Key Points
Question
What are the associations of daily total physical activity, total moderate-to-vigorous physical activity (MVPA), and leisure-time MVPA with risk of dementia in the general population in Japan?
Findings
This cohort study including 43 896 participants investigated associations of daily total physical activity and total MVPA with risk of disabling dementia. However, a higher level of leisure-time MVPA was statistically significantly associated with decreased risk of disabling dementia in men.
Meaning
These findings suggest that higher leisure-time MVPA may be associated with the prevention of disabling dementia in men.
This cohort study assesses the association between physical activity and risk of disabling dementia in Japan.
Abstract
Importance
The associations of daily total physical activity and total moderate to vigorous physical activity (MVPA) with dementia are still unclear.
Objective
To investigate the association between daily total physical activity and subsequent risk of disabling dementia in large-scale, extended follow-up prospective study.
Design, Setting, and Participants
This prospective cohort study used data from questionnaires collected between 2000 and 2003 from 8 areas from the Japan Public Health Center-based Prospective Disabling Dementia Study. Participants included adults aged 50 to 79 years in with available follow-up data on disabling dementia. Data analysis was performed from February 1, 2019, to July 31, 2021.
Exposures
Daily total physical activity, total MVPA, and leisure-time MVPA.
Main Outcomes and Measures
The main outcome was incidence of disabling dementia during the dementia ascertainment period between 2006 and 2016, based on the national long-term care insurance system. Risks of dementia in association with daily total physical activity, total MVPA, and leisure time MVPA were calculated using multivariable adjusted hazard ratios (aHRs).
Results
Among 43 896 participants (mean [SD] age, 61.0 [7.5] years; 23 659 [53.9%] women), 5010 participants were newly diagnosed with disabling dementia during a mean (SD) of 9.5 (2.8) years in the dementia ascertainment period. In the highest daily total physical activity group, compared with the lowest activity group, risk of dementia was lower in men (aHR, 0.75 [95% CI, 0.66-0.85]; P for trend < .001) and women (aHR, 0.75 [95% CI, 0.67-0.84]; P for trend < .001). Similar inverse associations were observed in men and women for total MVPA (men: aHR, 0.74 [95% CI, 0.65-0.84]; P for trend < .001; women: aHR, 0.74 [95% CI, 0.66-0.83]; P for trend < .001) and leisure-time MVPA (men: aHR, 0.59 [95% CI, 0.53-0.67]; P for trend < .001; women: aHR, 0.70 [95% CI, 0.63-0.78]; P for trend < .001). However, these inverse associations disappeared when participants diagnosed with disabling dementia within 7 years of the starting point were excluded in men (aHR, 0.93 [95%CI, 0.77-1.12]) and within 8 years were excluded in women (aHR, 0.86 [95%CI, 0.71-1.04]). The association remained significant among men in the highest vs lowest group of leisure-time MVPA, after excluding participants diagnosed within the first 9 years (aHR, 0.72 [95% CI, 0.56-0.92]; P for trend = .004).
Conclusions and Relevance
This cohort study examined associations of daily total physical activity and total MVPA with risk of disabling dementia. The findings suggest that a high level of leisure-time MVPA was associated with decreased risk of disabling dementia in men.
Introduction
Dementia is one of the major causes of disability and dependency among older people. Approximately 50 million people worldwide currently have dementia, and nearly 10 million new cases are diagnosed every year. The World Health Organization has listed dementia as a public health priority.1
Physical activity is a potential preventive factor for dementia1 and has been shown to have an inverse association with dementia incidence in several epidemiological studies.2,3,4,5,6,7,8,9,10 However, these studies were conducted with short-term follow-up periods. In contrast, some cohort studies11,12,13 with long-term follow-up reported no association between leisure-time physical activity and risk of dementia and suggested that the observed inverse association between them may be attributable to a reverse causation bias, defined in epidemiology as when exposure to a disease process is reversed.14 In dementia studies, for example, a decline in physical activity may not be the cause of dementia, but rather a consequence arising in the preclinical phase of dementia. However, because the preclinical phase of dementia is relatively long, studies with shorter follow-up are unable to identify this association.
Similarly, a meta-analysis that included 1300 incident cases of all-cause dementia occurring after 10 years from the start of follow-up also found a null association between leisure-time physical activity and dementia, and suggested a reverse causation bias.15 In contrast, although daily total physical activity, including leisure-time physical activity and nonexercise physical activity, contributes to total daily energy expenditure and thereby also confers important health benefits,16,17 only a few epidemiological studies3,8,9 have focused on the association between daily total physical activity and risk of dementia. These studies reported an association between a high level of daily total physical activity and risk of dementia but did not consider the possibility of reverse causation bias because of their short follow-up. In addition, it remains unclear whether the association of daily total moderate to vigorous physical activity (MVPA) with incident dementia may have a potential reverse causation bias, although many studies report that MVPA is beneficial for dementia3,4 and brain health.18,19,20,21 In this study, to investigate whether daily total physical activity and MVPA in daily total time and in leisure-time are associated with the subsequent risk of dementia, and whether the associations may be subject to reverse causation bias, we analyzed a large number of adults with disabling dementia, as certified under Japan’s national long-term care insurance (LTCI) system within a large prospective cohort study with long-term follow-up.
Methods
This cohort study was approved by the institutional review board of the National Cancer Center, Tokyo, Japan. Study participants were informed of the purpose of the study, and those completing the survey questionnaire were regarded as consenting to participation. This study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline.
Study Population
The Japan Public Health Center-based Prospective Study (JPHC Study) was launched in 1990 for cohort I and in 1993 for cohort II. Details of the study are reported elsewhere.22 Cohort I included residents aged 40 to 59 years in 5 public health center (PHC) areas (Iwate, Akita, Nagano, Okinawa, and Tokyo) and cohort II included residents aged 40 to 69 years in 6 PHC areas (Ibaraki, Niigata, Kochi, Nagasaki, Okinawa, and Osaka). The 2 cohorts included a total of 140 420 men and women. Data analysis was performed from February 1, 2019, to July 31, 2021.
In the JPHC Disabling Dementia Study, among 140 420 total participants, we included 62 401 participants in 8 PHC areas with available follow-up data on disabling dementia: Omonogawa and Yokote districts in Yokote in Akita Prefecture; Iwase district in Sakuragawa and Tomobe district in Kasama in Ibaraki Prefecture; Usuda district in Saku in Nagano Prefecture; Kagami and Noichi districts in Konan in Kochi Prefecture; and Gushikawa district in Uruma in Okinawa Prefecture. Of 62 401 participants, we excluded 134 participants who were ineligible (non-Japanese nationality, not present from baseline, incorrect birth data, or duplicate enrollment) and 11 591 participants who moved away or died before the starting point for follow-up of case ascertainment, leaving 50 676 participants for analysis. Among these 50 676, 45 043 responded to the 10-year follow-up questionnaire (response rate, 88.9%), of whom 472 with a severe physical limitation who had difficulty going out independently and 675 with missing information on physical activity were excluded (eFigure in the Supplement).
