Abstract
Background
Factors associated with outcome in dogs diagnosed with infective endocarditis (IE) are not well characterized.
Objectives
Evaluate outcome and prognostic factors in dogs with IE.
Animals
One hundred and thirteen dogs with IE.
Methods
Medical records for dogs that fulfilled the modified Duke criteria between 2005 and 2020 were retrospectively reviewed. Signalment, preexisting conditions, clinicopathologic findings, treatment regimen, and outcomes were recorded. Univariate logistic regression was performed to identify categorical factors associated with mortality, and then multivariate analysis was performed.
Results
Dogs were categorized as survivors (n = 47), non‐survivors (n = 57), or lost to follow‐up (n = 9). Survival to discharge and at 1 month was documented in 79 (70%) of 113 and 56 (54%) of 104 dogs, respectively, with median survival time (MST) of 72 days. Risk factors associated with mortality included development of congestive heart failure (odds ratio [OR], 11.8; 95% confidence interval [CI], 1.4‐97.8), thromboembolic events (OR, 5.7; 95% CI, 2.3‐14.4), and acute kidney injury (OR, 6.2; 95% CI, 2.0‐18.8). Administration of antithrombotic medications was associated with survival (OR, 0.35; 95% CI, 0.13‐0.97). Dogs that were not treated with antithrombotics had MST of 92 days, whereas dogs treated with antithrombotics did not reach MST during the study period. The heart valves involved and etiologic agent identified did not correlate with outcome.
Conclusion and Clinical Importance
Dogs with IE that had thromboembolic events, acute kidney injury, or congestive heart failure had higher risk of mortality. Administration of antithrombotics was associated with prolonged survival time.
Keywords: bacterial, Bartonella, cardiac infection, endomyocarditis
Abbreviations
- CKD
chronic kidney disease
- IE
infective endocarditis
- MST
median survival time
- OR
odds ratio
1. INTRODUCTION
Infective endocarditis (IE) is a rare but severe condition caused by infection of the endocardium and cardiac valves. Although the prevalence of bacterial IE in dogs is <1%, 1 , 2 the consequences of infection are severe, with a reported mortality rate of up to 78%. 1 However, substantial improvements have been made in the proportion of dogs surviving IE in the past 5 decades, with reports in the 1980s showing survival rates of 20% whereas studies from 15 years ago reported survival rates of 44%. 2 , 3
Complications associated with IE include congestive heart failure (CHF), cardiac arrhythmias, thromboembolic disease, and immune‐complex disease. 1 Congestive heart failure can develop secondary to direct damage to cardiac valves and is often refractory to medical treatment. 4 Thromboembolic disease has been noted in up to 80% of dogs with IE and can manifest in various organ, including the kidney, myocardium, and brain. 1 , 5 , 6 Thromboembolic complications and their associated clinicopathologic findings previously have been associated with fatality. 2 Other potential clinical factors associated with mortality that have been identified include causative etiologic agent and cardiac valves involved, 2 , 7 but these findings have not been evaluated recently.
There have been advances in the quality of veterinary diagnostic imaging, clinician awareness of the disease, and the ability of clients to pursue advanced diagnostic testing and treatment. Understanding prognosis and expected outcomes is a critical component of an owner's decision to pursue treatment. 8 Therefore, an updated evaluation of prognostic indicators for IE is needed. We aimed to describe the clinical features of dogs diagnosed with IE and determine factors associated with survival.
2. METHODS
2.1. Population selection
Electronic medical records from University of California, Davis Veterinary Medical Teaching Hospital, were searched for dogs diagnosed with endocarditis from 2005 to 2020. Medical records were evaluated to determine if the dog fulfilled criteria for a definitive diagnosis of IE based on modified Duke criteria (Table 1) by meeting 2 major criteria or 1 major and 2 minor criteria. 2 , 7 , 9 The medical history was reviewed for a previous diagnosis of diabetes mellitus, surgical implants such as bone plates or vascular implants, previously diagnosed malignant neoplasia confirmed by cytology or histopathology, and administration of any antimicrobial or immunosuppressive medication in the 30 days before diagnosis. Dogs were classified as having chronic kidney disease (CKD) if there was a previous diagnosis of CKD or if there were ultrasonographic renal changes consistent with CKD at the time of IE diagnosis.
TABLE 1.
Modified Duke criteria
| Major criteria | Minor criteria |
|---|---|
| Echocardiogram consistent with IE | Rectal temperature ≥39°C |
| Vegetative lesions | New or worsening heart murmur |
| Erosive lesion | Predisposing cardiac disease (subaortic stenosis) |
| Abscess | Evidence of thromboembolic disease |
| Greater than trivial valvular insufficiency | Evidence of secondary immune‐mediated disease |
| Microbiologic findings not meeting major criteria | |
| Positive blood cultures with | Positive Bartonella serology |
| ≥2 bottles with typical organism | Blood cultures not meeting major criteria |
| ≥3 bottles with common skin contaminant | |
| Persistent positive cultures over ≥12 hours |
Clinical data, including signalment, preexisting conditions, physical examination findings, concurrent diagnoses, diagnostic testing, imaging results, treatment, and outcome, were recorded from the medical record. The type and duration of antimicrobial administered was documented along with route of administration (IV or PO). Duration of hospitalization and non‐antimicrobial treatments also were recorded. Cost of hospitalization was recorded and adjusted using an average 2% inflation rate.
Echocardiography reports were reviewed for each dog. The presence, location, and subjective size of vegetative lesions were recorded for each patient. Additional diagnostic information was recorded when available including CBC, serum biochemistry panel, urinalysis, cardiac troponin I concentration (Immulite 2000 troponin I; Siemens Healthcare), thoracic radiographs, and abdominal ultrasound examination findings.
The institution protocol for collection of blood cultures utilized aseptic collection of 3 to 6 aliquots of blood (1‐10 mL) while wearing sterile gloves at 0‐, 10‐, and 60‐minute time points from 3 different anatomic locations. Whole blood was transferred to aerobic or anaerobic blood culture vials, incubated at 37°C, and subcultured on blood agar. Bacterial colonies were identified using a combination of biochemical testing and matrix‐assisted laser desorption ionization time‐of‐flight mass spectrometry analysis. Results from blood cultures were reviewed independently by 2 authors (K.L.R. and S.E.E.) and interpreted as positive when considered unlikely to represent growth of contaminants based on presence in multiple blood culture vials and speciation of a typical IE pathogen.
