6 mg/l and inadequate response or intolerance to one prior csDMARD; TNFi-experienced patients were excluded from RA-BUILD [2]. Patients enrolled in RA-BEACON had high sensitivity CRP levels greater than 3 mg/l and prior treatment with at least one TNFi discontinued due to an insufficient response after ≥ 3 months on therapy or intolerance [3]. RA-BUILD and RA-BEACON were conducted in accordance with the principles of the Declaration of Helsinki and Good Clinical Practice Guidelines and were approved by each center’s institutional review board (IRB): Quorum Review IRB, #27258 and #27259. Written informed consent was provided by all patients.
Assessments
Pain was measured by the Pain Visual Analogue Scale (VAS) on a scale of 0 to 100 mm, with higher scores indicating worse pain. Physical functioning was measured by the Health Assessment Questionnaire-Disability Index (HAQ-DI) on a scale of 0 to 3, with higher scores indicating worse physical function and disability. Fatigue was measured by the Functional Assessment of Chronic Illness Therapy-Fatigue (FACIT-Fatigue) on a scale of 0 to 52, with lower scores indicating worse fatigue. The Medical Outcomes Study Short-Form-36 (SF-36; V.2, Acute) was used to assess HRQoL in eight domains scored from 0 to 100 that are normalized into physical (PCS) and mental (MCS) component scores, with lower scores indicating worse quality of life. SF-36 PCS was used to measure physical functioning. Patient global assessment was measured by the Patient’s Global Assessment of Disease Activity (PtGA) on a scale of 0 to 100 mm, with higher scores indicating worse disease activity.
The PRO maintenance analyses used MCIDs as cutoffs. The MCID for Pain VAS was defined as a reduction greater than or equal to 10 mm [12]. The MCID for HAQ-DI was defined as a score reduction greater than or equal to 0.22 units [13]. The MCID for FACIT-Fatigue was defined as improvement greater than or equal to 3.56 points [14, 15]. The MCID for SF-36 PCS was defined as improvement greater than or equal to 5 units [16, 17]. The MCID for PtGA was defined as a score reduction greater than or equal to 10 mm [12].
Substantial response in PROs was defined as: (1) a decrease from baseline ≥ 50% in Pain VAS, (2) a normative score ≤ 0.5 in HAQ-DI, (3) a normative score ≥ 43.5 in FACIT-Fatigue, (4) an improvement from baseline ≥ 5 in SF-36 PCS (equivalent to MCID), and (5) a decrease from baseline ≥ 50% in PtGA [9, 18–20]. The time to substantial response was assessed for Pain VAS, HAQ-DI, FACIT-Fatigue, SF-36 PCS, and PtGA.
Statistical Analyses
These post hoc analyses used data from patients randomized to placebo and baricitinib 2-mg in RA-BUILD and RA-BEACON. Analyses included the proportion of patients achieving MCID for each PRO at weeks 4, 12, and 24, the number needed to treat (NNT) for PRO responders, the proportion of patients who maintained PRO improvements greater than or equal to MCID at week 24 (calculated among PRO responders at weeks 4 or 12), and median times to substantial response or achievement of normative score.
For the proportion of patients achieving MCID, p values for comparisons of baricitinib 2-mg versus placebo were determined for the difference in MCID response rates using the Newcombe–Wilson method, without continuity correction.
The NNT for PRO responders was calculated as the reciprocal of the difference in response rates, with missing data imputed by NRI.
For MCID maintenance comparisons of placebo and baricitinib 2-mg, p values were determined by Chi-squared tests without multiplicity adjustment. Patients with baseline PRO scores less than the MCID definitions for each measure were excluded from the PRO maintenance analyses, as the available PRO changes were lower than the MCID threshold. Missing data for the PRO maintenance analyses were imputed by non-responder imputation (NRI).
Median times to substantial response (in Pain VAS, HAQ-DI, FACIT-Fatigue, SF-36 PCS, and PtGA) were calculated from baseline to week 24 and assessed using a Kaplan–Meier estimator. Cox proportional hazard models were used to calculate the hazard ratios and p values for baricitinib 2-mg vs. placebo, without multiplicity adjustment. The model adjusted for geographical region, baseline joint erosion status (yes/no), and baseline PRO score.
Results
Baseline Characteristics
In RA-BUILD, 457 csDMARD-IR patients were randomized to placebo (N = 228) or baricitinib 2-mg (N = 229). In RA-BEACON, 350 bDMARD-IR patients were randomized to placebo (N = 176) or baricitinib 2-mg (N = 174). Baseline characteristics were similar between treatment groups in each trial (Table 1). Compared to patients enrolled in RA-BUILD (csDMARD-IR), patients enrolled in RA-BEACON (bDMARD-IR) were older, had longer duration of RA, had higher swollen joint count, higher high-sensitivity CRP levels, and had worse PRO baseline scores. Patients in both trials reported substantial disease burden as evaluated by the baseline PROs, which aligned with baseline clinical disease activity as measured by CRP levels and swollen joint count.