Follow-up and Identification of Disabling Dementia
We used certification records in the national LTCI system to identify study participants with disabling dementia. Criteria were the same as those used in previous studies in Japan.23,24 The LTCI system is a compulsory insurance system introduced by the Ministry of Health, Labor and Welfare of Japan in 2000 that is administered by municipalities.25 Residents aged 65 years and older and those with disability aged 40 to 64 years wishing to receive long-term care services apply as functionally disabled with the municipality. The municipal government assesses the applicant’s functional health status by a comprehensive assessment and obtains a primary care physician’s written opinion of the disability. A physician completes the dementia rating scale according to the manual issued by the national government. We defined disabling dementia as certification at any level of needed long-term care, within the range of severity of cognitive disability (grade IIa, IIb, IIIa, IIIb, IV, or M) on the dementia rating scale derived from a primary care physician’s written opinion. Regarding validation of the dementia rating scale, a 2009 study26 reported that this is well correlated with the Mini-Mental State Examination score (r = −0.74).
Because certification records in the LTCI system were available from 2006, the starting point was defined as January 1, 2006. Records of participants in the system were collected during the dementia ascertainment period from the starting point until December 31, 2016.
Assessment of Physical Activity
This study consisted of a baseline survey (survey 1), and follow-up surveys at 5 years (survey 2) and 10 years (survey 3). The questionnaire item on physical activity item differed in each of the 3 surveys. We assessed physical activity as the main exposure variable using the 10-year follow-up survey conducted in 2000 to 2003, because it included more comprehensive information on physical activity than the other 2 and allowed evaluation of daily total MVPA and leisure-time MVPA in the 10-year follow-up survey only. Participants were asked the number of hours spent at sitting, standing, walking, and strenuous work in nonleisure time on a typical day in the last year, and the frequency and number of hours spent walking slowly, such as when taking a walk; walking quickly; light to moderate exercise, such as in golf or gardening; and strenuous exercise, such as tennis, jogging, aerobics, or swimming, in leisure time. We assigned metabolic equivalents (METs)27 as 1.3 METs for sitting, 2.0 METs for standing, 3.0 METs for walking, and 6.0 METs for strenuous work in nonleisure time. For leisure time, we assigned 2.8 METs for walking slowly, 4.0 METS for walking quickly, 3.0 METs for light to moderate exercise, and 6.0 METs for strenuous exercise.27 We assigned 0.9 METs for sleep and 1.3 METs for other activities. Finally, we calculated daily total physical activity by the sum of lengths of time spent for the respective activities multiplied by the assigned METs. If the total time spent in the respective activities exceeded 24 hours, hourly METs were first calculated by dividing the sum of METs by the total time spent, and then converted to daily METs by multiplying the number obtained by 24. Further, we calculated total MVPA and leisure-time MVPA by considering activities with an intensity of 3.0 METs or higher. Spearman rank correlation coefficient for daily total physical activity and 24-hour activity record was 0.672, and for total MVPA and 24-hour activity record was 0.610.28
Statistical Analysis
Person-years of the dementia ascertainment period were calculated for each participant from the starting point until the date of disabling dementia diagnosis, date of migration from a study area to a nonstudy area, date of death, or end of follow-up (December 31, 2016), whichever occurred first.
Adjusted hazard ratios (aHRs) and 95% CIs for disabling dementia according to physical activity, including daily total physical activity, total MVPA and leisure-time MVPA, were calculated using Cox proportional hazard regression models in men and women. The basic models stratified by area (8 city-level municipalities) and were adjusted for age (continuous). Multivariable models included covariates in the same questionnaire with primary exposure at the 10-year follow-up survey, such as smoking status (never, former, 1 to 19 cigarettes/d, ≥20 cigarettes/d), alcohol intake status (nondrinker or occasional drinker, 1 to <150 g/wk, 150 to <300 g/wk, ≥300 g/wk), body mass index (BMI; calculated as weight in kilograms divided by height in meters squared and categorized as <18.5, 18.5-24.9, 25-29, and ≥30), past history of diabetes (yes or no), use of medication for hypertension (yes or no), and occupation (primary industry, secondary or tertiary industry, unemployed, or household duties). These covariates are collected at same time with primary exposure. Linear trends were assessed by assigning ordinal numbers to categories of physical activity. Furthermore, we performed analyses after sequential exclusion of incident disabling dementia arising each year from the first year of the dementia ascertainment period to assess for potential reverse causation bias owing to a decline in physical activity level before the incidence of dementia. Additionally, we performed 4 sensitivity analyses. First, we performed the same analyses in participants aged 65 years and older. Second, we analyzed competing risk using the Fine and Gray subdistribution hazards model,29 on the basis that death (during 2006 to 2016) before disabling dementia incidence can be a competing event. Third, we analyzed the association between the risk of disabling dementia and change in daily total physical activity between the 5-year and 10-year questionnaires by considering changes between categories as determined by tertile of distribution of daily total physical activity, albeit that we did not assess total MVPA and leisure-time MVPA using the 5-year questionnaire because of the simplicity of its questions. Fourth, we also conducted a subgroup analysis in a multivariable model with the addition of education level (junior high school or higher education) to covariates in cohort I that included information on education. Multiple imputations were performed for missing covariate values using the full-conditional specification method with arbitrary missing patterns, creating 10 imputed data sets. All P values were 2-sided, and significance level was set at P < .05. All statistical analyses were performed with SAS software versions 9.3 and 9.4 (SAS Institute).
Results
The final cohort included 43 896 participants (mean [SD] age, 61.0 [7.5] years; 23 659 [53.9%] women) during 417 027 person-years of follow-up, and 5010 participants (11.4%) were newly diagnosed with disabling dementia during a mean (SD) dementia ascertainment period of 9.5 (2.8) years (eFigure in the Supplement). During the dementia ascertainment period, 11 077 participants (21.9%) died, 2287 participants (4.5%) moved away, and 6 participants (0.01%) were lost to follow-up before incident disabling dementia. Participant characteristics at the 10-year follow-up survey by daily total physical activity in men and women are shown in Table 1. In both sexes, participants with a high daily total physical activity level were younger, had a lower BMI, higher proportion of never smoking, higher proportion of drinking, lower proportion of unemployment, and lower prevalence of diabetes and hypertension.
Table 1. Characteristics of Participants According to Daily Total Physical Activity in Men and Women.