In some dogs, Bartonella serology for 3 Bartonella species (Bartonella vinsonii subsp. berkhoffii, B. clarridgeia, and B. henselae), Bartonella culture, and PCR all were performed as previously described. 1 Culture‐enriched, Bartonella alpha proteobacteria growth medium (BAPGM), PCR was performed in selected cases as previously described by a reference laboratory (Galaxy Diagnostics, Research Triangle Park, North Carolina). 10 Dogs with serologic titers >1:64 were recorded as positive. Dogs were determined to have presumed Bartonella as an etiologic agent of IE if (a) serologic titers were >1 : 512, (b) if Bartonella culture or PCR was positive, or (c) if serologic titers were positive and conventional blood cultures were negative.
Development of concurrent diseases was recorded for each patient. Acute kidney injury was defined as a 0.3 mg/dL increase in serum creatinine concentration during hospitalization or serum creatinine concentration above the normal reference interval in animals without a previous diagnosis of CKD. 11
Congestive heart failure was diagnosed by characteristic findings of distension of pulmonary vasculature and infiltrative pulmonary patterns consistent with cardiogenic pulmonary edema on thoracic radiographs identified by a board‐certified veterinary radiologist.
Thromboembolic disease was defined as (a) visualization of thrombosis on abdominal ultrasound examination or echocardiogram conducted by a board‐certified veterinary radiologist or cardiologist, respectively, (b) identification of thrombus on necropsy examination, (c) palpable identification of thrombosis on physical examination, (d) acute onset of neurologic signs with no other identified underlying cause, or (e) acute onset of tachypnea or respiratory distress in a patient with thoracic radiographs ruling out evidence of CHF or aspiration pneumonia with clinicopathologic evidence of thromboembolic disease. 12 , 13
Polyarthritis was defined as the presences of non‐degenerate neutrophilic inflammation in synovial fluid obtained by arthrocentesis as determined by a board‐certified veterinary clinical pathologist.
2.2. Definition of survival classification
Survival status was recorded at discharge from the hospital and a minimum of 1 month after diagnosis, regardless of the number of days spent in the hospital. Dogs were classified as survivors if they were alive at a minimum of 1 month after diagnosis or non‐survivors if the death was noted at any time after diagnosis and attributed to IE. Dogs that were lost to follow‐up before 1 month after diagnosis were categorized as an unknown outcome and censored in survival analysis.
2.3. Statistical analysis
Descriptive non‐parametric statistics were used to describe the population. A univariate exact logistic regression model was used to assess odds ratios (OR) to identify categorical factors associated with mortality. Factors that were assessed a priori included sex, neuter status, age, weight, preexisting conditions (neoplasia, surgical implants, immunosuppressive medications, antimicrobial administration in the preceding 30 days, or CKD), number of positive blood culture bottles (0, 1, 2, or ≥3), Bartonella or non‐Bartonella etiology, cardiac valves involved, and presence of clinicopathologic abnormalities (anemia, neutrophilia, thrombocytopenia, hyperbilirubinemia, azotemia, hypoalbuminemia, and hypoglycemia). Furthermore, therapeutic interventions were assessed for association with mortality including IV administration of antimicrobials, antithrombotic medications, or cardiac medications. Development of CHF, acute kidney injury, thromboembolic disease, neutrophilic polyarthritis, and hospitalization in the ICU also were tested a priori. Variables with a P < .05 in the univariable model were assessed using a backwards stepwise multivariable logistic regression analysis (statsmodel, Python3). 14 Median survival time (MST) from diagnosis was determined and Kaplan‐Meier survival curves were made for the total population and sub‐populations, including etiology (Bartonella vs non‐Bartonella), cardiac valves involved (mitral vs aortic), development of CHF, and administration of antithrombotic medications a priori, using commercial software (Prism 9.0.0, GraphPad). Survivors were censored on the last date of follow‐up. Significance was set at P < .05. A post hoc‐analysis assessing association of antimicrobial administration in the preceding 30 days with positive blood culture results was performed using a Fischer's exact test.
3. RESULTS
3.1. Population description
A medical record search of 120 150 dogs presented to University of California, Davis Veterinary Medical Teaching Hospital, from 2005 to 2020 identified 233 dogs with endocarditis in the clinical diagnosis field (Figure 1). After review of each medical record, 113 dogs fulfilled the modified Duke criteria for IE diagnosis. The distribution of major and minor Duke criteria findings is presented in Table 2. The prevalence of IE in this population was 0.09%. The IE case numbers by year are presented in Figure 2.
FIGURE 1.
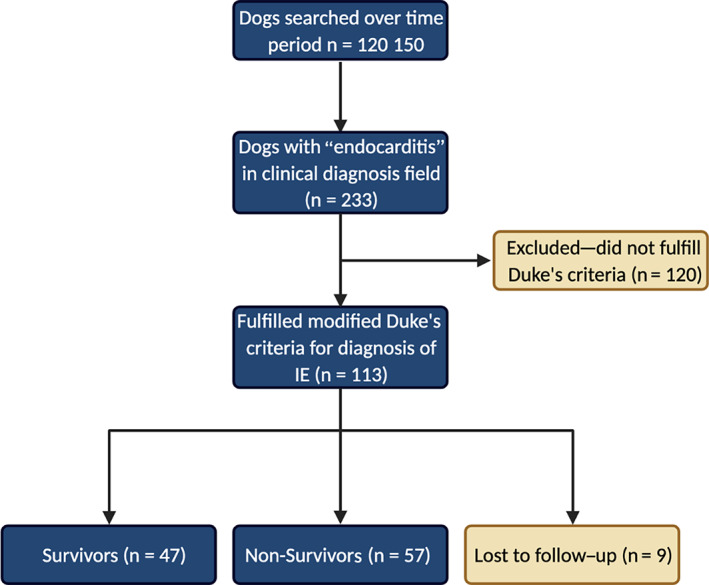
Consort diagram describing patient population. Created with BioRender.com
TABLE 2.
Characterization of modified Duke criteria findings in patient population
| Characteristic | Total population, n (%) | Survivors, n (%) | Non‐survivors, n (%) |
|---|---|---|---|
| Major criteria | |||
| Vegetative lesion | 106 (93.8) | 42 (89.4) | 55 (96.5) |
| Greater than trivial valvular insufficiency | 103 (91.1) | 44 (93.6) | 50 (87.7) |
| ≥2 positive blood culture (typical organism) | 29 (25.7) | 11 (23.4) | 17 (29.8) |
| ≥3 positive blood culture (skin commensal) | 8 (7.1) | 3 (6.4) | 4 (7.0) |
| Minor criteria | |||
| Fever | 59 (52.2) | 23 (48.9) | 32 (56.1) |
| New or worsening murmur | 82 (72.6) | 36 (76,6) | 41 (71.9) |
| Secondary disease process | 57 (50.4) | 19 (40.4) | 36 (63.2) |
| Microbiologic criteria not meeting major criteria | 17 (15.0) | 4 (8.5) | 13 (22.8) |
FIGURE 2.

