Abstract
Prosthetic hip-associated cobalt toxicity (PHACT) is caused by elevated blood cobalt concentrations after hip arthroplasty.
The aim of this study is to determine which symptoms are reported most frequently and in what type of bearing. We also try to determine the blood level of cobalt concentrations associated with toxicological symptoms.
A systematic review was conducted on the 10th of July according to Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. A methodological quality assessment (risk of bias (RoB)) was performed. Primary outcomes were the reported symptoms of cobalt toxicity and the level of cobalt concentrations in blood. These levels were associated with toxicological symptoms. A total of 7645 references were found of which 67 relevant reports describing 79 patients.
The two most used bearings in which PHACT was described were metal-on-metal (MoM) bearings (38 cases) and revised (fractured) ceramic-on-ceramic (CoC) bearings where the former ceramic head was replaced by a metal head (32 cases).
Of all reported symptoms, most were seen in the neurological system, of which 24% were in the sensory system and 19.3% were in central/peripheral system, followed by the cardiovascular (22.1%) system.
The mean cobalt concentration for MoM-bearings was 123.7 ± 96.8 ppb and 1078.2 ± 1267.5 ppb for the revised fractured CoC-bearings.
We recommend not to use a metal-based articulation in the revision of a fractured CoC bearing and suggest close follow-up with yearly blood cobalt concentration controls in patients with a MoM bearing or a revised fractured CoC bearing.
Level of Evidence: Level V, systematic review
Keywords: hip arthroplasty, prosthetic hip-associated cobalt toxicity, cobalt
Introduction
Exposure to metal ions after hip arthroplasty surgery is a widely reported phenomenon. Multiple studies have shown that an increase in metal ions can result in local soft tissue reactions described as an adverse reaction to metal debris (ARMD) (1, 2, 3, 4). There is also an increasing number of case reports describing systemic reactions in relation to elevated blood cobalt concentrations known as prosthetic hip-associated cobalt toxicity (PHACT) (5, 6).
Increased cobalt concentrations are often seen after implantation of metal-on-metal (MoM) hip bearings (7). This can be due to the release of ions from the metal (cobalt–chromium) surface either directly (corrosion) or during sliding under load, which may create wear particles (adhesion). Another source of significant metal particle release is the application of a metal component for the revision of a fractured ceramic head and/or a fractured ceramic acetabular liner. In this scenario, massive three-body abrasive wear can be created, as small remaining particles of the fractured ceramic bearing lead to abrasion of the metal surface (8, 9).
The systemic effects of cobalt toxicity are historically well documented from industrial exposure, iatrogenic use of oral cobalt chloride tablets and from the beer industry as a foam stabilizing agent (10, 11, 12). The toxicity of cobalt is related to the unbound (free) form of cobalt (Co2+) and certain patient conditions. Unice et al. (13) stated that kidney failure, iron deficiencies, sepsis, malnutrition and use of certain medication increased the toxicity of cobalt at lower concentrations. The systemic complaints in patients with PHACT may lead to a variety of symptoms: neuro-ocular toxicity (e.g. tinnitus, vertigo, deafness, blindness, convulsions, headaches and peripheral neuropathy), cardiotoxicity and thyroid toxicity (14). Nausea, anorexia and unexplained weight loss have also been described (6, 15, 16, 17). Initially, there were concerns that high cobalt and chromium concentrations increased the risk of cancer; however, this was not proven in large comparative studies (18, 19).
It is still unknown which of these systemic symptoms are mostly reported in PHACT and at what blood cobalt concentration toxicity occurs. The present study is a systematic review of the current literature reporting systemic cobalt toxicity symptoms after any type of hip arthroplasty. The aim is to define and present the most reported systemic symptoms related to PHACT and to determine blood cobalt levels associated with toxicity.
Methods
The study protocol of this systematic review on case reports was registered in PROSPERO, the international prospective register of systematic review, with registration number: CRD42020215827.
Criteria for considering studies for this review
Types of studies and participants
Case reports concerning cobalt toxicity after hip arthroplasty were included. Patients with any type of bearing (MoM, CoC, metal-on-polyethylene (MoP) and ceramic-on-polyethylene (CoP)) and any type of hip arthroplasty design (hip resurfacing arthroplasty (HRA), short stem hip arthroplasty, and ‘conventional’ stemmed total hip arthroplasty, both uncemented and cemented) were included. Articles describing allergic reactions on hip prosthesis and/or cobalt and articles reporting only local problems around the hip such as adverse local tissue reactions (ALTR), ARMD and aseptic lymphocytic vasculitis-associated lesion (ALVAL) were excluded.
Types of interventions
The description of intervention was not necessary for inclusion, as patients may have died from cobalt toxicity before intervention could be initiated. In some cases, a revision arthroplasty or chelation therapy was the intervention of choice of the attending physicians.
Types of outcome measures
Primary outcomes were the reported symptoms of cobalt toxicity and the blood cobalt concentration at which these symptoms were seen. All reported symptoms were counted and divided into nine different categories based on the physiological system related to the occurrence of the symptoms. We followed the categories used in the study of Devlin et al., with some minor adjustments (6). Cobalt concentrations in blood were reported in nmol/L, µg/L and parts per billion (ppb). Cobalt concentrations in nmol/L were converted to ppb where 1 nmol/L = 0.059 ppb.
Search methods for identification of studies
The search was performed on July 10, 2020, in PubMed, EMBASE, Cochrane Library/Wiley, CINAHL (EBSCO), Web of Science (Clarivate Analytics) and Trial registers (PROSPERO by one author (JJ). The following (MeSH) search terms were used: ‘Hip Prosthesis’, ‘Arthroplasty’, ‘Replacement’, ‘Hip and Cobalt’. The full search strategy and terms can be found in Supplementary data 3 (see section on supplementary materials given at the end of this article). Articles published in Dutch, English, German or Spanish were included. There were no further restrictions for publication type or date. Reference lists of included articles were screened for missing items. In addition, also posters presented at congresses and published abstracts were included. Duplicates were identified by one author (JJ) in RefWorks. All records were independently screened on the title and abstract by two authors (JJ, MGMS) and disagreement was resolved by mutual discussion. Full-text articles were assessed for eligibility by two authors (JRWC, MCK), differences were resolved in a consensus meeting and if necessary, through discussion with another author (JJ).
Data collection and analysis
Data were extracted and stored in a Microsoft Excel 2019 file (Microsoft).The following data of the included studies were extracted: study ID (author, year of online publication), number of patients (n), patient characteristics at onset of symptoms (age in years, sex), primary intervention and indication for the primary procedure, secondary intervention and indication (if applicable), follow-up (in months) since surgery, cobalt ion concentration in any type of amount (e.g. nmol/L, µg/L, ppb) when symptoms were seen, symptoms reported and outcome after treatment, regardless of the type of treatment. All results are presented as total (percentage) or as mean (s.d.).
Quality assessment
The risk of bias (RoB) tool of the Cochrane Handbook for Systematic Reviews of Interventions was used and the Newcastle–Ottawa Scale (NOS) was chosen to assess the quality of the articles (20, 21, 22). This checklist was used to determine quality of non-randomized studies, including case-controlled and cohort studies, in three areas: selection, comparability and the ascertainment of either the exposure or outcome of interest. An assessment scale was available to award stars with a maximum score of 9: 1 for each question in the selection and outcome scale and 2 for the comparability domain (Supplementary data 1) (21). The follow-up as described in question 6 was determined to be at least 3 months in agreement with all authors. A score of less than 5 stars represents a high RoB (23).
In addition, a checklist suggested by Murad et al. was also used to obtain RoB (24). This checklist is especially designed for case reports and exists of an eight-item tool categorized in four domains: selection, ascertainment, causality and reporting. It is a modification of the tools by Pierson, Bradford Hills and the NOS (Supplementary data 2) (24). The eight items of the tool were scored yes or no. Like the NOS, the adequate follow-up was determined to be 3 months. Questions 5 and 6 of the questionnaire were not taken into account since they were mostly relevant to cases of adverse drug events. Quality of the articles was defined ‘good’ when ‘yes’ was scored ≥4 times, 3–2 times ‘yes’ was defined ‘moderate’ and ≤1 time ‘yes’ as ‘poor’. All eligible case reports were included in the review irrespective of their methodological quality.
