Abstract
Study Design:
Retrospective cost-effectiveness analysis.
Objectives:
While the incidence of traumatic spine injury (TSI) is high in low-middle income countries (LMICs), surgery is rarely possible due to cost-prohibitive implants. The objective of this study was to conduct a preliminary cost-effectiveness analysis of operative treatment of TSI patients in a LMIC setting.
Methods:
At a tertiary hospital in Tanzania from September 2016 to May 2019, a retrospective analysis was conducted to estimate the cost-effectiveness of operative versus nonoperative treatment of TSI. Operative treatment included decompression/stabilization. Nonoperative treatment meant 3 months of bed rest. Direct costs included imaging, operating fees, surgical implants, and length of stay. Four patient scenarios were chosen to represent the heterogeneity of spine trauma: Quadriplegic, paraplegic, neurologic improvement, and neurologically intact. Disability-adjusted-life-years (DALYs) and incremental-cost-effectiveness ratios were calculated to determine the cost per unit benefit of operative versus nonoperative treatment. Cost/DALY averted was the primary outcome (i.e., the amount of money required to avoid losing 1 year of healthy life).
Results:
A total of 270 TSI patients were included (125 operative; 145 nonoperative). Operative treatment averaged $731/patient. Nonoperative care averaged $212/patient. Comparing operative versus nonoperative treatment, the incremental cost/DALY averted for each patient outcome was: quadriplegic ($112-$158/DALY averted), paraplegic ($47-$67/DALY averted), neurologic improvement ($50-$71/DALY averted), neurologically intact ($41-$58/DALY averted). Sensitivity analysis confirmed these findings without major differences.
Conclusions:
This preliminary cost-effectiveness analysis suggests that the upfront costs of spine trauma surgery may be offset by a reduction in disability. LMIC governments should consider conducting more spine trauma cost-effectiveness analyses and including spine trauma surgery in universal health care.
Keywords: Tanzania, low-middle income countries, neurotrauma, traumatic spinal cord injury, East Africa, global neurosurgery
Introduction
Traumatic spine injury (TSI), comprising of fractures to the spinal column and spinal cord injury (SCI), is a major cause of morbidity and mortality worldwide, often affecting young, wage-earning males. 1 Low- and middle-income countries (LMICs) bear the brunt of this public health problem, and surgery is often not possible due to cost-prohibitive spinal implants.2,3 However, nonoperative treatment is associated with a 4-fold increase in mortality. 4
Prior to undergoing spine trauma surgery in most LMICs, patients must gather funds to purchase implants, leading to surgical delays, undertreatment of complex fractures, and gross inequity in treatment. 5 Due to insufficient funds, many patients are relegated to nonoperative care, consisting of bed rest for 3 months.6-8 Case series from Nigeria 9 and Ghana 10 report operative rates ranging from 47% to 57% in patients with surgical indications. While implants have a high upfront cost, the value of spine trauma surgery may be realized through achieving early mobilization, neurologic improvement, and return to wage-earning jobs. Due to the heterogeneity of traumatic spine injuries, complexity of spine trauma surgery, and poor research infrastructure in most LMICs, no cost-effectiveness studies determining the value of spine trauma surgery have been attempted. A more complete understanding of the economic benefits of operative care for TSI in LMICs is warranted.
Using a large cohort from a major East African referral hospital as a pilot study, we undertook a cost-effectiveness analysis comparing the benefit of operative versus nonoperative treatment in TSI patients. It is our hope that this preliminary analysis will generate future cost-effectiveness studies and facilitate comparison of spine trauma surgery to other public health interventions.
Materials and Methods
Population and Setting
We prospectively collected de-identified data from a single institution, Muhimbili Orthopaedic Institute (MOI), a major referral hospital in Dar es Salaam, Tanzania. MOI houses approximately 380 general ward, 16 intensive care unit (ICU), and 10 emergency department (ED) beds. Onsite imaging includes X-ray, computed tomography (CT), and magnetic resonance imaging (MRI). Payment is required prior to imaging and surgery. Hospital costs are out-of-pocket for uninsured patients; approximately 86% of TSI patients treated at MOI do not have insurance. 11 For our analysis, all patients were considered uninsured to assume the worst-case scenario and maximize external validity.
MOI is the only center in Tanzania and neighboring countries that routinely offers surgery for TSI. While other centers offer decompressive spinal surgery, this does not include the use of spinal implants to treat acute spinal trauma.12,13 We used prior reports to estimate the TSI burden in Tanzania and neighboring countries of 154 patients per year with a 40% surgical rate. 11
Data Collection
Data was collected from September 2016 to May 2019 (33 months). Pediatric patients (<14 years) and those with simultaneous brain and spine injuries were excluded. From a total of 270 patients, we collected age, gender, days from injury to admission, distance from injury to hospital, location (cervical/thoracic/lumbar) and severity of injury at admission and discharge using the American Spinal Injury Association (ASIA) Impairment Scale, 14 type of surgery, number of levels stabilized, and mortality. Appreciating that patients pay for the majority of their care out of pocket, we adopted a health care sector perspective with the assumption that a third-party payor (i.e., government or other) would ultimately incur the costs described. We obtained ethical approval from local and international institutional review boards. Patient consent was not required as all clinical data was de-identified.
