Abstract
9-Deoxy-Δ9,Δ12-13,14-dihydro-prostaglandin D2 (Δ12-PGJ2), a natural cyclopentenone metabolite of prostaglandin D2, is shown to possess therapeutic efficacy against influenza A virus A/PR8/34 (H1N1) infection in vitro and in vivo. The results indicate that the antiviral activity is associated with induction of cytoprotective heat shock proteins and suggest novel strategies for treatment of influenza virus infection.
Despite large immunization programs, viral influenza remains a serious source of morbidity and mortality throughout the world and a significant cause of illness and death among people with immunodeficiency associated with aging or different clinical conditions (2). Antiviral chemotherapy with amantadine and rimantadine reduces the duration of symptoms of clinical influenza, but major side effects and the emergence of drug-resistant variants were described (2, 5). A new class of antiviral agents designed to inhibit influenza neuraminidase is presently being developed for use in prophylaxis and treatment of influenza A and B infections (4). However, the ability of viruses to mutate the target proteins presents an obstacle for effective treatment with molecules which selectively inhibit the function of specific viral polypeptides.
One successful approach in combating viral diseases appears to be the simultaneous use of two or more drugs that affect different targets during the virus life cycle. A group of prostaglandins (PG) and PG derivatives containing an α,β-unsaturated carbonyl group in the cyclopentane ring (cyclopentenone PG [cyPG]) have the interesting ability to interfere with virus replication at multiple levels (12). PG of the A and J types (PGAs and PGJs) inhibit the replication of a variety of RNA viruses, including paramyxoviruses, rhabdoviruses, rotaviruses, and retroviruses in cultured cells (reviewed in reference 12). They act differently from any other known antiviral agent, by turning on an intracellular defense response which includes the induction of cytoprotective heat shock proteins (HSP) (7, 12). Whereas there is extensive literature on the antiviral activity of cyclopentenone prostanoids in in vitro experimental models, little is known about the therapeutic efficacy of these molecules in in vivo viral infection.
We now report that 9-deoxy-Δ9,Δ12-13,14-dihydro-PGD2 (Δ12-PGJ2), a natural cyclopentenone metabolite of PGD2 physiologically present in human body fluids, is a potent inhibitor of influenza A virus replication in Madin-Darby canine kidney (MDCK) cells. We also describe the therapeutic efficacy of Δ12-PGJ2 in mice infected with influenza A virus.
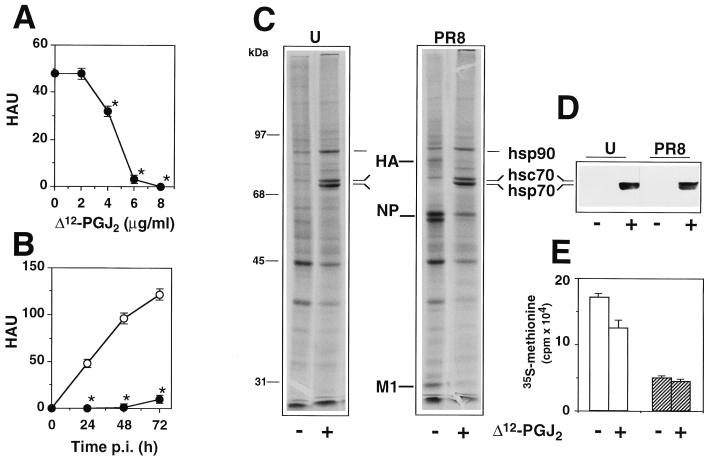
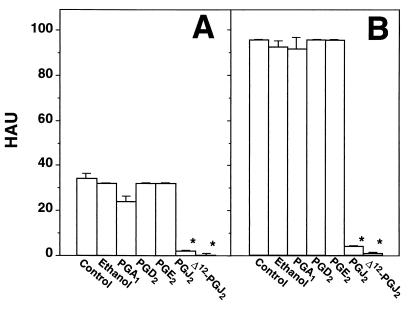
MDCK cells were grown at 37°C in RPMI 1640 medium supplemented with 5% fetal calf serum (Gibco) and antibiotics. Cell viability was determined by the dye exclusion technique and by the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay, as described previously (14). Influenza A virus A/PR8/34 (H1N1) (PR8 virus) (13) was grown in the allantoic cavities of 10-day-old embryonated eggs. Virus titers were determined by hemagglutinin titration, according to standard procedures. One hemagglutinating unit (HAU) corresponded to 106 PFU in this model. Confluent MDCK monolayers were infected with PR8 virus (5 HAU/105 cells) for 1 h at 37°C, after which the viral inoculum was removed and cells were treated with different concentrations of Δ12-PGJ2 (Cayman Chemical Co.) or