Abstract
Background
Lymphoma is the most common spinal cord neoplasm and second most common intracranial tumor in cats, but description of specific magnetic resonance imaging (MRI) features is lacking.
Objective
Describe the clinical and MRI features of lymphoma affecting the central (CNS) or peripheral (PNS) nervous system or both in cats.
Animals
Thirty‐one cats with confirmed cytological or histopathological diagnosis or both of lymphoma involving the CNS or PNS or both, and MRI findings of the lesions.
Methods
Multicenter retrospective descriptive study. Signalment and medical information were recorded. Magnetic resonance imaging findings were reviewed by 3 observers following a list of predefined criteria and consensus was sought. Frequency distributions of the different categorical data were reported.
Results
Median duration of clinical signs at time of presentation was 14 days (range, 1‐90). Neurological examination was abnormal in 30/31 cats. On MRI, lesions affecting the CNS were diagnosed in 18/31 cats, lesions in both CNS and PNS in 12/31, and lesions in the PNS only in 1/31. Intracranial lesions were diagnosed in 22 cats (extra‐axial, 7/22; intra‐axial, 2/22; mixed, 13/22), and spinal lesions were diagnosed in 12 (6/12 involving the conus medullaris and lumbosacral plexuses). Infiltration of adjacent extra‐neural tissue was present in 11/31 cases. Contrast enhancement was seen in all lesions, being marked in 25/30. Meningeal enhancement was present in all but 2 cases. Several distinct MRI patterns were observed.
Conclusions and Clinical Importance
Nervous system lymphoma in cats has a wide range of MRI features, of which none is pathognomonic. However, together with clinical data and cerebrospinal fluid (CSF) analysis, MRI may provide a strong tentative antemortem diagnosis.
Keywords: central nervous system, feline, lymphosarcoma, neurolymphomatosis, peripheral nervous system
Abbreviations
- ADC
apparent diffusion coefficient
- CN
cranial nerve
- CNS
central nervous system
- CSF
cerebrospinal fluid
- DWI
diffusion weighted Imaging
- FeLV
feline leukemia virus
- FIV
feline immunodeficiency virus
- FLAIR
fluid attenuated inversion recovery
- FNA
fine‐needle aspirate
- MRI
magnetic resonance imaging
- NCC
nucleated cell count
- PARR
PCR for antigen receptor rearrangement
- PNS
peripheral nervous system
- SPIR
spectral presaturation with inversion recovery
- SSH‐TSE
single‐shot turbo spin echo
- STIR
Short Tau Inversion Recovery
- T1w
T1‐weighted
- T2*w
T2*‐weighted
- T2w
T2‐weighted
- TP
total protein
1. INTRODUCTION
Lymphoma is the most common spinal neoplasm and the second most common intracranial tumor in cats. It accounts for 7% to 10% of spinal diseases in cats, 1 , 2 , 3 27% to 39% of spinal neoplasia, 1 , 2 , 3 , 4 and 13% to 31% of intracranial neoplasia. 5 , 6 , 7 Involvement of the central nervous system (CNS) is reported in up to 12% of cats with lymphoma, 8 , 9 , 10 whereas involvement of the peripheral nervous system (PNS) is described less frequently. 1 , 8 , 10 , 11 , 12 , 13 A definitive antemortem diagnosis is often difficult to obtain, because of the low yield of cerebrospinal fluid (CSF) analysis, 14 , 15 and the invasiveness and potential risks of spinal cord and brain biopsy.
Magnetic resonance imaging (MRI) is the imaging modality of choice to assess nervous system pathology, being a valuable noninvasive technique to reach reliable presumptive antemortem diagnoses. Despite increased availability of MRI in veterinary medicine, and the high incidence of nervous system lymphoma in cats, MRI features have been sparsely described in several case reports, 16 , 17 , 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25 and in cases series including low numbers of cats. 2 , 6 , 26 , 27 Although the histopathologic distribution of nervous system lymphoma in cats recently has been described, 10 , 13 a description of specific MRI features in a larger cohort of affected cats is lacking. The aim of our multicenter retrospective study was to describe the clinical and MRI features of lymphoma affecting the CNS and PNS in a large cohort of cats.
2. MATERIALS AND METHODS
2.1. Data collections and inclusion criteria
Medical records of 4 centers were retrospectively searched over a 15‐year‐period for cats with a diagnosis of lymphoma involving the CNS or PNS or both, confirmed either by cytology (fine‐needle aspirates [FNA]) or smears of either the lesion or cerebrospinal fluid [CSF]) or histopathology (biopsy or necropsy examination of the lesion), and that underwent a brain, vertebral column, or brachial or lumbar plexus MRI, or some combination of these. Search terms included “cat,” “MRI,” “lymphoma,” “lymphosarcoma,” “nervous system lymphoma/lymphosarcoma,” “CNS lymphoma/lymphosarcoma,” and “PNS lymphoma/lymphosarcoma.” Low‐ and high‐field MRI were included. Signalment and medical information were recorded, including breed, sex, age at the time of presentation, feline leukemia virus (FeLV) and feline immunodeficiency virus (FIV) status, presenting complaints, onset, duration and progression of clinical signs before presentation, neurological signs at initial examination, imaging findings, CSF analysis, cytologic or histopathologic diagnosis or both, treatment, survival time and cause of death.
2.2. Onset, duration, and progression of clinical signs
The duration of clinical signs before presentation was based on the owners' observations as described in the medical records. The onset was defined as acute (<1 day), subacute (>1 day, <2 weeks), or chronic (≥2 weeks). The clinical signs also were described as progressive (worsening or additional clinicals signs noticed over time), static (no progression or improvement), or waxing and waning (alternating improvement and worsening over time).
2.3. MRI analysis
Magnetic resonance imaging studies were retrospectively and independently reviewed by 2 board‐certified radiologists (AD, EK) and 1 board‐certified neurologist (RG), using a predefined list of criteria as previously described, 6 , 26 , 28 , 29 , 30 including lesion number, 28 neuroanatomic location, invasive behavior, lesion extension, shape and margins, 6 signal intensity and homogeneity, 6 degree 6 and pattern of contrast enhancement, meningeal and ependymal enhancement, intralesional mineralization, hemorrhage, cyst‐like formation, 6 degree of mass effect, 26 perilesional edema, 6 signs of increased intracranial pressure, 29 ventricular enlargement, 6 syringohydromyelia, 30 and presence of any additional lesions. 28 Lesions were classified as “CNS only” if only the brain parenchyma, optic nerves, spinal parenchyma, adjacent meninges, or some combination of these were affected. Lesions were classified as “PNS only,” if only the cranial nerves, excluding optic nerves, or spinal nerves or both were affected. Lesions involving both cranial or spinal nerves and brain or spinal parenchyma or meninges, were classified as “both CNS and PNS.” Intracranial axial 6 and spinal compartment 31 (extradural, intradural extramedullary, intramedullary) origins were recorded. Lesions were described as invasive if infiltrating into the adjacent extra‐neural tissue, such as bone or soft tissue. They were described as extensive if extending along ≥1 nerve root, over the length of >2 vertebral bodies for spinal lesions, or >3 brain regions (including individual cerebral lobes, diencephalon, brainstem, cerebellum) for intracranial lesions. Lesions extending along several nerve roots of the same plexuses were considered focal extensive lesions. Signal intensity was assessed relative to normal neighboring cortical or spinal gray matter in T2‐weighted (T2w), T2w‐fluid attenuated inversion recovery (FLAIR), T1‐weighted (T1w), short tau inversion recovery (STIR) or T2w‐spectral presaturation with inversion recovery (SPIR) sequences, diffusion weighted imaging (DWI) and apparent diffusion coefficient (ADC) mapping, when available. Signal homogeneity was described in T2w sequences. Signal intensity and homogeneity were determined by the most prominent characteristics of the main lesion. Contrast enhancement pattern was characterized as either uniform or nonuniform. A uniform pattern was defined as a diffuse and homogeneous contrast enhancing intensity. Nonuniform patterns were subcategorized as heterogeneous (contrast enhancement with mixed intensities), focal (single discrete area of contrast enhancement), multifocal (multiple contrast enhancing areas anatomically separated by noncontrast enhancing areas), or rim‐like (more pronounced peripheral enhancement as compared to the center of the lesion). Meningeal enhancement was characterized as none, focal (dural tail sign), 32 regional (extending well beyond the lesion but not to the entire brain or spinal cord), or diffuse. When multiple lesions were present, the above criteria were assessed for the larger or main lesion. Final results were achieved by consensus of the 3 observers.
2.4. CSF analysis
When performed, the site of CSF puncture (cisternal vs lumbar) was recorded. The analysis was classified as abnormal when albuminocytologic dissociation (reference total protein [TP] concentration for cisternal puncture <25 mg/dL, and for lumbar puncture <45 mg/dL), pleocytosis (reference nucleated cell count [NCC] <5 cells/μL) with or without increased TP, or abnormal NCC distribution were present. 33 Cell count differential was reported when available, and diagnosis of lymphoma recorded.
