Abstract
Purpose
Only a few Asian studies have discussed the impact of statin intensity on clinical outcomes in patients with peripheral artery disease (PAD). We aimed to investigate the clinical impact of statin intensity in patients with PAD after endovascular revascularization.
Materials and Methods
From April 2009 to June 2019, 376 patients with lower extremity PAD treated with endovascular revascularization were enrolled. They were classified into three groups according to statin intensity: no-statin, low-to-moderate intensity (LMI), and high-intensity (HI). The primary outcomes were major adverse cardiovascular events (MACE) and major adverse limb events (MALE).
Results
During the 40-month follow-up, MACE occurred less frequently in the HI and LMI groups than the no-statin group (11.4% vs. 16.0% vs. 39%, p<0.001). In adjusted Cox models, the HI group had the fewest MACE [hazard ratio (HR): 0.447; 95% confidence interval (CI): 0.244–0.834; p=0.018] and MALE (HR: 0.360; 95% CI: 0.129–1.006; p=0.051) events, while the LMI group had fewer MACE (HR: 0.571; 95% CI: 0.326–1.0; p=0.050) events than the no-statin group. HI statin therapy was associated with better outcomes in terms of MALE (HR: 0.432; 95% CI: 0.223–0.837; p=0.003) than LMI statin therapy after inverse probability treatment weighting analysis.
Conclusion
HI and LMI statin use is associated with a significant reduction in MACE events than no-statin use. HI statin use was associated with better MALE outcomes than no-statin or LMI statin use.
Keywords: Peripheral artery disease, statin, endovascular revascularization, major adverse cardiovascular event
INTRODUCTION
Peripheral artery disease (PAD) is characterized by atherosclerotic obstruction of the arteries of the lower extremities. In 2010, more than 200 million persons were diagnosed with PAD worldwide.1 According to previous studies, PAD is more severe than coronary heart disease (CHD) or cerebrovascular disease (CVD).2 Although PAD, CHD, and CVD have similar major risk factors, PAD is an independent risk factor of mortality and morbidity from coronary and CVDs after adjustment for other known risk factors.3
Recent guidelines have recommended that control of cardiovascular risk factors should be incorporated in the management of PAD, including support for cessation of smoking and strict control of hypertension and diabetes (treatment with a statin and low-dose clopidogrel or aspirin).4 Regarding statin therapy, current American and European guidelines provide a class 1 recommendation supporting high-dose statin therapy.5,6 While knowledge of PAD is increasing, the use of guideline-directed therapy is still rare among patients with CHD or CVD. A recent study showed that only 33.9% patients with PAD were prescribed statins.7
To date, data on optimization of guideline-directed statin therapy and its impact on clinical outcomes in patients with PAD are limited. Thus, we investigated the clinical impact of statin intensity in patients with PAD after endovascular revascularization. We hypothesized that high-intensity (HI) statin therapy would be associated with improved survival and fewer major adverse cardiovascular events (MACE) than no or low-to-intermediate-intensity statin therapy in these patients.
MATERIALS AND METHODS
Subjects and study design
Between April 2009 and June 2019, 461 patients diagnosed clinically with PAD who received endovascular treatment at Sanggye-Paik Hospital in Seoul, South Korea were included in the study. Eighty-five patients were excluded due to non-atherosclerotic causes (n=8), incomplete data including drug history (n=35), non-endovascular therapy (n=16), and loss to follow-up before 6 months (n=26). In total, 376 patients with symptomatic PAD [claudication or critical limb ischemia (CLI)] were included in the study. The study protocol was approved by the Institutional Review Board at Sanggye Paik Hospital (2019-10-010).
Data were collected from the patients’ electronic medical records and angiography findings. The Rutherford classification was used. Claudication was classified as Categories 1–3 (mild, moderate, or severe claudication, respectively), while CLI was classified as Categories 4–6 (ischemic rest pain, minor tissue loss, or major tissue loss, respectively). The drug prescriptions given to the patients before endovascular treatment and during follow-up were verified. The statin intensity was categorized as low, moderate, and high according to the 2013 American College of Cardiology/American Heart Association (ACC/AHA) cholesterol guidelines.5 Patients whose statin doses were changed following a prescription change at discharge or during the 3-month follow-up were excluded from the final analysis. For the included patients, the prescription on discharge was considered. At 1- and 6-month follow-up evaluations, low-density lipoprotein-cholesterol (LDL-C) levels were measured, and the lowest value was defined as the follow-up LDL-C level. Patients were categorized into two groups [dual antiplatelet therapy (DAPT) <6 months or single antiplatelet therapy (SAPT) vs. DAPT ≥6 months]. SAPT was defined as taking aspirin (100 mg/day) or clopidogrel (75 mg/day), while DAPT was defined as taking aspirin (100 mg/day) plus clopidogrel (75 mg/day).8
The variables obtained during the endovascular procedure included the target lesion, TransAtlantic Inter-Society Consensus for the Management of Peripheral Arterial Disease classification (TASC; TASC II: aortoiliac and femoropopliteal levels, TASC I: infrapopliteal level),9 number of diseased vessels, intervention type (balloon angioplasty, atherectomy, or stent insertion), and pre- and post-intervention ankle-brachial index values. Multilevel disease was defined as the presence of significantly obstructed lesions at >1 level in the same limb. Follow-up was performed at two postoperative weeks and then every 1–3 months thereafter, during which a physical examination was performed.
Clinical outcomes
The primary end points were MACE (composite occurrence of all-cause death, myocardial infarction, and stroke) and major adverse limb events (MALE; composite occurrence of unplanned repeat revascularization and major amputation). Myocardial infarction was defined by the following: the presence of ischemic symptoms, electrocardiographic changes, abnormal angiographic findings indicative of myocardial infarction, an increase in creatine kinase–myocardial band fraction above the normal upper limit, or an increase in troponin above the 99th percentile of the normal upper limit.10 Stroke, indicated by neurological deficits, was confirmed by a neurologist on the basis of imaging studies. Repeat revascularization was defined as repeated open or endovascular intervention on the target limb. Major amputation was defined as above-the-ankle amputation of the index limb.11 If the patient had multiple events, we classified the first event as MACE or MALE. All events were diagnosed by experienced attending physicians, and the patients were reviewed by three cardiologists.
