Abstract
It was reported previously that circulation levels of kallistatin, an endogenous Wnt signaling inhibitor, are increased in patients with diabetes. The current study was conducted to determine the role of kallistatin in delayed wound healing in diabetic corneas. Immunostaining and Western blot analysis showed kallistatin levels were upregulated in corneas from humans and rodents with diabetes. In murine corneal wound healing models, the canonical Wnt signaling was activated in nondiabetic corneas and suppressed in diabetic corneas, correlating with delayed wound healing. Transgenic expression of kallistatin suppressed the activation of Wnt signaling in the cornea and delayed wound healing. Local inhibition of Wnt signaling in the cornea by kallistatin, an LRP6-blocking antibody, or the soluble VLDL receptor ectodomain (an endogenous Wnt signaling inhibitor) delayed wound healing. In contrast, ablation of the VLDL receptor resulted in overactivation of Wnt/β-catenin signaling and accelerated corneal wound healing. Activation of Wnt signaling in the cornea accelerated wound healing. Activation of Wnt signaling promoted human corneal epithelial cell migration and proliferation, which was attenuated by kallistatin. Our findings suggested that diabetes-induced overexpression of kallistatin contributes to delayed corneal wound healing by inhibiting the canonical Wnt signaling. Thus, kallistatin and Wnt/β-catenin signaling in the cornea could be potential therapeutic targets for diabetic corneal complications.
Introduction
Diabetes mellitus (DM) is a metabolic disorder of complex etiology characterized by chronic hyperglycemia with disturbed metabolism of carbohydrate, fat, and protein resulting from defects in insulin secretion, insulin action, or both (1). DM is associated with long-term damage, dysfunction, and failure of various organs, especially the eyes, kidneys, nerves, heart, and blood vessels (1). A smooth and continuous surface of the cornea is essential for normal vision. However, compared with other diabetic complications, diabetic keratopathy (DK), one of the blinding diabetic ocular complications, receives less attention. Diabetes results in reduced corneal epithelial cell density and subbasal nerve plexus alterations (2–5). The effect and underlying mechanism of altered metabolism in patients with diabetes on ocular surface wound healing remains unclear. Previous studies have reported that elevated serum levels of tumor necrosis factor-α (TNF-α), interleukin (IL)-6 and IL-8 in patients with diabetes could contribute to the diabetic corneal complication (5,6). On the other hand, hyperglycemia changed the expression of cytokines in the cornea, such as reduced levels of insulin growth factor-1 (IGF-1), transforming growth factor-β3 (TGFβ3), epidermal growth factor receptor (EGFR), and ciliary neurotrophic factor (CNTF), which may contribute to the delayed cornea wound healing in the diabetic condition (7,8). However, the pathogenic pathways mediating DK remain uncertain.
The Wnt/β-catenin signaling pathway is known to mediate cell proliferation, differentiation, and migration (9). Wnt signaling is a tightly regulated pathway comprising Wnt ligands, frizzled (Fzd) receptors, and coreceptors, including LDL receptor-related protein 5/6 (LRP5/6), an intracellular signaling molecule cascade, and the effector β-catenin (10). Nonphosphorylated β-catenin plays an essential role in the canonical Wnt pathway (or Wnt/β-catenin pathway). Upon binding of Wnt ligands to the Wnt receptor complex, β-catenin becomes unphosphorylated, and the unphosphorylated β-catenin is accumulated in the cytosol and then translocated into the nucleus to activate transcription of target genes (11).
Wnt signaling dysregulation in diabetic conditions is tissue specific. For example, we found that Wnt signaling is overactivated in the retina from patients with diabetes or diabetic animal models (11–13). Furthermore, aberrant activation of canonical Wnt signaling leads to retinal inflammation and neovascularization (11–15). However, diabetes suppressed Wnt signaling in the skin, contributing to delayed skin wound healing (16). Recently, a study suggests that Wnt signaling may mediate the beneficial effect of insulin on corneal wound healing (17). However, the mechanism for the dysregulation of Wnt signaling in the cornea in diabetes is unclear.
Many endogenous proteins have been identified as Wnt inhibitors, such as DKK1, VLDL receptor (VLDLR), kallistatin, and pigment epithelium-derived factor (PEDF) (11). We also generated a monoclonal antibody specific for the LRP6 E1E2 domains (Mab2F1), which blocks the Wnt/β-catenin signaling at the receptor level (13). Our previous study showed that VLDLR inhibits Wnt signaling by dimerizing with LRP6 through its extracellular domain (VLN) and that VLDLR ablation results in Wnt signaling overactivation (18–20). However, the impacts of kallistatin, Mab2F1, and VLDLR on the canonical Wnt pathway activity in the diabetic cornea and their roles in corneal wound healing remain elusive.
Kallistatin is a serine proteinase inhibitor (21,22). We reported previously that kallistatin protein functions as an endogenous antagonist of LRP6 and inhibitor of Wnt signaling in the retina (23). Decreased kallistatin levels were found in the vitreous of patients with diabetes and in the retina of diabetic animal models (24). However, circulating kallistatin levels are elevated in patients with type 1 and type 2 diabetes with complications (25). The elevated kallistatin level is responsible, at least in part, for the delayed skin wound healing in diabetes through inhibition of Wnt signaling (16). However, the impact of kallistatin on diabetic corneas has not been well studied.
