Abstract
Background
Mapping techniques are frequently used to preserve neurological function during glioma surgery. There is, however, no consensus regarding the use of many variables of these techniques. Currently, there are almost no objective data available about potential heterogeneity between surgeons and centers. The goal of this survey is therefore to globally identify, evaluate and analyze the local mapping procedures in glioma surgery.
Methods
The survey was distributed to members of the neurosurgical societies of the Netherlands (Nederlandse Vereniging voor Neurochirurgie—NVVN), Europe (European Association of Neurosurgical Societies—EANS), and the United States (Congress of Neurological Surgeons—CNS) between December 2020 and January 2021 with questions about awake mapping, asleep mapping, assessment of neurological morbidity, and decision making.
Results
Survey responses were obtained from 212 neurosurgeons from 42 countries. Overall, significant differences were observed for equipment and its settings that are used for both awake and asleep mapping, intraoperative assessment of eloquent areas, the use of surgical adjuncts and monitoring, anesthesia management, assessment of neurological morbidity, and perioperative decision making. Academic practices performed awake and asleep mapping procedures more often and employed a clinical neurophysiologist with telemetric monitoring more frequently. European neurosurgeons differed from US neurosurgeons regarding the modality for cortical/subcortical mapping and awake/asleep mapping, the use of surgical adjuncts, and anesthesia management during awake mapping.
Discussion
This survey demonstrates the heterogeneity among surgeons and centers with respect to their procedures for awake mapping, asleep mapping, assessing neurological morbidity, and decision making in glioma patients. These data invite further evaluations for key variables that can be optimized and may therefore benefit from consensus.
Keywords: awake craniotomy, glioma, intraoperative stimulation mapping, survey
Gliomas are the most common form of primary brain malignancy in adults and the current standard treatment consists of maximum safe surgery.1,2 For gliomas that are located in or near eloquent areas, the oncological goal of resection—tumor cytoreduction—is often in conflict with the functional goal—preventing neurological deficits.3–11 The surgeon can choose from a wide array of surgical and nonsurgical modalities to help him balance between both goals. For this purpose, mapping techniques are one of the most frequently used modalities. There is, however, no consensus regarding the choice of surgical modality and there are no existing guidelines regarding the indications for mapping techniques, tools for choosing between different mapping modalities, specific settings for intraoperative mapping techniques, and so forth. This lack of consensus may have resulted in a large heterogeneity between surgeons and centers with respect to these variables. The extent of this heterogeneity has never been assessed objectively, although certain aspects of the procedure may very well benefit from consensus, which may be advantageous for future collaborative efforts as well.
The goal of this survey is therefore to globally identify, evaluate and analyze the local procedures of mapping techniques in glioma surgery. The results will subsequently serve as a first stepping-stone toward potential consensus on certain aspects and as a starting point for future collaboration.
Materials and Methods
Survey Design
The questionnaire was constructed by a panel of neurosurgeons from Europe and the United States with ample experience with mapping techniques for glioma resections as part of the ENCRAM Research Consortium.12 It has been conducted in compliance with the principles of the Declaration of Helsinki (2013) and the General Data Protection Regulation (GDPR) (2018). Question subgroups included awake mapping, asleep mapping, the assessment of neurological morbidity, and intraoperative decision making. Questions were aimed to evaluate the local mapping procedures, especially regarding equipment and its settings, intraoperative assessment of eloquent areas, use of surgical adjuncts, anesthesia techniques for mapping procedures, assessment and registration of neurological morbidity, management of mapping-induced seizures, and intraoperative decision making. The target audience included consultant neurosurgeons (attendings) and neurosurgery fellows. These providers were divided into 3 groups: neurosurgery consultants/attendings with >5 years as experience as a neurosurgeon after their residency, neurosurgery consultants/attendings with <5 years as experience, and neurosurgery fellows. Additional baseline characteristics included country, gender, number of glioma resections performed, and affiliation.
Survey Distribution
The survey was made available by a link to the online LimeSurvey questionnaire platform (LimeSurvey GmbH, Hamburg, Germany) and was distributed twice by electronic mailing lists of the Congress of Neurological Surgeons (CNS) and the Dutch Neurosurgical Association (Nederlandse Vereniging voor Neurochirurgie—NVVN) with Mailchimp (Atlanta, GA, USA). It was included twice in the monthly newsletter of the European Association of Neurological Societies (EANS). Participation in the survey was anonymous, voluntary, and without remuneration. Response rate was 3.7% among CNS members and 17.8% among NVVN members. Response rate among EANS members could not be assessed due to the nature of the survey’s dispersal. The survey was open for entries between December 2020 and January 2021.
