Abstract
Background
Reports of clinicopathologic features of bronchomalacia (BM) differ because of inconsistent definitions and frequent prevalence of comorbid cardiopulmonary disease. Pulmonary hypertension (PH) secondary to BM is poorly described.
Objectives
Dogs with BM will be older but of any somatotype, and increased expiratory effort, ≥1 comorbid disease, and PH will be more common than in dogs without BM.
Animals
Client‐owned dogs (n = 210) evaluated for respiratory signs.
Methods
Medical records of dogs with paired inspiratory: expiratory‐breath‐hold computed tomography, tracheobronchoscopy, or both between January 2016 and December 2019 were retrospectively reviewed. Comparisons between dogs with and without BM using Mann‐Whitney rank sum or χ 2 tests (P < .05 significant were made). Because of high numbers of variables, criteria with high prevalence (>25%) were identified (n = 10) for univariate analysis (P < .005 significant). Significant variables were submitted for multivariate analysis.
Results
Bronchomalacia was identified in 41% of dogs of all sizes/somatotypes; 38% were >10 kg. All dogs with BM had ≥1 comorbid cardiopulmonary disorder. Dogs with BM were significantly older (P < .001), smaller (P < .001), and were more likely diagnosed with tracheal or mainstem bronchial collapse (P < .001) or bronchiectasis (P < .001). Multivariate analysis confirmed associations with age, tracheal or mainstem bronchial collapse, and bronchiectasis. In dogs with BM, PH was more prevalent.
Conclusions and Clinical Importance
Although significantly more common in older, smaller dogs, BM occurs in dogs of all sizes and in all instances with comorbidities. Echocardiography should be considered in dogs with BM to identify PH.
Keywords: chronic bronchitis, computed tomography, echocardiography, dynamic airway collapse, thoracic imaging
ABBREVIATIONS
- BM
bronchomalacia
- CB
chronic bronchitis
- CVC
caudal vena cava
- EB
eosinophilic bronchitis
- EBP
eosinophilic bronchopneumopathy
- EP
eosinophilic pneumonia
- I/E
inspiratory/expiratory
- I/E‐BH CT
inspiratory : expiratory‐breath‐hold computed tomography
- NA
not applicable
- PA/Ao
pulmonary artery to aorta
- RA
right atrial
- RPAD
right pulmonary artery distensibility
- RV
right ventricular
- TAPSE
tricuspid annular plane systolic excursion
- TR
tricuspid regurgitation
- VFSS
videofluoroscopic swallow study
1. INTRODUCTION
Static or dynamic collapse of the trachea, mainstem bronchi, lobar bronchi, or segmental and subsegmental bronchi causes a spectrum of clinical signs in dogs ranging from mild to life‐threatening. In humans, tracheomalacia is defined as weakness of the tracheal cartilage leading to airway collapse whereas tracheobronchomalacia implies extension to the principal bronchi. 1 , 2 Definitions of bronchomalacia (BM) in dogs vary, including collapse of ≥1 of principal, lobar, segmental, or subsegmental bronchi 3 , 4 , 5 Because of these various definitions, previously published reports summarizing clinical characteristics of this condition 3 , 4 , 5 lack consistency. A recent review of BM in dogs proposed a strict definition of BM: “regional to diffuse dynamic airway collapse of segmental and/or subsegmental bronchi with associated clinical signs due to airflow limitation.” 6 Using a more discrete definition aims to identify and distinguish BM from other causes of airway collapse, with relevance to clinicopathologic features, diagnostic testing, treatment, and prognosis.
Bronchoscopy is the primary modality used to diagnose BM in dogs, but identification of dynamic airway narrowing can be hampered by apnea or shallow breaths. Paired inspiratory : expiratory‐breath‐hold computed tomography (I/E‐BH CT) can capture airway caliber changes at peaks of inspiration and expiration independent of spontaneous respiration. 7 Previously, a scoring system was proposed to describe and grade the severity of BM using I/E‐BH CT but no direct comparisons to bronchoscopic scoring were made. 8 Another advantage of CT is that it allows assessment of comorbid respiratory 7 , 8 , 9 and cardiac disorders, 10 , 11 , 12 something that tracheobronchoscopy cannot. Although CT has documented bronchial collapsibility in dogs, prior studies assessed lobar bronchial collapse rather than BM as defined herein. 13 , 14 Additionally, the technique to acquire CT images should reflect relevant physiology, which makes artificial forced expiratory maneuvers reporting a high degree of airway collapsibility in healthy dogs somewhat suspect. 14 Therefore, reliance on an expiratory series (by manual hyperventilation to induce apnea on exhalation or with ventilator‐assisted expiratory breath‐hold) better mimics changes occurring tidal breathing. Thus, optimal assessment of collapse of segmental and subsegmental bronchi ideally requires tracheobronchoscopy, I/E‐BH CT, or both. By using these diagnostic tests as inclusion criteria, we aimed to provide a refined understanding of the clinicopathologic features and (using other diagnostic testing) to identify comorbid conditions associated with BM.
In dogs, pulmonary hypertension (PH) occurs secondary to obstructive airway disease 15 and is associated with decreased survival time. 16 Treatment with sildenafil can improve clinical signs and quality of life. 3 A recent consensus statement for diagnosis, classification, and treatment of PH established a clarified definition of PH based on multiple echocardiographic and clinical criteria, allowing greater correlation between clinical signs and intermediate or high probability of PH. 15
To characterize BM in dogs using the definition of dynamic segmental or subsegmental bronchial collapse, our primary objective was to describe the signalment, clinicopathologic features, and comorbid conditions using I/E‐BH CT, tracheobronchoscopy, or both. We also aimed to characterize prevalence and severity of PH using updated guidelines. 15 We hypothesized that dogs with BM would be older but of various sizes, have increased expiratory effort, have ≥1 comorbid cardiac or respiratory disease, and have a higher prevalence of PH compared to dogs without BM.
2. MATERIALS AND METHODS
2.1. Case selection
Ours was a retrospective cohort study covered under IACUC #9866 of client‐owned dogs having advanced respiratory diagnostic testing at a tertiary referral hospital. Owners provided informed consent for use of medical records in future studies at the time of admission. Medical records of dogs that underwent thoracic CT, tracheobronchoscopy, or both at the University of Missouri Veterinary Health Center between 1 January 2016 and 31 December 2019 were retrospectively reviewed. To be included dogs had to have had a paired I/E‐BH CT, tracheobronchoscopy, or both to definitively assess for BM. Dogs subsequently were grouped into those with BM or those without BM. If an individual dog was seen on more than 1 visit for the same clinical signs, diagnostic test results extracted from the medical record were accepted within a 6‐week period of either I/E‐BH CT or tracheobronchoscopy.
2.2. Medical record review
Data extracted from the medical record included history, signalment (age, breed, and sex), cephalic index (brachycephalic, mesocephalic, or dolichocephalic), body weight, owner reported clinical signs, physical examination findings, and results of respiratory diagnostic tests. Historical variables included owner‐reported cough, sneeze, nasal discharge, and respiratory sounds. Additionally, as outlined in the American College of Veterinary Internal Medicine (ACVIM) consensus statement on PH in dogs, owner reported clinical characteristics potentially suggestive of PH (eg, syncope, increased respiratory effort or distress at rest, respiratory distress after exercise, and cyanosis), 15 were recorded, with the understanding that these clinical characteristics could reflect other cardiopulmonary disorders. Chronicity of signs was documented. Cephalic index was determined based on American Kennel Club definitions of the breed standard (https://www.akc.org/dog-breeds/). Physical examination findings included temperature, pulse, respiration, respiratory pattern, and mucous membrane color. Results of all respiratory diagnostic tests including pulse oximetry, heartworm testing (IDEXX 3Dx or 4Dx SNAP), thoracic radiography, fluoroscopy, thoracic CT, tracheobronchoscopy, bronchoalveolar fluid cytology and culture, and histopathology (lung lobe biopsy or necropsy evaluation) were recorded. Angiostrongylus spp. is not endemic in the study area and is not routinely tested, and so was not included.
