Abstract
Background
Reticulocyte indices have been suggested as alternatives to transferrin saturation (TSAT) for iron status assessment in humans and dogs but they have not been evaluated thoroughly in cats.
Objectives
To assess the value of the reticulocyte indices for the diagnosis of iron deficiency in cats with chronic kidney disease (CKD) and chronic hematuria associated with subcutaneous ureteral bypasses (SUBs).
Animals
Sixty‐four cats: 16 healthy, 14 CKD without SUB, and 34 CKD with SUB.
Methods
Prospective observational cross‐sectional study of cats presented for routine nephrology visits. Primary outcomes included assessment of the diagnostic values of erythrocyte indices (mean corpuscular volume, hemoglobin, and hemoglobin concentration: MCV, MCH, and MCHC) and reticulocyte indices (mean corpuscular volume, MCVr; corpuscular hemoglobin, CHr), using TSAT as reference.
Results
Iron deficiency was diagnosed in 9/64 cats (14%). A receiver‐operating characteristic curve analysis yielded a moderate discriminatory value for CHr in this diagnosis: area under the curve [AUC] = .75 (95% confidence interval, 0.48‐0.89); P = .006; sensitivity 67%, specificity 82% for a cutoff of 15.9 pg. This compared favorably to MCVr (AUC = .63; P = .29), MCV (AUC = .58; P = .45), MCH (AUC = .64; P = .19), and MCHC (AUC = .7; P = .03).
Conclusion and Clinical Importance
CHr added moderate value to the diagnosis of iron deficiency in cats with CKD.
Keywords: CHr, iron deficiency, MCVr, serum iron concentration, transferrin saturation
Abbreviations
- CBC
complete blood count
- CHr
corpuscular hemoglobin of reticulocytes
- CI
confidence interval
- CKD
chronic kidney disease
- ID
iron deficiency
- IQR
interquartile range
- IRIS
international renal interest society
- MCH
mean corpuscular hemoglobin
- MCHC
mean corpuscular hemoglobin concentration
- MCV
mean corpuscular volume
- MCVr
mean corpuscular volume of reticulocytes
- ROC
receiver‐operating characteristic
- SUB
subcutaneous ureteral bypass
- TIBC
total iron binding capacity
- TSAT
transferrin saturation
- USG
urine specific gravity
1. INTRODUCTION
Iron deficiency (ID) affects important pathways of energy metabolism, DNA synthesis, and cellular immune response, causing nonspecific clinical signs recognized in humans as lethargy, decreased quality of life, and nonregenerative microcytic hypochromic anemia in later stages. 1 , 2 , 3 , 4 , 5 , 6 Chronic external blood loss is the main cause of ID in small animals and includes gastrointestinal and urinary bleeding, or infestation with endo‐ or ectoparasites. 7 , 8 , 9 , 10 Increased iron demand during the treatment with erythropoiesis‐stimulating agents further worsens ID when not supplemented adequately. 11 , 12 , 13
The semiquantitative evaluation of iron stores in the bone marrow or in the liver with Prussian blue stain for ferric (3+) iron (including hemosiderin) and the quantitative measurement of hepatic iron by dry matter analysis are considered gold standards for the assessment of the iron status in humans and dogs. 8 , 14 In cats the bone marrow does not contain hemosiderin and liver biopsies are rarely performed for this purpose. 7 , 8 Serum ferritin concentration is a good predictor of the body iron stores in humans and used as the sole marker of ID by some authors. 14 , 15 However, since ferritin is a positive acute phase protein, it can be elevated into the normal range when systemic inflammation is associated with ID, limiting its diagnostic utility. 14 , 16 , 17 The serum iron concentration and the total iron‐binding capacity (TIBC) are used to calculate the transferrin saturation (TSAT), which serves to evaluate the iron status in various species, including cats. 7 , 8 The routinely available erythrocyte indices mean corpuscular volume (MCV) and mean corpuscular hemoglobin concentration (MCHC) are often used clinically when screening for ID. However, they are late and nonspecific indicators of the iron status and poorly reflect early nonanemic ID. 18 The reticulocyte indices mean cell volume (MCVr) and hemoglobin content (CHr) have been proposed to better reflect early iron‐deficient erythropoiesis due to the shorter lifespan of the aggregate reticulocytes (1‐2 days) compared to the erythrocytes (70 days in cats). 19 , 20 There is a clear relationship between reticulocytes indices and ID in humans, 16 , 21 , 22 , 23 , 24 but the results are more ambiguous in dogs. 25 , 26 , 27 , 28 In cats, a low CHr is associated with functional ID in chronic gastrointestinal disease 29 and this variable is highly sensitive (94%) and moderately specific (77%) for the diagnosis of ID using a cutoff of 14.2 pg. 30 Another study found that a low CHr is specific but not sensitive to detect iron‐limited erythropoiesis in diseased cats. 20
The primary objectives of our study were: (1) to assess the value of the reticulocyte indices CHr and MCVr for the diagnosis of ID in a sample of cats with chronic kidney disease (CKD), with CKD and chronic hematuria associated with a subcutaneous ureteral bypass (SUB) and healthy cats, and (2) to compare the diagnostic value of the reticulocyte indices with that of the erythrocyte indices.
We hypothesized that the reticulocyte indices reflect the iron status of these cats adequately and more accurately than the erythrocyte indices.
2. MATERIALS AND METHODS
2.1. Study design
This study was designed as a prospective observational cross‐sectional study. It was conducted in the Division of Small Animal Internal Medicine—Nephrology of the Vetsuisse Faculty University of Bern (Switzerland). Client‐ and staff‐owned cats were recruited prospectively during 2 periods (November 2014‐November 2016 and January 2019‐October 2019). Animals were cared for according to national and institutional guidelines. Ethical approval was granted by the Cantonal Committee on Animal Experiments (projects BE 69/14+ and BE 72/19) and written owner consent was obtained for all cats enrolled in the study.
2.2. Animals and disease definition
Three groups of cats were recruited to obtain a wide range of results in iron status: healthy cats (expected normal iron status), cats with CKD (expected low‐level ID in approximately 10% to 20% of the cats 31 ), and cats with CKD and chronic hematuria after treatment of ureteral obstruction with SUBs (expected more prevalent and more pronounced ID due to additional ongoing blood loss 10 ).
Healthy cats: adult cats (>1 year old) from the staff or students and cats presented for routine health maintenance were screened for enrollment in the healthy control group. They were deemed healthy based on history, physical examination, and clinicopathological results (hematology, chemistry). Cats were excluded if they had received any medication in the previous 2 months, except for vaccines and flea and tick preventatives; this criterion was used in this group of cats to ensure the absence of any underlying chronic or acute disease. Cats with a serum creatinine up to 25% above the upper limit of the reference range and a urine specific gravity (USG) suggestive of prerenal azotemia (>1.035) were screened a second time within 8 weeks and included if they satisfied the criteria.