Interventions: Operative Versus Nonoperative Treatment
We investigated the value of operative compared to nonoperative care for all TSI patients. During the study period, no official spine trauma protocol was in place, and a pragmatic surgical decision-making approach was taken. Both the decision to operate and the surgical plan were based heavily on the patient’s ability to pay for implants. 5
Operative treatment consisted of decompression of the neural elements and stabilization of the spinal column with 1 of 4 operations: anterior cervical discectomy and fusion (ACDF), anterior cervical corpectomy with tricortical iliac crest graft and plate (ACC), posterior cervical laminectomy and fusion with lateral mass screws and rods (PCLF), and posterolateral thoracic/lumbar fusion with pedicle screws and rods (PLF). Postoperatively, patients immediately began ambulating, wheelchair use, physiotherapy, and mobilization.
Nonoperative treatment consisted of bed rest for a minimum of 3-months, which included laying supine without walking or wheelchair use until the 3-month period was over and the fracture had healed. Ambulation, wheelchair use, or physiotherapy was not provided during this period of bed rest.
Cost Data
We included all costs within the formal health care sector defined as the sum of direct costs to the patient during admission. For nonoperative patients, this was calculated by adding the cost of 2 variables: initial imaging and length of stay (LOS). For operative patients, costs followed the same structure with the addition of operating room (OR) fee, surgical implants, and postoperative imaging. Imaging costs included: X-ray ($11.17), CT ($66.99), or MRI ($93.79). LOS costs included: LOS was multiplied by the daily fee charged for a general ward bed. OR costs included: Anesthetic medications, OR tech labor, and operative instruments used for each patient. Surgical implant costs included: Screws ($69.23), rods ($20.10), or cages/plates ($29.03). No cost distinction was made between lateral mass, anterior cervical, and pedicle screws. Using the foreign exchange rate method, all costs in Tanzanian Shillings were converted to 2018 US$. We calculated total costs with and without a 3% annual discount rate for years 2 and 3 and presented cost values as a range.
Disability-Adjusted Life-Year Data
We translated morbidity associated with TSI into disability-adjusted life-years (DALYs) averted and calculated the incremental difference between intervention scenarios. Health effects in LMICs are typically measured by DALYs averted, a measure of disease burden that combines both changes in life expectancy and morbidity. Our principal outcome measure was cost/DALY averted, a metric that quantifies the amount of money spent to avoid years of life lost due to ill health from a given disease. In the current study, cost/DALY averted meant the amount of money required to avoid losing one year of healthy life due to TSI. To calculate total cost, the patient sample was used to calculate the average cost for both operative and nonoperative treatment. For the cost-effectiveness analysis, to ensure comparison of equal groups, DALYs were calculated for those who underwent operative intervention, which was then compared to a hypothetical scenario that assumed the same operative cohort was instead managed nonoperatively, consistent with prior neurosurgical studies and considered standard among DALY analyses. 15 The hypothetical nonoperative group was chosen because it facilitated comparison of groups with equal sample sizes, demographics, and type of spine injury, which limited confounders and allowed for direct study of whether or not the intervention was cost-effective. That said, cost data for the hypothetical group was taken directly from the actual non-operative cohort. In other words, though the demographic and injury characteristics for the non-operative group were hypothetical to facilitate a direct comparison, all cost data used came from actual patient data.
DALYs are defined by 2 components: (1) the quality of life lost due to a specific disability and (2) the years of life lost due to premature death from said disease. Combined with age of onset, DALYs are calculated with disability weights (DWs), developed by the Global Burden of Disease Studies.16,17 DALYs range from 0 to 1, where 0 is equivalent to full health without any disease or disability, and 1 is equivalent to death. Each DW quantifies the added disability, or quality of life lost, from a particular health state.18,19
We used the Tufts Global Health Cost-Effectiveness Analysis (CEA) Registry DALY Calculator, a validated tool that converts non-DALY health metrics into DALYs, to compare cost-effectiveness ratios of interventions using DWs, age of onset, and age of premature death. 20 Age of onset was the average age of each subgroup below. Limited data exists on the life expectancy of TSI patients in LMICs, so we used the average Tanzanian life expectancy of 66 years from the World Bank. 21
DALY calculations vary based on 2 concepts: discounting and age-weighting. Discounting means more value is placed on a healthy year in the present compared the future. Age-weighting assumes that health is more valuable in the earlier rather than later stages of life. We used a discount rate of 3% and an age-weighting factor of 0.04, which aligns with World Health Organization (WHO) methodology 22 and other neurosurgical studies.15,23 Because TSI affects young, wage-earning males, age-weighting was an important factor to consider when determining long-term societal costs of younger individuals. We presented DALYs with and without discounting and age-weighting for each intervention. 24
Because surgery for TSI can lead to a variety of neurologic outcomes, all with varying levels of societal contribution and quality of life, a simple comparison of operative versus nonoperative treatment for all patients would not reflect the reality of spinal trauma. Therefore, we divided patients into four subgroups that represented a range of possible outcomes including quadriplegic, paraplegic, neurologically improved, and neurologically intact. In the absence of DWs and prior validated studies for these spine-specific scenarios,18,19,25,26 we chose available DWs from the Global Burden of Disease 2016 study. 17 Selections were based on clinical judgment of authors. A full explanation of each DW is noted in Supplementary Appendix 1 and presented below:
-
Quadriplegics: Compared operative versus nonoperative patients with cervical injuries discharged with ASIA A-C exams. Though ASIA C patients have motor function, they are still functionally quadriplegic, unable to care for themselves, or ambulate independently.