Table 1.
Baseline characteristics and PRO scores of patients enrolled in RA-BUILD (csDMARD-IR) and RA-BEACON (bDMARD-IR)
| RA-BUILD (csDMARD-IR) | RA-BEACON (bDMARD-IR) | |||
|---|---|---|---|---|
| Placebo (N = 228) | Baricitinib 2-mg (N = 229) | Placebo (N = 176) | Baricitinib 2-mg (N = 174) | |
| Age, years | 51.4 (12.5) | 52.2 (12.3) | 56.0 (10.7) | 55.1 (11.1) |
| Female, n (%) | 189 (82.9) | 184 (80.3) | 145 (82.4) | 137 (78.7) |
| Time since RA diagnosis, years | 5.9 (6.8) | 6.5 (7.6) | 12.8 (9.4) | 12.3 (7.5) |
| SJC, of 66 joints examined | 13.1 (7.2) | 13.6 (8.7) | 17.2 (10.8) | 18.6 (12.3) |
| High-sensitivity CRP | 17.7 (20.4) | 18.2 (21.5) | 20.6 (25.3) | 29.9 (22.5) |
| Pain VAS | 57.1 (23.1) | 59.5 (21.2) | 64.7 (19.3) | 62.4 (21.5) |
| HAQ-DI | 1.5 (0.6) | 1.5 (0.6) | 1.8 (0.6) | 1.7 (0.6) |
| FACIT-F | 26.6 (11.1) | 26.6 (11.5) | 22.2 (10.6) | 22.5 (10.0) |
| SF-36 PCS | 32.2 (8.5) | 32.5 (8.4) | 28.2 (7.7) | 28.7 (8.1) |
| PtGA | 60.4 (21.4) | 61.6 (20.2) | 66.1 (18.8) | 67.4 (19.3) |
Data are mean (SD), unless otherwise stated
BARI baricitinib, CRP C-reactive protein, bDMARD biologic disease-modifying anti-rheumatic drug, csDMARD conventional synthetic disease-modifying anti-rheumatic drug, FACIT-F Functional Assessment of Chronic Illness Therapy-Fatigue, HAQ-DI Health Assessment Questionnaire-Disability Index, IR inadequate responder, PBO placebo, PtGA Patient’s Global Assessment of Disease Activity, RA rheumatoid arthritis, SF-36 PCS Short Form-36 Physical Component Score, SJC swollen joint count, VAS visual analog scale
Achievement of MCID in PROs at Weeks 4, 12, and 24 and Associated NNTs
As reported previously, csDMARD-IR and bDMARD-IR patients enrolled in RA-BUILD and RA-BEACON, respectively, treated with baricitinib 2-mg had significantly greater improvements in PROs than those receiving placebo at week 12. [2, 3] The proportions of patients in each treatment group with MCID responses for PROs at weeks 4, 12, and 24 with associated NNTs are described in Table 2. Across both trials at week 12, the incremental NNT ranged from 4 to 7 for all PROs, with the exception of FACIT-Fatigue in RA-BUILD. At week 24, the incremental NNT ranged from 5 to 8 in all PROs across both trials.
Table 2.
Patients reporting PRO improvement greater than or equal to MCID at weeks 4, 12, and 24
| RA-BUILD (csDMARD-IR) | RA-BEACON (bDMARD-IR) | |||||
|---|---|---|---|---|---|---|
| PBO (N = 228), n (%) | BARI 2-mg (N = 229), n (%) | NNT, (95% CI) | PBO (N = 176), n (%) | BARI 2-mg (N = 174), n (%) | NNT, (95% CI) | |
| Week 4 | ||||||
| Pain VAS | 118 (51.8) | 146 (63.8)** | 8.3 (4.8, 33.2) | 79 (44.9) | 85 (48.9) | 25.2 (N/A) |
| HAQ-DI | 120 (52.6) | 141 (61.6) | 11.2 (N/A) | 76 (43.2) | 102 (58.6)** | 6.5 (3.9, 19.6) |
| FACIT-Fatigue | 109 (47.8) | 129 (56.3) | 11.7 (N/A) | 86 (48.9) | 104 (59.8)* | 9.2 (4.7, 187.9) |
| SF-36 PCS | 73 (32.0) | 104 (45.4)** | 7.5 (4.5, 22.0) | 52 (29.5) | 81 (46.6)** | 5.9 (3.7, 14.3) |
| PtGA | 124 (54.4) | 150 (65.5)* | 9.0 (5.0, 45.7) | 76 (43.2) | 97 (55.7)* | 8.0 (4.4, 46.0) |
| Week 12 | ||||||
| Pain VAS | 120 (52.6) | 163 (71.2)*** | 5.4 (3.7, 10.2) | 69 (39.2) | 95 (54.6)** | 6.5 (3.9, 19.8) |
