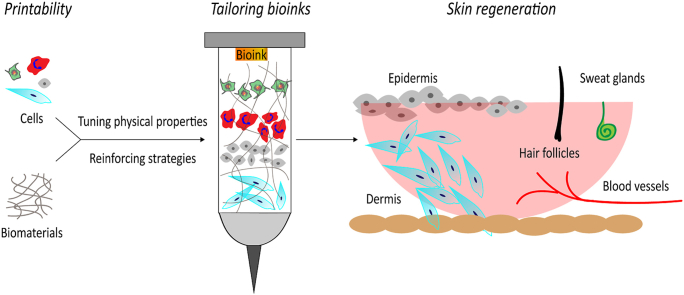
Abstract
Extrusion-based bioprinting (EBB) holds potential for regenerative medicine. However, the widely-used bioinks of EBB exhibit some limitations for skin regeneration, such as unsatisfactory bio-physical (i.e., mechanical, structural, biodegradable) properties and compromised cellular compatibilities, and the EBB-based bioinks with therapeutic effects targeting cutaneous wounds still remain largely undiscussed. In this review, the printability considerations for skin bioprinting were discussed. Then, current strategies for improving the physical properties of bioinks and for reinforcing bioinks in EBB approaches were introduced, respectively. Notably, we highlighted the applications and effects of current EBB-based bioinks on wound healing, wound scar formation, vascularization and the regeneration of skin appendages (i.e., sweat glands and hair follicles) and discussed the challenges and future perspectives. This review aims to provide an overall view of the applications, challenges and promising solutions about the EBB-based bioinks for cutaneous wound healing and skin regeneration.
Keywords: Bioink, Extrusion-based bioprinting, Cutaneous wound healing, Skin regeneration
Graphical abstract
Highlights
-
•
Bioink performance is improved by tuning physical cues and reinforcing strategies.
-
•
Skin bioprinting promoted wound healing, angiogenesis, and appendages regeneration.
-
•
Bionic strategies, 4D bioprinting, and skin organoids are prospective hotspots.
Abbreviations
- 3D
three-dimensional
- 4D
four-dimensional
- DPC
dermal papilla cell
- EBB
extrusion-based bioprinting
- ECM
extracellular matrix
- ELR
elastin-like recombinamer
- FRESH
Freeform Reversible Embedding of Suspended Hydrogels
- GelMA
gelatin methacryloyl
- MSC
mesenchymal stem cell
- TAZ
tafazzin
- YAP
yes-associated protein
1. Introduction
Cutaneous wounds are becoming a growing worldwide medical burden. In developed countries, about 1–2% of the general population suffer from chronic wounds throughout their lifetime [1]. In United States, the Medicare cost estimates of acute and chronic wound care ranged from $28.1 billion to $96.8 billion [2]. Among all wound kinds, surgical wounds and diabetic foot ulcers cost the highest expenses [3]. In China, a recent cross-sectional study identified that the prevalence rate of chronic wounds was as high as 1.7‰ among patients in hospitals [4].
Current progress highlighted the versatile roles of three-dimensional (3D) bioprinting for cutaneous wound healing. Based on their native prototypes, 3D bioprinting techniques are categorized as extrusion-based, droplet-based, laser-based and stereolithography bioprinting [5]. Among them, extrusion-based bioprinting (EBB) integrated with cell-loaded bioinks is the most prevalent used techniques for cutaneous wound care because of its accessibility, cost-effectiveness and capacity to replicate tissue complexity [6]. Specifically, EBB extrudes or dispenses continuous strands of “bioinks”, which are mainly comprised of biomaterials, living cells and/or bioactive molecules, onto a free-moving platform in a predesigned layer-by-layer manner to fabricate geometrically well-defined 3D complex structures. Usually, bioinks are extruded through a nozzle in an EBB system powered by pneumatic-, piston-, screw-based, or microfluidic mechanisms [7].
Compared with other 3D bioprinting techniques, EBB can process a much wider variety of biomaterials or cell types [8] and cause relatively less process-induced cell damages [9]. The EBB-related bioinks, which are generally comprised of natural-derived (i.e., collagen, fibrin, gelatin, hyaluronic acid, chitosan, alginate, agarose, etc.) or synthetic biomaterials (i.e., polycaprolactone, polyethylene glycol, polylactic acid, etc.) [10], are a mixture of high-molecular polymers possess shear-thinning properties and can be further crosslinked for stabilizing [11]. By tuning biophysical mechanical parameters or adding functional growth factors or drugs into bioinks, EBB approaches can promote cell growth or even direct cell fates toward targeted regeneration applications [12].
However, current EBB-based bioinks exhibit some limitations, such as unsatisfactory bio-physical (i.e., mechanical, structural, biodegradable) properties for skin regeneration, and a compromise between the printability and cellular compatibility of EBB-based bioinks usually has to be made. Despite a few published reviews [10], EBB-based bioinks with therapeutic effects targeting cutaneous wounds still remain largely undiscussed.
Here, we discussed the four key features about the printability (i.e., extrudability, filament classification, shape fidelity and printing accuracy) of EBB-based bioinks. Then, current strategies for improving the physical properties (i.e., mechanical properties, structural properties and biodegradability) of bioinks and possible solutions for reinforcing EBB-based bioinks (i.e., reinforcing bioinks with functionalized polymers, supramolecular hydrogels, nanocomposites and surface modifications) were introduced, respectively. Notably, we highlighted the applications and effects of current EBB-based bioinks on wound healing, wound scar formation, vascularization and the regeneration of skin appendages (i.e., sweat glands and hair follicles) and discussed the challenges and future perspectives. This review aims to provide an overall view of the features and applications targeting cutaneous wound healing and skin regeneration, and to discuss current challenges and promising solutions about the EBB-based bioinks.
2. Printability considerations for skin bioprinting
Skin is the largest organ around body compared to other tissues, and the regional heterogeneity of skin requires different physical and cellular parameters such as stiffness, pore size and cell types for 3D bioprinting approaches. An ideal bioprinted skin substitute should exhibit the following properties [13]: (i) a biocompatible surface chemistry for cell attachment, (ii) a highly porous inner architecture with interconnected pores for cell immigration, nutrients transportation, and wound exudates absorption, (iii) a large surface area to volume ratio, (iv) suitable pore sizes (usually 200–400 μm) to promote cellular activities and protect the wound from microbial invasion [14], (v) a proper biodegrading speed to maintain its 3D structures for at least 3 weeks for fibroblast and epithelial cell proliferation and for allowing ingrowths of blood vessels [15].
The printability, a crucial parameter of bioinks for skin bioprinting, generally refers to the ability of bioinks to be printed and is greatly influenced by a range of biophysical and biochemical attributes of bioinks, such as shear-thinning, gelation kinetics, recoverability, biodegradation and biocompatibility [16]. During EBB-based skin biopriting, bioink filaments were continuously extruded from a nozzle at a well-regulated printing speed between 700 mm/s−1 and 10 μm/s−1 [17], and were deposited in predefined geometries with the resolution ranged from 50 to 1000 mm [16]. Specifically, the bioink should possess a lower viscosity to protect cells from excessive fluid shear stress and prevent possible clogging at the extrusion nozzles, and should undergo rapid solidification to maintain the deposited shape upon deposition on the printer bed [18]. The viscosities of EBB-based bioinks are usually ranged from 30 to 6 × 107 mPa s [19].
Currently available hydrogel-related bioinks for skin regeneration or wound healing are outlined based on four key features targeting printability, i.e., (i) extrudability, (ii) filament classification, (iii) shape fidelity and (iv) printing accuracy.
Extrudability refers to the ability that a bioink is extruded through a micrometer-scale nozzle. Notably, viscosity is the main rheological measure of extrudability because higher viscosity directly results in lower extrudability. Bioinks are generally a mixture of high-molecular-weight polymers exhibiting shear-thinning properties, i.e., the viscosity decreases under shear strains. Shear-thinning effects of bioinks are caused by the disentanglement of polymer chains and the alignment along the direction of shear during flow, which prevent clogging at the nozzle during printing processes and contribute to regain immediate structural consistency post-printing [20]. The cell viability during an EBB approach is closely related to the pressure loaded on the piston, the shear force at the needle and the residence time of the cells in the syringe [21]. Hence, the extrudability of bioinks can be quantified by rheological properties or measuring the pressures required to achieve a specified flow rate [22]. In other cases, the extrudability of a bioink is also indirectly evaluated by live/dead assay following the bioprinting process [23].
A filament classification system should be established in order to accurately describe different types of deposited filaments because bioinks will form a variety of filament types after being extruded from the nozzle in an EBB approach. Water droplets and filaments are two main shapes formed after bioinks being extruded into the air [24]. Specifically, the filaments can be sub-categorized by either smooth or nonuniform exteriors [24]. Smooth filaments deposition is precisely controlled by the 3D printer, but nonuniform filaments often deviate from the predetermined printing paths [25]. In addition, the deposited filaments can be quantitatively measured by the following parameters, e.g. the perimeter of single lattice of bioprinting structures relative to perfect square [24], the inhomogeneity of single filament width [26], etc.
Shape fidelity represents the ability of bioinks to maintain structural integrity after stacking layers, which directly determines the ultimate size and complexity of the shapes that a specific bioink can generate [27]. As multiple new layers are deposited on top of previous layers, bioinks with poor shape fidelity will collapse due to the weight of those layers. Ouyang et al. [24] developed the Pr value to characterizes the shape fidelity of bioinks, which is positively correlated with the gelation degree of bioinks. Although the Pr value is only applicable to the evaluation of the two-layer printing model, it has a positive guiding role in the deposition processes of bioinks. Moreover, printing in support gel was required to maintain the integrity of delicate structures.
Printing accuracy refers to the similarity between the actually printed structures and the ideal dimensions, which is similar to shape fidelity in terms of measurement methods. The key distinction is that the printing accuracy emphasizes different printing conditions, while the shape fidelity emphasizes different bioinks. Filament height, width and pore size are most commonly used to evaluate printing accuracy in an EBB approach. In addition, microcomputed tomography measurements and weight/volume of printed construct measurements provide more reliable evidence despite that they are time and cost consuming [28]. Despite of great progress, printing soft biomaterials into air inevitably results in poor fidelity. Most recently, the emerging Freeform Reversible Embedding of Suspended Hydrogels (FRESH) method has gained great attentions for achieving high printing accuracy and building much complex 3D architectures. Specially, the FRESH method is an embedded printing approach that extrudes bioinks within a yield-stress support bath that holds the bioinks in place until cured [29]. Coaxial nozzle-assisted 3D bioprinting or 3D-bioprint collagen using FRESH methods enabled rapid shape and high printing accuracy, which holds potential for designing more complex skin scaffolds.
3. Strategies for improving the bioink performances
The bio-hospitable nature of hydrogels has made them the best candidate of bioinks for printing 3D cell-loaded constructs [30]. Controlling the characteristics (i.e., pore size, structure, mechanics, biodegradation, etc.) of bioprinted scaffolds is a major focus of bioprinting research. In this section, we discussed how the properties of bioinks are related to its performances, and reviewed currently available strategies for improving the performances of bioinks.
3.1. Improving physical properties of bioinks
3.1.1. Tuning mechanical properties
Mechanical properties are crucial guarantees for the guiding effects of printed skin constructs at both macro- and micro-levels.
Macroscopically, the mechanical features of the implants should be consistent with the target tissues. For example, bioprinted skin constructs are implanted into the body aiming for skin regeneration. Hence, the mechanical properties should match those of native skin, such as stiffness (1–5 kPa) and yield stress/strain [31]. Due to high-water content and tunability, the elastic modulus of hydrogels can be adjusted in a wide range, e.g., soft collagen gels (<1 kPa) [32], stiff reinforced double network hydrogels (>1 MPa) [33], etc. A printable hydrogel-based bioink should exhibit a storage modulus between 102 and 103 Pa to successfully maintain the stability of printed constructs [34].
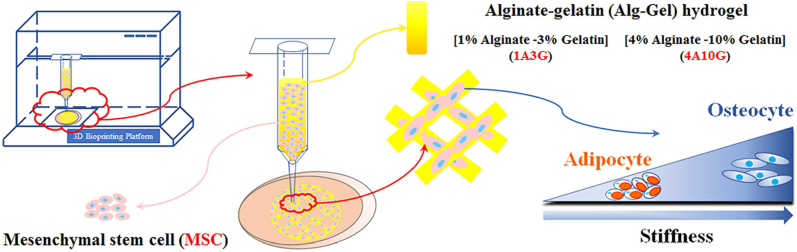
Microscopically, the stiffness of bioinks significantly regulates the behaviors of encapsulated cells. Multiple studies have shown that cells could sense and response to stiffness of surrounding extracellular matrix (ECM) [35]. For example, matrix stiffness can guide fibroblasts and mesenchymal stem cells (MSCs) to migrate to damaged tissues and promote tissue repair [36], and normal mammary cells would transform into malignant cancer cells under high stiffness matrix treatment [37]. In addition, the matrix stiffness is able to regulate the differentiation direction of stem cells. Mammary gland progenitor cells prefer to differentiate into luminal epithelial cells when cultured on the surface with lower stiffness, while turned into myoepithelial cells with higher stiffness stimulus [38]. Gilbert et al. [39] identified that muscle stem cells could maintain the regeneration capabilities and self-renew in vitro when cultured on soft hydrogel substrates that mimic the elasticity of natural muscle tissues. The differentiation of neural stem cells is also vulnerable to the changes of stiffness, i.e., neural stem cells prefer to commit the neurons fate on matrix with lower elasticity similar to brain tissues, while they are likely to commit the glial cells fate on a rigid matrix [39]. Similarly, neonatal cardiomyocytes or induced cardiomyocytes usually failed to function like normal myocardium, while they were able to beat well and retrieve their differentiated states once cultured on a physiologically stiff matrix with heart-like elasticity [40]. Moreover, stiffness could individually induce MSCs differentiating to the osteoblasts or adipocytes in 3D printed matrices [41] (Fig. 1).
Fig. 1.
MSCs in constructs show adipocyte differentiation with low stiffness and show osteocyte differentiation with high stiffness, respectively. Cited from Ref. [41].
3.1.2. Tuning structural properties
Structural characteristics (i.e., pore size, isotropy, topology, etc.) are closely related with mechanical properties and play an indispensable role in guiding or even determining cell behaviors [36]. For example, the increases of the diameter of the cardiac collagen fibers are related to age-associated changes in cardiovascular dysfunctions [42]. Generally, small pore sizes repress cell migration and reduce nutrient diffusion, while larger pores are facilitated with cell proliferation and cell extension but decrease mechanical properties [36].
The structural properties of bioinks are closely related to skin bioprinting, especially hypertrophic scar or keloids, which are characterized by disordered dermal microarchitectures and myofibroblasts. Myofibroblasts are highly contractile and can secret a large amount of ECM resulting in collagen network remodeling with higher stiffness, which greatly affect cell behaviors [43]. Isotropic features like aligned structures can maintain the stemness of muscle stem cells and activate the myofibroblast to promote scar formation [44]. Another study also reported that adipose stroma cells embedded in collagen gels with increased fiber thickness and pore size developed a more contractile phenotype, mechanically triggering myofibroblast differentiation [43].
3.1.3. Tuning biodegradability
Biodegradability generally refers to the ability of materials to be degraded or broken-down over time in the host bodies [45]. Cell growth in the bioprinted scaffold requires gradually expanded inner spaces and the implanted construct is meant to be replaced with functional neo-tissues. In ideal conditions, the biodegradation rate should be balanced with the rate of the tissue regeneration process, whilst the degradation products should not trigger violent foreign body responses [45]. During wound healing, skin substitutes should be not only biodegradable but also stable enough to maintain its composite structures for at least three weeks to guide ingrowths of loose connective tissue and blood vessels [46].
In 3D printing, the bioinks for skin substitutes are usually hydrogels, and degradability of these constructs mainly depends on the selected bioinks and external conditions (e.g., hydrogel components, concentrations, pH values, temperature, external additives, in vitro or in vivo, etc.) [47]. Generally, peptide-based materials are degraded by enzymes produced by cells and polysaccharide-based materials are degraded through ion exchanges in the hydrogel. Rodrigues et al. [48] also found a positive correlation between the oxidation level and the degradability of the bioprinted scaffolds. In another study, Madl et al. [49] generated a family of hydrogels with a range of degradability and stiffness to demonstrate that degradation rate of hydrogel was necessary for maintenance of the stemness of neural progenitor cells.
Bioinks derived from natural polymers (e.g., collagen, gelatin, fibrinogen, etc.) exhibit an ideal biodegradability. For example, collagen exhibits high degradation rates, proper pore sizes and marginal mechanical strengths. In tissue-engineered bone or cartilage, collagen is often used in combination with synthetic polymers or bio-inert materials to slow down the overall degradation rate of bioinks [50]. While in tissue-engineered skin, the composite bioinks of natural collagen and gelatin exhibits a good biodegradability and printability, which have been successfully applied for cutaneous wound care [51].
