Abstract
Purpose
To assess the role of ultrasound (US) in detecting and characterizing ductal carcinoma in situ (DCIS) of the breast and to investigate the correlation between ultrasonographic and biological features of DCIS.
Methods
In total, 171 patients (mean age 44; range 39–62) with 178 lesions were retrospectively evaluated by two independent radiologists searching for US mass or non-mass lesions. Immunohistochemistry analysis was performed to determine estrogen receptor (ER), progesterone receptor (PR), and human epidermal growth factor receptor 2 (HER2) expression. The US detection rate and pattern distribution among the lesion types were evaluated. The χ2 test was used to evaluate the correlation between the US findings and the biological factors. Statistical significance was indicated by p values < 0.05. Inter-observer agreement was calculated by Kohen’s k test.
Results
US detected 35% (63/178) of all lesions. Fifty-two (83%) lesions were classified as mass lesions, and 11 (17%) as non-mass lesions (p < 0.0001). Among the mass lesions, the most common shape was irregular (79%; p < 0.0001), with 45 (87%) lesions having indistinct margins. Hypoechogenicity was the most common echo pattern (49 cases, 94%; p < 0.0001). Microcalcifications were found in 23 cases (37%; p = 0.004) and were associated with mass lesions in 15 cases (65%) and with non-mass lesions in 8 cases (35%) (p = 0.21). An almost perfect inter-observer agreement (k = 0.87) was obtained between the two radiologists. A significant ER expression was found in mass lesions (83%; p < 0.0001), with no significant PR (p = 0.89) or HER2 expression (p = 0.81). Among the lesions with microcalcifications, only 7 out of 23 cases (30%) were positive for HER2 (p = 0.09).
Conclusion
DCIS represents a heterogeneous pathological process with variable US appearance (mass-like, non-mass-like, or occult). The most common US finding is represented by mass-type, hypoechogenic lesions with indistinct margins. A significant ER expression exists among mass-type lesions, while microcalcifications seem not to be associated with HER2 expression.
Keywords: Breast cancer, DCIS, US, Ultrasound
Introduction
Ductal carcinoma in situ (DCIS) of the breast represents a neoplastic proliferation of epithelial cells of the ducto-lobular unit not exceeding the basement membrane. DCIS is considered to be a true (non-obligatory) precursor of invasive breast cancer [1–3]. It is classified according to the architectural model (solid, cribriform, papillary, micropapillary, and comedogenic type), tumor grading (high, intermediate, and low), and the presence or absence of necrosis. The advent of mammography screening has led to a dramatic increase in the diagnosis of early-stage breast cancer—including DCIS, going up from 5 to 25%—and its incidence is increasing in young women [4].
The most typical finding of DCIS is the presence of clusters of microcalcifications in asymptomatic patients (50–75% of cases) [4–7]. Other manifestations include a soft-tissue opacity either with or without associated calcifications, areas of architectural distortions, and focal asymmetry. Ultrasound (US) has been shown to be helpful in detecting DCIS in patients with dense breasts and in DCIS presenting without microcalcifications [8]. Technological advances in US have improved the ability not only to characterize mammographic lesions and asymmetries but also to detect calcifications, visible as intralesional or intraductal hyperechoic foci.
Although it is impossible to predict whether DCIS will progress to invasive disease, biological markers, such as the estrogen receptor (ER), the progesterone receptor (PR), the human epidermal growth factor receptor 2 (HER2), and the Ki-67 index, can provide useful information for predicting the biological response, pathologic progression, treatment response, and tumor recurrence in patients with DCIS [9–12].
The purpose of this study is to evaluate the role of US in detecting and characterizing breast DCIS and to investigate the correlation between ultrasonographic and biological features of DCIS.
