Abstract
Purpose
The purpose of this study was to report the author’s experiences in treating large (10–25 mm) and giant (>25 mm) intracranial aneurysms (IAs) using a single Flow Re-direction Endoluminal Device (FRED) without assistant coiling, with a focus on procedure-related complications.
Materials and Methods
A total of 33 patients who were treated with FRED between January 2018 and July 2020 were retrospectively reviewed. The timing of procedure-related complications was chronologically categorized as acute (within 7 days), subacute (8 to 21 days), and delayed (after 21 days) periods. Follow-up angiography was performed at 2 to 27 months (mean 9.7 months), and clinical follow-up was performed at 1 to 31 months (mean 14.1 months) in all patients.
Results
Six (18.2%) patients experienced procedure-related complications, including 2 (6.1%) in acute period, 1 (3.0%) in subacute period, and 3 (9.1%) in delayed period. Thromboembolic complications occurred in 5 (15.2%) patients and hemorrhagic complications in 1 (3.0%). Permanent morbidity and mortality rates were 3.0% each. Non-internal carotid artery (ICA) location of IAs (odds ratio 6.532; 95% confidence interval, 1.335–17.816; p=0.034) was the only independent risk factor for procedure-related complications on multivariate logistic regression analysis.
Conclusion
The procedure-related complication rate was 18.2% in this study. Procedure-related complications might increase when treating large and giant IAs located on a non-ICA, especially on the middle cerebral artery. Therefore, it may be suggested that neurointerventionists and endovascular neurosurgeons should pay attention to the location of IAs when treating large and giant IAs with a single FRED.
Keywords: Endovascular treatment, flow diverter, intracranial aneurysm, stent
INTRODUCTION
Endovascular coiling has become an effective therapeutic option for all intracranial aneurysms (IAs). However, endovascular coiling for large (10–25 mm) and giant (>25 mm) IAs remains controversial due to the high recurrence and retreatment rates with the risk of perioperative complications.1,2,3 Recently, the development of flow diverting stent (FDS) have offered a simple way to treat these difficult lesions with acceptable morbidity and mortality rates. Since the approval of Pipeline embolization device (PED, Medtronic, Minneapolis, MN, USA) in 2011 and the publication of good results of Pipeline for Uncoilable or Failed Aneurysms study, FDS has been widely used for complex IAs, including large and giant aneurysms.4 In addition, indication for the use of FDS has expanded to various IAs regardless of size, location, or complexity.5,6,7,8
The Flow Re-direction Endoluminal Device (FRED, Microvention, Aliso Viejo, CA, USA) has a unique integrated double-layered system that consists of 16-strand stent-like outer layer and 48-strand inner layer for flow diversion. This design has some advantages, including 1) navigability improvement, 2) improved device opening, as well as 3) good wall apposition and easy deployment. One year follow-up results of the Safety and efficacy Analysis of FRED Embolic device in aneurysm treatment (SAFE) trial have shown that FRED is an effective (81.1% of adequate occlusion rate) and safe (2.9% of morbidity and 1.9% of mortality) device for aneurysm treatment.9 However, most aneurysms included in the SAFE trial were small, with diameters of less than 10 mm (71 out of 103 patients, 68.9%).9 Several investigators have demonstrated that the complication rate of FDS is significantly increased in large and giant IAs.10,11,12,13 To the best of our knowledge, there has been no report that mainly focuses on procedure-related complications after the treatment of large and giant IAs with FRED. In this study, we reported our experiences in using a single FRED for treating large and giant IAs, and evaluated the incidence and risk factors of procedure-related complications.
MATERIALS AND METHODS
Study population and indication for FRED
This retrospective study was approved by Yonsei University Severance Hospital Institutional Review Board (4-2021-0867), and the requirement for informed consent was waived. A total of 33 unruptured aneurysms in 33 patients who were treated with FRED in two tertiary centers between January 2018 and July 2020 were included in this study. The indication for the use of FDS in South Korea is very strict, under the national insurance system. FDS can only be used for an unruptured aneurysm. The maximal diameter of the aneurysm should be over 15 mm, with the exception of fusiform aneurysm or vertebral artery dissecting aneurysm. Additional coil insertion along with FDS is not allowed. Moreover, one FDS is permitted in a single procedure. Therefore, our indications for FRED in this study were as follows: 1) large and giant unruptured saccular aneurysms with maximal diameter greater than 15 mm regardless of aneurysm location on the anterior circulation and 2) unruptured fusiform aneurysm over 10 mm in maximal diameter. The clinical and radiologic characteristics and outcomes were retrospectively reviewed from each center’s database, including the clinical information, radiological findings, outcome, and complications.