ethanol diluent, which did not affect cell metabolism or virus replication. Viral yields were determined 24 h postinfection (p.i.). As shown in Fig. 1A, Δ12-PGJ2 reduced PR8 virus production dose dependently; a level of inhibition of more than 95% compared to the control was obtained at a concentration of 6 μg/ml, and virus yield was not detectable at higher concentrations. In a separate experiment, PR8 virus-infected monolayers were treated with 6 μg of Δ12-PGJ2 per ml (18 μM) after virus adsorption. Treatment was not repeated for the following 72 h. Figure 1B shows that the antiviral activity of Δ12-PGJ2 is sustained for a period of at least 72 h p.i. In a different experiment, MDCK cells were infected with PR8 virus (1 HAU/105 cells) and treated with 6 μg of Δ12-PGJ2 per ml or ethanol diluent soon after the 1-h adsorption period. Virus titers were determined in triplicate samples at 24 h p.i. by both HAU and 50% cytopathic effect (CPE50%) assay on confluent MDCK monolayers, as described previously (8). Treatment with Δ12-PGJ2 caused the expected decrease in HAU (titers were as follows: for control cells, 16 ± 0 HAU; and for Δ12-PGJ2-treated cells, 0 HAU), and it was effective in reducing infectious virus titers by more than 99% compared to the control (titers were as follows: for control cells, 3.6 × 104 ± 0.4 × 104 CPE50% U/ml; and for Δ12-PGJ2-treated cells, 3.2 × 101 ± 0.6 × 101 CPE50% U/ml). When the antiviral activity of Δ12-PGJ2 was compared with that of other PG, Δ12-PGJ2 was found to be the most effective cyclopentenone PG, whereas the noncyclopentenone PG of the E and D type did not affect PR8 replication (Fig. 2). It should be noted that PGA1, which possesses antiviral activity against several RNA viruses (12), only modestly and transiently inhibited PR8 replication at the concentration of 6 μg/ml; however, at higher concentrations (10 μg/ml), PGA1 was effective in decreasing PR8 virus yield by more than 90% compared to the control up to 48 h p.i. (data not shown).
FIG. 1.
Effect of Δ12-PGJ2 on PR8 influenza virus replication and protein synthesis. (A) MDCK cells infected with PR8 influenza virus were treated with different concentrations of Δ12-PGJ2 soon after the 1-h adsorption period. Virus yield was determined 24 h p.i. by HAU titration. (B) Under the same conditions, PR8 virus replication was inhibited by more than 95% up to 72 h p.i. after a single treatment with 6 μg of Δ12-PGJ2 per ml (●) compared to the control (○). Data in panels A and B are means ± standard deviations of at least duplicate samples of a representative experiment. Each experiment was repeated at least three times. The Student's t test was used for unpaired data analysis and P values of <0.05 were considered significant. ∗, P < 0.05. For panels C to E, MDCK cells mock infected (U) or infected with PR8 virus (PR8) were treated with Δ12-PGJ2 (6 μg/ml) (+) or control diluent (−) soon after the 1-h adsorption period and labeled with [35S]methionine for the following 24 h. (C) Samples containing equal amounts of radioactivity were processed for SDS-PAGE and autoradiography. Viral proteins HA, NP and M1 are indicated. (D) Samples containing equal amounts of protein were processed for IB analysis with anti-hsp70 antibodies which recognize both the 72-kDa constitutive hsc70 and the 70-kDa inducible hsp70. (E) Protein synthesis in uninfected (□) or PR8-infected (▨) cells, as determined by [35S]methionine incorporation into trichloroacetic acid-insoluble material.