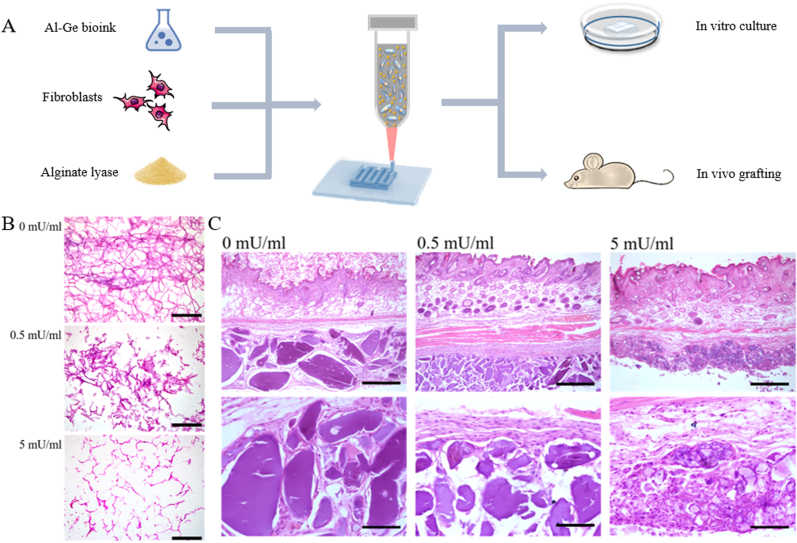
Specifically, alginate is one of the most widely used natural materials in 3D bioprinting due to its excellent biocompatibility and great capability of patterning. However, the slow and difficult degradation of alginate has restricted its applications. By adding alginate lyase, enzymatically degradable alginate/gelatin bioink was used for establishment of 3D bioprinted constructs which can be degraded both in vitro and in vivo. Cell adhesion, extension, proliferation and retention are promoted in degradable bioinks and the enhanced cell communication between grafted and host tissues meets the requirements of cytotherapy (Fig. 2) [52]. In addition, Liu et al. [53] introduced alginate lyase into composite gelatin methacryloyl (GelMA)/alginate hydrogel and the bioink with proper lyase showed excellent movability and biocompatibility and promoted the assembly 3D constructs. Another attempt for accelerating the degradation rates of alginate is focused on chemically modifying its molecular structures. For example, the partially oxidized alginate [54], which is produced by employing sodium periodate to oxidize alginate resulting in newly-generated aldehyde groups, exhibits significantly accelerated degradation rates than non-modified alginates [55].
Fig. 2.
Alginate lyase-contained bioinks promote the degradation of 3D constructs in vitro and in vivo. A. Schematic illustration of bioprinting process using alginate lyase-contained bioinks; B. H&E staining of bioprinted lattice with different concentrations of alginate lyase cultured for 14 days (Scale bars: 500 μm); C. H&E staining of subcutaneously engrafted bioprinted constructs with alginate lyase of different concentrations at 14 days post-operatively (scale bar: 500 μm). Cited from Ref. [52].
3.2. Reinforcing bioinks
The complex and diverse needs of bioinks have led to a powerful drive to invent new ways to incorporate the best possible qualities into bioinks. At the early stage, 3D printed hydrogel-based bioinks made a compromise between the printability and the cellular compatibility. With the development of 3D bioprinting, the demand of reinforcing the bioinks in EBB approaches emerges rapidly.
3.2.1. Reinforcing bioinks with functionalized polymers
Synthetic-polymer-based hydrogels are usually covalently crosslinked, while natural-polymer-based hydrogels are physically crosslinked by conformational changes and physical interactions [56]. Generally, physical interactions are reversible and much weaker than covalent bonds, which are easily affected by the environmental factors such as temperature or pH values [57].
Functionalization of polymers serves as promising solutions to solve these problems. By covalently crosslinking natural-polymer-based hydrogels, the mechanical properties can be improved and the sensitivities to environmental factors can be reduced [58]. The properties of bioinks including degradation, mechanical strength, stability and cell attachment could be regulated by polymer functionalization [59]. Compared to single-crosslinked networks, polymer functionalization can produce stronger crosslinking and sometimes various crosslinking modes can be interacted with each other to form a dual-crosslinked network. Therefore, functionalization of polymers can sometimes introduce new biological activities into bioinks.
One of the most popular approaches for functionalized polymers is modifying the polymer backbones with methacrylic groups and the functional groups of methacrylate can be photo-crosslinked at the presence of a photo-initiator [60]. Various kinds of hydrogels (gelatin, alginate, hyaluronic acid, etc.) have been subject to methacrylation by now without affecting their bioactive properties, such as cell attachments or enzymatic degradations. GelMA is stable at room temperature and resistant to rapid degradation, and the mechanical properties of 3D printed structures based on GelMA bioinks could be readily regulated by changing polymer concentration and crosslinking time [61].
3.2.2. Reinforcing bioinks with supramolecular hydrogels
Supramolecular hydrogels are composed of environmental-sensitive short polymer strands and are self-assembled by non-covalent interactions between functional end groups. Incorporating supramolecular hydrogels with bioinks can rapidly strengthen crosslinked networks, which holds great potential for reinforcing bioinks.
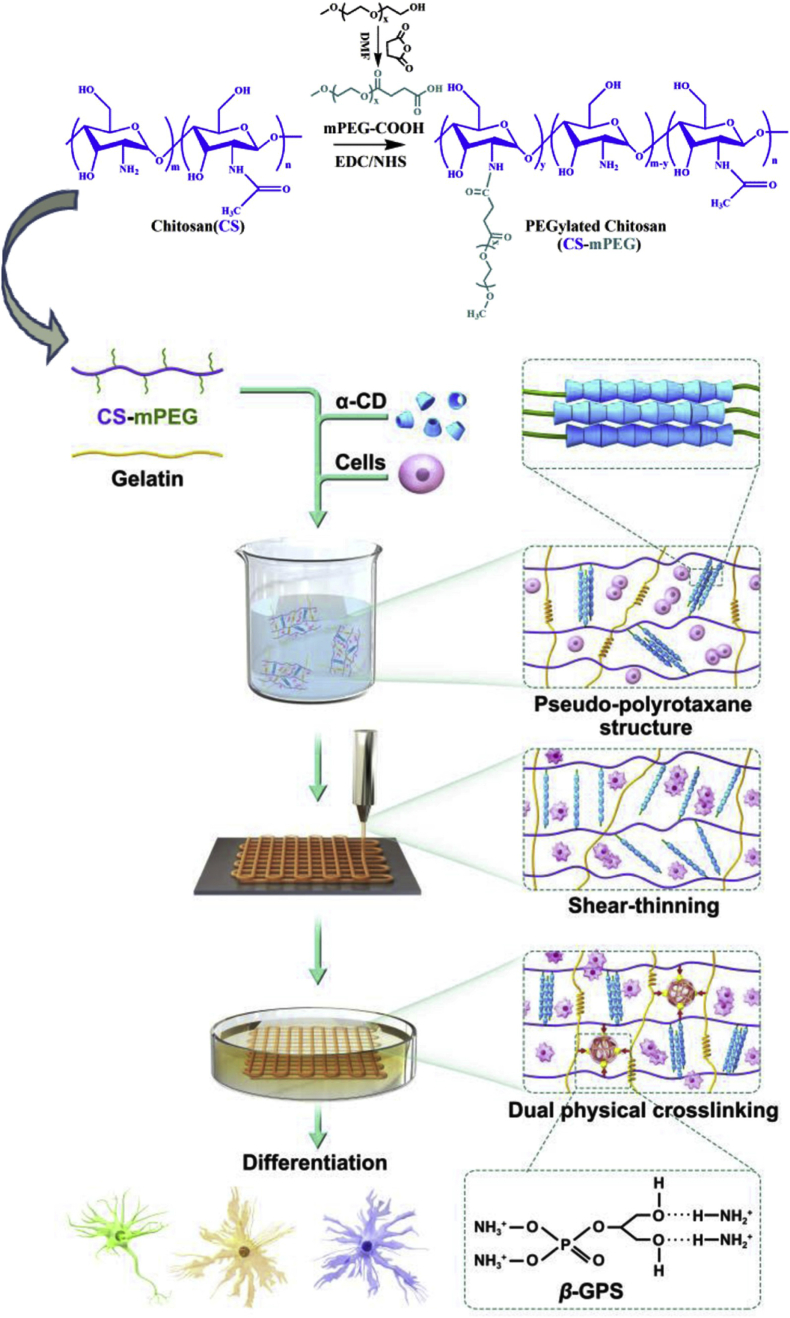
Polyrotaxanes or pseudo-polyrotaxanes, whose structures are mainly the inclusion of the polymers and cyclodextrins through host-guest interactions, have been widely used in molecular machines, energy storage and biomedicine [62]. In our previous studies, chitosan and gelatin are utilized for bioinks due to their biodegradability and biocompatibility [63]. Chitosan was modified with polyethylene glycol to make it water soluble, and then forms pseudo-polyrotaxanes structures with α-cyclodextrin. Introduction of gelatin and the aggregation of pseudo-polyrotaxanes structure provide increased viscosity and good shear-thinning property for 3D bioprinting of the supramolecular hydrogel (Fig. 3). The bioink exhibited ideal biocompatibility and tunable strength which would determine stem cell differentiation [64].
Fig. 3.
Schematic illustration and flow chart of chitosan/polyethylene glycol formulation, bioink preparation, dual physical crosslinking and 3D bioprinting process. Cited from Ref. [64].
3.2.3. Reinforcing bioinks with nanocomposites
The lack of bioinks with tailored bioactivity are becoming one of the major obstacles that restrict the development of 3D bioprinting [65]. Presently, bioinks are mainly composed of hydrogels to mimic natural ECM for cell adhesion and function. However, the potential of pure hydrogels as bioinks usually compromises with fewer cell binding sites (especially synthetic polymers), poor mechanical strengths and uncontrollable degradation rates [66].
Currently, new composite bioinks have been developed to not only improve the physical properties (e.g., printability, mechanical strength, etc.) but also to add bioactive ingredients for facilitating cell behavior and tissue regeneration [67]. One representative type of such strategies is hydrogel-inorganic composite system. By adding nano-sized fillers (e.g., hydroxyapatite, bioactive glasses, silica nanoparticles, etc.) to the hydrogel, the mechanical properties and bioactivity have been significantly improved [68]. Furthermore, due to the unique microstructure and chemical composition of these inorganic fillers, these materials are able to promote cell adhesion, proliferation and some of them even enable to regulate the cell fate of stem cells or progenitor cells [69].
Skin bioinks should mimic the characteristic of the ECM to maintain cell viability and function. In the field of skin tissue engineering, composite hydrogels are able to prepare tunable bioinks to match the features of native skin [11]. Because of the high surface property of nanomaterials, the performances of hydrogel could be influenced by a low concentration of nanoparticles [70]. Nanomaterials are being used to introduce new functions to bioinks such as mechanical reinforcement, printability improvement and cell behavior regulation [71].
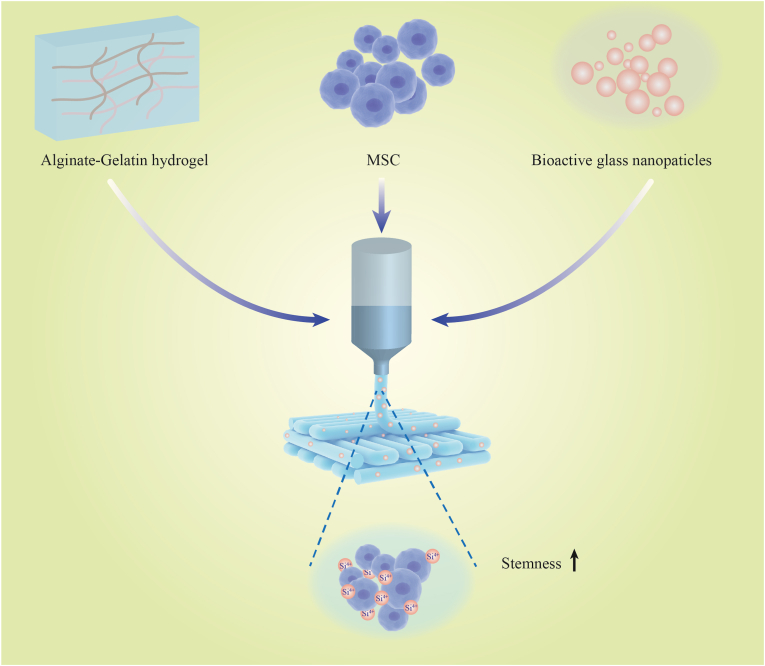
Bioactive nanoparticles are several kinds of silica-based bioactive ceramics and glasses, which has significant effects on the structural, chemical and biological properties in bioinks [72]. Incorporation of bioactive nanoparticles in alginate/gelation hydrogel would improve the printability of bioink and promote cell proliferation, and the expression level of Nanog and Oct4 which represents stemness of MSCs was upregulated by the silicon ions released from the bioactive nanoparticles [73]. Interestingly, bioactive nanoparticles did not change pore properties of the bioink and the influence on stem cell regulation is attributed to the biochemical signals of bioactive nanoparticles (Fig. 4) [69]. Zhai et al. [74] mixed laponite nanoclay in bioinks which could release Mg2+ and Si4+ to support osteogenic ability in vitro and in vivo. Recently, graphene oxide serves as an emerging nanofiller for enhancing the mechanical strength and electrical conductivity of bioinks due to its high mechanical strengths, large surface areas (2300 m2/g) [75], and high hydrophilicity with the presence of oxy-functional groups [76]. The incorporation of graphene oxide into chitosan- or alginate-based bioinks was reported to significantly improve both the G′ and G″ and ε′ and ε″ values of bioinks but did not influence the gel point, which resulted in increased rheology and printability [77]. The underlying mechanisms were probably that graphene oxide enhanced the thixotropic behaviors with a faster viscosity recovery post-extrusion which resulted in the improved shape fidelity and resolution [78]. Moreover, the liquid crystalline properties of graphene oxide contributed to generate anisotropic threads after printing processes [78], which holds potentials for bioprinting skin basal layers or dermal nerve fibers where cellular alignment is required.
Fig. 4.
Incorporation of bioactive nanoparticles in alginate/gelation hydrogel upregulated the stemness of MSCs. Cited from Ref. [69].
3.2.4. Reinforcing bioinks with surface modifications
Although EBB approaches enable us to control geometric or architecture parameters of printed constructs, such as shapes, pore sizes and spatial distributions, it remains quite challenging to tailor their surface properties [79].
Surface modification strategies present promising solutions by tuning the physical or chemical properties on the surfaces of implanted biomaterials in order to offer a favorable interaction between the implants and cells. Generally, surface modification strategies are categorized as physical- and chemical-modifications [80] serving as a follow-up processing post printing or being employed synchronously along with printing procedures [47]. Physical modifications (also called architectural reconfiguration) include changes in the morphology or topography of the surface without changes in the chemistry, such as machining, etching and grit-blasting [80]. Chemical modifications (also called surface functionalization) utilize a variety of chemical reactions to endow surfaces with defined biofunctions [81].
Based on currently available literature, chemical modification strategies are most widely used and are even regarded as the promising solutions for closing the gap between EBB approaches and optimal therapeutic effects of wound healing [80]. In this section, we briefly discussed three promising chemical modification strategies, i.e., mussel-inspired strategy, antifouling strategy and antimicrobial peptides (AMPs), which holds potential for reinforcing bioinks to favor wound healing and skin regeneration.
3.2.4.1. Mussel-inspired strategy
Mussel-inspired strategy has opened a new avenue for skin bioprinting by enhancing cellular attachments as commonly-used bioinks only offer limited cell adhesion sites. Mussel-inspired strategy is inspired by mussels’ secreted proteins containing lysine and 3,4-dihydroxyphenyl-l-alanine with functional catechol groups, which enable mussels to toughly adhere onto moist surfaces [82]. Under alkaline conditions, dopamine can undergo oxidative self-polymerization to form polydopamine films, which react with amino- and thiol-containing compounds acting as platforms allowing for secondary reactions generating numerous ad-layers (e.g., macromolecules grafting, metal films, self-assembly monolayers, etc.) [83]. Moreover, the biochemical reactions are tunable utilizing artificially-modified amino compounds or metal ions to further impact the adhesion capacities [84]. Exploiting the adhesion capacity of dopamine and reductivity of self-polymerized polydopamine film, various biomolecules (amino acids, peptides, growth factors, proteins, etc.) and some inorganic substances containing amino or thiol compounds are able to be immobilized and deposited on substrates in-situ [83].
As catechol and amine groups are key molecules that contribute to the adhesive capacities of mussel-inspired coatings, Lee et al. [85] developed a chitosan-catechol bioink that contains catechol and amine groups, which achieved rapid complexation by using serum proteins and generated 3D constructs without any external stimuli for cross-linking. Guo et al. [86] utilized mussel-inspired polydopamine to promote the adhesive properties of 3D printable bioinks in an EBB approach. The bioinks are double-network hydrogels composed of hyaluronic acid and alginate. In vitro cell culture experiments identified that this bioink formulation significantly enhanced the adhesive properties and are compatible with human umbilical vein endothelial cells. Another study from Li et al. [87] reported a 3D printed bioceramic scaffolds with a mussel-inspired surface coating which aimed to accelerate tissue regeneration by regulating the paracrine-signaling network of adipose-derived MSCs.