Periprocedural angiographic evaluation and endovascular procedure
All patients underwent preprocedural diagnostic four-vessel digital subtraction angiography (DSA) with ipsilateral external carotid artery angiography. Morphological characteristics of the aneurysm, including the shape, size, location, and the presence of intramural thrombus formation, the relationship between aneurysm and parent artery, and parent artery diameter, were evaluated using 3-dimensional rotational angiographic images.
All procedures were conducted under general anesthesia. Preprocedural dual antiplatelet agents (aspirin 100 mg and clopidogrel 75 mg qd) were administered for at least 7 days in all patients. Platelet function test (PFT) using the VerifyNow (Accumetrics, San Diego, DA, USA) system was done in all patients, but we did not change the antiplatelet regimen according to the result of PFT. Systemic heparinization (50 IU/kg) was routinely conducted after the placement of femoral sheath. If the procedure was prolonged, additional heparin (1000 IU) was injected intravenously according to the activated clotting time that was maintained at twice that of the baseline. A co-axial guiding system using 5- or 6-French Sofia (Microvention, Aliso Viejo, CA, USA) within 6-French shuttle and Headaway 27 (Microvention) microcatheter were mainly used for our procedure. Adjunctive balloon angioplasty was performed by the treating physicians, if necessary. After completing the procedure, we checked the two-dimensional DSA and three-dimensional rotational angiography as well as VasoCT to confirm the appropriate placement and wall apposition of FRED. Dual antiplatelet agents were maintained for 6 months after the procedure and were changed to aspirin monotherapy for at least 2 years.
Follow-up protocol and outcome assessment
Clinical follow-up was performed at 1 to 31 months (mean 14.1 months) in all patients. Clinical outcomes were assessed by the modified Rankin Scale (mRS) at the last follow-up day. Good clinical outcome was defined as either 1) mRS=0 or 2) the same mRS score as pre-treatment status. Diffusion weighted image with gradient echo image within 24 h after the procedure was conducted to confirm postprocedural ischemic and hemorrhagic complications. All procedure-related and periprocedural complications were registered in each center’s database. We chronologically categorized the timing of procedure-related complications as acute (within 7 days), subacute (8–21 days), and delayed (after 21 days) periods. Procedure-related complication was defined as 1) development of new neurological deficits after treatment, 2) occlusion of related large vessels after treatment regardless of symptom development, or 3) neurological death.
We conducted magnetic resonance angiography with time-of-flight image or computed tomography angiography as the initial follow-up modalities. These first follow-up examinations were performed at 3 to 6 months after the procedure in the outpatient clinic. Follow-up DSA was performed at 2 to 27 months (mean 9.7 months) in all patients. The angiographic results were classified by the O’Kelly-Marotta filling grade (OKMG) system as follows: 1) total filling of aneurysm sac (A), 2) subtotal filling with 5%–95% filling of aneurysm sac (B), 3) entry remnant with less than 5% filling of aneurysm sac (C), and 4) no filling (D).14
Statistical analysis
Statistical analysis was performed by SPSS version 24 (IBM Corp., Armonk, NY, USA). We performed chi-square test or Fisher’ exact test for categorical variables and independent t-test or Mann-Whitney U test for continuous variables. Multivariate logistic analysis was conducted after including variables with p values <0.1 in univariate analysis to identify the risk factors for periprocedural and delayed complications. P values <0.05 were considered statistically significant.
RESULTS
Patient baseline characteristics
Of the 33 patients who were treated with FRED, 23 (69.7%) patients were female, and the mean age of all patients was 57.8 years. Of 33 lesions, 16 (48.5%) were incidentally detected. The most common presenting symptoms were eye symptoms, including visual disturbance (n=4) and ophthalmoplegia (n=4), followed by cerebral infarction (n=3), and headache (n=3). Recanalization after initial treatment on follow-up examination was observed in four patients. Distal internal carotid artery (ICA, n=22), including ICA-ophthalmic segment (n=11), ICA-cavernous segment (n=9), and posterior communicating artery (n=2), was the most common location, followed by the middle cerebral artery (MCA, n=7) and anterior cerebral artery (n=4). Of the 33 aneurysms, 24 were saccular type and 9 were fusiform. Intramural thrombosis was found in 10 of 33 aneurysms (30.3%). The mean aneurysm size was 19.5±5.2 mm, and there were six giant aneurysms that were greater than 25 mm in size. The mean neck size was 10.5±4.9 mm.
