Abstract
Study Design:
Controlled laboratory study.
Objective:
To investigate the impact of exposure to physiologically relevant caffeine concentrations on intervertebral disc (IVD) cell viability and extracellular matrix composition (ECM) in a whole organ culture model as potential contributing mechanisms in development and progression of IVD disorders in humans. Primary outcome measures were IVD viable cell density (VCD) and ECM composition.
Methods:
A total of 190 IVD whole organ explants from tails of 16 skeletally mature rats—consisting of cranial body half, endplate, IVD, endplate, and caudal body half—were harvested. IVD explants were randomly assigned to 1 of 2 groups: uninjured (n = 90) or injured (20G needle disc puncture/aspiration method, n = 100). Explants from each group were randomly assigned to 1 of 3 treatment groups: low caffeine (LCAF: 5 mg/L), moderate caffeine (MCAF: 10 mg/L), and high caffeine (HCAF: 15 mg/L) concentrations.
Results:
Cell viability was significantly higher in the low-caffeine group compared with the high-caffeine group at day 7 (P = .037) and in the low-caffeine group compared with the medium- and high-caffeine groups at day 21 (P ≤ .004). Analysis of ECM showed that all uninjured and control groups had significantly higher (P < .05) glycosaminoglycan concentrations compared with all injured groups. Furthermore, we observed a temporal, downward trend in proteoglycan to collagen ratio for the caffeine groups.
Conclusions:
Caffeine intake may be a risk factor for IVD degeneration, especially in conjunction with disc injury. Mechanisms for caffeine associated disc degeneration may involve cell and ECM, and further studies should elucidate mechanistic pathways and potential benefits for caffeine restriction.
Keywords: intervertebral disc, caffeine, nicotine, disc degeneration, degenerative disc, cell viability, whole organ culture
Introduction
Intervertebral disc (IVD) disorders are prevalent and often associated with pain and dysfunction.1,2 In the United States alone, direct and indirect medical costs of these conditions are estimated to be between $50 billion and $100 billion each year, highlighting the importance of pathophysiologic understanding and interventional development.3-7 Although the precise mechanisms driving disc degeneration have not been fully characterized, a number of suspected contributors have been described, including patient age, genetics, nutrition, metabolism, environmental factors, mechanical stress, injury, and comorbidities.6-9 Importantly, nutritional and environmental factors may contribute to the pathologic processes and represent modifiable risk factors, in contrast to other factors such as age, genetics, or injury.
Among the environmental factors associated with IVD disorders, nicotine use has long been considered a potential contributor in IVD degeneration and more recently, its relationship with back pain and disc disease have been more elegantly described and understood.2,10-23 Recently, our laboratory used a novel whole organ culture model to assess the effects of nicotine exposure, as well as its metabolite cotinine, on IVD health and cell viability, and found significantly decreased cell viability and glycosaminoglycan (GAG) content in IVDs exposed to nicotine or cotinine for as little as 7 days. Importantly, these detrimental changes were profoundly more rapid and severe in injured discs. 2
Although structurally different and with unique receptors and mechanisms of actions, caffeine and nicotine have a number of shared physiologic effects, pathways, and end-results.24-26 While caffeine has not been directly implicated as a risk factor for IVD degeneration, studies have reported an association between caffeine intake and back pain.27,28 Historically, moderate caffeine consumption has not been associated with proven long-term adverse health effects. However, consumption patterns and caffeine concentrations in beverages and supplements have continued to rise over the past 2 decades such that dependence syndromes have been well described.29-33 Importantly, while only 15.5% of US citizens are current smokers, up to 89% of US citizens consume at least one caffeinated beverage daily.27,34,35 Given the known detrimental effects of nicotine on IVDs and shared physiologic effects of caffeine and nicotine in conjunction with the growing prevalence and amount of caffeine consumption, it is germane to characterize the effects of caffeine on IVD health at the cell and tissue levels in order to understand the potential clinical ramifications with regard to IVD degeneration, pain, and disability. While controlled laboratory studies cannot fully mimic cell and matrix responses that occur in patients, the whole organ rat tail IVD model provides an ethical and valid method for providing initial data for subsequent translational studies. Therefore, the purpose of this study was to investigate the effects of caffeine on IVD cell viability and extracellular matrix (ECM) composition using our validated whole organ IVD culture model. 7 The study was designed to test the hypothesis that caffeine exposure will be associated with significant dose-dependent loss of IVD cell viability and ECM composition, which will be further exacerbated by injury, when compared with controls.
