Summary
Background
A major challenge of the SARS-CoV-2 pandemic is to better define “protective thresholds” to guide the global response. We aimed to characterize the longitudinal dynamics of the antibody responses in naturally infected individuals in Chile and compared them to humoral responses induced after immunization with CoronaVac-based on an inactivated whole virus -or the BNT162b2- based on mRNA-vaccines. We also contrasted them with the respective effectiveness and efficacy data available for both vaccines.
Methods
We determined and compared the longitudinal neutralizing (nAb) and anti-nucleocapsid (anti-N) antibody responses of 74 COVID-19 individuals (37 outpatient and 37 hospitalized) during the acute disease and convalescence. We also assessed the antibody boosting of 36 of these individuals who were immunized after convalescence with either the CoronaVac (n = 30) or the BNT162b2 (n = 6) vaccines. Antibody titres were also measured for 50 naïve individuals immunized with two doses of CoronaVac (n = 35) or BNT162b2 (n = 15) vaccines. The neutralizing level after vaccination was compared to those of convalescent individuals and the predicted efficacy was estimated.
Findings
SARS-CoV-2 infection induced robust nAb and anti-N antibody responses lasting >9 months, but showing a rapid nAb decay. After convalescence, nAb titres were significantly boosted by vaccination with CoronaVac or BNT162b2. In naïve individuals, the calculated mean titre induced by two doses of CoronaVac or BNT162b2 was 0·2 times and 5.2 times, respectively, that of convalescent individuals, which has been proposed as threshold of protection. CoronaVac induced no or only modest anti-N antibody responses. Using two proposed logistic models, the predicted efficacy of BNT162b2 was estimated at 97%, in close agreement with phase 3 efficacy studies, while for CoronaVac it was ∼50% corresponding to the lowest range of clinical trials and below the real-life data from Chile (from February 2 through May 1, 2021 during the predominant circulation of the Gamma variant), where the estimated vaccine effectiveness to prevent COVID-19 was 62·8–64·6%.
Interpretation
The decay of nAbs titres in previously infected individuals over time indicates that vaccination is needed to boost humoral memory responses. Immunization of naïve individuals with two doses of CoronaVac induced nAbs titres that were significantly lower to that of convalescent patients, and similar to vaccination with one dose of BTN162b2. The real life effectiveness for CoronaVac in Chile was higher than estimated; indicating that lower titres and additional cellular immune responses induced by CoronaVac might afford protection in a highly immunized population. Nevertheless, the lower nAb titre induced by two doses of CoronaVac as compared to the BTN162b2 vaccine in naïve individuals, highlights the need of booster immunizations over time to maintain protective levels of antibody, particularly with the emergence of new SARS-CoV-2 variants.
Funding
FONDECYT 1161971, 1212023, 1181799, FONDECYT Postdoctorado 3190706 and 3190648, ANID Becas/Doctorado Nacional 21212258, PIA ACT 1408, CONICYT REDES180170, Centro Ciencia & Vida, FB210008, Financiamiento Basal para Centros Científicos y Tecnológicos de Excelencia grants from the Agencia Nacional de Investigación y Desarrollo (ANID) of Chile; NIH-NIAD grants U19AI135972, R01AI132633 and contracts HHSN272201400008C and 75N93019C00051; the JPB Foundation, the Open Philanthropy Project grant 2020-215611 (5384); and by anonymous donors. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Keywords: COVID-19, Serological response, Neutralizing antibody persistence, SARS-CoV-2 vaccines, Vaccination boost
Research in context.
Evidence before this study
The duration of immune protection against SARS-CoV-2 by natural infection or vaccination remains to be elucidated during the current pandemic. As a central parameter of protection, the titre of circulating neutralizing antibodies has been characterized and compared with the efficacy and effectiveness of vaccines to protect from symptomatic disease. We searched in the PubMed database for articles published up to July 26th 2021, using the terms “SARS-CoV-2” or “COVID-19” and “neutralizing antibodies”, “long-lasting response”, “CoronaVac vaccine” or “BNT162b2 vaccine” to identify articles related with antibody decay over time after natural infection and initial antibody titres upon vaccination. There was data available on spike-specific antibody up to 11 months after onset of symptoms. Numerous data was also available on mRNA vaccine studies, however; little independent data was available on the inactivated virus based CoronaVac vaccine. Of note, the assays for measuring neutralization varied widely and to express data as ratio of convalescence sera, the time of convalescence since the onset of symptoms was not standardized either.
Added value of this study
This study provides a direct comparison of longitudinal convalescent nAb titres after SARS-CoV-2 natural infection and those of individuals immunized with two different vaccine formulations, CoronaVac and BNT162b2. Based on the maximal response curves to SARS-CoV-2 infection we compared the mean titre of nAb response using different time frames and used them as fold comparison with titres found in naïve immunized individuals. The data was further contrasted with the estimated real-life vaccine effectiveness and efficacy to prevent COVID-19, available for these vaccines.
Implications of all the available evidence
Understanding the “threshold” of neutralizing antibody titres that confer protection against symptomatic COVID-19 would help in the management of the pandemic. This is of particular importance because of the decay of antibody levels observed over time after natural infection and vaccination, and due to the emergence of SARS-CoV-2 variants. In this study we showed that two doses CoronaVac immunization leads to initial neutralizing antibody titres that are significantly lower than that of convalescent patients and equivalent to one dose of BNT162b2. However, the real life effectiveness for CoronaVac in Chile was higher than estimated from current logistic models. Hence, further studies are required to assess if lower titres or additional cellular immune responses, might contribute to effective protection in a population with high vaccine coverage.
Alt-text: Unlabelled box
Introduction
The durability of circulating neutralizing antibody (nAb) responses to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection or vaccination has become a central question during the current pandemic to determine correlates of immune protection against disease. While the antibody dynamics during the acute phase have been described, many studies vary considerably in the methods used.1 Increasing evidence suggest that infected individuals can mount long-term SARS-CoV-2 spike-specific nAbs that can remain detectable for up to 11 months.2, 3, 4, 5 However, a “threshold” of nAb titres related with protective activity remains to be defined.6 This definition is of particular importance where vaccine doses are sparse and for less studied vaccines that are being used widely in middle- and low-income countries.
As of June 2021, the World Health Organization (WHO) has authorized the emergency use of six vaccines, which are now also considered for distribution through the coronavirus disease 2019 (COVID-19) Vaccines Global Access (COVAX) program (https://www.who.int/initiatives/act-accelerator/covax). Limited information is currently available on the longevity of the humoral response after vaccination7 or natural infection, and whether a vaccination boost is required for previously infected individuals, including when this should be recommended, particularly in the context of new variants of concern.8, 9, 10 Of the authorized vaccines, limited data is available on the induction of nAbs by the inactivated virus CoronaVac vaccine (Sinovac Life Sciences Co., LTD, Beijing, China), which has been used widely in over 50 countries in the developing world, such as Brazil, Chile, Indonesia and Turkey, with a reported efficacy in protection against symptomatic COVID-19 ranging from 50 to 84%.11 In general, there is limited information on the correlates of protection and the relationship between nAbs levels and the efficacy against symptomatic SARS-CoV-2 infection when immunized with any of the available vaccines.12,13 To provide a framework to implement improved global vaccination strategies, it is imperative to establish correlates of protection that are evaluated and compared simultaneously across different vaccine formulations and dose schedules.14 Hence, additional longitudinal data are needed to characterize the medium- and long-term nAb dynamics, as well as the CD4+ T cells and CD8+ T immune response15 and the Fc- effector functions,16 starting from the acute phase of disease of patients with mild and moderate/severe outcome. It is also important to determine and compare their memory responses upon immunization with the different vaccines currently in use (e.g. inactivated versus mRNA vaccines).
In this study we aimed to analyse the longitudinal neutralizing and anti-nucleocapsid (anti-N) antibody responses after natural infection in convalescent COVID-19 individuals, including analyses of the temporal induction and decay dynamics of these humoral responses. Using these data as a framework, we then compared these titres to those of naïve individuals vaccinated with the CoronaVac vaccine or the BNT162b2 vaccine based on spike protein-encoding messenger RNA (BioNTech/Pfizer), which we then used to contrast them with the respective effectiveness and efficacy data available for both vaccines.
Methods
Study population and clinical metadata
The individuals included in the study are part of the CHILE COVID-19 cohort, which was established in late February of 2020, as part of a CEIRS Cross-Centre project funded by the NIH-NIAID, to study the natural history of SARS-CoV-2 in the Southern Hemisphere (Supplementary Figure 1). Of a total of 168 participants (n = 81 outpatients and n = 87 hospitalized), 74 individuals with a confirmed diagnosis for SARS-CoV-2 infection were recruited prospectively between March 5 and October 22, 2020, and were selected for longitudinal convalescent serology analyses if they had 2 or more samples during 12 months since onset of symptoms. Given that convalescent samples were obtained prior to the appearance of virus variants, in this study we assessed antibody titres against the Wuhan-like virus strain. Due to the rapid vaccination campaign in Chile, 36 of these 74 participants were immunized with 1 or 2 doses of either the CoronaVac or BNT162b2 vaccines during the follow up period within 127–398 days (4.2–13.3 months) since onset of symptoms (Supplementary Figure 2 and Supplementary Table 1). Hence, they were re-consented and followed up for an additional time period (31–126 days). Extensive metadata is collected at each visit and samples are clearly identified as being part of the convalescent period or post-vaccination period. No samples taken after vaccination were included in the longitudinal (persistent) analyses (Figures 1 and 2). The post-vaccination samples are only included in (Figures 3 and 4). We also enrolled healthy individuals (n = 50) who were recruited as controls and received two doses of the CoronaVac (n = n = 35; Sinovac Life Sciences Co., LTD, Beijing, China) or BNT162b2 (n = 15; Pfizer Manufacturing Belgium NV, Puurs, Belgium) vaccines at time intervals of 28 or 21 days, respectively. The analysis were performed considering two major groups of individuals, hospitalized and outpatients: Hospitalized individuals (n = 37) were either severe patients (n = 14), defined as those who developed pneumonia with one of the following three conditions: (1) acute respiratory failure that required invasive mechanical ventilation or a high-flow nasal cannula (HFNC) with prone position, (2) septic shock or (3) multiple organ dysfunction; moderate cases (n = 23) consisted of inpatients with pneumonia without these conditions. Outpatients (n = 37) were individuals that had mild symptoms of COVID-19 but did not meet the criteria mentioned above. Peripheral blood samples, nasopharyngeal swabs and sputum samples were collected between 2 and 437 days after the onset of symptoms. For naïve individuals, samples were collected 1–2 days prior to vaccination and between 10 and 30 days after the first dose but prior to the second dose and 6–31 days after the second dose. For previously infected individuals, samples were collected at time intervals corresponding to weeks 1, 2, 3, 4, and months 3, 6, 9 and 12–14 months after onset of symptoms as shown in Figs. 1 and 2. Demographic data for all patients and controls, obtained through a clinical questionnaire, are shown in Table 1.