Statistical analysis
The chi-square test or Fisher’s exact test was used to compare categorical variables, which are reported as numbers (percentages). Student’s t-test was used to compare continuous variables, which are as mean and standard deviation. Kaplan-Meier survival analysis was used to compare 3-year event rates. Hazard ratios (HRs) were calculated using Cox regression analysis. Univariate and multivariate analyses were conducted to determine predictors of clinical outcomes. Multivariate Cox regression analysis was performed using propensity score (PS) matching variables as mentioned below. We applied a stepwise method, and the variables that showed p values of less than 0.10 in univariate analysis were included in the final multivariate analysis. HRs are provided with 95% confidence intervals (CIs). For all tests, a p value<0.05 was considered significant. All statistical analyses were performed with Statistical Package for the Social Sciences for Windows, release 25.0 (IBM Corp., Armonk, NY, USA). To compare the clinical impact between the HI and low-to-moderate-intensity (LMI) groups, inverse probability treatment weighting (IPTW) using the PS was performed using the demographic, laboratory, and treatment characteristics of the patients.12 The matching variables for IPTW included age, sex, CLI, hypertension, diabetes, dyslipidemia, heart failure, chronic kidney disease, coronary artery disease, stroke, use of angiotensin-converting enzyme inhibitor or angiotensin receptor blocker, use of beta blocker, smoking status, body mass index (BMI), hemoglobin level, serum albumin level, follow-up LDL level, duration of DAPT, and lesion characteristics, including TASC classification and target lesion. To measure the balancing, we calculated the standardized bias for each measured covariate of the weighted samples. SAS software version 9.4 (SAS Institute Inc., Cary, NC, USA) was used for the IPTW analysis to automatically compute the PS scores and to conduct a balance check using generalized boosted regression.
RESULTS
Baseline characteristics
A total of 376 patients with claudication or CLI was included in the overall population, and 309 (82%) of these patients were prescribed a statin medication. Among the patients treated with a statin, 79 (21.0%) were prescribed an HI statin, and 238 (63.3%) were prescribed LMI statins. The mean follow-up duration was 40 months. The patients’ baseline clinical characteristics are shown in Table 1. Patients in the HI statin group were younger than those in the LMI group. Most of the patients in both groups were male. There was no significant difference in underlying diseases including hypertension, diabetes, chronic kidney disease, and other cardiovascular disease. The prescription rate of aspirin and clopidogrel were lower in the patients who were not on statins (no-statin group). Compared to the HI and LMI groups, BMI and hemoglobin and albumin levels were lower in the no-statin group. Meanwhile, aspartate aminotransferase (AST) and alanine aminotransferase (ALT) levels were higher in the no-statin group. Baseline LDL-C levels were not different among the three groups; however, the highest follow-up LDL-C levels were found in the in no-statin group. When the target LDL-C was defined as 70 mg/dL or less, 38.3% of the patients overall reached the target LDL, and 46.8% of the patients in the HI group corresponded to the target.
Table 1. Baseline Characteristics on the Basis of Statin Treatment Intensity.
| Variables | Total (n=376) | No-statin (n=59) | LMI (n=238) | HI (n=79) | p value | |
|---|---|---|---|---|---|---|
| Age, yr | 70±11 | 71±10 | 71±10 | 68±11 | 0.139 | |
| Male | 285 (75.8) | 46 (78.0) | 175 (73.5) | 64 (81.0) | 0.571 | |
| Body mass index (kg/m2) | 22.8±4.1 | 21±5.1* | 23±3.6† | 23±4.2† | 0.002 | |
| Current smoker | 129 (34.3) | 18 (30.5) | 79 (33.2) | 32 (40.5) | 0.199 | |
| Hypertension | 284 (75.5) | 45 (76.3) | 187 (78.6) | 52 (65.8) | 0.108 | |
| Diabetes mellitus | 197 (52.4) | 33 (55.9) | 128 (53.8) | 36 (45.6) | 0.201 | |
| Chronic kidney disease | 109 (29.0) | 22 (37.3) | 66 (27.7) | 21 (26.6) | 0.201 | |
| Congestive heart failure | 50 (13.3) | 12 (20.3) | 31 (13.0) | 7 (8.9) | 0.054 | |
| Coronary artery disease | 182 (48.4) | 22 (37.3) | 122 (51.3) | 38 (48.1) | 0.281 | |
| Previous myocardial infarction | 37 (9.8) | 4 (6.8) | 29 (12.2) | 4 (5.1) | 0.573 | |
| Previous stroke | 71 (18.9) | 10 (16.9) | 51 (21.4) | 10 (12.7) | 0.410 | |
| Previous percutaneous transluminal angioplasty | 25 (6.6) | 7 (11.9) | 14 (5.9) | 4 (5.1) | 0.138 | |
| Medication | ||||||
| Aspirin | 322 (85.6) | 45 (76.3) | 204 (85.7) | 73 (92.4) | 0.008 | |
| Clopidogrel | 275 (73.1) | 27 (45.8) | 181 (76.1) | 67 (84.8) | <0.001 | |
| Cilostazol | 215 (57.2) | 38 (64.4) | 134 (56.3) | 43 (54.4) | 0.267 | |
| ACEI or ARB | 164 (43.6) | 21 (35.6) | 112 (47.1) | 31 (39.2) | 0.826 | |
| Calcium channel blocker | 149 (39.6) | 20 (33.9) | 101 (42.4) | 28 (35.4) | 0.990 | |
| Beta blocker | 137 (36.4) | 15 (25.4) | 93 (39.1) | 29 (36.7) | 0.234 | |
| Insulin | 50 (13.3) | 8 (13.6) | 32 (13.4) | 10 (12.7) | 0.868 | |
| Hemoglobin (g/dL) | 12.1±2.1 | 11.3±2.1* | 12.2±2.1† | 12.3±2.1† | 0.005 | |
| White blood cell (103/mm3) | 8.8±3.5 | 10.1±4.7* | 8.4±3.2† | 8.8±2.9† | 0.004 | |
| Platelet (103/mm3) | 235±85 | 233±83 | 232±79 | 246±100 | 0.417 | |
| Creatinine (mg/dL) | 1.6±2.1 | 2.1±2.7 | 1.6±2.0 | 1.5±2.0 | 0.273 | |
| Total cholesterol (mg/dL) | 148±43 | 142±38 | 148±42 | 155±48 | 0.259 | |
| Triglyceride (mg/dL) | 144±103 | 134±120 | 143±84 | 153±141 | 0.609 | |
| Low-density lipoprotein (mg/dL) | 96±32 | 93±29 | 95±33 | 99±32 | 0.468 | |
| High-density lipoprotein (mg/dL) | 38±11 | 37±13 | 38±10 | 41±12 | 0.160 | |
| AST (U/L) | 33±77 | 61±179* | 28±29† | 31±46† | 0.014 | |
| ALT (U/L) | 22±34 | 39±80* | 18±13† | 21±20† | <0.001 | |
| Albumin (g/dL) | 3.7±0.6 | 3.3±0.72* | 3.8±0.6† | 3.8±0.4† | <0.001 | |
| Follow up low-density lipoprotein (mg/dL) | 83±28 | 99±36* | 80±25† | 83±27† | 0.001 | |
| Follow up low-density lipoprotein <70 mg/dL | 142 (38.3) | 17 (29.8) | 88 (37.4) | 37 (46.8) | 0.040 | |
| Follow up duration (days) | 1199±1007 | 1098±1154 | 1228±995 | 1188±32 | 0.671 | |
ACEI, angiotensin converting enzyme inhibitor; ALT, alanine aminotransferase; ARB, angiotensin receptor blocker; AST, aspartate aminotransferase; HI, high-intensity; LMI, low-to-moderate intensity.
