Abstract
Cuprous oxide (Cu2O) nanorods have been deposited on soda-lime glass substrates by the modified successive ionic layer adsorption and reaction technique by varying the concentration of NaCl electrolyte into the precursor complex solution. The structural, electrical and optical properties of synthesized Cu2O nanorod films have been studied by a variety of characterization tools. Structural analyses by X-ray diffraction confirmed the polycrystalline Cu2O phase with (111) preferential growth. Raman scattering spectroscopic measurements conducted at room temperature also showed characteristic peaks of the pure Cu2O phase. The surface resistivity of the Cu2O nanorod films decreased from 15 142 to 685 Ω.cm with the addition of NaCl from 0 to 4 mmol and then exhibited an opposite trend with further addition of NaCl. The optical bandgap of the synthesized Cu2O nanorod films was observed as 1.88–2.36 eV, while the temperature-dependent activation energies of the Cu2O films were measured as about 0.14–0.21 eV. Scanning electron microscope morphologies demonstrated Cu2O nanorods as well as closely packed spherical grains with the alteration of NaCl concentration. The Cu2O phase of nanorods was found stable up to 230°C corroborating the optical bandgap results of the same. The film fabricated in presence of 4 mmol of NaCl showed the lowest resistivity and activation energy as well as comparatively uniform nanorod morphology. Our studies demonstrate that the nominal presence of NaCl electrolytes in the precursor solutions has a significant impact on the physical properties of Cu2O nanorod films which could be beneficial in optoelectronic research.
Keywords: cuprous oxide, NaCl, physical properties, successive ionic layer adsorption and reaction, nanorod
1. Introduction
Cuprous oxide (Cu2O) is a p-type intrinsic semiconductor due to copper vacancies in the crystal lattice with the bandgap of approximately 2.17 eV [1,2] having several promising advantages such as high abundance, low-cost production, visible-light harvesting and non-toxicity. Cu2O has attracted interest as a good candidate material for photocatalysis [3,4] chemo sensing [5,6], electrode materials in lithium-ion batteries [7,8], photovoltaics [9,10] and photoelectrochemical water splitting [4,11]. Still, there are numerous methodological challenges in the research and application of Cu2O including the consistent synthesis of nanostructured Cu2O materials as well as the unavoidable formation of metallic Cu at the p-n heterojunction [12,13]. Moreover, the maximum theoretical limit of the efficiency of single-junction Cu2O is as high as 20% under air mass 1 solar illumination [13], which is far from the achieved results. Recently, Minami et al. [9] fabricated heterojunction solar cells by inserting an n-type zinc-germanium-oxide (Zn1−XGeXO) thin film between an Al-doped ZnO thin film and a p-type Na-doped Cu2O (Cu2O : Na) sheet prepared by thermally oxidized Cu sheets and reported the conversion efficiency of the cell as 8.1% [9]. On the other hand, Ci et al. [14] fabricated Cl-doped n-type Cu2O films by chemical bath deposition by using CuSO4 solution with the addition of CuCl2 as a Cl− source. Therefore, it looks crucial to study the influence of Na and Cl individually or the electrolytic behaviour of NaCl in a broad spectrum on Cu2O film deposition.
There are various methods to synthesis Cu2O thin films such as atomic layer deposition [15], electrochemical deposition [16–19], metal-organic chemical vapour deposition [20], molecular beam epitaxy [21], successive ionic layer adsorption and reaction (SILAR) [22–24], the direct oxidation of Cu sheets [25], sputtering [26], vapour phase epitaxy [27] and sol–gel technique [28]. However, towards the preparation of nanostructured Cu2O, the electrodeposition of Cu2O thin films allows the greatest control yet [23–26]. To the best of our knowledge, there is no study about the SILAR deposition of Cu2O nanorod films until now. Moreover, chemical bath optimization for epitaxial growth of Cu2O nanorods from surfaces has not yet been reported [29].
SILAR is basically one of the most simple and cost-effective methods since it does not require sophisticated apparatus as required in electrodeposition or other methods. In this study, a modified SILAR method was used to synthesize Cu2O nanostructured thin films. In our prior report, we have shown the modification [22–24] of SILAR by eliminating the rinsing steps during the growth of pure Cu2O thin films and named the method as the modified (m)-SILAR technique. Therefore, we synthesized Cu2O nanorods on SLG substrates by the m-SILAR method in the nominal presence of NaCl into the precursor solution complex. The objective of this work was to study the impact of the NaCl electrolyte concentrations on the growth of Cu2O nanorod films through the investigation of their structural, morphological, optical and electrical properties for photovoltaic applications.
2. Materials
In this work, sodium thiosulfate pentahydrate (Na2S2O3.5H2O; Scharlau: purity approx. 99.0%), copper (II) sulfate pentahydrate (CuSO4.5H2O; Merck Millipore: purity approx. 99.0%), sodium hydroxide (NaOH; Active fine chemicals: purity approx. 98.0%) and sodium chloride (NaCl; Merck Millipore: purity approx. 99%) were collected from the local market and used without further refinement. Soda-lime glass (SLG) microscopy slides (25 × 25 × 1 mm3) were used as substrates to deposit copper oxide thin films.