FIG. 2.
Effect of different PG on PR8 virus replication. MDCK cells infected with PR8 virus (1 HAU/ml) were treated with 6 μg/ml of PGA1, PGD2, PGE2, PGJ2, Δ12-PGJ2, or ethanol diluent soon after the 1-h adsorption period. Virus yield was determined at 24 h (A) and 48 h (B) p.i. by HAU titration. Data are the means + standard deviations (error bars) of triplicate samples. ∗, P < 0.05.
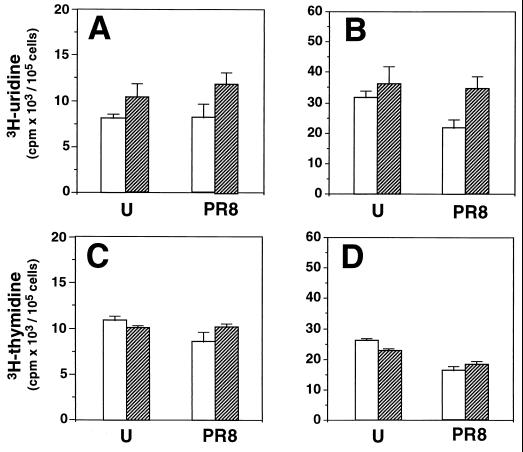
Under the conditions described, Δ12-PGJ2 was nontoxic to uninfected cultures, as determined by microscopic examination and by MTT assay. For MTT assay uninfected MDCK cells were treated with 6 μg of Δ12-PGJ2 per ml or ethanol diluent for 24 h, after which 10 μl of a 0.5% MTT solution in phosphate-buffered saline was added to the monolayers and the mixture was incubated for 2 h at 37°C. Reduced MTT (formazan) was extracted from cells by adding 100 μl of acidic isopropanol containing 10% Triton X-100, and formazan absorbance was measured in an enzyme-linked immunosorbent assay microplate reader at two different wavelengths (540 and 690 nm). The results from quadruplicate samples show that Δ12-PGJ2 did not affect cell viability (results were as follows: for ethanol control cells, 292 ± 21; and for Δ12-PGJ2-treated cells, 332 ± 44). Moreover, treatment with Δ12-PGJ2 did not inhibit nucleic acid synthesis in MDCK cells. Uninfected or PR8 virus-infected MDCK cells were treated with Δ12-PGJ2 (6 μg/ml) or control diluent soon after the 1-h adsorption period and labeled for the following 24 h with [3H]thymidine or [3H]uridine (5 μCi/105 cells) for DNA or RNA synthesis, respectively. The radioactivity incorporated into trichloroacetic acid (TCA)-soluble (uptake) and -insoluble (incorporation) material was determined as described previously (11). As shown in Fig. 3, Δ12-PGJ2 treatment did not inhibit the uptake of precursors or DNA or RNA synthesis in either uninfected or PR8 virus-infected cells. Δ12-PGJ2 treatment actually prevented the virus-induced inhibition of cellular RNA synthesis (Fig. 3B).
FIG. 3.
Effect of Δ12-PGJ2 on DNA and RNA synthesis in uninfected or PR8 virus-infected MDCK cells. Confluent MDCK monolayers mock infected (U) or infected with PR8 virus (PR8) were treated with Δ12-PGJ2 (6 μg/ml) (▨) or control diluent (□) soon after the 1-h adsorption period and labeled with [3H]uridine (A and B) or [3H]thymidine (C and D). The amount of radioactivity incorporated into the TCA-soluble (uptake) (A and C) or -insoluble (incorporation) (B and D) material was determined after 24 h. Data are the means + standard deviations (error bars) of at least duplicate samples of a representative experiment.