Data are presented as a mean±SD or n (%).
*†Difference in post-hoc analysis.
There was no significant difference in multilevel disease among the groups (Table 2). However, the number of CLI and infra-popliteal lesions and TASC C or D lesions were highest in the no-statin group. Regarding the treatment type, there was no difference among the three groups. Pre-treatment ankle-brachial index values were lowest in the no-statin group, although there was no difference after treatment.
Table 2. Procedural Data on the Basis of Statin Treatment Intensity.
| Variables | Total (n=376) | No-statin (n=59) | LMI (n=238) | HI (n=79) | p value | |
|---|---|---|---|---|---|---|
| Critical limb ischemia | 144 (38.5) | 34 (57.6) | 89 (37.4) | 23 (29.1) | 0.001 | |
| Target lesion | ||||||
| Aorto-iliac | 149 (39.5) | 18 (30.5) | 97 (40.8) | 34 (43.0) | 0.159 | |
| Femoro-popliteal | 237 (63.0) | 43 (72.9) | 149 (62.6) | 45 (57.0) | 0.061 | |
| Below knee | 120 (31.9) | 29 (49.2) | 72 (30.3) | 19 (24.1) | 0.003 | |
| Multilevel disease (target lesion) | 111 (29.5) | 21 (35.6) | 70 (29.4) | 20 (25.3) | 0.196 | |
| TASC classification | 0.006 | |||||
| A | 72 (19.2) | 6 (10.2) | 50 (21.1) | 16 (20.3) | ||
| B | 100 (26.7) | 11 (18.6) | 65 (27.4) | 24 (30.4) | ||
| C | 116 (30.9) | 19 (32.2) | 72 (30.4) | 25 (31.6) | ||
| D | 87 (23.2) | 23 (39.0) | 50 (21.1) | 14 (17.7) | ||
| Type of intervention | ||||||
| Balloon | 362 (96.3) | 57 (96.6) | 230 (96.6) | 75 (94.9) | 0.572 | |
| Stent | 245 (65.2) | 35 (59.3) | 155 (65.1) | 55 (69.6) | 0.212 | |
| Atherectomy | 20 (5.3) | 1 (1.7) | 16 (6.7) | 3 (3.8) | 0.722 | |
| Hemodynamics | ||||||
| Pre-ABI | 0.7/0.7 | 0.6./0.5 | 0.7/0.7 | 0.8/0.7 | 0.041 | |
| Post-ABI | 0.9/0.9 | 0.8/0.8 | 0.9/0.9 | 0.9/0.9 | 0.390 | |
ABI, ankle-brachial index; HI, high-intensity; LMI, low-to-moderate intensity; TASC, Trans-Atlantic Inter-Society Consensus for the Management of Peripheral Arterial Disease.
Data are presented as a n (%).
Clinical outcomes
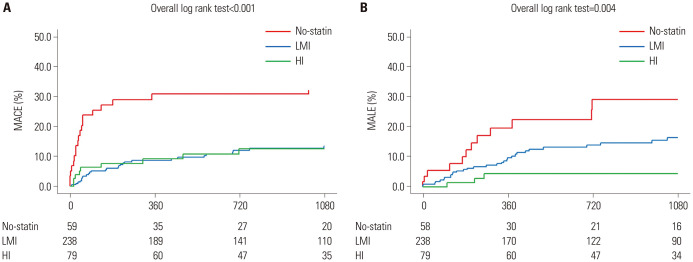
The clinical outcomes based on statin intensity are summarized in Table 3. Compared with the no-statin group, the occurrence of MACE was lower in the LMI (HR: 0.347; 95% CI: 0.206–0.583; p<0.001) and HI statin groups (HR: 0.256; 95% CI: 0.118–0.555; p=0.001). The findings were similar with the occurrence of all-cause death. The number of MALE events was lower in the HI statin group than in the no-statin group (HR: 0.300; 95% CI: 0.111–0.812; p=0.018); the incidence of target vessel revascularization (HR: 0.372; 95% CI: 0.132–1.048; p=0.061) was lower in the HI statin group. There was no statistical difference in MALE events between the no and LMI statin group. Similar results were found in the Kaplan-Meier survival analysis (Fig. 1).
