Abstract
Background
Resting cortisol concentrations are routinely measured in dogs with chronic gastrointestinal signs to rule out hypoadrenocorticism based on a concentration >2 μg/dL (>55 nmol/L).
Hypothesis/Objectives
To assess the cross‐sectional prevalence of hypoadrenocorticism in a group of dogs with chronic gastrointestinal signs presented to a referral internal medicine service.
Animals
Two‐hundred and eighty‐two client‐owned dogs with chronic gastrointestinal signs and with resting cortisol concentration testing performed.
Methods
Retrospective review of medical records (final diagnosis, resting cortisol concentration, and adenocorticotropic hormone [ACTH] stimulation test results) of a referral population of dogs between May 2013 and September 2017.
Results
Resting cortisol concentration was <2 μg/dL (<55 nmol/L) in 79 patients (28%). Repeated resting cortisol concentration measurements were performed in 28 dogs, and in 8, resting cortisol concentrations remained <2 μg/dL (<55 nmol/L). Post‐ACTH cortisol concentration was <2 μg/dL (<55 nmol/L) in 1 dog, consistent with a diagnosis of hypoadrenocorticism and giving a prevalence estimate of hypoadrenocorticism in this population of dogs of 0.3% (95% confidence interval [95CI], 0.03‐1.5%). In 19 dogs with an initial resting cortisol concentration <2 μg/dL (<55 nmol/L), hypoadrenocorticism was excluded based on a repeat resting cortisol concentration >2 μg/dL (>55 nmol/L). Overall, the most common diagnosis was chronic primary inflammatory enteropathy (176/282, 62.4%), followed by extragastrointestinal neoplasia (17/282, 6%), protein‐losing enteropathy, pancreatitis and megaesophagus (10/282, 3.5% each).
Conclusions and Clinical Importance
Although dogs with hypoadrenocorticism can present with chronic gastrointestinal signs, it was the final diagnosis in only 1 of 282 dogs presenting to a referral internal medicine service for signs of chronic enteropathy. Repeated resting cortisol concentration may be considered as a test to try and exclude hypoadrenocorticism.
Keywords: ACTH stimulation test, Addison's disease, chronic enteropathy, hypoadrenocorticism, resting cortisol
Abbreviation
- ACTH
adenocorticotropic hormone
1. INTRODUCTION
Hypoadrenocorticism is an uncommon endocrine disease of dogs, characterized by insufficient corticosteroid hormone secretion from the adrenal cortex. 1 The estimated cross‐sectional prevalence of hypoadrenocorticism is between 0.06% and 1.1%. 2 , 3 , 4 , 5 Predisposed breeds have higher prevalence estimates, including bearded collies (3.4%), standard poodles (10%), Portuguese water dogs (1.5%) 6 and Nova Scotia duck tolling retrievers (1.4%), 1 and it is considered to be an inherited disease in these breeds. 1 Primary hypoadrenocorticism, a lack of both glucocorticoid and mineralocorticoid secretion caused by immune‐mediated adrenal gland damage is most common. 1 Glucocorticoid deficiency is described as responsible for the majority of waxing and waning clinical signs, with mineralocorticoid deficiency responsible for the classical electrolyte imbalances of hyponatremia and hypokalemia. 1 Eunatremic, eukalemic hypoadrenocorticism (previously known as atypical hypoadrenocorticism; Agreed Language In Veterinary Endocrinology [ALIVE] criteria) 7 describes a clinical presentation of hypoadrenocorticism in dogs with normal serum electrolyte concentrations. These patients may be more likely to be presented with gastrointestinal signs. 8 , 9 Dogs presented with eunatremic, eukalemic hypoadrenocorticism may have isolated hypocortisolism, either primary or secondary, or hypoadrenocorticism with compensatory mechanisms protecting against changes in serum electrolyte concentrations secondary to hypoaldosteronism. 10
Clinical signs of hypoadrenocorticism can be vague and nonspecific, mimicking other diseases, and making diagnosis challenging. 1 The most common clinical signs reported are lethargy, anorexia, vomiting, diarrhea, weakness, and weight loss 1 , 5 , which may be gradual in onset and commonly wax and wane. 1 , 11 , 12
Hypoadrenocorticism is diagnosed by a lack of appropriate cortisol response to an adenocorticotropic hormone (ACTH) stimulation test. However, resting cortisol concentration <2 μg/dL (<55 nmol/L) has excellent sensitivity for hypoadrenocorticism (99.4%‐100%). 13 , 14 , 15 As a result, a resting cortisol concentration >2 μg/dL (>55 nmol/L) is commonly used as a screening test to rule out hypoadrenocorticism.