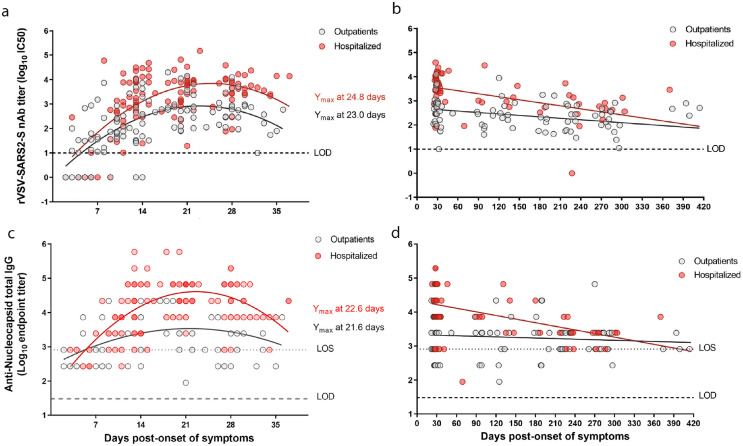
Figure 1.
Longitudinal dynamics of neutralizing and anti-N antibody responses to SARS-CoV-2 infection from outpatient and hospitalized individuals. a,b. The half-maximum inhibitory concentration (IC50) of sera was determined by microneutralization assay of recombinant vesicular stomatitis virus carrying SARS-CoV-2 spike protein (rVSV-SARS2-S). a. Neutralizing antibody (nAb) titres (log10 IC50) from n = 30 outpatients (116 samples; grey circles) and n = 35 hospitalized (112 samples; red circles) at 2 to 37 days post-symptom onset. c. Longitudinal nAb titres (log10 IC50) from n = 36 outpatients (85 samples) and n = 31 hospitalized (58 samples) taken from day 23 (outpatients) or day 25 (hospitalized) until day 414 post-symptom onset. c,d. The end-point titres of anti-N IgG were determined by ELISA using a recombinant SARS-CoV-2 nucleocapsid protein. Samples and time points are the same as those in A and B. a-c. The second order polynomial (quadratic) curve fitting was used to establish the days at which peak titres occurred (Ymax). b–d. Continuous decay fit is shown with the red and gray line for the corresponding patient group. Every data point represents results from two technical replicates.
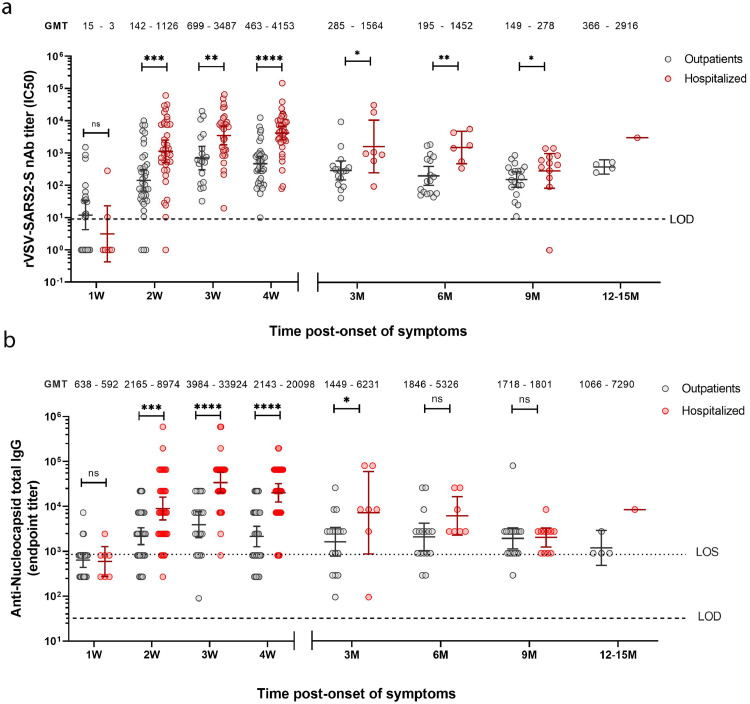
Figure 2.
Comparison of neutralizing and anti-N antibody responses after SARS-CoV-2 infection of outpatient and hospitalized individuals over a 12 months period. a. nAb IC50 titres were determined by microneutralization assay of recombinant vesicular stomatitis virus carrying SARS-CoV-2 spike protein (rVSV-SARS2-S). b. End-point titres of anti-N IgG were determined by ELISA using a recombinant SARS-CoV-2 nucleocapsid protein. a-b. Samples were obtained for n = 37 outpatients (172 samples; grey circles) and n = 37 hospitalized (139 samples; red circles) grouped by weeks (W) or months (M) post-symptom onset (serum samples from: 1W = 1–7 days; 2W = 8–14 days; 3W = 15–21 days; 4W = 22–45 days; 3M = 46–135 days; 6M = 136–225 days; 9M = 226–315 days and 12-14M = 316–414 days). The bars indicate geometric mean titres (GMT) with 95% confidence intervals. GMTs are indicated above each data set. Dashed line represents the limit of detection (LOD) of each assay. Statistical analyses shown at the indicated time points were performed between nAb titres of outpatient and hospitalized using the unpaired two-tailed Mann-Whitney test (*P < 0·05; **P < 0·01; **P < 0·001; ****P < 0·0001; ns, non-significant). Every data point represents results from two technical replicates.
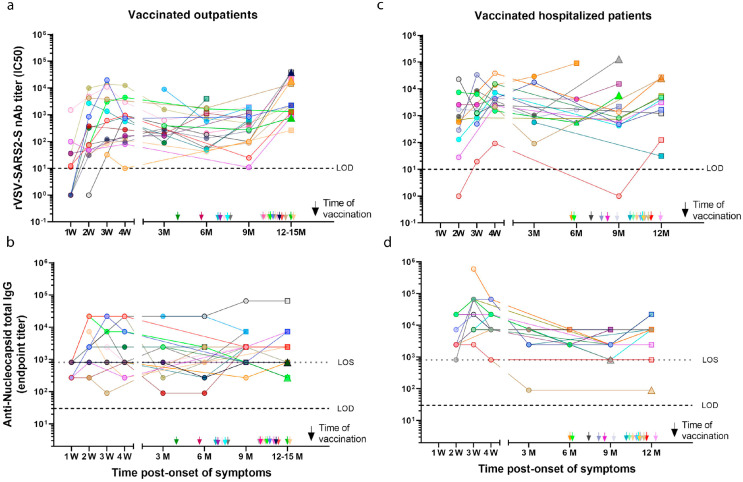
Figure 3.
Longitudinal neutralizing and anti-N antibody titres to SARS-CoV-2 in previously infected before and after CoronaVac or BNT162b2 vaccination. nAb titres (IC50) obtained using a rVSV-SARS2-S microneutralization assay and end-point titres of anti-N IgG were determined by ELISA using a recombinant SARS-CoV-2 nucleocapsid protein for vaccinated previously infected outpatients (a-b; 20 participants) or vaccinated hospitalized patients (c-d; 16 participants) at different time points grouped by weeks (W) or months (M) post-symptom onset (serum samples from: 1W = 1-7 days; 2W = 8-14 days; 3W = 15-21 days; 4W = 22-45 days; 3M = 46-135 days; 6M = 136-225 days; 9M = 226-315 days and 12M = 316-405 days/12-15M = 316-495). The arrows indicate time of vaccination post-onset of symptoms (see Supplementary Table 1 for specific days of vaccination and sample collections). Circles, non-vaccinated; squares, vaccinated with CoronaVac; triangles, vaccinated with BNT162b2. Conv: convalescent; Vacc: vaccine; 0: indicates pre-vaccination samples; 1: first dose; 2: second dose. Dashed line indicates the limit of detection (LOD) of the microneutralization assay. Every data point represents results from two technical replicates.
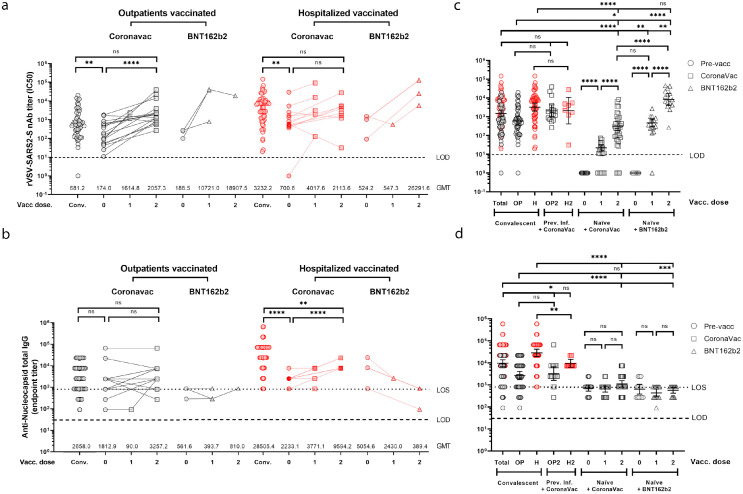
Figure 4.