2.1. Synthesis of copper (I) oxide thin films
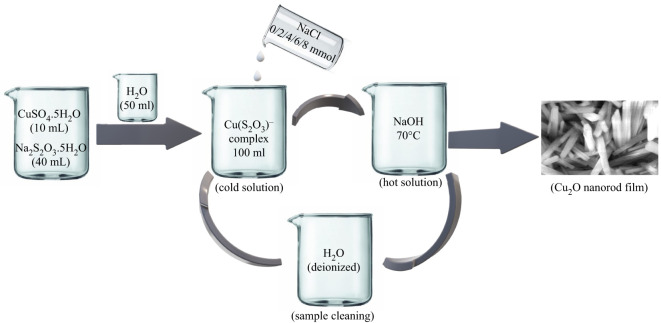
Cu2O thin films were deposited on SLG substrates as shown in figure 1 by using the same method described in our previous work [22]. Briefly, the SLG substrates were initially cleaned by detergent to remove loosely attached visible dust particles. Then, the substrates were successively cleaned in an ultrasonic water bath through deionized (DI) water, ethanol, toluene and isopropanol for 15 min in each case. Prior to the film deposition, 10 ml 1 M copper (II) sulfate and 40 ml 1 M sodium thiosulfate solution were added into a 100 ml volumetric flask until the colourless solution of copper-thiosulfate complex appeared. Then, a 2 mmol NaCl electrolyte was added into the same flask and after shaking the remaining portion was filled with DI water. This complex solution was labelled as a cold solution. A 2 M NaOH solution was kept at 70°C (hot solution). After that, the SLG substrate was alternatively immersed in hot and cold solution respectively for 23 s each and had completed one SILAR cycle. This process was repeated for up to 40 immersion cycles. OH− and Cu+ ions were adsorbed on the substrate respectively when immersed in a hot and cold solution. Consequently, Cu2O thin films were deposited on the substrate owing to the following chemical reactions:
and
Figure 1.
Synthesis of copper (I) oxide nanorod thin films.
After deposition, this as-made sample was washed through DI water to eliminate loosely bound particles and dried naturally in the laboratory ambient. Other samples were prepared in a similar way and stored safely in an air-tight sample box for future characterization purposes. The growth mechanism of Cu2O nanorods are discussed later in the surface morphology section with the support of scanning electron microscope (SEM) micrographs.
2.2. Characterization process
The crystal structure and phase present in the samples were examined through an X-ray diffraction (XRD) spectrometer (Philips PANalytical X'Pert MRD) under θ–2θ coupled mode with CuKα radiation source of wavelength, λ = 0.15406 nm as well as a sensitive Raman scattering spectrometer (Horiba HR800) where the excitation radiation was 488 nm laser source. SEM (Philips XL30 EEG) was employed to investigate the morphological properties of the samples. An ultraviolet-visible-near-infrared (UV-Vis-NIR) spectrophotometer (Shimadzu UV 2600 ISR Plus) of wavelength, λ = 220–1400 nm was applied to study the optical response of the deposited samples. A homemade four-point collinear probe coupled with a Keithley SMU2450 was used to measure the surface resistivity of the samples. Temperature-dependent surface resistivity was measured by air annealing the samples from 30°C to 230°C through a homemade two-probe system coupled with a digital multimeter (BK Precision 2704C).
3. Results and discussion
3.1. Crystal structure and phase identification
The crystal structure and phases present in the samples deposited on SLG substrate have been examined through XRD spectroscopy under θ−2θ coupled mode in the range 30°−70°, and the relevant XRD pattern is illustrated in figure 2a. All the samples exhibited distinguished peaks at 2θ ≈ 36.5°, 42.5° and 61.5°, respectively, which were assigned to the (111), (200) and (220) plane of pure cubic phase of Cu2O only that matches to the high-quality inorganic crystal structure database (ICSD) of phase pure Cu2O (ICSD PDF no. 180846) [22], and none of the peaks from Cu or CuO phases were present. Figure 2a reveals that all the films were polycrystalline in nature with (111) preferential growth. The intensity of the (111) plane of Cu2O is increased with increasing the concentration of NaCl electrolyte (2–8 mmol) which indicates the improvement of the crystalline quality of the deposited films [23].
Figure 2.
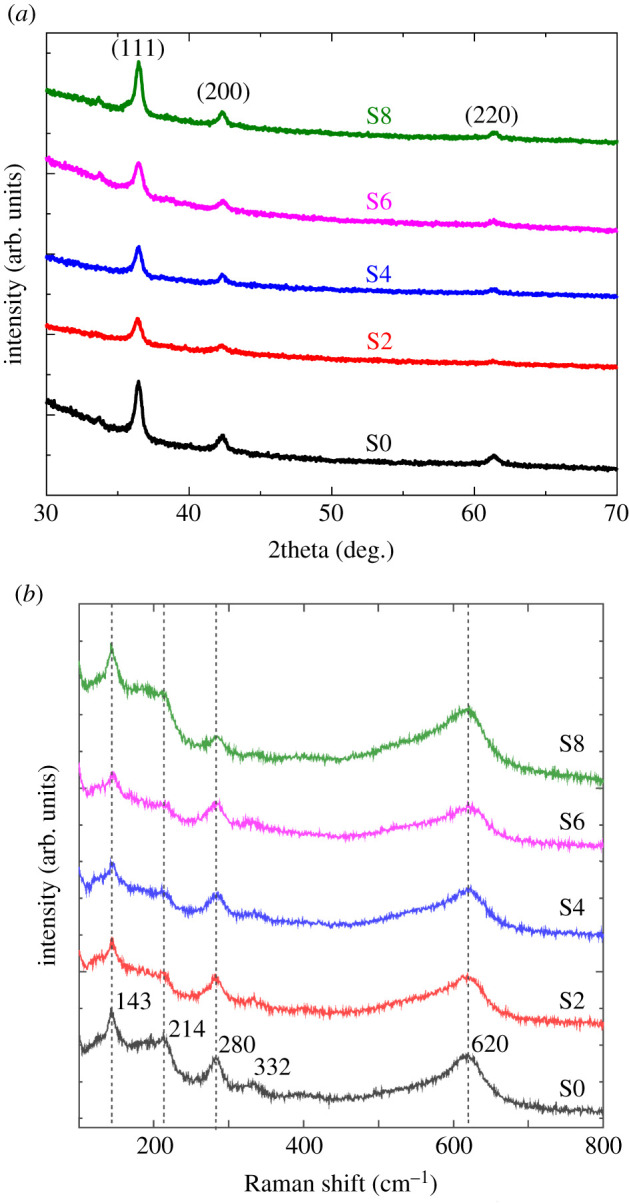
(a) XRD pattern and (b) Raman spectra of the samples deposited on SLG substrate in the presence of an NaCl electrolyte with various concentrations.