Operative: spinal cord lesion at neck, treated (DW = 0.589).
Nonoperative: spinal cord lesion at neck, untreated (DW = 0.732).
-
Paraplegics: Compared operative versus nonoperative patients with thoracolumbar spine injuries discharged with ASIA A-C exams.
Operative: spinal cord lesion below neck, treated (DW = 0.296).
Nonoperative: spinal cord lesion below neck, untreated (DW = 0.623).
-
Neurologically improved: Compared operative patients who improved to ASIA D/E versus nonoperative patients without neurologic improvement. This cohort represents how surgery can offer substantial improvement and help incomplete patients regain the ability to walk, though not to the point of being neurologically intact.
Operative: motor impairment, moderate (DW = 0.061), and musculoskeletal problems, legs, moderate (DW = 0.079) for the sensitivity analysis.
Nonoperative: motor impairment, severe (DW = 0.402).
-
Neurologically intact with unstable fracture: Compared operative versus nonoperative patients with an ASIA E exam who remained neurologically intact.
Operative: fractures, treated, long term (DW = 0.005).
Nonoperative: low back pain, most severe, without leg pain (DW = 0.372); neck pain, most severe (DW = 0.304) for the sensitivity analysis.
Statistical and Cost-Effectiveness Analysis
We reported descriptive statistics using means/medians and standard deviations (SDs)/ranges for continuous variables and counts with proportions for categorical variables. We conducted unpaired Student’s t tests, Mann-Whitney U tests, and chi-square tests for data comparison. We determined the cost per unit benefit of operative versus nonoperative treatment by comparing the 2 scenarios and calculating the incremental cost-effectiveness ratio (ICER; i.e., dividing the incremental cost of operative compared to nonoperative care by its incremental effectiveness, leading to our primary outcome variable of cost/DALYs averted). 15
Due to the preliminary nature of our study and uncertainty of DW inputs, we conducted a sensitivity analysis with the less straightforward clinical scenarios of: Neurological improvement and neurologically intact at discharge. Based on the Disease Control Priority Network’s cost-effectiveness threshold for low-income countries, 27 we used a cost-effectiveness threshold of $200 per DALY averted (Tanzania is considered low-income at <$1025 GDP/capita), which is a more conservative estimate than the previously used GDP/capita ($1020).21,28-30 The cost-effectiveness threshold of $200 per DALY averted was the most conservative estimate and minimized the chance of overestimating the cost-effectiveness of surgery. All analyses were conducted in RStudio, version 1.2.1335.
Results
Patient Cohort
A total of 270 patients met our inclusion criteria. The average (SD) age was 34.8 (11.6) years and 84% of the cohort was male (n = 226) (Table 1, panel A). Patients traveled a mean (SD) of 322 (319) km to hospital to receive treatment. Forty percent (n = 107) of injuries were to the cervical spine and 60% (n = 163) were to the thoracic/lumbar spine. Exactly half (n = 135) of the patients had ASIA A exams on admission. In total, 46% (n = 125) of patients underwent surgery: ACDF was performed in 3% (n = 4), ACC in 10% (n = 13), PCLF in 13% (n = 16), and PLF in 74% (n = 92). The rate of mortality among the entire cohort was 11% (n = 29).
Table 1.
(A) Demographics and Presentation Variables and (B) Cost Variables.
| Total (N = 270) | Operative (N = 125) | Nonoperative (N = 145) | P | |
|---|---|---|---|---|
| A: Demographics and presentation | ||||
| Age, years | ||||
| Mean (SD) | 34.8 (11.6) | 34.1 (11.7) | 35.5 (11.5) | .332 |
| Median (range) | 34 (8-74) | 32 (8-74) | 35 (15-67) | .237 |
| Male, n (%) | 226 (84) | 101 (81) | 125 (86) | .301 |
| Days from injury to MOI admission | ||||
| Mean, (SD) | 5.6 (10.6) | 5.1 (11.0) | 5.9 (10.2) | .560 |
| Median (range) | 2 (0-105) | 2 (0-105) | 2 (0-72) | .673 |
| Injury site to MOI distance (km) | ||||
| Mean (SD) | 322 (319) | 340 (325) | 307 (314) | .404 |
| Median (range) | 203 (0-1378) | 273 (0-1166) | 195 (0-1378) | .597 |
| Location, n (%) | ||||
| Cervical | 107 (40) | 33 (26) | 74 (51) | <.001* |
| Thoracic/lumbar | 163 (60) | 92 (74) | 71 (49) | — |
| Neurologic status, n (%) | ||||