Infective endocarditis diagnosis by year
Of dogs with IE, 73 (64.6%) of 113 were male and 97 (85.8%) of 113 were neutered, with a mean age of 8 ± 3.8 years and weight of 29.5 ± 15.2 kg (Table 3). Sporting (33/113; 29.2%) and herding (19/113; 16.8%) breeds were the most frequently affected purebred dogs. Mixed breed dogs accounted for 25 (22.1%) of 113 dogs with IE. Breed, neuter status, age, and weight were not significantly associated with mortality (Figure 3A).
TABLE 3.
Patient demographics
| Characteristic | Total population, n (%) | Survivors, n (%) | Non‐survivors, n (%) |
|---|---|---|---|
| Sex | |||
| Female | 4 (3.5) | 1 (2.1) | 2 (3.5) |
| Female spayed | 36 (31.9) | 16 (34.0) | 18 (31.6) |
| Male | 12 (10.6) | 3 (6.4) | 7 (12.3) |
| Male neutered | 61 (54.0) | 27 (57.4) | 30 (52.6) |
| Breed group | |||
| Herding | 19 (16.8) | 9 (19.1) | 9 (15.8) |
| Hound | 7 (6.2) | 4 (8.5) | 2 (3.5) |
| Mix | 25 (22.1) | 9 (19.1) | 13 (22.8) |
| Non‐sporting | 3 (2.7) | 1 (2.1) | 2 (3.5) |
| Other | 2 (1.8) | 0 (0) | 2 (3.5) |
| Sporting | 33 (29.2) | 16 (34.0) | 16 (28.1) |
| Terrier | 7 (6.2) | 4 (8.5) | 3 (5.3) |
| Toy | 7 (6.2) | 1 (2.1) | 3 (5.3) |
| Working | 10 (8.9) | 3 (6.4) | 7 (12.3) |
| Age category | |||
| Less than 3 years | 16 (14.2) | 7 (14.8) | 8 (14.0) |
| 3 to <6 years | 20 (17.7) | 9 (19.1) | 9 (18.8) |
| 6 to <9 years | 29 (25.7) | 9 (19.1) | 19 (33.3) |
| 9 to <12 years | 29 (25.7) | 13 (27.7) | 14 (24.6) |
| 12 years and older | 19 (16.8) | 9 (19.1) | 7 (12.3) |
| Weight category | |||
| <10 kg | 14 (12.7) | 5 (10.6) | 5 (9.1) |
| 10 to <20 kg | 7 (6.4) | 2 (4.3) | 5 (9.1) |
| 20 to <30 kg | 32 (29.1) | 18 (38.3) | 14 (25.5) |
| 30 to <40 kg | 39 (35.5) | 16 (34.0) | 22 (40.0) |
| 40 kg and above | 18 (16.4) | 6 (12.8) | 9 (16.4) |
| Preexisting conditions | |||
| Diabetes mellitus | 2 (1.8) | 2 (4.3) | 0 (0) |
| Surgical implants | 13 (11.5) | 5 (10.6) | 6 (10.5) |
| Neoplasia | 11 (9.7) | 6 (12.8) | 4 (7.0) |
| Immunosuppression | 27 (23.9) | 14 (29.8) | 12 (21.1) |
| Antibiotics in past 30 days | 80 (80.8) | 34 (72.3) | 41 (71.9) |
| Chronic kidney disease | 21 (18.6) | 9 (19.2) | 8 (14.0) |
FIGURE 3.
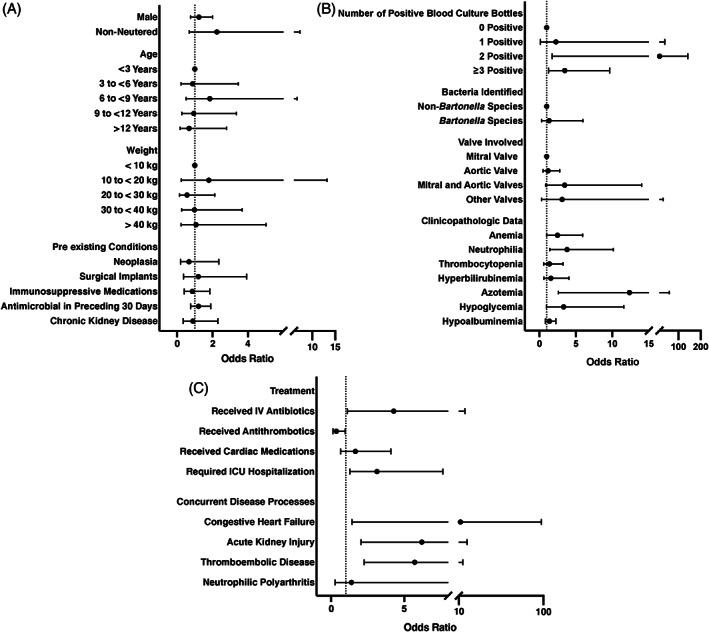
Univariate odds ratios for association with mortality. (A) Patient demographics. (B) Diagnostic findings. (C) Treatment and concurrent diagnosis
Preexisting medical conditions were identified in 58 of 113 dogs, with evidence of CKD in 21 (20.5%) of 102, surgical implants in 13 (11.5%) of 113, neoplasia in 11 (9.7%) of 113, and diabetes mellitus in 2 (1.7%) of 133 dogs. Immunosuppressive medications were being administered to 27 (23.9%) of 113 dogs at admission. Antimicrobial medications had been prescribed in the preceding 30 days for 80 (70.8%) of 113 dogs. Congenital subvalvular aortic stenosis was not diagnosed in any dogs. None of these preexisting conditions or drugs were significantly associated with mortality (Figure 3A; Table S1).
Survival to discharge was observed in 79/113 (69.9%) dogs. Of the 113 dogs, 47 (41.6%) survived beyond 1 month after diagnosis, 57 (50.4%) died, and 9 (8.0%) were lost to follow‐up. Death or euthanasia as a result of IE was reported between 0 and 1420 days after diagnosis. Median survival time was 72 days (Figure 4).
FIGURE 4.