2.3. Imaging protocols
All imaging was done at the University of Missouri Veterinary Health Center according to established protocols. Thoracic radiography included both lateral views and an orthogonal projection. Respiratory fluoroscopy was performed with the unrestrained patient in 1 of 4 sizes of freestanding polycarbonate kennels 4 during quiet breathing and with induced cough. Videofluoroscopic swallow studies (VFSSs) were performed after a 12‐hour fast using the same equipment with observation of ingestion of contrast‐containing food of 3 consistencies (liquid, slurry, kibble) according to a previously described protocol. 13
Medical records of dogs that underwent echocardiographic evaluation were retrospectively reviewed by a single author (KW). Standardized written reports (written by a cardiologist, a cardiology resident with direct cardiologist supervision, or a veterinarian residency‐trained in cardiology) were evaluated for the following information: left atrial size, presence or absence of tricuspid regurgitation (TR), velocity of TR (when available in report), right atrial (RA)‐to‐right ventricular (RV) pressure gradient (calculated based on TR velocity using the modified Bernoulli equation 17 ), septal flattening, decreased or underfilled left ventricular size, presence or absence of RV hypertrophy, RV systolic function (based on tricuspid annular plane systolic excursion [TAPSE]), main pulmonary artery‐to‐aorta (PA : Ao) ratio >1, presence of PR velocity >2.5 m/s, presence of right pulmonary artery distensibility (RPAD) index <30%, abnormalities in RV outflow profile (acceleration time <58 ms, acceleration‐to‐ejection time ratio <0.30, or systolic notching of the profile), presence of RA enlargement, and enlargement of the caudal vena cava (CVC). When available, raw echocardiographic images (originally obtained by a cardiologist, a cardiology resident with direct cardiologist supervision, or a veterinarian residency‐trained in cardiology) were reviewed for additional information that was not included in the written report. For purposes of data evaluation, a complete echocardiogram included all standard echocardiographic views, whereas a brief echocardiogram was a partial evaluation for the presence of PH and may have contained only some of the above information (at minimum, interrogation of the TR velocity, and potentially including any combination of the following: evaluation for the presence of septal flattening, left ventricular underfilling, presence or absence of RV hypertrophy, TAPSE, PA : Ao ratio, PR velocity, RPAD index, alterations in the pulmonary outflow tract profile, RA enlargement, and enlargement of the CVC).
For thoracic CT and tracheobronchoscopy, general anesthesia protocols were individually tailored under guidance of a board‐certified anesthesiologist. Drugs used for premedication and induction varied, but propofol at a constant rate infusion of 0.2‐0.4 mg/kg/min to effect was used to maintain general anesthesia.
Thoracic CT scans were performed during the inspiratory phase of spontaneous respiration or with I/E‐BH when a mechanical ventilator was used to provide breath‐holds for inspiratory and expiratory phases. If thoracic CT was performed without I/E‐BH, tracheobronchoscopy had to be performed for study inclusion. The protocol for I/E‐BH CT has been described elsewhere. 8 Diagnosis of BM was made using I/E‐BH CT scans assessing segmental and subsegmental bronchi and the surrounding parenchyma in the expiratory compared to inspiratory series. As proposed previously, 8 CT sub‐scores of 1, 2, or 3 were assigned to reflect severity of collapse of segmental and subsegmental airways: sub‐score 1, subtle flattening without peribronchovascular opacification; sub‐score 2, distorted circular appearance or >50% narrowing with mild to moderate peribronchovascular opacification; sub‐score 3, near disappearance of airway lumens with marked peribronchovascular opacification. Dogs were included in the BM group with a CT sub‐score of 1, 2, or 3.
Tracheobronchoscopy was performed in a systematic fashion using a standard tracheobronchial road map. 5 Endoscopic diagnosis of BM was made if there was regional to diffuse dynamic airway collapse (decrease of ≥25% of the airway caliber) of segmental and subsegmental bronchi. 6 After tracheobronchoscopy, the endoscope was gently wedged into a distal airway at the endoscopist's discretion to collect bronchoalveolar lavage fluid. One to two 20 mL aliquots of sterile saline were instilled via a 20 mL syringe through the biopsy channel of the endoscope and gentle suction applied. Lavage samples were transported to the laboratory for cytologic evaluation (cellular differential and microscopic description) and culture (aerobic and capnophilic) with antimicrobial sensitivity. If bronchoalveolar lavage was done without tracheobronchoscopic examination (and I/E : BH‐CT was used to diagnose BM), either a scope‐guided sample was obtained as described above, or a sterile 8Fr red rubber catheter was sterilely inserted through the endotracheal tube and gently wedged into a distal airway, and then samples were collected as described above.
2.4. Final clinical diagnoses
The comprehensive clinical picture including signalment, history, clinical signs, and diagnostic test results was reviewed by 2 internists with special interest in respiratory disease (AVP and CR) to assign final diagnoses and assign confidence to the diagnoses (suspected or definitive) for each case (Table S1).
2.5. Probability of pulmonary hypertension
When echocardiographic data were available, probability of PH (low, intermediate, high) was classified according to the metrics established in the ACVIM consensus statement on PH in dogs. 15 Initial grouping was based on TR velocity (<3 m/s or not detectable, 3.0‐3.4 m/s, or >3.4 m/s), and then further classification was based on additional signs of PH at 3 distinct anatomical sites: the ventricles, pulmonary artery, and RA/CVC (Figure 1). 15 Ventricular signs of PH included flattening of the interventricular septum, underfilling or decreased size of the left ventricle, RV hypertrophy, or RV systolic dysfunction. Pulmonary artery signs of PH included PA : Ao ratio >1.0, peak early diastolic pulmonary regurgitation velocity >2.5 m/s, RPAD index <30%, RV outflow Doppler acceleration time <58 ms or acceleration‐to‐ejection time ratio < 0.30, or systolic notching of the Doppler RV outflow profile. Right atrial signs of PH included RA enlargement or enlargement of the CVC. 15
FIGURE 1.
