Abstract
Objective
Faciocraniosynostoses (FCS) are malformations affecting the development of the bones of the skull and face, due to the premature closure of one or more craniofacial sutures, mostly secondary to activating Fibroblast Growth Factor Receptor (FGFR) 1–3 mutations. Gain-of-function FGFR3 mutations are also responsible for various conditions referred to as osteochondrodysplasia (OCD), characterized by structural and functional abnormalities of growth plate cartilages. We hypothesized that patients with FGFR-related faciocraniosynostoses may present extra-cranial growth anomalies.
Study design
We retrospectively collected height and weight data from a cohort of 70 patients. Included patients were admitted for FGFR-related FCS between 2000 and 2021 at the Craniofacial Unit of Necker – Enfants Malades University Hospital in Paris, France.
Results
We showed that FGFR-related faciocraniosynostoses had significantly reduced heights and weights relative to controls, and that two specific time periods (1–3 years and > 8 years of age) were associated with lower height and weight values. Four patients had received growth hormone treatment but remained below normal values for growth in height and weight.
Conclusions
Patients with FGFR-related faciocraniosynostoses have clinically significant extra-cranial anomalies which are not currently investigated and managed in usual protocols; these patients could benefit from a systematic pre-pubertal endocrine assessment. More generally, our results extend the scope of extracranial anomalies in FGFR-related faciocraniosynostoses and support the hypothesis that all conditions with activating FGFR mutations affect both membranous ossification and long bones.
Abbreviations: AS, Apert syndrome; BMI, body mass index; CS, Crouzon syndrome; FCS, faciocraniosynostosis; FGFR, fibroblast growth factor receptor; MS, Muenke syndrome; OCD, osteochondrodysplasia; PS, Pfeiffer syndrome; SD, standard deviation
Keywords: Craniofacial, Syndrome, Height, Weight
Highlights
-
•
FGFR mutations can cause syndromic faciocraniosynostoses (FCS) and are also responsible for osteochondrodysplasia.
-
•
Patients with FCS present with significant delays in development compared to the standard French population.
-
•
Patients with FCS could benefit from a systematic pre-pubertal endocrine assessment and hormonotherapy but more data is required to establish guidelines.
-
•
FCS and achondroplasia both affect membranous and endochondral ossification processes.
1. Introduction
Faciocraniosynostoses (FCS) are malformations affecting the development of the bones of the skull and face, due to the premature closure of one or more craniofacial sutures. FCS impair normal brain expansion and the growth of the midface, and are mostly due to activating FGFR1, FGFR2 and FGFR3 mutations, such as in Muenke, Crouzon, Pfeiffer, and Apert syndromes. Patients with Muenke syndrome (MS) generally present bi-coronal synostosis and extra-cranial symptoms including brachydactyly and carpal and tarsal bone fusions; MS prevalence is 0.8–1/10,000 births (Sabatino et al., 2004). Crouzon syndrome (CS) does not include known extra-cranial features and is mostly characterized by brachycephaly, hypertelorism and midface hypoplasia; CS affects 1/25,000 births (Cohen and Kreiborg, 1992). Apert syndrome (AS) is characterized by the triad of brachycephaly, hypoplasia of the midface and syndactyly of the feet and hands. AS affects 1/55,000 births (Cohen et al., 1992). Pfeiffer syndrome (PS) also associates brachycephaly with minor abnormalities of the extremities (broad thumbs), with a prevalence of 1/100.000 (Vogels and Fryns, 2006).
Fibroblast Growth Factor Receptors (FGFR) are activated by Fibroblast Growth Factors (FGF) and co-receptor, and play a major role in sutural bone formation. FGFR2 and FGFR3 are expressed by undifferentiated osteoprogenitor cells of cranial sutures. Upon ossification, the osteoprogenitor cells differentiate into osteoblasts expressing FGFR1, leading to the deposition of osteoid matrix along the sutures. The FGF/FGFR complex also regulates a signaling loop between mesenchymal and epithelial tissues that contributes to limb development (Teven et al., 2014).
FGFR1 or FGFR2 mutations have been identified in Pfeiffer syndrome, FGFR2 mutations in Crouzon and Apert syndromes, whereas FGFR3 mutations are described in Muenke syndrome and Crouzon syndrome with acanthosis nigricans (Meyers et al., 1995).
Interestingly, heterozygous gain-of-function FGFR mutations are also responsible for the clinical spectrum of osteochondrodysplasia (OCD), a group of conditions characterized by structural and functional abnormalities of growth plate cartilages. Three OCD are caused by activating FGFR3 mutations, namely achondroplasia, hypochondroplasia, and thanatophoric dysplasia. Furthermore, in Antley-Bixler syndrome (Antley and Bixler, 1975), caused by activating FGFR2 mutations, FCS is associated with abnormalities of the extremities and long bones.
It thus appears that FGFR genes play a central role in both craniofacial and long bone development and growth. We thus hypothesized that patients with FGFR-related FCS may present extra-cranial growth anomalies. There is currently no data on this question in the literature. To tackle this issue, we retrospectively collected height and weight data from a large FCS cohort (n = 70) and used hierarchical linear models to take repeated measurements into account and assess growth charts relative to data from a control age-matched French population.
2. Material and methods
This study was conducted according to the local ethical regulations. We included all patients admitted for FGFR-related FCS (MS, CS, AS, PS) between 2000 and 2021 at the Craniofacial Unit of Necker – Enfants Malades University Hospital in Paris, France (Table 1). Patients and their families were contacted via email to take part into this study and for authorizing the retrospective data to be used. Written consent was obtained from the parents or from the subjects. We collected growth and weight curves for each patient from the ‘Carnet de Santé’, the standard follow-up pediatric booklet mandatory for every child in France. Data collection took place between March 2020 and March 2021. We furthermore collected the following clinical parameters for all patients: sex, date of birth, type of FCS (MS, CS, AS, PS), weeks of amenorrhea at birth, parent heights and history of somatotropin hormonal therapy. We also compiled the dates of all craniofacial procedures: fronto-facial monobloc advancement (FFMBA), fronto-orbital advancement (FOA), posterior expansion, Le Fort III (LIII) and Le Fort I (LI) facial advancements, foramen magnum decompression (FM decompression), and ventriculoperitoneal shunt (VP). Finally, we collected data from sleep studies (polysomnography results with apnea/hypopnea index (AHI)), the use of nocturnal continuous positive airway pressure (CPAP), and the presence of tracheostomy. The 2018 French standard growth and weight curves were used as controls (Heude et al., 2019).
Table 1.
Patient characteristics.
| Patients characteristics | No. (%) girls | No. (%) boys |
|---|---|---|
| No. patients | 37 | 33 |
| Term (WA) | ||
| Mean (±SD) | 40 (2) | 40 (2) |
| Median | 41 | 40 |
| Minimum | 34 | 37 |
| Maximum | 43 | 42 |
| Target size (cm) | ||
| Mean (±SD) | 161 (4) | 179 (5) |
| Median | 162 | 180 |
| Minimum | 154 | 169 |
| Maximum | 167 | 188 |
| Syndrome | ||
| Crouzon | 12 (33%) | 20 (61%) |
| Pfeiffer | 9 (24%) | 6 (18%) |
| Apert | 7 (19%) | 4 (12%) |
| Muenke | 9 (24%) | 3 (9%) |
| Mutation | ||
| FGFR2 | 21 (57%) | 19 (58%) |
| Ala391Glu | 0 | 1 |
| Ala337Pro | 0 | 1 |
| Asn549Ser | 0 | 1 |
| Cys278Phe | 1 | 0 |
| Cys342Arg | 3 | 3 |
| Cys342Ser | 2 | 3 |
| Cys342Trp | 3 | 0 |
| Cys342Tyr | 1 | 2 |
| Gln289Pro | 1 | 0 |
| Ile288Asn | 1 | 0 |
| Phe276Val | 1 | 1 |
| Pro253Arg | 1 | 1 |
| Ser282Cys | 1 | 0 |
| Ser354Cys | 0 | 3 |
| Ser267Pro | 1 | 0 |
| Ser252Trp | 4 | 1 |
| Thr341Pro | 0 | 1 |
| Trp290Arg | 0 | 1 |
| Tyr340His | 1 | 0 |
| FGFR3 | 10 (29%) | 4 (12%) |
| Ala391Glu | 1 | 1 |
| Pro250Arg | 9 | 3 |
| Hormonal treatment | 2 (6%) | 2 (6%) |
| Follow-up (years) | ||
| Mean (±SD) | 17 (10) | 17 (7) |
| Median | 15 | 16.5 |
| Minimum | 3 | 5 |
| Maximum | 55 | 31 |
| Birth height (cm) | ||
| Mean (±SD) | 50.8 (2.1) | 48.9 (1.8) |
| Median | 51 | 48 |
| Minimum | 47 | 47 |
| Maximum | 54 | 53 |
| Birth weight (kg) | ||
| Mean (±SD) | 3.5 (0.6) | 3.3 (0.5) |
| Median | 3.6 | 3.2 |
| Minimum | 1.9 | 2.2 |
| Maximum | 4.2 | 4.4 |
| Birth BMI (kg/m2) | ||
| Mean (±SD) | 13.8 (1.1) | 13.1 (1.5) |
| Median | 13.9 | 13.6 |
| Minimum | 12.1 | 10.0 |
| Maximum | 15.5 | 15.2 |
| Birth head circumference (cm) | ||
| Mean (±SD) | 35.9 (1.4) | 34.3 (1.3) |
| Median | 35.8 | 34.0 |
| Minimum | 34 | 32 |
| Maximum | 39 | 36 |
Three hierarchical linear models were used to account for the repetition of the data over time and thus its non-independence. A random effect was introduced for each individual. The coefficients of the model were compared to 0 using Student t-tests. The predicted variables were: (1) height, (2) weight and (3) body mass index (BMI – weight in kg divided by the square of the height in meters). Univariate analyses were performed for each model and variables significantly increasing the likelihood were loaded into the respective multivariate models. Interaction parameters between variables were considered, as well as polynomial terms of degree 4 for the evolution of predicted variables as a function of time. The results of the multivariate models were reported in Table 2. The significance level was defined as p < 0.05. The assumptions of normality and homoscedasticity of the residuals were tested. Statistical analyses were performed with R version 3.6.2 (R Core Team, 2016) with the packages nlme (Pinheiro et al., 2017) and ggplot (Wickham, 2009).