Procedure-related complication and follow-up results
A total of 6 (18.2%) procedure-related complications occurred after treatment with FRED. The demographic and clinical characteristics are described in Table 1. Two (6.1%) complications developed in the acute period, 1 (3.0%) in the subacute period, and 3 (9.1%) in the delayed period. There were 5 (15.2%) thromboembolic complications, including symptomatic cerebral infarction (n=2) and transient ischemic attack (n=3). Among them, four recovered without neurologic deficit, and one had mild sequelae (mRS=2) (Fig. 1). All six patients with procedure-related complications were responders to aspirin [aspirin reaction unit (ARU) 494.3±47.2] and clopidogrel [P2Y12 reaction unit (PRU) 146.2±39.5]. One (3.0%) hemorrhagic complication occurred due to aneurysmal rupture in a delayed period, and the patient finally died. Intra-procedural foreshortening of FRED occurred in four patients, and additional FRED deployment was needed in one patient. The foreshortening occurred in the distal part of the device in all four patients. On multivariate logistic regression analysis, non-ICA location (odds ratio 6.532; 95% confidence interval, 1.335–17.816; p=0.034) was the only independent risk factor for procedure-related complication. Other clinical and radiologic features showed no statistical significance on uni- and multivariate analyses (Table 2). Additionally, ARU and PRU were not significantly associated with the prevalence of ischemic complications.
Table 1. Demographic and Clinical Data of Six Patients with Large and Giant Intracranial Aneurysms Who Had Procedure-Related Complications.
| Case No. | Presentation | Location | Type | Maximum diameter (mm) | Neck size (mm) | PreOP ARU | PreOP PRU | Causes and onset of complications | Clinical manifestation of complications | mRS at discharge | FU mRS | FU period (months) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Acute period (within 7 days) | ||||||||||||
| 1 | Infarction | MCA | Fusiform | 14.4 | 9.8 | 412 | 128 | Thromboembolism (POD 0) | TIA | 0 | 0 | 19 |
| 2 | Mass effect | ICA | Saccular | 17.7 | 6.4 | 503 | 125 | Thromboembolism (POD 0) | Hemiparesis | 1 | 0 | 18 |
| Subacute period (8 to 21 days) | ||||||||||||
| 3 | Incidental | MCA | Saccular | 18.9 | 11.4 | 531 | 100 | Thromboembolism (POD 11) | TIA | 0 | 0 | 6 |
| Delayed period (after 21 days) | ||||||||||||
| 4 | Infarction | MCA | Saccular | 34.5 | 25.8 | 526 | 152 | Thromboembolism (POD 23) | Hemiparesis | 2 (due to initial infarction) | 2 | 8 |
| 5 | Incidental | MCA | Fusiform | 10.1 | 9.8 | 528 | 215 | Thromboembolism (POD 176) | TIA | 0 | 0 | 6 |
| 6 | Mass effect | ICA | Saccular | 18.2 | 11.1 | 466 | 157 | Rupture (POD 275) | Death | 0 | 6 | 9 |
ARU, aspirin reaction units; FU, follow-up; ICA, internal carotid artery; MCA, middle cerebral artery; mRS, modified Rankin Scale; POD, post-operative day; PRU, P2Y12 reaction units; TIA, transient ischemic attack.
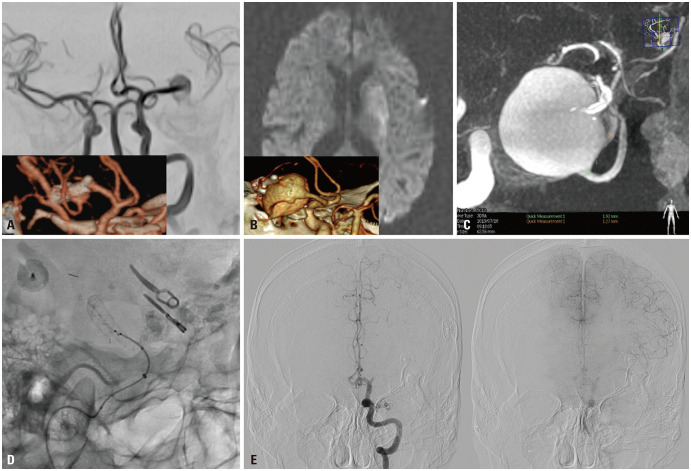
Fig. 1. Illustrative case. A 57-year-old male presenting with acute infarction on left basal ganglia. (A) In November 2003, the patient underwent surgical clipping for unruptured large aneurysm located at the left MCA. After the surgery, small neck remnant was observed on postoperative CTA (lower box). (B) In May 2019, the patient developed right hemiparesis with acute infarction on left basal ganglia on diffusion-weighted image, and follow-up CTA (lower box) showed major recurrence of the previously clipped aneurysm. (C) DSA showed a giant aneurysm involving MCA trifurcation and the inferior trunk had the largest diameter of about 1.92 mm. (D) We successfully deployed FRED from the inferior trunk to M1 without procedural complication in December 2019. (E) One month later, the patient had right hemiparesis again due to acute infarction on the left periventricular white mater. Follow-up DSA demonstrated left M1 occlusion with leptomeningeal collateral from the ACA. The aneurysm was completely occluded on DSA. ACA, anterior cerebral artery; CTA, computed tomography angiography; DSA, digital subtraction angiography; FRED, Flow Re-direction Endoluminal Device; MCA, middle cerebral artery.