| Characteristic | Physical activity quartile, men, No. (%) | P value | Physical activity quartile, women, No. (%) | P value | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 1 | 2 | 3 | 4 | |||
| No. | 5059 | 5059 | 5059 | 5060 | 5914 | 5915 | 5915 | 5915 | ||
| Age at survey, mean (SD), y | 62.6 (8.0) | 60.1 (7.3) | 60.4 (7.0) | 59.6 (6.8) | <.001 | 64.4 (8.1) | 61.3 (7.5) | 60.2 (6.9) | 59.3 (6.7) | <.001 |
| BMI | ||||||||||
| <18.5 | 185 (3.8) | 144 (2.9) | 128 (2.6) | 133 (2.7) | .009 | 274 (4.9) | 240 (4.1) | 212 (3.6) | 203 (3.5) | <.001 |
| 18.5-24.9 | 3243 (66.2) | 3355 (66.9) | 3383 (67.6) | 3528 (70.8) | 3491 (62.0) | 3889 (66.7) | 3981 (68.1) | 3932 (67.5) | ||
| 25.0-29.9 | 1346 (27.5) | 1411 (28.1) | 1380 (27.6) | 1228 (24.6) | 1582 (28.1) | 1517 (26.0) | 1489 (25.5) | 1507 (25.9) | ||
| ≥30 | 127 (2.6) | 106 (2.1) | 113 (2.3) | 96 (1.9) | 282 (5.0) | 185 (3.2) | 163 (2.8) | 185 (3.2) | ||
| Smoking status | ||||||||||
| Never | 1189 (24.2) | 1385 (27.6) | 1363 (27.2) | 1369 (27.4) | .001 | 5278 (93.3) | 5440 (93.3) | 5436 (93.3) | 5380 (92.4) | .02 |
| Past | 1729 (35.2) | 1658 (33.0) | 1643 (32.8) | 1333 (26.7) | 106 (1.9) | 103 (1.8) | 104 (1.8) | 82 (1.4) | ||
| 1-19 cigarettes/d | 654 (13.3) | 567 (11.3) | 582 (11.6) | 654 (13.1) | 182 (3.2) | 176 (3.0) | 189 (3.2) | 239 (4.1) | ||
| ≥20 cigarettes/d | 1340 (27.3) | 1408 (28.1) | 1416 (28.3) | 1634 (32.8) | 93 (1.6) | 110 (1.9) | 96 (1.7) | 121 (2.1) | ||
| Alcohol intake | ||||||||||
| None or occasional | 1128 (22.9) | 905 (18.2) | 877 (17.6) | 932 (18.7) | <.001 | 4540 (81.3) | 4357 (74.8) | 4285 (73.6) | 4218 (72.9) | <.001 |
| 1 to <150 ethanol g/week | 1571 (31.9) | 1601 (32.1) | 1485 (29.9) | 1293 (25.9) | 864 (15.5) | 1234 (21.2) | 1300 (22.3) | 1312 (22.7) | ||
| 15 to <300 ethanol g/week | 877 (17.8) | 1020 (20.5) | 1031 (20.7) | 945 (19.0) | 104 (1.9) | 154 (2.6) | 155 (2.7) | 170 (2.9) | ||
| ≥300 ethanol g/week | 1352 (27.4) | 1458 (29.3) | 1578 (31.7) | 1814 (36.4) | 74 (1.3) | 84 (1.4) | 84 (1.4) | 88 (1.5) | ||
| Occupation | ||||||||||
| Primary industry | 529 (12.2) | 512 (11.0) | 1060 (23.3) | 1345 (31.1) | <.001 | 644 (12.3) | 634 (11.3) | 886 (15.8) | 1584 (28.9) | <.001 |
| Secondary or tertiary industry | 2350 (54.3) | 3212 (69.1) | 2864 (62.8) | 2717 (62.7) | 1359 (25.9) | 2045 (36.4) | 2249 (40.1) | 2572 (46.9) | ||
| Household duty | NA | NA | NA | NA | 2103 (40.1) | 2395 (42.6) | 2105 (37.5) | 1107 (20.2) | ||
| Unemployed | 1452 (33.5) | 926 (19.9) | 636 (14.0) | 269 (6.2) | 1136 (21.7) | 552 (9.8) | 371 (6.6) | 223 (4.1) | ||
| Disease history | ||||||||||
| Diabetes | 532 (10.5) | 457 (9.0) | 414 (8.2) | 359 (7.1) | <.001 | 371 (6.3) | 302 (5.1) | 264 (4.5) | 254 (4.3) | <.001 |
| Hypertension | 1512 (29.9) | 1241 (24.5) | 1143 (22.6) | 1030 (20.4) | <.001 | 1966 (33.2) | 1576 (26.6) | 1391 (23.5) | 1193 (20.2) | <.001 |
| Physical activity, mean (SD), MET-h/d | ||||||||||
| Daily total physical activity | 29.6 (1.5) | 34.6 (1.5) | 42.6 (3.3) | 60.5 (7.4) | <.001 | 29.8 (1.6) | 34.5 (1.3) | 40.1 (2.3) | 55.0 (8.2) | <.001 |
| Total MVPA | 1.4 (1.6) | 6.4 (3.1) | 17.0 (5.5) | 44.8 (15.7) | <.001 | 1.3 (1.6) | 5.9 (2.9) | 13.2 (4.4) | 36.3 (16.0) | <.001 |
| Leisure-time MVPA | 0.5 (1.0) | 1.4 (2.1) | 1.9 (3.0) | 1.7 (4.3) | <.001 | 0.4 (0.8) | 1.0 (1.6) | 1.7 (2.4) | 2.0 (3.8) | <.001 |
Abbreviations: BMI, body mass index; MET, metabolic equivalent; MVPA, moderate to vigorous physical activity; NA, not applicable.
Table 2 shows that the higher level of daily total physical activity was inversely associated with disabling dementia risk in both sexes. Compared with participants in the first quarter (Q1) of daily total physical activity, risk of dementia decreased with increasing physical activity for men (Q2: aHR, 0.73 [95% CI, 0.65-0.82]; Q3: aHR, 0.69 [95% CI, 0.61-0.78]; Q4: aHR, 0.75 [95% CI, 0.66-0.85]; P for trend < .001), and in women (Q2: aHR, 0.76 [95% CI, 0.69-0.84]; Q3: aHR, 0.73 [95% CI, 0.66-0.81]; Q4: aHR, 0.75 [95% CI, 0.67-0.84]; P for trend < .001).
Table 2. Risk of Disabling Dementia Risk According to Physical Activity in Men and Women.
