Abstract
Background
The repeated administration of high doses of gabapentin may provide better analgesia in horses than current clinical protocols.
Hypothesis and Objectives
Administration of gabapentin at 40 and 120 mg/kg PO q 12 h for 14 days will not alter serum biochemistry findings or cause adverse effects. Our objectives were to evaluate the effect of gabapentin on serum biochemistry, physical examination, and plasma pharmacokinetics of gabapentin.
Animals
Six healthy adult mares.
Methods
Horses received 40 and 120 mg/kg of gabapentin orally q 12 h for 14 days. Horses were examined and scored for ataxia and sedation daily. Serum biochemistry variables were analyzed before treatment and days 7 and 14 after gabapentin administration. Plasma disposition of gabapentin was evaluated after the first and last drug administration. Pharmacokinetic parameters were estimated using noncompartmental analysis.
Results
No changes occurred in physiologic or biochemical variables. Median (range) maximal plasma gabapentin concentrations (μg/mL) after the last dose (day 15) were 7.6 (6.2‐11) and 22 (14‐33) for 40 mg/kg and 120 mg/kg doses respectively. Maximal concentration of gabapentin was reached within 1 hour after drug administration. Repeated administration of gabapentin resulted in a median (range) area under the curve (AUC0‐12 hours) last/first dose ratio of 1.5 (1.00‐2.63) and 2.92 (1.4‐3.8) for the 40 and 120 mg/kg regimens, respectively.
Conclusion and Clinical Importance
Our results suggest that horses tolerate gabapentin up to 120 mg/kg PO q 12 h for 14 days. The analgesic effect of the dosage regimens evaluated in our study warrants further research.
Keywords: analgesia, equine, neuropathic pain, plasma disposition, safety
Abbreviations
- ALKP
alkaline phosphatase
- AUC
area under the curve
- BUN
blood urea nitrogen
- CK
creatinine kinase
- C max
concentration maximum
- FDA
food and drug administration
- GGT
gamma‐glutamyl transferase
- HPLC
high performance liquid chromatography
1. INTRODUCTION
Limited scientific information exists regarding the effective and safe dosing of gabapentin in horses. Based on clinical experience and anecdotal reporting, 1 the most commonly used dosage of gabapentin is either 10 or 20 mg/kg 2 , 3 which by itself is often ineffective at controlling pain from laminitis or osteoarthritis. Higher dosages or more frequent administration may result in better drug exposure and efficacy at managing pain. 4 The pharmacodynamics and pharmacokinetics of gabapentin have not been elucidated, and results have been variable in dogs and cats, potentially because of inadequate dosing. 5 , 6 , 7 , 8 , 9 , 10 , 11 , 12 , 13 , 14
Repeated administration of relatively high doses of gabapentin may result in concentration‐dependent adverse effects. In humans, somnolence, dizziness and ataxia are frequent adverse effects of repeated dosing of gabapentin. 15 , 16 , 17 , 18 , 19 Although rare, more serious adverse consequences can occur, including cardiovascular effects (hypotension and bradycardia), renal failure, aggravation of chronic renal failure, colitis, and other gastrointestinal complications. 20 , 21 , 22 , 23 Sedation and ataxia can occur in dogs and cats receiving gabapentin, but no serious adverse effects have been reported. 5 , 6 , 7 , 8 , 9 , 10 , 11 , 12 , 13 , 14 In horses, administration of 10 and 20 mg/kg of gabapentin appears to be safe. 1 , 2 , 3 One report described mild sedation in a pregnant mare and in another report with IV dosing. 1 , 2 Single administration of up to 160 mg/kg of gabapentin did not cause adverse effects. 4 In silico simulation of gabapentin administration suggests that doses >20 mg/kg would maximize the analgesic effect of gabapentin in horses. 4 However, pharmacokinetics and occurrence of adverse effects of gabapentin after repeated administration of doses >20 mg/kg have not been determined.