| ASIA A | 135 (50) | 64 (51) | 71 (49) | .807 |
| ASIA B | 44 (16) | 28 (22) | 16 (11) | .018* |
| ASIA C | 22 (8) | 7 (6) | 15 (10) | .231 |
| ASIA D | 24 (9) | 13 (10) | 11 (8) | .551 |
| ASIA E | 45 (17) | 13 (10) | 32 (22) | .016* |
| Operation, n (%) | ||||
| Anterior cervical discectomy and fusion | 4 (3) | 4 (3) | — | — |
| Anterior cervical corpectomy | 13 (10) | 13 (10) | — | — |
| Posterior cervical laminectomy and fusion | 16 (13) | 16 (13) | — | — |
| Posterior thoracic/lumbar fusion | 92 (74) | 92 (74) | — | — |
| Levels stabilized |
— |
|||
| Mean (SD) | 2.1 (0.7) | 2.1 (0.7) | — | |
| Median (range) | 2 (1-4) | 2 (1-4) | — | — |
| Neurologic status at discharge, n (%)a | ||||
| Declined | 6 (2) | 2 (2) | 4 (3) | .684 |
| Stable | 201 (74) | 96 (82) | 105 (87) | .408 |
| Improved | 31 (11) | 19 (16) | 12 (10) | .209 |
| Mortality, n (%) | 29 (11) | 5 (4) | 24 (17) | .002* |
| B: Cost variables | ||||
| Initial imaging, n (%) | ||||
| X-ray | 206 (76) | 86 (69) | 120 (83) | .011* |
| CT | 84 (31) | 43 (34) | 41 (28) | .341 |
| MRI | 186 (69) | 90 (72) | 96 (66) | .234 |
| Implants, n | ||||
| Screws | — | 594 | — | — |
| Rods | — | 210 | — | — |
| Cages/plates | — | 21 | — | — |
| Postoperative imaging, n (%) | ||||
| X-ray | — | 57 (46) | — | — |
| CT | — | 1 (1) | — | — |
| MRI | — | 1 (1) | — | — |
| Length of stay, days | ||||
| Mean (SD) | 32.7 (22.9) | 38.5 (22.9) | 27.7 (21.8) | <.001* |
| Median (range) | 28 (1-190) | 35 (6-190) | 23 (1-120) | <.001* |
Abbreviations: MOI, Muhimbili Orthopaedic Institute; ASIA, American Spinal Injury Association; CT, computed tomography; MRI, magnetic resonance imaging.
a ASIA discharge values were unknown for 24 patients in the nonoperative group and 8 in the operative group.
* Denotes statistical significance at P < .05.
Intervention Costs
There was no significant difference in the number of CTs or MRIs between operative and nonoperative groups, but less patients in the operative group received initial X-rays (n = 86, 69% vs n = 120, 83%; P = .011) (Table 1, panel B). In total, the surgical group consumed 594 screws, 210 rods, and 21 cages/plates. Mean length of stay was longer for operative patients when compared with those managed nonoperatively (38.5 ± 22.9 vs 27.7 ± 21.8 days; P < .001).
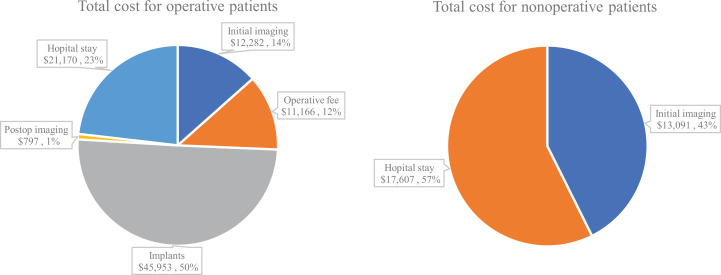
Total cost of treatment in the operative group was $87 515 to $91 369 vs. $29 404 to $30 698 in the nonoperative group (Figure 1). Cost per patient undergoing surgery was $700 to $731 compared with $203 to $212 per patient receiving nonoperative management. Within the operative group, implants accounted for most of the cost ($44 015-$45 953; 50%), followed by hospital stay ($20 277-$21 170; 23%), initial imaging ($11 764-$12 282; 14%), operative fee ($10 695-$11 166; 12%), and postoperative imaging ($763-$797; 1%). The majority of cost in the nonoperative group was from hospital stay ($16 865-$17 607; 57%), with the remaining portion from initial imaging ($12 539-$13 091; 43%). Total costs for each patient subgroup are presented in Table 2.
Figure 1.
Total costs for operative and nonoperative groups.
Table 2.
Cost/DALY Analysis Comparing Operative Versus Nonoperative Treatment in 4 Patient Scenarios.
|
Quadriplegic Cervical, ASIA A-C N = 20 DALYs (3, 1, 0.04)- DALYs (0, 0, 0)a |
Paraplegic Thoracic/lumbar, ASIA A-C N = 66 DALYs (3, 1, 0.04)- DALYs (0, 0, 0)a |
Neurologically improved Improved to ASIA D-E N = 18 DALYs (3, 1, 0.04)- DALYs (0, 0, 0)a |
Neurologically intact, unstable fracture Maintained ASIA E N = 13 DALYs (3, 1, 0.04)- DALYs (0, 0, 0)a |
|||||
|---|---|---|---|---|---|---|---|---|
| Operative | Nonoperativeb | Operative | Nonoperativeb | Operative | Nonoperativeb | Operative | Nonoperativeb | |
| Total costsc ($) | 14 004-14 620 | 4061-4240 | 46 212-48 246 | 13 402-13 992 | 12 603-13 158 | 3655-3816 | 9102-9503 | 2640-2756 |
| Total DALYs | 348-824 | 414-912 | 711-2138 | 1222-2829 | 98-444 | 230-622 | 49-300 | 165-458 |
| DALYs averted with surgery compared to without surgeryd | 66-89 | 511-691 | 132-178 | 116-158 | ||||
| Incremental cost-effectiveness ratio (cost/DALY averted)e ($) | 112-158 | 47-67 | 50-71 | 41-58 | ||||
Abbreviation: ASIA, American Spinal Injury Association.
