Abstract
Case series
Patients: Male, 53-year-old • Male, 65-year-old
Final Diagnosis: Ischemic optic neuropathy
Symptoms: Visual acuity loss • visual field defect
Medication: —
Clinical Procedure: —
Specialty: Ophthalmology
Objective:
Rare coexistence of disease or pathology
Background:
Since the start of vaccination efforts against COVID-19, several presumed secondary ocular events have been described. We present 2 cases of non-arteritic anterior ischemic optic neuropathy (NA-AION) in patients whose symptoms appeared in the first 2 weeks after administration of the Pfizer-BioNTech COVID-19 mRNA BNT162b2/ Cominarty vaccine.
Case Reports:
The first patient was a 53-year-old man who presented visual field disturbance in the right eye 7 days after the first vaccine dose, and who consulted a physician 10 days after the second dose, when he experienced loss of vision in the left eye. After a full examination, bilateral anterior optic disc neuropathy was diagnosed. The second patient was a 65-year-old man who presented anterior optic disc neuropathy 12 days after his first vacci-nation. In both cases, arteritic origin was ruled out due to absence of systemic symptoms and because of normal levels of C-reactive protein and erythrocyte sedimentation rates.
Conclusions:
Ischemic optic neuropathy is a rare adverse ocular secondary effect of COVID-19 vaccines. Further basic and clinical research is needed to elucidate the pathogenic mechanisms and better characterize the clinical picture of this entity.
Keywords: COVID-19 Vaccines; Optic Neuropathy, Ischemic; Pandemics
Background
Continued efforts to control the COVID-19 pandemic have led to the approval of multiple vaccines across the globe, including the Pfizer (BNT162b2 mRNA), Moderna (mRNA-1273), J&J (Ad26. COV2.S), and AstraZeneca (AZD1222) vaccines. These vaccines elicit an immune neutralizing response, preventing infection from acute