Methods
Patients
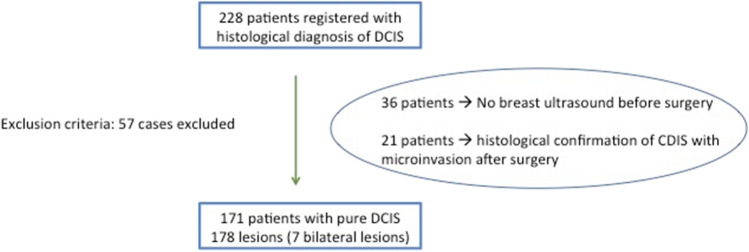
From December 2015 to November 2019, the US features of all patients registered in our Breast Unit database with histological diagnosis of pure DCIS were retrospectively evaluated. Thirty-six patients who did not undergo ultrasonography before surgical excision and 21 patients who had a histological confirmation of DCIS with micro-invasion after surgery were excluded. The final population included 171 patients (mean age 44; range 39–62) with 178 lesions (7 patients with bilateral lesions) (Fig. 1).
Fig. 1.
Inclusion and exclusion criteria. DCIS ductal carcinoma in situ
Ultrasonographic examinations
The US investigation was performed on both breasts by two breast radiologists with 10 years of experience in breast imaging, using a broadband 5–12-MHz linear array transducer (MyLab7, Esaote). A radial US technique from the periphery to the areolar region was used for both breasts. For each identified lesion, images were obtained on the axial and vertical planes. The US findings were described according to the lexicon of the American College of Radiology Breast Imaging Reporting and Data System (BI-RADS) [13]. In addition, to facilitate the description of the findings, the lesion pattern was classified as a mass, a non-mass, or an ultrasonographic occult lesion according to the guidelines of the Japan Society of Ultrasonics in Medicine [14]. Mass lesions consisted of well-defined and distinct lesions that stood out from the surrounding tissues, non-mass lesions were defined as space-occupying lesions (visible in two different US views) with no shape and well-defined margins (such as ductal changes and architectural distortions), and occult lesions were defined as lesions that were identified by mammography but that were not clearly recognizable on the US images. Mass lesions were analyzed in terms of size, shape, margin, echo pattern, and orientation. The presence of associated microcalcifications with mass or non-mass lesions was also assessed.
Histologic analysis
The following biological markers were examined by immunohistochemistry analysis as part of the routine pathologic assessment: ER, PR, and HER2 expression. ER and PR expression were considered positive if > 1% [1]. HER2 expression was scored as 0 (no staining), 1 + (weak and incomplete membrane staining), 2 + (strong, complete membrane staining in ≤ 30% of tumor cells or weak/moderate heterogeneous complete staining in ≥ 10% of tumor cells), or 3 + (strong, complete membrane staining in > 30% of tumor cells). When the score was 2 +, we used silver in situ hybridization, and the staining was considered positive if the ratio of HER2 gene copies to chromosome 17 signals was > 2.
Statistical analysis
The US detection rate and pattern distribution among the lesion types were evaluated. Statistical analysis was performed by comparing mass-type and non-mass-type lesions. The χ2 test was used to evaluate the correlation between the US findings and the biological factors. Statistical significance was indicated by p values < 0.05. Inter-observer agreement was calculated by Kohen’s k test. All calculations were performed using NCSS2007® statistical software.
Results
In total, 115 out of 178 (65%) lesions were defined as US occult with a US detection rate of 35%. Of the remaining 63 lesions detected by US, 52 (83%) were classified as mass lesions (Figs. 2 and 3) and 11 (17%) as non-mass lesions (6 with architectural distortions, 5 with ductal distortions) (p < 0.0001; Fig. 4). In 10 patients (6%), breast lesions were identified only by US (Table 1). Regarding mass lesions, the most common shape was irregular (41 cases, 79%; p < 0.0001). In addition, 7 cases (13%) were round, and 4 cases were oval (8%). Forty-five cases (87%) showed an indistinct margin, while only 7 cases (13%) showed circumscribed margins (p < 0.0001). Hypoechogenicity was the most common echo pattern (49 cases, 94%; p < 0.0001). The orientation of the mass was non-parallel in 31 cases (60%) and parallel in 21 cases (40%) (p = 0.21). Microcalcifications were found in 23 cases (37%; p = 0.004), and they were associated with mass lesions in 15 cases (65%) and with non-mass lesions in 8 cases (35%) (p = 0.21). An almost perfect inter-observer agreement (k = 0.87) was obtained between the two radiologists.