| Physical activity | Men | Women | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | P for trend | Q1 | Q2 | Q3 | Q4 | P for trend | |
| Daily total physical activity | ||||||||||
| MET-h/d, median (range) | 29.6 (22.4-32.1) | 34.5 (32.1-37.5) | 42.5 (37.6-49.0) | 59.7 (49.0-92.5) | NA | 29.9 (21.8-32.3) | 34.5 (32.3-36.8) | 39.8 (36.8-44.6) | 52.9 (44.6-93.4) | NA |
| Person-years, No. | 43 881 | 48 021 | 48 277 | 47 913 | NA | 53 221 | 57 776 | 58 623 | 59 314 | NA |
| Incident dementia, No. | 780 | 452 | 439 | 422 | NA | 1226 | 687 | 542 | 462 | NA |
| Model 1 HR (95% CI)a | 1 [Reference] | 0.71 (0.63-0.80) | 0.67 (0.60-0.76) | 0.73 (0.65-0.83) | <.001 | 1 [Reference] | 0.75 (0.68-0.83) | 0.71 (0.64-0.79) | 0.72 (0.64-0.80) | <.001 |
| Model 1 HR (95% CI)b | 1 [Reference] | 0.73 (0.65-0.82) | 0.69 (0.61-0.78) | 0.75 (0.66-0.85) | <.001 | 1 [Reference] | 0.76 (0.69-0.84) | 0.73 (0.66-0.81) | 0.75 (0.67-0.84) | <.001 |
| Daily total MVPA | ||||||||||
| MET-h/d, median (range) | 0.4 (0-3.2) | 6.0 (2.5-9.0) | 16.7 (9.0-19.0) | 42.0 (25.5-111.0) | NA | 0.3 (0-2.5) | 6.0 (2.5-9.0) | 12.1 (9.0-19.0) | 30.8 (19.1-108.0) | NA |
| Person-years, No. | 44 019 | 48 076 | 48 147 | 47 852 | NA | 53 857 | 51 337 | 64 391 | 59 350 | NA |
| Incident dementia, No. | 768 | 449 | 455 | 421 | NA | 1152 | 650 | 655 | 460 | NA |
| Model 1 HR (95% CI)a | 1 [Reference] | 0.72 (0.64-0.80) | 0.65 (0.58-0.73) | 0.72 (0.64-0.82) | <.001 | 1 [Reference] | 0.81 (0.73-0.89) | 0.74 (0.68-0.82) | 0.71 (0.64-0.80) | <.001 |
| Model 2 HR (95% CI)b | 1 [Reference] | 0.73 (0.65-0.82) | 0.66 (0.59-0.74) | 0.74 (0.65-0.84) | <.001 | 1 [Reference] | 0.82 (0.74-0.91) | 0.77 (0.69-0.85) | 0.74 (0.66-0.83) | <.001 |
| Leisure-time MVPA | ||||||||||
| MET-h/d, median (range) | 0 | 0.1 (0.03-0.3) | 0.9 (0.3-1.6) | 3.8 (1.6-58.5) | NA | 0 | 0.1 (0.03-0.3) | 0.8 (0.3-1.7) | 3.8 (1.7-58.5) | NA |
| Person-years, No. | 54 214 | 46 444 | 43 611 | 43 825 | NA | 76 689 | 50 942 | 49 662 | 51 642 | NA |
| Incident dementia, No. | 930 | 443 | 307 | 413 | NA | 1420 | 540 | 451 | 506 | NA |
| Model 1 HR (95% CI)a | 1 [Reference] | 0.89 (0.79-1.00) | 0.64 (0.56-0.73) | 0.60 (0.53-0.67) | <.001 | 1 [Reference] | 0.92 (0.83-1.02) | 0.75 (0.67-0.83) | 0.69 (0.62-0.76) | <.001 |
| Model 2 HR (95% CI)b | 1 [Reference] | 0.90 (0.80-1.01) | 0.65 (0.57-0.74) | 0.59 (0.53-0.67) | <.001 | 1 [Reference] | 0.94 (0.85-1.05) | 0.76 (0.68-0.85) | 0.70 (0.63-0.78) | <.001 |
Abbreviations: HR, hazard ratio; MET, metabolic equivalent; MVPA, moderate to vigorous physical activity; NA, not applicable; Q, quartile.
Adjusted for age and area.
Additionally adjusted for smoking status (never, former, 1-19 cigarettes/d, or ≥20 cigarettes/d), alcohol intake status (none or occasional drinkers, 1 to <150 g/week, 150 to <300 g/week, or ≥300 g/week), body mass index (calculated as weight in kilograms divided by height in meters squared; <18.5, 18.5-24.9, 25-29, or ≥30), past history of diabetes (yes or no), medication for hypertension (yes or no), and occupation (primary industry, secondary or tertiary industry, unemployed, or household duties).
Table 3 shows that an inverse association was present between daily total physical activity and risk of disabling dementia after excluding participants diagnosed within the first 3 and 6 years of the dementia ascertainment period in men and women, but that the associations were lost after exclusion of those diagnosed within the first 9 years of the dementia ascertainment period in both men (Q4 vs Q1: aHR, 0.99 [95% CI, 0.76-1.29]; P for trend = .69) and women (Q4 vs Q1: aHR, 0.93 [95% CI, 0.74-1.17]; P for trend = .51). Similar inverse associations were observed in men and women for total MVPA (men: aHR, 0.74 [95% CI, 0.65-0.84]; P for trend < .001; women: aHR, 0.74 [95% CI, 0.66-0.83]; P for trend < .001) and leisure-time MVPA (men: aHR, 0.59 [95% CI, 0.53-0.67]; P for trend < .001; women: aHR, 0.70 [95% CI, 0.63-0.78]; P for trend < .001). Regarding leisure-time MVPA, an inverse association with disabling dementia risk remained in men even after excluding participants diagnosed within first 9 years (Q4 vs Q1: aHR, 0.72 [95% CI, 0.56-0.92]; P for trend = .004), but not in women.
Table 3. Risk of Disabling Dementia Risk According to Physical Activity When Excluding Incident Disabling Dementia From the Starting Point.
| Physical activity | Men | Women | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | P for trend | Q1 | Q2 | Q3 | Q4 | P for trend | |
| Daily total physical activity | ||||||||||
| Excluding first 3 y | ||||||||||
| Incident dementia, No. | 570 | 356 | 364 | 333 | NA | 939 | 549 | 452 | 381 | NA |
| Multivariable HR (95% CI)a | 1 [Reference] | 0.78 (0.68-0.89) | 0.77 (0.67-0.88) | 0.78 (0.68-0.91) | <.001 | 1 [Reference] | 0.80 (0.72-0.89) | 0.79 (0.70-0.89) | 0.81 (0.71-0.92) | <.001 |
| Excluding first 6 y | ||||||||||
| Incident dementia, No. | 379 | 238 | 248 | 239 | NA | 653 | 414 | 319 | 282 | NA |
| Multivariable HR (95% CI)a | 1 [Reference] | 0.76 (0.65-0.90) | 0.77 (0.65-0.90) | 0.82 (0.69-0.98) | .02 | 1 [Reference] | 0.88 (0.77-0.99) | 0.78 (0.68-0.90) | 0.84 (0.72-0.98) | .002 |
| Excluding first 9 y | ||||||||||
| Incident dementia, No. | 151 | 103 | 100 | 114 | NA | 260 | 174 | 155 | 127 | NA |
| Multivariable HR (95% CI)a | 1 [Reference] | 0.81 (0.63-1.05) | 0.77 (0.59-1.00) | 0.99 (0.76-1.29) | .69 | 1 [Reference] | 0.92 (0.76-1.12) | 0.94 (0.77-1.16) | 0.93 (0.74-1.17) | .51 |
| Daily total MVPA | ||||||||||
| Excluding first 3 y | ||||||||||
| Incident dementia, No. | 566 | 351 | 374 | 332 | NA | 876 | 578 | 484 | 383 | NA |
| Multivariable HR (95% CI)a | 1 [Reference] | 0.75 (0.65-0.86) | 0.71 (0.62-0.81) | 0.75 (0.65-0.87) | <.001 | 1 [Reference] | 0.87 (0.78-0.97) | 0.83 (0.74-0.93) | 0.81 (0.71-0.92) | <.001 |
| Excluding first 6 y | ||||||||||
| Incident dementia, No. | 377 | 235 | 252 | 240 | NA | 623 | 418 | 343 | 284 | NA |
| Multivariable HR (95% CI)a | 1 [Reference] | 0.73 (0.62-0.85) | 0.69 (0.59-0.82) | 0.79 (0.67-0.94) | .002 | 1 [Reference] | 0.87 (0.77-0.99) | 0.79 (0.69-0.91) | 0.81 (0.70-0.95) | .001 |
| Excluding first 9 y | ||||||||||
| Incident dementia, No. | 147 | 101 | 107 | 113 | NA | 263 | 158 | 164 | 131 | NA |
| Multivariable HR (95% CI)a | 1 [Reference] | 0.80 (0.62-1.04) | 0.75 (0.59-0.97) | 0.98 (0.76-1.27) | .58 | 1 [Reference] | 0.80 (0.66-0.98) | 0.86 (0.70-1.05) | 0.87 (0.69-1.09) | .19 |
| Leisure-time MVPA | ||||||||||
| Excluding first 3 y | ||||||||||
| No. of cases | 695 | 351 | 243 | 334 | NA | 1085 | 430 | 393 | 413 | NA |
| Multivariable HR (95% CI)a | 1 [Reference] | 0.93 (0.82-1.07) | 0.67 (0.58-0.78) | 0.63 (0.55-0.72) | <.001 | 1 [Reference] | 0.97 (0.86-1.09) | 0.85 (0.75-0.95) | 0.73 (0.65-0.82) | <.001 |
| Excluding first 6 y | ||||||||||
| Incident dementia, No. | 470 | 239 | 167 | 228 | NA | 754 | 303 | 297 | 314 | NA |
| Multivariable HR (95% CI)a | 1 [Reference] | 0.92 (0.78-1.08) | 0.66 (0.55-0.79) | 0.61 (0.51-0.71) | <.001 | 1 [Reference] | 0.97 (0.85-1.12) | 0.90 (0.79-1.04) | 0.78 (0.68-0.90) | <.001 |
| Excluding first 9 y | ||||||||||
| Incident dementia, No. | 184 | 98 | 77 | 109 | NA | 290 | 132 | 145 | 149 | NA |
| Multivariable HR (95% CI)a | 1 [Reference] | 0.90 (0.69-1.16) | 0.73 (0.56-0.97) | 0.72 (0.56-0.92) | .004 | 1 [Reference] | 1.03 (0.83-1.28) | 1.08 (0.88-1.32) | 0.91 (0.75-1.12) | .59 |
Abbreviations: HR, hazard ratios; MVPA; moderate to vigorous physical activity; NA, not applicable; Q, quartile.