Statistical Analysis
Survey data were exported for further data analysis on January 19, 2021 from LimeSurvey into an Excel file and analyzed using R version 4.0.3 (the R Foundation, Vienna, Austria). Data were grouped according to the baseline characteristics gender, WHO region, affiliation, surgeon training level and the number of glioma resections the surgeon had performed. Overall response differences were analyzed using the χ 2 test for proportions with the Marascuillo procedure and Bonferroni correction for multiple testing. For responses with an observed count of <10 and/or expected count of <5, the Fisher’s exact test was used. Differences in survey responses based on the surgeon’s experience (in terms of number of glioma resections performed) were analyzed using the same statistical tests as for the overall response differences. Categorical survey responses were further analyzed for different subgroups using multivariate logistic (logit) regression with the type of institute and region (Europe/the United States) as the two independent variables. For variables with >2 response options, dummy coding was used for processing responses into dichotomous variables. For questions that allowed multiple answers, the McFadden MNL model was used as a mixed-effects model to analyze subgroup responses. Continuous survey outcomes were analyzed using multinominal linear regression. Statistical significance was set at 5%.
Results
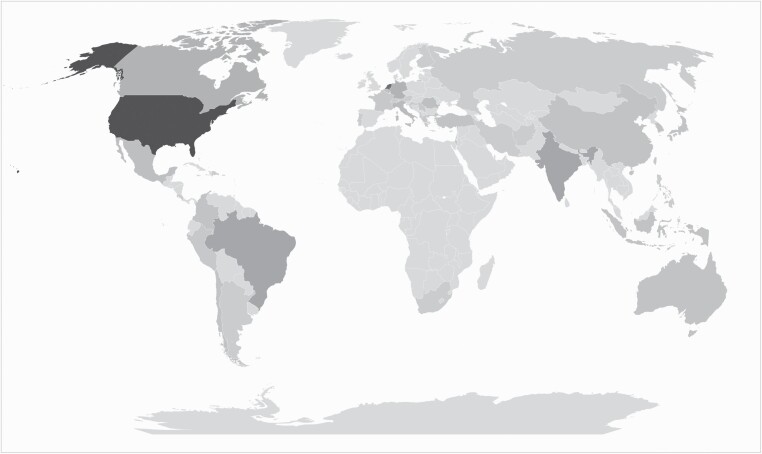
We obtained a total of 212 responses from 42 countries (Figure 1). Supplementary Table 1 shows the baseline characteristics of the respondents. 192 survey participants were male (90.1%) and 20 participants were female (9.9%). Forty percent of the responses originated from the United States and Canada (n = 85), 11.8% from Latin America (n = 25), 32.5% from Europe (n = 69), 0.94% from the Eastern Mediterranean Region (n = 2), 6.6% from South-East Asia (n = 14), 7.5% from the Western Pacific (n = 16), and 0.5% from the African Region (n = 1) (Supplementary Table 1, Supplementary Figure 1). 58.8% of participants was appointed at an academic practice/university hospital (n = 124), 18.9% worked at a nonacademic practice/community hospital (n = 40), 18.4% was appointed at a private practice (n = 39), and 4.2% selected “other” as their current appointment (n = 9) (Supplementary Table 1). The majority of survey respondents concerned consultant neurosurgeons with >5 years of practice after finishing their fellowship (79.9%, n = 169), 14.2% still had less than 5 years of experience (n = 30). 3.3% of respondents were currently appointed as neurosurgical fellow (n = 7), and 2.8% selected “other” as their current training level (n = 6) (Supplementary Table 1). Experience with glioma surgery differed between respondents: 28.8% had performed less than 100 glioma resections (n = 61), 47.2% had performed between 100 and 500 resections (n = 100) and 24.1% had performed more than 500 resections (n = 51) (Supplementary Table 1).
Figure 1.
Heatmap.
Overall Responses
Awake craniotomy—settings.
