Abstract
The aim of this report is to review the literature and shed light on the uncertainties surrounding the use of antiviral agents in general and remdesivir in COVID-19 patients. This review evaluated a battery of antiviral compounds and their effectiveness in the treatment of COVID-19 since the beginning of the pandemic. Remdesivir is the only antiviral approved by the EMA and FDA for the treatment of SARS-CoV-2 infection. This work extensively reviews remdesivir data generated from clinical trials and observational studies, paying attention to the most recent data, and focusing on outcomes to give readers a more comprehensive understanding of the results. This review also discusses the recommendations issued by official bodies during the pandemic in the light of the current knowledge. The use of remdesivir in the treatment of SARS-CoV-2 infection is justified because a virus is the causative agent that triggers the inflammatory responses and its consequences. More trials are needed to improve the management of this disease.
Keywords: COVID-19, anti-viral therapy, viral RNA polymerases, recommendations
Introduction
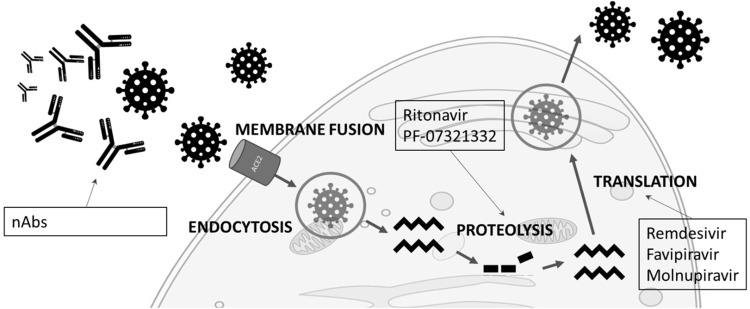
Coronavirus infectious disease 19 (COVID-19) is caused by a virus from the Coronaviridae family, and was first detected in the city of Wuhan in China at the end of 2019.1 The virus was named severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2).1 It is transported from infected individuals via droplets, aerosols or fomites and binds to the nasal epithelial cells, thus entering the cells through the highly expressed receptor ACE-2 with its cleaved spike protein (S) receptor-binding-domain.2 The conformational change of the S protein facilitates membrane fusion through the endosomal pathway in order to release the SARS-CoV-2 RNA inside the host cell, where it will be translated into viral replicase polyproteins by proteolysis. This is followed by replication of the viral genome and translation of viral structural proteins. Finally, the virion is assembled and released in the endoplasmic reticulum and Golgi.3 Figure 1 shows the SARS-CoV-2 life cycle and some of the drugs that inhibit the vital steps.
Figure 1.
SARS-Cov-2 life cycle and the antivirals that inhibit the different steps.
Abbreviation: nAbs, neutralizing antibodies.
COVID-19 clinical manifestations and complications were reviewed recently in a meta-analysis that included 41 studies and 16,495 patients.4 The most prevalent clinical manifestations were fever 78.1%, cough 64.6%, fatigue 40.8%, and dyspnea 38.6%. Severe COVID-19 patients were found to present significantly higher dyspnea (odds ratio (OR): 4.20, 95% CI: 3.09–5.72), cough (OR:1.45, 95% CI: 1.18–1.78) and fatigue (OR: 1.40, 95% CI: 1.14–1.72). The most prevalent comorbidities were hypertension 32.2%, diabetes 17.1%, and cardiovascular disease 15.3%. In the group with more severe disease, hypertension (OR: 1.98, 95% CI: 1.62–2.42), diabetes (OR: 2.04, 95% CI:1.67–2.50), cardiovascular disease (OR: 2.78, 95% CI: 2.00–3.86) and cancer (OR: 1.75, 95% CI: 1.40–2.18) were also significantly associated. Acute respiratory distress syndrome (ARDS) was present in 24.7% of patients.4
A meta-analysis5 focusing on the pathogenic effects observed in autopsies revealed diffuse alveolar damage (DAD) frequently associated with pulmonary thrombotic microangiopathy, caused by endothelial and myointimal growth with complement activation, as shown in skin lesions from COVID-19 patients.6 Evidence has shown endothelial cell damage and the presence of SARS-CoV-2 infection in the endothelium.7 Other organs, such as heart, liver, kidney, brain, spleen, skin and the adrenal glands, showed some inflammatory findings and vascular damage. The immunohistochemical analysis showed tissue inflammatory cell infiltration. The virus was detected by different techniques, such as RT-PCR, immunohistochemistry, and electron microscopy, showing that SARS-CoV-2 does not exclusively affect the lung.5 These results are consistent with those obtained from the first 100 autopsies of COVID-19-positive patients performed at Mount Sinai in New York, where 90% of all cases presented DAD. Other findings were microthrombi found in several organs, as well as presence of the virus confirmed by electron microscopy. Elevated cytokines, such as IL-16, IL-18, and TNFα were found as well as inflammatory markers and abnormal coagulation parameters.8
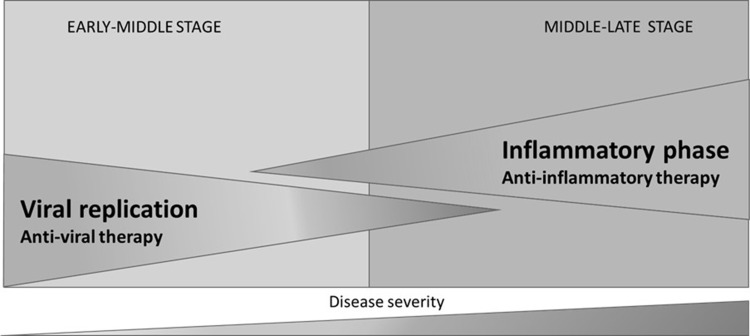
The damage caused by the virus in the lung is still poorly understood. But patients with high viral loads and long shedding periods are at higher risk for presenting severe forms of COVID-19.9 RNA load in plasma correlates with dysregulated host response involving chemokines, biomarkers of systemic inflammatory response, activation of NK cells, endothelial dysfunction, coagulation activation, tissue damage, neutrophil response, and immunodepression, suggesting that uncontrolled viral replication plays a major role in the pathogenesis of this disease.10 Massive epithelial and endothelial apoptosis together with vascular leakage and proinflammatory cytokine release may be caused by early onset viral replication.11 It is at this stage where antivirals, together with interferon-based therapies, may be more effective,12 although viral shedding has been detected in later stages of the disease.13,14 Later stages of the disease involve an exaggerated inflammatory response, where the host immune system reacts in an uncontrolled manner (Figure 2).15
Figure 2.
COVID-19 stages.
In the early-middle stage of COVID-19, when active replication of the virus is eventually present in all patients, the use of an antiviral to halt the propagation is justified. Moreover, the early use of these types of compounds may prevent progression to the inflammatory phase and subsequent complications, and can even reduce the risk of mortality, as demonstrated in real world practice.16,17 In the middle-late stage, anti-inflammatory/immunomodulatory therapy has demonstrated efficacy in diminishing mortality in patients receiving respiratory support.18 The main aim of this review is to garner expert opinion and shed light on the uncertainties surrounding the use of antiviral agents in general and remdesivir in particular in COVID-19 patients.
Antiviral Treatments Evaluated for COVID-19
In 2006, after the coronavirus outbreak of 2002, de Clercq proposed a series of viral targets for inhibiting SARS-CoV:19
The spike (S) glycoprotein involved in viral entry into the cells.
The processing of the replicase polyproteins by virus-encoded proteases.
The NTP/helicase and RNA-dependent RNA polymerase.