Our hypothesis was that administration of 40 and 120 mg/kg of gabapentin PO q 12 h for 14 days would not cause sedation or ataxia nor have adverse effects as determined by physical examination and serum biochemistry variables. In addition, it was expected that repeated administration of 40 and 120 mg/kg q12 h would achieve plasma concentrations reported to be analgesic in other species (half maximal effective concentration of gabapentin [EC50 ],16.7 μg/mL). 24 , 25
2. MATERIALS AND METHODS
Our study was approved by the Washington State Institutional Animal Care and Use Committee (ASAF #11023). Six clinically healthy adult mares were used in this randomized (Random Generator, Ireland; http://www.random.org/) cross‐over study. All mares were assessed before the beginning of the study to determine that no neurologic deficits or lameness was present. Complete blood counts and biochemistry profiles also were performed to ensure the horses were clinically healthy. The breeds consisted of 3 Arabian and 3 Quarter Horses. The mean ± SD weight of the mares was 496 ± 66.18 kg and the age ranged from 9 to 20 years with a mean age ± SD of 15.16 ± 5.34 years.
Forty‐eight hours before beginning gabapentin administration, the mares were brought into the teaching facility stalls. They were stalled separately and fed timothy hay twice daily, and selenium supplementation was provided once daily, with continuous access to water. The mares were not held off feed or water before gabapentin administration and were fed 30 minutes before gabapentin administration in the morning and evening. Feeding and sample collection were kept constant throughout the study.
The afternoon before the start of gabapentin administration, a 14‐gauge catheter was placed aseptically in the jugular vein of each mare and the catheter was heparin‐locked (2.5 mL normal saline, 2.5 mL of 100 units/mL heparin). The horses were evaluated at that time for lameness or any other health concerns. Plasma was obtained immediately after catheter placement for the serum biochemistry profile. The study began the next morning with physical examinations and confirmation of lack of ataxia or sedation. All gabapentin (600 mg tablets; Blue Point Labs, India) tablets were ground in a coffee grinder and placed in plastic bags with the air removed, sealed, and labeled with the horse's identification, date, and timing of administration (am or pm) for the 14‐day study. The sealed plastic bags were stored in closed cardboard boxes at room temperature during the study period. The ground gabapentin was mixed with 1/4‐1/3 cup corn oil (gabapentin is lipophilic) and applesauce or molasses and administered PO using a catheter tip syringe. The total volume of oil and molasses or applesauce varied between 60 mL for the 40 mg/kg dose and 120 mL for the 120 mg/kg dose. At the end of the study and during the 3‐week washout period, the mares were returned to the dry lot with the other teaching herd horses.
On day 1, blood samples were collected from the jugular catheter in heparinized tubes pregabapentin (T = 0), and at 0.25, 0.50, 1, 2, 4, 8, 12 hours postadministration of gabapentin. For each collection time, 5 mL of blood were drawn through the catheter and discarded, then a 5 mL sample of blood was collected and injected into a heparinized tube, and the catheter was irrigated with 5 mL of heparinized saline. On days with few sample collections, the catheter was irrigated 3 times a day with 50 U per heparin/5 mL normal saline. The catheter and catheter site were monitored 3 times per day for any signs of infection or inflammation. Blood also was obtained on days 7 and 14 for gabapentin concentrations and biochemistry profiles before the morning administration of gabapentin. On day 15, blood was collected starting at 12 hours after the last dose of gabapentin. After the initial blood was taken on day 15, blood was taken 15 minutes, 30 minutes, 1, 2, 4, 8, 16, 32, and 64 hours after the last dose of gabapentin administered on day 14. Blood collection time was the same as the initial sampling when the horses started the gabapentin day 1. Blood obtained for gabapentin analysis was centrifuged at 1800g for 5 minutes. Plasma was stored at −80°C until analysis.
At each sample time, the same 3 evaluators completed a physical examination that included heart rate, respiratory rate, body temperature, mucous membrane color, capillary refill time, gastrointestinal sounds, and observation of any feces or urination for each horse, along with being walked outside. In addition, the horses had a sedation score assigned to them using a response to loud clapping, observation and use of a sedation scoring system. 25 The scoring system was: 0‐no sedation (normal ear and neck position, normal posture, normal gait); 1‐mild sedation (relaxed facial muscles, decreased response to background activity, slight ear tip separation, mildly decreased movement); 2‐moderate sedation (limited movement, ear tip separation, neck position below the horizontal plane, eyelids partially closed, pendulous lower lip, no response to background activity); 3‐deep sedation (prolonged periods of no movement, base‐wide stance, pronounced ear tip separation, loss of postural tone, eyelids partially or totally closed, pendulous lower lip). 25 The horses were monitored continuously for sedation for 3 hours (peak concentration of gabapentin is 2 hours) and sedation scores were assigned at every data collection point throughout the entire 2‐week study.