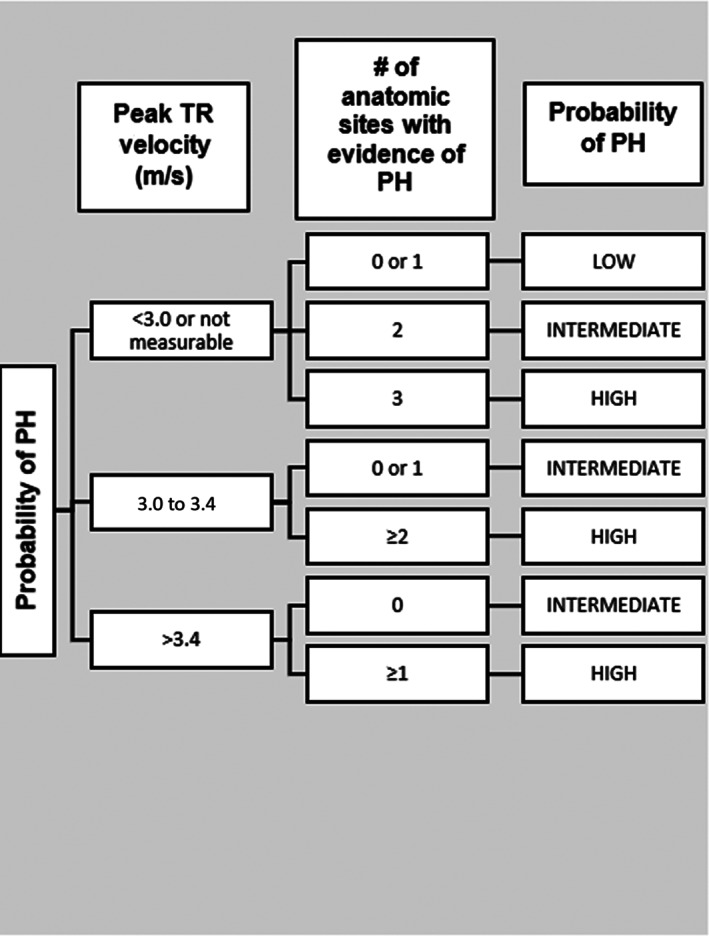
Flow chart to determine probability of pulmonary hypertension (PH) based on tricuspid regurgitation (TR) velocity and number of anatomic sites (ventricular, pulmonary artery, and right atrium/caudal vena cava (CVC)). Adapted from Reinero et al 15
2.6. Statistical analysis
Statistical analysis was done using commercially available software (SigmaPlot, Systat Software Inc. Chicago, Illinois). Patients were divided into groups (with or without BM), and history, signalment, physical examination findings, and diagnoses were compared between these 2 groups. Data were assessed for normality using the Shapiro‐Wilk test. Non‐normal data were compared using Mann‐Whitney rank sum tests. Categorical data were compared using χ 2 test. For univariate analysis of the categorical data, only variables (n = 10) with a prevalence >25%, but not <5% in either group, were considered (Table 1). Because of the number of interrelated variables evaluated, a Bonferroni correction was performed and thus significance for univariate analysis was a set at P < .005. The variables that were found to be significant under these conditions (n = 4) were assessed using multiple logistical regression (P < .05).
TABLE 1.
Prevalence of owner‐reported historical signs, physical examination findings, and diagnoses (suspected or definitive) in 210 dogs with and without bronchomalacia (BM) used in assessment of univariate analysis
| Variable | BM (n = 86) | % | No BM (n = 124) | % | P‐value | |
|---|---|---|---|---|---|---|
| History a | Acute onset | 11 | 12.8 | 31 | 25.0 | |
| Chronic onset | 68 | 79.1 | 86 | 69.4 | 1 | |
| Respiratory distress at rest | 14 | 16.3 | 20 | 16.1 | ||
| Exercise intolerance | 9 | 10.5 | 13 | 10.5 | ||
| Prolonged post‐activity tachypnea | 7 | 8.1 | 3 | 2.4 | ||
| Respiratory distress after exercise | 4 | 4.7 | 0 | 0 | ||
| Syncope | 6 | 7.0 | 5 | 4.0 | ||
| Tachypnea | 30 | 34.9 | 31 | 25.0 | .14 | |
| Coughing | 66 | 76.7 | 93 | 75.0 | .86 | |
| Sneeze | 5 | 5.8 | 11 | 8.9 | ||
| Wheeze | 7 | 8.1 | 9 | 7.3 | ||
| Stridor | 5 | 5.8 | 8 | 6.5 | ||
| Stertor | 9 | 10.5 | 7 | 5.6 | ||
| Physical exam | Crackles | 25 | 29.1 | |||
| Wheeze | 12 | 14.0 | 7 | 5.6 | ||
| Increased bronchovesicular sounds | 36 | 41.9 | 48 | 38.7 | .72 | |
| Respiratory noise (not characterized) | 8 | 9.3 | 14 | 11.3 | ||
| Abnormal respiratory effort | 29 | 33.7 | 36 | 29.0 | 1 | |
| Mixed breathing pattern | 6 | 7.0 | 7 | 5.6 | ||
| Paradoxical breathing pattern | 2 | 2.3 | 2 | 1.6 | ||
| Nasal discharge | 8 | 9.3 | 29 | 23.4 | ||
| Tachycardia | 12 | 14.0 | 25 | 20.2 | ||
| Fever b | 7 | 8.1 | 28 | 22.6 | ||
| Diagnosis (definitive or suspected) | BOAS | 6 | 7.0 | 6 | 4.8 | |
| Gastro‐esophageal reflux disease | 13 | 15.1 | 30 | 24.2 | ||
| Laryngeal paralysis | 5 | 5.8 | 13 | 10.5 | ||
| Tracheal/mainstem bronchial collapse c | 60 | 69.8 | 17 | 13.7 | .001b | |
| Chronic bronchitis | 41 | 47.7 | 39 | 31.5 | .02b | |
| Bronchiectasis | 55 | 64.0 | 28 | 22.6 | .001b | |
| Eosinophilic lung disease d | 8 | 9.3 | 19 | 15.3 | ||
| Aspiration pneumonia/aspiration pneumonitis | 11 | 12.8 | 23 | 18.5 | ||
| Bacterial pneumonia | 19 | 22.1 | 28 | 22.6 | ||
| Fungal pneumonia | 1 | 1.2 | 6 | 4.8 | ||
| Bronchiolar disease | 14 | 16.3 | 13 | 10.5 | ||
| Pulmonary fibrosis | 13 | 15.1 | 4 | 3.2 | ||
| Other interstitial lung disease | 10 | 11.6 | 9 | 7.3 | ||
| Neoplasia | 5 | 5.8 | 17 | 13.7 | ||
| Pulmonary thromboemboli | 2 | 2.3 | 3 | 2.4 | ||
| Other pulmonary vascular disease | 0 | 0 | 4 | 3.2 | ||
Note: Only criteria where at least 1 group had a prevalence of >25% (shaded boxes) but no less than 5% in either group were considered for univariate analysis. A Bonferroni correction was performed to account for the number of interrelated variables tested and significance (*) was a set at P < .005.
Abbreviations: BOAS, brachycephalic obstructive airway syndrome; GERD, gastroesophageal reflux disease.
Due to the retrospective nature of the study, historical and physical examination findings were not uniformly reported in all dogs. If the historical or physical examination finding was present (“yes”), it was recorded in this table. Absence of the finding could have been because the dog lacked the finding, the owner did not recognize the finding, or the finding did not get recorded in the medical record.
Fever defined as temperature >103F (39.4°C).
Tracheal and mainstem bronchial collapse were combined for the purpose of statistical analysis because of concern for co‐linearity.
Eosinophilic bronchitis, eosinophilic bronchopneumonopathy, and eosinophilic pneumonia (ie, eosinophilic lung disease) were also combined because of co‐linearity.
3. RESULTS
3.1. Animals
Of 265 records screened, 210 dogs met the inclusion criteria: 86 with and 124 without BM (Figure 2). Table 2 provides a summary of the signalment data for each group. Dogs with BM were significantly older (P < .001) and smaller (P < .001) than those without BM. However, a spectrum of weights and somatotypes was noted. In dogs with BM, 53 (62%) were ≤10 kg, 20 (23%) were between 10 and 20 kg, and 13 (15%) were ≥20 kg. In dogs without BM, 35 (28%) were ≤10 kg, 15 dogs (12%) were between 10 and 20 kg, and 74 dogs (60%) were ≥20 kg. In dogs with BM, there were 21 brachycephalic, 53 mesocephalic, and 2 dolichocephalic dogs, and 10 dogs without description of face shape. In dogs without BM, there were 20 brachycephalic, 71 mesocephalic, 17 dolichocephalic, and 16 dogs without face shape description. No significant difference was found between sex, breed, or face shape data between the 2 groups.