Results
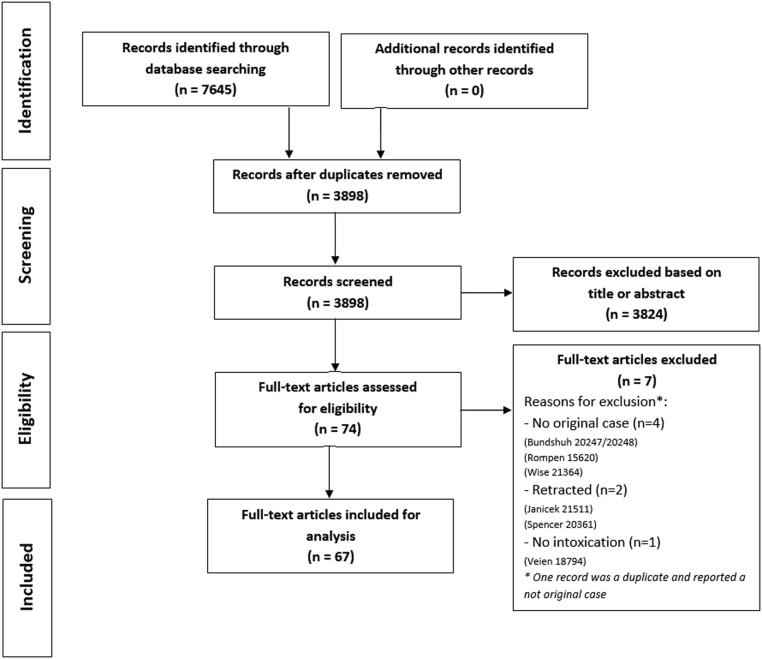
Our search identified 7645 references of which 3898 were screened after removal of duplicates (Supplementary data 3). A total of 3824 were excluded based on title or abstract, resulting in 74 eligible articles. Of these, a total of 67 were included for analysis after excluding another 7 studies, due to no original case description, retraction and no described toxicity (Fig. 1). The RoB classification according to the NOS checklist resulted in a 98.5% (n = 66) of low RoB and 1.5% (n = 1) of high-risk bias of the case reports (see Supplementary data 4). According to the checklist of Murad et al., 76.1% (n = 51) of the studies were classified as having good methodological quality. A full review of the Murad checklist is found in Supplementary data 5.
Figure 1.
Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) flowchart.
We identified a total of 79 patients with reported PHACT. Table 1 presents the most important data of all articles and methodological quality assessment score. The full overview is shown in Supplementary data 6. A total of 46 (58.2%) patients were male and 27 (34.2%) were female. Sex was not mentioned in six patients. The mean age at primary surgery was 53.2 ± 14.2 years. The main known reason for primary surgery was osteoarthritis (n = 28; 35.4%); however, in most reports, the primary indication was unknown (n = 36; 45.6%). Table 2 presents the demographic data of the entire group.
Table 1.
Most important details of the reviewed articles and quality assessment score results. For a complete overview see Supplementary data 2.
| Reference | Patients, n | Major reported systemic symptoms (classified) | Cobalt in ppb (sample) | Primary intervention | Secondary intervention | Indication revision | Quality score | |
|---|---|---|---|---|---|---|---|---|
| NOS | Murad | |||||||
| Allen et al. (32) | 1 | Cardiovascular | 287.6 (S) | MoM | CoP | Systemic symptoms | Low | Good |
| Apel et al. (33) | 1 | Neurological (sensory and C/P), cardiovascular | 355 (S) | CoC | MoC | Fracture CoC implant | Low | Good |
| Austin et al. (34) | 1 | Neurological (sensory), cardiovascular | 1351.4 (WB) | - | - | Systemic symptoms | Low | Good |
| Balbouzis et al. (35) | 1 | Cardiovascular | 22.2 (WB) | CoC | MoP | Fracture CoC implant | Low | Good |
| Bartholomeu et al. (36) | 1 | Neurological (sensory) | - | - | - | Systemic symptoms | Low | Good |
| Biglia et al. (37) | 1 | Metal/psychosocial | 14 (WB) | CoC | MoM | Fracture CoC implant | Low | Good |
| Bonilla & Bhimaray (38) | 1 | Cardiovascular (shock) | 100 (WB) | - | Systemic symptoms | Low | Good | |
| Briani et al. (39) | 1 | Neurological (C/P) | 14.3 (WB) | CoC | - | Systemic symptoms | Low | Good |
| Charette et al. (40) | 1 | Cardiovascular | 156 (S) | MoM bilateral | CoP | Systemic symptoms | Low | Good |
| Choi et al. (41) | 2 | Cardiovascular, neurological (sensory) | 489.5 (S) | CoP | MoP | Fracture CoC implant | Low | Good |
| Cardiovascular | 111.9 (S) | CoC bilateral | CoM | Fracture CoC implant | ||||
| Citak et al. (42) | 1 | Cardiovascular, neurological (sensory) | - | CoC | MoP | Fracture CoC implant | Low | Poor |
| Czekaj et al. (43) | 1 | Neurological (sensory and C/P) | 206 (WB) | MoM | CoP | Systemic symptoms | Low | Good |
| Dahms et al. (44) | 1 | Neurological (sensory), cardiovascular | 885 (WB) | CoC | MoP | Fracture CoC implant | Low | Good |
| Davies & Chareonthaitawee (45) | 1 | Neurological (sensory), cardiovascular | 953 (S) | CoC | MoP | Fracture CoC implant | Low | Poor |
| Dolliana & Nüesch (46) | 1 | Neurological (sensory), gastroenterology | 819.2 (WB) | CoC | MoM | Fracture CoC implant | Low | Moderate |
| Enseleit et al. (47) | 1 | Neurological (sensory), Cardiovascular | - | Bilateral | - | Systemic symptoms | Low | Poor |
| Sánchez & Cardona (48) | 1 | Neurological (sensory and C/P), cardiovascular |
1036 (S) | CoC | MoP | Fracture CoC implant | Low | Good |
| Fox (2016) (29) | 1 | Neurological (sensory and C/P), cardiovascular |
817 (WB) | CoC | MoP | Fracture CoC implant | Low | Good |
| Garcia et al. (49) | 1 | Neurological (sensory and C/P), Cardiovascular |
1000 (S) | CoC | MoP | Fracture CoC implant | Low | Good |
| Gautam et al. (50) | 1 | Cardiovascular | 373 (S) | CoC | MoP | Fracture CoC implant | Low | Good |
| Giampreti et al. (51) | 1 | Neurological (C/P) | 352.6 (S) | MoM | CoP | Hip pain | Low | Moderate |
| Giampreti et al. (52) | 4 | Neurological (sensory and C/P), cardiovascular |
50 -352.6 (WB) | MoM | - | Systemic symptoms | Low | Moderate |
| Gilbert et al. (53) | 1 | Cardiovascular | 1085 (S) | CoC bilateral | MoP | Fracture CoC implant | Low | Good |
| Goel & Hoskote (54) | 1 | Cardiovascular (shock) | 25 (S) | MoM bilateral | Systemic symptoms | Low | Moderate | |
| Grant et al. (55) | 1 | Neurological (sensory and C/P) | 2148(P) | CoC | MoM | Fracture CoC implant | Low | Good |
| Griffiths et al. (56) | 1 | Neurological (sensory and C/P), cardiovascular |
2006 (WB) | CoC | MoP | Fracture CoC implant | Low | Good |
| Grillo et al. (57) | 1 | Neurological (sensory), cardiovascular | 107.8 (S) | CoC | MoM | Fracture CoC implant | Low | Good |
| Grosso et al. (58) | 1 | Neurological (sensory) | 1076 (WB) | CoC | MoM | Fracture CoC implant | Low | Poor |
| Guevara et al. (59) | 1 | Neurological (sensory), cardiovascular |
- | - | - | Systemic symptoms | Low | Poor |
| Harris et al. (60) | 1 | Neurological (sensory and C/P), cardiovascular |
788.1 (WB) | CoC | MoP | Fracture CoC implant | Low | Good |
| Ho et al. (61) | 1 | Neurological (sensory and C/P), cardiovascular |
799 (S) | CoC | - | Systemic symptoms | Low | Moderate |
| Ikeda et al. (62) | 1 | Neurological (C/P) | 400 (WB) | CoC bilateral | MoP | Fracture CoC implant | Low | Good |
| Jones et al. (25) | 7 | Not classified | McKee hip | Girdlestone | Recurrent dislocations | Low | Good | |
| Not classified | McKee hip | MoP | Possible fracture | |||||
| Not classified | McKee hip | MoP | Persistent pain | |||||
| Skin/hair | McKee hip bilateral | Girdlestone | Protrusion acetabulum | |||||
| Not classified | McKee hip | None described | Hip pain | |||||
| Not classified | McKee hip | Recemented prosthesis | - | |||||
| Not classified | McKee hip | MoP | Recurrent dislocations | |||||
| Kao & Bunning (63) | 1 | Neurological (C/P) | 20 (WB) | Bilateral MoP | - | Systemic symptoms | Low | Moderate |
| Kim et al. (64) | 1 | Neurological (sensory and C/P), cardiovascular |