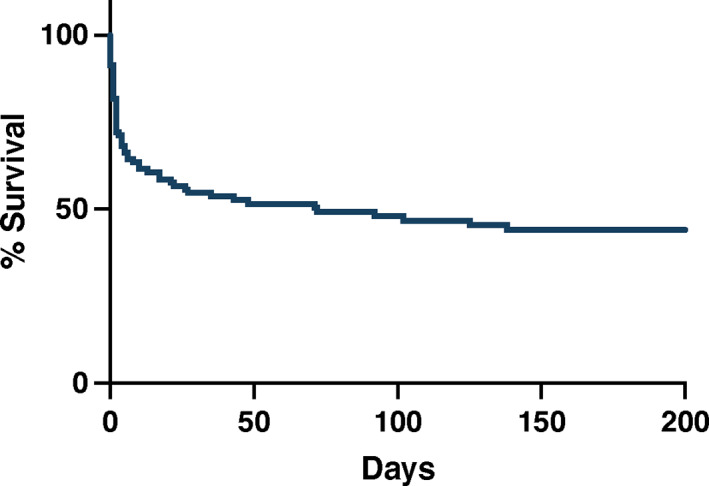
Overall survival of dogs diagnosed with infective endocarditis
3.2. Diagnosis of infective endocarditis
Modified Duke criteria for the diagnosis of IE were fulfilled in 113 of 233 dogs (48.5%) enrolled in the study (Table 2). Echocardiograms were performed on 109 (96.4%) of 113 dogs. Infective endocarditis was confirmed on necropsy evaluation in 27 (23.9%) of 113 dogs, which included the 4 dogs that did not have echocardiograms performed. Major diagnostic criteria that were observed included vegetative lesions in 106 dogs, more than trivial valvular insufficiency in 103 dogs, ≥2 blood culture bottles with a typical organism in 29 dogs, and ≥3 blood cultures bottles with a skin contaminant in 8 dogs. Minor criteria that were observed included fever in 59 dogs, new or worsening heart murmur in 82 dogs, evidence of a secondary disease in 11 dogs with neutrophilic polyarthritis, 1 dog with immune‐mediated hemolytic anemia, and 45 dogs with thromboembolic disease, and microbiologic criteria not meeting major criteria in 17 dogs.
3.3. Clinicopathologic findings
Complete blood count results were available from the time of diagnosis in 91 dogs. Anemia (HCT < 40%) was noted in 53 (58.2%) of 91 dogs, neutrophilia (> 11 000/μL) in 63 (69.2%) of 91, and band neutrophils in 48 (52.7%) of 91. A serum biochemistry panel was available in 78 dogs. Increased serum creatinine concentration (>1.4 mg/dL) was noted in 24 (30.8%) of 78 dogs, hypoglycemia (<80 mg/dL) in 25 (32.1%) of 78, hyperbilirubinemia (>0.2 mg/dL) in 31 (39.7%) of 78, and hypoalbuminemia (<3.4 g/dL) in 67 (85.9%) of 78. Urinalysis was performed in 44 dogs, 37 (84.1%) had proteinuria and 6 (13.6%) had bacteriuria on urine sediment examination. Aerobic urine cultures were performed in 56 dogs and 14 (25%) were positive for bacterial growth. Six of the 14 dogs with positive urine cultures had the same species of bacteria isolated from urine and blood cultures, 5 dogs had negative blood cultures but positive urine cultures, 2 dogs had different organisms isolated from blood cultures and urine culture, and 1 dog had a positive urine culture but no blood cultures performed. Cardiac troponin I concentration was measured in 10 dogs and ranged from 0.05 to 62.2 ng/mL (median, 0.96 ng/mL). Nine of 10 dogs had concentrations above the reference range (0.09‐0.17 ng/mL). The presence of neutrophilia and azotemia at diagnosis was associated with mortality with OR 3.8 (95% CI, 1.4‐10.1) and OR 12.4 (95% CI, 2.6‐59.0), respectively (Figure 3B). Azotemia was retained as a significant predictor of mortality in the multivariate model (P = .02; Table 5).
TABLE 5.
Multivariate model
| Characteristic | Odds ratio (95% CI) | P‐value |
|---|---|---|
| Signalment and initial diagnosis | ||
| 2 or more positive blood culture bottles | 1.8 (1.2‐2.8) | .01 |
| Azotemia | 5.6 (1.02‐31.1) | .02 |
| Treatment and concurrent disease | ||
| Thromboembolic disease | 6.2 (1.9‐20.2) | .002 |
| Received antithrombotics | 0.11 (0.03‐0.48) | .003 |
| Required ICU hospitalization | 4.2 (1.3‐12.2) | .02 |
| Congestive heart failure | 29.2 (2.3‐367.2) | .01 |
3.4. Cardiac and echocardiographic findings
Heart murmurs were noted in 86 (76.1%) of 113 dogs and were classified as grades 1, 2, 3, 4, and 5/6 in 2, 28, 29, 22, and 5 dogs, respectively. Infective endocarditis‐associated pathology, including vegetative or erosive lesions, was identified on the mitral valve in 58 (51.3%) of 113 dogs, on the aortic valve in 37 (32.7%) of 113 dogs, on both the mitral and aortic valve in 16 (14.1%) of 113 dogs, or on the tricuspid valve in 2 (1.8%) of 113 dogs. The MST for dogs with only mitral valve lesions was not reached. The MSTs for dogs with only aortic valve disease or those with both valves involved were 102 and 43 days, respectively (Figure 6A). The number and type of valves involved were not significantly correlated with outcome in the univariate model (Figure 3B).
FIGURE 6.
