Abstract
Background
Recent studies have investigated dogs with presumed diet‐associated dilated cardiomyopathy (daDCM), but prospective studies of multiple breeds are needed.
Hypothesis/Objectives
To evaluate baseline features and serial changes in echocardiography and cardiac biomarkers in dogs with DCM eating nontraditional diets (NTDs) or traditional diets (TDs), and in dogs with subclinical cardiac abnormalities (SCA) eating NTD.
Animals
Sixty dogs with DCM (NTD, n = 51; TDs, n = 9) and 16 dogs with SCA eating NTDs.
Methods
Echocardiography, electrocardiography, and measurement of taurine, cardiac troponin I, and N‐terminal pro‐B‐type natriuretic peptide were performed in dogs with DCM or SCA. Diets were changed for all dogs, taurine was supplemented in most, and echocardiography and cardiac biomarkers were reassessed (3, 6, and 9 months).
Results
At enrollment, there were few differences between dogs with DCM eating NTDs or TDs; none had low plasma or whole blood taurine concentrations. Improvement in fractional shortening over time was significantly associated with previous consumption of a NTD, even after adjustment for other variables (P = .005). Median survival time for dogs with DCM was 611 days (range, 2‐940 days) for the NTD group and 161 days (range, 12‐669 days) for the TD group (P = .21). Sudden death was the most common cause of death in both diet groups. Dogs with SCA also had significant echocardiographic improvements over time.
Conclusions and Clinical Importance
Dogs with DCM or SCA previously eating NTDs had small, yet significant improvements in echocardiographic parameters after diet changes.
Keywords: arrhythmia, congestive heart failure, grain‐free, heart disease, nutrition, pulses
Abbreviations
- 2D
2‐dimensional
- CHF
congestive heart failure
- daDCM
presumed diet‐associated dilated cardiomyopathy
- DCM
dilated cardiomyopathy
- FDA
United States Food and Drug Administration
- FS
fractional shortening
- hs‐cTnI
high‐sensitivity cardiac troponin I
- LA : Ao
ratio of the left atrial to aortic diameters (2D)
- LVIDdN
normalized left ventricular internal diameter in diastole
- LVIDsN
left ventricular internal diameter in systolc
- NTD
nontraditional diet
- NT‐proBNP
N‐terminal pro‐B‐type natriuretic peptide
- SCA
subclinical cardiac abnormalities
- TD
traditional diet
1. INTRODUCTION
Most cases of dilated cardiomyopathy (DCM) in dogs are thought to have a familial or genetic basis, affecting large and giant breeds. 1 However, secondary forms of DCM also can occur as a result of drugs, infectious agents, and nutritional causes. 2 Taurine deficiency is a form of secondary DCM in cats 3 and dogs, 4 , 5 , 6 , 7 , 8 , 9 , 10 , 11 but deficiencies of other nutrients such as thiamine or copper also can cause secondary DCM. 2 Other causes of secondary nutritional DCM include diet‐related toxins, such as heavy metals or monensin‐contaminated feed. 2
In 2018, the United States Food and Drug Administration (FDA) issued an alert regarding a possible connection between diet and DCM. 12 Since that time, there have been 2 FDA updates 13 , 14 and several peer‐reviewed research studies describing dogs with presumed diet‐associated DCM (daDCM). 15 , 16 , 17 , 18 Nontraditional diets (NTDs) eaten by dogs with daDCM have typically been grain‐free or rich in pulses (eg, peas, lentils, and chickpeas) or potatoes/sweet potatoes. 14 , 15 , 16 , 17 , 18 Breeds typically affected by primary DCM (eg, Doberman Pinschers) and breeds that do not commonly develop DCM (eg, Miniature Schnauzers) have been affected by daDCM. 14 , 15 , 16 , 17 , 18 This secondary form of DCM is unique because of the improvement in various echocardiographic variables and longer survival times after diet change, 14 , 15 , 16 , 17 , 18 , 19 whereas dogs with primary DCM typically have limited echocardiographic improvement and shorter survival times. 20 , 21 , 22 , 23 , 24 Published research studies on daDCM thus far have been retrospective 16 , 17 , 18 or conducted in a single breed, 15 and many questions remain.
The objectives of our prospective study were to: (a) compare the baseline characteristics of dogs with DCM eating NTDs versus traditional diets (TDs), (b) evaluate serial changes in echocardiographic measurements and cardiac biomarkers after medical treatment and change in diet, and (c) measure survival times in dogs with DCM eating NTD compared to TDs. In addition, dogs eating NTDs that had subclinical cardiac abnormalities (SCA) not meeting the definition of DCM were enrolled to evaluate serial echocardiographic and biomarker changes after dietary changes.
2. MATERIALS AND METHODS
2.1. Subjects
Dogs diagnosed with DCM were continuously enrolled between September 2018 and March 2020 from 2 universities (Tufts University and University of Florida). The study's definition of DCM consisted of M‐mode fractional shortening (FS) ≤25%, normalized left ventricular internal diameter in diastole (LVIDdN) ≥1.8, and normalized left ventricular internal diameter in systole (LVIDsN) ≥1.2 (or breed‐specific criteria for Doberman Pinschers or Boxers). 16 , 17 , 25 , 26 Eligible dogs had to be eating a commercial nontraditional or traditional extruded (kibble) diet as their main source of calories for at least 6 months. Baseline diets were categorized as NT if they were grain‐free or included pulses or potatoes/sweet potatoes in the top 10 ingredients and T if they were grain‐inclusive and had no pulses or potatoes/sweet potatoes in the top 10 ingredients. 14 , 17 , 18 Ingredients were determined based on the ingredient list of the diet providing the majority of calories to each dog. Grain‐free diets were defined as those not containing grains or grain‐derived ingredients. 14 , 17 , 27 Oils (eg, corn oil) were not classified as a grain product.
Dogs with SCA were identified during evaluation of dogs as potential healthy controls for the current study, screening of housemates of dogs diagnosed with DCM, or routine evaluation of dogs by the Cardiology Service. Dogs were eligible for enrollment in the SCA group if they were eating NTDs for at least 6 months and met 1 of the following 2 criteria:
M‐mode FS ≤25% plus either increased N‐terminal pro‐B‐type natriuretic peptide (NT‐proBNP) >900 pmol/L (>735 pmol/L in Doberman Pinschers) or increased high‐sensitivity cardiac troponin I (hs‐cTnI) >0.06 ng/mL (>0.12 in older dogs) concentrations.
M‐mode FS <35% plus increased NT‐proBNP and increased hs‐cTnI concentrations.
Dogs with >1+ (mild) mitral regurgitation or obvious thickening of the mitral valve were excluded from this group.
Required testing (ie, laboratory testing, echocardiogram, electrocardiogram, and genetic testing) and an initial supply of taurine supplement and dog food (to ensure early use and good compliance) were paid for by the study.
2.2. Baseline analyses
Owners signed informed consent and completed diet history forms. Diet pulse and diet pulse/potato scores were calculated for each dog's diet at enrollment (Table S1). Dogs had a diagnostic electrocardiogram and echocardiogram performed using standard techniques at baseline. 28 Echocardiograms were performed by board‐certified veterinary cardiologists or a supervised cardiology resident, with the same person performing serial measurements on an individual dog. Blood and buccal swabs were collected for the following variables at baseline: CBC; serum biochemistry profile; concentrations of NT‐proBNP, hs‐cTnI, plasma, and whole blood taurine; and, for Doberman Pinschers or Boxers, genetic mutation testing (Table S2). Taurine status was defined as low, borderline, normal, or high based on the laboratory's reference ranges (Table S2). Selected nutritional variables also were analyzed in small subgroups of the 60 dogs with DCM and compared to small subgroups of 18 control dogs determined to be healthy based on history, physical examination, CBC, serum biochemistry profile, and echocardiography (Table S3).