397.8 (WB) | CoP bilateral | MoP | Fracture CoP implant | Low | Good |
| Lapena Motilva et al. (65) | 1 | Neurological (sensory and C/P) | 892.8 (WB) | - | - | Systemic symptoms | Low | Good |
| Lecoanet et al. (66) | 1 | Neurological (sensory and C/P), cardiovascular |
1463.7 (S) | CoC | MoP | Fracture CoC implant | Low | Good |
| Leikin et al. (67) | 1 | Neurological (sensory and C/P) | 1096.5 (S) | CoC | MoM | Fracture CoC implant | Low | Poor |
| Machado et al. (68) | 1 | Cardiovascular | 13.6 (P) | MoM | - | Systemic symptoms | Low | Poor |
| Mao et al. (69) | 2 | Neurological (sensory and C/P) | 24.2 (S) | MoM | CoP | Systemic symptoms | Low | Good |
| Neurological (sensory and C/P) | 15.2 (S) | MoM | CoP | Systemic symptoms | ||||
| Marcus & Woodkotch (70) | 1 | Neurological (sensory and C/P) | - | MoM | MoP | Systemic symptoms | Low | Moderate |
| Martin et al. (71) | 1 | Cardiovascular | 192 (S) | MoM bilateral | Bilateral CoP | Systemic symptoms | Low | Good |
| Moniz et al. (72) | 1 | Cardiovascular | 169 (S) | MoM | CoP | Systemic symptoms | Low | Good |
| Mosier et al. (73) | 1 | Cardiovascular | 189 (S) | MoM bilateral | Dual mobility CoP | Systemic symptoms | Low | Good |
| Ng et al. (74) | 1 | Neurological (sensory and C/P) | 44.7 (S) | MoM bilateral | - | Systemic symptoms | Low | Good |
| Nogar & Bells (75) | 1 | Neurological (C/P), cardiovascular | 208 (S) | MoM | - | Systemic symptoms | Low | Good |
| Oldenburg et al. (26) | 1 | Neurological (sensory and C/P), cardiovascular |
625 (S) | CoP | MoP | Fracture CoP implant | Low | Good |
| Payen et al. (76) | 1 | Cardiovascular (shock) | 267.2 (WB) | MoM bilateral | - | Systemic symptoms | Low | Good |
| Pelayo-de Tomas et al. (77) | 1 | Neurological (sensory and C/P), cardiovascular |
651.2 (S) | CoC | MoP | Fracture CoC implant | Low | Good |
| Pelclova et al. (78) | 1 | Neurological (sensory and C/P) | 506 (S) | CoC | MoP | Fracture CoC implant | Low | Good |
| Peters et al. (79) | 1 | Neurological (sensory and C/P) | 596.5 (S) | CoC | MoP | Fracture CoC implant | Low | Good |
| Reich et al. (80) | 1 | Neurological (C/P) | 10.1 (S) | Revision MoM | 2nd revision MoP | Acetabular osteolysis | Low | Good |
| Reid et al. (81) | 1 | Cardiovascular | - | MoM | Unstable | Systemic symptoms | Low | Good |
| Rizzetti et al. (27) | 1 | Neurological (sensory and C/P) | 549 (WB) | CoC | MoP | Fracture CoC implant | Low | Good |
| Sanches Dalmau et al. (82) | 1 | Neurological (sensory and C/P) | 259 (P) | MoP bilateral | Chelationtherapy | Systemic symptoms | High | Poor |
| Sanz Perez et al. (83) | 1 | Cardiovascular (shock) | 652 (S) | CoC | MoP | Fracture CoC implant | Low | Good |
| Shapiro et al. (84) | 1 | Neurological (C/P) | 39 (S) | MoM | CoP | Systemic symptoms | Low | Good |
| Sotos & Tower (85) | 1 | Neurological (sensory and C/P) | 122 (S) | MoM | CoP | Systemic symptoms | Low | Good |
| Steens et al. (28) | 1 | Neurological (sensory and C/P) | - | CoC | CoM | Chronic pain | Low | Good |
| Tilney et al. (86) | 1 | Cardiovascular | 246.3(WB) | MoM | - | Systemic symptoms | Low | Good |
| Tower (87) | 1 | Neurological (sensory and C/P) | 74 (S) | MoM | - | Systemic symptoms | Low | Poor |
| Tower (88) | 2 | Neurological (sensory and C/P) cardiovascular |
122 (S) | MoM | - | Systemic symptoms | Low | Good |
| 23 (S) | MoM | - | Systemic symptoms | |||||
| Vasukutty et al. (89) | 1 | Cardiovascular | 44.9 (S) | CoC | MoP | Fracture CoC implant | Low | Good |
| Woelber et al. (90) | 1 | Neurological (sensory and C/P) | 116 (S) | MoM bilateral | BilateralCoP | Systemic symptoms | Low | Good |
| Wong & Nixon (91) | 1 | Skin/hair | 57.1 (S) | MoM | ToP | Systemic symptoms | Low | Good |
| Zeynalov et al. (92) | 1 | Metal/psychosocial | 1.6 (S) | MoM | CoM | Systemic symptoms | Low | Good |
| Zywiel et al. (93) | 1 | Cardiovascular | 6521 (WB) | CoC | MoP | Fracture CoC implant | Low | Good |
C/P, central and peripheral; CoC, ceramic-on-ceramic; CoP, ceramic-on-polyethylene; MoM, metal-on-metal; MoP, metal-on-polyethylene; NOS, Newcastle–Ottawa Scale; P, plasma; ppb, parts per billion; S, serum; ToP, titanium-on-polyethylene; WB, whole blood.
Table 2.
Demographics of all patients (n = 79). Data are presented as mean ± s.d. or as n (%).
| Demographics | Values |
|---|---|
| Primary surgery | |
| Mean age at primary surgery | 53.2 ± 14.2 |
| Indications for primary surgery | |
| Primary osteoarthritis | 28 (35.4%) |
| Avascular necrosis | 9 (11.4%) |
| Fracture | 3 (3.8%) |
| Dysplasia | 2 (2.5%) |
| Hip pain | 1 (1.3%) |
| Unknown | 36 (45.6%) |
| Male/female | 46/27 (58.2%/34.2%) |
| Primary bearing | |
| MoM | 38 (48%) |
| CoC | 32 (40.5%) |
| MoP | 2 (2.5%) |
| CoP | 2 (2.5%) |
| Unknown | 5 (6.5%) |
| Revision surgery | |
| Mean age at revision surgery | 58.6 ± 11.1 |
| Indication for revision surgery | |
| Systemic symptoms | 38 (48.1%) |
| Fracture CoC | 31 (39.2%) |
| (chronic) Pain | 4 (5.1%) |
| Recurrent dislocations | 2 (2.5%) |
| Protrusion acetabulum | 1 (1.3%) |
| Fracture | 1 (1.3%) |
| Osteolysis | 1 (1.3%) |
| Unknown | 1 (1.3%) |
| Male/female | 26/15 (63.4%/36.6%) |
| Cobalt toxicity | |
| Mean age at onset of symptoms | 59.0 ± 11.5 |
| Primary PHACT complaints | 38 (48%) |
| Revision PHACT complaints | 41 (52%) |
| Mean cobalt toxicity level in ppb | 572 ± 962.1 |
| Mean follow-up time in months | 12.7 ± 14.2 |
CoC, ceramic-on-ceramic; CoP, ceramic-on-polyethylene; MoM, metal-on-metal; MoP, metal-on-polyethylene; PHACT, prosthetic hip-associated cobalt toxicity; ppb, parts per billion.
PHACT related to type of bearing
The two most used bearings in the primary surgery were MoM (n = 38; 48.0%) and CoC (n = 32; 40.5%). Also, MoP (n = 2; 2.5%) and CoP (n = 2; 2.5%) were reported; in five cases (6.5%), no primary bearing was reported.
In 38 (48.0%) patients, the PHACT symptoms occurred after primary surgery; of which, in 34 (89.5%) after a primary MoM bearing. The mean time between the primary surgery and onset of symptoms was 2.1 (range: 0–13) years. A total of 41 (52.0%) patients developed PHACT symptoms after they had revision surgery. Especially, revision of a (fractured) CoC bearing for a MoP (n = 21) or MoM bearing (n = 6) caused the onset of cobalt toxicity symptoms. In this group, the mean time of developing PHACT was 8.8 (range: 4–15) years after the primary surgery and 2.4 (range: 0–9) years after the revision surgery (Table 3).
Table 3.