3.2.4.2. Anti-fouling strategy
The anti-fouling effect, also called “louts' effect”, represents the abilities of hindering the non-specific adsorption of cells or biomolecules onto the surface of biomaterials, which was inspired by the fact that lotus leaf repels water droplets. In clinical applications, wound dressings have to be frequently changed resulting in causing severe pains and possibly harming the newly-forming epithelium. Moreover, the adhesion of bacteria can also represent a hidden infectious threat with delayed wound healing and antibiotic resistances [88]. Despite of the fact that tissue engineered skin should favor cell attachments, wound dressings are preferred to exhibit antifouling capacities.
Zwitterionic hydrogels possess both negative and positive charges, which confer superhydrophilic properties of low bio-absorptions [89], low coefficient of friction [90] and high electrostatic attraction to water. Pan et al. [91] utilized a zwitterionic photochemistry and optical stereolithography to developed a novel tough and highly solvated hybrid hydrogels, which exhibits superior antifouling capacities with significantly less absorption of bovine serum albumin. However, zwitterionic hydrogels are not able to kill bacteria. Ghavami Nejad et al. [92] synthesized dually functional nano-silver particles and zwitterionic hydrogels which combine both antibacterial and antifouling capacities. The in vitro and in vivo experiments suggested that nano-silver particles coated with zwitterionic hydrogels can advance wound healing. Liu et al. [93] also reported cationic polycarbonate/PEG hydrogels endowed with both antibacterial and antifouling capacities which are synthesized through Michael addition chemistry. Other attempts integrated anti-bacterial with anti-fouling strategies for wound dressing which significantly increased surface hydrophobicity and provided a superior ability to kill bacteria [94]. Very recently, Yan et al. [95] reviewed the 3D printing techniques for fabricating construct surfaces with superamphiphobicity, i.e., superhydrophobicity and superoleophobicity. To our best knowledge, antifouling strategy has been rarely used with 3D bioprinting for wound care. It is predictable that antifouling strategy integrated with hydrogel-related EBB approaches are promising wound dressings, which are skilled at covering wounds rapidly and changing without much pain.
3.2.4.3. Antimicrobial peptides
Traditional 3D bioprinted constructs are highly susceptible to bacterial infection after being implanted into wound sites [96]. Antimicrobial peptides (AMPs), which act as natural immune defenses, are integral compounds secreted by natural organisms including animals, plants or invertebrates [97]. Usually, AMPs are endowed with broad-spectrum antibacterial activities, and only rarely cause bacterial resistance. Therefore, they have recently attracted an increasing attention as antibacterial agents [98]. Although there is a great diversity among AMPs families, AMPs share some common characteristics, one of which being that most of them are cationic peptides. Generally, cationic AMPs can target the bacterial surface via an electrostatic attraction between negatively charged cell surfaces and positively charged amino acids causing the physical disruption of cell membranes and eventually bacterial death [99]. Among them, the KR-12 peptide has exhibited a superior antibacterial activity against MRSA, which is a bacterium variant commonly found in wounds of burn patients [100]. The KR-12 peptide is the smallest antibacterial motif (residues 18–29) of human cathelicidin LL-37 [101], which can not only efficiently kill bacteria but also has several biological activities [102]. Liu et al. [103] identified that KR-12 antimicrobial peptides exhibited superior antibacterial activity and significantly promoted wound healing.
AMPs-integrated bioinks holds great potential to favor wound healing due to their superior antibacterial abilities. AMPs can be either embedded with bioinks or be coated onto the surface of printed implants afterwards. A controlled releasing fashion of AMPs is favored to achieve long-term antibacterial outcomes [96]. Fidan et al. [104] coated AMPs onto the surface of 3D-printed implants by surface modification using a mussel-inspired strategy or a plasma electrolytic oxidation protocol. Mohanraj et al. [105] utilized ink-jet printing to covalently attach AMPs on porous ultrafiltration membranes, which exhibit surface antibacterial activity against Pseudomonas aeruginosa and reduced biofilm growth. Other available approaches about AMPs integrated 3D bioprinting are involved of hydroxyapatite-related bone regeneration [106] and polycaprolactone-related patches for treating trachea injuries [107].
In particular, there are two limitations of AMPs, i.e. an instable molecular structure and a lack of selectivity in vivo. Hopefully, many solutions are available to overcome the innate AMPs deficits and to avoid the related cytotoxicity and side effects because AMPs are possessed of an easily tunable molecular skeleton. On one hand, structurally modified cyclic or branched AMPs exhibit a significantly more stable molecular structure, and the integration of AMPs with nanoparticles can increase their in vivo stability [108]. On the other hand, conjugating AMPs with antibodies represents a promising solution for improving the selectivity [97]. In a recent study, polymersomes were fabricated via self-assembly due to the hydrophobic interactions of admixed aqueous and organic substances, and then AMPs were ingeniously included at the peripheral hydrophilic region, while nano-silver particles were included inside the hydrophobic corona. In vitro tests indicated that the AMPs/nano-silver particles polymersomes exhibited a satisfactory bacteriostatic activity as well as a low cytotoxicity toward human dermal fibroblasts [109].
Despite of the fact that AMPs cause less frequent instances of bacterial resistance, various studies have proved beyond any doubt that many kinds of bacteria do resist AMPs even in the presence of high levels of locally delivered AMPs [108,110,111]. The resistance mechanisms exhibit a great diversity because most microbial species contain AMP-resistance genes [112,113]. Generally, the mechanisms of bacterial resistance to AMPs are the following: (1) secretion of exo-polymers or biofilms; (2) activation of efflux pumps; modification of cell surface charge; and changes in membrane fluidity; (3) secretion of trapping proteins or proteases; (4) generation of sensing systems via selective gene expression. As antibiotics-embedded bioinks for cutaneous wound care are rarely used, AMPs represents one of the limited alternative options for reinforcing printed implants with the antibacterial abilities and holds tremendous potential for EBB-based skin bioprinting.
4. Applications
4.1. Bioinks for acute or chronic wounds
4.1.1. In vitro bioprinting
Skin is composed of epidermis, dermis and hypodermis involving more than 50 different cell types (i.e., keratinocytes, fibroblasts, melanocytes, stem cells, etc.) [114], which are populated in a collagenous and anisotropic extracellular matrix (ECM) containing collagen, elastin, laminin, fibrillin, etc. [48]. Acute wound healing involves a spatiotemporal activation of various kinds of cells and proteins. A large family of growth factors is involved in wound healing, such as platelet derived growth factor, fibroblast growth factor, vascular endothelial growth factor, etc.
The healing processes of acute wounds are singled out in four stages, which successively appear and overlap one after the other, i.e., hemostasis, inflammation, novel tissue generation, and remodeling stage [115]. Once wound healing processes have not progressed for more than a month, it could be classified as chronic wounds [48]. For example, diabetic patients are usually characterized by high blood glucose levels due to reduced autologous insulin secretion or increased insulin resistance, which results in multiple metabolic dysfunctions, such as impaired microcirculation, peripheral neuropathy, tissue self-repairing deficits, as well as persistent bacterial infections, and possibly hamper the physiologic healing processes and bring about chronic wounds [116].
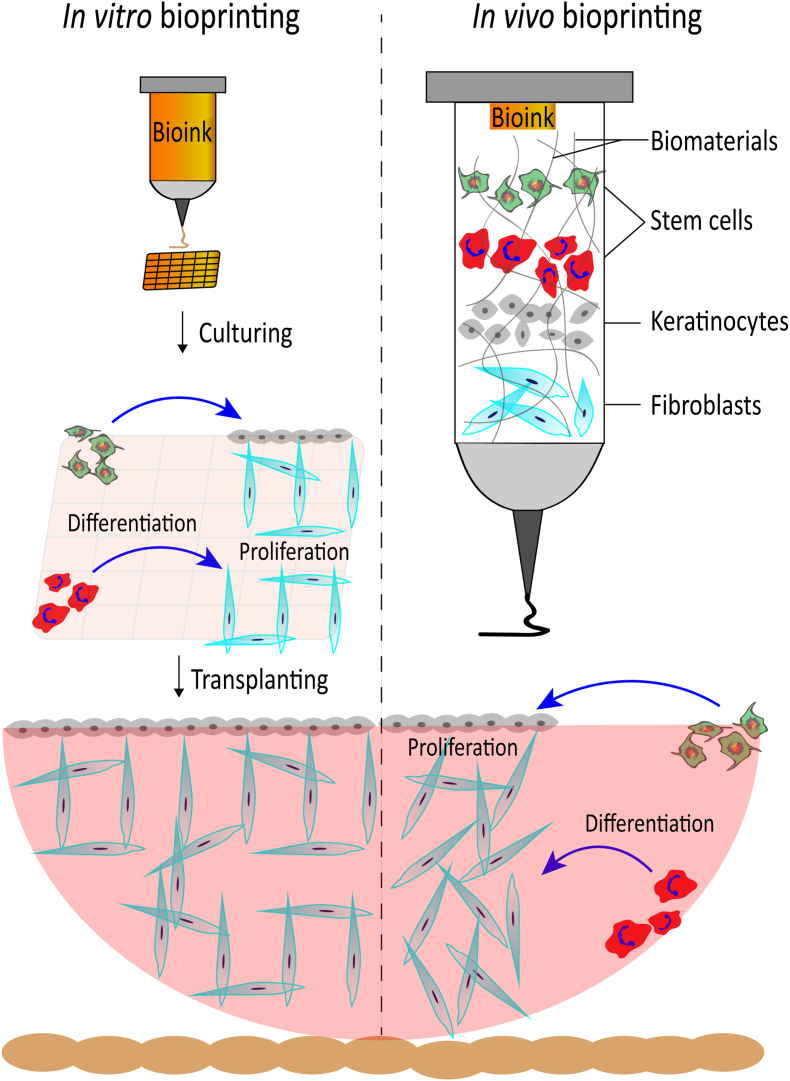
Traditional therapeutic approaches for acute or chronic wounds are unsatisfactory due to the weak resistance to bacterial infections, limited vascularization, the absence of major appendages (i.e., hair follicles and sweat glands) as well as unavoidable scarring and related physical dysfunctions [115]. EBB technologies serve as a promising strategy to overwhelm the current impasses. Despite the disadvantages of EBB (i.e., limited mass-production, the inconvenience of transportation and storage, etc.), EBB still exhibits remarkable advantages when compared with conventional wound patches, such as personalized customization, live cell processing and replicating the complexity of skin [6]. Specifically, skin-derived cells or stem cells can be appropriately isolated from donor sites and embedded in bioinks, then be printed layer-by-layer forming skin-mimetic constructs, which is quite challenging for conventional wound patches or other biomaterial fabrication methods [117] (Fig. 5).
Fig. 5.
Schematic diagram of in vitro and in vivo skin bioprinting.
Commonly used EBB strategy for wound care is in vitro bioprinting, i.e., the skin-like structures were printed, crosslinked and probably cultured for a period of time, then were transplanted into wounds. High swelling ratios of bioink can maintain moisture wound area to exchange nutrients and facilitate cell proliferation so as to restore the skin integrity and accelerate the wound healing processes [20]. Cells embedded in bioinks are categorized as adult cells, such as human- or animal-derived dermal fibroblasts and epidermal keratinocytes, as well as progenitor cells or stem cells, such as Wharton's jelly MSCs [118], amniotic epithelial cells [119], endothelial progenitor cells and adipose-derived or bone marrow-derived MSCs [120]. Especially, MSCs exhibit multilineage differentiation capacities and are able to differentiate into multiple kinds of skin-like cells. Moreover, circulating MSCs are found to be recruited and differential into skin cell phenotypes during physiological process of cutaneous wound healing. Despite the risks of stem cell-related tumorigenesis and the foreseeable shortage of donors, MSCs hold great potential for in vitro skin bioprinting.
4.1.2. In vivo bioprinting
Notably, more attentions have been paid to in vivo (also called in situ) bioprinting, i.e., bioinks are directly deposited into wounds and are crosslinked simultaneously with some rapid and non-toxic crosslink methods, such as visible light crosslink [121] or “two photon crosslinking”. At this situation, the spatiotemporal distribution of cells and biomaterials are achieved to replace the missing tissue in cutaneous wounds (Fig. 5).
In a recent study, autologous epidermal keratinocytes and dermal fibroblasts were embedded in a hydrogel-related bioinks. An integrated imaging technology were used to scan topography information of excisional wounds. The gathered information was encoded into computerized files guiding the EBB system to precisely delivery tailored bioinks directly into appropriate locations restore the layered skin structures. Results showed rapid wound closure and reduced contraction [122].
In vivo bioprinting enable fast coverage large area of cutaneous wounds with therapeutic live cells and standardized complex structures, which possesses a significant advantage over in vitro bioprinting. Highly automation of in vivo bioprinting processes holds great potentials in cutaneous wound care especially in mass casualty burn incidents. In addition, possible damages of the predesigned 3D topology caused by the transportation and culturing of the printed constructs are also successfully avoided.
As in vitro cultured stem cells are vulnerable to differentiate and subject to losing stemness, in vivo bioprinting enable bioink-embedded stem cells to choose a defined fate because these stem cells are cultured in a known wound environment at the very beginning. By tuning biochemical or biophysical cues of bioinks, stem cell fates are promisingly tailored for optimal skin regeneration.
4.2. Bioinks for reducing scar
Skin scaring is defined as a pathological process involves the fibroblasts-to-myofibroblasts trans-differentiation and the extensive deposition and contraction of skin ECM secretion from myofibroblasts. However, to our best knowledge, no available literature of formulating bioink aiming at directly reducing scar outcomes were found. Here, we talk about promising elastin-related bioinks with potentials of reducing scar outcomes.
Currently available elastin for bioinks are mainly natural tropoelastin or synthetic elastin-like recombinamers (ELRs) [123]. The tropoelastin precursors generate elastin monomers and then further are crosslink into polymeric elastic networks, while ELRs are genetically engineered polypeptides offering versatile elastic-tailored applications for wound care.
Recent studies identified that both tropoelastin and ELRs present beneficial effects to favor skin wound healing. Tayebi et al. [124] performed rheology analyses and selected a specific bioink (comprising 8% gelatin, 2% elastin and 0.5% sodium hyaluronate) as the most suitable composition for 3D printing guided tissue regeneration. Lee et al. [125] mixed the recombinant human tropoelastin into a highly elastic bioink for 3D printing to successfully generate a vascularized construct for establishing functional soft tissues. Chen et al. [126] integrated GelMA hydrogel with an ulvan type polysaccharide from a cultivated source of a specific Australian Ulvacean macroalgae. Results showed that this bioink embedded with human dermal fibroblasts promoted the deposition of elastin. Wu et al. [127] developed self-assembling bioink based on ELRs and graphene oxide with or without human umbilical vascular endothelial cells. The ELR-related bioink was printed in a 3D bioprinting approach to generate capillary-like structures.
Despite emerging versatile usages of tropoelastin and ELRs in bioinks, limited literature has been hitherto identified their therapeutic effect to recovery elasticity of the regenerated skin.
4.3. Bioinks for vascularization
Vascularization plays a critical role in skin regeneration and wound healing process. Vascular networks should be generated to connect wound bed tissues and printed constructs in order to provide nutrients and oxygen and remove metabolic wastes for bioink-embedded cells. Novel 3D bioprinting approaches for developing perfusable vascularized skin constructs mainly involve two aspects, i.e., vasculature structures with branched and convective channels, and stem cells integrated with chemical or mechanical cues [10].
4.3.1. Vasculature structures with branched and convective channels
Natural cutaneous vascular system is a hierarchical perfusable network with anatomically heterocellular structures, such as endothelium, vascular adventitia, vessel walls as well as widely distributed capillary vessels. Inspired by that, the most endeavor of bioprinting approaches for vascularization is devoted to generate lumen-containing strands with branched vasculature structures and convective diffusive transportation [128].
Recent approaches focused on embedding and removing sacrificial materials to generate vascular networks [129]. However, the topological channels are much different from natural vascular lumen. Moreover, this kind of approach can only fabricate limited vascularized tissue with temporally opened channels. With the proliferation and immigration of cells and the infiltration of tissue fluid, the channels were easily subject to block. Hence, attentions have to be paid to employ new bioinks to generate bioprinted vascular skin substitutes with small-diameter lumen and fully biological functions [130].
4.3.2. Stem cells integrated with chemical or mechanical cues
Various stem cells have been employed for creating vascularization in 3D bioprinted skin constructs, e.g., amniotic fluid-derived stem cells, human umbilical vascular endothelial cells, induced pluripotent stem cell-derived endothelial cells and MSCs [131].