In the current study, we first quantified the expression level of kallistatin in the corneas of humans with diabetes. We hypothesized that elevated kallistatin levels suppressed Wnt/β-catenin signaling in diabetic corneas, leading to delayed corneal wound healing. To investigate the pathogenic role of Wnt signaling in the context of corneal wound healing, we measured Wnt signaling activities in the corneas of mouse models of type 1 diabetes and the corneal wound healing rate. To further investigate the role of kallistatin and Wnt signaling in corneal wound healing, we modulated Wnt/β-catenin activities using genetic and pharmacological approaches and evaluated their impacts on the corneal wound healing rate. We also evaluated the roles of kallistatin and Wnt signaling in the proliferation and migration of human corneal epithelial cells (HCEC) in vitro.
Research Design and Methods
Ethical Approval and Informed Consent
The study adhered to the tenets of the Declaration of Helsinki and was performed with the approval from the University of Oklahoma Health Sciences Center Institutional Review Board (protocol No. 3450). Donor eyes from patients with diabetes and age-matched patients without diabetes were obtained from Lions Gift of Sight eye bank (Saint Paul, MN). All methods were performed in accordance with federal and institutional guidelines, and all human samples were deidentified prior to analysis. Clinical data for the donors without diabetes (NDM) and those with diabetes (DM) are as follows. NDM: three women and three men, all Caucasian, and the average age was 77.8 years. Cause of death included chronic obstructive pulmonary disease, cardiac arrest, sepsis, Alzheimer disease, intracranial bleeding, and intracerebral hemorrhage. DM: one woman and five men, whose average age was 75 years, all Caucasian. All of them had diabetes for >10 years. Causes of death included pancreatic cancer, sepsis, dementia, gastroesophageal junction adenocarcinoma, and pneumonia.
Animals
Male Brown Norway (BN) rats (8–10 weeks old; Charles River, Wilmington, MA), kallistatin transgenic (KS-Tg) mice in the C57/BL/6J background overexpressing human kallistatin (12–20 weeks old, generated as described previously [23]), wild-type (WT) C57BL/6J mice, Akita (Ins2akita) mice (16–20 weeks old), db/db (BKS.Cg- Leprdb/J) mice (16–20 weeks old), Vldlr−/− mice (12–20 weeks old), and Axin2-lacZ reporter mice (12–20 weeks old; Jackson Laboratories, Bar Harbor, ME) were used. All experiments were performed following the guidelines of the Association for Research in Vision and Ophthalmology (ARVO) Statement for the Use of Animals in Ophthalmic and Vision Research and approved by the University of Oklahoma Health Sciences Center Institutional Animal Care and Use Committee. In all procedures, animals were anesthetized with i.p. injection of 50 mg/kg ketamine hydrochloride mixed with 5 mg/kg xylazine (Vedco, St. Joseph, MO).
Induction of Diabetes by Streptozotocin Injection
Diabetes was induced in mice and rats at 8 weeks of age as described previously (26,27). Briefly, mice received five daily intraperitoneal injections of freshly prepared streptozotocin (STZ; 55 mg/kg in 10 mmol/L of citrate buffer, pH 4.5; Sigma-Aldrich), while BN rats received a single intraperitoneal injection of STZ following overnight fasting. Age-matched mice and rats that received citrate buffer injection alone were used for nondiabetic controls. Animals with blood glucose levels >350 mg/dL were considered diabetic.
Corneal Epithelial Debridement Wound
The corneal wound was induced following a documented protocol (28). After anesthesia, the central corneal epithelial layer was removed with an AlgerBrush II Corneal Rust Ring Remover (Alger, Largo Vista, TX) with a diameter of 2-mm in mice and 4-mm in rats. The abraded region was labeled with 0.1% sodium fluorescein and photographed daily with Micron IV (Phoenix Technology Group, Pleasanton, CA). The wound area was quantified with ImageJ software (National Institutes of Health, Bethesda, MD).
Immunohistochemistry of Human Eyes
Human donor eyes dissected within 12 h postmortem were immediately preserved in Davidson’s fixation solution for 24 h and then transferred to 10% buffered formalin for storage and paraffin section. Following the antigen retrieval with EDTA buffer (1 mmol/L EDTA, 0.05% Tween 20, pH 8.0) in steam bath and blocking, the sections were incubated with an anti-human kallistatin antibody (R&D, no. AF1669) overnight. After washes with PBS, the slides were incubated with A488-labeled donkey anti-goat IgG (Jackson ImmunoResearch, no. 705-545-003). The slides were mounted with Vectashield mounting buffer containing DAPI (Vector Laboratories, no. H-1200) and then photographed under a fluorescence microscope (Observer Z1, Pleasanton, CA).
Immunohistochemistry of Rat and Mouse Corneas
Anti-rat kallistatin antibody expressed in mouse hybridoma cells was generated through a contracted service at Proteintech (Rosemont, IL). The rat and mouse eyeballs were fixed in Davidson’s fixation solution for 48 h for the paraffin section. Following the antigen retrieval with sodium citrate buffer (10 mmol/L sodium citrate, 0.05% Tween 20, pH 6.0) in steam bath and blocking, the sections were incubated with anti-rat kallistatin antibody or anti-nonphosphorylated β-catenin antibody (Cell Signaling, no. 8814) overnight. After being washed with PBS, the slides were incubated with A488-labeled goat anti-mouse IgG (Jackson ImmunoResearch, no. 115-545-003) or A488-labeled goat anti-rabbit IgG (Jackson ImmunoResearch, no. 111-545-003). The slides were mounted with Vectashield mounting buffer containing DAPI (Vector Laboratories, no. H-1200) and photographed under a Zeiss Microscope (Observer Z1, Pleasanton, CA).