2.5. Diagnosis, outcome, and treatment
The method by which lymphoma was confirmed (CSF cytology, nervous system lesion cytology or histopathology), cell line origin, and other organs FNA or biopsy were recorded. When complete necropsy examination was available, lymphoma was classified as multicentric, primary nervous system, or primary nasal or nasopharyngeal with nervous system invasion, depending on the origin of the lesion, and presence or absence of lesions involving other organs elsewhere in the body. Survival time from the day of MRI acquisition and treatment undertaken (medical management, chemotherapy, radiation therapy, surgery, or combination of these) were recorded.
2.6. Statistical analysis
Statistical analyses were performed using commercially available software (StataCorp. 2017. Stata Statistical Software: Release 15. College Station, TX: StataCorp LLC). Normality of continuous data was assessed using the Shapiro‐Wilk test. Descriptive statistics were reported as mean and SD (SD) or median and range, depending on data normality. The Student t‐test was used to compare mean age at presentation of cats with and without spinal lesions. The Wilcoxon rank‐sum test was performed to compare CSF analyses between lumbar and cisternal puncture, and median survival time among treatment categories. For all analyses, P < .05 was considered significant.
3. RESULTS
Forty cats initially were identified. Two cats with confirmed CNS lymphoma were excluded, because of nondiagnostic MR images acquired in a low field system with low signal‐to‐noise ratio. Seven cats with confirmed round cell neoplasia were excluded because lymphoma could not be confidently confirmed, despite a high index of suspicion, based either on CSF cytology (2/7), lesion FNA (4/7), or necropsy examination (1/7).
3.1. Signalment, anamnesis, and neurologic examination
Thirty‐one cats were included. Twenty‐one were domestic short hair (67.7%), 3 were Maine Coon (9.7%), whereas domestic medium hair, domestic long hair, Birman, Himalayan, Siberian, Siamese, and Balinese breeds were represented by 1 cat each (3.2%). Twenty cats were male castrated (64.5%); 11 were female spayed (35.5%). The male‐to‐female ratio was 1.8. Overall mean age at the time of diagnosis was 10.1 ± 3.7 years (median, 10.1 years; range, 1.1‐16.3). No significant difference was observed in the mean age of cats with spinal lesions or brain lesions only (P = .91). Mean weight of the cats was 4.6 ± 1.2 kg (median, 4.5 kg; range, 2.6‐7.4). Feline leukemia virus and FIV status was evaluated in 15 cats. One cat was FeLV‐positive (6.7%), and 1 was FIV‐positive (6.7%).
Presenting complaints and respective numbers of cases are summarized in Table 1. The most common neurologic presenting complaints were gait abnormalities (61.3%). Respiratory signs were present in 5 cats (16.1%), being the main presenting complaint in 3. Eighteen cats (58.1%) presented with nonspecific signs. Median duration of clinical signs before initial neurological examination was 14 days (range, 1‐90). Based on history, clinical signs were progressive in 28 cats (90.3%), either chronic in 20 (64.5%) or subacute in 8 (25.8%). Signs were static in 2 cats (6.5%), respectively chronic and subacute, and chronic waxing and waning in 1 cat (3.2%). Two cats (6.5%) were diagnosed previously with lymphoma, respectively renal lymphoma 4 months prior and renal and intestinal lymphoma 2 years prior.
TABLE 1.
Presenting complaints in 31 cats with nervous system lymphoma
| Presenting complaints | Numbers of cases (%) | Presenting complaints | Numbers of cases (%) |
|---|---|---|---|
| Neurological signs | 30 (96.8) | Nonspecific clinical signs | 18 (58.1) |
| Gait abnormalities | 19 (61.3) | Inappetence/anorexia | 13 (41.9) |
| Paresis/plegia | 9 (29) | Lethargy | 9 (29) |
| Ataxia | 5 (16.1) | Weight loss | 4 (12.9) |
| Circling | 5 (16.1) | Vomiting | 4 (12.9) |
| Forelimb lameness | 4 (12.9) | Poor general condition | 2 (6.5) |
| Behavioral changes | 9 (29) | Respiratory signs | 5 (16.1) |
| Pacing and disorientation | 7 (22.6) | Sneezing and epistaxis | 1 (3.2) |
| Aggressivity | 1 (3.2) | Coughing | 1 (3.2) |
| Abnormal mentation | 4 (12.9) | Nasal discharge | 1 (3.2) |
| Anisocoria | 4 (12.9) | Tachypnoea | 1 (3.2) |
| Seizure | 2 (6.5) | Respiratory noise | 1 (3.2) |
| Spinal pain | 2 (6.5) | ||
| Blindness | 1 (3.2) |
At the time of presentation, 22 cats (71.0%) showed signs of brain disease, including 15 cats (48.4%) with signs of forebrain disease. Eleven cats (35.5%) showed signs of spinal disease, including 2 cats with concurrent signs of forebrain disease. Neurologic examination findings are summarized in Supplementary Table S1.
3.2. MRI features
Magnetic resonance imaging studies were performed using a low‐field MRI scanner in 5 cats (Hitachi Airis II 0.3T, Hitachi Medical Systems, Düsseldorf, Germany), and high‐field MRI scanners in 26 cats (Philips Panorama HFO 1.0T, Philips Medical Systems, Nederland B.V., Best, The Netherlands in 15/26; Siemens Symphony 1.5T, Siemens Medical Solutions USA, Inc., Malvern, PA, USA in 7/26; Siemens Magnetom Essenza 1.5T, Siemens Healthcare Ltd, Frimley, Surrey, UK in 3/26; GE Genesis Signa 1.5T, GE Medical Systems, Fairfield, CT, USA in 1/26). Twenty‐one cats (67.7%) had brain MRI, 8 (25.8%) MRI of the vertebral column (lumbar spine, 3/8; thoracolumbar spine, 2/8; cervical spine, 1/8; brachial plexuses region, 2/8), and 2 of both.
Topography and morphology of MRI lesions are summarized in Table 2. Intracranial lesions were present in 22 cats (71.0%), spinal lesions in 10 (32.3%), and brachial plexus lesions in 2 (6.5%). Most lesions were isolated to the CNS (18/31, 58.1%), but lesions affecting both CNS and PNS (12/31, 38.7%) also were commonly observed. Most intracranial lesions were classified as both extra‐ and intra‐axial (13/22, 59.1%; Figure 1), and multiple spinal compartments (extradural, intradural, intramedullary, or some combination of these) were involved in all but 1 cat with spinal cord or spinal nerve lesions (11/12, 91.7%). Most lesions were ill‐defined (24/31, 77.4%), focal (25/31, 80.6%), but extensive (20/31, 64.5%). Extra‐neural tissue invasion was present in 9 cats with intracranial lesions (9/22, 40.1%), but was uncommon in cats with spinal lesions (2/12, 16.7%). Distinct analogous lesion types were observed in several cats. Four distinct MRI patterns were observed in cats with invasive intracranial lesions: nasopharyngeal mass invading the calvarium floor (3/9, 33.3%; Figure 2), mass centered on the cribriform plate involving nasal cavity and olfactory lobes (3/9, 33.3%; Figure 3), rostral brain and adjacent frontal sinus infiltration (2/9, 22.2%), and extra‐axial mass with tympanic bulla infiltration (1/9, 11.1%; Figure 1A‐D). Other distinctive intracranial MRI patterns included midline ventral brain enhancement centered on the diencephalon (4/22, 18.2%; Figure 2), focal extra‐axial mass invading the underlying neuroparenchyma (4/22, 18.2%; Figure 1I‐L), and ill‐defined amorphous intra‐axial lesions involving lentiform and rostral thalamic nuclei (2/22, 9.1%; Figure 1E‐H).
TABLE 2.
