Abstract
We report the first case of organizing pneumonia (OP) associated with a new coronavirus disease (COVID‐19) vaccination. A 78‐year‐old woman developed cough and dyspnoea 10 days after receiving BNT162b2. Chest computed tomography (CT) revealed consolidation in the bilateral lower lobes of the lungs. Although antibiotic treatment did not improve her symptoms, she received a second vaccination as scheduled. She was referred to our hospital because of worsening dyspnoea on day 9 after the second vaccination, with reversed halo signs in the bilateral lower pulmonary lobes and new consolidation in the left lingual region on chest CT on day 15. She was diagnosed with OP based on bronchoalveolar lavage and transbronchial lung biopsy findings. Treatment with oral prednisolone 0.5 mg/kg/day immediately improved the symptoms and chest imaging findings. In the absence of other triggering factors, we considered this case as being COVID‐19 vaccine‐associated following the first and second vaccinations.
Keywords: adverse events, COVID‐19, interstitial lung disease, organizing pneumonia, vaccine
We report the first case of organizing pneumonia (OP) associated with a new coronavirus disease (COVID‐19) vaccination. The second vaccination in the present case caused further worsening of the clinical manifestation. It is important to diagnose vaccine‐associated OP/interstitial lung disease appropriately and avoid re‐vaccination in those cases.
INTRODUCTION
Under the new coronavirus disease (COVID‐19) global pandemic, the rapid development and distribution of efficient and safe COVID‐19 vaccine is an urgent issue worldwide. In Japan, three COVID‐19 vaccines, BNT162b2, mRNA‐1273 and ChAdOx1, have been approved, and the first approved BNT162b2 has been used most widely. Although the safety and efficacy of these vaccines have been confirmed via clinical trials, 1 post‐approval surveys are required to obtain data on delayed onset or rare adverse reactions. Herein, we report the first case of a COVID‐19 vaccine (BNT162b2)‐associated organizing pneumonia (OP) that developed and worsened following the first and second vaccinations.
CASE REPORT
An otherwise healthy 73‐year‐old woman presented with cough and dyspnoea 10 days after the first COVID‐19 vaccination (BNT162b2) and visited her local physician. Chest computed tomography (CT) revealed consolidation in the bilateral lower lobes of the lungs (Figure 1A). She was treated with antibiotics for the diagnosis of atypical pneumonia without clear improvement. Despite her persistent symptoms, she received a second vaccination as scheduled. Nine days after the second vaccination, her dyspnoea and fatigue worsened, and she was referred to our hospital. Her chest CT demonstrated reversed halo signs in the bilateral lower lobes and new consolidation in the left lingual region of the lung on day 15 of the second vaccination (Figure 1B). She was admitted to the hospital for further evaluation on day 25.
FIGURE 1.
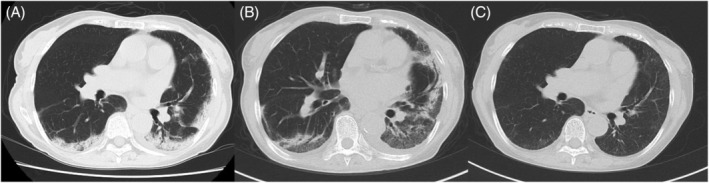
Chest computed tomography (CT) findings after the first and second coronavirus disease (COVID‐19) vaccinations. Chest CT is performed 12 days after the first COVID‐19 vaccination (A), 15 days after the second vaccination (B) and 2 months after the treatment with systemic corticosteroids (C)
Her body temperature was 35.8°C, respiratory rate was 14/min, pulse rate was 113/min and peripheral capillary oxygen saturation level was 93% on room air‐breathing. She had no rash or oedema; however, bilateral fine crackles were present on auscultation. The laboratory tests revealed mild peripheral blood eosinophilia (17.4%) with normal leukocyte counts (4300/μl) and modestly elevated levels of C‐reactive protein (0.46 mg/dl), Krebs von den Lungen 6 (919 U/ml) and surfactant protein‐D (178 ng/ml) in serum. Serum autoantibodies associated with collagen vascular diseases and a polymerase chain reaction of severe acute respiratory syndrome coronavirus 2 mRNA in nasopharyngeal swabs were negative. In bronchoalveolar lavage (BAL) fluid from the left lingual region, the total cell count was 525/μl with prominent lymphocytosis (79%) and mild eosinophilia (14%). The CD4/8 ratio was 0.21, and the bacterial culture was negative. Histopathology of transbronchial lung biopsy demonstrated mild‐to‐moderate chronic inflammatory cell infiltration in the alveolar wall, organization in the alveolar space and fibrosis of the alveolar wall, but lacked eosinophil infiltration (Figure 2).