To investigate the effect of Δ12-PGJ2 on cellular and viral protein synthesis, PR8 virus-infected MDCK monolayers were treated with Δ12-PGJ2 (6 μg/ml) or ethanol diluent after the 1-h adsorption period and labeled with [35S]methionine (5 μCi/105 cells) for the following 24 h. Uninfected cells were treated identically. After this time, the amount of radioactivity incorporated into proteins was quantified and samples containing equal amounts of radioactivity were analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) in a vertical slab gel apparatus (3% stacking gel, 10% resolving gel) and processed for autoradiography as described previously (9). Molecular weights were calculated by using Bio-Rad Mr markers. For immunoblot (IB) analysis, equal amounts of protein from each sample were separated by SDS-PAGE and blotted onto nitrocellulose, and filters were incubated with monoclonal anti-hsp70 antibodies (9). Virus proteins were identified by IB analysis with a polyclonal anti-WSN virus antiserum which recognizes PR8 virus HA, NP, and M1 proteins (1) and a monoclonal anti-HA antibody (kindly supplied by E. Rodriguez-Boulan, Cornell University, New York, N.Y.). In uninfected cells, Δ12-PGJ2 caused a modest reduction of protein synthesis (Fig. 1E) and did not alter the overall electrophoretic profile of cellular proteins, whereas it markedly induced the synthesis of two polypeptides of 70 and 72 kDa, respectively, which were identified as the constitutive (hsc70) and the inducible (hsp70) forms of hsp70 by IB analysis (Fig. 1C and D). Synthesis of hsp90 was also enhanced. PR8 virus infection caused a decrease in protein synthesis (Fig. 1E) and did not induce hsp70 synthesis in MDCK cells (Fig. 1C and D). Δ12-PGJ2 treatment caused a dramatic reduction of PR8 protein synthesis, which was associated with the synthesis of high levels of hsc70 and hsp70 (Fig. 1C). NP synthesis appeared to be reduced by a lesser extent than that of the other viral proteins.
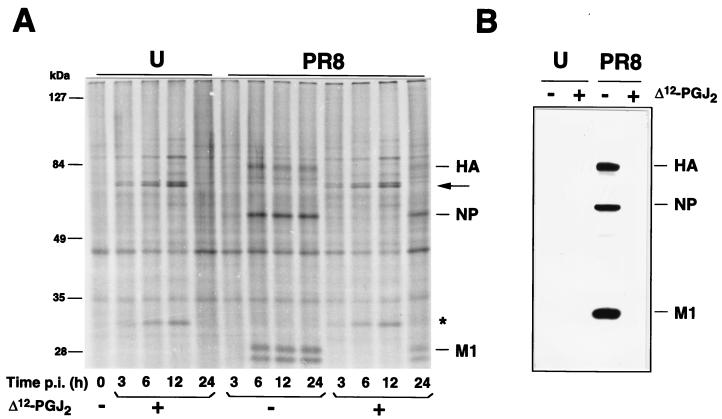
To investigate the kinetics of hsp70 and virus protein synthesis, PR8 virus-infected or mock-infected cells were treated with Δ12-PGJ2 (6 μg/ml) or ethanol diluent after the 1-h adsorption period and labeled with [35S]methionine (10 μCi/105 cells, 45-min pulse) at different times p.i. Samples containing the same amount of radioactivity were processed for SDS-PAGE and autoradiography (Fig. 4A). In the same experiment, equal amounts of protein from unlabeled uninfected or PR8 virus-infected cells at 24 h p.i. were processed for IB analysis with polyclonal anti-WSN virus antiserum (Fig. 4B) or monoclonal anti-HA antibodies (data not shown). hsp70 synthesis started 3 h after Δ12-PGJ2 treatment and continued for at least 12 h in both uninfected and PR8 virus-infected MDCK cells. As previously shown for other negative-strand RNA viruses (12), PR8 virus protein synthesis was inhibited as long as hsp70 was being synthesized by the host cell (Fig. 4A), and viral proteins were not detectable by IB analysis in Δ12-PGJ2-treated cells at 24 h p.i. (Fig. 4B).