FIGURE 2.
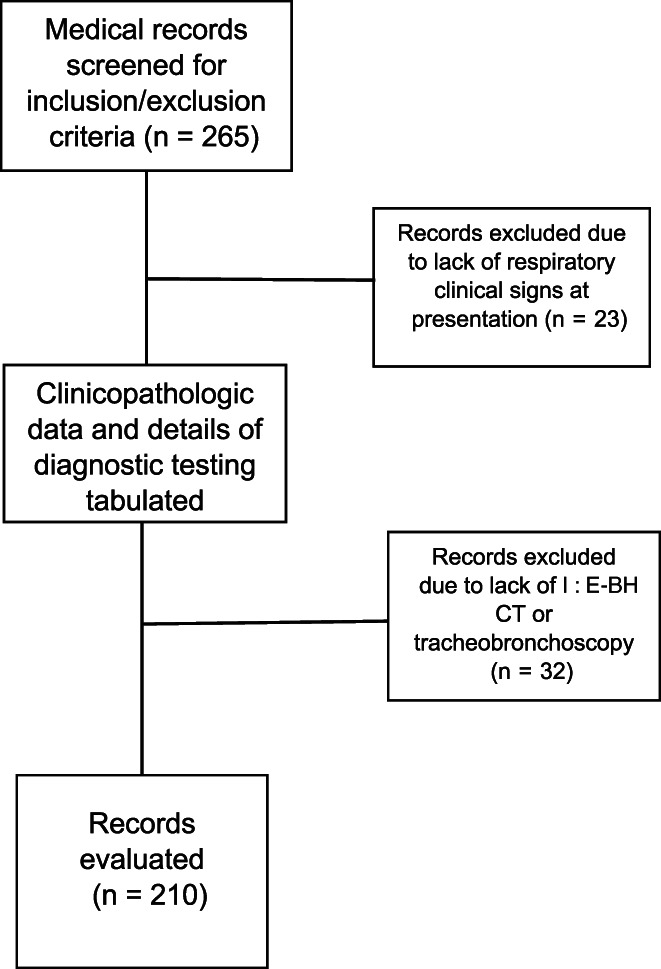
Flow chart indicating case selection for study inclusion
TABLE 2.
Signalment (age, weight, sex, and breed) in 210 dogs with and without bronchomalacia
| Bronchomalacia (n = 86) | No bronchomalacia (n = 124) | ||
|---|---|---|---|
| Age (mean ± SD years) | 10.0 ± 3.5 | 6.5 ± 4.0 | |
| Weight (mean ± SD kg) | 11.2 ± 8.0 | 23.5 ± 14.6 | |
| Sex | |||
| M | 4 | 16 | |
| MC | 35 | 53 | |
| F | 1 | 8 | |
| FS | 46 | 47 | |
| Breed | |||
| Mixed breed | 17 | 20 | |
| Labrador Retriever | 7 | 13 | |
| Yorkshire Terrier | 6 | 6 | |
| Shih‐Tzu | 6 | 4 | |
| Dachshund | 6 | 3 | |
| Beagle | 6 | 2 | |
| Siberian Husky | 1 | 7 | |
| German Shepherd | 1 | 5 | |
| Golden Retriever | 0 | 7 | |
| Boxer | 0 | 4 | |
| Others | 3 of each | Chihuahua, Pomeranian | American Staffordshire Terrier, Bulldog, Chihuahua, Great Dane, Standard Poodle |
| 2 of each | Australian Shepherd, Brussels Griffon, Corgi, Jack Russell Terrier, Maltese, Miniature Poodle, Miniature Schnauzer, Papillon, Pug, Toy Poodle, West Highland White Terrier | Basset Hound, Border Collie, Brittany Spaniel, Cavalier King Charles Spaniel, Cocker Spaniel, German Shorthair, Miniature Poodle, Miniature, Schnauzer, Toy Poodle, West Highland White Terrier | |
| 1 of each | Bulldog, Cavalier King Charles Spaniel, Cocker Spaniel, Miniature Pincher, Pekingese, Shetland Sheepdog, Silky Terrier, Wheaton Terrier | Airedale Terrier, Australian Cattle Dog, Australian Shepherd, Belgian Malinois, Brussels Griffon, Chinese Crested, Doberman Pinscher, English Setter, Flat Coated Retriever, Great Pyrenees, Lhasa Apso, Malamute, Pomeranian, Rhodesian Ridgeback, Schnauzer, Shetland Sheepdog, Weimaraner, Whippet | |
Abbreviations: F, female; FS, female spayed; M, male; MC, male castrated.
3.2. History and physical examination findings
Of the history and physical examination findings meeting the criteria for statistical analysis (chronic onset, cough, crackles, tachypnea, increased bronchovesicular sounds, and abnormal respiratory effort), none were significantly different between groups (Table 1). Other history or physical examination findings, including cyanosis, nasal discharge, respiratory sounds (stertor, stridor, pulmonary wheezes), respiratory pattern, clinical signs suggestive of PH (syncope, exercise intolerance, abdominal effusion, respiratory distress), occurred in <25% of dogs in either group and therefore were not considered for statistical analysis. Dogs with BM did not more commonly have increased expiratory effort, but only 7 (8%) dogs with BM and 9 (7%) dogs without BM had this specific respiratory pattern noted in the medical record.
3.3. Diagnostic testing
The number of dogs with and without BM having each respiratory diagnostic test is shown in Table 3. Overall, the average number of respiratory and cardiac diagnostic tests was 5 and 4 in dogs with and without BM, respectively. Fifty‐one dogs with BM had thoracic radiographs performed; 38 (75%) were abnormal. Seventy dogs without BM had thoracic radiographs; 61 (87%) were abnormal. The distribution of thoracic radiographic patterns is shown in Table 4.
TABLE 3.
Numbers of individual diagnostic tests performed in 210 dogs (86 with bronchomalacia, 124 without bronchomalacia) undergoing respiratory evaluation
| Diagnostic test | Bronchomalacia (n = 86 dogs) | No bronchomalacia (n = 124 dogs) | |
|---|---|---|---|
| Pulse oximetry | Single | 8 | 15 |
| 6‐minute walk test | 1 | 2 | |
| Heartworm test | Below detectable limits | 11 | 27 |
| Positive | 1 | 0 | |
| Thoracic radiographs | Normal | 13 | 9 |
| Abnormal | 38 | 61 | |
| Respiratory fluoroscopy | Normal | 3 | 2 |
| Abnormal | 28 | 8 | |
| VFSS | Normal | 3 | 4 |
| Abnormal | 14 | 15 | |
| Thoracic CT without breath‐hold assistance | Normal | 0 | 0 |
| Abnormal | 8 | 9 | |
| I : E‐BH CT | Normal | 0 | 4 |
| Abnormal | 64 | 87 | |
| Echocardiography | Partial | 32 | 21 |
| Complete | 23 | 12 | |
| Tracheobronchoscopy | Normal | 0 | 6 |
| Abnormal | 82 | 96 | |
| BALF cytology | Normal | 12 | 15 |
| Abnormal | 72 | 104 | |
| BALF culture | Growth | 42 | 61 |
| No growth | 39 | 53 | |
| Lung biopsy | 2 | 12 | |
| Lung fine‐needle aspirate | 2 | 5 | |
| Necropsy | 8 | 9 | |
Note: Only diagnostic tests performed within a 6‐week period of the requisite inspiratory : expiratory‐breath‐hold computed tomographic scan or tracheobronchoscopy needed for study inclusion were reported.