Table 3. Clinical Outcome Rates on the Basis of Statin Therapy Intensity.
| Variables | No-statin (n=59) | LMI (n=238) | HI (n=79) | HR (95% CI) No-stain vs. LMI | p value | HR (95% CI) No-stain vs. HI | p value | |
|---|---|---|---|---|---|---|---|---|
| MACE | 23 (39.0) | 38 (16.0) | 9 (11.4) | 0.347 (0.206–0.583) | <0.001 | 0.256 (0.118–0.555) | 0.001 | |
| Death | 20 (33.9) | 29 (12.2) | 7 (8.9) | 0.309 (0.175–0.548) | <0.001 | 0.232 (0.098–0.550) | 0.001 | |
| MI | 2 (3.4) | 2 (0.8) | 2 (2.5) | 0.209 (0.029–1.491) | 0.118 | 0.659 (0.093–4.697) | 0.678 | |
| Stroke | 1 (1.7) | 7 (2.9) | 1 (1.3) | 1.517 (0.185–12.414) | 0.697 | 0.640 (0.040–10.261) | 0.752 | |
| MALE | 12 (20.9) | 38 (16.0) | 6 (7.6) | 0.665 (0.338–1.308) | 0.238 | 0.300 (0.111–0.812) | 0.018 | |
| TVR | 9 (15.3) | 32 (13.4) | 6 (7.6) | 0.690 (0.327–1.455) | 0.330 | 0.372 (0.132–1.048) | 0.061 | |
| Major amputation | 3 (5.1) | 6 (2.5) | 1 (1.2) | 0.611 (0.123–3.032) | 0.547 | 0.532 (0.143–3.421) | 0.637 | |
CI, confidence interval; HI, high-intensity; HR, hazard ratio; LMI, low-to-moderate intensity; MACE, major adverse cardiovascular events; MALE, major adverse limb events; MI, myocardial infarction; TVR, target vessel revascularization.
Data are presented as a n (%).
Fig. 1. Kaplan-Meier survival curves for MACE, MALE according to statin intensity. (A) Kaplan-Meier survival curves for MACE according to statin treatment intensity. (B) Kaplan-Meier survival curves for MALE according to statin treatment intensity. HI, high-intensity; LMI, low-to-moderate intensity; MACE, major adverse cardiovascular events; MALE, major adverse limb events.
The adverse clinical outcomes for the entire population, predicted by univariate and multivariate Cox proportional survival analyses, are shown in Table 4. HI treatment was an independent risk factor for both MACE (HR: 0.447; 95% CI: 0.244–0.834; p=0.018) and MALE (HR: 0.360; 95% CI: 0.129–1.006; p=0.051) events. LMI treatment was associated with a lower occurrence of MACE (HR: 0.571; 95% CI: 0.326–1.004; p=0.050) events. In addition, age (HR: 1.049; 95% CI: 1.022–1.076; p<0.001), heart failure (HR: 2.879; 95% CI: 1.642–5.045; p<0.001), chronic kidney disease (HR: 2.647; 95% CI: 1.596–4.390; p<0.001), and serum albumin (HR 0.275; 95% CI: 0.184–0.411; p<0.001) were predictors of MACE events. Multiple lesions were an independent risk factor for MALE (HR: 3.011; 95% CI: 1.623–5.585; p<0.001) events.
Table 4. Predictors of MACE and MALE Events after Endovascular Revascularization.
| Univariate analysis HR (95% CI) |
p value | Multivariate analysis HR (95% CI) |
p value | ||
|---|---|---|---|---|---|
| MACE | |||||
| Age (per 1 yr) | 1.067 (1.042–1.093) | <0.001 | 1.049 (1.022–1.076) | <0.001 | |
| Male | 0.837 (0.489–1.434) | 0.518 | |||
| Current smoker | 3.095 (1.657–5.780) | <0.001 | 1.819 (0.908–3.644) | 0.092 | |
| Hypertension | 2.092 (1.071–4.087) | 0.031 | 1.552 (0.727–3.315) | 0.256 | |
| Diabetes mellitus | 0.883 (0.552–1.414) | 0.605 | |||
| Heart failure | 4.015 (2.415–6.675) | <0.001 | 2.879 (1.642–5.045) | <0.001 | |
| Chronic kidney disease | 3.983 (2.468–6.341) | <0.001 | 2.647 (1.596–4.390) | <0.001 | |
| Previous stroke | 0.954 (0.511–1.782) | 0.882 | |||
| Previous MI | 1.725 (0.881–3.377) | 0.112 | |||
| DAPT >6 months | 0.424 (0.254–0.708) | 0.001 | 0.579 (0.334–1.004) | 0.052 | |
| BMI | 0.924 (0.882–0.968) | 0.001 | 0.962 (0.912–1.015) | 0.161 | |
| Serum albumin | 0.226 (0.161–0.316) | <0.001 | 0.275 (0.184–0.411) | <0.001 | |
| Target LDL <70 mg/dL | 0.743 (0.444–1.244) | 0.259 | |||
| No-statin | 1 | ||||
| LMI | 0.347 (0.206–0.583) | <0.001 | 0.571 (0.326–1.004) | 0.050 | |
| HI | 0.256 (0.118–0.555) | 0.001 | 0.447 (0.244–0.834) | 0.018 | |
| RAAS-blocker | 0.554 (0.336–0.914) | 0.021 | 0.689 (0.401–1.185) | 0.178 | |
| Critical limb ischemia | 2.758 (1.700–4.473) | <0.001 | 1.460 (0.855–2.491) | 0.166 | |
| MALE | |||||
| Age (per 1 yr) | 0.989 (0.964–1.015) | 0.397 | |||
| Male | 0.934 (0.473–1.844) | 0.844 | |||
| Current smoker | 1.151 (0.670–1.978) | 0.610 | |||
| Hypertension | 0.926 (0.478–1.793) | 0.820 | |||
| Diabetes mellitus | 1.632 (0.893–2.984) | 0.111 | |||
| Heart failure | 0.048 (0.001–124.025) | 0.560 | |||
| Chronic kidney disease | 0.835 (0.413–1.688) | 0.616 | |||
| Previous stroke | 1.862 (0.977–3.549) | 0.059 | 1.052 (0.540–2.047) | 0.882 | |
| Previous MI | 1.256 (0.496–3.183) | 0.631 | |||
| DAPT >6 months | 1.033 (0.605–1.763) | 0.905 | |||
| Albumin | 0.616 (0.373–1.015) | 0.057 | 0.814 (0.466–1.422) | 0.470 | |
| Target LDL <70 mg/dL | 0.617 (0.318–1.197) | 0.153 | |||
| No-statin | |||||
| LMI | 0.665 (0.338–1.308) | 0.238 | 0.641 (0.311–1.322) | 0.229 | |
| HI | 0.300 (0.111–0.812) | 0.018 | 0.360 (0.129–1.006) | 0.051 | |
| RAAS-blocker | 1.023 (0.569–1.838) | 0.940 | |||
| Critical limb ischemia | 1.563 (0.868–2.815) | 0.137 | |||
| Multiple-lesion | 4.228 (2.455–7.280) | <0.001 | 3.011 (1.623–5.585) | <0.001 | |
| TASC C or D | 1.946 (1.102–3.438) | 0.022 | 1.144 (0.596–21.197) | 0.687 | |
BMI, body mass index; CI, confidence interval; DAPT, dual antiplatelet therapy; HI high-intensity; HR, hazard ratio; LMI, low-to-moderate intensity; LDL, low-density lipoprotein; MACE, major adverse cardiovascular events; MALE, major adverse limb events; MI, myocardial infarction; RAAS, renin angiotensin aldosterone system; TASC, Trans-Atlantic Inter-Society Consensus for the Management of Peripheral Arterial Disease.