Neutralizing and anti-N antibody titres to SARS-CoV-2 in previously infected and naïve individuals before and after CoronaVac or BNT162b2 vaccination. nAb (b) and anti-N IgG (c) titres from 20 outpatient (42 samples) or 16 hospitalized (33 samples) individuals immunized with one or two doses of CoronaVac (30 participants) or one or two doses of BNT162b2 (6 participants) vaccines. nAb (b) and anti-N IgG (d) titres from naïve individuals after the first and second dose of CoronaVac (35 participants) or BNT162b2 (15 participants) vaccines, compared to nAb titres from convalescent patients (samples taken between days 10 and 28 from 28 outpatients (49 samples) and 34 hospitalized (58 samples) participants) and previously infected individuals (31 participants) before (31 samples) or after receiving two doses (25 samples) of the CoronaVac vaccine. Black lines represent the geometric mean titres (c) or end-point titres (d) and bars show the 95% confidence intervals. Statistics were performed using unpaired two-tailed Mann-Whitney test ((*P < 0·05; **P < 0·01; ***P < 0·001; ****P < 0·0001; ns, non-significant), excluding non-seroconverted data determined as outliers. Circles, non-vaccinated; squares, vaccinated with CoronaVac; triangles, vaccinated with BNT162b2. Conv: convalescent; Vacc: vaccine; 0: indicates pre-vaccination samples; 1: first dose; 2: second dose. Dashed line indicates the limit of detection (LOD) of the microneutralization assay and dotted line represents the limit of sensitivity (LOS) of ELISA. Every data point represents results from two technical replicates.
Table 1.
Demographic and baseline characteristics of COVID-19 patients and vaccinated controls.
| Outpatients | Hospitalized | P value | CoronaVac | BNT162b2 | P value | Vaccinated | Vaccinated | p value | |
|---|---|---|---|---|---|---|---|---|---|
| (n = 37) | (n = 37) | (Outpatients vs. Hospitalized) | (n = 35) | (n = 15) | (CoronaVac vs. BNT162b2) | previously infected (n = 36) | naïve participant (n = 50) | (previously infected vs. naïve) | |
| Characteristics | |||||||||
| Male, n (%) | 17 (45.9) | 25 (67.6) | 0.0998 | 11 (31.4) | 3 (20) | 0.5067 | 14 (38.9) | 14 (28) | 0.3531 |
| Age, mean (range) | 37 (14-66) | 51 (16-83) | 0.0004 | 36 (21-80) | 34 (15-53) | 0.6981 | 44 (17-83) | 35 (15-80) | 0.0308 |
| >60 years, n (%) | 5 (13.5) | 12 (32.4) | 0.0956 | 1 (2.9) | 0 | >0.9999 | 8 (22.2) | 1 (2) | 0.0025 |
| Symptoms | |||||||||
| Respiratory | |||||||||
| Cough, n (%) | 27 (73) | 31 (83.8) | 0.3975 | NA | NA | NA | 30 (83.3) | NA | NA |
| Dyspnea, n (%) | 6 (16.2) | 19 (51.4) | 0.0028 | NA | NA | NA | 14 (38.9) | NA | NA |
| Odynophagia, n (%) | 21 (56.8) | 6 (16.2) | 0.0006 | NA | NA | NA | 13 (36.1) | NA | NA |
| Chest discomfort, n (%) | 3 (8.1) | 5 (13.5) | 0.7106 | NA | NA | NA | 4 (11.1) | NA | NA |
| Constitutional | |||||||||
| Fever, n (%) | 22 (59.5) | 31 (83.8) | 0.0377 | NA | NA | NA | 26 (72.2) | NA | NA |
| Headache, n (%) | 32 (86.5) | 14 (37.8) | < 0.0001 | NA | NA | NA | 23 (63.9) | NA | NA |
| Myalgia, n (%) | 25 (67.6) | 18 (48.6) | 0.157 | NA | NA | NA | 20 (55.6) | NA | NA |
| Severe fatigue, n (%) | 0 | 20 (54.1) | < 0.0001 | NA | NA | NA | 11 (30.6) | NA | NA |
| Altered mental status, n (%) | 0 | 3 (8.1) | 0.2397 | NA | NA | NA | 1 (2.8) | NA | NA |
| Gastrointestinal | |||||||||
| Diarrhea, n (%) | 12 (32.4) | 10 (27) | 0.7997 | NA | NA | NA | 12 (33.3) | NA | NA |
| Nausea/Vomiting, n (%) | 6 (16.2) | 9 (24.3) | 0.5642 | NA | NA | NA | 8 (22.2) | NA | NA |
| Sensorial | |||||||||
| Ageusia, n (%) | 18 (48.6) | 5 (13.5) | 0.0022 | NA | NA | NA | 14 (38.9) | NA | NA |
| Anosmia, n (%) | 24 (64.9) | 8 (21.6) | 0.0004 | NA | NA | NA | 18 (50) | NA | NA |
| Comorbidities or conditions | |||||||||
| Obesity (BMI ≥ 30), n (%) | 5 (13.5) | 14 (37.8) | 0.0317 | 5 (14.3) | 4 (26.7) | 0.4234 | 10 (27.8) | 9 (18) | 0.3038 |
| Hypertension, n (%) | 3 (8.1) | 13 (35.1) | 0.0095 | 3 (8.6) | 2 (13.3) | 0.6293 | 9 (25) | 5 (10) | 0.0797 |
| Metabolic conditions*, n (%) | 4 (10.8) | 12 (32.4) | 0.0459 | 2 (5.7) | 1 (6.7) | >0.9999 | 9 (25) | 3 (6) | 0.0239 |
| Hyperlipidemia, n (%) | 4 (10.8) | 7 (18.9) | 0.5151 | 1 (2.9) | 2 (13.3) | 0.2107 | 7 (19.4) | 3 (6) | 0.0865 |
| Cardiovascular disease, n (%) | 0 | 3 (8.1) | 0.2397 | 0 | 0 | NA | 1 (2.8) | 0 | 0.4186 |
| Chronic pulmonary disease, n (%) | 4 (10.8) | 3 (8.1) | 1 | 0 | 0 | NA | 4 (11.1) | 0 | 0.0277 |
| Asthma, n (%) | 6 (16.2) | 2 (5.4) | 0.2611 | 4 (11.4) | 4 (26.7) | 0.2195 | 5 (13.9) | 8 (16) | >0.9999 |
| Rheumatologic disease, n (%) | 0 | 3 (8.1) | 0.2397 | 0 | 1 (6.7) | 0.3 | 1 (2.8) | 1 (2) | >0.9999 |
| Immunocompromised, n (%) | 0 | 5 (13.5) | 0.0541 | 0 | 1 (6.7) | 0.3 | 2 (5.6) | 1 (2) | 0.5691 |
| Allergy⁎⁎, n (%) | 16 (43.2) | 6 (16.2) | 0.0209 | 17 (48.6) | 5 (33.3) | 0.3673 | 12 (33.3) | 22 (44) | 0.3751 |
| Neurologic disease, n (%) | 0 | 4 (10.8) | 0.1148 | 0 | 0 | NA | 2 (5.6) | 0 | 0.1724 |
| Smoker, n (%) | 8 (21.6) | 9 (24.3) | 1 | 6 (17.1) | 4 (26.7) | 0.4616 | 6 (16.7) | 10 (20) | 0.7836 |
Abbreviation: BMI, Body mass index; NA, Not applicable.
Metabolic conditions include insulin resistance, prediabetes, type 1/2 diabetes, non-alcoholic steatohepatitis and obstructive sleep apnea;
Allergy considered self-reported allergic rhinitis (by seasonal, perennial/year-round, or episodic allergens) and food allergy. [Fisher's exact test; Mann Whitney test].
For comparing seroconversion titres and correlates of protection, we used the same approach of Khoury et al.12 considered as a robust approach to associate nAbs and protection. Hence, took in to account the time ranges of seven vaccine studies (e.g. of the mRNA-1273, NVX-CoV2373, BNT162b2, rAd26-S+rAd5-S, ChAdOx1 nCoV-19, Ad26.COV2.S and CoronaVac vaccines) for determining neutralization titres. This time range was 10–60 days, or not specified, from which the neutralization and protection model was developed in the Khoury et al study. We also used our own data (Figure 1a,b) that showed that some individuals have high levels of nAb during week 1 (Figure 1b). We performed initial analyses considering convalescent titres obtained in our study using time ranges of 10–37, 14–28 and 14–21 days, which showed no significant differences (Supplementary Figure 3). With this context and for broad comparisons, we adopted a more dogmatic approach and used neutralizing data from 14 to 28 days post onset of symptoms as the period at which robust nAbs are generated upon natural infection.
Plasma and serum collection
Peripheral blood was collected in both plasma separating (EDTA/purple top) and serum separating (red top) tubes and was processed by centrifugation at 2000 × g for 5 min. Limited volume of plasma and serum samples were aliquoted and stored at −80 °C. Serum samples were heated at 56 °C for 1 h before use to eliminate the risk of any potential residual virus.
SARS-CoV-2 spike and nucleocapsid ELISAs
Overnight, 96-well plates (Immulon 4 HBX; Thermo Fisher Scientific #3355) were coated at 4 °C with 50 μL per well of a 2 μg/mL solution recombinant SARS-CoV-2 spike or nucleocapsid (GenScript #Z03488) proteins, as previously described.3,17,18 The next morning, the plates were blocked with 3% non-fat milk prepared in PBS with 0.1% Tween 20 (PBST) for 1 h. Serial dilutions of serum and antibody samples previously inactivated by heating at 56 °C for 1 h, were diluted starting 1:50 for spike and 1:30 for nucleocapsid SARS-CoV-2 proteins were prepared and 100 μL of each dilution was added to the plates for 2 h at room temperature. For primary antibody detection a 1:3,000 dilution of goat anti-human IgG–horseradish peroxidase (HRP) conjugated secondary antibody (Thermo Fisher Scientific # SA1-36011, RRID:AB_1075961) was added to each well for 1 h and SIGMAFAST OPD (o-phenylenediamine dihydrochloride; Sigma–Aldrich #P9187) was used as substrate. After 10 min the reaction was stopped by the addition 3 M hydrochloric acid and the optical density at 490 nm (OD490) was measured using a Synergy 4 (BioTek) plate reader. In some cases, end-point titres were calculated, with the end-point titre being the last dilution before reactivity dropped below an OD490 of <0.11. CR3022, a human monoclonal antibody reactive to the RBD of both SARS-CoV-1 and SARS-CoV-2,19,20 was used as control. Negative and positive controls were used to standardize each assay and normalize across experiments. The limit of detection (LOD) was defined as 1:50 for spike and 1:30 for nucleocapsid. Limit of sensitivity (LOS) for the nucleocapsid assay was established on the basis of the maximal serum reactivity of uninfected subjects using samples from 16 pre-pandemic donors never exposed to SARS-CoV-2. All data represent results from two technical replicates.