CKD: cats with stable CKD were enrolled during regular follow‐ups for their disease. CKD was diagnosed and staged according to the guidelines of the International Renal Interest Society (IRIS). 32 They had to fulfill ≥1 of the following criteria: (a) previous diagnosis of CKD based on persistently (>3 months) increased serum creatinine and a USG <1.035; (b) abdominal ultrasound signs compatible with CKD, with ≥2 of the following: kidney hyperechogenicity, markedly decreased corticomedullary differentiation, decreased kidney size or asymmetry, or irregular renal contour. Cats with serum creatinine >0.3 mg/dL over that of the previous visit (>1 month) were excluded.
SUB: cats with CKD treated with a SUB for ureteral obstruction were included during an appointment for maintenance of the system. The cats were enrolled at the earliest 90 days after SUB placement if they had documented hematuria (>10 red blood cells per high power field) in at least 2 of the 3 last visits or frequent episodes of red urine reported by the owner. All cats with a SUB also had CKD, documented with a stable and persistently elevated plasma creatinine concentration.
Cats with acute kidney injury, concurrent acute illness, active inflammatory disease other than uremia, or noncontrolled hyperthyroidism, and cats treated with iron supplementation, blood transfusion, or erythropoiesis‐stimulating agents <90 days before enrollment were excluded.
2.3. Collection of data and laboratory analyses
Age, sex, breed, detailed history with particular emphasis on treatments and the presence of urinary tract signs, and physical examination were recorded at inclusion for each cat. Blood was sampled by venipuncture, and urine either by cystocentesis or by puncture of the SUB port. Complete blood count, kidney chemistry profile, iron panel, and urinalysis (USG, dipstick, sediment ± bacteriological culture) were submitted for every cat. Additionally, serum thyroxine concentration was submitted for cats >6 years of age (IDEXX Diavet, Bäch, Switzerland). The CBC was performed on ethylenediaminetetraacetic acid (EDTA) anticoagulated blood with an automated Advia 2120i analyzer (Siemens Healthcare, Zürich, Switzerland) and included the microscopic evaluation of a blood smear. In addition to the usual CBC variables, the following reticulocyte variables were evaluated: automated reticulocyte count, mean corpuscular volume of reticulocytes (MCVr), and hemoglobin content of reticulocytes (CHr). The kidney chemistry panel was performed on lithium heparin anticoagulated plasma using a Cobas c501 analyzer (Roche Diagnostics, Basel, Switzerland) and included the following analytes: creatinine, urea, sodium, potassium, chloride, phosphate, total magnesium, total calcium, albumin, and serum amyloid A concentrations. For the iron panel, serum was collected from plain tubes immediately after centrifugation at 1400g for 10 minutes at room temperature. Serum was aliquoted in 1.5‐mL tubes and stored at −80°C until sent for batched analysis to the Kansas State Veterinary Diagnostic Laboratory (Kansas State University, Manhattan, Kansas). The iron panel included serum iron concentration, serum ferritin concentration, and total iron‐binding capacity (TIBC). The TIBC was calculated as the sum of the unbound iron binding capacity and the serum iron concentration; the unbound iron binding capacity was indirectly proportional to the unbound excess iron after addition of a known quantity of iron to the cats' samples. The transferrin saturation was calculated as TSAT (%) = serum iron concentration [μg/dL]/TIBC [μg/dL]. All samples were analyzed within 4 months of collection.
Anemia was defined as hematocrit <27%, based on the reference interval of our laboratory (27‐47). Iron deficiency was defined based on TSAT, using a cutoff of 20% as recommended for humans 33 and often adopted for small animals. 8
2.4. Statistical analysis
Data were organized with Microsoft Excel 2016 (Version 16.0.5134.1000, Microsoft Corporation, Redmond, Washington) and analyzed with the NCSS statistical software package (NCSS 9, 2013. NCSS, LLC, Kaysville, Utah). Statistical significance was set at P < .05.
All cats were analyzed as a single study sample independently of their group of origin. Continuous variables were tested for normality by visual inspection of histograms and a Shapiro‐Wilk test. Because most data were not normally distributed, their descriptive statistics were reported unless stated otherwise as median (interquartile range, IQR) and they were compared between groups (ID versus non‐ID; anemia vs nonanemia) using a nonparametric Mann‐Whitney U test. Categorical variables were reported as absolute numbers and proportions (95% confidence interval, 95% CI).
The correlations between the nonparametric variables possibly associated with ID (hematocrit, hemoglobin concentration, erythrocyte indices, reticulocyte count, reticulocyte indices, platelet count) and TSAT were assessed using the Spearman's correlation coefficient (r s). The strength of the relationship was qualified as weak for r s = 0 to .3; moderate for r s = .3 to .6; strong for r s = .6 to .9; and very strong for r s = .9 to 1. The predictive values of these variables for a diagnosis of ID were determined with receiver‐operating characteristic (ROC) curve analyses, using the area under the curve (AUC) and its 95% CI to indicate the strength of the predictive value. An AUC of .6 to .7 was considered to indicate a weak; .7 to .8, a moderate; .8 to .9, a strong; and .9 to 1, a very strong predictor.
3. RESULTS
3.1. Study sample
Sixty‐four cats met the inclusion criteria, including 16 healthy cats, 14 cats with CKD without a SUB, and 34 cats with CKD, chronic hematuria and a SUB. Six cats were excluded from the screening of 22 clinically healthy candidates because of hyperthyroidism (n = 1), persistent moderate azotemia (n = 3), or mild azotemia with USG <1.035 (n = 2). Concomitant diseases were identified in 9 cats among cats with CKD: hyperthyroidism controlled with methimazole (n = 1), primary hyperparathyroidism treated with alendronate (n = 1), idiopathic hypercalcemia treated with alendronate (n = 1), asymptomatic hypertrophic cardiomyopathy treated with atenolol (n = 1), chronic intermittent upper respiratory infection (n = 1, inactive when sampled), subclinical chronic bacteriuria (n = 2), diabetes mellitus, asthma and chronic pancreatitis (n = 1, no clinical evidence of active pancreatitis in the 2 months preceding blood sampling), and incidentally discovered lung mass of undetermined etiology (n = 1).
The study sample comprised 32 males (31 neutered and 1 intact) and 32 females (31 spayed and 1 intact). The median age was 8 years (range, 1‐16.6; IQR, 5.1‐10.7) and 3 cats were adults of unknown age. The breeds included Domestic Shorthair (n = 44), Domestic Longhair (n = 3), Maine Coon (n = 3), Siamese (n = 3), Persians (n = 2), and 1 each of the following: Balinese, Bengal, Birman, British Longhair, Chartreux, Devon Rex, Norwegian Forest Cat, Siberian Forest Cat, and Turkish Angora.