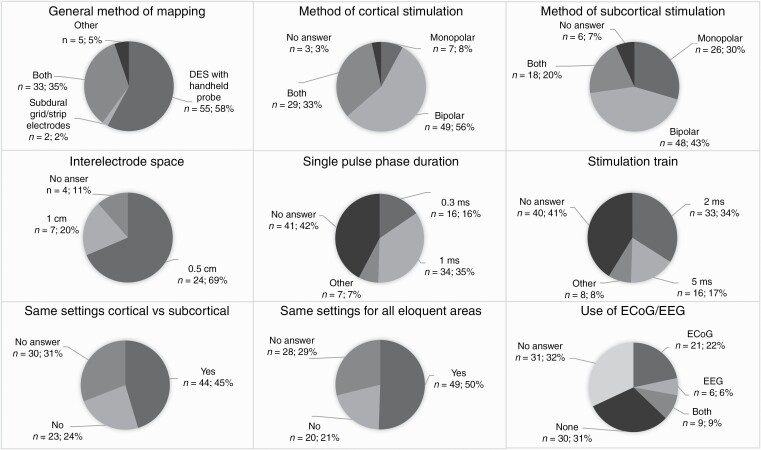
—Overall responses were significantly different for 21 of the 31 questions (Supplementary Table 2, Figure 2). Ninety-seven of the 212 neurosurgeons reported the use of awake craniotomies at their institution. Among them, the majority used direct electrostimulation with a handheld probe for cortical mapping (56.7%), whereas 34.9% preferred a subdural grid or strip electrodes (P < .0001). Most respondents used a bipolar stimulator for cortical stimulation (55.7%) or both a monopolar and bipolar (36.4%, P = .0104 for difference). For subcortical stimulation, 47.7% used only a bipolar stimulator, 29.5% used only a monopolar stimulator (P = .0134), and 20.5% used both (P = .0001). Among neurosurgeons who used subdural grid or strip electrodes, most of them used an interelectrode space of 0.5 cm (68.6%) (P < .0001). The median limits for current range during awake cortical mapping were 2-10 mA. Current’s increasing steps and stimulation frequencies did not differ significantly. The single pulse phase duration (SPPD) was more often 1.0 ms (35.1%) than 0.3 ms (15.5%) (P = .0017) and the majority of respondents reported a train of 2 seconds (34.0%) as opposed to 5 seconds (16.5%) (P = .0051). Most respondents used the same stimulation settings for all awake cortical mapping procedures (50.5%) and for cortical and subcortical mapping (45.4%) (P = .0015).
Figure 2.
Significant differences—awake mapping procedures.
Awake craniotomy—assessment of eloquent areas.
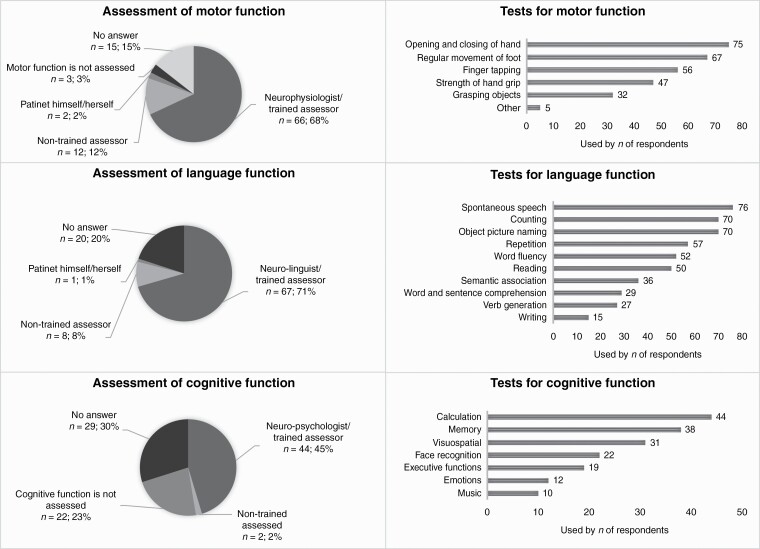
—The majority of respondents reported that eloquent areas were assessed by a trained assessor (68.0% for motor, 69.1% for speech, and 45.4% for cognition) (Supplementary Table 2, Figure 2). Motor function was most commonly assessed by opening and closing of the hand (79.8%), regular movement of the foot (71.3%); language function by spontaneous speech production (78.4%), counting (72.2%), and object picture naming (72.2%) and cognitive function with calculation (100%), memory (86.4%), and visuospatial functioning (70.5%).
Awake craniotomy—monitoring, surgical adjuncts, and anesthesia management.