Current studies highlighted the crucial roles of chemical cues endowed by cellular crosstalk or growth factors for directing stem cells towards vascular-specific lineages. For example, the interplay between these stem cells and surrounding cells (i.e., fibroblasts or epithelial cells) are indispensable for stem cell differentiation [132]. Another approach utilized laser-assisted bioprinting to pattern lines of cocultured endothelial cells and MSCs seeded onto a collagen hydrogel, which indicated that a sufficient local density of endothelial cells are indispensable for generating capillary-like structures [131]. Furthermore, adding exogenous vascular endothelial growth factors allowed capillary-like structures formation and preservation of the printed pattern over time.
In addition, mechanical cues (i.e., flow shear stress) involved in dynamic culture of blood vessels have long been neglected. Despite various chemical cues are able to direct stem cells to vascular-specific lineages, the newly-formed capillary networks are inaccessible with limited convective channels due to their spontaneous and random formation. Mori et al. [133] developed an endothelial-cell-coated vascular system under dynamic culturing environment by using a perfusion system composed of a peristaltic pump and silicone tubes, which highlighted that dynamic culturing with flow shear stress favored perfusable vascularization in vitro.
Above all, the cellular microenvironment comprised of both chemical cues (i.e., cellular crosstalk or growth factors) or mechanical cues (i.e., flow shear stress) play crucial roles for bio-functional blood vessels.
4.4. Bioinks for regeneration of skin appendages
4.4.1. Sweat gland regeneration
Sweat glands originate from epidermal progenitors and perform a crucial role of thermoregulatory function in mammals [134]. However, sweat glands exhibits limited regenerative potential and are very vulnerable to injuries. Patients suffer from a large area burn injury could result in sweat gland dysfunctions and are unable to tolerate temperature extremes. The regeneration of sweat glands remains challenging for traditional tissue engineering applications [135].
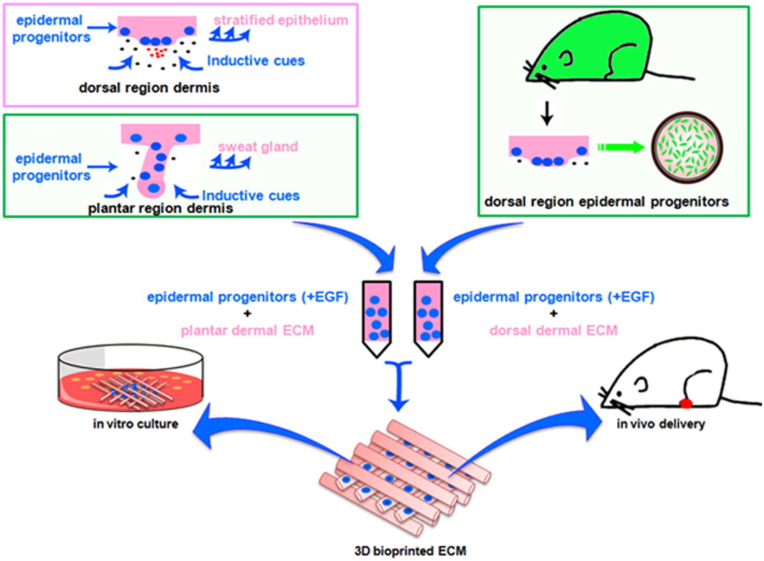
To our best knowledge, the first successful 3D-printed application of in vivo sweat gland regeneration in burned wounds came from Huang et al. [136]. As mouse sweat glands are restrictedly distributed in the paw pads of mouse, a home-made soluble dermal tissue homogenate was isolated from mouse plantar dermis, which is mainly comprised as dermis-derived ECM severing as one of the sweat gland-specific microenvironmental factors. Then, the dermal tissue homogenate together with epidermal growth factor were synchronously incorporated into gelatin-sodium alginate composite hydrogels. Epidermal progenitor cells were embedded in the hydrogel-related bioink for a BEE approach. Direct transplantation of the bioprinted constructs into burned paws of mice successfully resulted in functional restoration of sweat glands. In this research, the sweat gland-specific microenvironment or niche (i.e., the printed 3D porous constructs integrated with dermal tissue homogenate, epidermal growth factors and alginate/gelatin composite hydrogels) was able to direct the fate of epidermal progenitor cells into sweat gland lineages (Fig. 6). A later study of the same team identified that the artificial sweat gland-specific microenvironment also directly reprogramed mammary progenitor cells into functional sweat glands in vitro [137].
Fig. 6.
3D printed alginate/gelatin constructs integrated with dermal tissue homogenates and epidermal growth factors jointly directed the fate of epidermal progenitor cells into sweat gland lineages. Cited from Ref. [136].
Previously, chemical cues were mainly utilized to create sweat gland-specific microenvironment, i.e., by formulating a variety of soluble chemicals, such as growth factors, cytokines, drugs, etc. However, recent progress also highlighted the roles of physical cues for directing sweat gland fates. Liu et al. [138] found that the structural cues with defined 3D architecture are necessary for guiding the self-organized formation of epidermal progenitor-derived sweat glands in vitro. In another study, biophysical and biochemical cues synergistically work on MSC differentiation into sweat gland cells in a printed 3D skin constructs using alginate/gelatin bioinks. Higher stiffness probably activates the expression of Yes-associated protein (YAP) and its transcriptional co-activator tafazzin (TAZ), a pair of key proteins with synergistic effects on the pathways of mechano-transduction. Then, YAP/TAZ up-regulate proteins and genes related to sweat gland cells in MSCs or strengthen the biochemical signaling involved in MSC fate determination [35].
Hence, sweat gland-specific microenvironment integrated both chemical- and physical-cues is critical for restoring the biofunctions of regeneration sweat glands.
4.4.2. Hair follicle regeneration
Hair follicles are comprised of hair papillae, hair matrix, root sheath and hair bulges. Hair follicles reside in the dermal layer of the skin serving as important functional skin appendages and playing roles in barrier function, thermoregulation as well as wound healing [139]. Hair follicle loss caused by wound or trauma greatly affect cosmetic appearance. Hence, the endeavors devoted to restore or regenerate hair follicles are much more than any other skin appendage [140].
Dermal papilla cells (DPCs) and hair follicle stem cells serves as availably promising stem cells or progenitors for hair follicle regeneration [141]. Previous studies identified that the dissociated human DPCs induce hair follicle neogenesis in grafted dermal-epidermal composites [141]. Another study reported that intracutaneously transplanting of intact DPCs and epithelial cells resulted in a fully functional regeneration of hair follicles even with piloerection and restored hair cycles. Also, the newly isolated hair follicle stem cells mixed with dermal cells was identified to induce hair follicles regeneration [140].
Current research highlighted the indispensable role of 3D-cultured DPC spheroids and specific microenvironment for hair follicle regeneration. For example, the human DPC cultured in 3D spheroids can initiate the epidermal−mesenchymal interactions required for hair follicle morphogenesis and successfully induce de novo hair formation when placed in contact with human epidermis [142]. Notably, if the 3D cultured DPC spheroids was dissociated, hair follicles cannot be regenerated.
Due to the superior abilities of controlling inner microstructures and architectures of printed constructs, 3D bioprinting exhibits a powerful tool to achieve optimal hair follicle regeneration. In order to maintain DPCs-aggregated spheroids and precisely control their sizes, Abaci et al. [143] microfabricated plastic molds containing hair follicle-shaped extensions using 3D bioprinting. An array of microwells on a dermal fibroblast-loaded collagen gel was created by the plastic molds. Then, DPCs were seeded into the bottom of these microwells resulting in the DPCs-aggregated spheroids. The precise control of DPC aggregate size was achieved by adjusting the diameter of the microwells. Despite a great many successful attempts to restore bio-functional hair follicles using traditional fabrications, 3D bioprinting-based applications for regenerating hair follicles are so rare.
5. Challenges and future perspectives
5.1. Mimicking natural skin environment
In order to mimic natural skin environment, the optimal printed skin equivalent should be doped with a living pool of microenvironment factors (e.g., cells, chemokines, growth factors, oxygen, ECM, mechanical forces). However, some compromises have to be frustratingly made in order to achieve a balance between biomimicry and manufacturability [144]. Especially, the inclusion of skin microenvironment factors in bioink-related EBB applications is often neglected, broadening the disparity between the in vitro and in vivo environments.
The biofunctions of printed skin constructs are demonstrated by the complex spatial interplay between cells (i.e., exogenous cells from bioinks and endogenous cells immigrated from adjacent wound sites) and microenvironmental factors [145]. Hence, great efforts have been paid for adding a cocktail of microenvironmental factors into bioprinted skin substitutes. Recently, platelet-rich plasma (PRP) and decellularized ECM serves as cocktails of soluble and non-soluble microenvironmental factors exhibiting promising potential for creating biomimetic natural skin environment [146].
5.1.1. PRP-related bioinks
The PRP consists of highly concentrated platelets and the panoply of platelet-derived growth factors and cytokines [147]. The activation of the platelets suspended in PRP releases several growth factors and differentiating cytokines, setting off a chain of bio-reactions that accelerate wound healing [148]. Allogeneic PRP was identified to be superior to plasma or platelets alone in stimulating the proliferation of fibroblasts, the sprouting of endothelial cells in fibrin gels, and the chemotaxis of white blood cells [149]. Karina et al. [150] found that the combination of PRP with the stromal vascular fractions significantly promoted the regeneration of skin appendages, blood vessels, and hair growth in Sprague-Dawley rats. Moreover, clinical trials have indicated that PRP benefited wound healing and reduced scar outcomes. A recent clinical study enrolling 30 patients supported PRP's superior therapeutic outcome about wound healing and scar reduction at split-thickness skin graft donor sites [147]. Another prospective and randomized clinical study enrolling 60 patients also evidenced that allogeneic PRP applications significantly accelerated the healing of chronic wounds previously subjected to standard clinical treatments [151].
With the emerging concept of personalized medicine, bioinks containing autologous PRP attracted more attentions serving as a patient-specific source of autologous growth factors because of the convenience that PRP can be easily incorporated to hydrogel-related bioinks for 3D bioprinting applications. Faramarzi et al. [152] developed an bioink comprised of alginate and PRP that can be printed and crosslinked by calcium ions aiming to promote vascularization and stem cell migration. In another attempt to formulate biomimetic bioinks, gelatin-alginate and PRP were utilized to mimic the insoluble and soluble factors of natural microenvironment, respectively. The gelatin-alginate-PRP cocktails serve as promising bioinks in terms of printability, shape fidelity, and cell activities in cell-laden 3D bioprinting [153]. In addition, another group aimed to enhance the vascularization of 3D printed cell-laden bioinks because insufficient oxygen and nutrient supply hamper a long-term viability of encapsulated cells. They integrated PRP and mesenchymal stem cells (MSCs) together with biocompatible polylactic acid as novel bioinks, which contributed to extensive vascularization after being subcutaneously implanted either in rats or in nonhuman primates [154].
Despite the good therapeutic outcomes of PRP in terms of wound healing, conflicting results of autologous fat grafts arose some concerns about PRP's therapeutic effectiveness. A clinical trial study enrolling 10 patients investigated the safety and effectiveness of utilizing PRP combined with autologous fat grafts to face and hands [155]. The results only offered a third level of evidence and indicated the further requirements of longer follow-up periods and of a greater patients' number. Other concerns came from the pre-clinical report by Lopez et al. [156] that administering higher proportions of PRP and higher platelet levels together with micro-fat grafts associated with a poor graft survival. Moreover, the donor variation and PRP's changeable platelet content further contribute to its undocumented therapeutic efficiency.
5.1.2. ECM-related bioinks
ECM is a mixture of molecular components secreted by host's cells with tissue-specific spatial topology as well as soluble microenvironmental factors [157]. The skin-derived ECM, also called acellular dermal matrix (ADM), is generated by removing cellular components from the dermis, which has a low immunogenicity and provides natural scaffolds for cellular proliferation, differentiation, and migration. Compared with other man-made biomaterials, the skin-derived ECM is endowed with the best biomimetic components, ligands, chemical signals, and physical properties, which help reconstitute a natural skin microenvironment. Eweida et al. [158] reviewed twelve studies from 2010 to 2015 focusing on the therapeutic outcomes of ECM for dermal regeneration and identified that ECM scaffolds exhibit superior therapeutic effects for wound healing.
In terms of 3D bioprinting for skin regeneration, Kim et al. [159] formulated skin-derived ECM as bioinks for a full thickness 3D human skin model using cell-printing techniques. In vitro experiments identified the enhanced epidermal organization and dermal ECM secretion. Next, the ECM-related bioink integrated with endothelial progenitor cells and adipose-derived stem cells was further used to print 3D pre-vascularized skin patch, which significantly promoted in vivo wound re-epithelization and neovascularization. Similarly, Lee et al. [160] developed bio-ink containing decellularized porcine skin powder, which exhibited enhanced printability and biocompatibility for 3D bioprinting.
However, ADM is generated from decellularized skin tissue. Its clinical use is still confronted by lots of hurdles, such as shortage of raw materials, lack of a standardized production procedure, potential bio-risks of transmitting infectious diseases, etc.
5.2. Four-dimensional (4D) printing
The novel four-dimensional (4D) printing enables on-going control over the changing of the printed constructs, which seems like to integrate the fourth dimension of “time” to traditional 3D printing [161]. 4D printing (time as the fourth dimension) endows the printed constructs to transform biofunctions or to produce the desired shapes under specific stimuli to better adapt to the surrounding environment by preprogramming dynamic bioinks [162]. To achieve that, 4D printing should be integrated with smart biomaterials-related dynamic bioinks, which not only deliver targeted bioactive molecules but also respond to cellular behaviors over time.
5.2.1. Dynamic bioinks
The rationale of dynamic bioinks are categorized as three aspects, i.e., shape memory properties, self-healing abilities, or stimuli-responsive strategies [163], aiming at continuously regulating and programming the 3D printed constructs. Among them, stimuli-responsive strategies have been paid the most attention for the emerging 4D printing in the field of skin regeneration and wound care [164]. Dynamic bioinks that are enforced with stimuli-responsive strategies possess intelligently tailored properties which can be triggered by external or internal stimuli to exert newly-generated activities on cells and tissues [165]. Usually, they are composite constructs having the ability to respond to environmental agents triggering the release of growth factors, antibacterial compounds, anti-inflammatory drugs, etc.
Many kinds of growth factors, cytokines, molecules, enzymes are spatiotemporally involved in the wound healing process. Inspired by these natural processes, a spatiotemporal delivery of defined drugs during the different stages of the healing process might recreate an internal biomimetic microenvironment. Hence, dynamic bioinks integrated with spatiotemporal drug delivery hold great potentials to achieve better wound healing outcomes.
5.2.2. Stimuli-responsive strategies
The stimuli or triggers are categorized as external stimuli and internal stimuli [161]. External stimuli include light, pressures, voltage, piezoelectricity, shape changes, etc. While internal stimuli include pH levels, temperature, chemical molecules, etc. Regarding wound healing processes, these stimuli can trigger the on-demand therapeutic procedures, such as spatiotemporal drug delivery aiming at fighting resistant bacteria and/or at promoting angiogenesis [166].
The candidates of stimuli or triggers is generally referring to wounds' distinct target features due to ischemia, inflammation or bacterial activity. For example, the typical characteristics of diabetic patients’ wounds are higher amounts of lactic acids, glucose, proteases, and matrix metalloproteinases, as well as lower pH levels because of the concurrent harm to capillaries and peripheral nerves. Among all the peculiar characteristics, pH level changes play a vital role by revealing pathophysiological changes occurring during the transformation of acute wounds into chronic wounds due to infection, ischemia or inflammation [162]. Reportedly, pH levels exhibit a potential capacity for monitoring the alkalization process, which involves the typical pathophysiological pH changing when fresh wounds progress to chronic wounds in diabetic mice. Hence, pH level serves as the most commonly used stimulus.
Mirani et al. [167] developed a pH-responsive hydrogel-based wound dressing, which releases antibacterial agents to wounds triggered by a pH-dependent color change. Zhu et al. [168] utilized peptide-based bis-acrylate and acrylic acid to fabricate a novel hybrid hydrogel with pH-responsive swelling properties. In higher pH (alkaline) environments, the hydrogel swelling ratio significantly increased resulting in a controlled drug delivery. Another approach by Kiaee et al. [169] showed that a pH-responsive drug release can be controlled by applying a voltage. Specifically, a pH-sensitive poly(ethylene glycol)-diacrylate/laponite hydrogel incorporated with drug-loaded chitosan nanoparticles was coated on the surface of electrodes. Applying a voltage can change the environmental pH, which next triggers the release of defined amounts of drugs. Recently, combining a chemo-photothermal therapy with pH-responsive capabilities based on polydopamine-coated gold nanorods inspired another advanced design concept of wound dressings focusing on anti-infectious activity [170]. During the fabrication procedure, dopamine was first self-assembled into polydopamine and next coated onto the surface of gold nanorods to acquire a high loading efficiency in regard to glycol chitosan and Ag+ ions. Cy5-SE fluorescent agents were used to label the integrated system. When exposed to infectious environments with lowered pH levels (pH ∼ 6.3), the neutrally charged system starts to become positively charged which triggers the release of loaded Ag+ ions. Interestingly, a positive feedback loop then occurs. On the one hand, Ag+ ions damage bacterial membranes thus improving the antimicrobial efficiency of the photothermal therapy. On the other hand, the hyperthermia increases Ag+ ions release further improving Ag+ ions antibacterial efficiency.
