Abstract
Study Design:
Retrospective cohort study.
Objective:
To evaluate the clinical efficacy of minimally invasive endoscopic surgery in patients with spinal extradural and intradural-extramedullary tumors.
Methods:
This was a study of 15 consecutive patients with spinal extradural or intradural-extramedullary tumors up to 2 levels treated by minimal invasive surgery using a full endoscopic visualization and Caspar’s retraction system (for cervical, thoracic, and lumbar tumors) over a 4-year period between January 2015 to April 2019 at a tertiary center.
Results:
A gross total remove was achieved in all patients (100%), determined by postoperative contrast computed tomography scans and magnetic resonance imaging. There was no postoperative spinal instability. All patients had equal or better neurologic functions after surgery at follow-up. The average preoperative Nurick’s grade mean was 1.9 and the postoperative was 1.1. The average preoperative McCormick’s grade mean was 2.9 versus 1.3 in the postoperative period.
Conclusions:
Selective extradural or intradural-extramedullary tumors well localized and up to 2 levels can be safely and effectively treated by minimally invasive surgery using a full endoscopic visualization and the Caspar’s retractor. However, there is insufficient evidence to recommend this approach over the classical or other microsurgical approach described.
Keywords: endoscopy, minimally invasive, spinal tumors
Introduction
Minimally invasive surgery constitutes a good option to classical surgery in patients with spinal extradural and intradural-extramedullary tumors, which is associated with less morbidity and lower costs. 1 However, only a few case series have been reported.2-6 Otherwise, the surgical technique has been variable with different retraction systems and visualization methods. 1
Moreover, to our knowledge, no previous study has evaluated the use of the Caspar’s retractor as retraction system and the full endoscopic visualization as visualization method. The goal of this study was to determine the results of minimally invasive surgery for spinal extradural and intradural-extramedullary tumors using this technique.
Methods
Study Design
We performed a retrospective cohort study.
Objective
To evaluate the clinical efficacy of minimally invasive endoscopic surgery in patients with spinal extradural and intradural-extramedullary tumors.
Inclusion Criteria
A retrospective analysis of 15 consecutive adult patients was performed (equal to or older than 18 years) with spinal extradural or intradural-extramedullary tumors confirmed by postoperative excisional biopsy, up to 2 levels treated by minimally invasive surgery using a full endoscopic visualization and the Caspar’s retraction system (for cervical, thoracic, and lumbar tumors) over a 4-year period between January 2015 to April 2019 at a tertiary center.
Institutional Board Approval
This study has been approved by an institutional review board (register number: 207). All the subjects had signed the informed consent forms before enrollment.
Patient Population and Intervention
All patient selected were treated by the same technique describe above.
All procedures were done under a full endoscopic visualization using a 0°, 30°, and occasionally 45° 18-cm to 4-mm rod lens (Karl-Storz).
Patient’s position on the operating table was the standard one used for the cervical, thoracic, or lumbar laminectomy and the operation was performed under combined endotracheal anesthesia. The anesthesiology team was situated at the foot side of the patient. The surgeon and the assistant were situated at both sides of the surgical field and the monitor at the top of the patient side. All procedures were performed under general total intravenous anesthesia. The skin was infiltrated with epinephrine 1:100 000 and 0.5% bupivacaine to obtain a good hemostasis and a local anesthetic.
The minimally invasive approach starts with the fluoroscopic control for the center of the lesion, correlated with the preoperative magnetic resonance imaging (MRI), except in craniospinal junction lesions in which surgical landmarks were easier to identify and thoracic lesions in which a computed tomography (CT) scan with radiolucid markers were used as landmark for exact location of the lesion. The surgical technique was different between craniospinal junction tumors and cervical thoracic and lumbar tumors. In craniospinal junction tumors, a midline incision was performed from 2 cm below the inion to spinous process of C2. After the suboccipital muscles detached a foramen magnum region and posterior arch of C1 were exposed. A suboccipital keyhole approach was performed using a high-speed drill with the removal of the posterior border of the foramen magnum and the posterior arch of C1.