3.2. Iron profile
Median (IQR) serum iron concentration, TIBC, TSAT and serum ferritin concentration of all cats was 83.5 μg/dL (69‐96.3), 322 μg/dL (287‐347), 26.4% (22.4‐30.4), and 301 ng/mL (206‐372), respectively (Table 1). Based on a TSAT <20%, 9 cats were characterized as iron deficient (ID; 14%; 95% CI, 7‐25%). Of these 9 cats with ID, 7 had a decreased and 2 had a low‐normal serum iron concentration; 7 had a normal and 2 an elevated serum ferritin concentration. A comparison of the relevant CBC, chemistry, and iron profile variables between cats with and without ID is presented in Table 2 and Figures 1 and 2.
TABLE 1.
Relevant variables of the CBC, chemistry profiles, and iron profiles for the 64 cats included in the study
| Variable | Unit | Median | IQR | Range | RI |
|---|---|---|---|---|---|
| CBC | |||||
| Hematocrit | % | 35 | 29‐39 | 14‐48 | 27‐47 |
| Hemoglobin | g/dL | 12 | 9.6‐13.8 | 4.9‐16.6 | 8.2‐15.3 |
| RBC | 106/μL | 8.2 | 6.6‐9.2 | 3.2‐10.7 | 5.9‐11.2 |
| MCV | fL | 42.9 | 40.7‐44.9 | 32.9‐49.5 | 37‐55 |
| MCH | pg | 14.8 | 14.1‐15.6 | 11.2‐18.2 | 11.3‐17.2 |
| MCHC | g/dL | 34.6 | 34.1‐35 | 32.1‐36.9 | 26.3‐35.9 |
| RDW | % | 15.8 | 15.2‐16.7 | 14.2‐21.3 | 13.8‐21.1 |
| Reticulocytes | 103/μL | 25.3 | 15.8‐35.8 | 4.8‐79.8 | 3.7‐94.1 |
| MCVr | fL | 56.8 | 53.1‐59.8 | 45.1‐73.2 | 47.6‐72.8 |
| CHr | pg | 17.6 | 16‐18.6 | 14.1‐20.1 | >14.2 |
| Platelets | 103/μL | 215 | 139‐317 | 21‐494 | 180‐430 |
| MPV | fL | 15.6 | 13.6‐19.9 | 9.7‐26.4 | 10.2‐25.8 |
| Leukocytes | 103/μL | 6.5 | 5.2‐9 | 2.5‐28.9 | 6.5‐15.4 |
| Seg. neutrophils | 103/μL | 4.2 | 3.3‐5.8 | 1.4‐27.0 | 2.5‐12.5 |
| Bands | 103/μL | 0 | 0‐0.04 | 0‐0.29 | 0‐0.3 |
| Chemistry | |||||
| Urea | mg/dL | 73.3 | 56.5‐106.9 | 35.4‐287.1 | 39‐73.3 |
| Creatinine | mg/dL | 2.08 | 1.60‐2.87 | 0.96‐6.57 | 0.59‐1.56 |
| Phosphorus | mg/dL | 4.09 | 3.53‐4.71 | 1.64‐12.14 | 2.54‐5.91 |
| Iron profile | |||||
| Serum iron | μg/dL | 83.5 | 69.0‐96.3 | 28‐155 | 65‐162 |
| TIBC | μg/dL | 322 | 287‐347 | 233‐554 | 250‐470 |
| TSAT | % | 26.4 | 22.4‐30.4 | 10‐52.2 | 20‐60 |
| Ferritin | ng/mL | 301 | 206‐372 | 96‐880 | 82‐395 |
Note: The RI for the CBC and the chemistry variables were generated in‐house. Those of the iron profile (serum iron, TIBC, ferritin) were provided by the Kansas State Laboratory. The RI for TSAT was adapted from criteria commonly used in humans and often adopted in small animals.
Abbreviations: CHr, corpuscular hemoglobin of reticulocytes; IQR, interquartile range; MCH, mean corpuscular hemoglobin; MCHC, mean corpuscular hemoglobin concentration; MCV, mean corpuscular volume; MCVr, mean corpuscular volume of reticulocytes; MPV, mean platelet volume; RBC, red blood cell count; RDW, red blood cell distribution width; RI, reference interval; seg. neutrophils, segmented neutrophils; TIBC, total iron binding capacity; TSAT, transferrin saturation.
TABLE 2.
Complete blood count and iron profile variables in 9 cats with iron deficiency (ID) and 55 cats without iron deficiency (non‐ID)
| ID (n = 9) | Non‐ID (n = 55) | |||||
|---|---|---|---|---|---|---|
| Variable (RI) | Unit | Median | IQR | Median | IQR | P |
| Hematocrit (27‐47) | % | 31 | 24‐36 | 36 | 30‐40 | .06 |
| Hemoglobin (82‐153) | g/dL | 10.7 | 8.3‐12.4 | 12.6 | 10‐14 | .05 |
| RBC (5.9‐11.2) | 106/μL | 7.6 | 6.09‐8.02 | 8.56 | 6.67‐9.34 | .07 |
| MCV (37‐55) | fL | 42.1 | 39.8‐44.1 | 43.1 | 41.1‐44.9 | .44 |
| MCH (11.3‐17.2) | pg | 14.3 | 13.5‐15 | 14.8 | 14.1‐15.6 | .17 |
| MCHC (263‐359) | g/dL | 34.1 | 33.5‐34.8 | 34.6 | 34.2‐35.1 | .06 |
| Reticulocytes (3700‐94 100) | 103/μL | 25.3 | 15.1‐31.8 | 25.3 | 15.8‐38.2 | .85 |
| MCVr (47.6‐72.8) | fL | 51.4 | 50.5‐60.9 | 57 | 53.7‐59.8 | .21 |
| CHr (>14.2) | pg | 15.6 | 14.5‐17.7 | 17.7 | 16.3‐18.7 | .02* |
| Platelets (180‐430) | 103/μL | 209 | 163‐345 | 216 | 127‐311 | .44 |
| Serum iron (65‐162) | μg/dL | 63 | 48.5‐66.5 | 88 | 76‐101 | – |
| TIBC (250‐470) | μg/dL | 347 | 318‐374 | 313 | 283‐344 | – |
| TSAT (20‐60) | % | 18.1 | 14.1‐18.6 | 27.4 | 24‐31.6 | – |
| Ferritin (82‐395) | ng/mL | 300 | 188‐390 | 302 | 210‐372 | .71 |
Note: The P‐values refer to the comparison of cats with and without iron deficiency using a Mann‐Whitney U test.
Abbreviations: CHr, corpuscular hemoglobin of reticulocytes; IQR, interquartile range; MCH, mean corpuscular hemoglobin; MCHC, mean corpuscular hemoglobin concentration; MCV, mean corpuscular volume; MCVr, mean corpuscular volume of reticulocytes; RBC, red blood cell count; RI, reference interval; TIBC, total iron binding capacity; TSAT, transferrin saturation.
Denotes comparisons with a statistically significant difference set as a P < .05.
FIGURE 1.