respiratory syndrome coronavirus 2 (SARS-CoV-2). However, various countries have warned of secondary events after this novel vaccination, which, although rare, can be serious, including thromboembolic events that also appear in arteries [1–4]. The occurrence and significance of autoimmune manifestations after the administration of viral vaccines remain controversial [5]. We present 2 cases of non-arteritic anterior ischemic optic neuropathy (NA-AION) after administration of the BNT162b2/Cominarty vaccine.
Case Reports
Case 1
A 53-year-old man presented visual loss in his left eye (LE) 10 days after receiving the second dose of the BNT162b2/ Cominarty vaccine. He reported that 7 days after the first dose, he also had an altitudinal defect in his right eye (RE), but he did not consult a physician because he noticed spontaneous improvement, although without full recovery of vision.
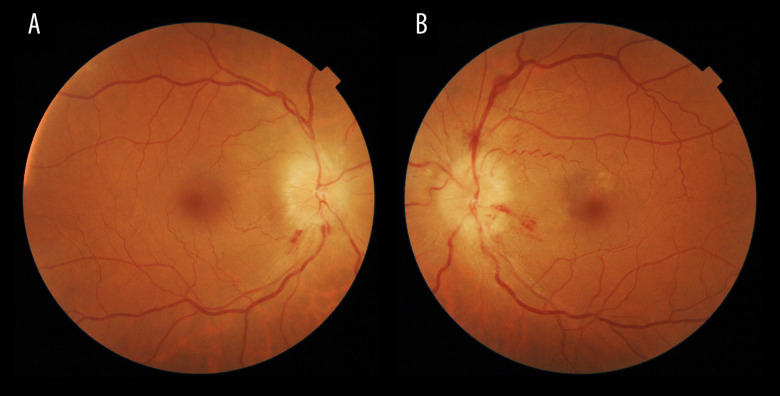
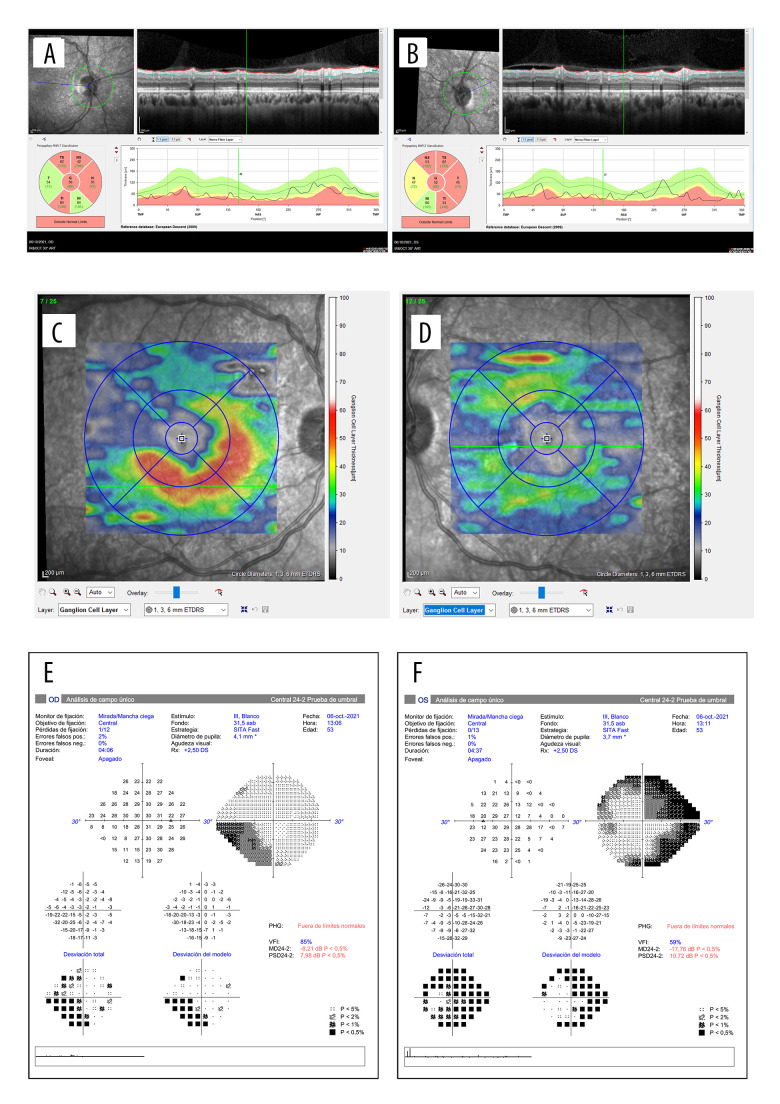
His past medical history was not relevant and he reported no other symptoms. Best-corrected visual acuity (BCVA, Snellen equivalent) in his LE was 20/40, a relative afferent pupillary defect was observed, and funduscopy showed a large optic disc swelling with some hemorrhages. In his RE, BCVA was 20/20 and a lazy pupil and mild papillary edema with 2 hemorrhages were also observed (Figure 1). The papillary edema in both eyes was confirmed by optical coherence tomography (OCT). The OCT also showed slight loss of ganglion cells in his RE but not yet in the left eye. The visual field was disturbed in both eyes (constriction of peripheral visual field in his left eye and an incomplete lower nasal scotoma in his right eye, Figure 2).
Figure 1.
A and B are funduscopy images of the first case. Right and left eyes, respectively, illustrating optic disc swelling predominantly in the left eye with some hemorrhages at the critical moment.