| HAQ-DI | 124 (54.4) | 158 (69.0)** | 6.8 (4.3, 17.3) | 75 (42.6) | 102 (58.6)** | 6.2 (3.8, 17.6) |
| FACIT-Fatigue | 134 (58.8) | 145 (63.3) | 22.0 (N/A) | 85 (48.3) | 111 (63.8)** | 6.5 (3.9, 19.1) |
| SF-36 PCS | 92 (40.4) | 130 (56.8)*** | 6.1 (3.9, 13.6) | 56 (31.8) | 86 (49.4)*** | 5.7 (3.6, 13.4) |
| PtGA | 124 (54.4) | 160 (69.9)*** | 6.5 (4.1, 14.9) | 67 (38.1) | 111 (63.8)*** | 3.9 (2.8, 6.4) |
| Week 24 | ||||||
| Pain VAS | 99 (43.4) | 148 (64.6)*** | 4.7 (3.3, 8.1) | 55 (31.3) | 80 (46.0)** | 6.8 (4.0, 21.5) |
| HAQ-DI | 95 (41.7) | 147 (64.2)*** | 4.4 (3.2, 7.3) | 52 (29.5) | 87 (50.0)*** | 4.9 (3.3, 9.6) |
| FACIT-Fatigue | 97 (42.5) | 135 (59.0)*** | 6.1 (3.9, 13.6) | 66 (37.5) | 87 (50.0)* | 8.0 (4.4, 45.7) |
| SF-36 PCS | 77 (33.8) | 127 (55.5)*** | 4.6 (3.3, 7.8) | 37 (21.0) | 68 (39.1)*** | 5.5 (3.6, 11.6) |
| PtGA | 100 (43.9) | 147 (64.2)*** | 4.9 (3.4, 8.8) | 56 (31.8) | 88 (50.6)*** | 5.3 (3.5, 11.6) |
Missing data were imputed by NRI
NNT = 1/(probability of achieving MCID with BARI 2-mg—probability of achieving MCID with PBO). When the difference in response rates was not statistically significant between treatments, the CI for NNT was not derived
p values were calculated for the difference in MCID response rates using the Newcombe–Wilson method without continuity correction. *p < 0.05; **p < 0.01; ***p < 0.001
BARI baricitinib, bDMARD biologic disease-modifying anti-rheumatic drug, CI confidence interval, csDMARD conventional synthetic disease-modifying anti-rheumatic drug, FACIT-Fatigue Functional Assessment of Chronic Illness Therapy-Fatigue, HAQ-DI Health Assessment Questionnaire-Disability Index, IR inadequate responder, MCID minimally clinically important difference, N/A not applicable, NNT number needed to treat, PBO placebo, RA rheumatoid arthritis, PtGA Patient’s Global Assessment of Disease Activity, SF-36 PCS Short Form-36 Physical Component Score
Maintenance of PRO MCID Improvement
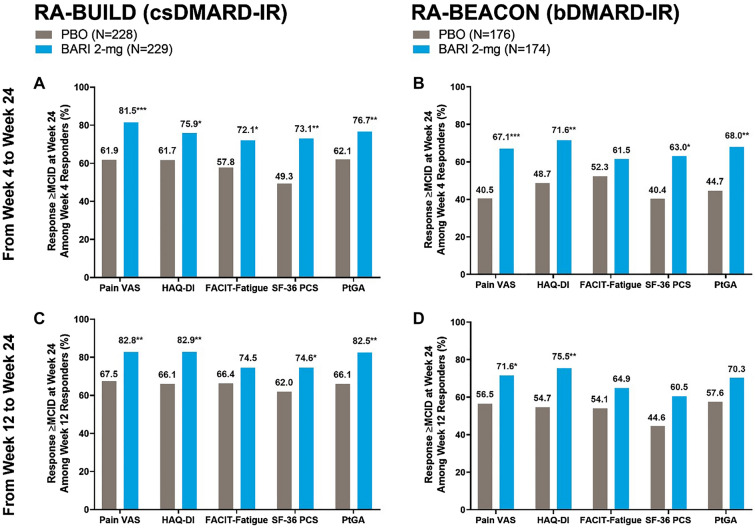
In both the RA-BUILD and RA-BEACON trials, significantly more patients treated with baricitinib 2-mg maintained MCID improvement in Pain VAS, HAQ-DI, SF-36 PCS, and PtGA at week 24 from week 4 responders than those receiving placebo (Fig. 1A, 1B). Significantly more baricitinib-treated patients maintained MCID improvement in Pain VAS, HAQ-DI, SF-36 PCS, and PtGA in RA-BUILD and Pain VAS and HAQ-DI in RA-BEACON at week 24 from week 12 responders compared to placebo (Fig. 1C, 1D). In RA-BUILD, significantly higher proportions of baricitinib-treated patients maintained improvement in FACIT-Fatigue from week 4 to 24 compared to placebo, and in RA-BEACON, numerically higher proportions of baricitinib-treated patients maintained MCID improvement in FACIT-Fatigue from week 4 to 24 compared to placebo. In RA-BUILD, numerically higher proportions of baricitinib-treated patients maintained MCID improvement in FACIT-Fatigue from week 12 to 24 than placebo, and in RA-BEACON, numerically higher proportions of baricitinib-treated patients maintained MCID improvement in FACIT-Fatigue, SF-36 PCS, and PtGA compared to placebo (Fig. 1).