Table 2. Risk Factors for Procedure-Related Complications in Patients with Large and Giant Aneurysms Treated by FRED.
| Variables | No. of patients | Procedure-related complications (%) | p, Univariate | p, Regression (OR, 95% CI) | ||
|---|---|---|---|---|---|---|
| Yes (n=6) | No (n=27) | |||||
| Age (yr) | 56.5±16.0 | 58.3±14.6 | 0.768* | |||
| Sex | 0.208 | |||||
| Male | 10 | 3 (30.0) | 7 (70.0) | |||
| Female | 23 | 3 (13.0) | 20 (87.0) | |||
| Hypertension | 0.467 | |||||
| Yes | 12 | 2 (16.7) | 10 (83.3) | |||
| No | 21 | 4 (19.0) | 17 (81.0) | |||
| Diabetes | 0.625 | |||||
| Yes | 5 | 1 (20.0) | 4 (80.0) | |||
| No | 28 | 5 (17.9) | 23 (82.1) | |||
| Smoking | 0.094 | 0.169 (1.682, 0.874–5.984) | ||||
| Yes | 4 | 0 (0) | 4 (100) | |||
| No | 29 | 6 (20.7) | 23 (79.3) | |||
| Dyslipidemia | 0.108 | |||||
| Yes | 3 | 0 (0) | 3 (100) | |||
| No | 30 | 6 (20.0) | 24 (80.0) | |||
| Location of aneurysm | 0.018 | 0.034 (6.532, 1.335–17.816) | ||||
| ICA | 22 | 2 (9.1) | 20 (90.9) | |||
| Non-ICA | 11 | 4 (36.4) | 7 (63.6) | |||
| Type of aneurysm | 0.352 | |||||
| Saccular | 24 | 4 (16.7) | 20 (83.3) | |||
| Non-saccular | 9 | 2 (22.2) | 7 (77.8) | |||
| Aspirin reaction units | 494.3±47.2 | 462.5±44.9 | 0.354* | |||
| P2Y12 reaction units | 146.2±39.5 | 176.4±50.2 | 0.286* | |||
| Maximum diameter (mm) | 19.0±8.3 | 19.7±4.6 | 0.889* | |||
| Neck size (mm) | 12.4±6.8 | 9.7±4.4 | 0.216* | |||
| Adjunctive procedure | 0.086 | 0.207 (0.885, 0.429–2.723) | ||||
| Yes | 5 | 0 (0) | 5 (100) | |||
| No | 28 | 6 (21.4) | 22 (78.6) | |||
| Intraaneurysmal thrombus | 0.296 | |||||
| Yes | 10 | 1 (10.0) | 9 (90.0) | |||
| No | 23 | 5 (21.7) | 18 (78.3) | |||
CI, confidence interval; ICA, internal carotid artery; OR, odds ratio; SD, standard deviation; FRED, Flow Re-direction Endoluminal Device.
Data are presented as mean±standard deviation or n (%).
*Mann-Whitney U test.
Follow-up DSA showed that complete (OKMG D, n=16) or near-complete (OKMG C, n=10) occlusion was achieved in 26 out of 33 patients (78.8%). There was no aneurysm with total filling (OKMG A) after a mean follow-up period of 9.4±6.8 months. The mean clinical follow-up period was 14.1±11.5 months, and the rate of good clinical outcome at the last follow-up was 93.9% (31 out of 33 patients).
DISCUSSION
The present study demonstrated that the total complication rate after treatment with FRED for large and giant IAs was 18.2% (6 out of 33 patients). Although the morbidity (n=1, 3.0%) and mortality (n=1, 3.0%) rates were low in this study, the complication rates were not negligible. Safety issue can be a potential limitation of flow diversion compared to the traditional coil embolization.4,15,16,17 Moreover, periprocedural complications of FDS occurred more frequently in cases of large and giant aneurysms rather than in small sized aneurysms.10,12 The results of the International Retrospective Study of the Pipeline Embolization Device have demonstrated that the complication rates were the lowest in the small ICA aneurysm (<10 mm) group.10 Aneurysms located at posterior circulation or other anterior circulation, and large ICA aneurysms (≥10 mm) showed higher complication rates.10 Morbidity and mortality rates (25.8%) were the highest in cases with giant IAs. In addition, the morbidity and mortality rates (26 out of 275 patients, 9.5%) in patients with large ICA aneurysm were much higher than in patients with small ICA aneurysm (12 out of 294 patients, 4.1%).10 Recently, SAFE study analysis at 1 year have shown that FRED device has excellent safety profile with low morbidity and mortality rates (2.9% and 1.9%, respectively).9 However, most aneurysms (71 out of 103 patients, 68.9%) included in the SAFE study were small-sized aneurysms (<10 mm).9 In addition, there were two delayed hemorrhage (one aneurysm rupture and one remote intraparenchymal hemorrhage) cases of large supraclinoid aneurysms.9 We thought that the complication rate was relatively high in this study for the following reasons: 1) we treated large and giant IAs with the maximal diameter larger than 15 mm, except for three fusiform aneurysms; and 2) the proportion of non-ICA locations, including the middle and anterior cerebral arteries (n=11, 33.3%), was relatively high. Choi, et al.18 have reported that the complication and mortality rates of FDS for large and giant IAs in a single-center series were 18.7% and 8.3%, respectively. These results were similar to the findings of the current study, as the indication for the use of FDS is the same in our country.