Demographics of all bearings (n = 79). Data are presented as mean ± s.d. or as n (%).
| Variable | Primary bearing | ||
|---|---|---|---|
| MoM | CoC | Others | |
| n | 38 | 32 | 9* |
| Primary surgery | |||
| Mean age at primary surgery in years | 56.2 ± 14.9 | 50.5 ± 13.1 | 54.4 ± 19.9 |
| Indications for primary surgery (%) | |||
| Primary osteoarthritis | 16 (42.1%) | 11 (34.4%) | 3 (33.3%) |
| Avascular necrosis | 2 (5.3%) | 4 (12.5%) | 1 (11.1%) |
| Fracture | 3 (7.9%) | 1 (3.1%) | 0 (0%) |
| Dysplasia | 2 (5.3%) | 0 (0%) | 0 (0%) |
| Hip pain | 0 (0%) | 1 (3.1%) | 0 (0%) |
| Unknown | 15 (39%.5) | 15 (46.9%) | 5 (55.6%) |
| Male/female (%) | 20/13 (52.6%/34.2%) | 20/12 (62.5%/37.5%) | 6/2 (66.7%/22.2%) |
| Primary PHACT complaints (%) | 34 (89.5%) | 1 (3.1%) | 3 (33.3%) |
| Revision PHACT complaints (%) | 4 (10.5%) | 31 (96.9%) | 6 (66.7%) |
| Cobalt toxicity level in ppb | 123.7 ± 96.8 | 1.078.2 ± 1.267.5 | 379.4 ± 369.3 |
| Mean age at onset of symptoms in years | 58.3 ± 12.9 | 59.3 ± 10.9 | 58.5 ± 11.5 |
| Mean time in years at onset of symptoms after primary surgery (range) | 2.1 (0–13) | 8.8 (4–15) | 4.1 (2–12) |
| Revision surgery | |||
| Mean age at revision surgery in years | 60.7 ± 11.2 | 56.9 ± 11.4 | 58.5 ± 8.8 |
| Indication for revision surgery (%) | |||
| Systemic symptoms | 29 (76.3%) | 1 (3.1%) | 2 (22.2%) |
| Fracture CoC | 0 (0%) | 29(90.6%) | 2 (22.2%) |
| (Chronic) pain | 3 (7.9%) | 1 (3.1%) | 0 (0%) |
| Recurrent dislocations | 2 (5.3%) | 0 (0%) | 0 (0%) |
| Protrusion acetabulum | 1 (2.6%) | 0 (0%) | 0 (0%) |
| Fracture | 1 (2.6%) | 0 (0%) | 0 (0%) |
| Osteolysis | 1 (2.6%) | 0 (0%) | 0 (0%) |
| Unknown | 1 (2.6%) | 1 (3.1%) | 5 (55.6%) |
| Bearing after revision (%) | |||
| MoM | 0 (0%) | 6 (18.8%) | 0 (0%) |
| CoC | 0 (0%) | 0 (0%) | 0 (0%) |
| MoP | 5 (13.2%) | 21 (65.6%) | 2 (22.2%) |
| CoP | 12 (31.6%) | 0 (0%) | 0 (0%) |
| ToP | 1 (2.6%) | 0 (0%) | 0 (0%) |
| CoM/MoC | 1 (2.6%) | 3 (9.4%) | 0 (0%) |
| Girdlestone | 2 (5.3%) | 0 (0%) | 0 (0%) |
| Not suitable | 16 (42.1%) | 0 (0%) | 0 (0%) |
| Unknown | 1 (2.6) | 2 (6.25) | 6 (66.7%) |
| Mean follow-up time in months | 13 ± 12.2 | 11 ± 13.5 | 15 ± 25.3 |
*MoP: 2; CoP: 2; unknown: 7.
CoC, ceramic-on-ceramic; CoM, ceramic-on-metal; CoP, ceramic-on-polyethylene; MoC, metal-on-ceramic; MoM, metal-on-metal; MoP, metal-on-polyethylene; PHACT, prosthetic hip-associated cobalt toxicity; ppb, parts per billion; ToP, titanium-on-polyethylene.
PHACT related systemic symptoms
A total of 321 symptoms were scored and divided into nine different categories: neurological, cardiovascular, gastroenterology, musculoskeletal, skin/hair, thyroid, mental/psychosocial and others. The neurological symptoms were subcategorized in central/peripheral and sensory. Some patients had more than one reported symptom during the first presentation. All documented symptoms were considered and scored as possible PHACT. Table 4 shows all the different symptoms in the nine different categories.
Table 4.
All systemic symptoms (n = 321; 100.0%) reported in 79 patients. Data are presented as n (%).
| Symptoms | Patients |
|---|---|
| Neurological | |
| Central and peripheral | 62 (19.3) |
| Cognitive/memory/concentration | 16 (20.3) |
| Paresthesiaanesthesia | 13 (16.5) |
| (Poly)neuropathy | 8 (10.1) |
| Proprioception loss/difficulty walking | 7 (8.9) |
| Headache | 4 (5.1) |
| Hyposthenia/asthenia | 3 (3.8) |
| Spasm/musclecramps | 3 (3.8) |
| Lower motor neuron syndromes | 2 (2.5) |
| Axonopathy | 1 (1.3) |
| Bulbarpalsy | 1 (1.3) |
| Convulsions | 1 (1.3) |
| Neuropaticpain | 1 (1.3) |
| Parkinson | 1 (1.3) |
| Tremors | 1 (1.3) |
| Sensory* | 77 (24.0) |
| Hearing impairment/loss | 34 (43.0) |
| Visual impairment/retinaldysfunction | 25 (31.6) |
| Dysgeusia/metallic taste | 9 (11.4) |
| Tinnitus | 5 (6.3) |
| Vertigo | 2 (2.5) |
| Loss of smell/anosmia | 1 (1.3) |
| Opticnervearthrophy | 1 (1.3) |
| Cardiovascular | 71 (22.1) |
| Dyspnoe/apnoe/orthopnea | 25 (31.6) |
| (Peri)cardiomyopathie | 12 (15.2) |
| Heart failure | 10 (12.7) |
| Tachycardia | 5 (6.3) |
| Cardiogenic shock | 4 (5.1) |
| Exertionalchest tightness/pain | 4 (5.1) |
| Oedema | 4 (5.1) |
| Pericarditis | 2 (2.5) |
| Hypertension | 2 (2.5) |
| Syncope | 2 (2.5) |
| Pericardial effusion | 1 (1.3) |
| Gastroenterology | 12 (3.7) |
| Diarrhea | 3 (3.8) |
| Nausea | 3 (3.8) |
| Vomiting | 3 (3.8) |
| Anorexia | 2 (2.5) |
| Liver failure | 1 (1.3) |
| Musculoskeletal | 5 (1.6) |
| Arthromyalgia | 1 (1.3) |
| Decreasedmusclemass | 1 (1.3) |
| Polyarthralgia | 1 (1.3) |
| Polymyalgia | 1 (1.3) |
| General stiffness | 1 (1.3) |
| Skin/hair | 8 (2.5) |
| Rash/dermatitis/sarcoid-like | 6 (7.6) |
| Diaphoresis | 1 (1.3) |
| Hair loss | 1 (1.3) |
| Thyroid | 9 (2.8) |
| Hypothyroidism/thyroiddysfunction | 9 (11.4) |
| Mental/pschosocial | 25 (7.8) |
| Fatigue | 17 (21.5) |
| Depression | 4 (5.1) |
| Anxious | 2 (2.5) |
| Insomnia | 2 (2.5) |
| Other | 20 (6.2) |
| Weight loss | 7 (8.9) |
| Weakness | 4 (5.1) |
| Fever | 2 (2.5) |
| Malaise | 2 (2.5) |
| Polydipsia | 2 (2.5) |
| Multi-organ failure | 1 (1.3) |
| Polycythemia | 1 (1.3) |
| Uncontrolled diabetes | 1 (1.3) |
*Visual, auditory, gustatory olfactory, somatosensory and vestibular.
The most identified symptoms were neurological related. Since most symptoms were especially related to the sensory system, we divided them into sensory system (n = 77; 24.0%) and central/peripheral-related symptoms (n = 62; 19.3%) .
Hearing impairment/loss and visual impairment/retinal dysfunction were the most mentioned problems in the sensory system, with a total of 34 (44.2%) and 25 (32.5%), respectively. Within the 79 described patients, hearing impairment/loss encounters for a total of 43.0% and visual impairment/retinal dysfunction for 31.6%. In the central/peripheral group, the most described symptoms were cognitive, memory, or concentration problems (n = 16; 12.6%) and paresthesia/anesthesia (n = 13; 16.5%).
The second most reported complaints were grouped in the cardiovascular origin. We found 71 suspected cobalt-induced cardiovascular complaints after primary and/or revision hip surgery. The described cardiovascular symptoms divers from dyspnea (n = 25; 31.6%), cardiomyopathy (n = 12; 15.2%), heart failure (n = 10; 12.7%) to cardiogenic shock (n = 4; 5.1%) (Table 3).
Another systemic problem, often related to cobalt toxicity, is hypothyroidism or thyroid dysfunction. We found nine patients (11.4%) with proven thyroid abnormalities. A total of 17 (21.5%) patients described fatigue and nine had thyroid dysfunction. Of these nine patients, only three patients had also proven thyroid dysfunction, whereas in all other patients, the cause of fatigue had not been investigated or described.
A total of 32 (40.5%) patients were recorded with hip pain as one of the symptoms. Despite this being no systemic complaint, we felt obligated to describe this symptom as it is most likely related to the (early) failure of the hip prosthesis.
In all patients who received treatment for the symptoms, by either removing the prosthesis or by medication, the symptoms reduced considerably.