Methods
Tissue Harvest and Culture
Experimental design methods are based on previous work.2,7 With institutional animal care and use committee (IACUC) approval, tails were harvested from skeletally mature Sprague-Dawley rats (n = 16) euthanatized for reasons unrelated to this study. Soft tissues were aseptically dissected from the caudal vertebrae, and whole organ explants consisting of cranial vertebral body half, cartilaginous endplate, IVD, cartilaginous endplate, and the caudal vertebral body half were harvested. Whole organ explants were then randomly assigned to either uninjured (n = 90) or injured (n = 100) groups. Uninjured discs (n = 22) were assessed immediately (time 0) as controls. For the injured group, the posterolateral annulus fibrosus (AF) of each explant was penetrated with a 20G needle to the approximated center of the nucleus pulposus (NP) based on needle penetration depth and tactile feedback. Aspiration of a portion of the NP was then performed using a 1-mL syringe with the plunger pulled back to 0.5-mL. Uninjured discs received no insult. Explants from each group were further randomly assigned to 1 of 3 treatment groups (n = 24/treatment uninjured and n = 32/treatment injured): low caffeine (LCAF) (5 mg/L), moderate caffeine (MCAF) (10 mg/L), and high caffeine (HCAF) (15 mg/L). Caffeine concentrations were determined based on serum levels reported for the average US consumer with normal serum concentrations of coffee drinkers being 2 to 10 mg/L and abuse defined as levels >15 mg/L. 36 IVDs were cultured in a rotating wall vessel (RWV) bioreactor (Synthecon, Inc) at 50 rpm in Dulbeccos’s modified Eagle’s medium supplemented with sodium pyruvate, l-glutamine, ascorbic acid, Minimum Essential Medium non-essential (MEM N-E) amino acid solution, insulin-transferrin-selenium acid (ITS), and penicillin-streptomyocin. Media were refreshed every 7 days and IVDs were harvested on day 7 (n = 6 uninjured and 8 injured from each of the 3 groups [low, medium, and high caffeine]), day 14 (again, n = 6 uninjured and 8 injured from each of the 3 groups), and day 21 (n = 12 uninjured and 16 injured from each of the 3 groups) and compared against the 22 control discs harvested at day 0. This accounts for 18 uninjured and 24 injured discs harvested at day 7 and again at day 14, and for 36 uninjured and 48 injured discs harvested at day 21, compared against 22 time-0 control discs, totaling 190 discs.
Assessments for Cell Viability and Extracellular Matrix Composition
A microscopic fluorescent cell viability stain assay (Invitrogen) was used to determine the viability of the IVD days 0, 7, 14, and 21 of culture. 37 For this assay, each whole organ explant was bisected through its center to expose the AF and NP in cross-section. The explant halves were placed in solution containing the live-cell stain calcein AM and the dead-cell stain ethidium homodimer and incubated at 37 °C for 30 minutes. After incubation, explant halves were washed in phosphate buffered saline (PBS) for 5 minutes at 25 °C, and then images of both halves of each IVD AF and NP were captured at 4× using a fluorescent microscope (Olympus BX51) with attached digital camera (F-View II 12 Bit B&W CCD). Using Microsuite software (Olympus), separate images for green-staining live cells and red-staining dead cells were obtained. The viable cell density (VCD) of the AF for each IVD was determined by counting the number of green-staining viable cells in AF using an in-house cell counting program. Minimum threshold level of pixel intensity was set to ensure background staining was eliminated. Difference in pixel intensity was used to delineate cell boundaries to verify accurate cell counting. The computer program counted total number of green-staining cells for each image. The VCD of the NP was not determined because the cells of the NP are too clumped to identify discrete cells and there was variability in NP content between injured and uninjured samples. The area of the respective tissue section counted was measured using MicroSuite (Olympus), and the VCD was determined by dividing the number of green-staining viable cells by the area of the tissue (number of viable cells/area of tissue in mm2). After cell viability assessments were completed, tissue sections were processed for biochemical evaluations of ECM composition.
Assessments for Extracellular Matrix Composition
AF and NP tissues were dissected from IVD bone with a scalpel blade and weighed to determine wet weight. The tissues were then digested in papain buffer (300 μg/mL dithioreitol; 300 μg/mL papain, 20 mM sodium phosphate pH 6.8; 1 mM ethylenediaminetetraacetic acid [EDTA]) overnight at 65 °C. Digested tissues were tested for total IVD proteoglycan (PG) content using the dimethylmethylene blue (DMMB) assay and total IVD collagen content was determined using α-chymotrypsin and hydroxyprolene (HP) assays, as previously described.38,39 Briefly, to determine proteoglycan content, a portion of the tissue digest was added the DMMB reagent, and then level of absorbance was measured at 525 nm and compared with a chondroitin sulfate standard curve to determine concentrations of sulfated proteoglycans in the digest. For determination of collagen content, a portion of the tissue digest was mixed with 4 N NaOH and the samples were hydrolyzed by autoclaving at 120 °C for 20 minutes. After autoclaving, samples were mixed with chloramine-T and incubated for 25 minutes at room temperature. Next, Ehrlich’s reagent was added to the samples and incubated for 30 minutes at 65 °C for color development. After incubation, absorbance level was determined at 550 nm and compared with a hydroxyproline standard to determine concentrations of collagen. In order to assess relative effects on ECM composition, the PG-to-HP ratio was calculated by dividing the PG content measured in the tissue to the HP content measured in the tissue.
Statistical Analysis
Statistical analysis was performed using SigmaPlot (Systat Software, Inc) using 1-way analysis of variance with the Bonferroni method to test for statistically significant differences among groups with significance set at P < .05.
Results
Cell Viability
Day 0 control IVDs had significantly (P < .05) higher VCD when compared with most injured and uninjured MCAF and HCAF groups at days 14 and 21 (Figures 1 and 2). The injured LCAF group had significantly (P < .05) higher VCD at day 7 than at days 14 and 21. Injured MCAF VCD at day 7 was also significantly (P < .05) higher than at days 14 and 21. For the HCAF groups, VCD was significantly (P < .05) higher in uninjured IVDs than in injured IVDs at days 7 and 14 (Figure 2). Taken together, this data indicates that AF puncture with NP aspiration injury had detrimental effects on IVD cell viability through 21 days in explant culture. In addition, significant caffeine dose-dependent loss of IVD viable cell density was noted, which magnified and expedited the detrimental effects of injury when compared with healthy (time 0) controls.
Figure 1.