Fig. 1.
Proportion of patients maintaining PRO improvements greater than or equal to MCID at week 24 among week 4 responders (A, B) and week 12 responders (C, D). N refers to the number of patients in each modified intent-to-treat arm. The percentages presented in Fig. 1 are fractions of the total number of MCID responders at weeks 4 (A, B) and 12 (C, D). For the number of MCID responders for each PRO at weeks 4 and 12, please see Table 2. p values were from Chi-square tests comparing the proportion of PRO MCID responders at week 24 between BARI 2-mg and PBO groups among those who responded at weeks 4 and 12; *p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001. BARI baricitinib, bDMARD biologic disease-modifying anti-rheumatic drug, csDMARD conventional synthetic disease-modifying anti-rheumatic drug, FACIT-Fatigue Functional Assessment of Chronic Illness Therapy-Fatigue, HAQ-DI Health Assessment Questionnaire-Disability Index, IR inadequate responder, MCID minimally clinically important difference, N number of patients in the treatment arm, PBO placebo, PtGA Patient’s Global Assessment of Disease Activity, RA rheumatoid arthritis, SF-36 PCS Short Form-36 Physical Component Score
Time to Substantial PRO Response
The median time to substantial PRO response was shorter for both csDMARD-IR and bDMARD-IR patients treated with baricitinib 2-mg compared to those receiving placebo (Table 3). Hazard ratios calculated for each PRO showed that patients treated with baricitinib 2-mg were overall more likely to achieve substantial response or normative value, except for FACIT-Fatigue in csDMARD-IR patients (p = 0.09) (Table 3).
Table 3.
Summary of median time to achieve substantial PRO responses and corresponding hazard ratios
| RA-BUILD (csDMARD-IR) | RA-BEACON (bDMARD-IR) | |||
|---|---|---|---|---|
| PBO (N = 228) | BARI 2-mg (N = 229) | PBO (N = 176) | BARI 2-mg (N = 174) | |
| Pain VAS | ||||
| Median time to response, weeks | 15.6 | 8.0 | N/Aa | 14.3 |
| Hazard ratio (95% CI) | – | 1.53 (1.21, 1.92)*** | – | 1.46 (1.08, 1.96)* |
| HAQ-DI | ||||
| Median time to response, weeks (SD) | N/A | N/Aa | N/Aa | N/Aa |
| Hazard ratio (95% CI) | – | 1.72 (1.27, 2.35)*** | – | 1.59 (1.03, 2.47)* |
| FACIT-Fatigue | ||||
| Median time to response, weeks (SD) | N/A | N/Aa | N/Aa | N/Aa |
| Hazard ratio (95% CI) | – | 1.29 (0.96, 1.74) | – | 1.75 (1.13, 2.70)* |
| SF-36 PCS | ||||
| Median time to response, weeks (SD) | 9.0 | 8.0 | 15.3 | 8.0 |
| Hazard ratio (95% CI) | – | 1.32 (1.08, 1.62)** | – | 1.69 (1.32, 2.17)*** |
| PtGA | ||||
| Median time to response, weeks (SD) | 14.0 | 8.0 | N/Aa | 14.0 |
| Hazard ratio (95% CI) | – | 1.38 (1.09, 1.73)** | – | 1.59 (1.18, 2.14)** |
Median time to response is defined as the time at which 50% of patients reached the response. This measure is not available if < 50% of patients reached the response at 24 weeks. Hazard ratio is defined as the ratio of the hazard rate (rate of reaching response) at any point in time
Hazard ratios and p values are from a Cox proportional hazards regression model adjusted for region, baseline joint erosion status (yes/no), and baseline score in RA-BUILD; region, history of bDMARD use (< 3, ≥ 3), and baseline score in RA-BEACON