The texture coefficient (TC) was assessed to describe the crystallographic nature of the deposited films by using the ratio, TC (hkl) : , where I(111) and I(200) were the intensity of (111) and (200) planes, respectively [22], and the calculated values are shown in table 1. The average crystallite size (D) was determined by using the Scherrer formula [30], and it was 14.91–16.51 nm. The values of some other structural parameters are listed in table 1. The increasing value of TC, similarly shifting of 2θ values to the higher diffraction angle with respect to the reference 2θ value (marked by footnote a (ref.a) in table 1 and the respective plot is inserted in figure 3b, line), signified the improvement of the crystalline quality of the deposited films in presence of a higher concentration of NaCl electrolyte [31]. Variations of crystallite size and TC are shown in figure 3a. Although the little amount of NaCl electrolyte (2 mmol) deteriorates the crystalline quality of the film in the case of sample S2 with respect to S0 (zero NaCl), but at higher NaCl concentration, the nano crystallinity of the films was improved which can be seen from figure 3a. In this case, sample S8 showed the highest crystallinity among all the samples having the largest crystallite size (16.51 nm) with the minimum dislocation density and micro strain.
Table 1.
Structural parameters of the deposited films. (FWHM, full width at half maximum.)
| sample | conc. of NaCl (mmol) | 2θ (°) | d(111) (nm) | a (nm) | TC (hkl) | FWHM (°) | crystallite (nm) | dislocation density δ × 10−3 (nm−2) | strain ε × 10−3 |
|---|---|---|---|---|---|---|---|---|---|
| ref.a | 0 | 36.42 | 0.2465 | 0.4270 | 0.75 | 0.33 | 27.00 | 1.372 | 1.37 |
| S0 | 0 | 36.46 | 0.2462 | 0.4265 | 0.65 | 0.50 | 15.94 | 3.94 | 2.17 |
| S2 | 2 | 36.40 | 0.2466 | 0.4272 | 0.62 | 0.52 | 15.24 | 4.30 | 2.27 |
| S4 | 4 | 36.47 | 0.2462 | 0.4264 | 0.63 | 0.53 | 14.91 | 4.50 | 2.31 |
| S6 | 6 | 36.47 | 0.2462 | 0.4264 | 0.63 | 0.56 | 14.14 | 5.00 | 2.45 |
| S8 | 8 | 36.47 | 0.2462 | 0.4264 | 0.66 | 0.48 | 16.51 | 3.67 | 2.09 |
aCu2O powder (purity: 99.99%).
Figure 3.

(a) Variation of crystallite size and texture coefficient with respect to NaCl concentration and (b) shifted 2θ values with respect to the reference one (indicated by the vertical line).
To further confirm the phases of the deposited films, the sensitive Raman scattering spectroscopy was also used, and the corresponding Raman spectra is given in figure 2b. Raman peaks at 143, 214, 280, 332 and 620 cm−1 corresponds to the Cu2O phase only [26,32,33] which perfectly coincided with the demonstrated XRD results and has an alike pattern with ref. [34]. Thus, the addition of an NaCl electrolyte to a cationic precursor solution unaltered the chemical environment of the deposited films while improving nano crystallinity.
3.2. Morphological analysis
Figure 4 depicts the SEM micrographs of the samples deposited in presence of an NaCl electrolyte at various concentrations. It is observed that the morphologies are crack-free and very well distributed on the substrate surface. The sample deposited without an NaCl electrolyte revealed pencil-thin nanorod surface morphology with an overgrown cluster in some regions, as also observed in our previous report [23]. When the electrolyte started to be added to the solution, such as 2 mmol of NaCl, the crowded nanorods developed, and with the rise of concentration of NaCl to 4 mmol, the nanorod formation enhanced, having a larger size and shape as distinguished in figure 4c. When NaCl electrolyte concentration reached 6 mmol, very rough, tiny and dense spherical grains as well as some overgrown clusters were seen. An overgrown cluster was formed owing to the coalescence of the particles [35]. Further addition of NaCl concentration was culminated at 8 mmol and exhibited distinctively distributed, clear and larger sized spherical grains. Thus, the increasing content of NaCl electrolyte changed surface morphologies from nanorods to spherical grains which have potential influences in electrical and optical properties described in the later sections.
Figure 4.
Surface morphologies of the samples deposited at (a) 0 mmol, (b) 2 mmol, (c) 4 mmol, (d) 6 mmol, and (e) 8 mmol of NaCl electrolyte.
The morphology of the Cu2O nanostructures was sensitive to the concentration of salts added as also reported for CuO [36]. When NaCl concentration was increased gradually, the growth of nanorods also increased but until a limit such as 4 mmol of NaCl addition. These phenomena indicate that NaCl concentration will result in similar morphology of the product and play key roles in controlling the size and shape of the Cu2O nanostructures. Besides the above-mentioned reasons, the steric hindrance effect caused by salt concentration should also influence the micelle aggregates, and these effects together result in the assemblies of the products. Further studies and work are ongoing to additional research of the mechanisms for the fabrication process caused by the new proposed route.
3.3. Electrical properties
The electrical resistivities of the samples were measured by using a homemade four-point collinear probe that was reported in our previous work [22]. Measurements were taken at several regions of the sample under investigation, and the results are the representative average values of all measurements summarized in table 2. Thickness as well as the type of conductivity of films was measured by a similar technique described in [22].
Table 2.