2.3. Interventions
Medical treatment of DCM was at the discretion of each dog's primary clinician. In most dogs, taurine supplementation was initiated at the baseline visit. Owners were instructed to administer taurine supplementation until laboratory results were available 2 to 4 weeks later and to continue supplementation if plasma or whole blood taurine concentrations were low or borderline. Owners were given the choice to continue or discontinue taurine supplementation if plasma and whole blood taurine concentrations were found to be normal or high (Table S4).
Owners of dogs with DCM in both diet groups and of dogs with SCA were instructed to change to 1 of 6 commercial extruded diets that were lower in sodium, grain‐inclusive, did not contain pulses or potatoes/sweet potatoes in the top 10 ingredients, and were made by manufacturers that met the World Small Animal Veterinary Association Global Nutrition Committee's guidelines. 29 Diet options had variable caloric densities, manufacturers, and costs to address different dog and owner needs. In some dogs with concurrent medical conditions, a diet different from the main 6 intervention diets (but meeting the same criteria) was selected to tailor the diet to the individual dog's needs (eg, higher fiber and lower fat). All dogs ate primarily an extruded diet as their main source of calories, but 3 canned options were available to supplement the extruded diet if desired by the owner or if dogs would not eat extruded food alone.
2.4. Serial assessment
Dogs with congestive heart failure (CHF) were re‐evaluated 1 to 2 weeks after diagnosis to assess their overall status, serum biochemical profile variables, and, if indicated, an electrocardiogram. Dogs were classified as having CHF based on a combination of clinical signs and echocardiographic findings, along with either radiographic evidence of cardiogenic pulmonary edema or presence of ascites or pleural effusion judged to be cardiogenic in origin. Dogs were re‐evaluated 3, 6, and 9 months after the diet changes, at which time an echocardiogram was performed and blood was collected for NT‐proBNP and hs‐cTnI analysis. Dogs with arrhythmias also had a 6‐lead electrocardiogram performed, and dogs with CHF had a serum biochemistry profile performed at each visit. Thoracic radiographs were performed as clinically indicated.
2.5. Statistical analysis
Differences in selected characteristics between dogs in the NTD and TD groups were compared at baseline using Fisher's exact tests (categorical variables) or Mann‐Whitney U tests (continuous variables). Spearman correlation tests were used to compare taurine and hs‐cTnI concentrations at the time of enrollment. In examining serial changes in various variables over time, the primary outcomes were FS, LVIDdN, LVIDsN, ratio of the left atrial‐to‐aortic diameters (LA : Ao), and hs‐cTnI and NT‐proBNP concentrations. Paired t tests (normally distributed data) or Wilcoxon signed‐rank tests (skewed data) were used to compare baseline and 9‐month variables for dogs that lived until the 9‐month time point. In addition, serial changes for all dogs (excluding 2 dogs that died or were euthanized during their initial hospitalization) were analyzed using mixed linear models adjusted for several possible confounding variables. Initially, a model was constructed that adjusted for key characteristics that were significantly different between the 2 diet groups at baseline (ie, age, sex, and body weight for most outcome variables; age and sex only for LVIDdN and LVIDsN). The second regression model added key clinical confounders (ie, presence of CHF, presence of any supraventricular or ventricular arrhythmia, and the intervention diet that dogs were changed to after enrollment [classified into the 4 most commonly fed diets and a 5th “other diet” category]).
For dogs that were no longer alive at the time of analysis (1 May 2021), the date and cause of death were recorded as either worsening CHF, sudden cardiac death, or noncardiac in origin. Cause of death in dogs with sudden cardiac arrest that underwent successful cardiopulmonary resuscitation and subsequently were euthanized within 1 hour of resuscitation were classified as sudden cardiac death (n = 2). Survival times were calculated from the time of diagnosis of DCM until the time of death or euthanasia (excluding 2 dogs that died or were euthanized during their initial hospitalization). Dogs that were still alive or that died from noncardiac causes were right‐censored. Kaplan‐Meier curves were constructed and the Fine and Gray proportional hazards models were utilized to examine differences in survival between the 2 diet groups after adjustment for several important, potentially confounding clinical and demographic factors, including age, presence of CHF or cardiac arrhythmia, intervention diet, serum hs‐cTnI concentration, and LA : Ao. Competing risk from noncardiac death was accounted for by calculating cause‐specific hazard ratios.
Commercial statistical software (SAS version 9.4, SAS Institute, Cary, NC; SPSS version 26.0, IBM Corp., Armonk, NY) was used for all analyses. P values ≤.05 were considered significant.
3. RESULTS
3.1. Dilated cardiomyopathy group
Between September 2018 and March 2020, 60 dogs with DCM were enrolled (51 [85%] eating NTDs, 9 [15%] eating TDs; Figure 1).
FIGURE 1.
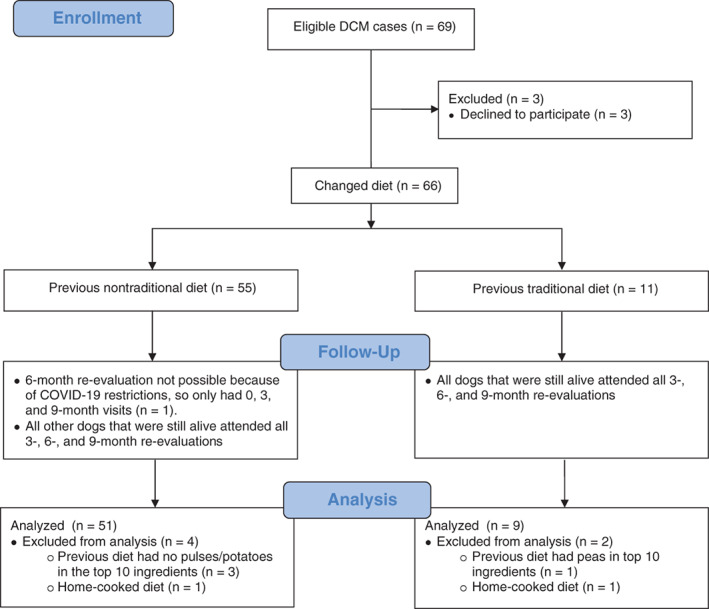
Flow diagram illustrating the enrollment of dogs with dilated cardiomyopathy (DCM), reasons for exclusion, missed re‐evaluations, and number of statistically evaluable cases
3.1.1. Baseline comparisons
Dogs in the nontraditional group weighed significantly less than dogs in the traditional group (P = .04; Table 1). Overall, breed was not significantly different between the 2 diet groups but breeds not typically affected by DCM were only found in the nontraditional group (eg, Chihuahua, Jack Russell Terrier, and Pit Bull). Congestive heart failure (NTD: 77%, TD: 78%; P = 1.00) and arrhythmias (NTD: 49%, TD: 67%; P = .47) were common in both diet groups but not significantly different between groups (Table 1). There was no difference in duration eating NTDs or TDs (Table 1). The only significant difference in CBC and serum biochemistry profile variables was a higher serum magnesium concentration in the NTD group (Table 1; P = .01; other variables not shown), although no dog had a serum magnesium concentration above the reference range.
TABLE 1.