Delayed aneurysmal rupture is one of the most fatal complications of FDS. We experienced one case of aneurysmal rupture in the delayed period (ICA aneurysm at postprocedural 275 days), and the patient finally died. The most effective method to prevent this fatal complication was adjunctive coil packing along with FDS. The efficacy and safety of partially dense coil packing with FDS were reported by Nossek, et al.19 They showed a high complete occlusion rate (23 out of 27 patients, 85.2%) without delayed aneurysmal ruptured at the 1-year follow-up period. Oishi, et al.20 also inserted additional coils when an aneurysm was located in subarachnoid space with jet flow, narrow neck, irregular shape, and size larger than 15 mm. They reported two cases of delayed rupture with carotid cavernous fistula, and there was no delayed subarachnoid hemorrhage in cases of FDS with additional coil packing. However, additional coil packing is not permitted in South Korea, as we previously described.18 Strict blood pressure control to lower direct hemodynamic stress on aneurysm wall and steroid therapy to reduce thrombus-related inflammation can be used to avoid this fatal complication.18
Ischemic complications are the most common complication of FDS treatment. Therefore, dual antiplatelet agents (aspirin and clopidogrel) should be administered prior to the procedure. Our previous study has demonstrated that clopidogrel resistance did not show significant relationship between thromboembolic complication and stent-assisted coil embolization.21 Ghandi, et al.22 have reported that a routine PFT prior to neurointerventional procedures was not routinely recommended due to insufficient data. Moreover, some studies have shown no significant association between the use of PFT and the rates of symptomatic ischemic complications for FDS treatment.23,24 Based on our experience and previous studies, we did not change the antiplatelet regimen according to the results of PFT. However, some investigators have suggested the importance of PRU results in neurointerventional field and the efficacy of changing medication from clopidogrel to prasugrel or ticagrelor in PRU hypo-response group.25,26,27 Although this issue is under debate, we thought that changing the antiplatelet agents based on the PFT results should be considered due to the increasing evidence regarding the efficacy of prasugrel or ticagrelor. Additionally, we found no significant difference in PRU values between the groups with and without ischemic complications. Moreover, since all six patients with procedure-related complications were responders to aspirin and clopidogrel, we think that tailor-made antiplatelet therapy cannot be a solution to avoid complications.
In the present study, ischemic/thromboembolism events occurred in four out of seven patients with MCA aneurysms. Generally, treatment morbidity after FDS is reported to be between 4%–10%.10,28,29 MCA aneurysms present with a particularly complex anatomy due to the frequency of wide-neck configuration with the incorporation of MCA branches. Recently, FDS has been used as an alternative technique for complex wide-neck MCA aneurysms. However, MCA location appears to be associated with a higher risk of ischemic injury compared to the general rate of ischemic complications related to FDS.30 Factors such as smaller diameters of the arteries, the technical challenges of distal navigation, and the coverage of bifurcation branches and perforators may increase the risk of treatment-related complications.30,31,32 A systematic review of treating MCA aneurysms with FDS showed that the rate of treatment-related complications was 20.7%, and approximately 10% of the complications were permanent. Ischemic/thromboembolic events were the most common type of complication (16.3%), followed by perianeurysmal inflammation (2.6%), hemorrhage (2%), and dissection/perforation (1.8%).30 A meta-analysis showed that the complication rate was higher for MCA aneurysms (18%) compared to anterior communicating artery aneurysms (8%) and distal anterior cerebral artery aneurysms (9%).33 Complication rates after the PED (9.2%) and Silk devices (8.2%) were slightly lower compared with the FRED (11.7%). Multivariate analysis confirmed larger size aneurysms (≥10 mm) and MCA location as factors independently associated with higher rates of complications.33 In our study, we treated seven large and giant MCA aneurysms, which resulted in MCA occlusion due to delayed in-stent thrombosis in one (mRS=2) and transient ischemic attack in three patients. Accordingly, FDS may present some limitations among MCA and large aneurysms, especially related to the higher rate of complications. Therefore, FDS for large and giant MCA aneurysms should only be considered as salvage therapy when traditional treatment methods are unfeasible.