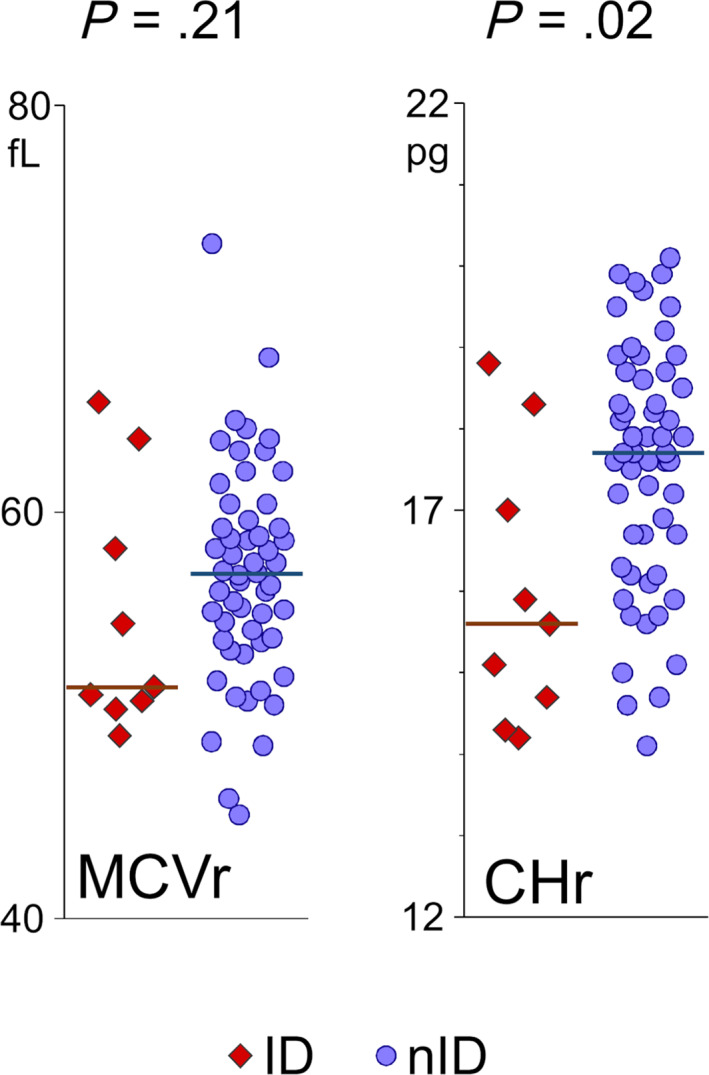
Dot plot representation of the reticulocyte indices (MCVr and CHr) stratified based on the presence or not of iron deficiency (ID vs non‐ID) in 64 cats. The horizontal bars represent the median value for each group. CHr, corpuscular hemoglobin of reticulocytes; ID, cats with iron deficiency; MCVr, mean corpuscular volume of reticulocytes; non‐ID, cats without iron deficiency
FIGURE 2.
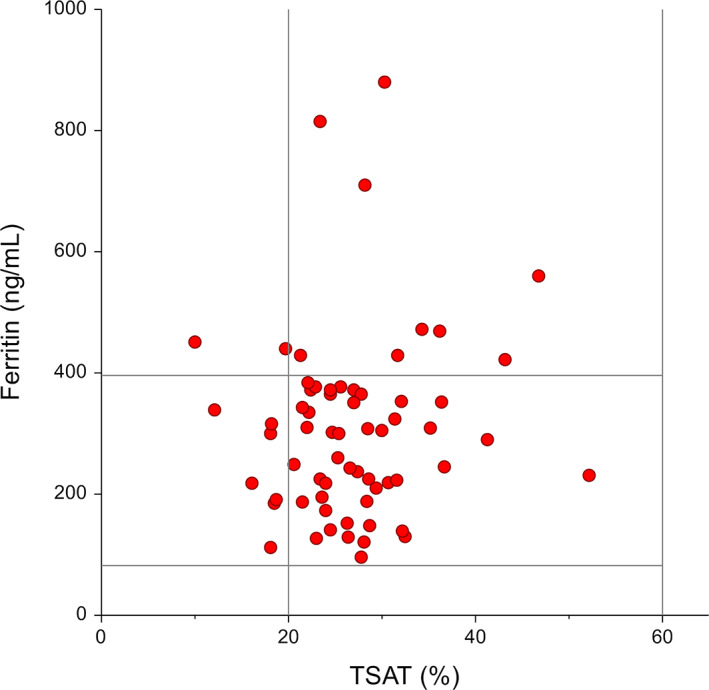
Scatter plot of the serum ferritin concentration (vertical) and the transferrin saturation (TSAT, horizontal) in 64 cats. The vertical and horizontal bars represent the limits of the reference intervals for the 2 variables: ferritin, 82 to 395 ng/mL; TSAT, 20% to 60%
3.3. CBC and chemistry
Relevant CBC and chemistry variables for the 64 study cats are summarized in Table 1. Eleven cats were anemic (17%; 95% CI, 9‐29%), including 4 cats with CKD and 7 cats with CKD and a SUB. Anemic cats had a lower reticulocyte count (15.7 × 103/μL; IQR, 13.6‐24.1) than cats without anemia (27.8 × 103/μL; 18.9‐39.7; P = .009). The anemia was normocytic and normochromic in all affected cats. Their MCV (42.8 fL, 40.6‐48), MCH (15 pg, 13.9‐15.8), and MCHC (346 g/L, 337‐351) were not different from that of nonanemic cats (MCV = 43 fL, 41.2‐44.7, P = .83; MCH = 14.8 pg, 14.1‐15.6, P = .88; MCHC = 345 g/L, 342‐350, P = .87).
The reticulocyte indices MCVr and CHr are reported for the whole study sample in Table 1. The CHr was lower in cats with ID compared to cats without ID (P = .02) but the MCVr was not different between both groups of cats (P = .2, Table 2, Figure 1). The reticulocyte indices were lower in cats with anemia (MCVr = 53.2 fL, 50.3‐58.2; CHr = 15.6 pg, 14.6‐16.9) than in cats without anemia (MCVr = 57.1 fL, 54‐60.9, P = .02; CHr = 17.9 pg, 16.7‐18.7, P = .002).
All healthy cats showed no evidence of kidney disease. Of the 48 cats with CKD, 5 were diagnosed with IRIS stage 1, 27 with stage 2, 11 with stage 3, and 5 with stage 4.
3.4. Correlation of CBC variables with TSAT and prediction of ID
Hematocrit, hemoglobin concentration, reticulocyte count, and platelet count were not correlated with TSAT (Table 3). The erythrocyte indices MCV (r s = .23; P = .03) and MCH (r s = .26; P = .03) were weakly correlated with TSAT, but not MCHC (r s = .2; P = .47). The reticulocyte indices CHr (r s = .29; P = .003) and MCVr (r s = .18; P = .02) both were weakly correlated with TSAT (Table 3, Figure 3).
TABLE 3.