a Disability-adjusted life-years (DALYs); DALYs (r, k, b): r is the discount rate, k is whether age-weighting was used (0 = no, 1 = yes), and b is the age-weighting value. DALYs are reported as a range, where DALYs (3, 1, 0.04) is the lower limit and DALYs (0, 0, 0) is the upper limit.
b Sample sizes are from the operative group. In accordance with standard DALY analyses, the nonoperative group is a hypothetical sample representative of the operative group had they not received surgery. Further description is provided in the article.
c Total costs presented as a range with and without a 3% annual discount rate for years 2 and 3.
d DALYs averted with surgery calculation: DALYs from nonoperative treatment minus the DALYs from operative treatment for a given scenario.
e Incremental cost-effectiveness ratio score calculation: total cost of operative treatment minus the total cost of nonoperative treatment for a given scenario, divided by the DALYs averted of operative treatment minus DALYs averted for nonoperative treatment.
Cost-Effectiveness Analysis Results
Of the 125 patients undergoing operative treatment, 8 were not included in the DALY analyses because of missing discharge neurological exams. In general, operative treatment had better outcomes at a lower cost/DALY averted than nonoperative care (Table 2). Surgery for neurologically intact patients with unstable fractures would prevent 116 to 158 DALYs and had a discounted, incremental cost of $497 per person (ICER compared with non-operative care: $41-$58). Surgery for paraplegic patients would prevent 511-691 DALYs and had a discounted, incremental cost of $497 per person (ICER compared with nonoperative care: $47-$67). Surgery for neurologically improved patients would prevent 132 to 178 DALYs and had a discounted, incremental cost of $497 per person (ICER compared with nonoperative care: $50-$71). Finally, surgery for quadriplegic patients would prevent 66 to 89 DALYs and had a discounted, incremental cost of $497 per person (ICER compared with nonoperative care: $112-$158). Taken together, compared with the a priori determined willingness to pay threshold of $200 per DALY averted, operative treatment of spinal trauma patients was cost-effective compared with nonoperative treatment in the low-income setting of Tanzania.
Sensitivity Analysis
Due to the exploratory nature of our investigation, we conducted a sensitivity analysis to assess how varying DW assignments would affect our cost-effectiveness results. While keeping all other assumptions within the DALY calculation constant, we altered DWs for 2 of the more complex patient subgroups: neurologically improved and neurologically intact. For neurologically improved patients, we alternatively assigned the operative group with the DW: musculoskeletal problems, legs, moderate. Under this instance, surgery for neurologically improved patients would prevent 125 to 169 DALYs and had a discounted, incremental cost of $497 per person (ICER compared with nonoperative care: $55-$75). For neurologically intact patients with unstable fractures, we alternatively assigned the nonoperative group with the DW: neck pain, most severe. Under this instance, surgery for neurologically intact patients with unstable fractures would prevent 95 to 128 DALYs and had a discounted, incremental cost of $497 per person (ICER compared with nonoperative care: $53-$71). While surgery had slightly greater ICERs for both scenarios, the alternative DWs did not change our overall results.
Discussion
In a study from a tertiary East African referral center, we undertook a preliminary cost-effectiveness analysis to evaluate the potential benefit of spine trauma surgery. Based on a willingness to pay cost/DALY averted threshold of $200, operative treatment compared to nonoperative care was found to be cost-effective in all four patient scenarios, with neurologically intact patients, those with neurologic improvement, and paraplegics being the most economically beneficial scenarios. Given the complexity of cost-effectiveness studies in a spine trauma population, and the dearth of these analyses in less resourced settings, the results of this pilot analysis should cautiously lend empirical support to the notion that spine trauma surgery is a priority intervention in low-income settings.
Little has been studied on the cost-effectiveness of spine trauma surgery, even in high income countries.31-33 Chan et al. 31 reviewed the economics of acute spine trauma and highlighted 11 cost-effectiveness studies, all from high-income countries, 3 of which evaluated operative versus nonoperative interventions.25,26,34 These studies addressed type-II odontoid 25 and thoracolumbar fractures26,34 in neurologically intact patients, which bears little resemblance to our population. Comparisons with an LMIC setting also prove difficult because previous studies compared early versus late surgery35,36 or elderly versus nonelderly groups. 32 Moreover, rehabilitation services are included in many previous studies,32,36 which are underutilized in Tanzania. Even fewer studies have assessed TSI costs in an LMIC setting. In Iran, Rahimi-Movaghar et al. 18 estimated the burden of SCI, while Moradi et al. 19 estimated the burden of spine fractures without neurologic deficit. Neither study discussed operative intervention nor conducted cost-effectiveness analyses.