Because of variable clinical signs and a presentation that can mimic chronic enteropathy, including protein‐losing enteropathy, hypoadrenocorticism always must be considered in dogs with chronic gastrointestinal signs. Therefore, resting cortisol concentration testing is routinely performed in dogs with chronic gastrointestinal disease to rule out hypoadrenocorticism. To our knowledge, only a single previous study has evaluated the prevalence of hypoadrenocorticism in dogs with chronic gastrointestinal signs, 9 with hypoadrenocorticism being the final diagnosis in 6/151 dogs, resulting in a prevalence of 4%.
Our primary objective was to assess the cross‐sectional prevalence of hypoadrenocorticism in a group of dogs with chronic gastrointestinal signs presented to a referral internal medicine service.
We hypothesized that: (a) the prevalence of hypoadrenocorticism in dogs with chronic gastrointestinal signs presented to a referral internal medicine service would be lower than previously reported, and (b) primary gastrointestinal disease would be the most common diagnosis.
2. MATERIAL AND METHODS
2.1. Design
Retrospective cohort study assessing patients with resting cortisol concentration as part of the assessment for chronic gastrointestinal signs.
2.2. Setting, location, dates, and data collection
Medical records at The Royal (Dick) School of Veterinary Studies (University of Edinburgh) between May 2013 and September 2017 were searched for dogs that had resting cortisol concentration testing, identified on the basis of billing codes and laboratory records. These records were retrospectively reviewed using the electronic client data management system (Tristan Veterinary Software Ltd, version 1.8.3.914, Orion Engineering Services, Orion House, Inverness, United Kingdom).
2.3. Case selection
All cases with resting cortisol concentration testing performed were reviewed. Dogs with a chronic history of gastrointestinal signs (vomiting, diarrhea, weight loss, hyporexia, anorexia, dysphagia, regurgitation, or a combination of these for ≥3 weeks) seen by the internal medicine service were included in the study. Dogs were excluded if cortisol concentration was part of ACTH stimulation or low dose dexamethasone suppression test to evaluate for hyperadrenocorticism, or for trilostane monitoring. Dogs with a history of recent glucocorticoid administration (1 week before presentation for short‐acting glucocorticoids and 4 weeks for long‐acting glucocorticoids) 15 also were excluded.
2.4. Extracted data
Signalment, presenting complaint(s), resting cortisol concentration measurement and final diagnosis (as documented by a European College of Veterinary Internal Medicine ‐ Companion Animal diplomate in the patient record) were evaluated in all dogs. Hematology, biochemistry values and ACTH stimulation test results were extracted when available.
2.5. Laboratory testing
Resting cortisol concentration measurements for all dogs were evaluated at The Royal (Dick) School of Veterinary Studies, using the Immulite 1000 Analyzer (Siemens Healthineers, United Kingdom). The ACTH stimulation test results for 63/70 cases were evaluated at The Royal (Dick) School of Veterinary Studies using the previously mentioned analyzer. The remaining 7 cases had ACTH stimulation tests performed at the primary care practice. Three of these results were reported by Capital Diagnostics Laboratory, United Kingdom, and for the remaining 4, this information was not known. Hematology results were unavailable for 8 dogs and serum biochemistry results unavailable for 3 dogs. For dogs with >1 resting cortisol concentration measurement, only testing associated with the first consultation was considered.
Final diagnosis was evaluated for all patients. A diagnosis of primary enteropathy was made if the patients either had endoscopic biopsy results consistent with an inflammatory enteropathy or if it was the main differential diagnosis suspected and the patients had a good clinical response to appropriate treatment (hydrolyzed diet, probiotics, immunosuppressive drugs, or antibiotics). Diagnosis was classified as suspected primary enteropathy if it was the main clinical suspicion, but no endoscopic biopsy samples were taken and the patients were lost to follow‐up.