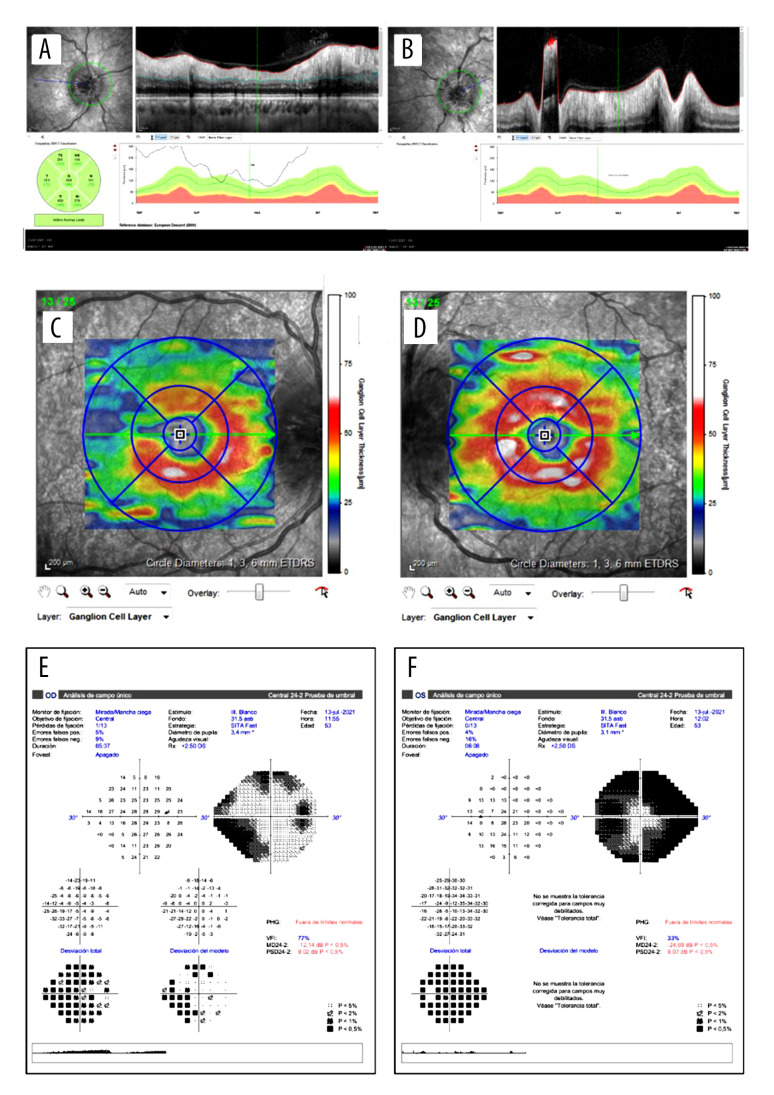
Figure 2.
OCT images and their functional correspondence by Humphrey 24-2 visual field. A and B show the retinal nerve fiber layer (RNFL) thickness of the right and left eye, respectively. It was not possible to measure the edema in the left eye at the critical moment. C and D illustrate the ganglion cell layer thickness of the right and left eye, respectively, showing a loss in the superotemporal quadrant of the right eye 3 weeks after the initial symptoms. The left eye remains at normal levels. E and F show the Humphrey 24-2 visual field of the right and the left eye, respectively. The right eye shows a lower nasal scotoma, while the left eye shows a constriction of the visual field.
A SARS-CoV-2 PCR nasal swab test was performed, and was negative. Bilateral anterior ischemic optic neuropathy was suspected and further evaluation was performed. A neurological examination was unremarkable. An extensive diagnostic work-up showed negative results for infectious serological tests, and based on a blood cell count, iron deficiency anemia was diagnosed, but his platelet count was within normal values. No active bleeding was detected. Erythrocyte sedimentation rate and C-reactive protein were normal. Antibodies against platelet factor 4 (PF4) were not tested for. Antineutrophil cytoplasmic antibody and antinuclear antibodies were negative. Tumor markers Alfa 1 fetoprotein, carcinoembryonic antigen, CA 19, CA 125, CYFRA 21.1, and NSE were also within normal values. An echo-Doppler of the supra-aortic vessels was also normal.
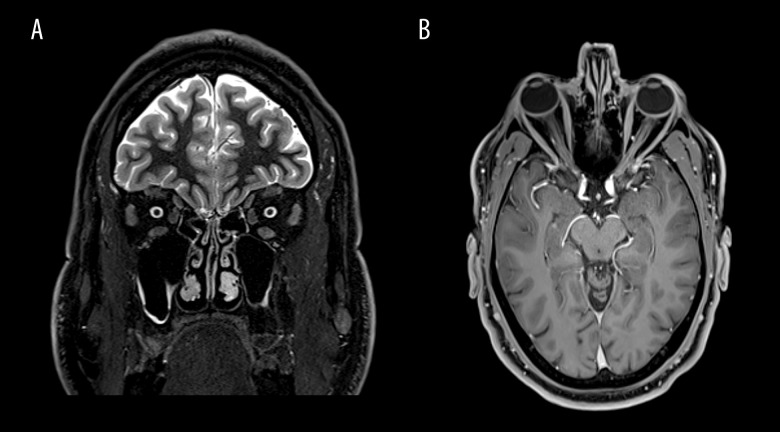
To rule out optic neuritis, an MRI of the brain and orbits with and without gadolinium was performed, without significant findings (Figure 3). A lumbar puncture was also performed. Opening pressure on the lumbar puncture was 38 cmH2O, and the cerebrospinal fluid showed normal protein and glucose levels, with infection serology tests showing negative. Whole-body computed tomography was also carried out, without remarkable findings.