Following this, several drugs have been tested in this pandemic to inhibit SARS-CoV-2 during various stages of the infection and reduce the total viral load (Figure 1).20 Several compounds were assessed as potential antiviral for COVID-19 at some point during the pandemic, but finally, their results showing a non-significant benefit in different clinical trials resulted in their withdraw. These include azithromycin,21 chloroquine/hydroxychloroquine (CQ/HCQ),22–24 convalescent plasma,25 lopinavir and ritonavir,26 interferon,27 ribavirin,28 Ivermectin,29 and nitazoxanide.30
The following drugs have shown efficacy in clinical trials or are under current investigation to prove efficacy:
Molnupiravir
Molnupiravir, a broad-spectrum antiviral that is an orally bioavailable prodrug of the nucleoside analogue β-D-N4-hydroxycytidine that can increase transition mutations during viral replication.31 An analysis of a Phase 3 clinical trial encompassing 1433 participants showed that molnupiravir significantly reduced the risk of hospitalization or death in at risk, non-hospitalized adult patients with mild-to-moderate COVID-19. After showing positive results in the interim analysis, in the analysis with all participants molnupiravir reduced the risk of hospitalization or death through day 29 was by approximately 30% compared to the placebo group (6.8% [48 of 709] vs 9.7% [68 of 699]; difference, −3.0%; 95% CI, −5.9 to −0.1). Results of subgroup analysis was largely consistent with these overall results, but in patients with previous SARS-CoV-2 infection, those with low baseline viral load, and those with diabetes the analysis favored the placebo group. In line with this data, at day 29 there was 1 death in the molnupiravir group, compared to the 9 deaths in the control group. Adverse events were reported in 30.4% in the molnupiravir group and 33.0% in the placebo group.32
PF-07321332 and Ritonavir
A combination of PF-07321332 and ritonavir has shown results from an interim analysis of the Phase II–III clinical trial. In this study patients receiving this combination within three day of symptoms onset showed 0.8% of hospital admission up to day 28 compared to the 7% showed by those patients receiving placebo, with none and 7 reported deaths respectively (p<0.0001). Also, in those patients receiving the combination within 5 days of disease onset, the hospitalization up to day 28 was 1% with no deaths, compared to a 6.7% of Hospitalizations and 10 deaths in the placebo group. Adverse effects were similar in both groups (19% and 21% in the paxlovid and placebo group, respectively).33
Favipiravir
A meta-analysis involving 11 studies (6 with favipiravir and 5 as comparator) found that this antiviral compound increased viral clearance at day 7 and provided clinical improvement at day 14, indicating a strong potential for treatment, especially in mild-to-moderate COVID-19 patients.34 Another meta-analysis encompassing 9 studies found significant clinical improvement and viral clearance in the Favipiravir group versus the control group after 7 and 14 days of hospitalization, respectively.35 This antiviral compound has received authorization for the treatment of COVID-19 in China and Russia.36
Anti-SARS-CoV-2 Neutralizing Antibodies
As mentioned above, SARS-CoV-2 enters the host cells through the ACE-2 receptor via the viral spike protein and its RBD.2 This interaction represents a theoretical target to block viral entry into the host cell. Several attempts have been made to generate antibodies that neutralize this interaction,37 and some have been approved for use against COVID-19. Bamlanivimab showed a significant reduction in mean viral load and the incidence of hospitalization or Emergency Department visits compared to controls.38 This antibody has also shown a prophylactic effect, reducing the likelihood of symptomatic COVID-19 by 57%.39 The combination of bamlanivimab with etesevimab in ambulatory COVID-19 patients was associated with a significant reduction in viral load versus placebo, and a reduction in the number of hospitalizations.40 The casirivimab and imdevimab combination reduced the risk of hospitalization and death by 70%.41 This antibody cocktail has also shown benefits in a prophylactic setting.42 Regdanvimab, also known as CT-P59, is a fully human anti-SARS-CoV-2 mAb that has shown promising results in a Phase I trial43 This neutralizing antibody (nAB) has shown neutralizing activity against the B.1.351 mutation containing variant.44 Sotrovimab has shown promising results in an interim analysis of the phase 3 trial COMET-ICE, reducing the risk of COVID-19 progression by 85% (97.24% CI, 44% - 96%; p = 0.002). Adverse events were reported by 17% and 19% of patients receiving sotrovimab and placebo, respectively; serious adverse events were less common with sotrovimab (2%) versus placebo (6%).45 Sotrovimab received a the approval from the EMA for use in COVID-19 patients with high risk of progression who do not require supplemental oxygen.46 One caveat of neutralizing antibodies is the differential capacity shown to block the different SARS-CoV-2 variants.47 In this regard sotrovimab has demonstrated its neutralizing capacity against the omicron variant.47
Remdesivir
Remdesivir was the first antiviral drug to be recommended for approval in the European Union (EU) and in the USA for the treatment of COVID-19,48,49 received final approval and has been recently reviewed by the EU regulatory authorities.50 This compound is an adenosine-analogue prodrug that inhibits viral RNA polymerases after metabolic activation.51 Remdesivir has demonstrated inhibitory in vitro activity in primary human airway epithelial cells and Calu-3 human lung cells with a half-maximal effective concentration of 9.9 nM and 280 nM, respectively.52 Therapeutic effects in vivo have also been demonstrated in rhesus monkeys,53,54 showing a significant decrease in clinical signs of respiratory disease, lung pathology, gross lesions, and RNA load compared to control animals.53 Recent preliminary clinical data showed a significant decrease in SARS-CoV-2 viral load in COVID-19 patients receiving remdesivir at day 5, and a significative increase of the Ct values of gRNA (genomic) and sgRNA (subgenomic) at day 2 and 5.55 This antiviral was formulated for intravenous (IV) administration after extensive first pass extraction.56 Intravenous remdesivir has 100% bioavailability, and peaks after 30 min.56 It presents moderate binding to plasma proteins, and is mainly metabolized by carboxylesterase 1, followed by cathepsin A, and cytochrome P450 (CYP) 3A.51 It is eliminated through urine (74%) and feces (18%).51
Several studies have evaluated the effectivity of remdesivir in the treatment of COVID-19 patients. This work reviews the main findings of clinical trials and observational studies of remdesivir in patients with COVID-19
Clinical Trials
Table 1 summarizes the results obtained in the different clinical trials assessing the effectiveness of this compound. An early randomized, double-blind, placebo-controlled, multicenter trial performed in 10 hospitals in Hubei, China, did not observe a clinical improvement (hazard ratio (HR): 1.23; 95% CI: 0.87–1.75). Patients receiving this drug presented a numerically faster time to clinical improvement compared to those who received placebo, with symptom duration of ≤ 10 days (HR: 1.52; 95% CI: 0.95–2.43). Treatment was stopped in 18 (12%) and 4 (5%) patients who received remdesivir and placebo, respectively. This study did not find significant reductions in SARS-CoV-2 RNA loads assayed in the upper respiratory tract and sputum. One limitation of this study was that it did not recruit the expected number of patients due to the restrictions imposed in the region in connection with the pandemic.57
Table 1.