FIGURE 2.
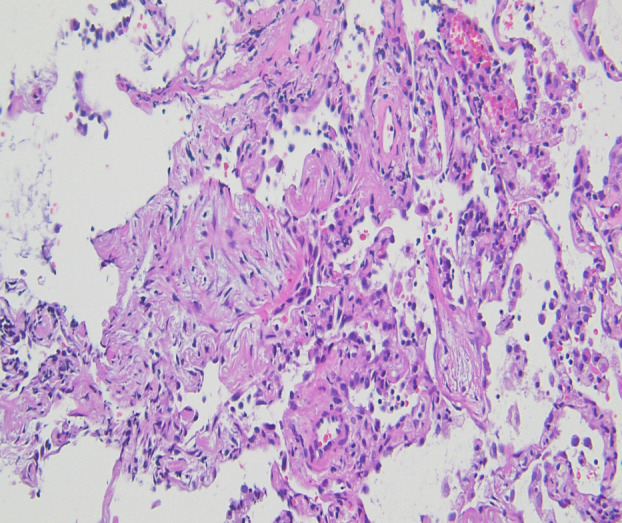
Pathological findings of the lung. Haematoxylin–eosin staining of the lung is obtained by transbronchial biopsy of the left lung (100×)
Based on clinical, radiological and pathological findings, the patient was diagnosed with OP and treated with oral prednisolone (0.5 mg/kg/day). Her respiratory symptoms and chest imaging improved immediately, and she was discharged on day 12 of hospitalization. Peripheral blood eosinophil counts were also normalized quickly after the treatment. Chest CT re‐examination 2 months after the treatment showed that the infiltrative shadows in the bilateral lungs had almost disappeared (Figure 1C). Corticosteroid treatment with low dose is still ongoing 6 months later.
DISCUSSION
In the present case, the first respiratory symptoms appeared 10 days after the first vaccination with BNT162b2 and worsened 9 days after the re‐vaccination. Although the drug‐induced lymphocyte stimulation test was not performed in the absence of an available vaccine for the test, the typical temporal relationship without other possible causes of OP, such as collagen vascular diseases and drugs, strongly suggests COVID‐19 vaccine‐associated OP.
Many factors can cause interstitial lung disease (ILD), and widely used vaccines such as the influenza virus vaccine are no exception. 2 Seven cases of ILD after COVID‐19 vaccination were reported 3 , 4 , 5 , 6 , 7 (Table 1). This is the first case of OP that was confirmed radiologically and histologically. The onset and exacerbation of ILD were slightly delayed (10 and 9 days after the first and second vaccinations, respectively) compared to the previous cases (1–4 days). Lymphocytosis in the BAL fluid was observed as in the previous cases; however, CD8‐positive T cells were more dominant in the present case. In agreement with previous reports, the prognosis was good with systemic corticosteroid therapy. The number of ILD cases has been increasing as per the adverse reaction survey conducted by the Japanese Ministry of Health, Labour and Welfare; 54 cases/141,442,370 injections for BNT162b2 and two cases/27,701,010 injections for mRNA‐1273 in the report on 22 October 2021.
TABLE 1.
Characteristics of patients with ILD associated with COVID‐19 vaccine
| Our case | Park 3 | Yoshifuji 4 | Miqdadi 5 | Piqueras 6 | Shimizu 7 | Shimizu 7 | Shimizu 7 | |
|---|---|---|---|---|---|---|---|---|
| Age/sex | 78/Female | 86/Male | 60/Male | 66/Male | 37/Male | 66/Male | 85/Male | 62/Male |
| Smoking | Never | Never | Ex‐smoker | — | — | Ex‐smoker | Ex‐smoker | Never |
| Underlying ILD | No | No | No | No | No | Yes | Yes | No |
| Onset | ||||||||
| Number of vaccinations | First, second | First | Second | First | Second | First | First | Second |
| Days after vaccination | 10, 9 | 2 | 3 | 1 | 1 | 2 | 4 | 3 |
| Re‐vaccination | Yes | No | — | — | — | No | Yes | — |
| KL‐6 (U/ml) | 919 | — | 800 | — | — | 1306 | 4084 | 297 |
| SP‐D (ng/ml) | 178 | — | 155 | — | — | 376 | 676 | 189 |
| BAL findings | ||||||||
| Macrophages (%) | 6 | — | 46.9 | — | — | 55 | 30.7 | — |
| Lymphocytes (%) | 79 | — | 31.3 | — | — | 42.3 | 62.7 | — |
| Neutrophils (%) | 1 | — | 21.9 | — | — | 1.7 | 0 | — |
| Eosinophils (%) | 14 | — | 0 | — | 60.3 | 1 | 6.7 | — |
| CD4/CD8 | 0.21 | — | 1.26 | — | — | 1.3 | 6.6 | — |
| Patterns of ILD | OP | — | — | AEP | AEP | — | — | — |
Abbreviations: AEP, acute eosinophilic pneumonia; BAL, bronchoalveolar lavage; COVID‐19, coronavirus disease; ILD, interstitial lung disease; KL‐6, Krebs von den Lungen 6 (normal range < 500 U/ml); OP, organizing pneumonia; SP‐D, surfactant protein D (normal range < 110 ng/ml).