In cervical, thoracic, and lumbar tumors an incision of 2.5 cm lateral to the midline (1.5 cm) and localized over the center of the tumor was performed. The fascia was incised in an arciform way with Metzenbaum’s scissor and retracted to midline, followed by blunt dissection and disinsertion of paravertebral muscles using a monopolar. Then, the appropriate Caspar’s retractor was inserted in a similar way of the lumbar microdiscectomy. Caspar’s contra separator was not necessary. At this moment, the assistant places the 18 × 4 mm, 0 grade endoscope (Karl Storz) in the surgical field and the remaining procedure is performed under full endoscopic visualization. An assistant held the endoscope in one corner while the surgeon performed the bimanual surgery. The remainder soft tissue was removed with electrocautery, and a curved high-speed drill was used to perform the hemilaminectomies. Additionally, the base of the spinous processes and contralateral lamina were decompressed with the drill and Kerrison’s rongeurs. The flavum ligament was resected. In intradural tumors the dura was opened in the midline with a long-handled No. 15 blade scalpel and opened with the Potts scissors with preservation of the arachnoid layer. The dural borders were tacked up to the paraspinal muscles with 4-0 Neurolon sutures. The arachnoid layer was opened and the tumor was resected with standard microsurgical techniques by using 0°, 30°, and 45° rod lens and the dura was closed with a running 4-0 polyester suture under endoscopic visualization with the technique previously described by Parihar et al. 6 The fascia was closed with absorbable 0 Vicryl sutures and the subcutaneous layer closed with 2-0 Vicryl sutures. The skin was closed with an interrupted 2-0 nylon suture. Figures 1 and 2 illustrate 2 representative cases.
Figure 1.
Tetraplegic patient with a C4-C5 epidural meningioma. (A) Photograph of the fluoroscopic control. (B) Photograph of the patient position and the endoscope equipment. (C, D) Transoperative captures: (C) during the microsurgical dissection of the tumor and (D) after the spinal cord decompression. (E) Postoperative computed tomography (CT) scan reconstruction showing the extension of bone removal. (F) Preoperative axial CT scan. A right extensive hyperdense well-circumscribed tumor is observed inside the spinal canal adjacent to the ipsilateral laminae. (G) Postoperative axial CT scan exhibiting a total removal of the tumor through the ipsilateral laminar defect. (H) Photograph of the patient walking on the third postoperative day.
Figure 2.
Paraplegic patient treated by a myxopapillary ependymoma. (A) Operative photograph exhibiting the paramedian 2.5 incision. (B-D) Transoperative captures: (B) after the dural incision the tumor is observed surrounded by the roots; (C) during the filum incision; and (D) after a total en bloc removal of the tumor, the intact nerve roots are observed. (E) Photograph of the tumor completely resected. The cephalic and caudal filum cord insertion is observed. (F) Preoperative sagittal T2-weighted imaging (WI) showing a hyperintense well-circumscribed intradural L2-L3 tumor. (G) Postoperative T2 WI exhibiting a total removal of the lesion. (H) The patient walking during the seventh postoperative day.
Outcomes and Analysis
Demographic and clinical data was recorded, including age, sex, clinical presentation, tumor’s location, size, number of involved spinal levels and the tumor’s histology. Baseline patient characteristics were recorded and listed in Tables 1 and 2.
Table 1.
Demographics, Clinical Presentation, Site of Lesion, Type of Pathologies, Approach Employed, Grade of Resection, and Complications of the Cohort.
| Patient No. | Age/sex | Clinical presentation | Level | Localization | Histology | Approach | Grade of resection | Complications |
|---|---|---|---|---|---|---|---|---|
| 1 | 50/F | Radicular pain | Lumbar | Intradural extramedullary | Myxopapillary ependymoma | Posterior laminectomy | Total | None |
| 2 | 49/F | Paraplegia | Thoracic | Extradural | Psammomatous meningioma | Posterior laminectomy | Total | None |
| 3 | 36/M | Quadriplegia | Cervical | Extradural | Lung metastases | Posterior laminectomy | Total | None |
| 4 | 49/F | Quadriparesis | Craniospinal junction | Extradural | Nerve sheath tumor | Suboccipital keyhole | Total | None |
| 5 | 18/M | Paraparesis | Craniospinal junction | Intradural extramedullary | Transitional meningioma | Suboccipital key hole | Total | None |
| 6 | 56/F | Radicular pain | Lumbar | Intradural extramedullary | Nerve sheath tumor | Posterior laminectomy | Total | None |
| 7 | 50/F | Radicular pain | Lumbar | Intradural extramedullary | Myxopapillary ependymoma | Posterior laminectomy | Total | None |
| 8 | 55/F | Radicular pain | Thoracic | Intradural extramedullary | Meningothelial meningioma | Posterior laminectomy | Total | None |
| 9 | 25/F | Radicular pain | Lumbar | Intradural extramedullary | Nerve sheath tumor | Posterior laminectomy | Total | Transient urinary incontinence |
| 10 | 48/F | Radicular pain | Thoracic | Extradural | Breast metastases | Posterior laminectomy | Total | None |
| 11 | 30/F | Radicular pain | Thoracic | Intradural extramedullary | Nerve sheath tumor | Posterior laminectomy | Total | None |
| 12 | 46/F | Quadriparesis | Craniospinal junction | Intradural extramedullary | Meningothelial meningioma | Suboccipital keyhole | Total | None |
| 13 | 52/F | Radicular pain | Lumbar | Intradural extramedullary | Nerve sheath tumor | Posterior laminectomy | Total | None |
| 14 | 28/M | Radicular pain | Craniospinal junction | Extradural | Nerve sheath tumor | Suboccipital keyhole | Total | None |
| 15 | 48/F | Radicular pain | Lumbar | Intradural extramedullary | Myxopapillary ependymoma | Posterior laminectomy | Total | None |
Table 2.