Adjusted for age, area, smoking status (never, former, 1-19 cigarettes/d, or ≥20 cigarettes/d), alcohol intake status (none or occasional drinkers, 1 to <150 g/week, 150 to <300 g/week, ≥300 g/week), body mass index (calculated as weight in kilograms divided by height in meters squared; <18.5, 18.5 to 24.9, 25 to 29, or ≥30), past history of diabetes (yes or no), medication for hypertension (yes or no), and occupation (primary industry, secondary or tertiary industry, unemployed or household duties).
Risks of disabling dementia in the highest vs lowest group of physical activity after excluding participants with disabling dementia diagnosed within the first 10 years of the dementia ascertainment period are shown in the Figure. The statistically significant associations between daily total physical activity and disabling dementia risk disappeared after excluding participants with disabling dementia diagnosed within the first 7 years in men (aHR, 0.93 [95%CI, 0.77-1.12]) and within the first 8 years in women (aHR, 0.86 [95%CI, 0.71-1.04]). In contrast, the inverse association between leisure-time MVPA and disabling dementia risk in men remained significant after excluding participants diagnosed within 10 years.
Figure. Risk of Disabling Dementia Risk in the Highest vs Lowest Groups of Physical Activity After Excluding Participants Diagnosed Within the First 10 Years of the Dementia Ascertainment Period.
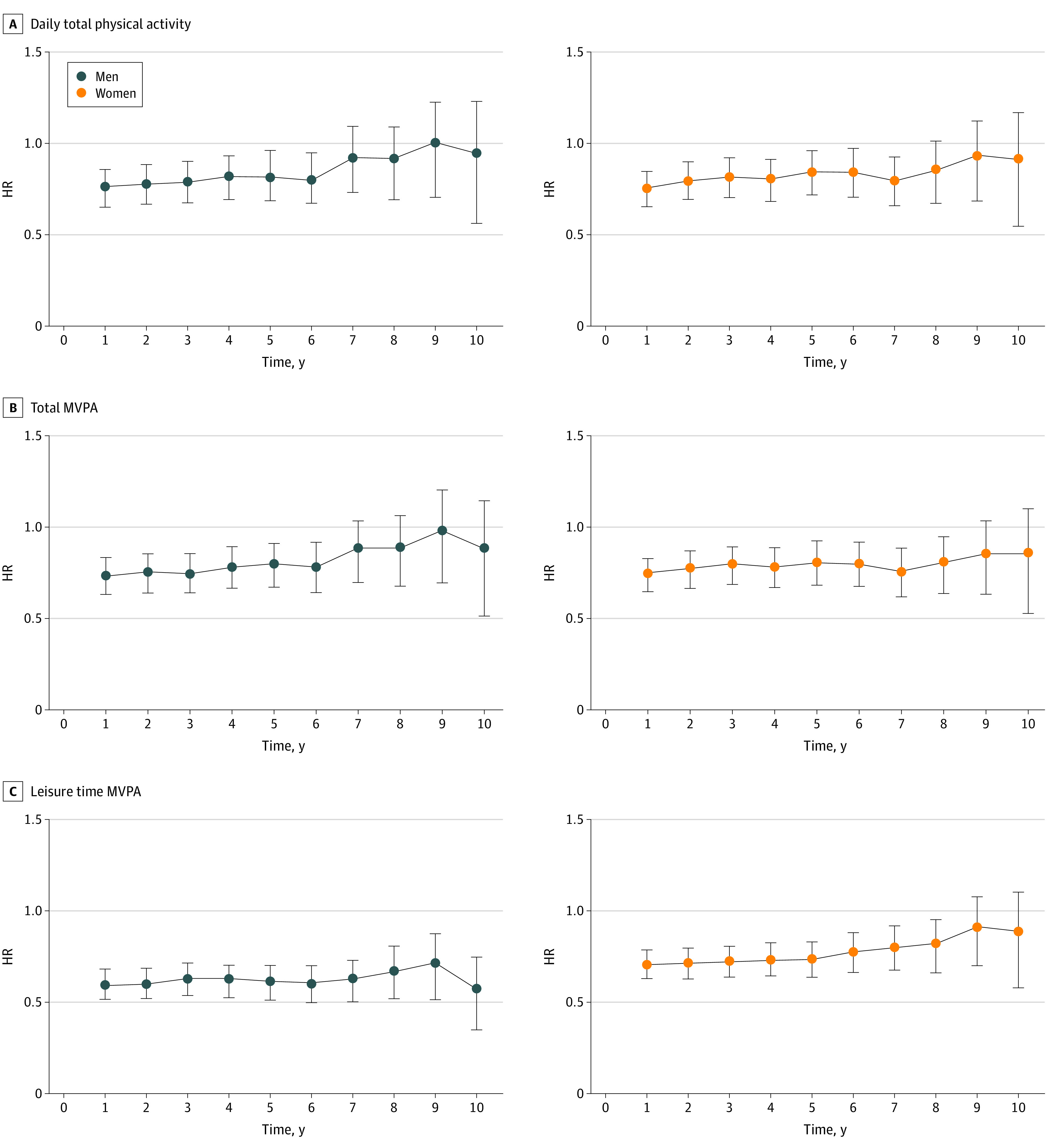
HR indicates hazard ratio; MVPA, moderate to vigorous physical activity. Time was calculated as number of years from starting point during which participants disabling dementia were excluded from analysis. HRs were adjusted for age, area, smoking status, alcohol intake status, body mass index, past history of diabetes, medication for hypertension, and occupation.