Summary of Results Obtained in Clinical Trials Testing Remdesivir
| Study | Treatment Extension1 | Severity | N | Design | Time from Symptom Onset (Median Time) | Primary Results | Ae | Mortality | Other Results |
|---|---|---|---|---|---|---|---|---|---|
| Wang et al57 | 10 d | Severe | T: 237 RDV: 158 P: 79 |
Randomized, double-blind, placebo-controlled, | RDV: 11 (9–12) P: 10 (9–12) |
Clinical improvement 2 points: remdesivir, 21 d P: 23 d (HR: 1.23 95% CI: 0.87–1.75) |
RDV: 66% P: 64% |
28 d RDV: 14% P: 13% |
Viral load 5d: RDV no difference vs P. more rapid decline in load (p=0.0672). |
| SIMPLE SEVERE58 | 5 or 10 d | Severe | T: 397 10d: 197 5d: 200 |
Randomized, open label | 5d: 8 (5–11) 10d: 9 (6–12) |
Clinical improvement: 5d: 64% 10d: 54% |
Nausea 9% worsening respiratory failure 8% AAT 7% constipation 7% |
5d: 8% 10d: 11% |
Discharge 5d: 60% 10d: 52% |
| SIMPLE MODERATE59 | 5 or 10 d | Moderate | 10d: 197 5d: 199 SOC: 200 |
Randomized, open label | 10d: 8 (5–11) 5d: 8 (5–11) SOC: 9 (6–11) |
Clinical status on day 11 5d: OR, 1.65; 95% CI, 1.09–2.48 p =0.02 10d P =0.8 |
Nausea 10% vs 3% Hypokalemia 6% vs 2% Headache 5% vs 3% |
5d: 1% 10d: 2% SOC: 2% |
Clinical status on day 14, 28 5d, 10d vs SOC p = 0.03 |
| ACTT-160 | 10d | Severe | 1062 RDV: 541 P:521 |
Randomized, double-blind, placebo-controlled | RDV: 9 (6–12) P: 9 (7–13) |
Time to recovery remdesivir vs P 10d vs 15d RR: 1.29; 95% CI, 1.12–1.49; P<0.001) |
RDV (SAE): 24.6% P (SAE): 31.6% |
d29 RDV: 11.4% P: 15.2% HR: 0.73; 95% CI, 0.52–1.03 |
43% fewer patients with RDV started invasive ventilation |
| SOLIDARITY27 | 10d | NR | RDV: 2743 C:2708 |
Randomized, open label | NR |
RR for death: 0.95 (0.81–1.11) |
NR | RDV: 301 C: 303 (RR: 0.95; 95% CI: 0.81–1.11; P = 0.50) |
No differences in start of mechanical ventilation |
| PINETREE61 | 3d | Moderate | RDV:279 P:283 |
Randomized, double-blind, placebo-controlled | 5 (3–6) |
COVID-19 hospitalization/all-cause death 28d HR: 0.13; 95% CI: 0.03–0.59; p = 0.008 |
Grade ≥3; SAE RDV: 4%; 2% P: 7%; 7% |
RDV: 0 P: 0 |
Risk of COVID-19 related MAV/all-cause death 28d HR: 0.19; 95% CI: 0.07–0.56; p = 0.002 |
Abbreviations: 1 load, 200 mg; 100 mg /day; AE, adverse effects; C, control; d, day; ECMO, extracorporeal membrane oxygenation; HR, hazard ratio, NR, not reported; P, placebo; OR, odds ratio; RDV, remdesivir; RR, risk ratio; Rx, radiographic; SAE, serious adverse effect; SOC, standard of care; SpO2, oxygen saturation; T, total; y, year.
In a randomized, open-label, phase 3 trial in which patients with severe COVID-19 not requiring mechanical ventilation were treated with remdesivir, no significant differences were detected between the 5-day course and the 10-day course of remdesivir. The magnitude of the benefit could not be determined due to the lack of a placebo control group. Regarding safety, the study showed that grade ≥3 laboratory abnormalities occurred in 27% and 34% of the patients who received the 5-day course and the 10-day course, respectively, but most were transient.58 Another study with the same design assessed the effects of remdesivir in patients with moderate disease. Major findings are summarized in Table 1. Comparing the 5-day and 10-day courses, no significant differences were found in time to ≥2-point improvement in clinical status, time to ≥1-point improvement in clinical status, time to recovery, time to modified recovery, and time to discontinuation of oxygen support. No differences were detected between remdesivir and SOC in duration of oxygen therapy or hospitalization. A Post hoc analysis showed that the clinical status in both remdesivir groups was significantly better compared to SOC (p = 0.03 for both groups) and remained significantly better in the 10-day course group by day 28 (p = 0.03).59
In a double-blind, randomized, placebo-controlled trial (ACTT-1), remdesivir achieved 50% clinical improvement (Table 1). In total, 23.0% (n=241) of all patients were treated with glucocorticoids and 35.6% (n=373) with HCQ. Benefits of remdesivir were more pronounced when the drug was given earlier in the disease course, but the benefits also persisted in most analysis of symptom duration (median 9 days). Remdesivir benefits were also significant in patients with comorbidities. Patients in the remdesivir group showed improvement in the ordinal scale score and a lower mortality rate at day 15 and 29 compared to placebo, especially those with a baseline ordinal score of 5 (hospitalized, requiring supplemental oxygen) (HR: 0.30; 95% CI: 0.14–0.64). The remdesivir group also required less respiratory support than the placebo group, specifically new use of oxygen (36% remdesivir vs 44% in placebo), new high-flow oxygen (17% remdesivir vs 24% placebo), and new mechanical ventilation or ECMO (13% remdesivir vs 23% placebo). Data also showed that treatment with remdesivir may have prevented progression to more severe respiratory disease, as shown by a lower incidence of serious adverse events related to respiratory failure.60 According to the model published by Soriano et al, based on the results obtained with remdesivir at the ACTT-1 trial, the use of this drug during the first wave of the pandemic in Spain would have prevented 2587 ICU admissions, thereby freeing up 5656 general hospital beds – a capacity increase of 17.53% [95% CI: 3.98–24.42%]) - and 1700 ICU beds – a capacity increase of 23.98% (95% CI: 21.33–28.22%) - for other patients. Overall, it has been predicted that the use of remdesivir during the first wave of COVID-19 in Spain would have prevented 7639 deaths – a reduction of 27.51% (95% CI: 14.25–34.07%).62 The World Health Organization (WHO) conducted a different randomized open-label non placebo controlled clinical trial of 4 antiviral drugs (remdesivir, hydroxychloroquine, lopinavir and interferon β-1a), involving 405 hospitals in 30 countries. The results in this case showed that remdesivir (RR: 0.95 [95% CI, 0.81–1.11]) and the other compounds had little or no effect on overall mortality, initiation of ventilation, or duration of hospitalization;27 however, patients in this study were stratified by need for respiratory support instead of the method used in other clinical trial (disease severity).27
In a recent double-blinded, placebo-controlled phase 3 study that included 64 sites in 4 countries and 584 randomized patients, the safety and efficacy of a 3-day course of remdesivir was evaluated in non-hospitalized participants at elevated risk of disease progression. Treatment with remdesivir resulted in an 87% reduction in risk of COVID-19 related hospitalization or all-cause death by day 28 compared to placebo (HR: 0.13; 95% CI: 0.03–0.59; p = 0.008). Remdesivir also resulted in a significant 81% reduction in risk of COVID-19 related medically attended visits or all-cause death by day 28 compared to placebo (HR: 0.19; 95% CI: 0.07–0.56; p = 0.002). No differences were observed in the safety profile of remdesivir compared to placebo. Interestingly, there was no reduction in nasopharyngeal viral load up to day 7 between groups, suggesting that this surrogate efficacy marker is inadequate.61
The effect of Remdesivir at other disease stages and in combination with other compounds is currently being evaluated in other clinical trials.