Clinical and Demographic Data of 15 Patients With Spinal Tumors Treated by Full Endoscopic Surgery.
| Variable | Value |
|---|---|
| Age, years, mean ± SD (range) | 42.6 ± 12 (18-56) |
| Sex, n (%) | |
| Female | 12 (80.0) |
| Male | 3 (20.0) |
| Clinical presentation, n (%) | |
| Radicular pain | 10 (66.7) |
| Quadriparesis | 2 (13.2) |
| Quadriplegia | 1 (6.7) |
| Paraparesis | 1 (6.7) |
| Paraplegia | 1 (6.7) |
| Pre-/postoperative Nurick grade | 1.9/1.1 |
| Pre-/postoperative McCormick grade | 2.9/1.3 |
| Spinal level, n (%) | |
| Lumbar | 6 (62.9) |
| Craniospinal junction | 4 (26.7) |
| Thoracic | 4 (26.7) |
| Cervical | 1 (6.7) |
| No. of spinal levels, n (%) | |
| 1 | 8 (53.3) |
| 2 | 6 (46.7) |
| Localization, n (%) | |
| Intradural-extramedullary | 10 (66.7) |
| Extradural | 5 (33.3) |
| Pathology, n (%) | |
| Nerve sheath tumor | 6 (40.0) |
| Meningioma | 4 (26.7) |
| Myxopapillary ependymoma | 3 (20.0) |
| Spinal metastases | 2 (13.3) |
| Follow-up, months, mean ± SD (range) | 22.7 ± 9 (3-40) |
Perioperative data was collected for the following: estimated blood loss; surgery’s duration; bed rest days; perioperative complications; pre- and postoperative clinical assessment (by means the Nurick’s, McCormick’s, and Frankel’s scale); and length of hospital stay.
Results
Data was analyzed in 15 consecutive patients. The mean age was 42.6 ± 12 years (range 18-56 years). Sex distribution was 12 females (80.0%) and 3 males (20.0%) with a female/male ratio of 4:1. The most common clinical presentation was radicular pain (66.7%) followed by different grades of muscular weakness (33.3%) (Table 2).
The commonest spinal level was lumbar (62.9%) followed by the craniospinal junction and thoracic level. Sagittal and axial diameter of the tumors ranged from 18 to 35 mm and from 15 to 22 mm. Eight tumors included 1 level (while in the remainders 6 tumors, 2 levels were included). The more common localization was intradural-extramedullary with 10 patients (66.7%).
The commonest diagnosis was nerve sheath tumor, followed by meningioma, myxopapillary ependymoma and 2 patients with extradural metastases from lung and breast cancer.
The surgical technique was a posterior laminectomy (73.3%) and a suboccipital keyhole approach (26.7%).
The mean operative time was 205.3 minutes (±24.6 minutes), the mean estimated blood loss was 121 mL (± 25.9mL). As result, no patients required perioperative blood transfusions.
A gross total resection was achieved in all patients (100%), determined by postoperative contrast-CT scans and MRI. There was not postoperative spinal instability.