X-Gal Staining
The X-gal staining was performed following the instruction of the manufacturer. Briefly, the corneas were fixed in 4% paraformaldehyde in 1× PBS, pH 7.4, for 30 min at 4°C and then stained with the X-gal staining solution (5 mmol/L potassium ferricyanide, 5 mmol/L potassium ferrocyanide, 2 mmol/L MgCl2, 0.02% NP-40, 0.01% sodium deoxycholate, 0.4 mg/mL X-gal in PBS) overnight (29,30). Corneas were flat-mounted with mounting media (Immu-Mount; Thermo Fisher Scientific, Kalamazoo, MI), and photographed under an Olympus Microscope (BX43F; Olympus, Tokyo, Japan).
HCEC Viability Assay
HCEC (ATCC, no. PCS700010) were cultured in Corneal Epithelial Cell Basal Medium (ATCC, no. PCS700030) in a 24-well plate. After incubation with 20% L cell-conditioned medium (LCM) or Wnt3a-conditioned medium (WCM) and different concentrations of human kallistatin protein, the cell suspension was stained with trypan blue (Thermo Fisher). Viable cells were counted using Cellometer Auto T4 Bright Field Cell Counter (Nexcelom, Lawrence, MA).
HCEC Migration Assay
HCEC were treated with WCM and various concentrations of kallistatin after 100% confluence. An in vitro “wound” was created by a straight-line scratch across the monolayer using a 200 µL pipette tip, as described previously (31). Six images of each scratch were taken by Cytation 1 Cell Imaging Multi-Mode Reader (BioTek, Winooski, VT) at different times. The acellular area was measured using the ImageJ program.
Western Blot Analysis
Western blot analysis was performed as described previously (32). Anti-human kallistatin monoclonal antibodies were gifts from Drs. L. Chao and J. Chao (21,22). GAPDH (Abcam, no. ab9485), rabbit anti-kallistatin antibody (Abcam, no. ab187656), anti-nonphosphorylated β-catenin antibody (Cell Signaling. no. 8814), anti-EGFR antibody (Abcam, no. ab52894), and mouse anti–β-actin antibody (Sigma-Aldrich, no. A5441) were used as primary antibodies at 1:1,000 dilution. Horseradish peroxidase-labeled secondary antibodies (12,000 dilution; Santa Cruz Biotechnology) were used for immunoblotting. Densitometry was performed using ImageJ software and normalized by β-actin levels.
Statistical Analysis
All statistical analyses were performed using GraphPad Prism 9 (GraphPad Software, San Diego, CA). Data are expressed as mean ± SEM. The paired Student t test was applied to compare differences between two groups. ANOVA was used to compare three or more groups.
Data and Resource Availability
All data are contained within this article. Reagents described in this manuscript are available upon request.
Results
Upregulated Expression of Kallistatin in Corneas From Humans, Rats, and Mice With Diabetes
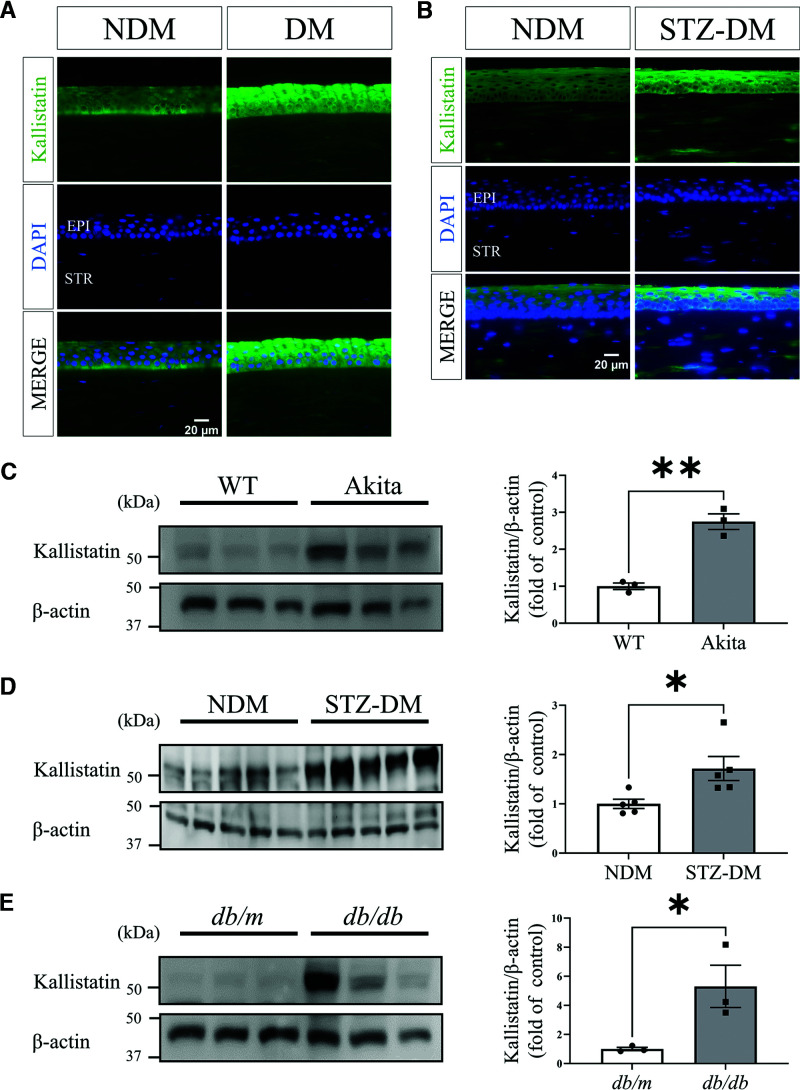
To study whether kallistatin is expressed in the cornea and its expression is altered in diabetes, we performed immunostaining of kallistatin in corneas from humans with diabetes. The result showed that kallistatin levels were upregulated in the cornea epithelium from human donors with diabetes compared with those in subjects without diabetes (Fig. 1A). We also performed Western blot analysis of kallistatin in tissue lysate from corneas from humans with diabetes. The result showed that there was a trend of upregulated kallistatin levels in the corneas from human donors with diabetes compared with those in the subjects without diabetes (Supplementary Fig. 1). Similarly, kallistatin levels were increased in the cornea epithelial layer of STZ-induced diabetic rats (3 months of diabetes) compared with that in the nondiabetic controls (Fig. 1B). As shown by Western blot analysis, the kallistatin level was upregulated in the corneas of type 1 diabetes mouse models (Akita mice and STZ-induced diabetic mice) and the type 2 diabetes mouse model (db/db mice) compared with those in the respective nondiabetic controls (Fig. 1C–E).