Correlation of relevant CBC variables with the transferrin saturation in 64 cats
| r s | 95% CI | P | Strength | |
|---|---|---|---|---|
| Hematocrit | .16 | −0.09 to 0.39 | .24 | |
| Hemoglobin | .19 | −0.06 to 0.41 | .23 | |
| MCV | .23 | −0.01 to 0.45 | .03* | Weak |
| MCH | .26 | 0.01 to 0.47 | .03* | Weak |
| MCHC | .2 | −0.05 to 0.42 | .47 | |
| Reticulocytes | −.07 | −0.31 to 0.18 | .35 | |
| MCVr | .18 | −0.06 to 0.42 | .02* | Weak |
| CHr | .29 | 0.05 to 0.5 | .003* | Weak |
| Platelets | −.06 | −0.3 to 0.19 | .89 |
Abbreviations: CHr, corpuscular hemoglobin of reticulocytes; CI, confidence interval; MCH, mean corpuscular hemoglobin; MCHC, mean corpuscular hemoglobin concentration; MCV, mean corpuscular volume; MCVr, mean corpuscular volume of reticulocytes; r s, Spearman correlation coefficient.
Denotes comparisons with a statistically significant difference set as a P < .05.
FIGURE 3.
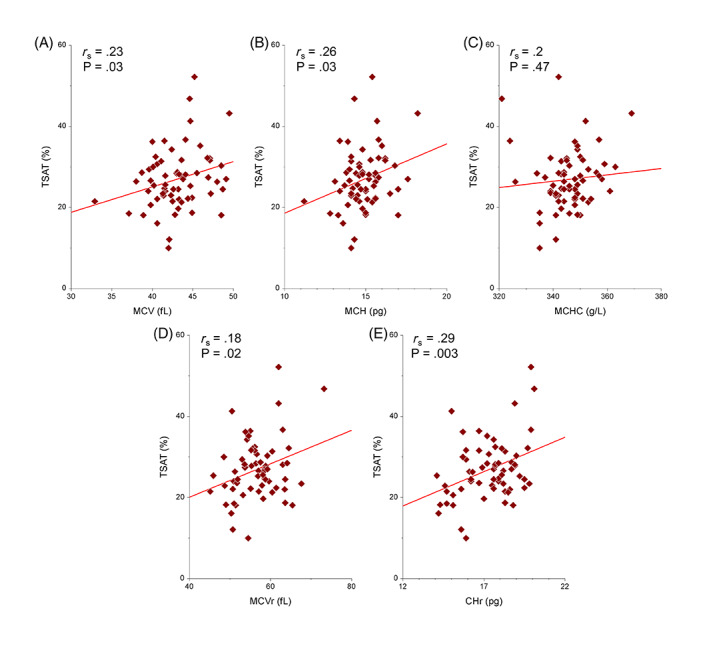
Scatter plots of the regression analyses of the erythrocyte indices and reticulocyte indices compared to transferrin saturation in 64 cats. (A: MCV, B: MCH, C: MCHC, D: MCVr, E: CHr). The solid line represents the regression line. CHr, corpuscular hemoglobin of reticulocytes; MCH, mean corpuscular hemoglobin; MCHC, mean corpuscular hemoglobin concentration; MCV, mean corpuscular volume; MCVr, mean corpuscular volume of reticulocytes; TSAT, transferrin saturation
The diagnostic value of these putative variables to predict ID was assessed with a ROC curve analysis and these results are summarized in Table 4 and Figure 4. Hematocrit, hemoglobin concentration, and MCHC were weak predictors, and CHr was a moderate predictor of ID. A CHr cutoff of 15.9 pg had a sensitivity of 82% and a specificity of 67% for predicting ID. Its positive predictive value was 38% and its negative predictive value was 94% in this sample of cats.
TABLE 4.
Receiver‐operating characteristic curve analysis of red blood cell indices and reticulocyte indices as predictors of iron deficiency in 64 cats
| AUC | 95% CI | P | Cutoff | Se (95% CI) | |
|---|---|---|---|---|---|
| Sp (95% CI) | |||||
| Hematocrit | .7 | 0.48‐0.84 | .02* | 37% |
Se 89% (57‐98) Sp 44% (31‐57) |
| Hemoglobin | .7 | 0.49‐0.84 | .02* | 13.2 g/dL |
Se 100% (70‐100) Sp 36% (25‐50) |
| MCV | .58 | 0.33‐0.75 | .45 | ||
| MCH | .64 | 0.38‐0.81 | .19 | ||
| MCHC | .7 | 0.47‐0.84 | .03* | 34.1 g/dL |
Se 56% (27‐81) Sp 80% (68‐88) |
| Reticulocytes | .52 | 0.28‐0.7 | .85 | ||
| MCVr | .63 | 0.32‐0.82 | .29 | ||
| CHr | .75 | 0.48‐0.89 | .01* | 15.9 pg |
Se 67% (35‐88) Sp 82% (70‐90) |
| Platelets | .58 | 0.31‐0.76 | .48 |
Abbreviations: AUC, area under the curve; CHr, corpuscular hemoglobin of reticulocytes; CI, confidence interval; MCH, mean corpuscular hemoglobin; MCHC, mean corpuscular hemoglobin concentration; MCV, mean corpuscular volume; MCVr, mean corpuscular volume of reticulocytes; Se, sensitivity; Sp, specificity.
Denotes comparisons with a statistically significant difference set as a P < .05.
FIGURE 4.
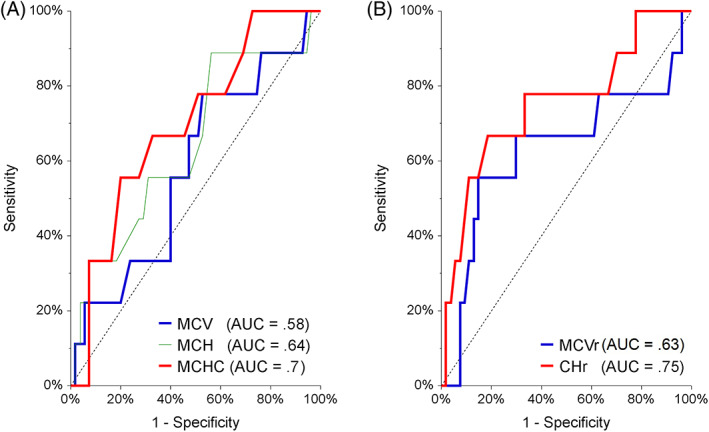
Receiver‐operating characteristic curve analysis of red blood cell indices and reticulocyte indices as predictors of iron deficiency in 64 cats. (A: MCV, MCH, MCHC; B: MCVr, CHr). AUC, area under the curve; CHr, corpuscular hemoglobin of reticulocytes; MCH, mean corpuscular hemoglobin; MCHC, mean corpuscular hemoglobin concentration; MCV, mean corpuscular volume; MCVr, mean corpuscular volume of reticulocytes
4. DISCUSSION
In this study sample of 64 cats, the reticulocyte index CHr was lower in cats with ID compared to nonaffected cats. It correlated weakly with TSAT and was a moderate predictor of ID in a ROC curve analysis. With a cutoff of 15.9 pg, its diagnostic value for ID was similar to that observed in humans and slightly lower than in dogs. 19 , 34 Erythrocyte indices and MCVr did not reliably predict ID in these cats.