Figure 3.
(A, B) Coronal and axial views of the brain on MRI, without any finding of neuritis.
With evidence from the lumbar puncture of increased intracranial pressure as a chance finding, the patient was started on acetazolamide 750 mg/day. The patient did not report any visual improvement during his admission or 3 months after the events.
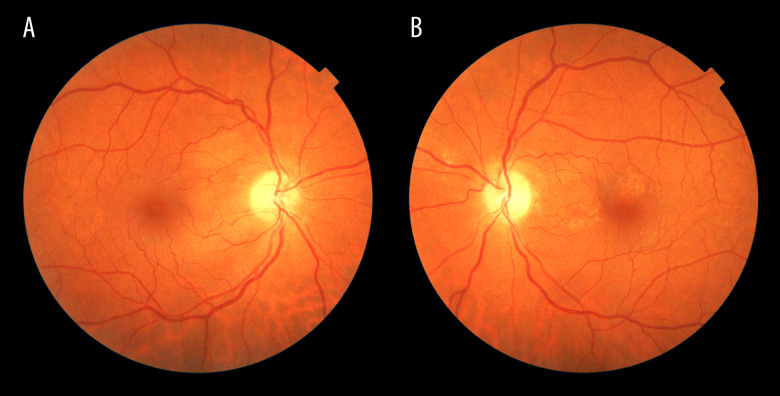
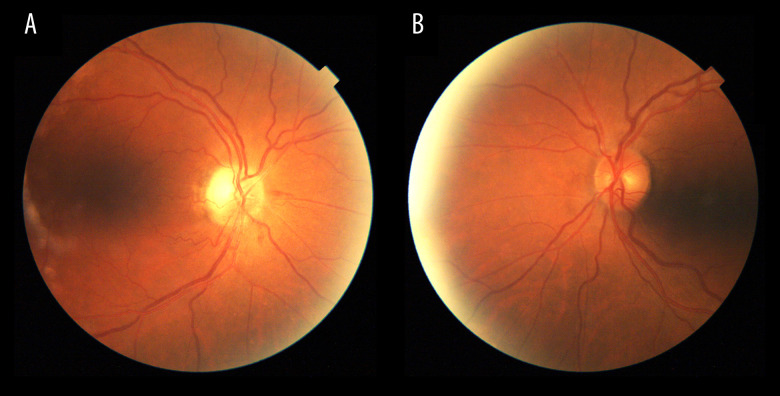
Three months later, clinical exploration revealed the following. The visual acuity was 20/20 in his RE and 20/40 in his LE, with lazy pupils and normal ocular movements. In the funduscopy, pallor disc was observed in both eyes, without any hemorrhages (Figure 4). The OCT showed slight loss of ganglion cells in both eyes and RNFL showed atrophy. The visual field did no change in the RE and in the LE was slightly better (Figure 5). Despite not having a typical altitudinal defect, with the results of the tests and the evolution, the diagnosis of non-arteritic anterior ischemic optic neuropathy was made.
Figure 4.
A and B are funduscopy images of the first case after 3 months of the event. Right and left eyes, respectively, illustrating pallor disc in both eyes, without any hemorrhages.
Figure 5.
Evolution of the OCT and Humphrey 24-2 visual field in the first case. Three months later, the RNFL shows atrophy of both eyes (A, B) with loss of superior quadrant of ganglion cell (C) and general loss of ganglion cells in the left eye (D). E and F show the Humphrey 24-2 visual field of the right and the left eye, respectively. A lower nasal inferior scotoma more defined is observed in the right eye and less constriction of the visual field is observed in the left eye.