Figure 1.
Kallistatin protein levels in nondiabetic and diabetic corneas. A: Representative immunostained images of kallistatin (green) and DAPI (blue) in the corneas from donors with DM and NDM donors (n = 6). Epi, epithelium; Str, stroma. B: Representative immunostained images of kallistatin (green) and DAPI (blue) in the corneas from STZ-induced DM rats (3 months of diabetes) and NDM controls (n = 3). Representative Western blots for kallistatin and densitometry quantification in the corneas from Akita mice (n = 3) (C), STZ-induced DM mice (n = 5) (D), and db/db mice (n = 3) (E), and their respective NDM controls. All values are mean ± SEM. *P < 0.05, **P < 0.01.
Increased Wnt/β-Catenin Signaling Activities in the Corneas With Wound Healing
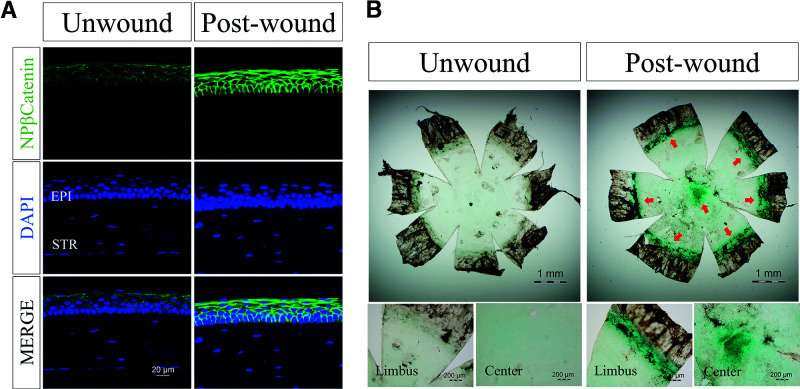
To examine the activation of the Wnt/β-catenin pathway in the corneal wound healing process, we measured nonphosphorylated β-catenin in wounded corneas in WT mice. As shown in Fig. 2A, elevated levels of nonphosphorylated β-catenin were detected in the wounded corneas compared with the unwounded corneas, suggesting that Wnt signaling was activated in the cornea in response to wounding. A canonical Wnt reporter mouse line, the Axin2-lacZ mouse line, was used to confirm the activation of corneal Wnt signaling during wound healing (30). At 48 h after epithelial debridement, the corneas were stained with X-gal. The wounded corneas showed more intense X-gal staining compared with unwounded corneas (Fig. 2B). These data indicated that the Wnt pathway is activated during the epithelial wound healing process.
Figure 2.
Increased Wnt/β-catenin signaling activities in the corneas with wound healing. A: Representative immunostained images of nonphosphorylated β-catenin (NP-β-catenin, green) and DAPI (blue) in corneal sections of 5-month-old C57BL/6J mice with corneal wound and age-matched unwound controls (n = 5). Epi, epithelium; Str, stroma. B: Representative images of X-gal staining (blue) of flat-mounted corneas from Axin2-LacZ mice with and without wound (n = 5).
Diabetic Corneas Showed Attenuated Wnt/β-Catenin Signaling Activation and Delayed Wound Healing
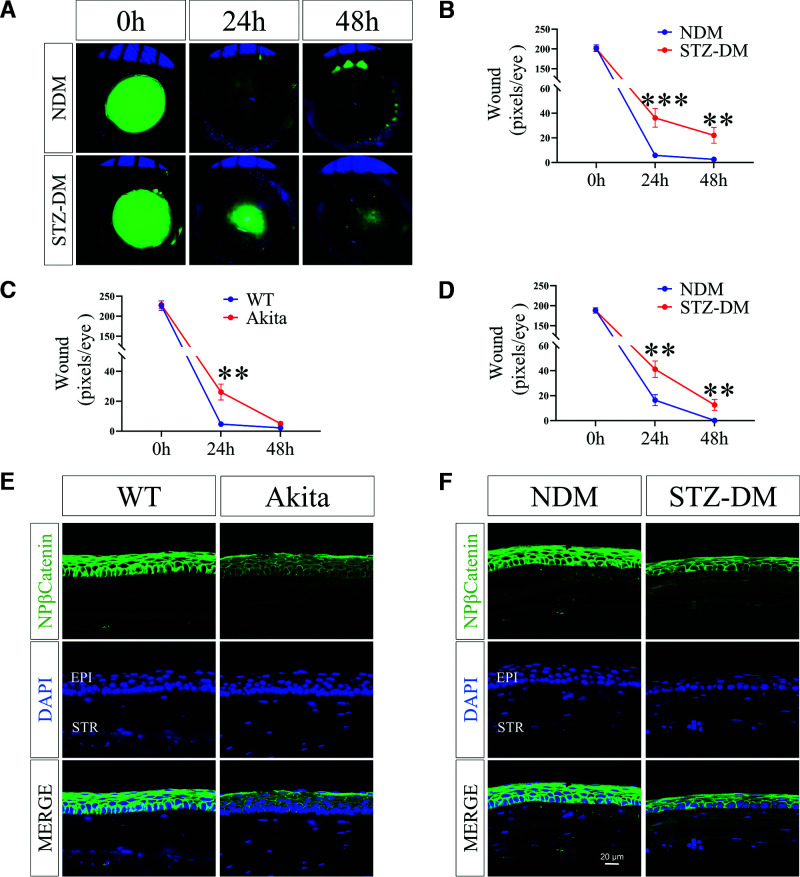
At 12 weeks after the onset of STZ-induced diabetes, diabetic C57BL/6J mice were subjected to corneal wound healing. On both day 2 and day 3 after wounding, the epithelial depletion areas were significantly larger in diabetic mice than those of age-matched nondiabetic mice (Fig. 3A and B). Similarly, the delayed epithelial wound closure was also observed in Akita mice, a genetic type 1 diabetic model (Fig. 3C), and in STZ-induced diabetic rats (Fig. 3D). These findings demonstrated delayed corneal epithelial wound healing in diabetic animals.