FIG. 4.
Effect of Δ12-PGJ2 on the kinetics of PR8 virus protein synthesis. MDCK cells mock-infected (U) or infected with PR8 virus (1 HAU/ml) (PR8) were treated with Δ12-PGJ2 (6 μg/ml) (+) or control diluent (−) soon after the 1-h adsorption period and labeled with [35S]methionine for 45 min at the times indicated. (A) Samples containing equal amounts of radioactivity were processed for SDS-PAGE and autoradiography. hsp70 is indicated by the arrow. Asterisk indicates a 32-kDa protein, which was identified as heme oxygenase by IB analysis (data not shown). (B) Samples containing equal amounts of protein were processed for IB analysis with a polyclonal anti-WSN virus antiserum which recognizes PR8 virus HA, NP and M1 proteins (1).
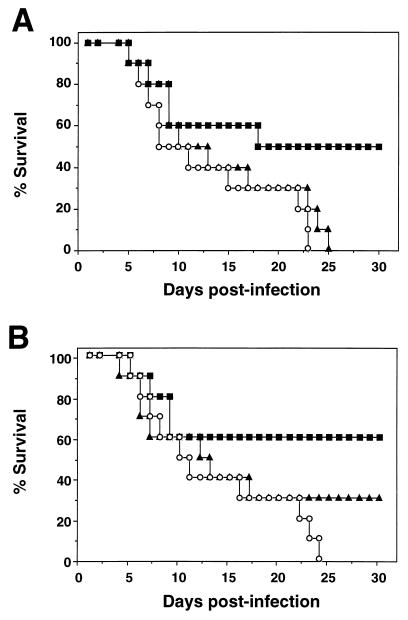
To evaluate whether Δ12-PGJ2 could also be effective in controlling influenza A infection in vivo, we performed a series of experiments using BALB/c mice as recipients for the PR8 virus. Depending on the dose, PR8 virus inoculation administered intranasally (i.n.) produces a damaging infection of the lungs, highly lethal to the animals; infection with 1 HAU of PR8 virus/mouse resulted in 100% death of 4-week-old BALB/c mice in the first month p.i. (13). Based on these results, 200 4-week-old BALB/c male mice were inoculated i.n. with 100 μl of PR8 virus suspension (12.5 HAU/ml) while they were under light ether anesthesia, and, 3 h after inoculation, they were randomly divided in groups of 10 or 20 and injected intraperitoneally (i.p.) with 100 μl of sterile saline solution containing 10% ethanol or Δ12-PGJ2-ethanolic solution, according to different schedules (Fig. 5). Mice were examined daily for survival for the following 4 months. Survival curves were compared using the Sign test (13), and α values of <0.05 were considered significant. Under these conditions, 100% of control animals were dead by day 24 p.i. Ethanol diluent did not significantly affect mouse survival after PR8 virus infection (13). Results of a representative dose-response experiment are shown in Fig. 5A. Treatment of PR8 virus-infected mice with 1 μg of Δ12- PGJ2/day/mouse for 7 days had no effect on mouse survival. Under the same conditions, treatment with 5 μg of Δ12-PGJ2/day/mouse for 7 days resulted in a significant increase in mice survival (50% on day 25 p.i.). In a different experiment, treatment with 5 μg of Δ12-PGJ2/day/mouse on days 0, 2, and 4 after PR8 virus infection was less effective, resulting in the survival of approximately 30% of the animals, compared to 60% survival when Δ12-PGJ2 was administered daily for 7 days (Fig. 5B). Mice that survived to day 25 p.i. did not show any sign of disease for the following 3 months and were considered cured. Compared to mock-infected controls, PR8 virus-infected mice showed a significative reduction in body weight at 7 days p.i. (weights were as follows: for control mice, 19.73 ± 0.61 g; and for PR8 virus-infected mice, 15.97 ± 0.98 g; P = 0.001). Loss of body weight at 7 days p.i. was significantly decreased in mice that received a 7-day Δ12-PGJ2 treatment (5 μg/day/mouse) (weights were as follows: for PR8 virus-infected mice, 15.97 ± 0.98 