2.6. Statistical evaluation
Data were organized in Microsoft Excel (Microsoft, USA); data analyses were performed in R (R version 4.0.3) using RStudio (version 1.3.1093). Data were assessed for normality by visual assessment of histograms and q‐q plots. Statistical significance was set at P < .05.
3. RESULTS
3.1. Dog data
A total of 1057 resting cortisol concentration measurements were performed in 856 dogs. The internal medicine service saw 508/856 patients. Two‐hundred and eighty‐two cases with chronic gastrointestinal signs met the inclusion criteria.
One‐hundred and eighty were male (111 neutered, 69 intact) and 102 were female (75 neutered, 27 intact). The most common breed was Labrador retriever (35), followed by boxer (13), Border collie (12), West highland white terrier (12), French bulldog (12), German shepherd (11) and golden retriever (11). Median age was 5.4 years (range, 1 month to 15.25 years; interquartile range [IQR], 1.8‐8.8 years) and median body weight was 18.2 kg (range, 1.35‐80 kg; IQR, 9‐29 kg). The most common clinical signs on presentation included diarrhea (145/282), vomiting (138/282) and weight loss (74/282, Table 1).
TABLE 1.
Most common presenting clinical signs in the 282 dogs that were finally included
| Clinical signs | Number of dogs (%) |
|---|---|
| Diarrhea | 145 (51.4) |
| Vomiting | 138 (48.9) |
| Weight loss | 74 (26.2) |
| Lethargy | 37 (13.1) |
| Hyporexia | 27 (9.6) |
| Regurgitation | 28 (9.9) |
| Anorexia | 14 (4.9) |
3.2. Resting cortisol concentrations and adrenal testing
Resting cortisol concentration was >2 μg/dL (>55 nmol/L) in 203/282 (71.9%) and resting cortisol concentration was <2 μg/dL (<55 nmol/L) in 79/282 (28%), no dog had a cortisol concentration of 2 μg/dL (55 nmol/L; Figure 1). Resting cortisol concentration was <1 μg/dL (<27.6 nmol/L) in 25/282 dogs (8.9%).
FIGURE 1.

Resting cortisol measurements for all 282 dogs with chronic gastrointestinal signs. Red dashed line represents 2 μg/dL (55 nmol/L) cut‐off for excluding hypoadrenocorticism
Of the patients with a resting cortisol concentration <2 μg/dL (<55 nmol/L), further adrenal testing was performed in 64/79 (81%) dogs associated with the initial consultation; 26/64 had an ACTH stimulation test performed, 37/64 had a post‐ACTH cortisol concentration measurement and 1 dog had a repeat resting cortisol concentration without subsequent ACTH stimulation, which was 2.5 μg/dL (68 nmol/L). Two ACTH stimulation tests were performed by the primary veterinarian and described as normal, which was assumed to be >2 μg/dL (>55 nmol/L), but actual results were not available for review. Post‐ACTH cortisol concentration measurement was <2 μg/dL (<55 nmol/L) in 1 dog, consistent with a diagnosis of hypoadrenocorticism. Post‐ACTH cortisol concentration measurement excluded hypoadrenocorticism in 57/58 dogs (Figure 2). In 6 dogs, initial resting cortisol concentration was undetectable at <0.5 μg/dL (<13.8 nmol/L), 3 had a repeat resting cortisol concentration measurement as part of an ACTH stimulation test, and 3 had post‐ACTH cortisol concentration measured only (Figure 3).
FIGURE 2.