Baseline comparison of signalment, clinical, laboratory, and echocardiographic variables for dogs with dilated cardiomyopathy eating nontraditional or traditional diets. Data are presented as number, mean ± SD, median (range), or number (percentage). P values are for comparison of nontraditional vs traditional groups, with significant P values in bold
| Variable | Nontraditional diet (n = 51) | Traditional diet (n = 9) | P value |
|---|---|---|---|
| Age (years) | 7.0 ± 2.7 | 8.9 ± 2.4 | .06 |
| Sex | .07 | ||
| Male | 27 (20 castrated) | 8 (5 castrated) | |
| Female | 24 (22 spayed) | 1 (1 spayed) | |
| Female (% of total) | 47% | 11% | |
| Breed | .98 | ||
| Doberman Pinscher | 11 (22%) | 2 (22%) | |
| Pit Bull | 6 (12%) | 0 (0%) | |
| Boxer | 5 (10%) | 1 (11%) | |
| Golden Retriever | 5 (10%) | 1 (11%) | |
| Great Dane | 4 (8%) | 1 (11%) | |
| Mixed breed | 4 (8%) | 0 (0%) | |
| Other | 16 (31%) | 4 (44%) | |
| DNA positive | 8/15 (53%) a | 2/3 (67%) | 1.00 |
| Body weight (kg) | 33.8 ± 14.6 | 44.8 ± 15.6 | .04 |
| Body condition score | 5.1 ± 1.3 | 4.9 ± 1.8 | .74 |
| Muscle condition score | .11 | ||
| Normal | 30 (59%) | 4 (44%) | |
| Mild muscle loss | 16 (31%) | 2 (22%) | |
| Moderate muscle loss | 4 (8%) | 1 (11%) | |
| Severe muscle loss | 1 (2%) | 2 (22%) | |
| Cardiac murmur intensity | 2 (0‐5) | 2 (0‐5) | .68 |
| Cardiac arrhythmia | |||
| Any arrhythmia | 25 (49%) | 6 (67%) | .47 |
| Supraventricular | 8 (16%) | 4 (44%) | .07 |
| Ventricular | 20 (39%) | 3 (33%) | 1.00 |
| Congestive heart failure | 39 (77%) | 7 (78%) | 1.00 |
| NT‐proBNP (pmol/L) | 4778 (461‐10 000) | 7997 (2811‐10 000) | .14 |
| hs‐cTnI (ng/mL) | 0.670 (0.024‐5.950) | 0.892 (0.167‐11.299) | .16 |
| Magnesium (mEq/L) | 2.0 ± 0.2 | 1.8 ± 0.3 | .01 |
| Duration eating diet (months) | 48 (6‐156) | 72 (12‐132) | .09 |
| Diet pulse score | 66 (0‐125) | 0 (0‐12) | <.001 |
| Diet pulse/potato score | 84 (23‐125) | 0 (0‐12) | <.001 |
| Plasma taurine (nmol/mL) | 145 (45‐411) | 115 (53‐202) | .07 |
| Plasma taurine categories | .28 | ||
| Low (<40 nmol/mL) | 0 (0%) | 0 (0%) | |
| Borderline (40‐59 nmol/mL) | 3 (6%) | 1 (11%) | |
| Normal (60‐120 nmol/mL) | 14 (28%) | 5 (56%) | |
| High (>120 nmol/mL) | 28 (55%) | 3 (33%) | |
| Whole blood taurine (nmol/mL) | 336 (192‐618) | 347 (240‐460) | .87 |
| Whole blood taurine categories | 1.00 | ||
| Low (<150 nmol/mL) | 0 (0%) | 0 (0%) | |
| Borderline (150‐199 nmol/mL) | 3 (6%) | 0 (0%) | |
| Normal (200‐350 nmol/mL) | 26 (51%) | 5 (56%) | |
| High (>350 nmol/mL) | 21 (41%) | 4 (44%) | |
| Echocardiography | |||
| M‐mode | |||
| Fractional shortening (%) | 14.12 ± 5.22 | 17.85 ± 6.82 | .06 |
| LVIDdN | 2.25 ± 0.39 | 2.05 ± 0.19 | .03 |
| LVIDsN | 1.80 ± 0.34 | 1.56 ± 0.21 | .04 |
| 2D | |||
| Left atrium : aorta | 2.17 ± 0.57 | 2.21 ± 0.58 | .83 |
| Sphericity index | 1.34 ± 0.21 | 1.30 ± 0.16 | .56 |
Abbreviations: 2D, 2‐dimensional; FS, fractional shortening; hs‐cTnI, high‐sensitivity cardiac troponin I; LA : Ao, ratio of the left atrial to aortic diameters (2‐dimensional); LVIDdN, normalized left ventricular internal diameter in diastole; LVIDsN, normalized left ventricular internal diameter in systole; NT‐proBNP, N‐terminal pro‐B‐type natriuretic peptide.
DNA tested only in Doberman Pinschers and Boxers. DNA was not tested in 1 Doberman Pinscher in the nontraditional group.
Plasma and whole blood taurine concentrations were not significantly different between diet groups, nor were the percentages of dogs in categories of plasma or whole blood taurine status (Table 1). No dogs had low plasma (<40 nmol/mL) or whole blood (<150 nmol/mL) taurine concentrations. Seven dogs (including 2 Golden Retrievers) had borderline low plasma or whole blood taurine concentrations, but no dog had both a borderline plasma and borderline whole blood concentration. Weak positive correlations were found for all 60 dogs at baseline between plasma taurine and hs‐cTnI (r = 0.33, P = .02) concentrations and between whole blood taurine and hs‐cTnI (r = 0.35, P = .01) concentrations. No measured vitamin or mineral concentrations were significantly different between dogs in the NTD group and healthy controls (Table S3). All dogs in the NTD group in which iron indices were measured had normal hematocrits, but 1 dog had low serum iron concentration and high ferritin concentration and 2 dogs had increased serum ferritin concentrations. At baseline, the only echocardiographic differences were a significantly larger LVIDdN (P = .03) and LVIDsN (P = .04) in the NTD group (Table 1).
3.1.2. Medications and supplements
Dogs with DCM in both diet groups received many cardiac medications throughout the study, without any between‐group differences (Table S4). Fifty‐one of the 58 discharged dogs (88%) received taurine supplementation after enrollment (NTD group: n = 44/49; TD group: n = 7/9). The median duration of taurine supplementation was not significantly different between diet groups (NTD group: median, 6.5 months; range, 0.4‐9.0 months; TD group: median, 4.9 months; range, 0.1‐9.0 months; P = .99).
3.1.3. Serial changes in cardiac biomarkers and echocardiographic measurements
Twenty‐nine of 51 dogs (57%) in the NTD group and 3/9 dogs (33%) in the TD group survived until the 9‐month re‐evaluation (P = .28). In the NTD group, a significant decrease was found in hs‐cTnI concentrations between 0 and 9 months (P = .03), but the change in NT‐proBNP was not significant (Table 2). Among dogs in the TD group, the change from baseline to 9 months was not significant for hs‐cTnI or NT‐proBNP concentrations (Table 2). The final mixed model for serial changes in hs‐cTnI concentrations showed that the interaction between diet group and time was not significant after adjusting for age, sex, weight, CHF, intervention diet, and arrhythmia (P = .06 for diet group × time interaction; Table 2). No significant diet group × time interaction was found for NT‐proBNP.
TABLE 2.