Iron deficiency is rarely diagnosed in cats although it occurs in chronic diseases, including CKD and chronic gastrointestinal diseases. 11 , 29 , 31 Despite the fact that the early phase of nonanemic ID is considered a major health issue in humans with severe long‐term consequences affecting the quality of life, 3 , 5 this has not been recognized in cats yet. The iron status of cats is typically only assessed when ID anemia is suspected. The limited availability of easy screening methods does not encourage wide testing of cats, even in groups of cats at risk. The widespread use of high‐quality hematology analyzers, however, has stimulated the evaluation of newer indicators of iron availability for the hematopoiesis such as the reticulocyte indices. Even though not limited by species‐specific assays, their diagnostic value for ID cannot be simply extrapolated from humans or other animal species, especially because cats are known for their singularities in erythropoiesis such as the existence of punctate and aggregate reticulocytes.
The question of the evaluation of the iron status is often raised in the clinical setting of cats with CKD, because of the tendency to develop a nonregenerative anemia, often of multifactorial origin (including functional ID, erythropoietin deficiency, and chronic gastrointestinal blood loss). 11 In cats treated with a SUB for ureteral obstruction, chronic irritation of the urinary tract by the SUB can cause persistent or intermittent hematuria and thus further exacerbate this tendency. Our case selection criteria aimed therefore to include cats with a wide range of iron status and to maximize the likelihood of selecting cats with ID. Moreover, diagnostic parameters need to be evaluated in the specific context of their future use, as their accuracy might depend on the cause of the ID. The results of the present study can therefore not necessarily be extrapolated to other causes of ID such as chronic gastrointestinal disease or parasitic infestation.
In addition to chronic gastrointestinal and urinary blood losses in cats with CKD, uremia can affect further the iron status by decreasing food intake and impairing the intestinal iron absorption. The presence of chronic uremic inflammation can also contribute to a functional ID by decreasing the iron availability for erythropoiesis. 35 , 36 A decreased TSAT with decreased serum ferritin concentration is typically used to define absolute ID while a decreased TSAT with normal to increased serum ferritin concentration characterizes a functional ID. 8 , 11 Because of the role of serum ferritin as an acute phase protein, this differentiation is particularly difficult in case of inflammation. 8 , 11 In the 9 cats with ID, serum ferritin was normal in 7 cats and elevated in 2 cats (Figure 2), suggesting a functional rather than an absolute ID in these cats, similarly to previous studies in cats with CKD. 31 , 37 We cannot exclude that the reticulocyte indices evaluated in this study would perform better for the evaluation of absolute ID, although these variables typically do not differentiate absolute from functional ID. 27
The definition of ID based on a TSAT <20% was motivated by criteria commonly used in humans 33 and often adopted in small animals, 8 although there is a lack of consensus in the definition. Other reports have used either higher TSAT cutoffs or definitions based on other variables such as a decreased serum iron concentration. 29 , 38 The iron status is commonly assessed in humans based on the serum ferritin concentration. 16 , 23 , 24 However, its use in humans with CKD is controversial because this disease causes a low‐grade chronic inflammation and thus can increase the concentration of the positive acute phase protein ferritin independently of the total iron stores. 33 The same chronic inflammatory state exists in cats with CKD and could mask hypoferritinemia. 37 Furthermore, a comparison of serum ferritin concentration with the gold standard liver iron concentration in cats showed only a weak correlation between the 2 variables and therefore does not support the use of serum ferritin concentration as a standard for the diagnosis of ID in this species. 39 Some authors therefore suggest the use of TSAT as a marker of ID in cats and humans with CKD. 8 , 33 , 38
Feline aggregate reticulocytes are younger with a short circulating maturation time (12 hours), compared to punctate reticulocytes, which stay in circulation for 10 to 12 days. 40 The small amount of RNA in punctate reticulocytes is not detected in automated analyzers, causing a discrepancy between manual and automated feline reticulocyte count. 41 Moreover, the capacity of Advia 2120i to detect aggregate reticulocytes might also be poor, according to Bauer et al 42 ; however, another study showed an excellent correlation between automated and manual count of aggregate reticulocytes on this analyzer. 41 Differences also exist between automated analyzers, as the feline reticulocyte count on the Sysmex XT‐2000iV includes a small proportion of punctate reticulocytes (approximately 9%) in addition to the aggregate reticulocytes, 43 leading to a positive bias in the reticulocyte count performed in Sysmex XT‐2000iV compared to Advia 2120i. 42 The correlation between the reticulocyte content in hemoglobin measured by Idexx ProCyte Dx (RETIC‐HGB) and Advia 2120i (CHr) is also only moderate, most likely due to the measurement of a small number of punctate reticulocytes by Idexx ProCyte Dx. 20 These observations highlight the possible discrepancies in measurement of reticulocyte indices due to variable measurements of reticulocyte counts by different analyzers. In our study, reticulocyte indices most likely only reflect iron status of the past 1 to 2 days, because of the inability of Advia 2120i to include punctate reticulocytes in the reticulocyte count. This observation is, however, not a major limitation to the use of CHr for the assessment of iron status, because iron homeostasis is a slow process with most of the total body iron stored in hepatocytes, macrophages and within heme (erythroid hemoglobin and muscle myoglobin), 8 and iron stores are not expected to significantly change from day to day.
A previous study aimed to determine a reference interval for feline CHr on Advia 120 and to assess its diagnostic value for the characterization of the iron status in this species. 30 Their proposed cutoff (14.2 pg) for ID anemia was lower than ours, a discrepancy that could be related to differences in techniques of hemoglobin measurement as the use of a cyanide‐free colorimetric method (eg, Advia 2120i, as in our study) overestimates CHr compared to a cyanmethemoglobin colorimetric method (eg, Advia 120). 30 , 44 Definition of ID also included the presence of anemia in this study 30 unlike in ours. Their cutoff was close to the cutoff proposed in another study (14.7 pg), where an Advia 2120i was also used 20 ; however, the sensitivity for detecting iron‐limited erythropoiesis was lower in the second study. 20 , 30 These few available reports illustrate the difficulties encountered when comparing studies, because of the differences in the measurement techniques among analyzers.