All patients had equal or better neurologic functions after surgery at follow-up according to Frankel’s scale (Table 1). The average preoperative Nurick’s grade mean was 1.9 and the postoperative was 1.1. The average preoperative McCormick’s grade mean was 2.9 versus 1.3 in the postoperative period.
The average bed rest days was 2.3 days (range 2-3 days) and the average hospital stay was 5.2 days (4-7 days).
The mean follow-up time was 22.7± 9 months (range 3-40 months). There were no deaths related to surgery in our series. The patient with lung cancer metastases died 6 months after the procedure due to disseminated oncological disease but there were not local recurrences. The patient with breast cancer metastases remained stable after 8 months follow-up period. The postoperative complication rate was very low (6.7%) and included 1 patient with a myxopapillary ependymoma who experienced transient urinary incontinence. Tumor recurrence was not detected during the follow-up period.
Discussion
Intradural-extramedullary spine tumors are uncommon, occurring in 5 to 10 per 1 00 000 people. The commonest lesions include meningiomas, schwannomas, and neurofibromas. 8
These tumors may present with local pain and radicular symptoms in general. Cervical and thoracic tumors can also present with myelopathy while lumbar region’s tumors may present with lower-extremity weakness, or bowel or bladder dysfunction. 8
The enthusiasm to develop minimally invasive strategies to treat spinal tumors is motivated by the significant complication rates associated with established surgical approaches to neoplastic spinal disease. High rates of gross-total resection with minimal long-term neurological deficit have been reported on traditional resection of intradural-extramedullary spinal tumors.9,10 However, these surgical approaches use an extended midline incision (2 levels above and below of the tumor), subperiosteal dissection of the paraspinal muscles, laminectomies, and intradural tumor resection. 11 As a result of their multilevel extensive soft-tissue dissection, and disruption of midline structures, a more postoperative discomfort, and a spinal instability can be appeared. At least 11% of patients undergoing open thoracotomy experience complications such as atelectasis, pulmonary contusion, pleural effusion, hemothorax, chylothorax, intercostal neuralgia, or significant postoperative pain from ribs resection and chest wall retraction (post-thoracotomy syndrome). 1
The application of minimally invasive techniques in treating intradural spinal tumors was first reported by Tredway et al 12 in 2006. Since then, several other reports have further demonstrated the safety and efficacy of minimally invasive surgery techniques when using tubular retractors in selected groups of patients with intra or extradural spinal neoplasms.5,13-15
Lately, minimally invasive spinal surgery has become increasingly popular for treatment of spinal pathology, principally for patients with degenerative pathology. These approaches have been associated with fewer operative blood loss, diminished narcotic use, shorter postoperative hospital stay, low rates of infections and lower costs of hospitalization comparative to open surgery.16,17 The hemilaminectomy not only preserves the motion and postoperative spinal stability but additionally reduces the stress and lowers the risk of postoperative disk degeneration. 18
Moreover, a biomechanical study performed by Ogden et al 19 suggested that minimally invasive approaches result in less spinal destabilization than open traditional approaches in patients with intradural pathology.
Successful minimally invasive resection of extradural and intradural spinal cord tumors has been previously described.1,7,12,20,21 However, there is a wide variability in terms of retraction systems and most of them include microscopic instead of endoscopic visualization.
In minimally invasive spinal tumor surgery, the most employed retraction systems have been the unexpansive tubular retractors. However, the use of unexpansive tubular retractors can be associated with a lack of completely exposure and, thus, the unnecessary retraction and manipulation of the tumor with higher risk of spinal cord lesion. 1 Instead, restricted exposure typically results in a piecemeal tumor resection, which is not recommended in myxopapillary ependymomas due the high risk of local or distant tumor recurrence. Although expandable tubular retractors have been employed, Caspar’s (B Braun-Aesculap AG) system is similar and could be a good option in undeveloped countries with limited resources. On the other hand, tubular retractors can reduce illumination offered by the microscope. This limitation could be reduced by using the expandable retractors or Caspar’s retractor (which are wider) and the endoscope (which improve the illumination and offered visualization angle). Konovalov et al 22 reported the effective use of Caspar’s retractor and the MAST Quadrant (Medtronic). In this context, the key is proper selection of patient in order to expose tumor’s limits.
The incision using our technique was considerably smaller than conventional incisions for spinal tumors. In spite of that, the minimally invasive concept does not include the size of the incision; moreover, the minimal soft tissue, muscles, ligaments manipulation, and a safe tumor dissection preserving neurovascular structures with a good visualization method rather than the incision size.