For Pain VAS, response was defined as a change from baseline of ≥ 50%. For HAQ-DI, response was defined as a normative score ≤ 0.5. For FACIT-Fatigue, response was defined as a normative score of ≥ 43.5. For SF-36 PCS, response was defined as equivalent to the MCID, a score improvement ≥ 5 units. For PtGA, response was defined as a change from baseline ≥ 50%
BARI baricitinib, bDMARD biologic disease-modifying anti-rheumatic drug, CI confidence interval, csDMARD conventional synthetic disease-modifying anti-rheumatic drug, FACIT-Fatigue Functional Assessment of Chronic Illness Therapy-Fatigue, HAQ-DI Health Assessment Questionnaire-Disability Index, IR inadequate responder, MCID minimally clinically important difference, PBO placebo, PtGA Patient’s Global Assessment of Disease Activity, RA rheumatoid arthritis, SF-36 PCS Short Form-36 Physical Component Score
*p < 0.05; **p < 0.01; ***p < 0.001
aN/A: unavailable or unable to be estimated because the response was not reached within 24 weeks
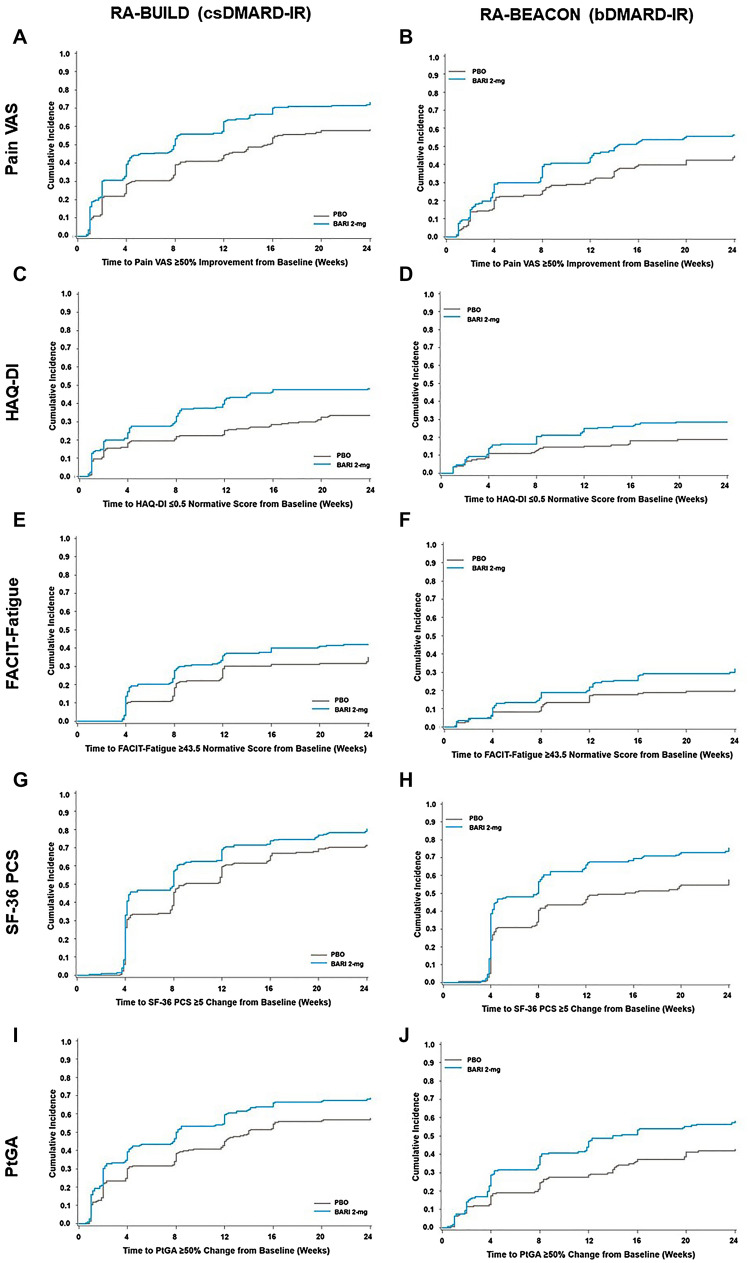
Patients treated with baricitinib 2-mg achieved substantial response or normative value more quickly than patients receiving placebo (Fig. 2).
Fig. 2.
Time to substantial PRO response. Cumulative incidence was estimated using a Kaplan–Meier approach. For Pain VAS, substantial response was defined as a change from baseline ≥ 50%. For HAQ-DI, substantial response was defined as a normative score ≤ 0.5. For FACIT-Fatigue, substantial response was defined as a normative score ≤ 43.5. For SF-36 PCS, substantial response was defined as equivalent to the MCID, a score improvement ≥ 5. For PtGA, substantial response was defined as a change from baseline ≥ 50%. BARI baricitinib, bDMARD biologic disease-modifying anti-rheumatic drug, csDMARD conventional synthetic disease-modifying anti-rheumatic drug, FACIT-Fatigue Functional Assessment of Chronic Illness Therapy-Fatigue, HAQ-DI Health Assessment Questionnaire-Disability Index, IR inadequate responder, MCID minimally clinically important difference, PBO placebo, PtGA Patient’s Global Assessment of Disease Activity, RA rheumatoid arthritis, SF-36 PCS Short Form-36 Physical Component Score
Discussion
The results as supported by previously published studies on the efficacy of baricitinib in RA suggest PRO improvements with baricitinib 2-mg are maintained over time [2, 3]. In addition, most PROs assessed at weeks 12 and 24 resulted in NNTs of less than 8 patients, which are generally considered favorable and demonstrate the value of baricitinib 2-mg treatment for both csDMARD-IR and bDMARD-IR patients with RA [21, 22]. Early MCID responses in pain, physical function, fatigue, HRQoL, and patient global assessment (as measured by Pain VAS, HAQ-DI, FACIT-Fatigue, SF-36 PCS, and PtGA, respectively) at weeks 4 and 12 were maintained up to week 24 in patients with active RA treated with baricitinib 2-mg. The largest differences in the proportions of placebo- and baricitinib-treated patients who maintained MCID PRO improvements were observed in pain and physical function. Lastly, time to achieve a PRO substantial response or normative score was shorter in patients treated with baricitinib 2-mg than patients receiving placebo over 24 weeks.