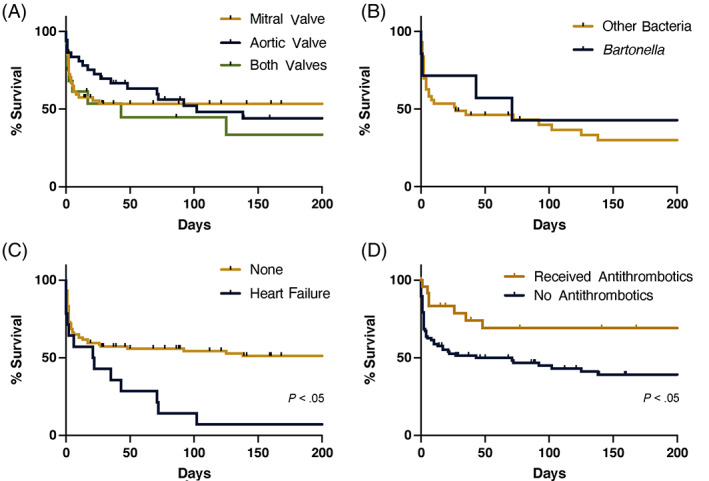
Survival curves of population subsets. (A) Cardiac valves involved. (B) Bartonella vs alternative bacterial species. (C) Diagnosis of left‐sided congestive heart failure. (D) Treatment with antithrombotics
3.5. Microbiologic data
Microbiologic blood cultures were performed in 89 (78.8%) of 113 dogs, 21 of which were aerobic bacterial cultures alone, and 68 that included aerobic and anaerobic bacterial cultures (Table 4). One or more blood culture bottles were positive for bacterial growth in 41 (46.1%) of 89 dogs. The number of blood culture specimens obtained from each dog ranged from 1 to 6, with a median of 5 bottles. In dogs with bacterial growth noted on blood culture, the proportion of positive blood culture bottles per dog ranged from 17% to 100% (median, 100%). Five of 27 dogs evaluated had positive bacterial cultures of the affected cardiac valves at the time of necropsy. Administration of antimicrobials in the preceding 30 days was not associated with negative growth on blood culture (P = .17). Bacterial species identified in dogs with IE are summarized in Figure 5. Of dogs that had blood cultures performed, those that had 2 or ≥3 positive blood culture bottles had increased odds of mortality with OR of 15.8 (95% CI, 1.7‐142.6) and 3.5 (95% CI, 1.3‐9.6) respectively (Figure 3B) and significance was retained in the multivariate model (P = .009; Table 5).
TABLE 4.
Microbiologic diagnostics
| Characteristic | Total population | Survivors | Non‐survivors |
|---|---|---|---|
| Blood cultures performed | |||
| Aerobic bacterial culture, n (%) | 21 (18.6) | 9 (19.1) | 9 (15.8) |
| Aerobic and anaerobic bacterial cultures, n (%) | 68 (60.2) | 35 (74.5) | 30 (52.6) |
| Blood cultures not performed, n (%) | 24 (21.2) | 3 (6.4) | 18 (31.6) |
| Blood culture characteristics per patient | |||
| No. of bottles collected (median, range) | 5 (1–6) | 5 (2–6) | 4 (1–6) |
| No. of bottles with bacterial growth (median, range) | 0 (0–6) | 0 (0–6) | 2 (0–6) |
| % positive bottles in dogs with bacterial growth (median, range) | 100 (17‐100) | 100 (17‐100) | 100 (17‐100) |
| Blood culture bacterial growth for population | |||
| No. of dogs with 1 positive bottle | 3 | 1 | 2 |
| No. of dogs with 2 positive bottles | 7 | 1 | 5 |
| No. of dogs with 3 or more positive bottles | 31 | 11 | 19 |
| No. of dogs with necropsy obtained positive valve cultures | 5 | 0 | 5 |
| Bartonella specific testing (no. of positive/no. of performed) | |||
| PCR | 0/6 | 0/4 | 0/1 |
| Serology | 6/36 | 2/22 | 4/12 |
| Culture | 0/14 | 0/9 | 0/4 |
| BAPGM‐enriched PCR | 1/2 | 1/1 | 0/0 |
FIGURE 5.
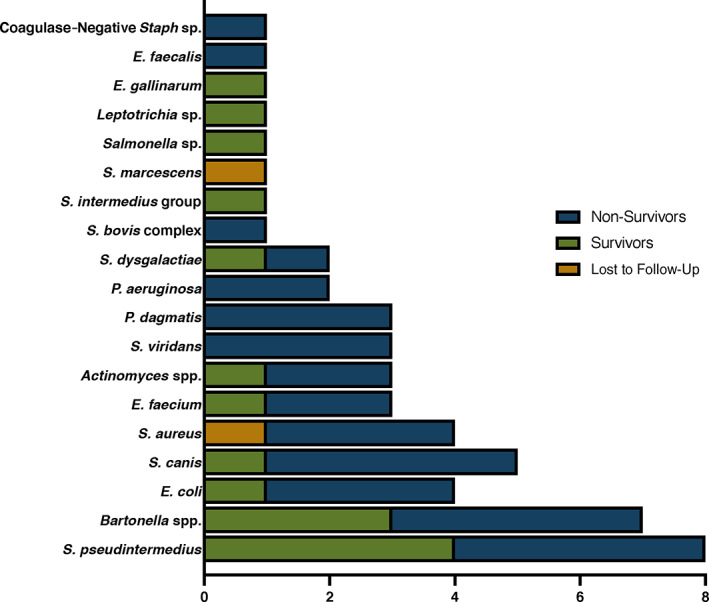
Bacterial species identified as causative agent of infective endocarditis
Bartonella testing was performed in 46 of 113 dogs and it was diagnosed as a presumed bacteriologic agent of IE in 7 (15.2%) dogs. Serology was positive in 6 of 36 dogs. Endpoint antibody titers were available for 5 dogs and ranged from 1 : 64 to 1 : 4096 with a median titer of 1 : 1024. One dog did not have an endpoint titer performed, and the results were reported as >1 : 64. The 3 dogs without a documented antibody titer >1 : 512 had concurrent negative microbiologic blood cultures. A BAPGM‐enriched PCR was positive in 1 of 2 dogs tested. Blood quantitative PCR and culture were performed in 6 and 14 dogs, respectively, and all were negative. The MST for dogs diagnosed with Bartonella IE was 71 days compared to 27 days in dogs diagnosed with IE associated with other bacterial species, and survival curves were not significantly different (P = .6; Figure 6B).
3.6. Treatment and development of subsequent disease processes
Antimicrobial treatment was initiated in 99 of 113 dogs (Table S2). The 14 dogs that were not treated were all non‐survivors. Of the dogs treated with antimicrobials, 82 of 99 were treated initially with parenterally administered antimicrobials for a median of 6 days (range, 1‐24 days). Of these dogs, 53 then were continued on PO antimicrobials after discharge from the hospital for a median of 64 days (range, 2‐530 days). Of the 47 dogs that survived, 36 initially were treated with parenteral antimicrobials for a median of 7 days, and then continued on PO antimicrobials for a median of 90 days (range, 21‐530 days). Eleven of the 47 dogs that survived were treated with PO antimicrobials (median, 64 days; range, 2‐135 days) without first receiving antimicrobials parenterally. Dogs in the non‐survivor group that were treated with antimicrobials generally were continued on treatment until the time of death. Of the dogs treated with antimicrobials, 83 of 99 were hospitalized, and 34 of 83 were in the intensive care unit. The median length and cost of hospitalization were 2 days (range, 0‐50 days) and $4186 USD (range, $163‐$70618) respectively. Treatment with IV antimicrobials (OR, 4.3; 95% CI, 1.1‐16.5) and ICU hospitalization (OR, 3.1; 95% CI, 1.3‐7.6) both were associated with mortality (Figure 3C), and the association of ICU hospitalization with mortality was retained in the multivariate model (P = .02; Table 5).