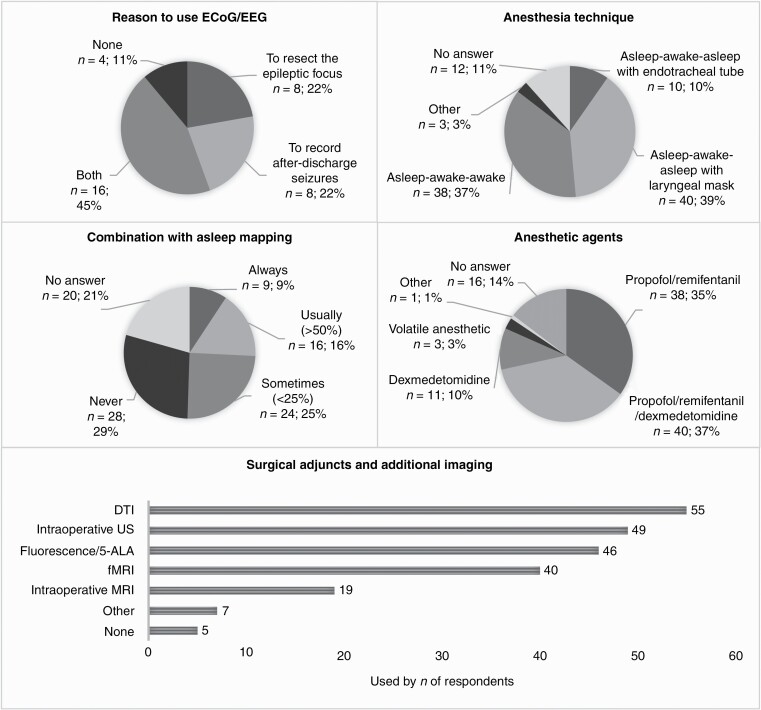
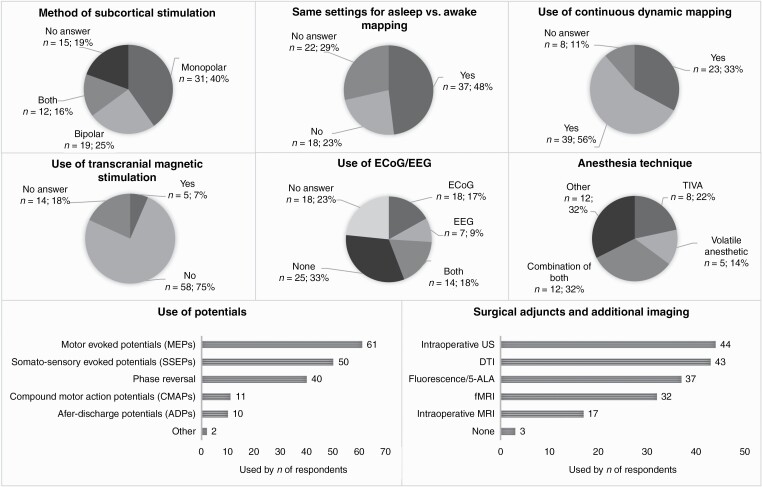
—Slightly more than half of the surgeons who performed awake craniotomies combine this sometimes (24.7%) or never (28.9%) with asleep mapping during the same resection (Supplementary Table 2, Figure 2). Diffusion tensor imaging (DTI) (56.7%) and intraoperative ultrasound (50.5%) are the most frequently used surgical adjuncts, followed by fluorescence/5-ALA (47.4), functional MRI (fMRI) (41.2%), and intraoperative MRI (19.6%) (P < .0001). Most neurosurgeons either use only ECoG (21.6%) or no electrophysiological monitoring at all (30.9%) (P = .0005). When ECoG and/or intraoperative EEG are used, they are most frequently used to record both after-discharge seizures and to resect the epileptic focus (44.4%) (P = .0011). For anesthesia management, the adjusted asleep-awake-asleep technique with laryngeal mask (41.2%) and awake-awake-awake technique (39.2%) were used most often (P < .0001). Either a combination propofol/remifentanil (39.2%) or propofol/remifentanil/dexmedetomidine (41.2%) were used the most frequently for anesthesia induction (P < .0001).
Asleep mapping.
—Overall responses were significantly different for 8 of the 18 questions (Supplementary Table 3, Figure 3). Seventy-seven (36.3%) respondents reported the use of asleep mapping techniques at their institute. For cortical mapping, a slight majority preferred the combination of a monopolar and bipolar stimulator (36.4%) or a bipolar stimulator alone (29.9%). For subcortical mapping, most neurosurgeons used the monopolar only (40.3%) (P = .0007). For the majority of them, the stimulation settings for asleep mapping were the same as for awake mapping (48.1% vs 23.4%, P = .0014).
Figure 3.
Significant differences—asleep mapping procedures.
The minority of neurosurgeons used continuous dynamic mapping (CDM) for asleep mapping techniques (29.9%) (P = .0090). Furthermore, only 6.5% of respondents reported that they used transcortical magnetic stimulation (TMS) for asleep mapping (P < .0001). Eloquent areas were most commonly identified using evoked potentials (motor evoked potentials—MEPs: 79.2%, somatosensory evoked potentials—SSEPs: 64.9%) or phase reversal (51.9%) (P < .0001). The majority of neurosurgeons reported the presence of a clinical neurophysiologist during asleep mapping procedures, either with telemetric monitoring (39.0%) or without (28.6%). The most common surgical adjuncts of modalities for additional imaging were fMRI (41.6%), fluorescence/5-ALA (48.1%), DTI (55.8%), and intraoperative ultrasound (57.1%) (P < .0001). A majority of neurosurgeons did not use ECoG or EEG intraoperatively during asleep mapping procedures (32.5%) (P = .0004), and general anesthesia was most frequently induced by total intravenous anesthesia (TIVA, 62.3%) (P < .0001).
Assessment of neurological morbidity.