Topography and morphology of the MRI lesions found in 31 cats with nervous system lymphoma
| Topographic and morphologic MRI features | Intracranial lesions | Spinal and spinal nerves lesions | Both intracranial and spinal lesions a | Total |
|---|---|---|---|---|
| Number of cases (%) | Number of cases (%) | Number of cases (%) | Number of cases (%) | |
| Total number of cases | 19 (61.3) | 9 (29) | 3 (9.7) | 31 (100) |
| CNS/PNS | ||||
| CNS only | 13 (68.4) | 2 (22.2) | 3 (100) | 18 (58.1) |
| PNS only | 1 (11.1) | 1 (3.2) | ||
| Both CNS/PNS | 6 (31.6) | 6 (66.7) | 12 (38.7) | |
| Intracranial lesions | 22 (71) | |||
| Extra‐axial | 6 (31.6) | – | 1 (33.3) | 7 (31.8) |
| Intra‐axial | 2 (10.5) | – | 2 (9.1) | |
| Extra‐ and intra‐axial | 11 (57.9) | – | 2 (66.7) | 13 (59.1) |
| Spinal lesions | 12 (38.7) | |||
| Extradural | – | 1 (11.1) | 1 (8.3) | |
| Extradural/intradural extramedullary | – | 5 (55.6) | 1 (33.3) | 6 (50) |
| Intradural/intramedullary | – | 1 (11.1) | 2 (66.7) | 3 (25) |
| Extradural/intradural/intramedullary | – | 2 (22.2) | 2 (16.7) | |
| Lesions number | ||||
| Focal | 17 (89.5) | 8 (88.9) | 25 (80.6) | |
| Multifocal | 2 (10.5) | 1 (11.1) | 3 (100) | 6 (19.4) |
| Lateralization | ||||
| Right | 3 (15.8) | 4 (44.5) | 1 (33.3) | 8 (25.8) |
| Left | 11 (57.9) | 2 (22.2) | 13 (41.9) | |
| Midline | 5 (26.3) | 3 (33.3) | 2 (66.7) | 10 (32.3) |
| Extensive behavior | ||||
| Yes | 12 (63.2) | 7 (77.8) | 1 (33.3) | 20 (64.5) |
| No | 7 (36.8) | 2 (22.2) | 2 (66.7) | 11 (35.5) |
| Invasive behavior | ||||
| Yes | 9 (47.4) | 1 (11.1) | 1 (33.3) | 11 (35.5) |
| No | 10 (52.6) | 8 (88.9) | 2 (66.7) | 20 (64.5) |
| Margins | ||||
| Well‐defined | 2 (10.5) | 5 (55.6) | 7 (22.6) | |
| Ill‐defined | 17 (89.5) | 4 (44.4) | 3 (100) | 24 (77.4) |
Abbreviations: CNS, central nervous system; MRI, magnetic resonance imaging; PNS, peripheral nervous system.
Including 2 cats with cranial cervical spinal lesions extending from brainstem lesions, and 1 cat with multifocal spinal and brain lesions.
FIGURE 1.
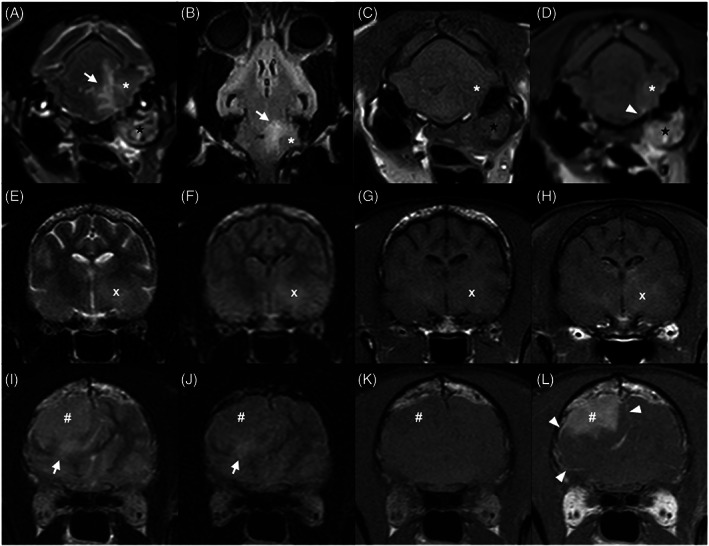
Transverse T2w (A, E, I), dorsal (B) and transverse FLAIR (F, J), transverse pre‐ (C, G, K) and postcontrast (D, H, L) T1w magnetic resonance images of feline nervous system lymphoma classified as extra‐axial (A‐D), intra‐axial (E‐H), and both extra‐ and intra‐axial (I‐L). (A‐D) An invasive ill‐defined extra‐axial T2w hyperintense, FLAIR isointense, T1w hypointense heterogeneously contrast enhancing extra‐axial mass (asterisk) is present along the left side of the brainstem and invades the ipsilateral tympanic cavity (black star). (E‐H) An ill‐defined T2w and FLAIR hyperintense, T1w isointense, faintly enhancing intra‐axial lesion is present in the region of the left rostral thalamus and basal nuclei (white cross). No meningeal enhancement is noted. (I‐L) A large T2w hyperintense, FLAIR isointense, T1w hypointense, strongly homogeneously enhancing mass, with ill‐defined irregular margins with the underlying parenchyma is present within the right frontal lobe (hash key). Regional meningeal enhancement was observed in both (D) and (L) (arrowheads). Respectively moderate (A, B), and mild (I, J) perilesional edema (white arrows) is present
FIGURE 2.

Sagittal T1w postcontrast (A, E), transverse T2w (B, F), T1w pre‐ (C, G) and T1w postcontrast (D, H) magnetic resonance images of the brain of a 7‐year‐old Domestic Short Hair (A‐D) and a 14‐year‐old Maine Coon (E‐H) showing infiltration of the hypothalamus and hypophysis (black asterisks). (A‐D) An ill‐defined strongly heterogeneously enhancing nasopharyngeal mass (black hash key), infiltrates the retropharyngeal muscles and middle cranial fossa, including the orbital fissures (white arrowhead). Meningeal enhancement is diffuse (dashed white arrows), most pronounced along the piriform lobes and focal rostroventral ependymal enhancement of the 3rd ventricle is noted (white arrow). (E‐F) Similar pattern of infiltration of the pituitary gland, hypothalamus and 3rd ventricle. A focal thickening of the dorsal nasopharyngeal wall is noted (dashed white arrows), without evident communication with the middle cranial fossa
FIGURE 3.
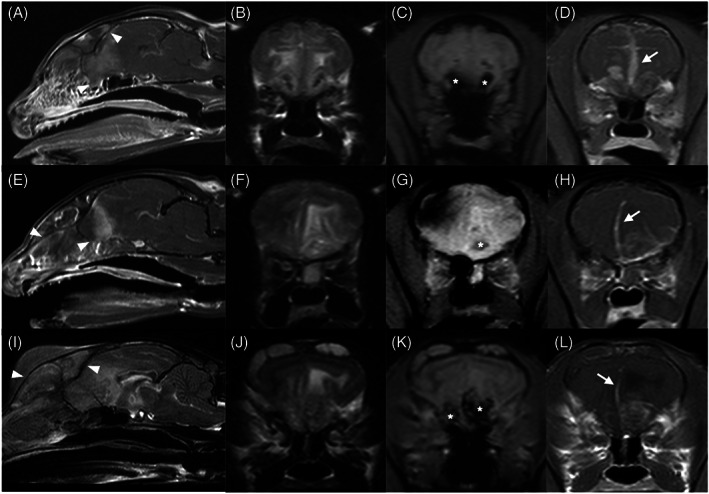
Sagittal T1w postcontrast (A, E) and T2w (I), and transverse T2w (B, F, J), T2*w (C, G, K), and T1w postcontrast (D, H, L) magnetic resonance of images of three cats (A‐D, E‐F, and I‐L) showing an ill‐defined heterogeneous mass centered on the cribriform plate invading the caudal nasal cavity, frontal sinuses, and rostral forebrain. The masses are classified as invasive and extensive, being both extra‐axial and intra‐axial, and involving the CNS only in all cases. T2*w signal voids are present in the invaded olfactory lobes (white asterisks), consistent with intralesional hemorrhages. The lesions show a strong inhomogeneous contrast enhancement, with strong regional leptomeningeal enhancement and thickening (white arrows). A severe mass effect associated with caudal transtentorial and foramen magnum brain herniations, consistent with signs of increased intracranial pressure, is present in all cases. Multifocal permeative lysis of the frontal and/or nasal bones and cribriform plates (white arrowheads), with extensive infiltration beyond the preserved bone margins is observed
The trigeminal nerves were affected in all intracranial lesions involving both CNS and PNS (6/22, 27.3%), with or without involvement of other cranial nerves. The PNS was involved in 7 cats with spinal lesions (7/12, 58.3%). In all, spinal nerve thickening or enhancement or both was present within the vertebral canal and lesions were classified with an extradural component. These included 5 cats (5/12, 41.7%) with caudal lumbar spinal lesions associated with lumbosacral plexus involvement (Figure 4), and 2 cats (2/12, 16.7%) with brachial plexus involvement (Figure 5), 1 showing additional involvement of the lumbosacral plexus.
FIGURE 4.