Intervertebral disc (IVD) explant viability. Representative 4× cell viability photomicrographs for injured and uninjured IVD explants on days 0, 7, 14, and 21 of culture for the low-caffeine group (LCAF), medium-caffeine group (MCAF), and high-caffeine group (H-CAF). Green staining indicates viable cells in the tissue (scale bar = 1 mm).
Figure 2.
Mean viable cell density (VCD) for all groups at all time points. (*) Significantly (P < .05) lower than day 0 control; (†) significantly (P < .05) lower than corresponding uninjured group; (‡) significantly (P < .05) lower than corresponding day 7 group.
Biochemical Extracellular Matrix Analyses
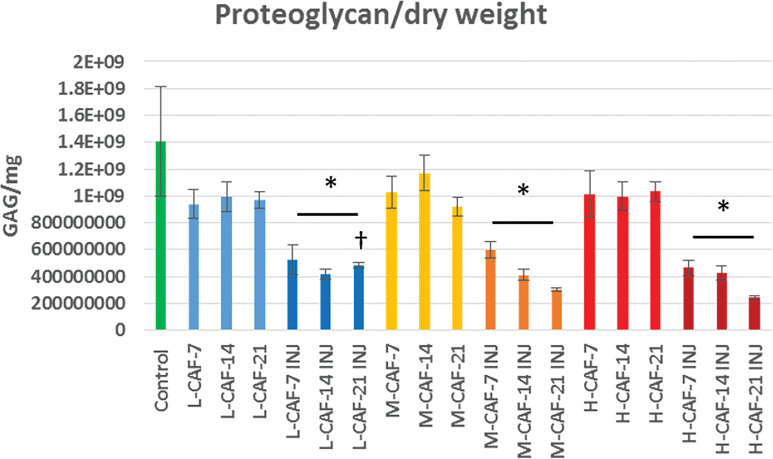
Proteoglycan concentrations were significantly (P < .05) higher in all control and uninjured groups compared to all concentration-matched injured groups (Figure 3). At day 21, the injured LCAF group demonstrated significantly (P < .05) greater PG content than injured MCAF and HCAF groups. Also at day 21, all injured groups had significantly (P < .05) lower PG-to-HP ratios compared with the control group (Figure 4). All injured groups, with the exception of HCAF at day 14, had significantly (P < .05) lower PG-to-HP ratios compared with all corresponding uninjured groups. Taken together, this data indicates that AF puncture with NP aspiration injury had detrimental effects on IVD proteoglycan content through 21 days in explant culture. In addition, significant caffeine dose-dependent loss of IVD proteoglycan was noted, which magnified and expedited the detrimental effects of injury when compared with healthy (time 0) controls. Based on PG-to-HP ratio data, IVD collagen was relatively spared from the effects of injury and caffeine.
Figure 3.
Mean proteoglycan content standardized to the dry weight of the tissue after days 7, 14, and 21. (*) Significantly (P < .05) less than the uninjured counterparts and the controls; (†) significantly (P < .05) greater than other injured day 21 groups.
Figure 4.
Mean ratio of tissue proteoglycan to hydroxyprolene (HP) content for the day 0 control and at days 14 and 21 of culture. (*) Significantly (P < .05) lower than day 0 control; (†) significantly (P < .05) lower than corresponding uninjured groups.
Discussion
To the authors’ knowledge, this is the first study to characterize the effects of caffeine on injured and uninjured IVDs using a whole organ explant model. The results of the present study suggest that caffeine exposure was associated with significant dose-dependent loss of IVD cell viability and ECM composition, which magnified and expedited the detrimental effects of injury, compared to controls. There was a negative, progressive effect on IVD health in culture with increasing concentrations of caffeine. Moderate and high caffeine concentrations were associated with detrimental effects on uninjured IVDs, suggesting that more excessive caffeine consumption may negatively affect even healthy IVDs. Higher levels of caffeine were associated with more rapid and more severe effects, and this dose-dependent relationship was magnified in injured IVDs.