Observational Studies
In a recent press release, the EMA noted that the evidence generated by high-quality “real world” data are fundamental to gain a near real-time understanding of the safety and effectiveness of drugs in everyday use, especially in this pandemic, in order to inform public health policies on treatment management and vaccinations.63 Several studies have analyzed the effects of remdesivir outside the clinical trial setting. In a preliminary study of the compassionate use of remdesivir, 53 confirmed COVID-19 patients with SpO2 < 94% received remdesivir at the standard regimen for up to 10 days. During the median 18-day follow-up, 68% (n = 36) of patients showed improvement in oxygen-support class (17 out of 30 were extubated), 47% (n = 25) were discharged, and 13% (n = 7) died.64 In another study evaluating 242 consecutive patients admitted for COVID-19, 123 (50.8%) with a median age of 58 years received remdesivir (48–69). Intensive care unit (ICU) admission and mechanical ventilation were needed in 19.5% and 7.3% of the patients, respectively. The 30-day mortality rate was 4.1% (5/123), like the 3.8% reported in the ACTT-1.60 The mortality rate was 8.3% (2/24) in those admitted to the ICU. Mortality among patients receiving concomitant dexamethasone with remdesivir was 16.7%, and 5.3% with tocilizumab. All non-survivors were ≥ 80 years of age and received concomitant anti-inflammatory drugs.65 Drug administration occurred earlier than in ACTT-1,60 which might explain the shorter hospital stay (8 vs 12 days) and less need for mechanical ventilation (7.3 vs 12.9%).65 Remdesivir was not discontinued due to severe adverse effects (including elevation of hepatic enzymes) in any of the patients, a finding that supports the good tolerability of this drug.65
A retrospective comparative effectiveness research study examined whether the administration of remdesivir with or without corticosteroids was associated with more rapid clinical improvement compared with matched individuals who did not receive the drug. Of the 2483 patients included with confirmed severe COVID-19, 342 received remdesivir, of whom 184 also received corticosteroids and 158 remdesivir alone. Patients who received remdesivir presented a shorter time to clinical improvement compared with those without remdesivir (median 5.0 days [interquartile range (IQR): 4.0–8.0 days] vs 7.0 days [IQR: 4.0–10.0 days]; adjusted hazard ratio (aHR), 1.47 [95% CI, 1.22–1.79]). Mortality at day 28 in the remdesivir group was 7.7% (22) compared to 14% (40) (aHR: 0.70; 95% CI: 0.38–1.28). The addition of corticosteroids did not reduce mortality (aHR: 1.94; 95% CI: 0.67–5.57). This study included a large proportion of non-Caucasian individuals (80%)66 compared to other clinical trials (30–47%).58–60 Another study analyzing real-world data from 2 population-based nationwide cohorts of individuals hospitalized with COVID-19 compared the effectiveness of SOC alone vs SOC plus remdesivir and dexamethasone. The 30-day mortality rate of patients treated with remdesivir and dexamethasone in addition to SOC was 12.6% compared to 19.7% for those receiving SOC alone (OR: 0.47; 95% CI: 0.38–0.57) for patients treated with remdesivir and dexamethasone compared to patients receiving SOC alone. Progression to mechanical ventilation was also reduced (OR: 0.36; 95% CI: 0.29–0.46).67
Three large observational studies using propensity score matching have recently observed benefits in patients hospitalized for COVID-19 who received remdesivir compared to those in the control group.16,68,69 A recent study assessed the mortality rate and time to discharge of hospitalized patients with COVID-19 who were treated with remdesivir (n=24,856) versus those in the control arm (n=24,856). It showed that patients treated with remdesivir had a 23% reduction in mortality risk (HR: 0.77; 95% CI: 0.73–0.81), regardless of the need for supplemental oxygen. Overall, a significant reduction in time to discharge was observed until day 28 in patients treated with a full 5-day course of remdesivir compared to those in the control arm (HR:1.19; 95% CI: 1.14–1.25).16 Mozaffari et al assessed the mortality of hospitalized patients treated with remdesivir (n=28,855) versus matched patients not treated with remdesivir (n=16,687). Patients were matched based on baseline O2, admission period, and not being discharged within 3 days of remdesivir start (excluding anticipated discharges within 72 h, to remain consistent with the ACTT-1 trial), concomitant medication and other clinical and demographic variables, among others. Patients treated with remdesivir had a significantly lower risk of mortality on both day 14 (HR:0.76, 95% CI:0.70–0.83, p<0.0001) and day 28 (HR:0.89, 95% CI:0.82–0.96, p=0.003) compared to patients who did not receive remdesivir.68 A retrospective study using data from the SIMPLE-Severe study analyzed 312 patients that received remdesivir and compared them with 818 patients from a real-life retrospective longitudinal cohort of hospitalized patients who did not receive remdesivir. The use of remdesivir was associated with significantly higher recovery and significantly lower mortality.69 The recovery rate at day 14 was 74.4% and 59.0% in the remdesivir and SOC groups, respectively (adjusted OR: 2.03; 95% CI: 1.34–3.08; p < 0.001). The mortality rate over the same period was 7.6% and 12.5% in the remdesivir and SOC groups, respectively (aOR: 0.38; 95% CI: 0.22–0.68; p = 0.001).69 This analysis was continued by Go et al with more patients with COVID-19 who received remdesivir (n=1974) and patients with COVID-19 who were not treated with remdesivir (n=1426). Treatment with remdesivir was associated with a statistically significant 54% lower risk of mortality at 28 days compared to those not treated with remdesivir, regardless of the patient’s initial oxygen requirements (HR: 0.46; 95% CI 0.39–0.54; p<0.001). Patients treated with a 10-day course of remdesivir had significantly less time to discharge at 28 days compared to those who did not receive remdesivir (HR:1.64, 95% CI:1.43–1.87, p<0.001). The time to discharge was not significant for patients receiving invasive mechanical ventilation or ECMO at baseline (HR:0.92, 95% CI: 0.62–1.36, p=0.68).70 A recent retrospective study that analyze data obtained from 2344 US veterans hospitalized with COVID-19 found no benefit of remdesivir in 30-day survival (HR: 1.06; 95% CI: 0.83–1.36) and an increase in median time to hospital discharge compared with matched controls (6 days [IR: 4–12 days] vs 3 days [IR: 1–7 days]; p < 0.001).71 The conflicting results obtained by this last work compared to the ACTT-1 trial60 could be attributed to the fact that patients with severe COVID-19 received remdesivir and finding more suitable matched controls was not possible, skewing the results toward less severe COVID-19 patients.72
Official Recommendations
Several health organizations have developed recommendations regarding the use of remdesivir in COVID-19 patients.73–78 Most of the current recommendations developed for the use of remdesivir in COVID-19 were established during the initial waves of the pandemic, when resources were scarce and administrative or pragmatic criteria took priority. Some of these recommendations have been updated and are in line with the prevailing body of evidence. Table 2 summarizes some of those recommendations.
Table 2.
Summary of Recommendations Regarding the Use of Remdesivir in COVID-19 Patients
| Recommendation | Grading1 | Updated On | |
|---|---|---|---|
| ESCMID78 | Conditional recommendation for use of RDV for COVID-19 in hospitalized patients not requiring mechanical ventilation or ECMO. |
QoE: Moderate | Nov 4, 2021 |
| IDSA73 | In hospitalized patients with severe COVID-19, suggests RDV over no antiviral treatment. | Conditional recommendation*, Moderate certainty of evidence | Nov 18, 2021 |
| In patients with COVID-19 on invasive ventilation and/or ECMO, suggests against the routine initiation of RDV | Conditional recommendation*, Very low certainty of evidence | ||
| In patients on supplemental O2 but not on mechanical ventilation or ECMO, suggests treatment with 5 days of RDV rather than 10 days of RDV. | Conditional recommendation*, Low certainty of evidence | ||
| In patients with COVID-19 admitted to the hospital without the need for supplemental oxygen and oxygen saturation >94% on room air, the IDSA panel suggests against the routine use of RDV. | Conditional recommendation*, Very low certainty of evidence | ||
| NICE77 | Consider RDV for up to 5 days for COVID-19 pneumonia in adults and young people 12 years and over weighing 40 kg or more, in hospital and needing low-flow supplemental oxygen. | Conditional recommendation* | Dec 16, 2021 |
| Do not use RDV for COVID-19 pneumonia in adults, young people, and children in hospital and on high-flow nasal oxygen, continuous positive airway pressure, non-invasive mechanical ventilation, or invasive mechanical ventilation, except as part of a clinical trial. | Only in research settings | ||
| NIH74 | For hospitalized patients who require minimal supplemental oxygen: recommend RDV for 5 days or until hospital discharge. | BIIa | Dec 16, 2021 |
| For hospitalized patients who require increasing volumes of supplemental O2: DEX + RDV. | BIIa | ||
| For hospitalized patients who require high-flow O2, non-invasive ventilation: recommend RDV + DEX | BIII | ||
| For recently intubated patients, RDV+DEX may be considered. RDV alone is not recommended | CIII | ||
| SEIMC75 | Hospitalized low flow pts RDV recommended if low-flow O2 if required to keep SpO2> 94%. Only in patients with symptoms ≤ 10 days. Early administration is recommended. If viral replication persists in immunocompromised patients consider repeated cycles. | NG | Nov 5, 2021 |
| WHO76 | Suggests against administering RDV in addition to usual care for the treatment of patients hospitalized with covid-19, regardless of disease severity | Weak or conditional | Jul 7, 2021 |
Notes: 1A = Strong; B = Moderate; C = Optional; I= One or more randomized trials with clinical outcomes and/or validated laboratory endpoints; IIa=Other randomized trials or subgroup analysis of randomized trials; II = One or more well-designed, non-randomized trials or observational cohort studies; III = Expert opinion; *Dependent on disease severity and oxygen requirements.