SARS-CoV-2 microneutralization assay
This assay was performed as previously described.21 Briefly, Vero E6 cells (ATCC #CRL-1586, RRID:CVCL_0574) were seeded at a density of 20,000 cells per well in a 96-well cell culture plate in complete Dulbecco's Modified Eagle Medium (cDMEM, Gibco Thermo Fisher Scientific #11995040). The following day, heat-inactivated serum samples (dilution of 1:10) were serially diluted threefold and 80 μL of each serum dilution were mixed with 80 μL of the authentic SARS-CoV-2 (USA-WA1/2020; GenBank: #MT020880) diluted to a concentration of 100 TCID50 (50% tissue culture infectious dose) and then added to a 96-well cell culture plate and allowed to incubate for 1 h at room temperature. After removing the cell culture media, the Vero E6 cells were incubated with 120 μL of the virus-serum mixture at 37 °C for 1 h. The virus-serum mixture was then removed from the cells and 100 μL of each corresponding serum dilution and 100 μL of 1 × MEM containing 1% fetal bovine serum (FBS, Corning # 35-010-CV) was added to the cells. After 48 h at 37 °C, the cells were fixed with 10% paraformaldehyde (Polysciences # 04018-1) for 24 h at 4 °C, permeabilized with PBS containing 0·1% Triton X-100 (Sigma-Aldrich # X100) and the plates were and blocked with 3% milk (American Bio # AB1010901000) in PBST. For detecting viral infection, a primary mAb 1C7 (anti-SARS nucleoprotein antibody generated in-house) was used at a 1:1,000 dilution and subsequently detected with a 1:3,000 dilution of a goat anti-mouse IgG–HRP (Rockland #KCB002, RRID:AB_10703407), and incubation with SIGMAFAST OPD (Sigma-Aldrich) as described above. A cut-off value of the average of the optical density values of blank wells plus three standard deviations established for each plate was used to calculate the microneutralization titre. Microneutralization assays were performed in a facility with a biosafety level of 3 at the Icahn School of Medicine at Mount Sinai. Each data point represents results obtained from two technical replicates.
rVSV SARS-CoV-2 spike protein (rVSV-SARS2-S) microneutralization assay
To determine the nAb titres of patient sera, we used a previously described the replication-competent recombinant vesicular stomatitis virus carrying the SARS-COV-2 spike protein and coding for an enhanced green fluorescent protein (eGFP).22 This recombinant virus has been shown to correlate well when compared to neutralization of convalescent serum with the authentic SARS-CoV-2, allows for rapid quantification, it enters cells through pathways of SARS-CoV-2, and does not require high biosafety containment. Briefly, Vero E6 cells (ATCC # CRL-1586, RRID:CVCL_0574) grown in 1X MEM (Gibco #11095-080) supplemented with 10% FBS (Gibco, #16000-044) were transfected with plasmid pCEP4-myc-ACE2 (Addgene catalog # 141185) and stable clones were selected by hygromycin (Invitrogen #10687010) (400 μg/mL). To assay nAb titres, serial dilutions of serum samples were incubated with rVSV-SARS2-S for 1 h at 37 °C. The serum-virus inoculum was added to Vero E6 hACE2 cells seeded the day before in optical bottom 96-well plates (Thermo Scientific #165305) at 80% confluence and adsorbed for 2 h at 37 °C. Next, the mixture was replaced by culture media and infection allowed to proceed for 20 h at 37 °C, 5% CO2 and 80% humidity. The cells were then fixed with 4% formaldehyde (Pierce #28906) and stained in with 4′,6-diamidino-2-phenylindole (DAPI) 300 nM (Invitrogen #D1306). Viral infectivity was quantified by automated enumeration of GFP-positive cells (normalizing against cells stained with DAPI) using a Cytation5 automated fluorescence microscope (BioTek) and segmentation algorithms applied from the ImageJ program. Alternatively, total GFP fluorescence per well was acquired using the Cytation5 fluorescence lector (wavelength for DAPI 360 nm for absorption, 460 nm for emission and for GFP, 485 nm for absorption, 526 nm for emission) and normalized against DAPI fluorescence. The half-maximum inhibitory concentration (IC50) of the sera, were calculated from data obtained with two technical replicates using non-linear regression analysis and the curve fitting was done using second-order polynomial (quadratic); and linear regression models (using log10 IC50 transformed data) were done with GraphPad Prism 5 software.
Statistical analysis
We used a convenience sampling approach and included n = 37 outpatients and n = 37 hospitalized SARS-CoV-2 individuals from a total pool of 168 recruited individuals representative of the population of the Metropolitan region of the country. Of the 74 infected individuals, we included all those that were vaccinated through the national COVID-19 immunization campaign during the longitudinal follow up period, and hence, there were no a priori criteria for selecting these individuals. A convenience sampling of uninfected individuals (n = 50) that were voluntarily vaccinated through the national COVID-19 immunization campaign, were also invited to participate in the study. The samples were assigned an anonymous code and all serological analyses were performed by scientists that were blinded in regards to the subject's clinical condition and time of sample collection. Our study did not have any a priori exclusion criteria and hence all individuals with a laboratory confirmed SARS-CoV-2 infection or that had been vaccinated during the study period were invited to participate in the study. Categorical variables were expressed as numbers or percentages. Association between categorical variables was examined with Chi-squared or Fisher's exact test. Continuous variables were expressed in mean, geometric mean and range and compared with unpaired two-tailed Mann-Whitney test. Correlation was evaluated calculating the Pearson correlation coefficient. GraphPad Prism 8 was used for statistical analysis: *P < 0·05; **P < 0·01; ***P < 0·001; ****P < 0·0001. We evaluated for potential cofounding effects on vaccinated individuals by first performing univariate analysis (Fisher's exact test) on all the demographics and clinical features. We then performed logistic regression with all the demographic variable and comorbidities. Variables that were found to be significant were used to perform a multivariate analysis, where the variables age and sex were included as common potential cofounder variables affecting immune responses. Relevant multivariate analyses were plotted as crude and adjusted odds ratio (OR) for vaccine responder capacity.
Ethics
Patient clinical and epidemiological data, along with their clinical specimens were collected after informed written consent was obtained under protocols 16-066 and 200829003, which were reviewed and approved by the Scientific Ethics Committee for Health Sciences (CECSaludUC, by its Spanish acronym) at Pontificia Universidad Católica de Chile (PUC).
Role of funders
The funders of this work had no role in the study design, management, data collection, data analysis, interpretation of the data nor the preparation, review, or approval of this manuscript and decision to submit the manuscript for publication.
Results
Longitudinal antibody titres induced by natural SARS-CoV-2 infections
To understand the long-term dynamics of antibody induction and decay after natural SARS-Cov-2 infection, we prospectively enrolled 74 individuals (overall mean age: 44 years [range 14–83, >60 23%]), of whom 37 were outpatient (mild disease mean age: 37 years [range 14–66]) and 37 were hospitalized (moderate [n = 23] and severe disease [n = 14], mean age: 51 years [range 16–83]) with a confirmed SARS-CoV-2 quantitative RT-PCR test. These individuals were followed longitudinally for up to 13·6 months from the onset of symptoms (demographic and baseline characteristics of the patients are summarized in Table 1; convalescent samples were collected between 2 and 414 days after the onset of symptoms).
To analyse humoral responses longitudinally, to determine nAb titres we used a microneutralization assay based on a recombinant vesicular stomatitis virus carrying a SARS-CoV-2 spike protein,22 that showed strong correlation (Pearson's r=0.80, R2 = 0.65, p < 0.001) with authentic SARS-CoV-2 microneutralization (Supplementary Figure 4a,b), and evaluated the induction of anti-N IgG antibodies by ELISA. Regardless of disease severity and age, infected individuals developed robust nAb and anti-N IgG responses during the first month. The nAb responses declined over time but were sustained for up to 13.6 months (Figure 1a,b), whereas the anti-N IgG titres were detectable at least for 9 months (Figure 1c,d). We performed kinetic analyses with samples from 65 individuals that were sampled weekly during the first month from symptom onset (Figure 1a,c; Supplementary Figure 4c,d). In agreement with previous reports,23,24 hospitalized individuals had significantly higher neutralization titres as compared to outpatients; with peak average nAb responses at day 25 and at day 23 post-symptom onset, respectively (Figure 1a and Supplementary Figure 4c,d). Similarly, the anti-N IgG titres peaked on days 23 for the hospitalized and day 22 for the outpatient individuals (Figure 1c). We included long-term longitudinal samples for 67 participants, which included samples from 58 individuals that were also analysed during the first month (Figure 1a) and performed nAb and anti-N IgG titre time decay analysis starting from the respective peak average responses (Figure 1a,b). Fitting our nAb data to a continuous decay model, estimated a half time of 147 days (95% CI = 68.7–322.5 days) for outpatients and 112 days (95% CI = 76.7–208.1 days) for hospitalized individuals (Figure 1b). For the anti-N IgG levels, the decay model for hospitalized individuals was 118 days (95% CI = 81–219.9 days) and for outpatients 600 days (95% CI = 203.8–635.6 days, Figure 1d). We then also compared longitudinally the antibody titres between hospitalized and outpatient individuals. Hospitalized individuals had significantly higher titres of nAbs at weeks 2–4 and at 3–9 months. However, between week four and month nine, the nAb GMT decrease of hospitalized individuals was 15 times compared to three times for outpatients (Figure 2a). A similar trend was observed when we assessed the anti-N IgG titres, which were significantly induced and remained higher in hospitalized individuals as compared to outpatients for the first 3 months since onset of symptoms (Figure 2b). However, these antibodies in the hospitalized group showed no significant differences to those observed for outpatients after 6 months. Noteworthy, in some individuals we detected basal levels of anti-N IgGs, above the limit of detection (LOD) but below the limit of sensitivity (LOS) for the assay, which suggest a potential previous exposure to seasonal coronaviruses that have shown to induce cross-reactive anti-N antibodies (e.g. HKU1 or OC43 strains).6 None of the individuals in the study had evidence of re-infections. Taken together, while the nAb and anti-N IgG titres remained higher in the hospitalized patients, these individuals had a more pronounced decrease over time. Nonetheless, despite this decrease in titres in both study groups, all individuals showed long-lasting responses of circulating nAbs 9–13.6 months after natural infection.