Abbreviations: BALF, bronchoalveolar lavage fluid; CT, computed tomography; I : E‐BH CT, inspiratory : expiratory‐breath‐hold CT; VFSS, videofluoroscopic swallow study .
TABLE 4.
Abnormal thoracic radiographic patterns from dogs with and without a diagnosis of bronchomalacia
| Radiographic pattern | Bronchomalacia (n = 51) | No bronchomalacia (n = 70) | ||
|---|---|---|---|---|
| n | % a | n | % a | |
| Bronchial | 11 | 22 | 6 | 9 |
| Unstructured interstitial | 6 | 12 | 10 | 14 |
| Structured interstitial (nodular) | 0 | 0 | 4 | 6 |
| Alveolar | 9 | 18 | 16 | 23 |
| Bronchointerstitial | 8 | 16 | 13 | 19 |
| Bronchoalveolar | 2 | 4 | 1 | 1 |
| Interstitial alveolar | 2 | 4 | 6 | 9 |
| Bronchointerstitial alveolar | 0 | 0 | 5 | 7 |
| Normal thoracic radiographs | 13 | 25 | 9 | 13 |
Note: Radiographs were taken in 51 out of 86 dogs with bronchomalacia and 70 out of 124 dogs without bronchomalacia.
Totals may equal >100% because of rounding.
3.4. Identification of co‐morbid diseases
Definitive and suspected final respiratory diagnoses for both groups are shown in Table 5. Overall, the mean ± SD number of definitive and suspect final diagnoses (ie, comorbid diseases) was 4 ± 1 definitive and 1 ± 1 suspect in dogs with BM and 2 ± 1 definitive and 1 ± 1 suspect in dogs without BM. All dogs with BM had at least 1 comorbid cardiovascular or respiratory disease. Eighty‐two dogs without BM had only a single definitive or suspect final diagnosis. Dogs with BM were more frequently diagnosed with concurrent tracheal collapse, mainstem bronchial collapse, or both (combined because of co‐linearity; P < .001) and bronchiectasis (P < .001) compared to dogs without BM.
TABLE 5.
Suspect and definitively diagnosed co‐morbid diseases in dogs with and without BM
| Diagnosis | Bronchomalacia (n = 86) | No bronchomalacia (n = 124) | |
|---|---|---|---|
| BOAS | Definitive | 6 | 6 |
| Suspect | 0 | 0 | |
| GERD | Definitive | 9 | 10 |
| Suspect | 4 | 20 | |
| Laryngeal paralysis | Definitive | 5 | 13 |
| Suspect | 0 | 0 | |
| Tracheal or mainstem bronchial collapse | Definitive | 54 | 13 |
| Suspect | 0 | 0 | |
| Chronic bronchitis | Definitive | 37 | 36 |
| Suspect | 4 | 3 | |
| Bronchiectasis | Definitive | 55 | 28 |
| Suspect | 0 | 0 | |
| Eosinophilic lung disease | Definitive | 8 | 17 |
| Suspect | 0 | 2 | |
| Aspiration pneumonia/pneumonitis | Definitive | 7 | 15 |
| Suspect | 4 | 8 | |
| Bacterial pneumonia | Definitive | 15 | 26 |
| Suspect | 4 | 2 | |
| Foreign body pneumonia | Definitive | 0 | 1 |
| Suspect | 0 | 2 | |
| Fungal pneumonia | Definitive | 0 | 5 |
| Suspect | 1 | 1 | |
| Viral pneumonia | Definitive | 0 | 0 |
| Suspect | 1 | 1 | |
| Bronchiolar disease | Definitive | 8 | 8 |
| Suspect | 6 | 5 | |
| Pulmonary fibrosis | Definitive | 3 | 2 |
| Suspect | 10 | 2 | |
| Other interstitial lung disease | Definitive | 0 | 2 |
| Suspect | 10 | 7 | |
| Neoplasia | Definitive | 2 | 8 |
| Suspect | 3 | 9 | |
| Pulmonary thromboemboli | Definitive | 1 | 1 |
| Suspect | 1 | 2 | |
| Other vascular disease | Definitive | 0 | 3 |
| Suspect | 0 | 1 | |
Note: See Table S1 for definitions.
Abbreviations: BOAS, brachycephalic obstructive airway syndrome; GERD, gastroesophageal reflux disease
Multiple logistic regression analysis was performed on the statistically significant variables (age, weight, presence of bronchiectasis, and tracheal collapse, mainstem bronchial collapse, or both [Table 6]). Older age, bronchiectasis, and tracheal collapse, mainstem bronchial collapse, or both were significantly (P < .05 for all) more common in dogs with BM. For each year of age, there was a 12% increase in odds of having BM. Dogs with tracheal collapse, mainstem bronchial collapse or both, and bronchiectasis were 9.5 and 5.3 times more likely to have BM, respectively.
TABLE 6.
Results of multivariate analysis using a logistic regression model comparing 4 variables in dogs with and without bronchomalacia
| Variable | P‐value | OR | 95% CI |
|---|---|---|---|
| Age | .02 | 1.128 | 1.019 to 1.249 |
| Weight | .13 | 0.970 | 0.933 to 1.009 |
| Bronchiectasis | <.001 | 5.356 | 2.407 to 11.914 |
| Airway collapse | <.001 | 9.51 | 3.859 to 23.476 |
Note: This model included variables with a prevalence greater than 25%, but no less than 5% in either group that were significantly different on univariate analysis.
Abbreviations: CI, confidence interval; OR, odds ratio.
3.5. Pulmonary hypertension
In total, 88 dogs (55/86 [64%] dogs with BM, 33/124 [26%] dogs without BM) were assessed for PH with complete or partial echocardiograms (Table 7). Of the dogs with BM, 22/55 (40%) had intermediate or high probability of PH and of the dogs without BM, 2/33 (6%) had intermediate or high probability of PH. Because of the low proportion of dogs without BM diagnosed with PH, statistical analysis was not performed to compare PH between groups. However, because only 26% of dogs without BM were screened for PH, the prevalence of PH in dogs without BM may be underestimated.
TABLE 7.
Echocardiographic findings and final diagnoses in dogs with and without bronchomalacia assessed for pulmonary hypertension with partial or complete echocardiograms
| BM (n = 55) | No BM (n = 33) | |||||
|---|---|---|---|---|---|---|
| Partial (n = 32) | Complete (n = 23) | Not assessed | Partial (n = 21) | Complete (n = 12) | Not assessed | |
| LA enlargement (mild, moderate, or severe) | 1 | 5 | 24 | 0 | 2 | 17 |
| Peak TRV >3.4 m/s | 5 | 8 | 5 | 1 | 0 | 4 |
| Septal Flattening | 0 | 4 | 17 | 1 | 0 | 11 |
| Underfilling or decreased LV size | 0 | 2 | 21 | 0 | 1 | 18 |
| RV hypertrophy | 2 | 7 | 11 | 1 | 0 | 7 |
| Right ventricular systolic dysfunction | 1 | 0 | 50 | 1 | 0 | 30 |
| PA/Ao >1 | 2 | 5 | 44 | 0 | 0 | 29 |
| PR velocity > 2.5 | 1 | 3 | 47 | 0 | 0 | 30 |
| RPAD index <30% | 3 | 1 | 41 | 1 | 0 | 30 |
| RV outflow Doppler acceleration time <58 ms or acceleration to ejection time ratio <0.30 | 7 | 6 | 16 | 0 | 0 | 14 |
| RA enlargement | 1 | 8 | 10 | 1 | 0 | 9 |
| CVC enlargement | 0 | 0 | 54 | 0 | 0 | 33 |
| Final diagnosis | ||||||
| Degenerative valve disease | 38 | 15 | ||||
| Other heart disease | 1 | 2 | ||||
| Pulmonary hypertension a | 22 | 3 | ||||
Abbreviations: CVC, caudal vena cava; LA, left atrial; LV, left ventricular; PA : Ao, pulmonary artery‐to‐aorta ratio; RA, right atrial; RPAD, right pulmonary artery distensibility; RV, right ventricular; TRV, tricuspid regurgitation velocity.