PROs are recognized to be important in evaluating treatment response alongside physician-reported measures [5, 8]. PRO measures have been validated in the context of RA and are similar in terms of reproducibility and sensitivity to change compared to physician-reported measures such as swollen and tender joint counts [8]. Given the importance of patient-centered care, the use of PROs to evaluate patients’ RA symptoms has become standard in randomized clinical trials and clinical settings [5, 8]. MCID values for each PROs, which are emphasized in the current analyses, allow for interpretation of the scores within the context of previously defined clinically meaningful improvement in changes from baseline over time and overall patient improvement [5]. The sensitivity of PRO measures and patients’ perceptions about their disease are particularly useful in helping physicians determine whether non-response to treatment is due to comorbidities or refractory disease [5].
RA-BUILD and RA-BEACON enrolled patients with inadequate responses or intolerance to csDMARDs, with a mean RA duration of 8 years, and bDMARDs, with a mean RA duration of 14 years, respectively; treatment with baricitinib led to improved outcomes regardless of previous csDMARD or bDMARD use [2, 3]. Previous studies have shown the long-term efficacy and safety of baricitinib 2-mg in RA [23–26].
Because RA treatment guidelines recommend shared decision-making that includes patient preferences, meeting these preferences is a significant part of achieving treatment satisfaction [1, 25]. Treatment satisfaction is an important consideration as it influences treatment adherence and continuation [26]. Patients expect sustained improvement in RA and its symptoms [26]. Improvement of the PROs pain, physical function, and fatigue have been associated with greater patient satisfaction and perception of RA remission [25, 27].
One of this study’s strengths is its comprehensive assessment on outcomes important to patients, conducted across csDMARD-IR and bDMARD-IR patients. Limitations of this study include the inability to separate confounding factors that can affect PROs, such as comorbidities. For instance, fatigue (measured here by FACIT-Fatigue) has a complex relationship with disease activity, as physical function and pain related to RA can cause fatigue along with psychological and behavioral factors that may or may not be related to RA [8]. Additionally, these findings may not be generalizable to real-world patients with RA, as patients were required to have moderate-to-severe active RA and meet other inclusion criteria for the RA-BUILD and RA-BEACON trials. These analyses were also limited as PRO maintenance was evaluated from weeks 4 or 12 to week 24 (trial period), which is a relatively short time in the context of RA, a chronic disease. These analyses also applied NRI for missing data, which may underestimate the true response rate.
Overall, in this study of csDMARD-IR and bDMARD-IR patients, early MCID in pain, physical function, fatigue, HRQoL, and patient global assessment at weeks 4 and 12 continued to week 24 in patients with active RA treated with baricitinib 2-mg. Additionally, the time to achieve a PRO substantial response or normative score was shorter in patients treated with baricitinib 2-mg than patients receiving placebo over 24 weeks. Together, these results suggest that treatment with baricitinib 2-mg allows patients to maintain quickly achieved PRO improvements over time.
Acknowledgements
Funding
Studies and the Journal’s Rapid Service Fee were sponsored by Eli Lilly and Company. The studies were sponsored by Eli Lilly and Company, under license from Incyte Corporation. The authors received no financial support for the research, authorship, and/or publication of this article.
Medical Writing, Editorial, and Other Assistance
The authors would like to thank Nicole Lipitz, Syneos Health, for medical writing support, funded by Eli Lilly and Company.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Author Contributions
DS contributed to acquisition of data for the work and critical revision of the manuscript for important intellectual content. JW contributed to conception of the work, design of the work, interpretation of data for the work, and critical revision of the manuscript for important intellectual content. BJ contributed to acquisition of data for the work, analysis of data for the work, interpretation of data for the work, and critical revision of the manuscript for important intellectual content. HZ contributed to analysis of data for the work and critical revision of the manuscript for important intellectual content. KG, JB, and HL contributed to interpretation of data for the work and critical revision of the manuscript for important intellectual content. PJSR contributed to analysis of data for the work, interpretation of data for the work, and critical revision of the manuscript for important intellectual content. COBIII contributed to conception of the work, analysis of data for the work, interpretation of data for the work, and critical revision of the manuscript for important intellectual content.