3. Results
A total of 294 patients were included and 70 patients could be contacted for data collection (Table 1). The mean patient age at data collection was 18.1 (2.8–56) years of age. We collected a total of 846 measurements for height and 863 for weight. The mean number of measurements per patient was 12 (1–35) for height and 12 (1–43) for weight.
Mean birth length was 51.3 cm (47–57) and 48.9 cm (47–53) for boys and girls respectively. Mean birth weight was 3.5 kg (1.9–4.2) and 3.3 kg (2.2–4.4) for boys and girls respectively. In the general population, the average birth measurements for boys were 49.9 ± 1.9 cm and 3.4 ± 0.49 kg, and the average birth measurements for girls were 49.2 ± 1.9 cm and 3.2 ± 0.46 kg (De Onis et al., 2009). All patients mean birth measurements were within normal values.
41/70 patients had reached their final height within the selected time frame, with 21/37 girls and 20/33 boys. Mean final adult height was 152 cm in girls compared to 165 cm in French charts, and mean adult height was 169 cm in boys compared to 177 cm in French charts.
55/70 patients had a confirmed genetic mutation: 41 patients with a FGFR2 mutation and 14 patients with a FGFR3 mutation. Patients without an identified genetic mutation had been tested outside Necker – Enfants Malades University Hospital, and they or their family were unable to provide a genetic test result.
In Apert syndrome, 7/11 patients had a confirmed FGFR2 mutation. In Crouzon syndrome, 23/32 patients had a confirmed FGFR2 (21/23) or FGFR3 (2/23) mutation. In Pfeiffer syndrome, 13/15 patients had a confirmed FGFR2 mutation. In Muenke syndrome, all patients had an identified FGFR3 mutation (12/12). No patient had both FGFR2 and FGFR3 mutations, and no other novel mutation was reported (Table 1).
Before 2014, genetic testing was made using Sanger sequencing. From 2014 onwards, Next Generation Sequencing was used. However for Apert syndrome, Sanger sequencing was continuously used because this syndrome is only related to two recurrent mutations.
Univariate analyses showed no significant difference in height (Table 2.1), weight and BMI (Supplemental table) between patients with hormonotherapy and controls, and between FGFR2-related and FGFR3-related FCS.
Table 2.1.
Parameters of the hierarchical models by syndrome.
| Parameters of the hierarchical models by syndrome Height | ||||
|---|---|---|---|---|
| Estimate | SD | p-Value | ||
| Intercept | 52.99 | 13.45 | ||
| Muenke | Intercept | +1.503 | 1.898 | 0.528 |
| Slope | +0.827 | 0.127 | <0.001 | |
| Apert | Intercept | +2.075 | 2.166 | 0.344 |
| Slope | +0.134 | 0.099 | 0.175 | |
| Pfeiffer | Intercept | −2.110 | 1.705 | 0.223 |
| Slope | −0.389 | 0.097 | <0.001 | |
| Female | Intercept | −0.068 | 1.433 | 0.962 |
| Slope | −5.501 | 0.552 | <0.001 | |
| Term | Intercept | +0.095 | 0.340 | 0.781 |
| Slope | +0.108 | 0.021 | <0.001 | |
| FOA | Intercept | −0.394 | 1.762 | 0.824 |
| Slope | +0.176 | 0.106 | 0.098 | |
| HT | +1.025 | 59.878 | 0.918 | |
| Target height | −0.270 | 0.282 | 0.347 | |
| FGFR3 | −6.197 | 8.537 | 0.471 | |
| FFMBA | −9.692 | 7.081 | 0.176 | |
| Posterior expansion | −9.439 | 6.745 | 0.162 | |
| LF3 | +2.098 | 7.063 | 0.767 | |
| LF1 | −0.518 | 15.09 | 0.973 | |
| FM decompression | −13.91 | 8.340 | 0.100 | |
| VP | −17.58 | 10.73 | 0.106 | |
| Tracheostomy | +13.53 | 7.313 | 0.065 | |
Regarding height, univariate analyses showed that patients with MS grew 0.83 cm more than patients with CS every year (p < 0.001). On the contrary, patients with PS grew less than patients with CS by 0.39 cm per year (p < 0.001). Female patients grew significantly less every year than male patients (p < 0.001). Patients who were born at a more advanced term grew more than the others by 0.11 cm every year (p < 0.001). There was no significant difference in height dynamics between patients with CS and AS, patients treated with hormonotherapy and controls, and between FGFR2 or FGFR3-related FCS. For patients who had reached adulthood, there was no significant difference between their target height and their final height. Univariate analyses showed no association between the timing of craniofacial surgery (FOA, FFMBA, posterior expansion, LIII, LI, FM decompression, and VP) and height increase. Finally, there was no significant difference between patients with tracheostomy and those without.
Regarding weight, univariate analyses also showed that patients with MS gained 1.13 kg more per year than patients with CS (p < 0.001), and that patients with AS gained 0.27 kg more per year than patients with CS (p = 0.001). The mean weight of female patients was significantly lower than male patients at birth (−2.77 kg; p = 0.022), but there was no significant difference in weight increase between female and male patients afterwards. Patients who were born at a more advanced term gained more weight by 0.12 kg every year (p < 0.001). There was no significant difference in weight increase between patients with PS and patients with CS, male and female patients, patients treated with hormonotherapy and controls, and FGFR2 or FGFR3-related FCS. Univariate analyses showed no association between the timing of craniofacial surgery and weight increase. Finally, there was no significant difference between patients with tracheostomy and those without.
Univariate analyses showed that the BMI of patients with MS increased significantly more than in patients with CS by 0.16 every year (p < 0.001). The mean BMI of female patients was significantly lower than in male patients at 1 year of age (−1.39; p = 0.036), but there was no significant difference in BMI increase between female and male patients afterwards. The BMI of patients who were born at a more advanced term increased more than the others by 0.02 every year (p = 0.002). There was no significant difference in the increase of BMI between patients with CS and patients with AS or PS, patients treated with hormonotherapy and controls, and patients with FGFR2 or FGFR3-related FCS. Univariate analyses showed no association between the timing of craniofacial surgery and BMI. Finally, there was no significant difference between patients with tracheostomy and those without.
Univariate analyses were also performed only in patients for whom a genetic mutation had been identified (55/70). Concerning height, patients with FGFR3 mutations grew significantly more each year than patients with an FGFR2 mutation (0.57 cm, p < 0.001 and 1.70 cm, p < 0.001 respectively) (Table 2.2). Again, girls grew significantly less than boys each year (p < 0.001), and patients who were born at a more advanced term grew more than the others by 0.19 cm every year (p < 0.001). There was no significant difference in height dynamics between patients treated with hormonotherapy and controls. For patients who had reached adulthood, there was no significant difference between their target height and their final height. Univariate analyses showed no association between the timing of craniofacial surgery and height increase. Finally, there was no significant difference between patients with a tracheostomy and those without.
Table 2.2.