The horses were walked in a straight line and tight circle to determine any signs of ataxia. The Mayhew ataxia scale was used: grade 0, normal; grade 1, subtle gait abnormality that may worsen with head elevation; grade 2, moderate gait abnormalities that are noted at a walk; grade 3, gait abnormalities that are easily recognizable and much worse when the horse is going around obstacles with elevation of the head; grade 4, gait abnormalities easily seen with potential for the horse to fall or nearly fall when prompted to walk or perform normal activities; and grade 5, recumbent horse. 26
2.1. Quantification of gabapentin
Gabapentin was extracted from plasma samples using a precolumn derivatization, solid phase extraction (SPE) method. 27 Frozen plasma samples were thawed and vortex‐mixed, and 100 μL were transferred to a clean tube internal standard (30 μL vigabatrin 10 μg/mL) and 1 mL of 0.1 N HCl was added. This mixture was loaded onto a preconditioned (per manufacturer's recommendation) MCX cartridge (Waters, Milford, MA). Samples were eluted with 2 mL of ammonia : water : acetonitrile (5 : 13 : 82) and evaporated to dryness using nitrogen gas for approximately 45 minutes at room temperature (72°F).
Analysis of gabapentin in plasma samples was conducted using reversed‐phase high performance liquid chromatography (HPLC) as reported previously. 4 The system consisted of a 2695 separation module and a 2475 fluorescence detector (Waters, Milford, MA). Separation was attained on a Waters Atlantis T3 4.6 × 250 mm (5 μm) column preceded by a 5 μm Atlantis T3 guard column. The mobile phase was a mixture of (A) 50 mM potassium phosphate dibasic buffer (pH 5.0) and (B) acetonitrile. Gradient elution was used to separate the analytes starting with 53% of solution A and 47% of solution B and was adjusted to 49% of solution A and 51% of solution B over 15 minutes, and back to initial conditions over 5 minutes. The flow rate was 1.1 mL/min. The fluorescence detector was set at an excitation of 300 and an emission of 500 with the gain at 10×. The column was kept at ambient temperature. The injection volume was 100 μL. Standard curves for plasma analysis were prepared by spiking untreated equine plasma with gabapentin, which produced a linear concentration range of 25 to 10 000 ng/mL. The quality control concentrations used were 75, 350, 3500, and 8000 ng/mL.
2.2. Biochemistry profile
An IDEXX Catalyst Dx Analyzer (Idexx Catalyst Chemistry Analyzer, Westbrook, ME) was used for biochemical profiles. The Equine 15 Clip (eIdexx Equine Clip 15, Westbrook, ME) was used for comprehensive biochemistry testing and included albumin, globulin, total protein, gamma‐glutamyl transferase (GGT), creatinine, blood urea nitrogen (BUN), BUN/creatinine ratio, calcium, aspartate aminotransferase (AST), alkaline phosphatase (ALKP) total bilirubin, and creatine kinase (CK). Each horse had biochemistry profiles run on pregabapentin plasma and on days 7 and 14 for each dose of 40 or 120 mg/kg.
2.3. Estimation of pharmacokinetic parameters
Noncompartmental analysis was used to calculate pharmacokinetic parameters as implemented by Phoenix WinNonlin v8.0, (Pharsight Corp. Mountain View, CA). Pharmacokinetic parameters included: area under the plasma concentration‐time curve from 0 hours to infinity after dosing (AUC0‐∞), area under the plasma concentration‐time curve from 0 hours to the last sampling after the last dosing (AUC0‐12), area under the plasma concentration‐time curve from 0 hours to last sampling time after the last dosing (AUClast), maximum concentration (C max), time to maximum concentration (T max), and half‐life of terminal portion of the curve after PO administration.
2.4. Statistical analysis
Biochemistry profile data was compared using chi‐squared, Student t‐test, and analysis of variance. The Shapiro‐Wilk test was used to assess normality of the data. For the pharmacokinetics of gabapentin, the median AUC0‐∞ and AUC0‐12 hours after the last dose of 40 and 120 mg/kg and trough concentrations at day 7 and 14 were compared statistically using a Mann‐Whitney U test. All the statistical comparisons were performed using GraphPad Prism v7.4 for Windows, (GraphPad Software, San Diego, CA). Plasma drug accumulation was estimated by the AUC0‐12 hours ratio after the last and first dose for each dose regimen. Significance was set at P < .05 for all comparisons.