Electrical properties of the deposited films.
| sample ID | thickness (nm) | sheet resistance (MΩ/square) | surface resistivity (Ω.cm) | activation energy Ea (eV) | types of conductivity | bandgap of samples Eg (eV) |
|
|---|---|---|---|---|---|---|---|
| as deposited | annealed | ||||||
| S0 | 1350 ± 80 | 33.46 ± 4.23 | 15 142 ± 33.84 | 0.16 ± 0.02 | p-type | 2.36 | 2.04 |
| S2 | 1270 ± 70 | 2.95 ± 1.14 | 1256 ± 7.98 | 0.21 ± 0.01 | p-type | 2.04 | 1.85 |
| S4 | 1610 ± 23 | 1.27 ± 0.11 | 685 ± 0.25 | 0.14 ± 0.02 | p-type | 1.96 | 1.96 |
| S6 | 1060 ± 10 | 4.40 ± 1.71 | 1563 ± 1.71 | 0.19 ± 0.02 | p-type | 2.0 | 2.02 |
| S8 | 630 ± 10 | 4.82 ± 0.97 | 1018 ± 0.97 | 0.15 ± 0.02 | p-type | 2.24 | 1.88 |
From table 2, the surface resistivity values were found in between 15,142 and 685 Ω.cm. Thicknesses were used to calculate the surface resistivity of the samples. The average thickness of the film is 1184 nm. The changes in surface resistivity with thicknesses is shown in figure 5a. It is clearly visible that sample S4 showed the maximum thickness with the minimum resistivity among all the samples. With increasing the content of the NaCl electrolyte into the cationic precursor solution, the surface resistivity (S2–S8) is reduced as compared to the sample deposited without NaCl (S0). The resistivity value dropped to 685 Ω.cm in the sample deposited at 4 mmol NaCl electrolyte (S4) which is about 22 times lower than sample S0.
Figure 5.
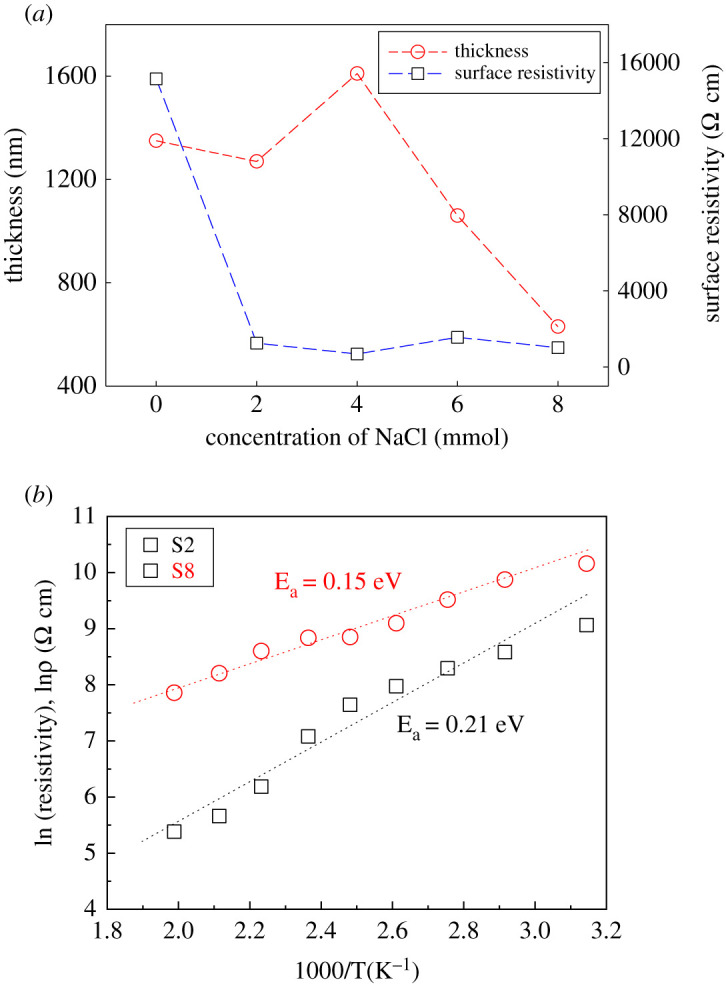
(a) Variation of surface resistivity with thickness and (b) temperature-dependent activation energy of the samples.
The change of surface resistivity with increasing content of NaCl electrolyte may be explained based on the SEM micrographs observed in figure 4. As the density, size and shape of nanorod were increased with increasing the content of NaCl up to 4 mmol as observed from figure 4a–c, the probability of passing the electrical current through the sample S4 is maximum and consequently the minimum order of surface resistivity (S4, ). Moreover, for the sample deposited at 6 mmol NaCl (S6), the surface was very rough and dense as seen from figure 4d, which may be the cause of a little bit of high resistivity in comparison to sample S4. Furthermore, sample S8 showed lower resistivity than S6 owing to the well-distributed and larger spherical grains, which is also understandable by the look of the micrographs in figure 4d,e.
The type of conductivity was determined by using the hot-probe method [22]. The temperature-dependent surface resistivity (ρ) was also measured to determine the activation energy of the samples. Resistivity value was measured for respective surface temperature of the deposited film in the range between 30°C and 230°C. The values were calculated by using the following Arrhenius equation [7]:
| 3.1 |
and
| 3.2 |
where ρ is the surface resistivity of the films at a specific temperature, is the activation energy, T is the temperature in Kelvin, is the proportionality constant and is the Boltzmann constant (8.617 × 10−5 eV K−1). By plotting log ρ versus 1000/T as shown in figure 5b, the activation energy Ea is obtained as listed in table 2. Likewise, the surface resistivity, the activation energy followed the same trend, and the values were found to be 0.14–0.21 eV. The measured values were perfectly matched with the reported results [24,37], and in this study, the sample deposited with 4 mmol NaCl electrolyte exhibited the lowest resistivity and activation energy as well as well-distributed nanorods. Hence, the sample S4 has preferentially shown better quality than the other deposited Cu2O samples. Also overall, the significant effect of adding NaCl electrolyte in the case of depositing Cu2O thin films has been pronounced.
3.4. Optical analysis
To assess the optical characteristics of the samples deposited at various NaCl electrolyte concentrations, the diffuse reflectance spectra of the samples has been taken. UV-Vis-NIR ranges of wavelength 220–1400 nm have been passed through the samples from the respective light sources, and the recorded diffuse reflectance spectra are shown in figure 6.
Figure 6.