g; for Δ12-PGJ2-treated, PR8 virus-infected mice, 18.25 ± 0.68 g; P = 0.001). To determine the effect of Δ12-PGJ2 on the virus titer in the lung, 10 4-week-old BALB/c mice were inoculated with 1 HAU of PR8 virus/mouse and treated daily with Δ12-PGJ2 (10 μg/mouse/day administered i.p., n = 5) or ethanol diluent (n = 5). Four days after virus infection, the mice were sacrificed and the virus titer in the lungs was determined by CPE50% assay on MDCK cells, as described previously (13). Δ12-PGJ2 treatment was found to cause a decrease in virus titer in the lungs (titers were as follows: for control mice, 6.44 × 104 ± 2.77 × 104 CPE50% U/g of lung tissue; and for Δ12-PGJ2-treated mice, 0.07 × 104 ± 0.03 × 104 CPE50% U/g of lung tissue; P < 0.001), indicating a direct action of Δ12-PGJ2 on virus replication in this organ. Animals treated with the higher dose of Δ12-PGJ2 (10 μg/day/mouse administered i.p. for 4 days) did not show any sign of toxicity; in fact, Δ12-PGJ2 treatment reduced the loss of body weight in infected animals (data not shown).
FIG. 5.
Effect of Δ12-PGJ2-treatment on the survival of PR8 virus-infected mice. BALB/c mice were each infected i.n. with a 100-μl volume of PR8 virus suspension (12.5 HAU/ml) on day 0 and then treated i.p. with different doses of Δ12-PGJ2. (A) ○, control diluent (n = 10); ▴, Δ12-PGJ2 (1 μg/day/mouse on days 1 to 7 p.i.; n = 10); ■, Δ12-PGJ2 (5 μg/day/mouse on days 1 to 7 p.i.; n = 10); (B) ○, control diluent (n = 20); ▴, Δ12-PGJ2 (5 μg/day/mouse on days 0, 2, and 4 p.i.; n = 20); ■, Δ12-PGJ2 (5 μg/day/mouse on days 1 to 7 p.i.; n = 20). Percent survival of mice treated with 5 μg of Δ12-PGJ2/day on days 1 to 7 was significantly increased compared to that of control mice for animals described for panels A and B (α <0.005).
PG participate in the regulation of a variety of physiological and pathological processes, including the immune response, cytoprotection, inflammation, the febrile response, and virus infection (17). The antiviral activity of cyclopentenone PG has been described in several experimental in vitro models (12). Their mechanism of action is distinct from any other known antiviral agent and involves the induction of HSP in the infected cell via activation of the heat shock transcription factor HSF1 (9). In the case of retroviruses, cyPG inhibit the replication of human immunodeficiency virus type 1 by blocking viral RNA transcription and translation (11). These events have been associated with hsp70 induction and inhibition of nuclear factor κB (NF-κB), an inducible eukaryotic transcription factor promoting the transcription of proinflammatory and viral genes (10). Δ12-PGJ2 and other cyPG were previously shown to inhibit herpes simplex virus replication in vitro by blocking viral RNA transcription (6, 18) and to reduce vesicular stomatitis virus yield by altering viral protein synthesis and maturation (8). In the case of orthomyxoviruses, whereas a protective activity of a synthetic analogue of PGA2 (16,16-dimethyl-PGA2-methylester) was reported during in vivo influenza A infection (13), there was no information on the effect of cyPG on influenza virus replication in tissue culture.