Abbreviations, b, breaths; CS, corticosteroids; CT, clinical trials; DEX, dexamethasone; ECMO, extracorporeal membrane oxygenation; ECSMID, European Society of Clinical Microbiology and Infectious Diseases; IDSA, Infectious Diseases Society of America; NG, not given; RDV, remdesivir; SEIMC, Spanish Society of Infectious Diseases and Clinical Microbiology SpO2, O2 saturation; PaO2/FIO2, ratio of partial pressure arterial oxygen and fraction of inspired oxygen; QoE, quality of evidence; WHO, World Health Organization.
Some government agencies have also issued regulations to indicate some directions to guide the utilization of remdesivir when availability was not guaranteed and resources were limited during the first wave of the pandemic.79
Discussion
The first wave of the pandemic has taken an important toll in several countries, due to limited understanding of how to manage a new disease and the lack of available effective treatments. In this regard, COVID-19 has represented a major challenge in several respects, including finding drugs that can ameliorate disease progression in infected patients. COVID-19 presents distinct stages that require different treatment approaches (Figure 1). Antivirals are designed to inhibit some of the stages of the virus life cycle (Figure 2)80 in order to decrease the total viral load.20 Later in the disease, and only in some cases as a consequence of a pathological immune system response, an exaggerated inflammatory state takes place and immunomodulatory drugs like dexamethasone are recommended to control the hyperinflammatory state, as shown in the RECOVERY trial.18 In severe cases, other treatments such as anticoagulants are recommended to avoid the formation of potentially lethal thrombi.81 Nevertheless, this multistage pathology is triggered by SARS-CoV-2, so administration of an antiviral, either alone or in combination with other treatments, is always justified unless there is a pharmacological contraindication or the individual risk-benefit indicates the need for a different approach.
To date, remdesivir is the only antiviral agent approved for COVID-19 treatment by the European Union.48 The clinical benefits of remdesivir in the treatment of COVID-19 have been validated in various clinical trials.60,61 The results demonstrated a reduction in disease progression, and a decline in mortality60,61 These results have been validated by the 2 SIMPLE randomized open-label clinical trials.58,59 In contrast, the Solidarity trial found that remdesivir did not show any improvement in mortality - the primary endpoint (12.5% remdesivir vs 12.7% SOC) - and patients receiving remdesivir were more likely to be hospitalized at day 7 (69%) that those receiving SOC (59%).27 The uncertainties generated in the scientific community and in prescribing physicians by these apparently contradictory results merit an explanation.
These differences can be partly attributed to the design of the trials. First, ACTT-1 was a double-blind placebo-controlled trial, while Solidarity was an open platform study. Patients in the ACTT-1 trial were discharged as soon as possible,60 but in the Solidarity trial patients receiving remdesivir often stayed in the hospital to receive the full 10-day course, thus increasing the risks inherent to prolonging hospital stay.27 Another difference is that the conditions in the ACTT-1 trial were more homogenous than in the Solidarity trial. While in the ACTT-1 trial the comparator was placebo,60 the control used in the Solidarity trial was the standard-of-care (SOC), which was highly variable and depended on the region or hospital where the patient was treated. In addition, the recruitment period was longer, implying that the SOC could differ during that period.27 Patient stratification criteria differed between the ACTT-1 and Solidarity trials,27,60 and the confidence intervals for mortality were 95% and 99%, respectively.
One limitation of the ACTT-1 trial is that the correlations between efficacy and the baseline ordinal scale score (as a continuous variable) were obtained by a post hoc analysis of the data.60 Post hoc analyses, which ignore randomization, have several limitations,82 but the findings could still be relevant and need to be confirmed in subsequent trials where they are validated using the correct design. Recent data obtained from observational studies in large cohorts of patients have validated the results obtained in ACCT-1 and SIMPLE,58–60 in terms of a reduction in mortality.16,17,64–66,70 The results obtained by the NIH sponsored ACTT-1 trial60 prompted several international health agencies to approve remdesivir for the treatment of COVID-19 and its temporary use in about 50 countries.73–76,79 The results obtained by the PINETREE study and other observational studies16,60,61,68,69 expanded the population that could benefit from remdesivir earlier in the disease course, possibly saving further costs due to the COVID-19 progression.
The resource burden placed on national health systems by the pandemic goes beyond direct COVID-19-related costs. For instance, COVID-19 patients that require ICU admission have a mortality rate of 35.5% (31.3–39.9%)83 and are at higher risk for presenting certain comorbidities, such as coagulation disorders. Patients in the ICU require more personnel and resources than those on the general ward, and far more than outpatients. The use of remdesivir, when indicated, would shorten hospital stays and reduce the risk of progression to the ICU, and therefore represents a saving in staff hours and other resources. Other pathologies have been neglected during the pandemic, among them cancer and chronic diseases, and patients with this condition may have a worst prognosis than before the pandemic and will require more resources.
The available data show that each patient needs to be evaluated individually before starting remdesivir. This antiviral is effective during viral replication, and it should be borne in mind that replication may be present at later stages of the disease13,14 and has been also detected in autopsies.8 Therefore, the therapeutic window for the use of an antiviral should be calculated based on the viral load and hyperactivation of the immune system.84 Viral load at diagnosis is not associated with severity of disease,85 but persistent viral loads in respiratory and blood samples indicate a more severe course.86 To sum up, there is a clear need to individualize the therapeutic window of remdesivir to ameliorate the disease course of COVID-19 patients.
In the initial waves of the pandemic resources were scarce; therefore, administrative, or pragmatic criteria took precedence over scientific evidence. This was when healthcare professionals and decision makers started to use “battlefield” terminology, hospitals were overcrowded and understaffed, new ICUs were opened whenever and wherever possible, and healthcare workers struggled to overcome an unknown enemy using all the resources available. Decisions were made under the pressure of this state of emergency. The situation has gradually been brought under control, and it is time to reformulate the non-evidence-based strategies that prevented patients from receiving the benefit of certain treatments that could have shortened their stay and reduced the risk of progression to ICU.
Vaccines appear to be a game changer in this pandemic. But while distribution remains unequal between developed and underdeveloped countries there is a risk for the development of SARS-CoV-2 variants like omicron containing vaccine-resistant mutations in the spike protein that are targeted by vaccine-generated neutralizing antibodies. Remdesivir acts by inhibiting the activity of RNA polymerase, a protein that is more conserved in different SARS-Cov-2 variants than the spike protein,87 and where remdesivir has also shown inhibitory activity against variants.88 Investigation should focus on improving the outcomes of existing viable antivirals and the discovery of new ones, as also update therapeutic strategies for the management of patients with COVID-19. Several molecules89 and nAbs37 have been investigated for the treatment of COVID-19 and the scientific community and society are awaiting the results.
Given the certainty that humanity will eventually have to face another pandemic, further research is needed to improve the management of existing antivirals and discover new ones.
Acknowledgments
The authors want to thank the writing and editorial assistance provided by Blanca Piedrafita and Antoni Torres from MSC consulting.
Funding Statement
Gilead provided the editorial support for the manuscript and was not involved in the discussions or the writing process.