The model used in the present study has been validated to represent changes observed in symptomatic IVD degeneration in humans, specifically with regard to cell viability, biochemical, and biomechanical changes. 7 Cadaveric studies have confirmed inflammatory, apoptotic, and PG degradation mechanisms of disease in patients with IVD degeneration.40-46 Loss of cell viability is a hallmark and other established criteria of IVD degeneration include (1) increased type I collagen production within the NP; (2) decreased PG, specifically aggrecan, production; (3) upregulation of matrix-degrading enzymes; (4) elevated levels of inflammatory cytokines; and (5) presence of neural growth promoters.46-48 The most significant biochemical transformation during disc degeneration is ECM loss through decreased production of proteoglycans and type II collagen within the NP. 46 Loss of normal ECM composition results in direct and immediate negative effects on the disc’s material properties, and the resultant mechanical overloading further drives loss of cell viability and ECM homeostasis.46,49-52 This cycle leads to the biomechanical changes that present as clinical manifestations of stiffness, pain, and dysfunction due to IVD protrusion, extrusion, and/or failure.46,53-55
The causes of disc degeneration appear to be multifactorial and include genetic, mechanical, and environmental contributions.46,47,56,57 Cigarette smoking, more specifically nicotine, is a modifiable environmental factor that is strongly associated with back pain, failed spinal fusions, and revision spine surgery.10-12,15-23 While this relationship is well established, the underlying mechanisms are incompletely understood, are multifactorial, and are likely accompanied by other contributors (eg, toxins, free radicals, carbon monoxide). 58 Nicotine appears to have indirect effects on IVD health through blood flow and nutrient restriction, while recent data suggest that nicotine and its breakdown product, cotinine, may have direct effects on the health and homeostasis of IVD cells and ECM.5,11,16,59-61 Data from our laboratory using the model described in the present study provided evidence in support of these direct effects by demonstrating dose-dependent negative effects on IVD cell viability and ECM composition (decreased GAG content and altered PG: HP ratios) with more rapid and severe changes in injured discs, and this mirrors the findings associated with caffeine exposure in the present study. 2
Caffeine is widely consumed throughout the world with reports of average US consumption rates between 85% and 89% of the population and average daily intake between 165 and 305 mg.27,35,62 Serum caffeine concentrations for the average US coffee drinker range between 2 and 10 mg/L with abuse of caffeine defined as levels >15 mg/L. 36 The relationship of caffeine with IVD health is not well established and while some studies report correlations between caffeine intake and back pain, others do not.27,28,63 Even in those studies that support a correlation, controversy exists with regard to the cause-and-effect nature of the relationship. 27 This controversy centers on the fact that caffeine serves to potentiate the effects of analgesics such that patients consuming higher levels of caffeine may be consciously or subconsciously self-medicating, which may confound the results of these studies.27,64 This potential confounder highlights the need for controlled laboratory studies, such as the present study, in order to delineate and differentiate direct and indirect effects, relationships, and mechanisms for caffeine in IVD health and disease. Interestingly, caffeine shares several physiologic effects with nicotine despite acting through different receptors and pathways. Nicotine primarily exerts its effects by binding to nicotinic receptors where it acts as an agonist, potentiating the effects of acetylcholine. Caffeine acts by antagonizing adenosine receptors, preventing adenosine-driven neuronal suppression and drowsiness. 25 Additionally, suppression of adenosine receptor activation is stimulatory at medullary centers of respiration, vagal, and vasomotor control, yielding vasoconstriction, increased heart rate, and respiration. 26 Caffeine also stimulates acetylcholine release and activates ryanodine receptors that mediate calcium release from the sarcoplasmic reticulum and endoplasmic reticulum of cells, potentiating muscle contraction. 24 While neither of these mechanisms of action explain caffeine’s seemingly direct, toxic effects on IVD health noted in the present study, they do provide potential pathways to investigate, which are the focus of ongoing studies in our laboratory.
Although the factors involved in the mechanisms of caffeine’s effects on IVD cell and ECM are not yet elucidated, it is clear from the present study that caffeine has the potential to contribute to IVD degeneration beyond a simple association with back pain. Based on previous studies, potential mechanistic factors for caffeine-related effects on cells and ECM production include mineralization and alkaline phosphatase activity, as well as decreased cartilage-specific matrix gene expression via the adenosine type 1 receptor pathway. 65 In addition, caffeine has been associated with increased apoptosis, chondrocyte phenotype alterations, and ECM perturbations in offspring exposed in utero.66-69 As such, these factors and pathways deserve further attention for elucidating caffeine-related disease mechanisms for IVD degeneration.
There are limitations to the present study that must be considered when interpreting and applying the results. The experimental design was based on the use of rodent tissues in culture for 21 days. While this design does not allow for direct translation to the clinical scenario, this model has demonstrated reproducible biomechanical and biologic changes reflective of the pathophysiologic processes known to occur in symptomatic IVD degeneration while allowing for control of important variables.7,70,71 Similarly, media concentrations of caffeine were based on reported serum levels in humans, which may not accurately reflect the concentrations to which IVDs are actually exposed. Therefore, it is possible that even the lowest caffeine concentration used in culture may be supraphysiologic, requiring further investigation using an in vivo model. The model also included only time 0 controls in order to maximize numbers for treatment groups. While it would have been ideal to have comprehensive time-matched controls, previous studies have demonstrated maintenance of cell viability over the duration of culture used in the present study, and time 0 controls represent the most accurate and stringent comparison to healthy cells and matrix. 2 In addition, media pH was not assessed. While caffeine is a base that could raise media pH, media color changes indicative of pH changes were not noted in any group at any time point.
The methods for inciting injury and subsequent loading must be considered experimental conditions. However, changes reflective of those observed in degenerative IVDs (ie, cell death, loss of ECM integrity and architecture, decreased disc height, water content, PG content, and increased stiffness) have been successfully reproduced using needle puncture to simulate disc injury.2,65-69,72 RWV bioreactors recreate the physiologic microgravity environment inducing sheer stresses on cultured tissues, generating laminar fluid-flow that reduces diffusional limitations, producing more efficient nutrient-waste exchange.7,73-76 Furthermore, this flow serves as a cell signaling and communication pathway.7,73,74 Using this model, we previously reported successful culture of whole-organ rat-tail IVDs such that they retained cell viability, tissue composition, and architecture, as well as material properties in both the AF and NP using a RWV bioreactor. 7 It should be reiterated that the injury used in the present study is associated with decreased cell viability and ECM changes. 72 Thus, the study was designed to compare the effects of caffeine on injured and noninjured discs over time. While the experimental design and its limitations require further study toward translational applicability, previous data in conjunction with the results of the present study suggest that the detrimental effects on IVD cells and ECM associated with caffeine exposure are valid and relevant.