Denotes clinically significant PH based on Reinero et al, 15 that is, intermediate or high probability of PH.
Echocardiographic data available for review were variable depending on the type of echocardiogram performed (full vs partial). Echocardiographic signs of PH in each group are shown in Table 7. Of the 88 dogs screened, 25 (28%) had clinically relevant (ie, intermediate or high probability) PH. Of these 25 dogs, most (15; 60%) were classified as Group 6 PH (multifactorial mechanisms); the remaining (10, 40%) dogs were classified as Group 3 PH (respiratory disease and hypoxia) based on the PH consensus statement. 15 Concurrent diseases in those 25 dogs included degenerative valve disease (10 definitive, 3 suspect), pulmonary thromboembolic disease (1 definitive, 1 suspect) along with various respiratory diseases, including 22 (88%) of 25 dogs with BM. Consistent with the PH consensus statement, 15 we did not identify any dogs with a sole diagnosis of chronic bronchitis (CB) and concurrent PH. All dogs with both CB and PH (n = 10) had concurrent diseases (8 with BM, 2 with suspect, or definitive interstitial lung disease).
4. DISCUSSION
In our study of dogs undergoing advanced respiratory diagnostic testing, BM was common, occurring in 86 (41%) of 210 of all dogs. Dogs with BM were significantly older and smaller. However, unlike dogs with tracheal collapse, 18 , 19 , 20 dogs with BM were of all ages and body weights (with 33/86 [38%] dogs weighing >10 kg). Face shape (somatotype) did not significantly differ between groups. Because of the retrospective nature of the study, characterization of the predominant phase of labored respiration rarely was recorded, leaving us unable to support the hypothesis that dogs with BM more frequently had increased expiratory effort. Tracheal collapse, mainstem bronchial collapse or both, and bronchiectasis were significantly (P < .001 for both) more common in dogs with BM. All dogs with BM had at least 1 comorbid cardiovascular or pulmonary disease. Because of the low number of dogs without BM screened for PH, statistical analysis could not be performed but a higher proportion of dogs with BM had intermediate or high probability of PH, suggesting that dogs with BM should be assessed for PH.
A recent review of BM in dogs highlighted inconsistent and unclear definitions of BM applied in prior studies, proposed a strict definition and new classification scheme, and called for additional studies to better understand this disorder. 6 Although some dogs previously reported to have BM (with definitions ranging from collapse of a single or both principal bronchi, lobar bronchi, segmental bronchi, subsegmental bronchi, or combinations of these 13 , 18 , 19 , 21 , 22 ) may fit the recently proposed strict definition, it is impossible to definitively identify only the dogs with segmental and subsegmental airway collapse in those studies. Many of the findings in our current study, such as significantly more dogs being older and smaller, and the high comorbid condition of tracheal collapse and mainstem bronchial collapse also were noted in older studies. To further clarify and refine the results of earlier studies, our current study focused on the definition of BM as regional to diffuse dynamic airway collapse of segmental, or subsegmental bronchi or both, 6 and showed a diverse clinical presentation (any age or somatotype, but with older and smaller dogs more common and with many different presenting respiratory signs and physical examination findings). Brachycephalic dogs previously have been documented to have bronchial abnormalities (some static 23 ), and represented a substantial proportion of the dogs described herein. The prior study on brachycephalic dogs primarily investigated right and left mainstem bronchial and lobar bronchial collapse, 23 and our study builds on those findings with a description of segmental and subsegmental airway collapse. Clinical signs associated with BM in the literature include chronic cough, pulmonary crackles, wheezing, cyanosis, and respiratory distress at rest. 13 , 18 , 19 , 24 , 25 The diversity in clinical signs likely supports the range in severity of BM and the high prevalence of comorbid conditions. Clinical signs strongly associated with PH specified in the ACVIM consensus statement include syncope, respiratory distress at rest, respiratory distress after exercise, and cardiogenic ascites. 15
The primary means of diagnosis of BM in dogs historically has been bronchoscopic examination, 18 , 24 but general anesthesia may induce apnea or shallow respiration, causing underestimation of the presence and severity of airway collapse during bronchoscopic evaluation. Ventilator‐assisted inspiratory and expiratory holds eliminate this effect during thoracic CT. Thoracic CT has proven to be a powerful tool complementary to tracheobronchoscopy, capable of detecting BM with paired I/E‐BH scans as well as detecting other types of airway, parenchymal and vascular pathology. 7 , 8 By anatomically identifying affected regions of the lung, CT can help guide subsequent cytologic and microbiologic sampling using tracheobronchoscopy. Limited studies using CT to diagnose BM are available. Some studies used CT to assess airway collapse of the mainstem bronchi, lobar bronchi or both, with no metrics applied to the segmental and subsegmental bronchi, which is the definition of BM used in our study. 13 , 14 , 26 , 27 Artificial forced expiration using syringes to aspirate air from intubated dogs does not mimic the physiology of tidal breathing 14 , 27 and differs from the paired I/E : BH CTs performed in our study. These ventilator assisted breath‐holds allow comparison of inspiratory and expiratory CT scans to assess changes in airway caliber and the peribronchial lung parenchyma. 6 , 7 , 8 Although not assessed in our study, manual inflation by holding an anesthetic bag behind a lead shield and passive exhalation after hyperventilation‐induced apnea also may highlight physiologically relevant changes in airway caliber with tidal breathing.
Advanced imaging and other comprehensive respiratory diagnostic tests are required to identify comorbid cardiac and respiratory diseases, which were identified in all dogs with BM and 42 of 124 dogs without BM in our study. Whether these diseases occur coincidentally with BM or if these diseases cause BM is, as yet, unknown. The average number of comorbid diseases was 5 and 2 in dogs with and without BM, respectively. Thoracic radiography, the most common imaging modality for respiratory disease in general practice, does not allow diagnosis of BM. When thoracic radiography provides a definitive (eg, tracheal collapse, hypoplastic trachea, bronchiectasis) or suspect diagnosis (eg, CB, eosinophilic bronchitis [EB], aspiration pneumonia, neoplasia), the diagnostic evaluation may end, which could prevent the diagnosis of concurrent BM or other comorbid diseases. Lack of knowledge of all diseases contributing to clinical signs prevents the development of a comprehensive therapeutic plan, may lead to therapeutic failure, and impacts accurate prognostic information. Although our current study is biased because cases are from a tertiary referral institution, 61% of this population presented with respiratory signs (both groups combined) had >1 respiratory or cardiovascular disorder.