The first limitation of this study is its sample size and especially the limited number of cats with ID. This was probably amplified by the strict definition of ID compared to other studies with a higher TSAT cutoff, but we considered it essential that the cats diagnosed with ID were true positives in order to optimize the specificity of the variables evaluated as surrogates for a complete iron panel. The inability to distinguish absolute from functional ID is unfortunately a limitation of most studies addressing this condition in cats. The evaluation of the iron stores in the liver can provide further insight in these mechanisms but it is rarely applicable in the clinical setting. The small sample size leads to the second limitation of the study, in which the evaluation of variables predicting the iron status of cats with CKD is limited to univariate analyses. It is conceivable that a more complex multivariable model including hematologic parameters and markers of inflammation would be more accurate. Furthermore, it is likely that CKD cats and SUB cats differ in their mechanisms causing ID: the respective influences of uremic microinflammation, malnutrition, and gastrointestinal or urinary blood losses might vary between these groups and affect differently the diagnostic accuracy of the reticulocyte indices. However, both disease groups are relevant clinical populations in which the question of the presence of ID is common and needs to be answered, hence their inclusion in this study. Finally, as discussed above, the results of this study cannot be extrapolated to other causes of ID without proper validation.
Based on these results, CHr added a moderate value to the diagnosis of ID in cats with CKD, similarly to what has been observed in humans and other species. It was significantly lower in cats with ID than in cats without ID (P = .02), the correlation of CHr with TSAT was weak (r S = .29), and CHr was a moderate predictor of ID in a ROC curve analysis (AUC = .75). A cutoff of 15.9 pg resulted in a sensitivity of 67% and a specificity of 82% for the diagnosis of ID. The MCVr and the erythrocyte indices, however, did not contribute to the diagnosis of ID in these cats. The development of a multivariable biological model might improve further the diagnosis of ID in cats with CKD when a complete iron panel is not available.
CONFLICT OF INTEREST DECLARATION
Authors declare no conflict of interest.
OFF‐LABEL ANTIMICROBIAL DECLARATION
Authors declare no off‐label use of antimicrobials.
INSTITUTIONAL ANIMAL CARE AND USE COMMITTEE (IACUC) OR OTHER APPROVAL DECLARATION
Approval from the regulatory authorities of the canton Bern, Switzerland (BE 69/14+ and BE 72/19).
HUMAN ETHICS APPROVAL DECLARATION
Authors declare human ethics approval was not needed for this study.
ACKNOWLEDGMENT
Funding provided by a grant from the specialization commission (SpezKo) of the Vetsuisse Faculty University of Bern, Switzerland.
Betting A, Schweighauser A, Francey T. Diagnostic value of reticulocyte indices for the assessment of the iron status of cats with chronic kidney disease. J Vet Intern Med. 2022;36(2):619‐628. doi: 10.1111/jvim.16367
Funding information Specialization commission (SpezKo) of the Vetsuisse Faculty University of Bern, Switzerland
REFERENCES
- 1. Pantopoulos K, Porwal SK, Tartakoff A, Devireddy L. Mechanisms of mammalian iron homeostasis. Biochemistry. 2012;51(29):5705‐5724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Clénin GE. The treatment of iron deficiency without anaemia (in otherwise healthy persons). Swiss Med Wkly. 2017;147:w14434. [DOI] [PubMed] [Google Scholar]
- 3. Houston BL, Hurrie D, Graham J, et al. Efficacy of iron supplementation on fatigue and physical capacity in non‐anaemic iron‐deficient adults: a systematic review of randomised controlled trials. BMJ Open. 2018;8:e019240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Firquet A, Kirschner W, Bitzer J. Forty to fifty‐five‐year‐old women and iron deficiency: clinical considerations and quality of life. Gynecol Endocrinol. 2017;33(7):503‐509. [DOI] [PubMed] [Google Scholar]
- 5. Pratt JJ, Khan KS. Non‐anaemic iron deficiency—a disease looking for recognition of diagnosis: a systematic review. Eur J Haematol. 2016;96(6):618‐628. [DOI] [PubMed] [Google Scholar]
- 6. Favrat B, Balck K, Breymann C, et al. Evaluation of a single dose of ferric carboxymaltose in fatigued, iron‐deficient women—PREFER a randomized, placebo‐controlled study. PLoS One. 2014;9(4):e94217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. McCown JL, Specht AJ. Iron homeostasis and disorders in dogs and cats: a review. J Am Anim Hosp Assoc. 2011;47(3):151‐160. [DOI] [PubMed] [Google Scholar]
- 8. Bohn AA. Diagnosis of disorders of iron metabolism in dogs and cats. Vet Clin North Am Small Anim Pract. 2013;43(6):1319‐1330. [DOI] [PubMed] [Google Scholar]
- 9. Giger U. Regenerative anemias caused by blood loss or hemolysis. In: Ettinger S, Feldman E, eds. Textbook of Veterinary Internal Medicine. 6th ed.; St Louis: Elsevier; 2005:1886‐1907. [Google Scholar]
- 10. Berent AC, Weisse CW, Bagley DH, Lamb K. Use of a subcutaneous ureteral bypass device for treatment of benign ureteral obstruction in cats: 174 ureters in 134 cats (2009–2015). J Am Vet Med Assoc. 2018;253(10):1309‐1327. [DOI] [PubMed] [Google Scholar]
- 11. Chalhoub S, Langston CE, Eatroff A. Anemia of renal disease: what it is, what to do and what's new. J Feline Med Surg. 2011;13(9):629‐640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chalhoub S, Langston CE, Farrelly J. The use of darbepoetin to stimulate erythropoiesis in anemia of chronic kidney disease in cats: 25 cases. J Vet Intern Med. 2012;26(2):363‐369. [DOI] [PubMed] [Google Scholar]
- 13. Cowgill LD, James KM, Levy JK, et al. Use of recombinant human erythropoietin for management of anemia in dogs and cats with renal failure. J Am Vet Med Assoc. 1998;212(4):521‐528. [PubMed] [Google Scholar]
- 14. Ali MAM, Luxton AW, Walker WHC. Serum ferritin concentration and bone marrow iron stores: a prospective study. Can Med Assoc J. 1978;118(8):945‐946. [PMC free article] [PubMed] [Google Scholar]
- 15. Ferraro S, Mozzi R, Panteghini M. Revaluating serum ferritin as a marker of body iron stores in the traceability era. Clin Chem Lab Med. 2012;50(11):1911‐1916. [DOI] [PubMed] [Google Scholar]
- 16. Urrechaga Igartua E, Hoffmann JJML, Izquierdo‐Álvarez S, Escanero JF. Reticulocyte hemoglobin content (MCHr) in the detection of iron deficiency. J Trace Elem Med Biol. 2017;43:29‐32. [DOI] [PubMed] [Google Scholar]