Large and giant IAs have tragic natural history and prone to rupture.18 In addition, those IAs have high postprocedural complication rates regardless of treatment modalities (coil embolization, FDS, surgery, or parent artery occlusion) due to specific pathological configurations, including brainstem compression, wide neck, complex branching vessels and perforators. Since the introduction and approval of PED, FDS has become a mainstream for the treatment of IAs regardless of the size, location, and complexity; however, the treatment outcome of FDS for large and giant IAs is still relatively unfavorable due to the high rate of procedure-related complications. However, with the evolution of new devices, techniques, and medications, procedure-related thromboembolic and hemorrhagic complications can be reduced. We thought that FRED would be an excellent device for the distal opening and wall apposition by its own radial forces among FDS. Additionally, post-deployment ballooning for making good wall apposition after using FRED seemed to be the least required. Due to its good navigability, ease of distal opening, and good wall apposition, FRED may be a good recommendation for beginners during their early experiences with FDS. Distal landing zone with single outer layer (external stent) can afford some space to allow accurate positioning of the device without the flow diverting effect and to anchor it to the vessel wall. Therefore, we tend to choose FRED for large and giant IAs in tortuous arteries with the segment that has acceptable size discrepancy. However, FRED seemed to have more foreshortening phenomenon in the arterial segment that has large size discrepancy between proximal and distal diameters. Since wall apposition may be challenging in tortuous anatomies and short distal landing zone, we do not recommend using FRED in a segment of the parent artery with two or more acute angles.
The current study had some limitations. First, this was not a population-based study, and the number of patients was relatively small. Consequently, the results of our study may not be sufficient to draw definitive conclusions. Second, this study was retrospectively analyzed, although the data were prospectively collected. Third, the follow-up protocol was not standardized and showed wide variation in the follow-up periods, which differed by each institution. Finally, our study mainly focused on the aneurysm size, as we presumed that the risk of procedure-related complications would not differ according to the aneurysm types (saccular or non-saccular) in large and giant aneurysms on the anterior circulation. Moreover, additional subgroup analysis according to vascular pathology could not be performed due to the small number of cases in this study. Further multi-center large-volume data are needed to analyze the clinical and radiologic outcomes of large and giant IAs treated with FRED.
In conclusion, the rate of procedure-related complications of FRED for large and giant IAs was not negligible in this study. In addition, procedure-related complication rate might increase when treating large and giant IAs located on the non-ICA, especially on the MCA. Therefore, neurointerventionists and endovascular neurosurgeons are recommended to pay attention to the location of IAs when treating large and giant IAs with a single FRED.
Footnotes
The authors have no potential conflicts of interest to disclose.
- Conceptualization: Jai Ho Choi and Joonho Chung.
- Data curation: all authors.
- Formal analysis: Jai Ho Choi and Joonho Chung.
- Investigation: all authors.
- Methodology: Jai Ho Choi and Joonho Chung.
- Project administration: Joonho Chung.
- Resources: all authors.
- Supervision: Joonho Chung.
- Validation: Jai Ho Choi and Joonho Chung.
- Visualization: Jai Ho Choi and Joonho Chung.
- Writing—original draft: Jai Ho Choi.
- Writing—review & editing: all authors.
- Approval of final manuscript: all authors.
References
- 1.Gruber A, Killer M, Bavinzski G, Richling B. Clinical and angiographic results of endosaccular coiling treatment of giant and very large intracranial aneurysms: a 7-year, single-center experience. Neurosurgery. 1999;45:793–803. doi: 10.1097/00006123-199910000-00013. [DOI] [PubMed] [Google Scholar]
- 2.Sluzewski M, Menovsky T, van Rooij WJ, Wijnalda D. Coiling of very large or giant cerebral aneurysms: long-term clinical and serial angiographic results. AJNR Am J Neuroradiol. 2003;24:257–262. [PMC free article] [PubMed] [Google Scholar]