Left‐sided CHF was diagnosed in 16 (14.2%) of 113 dogs with IE. These included 5 dogs with mitral valve lesions, 6 with aortic valve lesions, 4 with lesions on both the mitral and aortic valves, and 1 with a lesion on the tricuspid valve. Arrhythmias were noted in 21 (21.8%) of 96 dogs that had ECG performed. These included 8 dogs with premature ventricular complexes, 4 dogs with supraventricular tachycardia, 3 dogs with accelerated idioventricular rhythm, 2 dogs with ventricular tachycardia, and 1 dog each with atrial premature complexes, first degree atrioventricular block, atrial fibrillation, and atrial bigeminy. Cardiac medications were administered to 29 of the enrolled dogs. Dogs that were diagnosed with CHF had a MST of 21.5 days, where dogs without CHF had a MST of 1420 days (P < .05; Figure 6C). In dogs that were treated for IE, diagnosis of CHF was associated with mortality (OR, 11.83; 95% CI, 1.43‐97.8; Figure 3C) and CHF was retained as significant in the multivariate analysis (P = .01; Table 5).
Thromboembolic disease was diagnosed in 45 dogs and was associated with mortality (OR, 5.7; 95% CI, 2.3‐14.4). Thromboembolic disease was diagnosed on necropsy in 17 dogs and abdominal ultrasound examination in 16 dogs. Nine dogs were diagnosed with thromboembolic disease based on physical examination; 6 with compatible central nervous system (CNS) signs and 3 with distal limb edema or palpable thrombosis of a peripheral vessel. Two dogs were diagnosed with thromboembolic disease based on identification of thrombi on echocardiographic examination. Presumptive pulmonary thromboembolism was diagnosed in 2 dogs with acute onset tachypnea and no evidence of other pulmonary disease such as cardiogenic pulmonary edema or aspiration pneumonia on thoracic radiographs. One dog with presumptive pulmonary thromboembolism also had splenic venous thrombosis identified on abdominal ultrasound. The second dog with presumptive pulmonary thromboembolism had the diagnosis supported by an increased d‐dimer concentration and low antithrombin concentration.
Anti‐thrombotic medications (Table S3) were administered to 24 dogs of the 99 dogs in which treatment for IE was initiated, and use was significantly associated with survival (OR, 0.36; 95% CI, 0.13‐0.97; Figures 3C and 6D). Twelve of the dogs started on anti‐thrombotic medications also were diagnosed with thromboembolic disease. In the multivariate analysis, diagnosis of thromboembolic disease was associated with mortality (P = .002) and treatment with antithrombotics was associated with survival (P = .003; Table 5). Acute kidney injury was documented in 29 dogs and was significantly associated with mortality (OR, 6.2; 95% CI, 2.0‐18.8). Neutrophilic polyarthritis was documented in 11 of 19 dogs that had arthrocentesis performed and was not associated with mortality.
4. DISCUSSION
Our large‐scale retrospective study identified an increasing prevalence in the diagnosis of IE at an academic referral hospital and provides an updated retrospective description of dogs diagnosed with the disorder. Similar to a previous study, 2 50% of the dogs diagnosed with IE died or were euthanized because of the disease. However, MST for all dogs in our study was 72 days, approximately 33% longer than in the previous study. 2 This finding could represent earlier disease recognition, increased willingness of clients to consent to treatment, or possibly, improvements in therapeutic strategies, despite the fact that it was not reflected in overall mortality. Importantly, administration of anti‐thrombotic medication was strongly associated with survival, whereas diagnostic findings of CHF, azotemia, or thromboembolic disease were associated with mortality.
Of all dogs examined at our institution, 0.09% were diagnosed with IE. The number of diagnosed cases in our study steadily increased over the study period and the overall incidence was higher than previously reported. 2 This increase in prevalence at our institution could represent increased screening or recognition of disease as a result of clinician education or willingness of clients to pursue advanced diagnostic tests such as an echocardiogram or blood cultures. Although not specifically addressed in our study, it could also reflect an increase in possible predisposing conditions, such as the high use of potent antimicrobials before admission or increased diagnosis of immune‐mediated disease requiring use of immunosuppressant medications. A similar increase in diagnosis also has been noted in people, with a 2.4% increase annually between 1998 and 2009. 15
In our study, signalment of dogs with IE was similar to that reported in previous studies with male, large breed dogs predominating, but patient demographics or preexisting conditions were not predictive of treatment outcome. Congenital subaortic stenosis was not diagnosed in any of the dogs with IE in our study. The majority of dogs (70%) in our study had received antimicrobials in the month before the diagnosis of IE, but often the rationale for administration of the medication was not noted in the medical record. This practice could represent treatment of an infection that subsequently served as a nidus of IE infection or could reflect empirical antimicrobial treatment in an ill dog that had yet to be diagnosed with IE.
Dogs were diagnosed with IE based on fulfillment of modified Duke criteria or upon necropsy examination. The veterinary literature describes several variations of the modified Duke criteria. One of these variations is inclusion of body weight >15 kg as a minor criterion. 7 , 9 This criterion was not utilized in our study in an attempt to increase the stringency of diagnosis, but these variations emphasize the need for uniform diagnostic criteria in veterinary medicine and the difficulties associated antemortem diagnosis of IE. The most commonly observed major criterion of diagnosis was an echocardiographic lesion consistent with endocarditis. Transthoracic echocardiography is recommended as the first line imaging modality for people suspected of having IE, but false negative findings can be observed and more advanced imaging modalities such as transesophageal echocardiography are pursued in some cases. 16 This approach is complicated by the need for general anesthesia to perform this procedure. Bacterial blood cultures were performed in 76% of the enrolled dogs, and an etiologic agent thought to be associated with IE was identified in 51 (59%) of 86 dogs. Identification of bacteria in ≥2 or more blood culture specimens was associated with mortality. This finding could indicate that dogs with higher bacterial loads in the bloodstream have more serious disease, but it also could reflect the likelihood that clinicians often obtain blood cultures in more severely ill animals.