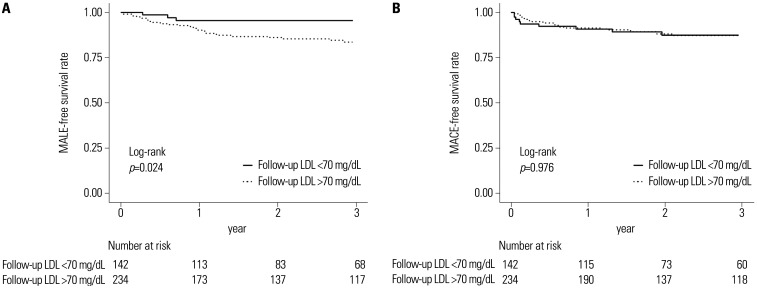
For head-to-head comparison between HI and LMI, we adjusted 20 variables for IPTW matching as mentioned in our Methods section. Baseline characteristics of the study population before and after PS matching is shown in Supplementary Table 1 (only online). HI statin therapy elicited better outcomes in terms of MALE (HR: 0.432; 95% CI: 0.223–0.837; p=0.013) events after IPTW-adjusted analysis as seen in Table 5. In addition, we compared clinical outcomes according to follow-up LDL-C levels (Fig. 2). Regarding MALE events, a follow-up LDL-C level less than 70 mg/dL showed favorable outcome. There was no significant difference in MACE events.
Table 5. Clinical Outcomes and Unadjusted and Inverse Probability-Weighted Adjusted HRs on the Basis of Statin Intensity.
| LMI (n=238) | HI (n=79) | Unadjusted | IPTW adjusted | ||||
|---|---|---|---|---|---|---|---|
| HR (95% CI) | p value | HR (95% CI) | p value | ||||
| MACE | 38 (16) | 9 (11.4) | 0.731 (0.353–1.511) | 0.397 | 1.145 (0.875–2.069) | 0.176 | |
| All-cause death | 29 (12.2) | 7 (8.9) | 0.737 (0.323–1.683) | 0.469 | 1.135 (0.680–1.862) | 0.137 | |
| Stroke | 7 (2.9) | 1 (1.3) | 0.418 (0.051–3.401) | 0.415 | 0.936 (0.452–1.938) | 0.859 | |
| Myocardial infarction | 2 (0.8) | 2 (2.5) | 3.148 (0.443–22.351) | 0.252 | 3.359 (0.680–13.051) | 0.137 | |
| MALE | 38 (16) | 6 (7.6) | 0.446 (0.188–1.057) | 0.067 | 0.432 (0.223–0.837) | 0.013 | |
| Repeat revascularization | 32 (13.5) | 6 (7.6) | 0.533 (0.022–1.279) | 0.159 | 0.649 (0.383–1.102) | 0.109 | |
CI, confidence interval; HI, high-intensity; HR, hazard ratio; IPTW, inverse probability of treatment weighting; LMI, low-to-moderate intensity; MACE, major adverse cardiovascular events; MALE, major adverse limb events.
Data are presented as a n (%).
Fig. 2. Kaplan-Meier survival curves for MALE, MACE according to target LDL-C level. (A) Kaplan-Meier survival curves for MALE according to target LDL-C level. (B) Kaplan-Meier survival curves for MACE according to target LDL-C level. LDL-C, low-density lipoprotein cholesterol; MACE, major adverse cardiovascular events; MALE, major adverse limb events.
DISCUSSION
The main findings of our analysis are as follows: 1) in real-world practice, statin prescriptions are related with a patient’s baseline characteristics and laboratory findings; 2) HI statin treatment is associated with significantly lower rates of MACE and MALE than no-statin use; 3) LMI statin treatment is an independent predictor of a reduced risk for MACE, but not MALE; 4) HI statin therapy elicits fewer MALE over LMI in IPTW-adjusted analysis.
Relative to CHD or CVD, PAD involves more vulnerable atherosclerotic plaques and poses an increased risk of adverse cardiovascular events.13 Therefore, when treating patients with PAD, it is important not only to relieve symptoms, but also to reduce the risk of atherosclerotic CVD. Statin use has been shown to be associated with a decreased risk of mortality, fewer MACE, and greater affected limb patency.14,15,16 Based on these findings, recent guidelines classify patients with PAD as very high-risk patients and strongly recommend the maximum tolerated dose of statins (Class 1A).6 However, the use of statin therapy might be lower among patients with PAD than those with CHD or CVD.17,18 This is consistent with a recent study, wherein the statin prescription rate in patients with PAD was as low as 34%.7 Although there are many studies on statin intensity in patients with coronary artery disease,19,20,21,22 there are few studies on the outcome of treatment according to statin intensity in patients with PAD. In addition, studies supporting the current statin guidelines primarily involve Western populations. However, considering the variation in responses to statin drugs by race and recommendations for a reduction in the statin dose in Asians,23,24,25 more studies among Asians are required.