Fig. 2.
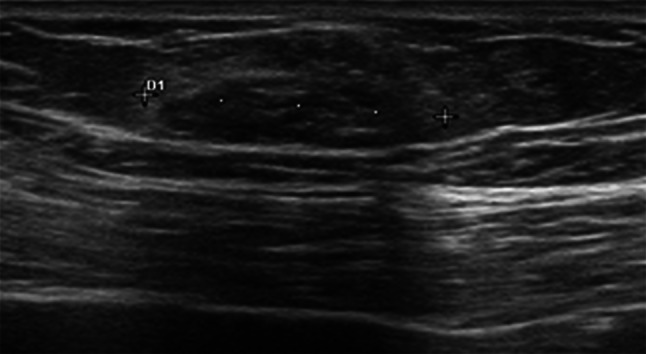
DCIS in a 46-year-old woman classified as a mass-like hypoechoic lesion with an oval shape and indistinct margins
Fig. 3.
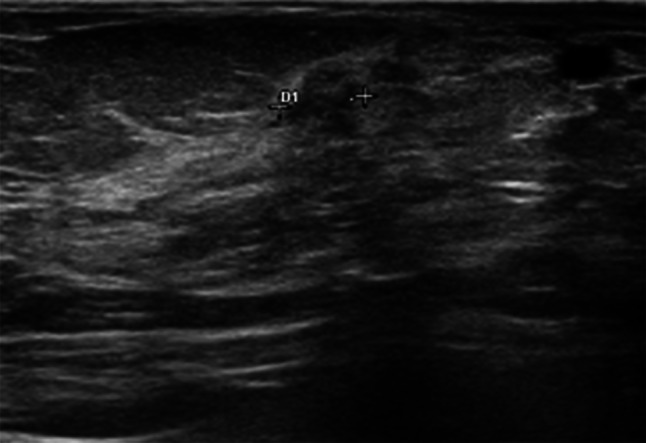
DCIS in a 52-year-old woman classified as a mass-like hypoechoic lesion with irregular margins
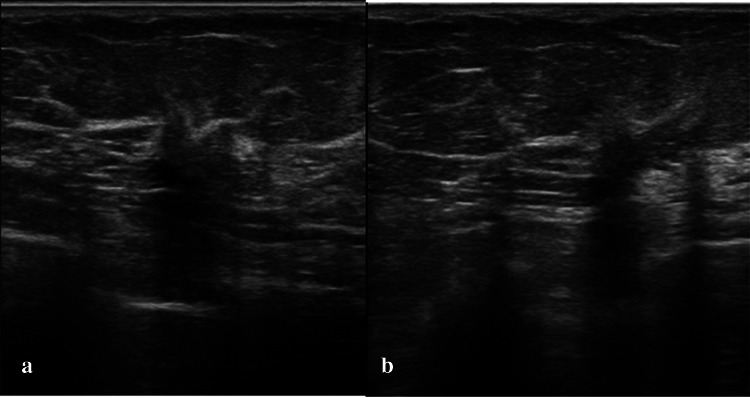
Fig. 4.
DCIS in a 49-year-old woman classified as a non-mass-like hypoechoic lesion with architectural distortion. a Axial plane. b Vertical plane
Table 1.
Ultrasonographic features of ductal carcinoma in situ (DCIS) lesions
| DCIS ultrasonographic features | Number of lesions (n = 178) |
|---|---|
| Lesion patterns | |
| Occult | 65% (115/178) |
| Non-occult | 35% (63/178) |
| Mass | 83% (52/63) |
| Non-mass lesions | 17%(11/63) |
| Microcalcifications | 37% (23/63) |
| Mass like | 29% (15/52) |
| Non-mass like | 72% (8/11) |
ER and PR expression showed a statistically significant difference (p < 0.0001) between mass-type and non-mass-type lesions: 43 out of 52 (83%) mass-type lesions expressed these biological markers.