An intermediate or high probability of PH was identified in 40% of patients with BM and in only 9% of patients without BM; because of the low numbers of dogs screened, statistical significance cannot be assigned to this finding. However, given the frequency with which PH was diagnosed in dogs with BM, we believe that all dogs with a high clinical suspicion for BM would benefit from echocardiography to screen for PH. Timely diagnosis of PH provides the opportunity for another treatment modality that could improve quality of life. 3 Additionally, BM should be a differential diagnosis in dogs with respiratory signs and concurrent PH. Because not all dogs with BM in our study had echocardiography performed, future prospective studies in dogs with BM are needed to determine the true prevalence of PH with this disease. It is also worthy of note that dogs with BM all had at least 1 other comorbid cardiopulmonary disorder, many of which have been associated with PH. 6 , 16 , 28 Other unidentified diseases may have contributed to PH but may have not been recognized because of a limited diagnostic evaluation. Although it is not possible to determine the individual contributions of each comorbid disease to PH in our study, it emphasizes recommendations in the ACVIM consensus statement on PH in dogs to specifically treat the underlying cause or factors contributing to PH as a part of the overall management strategy. 15
Historically, CB has been proposed as a cause of PH 29 , 30 but recently was disputed in the ACVIM consensus statement on PH. 15 Chronic bronchitis in dogs is an inflammatory airway disorder that by itself does not lead to clinically relevant airway obstruction, hypoxemia or respiratory failure. This situation is different from CB in humans, which, along with emphysema (and sometimes asthma), comprises the syndrome of chronic obstructive pulmonary disease (COPD). Dogs do not develop the same syndrome of COPD as do humans, and because of a lack of substantial functional lung impairment, it should not be surprising the dogs with CB do not develop PH. It is most likely that dogs with CB and PH have another comorbid disorder, and BM as an obstructive airway disorder should be strongly suspected in these cases. In support of this conclusion, in our study no dog with CB alone had PH. Every dog with CB that had PH also had either BM or another comorbid disease known to cause PH. 15 Over 30 years ago, the first extensive study of CB in dogs failed to recognize “partial collapse of mainstem or segmental bronchi during tidal volume expiration” in 9 (56%) of 16 dogs undergoing bronchoscopic examination as being diagnostic for BM. 31 Bronchiectasis, another cause of PH in dogs, 15 was noted radiographically in 4 (29%) of 14 dogs in that study. 31 The previous study of CB in dogs described abnormalities in PaO2 (44% hypoxemic), ventilation/perfusion scans (64% with inhomogeneities), and tidal breathing expiratory flow (clinically relevant decrease in dogs with CB compared to healthy controls) that in retrospect were influenced by the high comorbid rate of BM and bronchiectasis. 31 These results emphasize the need to perform thorough diagnostic respiratory evaluations in dogs with CB that display more serious signs of respiratory compromise to find a contributory comorbid condition, including PH. Given our study's findings, we propose that in dogs with CB and PH, additional diagnostic testing be performed to screen for comorbid diseases that could be contributing to (or be the sole cause of) PH. Other causes of PH not caused by left heart disease or secondary to respiratory disease, hypoxia, or both include pulmonary arterial hypertension, pulmonary thromboembolism, cardiac parasites and PH with multifactorial or unclear mechanisms. 15
Limitations of our study primarily are related to its retrospective nature. Clinical signs and historical data varied among patients because of disparities in medical records as recorded by multiple clinicians. Thoracic radiographic reports were used to summarize findings; results from thoracic CT scans were based on reports from board‐certified radiologists and were re‐reviewed by 2 of the authors with expertise in pulmonology (AVP and CR). Standardized tracheobronchoscopic reports were used to make a diagnosis of BM (subjective assessment of >25% collapse of segmental and subsegmental airways), but because many clinicians of different training levels provided the assessment, severity scores from those reports (mild, moderate or severe) were not included in our study. Future studies should provide more detailed information of location, distribution, and severity of collapse. To date, no comprehensive study has compared the diagnostic ability of bronchoscopy vs paired I/E‐BH CT scans for BM in dogs. This may be particularly relevant for dogs with mild BM because more subtle airway collapse could be missed by either modality. Additionally, it is unclear how much segmental and subsegmental airway collapse is present in dogs without respiratory disease when assessed by I/E BH‐ CT. A previous study 26 reported >50% collapse of lobar airways of apparently healthy dogs (including lobar bronchi leading to the cranial and caudal subsegments of the left cranial lobe which were termed subsegmental bronchi, but excluding all segmental and subsegmental bronchi as encompassed in the recent proposed definition of BM 6 ). Given that dogs with 25% to 50% segmental and subsegmental airway narrowing were included in the BM group in our study, any potential overlap with physiologic narrowing in healthy dogs needs further study. Investigation of comorbid diseases was impacted by a non‐standardized evaluation in all dogs, perhaps underestimating concurrent diseases, or leading to suspected but unconfirmed diagnoses. To provide clarity in data interpretation, a clear set of disease definitions, including criteria for definitive and suspect diagnoses, was provided in Table S1. Future prospective studies using standardized advanced diagnostic testing should be performed to confirm our results.
In conclusion, in dogs undergoing advanced respiratory diagnostic testing including but not limited to I/E‐BH CT, tracheobronchoscopy, or both, BM was identified in 41% of cases. A wide spectrum of ages and somatotypes was noted in dogs with BM, but significantly more dogs were older and smaller than dogs without BM. For every year older, there was a 12% increase in the odds of having BM. Comorbid cardiovascular and respiratory disorders were noted in 100% of dogs with BM, emphasizing the importance of a thorough diagnostic investigation. Dogs with tracheal, mainstem bronchial collapse or both, and bronchiectasis were 9.5 and 5.3 times more likely to have BM, respectively. A higher proportion of dogs with BM had intermediate or high probability of PH compared to those without BM, but more investigation is required to elucidate the nature of this finding. Because thoracic radiography does not permit identification of BM, diagnostic tests such as I/E BH CT, tracheobronchoscopy, or both should be considered in dogs undergoing evaluation for respiratory clinical signs. Additionally, given the apparent high prevalence of PH in dogs with BM, echocardiography should be recommended as an additional screening tool to provide information to allow a comprehensive management strategy and provide informed prognostication.
CONFLICT OF INTEREST DECLARATION
Authors declare no conflict of interest.
OFF‐LABEL ANTIMICROBIAL DECLARATION
Although some dogs were treated with antimicrobials, the selection of antimicrobial was at the discretion of the attending clinician and was not a part of the study.
INSTITUTIONAL ANIMAL CARE AND USE COMMITTEE (IACUC) OR OTHER APPROVAL DECLARATION
Approved by the University of Missouri College of Veterinary Medicine IACUC, number 9866.
HUMAN ETHICS APPROVAL DECLARATION
Authors declare human ethics approval was not needed for this study.
Supporting information
Table S1 Definitions, including criteria used to make definitive or suspect diagnoses, for respiratory diseases in the dog.
ACKNOWLEDGMENT
No funding was received for this study. The authors thank Dr. Amy DeClue for assistance with statistics. Preliminary results were presented as an extended research abstract at the Veterinary Comparative Respiratory Society Winter Research Forum 2020.