Antibody responses in previously-infected individuals after vaccination
Thirty-six of the previously infected individuals (mean age: 44 years [range 17–83]) included in our longitudinally cohort were immunized during the study period (Figure 3), while 38 individuals were not immunised (Supplementary Figure 5). Thus, we analysed the nAb and anti-N IgG response in these previously infected individuals after immunization with the two main vaccines used in Chile; the CoronaVac (Sinovac) or the BNT162b2 (BioNTech/Pfizer) vaccines. The previously infected individuals were vaccinated between 4.2 and 13.3 months (average 9.7 months, Supplementary Figure 2 and Supplementary Table 1) after the onset of symptoms for both, the outpatient (20 participants, Figure 3a,b) and hospitalized groups (16 participants, Figure 3c,d). Except for three cases, all the previously infected participants showed an increase in the nAb titres after receiving one or two doses of the vaccines, suggesting a significant induction of B cell memory response months after onset of symptoms. Strikingly, the only three individuals (age range 29–63 years) that lacked an induction of nAbs responses were obese (3/10 obese participants), including an outpatient (Figure 3a, light green patient) or two hospitalized participants (Figure 3c, grey and cyan patients). For these individuals we only had a previous sample 5.6 to 10.7 months prior to vaccination (Figure 3a–c), and hence no clear conclusions can be drawn about the trajectory of their nAb titres. Noteworthy, one of these participants had a marked decrease in nAb titre after two doses of the vaccine (IC50 563.9 to 31.0; Figure 3c, cyan patient). Univariate analysis of obesity as a cofounding factor for responding to vaccination in the previously infected group showed statistical significance (Table 1). Unexpectedly, regardless of the time of vaccination or severity anti-N IgG were only modestly boosted and in only in some previously infected patients upon immunization with the CoronaVac vaccine (Figure 3b–d).
To establish statistical comparisons of the antibodies induced by immunization in previously infected individuals with these two widely used vaccines, we grouped the nAbs and anti-N IgG titres before and after being vaccinated with CoronaVac or BNT162b2, and related them to antibody titres which these patients had reached during convalescence (Figure 4a,b). Given that there is yet no clear definition of the time frame in which protective nAb titres during convalescence should be considered, we took the peak maximal titres from our own data and its longitudinal decay, as well as the normalized data reported by Khoury et al.12 No statistical differences were observed when we considered days ranges from 14 to 21, 14 to 28 and 10 to 37 since the initiation of symptoms (Supplementary Figure 3). Hence, we included the more dogmatic seroconversion data for the first 14-28 days post-infection for outpatients and hospitalized individuals (outpatients GMT = 681.2 [95% CI = 430.7–1077 GMT]; hospitalized GMT = 3232 [95% CI = 1984–5,266 GMT]; Figure 4a–c). There were only 7 samples available from previously infected individuals vaccinated with one dose of CoronaVac, therefore analysis of additional samples would be needed to further evaluate the boosting capacity of a single dose. After the second dose of the CoronaVac vaccine in previously infected individuals, the average nAb increase since the pre-vaccine time point was 12 times among outpatients (pre-vaccine GMT = 174 [95% CI = 81.2–372 GMT], second dose GMT = 2057.3 [95% CI = 987.7–4,285 GMT]) and five times among hospitalized (pre-vaccine GMT=700.8 [95% CI=171.9–2,856.8 GMT], second dose GMT = 2113.6 [95% CI = 412.9–10,018.7 GMT]; Figure 4a). When compared to the 14–28 day convalescent titre, the pre-vaccination titres (Vacc Dose 0) of outpatients and hospitalized individuals were significantly lower. However, only the previously infected outpatients group immunized with two doses of CoronaVac generated a significant increase in titre, which re-established them to levels comparable to the convalescent titres (Figure 4a). In general, hospitalized individuals had sustained higher antibody levels at the time of vaccination, however; while immunization with CoronaVac generated a measurable nAb titre increase in most of these individuals, the overall level of induction was not significant (Figure 4a).
When we assessed the induction of anti-N IgG of these previously infected individuals after immunization with CoronaVac, surprisingly there was no increase in titre in the outpatients as compared to their convalescent levels, and only a modest increase in titres was observed in the hospitalized group (Figure 4b), suggesting that this inactivated virus vaccine is a poor inducer of anti-N antibodies. There were only 6 cases of previously infected individuals that were immunized with BNT162b2 (3 outpatient and 3 hospitalized) and therefore we had insufficient statistical power to perform any further analyses. Nonetheless, as previously reported, the general pattern in these individuals showed an induction in their nAbs (Figure 4a)25 and as expected no increases in anti-N IgGs were observed (Figure 4b).
Induction of antibody responses in naïve individuals through vaccination
To compare the antibody titres of previously infected individuals at convalescence and after vaccination to those of healthy naïve (SARS-CoV-2 seronegative) individuals immunized with one and two doses of either vaccine representing similar demographic characteristic (CoronaVac, 35 participants, mean age: 36 years [range 21–80] or BNT162b2, 15 participants, mean age: 34 years [range 15–53]; Figure 4c,d, Table 1), we determined the overall GMT antibody titre of both groups; outpatients (OP) and hospitalized (HP) individuals. For a broader point of comparison and to establish significant differences among all groups, in our analyses we also included the combined 14-28 day convalescent antibody titres from all previously infected individuals (Total, Figure 4c,d) representing the broad diversity of nAbs after natural infection (14–28 days GMT = 1596.9).
The induction of nAbs in naïve individuals vaccinated with CoronaVac (one dose GMT = 21.9; two doses GMT = 311.9) were lower to those of the combined titres of convalescent patients (Figure 4c). These lower levels were highly significant when we compared to those of hospitalized individuals (GTM = 3232.2) and to a lesser extent when compared to the outpatient group (GTM=681.2). Overall, this indicated that the nAb levels induced by CoronaVac were significantly lower to those generated after natural infection (Figure 4c), likely due to the high levels of viral replication in infected individuals.24 In addition, three out of 35 individuals immunized with CoronaVac did not seroconvert. Naïve individuals vaccinated with BNT162b had similar nAb titres after one dose (GMT = 465.7), but much higher titres after two doses (GMT = 8387.5) as compared to convalescent patients. This also indicated that one dose of the BNT162b vaccines induces similar nAb levels than immunization with two doses of CoronaVac. On the other hand, individuals with two doses of the BNT162b vaccine reached levels that were significantly higher to those of the convalescent outpatients and hospitalized combined, being most similar to those titres observed in the hospitalized group at convalescence or after these individuals were immunized with two doses of CoronaVac (Figure 4c). When we evaluated the induction of anti-N IgG through immunization with CoronaVac in naïve individuals, there were only a few individuals that had a detectable increase in titres after the second dose (Figure 4d). The overall anti-N IgG titres in vaccinees were significantly lower as compared to the combined or hospitalized convalescent titre but similar to that induced in convalescent outpatients after natural infection. As expected, no variation in anti-N titres was observed in individuals immunized with the BNT162b vaccine.
Since three naïve individuals did not respond to immunization, we assessed for potential cofounding factors affecting vaccine response. There were no demographic or clinical variables associated with either seroconvertion or lack of antibody induction in the naïve immunized group. We further analyzed all the vaccinee data, by including the previously infected and naïve-vaccinated participants together, two variables associated with a lack of vaccine response, age and obesity (Supplemental Table 2). Remarkably, logistic regression and multivariate analyses confirmed obesity as an underlying comorbidity affecting vaccine response (Supplemental Figure 6).
Neutralizing levels induced by CoronaVac and BNT162b2 vaccines and estimates of predictive efficacy
To assess the association of the nAbs titres from our study to the reported protection by the CoronaVac and BNT162b2 vaccines, we used the logistic models of Khoury et al.12 and Earle et al.13. In these models, the nAbs titres of the different studies were normalized to the mean convalescent titres of the same study, and compared against the corresponding protective efficacy reported from the phase 3 clinical trials. Hence, to analyse our data with these models, we calculated the mean neutralization level induced by the vaccines as a fold comparison to the combined convalescent titres of individuals at 14-28 days post-symptom onset (GMT = 1597). The mean titre induced by two doses of CoronaVac was 0·2 times that of convalescent individuals, whereas two doses of the BNT162b vaccine resulted in 5.25 times, representing a highly significant difference in the neutralization levels induced by both vaccines. By extrapolating these data to the mathematical models, the estimated predicted efficacy for CoronaVac was ∼50% and for BNT162b was ∼97%, suggesting that our independent data confirms the difference in predictive protection reported previously for both vaccines.26,27
Discussion
We found long-lasting nAb titres that persist for at least 13.6 months after the onset of symptoms in both, outpatient and hospitalized individuals. This is in agreement with the detection of SARS-CoV-2 spike-specific long-lived bone marrow plasma cells 7-8 months post-symptom onset.28 Our cohort study provides empirical data showing that long-lasting nAb responses induced through natural infection can be significantly boosted after immunization with CoronaVac or BNT162b2 vaccines, when administered up to 13.3 months since the onset of COVID-19 symptoms, suggesting that infection induces a robust B-cell memory response. Such responses have been well characterized in infected individuals28,29 and are also critical for the durability of protection in vaccinated individuals.30,31 Importantly, the decay of nAbs titres after infection seen over time in our study and reported by other groups,3,12,28 suggests that booster immunization strategies of previously infected individuals should be considered and might be required to control the pandemic and prevent re-infection with new variants of concern (VOC) in subsequent years.