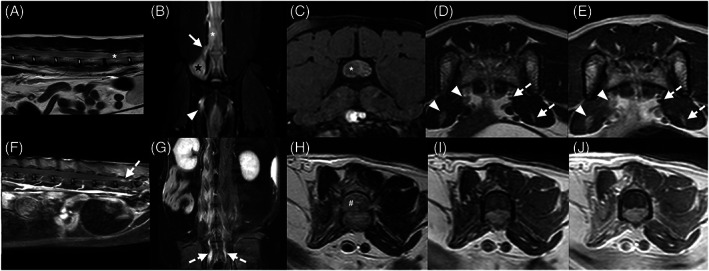
Magnetic resonance images of two cats (A‐E and F‐J) with caudal lumbar and lumbosacral plexus lesions. Sagittal T2w (A), dorsal SPIR (B), and transverse 3D T1w fat suppression postcontrast (C), T2w (D), and T1w postcontrast (E) magnetic resonance images of an 11‐year‐old Domestic Short Hair (DSH) showing a well‐defined homogeneous intradural and extradural mass at the level of L6 (white asterisk), that is T2w and SPIR hyperintense relative to normal spinal gray matter and shows strong heterogeneous contrast enhancement. The mass is continuous with the right L6 spinal nerve (white arrow), which is thickened and SPIR hyperintense. Thickening and enhancement of the right femoral and sciatic nerves (white arrow heads) are visible compared to the contralateral side (dashed white arrows). Focal right epaxial muscles SPIR hyperintensity (black star) is also noted. Sagittal T1w fat suppression postcontrast (F), dorsal SPIR (G), and transverse T2w (H), T1w pre‐ (I) and postcontrast (J) magnetic resonance images of a 15‐year‐old DSH of an extensive ill‐defined lumbar T2w and SPIR hyperintense intramedullary mass (white hash key). The mass is isointense to normal gray matter in T1w and none to faintly contrast enhancing. Thickening, SPIR hyperintensity, and enhancement of the conus medullaris meninges (not shown), lumbosacral spinal nerve roots and sciatic nerves (white dashed arrows) are present, most pronounced on the right. Severe atrophy of the right lumbar epaxial and hypaxial muscles is visible
FIGURE 5.
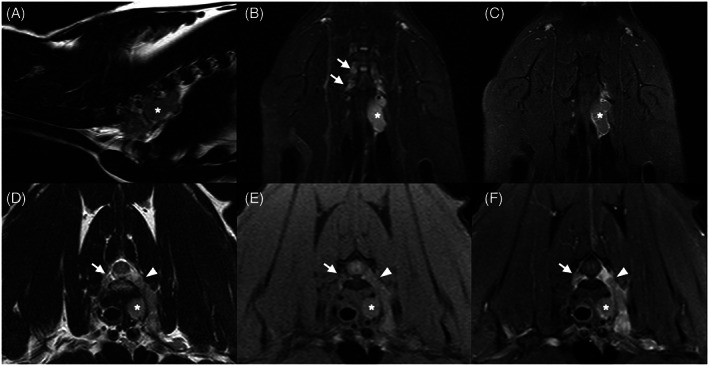
Parasagittal T2w (A), dorsal STIR (B) and T1w fat saturation postcontrast (C), transverse T2w (D), T1w fat saturation pre‐ (E) and postcontrast (F) magnetic resonance images of a 4‐year‐old Himalayan cat, showing a large well‐defined lobulated mass (white asterisk) affecting the left brachial plexus and extending into the vertebral foramen (white arrowhead). The lesion is T2w homogeneous, T2w and STIR hyperintense, and T1w isointense relative to spinal gray matter, and shows heterogeneous contrast enhancement. Mild left forelimb muscle atrophy is noted. STIR hyperintensity, thickening and enhancement of the right brachial plexus nerve roots are also visible (white arrows). No meningeal enhancement was observed, the lesion was classified as PNS only
Lesion MRI signal characteristics, enhancing pattern, and perilesional changes are summarized in Table 3. All lesions were T2w‐isointense to hyperintense relative to normal gray matter, most were FLAIR‐hyperintense (15/22, 68.2%) and T1w‐isointense to hypointense (28/30, 93.3%). Intralesional signal voids in T2*w sequence, consistent with intralesional hemorrhage, were observed only in 3 cats (3/20, 15%), all showing the same lesion pattern (Figure 3). Neoplastic infiltration of vessels was histopathologically confirmed in 1 of these cases. Diffusion‐weighted imaging was performed in 6 cats, using a b‐value of 800 s/mm2. Four lesions were hyperintense in the b800 diffusion‐weighted images but only 2 showed low ADC values, consistent with restricted diffusion (2/6, 33.3%). Contrast enhancement was present in all cases but was faint in 3 cases (Figure 4). Meningeal enhancement was noted in all but 2 cases (29/31, 93.6%), being most commonly regional (19/31, 61.3%). Ependymal enhancement of the 3rd ventricle was visible in all 4 cats with infiltration of the hypophysis‐hypothalamus axis (Figure 2). Thickening and enhancement of the choroid plexuses was seen in 1 case.
TABLE 3.
MRI signal characteristics and enhancement patterns in 31 cats with nervous system lymphoma
| MRI features | Intracranial lesions | Spinal and spinal nerves lesions | Both intracranial and spinal lesions b | Total |
|---|---|---|---|---|
| Number of cases (%) | Number of cases (%) | Number of cases (%) | Number of cases (%) | |
| Total number of cases | 19 (61.3) | 9 (29) | 3 (9.7) | 31 (100) |
| Signal characteristics a | ||||
| T2w homogeneous | 8 (42.1) | 9 (100) | 3 (100) | 20 (64.5) |
| Heterogeneous | 11 (57.9) | 11 (35.5) | ||
| T2w hyperintense | 14 (73.7) | 8 (88.9) | 3 (100) | 25 (80.7) |
| Isointense | 5 (26.3) | 1 (11.1) | 6 (19.3) | |
| Hypointense | 0 (0) | |||
| FLAIR Hyperintense | 10 (58.8) | 2 (100) | 3 (100) | 15 (68.2) |
| Isointense | 6 (35.3) | 6 (27.3) | ||
| Hypointense | 1 (5.9) | 1 (4.5) | ||
| T1w Hyperintense | 1 (5.2) | 1 (33.3) | 2 (6.7) | |
| Isointense | 6 (31.6) | 8 (100) | 2 (66.7) | 16 (53.3) |
| Hypointense | 12 (63.2) | 12 (40) | ||
| T2*w signal voids | ||||
| Yes | 3 (21.4) | 3 (15) | ||
| No | 11 (78.6) | 4 (100) | 2 (100) | 17 (85) |
| Cyst‐like lesions | ||||
| Yes | 1 (5.3) | 1 (3.2) | ||
| No | 18 (94.7) | 9 (100) | 3 (100) | 30 (96.8) |
| Contrast enhancement | ||||
| Strength | ||||
| None | 0 (0) | |||
| Mild | 2 (10.5) | 1 (12.5) | 3 (10) | |
| Moderate | 1 (5.3) | 1 (33.3) | 2 (6.7) | |
| Strong | 16 (84.2) | 7 (87.5) | 2 (66.7) | 25 (83.3) |
| Pattern | ||||
| Homogeneous | 6 (31.6) | 4 (50) | 1 (33.3) | 11 (36.7) |
| Heterogeneous | 9 (47.3) | 2 (25) | 11 (36.7) | |
| Focal | 1 (5.3) | 1 (12.5) | 2 (6.7) | |
| Multifocal | 2 (10.5) | 1 (12.5) | 2 (66.7) | 5 (16.7) |
| Rim | 1 (5.3) | 1 (3.3) | ||
| Meningeal enhancement | ||||
| None | 1 (5.3) | 1 (11.1) | 2 (6.4) | |
| Focal (dural tail) | 4 (21.1) | 3 (33.3) | 7 (22.6) | |
| Regional | 12 (63.1) | 4 (44.5) | 3 (100) | 19 (61.3) |
| Diffuse | 2 (10.5) | 1 (11.1) | 3 (9.7) | |
| Ependymal enhancement | ||||
| Yes | 4 (21.1) | 1 (33.3) | 5 (16.1) | |
| No | 15 (78.9) | 9 (100) | 2 (66.7) | 26 (83.9) |
| Mass effect | ||||
| None | 1 (5.3) | 1 (3.2) | ||
| Mild | 2 (10.5) | 3 (33.3) | 2 (66.7) | 7 (22.6) |
| Moderate | 4 (21.1) | 3 (33.3) | 7 (22.6) | |
| Severe | 12 (63.1) | 3 (33.3) | 1 (33.3) | 16 (51.6) |
| Perilesional edema | ||||
| None | 3 (15.8) | 3 (33.3) | 1 (33.3) | 7 (22.6) |
| Mild | 4 (21) | 2 (22.2) | 2 (66.7) | 8 (25.8) |
| Moderate | 6 (31.6) | 4 (44.5) | 10 (32.3) | |
| Severe | 6 (31.6) | 6 (19.3) | ||
| Signs of increased ICP | ||||
| Yes | 12 (63.2) | – | 1 (33.3) | 13 (59.1) |
| No | 7 (36.8) | – | 2 (66.7) | 9 (40.9) |
| Ventriculomegaly | ||||
| None | 8 (42.1) | – | 2 (66.7) | 10 (45.4) |
| Mild | 8 (42.1) | – | 1 (33.3) | 9 (40.1) |
| Moderate | 3 (15.8) | – | 3 (13.6) | |
| Syringohydromyelia | ||||
| None | 16 (84.2) | 5 (55.6) | 2 (66.7) | 23 (74.2) |
| Mild | 2 (10.5) | 4 (44.4) | 1 (33.3) | 7 (22.6) |
| Moderate | 1 (5.3) | 1 (3.2) | ||
| SSH‐TSE CSF signal loss | ||||
| None | – | 1 (12.5) | 1 (12.5) | |
| Focal | – | 2 (25) | 2 (25) | |
| Multifocal | – | 1 (12.5) | 1 (12.5) | |
| Extensive (> length of 7 vertebrae) | – | 4 (50) | 4 (50) |
Abbreviations: CSF, cerebrospinal fluid; ICP, intracranial pressure; MRI, magnetic resonance imaging; SSH‐TSE, single‐shot turbo spin echo.