Disclosures
Dalton Sholter has been a consultant for and/or has received research support from: AbbVie, Amgen, Bristol Myers Squibb, Eli Lilly and Company, Gilead Sciences, Janssen, Merck, Pfizer, Roche, Sanofi Genzyme, and UCB Pharma; Jianmin Wu, Bochao Jia, Kirstin Griffing, Julie Birt, and Paulo Jorge Simoes Reis are current employees and shareholders of: Eli Lilly and Company; Hong Zhang is a consultant from: TechData Service; Huaxiang Liu has no conflicts of interest to report; Clifton O. Bingham III has been a consultant for and/or has received research support from: AbbVie, Bristol Myers Squibb, Eli Lilly and Company, Gilead Sciences, Janssen, Pfizer, Regeneron, and Sanofi.
Prior Presentation
Preliminary results of this analysis were presented at the Canadian Rheumatology Association 2021 Annual Scientific Meeting.
Compliance with Ethics Guidelines
RA-BUILD and RA-BEACON were conducted in accordance with the principles of the Declaration of Helsinki and Good Clinical Practice Guidelines and were approved by each center’s institutional review board (IRB): Quorum Review IRB, #27258 and #27259. Written informed consent was provided by all patients.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
- 1.Singh JA, Saag KG, Bridges SL, Jr, Akl EA, Bannuru RR, Sullivan MC, et al. 2015 American College of Rheumatology Guideline for the Treatment of Rheumatoid Arthritis. Arthritis Care Res. 2016;68(1):1–25. doi: 10.1002/acr.22783. [DOI] [PubMed] [Google Scholar]
- 2.Dougados M, van der Heijde D, Chen YC, Greenwald M, Drescher E, Liu J, et al. Baricitinib in patients with inadequate response or intolerance to conventional synthetic DMARDs: results from the RA-BUILD study. Ann Rheum Dis. 2017;76(1):88–95. doi: 10.1136/annrheumdis-2016-210094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Genovese MC, Kremer J, Zamani O, Ludivico C, Krogulec M, Xie L, et al. Baricitinib in patients with refractory rheumatoid arthritis. N Engl J Med. 2016;374(13):1243–1252. doi: 10.1056/NEJMoa1507247. [DOI] [PubMed] [Google Scholar]
- 4.Strand V, Khanna D. The impact of rheumatoid arthritis and treatment on patients' lives. Clin Exp Rheumatol. 2010;28(3 Suppl 59):S32–40. [PubMed] [Google Scholar]
- 5.Orbai AM, Bingham CO., 3rd Patient reported outcomes in rheumatoid arthritis clinical trials. Curr Rheumatol Rep. 2015;17(4):28. doi: 10.1007/s11926-015-0501-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bingham CO, 3rd, Gaich CL, DeLozier AM, Engstrom KD, Naegeli AN, de Bono S, et al. Use of daily electronic patient-reported outcome (PRO) diaries in randomized controlled trials for rheumatoid arthritis: rationale and implementation. Trials. 2019;20(1):182. doi: 10.1186/s13063-019-3272-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Emery P, Blanco R, Maldonado Cocco J, Chen YC, Gaich CL, DeLozier AM, et al. Patient-reported outcomes from a phase III study of baricitinib in patients with conventional synthetic DMARD-refractory rheumatoid arthritis. RMD Open. 2017;3(1):e000410. doi: 10.1136/rmdopen-2016-000410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gossec L, Dougados M, Dixon W. Patient-reported outcomes as end points in clinical trials in rheumatoid arthritis. RMD Open. 2015;1(1):e000019. doi: 10.1136/rmdopen-2014-000019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Smolen JS, Kremer JM, Gaich CL, DeLozier AM, Schlichting DE, Xie L, et al. Patient-reported outcomes from a randomised phase III study of baricitinib in patients with rheumatoid arthritis and an inadequate response to biological agents (RA-BEACON) Ann Rheum Dis. 2017;76(4):694–700. doi: 10.1136/annrheumdis-2016-209821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fraenkel L, Bathon JM, England BR, St Clair EW, Arayssi T, Carandang K, et al. 2021 American College of Rheumatology Guideline for the Treatment of Rheumatoid Arthritis. Arthritis care & research. 2021. [DOI] [PMC free article] [PubMed]
- 11.Company ELa. Olumiant (baricitinib) [package insert]. U.S. Food and Drug Administration website2018.