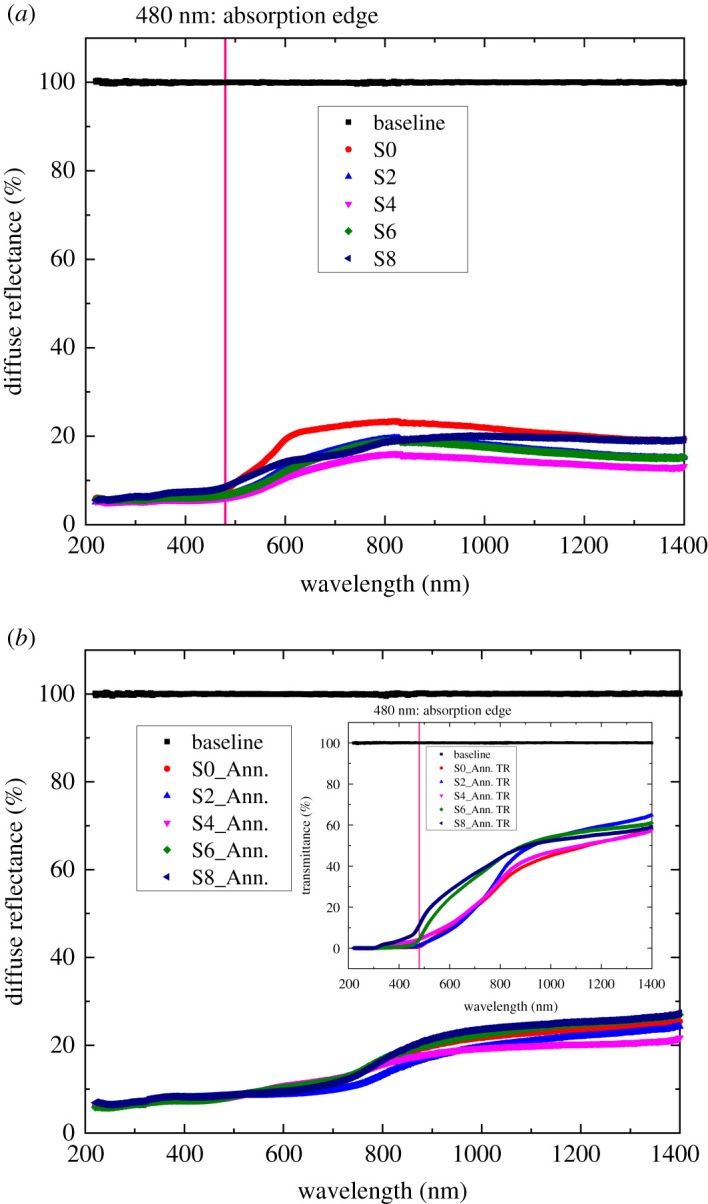
Diffuse reflectance spectra of (a) as-deposited and (b) annealed samples. (The absorption edge is drawn at λ ≈ 480 nm by the pink line, and the transmittance data are shown inset of (b) to clarify the phase present in the annealed samples.)
From figure 6a, it is observed that a strong absorption edge occurs at wavelength, λ ≈ 480 nm which corresponds to the Cu2O phase only. No other phase such as CuO is absent which was preliminarily confirmed from both XRD and Raman spectra, those were shown in figure 2a,b. In our study, the diffuse reflectance spectra were mainly taken to eliminate any effect that may come from the SLG substrates. The reflectance was seen about 10–20%. To investigate the NaCl electrolyte concentration on the optical band gap (Eg) of the deposited films, a Tauc plot was drawn by using reflectance data in correlation with the Kubelka–Munk function F(R∞) of the following equation [3]:
| 3.3 |
where, h is the Planck's constant (6.626 × 10−34 JS), is the optical bandgap, is the diffuse reflectance, A is the proportionality constant, is the frequency of the incident photon, n = 1/2 for indirect band gap semiconductor and n = 2 for direct band gap semiconductor. By plotting versus hν (putting n = 2 for direct band gap Cu2O semiconductor) and subsequently extrapolated to the x-axis, the curves of the following types were found for as-deposited and annealed samples, and the respective values are shown in table 2.
From table 2, it is seen that for as-deposited samples the values were 2.0–2.36 eV [38], whereas it was 1.88–2.04 eV for annealed samples [23]. In the case of as-deposited samples (figure 7a), the value dropped from 2.36 to 1.96 eV and then again increased up to 2.24 eV. The sample deposited at 4 mmol NaCl electrolyte showed the lowest bandgap among all the samples having the smallest surface resistivity (685 Ω.cm) and activation energy (0.14 eV) as well as the well-distributed nanorods that are observed from figure 4c. On the other hand, air annealing lowered the values those observed from table 2 and figure 7b, and the value was approximately 1.95 eV. The former reports [23,39] and current observed values can conclude that air annealing lowered the band gap but did not change the Cu2O phase into CuO up to annealing at 230°C (cleared from the inset transmittance absorption spectra shown in figure 6b). Hence, air annealing is beneficial to lower the band gap.
Figure 7.
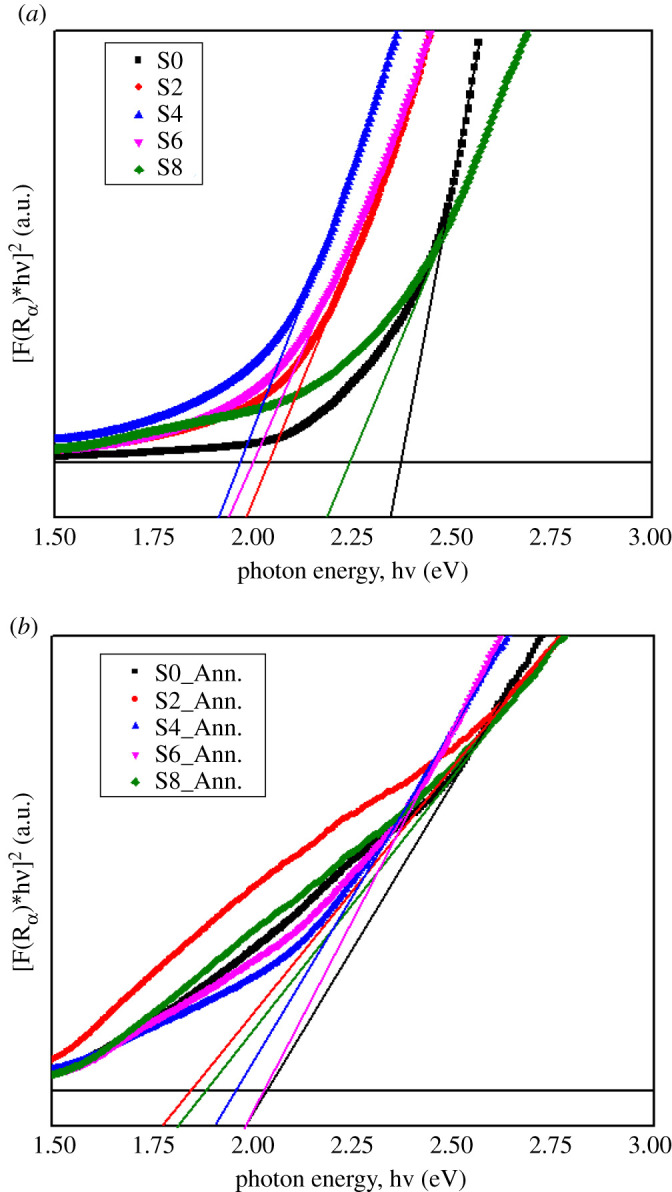
Optical band gap plot of (a) as-deposited and (b) annealed samples deposited at various NaCl electrolyte concentrations.