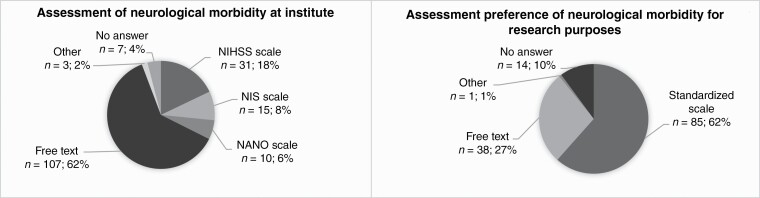
—Most of the respondents reported that neurological morbidity is documented as free text in the electronic patient system at their institute (77.5%) (P < .0001) (Supplementary Table 4, Figure 4). In contrast, a majority of survey participants reported that they would prefer to assess neurological morbidity using a standardized scale (61.6%) (P < .0001). Neurosurgeons were the most common assessors of neurological morbidity in our survey (92.0%), followed by neurosurgical residents (40.6%), neurologists (29.7%), and physician assistants (25.4%).
Figure 4.
Significant differences—assessment of neurological morbidity.
Decision making.
—The most common reason among respondents to perform an awake craniotomy in glioma patients was the possibility to perform mapping or monitoring in an awake setting (56.2%, P < .0001) (Supplementary Table 5, Figure 5). Documentation of the stimulation threshold and intensity in relation to eloquent mapping sites (39.7%) was most common, followed by information regarding the neuronavigation (29.8%), and information regarding the evoked potentials (28.1%) (Supplementary Table 3). On a scale of 1-10, the most important information on which neurosurgeons based their decision to end the resection is the patient’s task performance (median 10) followed by the evoked potentials (median 9), the imaging (median 8), and the macroscopical view (median 8).
Figure 5.
Significant differences—decision making.
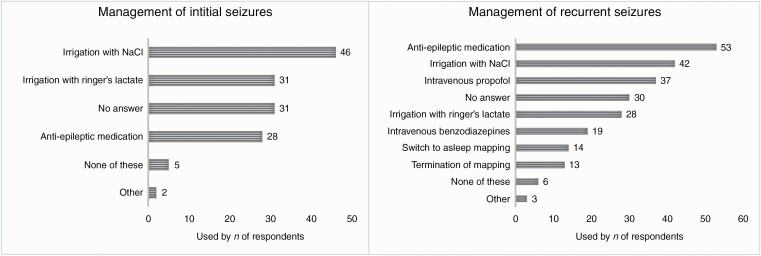
Initial stimulation-induced seizures were most commonly suppressed by irrigation of the exposed brain surface with chilled sodium chloride (NaCl) or Ringer’s lactate solution (38.0% and 25.6%), or administration of anti-epileptic medication (23.1%) (P = .0120).
Recurrent stimulation-induced seizures were more commonly treated with anti-epileptic medications (43.8%) than irrigation of chilled NaCl (34.7%) or Ringer’s lactate solution (23.1%) (P = .0271).
Subgroup Responses
Responses were further analyzed according to the respondent’s affiliation and region: academic practice/university hospital vs nonacademic practice/community hospital or private practice; and Europe vs the United States.
Responses by center (academic vs nonacademic or private practice).
—For 5 of the 57 questions, significant differences were found between subgroups (Supplementary Tables 2–5). Academic neurosurgeons were more than five times as likely to perform awake craniotomies (OR = 5.15, P = .0007). Academic neurosurgeons also reported the use of asleep mapping techniques more than three times as often (OR = 3.56, P = .0094). In academic centers, it was more common to have a clinical neurophysiologist present with telemetric monitoring during asleep mapping procedures: OR = 6.00 (P = .0278).
Responses by region (Europe vs the United States).
—For 9 of the 57 questions, significant differences were found between subgroups (Supplementary Tables 2–5). European neurosurgeons were less likely to report using a subdural grid/strip electrode alone (OR = 0.28, P = .0144) or in combination with a handheld probe for direct electrostimulation (OR = 0.31, P = .0245) during awake craniotomy. They assessed cognitive function more often during awake craniotomy (OR = 4.03, P = .0313) and used fluorescence/5-ALA intraoperatively (OR = 3.77, P = .0124) more frequently. They were less likely to use intraoperative MRI though (OR = 0.23, P = .0343). With respect to anesthesia management, European colleagues more often used propofol/remifentanil (OR = 3.97, P = .0124), whereas in the United States they preferred the addition of dexmedetomidine to this regimen more commonly (OR = 0.15, 95% CI = 0.050-0.48, P = .0012). During asleep mapping, European neurosurgeons were more likely to use CDM (OR = 6.26, P = .0314), but less likely to use compound motor action potentials (CMAPs; OR = 0.17, P = .0493) or phase reversal (OR = 0.21, P = .0103) for the identification of eloquent areas. They also more often reported to have the clinical neurophysiologist present without telemetric monitoring during asleep mapping procedures (OR = 5.91, P = .0485).