- 12.Strand V, Pope J, Tundia N, Friedman A, Camp HS, Pangan A, et al. Upadacitinib improves patient-reported outcomes in patients with rheumatoid arthritis and inadequate response to conventional synthetic disease-modifying antirheumatic drugs: results from SELECT-NEXT. Arthritis Res Ther. 2019;21(1):272. doi: 10.1186/s13075-019-2037-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wells GA, Tugwell P, Kraag GR, Baker PR, Groh J, Redelmeier DA. Minimum important difference between patients with rheumatoid arthritis: the patient's perspective. J Rheumatol. 1993;20(3):557–560. [PubMed] [Google Scholar]
- 14.Cella D, Yount S, Sorensen M, Chartash E, Sengupta N, Grober J. Validation of the Functional Assessment of Chronic Illness Therapy Fatigue Scale relative to other instrumentation in patients with rheumatoid arthritis. J Rheumatol. 2005;32(5):811–819. [PubMed] [Google Scholar]
- 15.Keystone E, Burmester GR, Furie R, Loveless JE, Emery P, Kremer J, et al. Improvement in patient-reported outcomes in a rituximab trial in patients with severe rheumatoid arthritis refractory to anti-tumor necrosis factor therapy. Arthritis Rheum. 2008;59(6):785–793. doi: 10.1002/art.23715. [DOI] [PubMed] [Google Scholar]
- 16.Strand V, Singh JA. Newer biological agents in rheumatoid arthritis: impact on health-related quality of life and productivity. Drugs. 2010;70(2):121–145. doi: 10.2165/11531980-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 17.Kosinski M, Zhao SZ, Dedhiya S, Osterhaus JT, Ware JE., Jr Determining minimally important changes in generic and disease-specific health-related quality of life questionnaires in clinical trials of rheumatoid arthritis. Arthritis Rheum. 2000;43(7):1478–1487. doi: 10.1002/1529-0131(200007)43:7<1478::AID-ANR10>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 18.Strand V, Kaine J, Alten R, Wallenstein G, Diehl A, Shi H, et al. Associations between Patient Global Assessment scores and pain, physical function, and fatigue in rheumatoid arthritis: a post hoc analysis of data from phase 3 trials of tofacitinib. Arthritis Res Ther. 2020;22(1):243. doi: 10.1186/s13075-020-02324-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dworkin RH, Turk DC, Wyrwich KW, Beaton D, Cleeland CS, Farrar JT, et al. Interpreting the clinical importance of treatment outcomes in chronic pain clinical trials: IMMPACT recommendations. J Pain. 2008;9(2):105–121. doi: 10.1016/j.jpain.2007.09.005. [DOI] [PubMed] [Google Scholar]
- 20.Strand V, Lee EB, Yazici Y, Dikranian A, Wilkinson B, Takiya L, et al. Evaluation of disease activity in patients with rheumatoid arthritis treated with tofacitinib by RAPID3: post hoc analyses from two phase 3 trials. Clin Rheumatol. 2018;37(8):2043–2053. doi: 10.1007/s10067-018-4077-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Laupacis A, Sackett DL, Roberts RS. An assessment of clinically useful measures of the consequences of treatment. N Engl J Med. 1988;318(26):1728–1733. doi: 10.1056/NEJM198806303182605. [DOI] [PubMed] [Google Scholar]
- 22.Siwek J, Newman DH. Introducing medicine by the numbers: a collaboration of the NNT group and AFP. Am Fam Physician. 2015;91(7):434–435. [PubMed] [Google Scholar]
- 23.Genovese MC, Smolen JS, Takeuchi T, Burmester G, Brinker D, Rooney TP, et al. Safety profile of baricitinib for the treatment of rheumatoid arthritis over a median of 3 years of treatment: an updated integrated safety analysis. Lancet Rheumatol. 2020;2(6):e347–e357. doi: 10.1016/S2665-9913(20)30032-1. [DOI] [PubMed] [Google Scholar]
- 24.Wells AF, Jia B, Xie L, Valenzuela GJ, Keystone EC, Li Z, et al. Efficacy of long-term treatment with once-daily baricitinib 2 mg in patients with active rheumatoid arthritis: post hoc analysis of two 24-week, phase III, randomized, controlled studies and one long-term extension study. Rheumatol Therapy. 2021;8(2):987–1001. doi: 10.1007/s40744-021-00317-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schäfer M, Albrecht K, Kekow J, Rockwitz K, Liebhaber A, Zink A, et al. Factors associated with treatment satisfaction in patients with rheumatoid arthritis: data from the biological register RABBIT. RMD Open. 2020;6(3):e001290. doi: 10.1136/rmdopen-2020-001290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Taylor PC, Ancuta C, Nagy O, de la Vega MC, Gordeev A, Janková R, et al. Treatment satisfaction, patient preferences, and the impact of suboptimal disease control in a large international rheumatoid arthritis cohort: SENSE study. Patient Prefer Adherence. 2021;15:359–373. doi: 10.2147/PPA.S289692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.van Tuyl LH, Sadlonova M, Hewlett S, Davis B, Flurey C, Goel N, et al. The patient perspective on absence of disease activity in rheumatoid arthritis: a survey to identify key domains of patient-perceived remission. Ann Rheum Dis. 2017;76(5):855–861. doi: 10.1136/annrheumdis-2016-209835. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.