Serial changes in key outcome variables in dogs with dilated cardiomyopathy (DCM) eating nontraditional or traditional diets. Data are presented as mean ± SD or median (range). Significant P values are in bold
| Variable | P value (diet group × time) a | Nontraditional diet group | Traditional diet group | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 month | 3 months | 6 months | 9 months | P value (within‐group change) b | 0 month | 3 months | 6 months | 9 months | P value (within‐group change) b | ||
| n | — | 51 | 37 | 30 | 29 | — | 9 | 5 | 5 | 3 | — |
| Biomarkers | |||||||||||
| hs‐cTnI (ng/mL) | .06 | 0.670 (0.024‐5.95) | 0.306 (0.025‐2.411) | 0.394 (0.025‐3.270) | 0.374 (0.016‐3.296) | .03 | 0.892 (0.167‐11.299) | 0.642 (0.122‐0.695) | 0.650 (0.090‐1.514) | 0.178 (0.090‐0.757) | .59 |
| NT‐proBNP (pmol/L) | .13 | 4778 (461‐10 000 | 3015 (370‐10 000) | 3607 (363‐10 000) | 4061 (336‐10 000) | .28 | 7997 (2811‐10 000) | 3868 (1177‐8031) | 4536 (674‐10 000) | 4012 (666‐10 000) | .18 |
| Echocardiographic variables | |||||||||||
| FS (%) | .005 | 14.12 ± 5.22 | 16.26 ± 6.51 | 16.60 ± 6.54 | 18.70 ± 7.53 | <.001 | 17.85 ± 6.82 | 20.98 ± 8.42 | 14.95 ± 3.88 | 18.02 ± 4.51 | .58 |
| LVIDdN | .70 | 2.25 ± 0.39 | 2.11 ± 0.36 | 2.07 ± 0.35 | 2.04 ± 0.41 | .005 | 2.05 ± 0.19 | 1.98 ± 0.18 | 1.88 ± 0.14 | 1.79 ± 0.33 | .31 |
| LVIDsN | .60 | 1.80 ± 0.34 | 1.65 ± 0.35 | 1.63 ± 0.38 | 1.56 ± 0.39 | <.001 | 1.56 ± 0.21 | 1.44 ± 0.17 | 1.47 ± 0.12 | 1.36 ± 0.31 | .17 |
| LA : Ao | .23 | 2.17 ± 0.57 | 1.89 ± 0.55 | 1.74 ± 0.47 | 1.72 ± 0.52 | .004 | 2.21 ± 0.58 | 1.67 ± 0.60 | 1.86 ± 0.51 | 1.98 ± 1.17 | .86 |
Abbreviations: 2D, 2‐dimensional; FS, fractional shortening; hs‐cTnI, high‐sensitivity cardiac troponin I; LA : Ao, ratio of the left atrial to aortic diameters (2‐dimensional); LVIDdN, normalized left ventricular internal diameter in diastole; LVIDsN, normalized left ventricular internal diameter in systole; NT‐proBNP, N‐terminal pro‐B‐type natriuretic peptide.
Mixed models analysis of diet x time interaction for all 58 dogs (both non‐traditional and traditional diets) discharged from the hospital after adjustment for age, sex, body weight, intervention diet, congestive heart failure, and arrhythmia.
Comparing 0 to 9 months for the 29 dogs in the non‐traditional group and 3 dogs in the traditional group that completed the study.
For the echocardiographic endpoints, the 29 dogs that completed the study in the NTD group had a significant within‐group increase from 0 to 9 months in FS (P < .001) and significant decreases in LVIDdN (P = .005), LVIDsN (P < .001), and LA : Ao (P = .004; Table 2). Within‐group changes for the 3 dogs in the TD group that completed the study were not significant for any of the echocardiographic variables examined (Table 2). Mixed models analysis incorporating all time points (0, 3, 6, and 9 months) for dogs in both diet groups showed that the interaction between diet group and time was significantly associated with improvements in FS (P = .005), with greater improvement in the NTD group after adjustment for age, sex, weight, intervention diet, arrhythmia, and CHF (Table 2). No diet group x time interaction was found for LVIDdN, LVIDsN, or LA : Ao after adjustment (Table 2).
3.1.4. Survival
At the time of analysis (1 May 2021), 19 dogs in the NTD group (37%) and 2 dogs in the TD group (22%) were still alive (P = .47). The most common cause of death was sudden death (16/32 [50%] in the NTD group and 5/7 [72%] in the TD group). Euthanasia for worsening CHF occurred in 12/32 dogs in the NTD group [38%] and in 1/7 dogs in the TD group [14%]. Four of 32 dogs in the NTD group (13%) and 1/7 dogs in the TD group (14%) were euthanized for noncardiac causes. The cause of death was not significantly different between dogs in the NTD vs TD groups (P = .47).
For dogs with DCM that were discharged after their initial visit (n = 58), median survival time was 611 days (range, 2‐940 days) for the NTD group and 161 days (range, 12‐669 days) for the TD group (P = .21; Figure 2). After adjusting for age, CHF, arrhythmia, intervention diet, hs‐cTnI, and LA : Ao, diet group still was not significantly associated with survival time (Figure 2).
FIGURE 2.
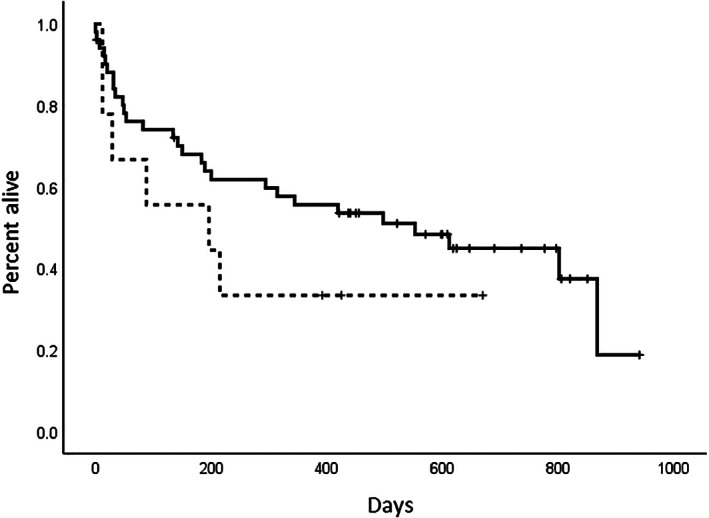
Kaplan‐Meier survival curves comparing survival time in 60 dogs with dilated cardiomyopathy (DCM) after diet change. Median survival time of the 51 dogs originally eating a nontraditional diet (611 days, range, 2‐940 days; solid line) was not significantly longer than that of the 9 dogs originally eating a traditional diet (161 days, range, 12‐669 days; dashed line; P = .21)
3.2. Subclinical cardiac abnormalities group
Sixteen dogs comprised the SCA group (Table 3). The median duration eating NTDs was significantly shorter in dogs with SCA compared to dogs with DCM eating NTDs (P < .001), but median diet pulse (P = 1.00) and diet pulse/potato (P = .62) scores for NTDs were not significantly different between dogs with SCA and dogs with DCM (Tables 1 and 3). None of the 16 dogs with SCA had observable clinical signs of heart disease at the time of enrollment. Cardiac arrhythmias were present in 4/16 (25%) of SCA dogs (known before arrhythmia [1 dog] or detected at enrollment [3 dogs]).
TABLE 3.