Responses by surgeon’s experience.
—For 2 of the 40 questions, significant differences were found between subgroups (Supplementary Tables 6–8). During awake mapping, the most experienced neurosurgeons (>500 glioma resections performed) used less often direct electrostimulation (DES) with a handheld probe (37.1%) than less experienced neurosurgeons (100-500 glioma resections performed: 65%; <100 glioma resections performed: 72.7%). Conversely, they used a combination of a handheld probe and a subdural grid or strip electrodes (54.3% vs 30.0% for the 100-500 subgroup and 9.1% for the <100 subgroup) (P = .0009). Second, when more experienced neurosurgeons used a subdural grid or strip electrodes, more often the interelectrode space of the grid or strip was 1 cm (65.0%) than was the case during resections done by less experienced neurosurgeons (100-500 subgroup: 16.7%; <100 subgroup: 33.3%) (P = .0085).
Discussion
Key Results
This survey is the first study that investigates the local mapping procedures used in glioma resections on a global scale, and further analyzed by institute and region.
We found an evident heterogeneity among surgeons and centers with respect to their local procedures. Overall, the most notable differences were observed for the kinds of equipment and its settings that are used for both awake and asleep mapping, the intraoperative assessment of eloquent areas, the use of surgical adjuncts, the use of monitoring, the anesthesia management, the assessment of neurological morbidity and the perioperative decision making. Academic practices more often performed awake and asleep mapping procedures and more often employ a clinical neurophysiologist with telemetric monitoring. There were significant differences in preference among European vs US neurosurgeons regarding the modality for cortical and subcortical mapping, the use of surgical adjuncts, and anesthesia management for awake mapping. Furthermore, for asleep mapping, there were differences regarding the use of CDM, the kind of evoked potentials that is being used, and the addition of telemetric monitoring. Last, more experienced neurosurgeons (in terms of glioma resections performed) used more often a combination of a probe and subdural grid/strip for awake mapping whereas less experienced neurosurgeons more frequently used a probe only.
Interpretation and Comparison With the Literature
The results from this survey should be interpreted from the perspective of previously conducted surveys. In 2017, Spena et al found in a survey among 20 European centers a substantial amount of heterogeneity between centers: some only performed awake mapping, and some only asleep mapping.13 In our survey, 40.4% performed both awake and asleep mapping, 23.8% only awake mapping, and 9.9% only asleep mapping. Furthermore, Spena et al found that 53% used ECoG or EEG, which corresponded well with our results (37.1% during awake mapping, 44.2% during asleep mapping). Hamberger et al found in a survey among 56 epilepsy centers evident variability in all aspects of the procedure.14 We found similar variability in our survey and share their conclusion that “this will influence mapping results, which directly affect the boundaries of cortical resection and, consequently, might worsen either seizure or functional outcomes.” We would like to add that increased consensus on certain aspects would be beneficial in terms of collaborative scientific efforts between centers. A recent survey conducted by Arzoine et al explored among 20 European centers the local practices in anesthetic management during low-grade glioma surgery.15 Their results were relatively similar to ours (ours in parenthesis): for awake surgery, 56% used the asleep-awake-asleep technique (51.5%), and 40% the awake-awake-awake technique (39.2%). For asleep surgery, 82% used a laryngeal mask (80%).
Few studies exist that have compared the outcomes of different mapping settings. Szelényi et al reported that stimulation-induced seizures are more frequent with the 50/60 Hz bipolar stimulation than with the train-of-five technique using strip electrodes or a monopolar stimulator.16 They promote the use of this technique for both cortical and subcortical mapping and state that monopolar stimulation is more effective for subcortical mapping of the corticospinal tract than bipolar stimulation.17 In contrast, Yamaguchi et al reported that the use of a bipolar stimulator for subcortical stimulation can be performed safely, which has been described by Berger et al as early as in 1990 and has since then become the gold standard for cortical mapping.18,19 In our survey, we observed the contrasts between these studies as well: we found that 90.1% used a monopolar or bipolar for mapping during awake craniotomies. Among them, the majority used a bipolar for cortical (55.7%) and subcortical (47.7%) stimulation. For asleep mapping, comparable proportions of respondents used a monopolar (23.4%), bipolar (29.9%), or both (36.4%) for cortical mapping. For subcortical mapping, the majority used a monopolar (40.3%), rather than a bipolar (24.7%) or both (15.6%).
Our results are in line with the 2012 recommendations from the Japan Awake Surgery Conference,20,21 in which they state that cortical stimulation should be performed with a bipolar stimulator (current range 2-8 mA with 1 mA increments, SPPD 0.5 ms, frequency 50 Hz, duration 1-2 seconds) with seizure monitoring using ECoG. In our survey, the majority used a bipolar stimulator with a median current range of 2-12 with 1 or 2 mA increments, SPPD 1 ms, frequency 50 Hz (Europe) or 60 Hz (the United States) with a train of 2 seconds.