3. RESULTS
No adverse effects were noted in any of the horses for any of the dosage regimens. Sedation and ataxia scores were rated 0 throughout the study. Food and water consumption, urination, defecation, and vital signs remained normal throughout the study. Biochemistry variables also remained within normal limits throughout the study (Table S1).
The chromatographic method used to quantify gabapentin in horse plasma has an average recovery of 87%. Intra‐assay coefficient of variation ranged from 1.0% to 5.4% whereas interassay variability ranged from 0.4% to 11%. The lower limit of quantification was 25 ng/mL.
Pharmacokinetic parameters were determined by noncompartmental analysis (Table 1) and compartmental analysis (Table S2). For 1 horse at the 120 mg/kg dose, no model fit the concentration data because of an atypical plasma concentration vs time profile after the first dose. The reasons for this atypical profile are unknown, and this profile was excluded from the pharmacokinetic analysis. The peak concentration of gabapentin occurred within 2 hours. The administration of 120 mg/kg of gabapentin q 12 h resulted in higher exposure to the drug than did the administration of 40 mg/kg as reflected by a higher C max and AUC. The median AUC0‐∞ after the last administration of gabapentin at 40 mg/kg was lower than the median AUC0‐∞ obtained after the last administration of gabapentin at 120 mg/kg (P < .05). The median (range) gabapentin trough concentrations at days 7 were 5.2 μg/mL (4.4‐6.1) and 16 μg/mL (8.1‐28) for 40 and 120 mg/kg dosage protocols, respectively. The median (range) gabapentin trough concentration after 14‐days was 6.4 μg/mL (5.2‐8.7) and 17 μg/mL (9.5‐30) for 40 and 120 mg/kg dosage protocols, respectively (Figure 1).
TABLE 1.
Plasma pharmacokinetic parameters (median [range]) derived from noncompartmental analysis in horses after PO administration at 40 and 120 mg of body weight q 12 h for 14 days
| PK parameter | Unit | 40 mg/kg first dose (n = 6) | 40 mg/kg last dose (n = 6) | 120 mg/kg first dose (n = 5) | 120 mg/kg last dose (n = 6) |
|---|---|---|---|---|---|
| HL_Lambda_z | hours | 11 (6‐30) | 15 (13‐29) | 7.2 (6.2‐19) | 20 (12‐22) |
| T max | hours | 1 (1‐2) | 0.4 (0‐8) | 1 (1‐2) | 0.0 (0.0‐0.3) |
| C max | ng/mL | 7.6 (6.2‐11) | 8 (6.6‐11) | 9.9 (6.1‐27) | 22 (14‐33) |
| AUClast | h*ng/mL | 39 (25‐72) | 140 (100‐320) | 62 (320‐160) | 480 (260‐650) |
| AUCINF_obs | h*ng/mL | N/A | 146 (104‐400) | N/A | 550 (270‐650) |
| AUC0‐12h | ng*h/mL | 39a (25‐72) | 62b (48‐98) | 63b (45‐160) | 180c (120‐220) |
| AUC0‐12h‐last/first dose | n/a | 1.5 (1.00‐2.63) | 2.92 (range, 1.4‐3.8) | ||
Note: The AUC0‐12 hours was lower after the first dose relative to the last dose administration for the 40 mg/kg (P = .01) and 120 mg/kg regimen (P = .009). The % extrapolated ranged between 5% and 21% and from 2.4% to 13% for the 40 and 120 mg/kg, respectively. The different superscripts indicate P < .05. AUC0‐12h was only compared within each dose level.
Abbreviations: AUC0‐12h, area under the plasma concentration‐time curve from 0 hours to infinity after dosing; AUC0‐12h‐last/first dose, represents the AUC0‐12h ratio after the last drug administration relative to the first dose administration for each dose level to measure drug accumulation after repeated drug administration; AUCINF_last, area under the plasma concentration‐time curve from 0 hours to last sample time; AUCINF_obs, area under the plasma concentration‐time curve from 0 hours to infinity after dosing; C max, maximum concentration; T max, time to maximum concentration.
FIGURE 1.