PHACT and blood cobalt concentrations
The mean cobalt concentration in blood at which the systemic symptoms were related was 572.0 ± 962.2 ppb for the total group. However, these concentrations differ greatly between the different bearings. The mean cobalt toxicity level for specific MoM, revised CoC, and other bearings were respectively 123.7 ± 96.8, 1078.2 ± 1267.5 and 379.4 ± 369.3 ppb. Table 5 described the mean cobalt concentration between the MoM and revised CoC bearings and three major systemic symptoms: neurological, central/peripheral and sensory and cardiovascular. There was no noticeable difference between the cobalt toxicity concentrations and the developed symptoms in the two bearings. After revision of the MoM bearing or a second revision of the earlier fractured CoC bearing, cobalt concentrations decreased in almost all reported patients.
Table 5.
The total number of the three most presented systemic symptoms in relation with the cobalt toxicity level in the two most reported bearings (MoM and CoC).
| Major systemic symptoms | Bearing type and cobalt Level (ppb) | |||
|---|---|---|---|---|
| MoM, n | Cobalt, mean ± s.d. | CoC, n | Cobalt, mean ± s.d. | |
| Neurological C/P | 17 | 127.2 ± 110.9 | 16 | 889.1 ± 574.9 |
| Neurological sensory | 13 | 119.4 ± 98.7 | 19 | 1000.1 ± 517.9 |
| Cardiovascular | 16 | 169.0 ± 100.2 | 19 | 778.4 ± 504.4 |
C/P, central and peripheral; CoC, ceramic-on-ceramic; MoM, metal-on-metal; ppb, parts per billion.
Discussion
The present review shows that PHACT is mostly seen in primary MoM and after revision of a (fractured) CoC bearings for an MoP or MoM articulation. PHACT is a relevant and serious complication with severe systemic symptoms in the neurological, cardiovascular and thyroid system.
It was only after the recall of several MoM prostheses in 2010 that PHACT was increasingly associated with this type of bearing (6, 15). Before that, only Jones et al. described several cases with cobalt-induced systemic issues in the McKee hip (firstgeneration MoM). In this case series (seven cases), the most frequently mentioned symptom was hip pain and there was increased concentrations of cobalt ions in urine and joint fluid (25). Three other reports before 2010 by Oldenburg et al., Rizzetti et al. and Steens et al. showed cobalt-related problems in revised ceramic bearings (26, 27, 28).
In primary MoM implants, the bearing surfaces can release metal particles through corrosion and adhesion (induced by wear). After revision of a (fractured) CoC bearing to a metal containing articulation (e.g. MoP or MoM), potentially remaining small ceramic particles in the soft tissue and joint space can cause massive abrasion on the metal surface through three-body wear. All mechanisms of particle release may contribute not only to local adverse reactions but also to potential systemic cobalt toxicity (8, 9, 29).
Limitations
There are some limitations that should be mentioned. Since there are no comparative studies, the present review consists mainly of case reports. Therefore, a publication bias is not ruled out and case reports are considered low-quality research. To minimize these limitations, we have assessed the articles on quality by two different methods as guidance for a systematic review methodology publication. As suggested by the Cochrane Handbook, we used the NOS to determine the RoB and assess the quality (22). Since this questionnaire is not entirely consistent with the assessment of case reports, we also used the checklist suggested by Murad et al. (24). A second major limitation is the lack of controlled comparison studies, no clear reported patient histories and a wide range of blood cobalt ion concentrations. Because of that, a direct relationship between the presented symptoms and elevated cobalt concentrations can not be proven. Some of the reported symptoms can also occur independent ofm cobalt toxicity and might relate to common health issues or are associated with age. However, we were able to describe and present as adequately as possible the most reported symptoms associated with cobalt toxicity and high probability.
PHACT related to type of bearing
The present review showed PHACT in 38 patients with an MoM bearing; of which, 34 (89.5%) were detected within 2.1 (range: 0–13) years after the primary surgery. This is in contrast with the 32 described revised CoC bearings. In these bearings, only 1 (3.1%) patient had PHACT related complications after primary surgery, whereas 31 (96.9%) patients experienced PHACT within 2.4 (range: 0–9) years after revision surgery. In 29 (93.5%) of these revision cases, the indication was a fractured CoC-bearing plus, all the bearings used in the revision surgery contained at least one metal component (Table 3).
PHACT-related systemic symptoms
The three most affected systems in patients with cobalt toxicity are in the sensory, neurological and cardiovascular systems. The neurotoxic effects of cobalt have already been well established in multiple animal studies (12, 27, 30). In addition, some case series describe the neurotoxicity in patients after the treatment with cobalt for anemia. Not only tinnitus and deafness but also paresthesia and ataxia seem to be associated with the use of cobalt (12).
All reviewed reports presume a direct relationship with increased blood cobalt concentrations. Within the sensory system, a total of 77 symptoms were described; of which, the most involved were hearing (n = 34; 44.2%) and visual impairment/loss. Most of these symptoms diminished after revision of the prosthesis and a decrease in blood cobalt concentrations was seen. The neurological problems contain mainly cognitive, memory and concentration dysfunction (n = 16; 25.8%), as well as paresthesia/anesthesia (n = 13; 21.0%). Patients with these symptoms also improved after explanting or revision of the hip prosthesis.
The second most reposted complaints were grouped in the cardiovascular origin (n = 71; 22.1%). Of these, dyspnea/apnea/orthopnea (n = 25; 31.6%), cardiomyopathy (n = 12; 15.2%), heart failure (n = 10; 12.7%) and cardiogenic shock (n = 4; 5.6%) were most described. The four patients with a cardiogenic shock showed cobalt concentrations from 25 to 652 ppb; however, a clear dose–response effect of the cobalt in these cases could not be established. Of these four patients, one died due to the cardiogenic shock, one needed heart transplantation and two others clinically recovered after explanting the hip prosthesis.
Thyroid dysfunction in relation to cobalt toxicity is also well described in the literature (31) and proven in nine reported patients (11.4%). Another symptom, often mentioned in relation to thyroid dysfunction, is fatigue. A total of 17 patients reported fatigue; of which, only 3 had proven thyroid dysfunction. In all other cases, there was no thyroid dysfunction described. If we combine the 2 different groups, a total of 23 patients (29.11%) may have cobalt-related thyroid issues. This will make the thyroid dysfunction a third major affected systemic system; however, we could not prove this.
PHACT and blood cobalt concentrations
Most published reports provide a toxicity level of cobalt concentration in their cases; however, this concentration divers between all patients and different bearings. The cobalt levels associated with systemic toxicity were considerably higher in patients with revised CoC bearings when compared to patients with a primary MoM bearing (mean of 1078.2 and 123.7 ppb, respectively see Table 5). Our assumption is that corrosion- and adhesion-related metal exposition in MoM bearings is more gradual and slower than the massive release of cobalt-containing metal wear through three-body-related abrasion in fractured CoC bearings, which have been revised with metal-containing components. Another possible explanation is the awareness of local and systemic problems of the metal ions in MoM bearings. As a result, clinicians are more likely to link sudden or unexplained systemic issues to the hip prosthesis.
Unfortunately, we found no controlled studies to definitively link the systemic clinical findings with the elevated blood cobalt concentrations and we were unable to determine a safe upper limit threshold for cobalt toxicity.
Conclusion
Since many MoM bearings are still in situ, we can expect more PHACT cases. This systematic review showed that wide blood cobalt concentrations are observed in the onset of systemic symptoms linked to serum cobalt levels. It was not possible to provide a clear threshold level for cobalt-related toxicity from this analysis.
Nevertheless, clinicians should be aware that patients with an MoM or revised CoC bearing are at risk for developing systemic problems. Especially, new-onset systemic diseases related to neurological, both central/peripheral and sensory, and cardiovascular-related symptoms could be provoked by elevated cobalt concentrations. We also recommend not to use a metal-based articulation in the revision of a fractured ceramic bearing and suggest keeping a close follow-up with yearly blood cobalt concentration controls in patients with an MoM or revised fractured CoC bearing.
Supplementary Material
ICMJE Conflict of Interest Statement
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of this study.
Funding Statement
This study was performed at the Department of Orthopaedic Surgery at the Zuyderland Medical Center, the VieCuri Medical Center and the Reinier HAGA Orthopaedic Center.