4. Conclusion
In this research, we synthesized Cu2O nanorod films by the variation of concentration of NaCl on top of simple SLG by the modified SILAR technique, and the structural, electrical and optical properties of the nanorod films have been investigated. Structural analysis by XRD and Raman validates the polycrystalline pure Cu2O phase with (111) preferential growth. The SEM micrographs reveal that the deposited films were nanorod structures and closely packed spherical grains, formed with the variation of NaCl concentration. The optical band gap of Cu2O films estimated by UV-VIS-NIR spectroscopy was observed to be in the range of 1.88–2.36 eV and consistent with the reported results in the literature. The resistivity of the Cu2O nanorod films decreased from 15 142 to 685 Ω.cm with the addition of NaCl from 0 to 4 mmol, while the temperature-dependent activation energies of the films were found as about 0.14–0.21 eV. The film optimized in presence of 4 mmol of NaCl demonstrated the lowest resistivity, activation energy and excellent nanorod growth. These results could eventually demand significant attention in the photovoltaic community and research into the development of ecofriendly as well as cost-effective Cu2O nanorod film-based optoelectronics.
Acknowledgements
M.A.M.P., M.A.R. and M.A.H. happily acknowledge the laboratory facilities at Physical Chemistry Research Laboratory of Comilla University, Cumilla, Bangladesh. S.F.U.F. and N.I.T. gratefully acknowledge the experimental support of the Energy Conversion and Storage Research (ECSR) Section, Industrial Physics Division (IPD), BCSIR Laboratories, Dhaka, Bangladesh. All the authors thankfully acknowledge the experimental support from the Optoelectronics laboratory of Saga University, Saga, Japan, and Centre for Nanotechnology, Department of Natural Sciences, Coppin State University, Baltimore, MD, USA.
Contributor Information
Syed Farid Uddin Farhad, Email: sf1878@my.bristol.ac.uk.
Md Abdul Majed Patwary, Email: mamajedp@gmail.com.
Ethics
The work has been started and approved by the Department of Chemistry, Comilla University, Bangladesh and Energy Conversion and Storage Research (ECSR) Section, Industrial Physics Division (IPD), BCSIR Laboratories, Dhaka, Bangladesh as one of the MS thesis research projects done by M. A. Hossain.
Data accessibility
Our data are deposited at Dryad Digital Repository: https://doi.org/10.5061/dryad.37pvmcvmm [40].
Authors' contributions
M.A.H.: data curation, formal analysis, investigation, methodology, writing—original draft, writing—review and editing; S.F.U.F.: funding acquisition, investigation, methodology, resources, writing—review and editing; N.I.T.: data curation, investigation, methodology, writing—review and editing; J.H.C.: data curation, formal analysis, investigation; M.A.R.: data curation, formal analysis, investigation, methodology; T.T.: investigation, methodology, resources, writing—review and editing; Q.G.: data curation, investigation, resources, writing—review and editing; J.U.: investigation, methodology, writing—review and editing; M.A.M.P.: conceptualization, data curation, funding acquisition, investigation, methodology, supervision, writing—review and editing.
All authors gave final approval for publication and agreed to be held accountable for the work performed therein.
Competing interests
We declare we have no competing interests.
Funding
M.A.M.P. and M.A.R. acknowledge the funding of Comilla University, Cumilla, and UGC, Bangladesh. S.F.U.F. and N.I.T. gratefully acknowledge the funding support of the Energy Conversion and Storage Research (ECSR) Section, Industrial Physics Division (IPD), BCSIR Laboratories, Dhaka under the scope of R&D project no. 100-FY2017-2021. S.F.U.F. also acknowledges the support of RSC research grant no. R20-3167 for ECSR, IPD.