In addition to pH-responsive strategies, usually molecules or enzymes exhibit biochemical activities that cleave ionic or covalent bonds, serving as a property enabling potential applications also in stimuli-responsive wound care. For example, wounds can contain higher amounts of ROS, which break thioketal bonds. Based on this mechanism, Tang et al. [171] developed ROS-responsive nanoparticles containing thioketals and stromal cell-derived factor-1α. When these nanoparticles are applied to wounded tissues, ROS break thioketal bonds and then release stromal cell-derived factor-1α. A mice model of full-thickness skin defect showed that the wound's higher amounts of ROS-released stromal cell-derived factor-1α did accelerate wound healing. Similarly, Li et al. [172] developed stimulus-triggered liposomal hydrogels that release Phospholipase A2, an enzyme enriched in wound exudates. Next, Phospholipase A2 hydrolyzes the liposomal lecithin, thereby destroying the liposomes and releasing curcumin to kill bacteria in infected wounds. Liu et al. [173] reported a near-infrared (NIR) laser triggered on-demand drug release for wound healing and cancer therapy. Briefly, a homogeneous layer of polycaprolactone was coated on a 3D printed alginate-gelatin hydrogel scaffolds aiming to reduce the free diffusion of drugs from the core gels. Then, polydopamine was coated throughout the outermost layer endowing the 3D printed structs with photothermal effects triggered by near-infrared (NIR) laser. In another study, a 3D printed temperature-responsive hydrogel achieved sustained delivery of antimicrobial agents, which demonstrated antimicrobial activity against a variety of commonly seen bacteria in wounds and exhibited non-cytotoxicity towards fibroblasts [174].
The cutting-edge 4D printing has opened up a new window to fabricate very complex constructs with dynamic revolutions or biofunctions to mimic natural skin architectures as well as pathological conditions, such as injuries and scars [175]. However, the potential bio-risks for stimuli-responsive strategy are the non-targeted delivery triggered by adverse analogues of stimuli. Moreover, brief burst releases of drugs pose a great risk. Generally, the spatiotemporally controlled delivery of suitable doses of drugs to targeted sites is highly dependent on the specific strategy used. Optimal pharmacokinetics require to ingeniously control the delivery of each drug. The more numerous are the incorporated drugs, the more complex the drug releasing platform needs to be.
5.3. Skin organoids and on-a-chip technologies
Nowadays, multidisciplinary approaches have been utilized for enforcing EBB, which also called hybrid 3D bioprinting [176] and have opened up new avenues toward advanced wound care or skin regeneration. Among these attempts, skin organoids [177] and on-a-chip technologies [71] integrated with EBB has presented an unprecedented potential for recapitulating key features during skin regeneration or creating more complex 3D constructs mimicking natural heterogeneous microenvironments.
Organoids are defined as the well-organized and dynamically changing in vitro 3D cell constructs with generally small-scale aiming to represent in vivo tissues or organs [178]. Very recently, the skin organoid bearing appendages (i.e., hair follicles and glands) were firstly reported, which were generated from pluripotent stem cells through step-wisely modulating transforming growth factor β (TGFβ) and fibroblast growth factor (FGF) signaling pathways [177]. Skin organoids represent the bio-processes that the dissociated stem cells or skin progenitors undergo in vitro self-organization resulting in a skin-mimetic phenotype [179], which is not identical to embryonic skin formation [135]. In other words, the morphologies of skin organoids are jointly specified by the cross talks between the genetic codes endowed by stem cells and the biophysical cues endowed by the microenvironments or specific architectures [180]. Hence, it is predictable that enormous progress of skin organoids will be contributed by 3D printing technologies due to their superior abilities about controlling spatial inner architectures. Moreover, 3D printing technologies are perfectly qualified in terms of the generally smaller sizes of skin organoids and the demands of the repeatability with high throughputs [181].
On this basis, the on-a-chip technologies enable the connection of multiple organoids of different tissue types through microfluidic devices, which will uncover a more complete mechanism of how the body as a whole may respond to therapeutics [178]. The currently related achievements are focused on multi-organ interactions [182], human disease models [183] and cancer metastatic models [184]. Inspired by that, different types of 3D-printed organoids, such as skin, subcutaneous tissues, capillary vessels, peripheral nerves, etc., could be connected on the microfluidic platforms to explore possible solutions of some key challenges, such as tailored drug screening, optimal wound healing with reduced scaring, or skin regeneration with fully restored appendages, etc.
6. Discussion
The final goal for cutaneous wound care is to generate functionally ideal skin equivalents. Although 3D bioprinting techniques stand out for unique advantages, some other limitations of EBB-based bioinks still exit in cutaneous wound care, such as restricted biomaterials choice, inadequate vascularization, and limited strand resolutions of the printed constructs. In addition, there is an antagonism between print fidelity and cell survival for hydrogel-related bioinks. As hydrogels exhibit comparatively insufficient mechanical strength, higher content of hydrogen polymers is favorable in order to increases the viscosity and mechanical properties of bioinks, which also limits the diffusion of nutrients or oxygen and decreases cell spreading [24]. Just like Morgan et al. [185] said that “current bioinks are often engineered from what we have, rather than designed for what we need”.
Great endeavor should be devoted into transforming laboratory 3D bioprinting into clinical applications. The global 3D bioprinting market is expected to reach $1.82 billion in 2022 [130]. Unfortunately, 3D bioprinting of skin equivalents is still in the early phase of laboratory research and are far from being available to clinical settings [144]. Also, it is such a pity that clinical trials concerning the use of bioprinting for skin conditions in humans are so rare. One of the insurmountable obstacles is human immune rejections against bioprinted transplants, which are mediated by numerous cell types such as B-cells, T-cells, neutrophils, dendritic cells, natural killer cells, macrophages, and molecular signals such as cytokines, antibodies, and reactive radical species [186]. Notably, human immune responses are against both biomaterials and embedded cells after the transplantation. Surface properties of implants, such as the surface roughness and wettability [187], and the surface-coated degrading products [188], generally dominate the processes of immune responses. Further researches focusing on mitigating immune rejections, such as macrophage activation, polarization, and immune modulation, are promising solutions for these challenges.
Contributor roles taxonomy author statement
Yuzhen Wang: Writing - original draft, Conceptualization, Visualization. Xingyu Yuan: Writing - original draft. Bin Yao: Writing - original draft, Writing - review & editing. Shuoji Zhu: Supervision. Ping Zhu: Supervision, Resources. Sha Huang: Writing - review & editing, Supervision, Resources, Funding acquisition.
Declaration of competing interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgements
This study was supported by the Beijing Natural Science Foundation (7204306), National Natural Science Foundation of China (82002056, 81830064, 81721092, 32000969, 81974019), National Key Research and Development Program of China (2018YFA0108700, 2017YFA0105602), NSFC Projects of International Cooperation and Exchanges (81720108004), China Postdoctoral Science Foundation (2020M673672), Key Support Program for Growth Factor Research (SZYZ-TR-03), Chinese PLA General Hospital for Military Medical Innovation Research Project (CX19026), the CAMS Innovation Fund for Medical Sciences (CIFMS, 2019-I2M-5-059), the Military Medical Research and Development Projects (AWS17J005), the Research Team Project of Natural Science Foundation of Guangdong Province of China (2017A030312007), the key program of guangzhou science research plan (201904020047). The Special Project of Dengfeng Program of Guangdong Provincial People's Hospital (DFJH201812, KJ012019119, KJ012019423).
Footnotes
Peer review under responsibility of KeAi Communications Co., Ltd.
Contributor Information
Ping Zhu, Email: tanganqier@163.com.
Sha Huang, Email: stellarahuang@sina.com.
References
- 1.Heyer K., Herberger K., Protz K., Glaeske G., Augustin M. Epidemiology of chronic wounds in Germany: analysis of statutory health insurance data. Wound Repair Regen. 2016;24(2):434–442. doi: 10.1111/wrr.12387. [DOI] [PubMed] [Google Scholar]
- 2.Sen C.K. Human wounds and its burden: an updated compendium of estimates. Adv. Wound Care. 2019;8(2):39–48. doi: 10.1089/wound.2019.0946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sen C.K. Human wound and its burden: updated 2020 compendium of estimates. Adv. Wound Care. 2021;10(5):281–292. doi: 10.1089/wound.2021.0026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jiang Y., Huang S., Fu X., Liu H., Ran X., Lu S., Hu D., Li Q., Zhang H., Li Y., Wang R., Xie T., Cheng B., Wang L., Liu Y., Ye X., Han C., Chen H. Epidemiology of chronic cutaneous wounds in China. Wound Repair Regen. 2011;19(2):181–188. doi: 10.1111/j.1524-475X.2010.00666.x. [DOI] [PubMed] [Google Scholar]
- 5.Fisch P., Holub M., Zenobi-Wong M. Biofabrication; 2020. Improved Accuracy and Precision of Bioprinting through Progressive Cavity Pump-Controlled Extrusion. [DOI] [PubMed] [Google Scholar]
- 6.Matai I., Kaur G., Seyedsalehi A., McClinton A., Laurencin C.T. Progress in 3D bioprinting technology for tissue/organ regenerative engineering. Biomaterials. 2020;226:119536. doi: 10.1016/j.biomaterials.2019.119536. [DOI] [PubMed] [Google Scholar]
- 7.Ning L., Chen X. A brief review of extrusion-based tissue scaffold bio-printing. Biotechnol. J. 2017;12(8) doi: 10.1002/biot.201600671. [DOI] [PubMed] [Google Scholar]
- 8.Pati F., Jang J., Ha D.H., Won Kim S., Rhie J.W., Shim J.H., Kim D.H., Cho D.W. Printing three-dimensional tissue analogues with decellularized extracellular matrix bioink. Nat. Commun. 2014;5:3935. doi: 10.1038/ncomms4935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Byambaa B., Annabi N., Yue K., Trujillo-de Santiago G., Alvarez M.M., Jia W., Kazemzadeh-Narbat M., Shin S.R., Tamayol A., Khademhosseini A. Bioprinted osteogenic and vasculogenic patterns for engineering 3D bone tissue. Adv. Healthc Mater. 2017;6(16) doi: 10.1002/adhm.201700015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Weng T., Zhang W., Xia Y., Wu P., Yang M., Jin R., Xia S., Wang J., You C., Han C., Wang X. 3D bioprinting for skin tissue engineering: current status and perspectives. J. Tissue Eng. 2021;12 doi: 10.1177/20417314211028574. 20417314211028574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Smandri A., Nordin A., Hwei N.M., Chin K.Y., Abd Aziz I., Fauzi M.B. Natural 3D-printed bioinks for skin regeneration and wound healing: a systematic review. Polymers (Basel) 2020;12(8) doi: 10.3390/polym12081782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Datta P., Ayan B., Ozbolat I.T. Bioprinting for vascular and vascularized tissue biofabrication. Acta Biomater. 2017;51:1–20. doi: 10.1016/j.actbio.2017.01.035. [DOI] [PubMed] [Google Scholar]
- 13.Wang Y., Xu R., Luo G., Lei Q., Shu Q., Yao Z., Li H., Zhou J., Tan J., Yang S., Zhan R., He W., Wu J. Biomimetic fibroblast-loaded artificial dermis with "sandwich" structure and designed gradient pore sizes promotes wound healing by favoring granulation tissue formation and wound re-epithelialization. Acta Biomater. 2016;30:246–257. doi: 10.1016/j.actbio.2015.11.035. [DOI] [PubMed] [Google Scholar]
- 14.Park Y.R., Ju H.W., Lee J.M., Kim D.K., Lee O.J., Moon B.M., Park H.J., Jeong J.Y., Yeon Y.K., Park C.H. Three-dimensional electrospun silk-fibroin nanofiber for skin tissue engineering. Int. J. Biol. Macromol. 2016;93(Pt B):1567–1574. doi: 10.1016/j.ijbiomac.2016.07.047. [DOI] [PubMed] [Google Scholar]
- 15.Augustine R. Skin bioprinting: a novel approach for creating artificial skin from synthetic and natural building blocks. Prog. Biomater. 2018;7(2):77–92. doi: 10.1007/s40204-018-0087-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Deo K.A., Singh K.A., Peak C.W., Alge D.L., Gaharwar A.K. Bioprinting 101: design, fabrication, and evaluation of cell-laden 3D bioprinted scaffolds, tissue engineering. Part A. 2020;26(5–6):318–338. doi: 10.1089/ten.tea.2019.0298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Duarte Campos D.F., Blaeser A., Weber M., Jäkel J., Neuss S., Jahnen-Dechent W., Fischer H. Three-dimensional printing of stem cell-laden hydrogels submerged in a hydrophobic high-density fluid. Biofabrication. 2013;5(1) doi: 10.1088/1758-5082/5/1/015003. [DOI] [PubMed] [Google Scholar]
- 18.Hong S., Kim J.S., Jung B., Won C., Hwang C. Coaxial bioprinting of cell-laden vascular constructs using a gelatin-tyramine bioink. Biomater. Sci. 2019;7(11):4578–4587. doi: 10.1039/c8bm00618k. [DOI] [PubMed] [Google Scholar]
- 19.Marga F., Jakab K., Khatiwala C., Shepherd B., Dorfman S., Hubbard B., Colbert S., Gabor F. Toward engineering functional organ modules by additive manufacturing. Biofabrication. 2012;4(2) doi: 10.1088/1758-5082/4/2/022001. [DOI] [PubMed] [Google Scholar]
- 20.Rastogi P., Kandasubramanian B. Review of alginate-based hydrogel bioprinting for application in tissue engineering. Biofabrication. 2019;11(4) doi: 10.1088/1758-5090/ab331e. [DOI] [PubMed] [Google Scholar]
- 21.Habib A., Sathish V., Mallik S., Khoda B. 3D printability of alginate-carboxymethyl cellulose hydrogel. Materials. 2018;11(3) doi: 10.3390/ma11030454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Giuseppe M.D., Law N., Webb B., R A.M., Liew L.J., Sercombe T.B., Dilley R.J., Doyle B.J. Mechanical behaviour of alginate-gelatin hydrogels for 3D bioprinting. J. Mech. Behav. Biomed. Mater. 2018;79:150–157. doi: 10.1016/j.jmbbm.2017.12.018. [DOI] [PubMed] [Google Scholar]
- 23.Wu Z., Xie S., Kang Y., Shan X., Li Q., Cai Z. Biocompatibility evaluation of a 3D-bioprinted alginate-GelMA-bacteria nanocellulose (BNC) scaffold laden with oriented-growth RSC96 cells. Mater. Sci. Eng. C, Mater. Biol. Appl. 2021;129:112393. doi: 10.1016/j.msec.2021.112393. [DOI] [PubMed] [Google Scholar]
- 24.Ouyang L., Yao R., Zhao Y., Sun W. Effect of bioink properties on printability and cell viability for 3D bioplotting of embryonic stem cells. Biofabrication. 2016;8(3) doi: 10.1088/1758-5090/8/3/035020. [DOI] [PubMed] [Google Scholar]