Baseline characteristics for signalment, clinical, laboratory, and echocardiographic variables for dogs with subclinical cardiac abnormalities eating a nontraditional diet. Data are presented as number, mean ± SD, or median (range)
| Variable | Nontraditional diet (n = 16) |
|---|---|
| Age (years) | 5.2 ± 2.2 |
| Sex | |
| Male | 7 (3 castrated) |
| Female | 9 (5 spayed) |
| Female (% of total) | 56% |
| Breed | |
| Boxer | 3 |
| Irish Wolfhound | 3 |
| Doberman Pinscher | 2 |
| English Bulldog | 2 |
| Golden Retriever | 2 |
| Other | 4 |
| DNA positive | 3/5 a |
| Weight (kg) | 29.7 (6.2‐82.7) |
| Body condition score | 5.8 ± 1.1 |
| Muscle condition score | |
| Normal | 14 |
| Mild | 2 |
| Moderate | 0 |
| Severe | 0 |
| Cardiac murmur intensity | 0 (0‐5) |
| Arrhythmia | |
| Any arrhythmia | 3 |
| Supraventricular | 2 |
| Ventricular | 2 |
| Congestive heart failure | 0 |
| NT‐proBNP (pmol/L) | 1367 (302‐3706) |
| hs‐cTnI (ng/mL) | 0.135 (0.017‐0.504) |
| Magnesium (mEq/L) | 2.1 ± 0.2 |
| Diet pulse score | 65 (16‐121) |
| Diet pulse/potato score | 81 (16‐121) |
| Duration eating diet (months) | 30 (7‐36) |
| Plasma taurine (nmol/mL) | 112 (19‐237) |
| Plasma taurine categories | |
| Low (<40 nmol/mL) | 1 |
| Borderline (40‐59 nmol/mL) | 1 |
| Normal (60‐120 nmol/mL) | 8 |
| High (>120 nmol/mL) | 4 |
| Whole blood taurine (nmol/mL) | 290 (168‐564) |
| Whole blood taurine categories | |
| Low (<150 nmol/mL) | 0 |
| Borderline (150‐199 nmol/mL) | 1 |
| Normal (200‐350 nmol/mL) | 11 |
| High (>350 nmol/mL) | 3 |
| Echocardiography | |
| M‐mode | |
| Fractional shortening (%) | 23.78 ± 4.62 |
| LVIDdN | 1.58 ± 0.13 |
| LVIDsN | 1.11 ± 0.11 |
| 2D | |
| Left atrium: aorta | 1.74 ± 0.38 |
| Sphericity | 1.67 ± 0.30 |
Abbreviations: 2D, 2‐dimensional; hs‐cTnI, high‐sensitivity cardiac troponin I; LVIDdN, normalized left ventricular internal diameter in diastole; LVIDsN, normalized left ventricular internal diameter in systole; NT‐proBNP, N‐terminal pro‐B‐type natriuretic peptide.
DNA tested only in Doberman Pinschers and Boxers.
No dogs in the SCA group had both low plasma and low whole blood taurine concentrations, but 1 dog had low plasma taurine concentration (19 nmol/mL) with borderline whole blood taurine concentration (168 nmol/mL). Another dog had borderline plasma taurine concentration (42 nmol/mL) and normal whole blood taurine concentration (304 nmol/mL). All others had normal or high taurine concentrations (Table 3). Over the course of the study, 5 dogs in the SCA group received ≥1 cardiac medications: pimobendan (n = 3), carvedilol (n = 2), and sotalol (n = 1). Fifteen dogs received taurine supplementation for a median of 3 months (range, 1‐9 months).
Two dogs in the SCA group could not be evaluated at the 6‐month visit because of COVID‐19 restrictions, and were evaluated at 0, 3, and 9 months. One dog died suddenly 95 days after the start of the study; all 15 other dogs survived the full 9 months of the study. At the time of analysis, 2 dogs had been euthanized for noncardiac reasons (osteosarcoma, n = 1; pneumonia, n = 1). The range of survival times for all dogs was 95 to 906 days.
A significant within‐group increase in FS (24.06 ± 4.65% vs 30.11 ± 5.81%; P = .005) and significant decreases in LVIDdN (P = .02), LVIDsN (P = .005), and LA : Ao (P = .04) were observed in these 15 SCA dogs between 0 and 9 months (Table 4). No significant association was found between changes in any of the echocardiographic measurements and either diet score or duration eating the NTD. Within‐group changes in NT‐proBNP and hs‐cTnI (Table 4) were not significant.
TABLE 4.
Changes in cardiac biomarkers and key echocardiographic variables after diet change in dogs with subclinical cardiac abnormalities that had been eating nontraditional diets. Values [presented as mean ± SD or median (range)] are for 15 of 16 dogs at baseline (0 months) and 9 months. One dog died suddenly 95 days after starting the study and is not included in the analysis. P‐values are for comparison of variables from 0 to 9 months, with significant P values in bold
| Variable | 0 months | 9 months | P value |
|---|---|---|---|
| Biomarkers | |||
| hs‐cTnI (ng/mL) | 0.126 (0.017‐0.504) | 0.121 (0.024‐0.363) | .49 |
| NT‐proBNP (pmol/L) | 1346 (302‐3706) | 1275 (250‐5533) | .87 |
| Echocardiographic variables | |||
| Fractional shortening (%) | 24.06 ± 4.65 | 30.11 ± 5.81 | .005 |
| LVIDdN | 1.59 ± 0.13 | 1.51 ± 0.19 | .02 |
| LVIDsN | 1.11 ± 0.12 | 0.99 ± 0.18 | .005 |
| LA : Ao | 1.70 ± 0.34 | 1.55 ± 0.18 | .04 |
Abbreviations: hs‐cTnI, high‐sensitivity cardiac troponin I; LA : Ao, ratio of the left atrial to aortic diameters (2‐dimensional); LVIDdN, normalized left ventricular internal diameter in diastole; LVIDsN, normalized left ventricular internal diameter in systole; NT‐proBNP, N‐terminal pro‐B‐type natriuretic peptide.
4. DISCUSSION
The main finding of our prospective study was that FS, an indicator of systolic function, significantly improved over a 9‐month period in dogs with DCM eating NTDs that underwent diet change in addition to standard medical treatment. This finding was consistent when variables were measured as within‐group changes for the 29 dogs that completed the 9‐month study or using mixed models analysis that included all dogs and time points for both diet groups.
Some echocardiographic improvements have been identified in each of the 4 published studies on this disease, but significant improvement in FS was only seen in a prospective study of Golden Retrievers with daDCM. 15 , 16 , 17 , 18 The lack of significant changes in FS in other previous studies might be related to their retrospective design or limited sample size. The 4 earlier studies also showed significant improvements in left ventricular and left atrial size, although not all of the studies found significant changes in all of these echocardiographic measurements. 15 , 16 , 17 , 18 In our study, LVIDdN, LVIDsN, and LA : Ao all showed significant within‐group improvements in the NTD group, but the mixed models diet group × time interaction for these echocardiographic variables was not significant. The small sample size of our study limited statistical power to detect serial differences in cardiac size or biomarkers after adjusting for other important potentially confounding variables. The findings from this relatively small study can be used to design more optimal studies in the future. Statistical adjustment for various confounding variables was not performed in previous studies. Cardiac medications, especially pimobendan and furosemide, can be associated with a decrease in cardiac size, and these medications also could have influenced the changes in cardiac size in the current and previous studies. However, in our study, no differences in cardiac medications received by dogs were found between the 2 diet groups. More detailed echocardiographic assessment, such as volume indices, 3D measurements, and global longitudinal strain would be valuable to include in future studies of this disease. In addition, because echocardiograms were not blinded, there is the potential for bias in performing echocardiographic measurements. This possibility is an important limitation of the study that must be considered in interpreting the results.
In our study, dogs in the TD group did not have significant within‐group improvement in any echocardiographic measurement, but the within‐group analysis was markedly limited by the fact that only 3 of 9 dogs in the traditional group survived until the final study visit for the within‐group analysis. Nevertheless, the mixed models analysis also showed improved FS only in the NTD group. Cardiac troponin I concentrations decreased significantly in the NTD group when comparing 0 to 9‐month results, but analysis over time using mixed models analysis did not reach statistical significance. Although not significant, hs‐cTnI concentrations also decreased in the TD group, and thus some of these changes may be associated with medical treatment and require further study. Concentrations of NT‐proBNP did not decrease significantly in either group, but serial changes in this biomarker may be limited by differences among breeds and week‐to‐week variability. 30 , 31 , 32
In our study, survival time in the dogs with DCM eating a NTD (611 days; range, 2‐940 days) was not significantly different from that of dogs in the TD group (161 days; range, 12‐669 days; Figure 2). This result is in contrast to findings from 2 retrospective studies, 17 , 18 which showed longer survival times in dogs with DCM eating NTDs after diet change. In 1 of these studies, eating a NTD was associated with a longer survival time after diet was changed, even after adjustment for the presence of CHF and cardiac arrhythmias. 17 In the second recent retrospective study of dogs with DCM and CHF, prior NTD was associated with longer survival, after adjustment for breed, atrial fibrillation, and age of diagnosis. 18 However, both studies used all‐cause mortality and Cox proportional hazards analysis, which does not account for competing risks, whereas we analyzed for cardiac mortality and adjusted for competing risks. In addition, 1 study had some different inclusion criteria (eg, smaller LVIDdN, required presence of CHF) that could account for the different results. 18 Finally, as previously noted, an important limitation of our study is sample size. It is likely that the relatively small number of dogs (especially in the TD group [n = 9]) might have contributed to the lack of significant differences in survival times between the 2 diet groups.