- 3.van Rooij WJ, Sluzewski M, Slob MJ, Rinkel GJ. Predictive value of angiographic testing for tolerance to therapeutic occlusion of the carotid artery. AJNR Am J Neuroradiol. 2005;26:175–178. [PMC free article] [PubMed] [Google Scholar]
- 4.Becske T, Kallmes DF, Saatci I, McDougall CG, Szikora I, Lanzino G, et al. Pipeline for uncoilable or failed aneurysms: results from a multicenter clinical trial. Radiology. 2013;267:858–868. doi: 10.1148/radiol.13120099. [DOI] [PubMed] [Google Scholar]
- 5.Becske T, Brinjikji W, Potts MB, Kallmes DF, Shapiro M, Moran CJ, et al. Long-term clinical and angiographic outcomes following pipeline embolization device treatment of complex internal carotid artery aneurysms: five-year results of the pipeline for uncoilable or failed aneurysms trial. Neurosurgery. 2017;80:40–48. doi: 10.1093/neuros/nyw014. [DOI] [PubMed] [Google Scholar]
- 6.Brinjikji W, Lanzino G, Cloft HJ, Siddiqui AH, Boccardi E, Cekirge S, et al. Risk factors for ischemic complications following Pipeline embolization device treatment of intracranial aneurysms: results from the IntrePED study. AJNR Am J Neuroradiol. 2016;37:1673–1678. doi: 10.3174/ajnr.A4807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ye G, Zhang M, Deng L, Chen X, Wang Y. Meta-analysis of the efficiency and prognosis of intracranial aneurysm treated with flow diverter devices. J Mol Neurosci. 2016;59:158–167. doi: 10.1007/s12031-016-0723-x. [DOI] [PubMed] [Google Scholar]
- 8.Zhou G, Su M, Zhu YQ, Li MH. Efficacy of flow-diverting devices for cerebral aneurysms: a systematic review and meta-analysis. World Neurosurg. 2016;85:252–262. doi: 10.1016/j.wneu.2015.09.088. [DOI] [PubMed] [Google Scholar]
- 9.Pierot L, Spelle L, Berge J, Januel AC, Herbreteau D, Aggour M, et al. SAFE study (safety and efficacy analysis of FRED embolic device in aneurysm treatment): 1-year clinical and anatomical results. J Neurointerv Surg. 2019;11:184–189. doi: 10.1136/neurintsurg-2018-014261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kallmes DF, Hanel R, Lopes D, Boccardi E, Bonafé A, Cekirge S, et al. International retrospective study of the pipeline embolization device: a multicenter aneurysm treatment study. AJNR Am J Neuroradiol. 2015;36:108–115. doi: 10.3174/ajnr.A4111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rouchaud A, Brinjikji W, Lanzino G, Cloft HJ, Kadirvel R, Kallmes DF. Delayed hemorrhagic complications after flow diversion for intracranial aneurysms: a literature overview. Neuroradiology. 2016;58:171–177. doi: 10.1007/s00234-015-1615-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lv X, Ge H, He H, Jiang C, Li Y. A systematic review of pipeline embolization device for giant intracranial aneurysms. Neurol India. 2017;65:35–38. doi: 10.4103/0028-3886.198200. [DOI] [PubMed] [Google Scholar]
- 13.Sheen JJ, Park W, Kwun BD, Park JC, Ahn JS. Microsurgical treatment strategy for large and giant aneurysms of the internal carotid artery. Clin Neurol Neurosurg. 2019;177:54–62. doi: 10.1016/j.clineuro.2018.12.014. [DOI] [PubMed] [Google Scholar]
- 14.O’kelly CJ, Krings T, Fiorella D, Marotta TR. A novel grading scale for the angiographic assessment of intracranial aneurysms treated using flow diverting stents. Interv Neuroradiol. 2010;16:133–137. doi: 10.1177/159101991001600204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kallmes DF, Brinjikji W, Cekirge S, Fiorella D, Hanel RA, Jabbour P, et al. Safety and efficacy of the Pipeline embolization device for treatment of intracranial aneurysms: a pooled analysis of 3 large studies. J Neurosurg. 2017;127:775–780. doi: 10.3171/2016.8.JNS16467. [DOI] [PubMed] [Google Scholar]
- 16.Pumar JM, Banguero A, Cuellar H, Guimaraens L, Masso J, Miralbes S, et al. Treatment of intracranial aneurysms with the SILK embolization device in a multicenter study. A retrospective data analysis. Neurosurgery. 2017;81:595–601. doi: 10.1093/neuros/nyw123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wakhloo AK, Lylyk P, de Vries J, Taschner C, Lundquist J, Biondi A, et al. Surpass flow diverter in the treatment of intracranial aneurysms: a prospective multicenter study. AJNR Am J Neuroradiol. 2015;36:98–107. doi: 10.3174/ajnr.A4078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Choi JH, Lee KS, Kim BS, Shin YS. Treatment outcomes of large and giant intracranial aneurysms according to various treatment modalities. Acta Neurochir (Wien) 2020;162:2745–2752. doi: 10.1007/s00701-020-04540-1. [DOI] [PubMed] [Google Scholar]
- 19.Nossek E, Chalif DJ, Chakraborty S, Lombardo K, Black KS, Setton A. Concurrent use of the Pipeline embolization device and coils for intracranial aneurysms: technique, safety, and efficacy. J Neurosurg. 2015;122:904–911. doi: 10.3171/2014.12.JNS141259. [DOI] [PubMed] [Google Scholar]