Parameters of the hierarchical models by mutation.
| Parameters of the hierarchical models by mutation Height | ||||
|---|---|---|---|---|
| Estimate | SD | p-Value | ||
| Intercept | 47.38 | 12.42 | ||
| FGFR3 | Intercept | +1.898 | 1.440 | 0.196 |
| Slope | +0.573 | 0.081 | <0.001 | |
| Female | Intercept | −0.850 | 1.384 | 0.543 |
| Slope | −2.501 | 0.631 | <0.001 | |
| Term | Intercept | +0.218 | 0.310 | 0.488 |
| Slope | +0.185 | 0.022 | <0.001 | |
| Tracheostomy | Intercept | −1.679 | 1.129 | 0.138 |
| Slope | −0.098 | 0.089 | 0.272 | |
| HT | +3.203 | 10.16 | 0.754 | |
| Target height | −0.264 | 0.300 | 0.390 | |
| FFMBA | −3.046 | 7.755 | 0.696 | |
| Posterior expansion | −9.654 | 7.732 | 0.217 | |
| FOA | −14.59 | 7.727 | 0.065 | |
| LF3 | +12.16 | 8.968 | 0.181 | |
| LF1 | +9.595 | 17.06 | 0.576 | |
| FM decompression | −8.232 | 8.698 | 0.348 | |
| VP | −13.15 | 10.65 | 0.223 | |
Regarding weight, patients with FGFR3 mutations gained significantly more weight each year than patients with an FGFR2 mutation (0.65 kg, p < 0.001 and 0.96 kg, p < 0.001 respectively) (Supplemental table). Univariate analyses showed that male patients gained 1.28 kg more per year than female patients (p = 0.003). Patients who were born at a more advanced term gained more weight by 0.22 kg every year (p < 0.001). Patients without tracheostomy gained more weight per year than patients with tracheostomy (0.19 kg, p = 0.003). There was no significant difference in weight increase between patients treated with hormonotherapy and controls. Univariate analyses showed no association between the timing of craniofacial surgery and weight increase.
Finally, analyses showed that the BMI of patients with an FGFR3 mutation increased significantly more each year than patients with an FGFR2 mutation (0.10, p = 0.002) (Supplementary table). Univariate analyses also showed that the BMI of female patients was significantly lower than in male patients at 1 year of age (−2.02; p = 0.013), but there was no significant difference in BMI increase between female and male patients afterwards. The BMI of patients who were born at a more advanced term increased more than the others by 0.04 every year (p < 0.001). There was no significant difference in the increase of BMI between patients treated with hormonotherapy and controls. Univariate analyses showed no association between the timing of craniofacial surgery and BMI. Finally, there was no significant difference between patients with tracheostomy and those without.
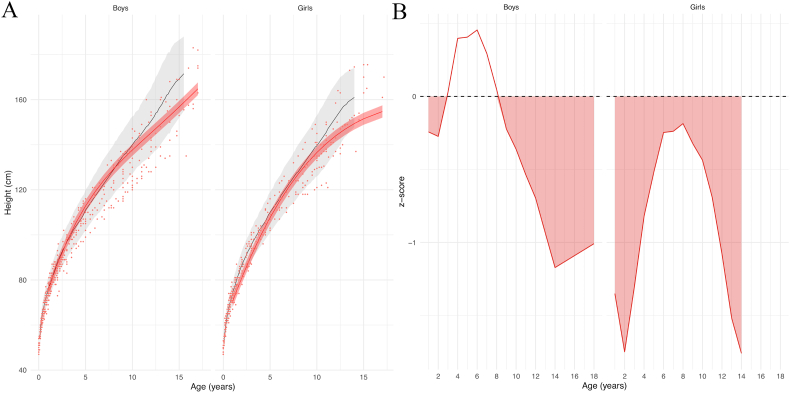
Patients mean height was either close to the mean standard height or under the mean standard height and above −2SD (standard deviations) for boys; for girls, the mean height curve stayed under the mean standard height and above −2SD (Fig. 1A). More precisely, average height in boys with FCS was significantly lower than controls in between 1 and 3 years or age and after 8 years of age. Average height in girls was significantly lower than average height of controls from the age of 1 onwards (Fig. 1B).
Fig. 1.
(A) Model prediction of height according to sex relative to the reference chart (confidence interval: −2DS to +2DS). Patient data in red; reference chart in black (confidence interval: −2DS to +2DS). (B) z-scores. Shaded areas: time periods when subjects had lower heights than controls.
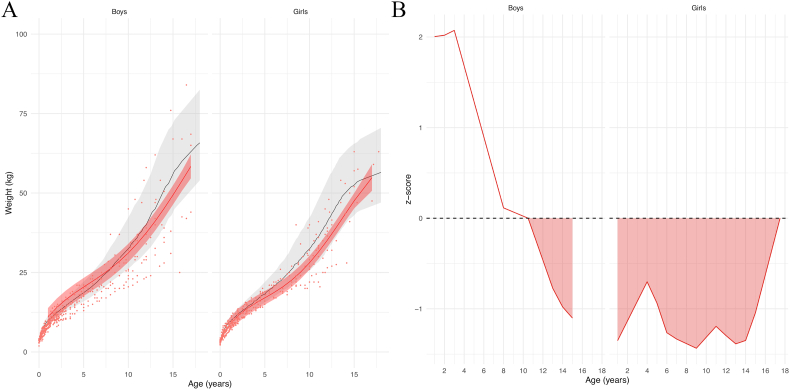
The mean weight curves for boys and girls with FCS were located close to the controls mean weight curve (Fig. 2A). In boys, the average weight was significantly lower than controls mean after 10.5 years of age. In girls, the average weight was significantly lower than controls from the age of 1 onwards (Fig. 2B).
Fig. 2.
(A) Model prediction of weight according to sex relative to the reference chart (confidence interval: −2DS to +2DS). Patient data in red; reference chart in black (confidence interval: −2DS to +2DS). (B) z-scores. Shaded areas: time periods when subjects had lower weights than controls.
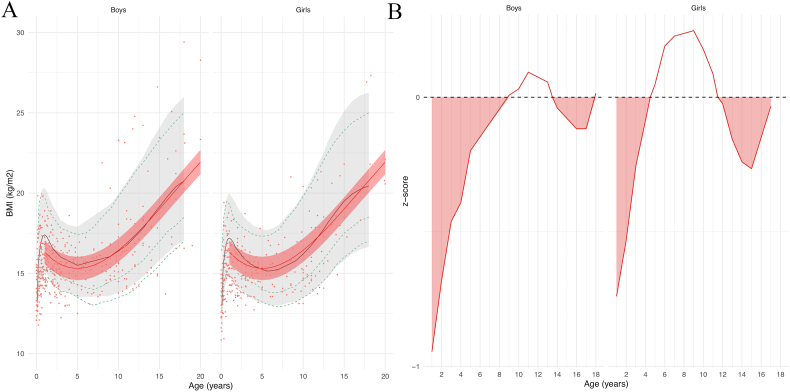
Regarding BMI, the mean curves for boys and girls were also close to controls mean curves (Fig. 3A). In boys from 1 to 9 years of age and from 13.5 to 18 years of age, and in girls from 1 to 4.5 years of age and above 11.5 years old, mean BMI was significantly lower than controls (Fig. 3B).
Fig. 3.
(A) Model prediction of body mass index (BMI) according to sex relative to the reference chart (confidence interval: −2DS to +2DS). Patient data in red; reference chart in black (confidence interval: −2DS to +2DS). (B) z-scores. Shaded areas: time periods when subjects had lower BMIs than controls.