Relative to normal gray matter.
Including 2 cats with cranial cervical spinal lesions extending from brainstem lesions, and 1 cat with multifocal spinal and brain lesions.
Additional MRI findings are summarized in Table 4. All 5 cats with infiltration of the nasal cavity or frontal sinuses or both showed permeative to moth‐eaten lysis of at least the frontal bones, with or without lysis of the cribriform plate, nasal turbinates, and nasal and maxillary bones. The bone lysis was considered mild in relation to the extent of the lesion (Figure 3). Two cats with confirmed bone marrow infiltration showed mild signal changes in vertebral bodies, including 1 with multifocal epidural and hypaxial lesions, leaving apparently intact vertebral cortices.
TABLE 4.
Additional MRI findings in 31 cats with nervous system lymphoma
| Additional MRI findings | Intracranial lesions | Spinal and spinal nerves lesions | Both intracranial and spinal lesions b | Total |
|---|---|---|---|---|
| Number of cases (%) | Number of cases (%) | Number of cases (%) | Number of cases (%) | |
| Total number of cases | 19 (61.3) | 9 (29) | 3 (9.7) | 31 (100) |
| Bone changes | 10 (52.6) | 5 (55.6) | 1 (33.3) | 16 (51.6) |
| Lysis | 7/10 | 7 | ||
| MRI signal changes a | 3/10 | 2/5 | 1/1 | 6 |
| Neuroforamen widening | 5/10 | 2/5 | 7 | |
| VB pressure atrophy | 1/5 | 1 | ||
| Muscles changes | 4 (21.1) | 9 (100) | 1 (33.3) | 14 (45.2) |
| Neurogenic muscle atrophy | 3/4 | 5/9 | 8 | |
| MRI signal changes a | 4/4 | 9/9 | 1/1 | 14 |
| Hypaxial masses | 1/1 | 1 | ||
| Lymphadenomegaly | 8 (42.1) | 3 (33.3) | 2 (66.7) | 13 (41.2) |
| Medial retropharyngeal | ||||
| Mild | 5/8 | 5 | ||
| Marked | 2/8 | 1/3 | 3 | |
| Mandibular and parotid | ||||
| Mild | 1/8 | 1 | ||
| Moderate | 1/8 | 1/2 | 2 | |
| Hepatic, marked | 1/2 | 1 | ||
| Sacral, mild | 1/3 | 1 | ||
| Colic, mild | 1/3 | 1 | ||
| Additional lesions | 2 (10.5) | 4 (33.3) | 6 (19.4) | |
| Focal NP wall thickening | 2/2 | 2 | ||
| Renal mass, RP effusion | 1/4 | 1 | ||
| Duodenal mass, P effusion | 1/4 | 1 | ||
| Intestinal mass, P effusion | 1/4 | 1 | ||
| Diffuse SI wall thickening | 1/4 | 1 |
Abbreviations: MRI, magnetic resonance imaging; NP, nasopharyngeal; P, peritoneal; RP, retroperitoneal; SI, small intestinal; VB, vertebral body.
Including enhancement.
Including 2 cats with cranial cervical spinal lesions extending from brainstem lesions, and 1 cat with multifocal spinal and brain lesions.
Only 6/31 cases (19.4%) showed multifocal lesions, involving the brain in 2, the brain and cranial cervical spine in 2, the brain and entire thoracolumbar spine in 1, and the brachial and lumbar plexuses in 1 cat. The lesions showed overall similar MRI characteristics in these cases.
3.3. CSF analysis
Cerebrospinal fluid analysis was abnormal in 20/25 cats (80%; cisternal, 12/15; lumbar, 7/9; unknown location, 1). Median TP and NCC in abnormal CSF were 93 mg/dL (range, 30‐1106) and 16.5 cells/μL (range, 0‐341), respectively. No significant differences were found in CSF TP (P = .64) and NCC (P = .32) between cisternal and lumbar punctures. Albuminocytologic dissociation was present in 6 cats (35%). Differential cytology was performed on 19 CSF samples. Lymphoma was diagnosed on CSF cytology in 5 cats (20%), including 1 immediately postmortem, and was suspected in another cat. Pleocytosis associated with increased TP was seen in 13 cats (65%; mononuclear, 4/13; mixed, 4/13; lymphocytic, 3/13; neutrophilic, 1/13). In 2 cats, the NCC was normal but showed an increased percentage of neutrophils.
3.4. Diagnosis, treatment, and outcome
Antemortem diagnosis was achieved in 10 cases (32.3%), based on CSF cytology (4/10), nervous system lesion cytology (3/10), or histopathology (biopsy; 4/10). Lymphoma was confirmed by both lesion cytology and histopathology in 1 case. Overall, nervous system lesion FNA or smears were obtained in 9 cats, being diagnostic for lymphoma in 3/9 (33.3%), suspicious in 2/9 (22.2%), and inconclusive in 4/9 (44.4%). Antemortem surgical biopsies were performed in 7 cats, being diagnostic in 4/7 (57.1%). Lymph node FNA (medial retropharyngeal, mandibular, ileocolic) were performed in 4 cats, being diagnostic for lymphoma in 1 cat with diagnostic CSF cytology.
Lymphoma was confirmed on necropsy histopathologic examination of the nervous system in 24 cats (77.4%), including 2 cats with postmortem biopsies only, and 3 of the 10 cats with an antemortem diagnosis. Complete brain histopathology was performed in 16 cats with intracranial lesions. Fourteen cats had concurrent neuroparenchymal and adjacent meningeal infiltration. Three of these lesions were classified as extra‐axial only on MRI. One cat with ill‐defined intra‐axial lesions and no visible meningeal enhancement on MRI showed infiltration of both the neuroparenchyma and meninges on histopathology. The lesions of the remaining 2 cats were correctly classified on MRI as intra‐axial and extra‐axial, respectively. Additionally, 1 cat with no visible MRI brain lesion showed leptomeningeal and optic nerve infiltration on histopathology.
Seven cats with spinal or brachial plexus involvement or both had complete histopathologic examination of the spinal cord and nerves, and 2 had biopsies performed. One cat had multifocal compressive and strictly epidural lesions, which were classified as both extradural and intradural on MRI based on meningeal enhancement. Lymphomatous infiltration of the nerves was confirmed histopathologically in all MRI cases that had nerve thickening and enhancement.
Complete necropsy examination was available in 20 cats (64.5%). Lymphoma was classified as multicentric in 11 (55%), primary nervous system in 8 (40%), and primary nasopharyngeal with local nervous system invasion in 1. The cell line was classified as B‐cell in 9/10 cats and T‐cell in 1.
Thirty cats (96.8%) were euthanized because of lymphoma, and 1 was lost to follow up. Median survival time after MRI acquisition was 1 day (range, 0‐218; mean ± SD, 30 ± 58 days). Twenty‐one cats (67.7%) were euthanized within 2 weeks of MRI, including 14 (45.2%) on the day of MRI acquisition, and 6 (19.4%) within the next 5 days. Survival times for the different treatment categories are summarized in Table 5. A significant difference in median survival time was found between cats treated palliatively (steroids, antibiotics, analgesic, or some combination of these) and cats treated by chemotherapy (P = .02). The other categories could not be compared given the small number of cats in each category.
TABLE 5.
Survival time in 31 cats with nervous system lymphoma by treatment categories
| Treatment | Numbers of cases (%) | Survival time in days | |
|---|---|---|---|
| Median (range) | Mean ± SD | ||
| Euthanasia on the day of MRI | 14 (45.2) | 0 | 0 |
| Medical management | 9 (29) | 4 (1‐36) | 10.5 ± 13.1 |
| Chemotherapy | 4 (12.9) | 83.5 (12‐218) | 99.3 ± 86.2 |
| Radiation therapy | 1 (3.2) | 181 | |
| Radiation therapy and chemotherapy | 1 (3.2) | 146 | |
| Debulking surgery and radiation therapy | 1 (3.2) | 90 | |
4. DISCUSSION
In our group of 31 cats with nervous system lymphoma, lesions commonly affected the CNS alone or both CNS and PNS. In more than two‐thirds of the cases, lesions were focal, showing ill‐defined borders, with widespread extension. They frequently affected the 2 intracranial axial compartments, or multiple spinal compartments. All lesions were enhancing, and regional meningeal enhancement was common. Infiltration into adjacent extra‐neural tissue was common with intracranial lesions because of the inclusion of nasopharyngeal and nasal lymphoma that invaded the nervous system.