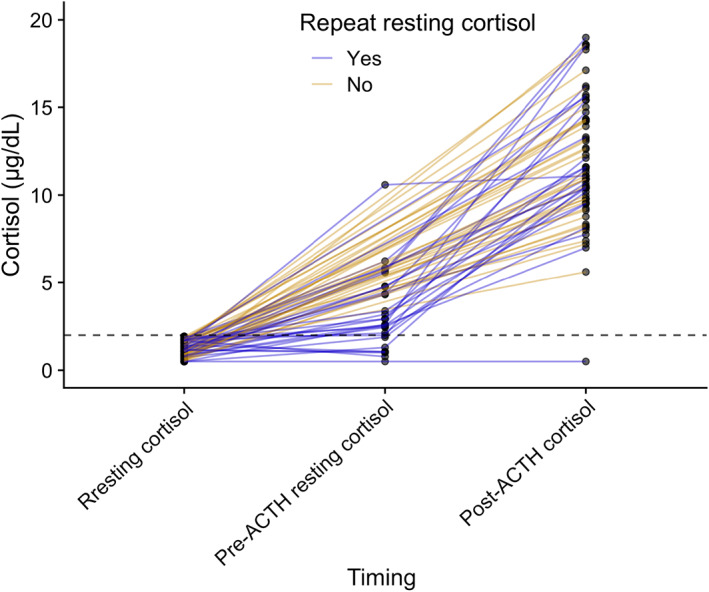
Cortisol measurements in 58 dogs with resting cortisol <55 nmol/L (<2 μg/dL) with a post‐ACTH measured cortisol. Twenty‐three dogs had also pre‐ACTH resting cortisol concentration measurement. Dashed line represents 2 μg/dL (55 nmol/L) cut‐off for excluding hypoadrenocorticism
FIGURE 3.

Cortisol measurements in 6 dogs with undetectable initial resting cortisol (<0.5 μg/dL) with a post‐ACTH measured cortisol. Three dogs also had a pre‐ACTH resting cortisol concentration measurement. Dashed line represents 2 μg/dL (55 nmol/L) cut‐off for excluding hypoadrenocorticism
In 6 dogs with initial resting cortisol concentration >2 μg/dL (>55 nmol/L; range, 2.03‐4.9 μg/dL [56.6‐136 nmol/L]) further adrenal testing was performed; 1 dog had an ACTH stimulation test and 5 post‐ACTH cortisol concentrations, and none were consistent with hypoadrenocorticism (Figure 4).
FIGURE 4.

Cortisol measurements in 6 dogs with initial resting cortisol >55 nmol/L (>2 μg/dL) with a post‐ACTH measured cortisol. One dog also had a pre‐ACTH resting cortisol concentration measurement. Dashed line represents 2 μg/dL (55 nmol/L) cut‐off for excluding hypoadrenocorticism
In total, repeat resting cortisol concentrations were measured in 28 dogs (Figure 5). Eight of these dogs had resting cortisol concentrations <2 μg/dL (<55 nmol/L) twice, in 19/28 dogs the second concentration was >2 μg/dL (>55 nmol/L) and in 1 dog the concentrations were >2 μg/dL (>55 nmol/L) on both occasions. Eight dogs with resting cortisol concentrations <1 μg/dL (<27.6 nmol/L) had repeat resting cortisol concentrations measured, and of these only the dog with hypoadrenocorticism had resting cortisol concentration <1 μg/dL (<27.6 nmol/L) twice.
FIGURE 5.

Comparison of initial and follow‐up resting cortisol measurements in 28 dogs that had repeat resting cortisol concentration measurement. Black dashed lines represent 2 μg/dL (55 nmol/L) cut‐off for excluding hypoadrenocorticism. Red dashed lines represents 1 μg/dL (27.6 nmol/L)
3.3. No follow‐up adrenal testing dogs <2 μg/dL (<55 nmol/L)
Follow‐up adrenal test results were not available in 15/79 (19%) dogs with resting cortisol concentrations <2 μg/dL (<55 nmol/L). Final diagnoses in this group of patients included primary inflammatory enteropathy (6), suspected primary inflammatory enteropathy (2), lymphangiectasia (2), protein‐losing nephropathy and chronic kidney disease (1), diaphragmatic hernia (1), gastroenteritis (1), gastric foreign body (1), gastrointestinal lymphoma (1), giardiasis (1), and unknown diagnosis (1). One of the patients diagnosed initially with primary inflammatory enteropathy was diagnosed 2 months later with gastrointestinal lymphoma. All of these patients had resting cortisol concentration, hematology, and serum biochemistry performed. Other additional tests included abdominal ultrasound examination (14), thoracic radiographs (8), serum cobalamin concentration (6), urinalysis (7), and gastrointestinal endoscopy (7). However, it cannot be fully excluded that all of these patients had hypoadrenocorticism because of the retrospective nature of the study.