Our study outlined the prescription patterns of statins in patients with PAD in a real-world setting. The patients in the no-statin group had lower BMI and significantly lower levels of hemoglobin and albumin. In addition, although there was no statistical significance, creatinine levels were higher and the prevalence of chronic kidney disease was high in the no-statin group. Since these factors are risk factors for statin intolerance,26 it can be assumed that there might be a tendency not to prescribe statins. This may be reflected by the lower prescription rate of aspirin or clopidogrel in the no-statin group. This may also be explained by their AST and ALT levels: liver enzyme levels were highest in the no-statin group, and the levels were higher than the normal range of values. Considering that elevated liver enzymes are common side effects of statin therapy, the patients with elevated liver enzyme might be less prescribed the statin.
Our study also showed that MACE and MALE may be prevented by HI statin use in patients with PAD. Several previous studies have reported that any statin dose in patients with PAD is associated with lower mortality and cardio-vascular events.27,28 However, data are limited, and there are no randomized clinical trials evaluating the clinical impact of statin intensity on clinical outcomes in PAD. To the best of our knowledge, only one study found a clinical impact of statin intensity in patients with clinically significant PAD referred for endovascular intervention.29 They also reported that guideline-directed HI statin therapy was not prescribed frequently (13.6%), although HI statin therapy was associated with improved survival and fewer MACEs than LMI. However, our study involved Asians, unlike other studies, which involved Western populations and excluded patients who were not receiving statins. In addition, follow-up LDL-C levels were not measured. Arya, et al.30 conducted a population-based study using national Veterans Health administration data in the United States in which HI statin use at the time of PAD diagnosis was associated with a significantly higher reduction in limb loss and mortality than LMI statin use. In their study, when compared to the no-statin group, any dose of statin use improved mortality and limb patency, and when comparing the HI and LMI groups, the HI group had better outcomes, which is consistent with our findings. However, most patients in their study were male (97.9%) and Caucasians (82.6%). In addition, PAD was based on a diagnosis in the medical records, and in fact, information on the severity of PAD could not be obtained in 68.5%. Therefore, the accuracy of the clinical diagnosis of PAD was not conclusive, which may be an important limitation to the study.
In our study, there was a strong association of statin use with decreased overall mortality and fewer MACEs. The incremental benefit of using HI statins, rather than LMI, for MALEs was shown. Possible mechanisms of this overall benefit of statins include their lipid lowering and pleiotropic effects in stabilizing and regressing plaques, which result in an anti-inflammatory effect.28,31,32 There was no difference in baseline LDL-C levels among the three groups, but follow-up LDL-C levels were highest in the no-statin group. Also, the achievement of target LDL-C rate was highest in the HI group. Therefore, it is possible that the LDL-C lowering effect of statins influenced the better outcome of both statin-treated groups shown in Table 3. In the HI group, MALEs significantly decreased, compared with the LMI group and no-statin group. Considering that follow-up LDL-C levels and target LDL-C rates were not significantly different in comparison with the LMI group, this could be correlated with the pleiotropic effect of HI statin. Compared with recent studies,20 our study showed higher LDL-C levels and lower target LDL-C achievement rates in the HI group. There may be several factors involved, but it is thought that a change in the guidelines for cholesterol treatment during the study enrollment period had a major impact. In the 2013 ACC/AHA guidelines, high and moderate intensity statin use was recommended, but there was no target LDL-C goal.33 However, in the 2018 ACC/AHA and 2018 Korean guidelines,34,35 it was recommended to reduce the LDL-C levels to <70 mg/dL or by >50% from the baseline level for secondary prevention and <55 mg/dL in 2019 ESC guidelines.6 Therefore, since this study was conducted with patients between 2009 and 2019, it is thought that active efforts to achieve target LDL-C were insufficient.
We also analyzed outcomes according to the achievement of target LDL-C levels using multivariate Cox analysis and Kaplan-Meier curves. In Cox analysis, there was no significant difference in the incidence of MACE or MALE according to the target LDL-C. However, Kaplan-Meier curves indicated that reaching the target LDL-C elicited good clinical outcomes in terms of MALE. Considering that the frequency of reaching target LDL-C levels increased with more intense stating therapy, from the no-statin group to the HI statin group, we think that the cholesterol lowering effect of statin has an important effect on outcomes. However, as mentioned above, in this study, the target LDL-C reach rate was low, even in the HI group, and the size of the overall population was small. Therefore, it is thought that these issues may have influenced the clinical events according to the target LDL-C, and it is considered that additional studies are needed to evaluate the effect of the target LDL-C, as well as statin intensity, in PAD patients.
This study has several limitations. First, we reported outcomes from a single center. Therefore, our findings are not generalizable. In addition, this was a retrospective, non-randomized study, which pose selection bias and unmeasured data. However, we reduced this bias by performing multivariate Cox and IPTW analyses. Third, in our study, the outcomes were clinical events or revascularization, since angiography and CT were not performed routinely. Thus, there is a high possibility that the vascular outcome was underestimated. Fourth, follow-up duration and lab examinations were left to the physician’s discretion. In particular, since lab follow up, including LDL-C levels, did not follow a set schedule, there was difficulty in judging the effectiveness of statins. Finally, although there was no-statin toxicity event, the side effects of statins could not be evaluated since laboratory tests, such as muscle enzymes or liver function tests, were not performed routinely.
In conclusion, use of HI statins appears to be associated with improved overall survival and fewer MACE events than no-statin use. LMI statins also reduced the risk of mortality and MACEs more than no-statin use, and may have an important role in patients who cannot tolerate HI statins. Finally, the results of HI statin therapy were superior to those achieved with no-statin or LMI statin therapy.
ACKNOWLEDGEMENTS
This work was supported by a National Research Foundation of Korea (NRF) grant funded by the Korean government (Ministry of Science and ICT, MSIT) (No. 2019R1G1A1100442).