On the other hand, there was no statistically significant difference between the two lesions types regarding PR expression (37 out of 52 [71%] mass-type lesions, 7 out of 11 [63%] non-mass-type lesions; p = 0.89) and HER2 expression (19 out of 52 [36%] mass-type lesions, 3 out of 11 [27%] non-mass-type lesions; p = 0.81).
Among the lesions with microcalcifications, only 7 of 23 cases (30%) were positive for HER2 expression (p = 0.09).
Discussion
The US features of DCIS have previously been described in the medical literature [15–19].
Scoggins et al. [8] evaluated the US appearance of 691 cases of pure DCIS and found that the most common sonographic appearance of DCIS was an irregular hypoechoic mass with indistinct margins and normal posterior features. Similarly, other studies on the US appearance of DCIS have shown that most cases of DCIS appear as a mass with indistinct margins [20–26]. Shin et al. compared the sonographic features of screening-detected and symptomatic DCIS; overall, the most common morphological lesion type was the mass type (84%) with irregular morphology and indistinct margins [18].
Regarding the comparison between symptomatic and screening patients, masses and associated ductal changes were more common in symptomatic patients, whereas associated microcalcifications and posterior shadowing were mostly found in screening-detected DCIS. According to our results, the most common ultrasonographic appearance of pure DCIS was a mass-type lesion (83%, against 17% non-mass-type lesions), which is concordant with the results of prior studies. Among the mass lesions, the most common features were irregular shape (79%), indistinct margins (87%), and hypoechogenicity (94%).
In our study, we found that 17% of all lesions were non-mass like (duct changes and architectural distortions). Although these sonographic findings are suspicious, they may be subtle and overlap with the findings of both benign and malignant entities [24–26]. Regarding microcalcifications, they were visible in 29% of mass lesions, similar to what is reported by Cha et al. [26], and in 72% of non-mass lesions. In our study, we found a US recognition rate of 35%, which is lower than that reported in other studies [16–18]. It could be related to the studies’ different percentages of symptomatic patients; indeed, our study included only asymptomatic patients, while the percentages of symptomatic women reported in the studies by Scoggins et al. [8] and Park et al. [16] were 23% and 33%, respectively.
In our study, we evaluated the presence of statistically significant differences in the expression of biological markers (ER, PR, and HER2 positivity), which represent important prognostic factors, between mass- and non-mass-type lesions. The only statistically significant difference was found in ER expression.
Moreover, unlike what was reported in the study by Cha et al. [26], in which microcalcifications found on ultrasonography scans showed a statistically significant correlation with HER2 positivity, we did not find such an association in our results.
Finally, our study confirms the role of US in breast imaging for characterizing breast lesions regardless of the age of patients, and more studies are needed to further investigate the potential role of US elastography in the field of DCIS diagnosis [27–30].
This study has several limitations. First, our study was limited by its retrospective nature. Second, there was likely a selection bias because not all patients with DCIS lesions underwent preoperative whole-breast sonography. Third, unlike other studies, our study did not assess the Ki-67 index.
In conclusion, DCIS represents a heterogeneous pathological process that can have a variable US appearance (mass-like, non-mass-like, or occult). The most common US finding is represented by mass-type, hypoechogenic lesions with indistinct margins. A significant ER expression exists among mass-type lesions, while microcalcifications seem not to be associated with HER2 expression.