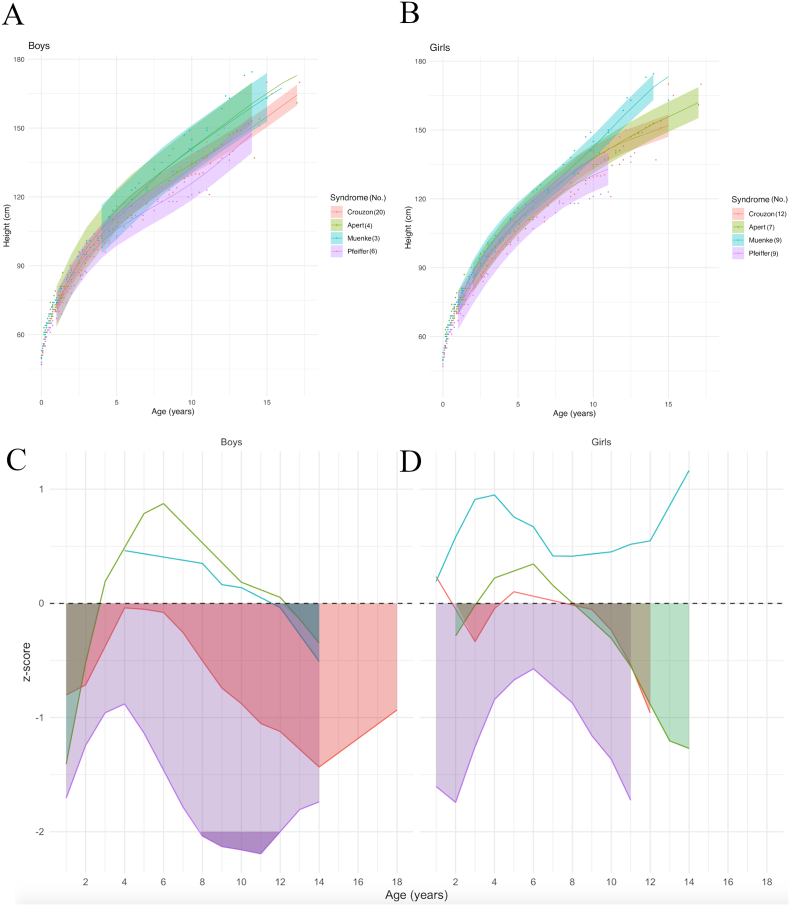
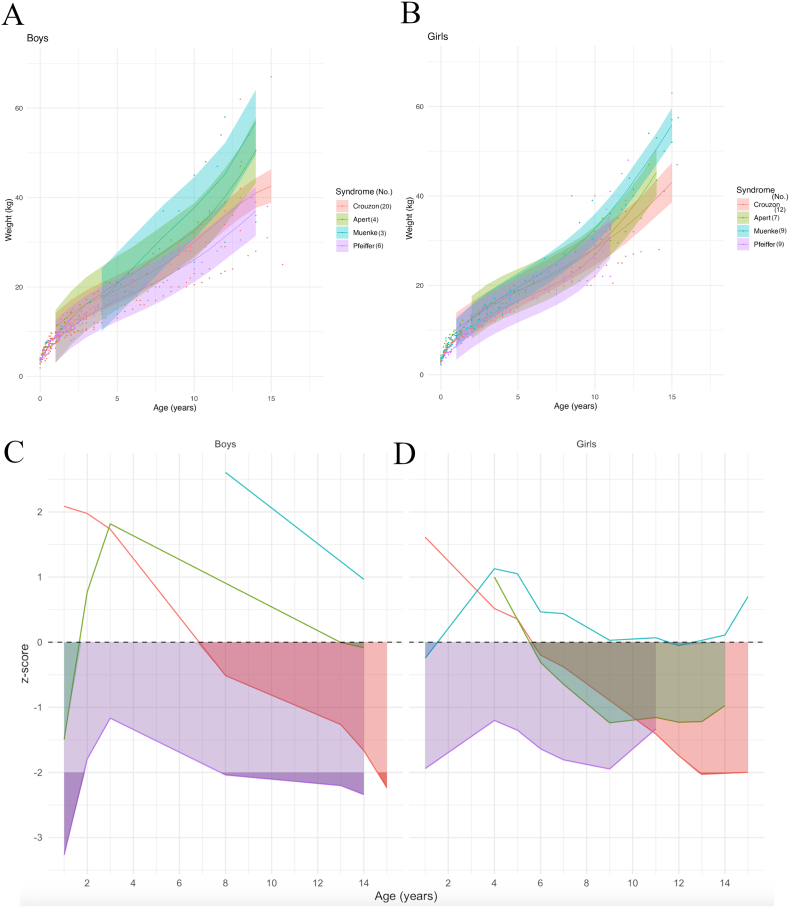
Model prediction curves by syndrome showed that MS and AS have similar slopes for height increase in boys, and that the CS and PS curves for height were located below the other FCS curves (Fig. 4A). In girls, AS, CS and MS height curves initially followed a similar growth speed, but around 8 years of age patients with MS seemed to grow taller than the other syndromes; height curve for girls with PS was located below the other FCS curves (Fig. 4B).
Fig. 4.
Model prediction of height according to sex (A,B) and to syndrome, relative to the reference chart (confidence interval: −2DS to +2DS). (C, D): z-scores compared to controls according to sex. Shaded areas: time periods when subjects had lower weights than controls.
Compared to controls, boys' z-scores for height differed by syndrome: z-score for boys with PS was the lowest of all FCS and stayed under the controls means, ranging from −0.8 to <−2 at its lowest; z-score for boys with CS also stayed under the controls mean, varying between near below zero and −1.4; the mean height boys with AS was below the controls mean from 1 to 2.5 years of age (z-score = −1.4 at 1 year of age), above the controls mean from 2.5 to 12 years of age (with a peak z-score of 0.8), and then fell back below the controls mean after 12 years of age; boys with MS initially had a mean height above the controls mean, but then dropped significantly below the controls mean after 11.5 years of age (Fig. 4C). In girls, the mean height curve for PS was also below the controls mean, with a z-score ranging from −0.8 to −1.5; the curves for AS and CS hovered around the controls mean, before both dropping below it after 8 years of age; MS patients maintained a mean curve above the controls mean (Fig. 4D).
Weight curves by syndrome showed similar slopes for all syndromes in boys; the MS curve was above the AS curve, then the CS curve, with the PS curve below all others (Fig. 5A). Weight curve modeling by syndromes was similar in girls, with the curves following similar slopes and the order of the curves being the same: MS, AS, CS, and then PS (Fig. 5B).
Fig. 5.
Model prediction of weight according to sex (A, B) and to syndrome, relative to the reference chart (confidence interval: −2DS to +2DS). (C, D): z-scores (compared to controls) according to sex. Shaded areas: time periods when subjects had lower weights than controls.
The z-score of the weight of PS patients relative to controls ranged from −1.8 to −3.2 and was the lowest of all syndromes, while that of MS patients remained above controls; the mean weight curve of CS patients was initially above controls(with a z-score > 2 at 1 year old) then after 7 years of age fell below controls (z-score < −2 at 15 years of age); the AS curve was initially below controls(z-score = −1.5 at 1 year of age), rose above control values from 1.5 years of age onward, and then fell back below control values after 13 years of age (Fig. 5C). In girls, only the mean weight curve for MS remained above controls almost all the time; the z-score for PS relative to controls varied between −2 and −1.2 and remained below all other curves; the curves for AS and CS were initially above the control mean and then fell below control values by 5.5 years of age (Fig. 5D).
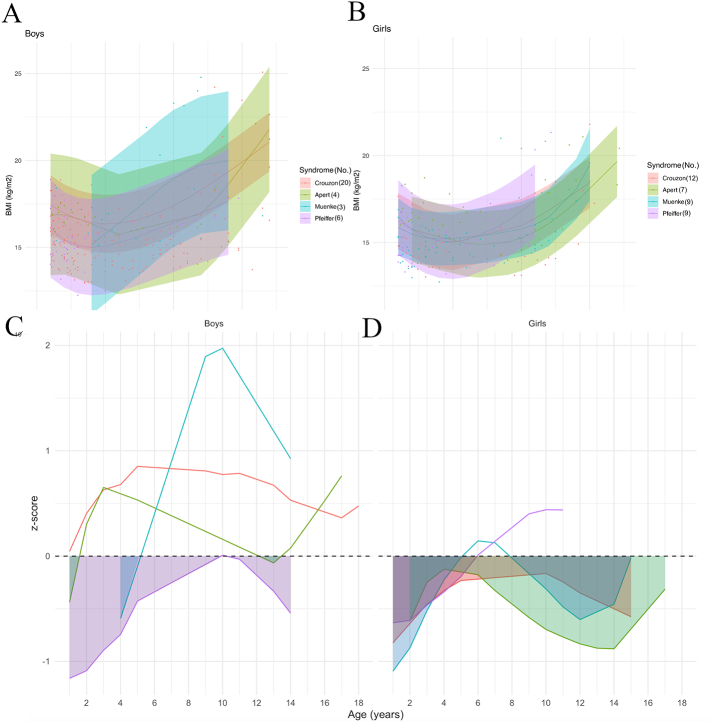
Finally, BMI curves showed different slopes for each syndrome: in boys, the MS curve was above the other curves, the PS curve was below the other curves, and all curves intersected at different times (Fig. 6A); in girls, BMI curves by syndrome showed similar slopes for AS, CS and MS, and the PS curve was above other curves until 11 years old (Fig. 6B).
Fig. 6.
Model prediction of BMI according to sex (A, B) and to syndrome, relative to the reference chart (confidence interval: −2DS to +2DS). (C, D): z-scores (compared to controls) according to sex. Shaded areas: time periods when subjects had lower weights than controls.
More precisely, boys with PS had a mean BMI that stayed below controls, with a z-score varying from −1.2 to 0; boys with MS initially had a z-score of −0.6 compared to control BMI, but their curve then overtook control mean values after 5 years old; AS curve was below the control mean values from 1 to 1.5 years of age and from 12 to 13.5 years of age; BMI curve for CS stayed above control mean values (Fig. 6C). For girls, all BMI curves per syndrome stayed under control mean values, except for the PS curve that went above the controls mean after 6 years of age, and the MS curve that briefly went above the control mean values between 5 and 8 years old (Fig. 6D).