Figure 3.
Comparison of corneal wound healing rates of DM animal models and NDM controls. A: Representative fluorescence stained images show corneal wounds in STZ-induced DM mice and NDM mice. The postinjury corneas were stained with sodium fluorescein (green) and photographed at indicated time points postwounding. B: Quantification of the corneal wound. The progress of wound healing was quantified by measuring wound severity using the pixels of green fluorescence per eye with ImageJ (n = 16). C: Comparison of corneal wound healing in Akita mice and WT controls (n = 5). D: Comparison of corneal wound healing in STZ-induced DM rats (n = 12). Representative immunohistochemistry images with an antibody for nonphosphorylated β-catenin (NP-β-catenin) in the corneas 48 h postwounding from 5-month-old Akita mice (E) and STZ-induced DM mice (3 months of diabetes) (F) and their respective NDM controls (n = 5). Scale bars, 20 µm. All values are mean ± SEM. **P < 0.01; ***P < 0.001.
As shown by immunostaining, the nonphosphorylated β-catenin level declined in the wounded corneal epithelium of Akita mice or STZ-induced diabetic mice compared with that in the respective nondiabetic controls (Fig. 3E and F).
Taken together, these data revealed that the activation of Wnt/β-catenin signaling is suppressed in diabetic corneas during wound healing.
Corneal Wound Healing Rates Correlated With Wnt/β-Catenin Signaling Activities in Kallistatin Transgenic Mice
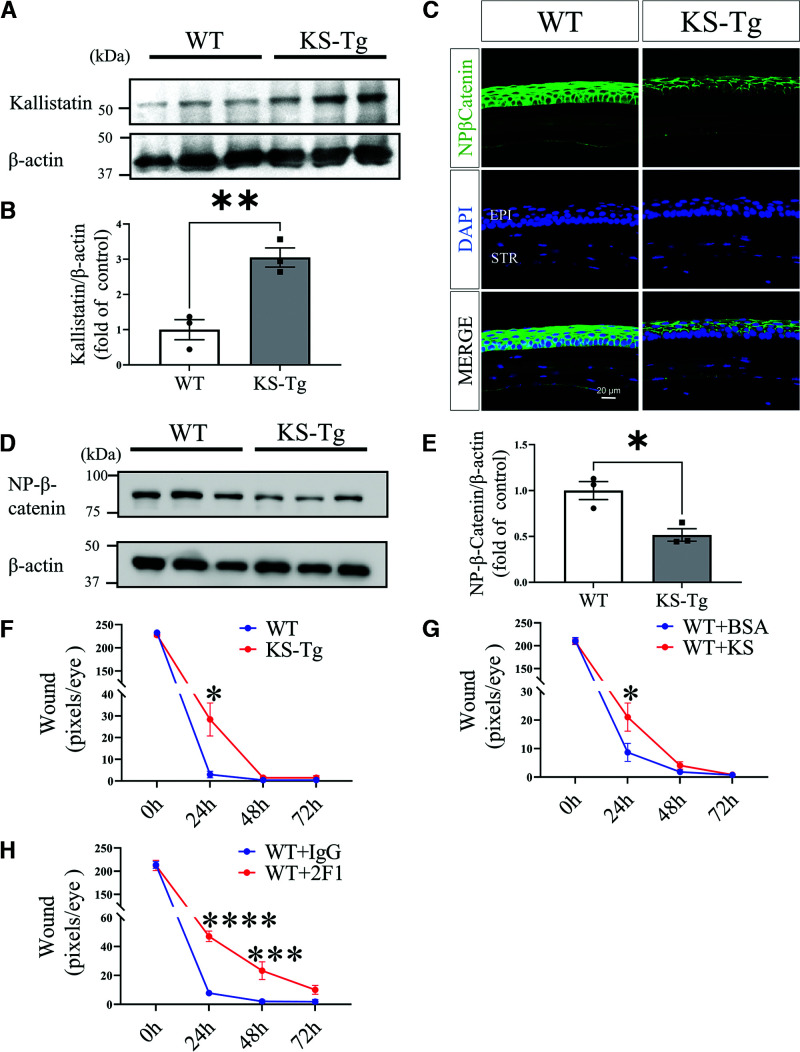
To explore the role of kallistatin and Wnt signaling in cornea wound healing, we used KS-Tg mice. KS-Tg mice showed circulation kallistatin levels threefold higher than WT mice, comparable to the increases in patients with diabetes (16). Western blot analysis showed that kallistatin levels in the corneas of KS-Tg mice were significantly higher than those of WT controls (Fig. 4A and B). As shown by immunostaining (Fig. 4C) and Western blotting (Fig. 4D and E), KS-Tg mice had lower nonphosphorylated β-catenin levels in corneal wound healing compared with WT mice. In addition, the rate of corneal epithelial wound healing was significantly lower in KS-Tg mice than in WT mice (Fig. 4F). Thus, the results suggested that kallistatin expression resulted in lower Wnt/β-catenin signaling in the cornea and delayed corneal wound healing, similar to the phenotype in diabetic mice.