Funding
Nothing to declare.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Allred DC. Ductal carcinoma in situ: terminology, classification, and natural history. J Natl Cancer Inst Monogr. 2010;2010:134–138. doi: 10.1093/jncimonographs/lgq035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Burstein HJ, Polyak K, Wong JS, Lester SC, Kaelin CM. Ductal carcinoma in situ of the breast. N Engl J Med. 2004;350:1430–1441. doi: 10.1056/NEJMra031301. [DOI] [PubMed] [Google Scholar]
- 3.Erbas B, Provenzano E, Armes J, Gertig D. The natural history of ductal carcinoma in situ of the breast: a review. Breast Cancer Res Treat. 2006;97:135–144. doi: 10.1007/s10549-005-9101-z. [DOI] [PubMed] [Google Scholar]
- 4.Barreau B, de Mascarel I, Feuga C, et al. Mammography of ductal carcinoma in situ of the breast: review of 909 cases with radiographic–pathologic correlations. Eur J Radiol. 2005;54:55–61. doi: 10.1016/j.ejrad.2004.11.019. [DOI] [PubMed] [Google Scholar]
- 5.Houssami N, Ciatto S, Irwig L, et al. The comparative sensitivity of mammography and ultrasound in women with breast symptoms: an age-specific analysis. Breast. 2002;11:125–130. doi: 10.1054/brst.2001.0391. [DOI] [PubMed] [Google Scholar]
- 6.Dershaw DD, Abramson A, Kinne DW. Ductal carcinoma in situ: mammographic findings and clinical implications. Radiology. 1989;170:411–415. doi: 10.1148/radiology.170.2.2536185. [DOI] [PubMed] [Google Scholar]
- 7.Evans A, Pinder S, Wilson R, Sibbering M, Poller D, Elston C, et al. Ductal carcinoma in situ of the breast: correlation between mammographic and pathologic findings. Am J Roentgenol. 1994;162:1307–1311. doi: 10.2214/ajr.162.6.8191988. [DOI] [PubMed] [Google Scholar]
- 8.Scoggins ME, Fox PS, Kuerer HM, Rauch GM, Benveniste AP, Park YM, et al. Correlation between sonographic findings and clinicopathologic and biologic features of pure ductal carcinoma in situ in 691 patients. Am J Roentgenol. 2015;204:878–888. doi: 10.2214/AJR.13.12221. [DOI] [PubMed] [Google Scholar]
- 9.Lari SA, Kuerer HM. Biological markers in DCIS and risk of breast recurrence: a systematic review. J Cancer. 2011;2:232–261. doi: 10.7150/jca.2.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Allred DC, Anderson SJ, Paik S, Wickerham DL, Nagtegaal ID, Swain SM, et al. Adjuvant tamoxifen reduces subsequent breast cancer in women with estrogen receptor-positive ductal carcinoma in situ: a study based on NSABP protocol B-24. J Clin Oncol. 2012;30:1268–1273. doi: 10.1200/JCO.2010.34.0141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Davis JE, Nemesure B, Mehmood S, Nayi V, Burke S, Brzostek SR, et al. Her2 and Ki67 biomarkers predict recurrence of ductal carcinoma in situ. ApplImmunohistochemMolMorphol. 2016;24:20–25. doi: 10.1097/PAI.0000000000000223. [DOI] [PubMed] [Google Scholar]
- 12.Irshad A, Leddy R, Pisano E, Baker N, Lewis M, Ackerman S, et al. Assessing the role of ultrasound in predicting the biological behavior of breast cancer. Am J Roentgenol. 2013;200:284–290. doi: 10.2214/AJR.12.8781. [DOI] [PubMed] [Google Scholar]
- 13.D'Orsi CJ, Sickles EA, Mendelson EB, Morris EA, et al. ACR BI-RADS® Atlas, breast imaging reporting and data system. Reston: American College of Radiology; 2013. [Google Scholar]
- 14.Ueno E, Endo T, Kubota M, Kawauchi A, Kato Y, Konishi Y, et al. Draft JSUM diagnostic guidelines for mass image-forming lesions. In: Ueno E, Shiina T, Kubota M, Sawai K, et al., editors. Research and development in breast ultrasound. Tokyo: Springer; 2005. pp. 76–88. [Google Scholar]