We found no substantial difference in the associations of physical activity with disabling dementia in participants aged 65 years and older (eTable 1 in the Supplement). Regarding the analysis using death as a competing risk, no substantial differences were observed (eTable 2 in the Supplement). We found an increased risk of disabling dementia among participants who were in the lower tertiles of daily total physical activity in the 5-year questionnaire and the lower, middle, and upper tertiles in the 10-year questionnaire, as well as in those who were in the upper and middle tertiles of daily total physical activity in the 5-year questionnaire and the lower tertiles in the 10-year questionnaire (eTable 3 in the Supplement). Additionally, there were no substantial differences in the associations after addition of education level to the multivariable model in cohort I (eTable 4 in the Supplement).
Discussion
In this prospective cohort study, we found that a higher level of physical activity was significantly associated with decreased risk of disabling dementia in men and women. However, after excluding participants diagnosed within 7 years from the starting point in men and within 8 years in women, the inverse associations of daily total physical activity and total MVPA with risk of disabling dementia incidence disappeared. These results suggest the potential presence of reverse causation bias. To our knowledge, this is the first prospective cohort study to suggest a potential for reverse causation bias between daily total physical activity and total MVPA and dementia. In contrast, a higher level of leisure-time MVPA was associated with reduced risk of disabling dementia in men.
Although 2 cohort studies8,9 have reported an inverse association between daily total physical activity and dementia risk, neither examined reverse causation bias. In the Honolulu-Asia Aging Study, Taaffe et al8 showed that a low level of daily total physical activity was associated with high risk of dementia in 173 patients with incident dementia with a mean follow-up of 6.1 years. In the Framingham study, Tan et al9 reported that a low level of daily total physical activity was associated with a higher risk of dementia in 236 patients with a longer follow-up of up to 22 years, but the study did not stratify results by sex owing to the low number of patients with incident dementia. To our knowledge, therefore, our study is the first large prospective study involving middle-aged and older participants with a longer dementia ascertainment period (9.5 years) and large number of incident diagnoses to observe potential reverse causation bias in the association between daily total physical activity and dementia risk. Given that 2 randomized clinical trials30,31 and a systematic review32 found that physical activity had little associations with improving cognitive function or the incidence of dementia, our results of no association between total physical activity and risk of disabling dementia after controlling for potential reverse causation bias may be plausible. One reason for potential reverse causation bias in this association was that daily total physical activity had already declined at the starting point in persons who would develop disabling dementia in the near future. In the preclinical phase of dementia, apathy is commonly observed as a neuropsychiatric feature,33,34 and persons with cognitive deficit35 and mild cognitive impairment (MCI)36 show features of behavioral inhibition.37 In fact, it has been reported that persons with MCI have a significantly lower physical activity level and a lower exercise capacity, which are associated with behavioral inhibition.37 Our results therefore suggest the presence of reverse causation bias in the association between daily total physical activity and risk of disabling dementia, as previously reported.12 Additionally, our results showing that a decline in daily total physical activity in the 5-year to 10-year questionnaire was positively associated with the risk of disabling dementia could also be considered as suggesting reverse causation bias. A further reason is that participants with a low level of physical activity may already have had other conditions that were likely to convert to or promote dementia or were associated with it.
Regarding the intensity of physical activity, a cohort study by Iso-Markku et al38 reported that the association of physical activity on cognitive function did not differ by the intensity of activity. This is consistent with our finding that the presence or disappearance of an association by exclusion of participants did not differ by the intensity of activity in both daily total physical activity and total MVPA.
In contrast, our results show that the linear trend of an inverse association between leisure-time MVPA and disabling dementia risk remained even after excluding participants diagnosed within the first 10 years in men. Therefore, a high level of physical activity in leisure-time may be associated with a decreased risk of disabling dementia in men. The findings of the Whitehall II cohort study12 suggested a reverse causation bias, namely that leisure-time physical activity was not associated with the risk of dementia in 329 participants and that preclinical decline in physical activity occurred in the 9 years before the diagnosis of dementia, while a meta-analysis by Kivimäki15 of 2044 participants with incident dementia also concluded that physical inactivity was not associated with all-cause dementia in men. An animal study by Barha et al39 reported that voluntary physical activity, such as in leisure-time, was associated with improving some cognitive functions, including memory, to a greater extent in males. Leisure-time MVPA measured in this study was derived from activities involving cognitive activity, such as golf and tennis.28 Leisure activities that include cognitive activity have a protective association against cognitive decline and dementia,40 and a randomized clinical trial reported that combined cognitive and exercise training could improve the cognitive functions of community-dwelling older adults.41 In addition, the social activity42,43 that accompanies leisure-time physical activities, such as participation in golf competitions and enrollment in tennis circles, also has a protective association against cognitive decline and dementia. The men in this study might therefore have been subject to different protective associations against disabling dementia through habitual leisure-time MVPA involving cognitive activity and social activity compared with men with less leisure-time MVPA. In contrast, this association of leisure-time MVPA may have been attenuated in women participants because women already engage in many cognitive activities through daily housework activities,44 and are likely to have a larger social network than men.45 Accordingly, our findings suggesting no reverse causation bias in the association between leisure-time MVPA and disabling dementia in men may be plausible.
The strengths of this study include its prospective design in participants aged 50 to 79 years at the survey point of exposure, long follow-up, large sample size, systematic registration of disabling dementia and comprehensive assessment of lifestyle factors, such as smoking status and alcohol consumption. These strengths allowed us to examine possible reverse causation bias in the association between daily total physical activity and disabling dementia, which few previous studies have investigated.
Limitations
This study has several limitations. First, we could not assess the association between physical activity and specific types of dementia, such as Alzheimer disease and vascular dementia, because data from the LTCI system did not include type of dementia. In addition, several previous studies reported that type of dementia, such as Alzheimer disease, was not associated with mediating the association between physical activity and dementia risk.9,11,18 Accordingly, the risk of disabling dementia in our study participants was unlikely to have been associated with dementia type. Second, we could not completely eliminate misclassification in the diagnosis of disabling dementia conducted by attending physicians, albeit that a previous validation showed that such diagnosis had high specificity for the diagnosis of disabling dementia and was less likely to be affected by false-positive diagnoses.23 Third, we did not obtain information on education from all participants. We conducted a subanalysis of participants with a reported education level, but the results did not substantially differ when education level was included in the multivariable model. Fourth, the approach used to evaluate for potential reverse causation bias may have led to selection bias. Fifth, because we used information on physical activity at a single time point only (10-year follow-up survey), misclassification of exposure due to changes in physical activity during the dementia ascertainment period might have occurred. If present, however, misclassification of exposure would likely influence the true relative risk toward null.