Case 2
A 65-year-old male patient consulted emergencies services for blurry vision in his RE 12 days after having been vaccinated with the first dose of the BNT162b2/Cominarty vaccine. The patient was in treatment for arterial hypertension, with good results. He did not report other relevant episodes in his medical history; furthermore, he reported no prior similar episodes or any other symptoms. The ophthalmological examination showed low visual acuity (20/200 BCVA, Snellen equivalent), a relative afferent pupillary defect, and optic disc swelling suggestive of anterior ischemic optic neuropathy. The LE was normal on examination, with neither pupillary defect nor crowded axonal disc. Neurological examination did not show any relevant data. Furthermore, he had normal blood parameters, since blood glucose, erythrocyte sedimentation rate, C-reactive protein, and platelet count were within normal limits. Antibodies against PF4 were not tested for. Brain computed tomography was performed as a matter of emergency and did not present any abnormality, and an echo-Doppler of the supra-aortic vessels was also normal. No specific treatment was given.
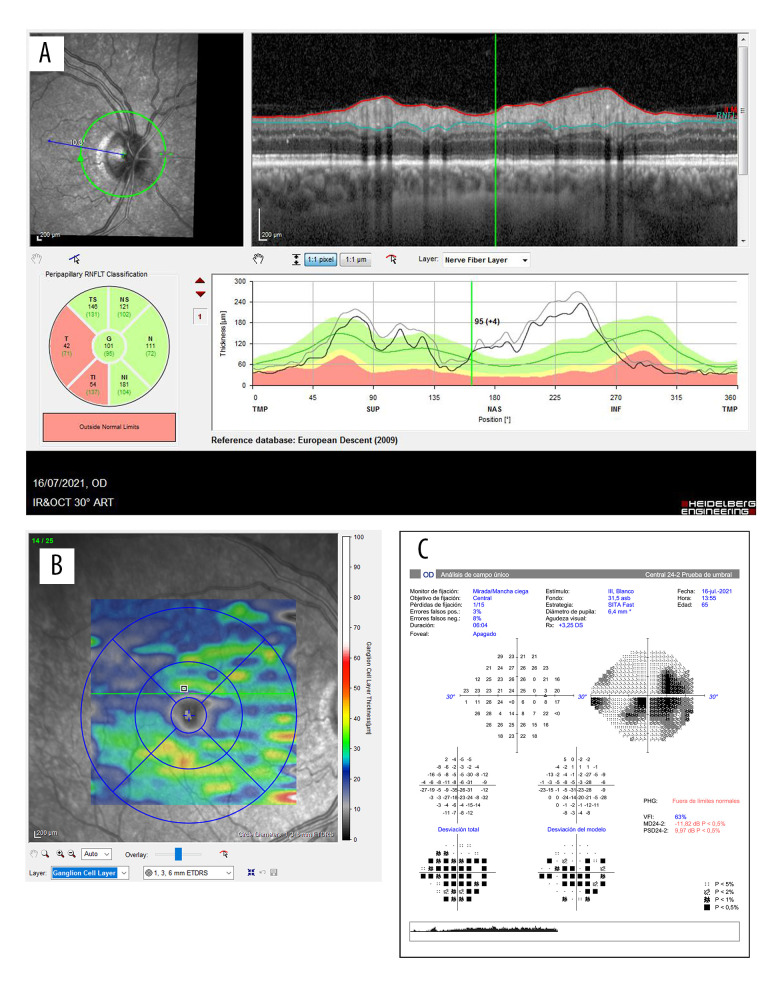
One month later, he came to our neuro-ophthalmology consultations for follow-up. Low visual acuity persisted (20/200), with dyschromatopsia (Ishihara test: n1/17), a relative afferent pupillary, pallor of temporal optic disc was evident in funduscopy in the RE (Figure 6), defect and a visual field defect were detected on Humphrey automated perimetry (blind spot enlargement with centrocecal scotoma). OCT showed atrophy of the temporal quadrants of RNFL and a general loss of ganglion cells in the affected eye (Figure 7). Although a typical visual field defect was not detected, given the result of the other tests, the diagnosis of non-arteritic anterior ischemic optic neuropathy was made.
Figure 6.