Disclosure
SM has been involved in speaking activities and has received grants for research from Gilead, Janssen Cilag, Merck Sharp&Dohme, and ViiV Healthcare. BAN reports personal fees and non-financial support from Gilead, Boehringer- Ingelheim, AstraZeneca, Novartis AG, and Chiesi, grants, personal fees, and non-financial support from GSK, and grants and personal fees from Menarini, not related to this study. BAN holds no stock options in companies mentioned in this study. CD reports grants from commercial sponsors, outside the submitted work, and has received grants for research, fees for participating in advisory boards and educational activities from Gilead Sciences, Janssen Cilag, Menarini, Merck Sharp & Dohme, Pfizer, Shionogi, and ViiV healthcare. JGC received research and educational grants from Gilead, GSK, Merck, and Thermofisher. JOS has been involved in speaking activities and has received grants for research from Gilead, Janssen Cilag, Merck Sharp&Dohme, and ViiV Healthcare. AA reports non-financial support from Gilead, during the conduct of the study; and has received grants for research and fees for participating in clinical trials, advisory boards, and educational activities from Gilead Sciences, Janssen Cilag, Merck Sharp & Dohme, and ViiV Healthcare, outside the submitted work. The authors report no other potential conflicts of interest for this work.
References
- 1.Parasher A. COVID-19: current understanding of its pathophysiology, clinical presentation and treatment. Postgrad Med J. 2021;97(1147):312–320. doi: 10.1136/postgradmedj-2020-138577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wan Y, Shang J, Graham R, Baric RS, Li F. Receptor recognition by the novel coronavirus from wuhan: an analysis based on decade-long structural studies of SARS coronavirus. J Virol. 2020;94(7). doi: 10.1128/JVI.00127-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shereen MA, Khan S, Kazmi A, Bashir N, Siddique R. COVID-19 infection: emergence, transmission, and characteristics of human coronaviruses. J Adv Res. 2020;24:91–98. doi: 10.1016/j.jare.2020.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Giri M, Puri A, Wang T, Guo S. Comparison of clinical manifestations, pre-existing comorbidities, complications and treatment modalities in severe and non-severe COVID-19 patients: a systemic review and meta-analysis. Sci Prog. 2021;104(1):368504211000906. doi: 10.1177/00368504211000906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mondello C, Roccuzzo S, Malfa O, et al. Pathological findings in COVID-19 as a tool to define SARS-CoV-2 pathogenesis. a systematic review. Front Pharmacol. 2021;12:614586. doi: 10.3389/fphar.2021.614586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Valtueña J, Martínez-García G, Ruiz-Sánchez D, et al. Vascular obliteration because of endothelial and myointimal growth in COVID-19 patients. Int J Dermatol. 2021;60(1):73–80. doi: 10.1111/ijd.15300 [DOI] [PubMed] [Google Scholar]
- 7.Varga Z, Flammer AJ, Steiger P, et al. Endothelial cell infection and endotheliitis in COVID-19. Lancet. 2020;395(10234):1417–1418. doi: 10.1016/S0140-6736(20)30937-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bryce C, Grimes Z, Pujadas E, et al. Pathophysiology of SARS-CoV-2: the Mount Sinai COVID-19 autopsy experience. Mod Pathol. 2021;34:1456–1467. doi: 10.1038/s41379-021-00793-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu Y, Yan LM, Wan L, et al. Viral dynamics in mild and severe cases of COVID-19. Lancet Infect Dis. 2020;20(6):656–657. doi: 10.1016/s1473-3099(20)30232-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bermejo-Martin JF, González-Rivera M, Almansa R, et al. Viral RNA load in plasma is associated with critical illness and a dysregulated host response in COVID-19. Crit Care. 2020;24(1):691. doi: 10.1186/s13054-020-03398-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yang M. Cell pyroptosis, a potential pathogenic mechanism of 2019-nCoV infection. SSRN Electronic J. 2020. doi: 10.2139/ssrn.3527420 [DOI] [Google Scholar]
- 12.Jamilloux Y, Henry T, Belot A, et al. Should we stimulate or suppress immune responses in COVID-19? Cytokine and anti-cytokine interventions. Autoimmun Rev. 2020;19(7):102567. doi: 10.1016/j.autrev.2020.102567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van Kampen JJ, van de Vijver DA, Fraaij PL, et al. Shedding of Infectious virus in hospitalized patients with coronavirus disease-2019 (COVID-19): duration and key determinants. MedRxiv. 2020;4:54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Morone G, Palomba A, Iosa M, et al. Incidence and persistence of viral shedding in COVID-19 post-acute patients with negativized pharyngeal swab: a systematic review. Front med. 2020;7:562. doi: 10.3389/fmed.2020.00562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ragab D, Salah Eldin H, Taeimah M, Khattab R, The SR. COVID-19 cytokine storm; what we know so far. Front Immunol. 2020;11:1446. doi: 10.3389/fimmu.2020.01446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chokkalingam AP, Li H, Asubonteng J, et al. Comparative effectiveness of remdesivir treatment in patients hospitalized with COVID-19. Presented at: WMF; Session Poster; 2021:WMF21–2970.
- 17.Mozaffari E, Chandak A, Zhang Z, et al. Remdesivir treatment is associated with improved survival in hospitalized patients with COVID-19. Presented at: WMF; Session Poster; 2021: WMF21–2507.
- 18.Horby P, Lim WS, Emberson JR, et al. Dexamethasone in hospitalized patients with Covid-19. N Engl J Med. 2021;384(8):693–704. doi: 10.1056/NEJMoa2021436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.De Clercq E. Potential antivirals and antiviral strategies against SARS coronavirus infections. Expert Rev Anti Infect Ther. 2006;4(2):291–302. doi: 10.1586/14787210.4.2.291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Espano E, Kim D, Kim J, Park SK, Kim JK. COVID-19 antiviral and treatment candidates: current status. Immune Netw. 2021;21(1):e7. doi: 10.4110/in.2021.21.e7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Group PTC, Dorward J, Yu L-M. Azithromycin for community treatment of suspected COVID-19 in people at increased risk of an adverse clinical course in the UK (PRINCIPLE): a randomised, controlled, open-label, adaptive platform trial. Lancet. 2021;397(10279):1063–1074. doi: 10.1016/S0140-6736(21)00461-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Geleris J, Sun Y, Platt J, et al. Observational study of hydroxychloroquine in hospitalized patients with Covid-19. N Engl J Med. 2020;382(25):2411–2418. doi: 10.1056/NEJMoa2012410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Horby P, Mafham M, Linsell L, et al. Effect of hydroxychloroquine in hospitalized patients with COVID-19: preliminary results from a multi-centre, randomized, controlled trial. medRxiv. 2020:2020.07.15.20151852. doi: 10.1101/2020.07.15.20151852 [DOI] [Google Scholar]
- 24.Singh B, Ryan H, Kredo T, Chaplin M, Fletcher T. Chloroquine or hydroxychloroquine for prevention and treatment of COVID-19. Cochrane Database Syst Rev. 2021;2:CD013587. doi: 10.1002/14651858.CD013587.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Piechotta V, Iannizzi C, Chai KL, et al. Convalescent plasma or hyperimmune immunoglobulin for people with COVID-19: a living systematic review. Cochrane Database Syst Rev. 2021;5(5):CD013600. doi: 10.1002/14651858.CD013600.pub4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Alhumaid S, Al Mutair A, Al Alawi Z, Alhmeed N, Zaidi ARZ, Tobaiqy M. Efficacy and safety of lopinavir/ritonavir for treatment of COVID-19: a systematic review and meta-analysis. Trop Med Infect Dis. 2020;5(4):180. doi: 10.3390/tropicalmed5040180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Consortium WHOST, Pan H, Peto R, et al. Repurposed antiviral drugs for Covid-19 - interim WHO solidarity trial results. N Engl J Med. 2021;384(6):497–511. doi: 10.1056/NEJMoa2023184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tong S, Su Y, Yu Y, et al. Ribavirin therapy for severe COVID-19: a retrospective cohort study. Int J Antimicrob Agents. 2020;56(3):106114. doi: 10.1016/j.ijantimicag.2020.106114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.World Helath Organization. Therapeutics and COVID-19: living guideline; 2021. Available from: https://www.who.int/publications/i/item/WHO-2019-nCoV-therapeutics-2021.3. Accessed March 5, 2021. [PubMed]
- 30.Nitazoxanide. COVID-19 treatment guidelines. @NIHCOVIDTxGuide. Available from: https://www.ncbi.nlm.nih.gov/pubmed/. Accessed March 5, 2022.