Gram‐positive bacterial organisms predominated in this population, as has been reported previously in dogs and people. 7 , 17 Culture‐negative endocarditis was diagnosed in 40% of the dogs in which blood cultures were performed, which is similar to previous studies in veterinary and human medicine. 7 , 18 High rates of empirical antimicrobial administration before obtaining blood cultures or infection with fastidious organisms, such as Bartonella, likely contribute to this phenomenon.
Bartonella spp. was implicated as an etiologic agent in 7 cases, 4 of which survived and 3 of which died. The diagnostic criteria utilized in this study were similar to those used in previous studies. 7 Three dogs in our population had an antibody titers <1 : 512, which has been proposed as a potential diagnostic cutoff for the diagnosis of bartonellosis. 1 The MST and overall survival for dogs diagnosed with Bartonella IE were not different from dogs diagnosed with other etiologic agents. Only a minority of the population was tested for bartonellosis however, and therefore the prevalence of disease is likely underestimated. The MST noted in our population (71 days) is in contrast to a previous report that associated Bartonella IE in dogs with a poorer prognosis, with a MST of 3 days. 1 This poorer prognosis has been attributed to the fact that Bartonella IE is more commonly associated with lesions on the aortic valve, which historically has been associated with a poorer outcome. 7 Indeed, all dogs diagnosed with Bartonella IE in our study did have aortic valve involvement. However, the cardiac valves involved in the disease process were not strong predictors of survival in our population. The MST for dogs with aortic valve IE was 102 days in our population, whereas the MST for dogs with mitral valve IE was not reached. This observation may reflect earlier detection of disease or willingness of owners to pursue treatment. Dogs that have multiple valves involved fared the worst, with a MST of 43 days. This observation could represent diagnosis later in the disease process with more structural changes on the cardiac valves, thus worsening the prognosis.
Antimicrobial treatment was pursued in 87% of dogs diagnosed with IE. Antimicrobial treatment was not standardized in our retrospective study, and comparisons between outcome for dogs that received parenteral treatment and PO treatment were difficult because of multiple confounding factors. Dogs that received antimicrobials parenterally were more likely to die or be euthanized as a result of IE, which likely reflects a more severe clinical course in these dogs that required intensive care treatment and thus parenteral administration of medications. Effective antimicrobial treatment of IE results in sterilization of the vegetative lesion and requires use of a bactericidal antibiotic that will effectively penetrate the biofilm and thrombotic material on the valve. 19 In human medicine, duration of antimicrobial treatment ranges from 2 to 6 weeks depending on the presence of a prosthetic valve. 19 The optimal duration of treatment in dogs has not been established and is reflected in the wide range of treatment durations in our study.
Documentation of an increased serum creatinine concentration either at the time of initial diagnosis or during treatment was strongly associated with mortality. This finding has been previously reported in the veterinary and human medical literature. 2 , 19 Acute kidney injury in dogs with IE is likely multifactorial and could result from sepsis, hypotension, decreased glomerular filtration rate secondary to heart failure, thromboembolic disease, or administration of nephrotoxic medications such as aminoglycosides. Prospective investigation of the causes for acute kidney injury is necessary to allow implementation of strategies that avoid this complication and improve overall survival.
The subset of dogs diagnosed with CHF had the lowest overall survival. In this population, 14.2% of dogs with IE were diagnosed with subsequent CHF. This finding is in contrast to previous studies that have reported rates of CHF of 30%. 2 This difference may reflect diagnosis earlier in the course of disease before severe and irreversible valvular damage or development of better management strategies for patients at risk for CHF. In people with IE, development of CHF is an indication for placement of a prosthetic valve, because they are often refractory to medical treatment. 20 This finding also has been documented in a cat with endocarditis that experienced marked improvement in the infectious component of the disease, but the resultant structural valvular damage resulted in refractory CHF and euthanasia. 4
Thromboembolic disease has been recognized in up to 80% of dogs with IE. 1 , 5 In our study, diagnosis of thromboembolic disease was a predictor of mortality. Platelet aggregation is critical in the formation of IE lesions, and administration of anti‐thrombotic medications can decrease lesion weight and decrease bacterial loads within vegetative lesions. 21 , 22 Dogs that were treated with anti‐thrombotic medications had markedly better outcomes, but some of these dogs might have been treated with these medications only after discovering thromboembolic disease. Administration of antiplatelet medications has been shown to benefit people diagnosed with IE. Therefore, further assessment of coagulation dysfunction and treatment is needed to optimize treatment of dogs with IE. 23
Retrospective case studies have inherent limitations, including care and diagnostic testing that were not standardized and the potential for missing case data in the medical record. Additionally, confounding factors may have that impacted the risk factors identified in our study, including owners' willingness to treat their pets in light of previously identified negative prognostic indicators. Furthermore, our study spanned 15 years during which improvements in diagnostic capabilities changed and perhaps improved the sensitivity of diagnosing IE. Lastly, the small sample size for some assessed variables may have led to type‐II statistical errors in our evaluation, preventing discovery of potential risk factors in our population.
In conclusion, we determined that the prevalence of IE increased throughout the study period and carried a guarded prognosis, with an overall survival rate of approximately 50%. The demographics of affected dogs were similar to previous studies with large breed, male dogs predominating, and typical bacteria were involved in the disease process more often than Bartonella. A thorough echocardiographic assessment was integral to the diagnosis in most cases. Risk factors associated with mortality included the development of CHF, thromboembolic disease, and acute kidney injury, similar to previous studies. Interestingly, neither the valve affected nor the bacterial organism identified in our population was correlated with outcome. Our study also found that administration of anti‐thrombotic medications was strongly associated with survival, indicating that further investigation into the role of anti‐thrombotic medications in the treatment of IE should be undertaken.
CONFLICT OF INTEREST DECLARATION
Authors declare no conflict of interest.
OFF‐LABEL ANTIMICROBIAL DECLARATION
Many antimicrobials used to treat infective endocarditis are done so off‐label.
INSTITUTIONAL ANIMAL CARE AND USE COMMITTEE (IACUC) OR OTHER APPROVAL DECLARATION
Authors declare no IACUC or other approval was needed.
HUMAN ETHICS APPROVAL DECLARATION
Authors declare human ethics approval was not needed for this study.
Supporting information
Table S1. Potential risk factors for survival in dogs with IE.
Table S2. Antimicrobial treatment administered to dogs with IE.
Table S3. Antithrombotic treatment administered to dogs with IE.
ACKNOWLEDGMENT
No funding was received for this study.