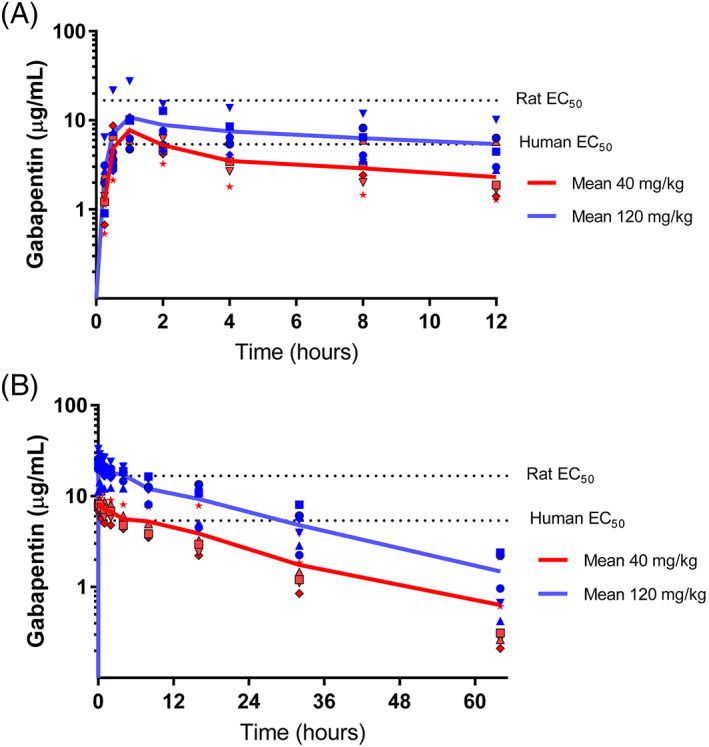
Plasma gabapentin concentration (individual and mean values) vs time profile in adult horses after PO administration of gabapentin at 40 (n = 6) and 120 (n = 5) mg of body weight q 12 h for 14 days. The dotted lines correspond to estimated gabapentin effective concentration 50% for rats 28 and humans. 24 (A) Disposition of gabapentin in plasma after the first PO administration (day 1). (B) Disposition of gabapentin in plasma after the last oral administration (day 15)
4. DISCUSSION
The disposition of gabapentin in plasma was comparable to previous reports. 2 , 3 , 5 The exposure to gabapentin was larger after administration of gabapentin at 120 mg/kg than after 40 mg/kg. The dosage regimen evaluated in our study resulted in accumulation of gabapentin (Figure 1). Plasma drug accumulation, as reflected by the AUC0‐12 hours, was larger after the administration of 120 mg/kg than after the administration of 40 mg/kg (Table 1). In addition, the trough plasma concentration before day 7 and after the last dose was comparable following the administration of 120 mg/kg, suggesting that steady‐state plasma concentrations had been attained. In contrast, the trough plasma concentrations for the 40 mg/kg dosage suggest that steady state has not been attained. The reason for the relatively lower accumulation and unstable trough plasma concentrations after repeated administration of 40 mg/kg remains unknown. This finding could be the result of large inter‐inter‐individual variability in absorption, distribution or elimination of gabapentin and the small sample size in our study. How these results impact the analgesic effect of the dosage regimens evaluated remains to be determined. Importantly, these findings suggest that a fixed dosage regimen may not be equally effective for all horses even if pain intensity were the same in all animals over time, considerable variability exists among individuals. Optimization of dosage regimens would be necessary to maximize the chances of controlling pain in some horses.
Unfortunately, optimal analgesic plasma concentrations for gabapentin in horses are unknown. 29 , 30 , 31 , 32 In humans, the half‐maximal effective concentration (EC50) for treating neuropathic pain is 5.4 μg/mL. 16 , 17 , 18 , 24 Studies in rats suggest that the EC50 ranges from 1.4 to 16.7 μg/mL for the treatment of inflammatory hyperalgesia. 28 In our study, the median (range) trough plasma concentration of gabapentin before the last administration of 40 mg/kg was 7.3 μg/mL (5.2‐8.7 μg/mL) and did not attain the theoretical EC50 (16 μg/mL) in humans, 24 suggesting that a more frequent dose interval might be required to reach theoretical effective analgesic plasma concentrations at 40 mg/kg. These findings contrast with previous single‐dose gabapentin concentration simulations that suggested that doses ≥20 mg/kg administered q 12 h to horses would maintain plasma concentrations of 16.7 μg/mL during a 12‐hour dose interval. 4 Mathematical simulations are based on several assumptions, including the representativeness of the input data. Interindividual variability of gabapentin pharmacokinetics between studies would explain the discrepancies. In contrast, the mean (range) trough plasma concentrations of gabapentin before the last administration of 120 mg/kg of gabapentin were 22.3 μg/mL (11‐33 μg/mL) and attained the theoretical EC50 of 16.7 μg/mL in humans (represented as the theoretical EC50 in Figure 1) in 5 of 6 horses as predicted previously. 4 This finding suggests that administration of 120 mg/kg of gabapentin q 12 h would provide analgesic effects.