Conclusions
In this whole organ culture model, caffeine exposure was associated with marked loss of IVD cell viability and altered ECM composition in a dose-dependent manner, which was magnified by needle puncture and aspiration injury. These negative effects seen in conjunction with caffeine exposure are similar to those reported for symptomatic IVD degeneration in patients. As such, caffeine intake may be a risk factor for IVD disorders, especially if the disc is already injured or degenerative with potentially detrimental effects on both cells and ECM. Further studies are warranted to confirm these results in vivo, elucidate mechanisms of disease, and investigate potential benefits of education regarding caffeine restriction or avoidance for prevention and treatment of patients with or at risk for IVD injury and degeneration.
Footnotes
Authors’ Note: This research was presented as a poster at the 2017 Orthopaedic Research Society’s Annual Meeting, March 19-22, in San Diego, CA.
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Dr Stoker reports personal fees from Arthrex, Inc, personal fees from Musculoskeletal Transplant Foundation, outside the submitted work. Dr Choma reports other from AO Spine North America, personal fees from Gentis, Inc, other from North American Spine Society, other from Scoliosis Research Society, outside the submitted work. Dr Cook reports grants and personal fees from Arthrex, Inc, personal fees from AthleteIQ, grants from ConforMIS, personal fees from CONMED Linvatec, grants from Coulter Foundation, grants from DePuy Synthes, grants and personal fees from Eli Lilly, other from Journal of Knee Surgery, grants from Merial, other from Midwest Transplant Network, grants, personal fees, and other from Musculoskeletal Transplant Foundation, grants from National Institutes of Health (NIAMS and NICHD), grants from Purina, grants from Sites Medical, personal fees and other from Thieme, grants from TissueGen Inc, personal fees from Trupanion, grants from the US Department of Defense, grants from Zimmer-Biomet, outside the submitted work.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Aaron M. Stoker, PhD  https://orcid.org/0000-0001-6403-3969
https://orcid.org/0000-0001-6403-3969
James L. Cook, DVM, PhD  https://orcid.org/0000-0002-0862-995X
https://orcid.org/0000-0002-0862-995X
References
- 1.Samartzis D, Karppinen J, Mok F, Fong DY, Luk KD, Cheung KM. A population-based study of juvenile disc degeneration and its association with overweight and obesity, low back pain, and diminished functional status. J Bone Joint Surg Am. 2011;93:662–670. doi:10.2106/JBJS.I.01568 [DOI] [PubMed] [Google Scholar]
- 2.Stannard JT, Stoker A, Choma TJ, Reinsel TE, Cook JL. A whole organ culture model for intervertebral disc in the presence of nicotine and cotinine using rat tail explants in a rotating bioreactor. Spine J. 2013;13(9 suppl):S29–S30. doi:10.1016/j.spinee.2013.07.102 [Google Scholar]
- 3.Gawri R, Mwale F, Ouellet J, et al. Development of an organ culture system for long-term survival of the intact human intervertebral disc. Spine (Phila Pa 1976). 2011;36:1835–1842. doi:10.1097/BRS.0b013e3181f81314 [DOI] [PubMed] [Google Scholar]
- 4.Frymoyer JW. Predicting disability from low back pain. Clin Orthop Relat Res. 1992;(279):101–109. http://www.ncbi.nlm.nih.gov/pubmed/1534720. [PubMed]
- 5.Maniadakis N, Gray A. The economic burden of back pain in the UK. Pain. 2000;84:95–103. [DOI] [PubMed] [Google Scholar]
- 6.Urban JP, Roberts S. Degeneration of the intervertebral disc. Arthritis Res Ther. 2003;5:120–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stannard JT, Edamura K, Stoker AM, et al. Development of a whole organ culture model for intervertebral disc disease. J Orthop Translat. 2016;5:1–8. doi:10.1016/j.jot.2015.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gantenbein B, Grünhagen T, Lee CR, van Donkelaar CC, Alini M, Ito K. An in vitro organ culturing system for intervertebral disc explants with vertebral endplates: a feasibility study with ovine caudal discs. Spine (Phila Pa 1976). 2006;31:2665–2673. doi:10.1097/01.brs.0000244620.15386.df [DOI] [PubMed] [Google Scholar]
- 9.Iatridis JC, Michalek AJ, Purmessur D, Korecki CL. Localized intervertebral disc injury leads to organ level changes in structure, cellularity, and biosynthesis. Cell Mol Bioeng. 2009;2:437–447. doi:10.1007/s12195-009-0072-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Battié MC, Videman T, Gill K, et al. 1991 Volvo Award in clinical sciences. Smoking and lumbar intervertebral disc degeneration: an MRI study of identical twins. Spine (Phila Pa 1976.) 1991;16:1015–1021. [PubMed] [Google Scholar]
- 11.Eriksen W, Natvig B, Bruusgaard D. Smoking, heavy physical work and low back pain: a four-year prospective study. Occup Med (Lond). 1999;49:155–160. doi:10.1093/occmed/49.3.155 [DOI] [PubMed] [Google Scholar]