- 25.Kirchmajer D.M., Gorkin R., Iii M., Het Panhuis In. An overview of the suitability of hydrogel-forming polymers for extrusion-based 3D-printing. J. Mater. Chem. B. 2015;3(20):4105–4117. doi: 10.1039/c5tb00393h. [DOI] [PubMed] [Google Scholar]
- 26.Göhl J., Markstedt K., Mark A., Håkansson K., Gatenholm P., Edelvik F. Simulations of 3D bioprinting: predicting bioprintability of nanofibrillar inks. Biofabrication. 2018;10(3) doi: 10.1088/1758-5090/aac872. [DOI] [PubMed] [Google Scholar]
- 27.Lee S.C., Gillispie G., Prim P., Lee S.J. Physical and chemical factors influencing the printability of hydrogel-based extrusion bioinks. Chem. Rev. 2020;120(19):10834–10886. doi: 10.1021/acs.chemrev.0c00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Klar V., Pere J., Turpeinen T., Kärki P., Orelma H., Kuosmanen P. Shape fidelity and structure of 3D printed high consistency nanocellulose. Sci. Rep. 2019;9(1):3822. doi: 10.1038/s41598-019-40469-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shiwarski D.J., Hudson A.R., Tashman J.W., Feinberg A.W. Emergence of FRESH 3D printing as a platform for advanced tissue biofabrication. APL Bioeng. 2021;5(1) doi: 10.1063/5.0032777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vedadghavami A., Minooei F., Mohammadi M.H., Khetani S., Rezaei Kolahchi A., Mashayekhan S., Sanati-Nezhad A. Manufacturing of hydrogel biomaterials with controlled mechanical properties for tissue engineering applications. Acta Biomater. 2017;62:42–63. doi: 10.1016/j.actbio.2017.07.028. [DOI] [PubMed] [Google Scholar]
- 31.Ozbolat I.T., Yu Y. Bioprinting toward organ fabrication: challenges and future trends. IEEE Trans. Biomed. Eng. 2013;60(3):691–699. doi: 10.1109/TBME.2013.2243912. [DOI] [PubMed] [Google Scholar]
- 32.Raub C.B., Putnam A.J., Tromberg B.J., George S.C. Predicting bulk mechanical properties of cellularized collagen gels using multiphoton microscopy. Acta Biomater. 2010;6(12):4657–4665. doi: 10.1016/j.actbio.2010.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hou J., Ren X., Guan S., Duan L., Gao G.H., Kuai Y., Zhang H. Rapidly recoverable, anti-fatigue, super-tough double-network hydrogels reinforced by macromolecular microspheres. Soft Matter. 2017;13(7):1357–1363. doi: 10.1039/c6sm02739c. [DOI] [PubMed] [Google Scholar]
- 34.McBeth C., Lauer J., Ottersbach M., Campbell J., Sharon A., Sauer-Budge A.F. 3D bioprinting of GelMA scaffolds triggers mineral deposition by primary human osteoblasts. Biofabrication. 2017;9(1) doi: 10.1088/1758-5090/aa53bd. [DOI] [PubMed] [Google Scholar]
- 35.Liu Y., Li J., Yao B., Wang Y., Wang R., Yang S., Li Z., Zhang Y., Huang S., Fu X. The stiffness of hydrogel-based bioink impacts mesenchymal stem cells differentiation toward sweat glands in 3D-bioprinted matrix. Mater. Sci. Eng. C Mater. Biol. Appl. 2021;118:111387. doi: 10.1016/j.msec.2020.111387. [DOI] [PubMed] [Google Scholar]
- 36.Huang G., Li F., Zhao X., Ma Y., Li Y., Lin M., Jin G., Lu T.J., Genin G.M., Xu F. Functional and biomimetic materials for engineering of the three-dimensional cell microenvironment. Chem. Rev. 2017;117(20):12764–12850. doi: 10.1021/acs.chemrev.7b00094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Paszek M.J., Zahir N., Johnson K.R., Lakins J.N., Rozenberg G.I., Gefen A., Reinhart-King C.A., Margulies S.S., Dembo M., Boettiger D., Hammer D.A., Weaver V.M. Tensional homeostasis and the malignant phenotype. Cancer Cell. 2005;8(3):241–254. doi: 10.1016/j.ccr.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 38.Lui C., Lee K., Nelson C.M. Matrix compliance and RhoA direct the differentiation of mammary progenitor cells. Biomech. Model. Mechanobiol. 2012;11(8):1241–1249. doi: 10.1007/s10237-011-0362-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gilbert P.M., Havenstrite K.L., Magnusson K.E., Sacco A., Leonardi N.A., Kraft P., Nguyen N.K., Thrun S., Lutolf M.P., Blau H.M. Substrate elasticity regulates skeletal muscle stem cell self-renewal in culture. Science. 2010;329(5995):1078–1081. doi: 10.1126/science.1191035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Engler A.J., Carag-Krieger C., Johnson C.P., Raab M., Tang H.Y., Speicher D.W., Sanger J.W., Sanger J.M., Discher D.E. Embryonic cardiomyocytes beat best on a matrix with heart-like elasticity: scar-like rigidity inhibits beating. J. Cell Sci. 2008;121(Pt 22):3794–3802. doi: 10.1242/jcs.029678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liu Y., Li Z., Li J., Yang S., Zhang Y., Yao B., Song W., Fu X., Huang S. Stiffness-mediated mesenchymal stem cell fate decision in 3D-bioprinted hydrogels. Burns Trauma. 2020;8 doi: 10.1093/burnst/tkaa029. tkaa029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Xue M., Jackson C.J. Extracellular matrix reorganization during wound healing and its impact on abnormal scarring. Adv. Wound Care. 2015;4(3):119–136. doi: 10.1089/wound.2013.0485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sapudom J., Kalbitzer L., Wu X., Martin S., Kroy K., Pompe T. Fibril bending stiffness of 3D collagen matrices instructs spreading and clustering of invasive and non-invasive breast cancer cells. Biomaterials. 2019;193:47–57. doi: 10.1016/j.biomaterials.2018.12.010. [DOI] [PubMed] [Google Scholar]
- 44.De France K.J., Xu F., Hoare T. Structured macroporous hydrogels: progress, challenges, and opportunities. Adv. Healthc Mater. 2018;7(1) doi: 10.1002/adhm.201700927. [DOI] [PubMed] [Google Scholar]
- 45.Raut H.K., Das R., Liu Z., Liu X., Ramakrishna S. Biocompatibility of biomaterials for tissue regeneration or replacement. Biotechnol. J. 2020;15(12) doi: 10.1002/biot.202000160. [DOI] [PubMed] [Google Scholar]
- 46.Sekine H., Shimizu T., Sakaguchi K., Dobashi I., Wada M., Yamato M., Kobayashi E., Umezu M., Okano T. In vitro fabrication of functional three-dimensional tissues with perfusable blood vessels. Nat. Commun. 2013;4:1399. doi: 10.1038/ncomms2406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hospodiuk M., Dey M., Sosnoski D., Ozbolat I.T. The bioink: a comprehensive review on bioprintable materials. Biotechnol. Adv. 2017;35(2):217–239. doi: 10.1016/j.biotechadv.2016.12.006. [DOI] [PubMed] [Google Scholar]
- 48.Rodrigues M., Kosaric N., Bonham C.A., Gurtner G.C. Wound healing: a cellular perspective. Physiol. Rev. 2019;99(1):665–706. doi: 10.1152/physrev.00067.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Madl C.M., LeSavage B.L., Dewi R.E., Dinh C.B., Stowers R.S., Khariton M., Lampe K.J., Nguyen D., Chaudhuri O., Enejder A., Heilshorn S.C. Maintenance of neural progenitor cell stemness in 3D hydrogels requires matrix remodelling. Nat. Mater. 2017;16(12):1233–1242. doi: 10.1038/nmat5020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Donderwinkel I., van Hest J.C.M., Cameron N.R. Bio-inks for 3D bioprinting: recent advances and future prospects. Polym. Chem. 2017;8(31):4451–4471. [Google Scholar]
- 51.Jeong K.H., Park D., Lee Y.C. Polymer-based hydrogel scaffolds for skin tissue engineering applications: a mini-review. J. Polym. Res. 2017;24(7):10. [Google Scholar]
- 52.Yao B., Hu T., Cui X., Song W., Fu X., Huang S. Enzymatically degradable alginate/gelatin bioink promotes cellular behavior and degradation in vitro and in vivo. Biofabrication. 2019;11(4) doi: 10.1088/1758-5090/ab38ef. [DOI] [PubMed] [Google Scholar]
- 53.Liu X., Zuo Y., Sun J., Guo Z., Fan H., Zhang X. Degradation regulated bioactive hydrogel as the bioink with desirable moldability for microfluidic biofabrication. Carbohydr. Polym. 2017;178:8–17. doi: 10.1016/j.carbpol.2017.09.014. [DOI] [PubMed] [Google Scholar]
- 54.Khalighi S., Saadatmand M. Bioprinting a thick and cell-laden partially oxidized alginate-gelatin scaffold with embedded micro-channels as future soft tissue platform. Int. J. Biol. Macromol. 2021 doi: 10.1016/j.ijbiomac.2021.11.046. [DOI] [PubMed] [Google Scholar]
- 55.Verbeke C.S., Mooney D.J. Injectable, pore-forming hydrogels for in vivo enrichment of immature dendritic cells. Adv. Healthc Mater. 2015;4(17):2677–2687. doi: 10.1002/adhm.201500618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Abasalizadeh F., Moghaddam S.V., Alizadeh E., Akbari E., Kashani E., Fazljou S.M.B., Torbati M., Akbarzadeh A. Alginate-based hydrogels as drug delivery vehicles in cancer treatment and their applications in wound dressing and 3D bioprinting. J. Biol. Eng. 2020;14:8. doi: 10.1186/s13036-020-0227-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lee J.M., Yeong W.Y. Design and printing strategies in 3D bioprinting of cell-hydrogels: a review. Adv. Healthc Mater. 2016;5(22):2856–2865. doi: 10.1002/adhm.201600435. [DOI] [PubMed] [Google Scholar]
- 58.Zhang Y.S., Khademhosseini A. Advances in engineering hydrogels. Science. 2017;356(6337) doi: 10.1126/science.aaf3627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chimene D., Lennox K.K., Kaunas R.R., Gaharwar A.K. Advanced bioinks for 3D printing: a materials science perspective. Ann. Biomed. Eng. 2016;44(6):2090–2102. doi: 10.1007/s10439-016-1638-y. [DOI] [PubMed] [Google Scholar]
- 60.Shirahama H., Lee B.H., Tan L.P., Cho N.J. Precise tuning of facile one-pot gelatin methacryloyl (GelMA) synthesis. Sci. Rep. 2016;6:31036. doi: 10.1038/srep31036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bertassoni L.E., Cardoso J.C., Manoharan V., Cristino A.L., Bhise N.S., Araujo W.A., Zorlutuna P., Vrana N.E., Ghaemmaghami A.M., Dokmeci M.R., Khademhosseini A. Direct-write bioprinting of cell-laden methacrylated gelatin hydrogels. Biofabrication. 2014;6(2) doi: 10.1088/1758-5082/6/2/024105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Preman N.K., E S.S., Prabhu A., Shaikh S.B., C V., Barki R.R., Bhandary Y.P., Rekha P.D., Johnson R.P. Bioresponsive supramolecular hydrogels for hemostasis, infection control and accelerated dermal wound healing. J. Mater. Chem. B. 2020;8(37):8585–8598. doi: 10.1039/d0tb01468k. [DOI] [PubMed] [Google Scholar]
- 63.Li H., Tan Y.J., Li L. A strategy for strong interface bonding by 3D bioprinting of oppositely charged κ-carrageenan and gelatin hydrogels. Carbohydr. Polym. 2018;198:261–269. doi: 10.1016/j.carbpol.2018.06.081. [DOI] [PubMed] [Google Scholar]
- 64.Hu T., Cui X., Zhu M., Wu M., Tian Y., Yao B., Song W., Niu Z., Huang S., Fu X. 3D-printable supramolecular hydrogels with shear-thinning property: fabricating strength tunable bioink via dual crosslinking. Bioact. Mater. 2020;5(4):808–818. doi: 10.1016/j.bioactmat.2020.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Jungst T., Smolan W., Schacht K., Scheibel T., Groll J. Strategies and molecular design criteria for 3D printable hydrogels. Chem. Rev. 2016;116(3):1496–1539. doi: 10.1021/acs.chemrev.5b00303. [DOI] [PubMed] [Google Scholar]
- 66.Müller M., Öztürk E., Arlov Ø., Gatenholm P., Zenobi-Wong M. Alginate sulfate-nanocellulose bioinks for cartilage bioprinting applications. Ann. Biomed. Eng. 2017;45(1):210–223. doi: 10.1007/s10439-016-1704-5. [DOI] [PubMed] [Google Scholar]
- 67.Cross L.M., Shah K., Palani S., Peak C.W., Gaharwar A.K. Gradient nanocomposite hydrogels for interface tissue engineering. Nanomed. Nanotechnol. Biol. Med. 2018;14(7):2465–2474. doi: 10.1016/j.nano.2017.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Means A.K., Grunlan M.A. Modern strategies to achieve tissue-mimetic, mechanically robust hydrogels. ACS Macro Lett. 2019;8(6):705–713. doi: 10.1021/acsmacrolett.9b00276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Li J., Zhang Y., Enhe J., Yao B., Wang Y., Zhu D., Li Z., Song W., Duan X., Yuan X., Fu X., Huang S. Bioactive nanoparticle reinforced alginate/gelatin bioink for the maintenance of stem cell stemness. Mater. Sci. Eng. C, Mater. Biol. Appl. 2021;126:112193. doi: 10.1016/j.msec.2021.112193. [DOI] [PubMed] [Google Scholar]
- 70.Zeimaran E., Pourshahrestani S., Fathi A., Razak N., Kadri N.A., Sheikhi A., Baino F. Advances in bioactive glass-containing injectable hydrogel biomaterials for tissue regeneration. Acta Biomater. 2021 doi: 10.1016/j.actbio.2021.09.034. [DOI] [PubMed] [Google Scholar]
- 71.Bonaccorso F., Colombo L., Yu G., Stoller M., Tozzini V., Ferrari A.C., Ruoff R.S., Pellegrini V. 2D materials. Graphene, related two-dimensional crystals, and hybrid systems for energy conversion and storage. Science. 2015;347(6217):1246501. doi: 10.1126/science.1246501. [DOI] [PubMed] [Google Scholar]
- 72.Oudadesse H., Najem S., Mosbahi S., Rocton N., Refifi J., El Feki H., Lefeuvre B. Development of hybrid scaffold: bioactive glass nanoparticles/chitosan for tissue engineering applications. J. Biomed. Mater. Res. 2021;109(5):590–599. doi: 10.1002/jbm.a.37043. [DOI] [PubMed] [Google Scholar]
- 73.Ouyang L., Armstrong J.P.K., Lin Y., Wojciechowski J.P., Lee-Reeves C., Hachim D., Zhou K., Burdick J.A., Stevens M.M. Expanding and optimizing 3D bioprinting capabilities using complementary network bioinks. Sci. Adv. 2020;6(38) doi: 10.1126/sciadv.abc5529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zhai X., Ruan C., Ma Y., Cheng D., Wu M., Liu W., Zhao X., Pan H., Lu W.W. 3D-Bioprinted osteoblast-laden nanocomposite hydrogel constructs with induced microenvironments promote cell viability, differentiation, and osteogenesis both in vitro and in vivo. Adv. Sci. 2018;5(3):1700550. doi: 10.1002/advs.201700550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lee W.C., Lim C.H., Shi H., Tang L.A., Wang Y., Lim C.T., Loh K.P. Origin of enhanced stem cell growth and differentiation on graphene and graphene oxide. ACS Nano. 2011;5(9):7334–7341. doi: 10.1021/nn202190c. [DOI] [PubMed] [Google Scholar]
- 76.Hermenean A., Codreanu A., Herman H., Balta C., Rosu M., Mihali C.V., Ivan A., Dinescu S., Ionita M., Costache M. Chitosan-graphene oxide 3D scaffolds as promising tools for bone regeneration in critical-size mouse calvarial defects. Sci. Rep. 2017;7(1):16641. doi: 10.1038/s41598-017-16599-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ahmed J., Mulla M., Maniruzzaman M. Rheological and dielectric behavior of 3D-printable chitosan/graphene oxide hydrogels. ACS Biomater. Sci. Eng. 2020;6(1):88–99. doi: 10.1021/acsbiomaterials.9b00201. [DOI] [PubMed] [Google Scholar]
- 78.Olate-Moya F., Arens L., Wilhelm M., Mateos-Timoneda M.A., Engel E., Palza H. Chondroinductive alginate-based hydrogels having graphene oxide for 3D printed scaffold fabrication. ACS Appl. Mater. Interfaces. 2020;12(4):4343–4357. doi: 10.1021/acsami.9b22062. [DOI] [PubMed] [Google Scholar]
- 79.Chimene D., Kaunas R., Gaharwar A.K. Hydrogel bioink reinforcement for additive manufacturing: a focused review of emerging strategies. Adv. Mater. 2020;32(1) doi: 10.1002/adma.201902026. [DOI] [PubMed] [Google Scholar]
- 80.Bose S., Robertson S.F., Bandyopadhyay A. Surface modification of biomaterials and biomedical devices using additive manufacturing. Acta Biomater. 2018;66:6–22. doi: 10.1016/j.actbio.2017.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hölzl K., Lin S., Tytgat L., Van Vlierberghe S., Gu L., Ovsianikov A. Bioink properties before, during and after 3D bioprinting. Biofabrication. 2016;8(3) doi: 10.1088/1758-5090/8/3/032002. [DOI] [PubMed] [Google Scholar]
- 82.Madhurakkat Perikamana S.K., Lee J., Lee Y.B., Shin Y.M., Lee E.J., Mikos A.G., Shin H. Materials from mussel-inspired chemistry for cell and tissue engineering applications. Biomacromolecules. 2015;16(9):2541–2555. doi: 10.1021/acs.biomac.5b00852. [DOI] [PubMed] [Google Scholar]