High doses of gabapentin can result in adverse effects in humans. 19 , 20 , 21 , 22 Acute renal failure, exacerbation of chronic renal failure, and hepatotoxicity have been reported in human patients taking gabapentin. 19 , 20 , 21 , 22 In cats, dogs, and humans, gabapentin has been found to cause sedation ataxia, and dizziness (in human patients) 21 , 22 , 23 In a previous study of horses, 4 1 of 15 horses became slightly sedated for 2 hours after a single PO administration of both 120 mg/kg and 160 mg/kg of gabapentin. One study noted variable degrees of sedation after IV gabapentin 3 and another study reported mild sedation in a pregnant mare after 2.5 mg/kg of gabapentin. 1 In our study, neither sedation nor ataxia were observed in any of the mares during the 2‐week administration of either dose. In addition, the dosage regimens used in our study did not alter the biochemistry profiles of treated horses.
Our results provide a foundation for optimizing analgesic treatment with gabapentin in horses. Collectively, the pharmacokinetic data suggests therapeutic plasma concentrations could be attained in horses when treated with doses up to 120 mg/kg q 12 h. Based on this information, along with other published information, clinicians would be able to individualize gabapentin treatment by escalating or de‐escalating dosage regimens between 5 and 120 mg/kg q 12 h to meet individual analgesic needs. At current market prices of gabapentin, long‐term administration of 120 mg/kg to an adult horse would be relatively expensive but still would be a reasonable option in some horses. A limitation of our study was the relatively small number of healthy adult mares treated for only 14 days. Therefore, our findings should be confirmed with a larger and more diverse population of horses receiving treatments over a longer period of time, as would be necessary to manage chronic and or neuropathic pain.
In conclusion, we report the plasma disposition of gabapentin after the administration of 40 and 120 mg/kg PO q 12 over 14 days to adult horses. No clinical or biochemical adverse effects were noted in any horses in the study. Although the results of our study are promising, the analgesic efficacy of the administration of 40 or 120 mg/kg needs further research.
CONFLICT OF INTEREST DECLARATION
Authors declare no conflict of interest.
OFF‐LABEL ANTIMICROBIAL DECLARATION
Authors declare no off‐label use of antimicrobials.
INSTITUTIONAL ANIMAL CARE AND USE COMMITTEE (IACUC) OR OTHER APPROVAL DECLARATION
Approved by the Washington State University IACUC, ASAF #11023.
HUMAN ETHICS APPROVAL DECLARATION
Authors declare human ethics approval was not needed for this study.
Supporting information
Table S1 Biochemistry parameters (mean and SD) for trial 1 and trial 2. No significant difference was found between any of the parameters.
Table S2 Plasma pharmacokinetic parameters (median [range]) derived from a 1‐or 2‐compartmental model with first‐order absorption for gabapentin in horses after oral administration at 40 and 120 mg/kg of body weight q 12 h for 14 days.
ACKNOWLEDGMENT
No funding was received for this study. The authors thank Dr. Alyssa Marre, Jonathon Heinrich and Emily Richardson for the help on this project. A synopsis of this work was presented at 2020 ACVIM Forum On Demand.
Gold JR, Grubb TL, Cox S, Malavasi L, Villarino NL. Pharmacokinetics and pharmacodynamics of repeat dosing of gabapentin in adult horses. J Vet Intern Med. 2022;36(2):792‐797. doi: 10.1111/jvim.16386
[Correction added on 18 February 2022, after first online publication: Author name Tamera L. Grubb has been corrected as Tamara L. Grubb.]