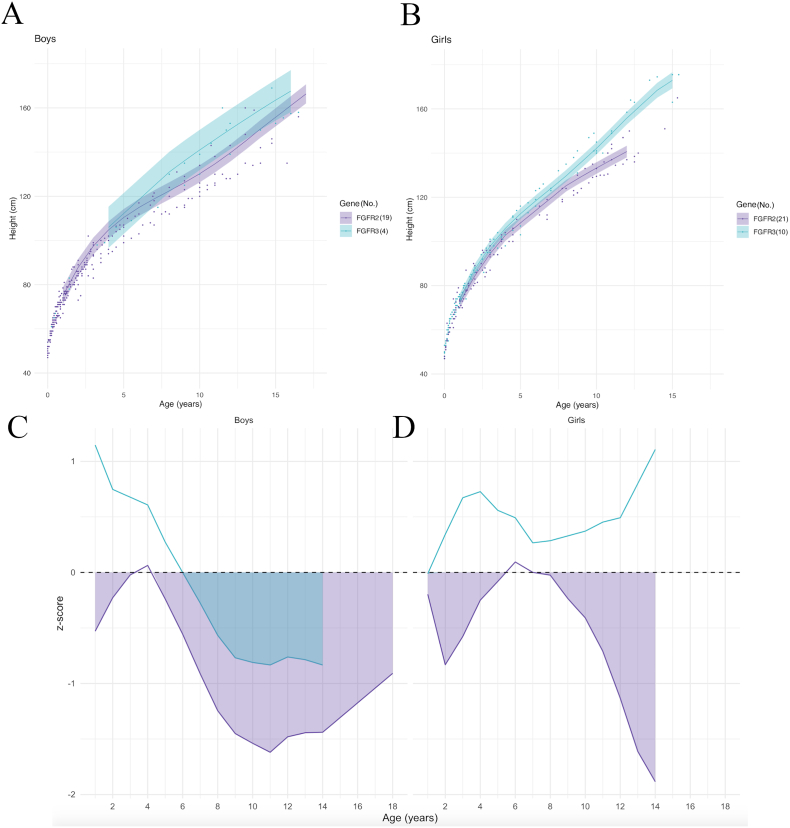
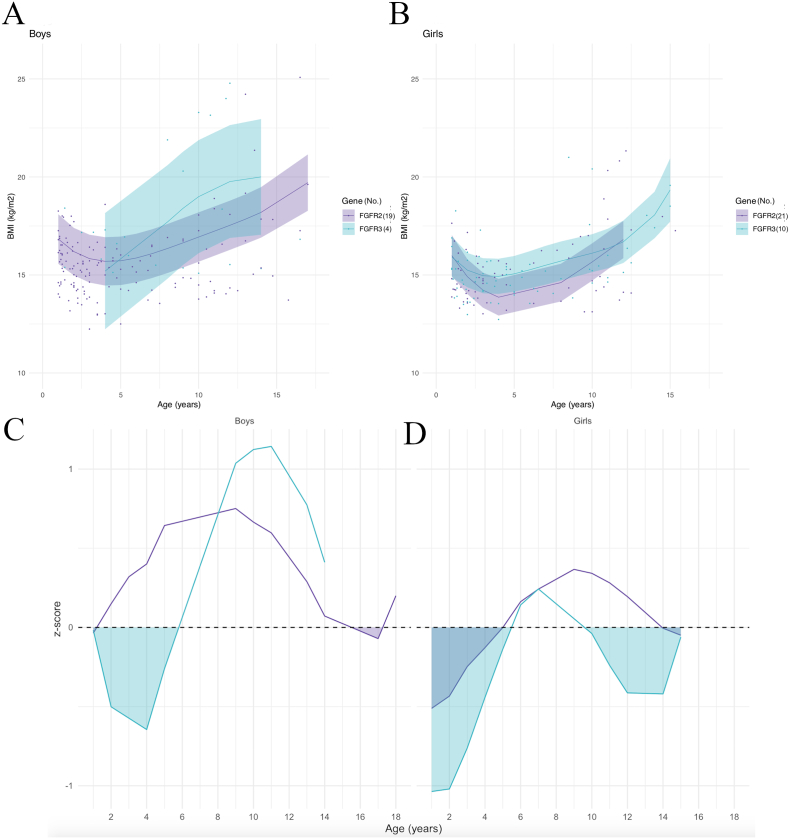
Model prediction curves by genetic mutation showed similar slopes for height growth in boys with FGFR2 and FGFR3 mutations (Fig. 7A); in girls, the curves initially followed the same slope and then curves for FGFR3 mutations clearly exceeded FGFR2 after 10 years of age (Fig. 7B).
Fig. 7.
Model prediction of height according to sex (A, B) and to gene, relative to the reference chart (confidence interval: −2DS to +2DS). (C, D): z-scores (compared to controls) according to sex. Shaded areas: time periods when subjects had lower weights than controls.
The z-scores showed that in boys, the mean height of patients with FGFR2 mutations was significantly lower than the mean height of controls except between 3 and 4.5 years of age; the mean height of FGFR3 patients was initially above controls, then became significantly lower after 6 years of age (Fig. 7C). In girls, the mean height of FGFR3 mutation patients remained above the control mean, whereas the mean height of FGFR2 mutation patients was significantly below the control mean except from 5.5 to 7 years of age (Fig. 7D).
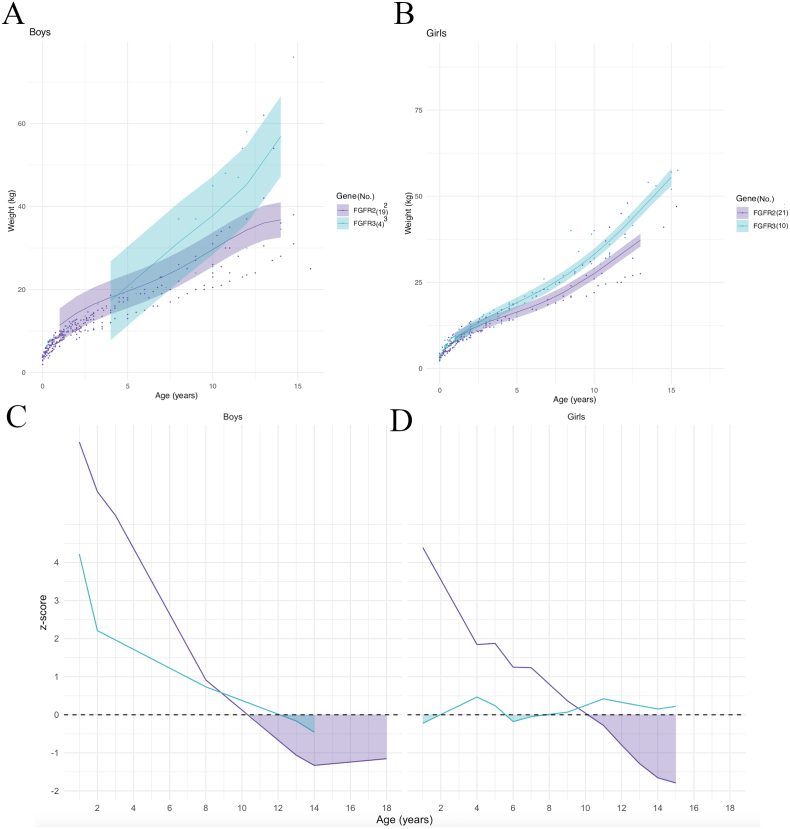
Weight modeling curves by syndrome are shown in Fig. 8A for boys and 8B for girls. Calculation of the z-scores showed that in boys, the mean weight for FGFR2 and FGFR3 mutations was initially above the controls mean and then fell below it after 10.5 years of age in FGFR2 and 12 years of age in FGFR3 Fig. 8C). In girls, the mean weight in patients with FGFR2 mutations was also initially above the controls mean, then progressively decreased to fall below controls after 10 years of age; in FGFR3 patients, the mean weight curve fluctuated around the control mean values, with few decreases between 1 and 2 years of age and then between 5.5 and 7 years of age (Fig. 8D).
Fig. 8.
Model prediction of weight according to sex (A, B) and to gene, relative to the reference chart (confidence interval: −2DS to +2DS). (C, D): z-scores (compared to controls) according to sex. Shaded areas: time periods when subjects had lower weights than controls.
Finally, model prediction curves for BMI by syndrome are shown in Fig. 9A for boys and Fig. 9B for girls. Calculation of the z-scores showed that in boys, the mean BMI for FGFR2 was above controls except between 15.5 and 17.5 years of age, and the mean BMI for FGFR3 mutations was initially significantly lower than controls from 1 to 6 years of age, before rising above controls after 6 years of age (Fig. 9C). In girls, the mean BMI in patients with FGFR2 and FGFR3 mutations showed a similar evolution: both patient groups were initially below control values until 5 years of age in FGFR2 mutations and 5.5 years of age in FGFR3 mutations, then were both above controls before falling behind after 14 years of age in FGFR2 mutations and 9.5 years of age in FGFR3 mutations (Fig. 9D).
Fig. 9.
Model prediction of BMI according to sex (A, B) and to gene, relative to the reference chart (confidence interval: −2DS to +2DS). (C, D): z-scores (compared to controls) according to sex. Shaded areas: time periods when subjects had lower weights than controls.