References
- 1.Pandit H, Glyn-Jones S, McLardy-Smith P, Gundle R, Whitwell D, Gibbons CLM, Ostlere S, Athanasou N, Gill HS, Murray DW. Pseudotumours associated with metal-on-metal hip resurfacings. Journal of Bone and Joint Surgery: British Volume 2008. 90 847–851. ( 10.1302/0301-620X.90B7.20213) [DOI] [PubMed] [Google Scholar]
- 2.Carlson BC, Bryan AJ, Carrillo-Villamizar NT, Sierra RJ. The utility of metal ion trends in predicting revision in metal-on-metal total hip arthroplasty. Journal of Arthroplasty 2017. 32 S214–S219. ( 10.1016/j.arth.2017.02.031) [DOI] [PubMed] [Google Scholar]
- 3.Chalmers BP, Perry KI, Taunton MJ, Mabry TM, Abdel MP. Diagnosis of adverse local tissue reactions following metal-on-metal hip arthroplasty. Current Reviews in Musculoskeletal Medicine 2016. 9 67–74. ( 10.1007/s12178-016-9321-3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Matharu GS, Eskelinen A, Judge A, Pandit HG, Murray DW. Revision surgery of metal-on-metal hip arthroplasties for adverse reactions to metal debrisaclinicalupdate. Acta Orthopaedica 2018. 89 278–288. ( 10.1080/17453674.2018.1440455) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brent J, Devlin JJ. Dilemmas about the toxicological consequences of metal-on-metal hip prostheses – what we do and do not know, and what we should do? Clinical Toxicology 2013. 51 195–198. ( 10.3109/15563650.2013.784326) [DOI] [PubMed] [Google Scholar]
- 6.Devlin JJ, Pomerleau AC, Brent J, Morgan BW, Deitchman S, Schwartz M. Clinical features, testing, and management of patients with suspected prosthetic hip-associated cobalt toxicity: a systematic review of cases. Journal of Medical Toxicology 2013. 9 405–415. ( 10.1007/s13181-013-0320-0) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Daniel J, Ziaee H, Pradhan C, Pynsent PB, McMinn DJ. Blood and urine metal ion levels in young and active patients after Birmingham hip resurfacing arthroplasty: four-year results of a prospective longitudinal study. Journal of Bone and Joint Surgery: British Volume 2007. 89 169–173. ( 10.1302/0301-620X.89B2.18519) [DOI] [PubMed] [Google Scholar]
- 8.McKellop HA, Hart A, Park SH, Hothi H, Campbell P, Skinner JA. A lexicon for wear of metal‐on‐metal hip prostheses. Journal of Orthopaedic Research 2014. 32 1221–1233. ( 10.1002/jor.22651) [DOI] [PubMed] [Google Scholar]
- 9.Berber R, Skinner JA, Hart AJ. Management of metal-on-metal hip implant patients: who, when and how to revise? World Journal of Orthopedics 2016. 7 272–279. ( 10.5312/wjo.v7.i5.272) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gross RT, Kriss JP, Spaet TH. The hematopoletic and goltrogenic effects of cobaltous chloride in patients with sickle cell anemia. Pediatrics 1955. 15 284–290. ( 10.1542/peds.15.3.284) [DOI] [PubMed] [Google Scholar]
- 11.Curtis JR, Goode GC, Herrington J, Urdaneta LE. Possible cobalt toxicity in maintenance hemodialysis patients after treatment with cobaltous chloride: a study of blood and tissue cobalt concentrations in normal subjects and patients with terminal and renal failure. Clinical Nephrology 1976. 5 61–65. [PubMed] [Google Scholar]
- 12.Cheung AC, Banerjee S, Cherian JJ, Wong F, Butany J, Gilbert C, Overgaard C, Syed K, Zywiel MG, Jacobs JJet al. A mont systemic cobalt toxicity from total hip arthroplasties: review of a rare condition part 1 – history, mechanism, measurements, and pathophysiology. Bone and Joint Journal 2016. 98-B 6–13. [DOI] [PubMed] [Google Scholar]
- 13.Unice KM, Kerger BD, Paustenbach DJ, Finley BL, Tvermoes BE. Refined biokinetic model for humans exposed to cobalt dietary supplements and other sources of systemic cobalt exposure. Chemico-Biological Interactions 2014. 216 53–74. ( 10.1016/j.cbi.2014.04.001) [DOI] [PubMed] [Google Scholar]
- 14.Leyssens L, Vinck B, Van Der Straeten C, Wuyts F, Maes L. Cobalt toxicity in humans – a review of the potential sources and systemic health effects. Toxicology 2017. 387 43–56. ( 10.1016/j.tox.2017.05.015) [DOI] [PubMed] [Google Scholar]
- 15.Bradberry SM, Wilkinson JM, Ferner RE. Systemic toxicity related to metal hip prostheses. Clinical Toxicology 2014. 52 837–847. ( 10.3109/15563650.2014.944977) [DOI] [PubMed] [Google Scholar]
- 16.Leikin JB, Karydes HC, Whiteley PM, Wills BK, Cumpston KL, Jacobs JJ. Outpatient toxicology clinic experience of patients with hip implants. Clinical Toxicology 2013. 51 230–236. ( 10.3109/15563650.2013.768343) [DOI] [PubMed] [Google Scholar]
- 17.Ho JH, Leikin JB, Dargan PI, Archer JRH, Wood DM, Brent J. Metal-on-metal hip joint prostheses: a retrospective case series investigating the association of systemic toxicity with serum cobalt and chromium concentrations. Journal of Medical Toxicology 2017. 13 321–328. ( 10.1007/s13181-017-0629-1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Haddad FS.Primary metal-on-metal hip arthroplasty was not associated with increased cancer risk. Journal of Bone and Joint Surgery: American Volume 2013. 95 364. ( 10.2106/JBJS.9504.ebo313) [DOI] [PubMed] [Google Scholar]
- 19.Kovochich M, Finley BL, Novick R, Monnot AD, Donovan E, Unice KM, Fung ES, Fung D, Paustenbach DJ. Understanding outcomes and toxicological aspects of second generation metal-on-metal hip implants: a state-of-the-art review. Critical Reviews in Toxicology 2018. 48 853–901. ( 10.1080/10408444.2018.1563048) [DOI] [PubMed] [Google Scholar]
- 20.Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA. Cochrane Handbook for Systematic Reviews of Interventions, 2nd ed. Chichester (UK): John Wiley & Sons, 2019. [Google Scholar]
- 21.Wells GA, Shea B, O’Connell D, Peterson J, Welch V, Losos M, Tugwell P. The Newcastle-Ottawa Scale (NOS) for assessing the quality if nonrandomized studies in meta-analyses, 2012. (available from: http://wwwohrica/programs/clinical_epidemiology/oxfordasp) [Google Scholar]
- 22.Higgins JPT, Altman DG, Sterne JAC. & on behalf of the Cochrane Statistical Methods Group and the Cochrane Bias Methods Group. Chapter 8: Assessing risk of bias in included studies. In Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.1 (updated September 2008). The Cochrane Collaboration, 2008. (available at: www.cochrane-handbook.org) [Google Scholar]
- 23.Veronese N, Cereda E, Solmi M, Fowler SA, Manzato E, Maggi S, Manu P, Abe E, Hayashi K, Allard JPet al. Inverse relationship between body mass index and mortality in older nursing home residents: a meta-analysis of 19,538 elderly subjects. Obesity Reviews 2015. 16 1001–1015. ( 10.1111/obr.12309) [DOI] [PubMed] [Google Scholar]
- 24.Murad MH, Sultan S, Haffar S, Bazerbachi F. Methodological quality and synthesis of case series and case reports. BMJ Evidence-Based Medicine 2018. 23 60–63. ( 10.1136/bmjebm-2017-110853) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jones DA, Lucas HK, O'Driscoll M, Price CH, Wibberley B. Cobalt toxicity after McKee hip arthroplasty. Journal of Bone and Joint Surgery: British Volume 1975. 57 289–296. ( 10.1302/0301-620X.57B3.289) [DOI] [PubMed] [Google Scholar]
- 26.Oldenburg M, Wegner R, Baur X. Severe cobalt intoxication due to prosthesis wear in repeated total hip arthroplasty. Journal of Arthroplasty 2009. 24 825.e15–825.e20. ( 10.1016/j.arth.2008.07.017) [DOI] [PubMed] [Google Scholar]
- 27.Rizzetti MC, Liberini P, Zarattini G, Catalani S, Pazzaglia U, Apostoli P, Padovani A. Loss of sight and sound. Could it be the hip? Lancet 2009. 373 1052. ( 10.1016/S0140-6736(09)60490-6) [DOI] [PubMed] [Google Scholar]
- 28.Steens W, Loehr JF, von Foerster G, Katzer A. Chronic cobalt poisoning in endoprosthetic replacement. Orthopade 2006. 35 860–864. ( 10.1007/s00132-006-0973-3) [DOI] [PubMed] [Google Scholar]