We have now shown that the natural cyclopentenone Δ12-PGJ2 is a potent inhibitor of influenza A virus replication. A single treatment at a nontoxic concentration, which does not inhibit cellular DNA and RNA synthesis, is effective in reducing virus yield by more than 95% for a period of at least 72 h. As previously shown for other negative-strand RNA viruses, the antiviral activity is associated with inhibition of viral protein synthesis, but it remains to be established whether Δ12-PGJ2 acts at the transcriptional or translational level. Inhibition of PR8 virus protein synthesis by Δ12-PGJ2 is associated with the synthesis of elevated intracellular levels of hsp70. Differently from poliovirus and rotavirus (3, 16), influenza A virus did not interfere with the ability of the infected cell to synthesize hsp70 after cyPG treatment. Two different cyclopentenone PG, PGA1 and PGJ2, also inhibited PR8 virus replication in vitro, though to a minor extent compared to Δ12-PGJ2. The antiviral activity of PGA1 and PGJ2 was also found to be correlated with the level of hsp70 synthesis in MDCK cells (data not shown). Noncyclopentenone PG of the E and D type, which do not activate HSF1 and are not able to induce hsp70 synthesis (9, 10), did not affect PR8 virus replication.
A role for hsp70 as the cellular mediator interfering with viral protein synthesis during negative-strand RNA virus infection was suggested, as different hsp70 inducers, including sodium arsenite, cadmium, azetidine, and heat shock, all selectively inhibit viral protein synthesis (12); conversely, cyPG treatment has no effect on viral protein synthesis in cells lacking the ability to synthesize hsp70 or during infection with viruses that shut off hsp70 synthesis (3, 12, 16). The mechanism by which HSP can interfere with viral protein synthesis remains to be elucidated. hsp70 could directly interact with the nascent viral polypeptides, causing a translational block. Alternatively, it was hypothesized that HSP and virus messages could possess similar mechanisms for preferential translation and compete with each other (12).
Finally, we have shown that i.p. administration of Δ12-PGJ2 to PR8 virus-infected mice is effective in protecting them from viral infection and decreasing virus titers in the lung. As compared to the control group, where there were no survivors by day 25 after infection, 50 to 60% of the animals that received a 7-day Δ12-PGJ2 treatment (5 μg/mouse/day) survived the infection. Δ12-PGJ2-treated mice that survived to day 25 p.i. did not show any sign of disease for the following 3 months and were considered cured.
PG are used clinically as cytoprotective drugs for gastroduodenal ulcers, in the treatment of congenital heart disease and erectile dysfunction, and to facilitate labor, and are generally effective and well tolerated (17). PGE infusion was also shown to be beneficial in patients with fulminant viral hepatitis (15). The results described suggest a possible therapeutic use of cyclopentenone prostanoids or prostanoid-derived molecules during clinical complications of influenza virus infection.
Acknowledgments
We gratefully acknowledge R. Schneider (New York University, New York, N.Y.) and P. Palese (Mount Sinai School of Medicine, New York, N.Y.) for polyclonal anti-WSN virus antiserum and E. Rodriguez-Boulan (Cornell University) for monoclonal anti-HA antibodies.
This work was supported by grants from the Italian Ministry of Public Health, the 1999 AIDS Research Project, and CNR, P.F. Biotechnology.