Most COVID‐19 vaccines, except Ad26.COV2.S, require multiple vaccinations, and booster vaccinations are being promoted now. 8 ILD developed in five cases after the first vaccination and in three cases following the second; however, there were no cases of re‐vaccination after ILD was confirmed with chest CT. The second vaccination in the present case caused further worsening of the clinical manifestation. Therefore, it is important to diagnose vaccine‐associated OP/ILD appropriately and avoid re‐vaccination in those cases.
We encountered a case of OP, an ILD, after the COVID‐19 vaccination. The assessment of safety in clinical trials is limited, and the evaluation of rare adverse events is inadequate. Therefore, further studies on vaccine‐related ILD are required to ensure prompt diagnosis, as well as appropriate treatment and management.
CONFLICT OF INTEREST
None declared.
AUTHOR CONTRIBUTION
Tomohiro Yoshikawa and Katsuyoshi Tomomatsu contributed to the conception of the work and drafting of the manuscript. Eriko Okazaki, Tomoe Takeuchi and Yukihiro Horio contributed to the acquisition and analysis of clinical data. Yusuke Kondo contributed to the analysis of histological data for the study. Tsuyoshi Oguma and Koichiro Asano revised the draft critically for important intellectual content. All the authors approved the final version of the manuscript.
ETHICS STATEMENT
The authors declare that appropriate written informed consent was obtained for publication of this manuscript and the accompanying images.
ACKNOWLEDGMENT
We would like to thank Editage (www.editage.com) for English language editing.
Yoshikawa T, Tomomatsu K, Okazaki E, Takeuchi T, Horio Y, Kondo Y, et al. COVID‐19 vaccine‐associated organizing pneumonia. Respirology Case Reports. 2022;10:e0944. 10.1002/rcr2.944
Tomohiro Yoshikawa and Katsuyoshi Tomomatsu contributed equally to this study.
Associate Editor: Arata Azuma
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
REFERENCES
- 1. Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, Lockhart S, et al. Safety and efficacy of the BNT162b2 mRNA COVID‐19 vaccine. N Engl J Med. 2020;383:2603–15. 10.1056/NEJMoa2034577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Watanabe S, Waseda Y, Takato H, Inuzuka K, Katayama N, Kasahara K, et al. Influenza vaccine‐induced interstitial lung disease. Eur Respir J. 2013;41:474–7. 10.1183/09031936.00146912 [DOI] [PubMed] [Google Scholar]
- 3. Park JY, Kim JH, Lee IJ, Kim HI, Park S, Hwang YI, et al. COVID‐19 vaccine‐related interstitial lung disease: a case study. Thorax. 2021;7:102–4. 10.1136/thoraxjnl-2021-217609 [DOI] [PubMed] [Google Scholar]
- 4. Yoshifuji A, Ishioka K, Masuzawa Y, Suda S, Murata S, Uwamino Y, et al. COVID‐19 vaccine induced interstitial lung disease. J lnfect Chemother. 2022;28:95–8. 10.1016/j.jiac.2021.09.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Miqdadi A, Herrag M. Acute eosinophilic pneumonia associated with the anti‐COVID‐19 vaccine AZD1222. Cureus. 2021;13:e18959. 10.7759/cureus.18959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Piqueras MB, Casajús AE, Rodero CF, Iriarte CU, Frisco IMD, Larrache JC, et al. Acute eosinophilic pneumonia following mRNA COVID‐19 vaccination: a case report. Arch Bronconeumol. 2021. 10.1016/j.arbres.2021.11.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Shimizu T, Watanabe S, Yoneda T, Kinoshita M, Terada N, Kobayashi T, et al. Interstitial pneumonitis after COVID‐19 vaccination: a report of three cases. Allergol Int. 2021;S1323‐8930(21)00138‐6. 10.1016/j.alit.2021.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bar‐On YM, Goldberg Y, Mandel M, Bodenheimer O, Freedman L, Kalkstein N, et al. Protection of BNT162b2 vaccine booster against COVID‐19 in Israel. N Engl J Med. 2021;385:1393–400. 10.1056/NEJMoa2114255 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.