A and B show funduscopy images of the second case, right and left eye, respectively. A shows disc pallor in the superotemporal quadrants after a month of the critical moment and B illustrates no predisposing, axonal crowding in the disc of the left eye.
Figure 7.
Evolution of the OCT and Humphrey 24-2 visual field in the second case. A shows the RNFL of the right eye and the correspondence of the atrophic quadrants in the affected eye after a month of the symptoms. B illustrates general loss of ganglion cells of the right eye. Image C illustrates the Humphrey 24-2 visual field of the right eye, showing a centrocecal scotoma.
These 2 cases were reported to the Spanish pharmacovigilance system, which recorded the events.
Discussion
Here we describe 2 cases of non-arteritic anterior ischemic optic neuropathy that might be attributed to the BNT162b2/ Cominarty vaccine. Although we acknowledge that this progression could be unrelated to this patient’s vaccination, the time sequence between the vaccine and the development of the ischemic optic neuropathy (especially in the first case where the ischemic optic neuropathy developed in one eye after the first dose and in the other eye after the second dose) makes a potential role of the COVID-19 vaccines in the pathogenesis of this condition plausible in these 2 cases. We acknowledge that this case report describes an association, not causation, between NA-AION and COVID-19 vaccination [17].
In our first case, although elevated intracranial pressure was found and this may be associated with bilateral optic disc edema (ie, papilledema), this condition typically does not show only acute visual symptoms, so we believe that the patient indeed presented an ischemic optic neuropathy or that the vaccine may have precipitated an ischemic event in prone optic nerves, with crowded structure due to elevated intracranial pressure.
In the second case, the patient only had arterial hypertension as a risk factor for vasculopathic disease, which was well controlled, nor did he present a predisposing optic disc.
In the United States, NA-AION affects between 2.3 and 10.3 people per 100 000 people per year, making it the most common cause of acute optic neuropathy in patients older than 50 years. The vast majority of NA-AION cases are idiopathic, but some specific etiologies have been reported to be associated with NAION (eg, sleep apnea syndrome, medications such as interferons or Sildenafil, and optic disc drusen), as well as predisposing risk factors in the pathophysiology, such as optic disc anatomy, nocturnal hypotension, or vasculopathic risk factors. Regarding these cases, the close temporal relationship with vaccination makes us think that it was the predisposing event that triggered non-arteritic anterior ischemic neuropathy in these 2 patients. Although NA-AION after vaccination is very rare, 4 cases after influenza vaccination have been reported in the literature [18,19], and very recently, another case of NA-AION with a close temporal relationship with COVID-19 vaccination has been published [20].
Previous secondary ocular effects presumed to be associated with COVID-19 vaccines include episcleritis, scleritis, uveitis (both anterior and posterior), acute macular neuroretinopathy, paracentral acute medial maculopathy, corneal transplant rejection, central serous chorioretinopathy, and arteritic ischemic anterior optic neuropathy [5–16]. The pathogenesis of these complications is not fully understood and may be different in each type. Regarding the only arteritic anterior ischemic optic neuropathy case that has been published to date [15], the authors hypothesized cross-reactivity between the neutralizing antibodies against the SARS-CoV2 spike proteins and arterial antigens, resulting in a presumed autoimmune phenomenon. In fact, this patient presented fever, elevated C-reactive protein, and elevated erythrocyte sedimentation rate, and was diagnosed with Horton disease after a positive temporal artery biopsy, so the above-mentioned mechanism is plausible. However, our 2 patients did not show systemic manifestations and the blood analysis was normal, so this autoimmune mechanism seems unlikely, although a local ophthalmic autoimmune event may still be considered. Another mechanism that should be considered is of thrombotic origin. One of the rare but serious effects described after SARS-CoV-2 vaccination is cerebral venous sinus thrombosis (CVST) because of immune thrombotic thrombocytopenia (VITT). VITT is caused by antibodies that PF4, also called CXCL4, binds to platelets. These antibodies are immunoglobulin G (IgG) molecules that activate platelets via low-affinity platelet FcγIIa receptors (receptors on the platelet surface that bind the Fc portion of the IgG molecule). Ultimately, platelet activation (and possibly activation of other cells such as neutrophils) results in marked stimulation of the coagulation system and clinically significant thromboembolic complications. Although they have been more frequently described with the SARS-CoV-2 infection, they are already well known to occur after COVID-19 vaccination. Previous cases of ischemic optic neuropathy have also been reported in patients with COVID-19 [21–26]. Recently, Al-Mayhani et al [2] also described VITT characteristics with arterial thrombosis; they reported 3 cases of ischemic stroke associated with COVID-19 vaccination. This was explained as immune-mediated coagulopathy that can also cause arterial thrombosis, which often affects multiple arterial territories, although small-artery stroke is less common. VITT has been described more frequently with the adenovirus vector vaccine. For the moment, there is only 1 report, by Sangli and colleagues, who describe a catastrophic thrombosis after a second dose of the Moderna SARS-CoV-2 messenger RNA (mRNA) vaccine [14]. However, antibodies against PF4 were not determined in our cases; hence this pathogenic mechanism cannot be completely ruled out.