- 83.Liu M., Zeng G., Wang K., Wan Q., Tao L., Zhang X., Wei Y. Recent developments in polydopamine: an emerging soft matter for surface modification and biomedical applications. Nanoscale. 2016;8(38):16819–16840. doi: 10.1039/c5nr09078d. [DOI] [PubMed] [Google Scholar]
- 84.Messersmith P.B. Materials science. Multitasking in tissues and materials. Science. 2008;319(5871):1767–1768. doi: 10.1126/science.1155122. [DOI] [PubMed] [Google Scholar]
- 85.Lee D., Park J.P., Koh M.Y., Kim P., Lee J., Shin M., Lee H. Chitosan-catechol: a writable bioink under serum culture media. Biomater. Sci. 2018;6(5):1040–1047. doi: 10.1039/c8bm00174j. [DOI] [PubMed] [Google Scholar]
- 86.Guo Z., Xia J., Mi S., Sun W. Mussel-inspired naturally derived double-network hydrogels and their application in 3D printing: from soft, injectable bioadhesives to mechanically strong hydrogels. ACS Biomater. Sci. Eng. 2020;6(3):1798–1808. doi: 10.1021/acsbiomaterials.9b01864. [DOI] [PubMed] [Google Scholar]
- 87.Li T., Ma H., Ma H., Ma Z., Qiang L., Yang Z., Yang X., Zhou X., Dai K., Wang J. Mussel-inspired nanostructures potentiate the immunomodulatory properties and angiogenesis of mesenchymal stem cells. ACS Appl. Mater. Interfaces. 2019;11(19):17134–17146. doi: 10.1021/acsami.8b22017. [DOI] [PubMed] [Google Scholar]
- 88.Shi G., Wang Y., Derakhshanfar S., Xu K., Zhong W., Luo G., Liu T., Wang Y., Wu J., Xing M. Biomimicry of oil infused layer on 3D printed poly(dimethylsiloxane): non-fouling, antibacterial and promoting infected wound healing. Mater. Sci. Eng. C Mater. Biol. Appl. 2019;100:915–927. doi: 10.1016/j.msec.2019.03.058. [DOI] [PubMed] [Google Scholar]
- 89.Xu T., Yang J., Zhang J., Zhu Y., Li Q., Pan C., Zhang L. Facile modification of electrospun fibrous structures with antifouling zwitterionic hydrogels. Biomed. Mater. 2017;13(1) doi: 10.1088/1748-605X/aa89b2. [DOI] [PubMed] [Google Scholar]
- 90.Osaheni A.O., Finkelstein E.B., Mather P.T., Blum M.M. Synthesis and characterization of a zwitterionic hydrogel blend with low coefficient of friction. Acta Biomater. 2016;46:245–255. doi: 10.1016/j.actbio.2016.09.022. [DOI] [PubMed] [Google Scholar]
- 91.Pan W., Wallin T.J., Odent J., Yip M.C., Mosadegh B., Shepherd R.F., Giannelis E.P. Optical stereolithography of antifouling zwitterionic hydrogels. J. Mater. Chem. B. 2019;7(17):2855–2864. doi: 10.1039/c9tb00278b. [DOI] [PubMed] [Google Scholar]
- 92.GhavamiNejad A., Park C.H., Kim C.S. In situ synthesis of antimicrobial silver nanoparticles within antifouling zwitterionic hydrogels by catecholic redox chemistry for wound healing application. Biomacromolecules. 2016;17(3):1213–1223. doi: 10.1021/acs.biomac.6b00039. [DOI] [PubMed] [Google Scholar]
- 93.Liu S.Q., Yang C., Huang Y., Ding X., Li Y., Fan W.M., Hedrick J.L., Yang Y.Y. Antimicrobial and antifouling hydrogels formed in situ from polycarbonate and poly(ethylene glycol) via Michael addition. Adv. Mater. 2012;24(48):6484–6489. doi: 10.1002/adma.201202225. [DOI] [PubMed] [Google Scholar]
- 94.Menglong Liu Y.W., Hu Xiaodong, He Weifeng, Gong Yali, Hu Xiaohong, Liu Meixi, Luo Gaoxing, Xing Malcolm, Wu Jun, Janus N. N-dimethylformamide as a solvent for a gradient porous wound dressing of poly(vinylidene fluoride) and as a reducer for in situ nano-silver production: anti-permeation, antibacterial and antifouling activities against multi-drug-resistant bacteria both in vitro and in vivo. RSC Adv. 2018;8:26626–26639. doi: 10.1039/c8ra03234c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Yan C., Jiang P., Jia X., Wang X. 3D printing of bioinspired textured surfaces with superamphiphobicity. Nanoscale. 2020;12(5):2924–2938. doi: 10.1039/c9nr09620e. [DOI] [PubMed] [Google Scholar]
- 96.Mofazzal Jahromi M.A., Sahandi Zangabad P., Moosavi Basri S.M., Sahandi Zangabad K., Ghamarypour A., Aref A.R., Karimi M., Hamblin M.R. Nanomedicine and advanced technologies for burns: preventing infection and facilitating wound healing. Adv. Drug Deliv. Rev. 2018;123:33–64. doi: 10.1016/j.addr.2017.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kokel A., Torok M. Recent advances in the development of antimicrobial peptides (AMPs): attempts for sustainable medicine? Curr. Med. Chem. 2018;25(21):2503–2519. doi: 10.2174/0929867325666180117142142. [DOI] [PubMed] [Google Scholar]
- 98.Ciandrini E., Morroni G., Arzeni D., Kamysz W., Neubauer D., Kamysz E., Cirioni O., Brescini L., Baffone W., Campana R. Antimicrobial activity of different antimicrobial peptides (AMPs) against clinical methicillin-resistant Staphylococcus aureus (MRSA) Curr. Top. Med. Chem. 2018;18(24):2116–2126. doi: 10.2174/1568026618666181022140348. [DOI] [PubMed] [Google Scholar]
- 99.Farkas A., Maroti G., Kereszt A., Kondorosi E. Comparative analysis of the bacterial membrane disruption effect of two natural plant antimicrobial peptides. Front. Microbiol. 2017;8:51. doi: 10.3389/fmicb.2017.00051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kim E.Y., Rajasekaran G., Shin S.Y. LL-37-derived short antimicrobial peptide KR-12-a5 and its d-amino acid substituted analogs with cell selectivity, anti-biofilm activity, synergistic effect with conventional antibiotics, and anti-inflammatory activity. Eur. J. Med. Chem. 2017;136:428–441. doi: 10.1016/j.ejmech.2017.05.028. [DOI] [PubMed] [Google Scholar]
- 101.Jacob B., Park I.S., Bang J.K., Shin S.Y. Short KR-12 analogs designed from human cathelicidin LL-37 possessing both antimicrobial and antiendotoxic activities without mammalian cell toxicity. J. Pept. Sci. 2013;19(11):700–707. doi: 10.1002/psc.2552. [DOI] [PubMed] [Google Scholar]
- 102.Song D.W., Kim S.H., Kim H.H., Lee K.H., Ki C.S., Park Y.H. Multi-biofunction of antimicrobial peptide-immobilized silk fibroin nanofiber membrane: implications for wound healing. Acta Biomater. 2016;39:146–155. doi: 10.1016/j.actbio.2016.05.008. [DOI] [PubMed] [Google Scholar]
- 103.Liu M., Liu T., Zhang X., Jian Z., Xia H., Yang J., Hu X., Xing M., Luo G., Wu J. Fabrication of KR-12 peptide-containing hyaluronic acid immobilized fibrous eggshell membrane effectively kills multi-drug-resistant bacteria, promotes angiogenesis and accelerates re-epithelialization. Int. J. Nanomed. 2019;14:3345–3360. doi: 10.2147/IJN.S199618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Fidan S., Muhaffel F., Riool M., Cempura G., de Boer L., Zaat S.A.J., Filemonowicz A.C., Cimenoglu H. Fabrication of oxide layer on zirconium by micro-arc oxidation: structural and antimicrobial characteristics. Mater. Sci. Eng. C Mater. Biol. Appl. 2017;71:565–569. doi: 10.1016/j.msec.2016.11.035. [DOI] [PubMed] [Google Scholar]
- 105.Mohanraj G., Mao C., Armine A., Kasher R., Arnusch C.J. Ink-jet printing-assisted modification on polyethersulfone membranes using a UV-reactive antimicrobial peptide for fouling-resistant surfaces. ACS Omega. 2018;3(8):8752–8759. doi: 10.1021/acsomega.8b00916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Zhongxing L., Shaohong W., Jinlong L., Limin Z., Yuanzheng W., Haipeng G., Jian C. Three-dimensional printed hydroxyapatite bone tissue engineering scaffold with antibacterial and osteogenic ability. J. Biol. Eng. 2021;15(1):21. doi: 10.1186/s13036-021-00273-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Townsend J.M., Hukill M.E., Fung K.M., Ohst D.G., Johnson J.K., Weatherly R.A., Detamore M.S. Biodegradable electrospun patch containing cell adhesion or antimicrobial compounds for trachea repair in vivo. Biomed. Mater. 2020;15(2) doi: 10.1088/1748-605X/ab5e1b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Moravej H., Moravej Z., Yazdanparast M., Heiat M., Mirhosseini A., Moosazadeh Moghaddam M., Mirnejad R. Antimicrobial peptides: features, action, and their resistance mechanisms in bacteria. Microb. Drug Resist. 2018;24(6):747–767. doi: 10.1089/mdr.2017.0392. [DOI] [PubMed] [Google Scholar]
- 109.Bassous N.J., Webster T.J. The binary effect on methicillin-resistant Staphylococcus aureus of polymeric nanovesicles appended by proline-rich amino acid sequences and inorganic nanoparticles. Small. 2019;15(18) doi: 10.1002/smll.201804247. [DOI] [PubMed] [Google Scholar]
- 110.Ernst R.K., Guina T., Miller S.I. Salmonella typhimurium outer membrane remodeling: role in resistance to host innate immunity. Microb. Infect. 2001;3(14–15):1327–1334. doi: 10.1016/s1286-4579(01)01494-0. [DOI] [PubMed] [Google Scholar]
- 111.Joo H.S., Otto M. Mechanisms of resistance to antimicrobial peptides in staphylococci. Biochim. Biophys. Acta. 2015;1848(11 Pt B):3055–3061. doi: 10.1016/j.bbamem.2015.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Weidenmaier C., Kristian S.A., Peschel A. Bacterial resistance to antimicrobial host defenses--an emerging target for novel antiinfective strategies? Curr. Drug Targets. 2003;4(8):643–649. doi: 10.2174/1389450033490731. [DOI] [PubMed] [Google Scholar]
- 113.Bechinger B., Gorr S.U. Antimicrobial peptides: mechanisms of action and resistance. J. Dent. Res. 2017;96(3):254–260. doi: 10.1177/0022034516679973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Abaci H.E., Coffman A., Doucet Y., Chen J., Jacków J., Wang E., Guo Z., Shin J.U., Jahoda C.A., Christiano A.M. Tissue engineering of human hair follicles using a biomimetic developmental approach. Nat. Commun. 2018;9(1):5301. doi: 10.1038/s41467-018-07579-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Wang Y., Armato U., Wu J. Targeting tunable physical properties of materials for chronic wound care. Front. Bioeng. Biotechnol. 2020;8:584. doi: 10.3389/fbioe.2020.00584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Nuutila K., Samandari M., Endo Y., Zhang Y., Quint J., Schmidt T.A., Tamayol A., Sinha I. In vivo printing of growth factor-eluting adhesive scaffolds improves wound healing. Bioact. Mater. 2022;8:296–308. doi: 10.1016/j.bioactmat.2021.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Ozbolat I.T., Hospodiuk M. Current advances and future perspectives in extrusion-based bioprinting. Biomaterials. 2016;76:321–343. doi: 10.1016/j.biomaterials.2015.10.076. [DOI] [PubMed] [Google Scholar]
- 118.Messaoudi O., Henrionnet C., Bourge K., Loeuille D., Gillet P., Pinzano A. Stem cells and extrusion 3D printing for hyaline cartilage engineering. Cells. 2020;10(1) doi: 10.3390/cells10010002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Liu P., Shen H., Zhi Y., Si J., Shi J., Guo L., Shen S.G. 3D bioprinting and in vitro study of bilayered membranous construct with human cells-laden alginate/gelatin composite hydrogels. Colloids Surf. B Biointerfaces. 2019;181:1026–1034. doi: 10.1016/j.colsurfb.2019.06.069. [DOI] [PubMed] [Google Scholar]
- 120.Kim B.S., Kwon Y.W., Kong J.S., Park G.T., Gao G., Han W., Kim M.B., Lee H., Kim J.H., Cho D.W. 3D cell printing of in vitro stabilized skin model and in vivo pre-vascularized skin patch using tissue-specific extracellular matrix bioink: a step towards advanced skin tissue engineering. Biomaterials. 2018;168:38–53. doi: 10.1016/j.biomaterials.2018.03.040. [DOI] [PubMed] [Google Scholar]
- 121.Ng W.L., Wang S., Yeong W.Y., Naing M.W. Skin bioprinting: impending reality or fantasy? Trends Biotechnol. 2016;34(9):689–699. doi: 10.1016/j.tibtech.2016.04.006. [DOI] [PubMed] [Google Scholar]
- 122.Albanna M., Binder K.W., Murphy S.V., Kim J., Qasem S.A., Zhao W., Tan J., El-Amin I.B., Dice D.D., Marco J., Green J., Xu T., Skardal A., Holmes J.H., Jackson J.D., Atala A., Yoo J.J. In situ bioprinting of autologous skin cells accelerates wound healing of extensive excisional full-thickness wounds. Sci. Rep. 2019;9(1):1856. doi: 10.1038/s41598-018-38366-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Rodriguez-Cabello J.C., Gonzalez de Torre I., Ibanez-Fonseca A., Alonso M. Bioactive scaffolds based on elastin-like materials for wound healing. Adv. Drug Deliv. Rev. 2018;129:118–133. doi: 10.1016/j.addr.2018.03.003. [DOI] [PubMed] [Google Scholar]
- 124.Tayebi L., Rasoulianboroujeni M., Moharamzadeh K., Almela T.K.D., Cui Z., Ye H. 3D-printed membrane for guided tissue regeneration. Mater. Sci. Eng. C Mater. Biol. Appl. 2018;84:148–158. doi: 10.1016/j.msec.2017.11.027. [DOI] [PubMed] [Google Scholar]
- 125.Lee S., Sani E.S., Spencer A.R., Guan Y., Weiss A.S., Annabi N. Human-recombinant-elastin-based bioinks for 3D bioprinting of vascularized soft tissues. Adv. Mater. 2020;32(45) doi: 10.1002/adma.202003915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Chen X., Yue Z., Winberg P.C., Lou Y.R., Beirne S., Wallace G.G. 3D bioprinting dermal-like structures using species-specific ulvan. Biomater. Sci. 2021;9(7):2424–2438. doi: 10.1039/d0bm01784a. [DOI] [PubMed] [Google Scholar]
- 127.Wu Y., Fortunato G.M., Okesola B.O., Brocchetti F., Suntornnond R., Connelly J., De Maria C., Rodriguez-Cabello J.C., Vozzi G., Wang W., Mata A. An interfacial self-assembling bioink for the manufacturing of capillary-like structures with tuneable and anisotropic permeability. Biofabrication. 2021;13(3) doi: 10.1088/1758-5090/abe4c3. [DOI] [PubMed] [Google Scholar]
- 128.Athirasala A., Lins F., Tahayeri A., Hinds M., Smith A.J., Sedgley C., Ferracane J., Bertassoni L.E. A novel strategy to engineer pre-vascularized full-length dental pulp-like tissue constructs. Sci. Rep. 2017;7(1):3323. doi: 10.1038/s41598-017-02532-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Hinton T.J., Jallerat Q., Palchesko R.N., Park J.H., Grodzicki M.S., Shue H.J., Ramadan M.H., Hudson A.R., Feinberg A.W. Three-dimensional printing of complex biological structures by freeform reversible embedding of suspended hydrogels. Sci. Adv. 2015;1(9) doi: 10.1126/sciadv.1500758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Askari M., Afzali Naniz M., Kouhi M., Saberi A., Zolfagharian A., Bodaghi M. Recent progress in extrusion 3D bioprinting of hydrogel biomaterials for tissue regeneration: a comprehensive review with focus on advanced fabrication techniques. Biomater. Sci. 2021;9(3):535–573. doi: 10.1039/d0bm00973c. [DOI] [PubMed] [Google Scholar]
- 131.Kérourédan O., Bourget J.M., Rémy M., Crauste-Manciet S., Kalisky J., Catros S., Thébaud N.B., Devillard R. Micropatterning of endothelial cells to create a capillary-like network with defined architecture by laser-assisted bioprinting. J. Mater. Sci. Mater. Med. 2019;30(2):28. doi: 10.1007/s10856-019-6230-1. [DOI] [PubMed] [Google Scholar]
- 132.Zhou F., Hong Y., Liang R., Zhang X., Liao Y., Jiang D., Zhang J., Sheng Z., Xie C., Peng Z., Zhuang X., Bunpetch V., Zou Y., Huang W., Zhang Q., Alakpa E.V., Zhang S., Ouyang H. Rapid printing of bio-inspired 3D tissue constructs for skin regeneration. Biomaterials. 2020;258:120287. doi: 10.1016/j.biomaterials.2020.120287. [DOI] [PubMed] [Google Scholar]
- 133.Mori N., Morimoto Y., Takeuchi S. Skin integrated with perfusable vascular channels on a chip. Biomaterials. 2017;116:48–56. doi: 10.1016/j.biomaterials.2016.11.031. [DOI] [PubMed] [Google Scholar]