There have been some reports of the use of the endoscope for assistance in the removal of spinal tumors but limited to very scarce patients series or case reports.6,23-27 The advantages of endoscope visualization in terms of illumination and vision angle have been demonstrated in other surgical approaches. Additionally, some structures like facet joints can be preserved. Unfortunately, it requires a steep learning curve and surgeons should have experience in both spinal surgery and endoscopic visualization. Perhaps this is one of the reasons for the scarce articles related to endoscopic visualization in spinal tumors (Table 3). However, this article shows that endoscopic visualization can be a good method in the removal of extradural and also intradural-extramedullary spinal tumors from the craniospinal junction to the lumbar spine.
Table 3.
A Comparation Between the Largest Series (More Than 10 Patients) Using an Endoscopic Visualization and the Actual Study.
| Authors | Year | Sample | Type of study | Retraction system | Gross total resection rate |
|---|---|---|---|---|---|
| Parihar et al 6 | 2016 | 20 | Retrospective | Destandau (Karl Storz Inc) | 100% |
| Dhandapani et al 28 | 2018 | 16 | Retrospective | X tube/Quadrant (Medtronics Inc), Destandau (Karl Storz Inc) | 100% |
| Caballero-García et al (present study) | 2019 | 15 | Retrospective | Caspar system | 100% |
In 2015, Wong et al 1 published a comparative study of open and minimally invasive surgery for intradural-extramedullary spine tumors. They founded significant differences in operative blood loss, postoperative cerebrospinal fluid leak, lumbar drain placement and duration, change in ASIA (American Spinal Injury Association) score and length of hospital stay that favored minimally invasive surgery.
The mean blood loss in the minimally invasive group was 133.7 mL, similarly of our results. There are several reasons of the less blood loss founded in minimally invasive spinal surgery: the smaller incision; the paramedial muscle splitting; the tamponade effects offered by retractors; the improvement of visualization, which helps identify the source of bleeding; and the shortest surgical time, which decrease the total amount of blood loss. We agree that hospital stay could be already lower, but it is necessary take into account that we worked at a public tertiary center of an underdeveloped country, so we needed to ensure that there were not complications in patients with limited economic resources before been discharged.
Differences in gross total resection can be explained due the fact that subtotal resection was performed in patients with metastasis cancer with the objective of a palliative treatment. 1 Our series included two patients with spinal metastasis but limited to the epidural space.
We have founded that one disadvantage of minimally invasive approach is that the dural closure is more hazardous than in conventional approaches which offered a wider working angle. However, a complete dural closure was performed in our patients and none of them developed cerebrospinal fluid fistulae. It is necessary to take in count that the limited soft tissue dissection guaranty a good closure of surgical layers with less possibility to develop a cerebrospinal fistulae or a pseudomeningocele.
Another disadvantage includes the size of the lesion. It has been known that minimally invasive spinal surgery is convenient for intradural extramedullary lesions with less than 6.8 cm 28 and with no more than 2 levels of extension. 6 On the other hand, small mid-anterior thoracic spinal tumors would be technically difficult similar to in open surgical procedure. Open surgery constitutes the goal standard treatment, and minimally invasive surgery is only a valuable advantageous alternative in such selected patients.
The short average of bed rest days and hospital stay are explained by the decreased soft-tissue dissection and lower rate of complications. Moreover, the shortest surgical time and the minimally tissue trauma helps to reduce the recovery period. The hospital stay could be already lower in our series, but it is necessary to take into account that we worked in a public tertiary center of an underdeveloped country, so we needed to ensure that there were no complications in patients with limited economic resources before being discharged.