REFERENCES
- 1.Basler C F, García-Sastre A, Palese P. Mutation of neuraminidase cysteine residues yields temperature-sensitive influenza viruses. J Virol. 1999;73:8095–8103. doi: 10.1128/jvi.73.10.8095-8103.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention. Prevention and control of influenza: recommendations of the Advisory Committee on Immunization Practices. Morbid Mortal Weekly Rep. 1996;45(RR-5):1–24. [PubMed] [Google Scholar]
- 3.Conti C, Mastromarino P, Tomao P, De Marco A, Pica F, Santoro M G. Inhibition of poliovirus replication by prostaglandins A and J in human cells. Antimicrob Agents Chemother. 1996;40:367–372. doi: 10.1128/aac.40.2.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Couch R B. A new antiviral agent for influenza—is there a clinical niche? N Engl J Med. 1997;337:927–928. doi: 10.1056/NEJM199709253371310. [DOI] [PubMed] [Google Scholar]
- 5.Hayden F G. Amantadine and rimantadine—clinical aspects. In: Richman D D, editor. Antiviral drug resistance. Chichester, United Kingdom: John Wiley; 1996. pp. 59–77. [Google Scholar]
- 6.Hughes-Fulford M, McGrath M S, Hanks D, Erickson S, Pulliam L. Effects of dimethyl prostaglandin A1 on herpes simplex virus and immunodeficiency virus replication. Antimicrob Agents Chemother. 1992;36:2253–2258. doi: 10.1128/aac.36.10.2253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Morimoto R I, Santoro M G. Stress-inducible responses and heat shock proteins: new pharmacologic targets for cytoprotection. Nat Biotechnol. 1998;16:833–838. doi: 10.1038/nbt0998-833. [DOI] [PubMed] [Google Scholar]
- 8.Pica F, De Marco A, De Cesare F, Santoro M G. Inhibition of vesicular stomatitis virus replication by Δ12-prostaglandin J2 is regulated at two separate levels and is associated with induction of stress protein synthesis. Antivir Res. 1993;20:193–208. doi: 10.1016/0166-3542(93)90020-j. [DOI] [PubMed] [Google Scholar]
- 9.Rossi A, Elia G, Santoro M G. 2-Cyclopenten-1-one, a new inducer of heat shock protein 70 with antiviral activity. J Biol Chem. 1996;271:32192–32196. doi: 10.1074/jbc.271.50.32192. [DOI] [PubMed] [Google Scholar]
- 10.Rossi A, Elia G, Santoro M G. Inhibition of nuclear factor κB by prostaglandin A1: an effect associated with heat shock transcription factor activation. Proc Natl Acad Sci USA. 1997;94:746–750. doi: 10.1073/pnas.94.2.746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rozera C, Carattoli A, De Marco A, Amici C, Giorgi C, Santoro M G. Inhibition of HIV-1 replication by cyclopentenone prostaglandins in acutely infected human cells: evidence for a transcriptional block. J Clin Investig. 1996;97:1795–1803. doi: 10.1172/JCI118609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Santoro M G. Antiviral activity of cyclopentenone prostanoids. Trends Microbiol. 1997;5:276–281. doi: 10.1016/S0966-842X(97)01066-4. [DOI] [PubMed] [Google Scholar]
- 13.Santoro M G, Favalli C, Mastino A, Jaffe B M, Esteban M, Garaci E. Antiviral activity of a synthetic analog of prostaglandin A in mice infected with influenza A virus. Arch Virol. 1988;99:89–100. doi: 10.1007/BF01311026. [DOI] [PubMed] [Google Scholar]
- 14.Shigeta S, Mori S, Watanabe J, Soeda S, Takahashi K, Yamase T. Synergistic anti-influenza virus A (H1N1) activities of PM-523 (polyoxometalate) and ribavirin in vitro and in vivo. Antimicrob Agents Chemother. 1997;41:1423–1427. doi: 10.1128/aac.41.7.1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sinclair S B, Greig P D, Blendis L M, et al. Biochemical and clinical response of fulminant viral hepatitis to administration of prostaglandin E. J Clin Investig. 1989;84:1063–1069. doi: 10.1172/JCI114268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Superti F, Amici C, Tinari A, Donelli G, Santoro M G. Inhibition of rotavirus replication by prostaglandin A: evidence for a block of virus maturation. J Infect Dis. 1998;178:564–568. doi: 10.1086/517475. [DOI] [PubMed] [Google Scholar]
- 17.Vane J, O'Grady J. Therapeutic applications of prostaglandins. Sevenoaks, United Kingdom: Edward Arnold; 1993. pp. 1–288. [Google Scholar]
- 18.Yamamoto N, Fukushima M, Tsurumi T, Maeno K, Nishiyama Y. Mechanism of inhibition of herpes simplex virus replication by Δ7-prostaglandin A1 and Δ12-prostaglandin J2. Biochem Biophys Res Commun. 1987;146:1425–1431. doi: 10.1016/0006-291x(87)90809-6. [DOI] [PubMed] [Google Scholar]