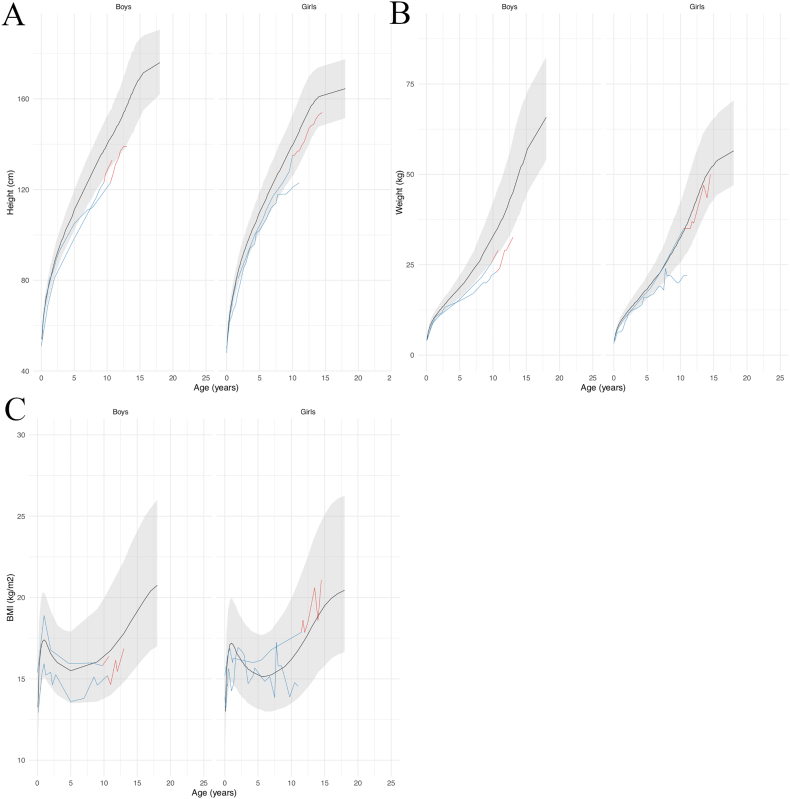
4 patients (2 girls and 2 boys) had received growth hormone (GH) treatment. One had Apert syndrome, two had Pfeiffer syndrome and one had Crouzon syndrome with acanthosis nigricans. No hypothalamic-pituitary axis involvement has been reported in the literature for any of these conditions. All patients were treated with recombinant somatotropin. The indication for GH treatment was an inflection of the growth curve with repercussions on the social life of the patients. The biological workup did not reveal any GH deficiency, and the IGF1 level was normal in all 4 patients. Their height, weight and BMI growth curves are shown in Fig. 10.
Fig. 10.
Growth curves for height (A), weight (B) and BMI (C) of the 4 patients treated with growth hormone according to sex, relative to the reference chart (confidence interval: −2DS to +2DS). Patient data in blue for the period without GH, and in red for the period with GH treatment; reference chart in black (confidence interval: −2DS to +2DS).
More precisely, for one female patient who had Pfeiffer syndrome with an identified FGFR2 mutation, the height curve had changed from −1SD until 8 years of age to −3SD at age 11, and the weight curve had changed from average to −2SD after 8 years of age. GH treatment was started at 11.5 years old: at this time, the height curve was at −3SD, the patient did not exhibit any sign of puberty onset and reported major psychological suffering due to her short height. Seven months after treatment initiation, the patient had moderate height increase acceleration (+2.5 cm) and a weight gain of 3 kg; the absence of puberty persisted. Interestingly, investigations revealed that the patient had a central corticotropic insufficiency of unknown origin; other pituitary hormones were at normal levels. Hydrocortisone therapy was initiated, and the patient is still followed for growth and puberty onset monitoring and for her corticotropic insufficiency.
The other female patient had Apert syndrome with an identified FGFR2 mutation. She was 11.5 years old and presented no pubic hair or metrorrhagia although her breast had started developing 2 years prior. She was 141 cm tall (−1SD), and her height curve showed a recent break at 11 years of age: her final height depended on the onset of menarche, with a prognosis of less than 150 cm. At the age of 14, she presented her first menstrual period; she was 152 cm in height and radiographs showed that the femoral cartilage was almost fused and that she was therefore reaching the end of her growth. Treatment was stopped at age 15 with a final height of 154.5 cm.
For one of the male patients who had Crouzon syndrome with acanthosis nigricans with an identified FGFR3 mutation, the height curve progressively declined over the years: normal until the age of 2, then inflection behind between 4 and 7 years of age, and finally −2.5SD at 8.5 years of age. GH treatment was started at the age of 10, when the height curve was at −2.5 SD and the social impact was beginning to be significant. Three years after the initiation of the treatment, the height curve was at −1.7SD and puberty had started recently with an increase in testicular volume. Treatment was continued and the patient is still being followed for growth and puberty.
For the other male patient who had Pfeiffer syndrome with an identified FGFR2 mutation, growth monitoring showed that the curve was progressively decreasing from −1SD at 5 years old to −2SD at 8 years old. Treatment was initiated at age 9 to potentially improve the prognosis for final height. At the last visit, the curve had risen back to −1SD. Treatment is currently pursued and patient follow-up continues.
All patients tolerated their treatment well, with no significant side effects and good compliance.
4. Discussion
Based on a retrospective series of 70 patients, we showed that FGFR-related FCS had significantly reduced mean heights and weights relative to controls.
Growth delay is frequently observed in children with chronic diseases (Preece et al., 1986; Kyle et al., 2015). In such cases, catch-up growth has been described during the 0–3 years period: when nutrient intakes are adjusted and adapted, children grow rapidly and growth curves cross percentile curves to catch up with normal values. Once the growth curve is straightened, the speed slows down to return to normal (Kyle et al., 2015). Similar observations have been obtained in children who have undergone solid organ transplantations: the greater the stunting prior to surgery, the greater the catch-up growth after transplantation (Laster and Fine, 2014). This phenomenon coincided with the 1–3 years for boys during which the average heights for boys were below control values with a z-score around −0.3, before secondary increase. A similar phenomenon could be noted for the average height of girls between 1 and 2–3 years of age, although their mean height did not reach control mean values. Thus, patients' height curves crossed percentile curves at the end of these catch-up periods. Interestingly, although stunted growth was observed in boys and girls during this time, there was no significant impact on their weight.
After this initial period of growth impairment, average height fell below control values again a few years before adolescence in boys, at 8 years of age. In girls, mean height fell from a z-score of −0.2 at 8 years old compared to controls to −1.8 at 14 years old. While pubescent children typically experience a growth spurt, this phenomenon appeared to be less obvious in patients with FCS. Similarly, the average weight for boys with FCS was significantly lower than controls a few years before adolescence (10.5 years of age). In girls, mean weight was below controls from 1 year onwards, but seemed to catch up in the midst of adolescence with a z-score that increased from −1.8 at 14 years of age to 0 at 17.5 years of age; however, their height remained significantly lower than controls.
At age 4, growth curves are expected to be on the curve of their target height. Mean boys and girls' values for height, weight and BMI were similar to the mean control values. At 8 years of age, mean values for FCS patients started to fall behind controls: mean height for boys with FCS was 129 cm [126–132] compared to 132 cm [117–147] for controls; mean weight for girls with FCS was 23 kg [20–26] compared to 28 kg [22−30] for controls and mean weight for all our patients was 24 kg [22–27] to 27 kg [22−32] for controls.
Twelve-year-old children are about to enter adolescence and start their growth spurt. In our series, mean height and weights at this age were all below control values, while BMIs were normal. The general growth pattern in FCS was thus initial growth delay, catch-up growth, secondary growth delay during adolescence and final definitive growth impairment.
Height modeling of the curves representing the average height according to genetic mutation reported different results by sex. In boys, those with an FGFR3 mutation initially had an average height above the controls average those with an FGFR2 mutation remained below the controls, except between 3 and 4.5 years of age. However, after 6 years of age, the mean height of the FGFR3 patients was also below control values. Girls with an FGFR3 mutation had an average height above the controls average, while girls with an FGFR2 mutation remained below controls, except between 5.5 and 7 years of age. Thus, FGFR mutations appeared to act differently over time on height in girls and boys. In our cohort, boys with an FGFR mutation all had a mean height below controls from childhood (6 years); in girls, on the contrary, patients with an FGFR3 mutation showed a significantly greater mean height than those with an FGFR2 mutation.
This difference could be explained by the small number of boys with an FGFR3 mutation (4 versus 10 in girls), which could be insufficient to show a significant difference.
The question of undetected pubertal delay in our patients should be addressed. In girls, average age at first menstruation is 12.5 years, and the adult height is reached maximum 2 years later. For girls for whom we had pubertal data (10 patients), follow-up was available up to 16.5 years of age: therefore, the height of these patients could not have been affected further by pubertal delay and most probably corresponded to the final height.
In boys, pubertal growth spurt happens later compared to girls. Adult height is reached around 17 to 17.5 years old. We cannot completely exclude that boys in our cohort could have grown more than the values we provide. Thus, it would be interesting to continue the follow-up of our male patients to confirm our results. More generally, evaluation of sexual maturation using bone age assessment is debatable in patients who may present bone dysplasia (Vajo et al., 2000), so that the issue of determining the end of pubertal growth is not easy to tackle.
Interestingly, the average term of birth for patients was 40 weeks of amenorrhea, so that most of the patients in our case series were born at term. Patient growth charts were therefore not influenced by premature birth and the differences observed during growth were not the consequences of initial discrepancies.
Achondroplasia patients also present with normal lengths at birth. However, growth velocity abruptly decreases before 1 year of age with no catch-up growth during childhood, and growth speed keeps slowing down during adolescence. Patients with achondroplasia also present with normal birth weights. Weight starts to fall below control values during the first year of life, but it is difficult to assess the correlation between height and weight in patients who suffer from pronounced body disproportions. Similarly, the use of BMI in these patients is questionable in terms of accuracy and relevance (Merker et al., 2018).