Other authors have explained these facts.1,6
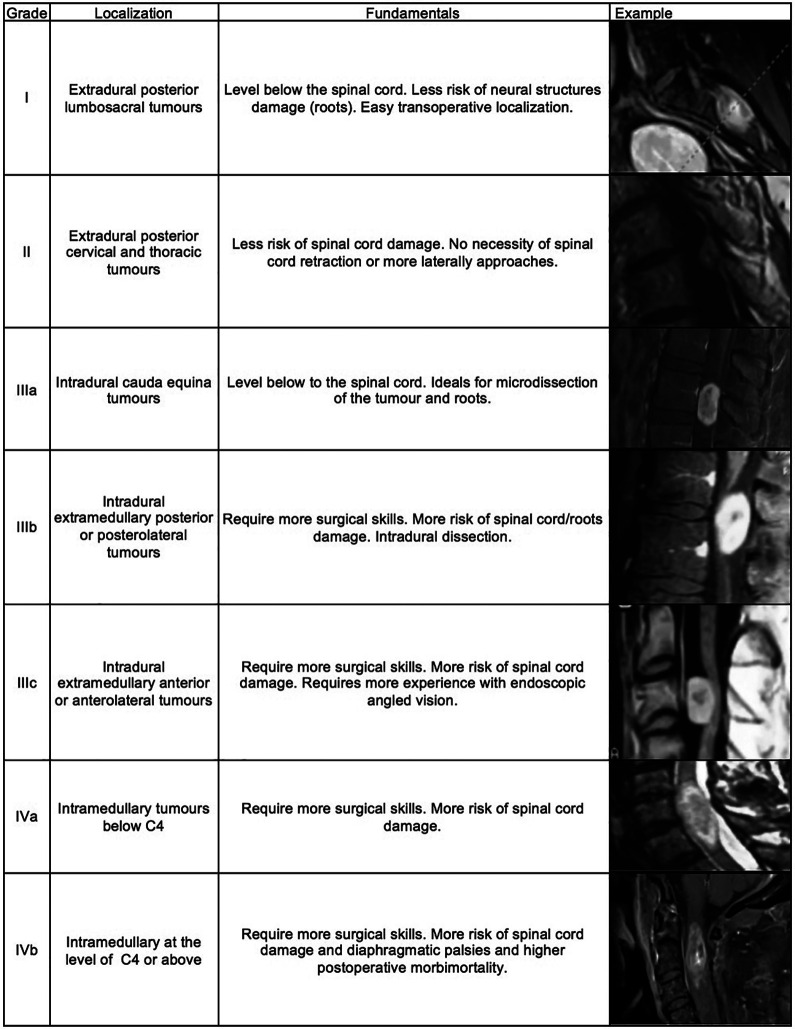
The paramedial approach has been described previously by some authors to minimize manipulation of the spinal cord and the potential risk of postoperative neurological deficit.29,30 We have suggested a classification of spinal tumor operated by minimally invasive surgery based on the fact that extradural tumors are easier to remove than intradural extramedullary tumors while intramedullary tumors are the most complex lesions; compression of spinal cord have less tolerance than compression of lumbosacral roots, and postoperative morbidity are higher in upper lesions than lowest lesions (Figure 3). For example, Mehta et al 31 reported a highest postoperative neurological deficit in patients with intradural-extramedullary tumors located in the upper thoracic spine probably secondary to the high spinal cord to canal space ratio and limited breakpoint blood supply zones. Wong et al 1 have supported these findings.
Figure 3.
Authors proposal grading classification of spinal tumors operated by minimally invasive endoscopic approach.
Thus, in theory, lumbosacral extradural tumors (grade I) are the best way to start minimally invasive spinal tumor resection followed by extradural tumors of other localizations (grade II). Intradural tumors are grade III. Selected intradural lumbar tumors surrounded by nerve roots of cauda equina are grade IIIa, posterior and posterolateral intradural extramedullary tumors are grade IIIb and anterior intradural extramedullary tumors are grade III c. Finally, intramedullary tumors (grade IV) are divided in lesions above the C4 level (Iva) and below (IVb), which are extremely complex to approach despite the surgical approach used.
It has described that most patients with intradural-extramedullary tumors present a good neurological function preoperatory. 1 However, in our series some patients (most of them with craniospinal or cervical tumors) had presented with bad neurological status and all of them have recovered after the surgery in a relative short period.
In spite of the promising results, this study has some limitations: This is a retrospective study with a short sample of patients. On the other hand, it has a heterogeneous sample which can allow some potential bias. Moreover, resection rate was estimated by postoperative radiological studies and this result should be cautiously interpreted by the fact that only a long follow-up period can demonstrate clearly if the surgical resection was total. Future prospective randomized studies comparing traditional and minimally invasive approaches, or microscopic and endoscopic minimally invasive approaches would be necessary to obtain a high level of evidence. Nevertheless, this study represents the largest reported series employing a full endoscopic visualization and the use of the Caspar’s retractor in spine tumors surgery. Further long-term prospective studies are needed.