Gamracy J, Wiggen K, Vientós‐Plotts A, Reinero C. Clinicopathologic features, comorbid diseases, and prevalence of pulmonary hypertension in dogs with bronchomalacia. J Vet Intern Med. 2022;36(2):417‐428. doi: 10.1111/jvim.16381
REFERENCES
- 1. Carden KA, Boiselle PM, Waltz DA, Ernst A. Tracheomalacia and tracheobronchomalacia in children and adults: an in‐depth review. Chest. 2005;127:984‐1005. [DOI] [PubMed] [Google Scholar]
- 2. Wallis C, Alexopoulou E, Antón‐Pacheco JL, et al. ERS statement on tracheomalacia and bronchomalacia in children. Eur Respir J. 2019;54:1900382. [DOI] [PubMed] [Google Scholar]
- 3. Brown AJ, Davison E, Sleeper MM. Clinical efficacy of sildenafil in treatment of pulmonary arterial hypertension in dogs. J Vet Intern Med. 2010;24:850‐854. [DOI] [PubMed] [Google Scholar]
- 4. Harris RA, Grobman ME, Allen MJ, et al. Standardization of a videofluoroscopic swallow study protocol to investigate dysphagia in dogs. J Vet Intern Med. 2017;31:383‐393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Amis TC, McKiernan BC. Systematic identification of endobronchial anatomy during bronchoscopy in the dog. Am J Vet Res. 1986;47:2649‐2657. [PubMed] [Google Scholar]
- 6. Reinero CR, Masseau I. Lower airway collapse: revisiting the definition and clinicopathologic features of canine bronchomalacia. Vet J. 2021;273:105682. [DOI] [PubMed] [Google Scholar]
- 7. Masseau I, Reinero CR. Thoracic computed tomographic interpretation for clinicians to aid in the diagnosis of dogs and cats with respiratory disease. Vet J. 2019;253:105388. [DOI] [PubMed] [Google Scholar]
- 8. Bianco Z, Bukoski A, Masseau I, Reich C, Schultz L, Reinero C. Risk factors and outcomes in dogs with respiratory disease undergoing diagnostic airway lavage. Front Vet Sci. 2020;7:165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Johnson EG, Wisner ER. Advances in respiratory imaging. Vet Clin North Am Small Anim Pract. 2007;37(879–900):vi‐900. [DOI] [PubMed] [Google Scholar]
- 10. Fries RC, Gordon SG, Saunders AB, Miller MW, Hariu CD, Schaeffer DJ. Quantitative assessment of two‐ and three‐dimensional transthoracic and two‐dimensional transesophageal echocardiography, computed tomography, and magnetic resonance imaging in normal canine hearts. J Vet Cardiol. 2019;21:79‐92. [DOI] [PubMed] [Google Scholar]
- 11. Andreis ME, Panopoulos I, Domenech O, et al. Novel coronary artery anomaly in a French bulldog with pulmonary stenosis. J Vet Cardiol. 2021;35:1‐7. [DOI] [PubMed] [Google Scholar]
- 12. Owens EJ, Scollan KF, Pereyda TX, LeBlanc NL. Single left coronary ostium with a prepulmonic right coronary artery course in a French Bulldog with congenital valvular pulmonary stenosis. J Vet Cardiol. 2021;36:1‐5. [DOI] [PubMed] [Google Scholar]
- 13. Hara Y, Teshima K, Yamaya Y. Arterial blood gas analysis in dogs with bronchomalacia. PLoS ONE. 2019;14:e0227194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kim H, Kim YJ, Lee H, et al. Computed tomographic and radiographic bronchial collapse may be a normal characteristic of forced expiration in dogs. Vet Radiol Ultrasound. 2018;59:551‐563. [DOI] [PubMed] [Google Scholar]
- 15. Reinero C, Visser LC, Kellihan HB, et al. ACVIM consensus statement guidelines for the diagnosis, classification, treatment, and monitoring of pulmonary hypertension in dogs. J Vet Intern Med. 2020;34:549‐573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Jaffey JA, Wiggen K, Leach SB, Masseau I, Girens RE, Reinero CR. Pulmonary hypertension secondary to respiratory disease and/or hypoxia in dogs: clinical features, diagnostic testing and survival. Vet J. 2019;251:105347. [DOI] [PubMed] [Google Scholar]
- 17. Boon JA. Evaluation of Size, Function and Hemodynamics. 2nd ed. Ames, IA: Wiley‐Blackwell; 2011. [Google Scholar]
- 18. Adamama‐Moraitou KK, Pardali D, Day MJ, et al. Canine bronchomalacia: a clinicopathological study of 18 cases diagnosed by endoscopy. Vet J. 2012;191:261‐266. [DOI] [PubMed] [Google Scholar]
- 19. Bottero E, Bellino C, De Lorenzi D, et al. Clinical evaluation and endoscopic classification of bronchomalacia in dogs. J Vet Intern Med. 2013;27:840‐846. [DOI] [PubMed] [Google Scholar]
- 20. Della MA. An update on tracheal and airway collapse in dogs. Vet Clin North Am Small Anim Pract. 2020;50:419‐430. [DOI] [PubMed] [Google Scholar]
- 21. Bernaerts F, Talavera J, Leemans J, et al. Description of original endoscopic findings and respiratory functional assessment using barometric whole‐body plethysmography in dogs suffering from brachycephalic airway obstruction syndrome. Vet J. 2010;183:95‐102. [DOI] [PubMed] [Google Scholar]
- 22. Dengate A, Culvenor JA, Graham K, Braddock JA, Churcher RK. Bronchial stent placement in a dog with bronchomalacia and left atrial enlargement. J Small Anim Pract. 2014;55:225‐228. [DOI] [PubMed] [Google Scholar]
- 23. De Lorenzi D, Bertoncello D, Drigo M. Bronchial abnormalities found in a consecutive series of 40 brachycephalic dogs. J Am Vet Med Assoc. 2009;235:835‐840. [DOI] [PubMed] [Google Scholar]
- 24. Johnson LR, Pollard RE. Tracheal collapse and bronchomalacia in dogs: 58 cases (7 /2001‐1 /2008). J Vet Intern Med. 2010;24:298‐305. [DOI] [PubMed] [Google Scholar]
- 25. Singh MK, Johnson LR, Kittleson MD, Pollard RE. Bronchomalacia in dogs with myxomatous mitral valve degeneration. J Vet Intern Med. 2012;26:312‐319. [DOI] [PubMed] [Google Scholar]
- 26. Oh D, Lee S, Kim S, Choen S, Choi M, Yoon J. Computed tomographic bronchial collapsibility values over 50% may be detected in healthy dogs. Vet Radiol Ultrasound. 2019;60:28‐37. [DOI] [PubMed] [Google Scholar]
- 27. Yoon H, Yu J, An G, et al. CT and radiographic evaluation of bronchial collapsibility at forced expiration in asymptomatic brachycephalic dogs. Vet Radiol Ultrasound. 2020;61:167‐180. [DOI] [PubMed] [Google Scholar]
- 28. Heikkilä HP, Lappalainen AK, Day MJ, Clercx C, Rajamäki MM. Clinical, bronchoscopic, histopathologic, diagnostic imaging, and arterial oxygenation findings in West Highland White Terriers with idiopathic pulmonary fibrosis. J Vet Intern Med. 2011;25:433‐439. [DOI] [PubMed] [Google Scholar]
- 29. Johnson L. Diseases of the small airways. In: Ettinger SJ, Feldman EC, eds. Textbook of Veterinary Internal Medicine. 6th ed. St. Louis, MO: Elsevier Saunders; 2005:1233‐1239. [Google Scholar]
- 30. McKiernan BC. Diagnosis and treatment of canine chronic bronchitis. Twenty years of experience. Vet Clin North Am Small Anim Pract. 2000;30:1267‐1278. vi–vii. [DOI] [PubMed] [Google Scholar]
- 31. Padrid PA, Hornof WJ, Kurpershoek CJ, Cross CE. Canine chronic bronchitis. A pathophysiologic evaluation of 18 cases. J Vet Intern Med. 1990;4:172‐180. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 Definitions, including criteria used to make definitive or suspect diagnoses, for respiratory diseases in the dog.