- 17. Ottenjann M, Weingart C, Arndt G, Kohn B. Characterization of the anemia of inflammatory disease in cats with abscesses, pyothorax, or fat necrosis. J Vet Intern Med. 2006;20(5):1143‐1150. [DOI] [PubMed] [Google Scholar]
- 18. Vora SM, Messina G, Pavord S. Utility of erythrocyte indices in identifying iron depletion in pregnancy. Obstet Med. 2019;14:23‐25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Mast AE, Blinder MA, Lu Q, Flax S, Dietzen DJ. Clinical utility of the reticulocyte hemoglobin content in the diagnosis of iron deficiency. Blood. 2002;99(4):1489‐1491. [DOI] [PubMed] [Google Scholar]
- 20. Keiner M, Fuchs J, Bauer N, Moritz A. Evaluation of reticulocyte hemoglobin content (RETIC‐HGB) for the diagnosis of iron‐limited erythropoiesis in cats. Vet Clin Pathol. 2020;49(4):557‐566. [DOI] [PubMed] [Google Scholar]
- 21. Cai J, Wu M, Ren J, et al. Evaluation of the efficiency of the reticulocyte hemoglobin content on diagnosis for iron deficiency anemia in Chinese adults. Nutrients. 2017;9(5):450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Nalado AM, Mahlangu JN, Duarte R, et al. Utility of reticulocyte haemoglobin content and percentage hypochromic red cells as markers of iron deficiency anaemia among black CKD patients in South Africa. PLoS One. 2018;13(10):e0204899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Toki Y, Ikuta K, Kawahara Y, et al. Reticulocyte hemoglobin equivalent as a potential marker for diagnosis of iron deficiency. Int J Hematol. 2017;106(1):116‐125. [DOI] [PubMed] [Google Scholar]
- 24. Malczewska‐Lenczowska J, Orysiak J, Szczepańska B, Turowski D, Burkhard‐Jagodzińska K, Gajewski J. Reticulocyte and erythrocyte hypochromia markers in detection of iron deficiency in adolescent female athletes. Biol Sport. 2017;34(2):111‐118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Steinberg JD, Olver CS. Hematologic and biochemical abnormalities indicating iron deficiency are associated with decreased reticulocyte hemoglobin content (CHr) and reticulocyte volume (rMCV) in dogs. Vet Clin Pathol. 2005;34(1):23‐27. [DOI] [PubMed] [Google Scholar]
- 26. Fuchs J, Moritz A, Grußendorf E, et al. Canine reticulocyte hemoglobin content (RET‐He) in different types of iron‐deficient erythropoiesis. Vet Clin Pathol. 2017;46(3):422‐429. [DOI] [PubMed] [Google Scholar]
- 27. Radakovich LB, Santangelo KS, Olver CS. Reticulocyte hemoglobin content does not differentiate true from functional iron deficiency in dogs. Vet Clin Pathol. 2015;44(4):511‐518. [DOI] [PubMed] [Google Scholar]
- 28. Schaefer DMW, Stokol T. The utility of reticulocyte indices in distinguishing iron deficiency anemia from anemia of inflammatory disease, portosystemic shunting, and breed‐associated microcytosis in dogs. Vet Clin Pathol. 2015;44(1):109‐119. [DOI] [PubMed] [Google Scholar]
- 29. Hunt A, Jugan MC. Anemia, iron deficiency, and cobalamin deficiency in cats with chronic gastrointestinal disease. J Vet Intern Med. 2021;35(1):172‐178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Prins M, Van Leeuwen MW, Teske E. Stability and reproducibility of advia 120‐measured red blood cell and platelet parameters in dogs, cats, and horses, and the use of reticulocyte haemoglobin content (CHr) in the diagnosis of iron deficiency. Tijdschr Diergeneeskd. 2009;134(7):272‐278. [PubMed] [Google Scholar]
- 31. Gest J, Langston C, Eatroff A. Iron status of cats with chronic kidney disease. J Vet Intern Med. 2015;29(6):1488‐1493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. International Renal Interest Society . IRIS Staging of CKD ; 2019. http://www.iris-kidney.com/pdf/IRIS_Staging_of_CKD_modified_2019.pdf. Accessed March 01, 2020.
- 33. Batchelor EK, Kapitsinou P, Pergola PE, Kovesdy CP, Jalal DI. Iron deficiency in chronic kidney disease: updates on pathophysiology, diagnosis, and treatment. J Am Soc Nephrol. 2020;31(3):456‐468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Fuchs J, Moritz A, Grußendorf E, et al. Evaluation of reticulocyte hemoglobin content (RET‐He) in the diagnosis of iron‐deficient erythropoiesis in dogs. Vet Clin Pathol. 2017;46(4):558‐568. [DOI] [PubMed] [Google Scholar]
- 35. von Roedern M, Buriko Y, Prittie J, Lamb K. Investigation of iron status and markers of inflammation in anaemic and non‐anaemic hospitalised cats. J Small Anim Pract. 2017;58(6):323‐329. [DOI] [PubMed] [Google Scholar]
- 36. Chikazawa S, Dunning MD. A review of anaemia of inflammatory disease in dogs and cats. J Small Anim Pract. 2016;57(7):348‐353. [DOI] [PubMed] [Google Scholar]
- 37. Javard R, Grimes C, Bau‐Gaudreault L, Dunn M. Acute‐phase proteins and iron status in cats with chronic kidney disease. J Vet Intern Med. 2017;31(2):457‐464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kidney Disease: Improving Global Outcomes (KDIGO) anemia work group. KDIGO clinical practice guideline for anemia in chronic kidney disease. Kidney inter, Suppl. 2012;2(4):279‐335. [Google Scholar]
- 39. Andrews GA, Chavey PS, Smith JE. Enzyme‐linked immunosorbent assay to measure serum ferritin and the relationship between serum ferritin and nonheme iron stores in cats. Vet Pathol. 1994;31(6):674‐678. [DOI] [PubMed] [Google Scholar]
- 40. Christian JA. Erythrokinetics and erythrocyte destruction. In: Weiss DJ, Wardrop KJ, Schalm OW, eds. Schalm's Veterinary Hematology. 6th ed. Ames, IA: Wiley‐Blackwell; 2010:136‐143. [Google Scholar]
- 41. Tvedten H, Moritz A. Reticulocyte and Heinz body staining and enumeration. In: Weiss DJ, Wardrop KJ, Schalm OW, eds. Schalm's Veterinary Hematology. 6th ed. Ames, IA: Wiley‐Blackwell; 2010:1067‐1073. [Google Scholar]
- 42. Bauer N, Nakagawa J, Dunker C, Failing K, Moritz A. Evaluation of the automated hematology analyzer Sysmex XT‐2000iV™ compared to the ADVIA® 2120 for its use in dogs, cats, and horses. Part II: accuracy of leukocyte differential and reticulocyte count, impact of anticoagulant and sample aging. J Vet Diagn Invest. 2012;24(1):74‐89. [DOI] [PubMed] [Google Scholar]
- 43. Lilliehöök I, Tvedten H. Validation of the Sysmex XT‐2000iV hematology system for dogs, cats, and horses. I. Erythrocytes, platelets, and total leukocyte counts. Vet Clin Pathol. 2009;38(2):163‐174. [DOI] [PubMed] [Google Scholar]
- 44. Bauer N, Moritz A. Evaluation of three methods for measurement of hemoglobin and calculated hemoglobin parameters with the ADVIA 2120 and ADVIA 120 in dogs, cats, and horses. Vet Clin Pathol. 2008;37(2):173‐179. [DOI] [PubMed] [Google Scholar]