Conclusions
Selective extradural or intradural-extramedullary tumors well localized and up to two levels can be safely and effectively treated by means minimally invasive surgery using a full endoscopic visualization and Caspar’s retractor. However, there is insufficient evidence to recommend this approach over the classical or other microsurgical approach described.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Joel Caballero-García, MD  https://orcid.org/0000-0002-0694-2408
https://orcid.org/0000-0002-0694-2408
Misael López-Sánchez, MD  https://orcid.org/0000-0001-5820-2340
https://orcid.org/0000-0001-5820-2340
References
- 1.Wong AP, Lall RR, Dahdaleh NS, et al. Comparison of open and minimally invasive surgery for intradural-extramedullary spine tumors. Neurosurg Focus. 2015;39:E11. [DOI] [PubMed] [Google Scholar]
- 2.Iacoangeli M, Gladi M, Di Rienzo A, et al. Minimally invasive surgery for benign intradural extramedullary spinal meningiomas: experience of a single institution in a cohort of elderly patients and review of the literature. Clin Interv Aging. 2012;7:557–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pompili A, Caroli F, Telera S, Occhipinti E. Minimally invasive resection of intradural-extramedullary spinal neoplasms. Neurosurgery. 2006;59:E1152. [DOI] [PubMed] [Google Scholar]
- 4.Smith ZA, Aoun SG, El Ahmadieh TY, et al. Minimally invasive resection of a high-thoracic intradural extramedullary tumor: an operative 3-D video. Neurosurgery. 2013;73(1 suppl operative):ons1. [DOI] [PubMed] [Google Scholar]
- 5.Gandhi RH, German JW. Minimally invasive approach for the treatment of intradural spinal pathology. Neurosurg Focus. 2013;35:E5. [DOI] [PubMed] [Google Scholar]
- 6.Parihar VS, Yadav N, Yadav YR, Ratre S, Bajaj J, Kher Y. Endoscopic management of spinal intradural extramedullary tumors. J Neurol Surg A Cent Eur Neurosurg. 2017;78:219–226. [DOI] [PubMed] [Google Scholar]
- 7.McCormick PC, Torres R, Post KD, Stein BM. Intramedullary ependymoma of the spinal cord. J Neurosurg.1990;72:523–532. [DOI] [PubMed] [Google Scholar]
- 8.Thavara BD, Kidangan GS, Rajagopalawarrier B. Analysis of the surgical technique and outcome of the thoracic and lumbar intradural spinal tumor excision using minimally invasive tubular retractor system. Asian J Neurosurg. 2019;14:453–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Levy WJ, Latchaw J, Hahn JF, Sawhny B, Bay J, Dohn DF. Spinal neurofibromas: a report of 66 cases and a comparison with meningiomas. Neurosurgery. 1986;18:331–334. [DOI] [PubMed] [Google Scholar]
- 10.Seppaülaü MT, Haltia MJ, Sankila RJ, Jaüaüskelaüinen JE, Heiskanen O. Long-term outcome after removal of spinal schwannoma: a clinicopathological study of 187 cases. J Neurosurg. 1995;83:621–626. [DOI] [PubMed] [Google Scholar]
- 11.Gu R, Liu JB, Xia P, Li C, Liu GY, Wang JC. Evaluation of hemilaminectomy use in microsurgical resection of intradural extra-medullary tumors. Oncol Lett. 2014;7:1669–1672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tredway TL, Santiago P, Hrubes MR, Song JK, Christie SD, Fessler RG. Minimally invasive resection of intradural-extramedullary spinal neoplasms. Neurosurgery. 2006;58(1 suppl):ONS52–ONS58. [DOI] [PubMed] [Google Scholar]
- 13.Haji FA, Cenic A, Crevier L, Murty N, Reddy K: Minimally invasive approach for the resection of spinal neoplasm. Spine (Phila Pa 1976). 2011;36:E1018–E1026. [DOI] [PubMed] [Google Scholar]
- 14.Lu DC, Chou D, Mummaneni PV: A comparison of miniopen and open approaches for resection of thoracolumbar in- tradural spinal tumors. J Neurosurg Spine. 2011;14:758–764. [DOI] [PubMed] [Google Scholar]
- 15.Mannion RJ, Nowitzke AM, Efendy J, Wood MJ. Safety and eficacy of intradural extramedullary spinal tumor removal using a minimally invasive approach. Neurosurgery. 2011;68:208–216. [DOI] [PubMed] [Google Scholar]