In total, 4 patients started GH therapy before the onset of puberty: one girl with PS at 11.5 years of age and two boys, with PS and with CS with acanthosis nigricans respectively, at 9 and 10 years of age. Their respective z-scores were −3SD, −2.5SD and −2SD for height. Even though GH treatment helped gaining 1SD on average, their height remained below controls means. As for the girl who started GH therapy 2 years after the first signs of puberty onset, her final height was at −1SD, which represents no change compared to values before treatment. Overall, GH treatment did not help heights to reach control values. However, hormonal therapy allowed maintaining height increase along the childhood curve.
GH treatment is commonly used for patients without any GH deficiency, for instance in Turner syndrome, Prader-Willi syndrome, chronic renal insufficiency, and for short children born small for gestational age, with better results when treatment is started early, and minor side-effects (Loche et al., 2014). Thus, it seems relevant to propose GH treatment to FCS patients with decreased growth rates compared to controls until further scientific confirmation of the validity of this strategy.
Not all hormone-naïve patients had benefited from endocrine assessment during childhood: one could assume that if they had received treatment, some of them could have followed a higher percentile curve. Our results suggest that a systematic pre-pubertal endocrine assessment in patients with FCS could be beneficial to detect and treat growth retardation with recombinant human growth hormone. Appendix 1 shows the standardized assessment performed in the pediatric endocrinology department at Necker-Enfants Malades Hospital in Paris. Further investigations are required to confirm this hypothesis but due to the little amount of data available in FCS, it seems reasonable to explore the hormonal profile of these patients prospectively until evidence-based results are available.
As mentioned earlier, activating FGFR3 mutations are also responsible for achondroplasia, hypochondroplasia, and thanatophoric dysplasia. Therapies are under development for these conditions and show encouraging results. The most promising option is a stabilized form of C-type natriuretic peptide (CNP), involved in longitudinal bone growth. Other potential treatments include FGFR3-selective tyrosine-kinase inhibitors, FGFR3-specific monoclonal antibodies, parathyroid hormone (PTH) and Meclozine (Ornitz and Legeai-Mallet, 2017). Assessing a potential effect of these molecules on growth in FGFR-related FCS is an exciting perspective.
We used the 2018 French height and weight growth curves as a reference. Previous curves dated back to 1979 and were based on measurements of 588 children born in the 1950s and followed-up until adulthood. Studies have shown that these curves were no longer suitable for monitoring the growth of children in France (Scherdel et al., 2015). The new growth curves were developed based on the height, weight and head circumference data of 261.000 French children aged 0 to 18. The ethnic background of our patients was not considered for several reasons. Most could be considered Caucasian based on their family names and on the physical appearance of their parents. Furthermore, it is forbidden in France to conduct studies based on the ethnic and racial backgrounds of patients. Article 6 of law 78-17, January 6th, 1978, relating to Data processing, Data Files and Individual Liberties states: “It is forbidden to process personal data revealing the alleged racial or ethnic origin […] of a physical person or to process genetic data, biometric data for the purpose of uniquely identifying a physical person”. The French Constitutional Council also stated on November 15th, 2007, the prohibition of processing personal data that directly or indirectly reveals the racial or ethnic origins of persons, the introduction of variables of race or religion in administrative files and the a priori definition of an “ethno-racial reference system”. Therefore, ethnicity could not be considered in our study.
All patients included in this study had benefited from numerous craniofacial surgical procedures including, in most cases, fronto-facial monobloc advancement (FFMBA) and Le Fort III facial advancement (LF3). During FFMBA and LF3, several steps such as temporal muscle disinsertion or pterygo-palatal disjunction could have an impact on the patient post-operative amplitude of mouth opening. Furthermore, prolonged management in intensive care could have interfered with normal growth. Therefore, it is appropriate to consider whether repeated craniofacial procedures and subsequent hospitalizations may have potentially interfered with the growth curves. However, our statistical analyses showed no association between the timing of craniofacial surgery and growth.
In addition, the link between feeding difficulties and delayed growth has also been demonstrated in several studies in children with cleft lip and palate, with higher rates of growth retardation in this population in the first months of life. Then, around the age of 5–6 months, catch-up growth occurs with a normalization of growth rates. This catch-up growth was observed in patients who have not yet undergone cleft repair surgery and has been attributed to an improvement and adaptation of infant feeding methods (Lee et al., 1997; Miranda et al., 2016; Marques et al., 2009). Therefore, congenital malformations such as cleft lip and palate or repeated surgeries involving the craniofacial region can indeed be associated with feeding difficulties leading to an initial growth delay. However, this delay is quickly caught up once the child resumes an adapted diet.
Another factor that could have impacted the growth of our patients is ventilation. We do not report significant differences in height increase between patients with/without tracheostomy. Univariate analyses by gene mutation showed that patients without tracheostomy gained more weight than those with tracheostomy; there were a total of 11 patients with tracheostomy, 10 with an FGFR2 mutation, and 1 with an FGFR3 mutation. This difference in weight growth was isolated because height growth is not impacted by tracheostomy. It could illustrate the fact that patients with tracheostomy were those with the most severe phenotype, where multiple surgeries were required or insufficient to correct the ventilation disorders and a greater number of hospital stays were necessary.
The patients in our cohort were all followed-up closely with regular polysomnography assessments since childhood to detect an obstructive sleep apnea (OSA) as early as possible. OSA is an intermittent airflow limitation in the upper airway during sleep. Its incidence is estimated at 4% in children (DeVries et al., 2020). The apnea + hypopnea index (AHI) indicates the severity of OSA. An AHI ≤ 1 event/h is considered normal; 1 < AHI ≤ 5 indicates mild OSA; 5 < AHI ≤ 10 indicates moderate OSA; AHI > 10 indicates severe OSA (Marcus et al., 1992).
Peripheral OSA has numerous causes including obesity, adenoid and/or tonsillar hypertrophy, upper airway abnormalities or inflammation, micrognathia, and neuromuscular diseases. Early diagnosis of OSA is particularly important in children as it can lead to psychological, cardiovascular and metabolic complications (DeVries et al., 2020; Li et al., 2016). This condition therefore requires multidisciplinary management in order to guide the family towards the most adapted treatment options.
In our cohort, 28 patients developed OSA during their follow-up managed with a combinaison of surgical methodsand/or the use of CPAP. At the time of our study, 12 patients still had OSA: 1 had mild OSA, 3 had moderate OSA and 8 had severe OSA. All of them used CPAP and some were listed for surgery to improve ventilation.
It is interesting to underline that few articles have been published about growth delay in children with OSA. Most authors report ‘catch-up growth’ after surgery with detected changes in IGF-1, IGFBP-3, ghrelin and leptin levels (Zaffanello et al., 2020). Although ventilation disorders are a significant part of the complications of FCS, we consider that this issue was tackled in our cohort based on regular follow-up and appropriate treatment. Thus, ventilation does not seem to be a major bias in the study of growth of our patients.
Of note, since our study was based on French patients, the BMI curves used for controls were that of the PNNS (Plan National Nutrition Santé or National Nutrition and Health Program), a French program developed for evaluating and monitoring children growth and weight. The curves were created as a tool for professionals to detect obesity. The PNNS BMI curves are divided into percentiles to establish thresholds (underweight, normal body weight zone, overweight).
This study was a case series, with many inherent methodological biases, such as a selection bias since the data we used were provided by patients and families who volunteered to respond by email. It is possible that many of them belonged to a higher socio-professional category, more health-conscious and more inclined to take part into such studies. The average follow-up was 17 years, with a mean patient age of 18.1 years at the time of the survey. It would be interesting to continue collecting growth charts until the end of adolescence for all patients to definitively assess the adult average height and weight in FCS.
5. Conclusion
To our knowledge, this is the first case series studying height and weight growth in patients with FCS due to FGFR2 and FGFR3 mutations. We show that these patients have impaired growth relative to the general French population, with discrepancy increasing at the beginning of adolescence.
While growth hormone treatment may have been successful in keeping patients within the norms in four cases, it has not been sufficient to restore height and weight growth up to control levels. Results from four cases have no scientific relevance. Nevertheless, FCS being a rare disease with years left before evidence-based endocrinologic management can be proposed to these patients, we believe that endocrine testing, including insulin-like growth factor (IGF-I), thyroid-stimulating hormone (TSH) and total thyroxine (T4) assays and a growth hormone (GH) stimulation test, should be considered as a first-line examination in early childhood.
Furthermore, it would be interesting to extend this work by looking more specifically into certain parameters that can influence the growth of patients and that have not been studied in depth, such as the existence of sleep apnea.
More generally, our results indicate that the phenotype of patients with FCS is not limited to the craniofacial region, and that conditions with activating FGFR mutations affect both membranous and long bones, both in FCS and in osteochondrodysplasia. Our results parallel a recent awareness of the craniofacial involvement in osteochondrodysplasia such as achondroplasia (Baujat et al., 2008; Di Rocco et al., 2014).
The following is the supplementary data related to this article.
Parameters of the hierarchical models for weight and BMI.
Funding/support
No specific support was secured for this study.
CRediT authorship contribution statement
Caroline Ea: Methodology, Investigation, Writing – original draft, Visualization. Quentin Hennocq: Formal analysis, Visualization. Arnaud Picard: Writing – review & editing. Michel Polak: Writing – review & editing. Corinne Collet: Resources. Laurence Legeai-Mallet: Writing – review & editing. Éric Arnaud: Resources. Giovanna Paternoster: Resources. Roman Hossein Khonsari: Conceptualization, Writing – review & editing, Visualization, Supervision, Project administration.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Appendix 1.