The most common cause of death in the dogs with DCM in both diet groups was sudden death, accounting for 54% of deaths overall, and cause of death was not different between diet groups. This finding suggests that dogs with DCM (whether primary or diet‐associated) are at high risk for sudden death. Arrhythmias were present in many dogs at the time of enrollment or over the course of the study in both diet groups, which may limit the time for cardiac improvement to be observed given the propensity for sudden cardiac death for those dogs with arrhythmias. The percentage of dogs in our study that died suddenly appeared relatively high compared to studies of dogs with primary DCM where sudden death was reported to account for 10% to 15% of deaths for dogs of multiple breeds and 29% to 42% for Doberman Pinschers. 20 , 21 , 22 , 23 , 24 , 33 , 34 , 35
The limited number of baseline differences between dogs in the different diet groups emphasizes the similar clinical findings of dogs with primary or secondary forms of DCM, which is consistent with previous studies. 16 , 17 , 18 However, it is important to acknowledge that a larger sample size might have provided additional statistical power to identify other differences in our study. Moreover, there was a wide range of breeds in the NTD group that included both atypical and typical breeds. Although some dogs of breeds typically associated with primary DCM in the NTD group had clinical and echocardiographic improvements and were therefore likely to have had daDCM, others in this group might have had primary DCM instead of daDCM, skewing the study's results.
Although the study numbers were small and provide only preliminary insights, no significant differences were found between dogs in the NTD group and healthy controls in concentrations of any measured vitamin, mineral, or other nutrients. In addition to the small numbers of dogs, only a limited number of nutrients were measured. These nutrients were selected for analysis based on their potential association with DCM, but a direct or indirect role for other nutrients in daDCM cannot be ruled out. Therefore, analysis of more nutrients in larger numbers of dogs is warranted.
Taurine deficiency is a possible cause of daDCM. However, no dogs with DCM in our study had low plasma or whole blood taurine concentrations; few had borderline taurine concentrations, and no differences in plasma or whole blood taurine concentrations were found between diet groups. In fact, many dogs with DCM in both the NTD and TD groups (and dogs in the SCA group) had high plasma or whole blood taurine concentrations. Taurine concentrations were weakly correlated with cTnI concentrations, suggesting that the high taurine concentrations might reflect myocardial damage. Some studies in rodents and humans have shown that myocardial ischemia is associated with decreased myocardial taurine concentrations and increased whole blood taurine concentrations. 36 , 37 , 38 , 39 , 40 Although the FDA's data did not show a difference in dietary taurine concentrations of grain‐free and grain‐containing diets, we did not analyze dietary taurine or taurine bioavailability. 13
The decision to initiate taurine supplementation at the time of study enrollment was caused by the delay between diagnosis and availability of plasma and whole blood taurine concentration results and our desire to optimize dogs' chances for clinical improvement while awaiting results, especially because, at the time the study was initiated, taurine deficiency was a popular hypothesis for the cause of daDCM. We elected to give owners the choice of continuing or discontinuing taurine supplementation once deficiency was ruled out. Nonetheless, no difference was identified between diet groups in the proportion of dogs that received taurine or the duration of taurine supplementation, making the differences in FS between groups more likely associated with diet than with taurine supplementation. Taurine, however, might exert some positive effects on myocardial function, even in the absence of deficiency, including mitochondrial tRNA modification for normal mitochondrial tRNA translation. 41 , 42 Therefore, further evaluation of taurine in primary and secondary forms of DCM is warranted.
The SCA group size in our study was small and did not include a control group, but findings from this group might be helpful for understanding daDCM because it could represent an earlier form of the disease. Dogs in the SCA group all were eating NTDs with diet pulse and pulse/potato scores similar to the diets fed to dogs with DCM eating NTDs. However, dogs in the SCA group had been eating the diets for a shorter time (30 months) compared to the DCM group (48 months), suggesting that exposure time to the diet might be associated with disease severity. This hypothesis is supported by findings from a retrospective study of dogs with daDCM that showed a longer duration on a grain‐free diet was associated with shorter survival time. 18 Taurine concentrations in the SCA group were similar to those in the DCM group, with no dogs having low plasma and whole blood taurine concentrations, and few having borderline taurine concentrations. This less severely affected SCA group had serial echocardiographic improvements that were similar to those seen in the DCM group, with significant increases in FS and significant decreases in LVIDdN, LVIDsN, and LA : Ao, although no dogs received diuretics and only 3 received pimobendan, suggesting dogs with SCA represent an earlier stage in the spectrum of disease. Three studies in apparently healthy dogs now have provided some evidence that NTDs are associated with negative cardiac effects (larger left ventricular diameter, lower left ventricular systolic function, higher hs‐cTnI concentrations, more arrhythmias) even in apparently healthy dogs. 43 , 44 , 45
Our study had some additional limitations. Although most dogs in both DCM diet groups had CHF, others had no clinical signs and, even in dogs with CHF, severity varied. Arrhythmias were common in both diet groups and impacted survival time, especially because sudden death was the most common cause of death in both groups. Some dogs with daDCM could have had disease that was too severe to allow for significant echocardiographic improvement. However, because primary DCM typically is a progressive disease, even stable echocardiographic measurements might be noteworthy for daDCM. Although the improvements in FS were significantly associated with diet, studying a more homogeneous population could have made it easier to identify differences in survival, cardiac biomarkers, and other echocardiographic variables as the result of diet. Another limitation is that 9 months might not have been long enough to detect echocardiographic changes in all dogs. Additional investigation of long‐term echocardiographic changes in dogs with daDCM, SCA, and in apparently healthy dogs eating NTDs after diet change would be valuable.
Eighty‐five percent of dogs with DCM enrolled in our study were eating NTDs, with only 15% eating TDs. This low percentage eating TDs is not surprising because so many dogs with DCM in recent years are eating NTDs (between 64% and 95% in 4 recent studies). 16 , 17 , 18 , 46 Nonetheless, the small numbers in the TD group made analysis of the data more challenging, especially for within‐group comparisons. The total number of dogs (n = 60) also limited the number of adjustments that could be considered in the mixed models and survival analyses.
Other limitations are related to the definitions used for diet groups and classification of dogs into diet groups. Definitions for NTDs have varied among studies and have been refined over time as additional data on this disease have accumulated. 14 , 15 , 16 , 17 , 18 , 43 , 44 , 45 The definition of NTDs used in our study focused on the presence or absence of ingredients, rather than subjective criteria. This definition still might not be optimal because, until the exact cause is known, it is impossible to specifically target ingredients or certain compounds that are lacking or in excess in the food. Therefore, definitions might need to be further refined over time. Diet pulse and potato/pulse scores were calculated to assess dogs' “dose” of pulses and potatoes, but these scores may not accurately reflect the exact amount of these ingredients in the diet, may not be related to clinical outcome, and have not been validated.