- 16.Khoo LT, Fessler RG. Microendoscopic decompressive laminotomy for the treatment of lumbar stenosis. Neurosurgery. 2002;51(5 suppl):S146–S154. [PubMed] [Google Scholar]
- 17.Wang MY, Cummock MD, Yu Y, Trivedi RA. An analysis of the differences in the acute hospitalization charges following minimally invasive versus open posterior lumbar interbody fusion. J Neurosurg Spine. 2010;12:694–699. [DOI] [PubMed] [Google Scholar]
- 18.Xie T, Qian J, Lu Y, Chen B, Jiang Y, Luo C. Biomechanical comparison of laminectomy, hemilaminectomy and a new minimally invasive approach in the surgical treatment of multilevel cervical intradural tumour: a finite element analysis. Eur Spine J. 2013;22:2719–2730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ogden AT, Bresnahan L, Smith JS, Natarajan R, Fessler RG. Biomechanical comparison of traditional and minimally invasive intradural tumor exposures using unite element analysis. Clin Biomech (Bristol, Avon). 2009;24:143–147. [DOI] [PubMed] [Google Scholar]
- 20.Nzokou A, Weil AG, Shedid D. Minimally invasive removal of thoracic and lumbar spinal tumors using a nonexpandable tubular retractor. J Neurosurg Spine. 2013;19:708–715. [DOI] [PubMed] [Google Scholar]
- 21.Smith ZA, Fessler RG. Nonexpandable tubular retractors and spinal tumors. J Neurosurg Spine. 2014;20:769–771. [DOI] [PubMed] [Google Scholar]
- 22.Konovalov NA, Shevelev IN, Nazarenko AG, et al. The use of minimally invasive approaches to resect intradural extramedullary spinal cord tumors [in Russian]. Zh Vopr Neirokhir Im N N Burdenko. 2014;78:24–36. [DOI] [PubMed] [Google Scholar]
- 23.Rosenthal D, Marquardt G, Lorenz R, Nichtweiss M. Anterior decompression and stabilization using a microsurgical endoscopic technique for metastatic tumors of the thoracic spine. J Neurosurg. 1996;84:565–572. [DOI] [PubMed] [Google Scholar]
- 24.Barami K, Dagnew E. Endoscope-assisted posterior approach for the resection of ventral intradural spinal cord tumors: report of two cases. Minim Invasive Neurosurg. 2007;50:370–373. [DOI] [PubMed] [Google Scholar]
- 25.Mobbs RJ, Maharaj MM, Phan K, Rao PJ. Unilateral hemilaminectomy for intradural lesions. Orthop Surg. 2015;7:244–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Burtscher J, Felber S, Twerdy K, Langmayr JJ. Endoscope-assisted interlaminar removal of an ependymoma of the cauda equine. Minim Invas Neurosurg. 2002;45:41–44. [DOI] [PubMed] [Google Scholar]
- 27.Chern JJ, Gordon AS, Naftel RP, Tubbs RS, Oakes WJ, Wellons JC, 3rd. Intradural spinal endoscopy in children. J Neurosurg Pediatr. 2011;8:107–111. [DOI] [PubMed] [Google Scholar]
- 28.Dhandapani S, Karthigeyan M. “Micro endoscopic” versus “pure endoscopic” surgery for spinal intradural mass lesions: a comparative study and review. Spine J. 2018;18:1592–1602. [DOI] [PubMed] [Google Scholar]
- 29.Acosta FL, Jr, Aryan HE, Chi J, Parsa AT, Ames CP. Modified paramedian transpedicular approach and spinal reconstruction for intradural tumors of the cervical and cervico- thoracic spine: clinical experience. Spine (Phila Pa 1976). 2007;32:203–210. [DOI] [PubMed] [Google Scholar]
- 30.Gambardella G, Gervasio O, Zaccone C.Approaches and surgical results in the treatment of ventral thoracic meningiomas. Review of our experience with a postero-lateral combined transpedicular-transarticular approach. Acta Neurochir (Wien). 2003;145:385–392. [DOI] [PubMed] [Google Scholar]
- 31.Mehta AI, Adogwa O, Karikari IO, et al. Anatomical location dictating major surgical complications for intradural extramedullary spinal tumors: a 10-year single-institutional experience. J Neurosurg Spine. 2013;19:701–707. [DOI] [PubMed] [Google Scholar]