Intracranial lymphoma may affect different parts of the brain, and has been classified as intraparenchymal lymphoma, extra‐axial lymphoma, angiotrophic lymphoma, lymphomatous choroiditis, and leptomeningeal lymphomatosis. 13 Contrary to previously published MRI findings, 6 , 26 in which lymphomatous intracranial lesions in cats were described as either extra‐ or intra‐axial, most intracranial lymphomas (59.1%) were classified as both extra‐ and intra‐axial in our study. In lesions with large extra‐axial components, 1 of the features classifying them as both intra‐ and extra‐axial was their ill‐defined and irregular border definition. Infiltration of the underlying neuroparenchyma was corroborated on necropsy examination, including cases classified as extra‐axial only on MRI. The most common intracranial neoplasm in cats and main differential diagnosis for a diffusely enhancing extra‐axial mass is meningioma, which usually has well‐defined regular margins with the underlying neuroparenchyma. 6 Tumor margination is therefore an important feature to take into consideration for differentiating intracranial lymphoma from meningioma in cats.
In cats with spinal lesions, the thoracic and lumbosacral regions were more commonly affected, and involvement of both extradural and intradural (including intramedullary) components was observed in most cases, similar to recent publications. 1 , 4 However, in our study >50% of the cases showed spinal nerve infiltration, which was more common than previously reported. 8 , 11 , 12 Neurolymphomatosis in cats mainly has been reported as single case reports, 16 , 22 , 24 , 34 , 35 , 36 , 37 brachial plexus involvement being most common, 16 , 35 either with 8 , 12 , 21 or without 11 , 35 infiltration of the subarachnoid space and spinal parenchyma, and alone 35 or in association with other peripheral nerve involvement. 22 , 34 Although less commonly reported, 1 , 24 , 34 , 38 lumbosacral plexus neurolymphomatosis was the most common lesion pattern in our group of cats. Similar to a recent publication, 24 MRI findings in this group included symmetrical or asymmetrical thickening and enhancement of the lumbosacral nerve roots or femoral and sciatic nerves or both, meningeal enhancement of the conus medullaris, with or without the presence of an extradural, intradural, or intramedullary mass, or some combination of these.
Extra‐neural tissue infiltration was observed in >40% of cats with intracranial lesions, with a permeative to moth‐eaten bone lysis growth pattern, leaving residual bone in situ with a preserved bone shape. This type of growth pattern recently has been described in cats with nasal lymphoma and carcinoma. 39 Although intracranial infiltration via the cribriform plate has been described in 7% to 34% of cats with nasal lymphoma, 39 , 40 , 41 to our knowledge ours is the first description of the peculiar infiltration of the rostral leptomeninges and olfactory lobes, associated with intralesional hemorrhages. The combination of this type of bone, leptomeningeal, and olfactory lobes infiltrative pattern on MRI may help to differentiate lymphoma from other neoplasms centered on the cribriform plate, such as nasal carcinomas, 39 , 40 , 42 esthesioneuroblastoma, 43 histiocytic sarcoma, 44 meningioma, 45 or from granulomatous rhinitis. 39 , 46 In contrast to brain lesions, bone lysis was not observed in our group of cats with spinal lesions, even in the presence of paravertebral lesions. This finding is consistent with previous publications on cats, 27 dogs, 27 , 28 and humans, 47 in which cortical sparing or cortical lysis with preserved vertebral shape were common features of round cell neoplasms, as opposed to other tumor types.
Intracranial invasion of nasopharyngeal lymphoma in cats with signs of cranial neuropathy has been described uncommonly, with similar MRI features. 48 , 49 , 50 Extension via the cranial foramina, lymphatic drainage pathways, or craniopharyngeal canal has been proposed as a possible mechanism for intracranial invasion of neoplastic cells. 48 In humans, the PNS is described as a common route for neoplasms, including lymphoma, to progress via the perineural or endoneural planes. 51 , 52 Typical MRI findings include cranial nerve thickening and enhancement, widening of the skull base foramina, enlargement of the cavernous sinus, neurogenic muscular atrophy, or some combination of these, 52 as seen in our group of cats. A similar growth pattern via the tympano‐occipital fissure was suspected in a cat with middle ear lymphomatous infiltration. Such lymphomatous infiltration of the middle ear has been described rarely in cats, 53 , 54 , 55 , 56 and should be taken into consideration as a differential diagnosis for infectious middle ear disease with intracranial empyema, given the shared MRI features.
The MRI signal characteristics and enhancement pattern of the lesions described here were similar to prior MRI studies of CNS lymphoma in dogs and cats. 6 , 26 Abnormal meninges adjacent to the lesions have been observed in 83% of cases, and diffuse meningeal enhancement has been described in half. 26 These results are comparable to our findings, in which 93.5% of the lesions showed abnormal meningeal enhancement, most commonly regional. However, leptomeningeal lymphomatosis was confirmed at necropsy in a cat without visible leptomeningeal enhancement on MRI, as previously described. 6 Focal ependymal enhancement of the third ventricle was noted in association with lesions centered on hypothalamic‐pituitary axis. Sellar or suprasellar lymphomatous masses have been described previously in cats, 19 , 20 , 57 although ependymal enhancement has not been reported. Lymphomatous infiltration of the hypothalamus and third ventricle, with or without infiltration of the hypophysis, optic chiasm, cavernous sinus and associated cranial nerves, is a rare but well described entity in human patients. 58 , 59 , 60 This infiltrative behavior may help to differentiate lymphoma from other sellar or suprasellar masses such as pituitary adenoma or carcinoma, although further study would be warranted for confirmation.
Diffusion weighted imaging, combined with conventional MRI, is considered valuable in the diagnostic of CNS lymphoma in humans. 61 , 62 Restricted diffusion is observed commonly because of high cellularity, appearing hyperintense in DWI trace images with a low ADC value. Similar findings have been described in a cat with B‐cell central nervous lymphoma, 23 and in cats with nasal 63 and nasopharyngeal 64 lymphoma. Interestingly, only 2/6 showed restrictive diffusion in our group of cats, although further studies are needed to evaluate the diagnostic and prognostic value of DWI in nervous system lymphoma of cats.
Similar to previous reports, 3 , 4 , 5 , 65 nonspecific clinical signs, such as anorexia, lethargy, and weight loss, were commonly reported in our cats. Neurological signs varied with location of the lesions, although altered consciousness, changes in behavior, paresis or plegia, spinal pain, and ataxia were commonly reported as reported previously. 1 , 4 , 5 , 8 , 65 , 66 Antemortem diagnosis was achieved in a third of the cases. Additionally, lymphoma was suspected on CSF in 1 cat, and on lesion cytology in 2. Cerebrospinal fluid was diagnostic for lymphoma in 20% of the cases and mononuclear pleocytosis with high TP was observed most commonly. This finding is similar to previous reports in which CSF cytology was diagnostic for lymphoma in only 9% to 35% of the cases, 4 , 5 , 8 because of the inflammatory nature of CSF, the difficulty in distinguishing between neoplastic and reactive lymphocytes, and frequent low cellularity. 14 , 15 , 67 Polymerase chain reaction for antigen receptor rearrangement (PARR) may assist in the diagnosis and prognosis of lymphoma. Fine‐needle aspiration or surgical biopsy of the lesion may be of greater diagnostic value, 14 although it remained relatively low in our group of cats.
Limitations of our study mainly reflect its retrospective and multi‐institutional nature, resulting in discrepancies in the available clinical, imaging, and pathological data. The inclusion criteria were based on a definitive diagnosis of lymphoma involving the nervous system, either by cytology or histopathology. Although a cytologic diagnosis of lymphoma was considered acceptable for inclusion, because this method is commonly used in routine clinical practice to achieve diagnosis, 68 most of the included cats were diagnosed by thorough necropsy and histopathologic examination. To further limit the inclusion of falsely diagnosed cats, 7 cases with a diagnosis of round cell neoplasia, being most likely but not confirmed lymphoma, were excluded from the study, most of them (6/7) being diagnosed by cytology only. This may, however, have introduced bias in the MRI findings, antemortem diagnosis rate, and outcome, because cats with more severe clinical signs and MRI changes were more likely to be euthanized and undergo necropsy examination, thus shortening median survival time. Information about cell characterization and body distribution of lymphoma was also lacking in some cats, leading to inability to differentiate MRI patterns or prognosis among the different cell types. Furthermore, MRI acquisition protocols and equipment were not standardized. The study was descriptive, and lesion classification was based on consensus evaluation of 3 observers. However, the consensus approach was chosen to optimize accuracy of MRI features and considered acceptable because interreader variability was not within the scope of the study. Finally, despite the inclusion of data from 4 institutions with high neurology caseloads over a large time period, the total number of included cases remained relatively small. The identified trends may not encompass all features of lymphoma in the nervous system of cats.