Conclusions
In this large prospective cohort study, we observed a potential reverse causation bias in the association of daily total physical activity and total MVPA with risk of disabling dementia in men and women. In contrast, however, leisure-time MVPA may have a protective association against disabling dementia in men.
eFigure. Flowchart of Study Participants
eTable 1. Risk of Disabling Dementia Risk According to Physical Activity in Men and Women Aged 65 Years and Older
eTable 2. Risk of Disabling Dementia Risk According to Physical Activity Using the Fine and Gray Subdistribution Hazards Model for Competing Risk of Death
eTable 3. Risk of Disabling Dementia Risk According to the Change of Daily Total Physical Activity in Men and Women
eTable 4. Risk of Disabling Dementia Risk According to Physical Activity in a Model Adding Education Level in Cohort I
eAppendix. Members of the Japan Public Health Center-based Prospective Study (JPHC) Study Group
References
- 1.World Health Organization . Dementia key facts. Updated September 2019. Accessed March 24, 2020. https://www.who.int/news-room/fact-sheets/detail/dementia
- 2.Akbaraly TN, Portet F, Fustinoni S, et al. Leisure activities and the risk of dementia in the elderly: results from the Three-City Study. Neurology. 2009;73(11):854-861. doi: 10.1212/WNL.0b013e3181b7849b [DOI] [PubMed] [Google Scholar]
- 3.Buchman AS, Boyle PA, Yu L, Shah RC, Wilson RS, Bennett DA. Total daily physical activity and the risk of AD and cognitive decline in older adults. Neurology. 2012;78(17):1323-1329. doi: 10.1212/WNL.0b013e3182535d35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Etgen T, Sander D, Huntgeburth U, Poppert H, Förstl H, Bickel H. Physical activity and incident cognitive impairment in elderly persons: the INVADE study. Arch Intern Med. 2010;170(2):186-193. doi: 10.1001/archinternmed.2009.498 [DOI] [PubMed] [Google Scholar]
- 5.Laurin D, Verreault R, Lindsay J, MacPherson K, Rockwood K. Physical activity and risk of cognitive impairment and dementia in elderly persons. Arch Neurol. 2001;58(3):498-504. doi: 10.1001/archneur.58.3.498 [DOI] [PubMed] [Google Scholar]
- 6.Lindsay J, Laurin D, Verreault R, et al. Risk factors for Alzheimer’s disease: a prospective analysis from the Canadian Study of Health and Aging. Am J Epidemiol. 2002;156(5):445-453. doi: 10.1093/aje/kwf074 [DOI] [PubMed] [Google Scholar]
- 7.Llamas-Velasco S, Contador I, Villarejo-Galende A, Lora-Pablos D, Bermejo-Pareja F. Physical activity as protective factor against dementia: a prospective population-based study (NEDICES). J Int Neuropsychol Soc. 2015;21(10):861-867. doi: 10.1017/S1355617715000831 [DOI] [PubMed] [Google Scholar]
- 8.Taaffe DR, Irie F, Masaki KH, et al. Physical activity, physical function, and incident dementia in elderly men: the Honolulu-Asia Aging Study. J Gerontol A Biol Sci Med Sci. 2008;63(5):529-535. doi: 10.1093/gerona/63.5.529 [DOI] [PubMed] [Google Scholar]
- 9.Tan ZS, Spartano NL, Beiser AS, et al. Physical activity, brain volume, and dementia risk: the Framingham Study. J Gerontol A Biol Sci Med Sci. 2017;72(6):789-795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Verdelho A, Madureira S, Ferro JM, et al. ; LADIS Study . Physical activity prevents progression for cognitive impairment and vascular dementia: results from the LADIS (Leukoaraiosis and Disability) study. Stroke. 2012;43(12):3331-3335. doi: 10.1161/STROKEAHA.112.661793 [DOI] [PubMed] [Google Scholar]
- 11.de Bruijn RF, Schrijvers EM, de Groot KA, et al. The association between physical activity and dementia in an elderly population: the Rotterdam Study. Eur J Epidemiol. 2013;28(3):277-283. doi: 10.1007/s10654-013-9773-3 [DOI] [PubMed] [Google Scholar]
- 12.Sabia S, Dugravot A, Dartigues JF, et al. Physical activity, cognitive decline, and risk of dementia: 28 year follow-up of Whitehall II cohort study. BMJ. 2017;357:j2709. doi: 10.1136/bmj.j2709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gross AL, Lu H, Meoni L, Gallo JJ, Schrack JA, Sharrett AR. Physical activity in midlife is not associated with cognitive health in later life among cognitively normal older adults. J Alzheimers Dis. 2017;59(4):1349-1358. doi: 10.3233/JAD-170290 [DOI] [PubMed] [Google Scholar]
- 14.Lash T, VanderWeele TJ, Haneuse S, Rothman KJ. Modern Epidemiology. 4th ed. Wolters Kluwer; 2020. [Google Scholar]
- 15.Kivimäki M, Singh-Manoux A, Pentti J, et al. ; IPD-Work consortium . Physical inactivity, cardiometabolic disease, and risk of dementia: an individual-participant meta-analysis. BMJ. 2019;365:l1495. doi: 10.1136/bmj.l1495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Matthews CE, Jurj AL, Shu XO, et al. Influence of exercise, walking, cycling, and overall nonexercise physical activity on mortality in Chinese women. Am J Epidemiol. 2007;165(12):1343-1350. doi: 10.1093/aje/kwm088 [DOI] [PubMed] [Google Scholar]
- 17.Inoue M, Iso H, Yamamoto S, et al. ; Japan Public Health Center-Based Prospective Study Group . Daily total physical activity level and premature death in men and women: results from a large-scale population-based cohort study in Japan (JPHC study). Ann Epidemiol. 2008;18(7):522-530. doi: 10.1016/j.annepidem.2008.03.008 [DOI] [PubMed] [Google Scholar]
- 18.Andel R, Crowe M, Pedersen NL, Fratiglioni L, Johansson B, Gatz M. Physical exercise at midlife and risk of dementia three decades later: a population-based study of Swedish twins. J Gerontol A Biol Sci Med Sci. 2008;63(1):62-66. doi: 10.1093/gerona/63.1.62 [DOI] [PubMed] [Google Scholar]
- 19.Iso-Markku P, Waller K, Kujala UM, Kaprio J. Physical activity and dementia: long-term follow-up study of adult twins. Ann Med. 2015;47(2):81-87. doi: 10.3109/07853890.2014.994675 [DOI] [PubMed] [Google Scholar]
- 20.Iso-Markku P, Waller K, Vuoksimaa E, et al. Midlife physical activity and cognition later in life: a prospective twin study. J Alzheimers Dis. 2016;54(4):1303-1317. doi: 10.3233/JAD-160377 [DOI] [PubMed] [Google Scholar]
- 21.Ravaglia G, Forti P, Lucicesare A, et al. Physical activity and dementia risk in the elderly: findings from a prospective Italian study. Neurology. 2008;70(19 Pt 2):1786-1794. doi: 10.1212/01.wnl.0000296276.50595.86 [DOI] [PubMed] [Google Scholar]
- 22.Tsugane S, Sawada N. The JPHC study: design and some findings on the typical Japanese diet. Jpn J Clin Oncol. 2014;44(9):777-782. doi: 10.1093/jjco/hyu096 [DOI] [PubMed] [Google Scholar]