- 29.Fox KA, Phillips TM, Yanta JH, Abesamis MG. Fatal cobalt toxicity after total hip arthroplasty revision for fractured ceramic components. Clinical Toxicology 2016. 54 874–877. ( 10.1080/15563650.2016.1214274) [DOI] [PubMed] [Google Scholar]
- 30.Catalani S, Rizzetti MC, Padovani A, Apostoli P. Neurotoxicity of cobalt. Human and Experimental Toxicology 2012. 31 421–437. ( 10.1177/0960327111414280) [DOI] [PubMed] [Google Scholar]
- 31.Lantin AC, Mallants A, Vermeulen J, Speybroeck N, Hoet P, Lison D. Absence of adverse effect on thyroid function and red blood cells in a population of workers exposed to cobalt compounds. Toxicology Letters 2011. 201 42–46. ( 10.1016/j.toxlet.2010.12.003) [DOI] [PubMed] [Google Scholar]
- 32.Allen LA, Ambardekar AV, Devaraj KM, Maleszewski JJ, Wolfel EE. Missing elements of the history. New England Journal of Medicine 2014. 370 559–566. ( 10.1056/NEJMcps1213196) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Apel W, Stark D, Stark A, O'Hagan S, Ling J. Cobalt–chromium toxic retinopathy case study. Documenta Ophthalmologica 2013. 126 69–78. ( 10.1007/s10633-012-9356-8) [DOI] [PubMed] [Google Scholar]
- 34.Austin E, Lazongas C, Thompson M. The tragic hip: a case of cobalt poisoning. In Clinical Toxicology. 4 Park Square, Milton Park, Abingdon, OXON …: Taylor & Francis Ltd, 2015. [Google Scholar]
- 35.Balbouzis T, Georgiadis T, Grigoris P. Granulomatous lung disease: a novel complication following metallosis from hip arthroplasty. Hip and Pelvis 2016. 28 249–253. ( 10.5371/hp.2016.28.4.249) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bartholomei D, Friang C, Betbeze M, Mensa C, Ohl X, Arndt C. Bilateral optic neuropathy confirmed with VEP in a case of chronic chrome/cobalt intoxication. Documenta Ophthalmologica 2018. 136 3–52. ( 10.1007/s10633-018-9641-2) [DOI] [PubMed] [Google Scholar]
- 37.Biglia A, Morandi V, Monti S, Delvino P, Cavagna Carlomaurizio L, Montecucco C. Cobalt hip prosthesis intoxication mimicking an autoimmune disease. Joint Bone Spine 2020. 87 652–654. ( 10.1016/j.jbspin.2020.05.014) [DOI] [PubMed] [Google Scholar]
- 38.Bonilla HMG, Bhimaraj A. A case of cobalt cardiomyopathy. Journal of the American College of Cardiology 2018. 71 A2386–A2386. ( 10.1016/S0735-1097(18)32927-9) [DOI] [Google Scholar]
- 39.Briani C, Cacciavillani M, Nicolli A, Trevisan A, Gasparotti R. Snake eyes MRI sign: possible role of cobalt toxicity. Journal of Neurology 2015. 262 471–472. ( 10.1007/s00415-014-7580-8) [DOI] [PubMed] [Google Scholar]
- 40.Charette RS, Neuwirth AL, Nelson CL. Arthroprosthetic cobaltism associated with cardiomyopathy. Arthroplasty Today 2017. 3 225–228. ( 10.1016/j.artd.2016.11.005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Choi HI, Hong JA, Kim MS, Lee SE, Jung SH, Yoon PW, Song JS, Kim JJ. Severe cardiomyopathy due to arthroprosthetic cobaltism: report of two cases with different outcomes. Cardiovascular Toxicology 2019. 19 82–89. ( 10.1007/s12012-018-9480-0) [DOI] [PubMed] [Google Scholar]
- 42.Citak M, Gehrke T, Thieme O. An extreme case of systemic metallosis after implantation of a hip prosthesis. Deutsches Ärzteblatt International 2018. 115 862. ( 10.3238/arztebl.2018.0862) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Czekaj J, Ehlinger M, Rahme M, Bonnomet F. Metallosis and cobalt – chrome intoxication after hip resurfacing arthroplasty. Journal of Orthopaedic Science 2016. 21 389–394. ( 10.1016/j.jos.2015.06.001) [DOI] [PubMed] [Google Scholar]
- 44.Dahms K, Sharkova Y, Heitland P, Pankuweit S, Schaefer JR. Cobalt intoxication diagnosed with the help of Dr House. Lancet 2014. 383 574. ( 10.1016/S0140-6736(14)60037-4) [DOI] [PubMed] [Google Scholar]
- 45.Davies D, Chareonthaitawee P. Beer-drinkers cardiomyopathy revisited: cobalt toxicity after a non-metal-on-metal hip arthroplasty. Journal of General Internal Medicine 2019. 34 S492–S493. [Google Scholar]
- 46.Dolliana P, Nüesch R. ‘Why am I deaf and my vision is blurred?’ Thinking of a horse or a zebra? Praxis 2016. 105 279–281. ( 10.1024/1661-8157/a002298) [DOI] [PubMed] [Google Scholar]
- 47.Enseleit F, Frank M, Naegele M, Flammer AJ, Ruschitzka F. Hip to be square: cardiomyopathy of unknown origin. European Journal of Heart Failure 2016. 18 (Supplement 1) 8–27. [Google Scholar]
- 48.Sánchez CME, Cardona LP. Cobalt intoxication in a patient with hip prosthesis. European Journal of Clinical Pharmacy: Atención Farmacéutica 2016. 18 189–190. [Google Scholar]
- 49.Garcia MD, Hur M, Chen JJ, Bhatti MT. Cobalt toxic optic neuropathy and retinopathy: case report and review of the literature. American Journal of Ophthalmology Case Reports 2020. 17 100606. ( 10.1016/j.ajoc.2020.100606) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gautam D, Pande A, Malhotra R. Fatal cobalt cardiomyopathy following revision total hip arthroplasty – a brief report with review of literature. Archives of Bone and Joint Surgery 2019. 7 379–383. [PMC free article] [PubMed] [Google Scholar]
- 51.Giampreti A, Lonati D, Locatelli CA. Chelation in suspected prosthetic hip-associated cobalt toxicity. Canadian Journal of Cardiology 2014. 30 465.e13. ( 10.1016/j.cjca.2013.12.009) [DOI] [PubMed] [Google Scholar]
- 52.Giampreti A, Petrolini VM, Vecchio S, Lonati D, Ronchi A, Locatelli CA. XXXIV International Congress of the European Association of Poisons Centres and Clinical Toxicologists (EAPCCT) 27–30 May 2014, Brussels, Belgium. Clinical Toxicology 2014. 52 295–443. ( 10.3109/15563650.2014.906213) [DOI] [Google Scholar]
- 53.Gilbert CJ, Cheung A, Butany J, Zywiel MG, Syed K, McDonald M, Wong F, Overgaard C. Hip pain and heart failure: the missing link. Canadian Journal of Cardiology 2013. 29 639.e1–639.e2. ( 10.1016/j.cjca.2012.10.015) [DOI] [PubMed] [Google Scholar]
- 54.Goel S, Hoskote S. Cobalt-induced cardiomyopathy requiring venoarterial ECMO. Critical Care Medicine 2016. 44 481. ( 10.1097/01.ccm.0000510298.48248.c4) [DOI] [Google Scholar]
- 55.Grant ML, Karp JK, Palladino M, Le N, Hall N, Herman JH. Does therapeutic plasma exchange have a role in the treatment of prosthetic hip-associated cobalt toxicity? A case report and literature review. Transfusion 2016. 56 2368–2373. ( 10.1111/trf.13720) [DOI] [PubMed] [Google Scholar]
- 56.Griffiths J, Colvin A, Yates P, Meyerkort D, Kop A, Prosser G. Extreme cobalt toxicity: bearing the brunt of a failed ceramic liner: a case report. JBJS Case Connector 2015. 5 e92. ( 10.2106/JBJS.CC.N.00242) [DOI] [PubMed] [Google Scholar]
- 57.Grillo LM, Nguyen HV, Tsang SH, Hood DC, Odel JG. Cobalt–chromium metallosis with normal electroretinogram. Journal of Neuro-Ophthalmology 2016. 36 383–388. ( 10.1097/WNO.0000000000000400) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Grosso M, Park P, Macaulay W. Multi-system manifestion of cobalt toxicity in the setting of metal-on-polyethylene total hip arthroplasty. Hip International 2018. 28 3–10. [Google Scholar]
- 59.Guevara G, Bail C, Logan P. Hip pain-an unusual cause of vision loss. Irish Journal of Medical Science 2018. 187 7–10.29536234 [Google Scholar]
- 60.Harris A, Johnson J, Mansuripur PK, Limbird R. Cobalt toxicity after revision to a metal-on-polyethylene total hip arthroplasty for fracture of ceramic acetabular component. Arthroplasty Today 2015. 1 89–91. ( 10.1016/j.artd.2015.09.002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ho VM, Arac A, Shieh PB. Hearing and vision loss in an older man. JAMA Neurology 2018. 75 1439–1440. ( 10.1001/jamaneurol.2018.1868) [DOI] [PubMed] [Google Scholar]
- 62.Ikeda T, Takahashi K, Kabata T, Sakagoshi D, Tomita K, Yamada M. Polyneuropathy caused by cobalt–chromium metallosis after total hip replacement. Muscle and Nerve 2010. 42 140–143. ( 10.1002/mus.21638) [DOI] [PubMed] [Google Scholar]