One patient was euthanized shortly after diagnosis (patient with protein‐losing nephropathy). The remaining 14 dogs were discharged from the hospital; of these, 9/14 were lost to follow‐up. Of the remaining 5 dogs, 2 were doing clinically well 6 months after discharge (1 with chronic enteropathy and 1 with chronic enteropathy and lymphangiectasia), and 1 was clinically well 2 years after discharge (chronic enteropathy and lymphangiectasia). Two dogs were humanly euthanized 2.5 months after discharge, both with gastrointestinal lymphoma.
3.4. Dog with hypoadrenocorticism
Hypoadrenocorticism was the final diagnosis in 1 dog, a 6‐year‐old, female neutered, labradoodle, with a 4‐week history of weight loss, hyporexia, dysphagia, polydipsia, and acute vomiting. Clinicopathological abnormalities are summarized in Table S1, and include eosinophilia and normal serum electrolytes concentrations. Both pre‐ and post‐ACTH stimulated cortisol concentrations were <0.5 μg/dL (<13.8 nmol/L).
This patient also had eosinophilia. Eosinophilia (eosinophils >1 × 10 9 /L) was present in 29/282 (10.3%) dogs in our study, including the patient diagnosed with hypoadrenocorticism. Common final diagnoses in this group of patients included chronic enteropathy (18), protein‐losing enteropathy (2) and megaesophagus (2; Table 2). A final diagnosis was not reached in 1 patient, but an ACTH stimulation test excluded hypoadrenocorticism.
TABLE 2.
Final diagnoses in 29 dogs with eosinophilia
| Final diagnosis | Number of dogs |
|---|---|
| Chronic enteropathy | 18 |
| PLE | 2 |
| Megaesophagus | 2 |
| Giardiasis | 1 |
| Hypoadrenocorticism | 1 |
| Multicentric lymphoma | 1 |
| Small intestinal mass | 1 |
| Hiatal hernia | 1 |
| Abscessation | 1 |
| Unknown | 1 |
Lymphocytosis was present in 14/282 (5%) dogs. In these 14, there were 17 final diagnoses. Primary diagnoses were primary inflammatory enteropathy or suspected primary inflammatory enteropathy in 9, lymphoma in 2 and brachycephalic airway obstructive syndrome, histiocytic ulcerative colitis and megaesophagus in 1 each. Concurrent diagnoses were esophagitis, and Campylobacter infection.
A lack of stress leukogram (neutrophils and monocytes within normal limits, lack of immature neutrophils and no lymphopenia) was present in 183/282 dogs (64.9%). None of these patients were diagnosed with hypoadrenocorticism.
A Na : K ratio <27 was present in 10 dogs. Three of these patients were diagnosed with primary inflammatory enteropathy or suspected primary inflammatory enteropathy, 2 with megaesophagus, 1 each with Mycoplasma canis infection, giardiasis, leishmaniosis, chronic kidney disease secondary to leptospirosis, and 1 dog did not have a final diagnosis.
3.5. Final diagnoses
In total, 337 diagnoses were made in 282 dogs; 1 dog had 4 diagnoses, 9 dogs had 3 diagnoses, 34 had 2 diagnoses, 231 had 1 diagnosis and in 7 dogs no final definitive diagnosis was made.
The most common final diagnosis in this population of dogs was primary inflammatory enteropathy in 176/282 (62.4%) dogs (confirmed in 154/282 and strongly suspected in 22/282). These included dogs with food‐responsive enteropathy, immunosuppressant‐responsive enteropathy, antibiotic‐responsive enteropathy, and histiocytic ulcerative colitis. Other frequent diagnoses included: extragastrointestinal neoplasia (17/282, 6%), gastrointestinal neoplasia (13/282, 4.6%), protein‐losing enteropathy (10/282, 3.5%), chronic pancreatitis (10/282, 3.5%), megaesophagus (10/282, 3.5%), and enteritis, gastritis or gastroenteritis (8/282, 2.8%).
When focusing only on dogs with a resting cortisol concentration <2 μg/dL (<55 nmol/L), the most common final diagnosis was chronic enteropathy (43/79 or strongly suspected in 9/79, 65.8%) followed by megaesophagus (4/79, 4.8%) and giardiasis (4/79, 4.8%).
Only 1/282 dog was diagnosed with hypoadrenocorticism, giving a prevalence estimate of hypoadrenocorticism in this population of dogs of 0.3% (95% confidence interval [95CI], 0.03%‐1.5%).