Our longitudinal data indicates that infected individuals generated robust nAb and anti-N IgG titres. Interestingly, hospitalized individuals had significantly higher titres when evaluated longitudinally throughout the study period, which is likely due to sustained higher viral loads observed in these individuals.24 There was a more pronounced decay of both antibody titres in the hospitalized population as compared to the outpatient group. Of note, individuals in the hospitalized group were ∼14 years older, which might explain the faster decay in this group (Figure 1 and Table 1). While nAbs were significantly boosted with two doses of CoronaVac in the previously infected group, surprisingly there was no or only moderate induction of anti-N IgG in any of the previously infected individuals (Figs. 3 and 4). Similarly, there was poor induction of anti-N antibodies after vaccination of naïve individuals with CoronaVac, overall confirming that this vaccine is a poor inducer of antibody responses against this protein.32, 33, 34 This is important to note, given that the protection afforded by CoronaVac is most likely due to the induction of nAbs responses, and additional immune mechanisms such as Fc-effector functions and T-cell immunity, which contribute to improve disease outcome.15,16
The correlates of protection against SARS-CoV-2 are currently unknown. However, current evidence of re-infections with the same virus variant remains limited.7,35, 36, 37 However this is a situation that continues to evolve given the emergence of VOCs such as Gamma, and Delta, which have shown significant reduction in cross-reactive neutralizing titre, and have generated increased rates of re-infection in some regions of the world,38, 39, 40 a scenario that remains to be fully evaluated with the new VOC, Omicron. To further strengthen models for protective correlates, additional comparative analyses of nAb titres of vaccinated individuals and better-defined standards for convalescent sera that incorporate titre variations due to disease severity and decay over time are needed. The current study provides a unique dataset and a direct comparison of longitudinal convalescent nAb titres to those of individuals immunized with two different vaccine formulations approved by the WHO and are currently being widely used. These comparative data is of crucial value to establish the relationship between neutralization level and efficacy against symptomatic SARS-CoV-2 infection, as recently proposed.12,13
The calculated mean GMT induced by vaccination of naïve individuals with CoronaVac and BNT162b2 were 0.2 and 5.5 times, respectively, that of convalescent titres at 14–28 days post-symptom onset. This suggests that the antibody response in previously uninfected individuals vaccinated with CoronaVac was significantly lower compared to individuals who have recovered from SARS-CoV-2 infection.2,3,41 As shown, more severe individuals had higher antibody titres, which other studies have been associated with increased replication and disease burden.24 Although lower titres are seen in Coronavac vaccinated individuals, as compared to natural infected individuals, this data indicates that immunization by this vaccine affords protection from COVID-19, as shown in recent vaccine effectiveness studies in Chile.42 The BNT162b2 vaccine induced titres that were higher and more similar to the titre of hospitalized convalescent patients. Based on these titres, the predicted efficacy by the mathematical model of Khoury et al. and Earle et al. suggested a ∼50% protection from symptomatic disease for CoronaVac and 97% for BNT162b2. While this predicted efficacy coincide well with the reported phase 3 trial of 95% for BNT162b2,43 for the CoronaVac vaccine the 50% prediction is lower compared to clinical data showing protection of 50–84% depending on the geographic location.11 Interestingly in our study, we determined nAb in sera collected from vaccinated individuals at 20–30 days post first dose and at 13–19 days after the second dose since immunization. The large real-life effectiveness data reported from Chile for BNT162b2 was 92.6% and for CoronaVac was 62.8–64.6%,42 which considered a similar timing post vaccination to evaluate effectiveness (e.g. those individuals who were partially immunized [≥14 days after receipt of the first vaccine dose and before receipt of the second dose], and those who were fully immunized [≥14 days after receipt of the second dose] allowing us to compare both parallel results. In contrast, in the CoronaVac clinical trial from Turkey, which represented a smaller sample size and included a large number of elderly individuals, among other differences, the reported protection was as high as 84%.44 While the prediction models correlated fairly well with the observed efficacy for most vaccines, Khoury et al. reported a less optimal correlation for the CoronaVac vaccine as these data points were towards the lower end of the logistic model. Hence, additional data such as the data provided from this study along side with real life efficacy data, may strengthen such models. Our study indeed suggests that lower nAb titres might still afford protection from disease. Moreover, approximately 10% of the individuals vaccinated with CoronaVac did not seroconvert, which has also been reported by others.45 This is in line with the notion that even lower nAb titres can be sufficient to protect from severe disease.12 Hence, additional cellular immune responses, such as T-cell immunity and Fc-effector mechanisms might also contribute significantly to protection. Thus, additional assessment of the correlates of protection induced by this and other vaccines warrants further investigations. Of note, our analyses revealed that obesity was a risk factor affecting seroconversion after immunization (Table 1 and Supplemental Figure 6 and Supplemental Table 2). Obesity has been reported to be a comorbidity associated with an increased risk of developing severe COVID-19,46,47 and increased body mass index (BMI) has been associated with decreased IgG levels.48 Hence, further studies to monitor the induction and decay of nAbs after vaccination in this population are needed.
Our study has some limitations. Firstly, it is a small longitudinal cohort representing a limited number of individuals tested out of the population diagnosed with COVID-19 in Chile during the study period. Moreover, it is currently uncertain how these results compare to the overall antibody levels induced by the CoronaVac and BTN162b2 vaccines in the general population, and hence, a larger study would be needed to draw further conclusions. In addition, we used a convenience sampling approach to rapidly recruit naïve vaccinated individuals. While the overall demographics of this group was highly similar to the previously infected immunized group, the average age of the naïve group was 9 years younger. Given that age is a known factor affecting vaccination response (Supplemental Figure 6), further assessment of the effect of age, obesity and other comorbidities in vaccines response in the general population are warranted. In addition, at the time of this study Chile had vaccinated >75% of its population, mainly with CoronaVac (https://deis.minsal.cl/), and saw a drastic reduction of COVID-19 cases (epidemiological weeks 24–31), even while the predominant circulating variants were Gamma (P.1 VOC; at 75% frequency) and Lambda (C.37 variant of interest; at 20% frequency) (https://vigilancia.ispch.gob.cl/app/varcovid). Noteworthy, in South America and Chile, there seemed to be distinct dynamics (apparently delayed) of the introduction of the Delta VOC as compared to other countries in the Northern Hemisphere. Thus, our data suggest that the immunity (humoral and B cell memory) induced by immunization with CoronaVac in the general population was capable of reducing the circulation of the SARS-CoV-2 strains including two recently emerged variants. However, this data also suggest that a larger proportion of the population would need to be immunized with CoronaVac to have an impact in the circulation of the virus and afford community level immunity, as compared to other vaccines. In fact, Chile has now (epidemiological week 9, 2022) reached >93% of its population vaccinated, and saw a very small peak of the Delta VOC in mid-November (epidemiological week 47) and a large peak of the Omicron VOC, but with reduced hospitalizations and severe cases. Nonetheless, Chile has also used other vaccines (Ad5-nCoV; Cansino, ChAdOx1 nCoV-19; AstraZeneca) and started to offer booster doses of BNT162b and ChAdOx1 nCoV-19 in mid-August (epidemiological week 33, 2021) to all individuals vaccinated 6 months earlier (https://deis.minsal.cl/). Hence, the direct independent effect of CoronaVac on herd immunity cannot be estimated.
Booster vaccination schemes seem fundamental, especially when the nAb decay over time is taken into consideration, as shown in our longitudinal study of infected individuals (half times ∼112 to ∼147 days) or longitudinal vaccine cohort studies.49 Such a decay is of particular concern when considering that the CoronaVac vaccine induces low initial nAb titers. This suggests that vaccination with CoronaVac will require booster doses within shorter time frames as compared to other vaccines, and therefore this data contributes to further defining the proper strategies and timing to implement boost immunizations for the general population.10
The WHO Strategic Advisory Group of Experts on Immunization (SAGE) in June 1st, 2021 authorized the CoronaVac for emergency use (https://www.who.int/news/item/01-06-2021-who-validates-sinovac-covid-19-vaccine-for-emergency-use-and-issues-interim-policy-recommendations). In this context, our data are highly relevant for the COVAX initiative and the developing world (e.g. 50 countries that have already authorized CoronaVac, including being extensively used in Chile, Turkey, Brazil, China and Indonesia). Further studies to determine and monitor the long-term duration of nAbs against SARS-CoV-2 induced by different vaccine formulations and against emergent variants are warranted.
Contributors
NAM and TGS collected and analyzed data, made figures and tables, interpreted data, and wrote the paper. CPR, EFS, JL, MJA, LIA, EP, and SS processed samples, performed experiments, analyzed data, and revised the paper. GV, ES, AM, CG, AR, recruited patients, collected clinical metadata, and revised the paper. RJ, KC, DH, MED, generated rVSV viral stocks, analyzed data and revised the paper. FK analyzed serological data, advised on data interpretation, and provided funding for the study and revised the paper. NT designed the study, collected, analyzed, and interpreted data, provided funding for the study, and wrote the paper. RAM conceived the longitudinal cohort design, recruited patients; collected, analyzed, and interpreted data, provided funding for the study, and wrote the paper. Data was verified by NAM, TGS. NT and RAM and made the decision to submit this manuscript. All authors read and approved the final version of the manuscript.
Data sharing
The individual-level data used in this study are sensitive and cannot be publicly shared. The data sets generated and that support the findings of this study are available from the corresponding authors on reasonable request.
Declaration of interests
The authors reported no potential conflict of interest. The Icahn School of Medicine at Mount Sinai has filed patent applications relating to SARS-CoV-2 serological assays and NDV-based SARS-CoV-2 vaccines which list Florian Krammer as co-inventor. Mount Sinai has spun out a company, Kantaro, to market serological tests for SARS-CoV-2. Florian Krammer has consulted for Merck and Pfizer (before 2020), and is currently consulting for Pfizer, Seqirus, Avimex and Third Rock Ventures. The Krammer laboratory is also collaborating with Pfizer on animal models for SARS-CoV-2. Kartik Chandran is a member of the scientific advisory boards of Integrum Scientific, LLC Biovaxys Technology Corp, and Celdera Medical, LLC; has received royalties from Q2 Solutions and has consulted for Axon Advisors, LLC. Denise Haslwanter, Maria Eugenia Dieterle, Rohit K Jangra and Kartik Chandran, are listed as inventors on a patent application covering the VSV-based SARS2 neutralization assay assigned to Albert Einstein College of Medicine. Rafael Medina has received funding from NIH-Centers of Excellence for Influenza Research and Response (CEIRR) Contract HHSN 75N9301R00028, NIH-Centers of Excellence for Influenza Research and Surveillance (CEIRS) - HHSN272201400008C, the Hope COVID-19 initiative, BHP – UC, FONDECYT 1212023 – ANID Chile and the NIH-NIAID 1U19AI135972: Fluomics: The Next Generation.