Conclusions
Ischemic optic neuropathy may be linked to mRNA-based COVID-19 vaccination. Further research is necessary to elucidate the pathophysiological mechanism.
Footnotes
Declaration of Figures’ Authenticity
All figures submitted have been created by the authors who confirm that the images are original with no duplication and have not been previously published in whole or in part.
References:
- 1.Markus HS. Ischaemic stroke can follow COVID-19 vaccination but is much more common with COVID-19 infection itself. J Neurol Neurosurg Psychiatry. 2021;92(11):1142. doi: 10.1136/jnnp-2021-327057. [DOI] [PubMed] [Google Scholar]
- 2.Al-Mayhani T, Saber S, Stubbs MJ, et al. Ischaemic stroke as a presenting feature of ChAdOx1 nCoV-19 vaccine-induced immune thrombotic thrombocitopenia. J Neurol Neurosurg Psychiatry. 2021;92(11):1247–48. doi: 10.1136/jnnp-2021-326984. [DOI] [PubMed] [Google Scholar]
- 3.Hunter PR. Thrombosis after COVID-19 vaccination. BMJ. 2021;373:n958. doi: 10.1136/bmj.n958. [DOI] [PubMed] [Google Scholar]
- 4.Sangli S, Virani A, Cheronis N, et al. Thrombosis with thrombocytopenia after the messenger RNA-1273 vaccine. Ann Intern Med. 2021;174(10):1480–82. doi: 10.7326/L21-0244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maleki A, Look-Why S, Manhapra A, Foster CS. COVID-19 Recombinant mRNA vaccines and serious ocular inflammatory side effects: Real or coincidence? J Ophthalmic Vis Res. 2021;16(3):490–501. doi: 10.18502/jovr.v16i3.9443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Abousy M, Bohm K, Prescott C, et al. Bilateral EK rejection after COVID-19 vaccine. Eye Contact Lens. 2021;47(11):625–28. doi: 10.1097/ICL.0000000000000840. [DOI] [PubMed] [Google Scholar]
- 7.Neri P, Pichi F. SARS-CoV-2 and the eye: The Pandora’s box of ocular immunology. J Ocul Pharmacol Ther. 2021;37(9):502–9. doi: 10.1089/jop.2021.0058. [DOI] [PubMed] [Google Scholar]
- 8.Koong LR, Chee WK, Toh ZH, et al. Vogt-Koyanagi-Harada disease associated with COVID-19 mRNA vaccine. Ocul Immunol Inflamm. 2021;29(6):1212–15. doi: 10.1080/09273948.2021.1974492. [DOI] [PubMed] [Google Scholar]
- 9.Valenzuela DA, Groth S, Taubenslag KJ, Gangaputra S. Acute macular neuroretinopathy following Pfizer-BioNTech COVID-19 vaccination. Am J Ophthalmol Case Rep. 2021;24:101200. doi: 10.1016/j.ajoc.2021.101200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pichi F, Aljneibi S, Neri P, Hay S, et al. Association of ocular adverse events with inactivated COVID-19 vaccination in patients in Abu Dhabi. JAMA Ophthalmol. 2021;139(10):1131–35. doi: 10.1001/jamaophthalmol.2021.3477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rallis KI, Ting DSJ, Said DG, Dua HS. Corneal graft rejection following COVID-19 vaccine. Eye (Lond) 2021 doi: 10.1038/s41433-021-01671-2. [Online ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.ElSheikh RH, Haseeb A, Eleiwa TK, Elhusseiny AM. Acute uveitis following COVID-19 vaccination. Ocul Immunol Inflamm. 2021;29(6):1207–9. doi: 10.1080/09273948.2021.1962917. [DOI] [PubMed] [Google Scholar]