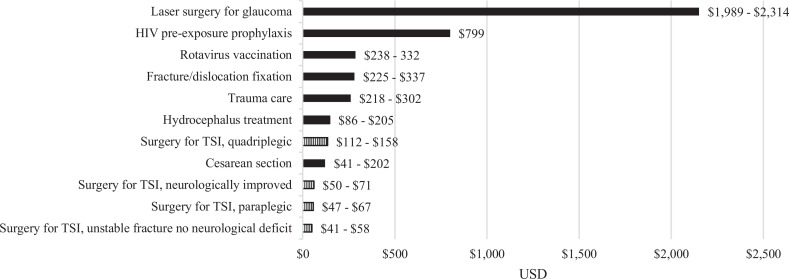
All 4 of our patient scenarios were deemed cost-effective, meaning the amount of money required to avoid 1 year of ill health due to spine trauma was below the willingness to pay threshold of $200 per DALY averted; neurologically intact, neurologically improved, and paraplegic patients were of the highest value, followed by quadriplegic patients. Cost-effectiveness of surgery in paraplegic patients was likely due to the ability to sit up, ambulate, and engage in physiotherapy. Without surgery and 3 months of bed rest, pressure ulcers and infections are almost inevitable. Surgically treated quadriplegic patients were the least cost-effective subgroup, and especially in elderly individuals, surgical patient selection is paramount. Conservative DWs were chosen for all 4 patient scenarios, but especially for the neurologically improved group. We assigned the following health weights for patients who improved to ASIA D-E with surgery: motor impairment, moderate (DW = 0.061), and musculoskeletal problems, legs, moderate (DW = 0.079) for the sensitivity analysis. With timely decompression and stabilization, some patients in this group, who often suffer incomplete spinal cord injuries, may gain complete return of function. However, we felt it was more appropriate to not assume return of full neurologic function. Therefore, surgery for patients who have the potential to improve neurologically may be even more cost-effective than shown herein. When taken in the context of other surgical procedures, the cost-effectiveness of spine trauma surgery is similar to other public health interventions in LMICs ( Figure 2 ).15,37-40 Given the population that spinal trauma disproportionately affects—young, wage-earning males supporting families—providing spine trauma surgery services for all patients, regardless of their ability to pay, may help mitigate the overall negative effects of spine trauma. Lastly, the cost-effectiveness of spine trauma surgery is predicated on adequate perioperative hospital care, including experienced anesthesia, nursing, intensive care unit, and rehabilitation services. Spine surgery does not exist in a vacuum, and these perioperative services are just as important as the surgical intervention.
Figure 2.
Comparison of cost/DALY for various public health interventions; lower cost equates to less money required to avert a single DALY and thus more cost-effective. DALY, disability-adjusted life-year.
Considering the preliminary nature of this study, the analysis is limited in several ways. First, societal/indirect costs such as patient travel costs, time lost from work (including both short and long-term loss of wages for nonoperative patients), and caretaker fees could not be included due to lack of patient and family data. We also did not have the breakdown of patients who were in the general ward versus ICU settings to determine cost differences between intervention groups. Additionally, postoperative costs for neck collar use, physiotherapy, and wheelchair use could not be accounted for, thus undervaluing the cost of surgery. Second, due to unequal groups, we created a hypothetical nonoperative scenario to compare groups of equal sizes, demographics, and spine injury types. The hypothetical scenario, still, imperfectly represented injury types in the nonoperative group, which had a greater number of cervical spine injuries than the operative group, likely due to fracture severity, poor neurologic function, complexity of surgery in this region, and lack of cervical instrumentation. Ideally, we would have conducted a randomized controlled or prospective trial with balanced groups of operative compared with nonoperative care; however, this was not feasible for reasons related to funding, research infrastructure, and equipment. Despite the hypothetical scenario, real costs generated from the nonoperative group were used to calculate the final cost-effectiveness results. Third, we used clinical judgment to choose imperfect DWs for each subgroup; however, sensitivity analysis altering DWs revealed no major differences in our overall conclusions. Exact disability quantified from spine trauma remains unknown and is an area for further research. Moreover, it is unknown if DWs can be universally applied to high- and low-income countries. Though the DWs used are the best estimates available and used heavily throughout the literature, they may not be applicable to the specific care provided in Tanzania. While all patients received adequate postoperative care in the hospital, their postoperative care at home was less controlled; thus, DWs depend heavily on adequate postoperative care, which could not be guaranteed in all settings. Lastly, though patients with complete SCI have a low chance of recovery, they were included to measure the benefit of stabilization and subsequent mobilization to wheelchair, sitting up in bed, and physical therapy compared with 3 months in the supine position.
Conclusions
In comparing operative versus nonoperative treatment across 4 patient scenarios from a low-income spine trauma population, the amount of money required to avoid 1 year of ill health due to TSI with operative treatment compared with nonoperative treatment was below the willingness to pay threshold of $200 per DALY averted. Surgery for neurologically intact, paraplegic, and neurologically improved patients had the highest economic gains. Though the results of our pilot study require confirmation in larger samples and should be cautiously extrapolated to other LMICs, we provide preliminary evidence that the upfront cost of spine trauma surgery may be offset by an overall reduction in adverse health effects. Therefore, LMIC governments should consider collecting more detailed cost data, conducting more definitive cost-effectiveness studies, and potentially including spine trauma surgery as a component of their priority interventions for universal health care. The results of this analysis require confirmation with additional and more robust studies.
Supplemental Material
Supplemental Material, Cost_appendix_(20-05-04) for Cost-Effectiveness of Operating on Traumatic Spinal Injuries in Low-Middle Income Countries: A Preliminary Report From a Major East African Referral Center by Noah L. Lessing, Scott L. Zuckerman, Albert Lazaro, Ashley A. Leech, Andreas Leidinger, Nicephorus Rutabasibwa, Hamisi K. Shabani, Halinder S. Mangat and Roger Härtl in Global Spine Journal
Acknowledgments
We would like to thank the entire Muhimbili Orthopaedic Institute for their ongoing commitment to research and excellence in clinical care in Dar es Salaam, Tanzania.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Noah L. Lessing, BS  https://orcid.org/0000-0002-3994-7977
https://orcid.org/0000-0002-3994-7977
Scott L. Zuckerman, MD, MPH  https://orcid.org/0000-0003-2951-2942
https://orcid.org/0000-0003-2951-2942
Ashley A. Leech, PhD, MS  https://orcid.org/0000-0001-6795-929X
https://orcid.org/0000-0001-6795-929X
Supplemental Material: Supplemental material for this article is available online.