REFERENCES
- 1. Davis JL, Posner LP, Elce Y. Gabapentin for the treatment of neuropathic pain in a pregnant mare. J Am Vet Med Assoc. 2007;231(5):755‐758. [DOI] [PubMed] [Google Scholar]
- 2. Dirikolu L, Dafalla A, Ely KJ, et al. Pharmacokinetics of gabapentin in horses. J Vet Pharmacol Ther. 2008;31(2):175‐177. PMID: 18307511. [DOI] [PubMed] [Google Scholar]
- 3. Terry RL, McDonnell SM, Van Eps AW, et al. Pharmacokinetic profile and behavioral effects of gabapentin in the horse. J Vet Pharmacol Ther. 2010;33(5):485‐494. PMID: 20840393. [DOI] [PubMed] [Google Scholar]
- 4. Gold JR, Grubb TL, Green S, Cox S, Villarino NF. Plasma disposition of gabapentin after the intragastric administration of escalating doses to adult horses. J Vet Intern Med. 2020;34:933‐940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Guedes AGP, Meadows JM, Pypendop BH, Johnson EG, Zaffarano B. Assessment of the effects of gabapentin on activity levels and owner‐perceived mobility impairment and quality of life in osteoarthritic geriatric cats. J Am Vet Med Assoc. 2018;253(5):579‐585. PMID: 30110208. [DOI] [PubMed] [Google Scholar]
- 6. Reid P, Pypendop BH, Ilkiw JE. The effects of intravenous gabapentin administration on the minimum alveolar concentration of isoflurane in cats. Anesth Analg. 2010;111(3):633‐637. PMID: 20547821. [DOI] [PubMed] [Google Scholar]
- 7. Pypendop BH, Siao KT, Ilkiw JE. Thermal antinociceptive effect of orally administered gabapentin in healthy cats. Am J Vet Res. 2010;71(9):1027‐1032. PMID: 20807141. [DOI] [PubMed] [Google Scholar]
- 8. van Haaften KA, Eichstadt Forsythe LR, Steelow EA, Bain MJ. Effects of single dose of gabapentin on signs of stress in cats during transportation and veterinary examination. J Vet Med Assoc. 2017;251(10):1175‐1181. [DOI] [PubMed] [Google Scholar]
- 9. Aghighi SA, Tipola A, Piechotta M, et al. Assessment of the effects of adjunctive gabapentin on postoperative pain after intervertebral disc surgery in dogs. Vet Anaesth Anal. 2012;39(6):636‐646. [DOI] [PubMed] [Google Scholar]
- 10. Crociolli GC, Cassu RN, Barbero RC, et al. Gabapentin as an adjuvant for postoperative pain management in dogs undergoing mastectomy. J Vet Med Sci. 2015;77(8):1011‐1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wagner AE, Mich PM, Uhrig SR, Hellyer PW. Clinical evaluation of perioperative administration of gabapentin as an adjunct for post‐operative analgesia in dogs undergoing amputation of a forelimb. J Am Vet Med Assoc. 2010;236(7):751‐756. [DOI] [PubMed] [Google Scholar]
- 12. Kukanich B, Cohen RL. Pharmacokinetics of oral gabapentin in greyhound dogs. Vet J. 2011;187(1):133‐135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Giudice E, Crino C, Barillaro G, et al. Clinical findings in degenerative lumbosacral stenosis in ten dogs—a pilot study on the activity of tramadol and gabapentin. J Vet Behav. 2019;33:7‐15. [Google Scholar]
- 14. Pankratz KE, Ferris KK, Griffith EH, Sherman BL. Use of single dose oral gabapentin to attenuate fear responses in cage‐trap community cats: a double‐blind, placebo‐controlled field trail. J Feline Med Surg. 2018;20(6):535‐543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Vollmer KO, von Hodernberg A, Kolle EU. Pharmacokinetics and metabolism of gabapentin in rat, dog and man. Fortschr Arzneimittelforsch. 1986;36(5):830‐839. [PubMed] [Google Scholar]
- 16.Gabapentin‐FDA Prescribing Information, Side Effects and Uses. https://www.drugs.com. Accessed 2019.