Acknowledgements
We would like to thanks Luis A. Diaz for excellent clinical assistance during the study period, Zandra Segovia for assistance in patient recruitment and follow-ups and to all individuals that selflessly participated in this study. We also express our deepest gratitude to all the individuals that participated in this study. Work in the Tischler Laboratory was partially funded by FONDECYT 1181799 and Centro Ciencia & Vida, FB210008, Financiamiento Basal para Centros Científicos y Tecnológicos de Excelencia grants from the Agencia Nacional de Investigación y Desarrollo (ANID) of Chile. Work in the Medina Laboratory was based on protocols and the study set-up used for influenza virus established in part with the support of the PIA ACT 1408, FONDECYT 1161971, 1212023, and CONICYT REDES180170 grants from ANID of Chile, the FLUOMICS Consortium (NIH-NIAD grant U19AI135972) and the Center for Research on Influenza Pathogenesis (CRIP), an NIAID Center of Excellence for Influenza Research and Surveillance (CEIRS, contract # HHSN272201400008C) to RAM and FK. Additionally, work in the Krammer laboratory was partially funded by the NIAID Collaborative Influenza Vaccine Innovation Centers (CIVIC) contract 75N93019C00051, by the generous support of the JPB Foundation and the Open Philanthropy Project (research grant 2020-215611 (5384); and by anonymous donors. Work in the Chandran laboratory was partially supported by NIH grant R01AI132633. CPR and JL conducted this work as part of their Postdoctoral grants FONDECYT 3190706 and 3190648, respectively. MJA and ES conducted this work as part of their Ph.D. Thesis, under Programa de Doctorado en Ciencias Biológicas mención Genética Molecular y Microbiología, Facultad de Ciencias Biológicas, Pontificia Universidad Cátolica de Chile. MJA was funded by the ANID Becas/Doctorado Nacional 21212258 scholarship and ES was funded by a scholarship from Vicerrectoría de Investigación de la Escuela de Graduados, Pontificia Universidad Católica de Chile. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.ebiom.2022.103972.
Contributor Information
Nicole D. Tischler, Email: ntischler@cienciavida.org.
Rafael A. Medina, Email: rmedinai@uc.cl.
Appendix. Supplementary materials
References
- 1.Post N., Eddy D., Huntley C., et al. Antibody response to SARS-CoV-2 infection in humans: a systematic review. PLoS ONE. 2020;15(12) doi: 10.1371/journal.pone.0244126. PubMed PMID: 33382764. PMCID: PMC7775097 www.icmje.org/coi_disclosure.pdf and declare: no support from any organisation for the submitted work; JM is chief scientific officer, shareholder and scientific founder of Leucid Bio, a spinout company focused on development of cellular therapeutic agents; no other relationships or activities that could appear to have influenced the submitted work. This does not alter our adherence to PLoS ONE policies on sharing data and materials. Epub 2021/01/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wajnberg A., Amanat F., Firpo A., et al. Robust neutralizing antibodies to SARS-CoV-2 infection persist for months. Science. 2020;370(6521):1227–1230. doi: 10.1126/science.abd7728. Dec 4PubMed PMID: 33115920. PMCID: PMC7810037. Epub 2020/10/30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dan J.M., Mateus J., Kato Y., et al. Immunological memory to SARS-CoV-2 assessed for up to 8 months after infection. Science. 2021;371(6529):1–13. doi: 10.1126/science.abf4063. Feb 5PubMed PMID: 33408181. PMCID: PMC7919858. Epub 2021/01/08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gaebler C., Wang Z., Lorenzi J.C.C., et al. Evolution of antibody immunity to SARS-CoV-2. Nature. 2021;591(7851):639–644. doi: 10.1038/s41586-021-03207-w. MarPubMed PMID: 33461210. Epub 2021/01/19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Anand S.P., Prevost J., Nayrac M., et al. Longitudinal analysis of humoral immunity against SARS-CoV-2 Spike in convalescent individuals up to 8 months post-symptom onset. Cell Rep Med. 2021;2(6):1–10. doi: 10.1016/j.xcrm.2021.100290. PubMed PMID: 33969322. PMCID: PMC8097665. Epub 2021/05/11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aydillo T., Rombauts A., Stadlbauer D., et al. Immunological imprinting of the antibody response in COVID-19 patients. Nat Commun. 2021;12(1):3781. doi: 10.1038/s41467-021-23977-1. PubMed PMID: 34145263. PMCID: PMC8213790. Epub 2021/06/20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Krammer F. Correlates of protection from SARS-CoV-2 infection. Lancet. 2021;397(10283):1421–1423. doi: 10.1016/S0140-6736(21)00782-0. 04PubMed PMID: 33844964. PMCID: PMC8040540. Epub 2021/04/09. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hodgson S.H., Mansatta K., Mallett G., Harris V., Emary K.R.W., Pollard AJ. What defines an efficacious COVID-19 vaccine? A review of the challenges assessing the clinical efficacy of vaccines against SARS-CoV-2. Lancet Infect Dis. 2021;21(2):e26–e35. doi: 10.1016/S1473-3099(20)30773-8. FebPubMed PMID: 33125914. PMCID: PMC7837315. Epub 2020/10/31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jeyanathan M., Afkhami S., Smaill F., Miller M.S., Lichty B.D., Xing Z. Immunological considerations for COVID-19 vaccine strategies. Nat Rev Immunol. 2020;20(10):615–632. doi: 10.1038/s41577-020-00434-6. OctPubMed PMID: 32887954. PMCID: PMC7472682. Epub 2020/09/06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Croda J., Ranzani OT. Booster doses for inactivated COVID-19 vaccines: if, when, and for whom. Lancet Infect Dis. 2021:430–432. doi: 10.1016/S1473-3099(21)00696-4. PubMed PMID: 34890538. PMCID: PMC8651253 from the Pan American Health Organization. Epub 2021/12/11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.WHO S . World Health Organization; 2021. SAGE Working Group on COVID-19 Vaccines - Evidence Assessment: Sinovac/CoronaVac COVID-19 Vaccine.https://cdn.who.int/media/docs/default-source/immunization/sage/2021/april/5_sage29apr2021_critical-evidence_sinovac.pdf [Google Scholar]
- 12.Khoury D.S., Cromer D., Reynaldi A., et al. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat Med. 2021:1205–1211. doi: 10.1038/s41591-021-01377-8. PubMed PMID: 34002089. Epub 2021/05/19. [DOI] [PubMed] [Google Scholar]
- 13.Earle K.A., Ambrosino D.M., Fiore-Gartland A., et al. Evidence for antibody as a protective correlate for COVID-19 vaccines. Vaccine. 2021;39(32):4423–4428. doi: 10.1016/j.vaccine.2021.05.063. PubMed PMID: 34210573. PMCID: PMC8142841. Epub 2021/07/03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.WHO RD . World Health Organization; 2021. COVID-19 Vaccines WHO Meeting on Correlates of Protection - R&DBlueprint.https://cdn.who.int/media/docs/default-source/blue-print/final-agenda-immunobridging.pdf?sfvrsn=8e074908_17 [Google Scholar]
- 15.Sette A., Crotty S. Adaptive immunity to SARS-CoV-2 and COVID-19. Cell. 2021;184(4):861–880. doi: 10.1016/j.cell.2021.01.007. PubMed PMID: 33497610. PMCID: PMC7803150 Epitogenesis, Gilead, and Avalia. S.C. is a consultant for Avalia. LJI has filed for patent protection for various aspects of T cell epitope and vaccine design work. Epub 2021/01/27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kaplonek P., Wang C., Bartsch Y., et al. Early cross-coronavirus reactive signatures of humoral immunity against COVID-19. Sci Immunol. 2021;6(64):eabj2901. doi: 10.1126/sciimmunol.abj2901. PubMed PMID: 34652962. Epub 2021/10/16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stadlbauer D., Amanat F., Chromikova V., et al. SARS-CoV-2 seroconversion in humans: a detailed protocol for a serological assay, antigen production, and test setup. Curr Protoc Microbiol. 2020;57(1):e100. doi: 10.1002/cpmc.100. PubMed PMID: 32302069. PMCID: PMC7235504. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Amanat F., Stadlbauer D., Strohmeier S., et al. A serological assay to detect SARS-CoV-2 seroconversion in humans. Nat Med. 2020;26(7):1033–1036. doi: 10.1038/s41591-020-0913-5. PubMed PMID: 32398876. Epub 2020/05/12. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.ter Meulen J., van den Brink E.N., Poon L.L., et al. Human monoclonal antibody combination against SARS coronavirus: synergy and coverage of escape mutants. PLoS Med. 2006;3(7):e237. doi: 10.1371/journal.pmed.0030237. PubMed PMID: 16796401. PMCID: PMC1483912. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tian X., Li C., Huang A., et al. Potent binding of 2019 novel coronavirus spike protein by a SARS coronavirus-specific human monoclonal antibody. Emerg Microbes Infect. 2020;9(1):382–385. doi: 10.1080/22221751.2020.1729069. PubMed PMID: 32065055. PMCID: PMC7048180. Epub 2020/02/17. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Amanat F., White K.M., Miorin L., et al. An in vitro microneutralization assay for SARS-CoV-2 serology and drug screening. Curr Protoc Microbiol. 2020;58(1):e108. doi: 10.1002/cpmc.108. PubMed PMID: 32585083. PMCID: PMC7361222. Epub 2020/06/26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dieterle M.E., Haslwanter D., Bortz R.H., et al. A replication-competent vesicular stomatitis virus for studies of SARS-CoV-2 spike-mediated cell entry and its inhibition. Cell Host Microbe. 