5. CONCLUSION
Lymphomatous nervous system infiltration in cats includes a wide range of MRI patterns, of which none is pathognomonic. Cytology or histopathology remain fundamental for a definitive diagnosis. However, together with clinical data and CSF analysis, MRI may provide a strong tentative antemortem diagnosis. Lymphoma should be considered in cases of extensive ill‐defined enhancing lesions affecting both the CNS and PNS, with or without involvement of multiple intracranial or spinal compartments, associated with focal or diffuse meningeal enhancement. Extra‐neural tissue infiltration may be seen in nerves passing through foramina or in bones, preserving most of their shape and contours. Such lesions should prompt sampling of CSF, of the lesion itself, lymph nodes, or any additional lesions to reach a definitive antemortem diagnosis. Further studies are needed to assess treatment, prognostic factors, and outcome in cats with lymphomatous infiltration of the nervous system.
CONFLICT OF INTEREST DECLARATION
Authors declare no conflict of interest.
OFF‐LABEL ANTIMICROBIAL DECLARATION
Authors declare no off‐label use of antimicrobials.
INSTITUTIONAL ANIMAL CARE AND USE COMMITTEE (IACUC) OR OTHER APPROVAL DECLARATION
Authors declare no IACUC or other approval was needed.
HUMAN ETHICS APPROVAL DECLARATION
Authors declare human ethics approval was not needed for this study.
Supporting information
Table S1. Neurologic examination findings in 31 cats with nervous system lymphoma.
ACKNOWLEDGMENT
No funding was received for this study.
Durand A, Keenihan E, Schweizer D, et al. Clinical and magnetic resonance imaging features of lymphoma involving the nervous system in cats. J Vet Intern Med. 2022;36(2):679‐693. doi: 10.1111/jvim.16350
REFERENCES
- 1. Marioni‐Henry K, Vite CH, Newton AL, et al. Prevalence of diseases of the spinal cord of cats. J Vet Intern Med. 2004;18:851‐858. [DOI] [PubMed] [Google Scholar]
- 2. Gonçalves R, Platt SR, Llabrés‐Díaz FJ, et al. Clinical and magnetic resonance imaging findings in 92 cats with clinical signs of spinal cord disease. J Feline Med Surg. 2009;11:53‐59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Mella SL, Cardy TJ, Volk HA, et al. Clinical reasoning in feline spinal disease: which combination of clinical information is useful? J Feline Med Surg. 2020;22:521‐530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Marioni‐Henry K, Van Winkle TJ, Smith SH, et al. Tumors affecting the spinal cord of cats: 85 cases (1980‐2005). J Am Vet Med Assoc. 2008;232:237‐243. [DOI] [PubMed] [Google Scholar]
- 5. Troxel MT, Vite CH, Van Winkle TJ, et al. Feline intracranial neoplasia: retrospective review of 160 cases (1985‐2001). J Vet Intern Med. 2003;17:850‐859. [DOI] [PubMed] [Google Scholar]
- 6. Troxel MT, Vite CH, Massicotte C, et al. Magnetic resonance imaging features of feline intracranial neoplasia: retrospective analysis of 46 cats. J Vet Intern Med. 2004;18:176‐189. [DOI] [PubMed] [Google Scholar]
- 7. Tomek A, Cizinauskas S, Doherr M, Gandini G, Jaggy A. Intracranial neoplasia in 61 cats: localisation, tumour types and seizure patterns. J Feline Med Surg. 2006;8:243‐253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lane SB, Kornegay JN, Duncan JR, Oliver JE Jr. Feline spinal lymphosarcoma—a retrospective evaluation of 23 cats. J Vet Intern Med. 1994;8:99‐104. [DOI] [PubMed] [Google Scholar]
- 9. Louwerens M, London CA, Pedersen NC, Lyons LA. Feline lymphoma in the post‐feline leukemia virus era. J Vet Intern Med. 2005;19:329‐335. [DOI] [PubMed] [Google Scholar]
- 10. Mello LS, Leite RV, Panziera W, et al. Feline lymphoma in the nervous system: pathological immunohistochemical, and etiological aspects in 16 cats. Pesqui Vet Bras. 2019;39:393‐401. [Google Scholar]
- 11. Zaki FA, Hurvitz AI. Spontaneous neoplasms of the central nervous system of the cat. J Small Anim Pract. 1976;17:773‐782. [DOI] [PubMed] [Google Scholar]
- 12. Spodnick GJ, Berg J, Moore FM, Cotter SM. Spinal lymphoma in cats—21 cases (1976‐1989). J Am Vet Med Assoc. 1992;200:373‐376. [PubMed] [Google Scholar]
- 13. Mandara MT, Motta L, Calo P. Distribution of feline lymphoma in the central and peripheral nervous systems. Vet J. 2016;216:109‐116. [DOI] [PubMed] [Google Scholar]
- 14. Twomey LN, Alleman AR. Cytodiagnosis of feline lymphoma. Comp Cont Educ Pract. 2005;27:17‐31. [Google Scholar]
- 15. Whitney MS, Coates JR. Cerebrospinal fluid analysis in the dog and cat. In: Sharkey LC, Radin MJ, Seelig D, eds. Veterinary Cytology. 1st ed. Hoboken, NJ, USA: John Wiley & Sons; 2021:638‐654. [Google Scholar]
- 16. Mellanby RJ, Jeffery ND, Baines EA, Woodger N, Herrtage ME. Magnetic resonance imaging in the diagnosis of lymphoma involving the brachial plexus in a cat. Vet Radiol Ultrasound. 2003;44:522‐525. [DOI] [PubMed] [Google Scholar]
- 17. Nakamoto Y, Ozawa T, Uchida K, et al. Primary intra‐axial B‐cell lymphoma in a cat. J Vet Med Sci. 2009;71:207‐210. [DOI] [PubMed] [Google Scholar]
- 18. Tsuboi M, Uchida K, Park ES, et al. Systemic T cell large granular lymphocyte lymphoma with multifocal white matter degeneration in the brain of a japanese domestic cat. J Vet Med Sci. 2010;72:795‐799. [DOI] [PubMed] [Google Scholar]
- 19. Simpson CJ, Mansfield CS, Milne ME, Hodge PJ. Central diabetes insipidus in a cat with central nervous system B cell lymphoma. J Feline Med Surg. 2011;13:787‐792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Guevar J, Gutierrez‐Quintana R, Peplinski G, Helm JR, Penderis J. Cavernous sinus syndrome secondary to intracranial lymphoma in a cat. J Feline Med Surg. 2014;16:513‐516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bray KY, Munana KR, Meichner K, et al. Eosinophilic meningomyelitis associated with T‐cell lymphoma in a cat. Vet Clin Pathol. 2016;45:698‐702. [DOI] [PubMed] [Google Scholar]
- 22. Sakurai M, Azuma K, Nagai A, et al. Neurolymphomatosis in a cat. J Vet Med Sci. 2016;78:1063‐1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Tanaka T, Akiyoshi H, Shimazaki H, et al. Apparent diffusion coefficient value for a B‐cell central nervous system lymphoma in a cat. JFMS Open Rep. 2018;4:2055116917750762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Beasley MJ, Hiebert EC, Daw DN, Alexander KJ, Gambino JM. Neurolymphomatosis caused by T‐cell lymphosarcoma in a cat: imaging description and treatment review. JFMS Open Rep. 2019;5:2055116919833534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hsueh CS, Tsai CY, Lee JCS, et al. CD56(+) B‐cell neurolymphomatosis in a cat. J Comp Pathol. 2019;169:25‐29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Palus V, Volk HA, Lamb CR, Targett MP, Cherubini GB. MRI features of CNS lymphoma in dogs and cats. Vet Radiol Ultrasound. 2012;53:44‐49. [DOI] [PubMed] [Google Scholar]
- 27. Auger M, Hecht S, Springer CM. Magnetic resonance imaging features of extradural spinal neoplasia in 60 dogs and seven cats. Front Vet Sci. 2021;7:610490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Allett B, Hecht S. Magnetic resonance imaging findings in the spine of six dogs diagnosed with lymphoma. Vet Radiol Ultrasound. 2016;57:154‐161. [DOI] [PubMed] [Google Scholar]
- 29. Bittermann S, Lang J, Henke D, Howard J, Gorgas D. Magnetic resonance imaging signs of presumed elevated intracranial pressure in dogs. Vet J. 2014;201:101‐108. [DOI] [PubMed] [Google Scholar]
- 30. Mai W. Syringomyelia. In: Mai W, ed. Diagnostic MRI in Dogs and Cats. 1st ed. Boca Raton, FL: CRC Press; 2018:595‐602. [Google Scholar]
- 31. Platt SR, Freeman AC. Neck and back pain. In: Platt SR, Olby NJ, eds. BSAVA Manual of Canine and Feline Neurology. 4th ed. Gloucester, UK: British Small Animal Veterinary Association; 2013:252‐270. [Google Scholar]
- 32. Graham JP, Newell SM, Voges AK, Roberts GD, Harrison JM. The dural tail sign in the diagnosis of meningiomas. Vet Radiol Ultrasound. 1998;39:297‐302. [DOI] [PubMed] [Google Scholar]