- 17. McLean MJ. Clinical pharmacokinetics of gabapentin. Neurology. 1994;44(6 Suppl 5):S17‐S22. [PubMed] [Google Scholar]
- 18. Tjandrawinata RR, Setaiawati E, Putri RSI, et al. Single dose of pharmacokinetics equivalence study of two gabapentin preparations in healthy subjects. Drug des Devel Ther. 2014;2014:1249‐1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Rose MA, Kam PCA. Gabapentin: pharmacology and its use in pain management. Anaesthesia. 2002;57:451‐462. [DOI] [PubMed] [Google Scholar]
- 20. Alhoda MAH, Perez A, Williams B, Emer J. Severe gabapentin toxicity after acute kidney injury in hospitalized patient with acute pain. Neurology Apr 2018, 90(15 Supplement) P6.020 https://n.neurology.org/content/90/15_Supplement/P6.020#:~:text=Conclusions%3A%20While%20gabapentin%20can%20be,in%20patients%20with%20renal%20failure. [Google Scholar]
- 21. Zand L, McKian KP, Qian Q. Gabapentin toxicity in patients with chronic kidney disease: preventable cause of morbidity. Am J Med. 2010;123(10):367‐373. [DOI] [PubMed] [Google Scholar]
- 22. Torregrosa‐de Juan E, Olague‐Diaz P, Royo‐Maicas P, et al. Acute renal failure due to gabapentin. A case report and literature review. Nefrologia. 2012;32(1):1‐132. doi: 10.3265/Nefrologia.pre2011.Nov.11087Fulltextaccess [DOI] [PubMed] [Google Scholar]
- 23. Jackson CD, Clanahan MJ, Joglekar K, Decha‐Umphai ST. Hold the Gaba: a case of gabapentin‐induced hepatotoxicity. Cureus. 2018;10(3):1‐4. doi: 10.7759/cureus.2269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lockwood PA, Cook JA, Ewy WE, Mandema JW. The use of clinical trial simulation to support dose selection:application to development of a new treatment for chronic neuropathic pain. Pharm Res. 2003;20(11):1133‐1143. [DOI] [PubMed] [Google Scholar]
- 25. Schavliege S, Cuypers C, Michielsen A, et al. How to score sedation and adjust the administration rate of sedatives in horses: a literature review and introduction of the Ghent sedation algorithm. Vet Anesth Analg. 2019;46:4‐13. [DOI] [PubMed] [Google Scholar]
- 26. Mayhew IG. Neurologic evaluation. In: Mayhew IG, ed. Large Animal Neurology. Philadelphia, PA: Lead and Fabier; 1989. [Google Scholar]
- 27. Mercolini L, Mandrioli R, Amore M, Raggi MA. Simultaneous HPLC‐F analysis of three recent antiepileptic drugs in human plasma. J Pharm Biomed Anal. 2010;53(1):62‐67. [DOI] [PubMed] [Google Scholar]
- 28. Larsen MS, Keizer R, Munro G, et al. Pharmacokinetic/pharmacodynamic relationship of gabapentin in a CFA‐induced inflammatory hyperalgesia rat model. Pharm Res. 2016;33(5):1133‐1143. [DOI] [PubMed] [Google Scholar]
- 29. Jones E, Viñuela‐Fernandez I, Eager RA, et al. Neuropathic changes in equine laminitis pain. Pain. 2007;132(3):321‐331. PMID: 17935886. [DOI] [PubMed] [Google Scholar]
- 30. Readford PK, Lester GD, Secombe CJ. Temporhyoid osteoarthropathy in two young horses. Aust Vet Journal. 2013;91(5):209‐212. [DOI] [PubMed] [Google Scholar]
- 31. Caldwell FJ, Taintor J, Waguespack RW, Sellers G, Johnson J, Lin HC. Effect of PO administration of gabapentin in chronic lameness in horses. J Equine Veterinary Science. 2015;35(6):536‐540. [Google Scholar]
- 32. Young JY, Schoonover MJ, Kembel SL, et al. Efficacy of orally administered gabapentin in horses with chronic thoracic limb lameness. Vet Anaseth Analg. 2020;47(2):259‐266. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 Biochemistry parameters (mean and SD) for trial 1 and trial 2. No significant difference was found between any of the parameters.
Table S2 Plasma pharmacokinetic parameters (median [range]) derived from a 1‐or 2‐compartmental model with first‐order absorption for gabapentin in horses after oral administration at 40 and 120 mg/kg of body weight q 12 h for 14 days.