Reagan KL, Visser LC, Epstein SE, Stern JA, Johnson LR. Outcome and prognostic factors in infective endocarditis in dogs: 113 cases (2005‐2020). J Vet Intern Med. 2022;36(2):429‐440. doi: 10.1111/jvim.16380
REFERENCES
- 1. MacDonald KA, Chomel BB, Kittleson MD, et al. A prospective study of canine infective endocarditis in northern California (1999–2001): emergence of Bartonella as a prevalent etiologic agent. J Vet Intern Med. 2004;18:56‐64. [DOI] [PubMed] [Google Scholar]
- 2. Sykes JE, Kittleson MD, Chomel BB, MacDonald KA, Pesavento PA. Clinicopathologic findings and outcome in dogs with infective endocarditis: 71 cases (1992–2005). J Am Vet Med Assoc. 2006;228:1735‐1747. [DOI] [PubMed] [Google Scholar]
- 3. Peddle G, Sleeper MM. Canine bacterial endocarditis: a review. J Am Anim Hosp Assoc. 2007;43:258‐263. [DOI] [PubMed] [Google Scholar]
- 4. Wood J, Reagan KL, Gunther‐Harrington C, Sykes JE. Identification of Streptococcus suis in a cat with endomyocarditis. J Feline Med Surg Open Rep. 2021;7:20551169211012346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cook LB, Coates JR, Dewey CW, Gordon S, Miller MW, Bahr A. Vascular encephalopathy associated with bacterial endocarditis in four dogs. J Am Anim Hosp Assoc. 2005;41:252‐258. [DOI] [PubMed] [Google Scholar]
- 6. Ellison GW, King RR, Calderwood‐Mays M. Medical and surgical management of multiple organ infarctions secondary to bacterial endocarditis in a dog. J Am Vet Med Assoc. 1988;193:1289‐1291. [PubMed] [Google Scholar]
- 7. Sykes JE, Kittleson MD, Pesavento PA, Byrne BA, MacDonald KA, Chomel BB. Evaluation of the relationship between causative organisms and clinical characteristics of infective endocarditis in dogs: 71 cases (1992‐2005). J Am Vet Med Assoc. 2006;228:1723‐1734. doi: 10.2460/javma.228.11.1723 [DOI] [PubMed] [Google Scholar]
- 8. Brockman BK, Taylor VA, Brockman CM. The price of unconditional love: consumer decision making for high‐dollar veterinary care. J Bus Res. 2008;61:397‐405. doi: 10.1016/j.jbusres.2006.09.033 [DOI] [Google Scholar]
- 9. MacDonald K. Infective endocarditis in dogs: diagnosis and therapy. Vet Clin Small Anim Prac. 2010;40:665‐684. [DOI] [PubMed] [Google Scholar]
- 10. Pérez C, Maggi R, Diniz P, et al. Molecular and serological diagnosis of Bartonella infection in 61 dogs from the United States. J Vet Intern Med. 2011;25:805‐810. [DOI] [PubMed] [Google Scholar]
- 11. Cowgill L. IRIS guideline recommendations for grading of AKI in dogs and cats, http://www.iris-kidney.com/pdf/4_ldc-revised-grading-of-acute-kidney-injury.pdf. 2016. Accessed June 9, 2021.
- 12. Johnson LR, Lappin MR, Baker DC. Pulmonary thromboembolism in 29 dogs: 1985–1995. J Vet Intern Med. 1999;13:338‐345. [DOI] [PubMed] [Google Scholar]
- 13. Epstein SE, Hopper K, Mellema M, et al. Diagnostic utility of D‐dimer concentrations in dogs with pulmonary embolism. J Vet Intern Med. 2013;27:1646‐1649. [DOI] [PubMed] [Google Scholar]
- 14. Seabold S, Perktold J. Statsmodels: econometric and statistical modeling with python. In: Proceedings of the 9th Python in Science Conference 2010, p. 61. Austin, TX.
- 15. Bor DH, Woolhandler S, Nardin R, Brusch J, Himmelstein DU. Infective endocarditis in the US, 1998–2009: a nationwide study. PLoS ONE. 2013;8:e60033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Baddour LM, Wilson WR, Bayer AS, et al. Infective endocarditis in adults: diagnosis, antimicrobial therapy, and management of complications: a scientific statement for healthcare professionals from the American Heart Association. Circulation. 2015;132:1435‐1486. [DOI] [PubMed] [Google Scholar]
- 17. Murdoch DR, Corey GR, Hoen B, et al. Clinical presentation, etiology, and outcome of infective endocarditis in the 21st century: the International Collaboration on Endocarditis–Prospective Cohort Study. Arch Intern Med. 2009;169:463‐473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Fournier P‐E, Thuny F, Richet H, et al. Comprehensive diagnostic strategy for blood culture‐negative endocarditis: a prospective study of 819 new cases. Clin Infect Dis. 2010;51:131‐140. [DOI] [PubMed] [Google Scholar]
- 19. Habib G, Hoen B, Tornos P, et al. Guidelines on the prevention, diagnosis, and treatment of infective endocarditis (new version 2009): The task force on the prevention, diagnosis, and treatment of infective endocarditis of the european society of cardiology (ESC). Eur. Heart J. 2009;30(19):2369–2413. 10.1093/eurheartj/ehp285 [DOI] [PubMed] [Google Scholar]
- 20. Tornos P, Iung B, Permanyer‐Miralda G, et al. Infective endocarditis in Europe: lessons from the Euro heart survey. Heart. 2005;91:571‐575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Jung C‐J, Yeh C‐Y, Shun C‐T, et al. Platelets enhance biofilm formation and resistance of endocarditis‐inducing streptococci on the injured heart valve. J Infect Dis. 2012;205:1066‐1075. doi: 10.1093/infdis/jis021 [DOI] [PubMed] [Google Scholar]
- 22. Kupferwasser LI, Yeaman MR, Shapiro SM, et al. Acetylsalicylic acid reduces vegetation bacterial density, hematogenous bacterial dissemination, and frequency of embolic events in experimental Staphylococcus aureus endocarditis through antiplatelet and antibacterial effects. Circulation. 1999;99:2791‐2797. [DOI] [PubMed] [Google Scholar]
- 23. Anavekar NS, Tleyjeh IM, Anavekar NS, et al. Impact of prior antiplatelet therapy on risk of embolism in infective endocarditis. Clin Infect Dis. 2007;44:1180‐1186. doi: 10.1086/513197 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Potential risk factors for survival in dogs with IE.
Table S2. Antimicrobial treatment administered to dogs with IE.
Table S3. Antithrombotic treatment administered to dogs with IE.