- 12.Zvolensky MJ, McMillan K, Gonzalez A, Asmundson GJG. Chronic pain and cigarette smoking and nicotine dependence among a representative sample of adults. Nicotine Tob Res. 2009;11:1407–1414. doi:10.1093/ntr/ntp153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Weingarten TN, Moeschler SM, Ptaszynski AE, Hooten WM, Beebe TJ, Warner DO. An assessment of the association between smoking status, pain intensity, and functional interference in patients with chronic pain. Pain Physician. 2008;11:643–653. [PubMed] [Google Scholar]
- 14.Goldberg MS, Scott SC, Mayo NE. A review of the association between cigarette smoking and the development of nonspecific back pain and related outcomes. Spine (Phila Pa 1976). 2000;25:995–1014. doi:10.1097/00007632-200004150-00016 [DOI] [PubMed] [Google Scholar]
- 15.Stienen MN, Joswig H, Smoll NR, et al. Short- and long-term effects of smoking on pain and health-related quality of life after non-instrumented lumbar spine surgery. Clin Neurol Neurosurg. 2016;142:87–92. doi:10.1016/j.clineuro.2016.01.024 [DOI] [PubMed] [Google Scholar]
- 16.Alkherayf F, Agbi C. Cigarette smoking and chronic low back pain in the adult population. Clin Invest Med. 2009;32:E360–E367. [DOI] [PubMed] [Google Scholar]
- 17.Alkherayf F, Wai EK, Tsai EC, Agbi C. Daily smoking and lower back pain in adult Canadians: the Canadian Community Health Survey. J Pain Res. 2010;3:155–160. doi:10.2147/JPR.S11031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Boshuizen HC, Verbeek JH, Broersen JP, Weel AN. Do smokers get more back pain? Spine (Phila Pa 1976). 1993;18:35–40. doi:10.1097/00007632-199301000-00007 [DOI] [PubMed] [Google Scholar]
- 19.Wang D, Nasto LA, Roughley P, et al. Spine degeneration in a murine model of chronic human tobacco smokers. Osteoarthritis Cartilage. 2012;20:896–905. doi:10.1016/j.joca.2012.04.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Palmer KT, Syddall H, Cooper C, Coggon D. Smoking and musculoskeletal disorders: findings from a British national survey. Ann Rheum Dis. 2003;62:33–36. doi:10.1136/ard.62.1.33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ditre JW, Kosiba JD, Zale EL, Zvolensky MJ, Maisto SA. Chronic pain status, nicotine withdrawal, and expectancies for smoking cessation among lighter smokers. Ann Behav Med. 2016;50:427–435. doi:10.1007/s12160-016-9769-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Glassman SD, Anagnost SC, Parker A, Burke D, Johnson JR, Dimar JR. The effect of cigarette smoking and smoking cessation on spinal fusion. Spine (Phila Pa 1976). 2000;25:2608–2615. doi:10.1097/00007632-200010150-00011 [DOI] [PubMed] [Google Scholar]
- 23.Andersen T, Christensen FB, Laursen M, Høy K, Hansen ES, Bünger C. Smoking as a predictor of negative outcome in lumbar spinal fusion. Spine (Phila Pa 1976). 2001;26:2623–2628. doi:10.1097/00007632-200112010-00018 [DOI] [PubMed] [Google Scholar]
- 24.Santulli G, Marks AR. Essential roles of intracellular calcium release channels in muscle, brain, metabolism, and aging. Curr Mol Pharmacol. 2015;8:206–222. [DOI] [PubMed] [Google Scholar]
- 25.Froestl W, Muhs A, Pfeifer A. Cognitive enhancers (nootropics). Part 1: drugs interacting with receptors. J Alzheimers Dis. 2012;32:793–887. doi:10.3233/JAD-2012-121186 [DOI] [PubMed] [Google Scholar]
- 26.National Cancer Institute (NCI). Thesaurus (search terms “caffeine” and “nicotine”). Accessed March 30, 2020. https://ncit.nci.nih.gov/ncitbrowser/ConceptReport.jsp?dictionary=NCIThesaurus&code=C328
- 27.Bryan CJ, Wolfe AL, Morrow CE, Stephenson JA, Haskell J, Bryan AO. Associations among back and extremity pain with alcohol, tobacco, and caffeine use among US Air Force pararescuemen. J Spec Oper Med. 2015;15:66–71. [DOI] [PubMed] [Google Scholar]
- 28.McPartland JM, Mitchell JA. Caffeine and chronic back pain. Arch Phys Med Rehabil. 1997;78:61–63 [DOI] [PubMed] [Google Scholar]
- 29.Phillips JG, Currie J, Ogeil RP. Consumption and foraging behaviors for common stimulants (nicotine, caffeine). J Addict Dis. 2016;35:15–21. doi:10.1080/10550887.2015.1094721 [DOI] [PubMed] [Google Scholar]
- 30.Freedman ND, Park Y, Abnet CC, Hollenbeck AR, Sinha R. Association of coffee drinking with total and cause-specific mortality. N Engl J Med. 2012;366:1891–1904. doi:10.1056/NEJMoa1112010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Reissig CJ, Strain EC, Griffiths RR. Caffeinated energy drinks—a growing problem. Drug Alcohol Depend. 2009;99:1–10. doi:10.1016/j.drugalcdep.2008.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Juliano LM, Evatt DP, Richards BD, Griffiths RR. Characterization of individuals seeking treatment for caffeine dependence. Psychol Addict Behav. 2012;26:948–954. doi:10.1037/a0027246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Meredith SE, Juliano LM, Hughes JR, Griffiths RR. Caffeine use disorder: a comprehensive review and research agenda. J Caffeine Res. 2013;3:114–130. doi:10.1089/jcr.2013.0016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Centers for Disease Control and Prevention. Smoking is down, but almost 38 million American adults still smoke. Accessed March 30, 2020. https://www.cdc.gov/media/releases/2018/p0118-smoking-rates-declining.html