Figure 4.
Delayed corneal wound healing in KS-Tg mice by inhibition of the Wnt signaling pathway. A: Western blot analysis of kallistatin in the cornea of 5-month-old KS-Tg mice and WT littermates. B: Densitometry analysis of kallistatin in panel A and normalized by β-actin levels (n = 3). C: Representative images of nonphosphorylated β-catenin (NP-β-catenin) in the corneas of 5-month-old KS-Tg mice and WT littermates (n = 5). D: Western blot analysis of NP-β-catenin in the corneas. E: Densitometry analysis of NP-β-catenin in the cornea in panel D and normalized by β-actin levels (n = 3). F: The wound in the cornea from KS-Tg and WT mice was quantified after fluorescein staining using the pixel per eye with ImageJ (n = 10). G: Subconjunctival injection of kallistatin protein (KS, 10 µg/eye) into WT mice, with BSA for control. Wound severity was quantified at postwounding time as indicated (n = 10). H: WT mice received a subconjunctival injection of 10 µg of Mab2F1 with IgG for control, and then wound severity was quantified at the indicated time points (n = 8). All values are mean ± SEM. *P < 0.05; ***P < 0.001; ****P < 0.0001.
Local Inhibition of the Wnt Signaling Pathway Delayed Corneal Wound Healing
We injected purified recombinant kallistatin protein into subconjunctival space of WT mice to exclude potential impacts of systemic inhibition of Wnt signaling in KS-Tg mice on corneal wound healing, with the same amount BSA as control. As shown by Fig. 4G, the recombinant kallistatin protein delayed corneal wound healing compared with the controls.
To further investigate whether the regulatory role of kallistatin in corneal wound healing is through the Wnt signal pathway, we injected Mab2F1, a specific LRP6-blocking antibody, into the subconjunctival space of the WT mice, with the same dose of nonspecific IgG for control. As shown by Fig. 4H, the subconjunctival injection of Mab2F1 significantly delayed corneal wound healing relative to the IgG control.
Cornea Wound Healing Rate Was Promoted in Vldlr−/− Mice and Suppressed by Soluble VLDLR Ectodomain
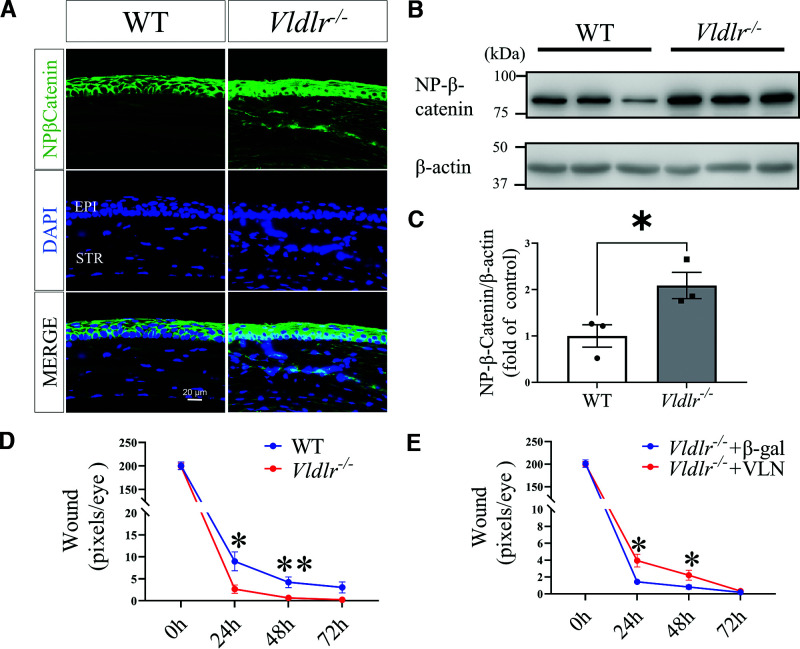
Our previous study showed that VLDLR deficiency results in Wnt signaling overactivation in the retina (18–20). Therefore, we used Vldlr−/− mice for the gain-of-function study of the canonic Wnt pathway activation on corneal wound healing. We measured nonphosphorylated β-catenin levels in the corneas of Vldlr−/− mice. As shown by immunostaining and Western blot analysis, Vldlr−/− mice had higher Wnt/β-catenin signaling activities in the cornea with wound relative to those in the age-matched WT mice with corneal wound (Fig. 5A–C). In addition, the corneal wound healing assay showed that cornea wound healing was significantly accelerated in Vldlr−/− mice relative to WT mice (Fig. 5D).
Figure 5.