PRESCRIPTION
Date:
Name:
Surname:
Complete blood count
Sedimentation rate, CRP
Na, K
Urea
Creatinine
Calcemia, PTH, 25-OH-D
IgA quantitative measurement
Anti-endomysial antibodies
Anti-transglutaminase antibodies
T3, T4, TSH
IGF-1
If pubarche and/or puberty has started:
- sDHA
- testosterone levels (boys) or estradiol levels (girls)
- LH, FSH
For girls: X chromosome abnormalities
If the growth rate is slowing down: pharmacological test for growth hormone secretion.
References
- Antley R., Bixler D. Trapezoidocephaly, midfacial hypoplasia and cartilage abnormalities with multiple synostoses and skeletal fractures. Birth Defects Orig. Artic. Ser. 1975;11(2):397–401. [PubMed] [Google Scholar]
- Baujat G., Legeai-Mallet L., Finidori G., Cormier-Daire V., Le Merrer M. Achondroplasia. Best Pract. Res. Clin. Rheumatol. 2008;22(1):3–18. doi: 10.1016/j.berh.2007.12.008. [DOI] [PubMed] [Google Scholar]
- Cohen M.M., Kreiborg S. Birth prevalence studies of the Crouzon syndrome: comparison of direct and indirect methods. Clin. Genet. 1992;41(1):12–15. doi: 10.1111/j.1399-0004.1992.tb03620.x. Jan. [DOI] [PubMed] [Google Scholar]
- Cohen M.M., Kreiborg S., Lammer E.J., Cordero J.F., Mastroiacovo P., Erickson J.D., et al. Birth prevalence study of the Apert syndrome. Am. J. Med. Genet. 1992;42(5):655–659. doi: 10.1002/ajmg.1320420505. March. [DOI] [PubMed] [Google Scholar]
- De Onis M., Garza C., Onyango A.W., Rolland-Cachera M.-F. Les standards de croissance de l’organisation mondiale de la santé pour les nourrissons et les jeunes enfants. Arch. Pediatr. 2009;16(1):47–53. doi: 10.1016/j.arcped.2008.10.010. [DOI] [PubMed] [Google Scholar]
- DeVries J.K., Nation J.J., Nardone Z.B., Lance S.H., Stauffer J.A., Abichaker G.M., et al. Multidisciplinary clinic for care of children with complex obstructive sleep apnea. Int. J. Pediatr. Otorhinolaryngol. 2020;1(138) doi: 10.1016/j.ijporl.2020.110384. Nov. [DOI] [PubMed] [Google Scholar]
- Di Rocco F., Biosse Duplan M., Heuzé Y., et al. FGFR3 mutation causes abnormal membranous ossification in achondroplasia. Hum. Mol. Genet. 2014;23(11):2914–2925. doi: 10.1093/hmg/ddu004. [DOI] [PubMed] [Google Scholar]
- Heude B., Scherdel P., Werner A., Le Guern M., Gelbert N., Walther D., et al. A big-data approach to producing descriptive anthropometric references: a feasibility and validation study of paediatric growth charts. Lancet Digit Health. 2019;1(8):e413–e423. doi: 10.1016/S2589-7500(19)30149-9. Dec. [DOI] [PubMed] [Google Scholar]
- Kyle U.G., Shekerdemian L.S., Coss-Bu J.A. Growth failure and nutrition considerations in chronic childhood wasting diseases. Nutr. Clin. Pract. 2015;30(2):227–238. doi: 10.1177/0884533614555234. [DOI] [PubMed] [Google Scholar]
- Laster M.L., Fine R.N. Growth following solid organ transplantation in childhood. Pediatr. Transplant. 2014;18(2):134–141. doi: 10.1111/petr.12219. [DOI] [PubMed] [Google Scholar]
- Lee J., Nunn J., Wright C. Height and weight achievement in cleft lip and palate. Arch. Dis. Child. 1997;76(1):70–72. doi: 10.1136/adc.76.1.70a. Jan. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z., Celestin J., Lockey R.F. Pediatric sleep apnea syndrome: an update. J Allergy Clin Immunol Pract. 2016;4(5):852–861. doi: 10.1016/j.jaip.2016.02.022. Sep 1. [DOI] [PubMed] [Google Scholar]
- Loche S., Carta L., Ibba A., Guzzetti C. Growth hormone treatment in non-growth hormone-deficient children. Ann. Pediatr. Endocrinol. Metab. 2014;19(1):1–7. doi: 10.6065/apem.2014.19.1.1. mars. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcus C.L., Omlin K.J., Basinki D.J., Bailey S.L., Rachal A.B., Von Pechmann W.S., et al. Normal polysomnographic values for children and adolescents. Am. Rev. Respir. Dis. 1992;146(5 Pt 1):1235–1239. doi: 10.1164/ajrccm/146.5_Pt_1.1235. Nov. [DOI] [PubMed] [Google Scholar]
- Marques I.L., Nackashi J.A., Borgo H.C., Martinelli Â.P.M.C., Pegoraro-Krook M.I., Williams W.N., et al. Longitudinal study of growth of children with unilateral cleft-lip palate from birth to two years of age. Cleft Palate Craniofac. J. 2009;46(6) doi: 10.1597/08-105. Nov 603-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merker A., Neumeyer L., Hertel N.T., Grigelioniene G., Mäkitie O., Mohnike K., et al. Growth in achondroplasia: development of height, weight, head circumference, and body mass index in a European cohort. Am. J. Med. Genet. A. 2018;176(8):1723–1734. doi: 10.1002/ajmg.a.38853. Aug. [DOI] [PubMed] [Google Scholar]
- Meyers G.A., Orlow S.J., Munro I.R., Przylepa K.A., Jabs E.W. Fibroblast growth factor receptor 3 (FGFR3) transmembrane mutation in crouzon syndrome with acanthosis nigricans. Nat. Genet. 1995;11(4):462–464. doi: 10.1038/ng1295-462. Dec. [DOI] [PubMed] [Google Scholar]
- Miranda G.S., Marques I.L., De Barros S.P., Arena E.P., De Souza L. Weight, length, and body mass index growth of children under 2 years of age with cleft lip and palate. Cleft Palate Craniofac. J. 2016;53(3):264–271. doi: 10.1597/14-003. May 1. [DOI] [PubMed] [Google Scholar]
- Ornitz D.M., Legeai-Mallet L. Achondroplasia: development, pathogenesis, and therapy. Dev. Dyn. 2017;246(4):291–309. doi: 10.1002/dvdy.24479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinheiro J., Bates D., Sarkar D., DebRoy S., R Core Team nlme: Linear and nonlinear mixed effects models. R package version 3.1-131. 2017. https://CRAN.R-project.org/package=nlme/ URL.
- Preece M.A., Law C.M., Davies P.S. The growth of children with chronic paediatric disease. Clin. Endocrinol. Metab. 1986;15(3):453–477. doi: 10.1016/s0300-595x(86)80006-8. [DOI] [PubMed] [Google Scholar]
- R Core Team . R Foundation for Statistical Computing; Vienna, Austria: 2016. R: a language and environment for statistical computing.https://www.R-project.org/ URL. [Google Scholar]
- Sabatino G., Di Rocco F., Zampino G., Tamburrini G., Caldarelli M., Di Rocco C. Muenke syndrome. Childs Nerv. Syst. 2004;20(5):297–301. doi: 10.1007/s00381-003-0906-y. May. [DOI] [PubMed] [Google Scholar]
- Scherdel P., Botton J., Rolland-Cachera M.-F., et al. Should the WHO growth charts be used in France? PLoS One. 2015;10(3) doi: 10.1371/journal.pone.0120806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teven C.M., Farina E.M., Rivas J., Reid R.R. Fibroblast growth factor (FGF) signaling in development and skeletal diseases. Genes Dis. 2014;1(2):199–213. doi: 10.1016/j.gendis.2014.09.005. Dec. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vajo Z., Francomano C.A., Wilkin D.J. The molecular and genetic basis of fibroblast growth factor receptor 3 disorders: the achondroplasia family of skeletal dysplasias, muenke craniosynostosis, and crouzon syndrome with acanthosis nigricans. Endocr. Rev. 2000;21(1):23–39. doi: 10.1210/edrv.21.1.0387. Feb. [DOI] [PubMed] [Google Scholar]
- Vogels A., Fryns J.-P. Pfeiffer syndrome. Orphanet J. Rare Dis. 2006;1(1):19. doi: 10.1186/1750-1172-1-19. Jun. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickham H. Springer-Verlag; New York: 2009. ggplot2: Elegant Graphics for Data Analysis. [Google Scholar]
- Zaffanello M., Piacentini G., La Grutta S. Beyond the growth delay in children with sleep-related breathing disorders: a systematic review. Panminerva Med. 2020;62(3):164–175. doi: 10.23736/S0031-0808.20.03904-X. Sep. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Parameters of the hierarchical models for weight and BMI.