- 35.Mitchell DC, Knight CA, Hockenberry J, Teplansky R, Hartman TJ. Beverage caffeine intakes in the US. Food Chem Toxicol. 2014;63:136–142. doi:10.1016/j.fct.2013.10.042 [DOI] [PubMed] [Google Scholar]
- 36.Baselt RC. Disposition of Toxic Drugs and Chemicals in Man. Biomedical; 2014:236–239. [Google Scholar]
- 37.Kuroki K, Stoker AM, Stannard JP, et al. Biologic joint repair strategies: the Mizzou BioJoint story. Toxicol Pathol. 2017;45:931–938. doi:10.1177/0192623317735786 [DOI] [PubMed] [Google Scholar]
- 38.Farndale RW, Buttle DJ, Barrett AJ. Improved quantitation and discrimination of sulphated glycosaminoglycans by use of dimethylmethylene blue. Biochim Biophys Acta. 1986;883:173–177. doi:10.1016/0304-4165(86)90306-5 [DOI] [PubMed] [Google Scholar]
- 39.Reddy GK, Enwemeka CS. A simplified method for the analysis of hydroxyproline in biological tissues. Clin Biochem. 1996;29:225–229. [DOI] [PubMed] [Google Scholar]
- 40.Fields AJ, Sahli F, Rodriguez AG, Lotz JC. Seeing double: a comparison of microstructure, biomechanical function, and adjacent disc health between double- and single-layer vertebral endplates. Spine (Phila Pa 1976). 2012;37:E1310–E1317. doi:10.1097/BRS.0b013e318267bcfc [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Adams MA, Freeman BJ, Morrison HP, Nelson IW, Dolan P. Mechanical initiation of intervertebral disc degeneration. Spine (Phila Pa 1976). 2000;25:1625–1636. doi:10.1097/00007632-200007010-00005 [DOI] [PubMed] [Google Scholar]
- 42.Choi YS, Cohen NA, Potter HG, Mintz DN. Magnetic resonance imaging in the evaluation of osteochondritis dissecans of the patella. Skeletal Radiol. 2007;36:929–935. doi:10.1007/s00256-007-0357-8 [DOI] [PubMed] [Google Scholar]
- 43.Tschoeke SK, Hellmuth M, Hostmann A, et al. Apoptosis of human intervertebral discs after trauma compares to degenerated discs involving both receptor-mediated and mitochondrial-dependent pathways. J Orthop Res. 2008;26:999–1006. doi:10.1002/jor.20601 [DOI] [PubMed] [Google Scholar]
- 44.Weiler C, Nerlich AG, Schaaf R, Bachmeier BE, Wuertz K, Boos N. Immunohistochemical identification of notochordal markers in cells in the aging human lumbar intervertebral disc. Eur Spine J. 2010;19:1761–1770. doi:10.1007/s00586-010-1392-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nerlich AG, Schaaf R, Wälchli B, Boos N. Temporo-spatial distribution of blood vessels in human lumbar intervertebral discs. Eur Spine J. 2007;16:547–555. doi:10.1007/s00586-006-0213-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Amelot A, Mazel C. The intervertebral disc: physiology and pathology of a brittle joint. World Neurosurg. 2018;120:265–273. doi:10.1016/j.wneu.2018.09.032 [DOI] [PubMed] [Google Scholar]
- 47.Kepler CK, Ponnappan RK, Tannoury CA, Risbud MV, Anderson DG. The molecular basis of intervertebral disc degeneration. Spine J. 2013;13:318–330. doi:10.1016/j.spinee.2012.12.003 [DOI] [PubMed] [Google Scholar]
- 48.Gopal D, Ho AL, Shah A, Chi JH. Molecular basis of intervertebral disc degeneration. Adv Exp Med Biol. 2012;760:114–133. doi:10.1007/978-1-4614-4090-1_8 [DOI] [PubMed] [Google Scholar]
- 49.Li P, Gan Y, Wang H, et al. Dynamic compression effects on immature nucleus pulposus: a study using a novel intelligent and mechanically active bioreactor. Int J Med Sci. 2016;13:225–234. doi:10.7150/ijms.13747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Li P, Gan Y, Xu Y, et al. Matrix homeostasis within the immature annulus fibrosus depends on the frequency of dynamic compression: a study based on the self-developed mechanically active bioreactor. Biomech Model Mechanobiol. 2017;16:385–394. doi:10.1007/s10237-016-0823-0 [DOI] [PubMed] [Google Scholar]
- 51.Yan Z, Pan Y, Wang S, et al. Static compression induces ECM remodeling and integrin α2β1 expression and signaling in a rat tail caudal intervertebral disc degeneration model. Spine (Phila Pa 1976). 2017;42:E448–E458. doi:10.1097/brs.0000000000001856 [DOI] [PubMed] [Google Scholar]
- 52.Chan SC, Ferguson SJ, Gantenbein-Ritter B. The effects of dynamic loading on the intervertebral disc. Eur Spine J. 2011;20:1796–1812. doi:10.1007/s00586-011-1827-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Raj PP. Intervertebral disc: Anatomy-physiology-pathophysiology-treatment. Pain Pract. 2008;8:18–44. doi:10.1111/j.1533-2500.2007.00171.x [DOI] [PubMed] [Google Scholar]