- 63.Kao C, Bunning R. Toxicity from elevated cobalt and chromium blood levels after hip implant: a case report. Physcial Medicine and Rehabilitation 2014. 6 (Supplement) S208. ( 10.1016/j.pmrj.2014.08.470) [DOI] [Google Scholar]
- 64.Kim CH, Choi YH, Jeong MY, Chang JS, Yoon PW. Cobalt intoxication heart failure after revision total hip replacement for ceramic head fracture: a case report. Hip and Pelvis 2016. 28 259–263. ( 10.5371/hp.2016.28.4.259) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lapena Motilva J, de Lara Cadinanos PM, Duran GO, Diaz Diaz J, Requejo VH, Collado JB. Cobalt poisoning secondary to hip prosthesis. European Journal of Neurology 2019. 26 (Supplement 1) 347–935. [Google Scholar]
- 66.Lecoanet P, Blangis M, Garcia M, Legallois Y, Fabre T. Chromium-cobalt intoxication with intense systemic complications following total hip revision after per-operative ceramic fracture. Case Reports in Orthopedics 2019. 2019 4209796. ( 10.1155/2019/4209796) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Leikin JB, Gerlinger T, Balkissoon R, Urban RM, Jacobs JJ, Lazarevic V. Cobalt toxicity from hip arthroplasty with visual/hearing/neuropathy improvement within days post revision. Clinical Toxicology 2015. 53 760. ( 10.3109/15563650.2015.1071025) [DOI] [Google Scholar]
- 68.Machado C, Appelbe A, Wood R. Arthroprosthetic cobaltism and cardiomyopathy. Heart, Lung and Circulation 2012. 21 759–760. ( 10.1016/j.hlc.2012.03.013) [DOI] [PubMed] [Google Scholar]
- 69.Mao X, Wong AA, Crawford RW. Cobalt toxicity – an emerging clinical problem in patients with metal-on-metal hip prostheses. Medical Journal of Australia 2011. 194 649–651. ( 10.5694/j.1326-5377.2011.tb03151.x) [DOI] [PubMed] [Google Scholar]
- 70.Marcus S, Woodkotch D. Cobalt toxicity from hip joint replacement. Clinical Toxicology 2014. 52 682–818. ( 10.3109/15563650.2014.940163) [DOI] [Google Scholar]
- 71.Martin JR, Spencer-Gardner L, Camp CL, Stulak JM, Sierra RJ. Cardiac cobaltism: a rare complication after bilateral metal-on-metal total hip arthroplasty. Arthroplasty Today 2015. 1 99–102. ( 10.1016/j.artd.2015.10.002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Moniz S, Hodgkinson S, Yates P. Cardiac transplant due to metal toxicity associated with hip arthroplasty. Arthroplasty Today 2017. 3 151–153. ( 10.1016/j.artd.2017.01.005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mosier BA, Maynard L, Sotereanos NG, Sewecke JJ. Progressive cardiomyopathy in a patient with elevated cobalt ion levels and bilateral metal-on-metal hip arthroplasties. American Journal of Orthopedics 2016. 45 E132–E135. [PubMed] [Google Scholar]
- 74.Ng SK, Ebneter A, Gilhotra JS. Hip-implant related chorio-retinal cobalt toxicity. Indian Journal of Ophthalmology 2013. 61 35–37. ( 10.4103/0301-4738.105053) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Nogar J, Bellis M. A case of reversible cardiomyopathy associated with elevated cobalt and chromium levels. Clinical Toxicology 2018. 56 912–1092. ( 10.1080/15563650.2018.1506610) [DOI] [PubMed] [Google Scholar]
- 76.Payen C, Pulce C, Sapori JM, Vial T. Cobalt cardiotoxicity in a patient with bilateral metal-on-metal (MoM) arthroplasty. Clinical Toxicology 2015. 53 233–403. ( 10.3109/15563650.2015.1024953) [DOI] [Google Scholar]
- 77.Pelayo-de Tomás JM, Novoa-Parra C, Gómez-Barbero P. Cobalt toxicity after revision total hip replacement due to fracture of a ceramic head. Revista Espanola de Cirugia Ortopedica y Traumatologia 2017. 61 203–207. ( 10.1016/j.recot.2016.11.001) [DOI] [PubMed] [Google Scholar]
- 78.Pelclova D, Sklensky M, Janicek P, Lach K. Severe cobalt intoxication following hip replacement revision: clinical features and outcome. Clinical Toxicology 2012. 50 262–265. ( 10.3109/15563650.2012.670244) [DOI] [PubMed] [Google Scholar]
- 79.Peters RM, Willemse P, Rijk PC, Hoogendoorn M, Zijlstra WP. Fatal cobalt toxicity after a non-metal-on-metal total hip arthroplasty. Case Reports in Orthopedics 2017. 2017 9123684. ( 10.1155/2017/9123684) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Reich MS, Javidan P, Garg VK, Copp SN. Chronic systemic metal ion toxicity from wear on a revised cobalt-chromium trunnion. Journal of Orthopaedic Case Reports 2019. 9 48–51. ( 10.13107/jocr.2250-0685.1366) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Reid N, Mazer-Amirshahi M, Litovitz T. Cobalt, cardiomyopathy, and chelation in a patient with a metal-on-metal hip implant. Journal of Medical Toxicology 2014. 10 65–99. ( 10.1007/s13181-013-0376-x) [DOI] [Google Scholar]
- 82.Sanchez-Dalmau B, de Carvalho AM, Nieto C, Fontecilla C, Torres Torres R, Nogués Set al. Visual dysfunction induced by cobalt toxicity. Neuro-Ophthalmology 2015. 39 (Supplement 1) S1–S87. [Google Scholar]
- 83.Sanz Pérez MI, Villoras AMR, Velasco AM, García SB, Loarte JC. Heart transplant secondary to cobalt toxicity after hip arthroplasty revision. Hip International 2019. 29 NP1–NP5. ( 10.1177/1120700019834793) [DOI] [PubMed] [Google Scholar]
- 84.Shapiro JA, Eskildsen SM, Del Gaizo DJ. Systemic cobaltism manifesting as oral mucosal discoloration and metallic gustation after metal-on-metal hip resurfacing. Arthroplasty Today 2018. 4 436–440. ( 10.1016/j.artd.2018.06.005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Sotos JG, Tower SS. Systemic disease after hip replacement: aeromedical implications of arthroprosthetic cobaltism. Aviation, Space, and Environmental Medicine 2013. 84 242–245. ( 10.3357/asem.3262.2013) [DOI] [PubMed] [Google Scholar]
- 86.Tilney R, Burg MR, Sammut MA. Cobalt cardiomyopathy secondary to hip arthroplasty: an increasingly prevalent problem. Case Reports in Cardiology 2017. 2017 5434571. ( 10.1155/2017/5434571) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Tower SS.Arthroprosthetic cobaltism associated with metal on metal hip implants. BMJ 2012. 344 e430. ( 10.1136/bmj.e430) [DOI] [PubMed] [Google Scholar]
- 88.Tower SS.Arthroprosthetic cobaltism: neurological and cardiac manifestations in two patients with metal-on-metal arthroplasty: a case report. Journal of Bone and Joint Surgery: American Volume 2010. 92 2847–2851. ( 10.2106/JBJS.J.00125) [DOI] [PubMed] [Google Scholar]
- 89.Vasukutty NL, Minhas THA. Systemic effects of cobalt toxicity after revision hip replacement can manifest in intermediate to long term follow-up. Hip International 2016. 26 e31–e34. ( 10.5301/hipint.5000386) [DOI] [PubMed] [Google Scholar]
- 90.Woelber E, van Citters DW, Steck T, Glass GA, Tower S. Explant analysis from a patient exhibiting rapid acceleration of Parkinson disease symptoms and hypercobaltemia following metal-on-metal total hip arthroplasty: a case report. JBJS Case Connector 2016. 6 e45. ( 10.2106/JBJS.CC.15.00063) [DOI] [PubMed] [Google Scholar]
- 91.Wong CC, Nixon RL. Systemic allergic dermatitis caused by cobalt and cobalt toxicity from a metal on a metal hip replacement. Contact Dermatitis 2014. 71 113–114. ( 10.1111/cod.12267) [DOI] [PubMed] [Google Scholar]
- 92.Zeynalov E, Cutrufello N, Pierce A. Cobalt blues: a case report of cobalt intoxication associated obstrictive sleep apnea (OSA). Sleep 2018. 41 A415–A416. ( 10.1093/sleep/zsy063.1120) [DOI] [Google Scholar]
- 93.Zywiel MG, Brandt JM, Overgaard CB, Cheung AC, Turgeon TR, Syed KA. Fatal cardiomyopathy after revision total hip replacement for fracture of a ceramic liner. Bone and Joint Journal 2013. 95-B 31–37. ( 10.1302/0301-620X.95B1.30060) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.



 This work is licensed under a
This work is licensed under a