Extragastrointestinal disease was the primary diagnosis in 66/282 (23.4%) patients. Nongastrointestinal diseases included nongastrointestinal neoplasia (17/66), chronic pancreatitis (10/66), neurological disease (6/66), exocrine pancreatic insufficiency (3/66), chronic kidney disease (4/66), portal vein hypoplasia (3/66), and hepatopathy (2/66). Final diagnosis was not reached in 6/282 dogs (2.1%). Of these, 5 patients had a resting cortisol concentration <2 μg/dL (<55 nmol/L) and 1/5 did not have an ACTH stimulation test performed. This patient was also lost to follow‐up and returned to the referring veterinarian.
4. DISCUSSION
The prevalence of hypoadrenocorticism in this population of dogs with chronic gastrointestinal signs and presented to a referral internal medicine service was 0.3% (95CI, 0.03%‐1.5%), lower than previously described in a smaller population of dogs (prevalence estimate, 4%; 95CI, 1.6%‐8%). 9 One possible reason for the difference between studies is that the prevalence of hypoadrenocorticism in Germany is higher than in the United Kingdom. We believe this possibility seems unlikely, with prevalence in other geographically diverse populations reportedly being similar. 2 , 3 , 4 , 5 A further possibility is that referring practitioners in the United Kingdom are more likely than those in Germany to screen for hypoadrenocorticism before referral. This possible bias is intrinsic to studying a referral population of animals because the population is dependent on the referring veterinarians' routine practices. Finally, dogs with hypoadrenocorticism can present in acute crisis and in our hospital these cases are seen by the emergency service rather than the internal medicine service. Therefore, dogs with chronic gastrointestinal signs presenting in subsequent crisis would not be included in this population.
The prevalence of hypoadrenocorticism in the prior study was 4%. 9 Our clinical perception was that, in our referral population of dogs with chronic gastrointestinal signs, the prevalence of hypoadrenocorticism was lower, as was confirmed in our study. The prevalence estimate in our study more closely corresponds with the overall prevalence of hypoadrenocorticism in the general dog population, which has been estimated by previous studies as being between 0.06% and 1.1%. 2 , 3 , 4 , 5
Hypoadrenocorticism and other nongastrointestinal diseases (including pancreatic diseases, endocrinopathies, diseases of the liver and kidneys and neoplasia) can present clinically with chronic gastrointestinal signs and 23.4% (66/282) of the patients in our study had nongastrointestinal disease as the final diagnosis. Our findings are in agreement with those of a recent German study that assessed resting cortisol concentrations, with primary inflammatory enteropathy as the final diagnosis in more than half of the patients, both with resting cortisol concentrations higher and lower than 55 nmol/L. 9 Our study also agrees with a study that evaluated the final diagnosis in dogs with chronic diarrhea. 16 Inflammatory enteropathy was the most common diagnosis in that study with a prevalence estimate for hypoadrenocorticism of 0.73%. 16
Repeat resting cortisol concentration measurements were performed in 27 dogs with an initial resting cortisol <2 ug/dL (<55 nmol/L), including the patient finally diagnosed with hypoadrenocorticism. Out of 26 dogs with an initial resting cortisol concentration <2 μg/dL (<55 nmol/L) without hypoadrenocorticism, hypoadrenocorticism was excluded in 19 (19/26, 73%) based on a repeat resting cortisol concentration >2 μg/dL (55 nmol/L; Figure 5). Previous studies have found a resting cortisol concentration of <2 μg/dL (<55 nmol/L) to have excellent sensitivity for hypoadrenocorticism (99.4%‐100%). 13 , 14 , 15 As a result, a resting cortisol concentration >2 μg/dL (>55 nmol/L) is used as a screening test to exclude hypoadrenocorticism. Unfortunately, specificity is low (between 63.3% and 78.2%) and, as a result, a confirmatory test (ACTH stimulation test) is performed on many dogs that do not have hypoadrenocorticism, 13 , 14 , 15 with increased cost for the client. Based on our results, in some circumstances (eg, limited financial resources, lack of tetracosactide acetate to perform the ACTH stimulation test and its cost, low clinical suspicion, nonemergency presentation) repeated resting cortisol concentration measurement could be considered to try to rule out hypoadrenocorticism, but an ACTH stimulation test is always required to make a diagnosis of hypoadrenocorticism. Other diagnostic tests to consider in these circumstances include post‐ACTH stimulation cortisol concentration or cortisol‐to‐ACTH ratio to exclude or diagnose hypoadrenocorticism. 1