- 15.Hammond ME, Hayes DF, Wolff AC, Mangu PB, Temin S. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer. J Oncol Pract. 2010;6:195–197. doi: 10.1200/JOP.777003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Park JS, Park YM, Kim EK, Kim SJ, Han SS, Lee SJ, et al. Sonographic findings of high-grade and non-high-grade ductal carcinoma in situ of the breast. J Ultrasound Med. 2010;29:1687–1697. doi: 10.7863/jum.2010.29.12.1687. [DOI] [PubMed] [Google Scholar]
- 17.Wang LC, Sullivan M, Du H, Feldman MI, Mendelson EB. US appearance of ductal carcinoma in situ. Radiographics. 2013;33:213–228. doi: 10.1148/rg.331125092. [DOI] [PubMed] [Google Scholar]
- 18.Shin HJ, Kim HH, Kim SM, Kwon GY, Gong G, Cho OK. Screening detected and symptomatic ductal carcinoma in situ: differences in the sonographic and pathologic features. Am J Roentgenol. 2008;190:516–525. doi: 10.2214/AJR.07.2206. [DOI] [PubMed] [Google Scholar]
- 19.Yang WT, Tse GM. Sonographic, mammographic, and histopathologic correlation of symptomatic ductal carcinoma in situ. AJR. 2004;182:101–110. doi: 10.2214/ajr.182.1.1820101. [DOI] [PubMed] [Google Scholar]
- 20.Cho KR, Seo BK, Kim CH, et al. Noncalcified ductal carcinoma in situ: ultrasound and mammographic findings correlated with histological findings. Yonsei Med J. 2008;49:103–110. doi: 10.3349/ymj.2008.49.1.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Izumori A, Takebe K, Sato A. Ultrasound findings and histological features of ductal carcinoma in situ detected by ultrasound examination alone. Breast Cancer. 2010;17:136–141. doi: 10.1007/s12282-009-0134-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moon WK, Myung JS, Lee YJ, Park IA, Noh DY, Im JG. US of ductal carcinoma in situ. RadioGraphics. 2002;22:269–280. doi: 10.1148/radiographics.22.2.g02mr16269. [DOI] [PubMed] [Google Scholar]
- 23.Moon WK, Im JG, Koh YH, Noh DY, Park IA. US of mammographically detected clustered microcalcifications. Radiology. 2000;217:849–854. doi: 10.1148/radiology.217.3.r00nv27849. [DOI] [PubMed] [Google Scholar]
- 24.Rizzatto G, Chersevani R, Abbona M, Lombardo VL, Macorig D. High-resolution sonography of breast carcinoma. Eur J Radiol. 1997;24:11–19. doi: 10.1016/S0720-048X(96)01112-6. [DOI] [PubMed] [Google Scholar]
- 25.Stavros AT, Thickman D, Rapp CL, Dennis MA, Parker SH, Sisney GA. Solid breast nodules: use of sonography to distinguish between benign and malignant lesions. Radiology. 1995;196:123–134. doi: 10.1148/radiology.196.1.7784555. [DOI] [PubMed] [Google Scholar]
- 26.Cha H, Chang YW, Lee EJ, et al. Ultrasonographic features of pure ductal carcinoma in situ of the breast: correlations with pathologic features and biological markers. Ultrasonography. 2018;37(4):307–314. doi: 10.14366/usg.17039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Capasso R, Mamone R, Rossi E, Zeccolini M, Rotondo A. Breast intraductal papilloma as cause of bloody nipple discharge in a 2-year-old girl. Radiography. 2015;21(1):93–95. doi: 10.1016/j.radi.2014.11.001. [DOI] [Google Scholar]
- 28.Vestito A, Mangieri FF, Gatta G, Moschetta M, Turi B, Ancona A. Breast carcinoma in elderly women. Our experience. G Chir. 2011;32(10):411–416. [PubMed] [Google Scholar]
- 29.Elia D, Fresilli D, Pacini P, et al. Can strain US-elastography with strain ratio (SRE) improve the diagnostic accuracy in the assessment of breast lesions? Preliminary results. J Ultrasound. 2020 doi: 10.1007/s40477-020-00505-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pesce K, Binder F, Chico MJ, et al. Diagnostic performance of shear wave elastography in discriminating malignant and benign breast lesions. J Ultrasound. 2020 doi: 10.1007/s40477-020-00481-8. [DOI] [PMC free article] [PubMed] [Google Scholar]