- 54.Masuda K. Biological repair of the degenerated intervertebral disc by the injection of growth factors. Eur Spine J. 2008;17(suppl 4):441–451. doi:10.1007/s00586-008-0749-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.García-Cosamalón J, del Valle ME, Calavia MG, et al. Intervertebral disc, sensory nerves and neurotrophins: who is who in discogenic pain? J Anat. 2010;217:1–15. doi:10.1111/j.1469-7580.2010.01227.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Adams DS, Keller R, Koehl MA. The mechanics of notochord elongation, straightening and stiffening in the embryo of Xenopus laevis. Development. 1990;110:115–130. [DOI] [PubMed] [Google Scholar]
- 57.Mayer JE, Iatridis JC, Chan D, Qureshi SA, Gottesman O, Hecht AC. Genetic polymorphisms associated with intervertebral disc degeneration. Spine J. 2013;13:299–317. doi:10.1016/J.SPINEE.2013.01.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Church DF, Pryor WA. Free-radical chemistry of cigarette smoke and its toxicological implications. Environ Health Perspect. 1985;64:111–126. doi:10.1289/ehp.8564111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Holm S, Nachemson A. Nutrition of the intervertebral disc: acute effects of cigarette smoking. An experimental animal study. Ups J Med Sci. 1988;93:91–99. doi:10.1517/03009734000000042 [DOI] [PubMed] [Google Scholar]
- 60.Elmasry S, Asfour S, de Rivero Vaccari JP, Travascio F. Effects of tobacco smoking on the degeneration of the intervertebral disc: a finite element study. PLoS One. 2015;10:e0136137. doi:10.1371/journal.pone.0136137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Miller VM, Clouse WD, Tonnessen BH, et al. Time and dose effect of transdermal nicotine on endothelial function. Am J Physiol Circ Physiol. 2000;279:H1913–H1921. doi:10.1152/ajpheart.2000.279.4.H1913 [DOI] [PubMed] [Google Scholar]
- 62.Norton TR, Lazev AB, Sullivan MJ. The “buzz” on caffeine: patterns of caffeine use in a convenience sample of college students. J Caffeine Res. 2011;1:35–40. doi:10.1089/jcr.2010.0003 [Google Scholar]
- 63.Currie SR, Wilson KG, Gauthier ST. Caffeine and chronic low back pain. Clin J Pain. 1995;11:214–219. [PubMed] [Google Scholar]
- 64.Migliardi JR, Armellino JJ, Friedman M, Gillings DB, Beaver WT. Caffeine as an analgesic adjuvant in tension headache. Clin Pharmacol Ther. 1994;56:576–586. doi:10.1038/clpt.1994.179 [DOI] [PubMed] [Google Scholar]
- 65.Choi H, Choi Y, Kim J, Bae J, Roh J. Longitudinal bone growth is impaired by direct involvement of caffeine with chondrocyte differentiation in the growth plate. J Anat. 2017;230:117–127. doi:10.1111/joa.12530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Reis AMS, Oliveira KP, de Paula IHF, et al. Nonlinear effects of caffeine on the viability, synthesis and gene expression of chondrocytes from the offspring of rats treated during pregnancy. Acta Histochem. 2018;120:505–512. doi:10.1016/j.acthis.2018.06.001 [DOI] [PubMed] [Google Scholar]
- 67.Reis AMS, Raad RV, Ocarino NDM, Serakides R. In vitro effects of caffeine in growth cartilage of rats. Acta Ortop Bras. 2013;21:307–309. doi:10.1590/S1413-78522013000600001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Xie X, Tan Y, Xiao H, et al. Subchondral bone dysplasia mediates susceptibility to osteoarthritis in female adult offspring rats induced by prenatal caffeine exposure. Toxicol Lett. 2020;321:122–130. doi:10.1016/j.toxlet.2019.12.026 [DOI] [PubMed] [Google Scholar]
- 69.Qing-xian L, Lin-long W, Yi-zhong W, et al. Programming changes in GLUT1 mediated the accumulation of AGEs and matrix degradation in the articular cartilage of female adult rats after prenatal caffeine exposure. Pharmacol Res. 2020;151:104555. doi:10.1016/j.phrs.2019.104555 [DOI] [PubMed] [Google Scholar]
- 70.Alini M, Eisenstein SM, Ito K, et al. Are animal models useful for studying human disc disorders/degeneration? Eur Spine J. 2008;17:2–19. doi:10.1007/s00586-007-0414-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hunter CJ, Matyas JR, Duncan NA. Cytomorphology of notochordal and chondrocytic cells from the nucleus pulposus: a species comparison. J Anat. 2004;205:357–362. doi:10.1111/j.0021-8782.2004.00352.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hsieh AH, Hwang D, Ryan DA, Freeman AK, Kim H. Degenerative anular changes induced by puncture are associated with insufficiency of disc biomechanical function. Spine (Phila Pa 1976). 2009;34:998–1005. doi:10.1097/BRS.0b013e31819c09c4 [DOI] [PubMed] [Google Scholar]
- 73.Martin I, Wendt D, Heberer M. The role of bioreactors in tissue engineering. Trends Biotechnol. 2004;22:80–86. doi:10.1016/J.TIBTECH.2003.12.001 [DOI] [PubMed] [Google Scholar]
- 74.Lappa M. Organic tissues in rotating bioreactors: fluid-mechanical aspects, dynamic growth models, and morphological evolution. Biotechnol Bioeng. 2003;84:518–532. doi:10.1002/bit.10821 [DOI] [PubMed] [Google Scholar]
- 75.Unsworth BR, Lelkes PI. Growing tissues in microgravity. Nat Med. 1998;4:901–907. [DOI] [PubMed] [Google Scholar]
- 76.Duke PJ, Daane EL, Montufar-Solis D. Studies of chondrogenesis in rotating systems. J Cell Biochem. 1993;51:274–282. doi:10.1002/jcb.240510306 [DOI] [PubMed] [Google Scholar]