Footnotes
The authors have no potential conflicts of interest to disclose.
- Conceptualization: Byung Ok Kim.
- Data curation: Hye Young Lee and Moo-Nyun Jin.
- Formal analysis: Byung Gyu Kim.
- Funding acquisition: Gwang Sil Kim.
- Investigation: Gwang Sil Kim and Hye Young Lee.
- Methodology: Jongkwon Seo.
- Project administration: Gwang Sil Kim.
- Resources: Gwang Sil Kim.
- Software: Moo-Nyun Jin.
- Supervision: Young Sup Byun.
- Validation: Byung Gyu Kim.
- Visualization: Byung Gyu Kim.
- Writing—original draft: Gwang Sil Kim and Jongkwon Seo.
- Writing—review & editing: Young Sup Byun.
- Approval of final manuscript: all authors.
SUPPLEMENTARY MATERIAL
Baseline Characteristics of the Study Population according to the Intensity of Statin Therapy after Propensity Score Matching
References
- 1.Fowkes FG, Aboyans V, Fowkes FJ, McDermott MM, Sampson UK, Criqui MH. Peripheral artery disease: epidemiology and global perspectives. Nat Rev Cardiol. 2017;14:156–170. doi: 10.1038/nrcardio.2016.179. [DOI] [PubMed] [Google Scholar]
- 2.Caro J, Migliaccio-Walle K, Ishak KJ, Proskorovsky I. The morbidity and mortality following a diagnosis of peripheral arterial disease: long-term follow-up of a large database. BMC Cardiovasc Disord. 2005;5:14. doi: 10.1186/1471-2261-5-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Criqui MH, Aboyans V. Epidemiology of peripheral artery disease. Circ Res. 2015;116:1509–1526. doi: 10.1161/CIRCRESAHA.116.303849. [DOI] [PubMed] [Google Scholar]
- 4.Hinchliffe RJ, Forsythe RO, Apelqvist J, Boyko EJ, Fitridge R, Hong JP, et al. Guidelines on diagnosis, prognosis, and management of peripheral artery disease in patients with foot ulcers and diabetes (IWGDF 2019 update) Diabetes Metab Res Rev. 2020;36 Suppl 1:e3276. doi: 10.1002/dmrr.3276. [DOI] [PubMed] [Google Scholar]
- 5.Stone NJ, Robinson JG, Lichtenstein AH, Bairey Merz CN, Blum CB, Eckel RH, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines. J Am Coll Cardiol. 2014;63:2889–2934. doi: 10.1016/j.jacc.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 6.Mach F, Baigent C, Catapano AL, Koskinas KC, Casula M, Badimon L, et al. 2019 ESC/EAS guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. Eur Heart J. 2020;41:111–188. doi: 10.1093/eurheartj/ehz455. [DOI] [PubMed] [Google Scholar]
- 7.Colantonio LD, Hubbard D, Monda KL, Mues KE, Huang L, Dai Y, et al. Atherosclerotic risk and statin use among patients with peripheral artery disease. J Am Coll Cardiol. 2020;76:251–264. doi: 10.1016/j.jacc.2020.05.048. [DOI] [PubMed] [Google Scholar]
- 8.Cho S, Lee YJ, Ko YG, Kang TS, Lim SH, Hong SJ, et al. Optimal strategy for antiplatelet therapy after endovascular revascularization for lower extremity peripheral artery disease. JACC Cardiovasc Interv. 2019;12:2359–2370. doi: 10.1016/j.jcin.2019.08.006. [DOI] [PubMed] [Google Scholar]
- 9.Norgren L, Hiatt WR, Dormandy JA, Nehler MR, Harris KA, Fowkes FG TASC II Working Group. Inter-society consensus for the management of peripheral arterial disease (TASC II) J Vasc Surg. 2007;45 Suppl S:S5–S67. doi: 10.1016/j.jvs.2006.12.037. [DOI] [PubMed] [Google Scholar]
- 10.Thygesen K, Alpert JS, Jaffe AS, Simoons ML, Chaitman BR, White HD, et al. Third universal definition of myocardial infarction. Circulation. 2012;126:2020–2035. doi: 10.1016/j.gheart.2012.08.001. [DOI] [PubMed] [Google Scholar]
- 11.Patel MR, Conte MS, Cutlip DE, Dib N, Geraghty P, Gray W, et al. Evaluation and treatment of patients with lower extremity peripheral artery disease: consensus definitions from Peripheral Academic Research Consortium (PARC) J Am Coll Cardiol. 2015;65:931–941. doi: 10.1016/j.jacc.2014.12.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Braitman LE, Rosenbaum PR. Rare outcomes, common treatments: analytic strategies using propensity scores. Ann Intern Med. 2002;137:693–695. doi: 10.7326/0003-4819-137-8-200210150-00015. [DOI] [PubMed] [Google Scholar]
- 13.Grenon SM, Vittinghoff E, Owens CD, Conte MS, Whooley M, Cohen BE. Peripheral artery disease and risk of cardiovascular events in patients with coronary artery disease: insights from the Heart and Soul Study. Vasc Med. 2013;18:176–184. doi: 10.1177/1358863X13493825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stavroulakis K, Borowski M, Torsello G, Bisdas T CRITISCH collaborators. Association between statin therapy and amputation-free survival in patients with critical limb ischemia in the CRITISCH registry. J Vasc Surg. 2017;66:1534–1542. doi: 10.1016/j.jvs.2017.05.115. [DOI] [PubMed] [Google Scholar]
- 15.Westin GG, Armstrong EJ, Bang H, Yeo KK, Anderson D, Dawson DL, et al. Association between statin medications and mortality, major adverse cardiovascular event, and amputation-free survival in patients with critical limb ischemia. J Am Coll Cardiol. 2014;63:682–690. doi: 10.1016/j.jacc.2013.09.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kumbhani DJ, Steg PG, Cannon CP, Eagle KA, Smith SC, Jr, Goto S, et al. Statin therapy and long-term adverse limb outcomes in patients with peripheral artery disease: insights from the REACH registry. Eur Heart J. 2014;35:2864–2872. doi: 10.1093/eurheartj/ehu080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Welten GM, Schouten O, Hoeks SE, Chonchol M, Vidakovic R, van Domburg RT, et al. Long-term prognosis of patients with peripheral arterial disease: a comparison in patients with coronary artery disease. J Am Coll Cardiol. 2008;51:1588–1596. doi: 10.1016/j.jacc.2007.11.077. [DOI] [PubMed] [Google Scholar]