References
- 1.Badhiwala JH, Wilson JR, Fehlings MG. Global burden of traumatic brain and spinal cord injury. Lancet Neurol. 2019;18:24–25. [DOI] [PubMed] [Google Scholar]
- 2.GBD 2016 Traumatic Brain Injury; Spinal Cord Injury Collaborators. Global, regional, and national burden of traumatic brain injury and spinal cord injury, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019;18:56–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kumar R, Lim J, Mekary RA, et al. Traumatic spinal injury: global epidemiology and worldwide volume. World Neurosurg. 2018;113:e345–e363. [DOI] [PubMed] [Google Scholar]
- 4.Lessing NL, Lazaro A, Zuckerman SL, et al. Non-operative treatment of traumatic spinal injuries in Tanzania: who is not undergoing surgery and why? Spinal Cord. Published online April 29, 2020. doi:10.1038/s41393-020-0474-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Magogo J, Lazaro A, Mango M, et al. Operative treatment of traumatic spinal injuries in Tanzania: surgical management, neurologic outcomes, and time to surgery. Global Spine J. Published online January 21, 2020. doi:10.1177/2192568219894956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Adeolu AA, Komolafe EO. Outcome of a posterior spinal fusion technique using spinous process wire and vertical strut. Ann Afr Med. 2014;13:30–34. [DOI] [PubMed] [Google Scholar]
- 7.Rabiu TB. Clinical outcomes of posterior spinal stabilization with rigid vertical strut and spinal process wires (the Adeolu’s technique) in a developing country. Pan Afr Med J. 2017;26:84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Choi JH, Park PJ, Din V, Sam N, Iv V, Park KB. Epidemiology and clinical management of traumatic spine injuries at a major government hospital in Cambodia. Asian Spine J. 2017;11:908–916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nwankwo OE, Uche EO. Epidemiological and treatment profiles of spinal cord injury in southeast Nigeria. Spinal Cord. 2013;51:448–452. [DOI] [PubMed] [Google Scholar]
- 10.Ametefe MK, Bankah PE, Yankey KP, Akoto H, Janney D, Dakurah TK. Spinal cord and spine trauma in a large teaching hospital in Ghana. Spinal Cord. 2016;54:1164–1168. [DOI] [PubMed] [Google Scholar]
- 11.Leidinger A, Kim EE, Navarro-Ramirez R, et al. Spinal trauma in Tanzania: current management and outcomes. J Neurosurg Spine. 2019;31:103–111. [DOI] [PubMed] [Google Scholar]
- 12.Rashid SM, Jusabani MA, Mandari FN, Dekker MCJ. The characteristics of traumatic spinal cord injuries at a referral hospital in Northern Tanzania. Spinal Cord Ser Cases. 2017;3:17021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moshi H, Sundelin G, Sahlen KG, Sorlin A. Traumatic spinal cord injury in the north-east Tanzania—describing incidence, etiology and clinical outcomes retrospectively. Glob Health Action. 2017;10:1355604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kirshblum SC, Waring W, Biering-Sorensen F, et al. Reference for the 2011 revision of the International Standards for Neurological Classification of Spinal Cord Injury. J Spinal Cord Med. 2011;34:547–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hubbard ZS, Shah AH, Ragheb M, Wang S, Jernigan S, Ragheb J. Economic benefit of neurosurgical intervention for infant hydrocephalus in Haiti. J Neurosurg Pediatr. 2019;24:217–351. [DOI] [PubMed] [Google Scholar]
- 16.Salomon JA, Vos T, Hogan DR, et al. Common values in assessing health outcomes from disease and injury: disability weights measurement study for the Global Burden of Disease Study 2010. Lancet. 2012;380:2129–2143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Salomon JA, Haagsma JA, Davis A, et al. Disability weights for the Global Burden of Disease 2013 study. Lancet Glob Health. 2015;3:e712–e723. [DOI] [PubMed] [Google Scholar]
- 18.Rahimi-Movaghar V, Moradi-Lakeh M, Rasouli MR, Vaccaro AR. Burden of spinal cord injury in Tehran, Iran. Spinal Cord. 2010;48:492–497. [DOI] [PubMed] [Google Scholar]
- 19.Moradi-Lakeh M, Rasouli MR, Vaccaro AR, Saadat S, Zarei MR, Rahimi-Movaghar V. Burden of traumatic spine fractures in Tehran, Iran. BMC Public Health. 2011;11:789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Emerson J, Kim DD. Disability adjusted life year (DALY) calculator: Methodology. Center for the Evaluation of Value and Risk in Health, Tufts Medical Center, Boston, MA. Published 2018. Accessed July 11, 2020. http://ghcearegistry.org/ghcearegistry/Calculator_Methodology.pdf [Google Scholar]
- 21.The World Bank. World Bank Open Data. Accesses July 9, 2020. https://data.worldbank.org
- 22.Murray CJ, Lopez AD. Alternative projections of mortality and disability by cause 1990-2020: Global Burden of Disease Study. Lancet. 1997;349:1498–1504. [DOI] [PubMed] [Google Scholar]