One dog in our study had a final diagnosis of eunatremic, eukalemic hypoadrenocorticism. These patients usually have a longer course of clinical signs before diagnosis, 17 with anorexia, lethargy, diarrhea, vomiting and polydipsia more frequently reported. 17 , 18 Our patient's presenting complaints included a chronic history of lethargy, hyporexia, polydipsia and acute vomiting, but further conclusions could not be made in our study because of the low number of hypoadrenocorticism cases. Biochemical abnormalities in dogs with eunatremic, eukalemic hypoadrenocorticism are similar to those in typical cases, except for normal serum sodium and potassium concentrations. 17 Other biochemical abnormalities our patient had included eosinophilia and mild hypocholesterolemia. Hypocholesterolemia and hypoalbuminemia appeared to be more marked in dogs with eunatremic, eukalemic hypoadrenocorticism compared to cases with changes in serum electrolyte concentrations. 17 Eosinophilia is reported in up to 20% of hypoadrenocorticism cases. 1 Eosinophilia also is associated with inflammatory disease in organs with large epithelial surfaces, such as skin, intestine or lungs as well as in parasitic infections. 19 Eosinophilia was found in 10.3% (29/282) of the dogs in our study, with more than half of them having a final diagnosis of primary inflammatory enteropathy and only 1 dog diagnosed with hypoadrenocorticism.
Our study had some limitations, mainly as a consequence of its retrospective design. Dogs selected in our study are not necessarily representative of the general population of dogs with chronic gastrointestinal disease. Nineteen percent of dogs with resting cortisol concentration <2 μg/dL (<55 nmol/L) did not have an ACTH stimulation test performed. Six of 282 dogs (2.1%) did not have a final diagnosis and, of these, 1/5 did not have stimulation testing performed, and hypoadrenocorticism was not excluded. However, resting cortisol concentration in this patient was 1.92 μg/dL (53.2 nmol/L), close to the cutoff of 2 μg/dL (55 nmol/L) used to exclude hypoadrenocorticism.
In conclusion, prevalence of hypoadrenocorticism was 0.3% in a referral population of dogs with chronic gastrointestinal signs, lower than in a previous study and closer to overall reported prevalence estimates of hypoadrenocorticism in the general canine population. Repeated resting cortisol concentration measurement excluded hypoadrenocorticism in 19/27 dogs with a second resting cortisol concentration, meaning it could be of use as a test to exclude hypoadrenocorticism, especially if ACTH is not available.
CONFLICT OF INTEREST DECLARATION
Authors declare no conflict of interest.
OFF‐LABEL ANTIMICROBIAL DECLARATION
Authors declare no off‐label use of antimicrobials.
INSTITUTIONAL ANIMAL CARE AND USE COMMITTEE (IACUC) OR OTHER APPROVAL DECLARATION
Authors declare no IACUC or other approval was needed.
HUMAN ETHICS APPROVAL DECLARATION
Authors declare human ethics approval was not needed for this study.
Supporting information
Table S1 Clinicopathological abnormalities of the only dog diagnosed with hypoadrenocorticism.
ACKNOWLEDGMENT
No funding was received for this study.
Gallego AF, Gow AG, Boag AM. Evaluation of resting cortisol concentration testing in dogs with chronic gastrointestinal signs. J Vet Intern Med. 2022;36(2):525‐531. doi: 10.1111/jvim.16365
REFERENCES
- 1. Scott‐Moncrieff JC. Hypoadrenocorticism. In: Feldman EC, Nelson RW, Reusch C, et al., eds. Canine and Feline Endocrinology. 4th ed. St Louis, MO: Elsevier; 2015:485‐520. [Google Scholar]
- 2. Hanson JM, Tengvall K, Bonnett BN, Hedhammar Å. Naturally occurring adrenocortical insufficiency—an epidemiological study based on a Swedish‐insured dog population of 525,028 dogs. J Vet Intern Med. 2016;30:76‐84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bellumori TP, Famula TR, Bannasch DL, Belanger JM, Oberbauer AM. Prevalence of inherited disorders among mixed‐breed and purebred dogs: 27,254 cases (1995‐2010). J Am Vet Med Assoc. 2013;242:1549‐1555. [DOI] [PubMed] [Google Scholar]
- 4. Kelch WJ. Canine Hypoadrenocorticism (Canine Addison's Disease): History, Contemporary Diagnosis by Practicing Veterinarians, and Epidemiology [PhD dissertation]. University of Tennessee; 1996.