Limitations and Strengths
An important limitation of survey studies is self-selection sampling bias. We assume that this survey was subject to this kind of bias as well, since a number of surgeons and centers have not responded to the survey. Moreover, low- to middle-income counties may have to interpret the results of this study with caution since the responses were skewed toward Western high-income countries. The subgroup analyses that were conducted for institute and region focused on those countries as well which may have limited the external generalizability. Furthermore, a majority of respondents reported that the assessment of eloquent areas during mapping procedures was performed by highly trained personnel (neurophysiologists, neuro-linguists, or trained assessors). We acknowledge that this would have implications on the generalizability of best practices to centers or countries with a lower density of resources. Due to the survey design, we were not able to investigate the interplay between the surgeon’s personal preference and the institute’s tradition on the choice for certain variables. We were also not able to directly compare the impact of procedural heterogeneity on surgical outcomes; therefore, we chose to correlate survey responses with surgeon’s experience as a proxy for impact on outcomes as the experience has likely evolved over time toward Level 4 practice patterns. Last, we noticed a relatively high proportion of “no answers” to certain questions (in particular regarding the technical details of the mapping procedure) which may be explained by the inability of responders to invest a larger amount of time in completing the survey. A closer look at our data revealed that the percentage of “no answers” was lower when the respondent had more experience in terms of number of glioma resections performed. Consequently, we cannot state unequivocally that time constraints were the only factor at play and we cannot fully exclude a relative lack of technical understanding as a possible cause of this issue. Therefore, we imagine that the results of this survey could potentially serve as an instrument to gain insight into novel opportunities for education. Important strengths of this study include the scale of distribution, the width of the survey’s scope, the detail of the questions, the subgroup analyses between European and US neurosurgeons and academic vs nonacademic centers and the subgroup analyses between more and less experienced neurosurgeons.
Conclusions and Future Directions
This survey illustrates the evident heterogeneity between surgeons and centers regarding the specifics of mapping procedures and decision making. These results underline the importance of further research that addresses key aspects of mapping procedures and perioperative decision making. These aspects should be compared to identify the optimum framework for performing mapping procedures, taking into account local differences. The presented survey may serve as a first step toward a collaborative effort to investigate key variables that can be optimized and may therefore benefit from consensus. This will provide the neurosurgical field with the needed data on which clinical guidelines can be based in order to reach the full potential of mapping in glioma resections. Further studies should focus on (1) the impact of procedural variability on surgical outcomes, ideally accompanied with a comparison between high-income and low-income countries and (2) the correlation of this observed variability among neurosurgeons with neurophysiologists and anesthesiologists.
Supplementary Material
Acknowledgments
We gratefully thank our colleagues for their time and effort in completing the survey.
Contributor Information
Jasper K W Gerritsen, Department of Neurosurgery, Erasmus Medical Center, Rotterdam, the Netherlands.
Marike L D Broekman, Department of Neurosurgery, Haaglanden Medical Center, The Hague, the Netherlands.
Steven De Vleeschouwer, Department of Neurosurgery, University Hospital Leuven, Leuven, Belgium.
Philippe Schucht, Department of Neurosurgery, University Hospital Bern, Bern, Switzerland.
Christine Jungk, Department of Neurosurgery, University Hospital Heidelberg, Heidelberg, Germany.
Sandro M Krieg, Department of Neurosurgery, Technical University Munich, Munich, Germany.
Brian V Nahed, Department of Neurosurgery, Massachusetts General Hospital, Boston, Massachusetts, USA.
Mitchel S Berger, Department of Neurosurgery, University of California, San Francisco, San Francisco, California, USA.
Arnaud J P E Vincent, Department of Neurosurgery, Erasmus Medical Center, Rotterdam, the Netherlands.
Funding
None.
Conflict of interest statement. None.