- 23.Warf BC, Alkire BC, Bhai S, et al. Costs and benefits of neurosurgical intervention for infant hydrocephalus in sub-Saharan Africa. J Neurosurg Pediatr. 2011;8:509–521. [DOI] [PubMed] [Google Scholar]
- 24.Fox-Rushby JA, Hanson K. Calculating and presenting disability adjusted life years (DALYs) in cost-effectiveness analysis. Health Policy Plan. 2001;16:326–331. [DOI] [PubMed] [Google Scholar]
- 25.Barlow DR, Higgins BT, Ozanne EM, Tosteson AN, Pearson AM. Cost effectiveness of operative versus non-operative treatment of geriatric type-II odontoid fracture. Spine (Phila Pa 1976). 2016;41:610–617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Aras EL, Bunger C, Hansen ES, Sogaard R. Cost-effectiveness of surgical versus conservative treatment for thoracolumbar burst fractures. Spine (Phila Pa 1976). 2016;41:337–343. [DOI] [PubMed] [Google Scholar]
- 27.Horton S. Cost-effectiveness analysis in disease control priorities. In: Jamison DT, Gelband H, Horton S, et al. , eds. Disease Control Priorities: Improving Health and Reducing Poverty. Vol. 9. 3rd ed. The International Bank for Reconstruction and Development/The World Bank; 2017:147–156. [PubMed] [Google Scholar]
- 28.Leech AA, Kim DD, Cohen JT, Neumann PJ. Use and misuse of cost-effectiveness analysis thresholds in low- and middle-income countries: trends in cost-per-DALY studies. Value Health. 2018;21:759–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bertram MY, Lauer JA, De Joncheere K, et al. Cost-effectiveness thresholds: pros and cons. Bull World Health Organ. 2016;94:925–930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Robinson LA, Hammitt JK, Chang AY, Resch S. Understanding and improving the one and three times GDP per capita cost-effectiveness thresholds. Health Policy Plan. 2017;32:141–145. [DOI] [PubMed] [Google Scholar]
- 31.Chan BCF, Craven BC, Furlan JC. A scoping review on health economics in neurosurgery for acute spine trauma. Neurosurg Focus. 2018;44:E15. [DOI] [PubMed] [Google Scholar]
- 32.Furlan JC, Craven BC, Fehlings MG. Surgical management of the elderly with traumatic cervical spinal cord injury: a cost-utility analysis. Neurosurgery. 2016;79:418–425. [DOI] [PubMed] [Google Scholar]
- 33.Brauer CA, Rosen AB, Olchanski NV, Neumann PJ. Cost-utility analyses in orthopaedic surgery. J Bone Joint Surg Am. 2005;87:1253–1259. [DOI] [PubMed] [Google Scholar]
- 34.Siebenga J, Segers MJ, Leferink VJ, et al. Cost-effectiveness of the treatment of traumatic thoracolumbar spine fractures: nonsurgical or surgical therapy? Indian J Orthop. 2007;41:332–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Boakye M, Arrigo RT, Hayden Gephart MG, Zygourakis CC, Lad S. Retrospective, propensity score-matched cohort study examining timing of fracture fixation for traumatic thoracolumbar fractures. J Neurotrauma. 2012;29:2220–2225. [DOI] [PubMed] [Google Scholar]
- 36.Furlan JC, Craven BC, Massicotte EM, Fehlings MG. Early versus delayed surgical decompression of spinal cord after traumatic cervical spinal cord injury: a cost-utility analysis. World Neurosurg. 2016;88:166–174. [DOI] [PubMed] [Google Scholar]
- 37.Prinja S, Nandi A, Horton S, Levin C, Laxminarayan R. Costs, effectiveness, and cost-effectiveness of selected surgical procedures and platforms. In: Debas HT, Donkor P, Gawande A, Jamison DT, Kruk ME, Mock CN, eds. Disease Control Priorities: Essential Surgery. Vol. 1. 3rd ed. The International Bank for Reconstruction and Development/The World Bank; 2015:317–338. [PubMed] [Google Scholar]
- 38.Roberts G, Roberts C, Jamieson A, Grimes C, Conn G, Bleichrodt R. Surgery and obstetric care are highly cost-effective interventions in a sub-Saharan African District Hospital: a three-month single-institution study of surgical costs and outcomes. World J Surg. 2016;40:14–20. [DOI] [PubMed] [Google Scholar]
- 39.Nonvignon J, Atherly D, Pecenka C, et al. Cost-effectiveness of rotavirus vaccination in Ghana: examining impacts from 2012 to 2031. Vaccine. 2018;36:7215–7221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sarkar S, Corso P, Ebrahim-Zadeh S, Kim P, Charania S, Wall K. Cost-effectiveness of HIV prevention interventions in sub-Saharan Africa: a systematic review. EClinicalMedicine. 2019;10:10–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Material, Cost_appendix_(20-05-04) for Cost-Effectiveness of Operating on Traumatic Spinal Injuries in Low-Middle Income Countries: A Preliminary Report From a Major East African Referral Center by Noah L. Lessing, Scott L. Zuckerman, Albert Lazaro, Ashley A. Leech, Andreas Leidinger, Nicephorus Rutabasibwa, Hamisi K. Shabani, Halinder S. Mangat and Roger Härtl in Global Spine Journal