- 33. Dewey CW, Da Costa RC, Ducote JM. Neurodiagnostics. In: Dewey CW, Da Costa RC, eds. Practical Guide to Canine and Feline Neurology. 3rd ed. Hoboken, NJ, USA: John Wiley & Sons; 2016:61‐86. [Google Scholar]
- 34. Higgins MA, Rossmeisl JH, Saunders GK, et al. B‐cell lymphoma in the peripheral nerves of a cat. Vet Pathol. 2008;45:54‐57. [DOI] [PubMed] [Google Scholar]
- 35. Linzmann H, Brunnberg L, Gruber AD, Klopfleisch R. A neurotropic lymphoma in the brachial plexus of a cat. J Feline Med Surg. 2009;11:522‐524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Mandrioli L, Morini M, Biserni R, Gentilini F, Turba ME. A case of feline neurolymphomatosis: pathological and molecular investigations. J Vet Diagn Invest. 2012;24:1083‐1086. [DOI] [PubMed] [Google Scholar]
- 37. Mori M, Izawa T, Sasaki H, et al. A case of feline T‐cell lymphoma with tropism for striated muscle and peripheral nerve. J Comp Pathol. 2019;168:8‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Gory G, Couturier J, Cauvin E, Fournel‐Fleury C, Couturier L, Rault DN. Nervous system lymphoma with sciatic nerve involvement in two cats diagnosed using computed tomography and ultrasound guided fine needle aspiration. Vlaams Diergeneeskd Tijdschr. 2014;83:107‐112. [Google Scholar]
- 39. Bouyssou S, Hammond GJ, Eivers C. Comparison of CT features of 79 cats with intranasal mass lesions. J Feline Med Surg. 2021;23:987–995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Schoenborn WC, Wisner ER, Kass PP, Dale M. Retrospective assessment of computed tomographic imaging of feline sinonasal disease in 62 cats. Vet Radiol Ultrasound. 2003;44:185‐195. [DOI] [PubMed] [Google Scholar]
- 41. Haney SM, Beaver L, Turrel J, et al. Survival analysis of 97 cats with nasal lymphoma: a multi‐institutional retrospective study (1986‐2006). J Vet Intern Med. 2009;23:287‐294. [DOI] [PubMed] [Google Scholar]
- 42. Tromblee TC, Jones JC, Etue AE, et al. Association between clinical characteristics, computed tomography characteristics, and histologic diagnosis for cats with sinonasal disease. Vet Radiol Ultrasound. 2006;47:241‐248. [DOI] [PubMed] [Google Scholar]
- 43. Söffler C, Hartmann A, Gorgas D, et al. Magnetic resonance imaging features of esthesioneuroblastoma in three dogs and one cat. Tierarztl Prax Ausg K Kleintiere Heimtiere. 2016;44:333‐340. [DOI] [PubMed] [Google Scholar]
- 44. Santifort KM, Jurgens B, Grinwis GC, et al. Invasive nasal histiocytic sarcoma as a cause of temporal lobe epilepsy in a cat. JFMS Open Rep. 2018;4:2055116918811179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Pérez‐Accino J, Suñol A, Munro E, Philbey AW, Marioni‐Henry K. Feline meningioma with extensive nasal involvement. JFMS Open Rep. 2019;5:2055116919833732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Altuzarra R, Movilla R, Roura X, Espada Y, Majo N, Novellas R. Computed tomographic features of destructive granulomatous rhinitis with intracranial extension secondary to leishmaniasis in a cat. Vet Radiol Ultrasound. 2020;61:E64‐E68. [DOI] [PubMed] [Google Scholar]
- 47. Moulopoulos LA, Dimopoulos MA, Vourtsi A, Gouliamos A, Vlahos L. Bone lesions with soft‐tissue mass: magnetic resonance imaging diagnosis of lymphomatous involvement of the bone marrow versus multiple myeloma and bone metastases. Leuk Lymphoma. 1999;34:179‐184. [DOI] [PubMed] [Google Scholar]
- 48. Chang Y, Thompson H, Reed N, Penderis J. Clinical and magnetic resonance imaging features of nasopharyngeal lymphoma in two cats with concurrent intracranial mass. J Small Anim Pract. 2006;47:678‐681. [DOI] [PubMed] [Google Scholar]
- 49. Kmieciak L, Hague DW, Neumann ZL, Joslyn S. What is your neurologic diagnosis? Nasopharyngeal lymphoma. J Am Vet Med Assoc. 2016;248:613‐616. [DOI] [PubMed] [Google Scholar]
- 50. Osinchuk SC, Zwueste DM, Grahn BH. Peripheral cranial neuropathies consistent with cavernous sinus syndrome caused by extracranial nasopharyngeal lymphoma in a cat. Can Vet J. 2019;60:1156‐1160. [PMC free article] [PubMed] [Google Scholar]
- 51. Cruz AAV, Valera FCP, Carenzi L, Chahud F, Barros GE, Elias J. Orbital and central nervous system extension of nasal natural killer/T‐cell lymphoma. Ophthalmic Plast Reconstr Surg. 2014;30:20‐23. [DOI] [PubMed] [Google Scholar]
- 52. Saremi F, Helmy M, Farzin S, Zee CS, Go JL. MRI of cranial nerve enhancement. AJR Am J Roentgenol. 2005;185:1487‐1497. [DOI] [PubMed] [Google Scholar]
- 53. Trevor PB, Martin RA. Tympanic bulla osteotomy for treatment of middle‐ear disease in cats—19 cases (1984‐1991). J Am Vet Med Assoc. 1993;202:123‐128. [PubMed] [Google Scholar]
- 54. de Lorimier LP, Alexander SD, Fan TM. T‐cell lymphoma of the tympanic bulla in a feline leukemia virus‐negative cat. Can Vet J. 2003;44:987‐989. [PMC free article] [PubMed] [Google Scholar]
- 55. Santagostino SF, Mortellaro CM, Buchholz J, et al. Primary angiocentric/angioinvasive T‐cell lymphoma of the tympanic bulla in a feline leukaemia virus‐positive cat. JFMS Open Rep. 2015;1:2055116915593966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Kerns AT, Brakel KA, Premanandan C, Saffire A, Moore SA. Extranodal non‐B, non‐T‐cell lymphoma with bilateral tympanic bulla involvement in a cat. JFMS Open Rep. 2018;4:2055116918756724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Morrison JA, Fales‐Williams A. Hypernatremia associated with intracranial B‐cell lymphoma in a cat. Vet ClinPathol. 2006;35:362‐365. [DOI] [PubMed] [Google Scholar]
- 58. Pascual JM, Gonzalez‐Llanos F, Roda JM. Primary hypothalamic‐third ventricle lymphoma. Case report and review of the literature. Neurocirugia. 2002;13:305‐310. [DOI] [PubMed] [Google Scholar]
- 59. Tanki HN, Malik KN, Makhdoomi R, Feroz S, Ramzan AU. Primary hypothalamic lymphoma in an adult male: a case report and literature review. Oman Med J. 2018;33:346‐351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Shin DW, Kim JH, Kim YH, Cho YH, Hong SH. Primary central nervous system lymphoma involving the hypothalamic‐pituitary axis: a case series and pooled analysis. J Neurooncol. 2020;147:339‐349. [DOI] [PubMed] [Google Scholar]
- 61. Nabavizadeh SA, Vossough A, Hajmomenian M, Assadsangabi R, Mohan S. Neuroimaging in central nervous system lymphoma. Hematol Oncol Clin North Am. 2016;30:799‐821. [DOI] [PubMed] [Google Scholar]
- 62. Lu XY, Xu WL, Wei YY, et al. Diagnostic performance of DWI for differentiating primary central nervous system lymphoma from glioblastoma: a systematic review and meta‐analysis. Neurol Sci. 2019;40:947‐956. [DOI] [PubMed] [Google Scholar]
- 63. Tanaka T, Ashida K, Iimori Y, et al. MRI findings, including diffusion‐weighted imaging, in seven cats with nasal lymphoma and two cats with nasal adenocarcinoma. J Feline Med Surg. 2021;23:393‐399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Tanaka T, Akiyoshi H, Mie K, Nishida H. MRI findings, including diffusion‐weighted imaging and apparent diffusion coefficient value, in two cats with nasopharyngeal polyps and one cat with lymphoma. JFMS Open Rep. 2018;4:2055116918812254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Marioni‐Henry K. Feline spinal cord diseases. Vet Clin North Am Small Anim Pract. 2010;40:1011‐1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Taylor SS, Goodfellow MR, Browne WJ, et al. Feline extranodal lymphoma: response to chemotherapy and survival in 110 cats. J Small Anim Pract. 2009;50:584‐592. [DOI] [PubMed] [Google Scholar]
- 67. Singh M, Foster DJ, Child G, Lamb WA. Inflammatory cerebrospinal fluid analysis in cats: clinical diagnosis and outcome. J Feline Med Surg. 2005;7:77‐93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Burkhard MJ, Bienzle D. Making sense of lymphoma diagnostics in small animal patients. Vet Clin North Am Small Anim Pract. 2013;43:1331‐1347. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Neurologic examination findings in 31 cats with nervous system lymphoma.