Another limitation is that dogs were not all changed to the same diet for the 9‐month study. Although a single diet would have been ideal in terms of study design, it was not medically optimal for the dogs or practical in this clinical study, and thus a range of diet options was available so that diets could be individualized or account for pre‐existing concurrent disease. In addition, the study's goal was not to determine if dogs might have a better response with a specific diet over another, but rather to evaluate changes after NTDs had been discontinued. Statistical adjustment for the intervention diet (and other variables) did not change the findings in the mixed models and survival analysis, but further investigation into the role of diet in improvement from daDCM is warranted. Based on a perceived change in attitudes among dog owners and enhanced knowledge about this disease, a more consistent approach to the intervention diet likely could be used for future studies.
Despite the limitations, our results indicate that dogs with DCM eating NTDs have small but significant improvements in echocardiographic measurements during a 9‐month study period, and had a median survival time of 611 days. Nonetheless, they had a high risk of sudden death similar to that in dogs with primary DCM. Dogs with SCA likely represent an earlier form of the disease, and improvement of echocardiographic abnormalities occurred after diet change.
CONFLICT OF INTEREST DISCLOSURE
In the last 3 years, Dr Freeman has received research or residency funding from, given sponsored lectures for, or provided professional services for Aratana Therapeutics, Elanco, Guiding Stars Licensing Co, LLC, Hill's Pet Nutrition, Nestlé Purina PetCare, P&G Petcare (now Mars), and Royal Canin. In the past 3 years, Dr Rush has received research funding from, given sponsored lectures for, or provided professional services for Aratana Therapeutics, Boehringer Ingelheim, Elanco, IDEXX, Nestlé Purina PetCare, and Royal Canin. Dr Adin acknowledges research support from Nestle Purina PetCare and is a consultant and sponsored lecturer for Ceva Animal Health and Boehringer Ingelheim.
OFF‐LABEL ANTIMICROBIAL DECLARATION
Authors declare no off‐label use of antimicrobials.
INSTITUTIONAL ANIMAL CARE AND USE COMMITTEE (IACUC) OR OTHER APPROVAL DECLARATION
This study was approved by the Cummings School of Veterinary Medicine Clinical Studies Review Committee (006.18) and the University of Florida IACUC (201810504).
HUMAN ETHICS APPROVAL DECLARATION
Authors declare human ethics approval was not needed for this study.
Supporting information
Appendix S1: Supporting Information
ACKNOWLEDGMENT
The study was supported by Nestlé Purina PetCare and the Barkley Fund. Presented, in part, in abstract form at the 2020 ACVIM Forum On Demand. We gratefully acknowledge technical support from Melanie Borglund, Lana Fagman, Courtney Hanner, Viviana Heinekin, Michelle Maillet, and Melissa Pisaroglo de Carvalho; clinical management by Dr Amelie Beaumier; and analysis of DNA samples by Dr Kate Meurs. We also thank the owners of the dogs that participated in the study.
Freeman L, Rush J, Adin D, et al. Prospective study of dilated cardiomyopathy in dogs eating nontraditional or traditional diets and in dogs with subclinical cardiac abnormalities. J Vet Intern Med. 2022;36(2):451‐463. doi: 10.1111/jvim.16397
Funding information Nestlé Purina PetCare; The Barkley Fund
REFERENCES
- 1. Sisson D, O'Grady MR, Calvert CA. Myocardial diseases of dogs. In: Fox PR, Sisson D, Moise NS, eds. Textbook of Canine and Feline Cardiology. 2nd ed. Philadelphia, PA: W.B. Saunders Company; 1999:581‐619. [Google Scholar]
- 2. Van Vleet JF, Ferrans VJ. Myocardial diseases of animals. Am J Pathol. 1986;124:98‐178. [PMC free article] [PubMed] [Google Scholar]
- 3. Pion PD, Kittleson MD, Rogers QR, Morris JG. Myocardial failure in cats associated with low plasma taurine: a reversible cardiomyopathy. Science. 1987;237:764‐768. [DOI] [PubMed] [Google Scholar]
- 4. Kramer GA, Kittleson MD, Fox PR, Lewis J, Pion PD. Plasma taurine concentrations in normal dogs and in dogs with heart disease. J Vet Intern Med. 1995;9:253‐258. [DOI] [PubMed] [Google Scholar]
- 5. Kittleson MD, Keene B, Pion PD, Loyer CG. Results of the multicenter spaniel trial (MUST): taurine‐ and carnitine‐responsive dilated cardiomyopathy in American cocker spaniels with decreased plasma taurine concentration. J Vet Intern Med. 1997;11:204‐211. [DOI] [PubMed] [Google Scholar]
- 6. Sanderson SL, Gross KL, Ogburn PN, et al. Effects of dietary fat and L‐carnitine on plasma and whole blood taurine concentrations and cardiac function in healthy dogs fed protein‐restricted diets. Am J Vet Res. 2001;62:1616‐1623. [DOI] [PubMed] [Google Scholar]
- 7. Fascetti AJ, Reed JR, Rogers QR, Backus RC. Taurine deficiency in dogs with dilated cardiomyopathy: 12 cases (1997‐2001). J Am Vet Med Assoc. 2003;223:1137‐1141. [DOI] [PubMed] [Google Scholar]
- 8. Backus RC, Cohen G, Pion PD, Good KL, Rogers QR, Fascetti AJ. Taurine deficiency in Newfoundlands fed commercially available complete and balanced diets. J Am Vet Med Assoc. 2003;223:1130‐1136. [DOI] [PubMed] [Google Scholar]
- 9. Belanger MC, Ouellet M, Queney G, et al. Taurine‐deficient dilated cardiomyopathy in a family of golden retrievers. J Am Anim Hosp Assoc. 2005;41:284‐291. [DOI] [PubMed] [Google Scholar]
- 10. Backus RC, Ko KS, Fascetti AJ, et al. Low plasma taurine concentration in Newfoundland dogs is associated with low plasma methionine and cyst(e)ine concentrations and low taurine synthesis. J Nutr. 2006;136:2525‐2533. [DOI] [PubMed] [Google Scholar]
- 11. Basili M, Pedro B, Hodgkiss‐Geere H, Navarro‐Cubas X, Graef N, Dukes‐McEwan J. Low plasma taurine levels in English cocker spaniels diagnosed with dilated cardiomyopathy. J Small Anim Pract. 2021;62:570‐579. [DOI] [PubMed] [Google Scholar]
- 12. United States Food and Drug Administration . FDA investigating potential connections between diet and cases of canine heart disease; 2018. https://wayback.archive-it.org/7993/20201222194256/https:/www.fda.gov/animal-veterinary/cvm-updates/fda-investigating-potential-connection-between-diet-and-cases-canine-heart-disease
- 13. United States Food and Drug Administration . FDA investigation into potential link between certain diets and canine dilated cardiomyopathy; 2019. https://www.fda.gov/animal-veterinary/news-events/fda-investigation-potential-link-between-certain-diets-and-canine-dilated-cardiomyopathy-february
- 14. United States Food and Drug Administration . FDA investigation into potential link between certain diets and canine dilated cardiomyopathy; 2019. https://www.fda.gov/animal-veterinary/outbreaks-and-advisories/fda-investigation-potential-link-between-certain-diets-and-canine-dilated-cardiomyopathy
- 15. Kaplan JL, Stern JA, Fascetti AJ, et al. Taurine deficiency and dilated cardiomyopathy in golden retrievers fed commercial diets. PLoS One. 2018;13(12):e0209112. doi: 10.1371/journal.pone.0209112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Adin D, DeFrancesco TC, Keene B, et al. Echocardiographic phenotype of canine dilated cardiomyopathy differs based on diet type. J Vet Cardiol. 2019;21:1‐9. [DOI] [PubMed] [Google Scholar]