- 20.Oishi H, Teranishi K, Yatomi K, Fujii T, Yamamoto M, Arai H. Flow diverter therapy using a Pipeline embolization device for 100 unruptured large and giant internal carotid artery aneurysms in a single center in a Japanese population. Neurol Med Chir (Tokyo) 2018;58:461–467. doi: 10.2176/nmc.oa.2018-0148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Song J, Shin YS. Antiplatelet drug resistance did not increase the thromboembolic events after stent-assisted coiling of unruptured intracranial aneurysm: a single center experience of 99 cases. Neurol Sci. 2017;38:879–885. doi: 10.1007/s10072-017-2859-z. [DOI] [PubMed] [Google Scholar]
- 22.Gandhi CD, Bulsara KR, Fifi J, Kass-Hout T, Grant RA, Delgado Almandoz JE, et al. Platelet function inhibitors and platelet function testing in neurointerventional procedures. J Neurointerv Surg. 2014;6:567–577. doi: 10.1136/neurintsurg-2014-011357. [DOI] [PubMed] [Google Scholar]
- 23.Bender MT, Lin LM, Colby GP, Lubelski D, Huang J, Tamargo RJ, et al. P2Y12 hyporesponse (PRU>200) is not associated with increased thromboembolic complications in anterior circulation Pipeline. J Neurointerv Surg. 2017;9:978–981. doi: 10.1136/neurintsurg-2016-012618. [DOI] [PubMed] [Google Scholar]
- 24.Skukalek SL, Winkler AM, Kang J, Dion JE, Cawley CM, Webb A, et al. Effect of antiplatelet therapy and platelet function testing on hemorrhagic and thrombotic complications in patients with cerebral aneurysms treated with the pipeline embolization device: a review and meta-analysis. J Neurointerv Surg. 2016;8:58–65. doi: 10.1136/neurintsurg-2014-011145. [DOI] [PubMed] [Google Scholar]
- 25.Adeeb N, Griessenauer CJ, Foreman PM, Moore JM, Shallwani H, Motiei-Langroudi R, et al. Use of platelet function testing before pipeline embolization device placement: a multicenter cohort study. Stroke. 2017;48:1322–1330. doi: 10.1161/STROKEAHA.116.015308. [DOI] [PubMed] [Google Scholar]
- 26.Delgado Almandoz JE, Crandall BM, Scholz JM, Fease JL, Anderson RE, Kadkhodayan Y, et al. Pre-procedure P2Y12 reaction units value predicts perioperative thromboembolic and hemorrhagic complications in patients with cerebral aneurysms treated with the Pipeline embolization device. J Neurointerv Surg. 2013;5 Suppl 3:iii3–iii10. doi: 10.1136/neurintsurg-2012-010582. [DOI] [PubMed] [Google Scholar]
- 27.Texakalidis P, Bekelis K, Atallah E, Tjoumakaris S, Rosenwasser RH, Jabbour P. Flow diversion with the pipeline embolization device for patients with intracranial aneurysms and antiplatelet therapy: a systematic literature review. Clin Neurol Neurosurg. 2017;161:78–87. doi: 10.1016/j.clineuro.2017.08.003. [DOI] [PubMed] [Google Scholar]
- 28.Berge J, Biondi A, Machi P, Brunel H, Pierot L, Gabrillargues J, et al. Flow-diverter silk stent for the treatment of intracranial aneurysms: 1-year follow-up in a multicenter study. AJNR Am J Neuroradiol. 2012;33:1150–1155. doi: 10.3174/ajnr.A2907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brinjikji W, Cloft H, Cekirge S, Fiorella D, Hanel RA, Jabbour P, et al. Lack of association between statin use and angiographic and clinical outcomes after Pipeline embolization for intracranial aneurysms. AJNR Am J Neuroradiol. 2017;38:753–758. doi: 10.3174/ajnr.A5078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cagnazzo F, Mantilla D, Lefevre PH, Dargazanli C, Gascou G, Costalat V. Treatment of middle cerebral artery aneurysms with flow-diverter stents: a systematic review and meta-analysis. AJNR Am J Neuroradiol. 2017;38:2289–2294. doi: 10.3174/ajnr.A5388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lin N, Lanzino G, Lopes DK, Arthur AS, Ogilvy CS, Ecker RD, et al. Treatment of distal anterior circulation aneurysms with the pipeline embolization device: a US multicenter experience. Neurosurgery. 2016;79:14–22. doi: 10.1227/NEU.0000000000001117. [DOI] [PubMed] [Google Scholar]
- 32.Cagnazzo F, Cappucci M, Dargazanli C, Lefevre PH, Gascou G, Riquelme C, et al. Treatment of distal anterior cerebral artery aneurysms with flow-diverter stents: a single-center experience. AJNR Am J Neuroradiol. 2018;39:1100–1106. doi: 10.3174/ajnr.A5615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cagnazzo F, Perrini P, Dargazanli C, Lefevre PH, Gascou G, Morganti R, et al. Treatment of unruptured distal anterior circulation aneurysms with flow-diverter stents: a meta-analysis. AJNR Am J Neuroradiol. 2019;40:687–693. doi: 10.3174/ajnr.A6002. [DOI] [PMC free article] [PubMed] [Google Scholar]