References
- 1.Nolan M, Elliott SD. 2006. The p-type conduction mechanism in Cu2O: a first principles study. Phys. Chem. Chem. Phys. 8, 5350-5358. ( 10.1039/b611969g) [DOI] [PubMed] [Google Scholar]
- 2.Raebiger H, Lany S, Zunger A. 2007. Origins of the p-type nature and cation deficiency in Cu2O and related materials. Phys. Rev. B 76, 045209. ( 10.1103/PhysRevB.76.045209) [DOI] [Google Scholar]
- 3.Garrison A. 1924. The photo-chemical properties of cuprous oxide. J. Phys. Chem. 28, 279-284. ( 10.1021/j150237a009) [DOI] [Google Scholar]
- 4.Hara M, Kondo T, Komoda M, Ikeda S, Shinohara K, Tanaka A, Kondo JN, Domen K. 1998. Cu2O as a photocatalyst for overall water splitting under visible light irradiation. Chem. Commun. 1998, 357-358. ( 10.1039/a707440i) [DOI] [Google Scholar]
- 5.Zhang J, Liu J, Peng Q, Wang X, Li Y. 2006. Nearly monodisperse Cu2O and CuO nanospheres: preparation and applications for sensitive gas sensors. Chem. Mater. 18, 867-871. ( 10.1021/cm052256f) [DOI] [Google Scholar]
- 6.Deng S, Tjoa V, Fan HM, Tan HR, Sayle DC, Olivo M, Mhaisalkar S, Wei J, Sow CH. 2012. Reduced graphene oxide conjugated Cu2O nanowire mesocrystals for high-performance NO2 gas sensor. J. Am. Chem. Soc. 134, 4905-4917. ( 10.1021/ja211683m) [DOI] [PubMed] [Google Scholar]
- 7.Fu LJ, Gao J, Zhang T, Cao Q, Yang LC, Wu YP, Holze R, Wu HQ. 2007. Preparation of Cu2O particles with different morphologies and their application in lithium-ion batteries. J. Power Sources 174, 1197. ( 10.1016/j.jpowsour.2007.06.030) [DOI] [Google Scholar]
- 8.Poizot P, Laruelle S, Grugeon S, Dupont L, Taracon JM. 2000. Nano-sized transition-metal oxides as negative-electrode materials for lithium-ion batterie. Nature 407, 496-499. ( 10.1038/35035045) [DOI] [PubMed] [Google Scholar]
- 9.Minami T, Nishi Y, Miyata T. 2016. Efficiency enhancement using a Zn1-xGex-O thin film as an n-type window layer in Cu2O-based heterojunction solar cells. Appl. Phys. Exp. 9, 052301. ( 10.7567/APEX.9.052301) [DOI] [Google Scholar]
- 10.Rai BP. 1988. Cu2O solar cells: a review. Sol. Cells 25, 265-272. ( 10.1016/0379-6787(88)90065-8) [DOI] [Google Scholar]
- 11.Yuan WY, Yuan J, Xie JL, Li MC. 2016. Polymer-mediated self-assembly of TiO2@Cu2O core shell nanowire array for highly efficient photoelectrochemical water oxidation. ACS Appl. Mater. Interfaces 8, 6082-6092. ( 10.1021/acsami.6b00030) [DOI] [PubMed] [Google Scholar]
- 12.Olsen LC, Bohara RC, Urie MW. 1979. Explanation for low efficiency Cu2O Schottky barrier solar cells. Appl. Phys. Lett. 34, 47-49. ( 10.1063/1.90593) [DOI] [Google Scholar]
- 13.Olsen LC, Addis FW, Miller W. 1982. Experimental and theoretical studies of Cu2O solar cells. Sol. Cells 7, 247-279. ( 10.1016/0379-6787(82)90050-3) [DOI] [Google Scholar]
- 14.Ci JW, Tu WC, Uen WY, Lan SM, Zeng JX, Yang TN, Shen CC, Jhao JC. 2014. Chlorine-doped n-type cuprous oxide films fabricated by chemical bath deposition. J. Ele. Chem. Soc. 161, D321-D326. ( 10.1149/2.013406jes) [DOI] [Google Scholar]
- 15.Marin AT, Muñoz-Rojas D, Iza DC, Gershon T, Musselman KP, MacManus-Driscoll JL. 2013. Novel atmospheric growth technique to improve both light absorption and charge collection in ZnO/Cu2O thin film solar cells. Adv. Funct. Mater. 23, 3413-3419. ( 10.1002/adfm.201203243) [DOI] [Google Scholar]
- 16.Golden TD, Shumsky MG, Zhou Y, Vander Werf RA, Van Leeuwen RA, Switzer JA. 1996. Electrochemical deposition of copper (I) oxide films. Chem. Mater. 8, 2499-2504. ( 10.1021/cm9602095) [DOI] [Google Scholar]
- 17.Farhad SFU, Hossain MM, Tanvir NI, Islam S. 2020. Texture and bandgap tuning of phase pure Cu2O thin films grown by a simple potentiostatic electrodeposition technique. In ECS meeting abstracts. Vol. M A 2021-01. Canada: IOP Science. See 10.1149/MA2020-01191212mtgabs. [DOI] [Google Scholar]
- 18.Wang W, Wu D, Zhang Q, Wang L, Tao M. 2010. pH-dependence of conduction type in cuprous oxide synthesized from solution. J. Appl. Phys. 107, 123717. ( 10.1063/1.3452383) [DOI] [Google Scholar]
- 19.Septina W, Ikeda S, Khan MA, Hirai T, Harada T, Matsumura M, Peter LM. 2011. Potentiostatic electrodeposition of cuprous oxide thin films for photovoltaic applications. Electrochim. Acta. 56, 4882-4888. ( 10.1016/j.electacta.2011.02.075) [DOI] [Google Scholar]
- 20.Jeong S, Aydil ES. 2009. Heteroepitaxial growth of Cu2O thin film on ZnO by metal organic chemical vapor deposition. J. Cryst. Growth 311, 4188-4192. ( 10.1016/j.jcrysgro.2009.07.020) [DOI] [Google Scholar]
- 21.Oshima T, Nohara M, Hoshina T, Takeda H, Tsurumi T. 2014. Characterization of Cu2O thin film grown by molecular beam epitaxy. Key Eng. Mater. 582, 157-160. ( 10.4028/www.scientific.net/KEM.582.157) [DOI] [Google Scholar]
- 22.Farhad SFU, Hossain MA, Tanvir NI, Akter R, Patwary MAM, Shahjahan M, Rahman MA. 2019. Structural, optical, electrical, and photoelectrochemical properties of cuprous oxide thin films grown by modified SILAR method. Mater. Sci. Semicond. Proc. 95, 68. ( 10.1016/j.mssp.2019.02.014) [DOI] [Google Scholar]