- 17. Freid KJ, Freeman LM, Rush JE, et al. Retrospective study of dilated cardiomyopathy in dogs. J Vet Intern Med. 2021;35:58‐67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Walker AL, DeFrancesco TC, Bonagura JD, et al. Association of diet with clinical outcomes in dogs with dilated cardiomyopathy and congestive heart failure. J Vet Cardiol. 2021. doi: 10.1016/j.jvc.2021.02.001 [DOI] [PubMed] [Google Scholar]
- 19. Jones J, Carey L, Palmer LA. FDA update on dilated cardiomyopathy: fully and partially recovered cases. Scientific Forum Exploring Causes of Dilated Cardiomyopathy in Dogs. Manhattan, KS: Kansas State University; 2020. [Google Scholar]
- 20. Tidholm A, Svensson H, Sylvén C. Survival and prognostic factors in 189 dogs with dilated cardiomyopathy. J Am Anim Hosp Assoc. 1997;33:364‐368. [DOI] [PubMed] [Google Scholar]
- 21. Petric AD, Stabej P, Zemva A. Dilated cardiomyopathy in Doberman pinschers: survival, causes of death and a pedigree review in a related line. J Vet Cardiol. 2002;4:17‐24. [DOI] [PubMed] [Google Scholar]
- 22. Tidholm A. Survival in dogs with dilated cardiomyopathy and congestive heart failure treated with digoxin, furosemide and propranolol: a retrospective study of 62 dogs. J Vet Cardiol. 2006;8:41‐47. [DOI] [PubMed] [Google Scholar]
- 23. Martin MW, Stafford Johnson MJ, Celona B. Canine dilated cardiomyopathy: a retrospective study of signalment, presentation and clinical findings in 369 cases. J Small Anim Pract. 2009;50:23‐29. [DOI] [PubMed] [Google Scholar]
- 24. Laskary A, Fonfara S, Chambers H, et al. Prospective clinical trial evaluating spironolactone in Doberman pinschers with congestive heart failure due to dilated cardiomyopathy. J Vet Cardiol. 2021. doi: 10.1016/j.jvc.2021.06.001 [DOI] [PubMed] [Google Scholar]
- 25. Meurs KM, Stern JA, Sisson DD, et al. Association of dilated cardiomyopathy with the striatin mutation genotype in boxer dogs. J Vet Intern Med. 2013;27:1437‐1440. [DOI] [PubMed] [Google Scholar]
- 26. Wess G, Domenech O, Dukes‐McEwan J, Häggström J, Gordon S. European Society of Veterinary Cardiology screening guidelines for dilated cardiomyopathy in Doberman Pinschers. J Vet Cardiol. 2017;19:405‐415. [DOI] [PubMed] [Google Scholar]
- 27. Prantil LR, Heinze CR, Freeman LM. Comparison of carbohydrate content between grain‐containing and grain‐free dry cat diets and between reported and calculated carbohydrate values. J Feline Med Surg. 2018;20:349‐355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Thomas WP, Gaber CE, Jacobs GJ, et al. Recommendations for standards in transthoracic two‐dimensional echocardiography in the dog and cat. J Vet Intern Med. 1993;7:247‐252. [DOI] [PubMed] [Google Scholar]
- 29. World Small Animal Veterinary Association Global Nutrition Committee . Guidelines on selecting pet foods; 2021. https://wsava.org/wp-content/uploads/2021/04/Selecting-a-pet-food-for-your-pet-updated-2021_WSAVA-Global-Nutrition-Toolkit.pdf
- 30. Kellihan HB, Oyama MA, Reynolds CA, Stepien RL. Weekly variability of plasma and serum NT‐proBNP measurements in normal dogs. J Vet Cardiol. 2009;11:S93‐S97. [DOI] [PubMed] [Google Scholar]
- 31. Sjostrand K, Wess G, Ljungvall I, et al. Breed differences in natriuretic peptides in healthy dogs. J Vet Intern Med. 2014;28:451‐457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ruaux C, Scollan K, Suchodolski JS, Steiner JM, Sisson DD. Biologic variability in NT‐proBNP and cardiac troponin‐I in healthy dogs and dogs with mitral valve degeneration. Vet Clin Pathol. 2015;44:420‐430. [DOI] [PubMed] [Google Scholar]
- 33. Vollmar C, Vollmar A, Keene BW, Fox PR, Reese S, Kohn B. Dilated cardiomyopathy in 151 Irish Wolfhounds: characteristic clinical findings, life expectancy and causes of death. Vet J. 2019;245:15‐21. [DOI] [PubMed] [Google Scholar]
- 34. Summerfield NJ, Boswood A, O'Grady MR, et al. Efficacy of pimobendan in the prevention of congestive heart failure or sudden death in Doberman Pinschers with preclinical dilated cardiomyopathy (the PROTECT Study). J Vet Intern Med. 2012;26:1337‐1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Friederich J, Seuß AC, Wess G. The role of atrial fibrillation as a prognostic factor in doberman pinschers with dilated cardiomyopathy and congestive heart failure. Vet J. 2020;264:105535. [DOI] [PubMed] [Google Scholar]
- 36. Crass MF 3rd, Song W, Lombardini JB. Cardiac muscle taurine: effects of acute left ventricular ischemia in the dog and anoxic perfusion of the rat heart. Recent Adv Stud Cardiac Struct Metab. 1976;12:259‐263. [PubMed] [Google Scholar]
- 37. Lombardini JB, Cooper MW. Elevated blood taurine levels in acute and evolving myocardial infarction. J Lab Clin Med. 1981;98:849‐859. [PubMed] [Google Scholar]
- 38. Cooper MW, Lombardini JB. Elevated blood taurine levels after myocardial infarction of cardiovascular surgery: is there any significance? Adv Exp Med Biol. 1981;139:191‐205. [DOI] [PubMed] [Google Scholar]
- 39. Bhatnagar SK, Welty JD, al Yusuf AR. Significance of blood taurine levels in patients with first time acute ischaemic cardiac pain. Int J Cardiol. 1990;27:361‐366. [DOI] [PubMed] [Google Scholar]
- 40. Shi YR, Bu DF, Qi YF, et al. Dysfunction of myocardial taurine transport and effect of taurine supplement in rats with isoproterenol‐induced myocardial injury. Acta Pharmacol Sin. 2002;23:910‐918. [PubMed] [Google Scholar]
- 41. Bkaily G, Jaalouk D, Haddad G, et al. Modulation of cytosolic and nuclear Ca2+ and Na+ transport by taurine in heart cells. Mol Cell Biochem. 1997;170:1‐8. [DOI] [PubMed] [Google Scholar]
- 42. Fakruddin M, Wei FY, Suzuki T, et al. Defective mitochondrial tRNA taurine modification activates global proteostress and leads to mitochondrial disease. Cell Rep. 2018;22:482‐496. [DOI] [PubMed] [Google Scholar]
- 43. Ontiveros ES, Whelchel BD, Yu J, et al. Development of plasma and whole blood taurine reference ranges and identification of dietary features associated with taurine deficiency and dilated cardiomyopathy in golden retrievers: a prospective, observational study. PLoS One. 2020;15:e0233206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Adin D, Freeman L, Stepien R, et al. Effect of type of diet on blood and plasma taurine concentrations, cardiac biomarkers, and echocardiograms in 4 dog breeds. J Vet Intern Med. 2021;35:771‐779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Owens EJ, LeBlanc NL, Freeman LM, et al. Comparison of echocardiographic measurements and cardiac biomarkers in healthy dogs eating non‐traditional or traditional diets (abstract). In: American College of Veterinary Internal Medicine Forum (virtual); 2021. [DOI] [PMC free article] [PubMed]
- 46. Freid KJ, Freeman LM, Rush JE, et al. Retrospective investigation of diet and dilated cardiomyopathy (DCM) in dogs (abstract). In: American College of Veterinary Internal Medicine Forum (virtual); 2020.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1: Supporting Information