- 134.Lu C.P., Polak L., Keyes B.E., Fuchs E. Spatiotemporal antagonism in mesenchymal-epithelial signaling in sweat versus hair fate decision. Science. 2016;354(6319) doi: 10.1126/science.aah6102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Lei M., Schumacher L.J., Lai Y.C., Juan W.T., Yeh C.Y., Wu P., Jiang T.X., Baker R.E., Widelitz R.B., Yang L., Chuong C.M. Self-organization process in newborn skin organoid formation inspires strategy to restore hair regeneration of adult cells. Proc. Natl. Acad. Sci. U. S. A. 2017;114(34):E7101–E7110. doi: 10.1073/pnas.1700475114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Huang S., Yao B., Xie J., Fu X. 3D bioprinted extracellular matrix mimics facilitate directed differentiation of epithelial progenitors for sweat gland regeneration. Acta Biomater. 2016;32:170–177. doi: 10.1016/j.actbio.2015.12.039. [DOI] [PubMed] [Google Scholar]
- 137.Wang R., Wang Y., Yao B., Hu T., Li Z., Liu Y., Cui X., Cheng L., Song W., Huang S., Fu X. Redirecting differentiation of mammary progenitor cells by 3D bioprinted sweat gland microenvironment. Burns Trauma. 2019;7:29. doi: 10.1186/s41038-019-0167-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Liu N., Huang S., Yao B., Xie J., Wu X., Fu X. 3D bioprinting matrices with controlled pore structure and release function guide in vitro self-organization of sweat gland. Sci. Rep. 2016;6:34410. doi: 10.1038/srep34410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Schneider M.R., Schmidt-Ullrich R., Paus R. The hair follicle as a dynamic miniorgan. Curr. Biol. : CB. 2009;19(3):R132–R142. doi: 10.1016/j.cub.2008.12.005. [DOI] [PubMed] [Google Scholar]
- 140.Weng T., Wu P., Zhang W., Zheng Y., Li Q., Jin R., Chen H., You C., Guo S., Han C., Wang X. Regeneration of skin appendages and nerves: current status and further challenges. J. Transl. Med. 2020;18(1):53. doi: 10.1186/s12967-020-02248-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Thangapazham R.L., Klover P., Wang J.A., Zheng Y., Devine A., Li S., Sperling L., Cotsarelis G., Darling T.N. Dissociated human dermal papilla cells induce hair follicle neogenesis in grafted dermal-epidermal composites. J. Invest. Dermatol. 2014;134(2):538–540. doi: 10.1038/jid.2013.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Higgins C.A., Chen J.C., Cerise J.E., Jahoda C.A., Christiano A.M. Microenvironmental reprogramming by three-dimensional culture enables dermal papilla cells to induce de novo human hair-follicle growth. Proc. Natl. Acad. Sci. U. S. A. 2013;110(49):19679–19688. doi: 10.1073/pnas.1309970110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Abaci H.E., Coffman A., Doucet Y., Chen J., Jackow J., Wang E., Guo Z., Shin J.U., Jahoda C.A., Christiano A.M. Tissue engineering of human hair follicles using a biomimetic developmental approach. Nat. Commun. 2018;9(1):5301. doi: 10.1038/s41467-018-07579-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Perez-Valle A., Del Amo C., Andia I. Overview of current advances in extrusion bioprinting for skin applications. Int. J. Mol. Sci. 2020;21(18) doi: 10.3390/ijms21186679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Singh D., Singh D., Han S.S. 3D printing of scaffold for cells delivery: advances in skin tissue engineering. Polymers (Basel) 2016;8(1) doi: 10.3390/polym8010019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Scull G., Brown A.C. Development of novel microenvironments for promoting enhanced wound healing. Curr. Tissue Microenviron. Rep. 2020;1(3):73–87. doi: 10.1007/s43152-020-00009-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Fang Z., Yang X., Wu G., Liu M., Han J., Tao K., Hu D. The use of autologous platelet-rich plasma gel increases wound healing and reduces scar development in split-thickness skin graft donor sites. J. Plast. Surg. Hand Surg. 2019:1–5. doi: 10.1080/2000656X.2019.1635489. [DOI] [PubMed] [Google Scholar]
- 148.Martinez-Martinez A., Ruiz-Santiago F., Garcia-Espinosa J. Platelet-rich plasma: myth or reality? Radiologia. 2018;60(6):465–475. doi: 10.1016/j.rx.2018.08.006. [DOI] [PubMed] [Google Scholar]
- 149.van der Bijl I., Vlig M., Middelkoop E., de Korte D. Transfusion; 2019. Allogeneic Platelet-Rich Plasma (PRP) Is Superior to Platelets or Plasma Alone in Stimulating Fibroblast Proliferation and Migration, Angiogenesis, and Chemotaxis as Relevant Processes for Wound Healing. [DOI] [PubMed] [Google Scholar]
- 150.Karina, Samudra M.F., Rosadi I., Afini I., Widyastuti T., Sobariah S., Remelia M., Puspitasari R.L., Rosliana I., Tunggadewi T.I. Combination of the stromal vascular fraction and platelet-rich plasma accelerates the wound healing process: pre-clinical study in a Sprague-Dawley rat model. Stem Cell Invest. 2019;6:18. doi: 10.21037/sci.2019.06.08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Liao X., Liang J.X., Li S.H., Huang S., Yan J.X., Xiao L.L., Song J.X., Liu H.W. Allogeneic platelet-rich plasma therapy as an effective and safe adjuvant method for chronic wounds. J. Surg. Res. 2019;246:284–291. doi: 10.1016/j.jss.2019.09.019. [DOI] [PubMed] [Google Scholar]
- 152.Faramarzi N., Yazdi I.K., Nabavinia M., Gemma A., Fanelli A., Caizzone A., Ptaszek L.M., Sinha I., Khademhosseini A., Ruskin J.N., Tamayol A. Patient-specific bioinks for 3D bioprinting of tissue engineering scaffolds. Adv. Healthc Mater. 2018;7(11) doi: 10.1002/adhm.201701347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.L T.S., Kasoju N., Raju R., Bhatt A. Formulation and characterization of alginate dialdehyde, gelatin, and platelet-rich plasma-based bioink for bioprinting applications. Bioengineering (Basel) 2020;7(3) doi: 10.3390/bioengineering7030108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Paez-Mayorga J., Capuani S., Farina M., Lotito M.L., Niles J.A., Salazar H.F., Rhudy J., Esnaola L., Chua C.Y.X., Taraballi F., Corradetti B., Shelton K.A., Nehete P.N., Nichols J.E., Grattoni A. Enhanced in vivo vascularization of 3D-printed cell encapsulation device using platelet-rich plasma and mesenchymal stem cells. Adv. Healthc Mater. 2020;9(19) doi: 10.1002/adhm.202000670. [DOI] [PubMed] [Google Scholar]
- 155.Sasaki G.H. A preliminary clinical trial comparing split treatments to the face and hand with autologous fat grafting and platelet-rich plasma (PRP): a 3D, IRB-approved study. Aesthetic Surg. J. 2019;39(6):675–686. doi: 10.1093/asj/sjy254. [DOI] [PubMed] [Google Scholar]
- 156.Lopez M.A., Bertrand B., Kober F., Boucekine M., De Fromont De Bouailles M., Vogtensperger M., Bernard M., Casanova D., Magalon J., Sabatier F. Plast Reconstr Surg; 2019. The Use of Higher Proportions of Platelet-Rich Plasma to Enrich Microfat Has Negative Effects: A Pre-clinical Study. [DOI] [PubMed] [Google Scholar]
- 157.Mollica P.A., Booth-Creech E.N., Reid J.A., Zamponi M., Sullivan S.M., Palmer X.L., Sachs P.C., Bruno R.D. 3D bioprinted mammary organoids and tumoroids in human mammary derived ECM hydrogels. Acta Biomater. 2019;95:201–213. doi: 10.1016/j.actbio.2019.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Eweida A.M., Marei M.K. Naturally occurring extracellular matrix scaffolds for dermal regeneration: do they really need cells? BioMed Res. Int. 2015;2015:839694. doi: 10.1155/2015/839694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Kim B.S., Kwon Y.W., Kong J.S., Park G.T., Gao G., Han W., Kim M.B., Lee H., Kim J.H., Cho D.W. 3D cell printing of in vitro stabilized skin model and in vivo pre-vascularized skin patch using tissue-specific extracellular matrix bioink: a step towards advanced skin tissue engineering. Biomaterials. 2018;168:38–53. doi: 10.1016/j.biomaterials.2018.03.040. [DOI] [PubMed] [Google Scholar]
- 160.Lee S.J., Lee J.H., Park J., Kim W.D., Park S.A. Fabrication of 3D printing scaffold with porcine skin decellularized bio-ink for soft tissue engineering. Materials (Basel) 2020;13(16) doi: 10.3390/ma13163522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Chu H., Yang W., Sun L., Cai S., Yang R., Liang W., Yu H., Liu L. 4D printing: a review on recent progresses. Micromachines. 2020;11(9) doi: 10.3390/mi11090796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Zhou W., Qiao Z., Nazarzadeh Zare E., Huang J., Zheng X., Sun X., Shao M., Wang H., Wang X., Chen D., Zheng J., Fang S., Li Y.M., Zhang X., Yang L., Makvandi P., Wu A. 4D-Printed dynamic materials in biomedical applications: chemistry, challenges, and their future perspectives in the clinical sector. J. Med. Chem. 2020;63(15):8003–8024. doi: 10.1021/acs.jmedchem.9b02115. [DOI] [PubMed] [Google Scholar]
- 163.Lui Y.S., Sow W.T., Tan L.P., Wu Y., Lai Y., Li H. 4D printing and stimuli-responsive materials in biomedical aspects. Acta Biomater. 2019;92:19–36. doi: 10.1016/j.actbio.2019.05.005. [DOI] [PubMed] [Google Scholar]
- 164.Jacob J., More N., Kalia K., Kapusetti G. Piezoelectric smart biomaterials for bone and cartilage tissue engineering. Inflamm. Regen. 2018;38:2. doi: 10.1186/s41232-018-0059-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Zhang K., Wang S., Zhou C., Cheng L., Gao X., Xie X., Sun J., Wang H., Weir M.D., Reynolds M.A., Zhang N., Bai Y., Xu H.H.K. Advanced smart biomaterials and constructs for hard tissue engineering and regeneration. Bone Res. 2018;6:31. doi: 10.1038/s41413-018-0032-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Guo H.F., Li Z.S., Dong S.W., Chen W.J., Deng L., Wang Y.F., Ying D.J. Piezoelectric PU/PVDF electrospun scaffolds for wound healing applications. Colloids Surf. B Biointerfaces. 2012;96:29–36. doi: 10.1016/j.colsurfb.2012.03.014. [DOI] [PubMed] [Google Scholar]
- 167.Mirani B., Pagan E., Currie B., Siddiqui M.A., Hosseinzadeh R., Mostafalu P., Zhang Y.S., Ghahary A., Akbari M. An advanced multifunctional hydrogel-based dressing for wound monitoring and drug delivery. Adv. Healthc Mater. 2017;6(19) doi: 10.1002/adhm.201700718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Zhu J., Han H., Ye T.T., Li F.X., Wang X.L., Yu J.Y., Wu D.Q. Biodegradable and pH sensitive peptide based hydrogel as controlled release system for antibacterial wound dressing application. Molecules. 2018;23(12) doi: 10.3390/molecules23123383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Kiaee G., Mostafalu P., Samandari M., Sonkusale S. A pH-mediated electronic wound dressing for controlled drug delivery. Adv. Healthc Mater. 2018;7(18) doi: 10.1002/adhm.201800396. [DOI] [PubMed] [Google Scholar]
- 170.Liu M., He D., Yang T., Liu W., Mao L., Zhu Y., Wu J., Luo G., Deng J. An efficient antimicrobial depot for infectious site-targeted chemo-photothermal therapy. J. Nanobiotechnol. 2018;16(1):23. doi: 10.1186/s12951-018-0348-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Tang T., Jiang H., Yu Y., He F., Ji S.Z., Liu Y.Y., Wang Z.S., Xiao S.C., Tang C., Wang G.Y., Xia Z.F. A new method of wound treatment: targeted therapy of skin wounds with reactive oxygen species-responsive nanoparticles containing SDF-1alpha. Int. J. Nanomed. 2015;10:6571–6585. doi: 10.2147/IJN.S88384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Li D., An X., Mu Y. A liposomal hydrogel with enzyme triggered release for infected wound. Chem. Phys. Lipids. 2019;223:104783. doi: 10.1016/j.chemphyslip.2019.104783. [DOI] [PubMed] [Google Scholar]
- 173.Liu C., Wang Z., Wei X., Chen B., Luo Y. 3D printed hydrogel/PCL core/shell fiber scaffolds with NIR-triggered drug release for cancer therapy and wound healing. Acta Biomater. 2021;131:314–325. doi: 10.1016/j.actbio.2021.07.011. [DOI] [PubMed] [Google Scholar]
- 174.Nizioł M., Paleczny J., Junka A., Shavandi A., Dawiec-Liśniewska A., Podstawczyk D. 3D printing of thermoresponsive hydrogel laden with an antimicrobial agent towards wound healing applications. Bioengineering (Basel) 2021;8(6) doi: 10.3390/bioengineering8060079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Rafiee M., Farahani R.D., Therriault D. Multi-material 3D and 4D printing: a survey. Adv. Sci. 2020;7(12):1902307. doi: 10.1002/advs.201902307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Li R., McCarthy A., Zhang Y.S., Xie J. Decorating 3D printed scaffolds with electrospun nanofiber segments for tissue engineering. Adv. Biosyst. 2019;3(12) doi: 10.1002/adbi.201900137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Lee J., Rabbani C.C., Gao H., Steinhart M.R., Woodruff B.M., Pflum Z.E., Kim A., Heller S., Liu Y., Shipchandler T.Z., Koehler K.R. Hair-bearing human skin generated entirely from pluripotent stem cells. Nature. 2020;582(7812):399–404. doi: 10.1038/s41586-020-2352-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Serban M.A., Skardal A. Hyaluronan chemistries for three-dimensional matrix applications. Matrix Biol. 2019;78–79:337–345. doi: 10.1016/j.matbio.2018.02.010. [DOI] [PubMed] [Google Scholar]
- 179.Lee J., Koehler K.R. Skin organoids: a new human model for developmental and translational research. Exp. Dermatol. 2021;30(4):613–620. doi: 10.1111/exd.14292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Lee J., Bӧscke R., Tang P.C., Hartman B.H., Heller S., Koehler K.R. Hair follicle development in mouse pluripotent stem cell-derived skin organoids. Cell Rep. 2018;22(1):242–254. doi: 10.1016/j.celrep.2017.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Skardal A., Devarasetty M., Kang H.W., Seol Y.J., Forsythe S.D., Bishop C., Shupe T., Soker S., Atala A. Bioprinting cellularized constructs using a tissue-specific hydrogel bioink. JoVE. 2016;110 doi: 10.3791/53606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Rajan S.A.P., Aleman J., Wan M., Pourhabibi Zarandi N., Nzou G., Murphy S., Bishop C.E., Sadri-Ardekani H., Shupe T., Atala A., Hall A.R., Skardal A. Probing prodrug metabolism and reciprocal toxicity with an integrated and humanized multi-tissue organ-on-a-chip platform. Acta Biomater. 2020;106:124–135. doi: 10.1016/j.actbio.2020.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Gijzen L., Yousef Yengej F.A., Schutgens F., Vormann M.K., Ammerlaan C.M.E., Nicolas A., Kurek D., Vulto P., Rookmaaker M.B., Lanz H.L., Verhaar M.C., Clevers H. Culture and analysis of kidney tubuloids and perfused tubuloid cells-on-a-chip. Nat. Protoc. 2021;16(4):2023–2050. doi: 10.1038/s41596-020-00479-w. [DOI] [PubMed] [Google Scholar]
- 184.Aleman J., Skardal A. A multi-site metastasis-on-a-chip microphysiological system for assessing metastatic preference of cancer cells. Biotechnol. Bioeng. 2019;116(4):936–944. doi: 10.1002/bit.26871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Morgan F.L.C., Moroni L., Baker M.B. Dynamic bioinks to advance bioprinting. Adv. Healthcare Mater. 2020;9(15) doi: 10.1002/adhm.201901798. [DOI] [PubMed] [Google Scholar]
- 186.Elalouf A. Immune response against the biomaterials used in 3D bioprinting of organs. Transpl. Immunol. 2021;69:101446. doi: 10.1016/j.trim.2021.101446. [DOI] [PubMed] [Google Scholar]
- 187.Hotchkiss K.M., Clark N.M., Olivares-Navarrete R. Macrophage response to hydrophilic biomaterials regulates MSC recruitment and T-helper cell populations. Biomaterials. 2018;182:202–215. doi: 10.1016/j.biomaterials.2018.08.029. [DOI] [PubMed] [Google Scholar]
- 188.Chung L., Maestas D.R., Jr., Housseau F., Elisseeff J.H. Key players in the immune response to biomaterial scaffolds for regenerative medicine. Adv. Drug Deliv. Rev. 2017;114:184–192. doi: 10.1016/j.addr.2017.07.006. [DOI] [PubMed] [Google Scholar]