- 23.Farhad SFU, Majumder S, Hossain MA, Tanvir NI, Akter R, Patwary MAM. 2019. Effect of solution pH and post-annealing temperatures on the optical bandgap of the copper oxide thin films grown by modified SILAR method. MRS Adv. 4, 937. ( 10.1557/adv.2019.139) [DOI] [Google Scholar]
- 24.Majumder S, Tanvir NI, Ghos BC, Patwary MAM, Rahman MA, Hossain MA, Farhad SFU. 2020. Optimization of the growth conditions of Cu2O thin films and subsequent fabrication of Cu2O/ZnO heterojunction by m-SILAR method. IEEE WIECON-ECE 139-142. ( 10.1109/WIECON-ECE52138.2020.9397989) [DOI] [Google Scholar]
- 25.Wagner A, Stahl M, Ehrhardt N, Fahl A, Ledig J, Waag A, Bakin A. 2014. Oxides for sustainable photovoltaics with earth-abundant materials. In Proc. SPIE8987, Oxide-based materials and devices V, 898726–898729. USA: SPIE digital library. [Google Scholar]
- 26.Patwary MAM, Saito K, Guo Q, Tanaka T. 2019. Influence of oxygen flow rate and substrate positions on properties of Cu-oxide thin films fabricated by radio frequency magnetron sputtering using pure Cu target. Thin Solid Films 675, 59. ( 10.1016/j.tsf.2019.02.026) [DOI] [Google Scholar]
- 27.Wagner A, Scherg-Kurmes H, Waag A, Bakin A. 2013. Vapour phase epitaxy Cu2O a-plane Al2O3. Phys. Status Solidi C 10, 1284-1287. ( 10.1002/pssc.201200951) [DOI] [Google Scholar]
- 28.Kim SY, Ahn CH, Lee JH, Kwon YH, Hwang S, Lee JY, Cho HK. 2013. P-channel oxide thin film transistors using solution-processed copper oxide. ACS Appl. Mater. Interfaces 5, 2417-2421. ( 10.1021/am302251s) [DOI] [PubMed] [Google Scholar]
- 29.Haynes KM, et al. 2015. Templated electrodeposition, and photocatalytic activity of cuprous oxide nanorod arrays. ACS Appl. Mater. Interfaces 7, 830-837. ( 10.1021/am507244q) [DOI] [PubMed] [Google Scholar]
- 30.Daoudi O, Qachaou Y, Raidou A, Nouneh K, Lharch M, Fahoume M. 2019. Study of the physical properties of CuO thin films grown by modified SILAR method for solar cells applications. Superlattices Microstruct. 127, 93-99. ( 10.1016/j.spmi.2018.03.006) [DOI] [Google Scholar]
- 31.Visalakshi S, Kannan R, Valanarasu S, Kathalingam A, Rajashabala S. 2017. Studies on optical and electrical properties of SILAR-deposited CuO thin films. Mater. Res. Innov. 21, 146-151. ( 10.1080/14328917.2016.1194586) [DOI] [Google Scholar]
- 32.Farhad SFU, Cherns D, Smith JA, Fox NA, Fermín DJ. 2020. Pulsed laser deposition of single-phase n- and p-type Cu2O thin films with low resistivity. Mater. Des. 193, 108848. ( 10.1016/j.matdes.2020.108848) [DOI] [Google Scholar]
- 33.Patwary MAM, Ho CY, Saito K, Guo Q, Yu KM, Walukiewicz W, Tanaka T. 2020. Effect of oxygen flow rate on properties of Cu4O3 thin films fabricated by radio frequency magnetron sputtering. J. Appl. Phys. 127, 085302. ( 10.1063/1.5144205) [DOI] [Google Scholar]
- 34.Pastor LH, Becerril TD, Arellano MG, Sierra RP. 2020. Sodium doping of Cu2O layers by reactive annealing of Cu2O covered with a NaCl nano-film in a low-oxygen atmosphere. Thin Solid Films 693, 137711. ( 10.1016/j.tsf.2019.137711) [DOI] [Google Scholar]
- 35.Umeri RD, Osuji RU, Ezema FI. 2016. Synthesis and characterization of copper oxide thin films using successive ionic layer adsorption reaction (SILAR) method. Chem. Mater. Res. 8, 68-76. [Google Scholar]
- 36.Liu Y, Chu Y, Zhuo Y, Li M, Li L, Dong L. 2007. Anion-controlled construction of CuO honeycombs and flowerlike assemblies on copper foils. Cryst. Growth Des. 7, 467-470. ( 10.1021/cg060480r) [DOI] [Google Scholar]
- 37.Bose A, Basu S, Banerjee S, Chakravorty D. 2005. Electrical properties of compacted assembly of copper oxide nanoparticles. J. Appl. Phys. 98, 074307. ( 10.1063/1.2084311) [DOI] [Google Scholar]
- 38.Farhad SFU, Webster RF, Cherns D. 2018. Electron microscopy and diffraction studies of pulsed laser deposited cuprous oxide thin films grown at low substrate temperatures. Materialia 3, 230-238. ( 10.1016/j.mtla.2018.08.032) [DOI] [Google Scholar]
- 39.Nair MTS, Guerrero L, Arenas OL, Nair PK. 1999. Chemically deposited copper oxide thin films: structural, optical and electrical characteristics. Appl. Surf. Sci. 150, 143-151. ( 10.1016/S0169-4332(99)00239-1) [DOI] [Google Scholar]
- 40.Hossain MA, Farhad SFU, Tanvir NI, Chang JH, Rahman MA, Tanaka T, Guo Q, Uddin J, Majed Patwary MA. 2022. Data from: Facile synthesis of Cu2O nanorods in the presence of NaCl by successive ionic layer adsorption and reaction method and its characterizations. Dryad Digital Repository. ( 10.5061/dryad.37pvmcvmm) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Hossain MA, Farhad SFU, Tanvir NI, Chang JH, Rahman MA, Tanaka T, Guo Q, Uddin J, Majed Patwary MA. 2022. Data from: Facile synthesis of Cu2O nanorods in the presence of NaCl by successive ionic layer adsorption and reaction method and its characterizations. Dryad Digital Repository. ( 10.5061/dryad.37pvmcvmm) [DOI] [PMC free article] [PubMed]
Data Availability Statement
Our data are deposited at Dryad Digital Repository: https://doi.org/10.5061/dryad.37pvmcvmm [40].