Cornea wound healing rate was promoted in Vldlr−/− mice and delayed by soluble VLDLR ectodomain (VLN). A: Representative immunostaining images of nonphosphorylated β-catenin (NP-β-catenin) (green) and DAPI (blue) in corneal sections of 5-month-old Vldlr−/− mice and WT littermates (n = 5). B: Western blot analysis of NP-β-catenin in the corneas of Vldlr−/− mice and WT littermates. C: Densitometry analysis of NP-β-catenin in the cornea in panel B and normalized by β-actin levels (n = 3). D: The comparison of wound healing progress between Vldlr−/− and WT mice (n = 10). E: Subconjunctival injection of adenovirus expressing the soluble VLDLR ectodomain (VLN, 10 μL of 1 × 107 inclusion-forming units/mL each eye) into Vldlr−/− mice delayed corneal wound healing compared with adenovirus expressing β-galactosidase (β-gal) control (n = 10). All values are mean ± SEM. *P < 0.05; **P < 0.01.
We have identified that the shed soluble VLDLR ectodomain (VLN) can bind to the Wnt coreceptor LRP6 and subsequently inhibit Wnt signaling (19,32). To determine whether the promoted wound healing in the Vldlr−/− cornea results from the overactivation of the Wnt pathway, we subconjunctivally injected Vldlr−/− mice with an adenovirus expressing VLN (Ad-VLN), with adenovirus expressing β-galactosidase (Ad–β-gal) at the same titer as the control. Epithelial debridement was performed in mice 7 days after the injection. Ad-VLN significantly delayed the corneal wound closure in Vldlr−/− mice relative to the control virus group (Fig. 5E). The findings suggested that rate of corneal epithelial wound healing highly correlated with Wnt/β-catenin signaling activities in the cornea.
Local Activation of the Wnt Signaling Pathway Promoted Corneal Wound Healing
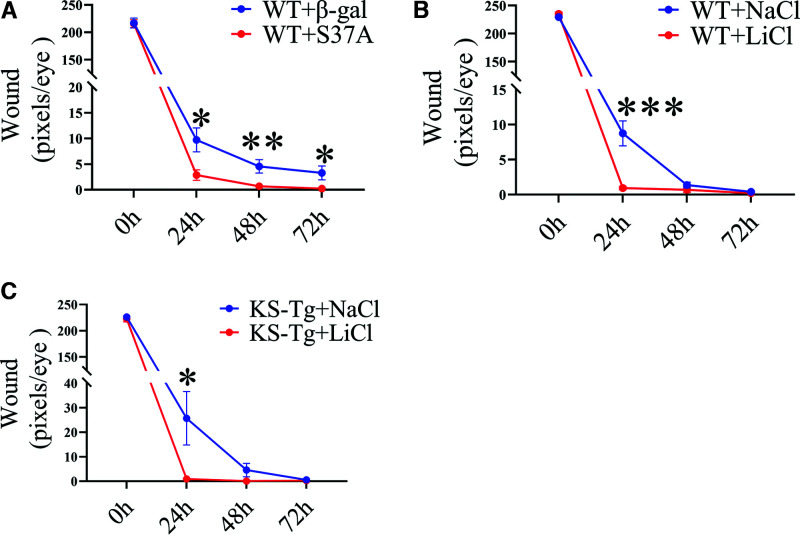
To exclude the potential secondary effect of systemic Wnt signaling changes in Vldlr−/− mice on corneal wound healing, we subconjunctivally injected an adenovirus expressing a constitutively active mutant of β-catenin (Ad-S37A) in which the phosphorylation site Ser37 in β-catenin was substituted by Ala (S37A), into C57BL/6J mice (33). Ad–β-gal at the same titer was used as the control. On the 7th day after the injection, we performed the wound healing assay and found that wound healing was accelerated in the Ad-S37A group relative to the control mice (Fig. 6A).
Figure 6.
Accelerated wound healing by local activation of the Wnt signaling pathway. A: Subconjunctival injection of a Wnt signaling activator Ad-S37A (10 µL of 1 × 107 inclusion-forming units/mL per eye) enhanced corneal wound closure in WT mice, relative to the control injected with the same titer of Ad-β-gal (n = 10). B: WT mice receiving LiCl (10 μL of 0.9% per eye) subconjunctival injection with NaCl for control (n = 10). C: KS-Tg mice receiving LiCl (10 μL of 0.9% per eye) subconjunctival injection with NaCl as control (n = 10). All values are mean ± SEM. *P < 0.05; **P < 0.01; ***P < 0.001.
Lithium chloride (LiCl) stabilizes β-catenin by inhibiting GSK-3β and is commonly used to activate canonical Wnt signaling intracellularly (34–36). LiCl (0.9%) was subconjunctivally administered to WT mice with wounded cornea once daily for 3 days. The corneal wound healing in the LiCl-treated group was significantly accelerated (Fig. 6B).
To determine whether intracellular Wnt pathway activation will rescue the delayed healing of corneal epithelial wound in KS-Tg mice, KS-Tg mice received daily subconjunctival injections of LiCl after corneal wounding, with NaCl as control. Our results showed that the corneal wound closure was significantly faster in the LiCl group relative to the NaCl group (Fig. 6C). This result suggested that activation of Wnt signaling downstream of the kallistatin interaction site will offset its inhibitory effect on wound healing.