- 13.Goyal M, Murthy SI, Annum S. Bilateral multifocal choroiditis following COVID-19 vaccination. Ocul Immunol Inflamm. 2021;29(4):753–57. doi: 10.1080/09273948.2021.1957123. [DOI] [PubMed] [Google Scholar]
- 14.Renisi G, Lombardi A, Stanzione M, et al. Anterior uveitis onset after bnt162b2 vaccination: Is this just a coincidence? Int J Infect Dis. 2021;110:95–97. doi: 10.1016/j.ijid.2021.07.035. [DOI] [PubMed] [Google Scholar]
- 15.Phylactou M, Li JO, Larkin DFP. Characteristics of endothelial corneal transplant rejection following immunisation with SARS-CoV-2 messenger RNA vaccine. Br J Ophthalmol. 2021;105(7):893–96. doi: 10.1136/bjophthalmol-2021-319338. [DOI] [PubMed] [Google Scholar]
- 16.Fowler N, Mendez Martinez NR, Pallares BV, Maldonado RS. Acute-onset central serous retinopathy after immunization with COVID-19 mRNA vaccine. Am J Ophthalmol Case Rep. 2021;23:101136. doi: 10.1016/j.ajoc.2021.101136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nachbor KM, Naravane AV, Adams OE, Abel AS. Nonarteritic anterior ischemic optic neuropathy associated with COVID-19 vaccination. J Neuroophthalmol. 2021 doi: 10.1097/WNO.0000000000001423. [Online ahead of print] [DOI] [PubMed] [Google Scholar]
- 18.Manasseh G, Donovan D, Shao EH, Taylor SR. Bilateral sequential non-arteritic anterior ischaemic optic neuropathy following repeat influenza vaccination. Case Rep Ophthalmol. 2014;5(2):267–69. doi: 10.1159/000366472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kawasaki A, Purvin VA, Tang R. Bilateral anterior ischemic optic neuropathy following influenza vaccination. J Neuroophthalmol. 1998;18(1):56–59. [PubMed] [Google Scholar]
- 20.Tsukii R, Kasuya Y, Makino S. Nonarteritic anterior ischemic optic neuropathy following COVID-19 vaccination: Consequence or coincidence. Case Rep Ophthalmol Med. 2021;2021:5126254. doi: 10.1155/2021/5126254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Deane K, Sarfraz A, Sarfraz Z, et al. Unilateral optic neuritis associated with SARS-CoV-2 infection: A rare complication. Am J Case Rep. 2021;22:e931665. doi: 10.12659/AJCR.931665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.François J, Collery AS, Hayek G, et al. Coronavirus disease 2019-associated ocular neuropathy with panuveitis: A case report. JAMA Ophthalmol. 2021;139(2):247–49. doi: 10.1001/jamaophthalmol.2020.5695. [DOI] [PubMed] [Google Scholar]
- 23.Sharma A, Kudchadkar US, Shirodkar R, et al. Unilateral inferior altitudinal visual field defect related to COVID-19. Indian J Ophthalmol. 2021;69(4):989–91. doi: 10.4103/ijo.IJO_3666_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Catharino AMS, Neves MAO, Nunes NSM, et al. COVID19 related optic neuritis: Case report. J Clin Neurol Neurosci. 2020;1(2):10. [Google Scholar]
- 25.Montesel A, Bucolo C, Mouvet V, et al. Case report: Central retinal artery occlusion in a COVID-19 patient. Front Pharmacol. 2020;11:588384. doi: 10.3389/fphar.2020.588384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sim R, Cheung G, Ting D, et al. Retinal microvascular signs in COVID-19. Br J Ophthalmol. 2021 doi: 10.1136/bjophthalmol-2020-318236. [Online ahead of print] [DOI] [PubMed] [Google Scholar]