- 5. Schofield I, Woolhead V, Johnson A, Brodbelt DC, Church DB, O'Neill DG. Hypoadrenocorticism in dogs under UK primary veterinary care: frequency, clinical approaches and risk factors. J Small Anim Pract. 2021;1–8:343‐350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Boag AM, Catchpole B. A review of the genetics of hypoadrenocorticism. Top Companion Anim Med. 2014;29:96‐101. [DOI] [PubMed] [Google Scholar]
- 7.Agreed Language In Veterinary Endocrinology (ALIVE) Project Definitions; 2021. https://www.esve.org/alive/search.aspx. Accessed July 2021.
- 8. Lyngby JG, Sellon RK. Hypoadrenocorticism mimicking protein‐losing enteropathy in 4 dogs. Can Vet J. 2016;57:757‐760. [PMC free article] [PubMed] [Google Scholar]
- 9. Hauck C, Schmitz SS, Burgener IA, et al. Prevalence and characterization of hypoadrenocorticism in dogs with signs of chronic gastrointestinal disease: a multicenter study. J Vet Intern Med. 2020;34:1‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Baumstark ME, Sieber‐Ruckstuhl NS, Müller C, Wenger M, Boretti FS, Reusch CE. Evaluation of aldosterone concentrations in dogs with hypoadrenocorticism. J Vet Intern Med. 2013;28:154‐159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Klein SC, Peterson ME. Canine hypoadrenocorticism: part I. Can Vet J. 2010;51:63‐69. [PMC free article] [PubMed] [Google Scholar]
- 12. Melian C, Peterson ME. Diagnosis and treatment of naturally occurring hypoadrenocorticism in 42 dogs. J Small Anim Pract. 1996;37:268‐275. [DOI] [PubMed] [Google Scholar]
- 13. Bovens C, Tennant K, Reeve J, Murphy KF. Basal serum cortisol concentration as a screening test for hypoadrenocorticism in dogs. J Vet Intern Med. 2014;28:1541‐1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gold AJ, Langlois DK, Refsal KR. Evaluation of basal serum or plasma cortisol concentrations for the diagnosis of hypoadrenocorticism in dogs. J Vet Intern Med. 2016;30:1798‐1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lennon EM, Boyle TE, Hutchins RG, et al. Use of basal serum or plasma cortisol concentrations to rule out a diagnosis of hypoadrenocorticism in dogs: 123 cases (2000–2005). J Am Vet Med Assoc. 2007;231:413‐416. [DOI] [PubMed] [Google Scholar]
- 16. Volkmann M, Steiner JM, Fosgate GT, Zentek J, Hartmann S, Kohn B. Chronic diarrhea in dogs—retrospective study in 136 cases. J Vet Intern Med. 2017;31:1043‐1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wakayama JA, Furrow E, Merkel LK, Armstrong PJ. A retrospective study of dogs with atypical hypoadrenocorticism: a diagnostic cut‐off or continuum? J Small Anim Pract. 2017;58:365‐371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Thompson AL, Scott‐Moncrieff JCR, Anderson JD. Comparison of classic hypoadrenocorticism with glucocorticoid‐deficient hypoadrenocorticism in dogs: 46 cases (1985‐2005). J Am Vet Med Assoc. 2007;230(8):1190‐1194. [DOI] [PubMed] [Google Scholar]
- 19. Lilliehöök I, Gunnarsson L, Zakrisson G, Tvedten H. Diseases associated with pronounced eosinophilia: a study of 105 dogs in Sweden. J Small Anim Pract. 2000;41(6):248‐253. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 Clinicopathological abnormalities of the only dog diagnosed with hypoadrenocorticism.