- 23.Ikeda A, Yamagishi K, Tanigawa T, et al. Cigarette smoking and risk of disabling dementia in a Japanese rural community: a nested case-control study. Cerebrovasc Dis. 2008;25(4):324-331. doi: 10.1159/000118377 [DOI] [PubMed] [Google Scholar]
- 24.Yamagishi K, Ikeda A, Chei CL, et al. ; CIRCS Investigators . Serum α-linolenic and other ω-3 fatty acids, and risk of disabling dementia: community-based nested case-control study. Clin Nutr. 2017;36(3):793-797. doi: 10.1016/j.clnu.2016.05.011 [DOI] [PubMed] [Google Scholar]
- 25.Tamiya N, Noguchi H, Nishi A, et al. Population ageing and wellbeing: lessons from Japan’s long-term care insurance policy. Lancet. 2011;378(9797):1183-1192. doi: 10.1016/S0140-6736(11)61176-8 [DOI] [PubMed] [Google Scholar]
- 26.Hisano S. The relationship between Revised Hasegawa Dementia Scale (HDS-R), Mini-Mental State Examination (MMSE) and Bed-fast Scale, Dementia Scale. Article in Japanese. Jpn J Geriatric Psychiatry. 2009;20:883-891. [Google Scholar]
- 27.Ainsworth BE, Haskell WL, Whitt MC, et al. Compendium of physical activities: an update of activity codes and MET intensities. Med Sci Sports Exerc. 2000;32(9)(suppl):S498-S504. doi: 10.1097/00005768-200009001-00009 [DOI] [PubMed] [Google Scholar]
- 28.Kikuchi H, Inoue S, Odagiri Y, et al. Intensity-specific validity and reliability of the Japan Public Health Center-based prospective study—physical activity questionnaire. Prev Med Rep. 2020;20:101169. doi: 10.1016/j.pmedr.2020.101169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94(446):496-509. doi: 10.1080/01621459.1999.10474144 [DOI] [Google Scholar]
- 30.Sink KM, Espeland MA, Castro CM, et al. ; LIFE Study Investigators . Effect of a 24-month physical activity intervention vs health education on cognitive outcomes in sedentary older adults: the LIFE randomized trial. JAMA. 2015;314(8):781-790. doi: 10.1001/jama.2015.9617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Andrieu S, Guyonnet S, Coley N, et al. ; MAPT Study Group . Effect of long-term omega 3 polyunsaturated fatty acid supplementation with or without multidomain intervention on cognitive function in elderly adults with memory complaints (MAPT): a randomised, placebo-controlled trial. Lancet Neurol. 2017;16(5):377-389. doi: 10.1016/S1474-4422(17)30040-6 [DOI] [PubMed] [Google Scholar]
- 32.Brasure M, Desai P, Davila H, et al. Physical activity interventions in preventing cognitive decline and alzheimer-type dementia: a systematic review. Ann Intern Med. 2018;168(1):30-38. doi: 10.7326/M17-1528 [DOI] [PubMed] [Google Scholar]
- 33.Robert P, Onyike CU, Leentjens AF, et al. Proposed diagnostic criteria for apathy in Alzheimer’s disease and other neuropsychiatric disorders. Eur Psychiatry. 2009;24(2):98-104. doi: 10.1016/j.eurpsy.2008.09.001 [DOI] [PubMed] [Google Scholar]
- 34.Ma L. Depression, anxiety, and apathy in mild cognitive impairment: current perspectives. Front Aging Neurosci. 2020;12:9. doi: 10.3389/fnagi.2020.00009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Palmer K, Di Iulio F, Varsi AE, et al. Neuropsychiatric predictors of progression from amnestic-mild cognitive impairment to Alzheimer’s disease: the role of depression and apathy. J Alzheimers Dis. 2010;20(1):175-183. doi: 10.3233/JAD-2010-1352 [DOI] [PubMed] [Google Scholar]
- 36.Richard E, Schmand B, Eikelenboom P, et al. ; Alzheimer’s Disease Neuroimaging Initiative . Symptoms of apathy are associated with progression from mild cognitive impairment to Alzheimer’s disease in non-depressed subjects. Dement Geriatr Cogn Disord. 2012;33(2-3):204-209. doi: 10.1159/000338239 [DOI] [PubMed] [Google Scholar]
- 37.Stuckenschneider T, Askew CD, Rüdiger S, et al. ; NeuroExercise Study Group . Cardiorespiratory fitness and cognitive function are positively related among participants with mild and subjective cognitive impairment. J Alzheimers Dis. 2018;62(4):1865-1875. doi: 10.3233/JAD-170996 [DOI] [PubMed] [Google Scholar]
- 38.Iso-Markku P, Waller K, Vuoksimaa E, et al. Objectively measured physical activity profile and cognition in Finnish elderly twins. Alzheimers Dement (N Y). 2018;4:263-271. doi: 10.1016/j.trci.2018.06.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Barha CK, Falck RS, Davis JC, Nagamatsu LS, Liu-Ambrose T. Sex differences in aerobic exercise efficacy to improve cognition: a systematic review and meta-analysis of studies in older rodents. Front Neuroendocrinol. 2017;46:86-105. doi: 10.1016/j.yfrne.2017.06.001 [DOI] [PubMed] [Google Scholar]
- 40.Verghese J, Lipton RB, Katz MJ, et al. Leisure activities and the risk of dementia in the elderly. N Engl J Med. 2003;348(25):2508-2516. doi: 10.1056/NEJMoa022252 [DOI] [PubMed] [Google Scholar]
- 41.Shimada H, Makizako H, Doi T, et al. Effects of combined physical and cognitive exercises on cognition and mobility in patients with mild cognitive impairment: a randomized clinical trial. J Am Med Dir Assoc. 2018;19(7):584-591. doi: 10.1016/j.jamda.2017.09.019 [DOI] [PubMed] [Google Scholar]
- 42.Shimada H, Makizako H, Lee S, Doi T, Lee S. Lifestyle activities and the risk of dementia in older Japanese adults. Geriatr Gerontol Int. 2018;18(10):1491-1496. doi: 10.1111/ggi.13504 [DOI] [PubMed] [Google Scholar]
- 43.Holtzman RE, Rebok GW, Saczynski JS, Kouzis AC, Wilcox Doyle K, Eaton WW. Social network characteristics and cognition in middle-aged and older adults. J Gerontol B Psychol Sci Soc Sci. 2004;59(6):278-284. doi: 10.1093/geronb/59.6.P278 [DOI] [PubMed] [Google Scholar]
- 44.Tsuchiya K, Mitsui S, Fukuyama R, et al. An acute bout of housework activities has beneficial effects on executive function. Neuropsychiatr Dis Treat. 2017;14:61-72. doi: 10.2147/NDT.S153813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nagayoshi M, Higashi M, Takamura N, et al. Social networks, leisure activities and maximum tongue pressure: cross-sectional associations in the Nagasaki Islands Study. BMJ Open. 2017;7(12):e014878. doi: 10.1136/bmjopen-2016-014878 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eFigure. Flowchart of Study Participants
eTable 1. Risk of Disabling Dementia Risk According to Physical Activity in Men and Women Aged 65 Years and Older
eTable 2. Risk of Disabling Dementia Risk According to Physical Activity Using the Fine and Gray Subdistribution Hazards Model for Competing Risk of Death
eTable 3. Risk of Disabling Dementia Risk According to the Change of Daily Total Physical Activity in Men and Women
eTable 4. Risk of Disabling Dementia Risk According to Physical Activity in a Model Adding Education Level in Cohort I
eAppendix. Members of the Japan Public Health Center-based Prospective Study (JPHC) Study Group