- 31.Gordon CJ, Tchesnokov EP, Schinazi RF, Gotte M. Molnupiravir promotes SARS-CoV-2 mutagenesis via the RNA template. J Biol Chem. 2021;297:100770. doi: 10.1016/j.jbc.2021.100770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jayk Bernal A, Gomes da Silva MM, Musungaie DB, et al. Molnupiravir for oral treatment of Covid-19 in nonhospitalized patients. New Engl J Med. 2021. doi: 10.1056/NEJMoa2116044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mahase E. Covid-19: pfizer’s paxlovid is 89% effective in patients at risk of serious illness, company reports. BMJ. 2021;375:n2713. doi: 10.1136/bmj.n2713 [DOI] [PubMed] [Google Scholar]
- 34.Manabe T, Kambayashi D, Akatsu H, Kudo K. Favipiravir for the treatment of patients with COVID-19: a systematic review and meta-analysis. BMC Infect Dis. 2021;21(1):489. doi: 10.1186/s12879-021-06164-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hassanipour S, Arab-Zozani M, Amani B, Heidarzad F, Fathalipour M, Martinez-de-hoyo R. The efficacy and safety of Favipiravir in treatment of COVID-19: a systematic review and meta-analysis of clinical trials. Sci Rep. 2021;11(1):11022. doi: 10.1038/s41598-021-90551-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ivashchenko AA, Dmitriev KA, Vostokova NV, et al. AVIFAVIR for treatment of patients with moderate COVID-19: interim results of a phase ii/iii multicenter randomized clinical trial. Clin Infect Dis. 2020. doi: 10.1093/cid/ciaa1176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.The Antibody Society. COVID-19 biologics tracker; 2021. Available from: https://www.antibodysociety.org/covid-19-biologics-tracker/. Accessed May 26, 2021.
- 38.Chen P, Nirula A, Heller B, et al. SARS-CoV-2 neutralizing antibody LY-CoV555 in outpatients with Covid-19. New Engl J Med. 2020;384(3):229–237. doi: 10.1056/NEJMoa2029849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Roche. Lilly’s neutralizing antibody bamlanivimab (LY-CoV555) prevented COVID-19 at nursing homes in the BLAZE-2 trial, reducing risk by up to 80 percent for residents; 2021. Available from: https://investor.lilly.com/news-releases/news-release-details/lillys-neutralizing-antibody-bamlanivimab-ly-cov555-prevented. Accessed June 1, 2021.
- 40.Gottlieb RL, Nirula A, Chen P, et al. Effect of bamlanivimab as monotherapy or in combination with etesevimab on viral load in patients with mild to moderate COVID-19: a Randomized Clinical Trial. JAMA. 2021;325(7):632–644. doi: 10.1001/jama.2021.0202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Roche. New Phase III data shows investigational antibody cocktail casirivimab and imdevimab reduced hospitalisation or death by 70% in non-hospitalised patients with COVID-19; 2021. Available from: https://www.roche.com/investors/updates/inv-update-2021-03-23.htm. Accessed June 1, 2021.
- 42.Regeneron. Regeneron reports positive interim data with regen-COV™ antibody cocktail used as passive vaccine to prevent COVID-19; 2021. Available from: https://investor.regeneron.com/news-releases/news-release-details/regeneron-reports-positive-interim-data-regen-covtm-antibody. Accessed June 1, 2021.
- 43.Celltrion announces positive interim results from phase i trial of CT-P59, an anti-COVID-19 monoclonal antibody treatment candidate. Available from: https://www.celltrionhealthcare.com/en-us/board/newsdetail?modify_key=364&keyword=&keyword_type=. Accessed March 5, 2022.
- 44.Ryu DK, Song R, Kim M, et al. Therapeutic effect of CT-P59 against SARS-CoV-2 South African variant. Biochem Biophys Res Commun. 2021;566:135–140. doi: 10.1016/j.bbrc.2021.06.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gupta A, Gonzalez-Rojas Y, Juarez E, et al. Early Covid-19 treatment with SARS-CoV-2 neutralizing antibody sotrovimab. medRxiv. 2021:2021.05.27.21257096. doi: 10.1101/2021.05.27.21257096 [DOI] [PubMed] [Google Scholar]
- 46.EMA. COVID-19: EMA recommends authorisation of antibody Medicine Xevud; 2021. Available from: https://www.ema.europa.eu/en/news/covid-19-ema-recommends-authorisation-antibody-medicine-xevudy. Accessed December 2021.
- 47.Tzou PL, Tao K, Nouhin J, et al. Coronavirus antiviral research database (CoV-RDB): an online database designed to facilitate comparisons between candidate anti-coronavirus compounds. Viruses. 2020;12(9):1006. doi: 10.3390/v12091006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.European Medicines Agency. First COVID-19 treatment recommended for EU authorisation. Available from: https://www.ema.europa.eu/en/news/first-covid-19-treatment-recommended-eu-authorisation. Accessed May 27, 2020.
- 49.Food and Drugs Administration. FDA approves first treatment for COVID-19. Available from: https://www.fda.gov/news-events/press-announcements/fda-approves-first-treatment-covid-19. Accessed May 27, 2021.
- 50.European Medicines Agency. Summary of opinion1 (post authorisation): veklury; 2021. Available from: https://www.ema.europa.eu/en/documents/smop/chmp-post-authorisation-summary-positive-opinion-veklury-ii-16_en.pdf. Accessed December 2021.
- 51.European Medicines Agency. Verklury, summary of product characteristics; 2021. Available from: https://www.ema.europa.eu/en/documents/product-information/veklury-epar-product-information_en.pdf. Accessed May 27, 2021.
- 52.Pruijssers AJ, George AS, Schafer A, et al. Remdesivir inhibits SARS-CoV-2 in human lung cells and chimeric SARS-CoV expressing the SARS-CoV-2 RNA polymerase in mice. Cell Rep. 2020;32(3):107940. doi: 10.1016/j.celrep.2020.107940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Williamson BN, Feldmann F, Schwarz B, et al. Clinical benefit of remdesivir in rhesus macaques infected with SARS-CoV-2. Nature. 2020;585(7824):273–276. doi: 10.1038/s41586-020-2423-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.de Wit E, Feldmann F, Cronin J, et al. Prophylactic and therapeutic remdesivir (GS-5734) treatment in the rhesus macaque model of MERS-CoV infection. Proc Natl Acad Sci U S A. 2020;117(12):6771–6776. doi: 10.1073/pnas.1922083117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Alonso Navarro R, Cuesta G, Camón AM, Rico V, Marcos MÁ, Viladomiu ÁS. Evolución de la carga viral normalizada (CVN), ARN genómico (ARNg) y sugenómico (ARNsg) en pacientes tratados con remdesivir (RDV). [Evolution of normalized viral load (NVC), genomic 460 RNA (gRNA) and subgenomic RNA (sgRNA) in patients treated with remdesivir (RDV)]. Presented at: XXIV CONGRESO NACIONAL SEIMC; 5–11 JUNIO 2021; 2021. Spanish. [Google Scholar]
- 56.Humeniuk R, Mathias A, Kirby BJ, et al. Pharmacokinetic, pharmacodynamic, and drug-interaction profile of REMDESIVIR, a SARS-CoV-2 replication inhibitor. Clin Pharmacokinet. 2021;60(5):569–583. doi: 10.1007/s40262-021-00984-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wang Y, Zhang D, Du G, et al. Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial. Lancet. 2020;395(10236):1569–1578. doi: 10.1016/S0140-6736(20)31022-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Goldman JD, Lye DCB, Hui DS, et al. Remdesivir for 5 or 10 days in patients with severe Covid-19. N Engl J Med. 2020;383(19):1827–1837. doi: 10.1056/NEJMoa2015301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Spinner CD, Gottlieb RL, Criner GJ, et al. Effect of remdesivir vs standard care on clinical status at 11 days in patients with moderate COVID-19: a Randomized Clinical Trial. JAMA. 2020;324(11):1048–1057. doi: 10.1001/jama.2020.16349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Beigel JH, Tomashek KM, Dodd LE, et al. Remdesivir for the treatment of Covid-19 - final report. N Engl J Med. 2020;383(19):1813–1826. doi: 10.1056/NEJMoa2007764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gottlieb RL, Vaca CE, Paredes R, et al. Early remdesivir to prevent progression to severe Covid-19 in outpatients. New Engl J Med. 2021. doi: 10.1056/NEJMoa2116846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Soriano A, Montejano R, Sanz-Moreno J, et al. Impact of remdesivir on the treatment of COVID-19 during the first wave in Spain. Adv Ther. 2021;38:4057–4069. doi: 10.1007/s12325-021-01804-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Agency EM. Global regulatory workshop on COVID-19 real-world evidence and observational studies; 2021. Available from: https://www.ema.europa.eu/en/news/global-regulatory-workshop-covid-19-real-world-evidence-observational-studies. Accessed June 16, 2021.