2020;28(3):486–496. doi: 10.1016/j.chom.2020.06.020. 09e6. PubMed PMID: 32738193. PMCID: PMC7332447. Epub 2020/07/03. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Legros V., Denolly S., Vogrig M., et al. A longitudinal study of SARS-CoV-2-infected patients reveals a high correlation between neutralizing antibodies and COVID-19 severity. Cell Mol Immunol. 2021;18(2):318–327. doi: 10.1038/s41423-020-00588-2. FebPubMed PMID: 33408342. PMCID: PMC7786875. Epub 2021/01/08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Carvalho T., Krammer F., Iwasaki A. The first 12 months of COVID-19: a timeline of immunological insights. Nat Rev Immunol. 2021;21(4):245–256. doi: 10.1038/s41577-021-00522-1. AprPubMed PMID: 33723416. PMCID: PMC7958099. Epub 2021/03/17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Krammer F., Srivastava K., Alshammary H., et al. Antibody responses in seropositive persons after a single dose of SARS-CoV-2 mRNA vaccine. N Engl J Med. 2021;384(14):1372–1374. doi: 10.1056/NEJMc2101667. PubMed PMID: 33691060. PMCID: PMC8008743. Epub 2021/03/10. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang Y., Zeng G., Pan H., et al. Safety, tolerability, and immunogenicity of an inactivated SARS-CoV-2 vaccine in healthy adults aged 18-59 years: a randomised, double-blind, placebo-controlled, phase 1/2 clinical trial. Lancet Infect Dis. 2021;21(2):181–192. doi: 10.1016/S1473-3099(20)30843-4. PubMed PMID: 33217362. PMCID: PMC7832443. Epub 2020/11/21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Walsh E.E., Frenck R.W., Falsey A.R., et al. Safety and Immunogenicity of two RNA-based COVID-19 vaccine candidates. N Engl J Med. 2020;383(25):2439–2450. doi: 10.1056/NEJMoa2027906. PubMed PMID: 33053279. PMCID: PMC7583697. Epub 2020/10/15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Turner J.S., Kim W., Kalaidina E., et al. SARS-CoV-2 infection induces long-lived bone marrow plasma cells in humans. Nature. 2021:421–425. doi: 10.1038/s41586-021-03647-4. PubMed PMID: 34030176. Epub 2021/05/25. [DOI] [PubMed] [Google Scholar]
- 29.Sakharkar M., Rappazzo C.G., Wieland-Alter W.F., et al. Prolonged evolution of the human B cell response to SARS-CoV-2 infection. Sci Immunol. 2021;6(56):1–14. doi: 10.1126/sciimmunol.abg6916. PubMed PMID: 33622975. PMCID: PMC8128290. Epub 2021/02/25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Goel R.R., Apostolidis S.A., Painter M.M., et al. Distinct antibody and memory B cell responses in SARS-CoV-2 naive and recovered individuals following mRNA vaccination. Sci Immunol. 2021;6(58):1–13. doi: 10.1126/sciimmunol.abi6950. PubMed PMID: 33858945. PMCID: PMC8158969. Epub 2021/04/17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sokal A., Barba-Spaeth G., Fernandez I., et al. mRNA vaccination of naive and COVID-19-recovered individuals elicits potent memory B cells that recognize SARS-CoV-2 variants. Immunity. 2021;54(12):2893–2907. doi: 10.1016/j.immuni.2021.09.011. PubMed PMID: 34614412. PMCID: PMC8452492. Epub 2021/10/07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Benjamanukul S., Traiyan S., Yorsaeng R., et al. Safety and immunogenicity of inactivated COVID-19 vaccine in health care workers. J Med Virol. 2021:1442–1449. doi: 10.1002/jmv.27458. PubMed PMID: 34783049. PMCID: PMC8661929. Epub 2021/11/17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Azak E., Karadenizli A., Uzuner H., Karakaya N., Canturk N.Z., Hulagu S. Comparison of an inactivated COVID19 vaccine-induced antibody response with concurrent natural Covid19 infection. Int J Infect Dis. 2021;113:58–64. doi: 10.1016/j.ijid.2021.09.060. PubMed PMID: 34597764. PMCID: PMC8479817. Epub 2021/10/02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Keskin A.U., Bolukcu S., Ciragil P., Topkaya AE. SARS-CoV-2 specific antibody responses after third CoronaVac or BNT162b2 vaccine following two-dose CoronaVac vaccine regimen. J Med Virol. 2022;94(1):39–41. doi: 10.1002/jmv.27350. PubMed PMID: 34536028. Epub 2021/09/19. [DOI] [PubMed] [Google Scholar]
- 35.Jeffery-Smith A., Rowland T.A.J., Patel M., et al. Reinfection with new variants of SARS-CoV-2 after natural infection: a prospective observational cohort in 13 care homes in England. Lancet Healthy Longev. 2021;2(12):e811–e8e9. doi: 10.1016/S2666-7568(21)00253-1. PubMed PMID: 34873592. PMCID: PMC8635459 England, which is a public body and an executive agency of the Department of Health and Social Care. Epub 2021/12/08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vitale J., Mumoli N., Clerici P., et al. Assessment of SARS-CoV-2 reinfection 1 year after primary infection in a population in Lombardy, Italy. JAMA Intern Med. 2021;181(10):1407–1408. doi: 10.1001/jamainternmed.2021.2959. PubMed PMID: 34048531. PMCID: PMC8164145. Epub 2021/05/29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Qureshi A.I., Baskett W.I., Huang W., Lobanova I., Naqvi S.H., Shyu CR. Re-infection with SARS-CoV-2 in patients undergoing serial laboratory testing. Clin Infect Dis. 2021:294–300. doi: 10.1093/cid/ciab345. PubMed PMID: 33895814. PMCID: PMC8135382. Epub 2021/04/26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Buss L.F., Prete C.A., Abrahim C.M.M., et al. Three-quarters attack rate of SARS-CoV-2 in the Brazilian Amazon during a largely unmitigated epidemic. Science. 2021;371(6526):288–292. doi: 10.1126/science.abe9728. PubMed PMID: 33293339. PMCID: PMC7857406. Epub 2020/12/10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sabino E.C., Buss L.F., Carvalho M.P.S., et al. Resurgence of COVID-19 in Manaus, Brazil, despite high seroprevalence. Lancet. 2021;397(10273):452–455. doi: 10.1016/S0140-6736(21)00183-5. PubMed PMID: 33515491. PMCID: PMC7906746. Epub 2021/01/31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chemaitelly H., Bertollini R., Abu-Raddad L.J. National study group for C-E. Efficacy of natural immunity against SARS-CoV-2 reinfection with the beta variant. N Engl J Med. 2021;385(27):2585–2586. doi: 10.1056/NEJMc2110300. PubMed PMID: 34910864. PMCID: PMC8693689. Epub 2021/12/16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gillot C., Favresse J., Maloteau V., Dogne J.M., Douxfils J. Dynamics of neutralizing antibody responses following natural SARS-CoV-2 infection and correlation with commercial serologic tests. A reappraisal and indirect comparison with vaccinated subjects. Viruses. 2021;13(11):1–9. doi: 10.3390/v13112329. PubMed PMID: 34835135. PMCID: PMC8621742. Epub 2021/11/28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jara A., Undurraga E.A., Gonzalez C., et al. Effectiveness of an Inactivated SARS-CoV-2 vaccine in Chile. N Engl J Med. 2021;385(10):875–884. doi: 10.1056/NEJMoa2107715. PubMed PMID: 34233097. PMCID: PMC8279092. Epub 2021/07/08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Polack F.P., Thomas S.J., Kitchin N., et al. Safety and efficacy of the BNT162b2 mRNA COVID-19 vaccine. N Engl J Med. 2020;383(27):2603–2615. doi: 10.1056/NEJMoa2034577. PubMed PMID: 33301246. PMCID: PMC7745181. Epub 2020/12/11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tanriover M.D., Doganay H.L., Akova M., et al. Efficacy and safety of an inactivated whole-virion SARS-CoV-2 vaccine (CoronaVac): interim results of a double-blind, randomised, placebo-controlled, phase 3 trial in Turkey. Lancet. 2021;398(10296):213–222. doi: 10.1016/S0140-6736(21)01429-X. PubMed PMID: 34246358. PMCID: PMC8266301. Epub 2021/07/12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bueno S.M., Abarca K., Gonzalez P.A., Galvez N.M.S., Soto J.A., Duarte L.F., et al. Safety and immunogenicity of an inactivated SARS-CoV-2 vaccine in a subgroup of healthy adults in Chile. Clin Infect Dis. 2021:1–13. doi: 10.1093/cid/ciab823. PubMed PMID: 34537835. Epub 2021/09/20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Epsi N.J., Richard S.A., Laing E.D., et al. Clinical, immunological, and virological SARS-CoV-2 phenotypes in obese and nonobese military health system beneficiaries. J Infect Dis. 2021;224(9):1462–1472. doi: 10.1093/infdis/jiab396. PubMed PMID: 34331541. PMCID: PMC8385847. Epub 2021/08/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Huang Y., Lu Y., Huang Y.M., et al. Obesity in patients with COVID-19: a systematic review and meta-analysis. Metabolism. 2020;113 doi: 10.1016/j.metabol.2020.154378. DecPubMed PMID: 33002478. PMCID: PMC7521361. Epub 2020/10/02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Frasca D., Reidy L., Cray C., et al. Influence of obesity on serum levels of SARS-CoV-2-specific antibodies in COVID-19 patients. PLoS ONE. 2021;16(3) doi: 10.1371/journal.pone.0245424. PubMed PMID: 33760825. PMCID: PMC7990309. Epub 2021/03/25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tartof S.Y., Slezak J.M., Fischer H., et al. Effectiveness of mRNA BNT162b2 COVID-19 vaccine up to 6 months in a large integrated health system in the USA: a retrospective cohort study. Lancet. 2021;398(10309):1407–1416. doi: 10.1016/S0140-6736(21)02183-8. PubMed PMID: 34619098. PMCID: PMC8489881. Epub 2021/10/08. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.