- 18.Hirsch AT, Criqui MH, Treat-Jacobson D, Regensteiner JG, Creager MA, Olin JW, et al. Peripheral arterial disease detection, awareness, and treatment in primary care. JAMA. 2001;286:1317–1324. doi: 10.1001/jama.286.11.1317. [DOI] [PubMed] [Google Scholar]
- 19.Xian Y, Navar AM, Li S, Li Z, Robinson J, Virani SS, et al. Intensity of lipid lowering with statin therapy in patients with cerebrovascular disease versus coronary artery disease: insights from the PALM Registry. J Am Heart Assoc. 2019;8:e013229. doi: 10.1161/JAHA.119.013229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Taguchi I, Iimuro S, Iwata H, Takashima H, Abe M, Amiya E, et al. High-dose versus low-dose pitavastatin in Japanese patients with stable coronary artery disease (REAL-CAD) a randomized superiority trial. Circulation. 2018;137:1997–2009. doi: 10.1161/CIRCULATIONAHA.117.032615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.O’Brien EC, Wu J, Schulte PJ, Christian A, Laskey W, Bhatt DL, et al. Statin use, intensity, and 3-year clinical outcomes among older patients with coronary artery disease. Am Heart J. 2016;173:27–34. doi: 10.1016/j.ahj.2015.11.014. [DOI] [PubMed] [Google Scholar]
- 22.Lee SY, Oh SJ, Kim EJ, Oum CY, Park SH, Oh J, et al. Statin intensity and clinical outcome in patients with stable coronary artery disease and very low LDL-cholesterol. PLoS One. 2016;11:e0166246. doi: 10.1371/journal.pone.0166246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Meor Anuar Shuhaili MFR, Samsudin IN, Stanslas J, Hasan S, Thambiah SC. Effects of different types of statins on lipid profile: a perspective on Asians. Int J Endocrinol Metab. 2017;15:e43319. doi: 10.5812/ijem.43319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liao JK. Safety and efficacy of statins in Asians. Am J Cardiol. 2007;99:410–414. doi: 10.1016/j.amjcard.2006.08.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nakamura H, Arakawa K, Itakura H, Kitabatake A, Goto Y, Toyota T, et al. Primary prevention of cardiovascular disease with pravastatin in Japan (MEGA Study): a prospective randomised controlled trial. Lancet. 2006;368:1155–1163. doi: 10.1016/S0140-6736(06)69472-5. [DOI] [PubMed] [Google Scholar]
- 26.Tomlinson B, Chan P, Liu ZM. Statin intolerance-an Asian perspective. J Atheroscler Thromb. 2020;27:485–488. doi: 10.5551/jat.50435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ramos R, García-Gil M, Comas-Cufí M, Quesada M, Marrugat J, Elosua R, et al. Statins for prevention of cardiovascular events in a low-risk population with low ankle brachial index. J Am Coll Cardiol. 2016;67:630–640. doi: 10.1016/j.jacc.2015.11.052. [DOI] [PubMed] [Google Scholar]
- 28.Feringa HH, Karagiannis SE, van Waning VH, Boersma E, Schouten O, Bax JJ, et al. The effect of intensified lipid-lowering therapy on long-term prognosis in patients with peripheral arterial disease. J Vasc Surg. 2007;45:936–943. doi: 10.1016/j.jvs.2007.01.024. [DOI] [PubMed] [Google Scholar]
- 29.Foley TR, Singh GD, Kokkinidis DG, Choy HK, Pham T, Amsterdam EA, et al. High-intensity statin therapy is associated with improved survival in patients with peripheral artery disease. J Am Heart Assoc. 2017;6:e005699. doi: 10.1161/JAHA.117.005699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Arya S, Khakharia A, Binney ZO, DeMartino RR, Brewster LP, Goodney PP, et al. Association of statin dose with amputation and survival in patients with peripheral artery disease. Circulation. 2018;137:1435–1446. doi: 10.1161/CIRCULATIONAHA.117.032361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.van Wissen S, Smilde TJ, de Groot E, Hutten BA, Kastelein JJ, Stalenhoef AF. The significance of femoral intima-media thickness and plaque scoring in the atorvastatin versus simvastatin on atherosclerosis progression (ASAP) study. Eur J Cardiovasc Prev Rehabil. 2003;10:451–455. doi: 10.1097/01.hjr.0000103277.02552.1e. [DOI] [PubMed] [Google Scholar]
- 32.Youssef F, Seifalian AM, Jagroop IA, Myint F, Baker D, Mikhailidis DP, et al. The early effect of lipid-lowering treatment on carotid and femoral intima media thickness (IMT) Eur J Vasc Endovasc Surg. 2002;23:358–364. doi: 10.1053/ejvs.2002.1611. [DOI] [PubMed] [Google Scholar]
- 33.Stone NJ, Robinson JG, Lichtenstein AH, Bairey Merz CN, Blum CB, Eckel RH, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines. Circulation. 2014;129:S1–S45. doi: 10.1161/01.cir.0000437738.63853.7a. [DOI] [PubMed] [Google Scholar]
- 34.Rhee EJ, Kim HC, Kim JH, Lee EY, Kim BJ, Kim EM, et al. 2018 guidelines for the management of dyslipidemia. Korean J Intern Med. 2019;34:723–771. doi: 10.3904/kjim.2019.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Grundy SM, Stone NJ, Bailey AL, Beam C, Birtcher KK, Blumenthal RS, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: a report of the American College of Cardiology/American Heart Association Task Force on clinical practice guidelines. Circulation. 2019;139:e1082–e1143. doi: 10.1161/CIR.0000000000000625. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Baseline Characteristics of the Study Population according to the Intensity of Statin Therapy after Propensity Score Matching