- 64.Grein J, Ohmagari N, Shin D, et al. Compassionate use of remdesivir for patients with severe Covid-19. N Engl J Med. 2020;382(24):2327–2336. doi: 10.1056/NEJMoa2007016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Garcia-Vidal C, Meira F, Cozar-Llisto A, et al. Real-life use of remdesivir in hospitalized patients with COVID-19. Rev Esp Quimioter. 2021;34(2):136–140. doi: 10.37201/req/018.2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Garibaldi BT, Wang K, Robinson ML, et al. Comparison of time to clinical improvement with vs without remdesivir treatment in hospitalized patients with COVID-19. JAMA Netw Open. 2021;4(3):e213071. doi: 10.1001/jamanetworkopen.2021.3071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Benfield T, Bodilsen J, Brieghel C, et al. Improved survival among hospitalized patients with COVID-19 treated with remdesivir and dexamethasone. A nationwide population-based cohort study. Clin Infect Dis. 2021. doi: 10.1093/cid/ciab536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mozaffari E, Chandak A, Zhang Z, et al. Remdesivir treatment in hospitalized patients with COVID-19: a comparative analysis of in-hospital all-cause mortality in a large multi-center observational cohort. Clin Infect Dis. 2021. doi: 10.1093/cid/ciab875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Olender SA, Perez KK, Go AS, et al. Remdesivir for severe COVID-19 versus a cohort receiving standard of care. Clin Infect Dis. 2020. doi: 10.1093/cid/ciaa1041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Go AS, Malenica I, Fusco D, et al. Remdesivir vs standard of care for severe COVID-19. presented at: WMF, Session Poster; 2021: WMF21–2969.
- 71.Ohl ME, Miller DR, Lund BC, et al. Association of remdesivir treatment with survival and length of hospital stay among us veterans hospitalized with COVID-19. JAMA Netw Open. 2021;4(7):e2114741–e2114741. doi: 10.1001/jamanetworkopen.2021.14741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Baracco GJ. Remdesivir use and hospital length of stay—the paradox of a clinical trial vs real-life use. JAMA Netw Open. 2021;4(7):e2116057–e2116057. doi: 10.1001/jamanetworkopen.2021.16057 [DOI] [PubMed] [Google Scholar]
- 73.Bhimraj A, Morgan RL, Shumaker AH, et al. Infectious diseases society of America guidelines on the treatment and management of patients with COVID-19. Available from: https://www.idsociety.org/COVID19guidelines#. Accessed March 5, 2022. [DOI] [PMC free article] [PubMed]
- 74.National Institutes of Health. COVID-19 treatment guidelines panel. coronavirus disease 2019 (COVID-19) treatment guidelines; 2021. Available from: https://www.covid19treatmentguidelines.nih.gov/. Accessed March 5, 2022. [PubMed]
- 75.Arribas JR, Garcia-Vidal C, Paño JR, et al. Recomendaciones SEIMC para el manejo Clínico de pacientes con COVID-19. Available from: https://seimc.org/contenidos/documentoscientificos/recomendaciones/seimc-rc-2020-COVID19-manejoclinico-v201209.pdf. Accessed June 2021.
- 76.World Health Organization. COVID-19 clinical management. Available from: https://www.who.int/publications/i/item/WHO-2019-nCoV-clinical-2021-1. Accessed June 2021.
- 77.Overview COVID-19 rapid guideline: managing COVID-19 | guidance | NICE. NICE; 2021. Available from: https://www.nice.org.uk/guidance/ng191. Accessed July 1, 2021.
- 78.Bartoletti M, Azap O, Barac A, et al. ESCMID COVID-19 Living guidelines: drug treatment and clinical management. Clin Microbiol Infect. 2021. doi: 10.1016/j.cmi.2021.11.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ministerio DS. Protocolo farmacoclínico del uso de remdesivir (veklury®) en el tratamiento de la enfermedad por covid-19 en el sistema nacional de salud. [Pharmacoclinical protocol for the use of remdesivir (veklury®) in the treatment of covid-19 disease in the national health system]. Available from: https://www.mscbs.gob.es/profesionales/farmacia/valtermed/docs/20200908_Protocolo_farmacoclinico_remdesivir2.pdf. Accessed June 2021. Spanish.
- 80.De Clercq E. Antivirals and antiviral strategies. Nat Rev Microbiol. 2004;2(9):704–720. doi: 10.1038/nrmicro975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Tang N, Bai H, Chen X, Gong J, Li D, Sun Z. Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. JTH. 2020;18(5):1094–1099. doi: 10.1111/jth.14817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Curran-Everett D, Milgrom H. Post-hoc data analysis: benefits and limitations. Curr Opin Allergy Clin Immunol. 2013;13(3):223–224. doi: 10.1097/ACI.0b013e3283609831 [DOI] [PubMed] [Google Scholar]
- 83.Armstrong RA, Kane AD, Kursumovic E, Oglesby FC, Cook TM. Mortality in patients admitted to intensive care with COVID-19: an updated systematic review and meta-analysis of observational studies. Anaesthesia. 2021;76(4):537–548. doi: 10.1111/anae.15425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Sundararaj Stanleyraj J, Sethuraman N, Gupta R, Thiruvoth S, Gupta M, Ryo A. Treating COVID-19: are we missing out the window of opportunity? J Antimicrob Chemother. 2021;76(2):283–285. doi: 10.1093/jac/dkaa442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lee S, Kim T, Lee E, et al. Clinical course and molecular viral shedding among asymptomatic and symptomatic patients with SARS-CoV-2 infection in a community treatment center in the Republic of Korea. JAMA Intern Med. 2020;180(11):1447–1452. doi: 10.1001/jamainternmed.2020.3862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Fajnzylber J, Regan J, Coxen K, et al. SARS-CoV-2 viral load is associated with increased disease severity and mortality. Nat Commun. 2020;11(1):5493. doi: 10.1038/s41467-020-19057-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Martin R, Li J, Parvangada A, et al. Genetic conservation of SARS-CoV-2 RNA replication complex in globally circulating isolates and recently emerged variants from humans and minks suggests minimal pre-existing resistance to remdesivir. Antiviral Res. 2021;188:105033. doi: 10.1016/j.antiviral.2021.105033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Reuschl AK, Thorne L, Zuliani Alvarez L, et al. Host-directed therapies against early-lineage SARS-CoV-2 retain efficacy against B.1.1.7 variant. bioRxiv. 2021. doi: 10.1101/2021.01.24.427991 [DOI] [Google Scholar]
- 89.BIO COVID-19 therapeutic development tracker | BIO; 2021. Available from: https://www.bio.org/policy/human-health/vaccines-biodefense/coronavirus/pipeline-tracker. Accessed June 1, 2021.