References
- 1. Stupp R, Mason WP, van den Bent MJ, et al. ; European Organisation for Research and Treatment of Cancer Brain Tumor and Radiotherapy Groups; National Cancer Institute of Canada Clinical Trials Group . Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352(10):987–996. [DOI] [PubMed] [Google Scholar]
- 2. DeAngelis LM. Brain tumors. N Engl J Med. 2001;344(2):114–123. [DOI] [PubMed] [Google Scholar]
- 3. Smith JS, Chang EF, Lamborn KR, et al. Role of extent of resection in the long-term outcome of low-grade hemispheric gliomas. J Clin Oncol. 2008;26(8):1338–1345. [DOI] [PubMed] [Google Scholar]
- 4. Stummer W, Reulen HJ, Meinel T, et al. ; ALA-Glioma Study Group . Extent of resection and survival in glioblastoma multiforme: identification of and adjustment for bias. Neurosurgery. 2008;62(3):564–576; discussion 564. [DOI] [PubMed] [Google Scholar]
- 5. Sanai N, Berger MS. Glioma extent of resection and its impact on patient outcome. Neurosurgery. 2008;62(4):753–764; discussion 264. [DOI] [PubMed] [Google Scholar]
- 6. Vourinen V, Hinkka S, Färkkilä M, Jääskeläinen J. Debulking or biopsy of malignant glioma in elderly people—randomized study. Acta Neurochir. 2003;145(1):5–10. [DOI] [PubMed] [Google Scholar]
- 7. Lacroix M, Abi-Said D, Fourney DR, et al. A multivariate analysis of 416 patients with glioblastoma multiforme: prognosis, extent of resection, and survival. J Neurosurg. 2001;95(2):190–198. [DOI] [PubMed] [Google Scholar]
- 8. Jeremic B, Grujicic D, Antunovic V, et al. Influence of extent of surgery and tumor location on treatment outcome of patients with glioblastoma multiforme treated with combined modality approach. J Neurooncol. 1994;21(2):177–185. [DOI] [PubMed] [Google Scholar]
- 9. Keles GE, Anderson B, Berger MS. The effect of extent of resection on time to tumor progression and survival in patients with glioblastoma multiforme of the cerebral hemisphere. Surg Neurol. 1999;52(4):371–379. [DOI] [PubMed] [Google Scholar]
- 10. Keles GE, Lamborn KR, Chang SM, et al. Volume of residual disease as a predictor of outcome in adult patients with recurrent supratentorial glioblastomas multiforme who are undergoing chemotherapy. J Neurosurg. 2004;100(1):41–46. [DOI] [PubMed] [Google Scholar]
- 11. Van den Bent MJ, Stupp R, Mason W. Impact of the extent of resection on overall survival in newly diagnosed glioblastoma after chemo-irradiation with temozolomide: further analyses of EORTC study 26981. Eur J Cancer Suppl. 2005;3:134. [Google Scholar]
- 12. Gerritsen JKW, Broekman MLD, De Vleeschouwer S, et al. Letter: the European and North American Consortium and Registry for intraoperative stimulation mapping: framework for a transatlantic collaborative research initiative. Neurosurgery. 2021;88(4):E369. [DOI] [PubMed] [Google Scholar]
- 13. Spena G, Schucht P, Seidel K, et al. Brain tumors in eloquent areas: a European multicenter survey of intraoperative mapping techniques, intraoperative seizures occurrence, and antiepileptic drug prophylaxis. Neurosurg Rev. 2017;40(2):287–298. [DOI] [PubMed] [Google Scholar]
- 14. Hamberger MJ, Williams AC, Schevon CA. Extraoperative neurostimulation mapping: results from an international survey of epilepsy surgery programs. Epilepsia. 2014;55(6):933–939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Arzoine J, Levé C, Pérez-Hick A, et al. ; Collaborators of the ELGGN . Anesthesia management for low-grade glioma awake surgery: a European Low-Grade Glioma Network survey. Acta Neurochir (Wien). 2020;162(7):1701–1707. [DOI] [PubMed] [Google Scholar]
- 16. Szelényi A, Joksimovic B, Seifert V. Intraoperative risk of seizures associated with transient direct cortical stimulation in patients with symptomatic epilepsy. J Clin Neurophysiol. 2007;24(1):39–43. [DOI] [PubMed] [Google Scholar]
- 17. Szelényi A, Senft C, Jardan M, et al. Intra-operative subcortical electrical stimulation: a comparison of two methods. Clin Neurophysiol. 2011;122(7):1470–1475. [DOI] [PubMed] [Google Scholar]
- 18. Yamaguchi F, Takahashi H, Teramoto A. Intra-operative detection of motor pathways using a simple electrode provides safe brain tumor surgery. J Clin Neurosci. 2007;14(11):1106–1110. [DOI] [PubMed] [Google Scholar]
- 19. Berger MS, Ojemann GA, Lettich E. Neurophysiological monitoring during astrocytoma surgery. Neurosurg Clin N Am. 1990;1(1):65–80. [PubMed] [Google Scholar]
- 20. Saito T, Muragaki Y, Maruyama T, et al. Intraoperative functional mapping and monitoring during glioma surgery. Neurol Med Chir (Tokyo). 2015;55(Suppl 1):1–13. [PubMed] [Google Scholar]
- 21. Kayama T; Guidelines Committee of the Japan Awake Surgery Conference . The guidelines for awake craniotomy guidelines committee of the Japan awake surgery conference. Neurol Med Chir (Tokyo). 2012;52(3):119–141. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.