EGFR Expression Levels Were Upregulated by Wnt/β-Catenin Signaling in the Corneas
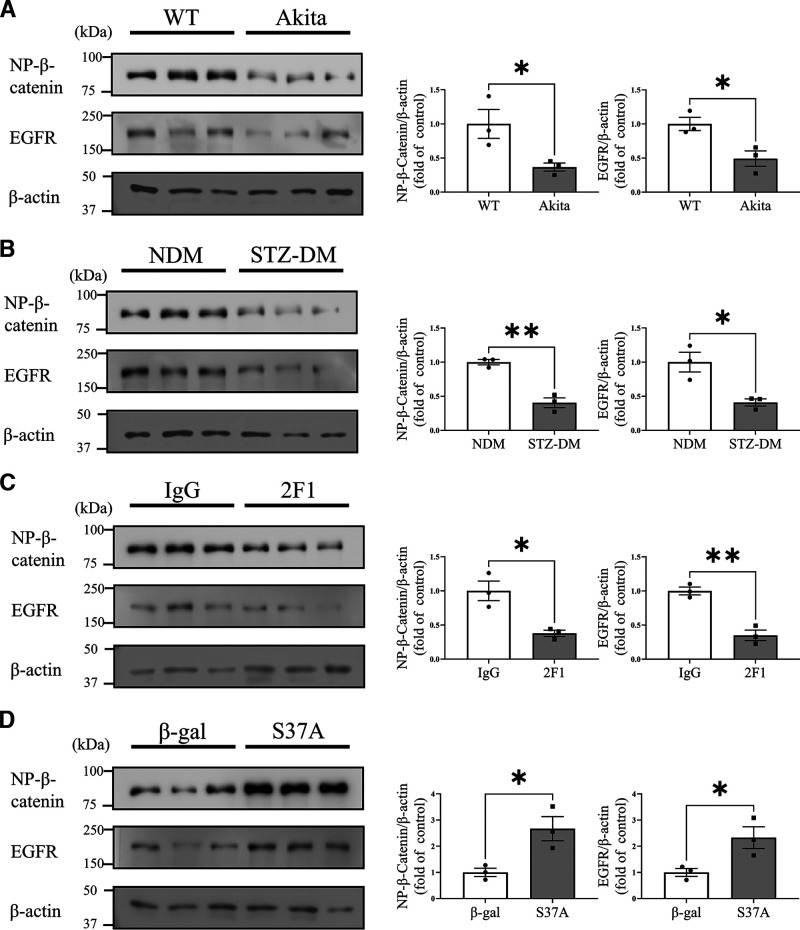
As shown by Western blot analysis, nonphosphorylated β-catenin levels in the cornea with wound were significantly lower in Akita mice and STZ-induced diabetic mice than their age-matched nondiabetic controls (Fig. 7A and B). In addition, reduced EGFR expression levels were demonstrated in diabetic mouse corneas, correlating with lower Wnt/β-catenin signaling activities (Fig. 7A and B). Inhibition of Wnt/β-catenin signaling in the cornea by subconjunctival injection of the Wnt signaling blocking antibody, Mab2F1, downregulated the expression of EGFR (Fig. 7C), while Ad-S37A substantially promoted the expression of EGFR (Fig. 7D).
Figure 7.
EGFR expression levels were associated with Wnt/β-catenin signaling in the corneas. Representative Western blot for nonphosphorylated β-catenin (NP-β-catenin) and EGFR and densitometry quantification in the corneas from 5-month-old Akita mice (A), STZ-induced DM mice (B), WT mice with a subconjunctival injection of Mab2F1 (C), and Ad-S37A (D) and their respective controls. Values are mean ± SEM (n = 3). *P < 0.05; **P < 0.01.
Kallistatin Blocked Wnt Signaling Enhanced HCEC Proliferation and Migration in a Concentration-Dependent Manner
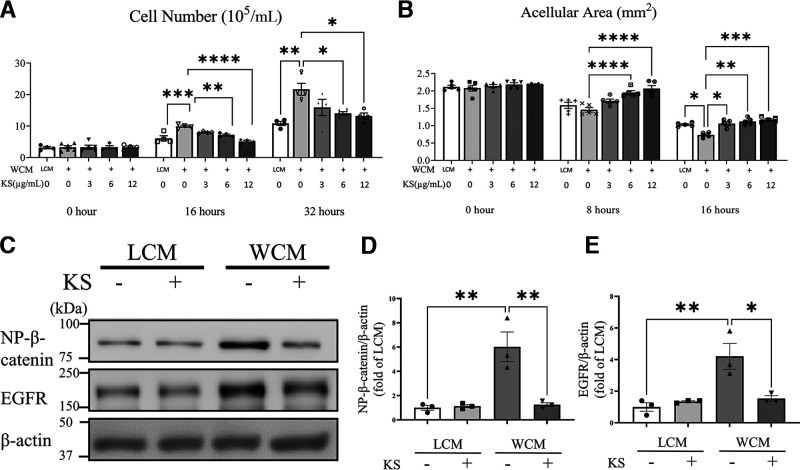
Epithelial cell proliferation and migration are key steps during the corneal wound healing process (37,38). To establish the direct effect of Wnt signaling on corneal epithelial cells, we evaluated the proliferation and migration of cultured primary HCEC treated with WCM, with LCM as control. As shown in Figs. 8A and B, WCM stimulated HCEC proliferation and migration. In contrast, kallistatin suppressed the HCEC proliferation and migration induced by WCM in a concentration-dependent manner. In correlation with this effect, Western blot analysis showed that kallistatin downregulated nonphosphorylated β-catenin and EGFR levels in HCEC (Fig. 8C and D), further confirming the in vivo findings.
Figure 8.
Effect of kallistatin on HCEC. A: HCEC were treated with 20% LCM or WCM, with or without different concentrations of human kallistatin, as indicated. Viable cells were quantified at 0, 16, and 32 h (n = 3–6). B: HCEC were treated with WCM and various concentrations of kallistatin after 100% confluence. An in vitro “wound” was created, and then 6 images of each scratch were captured at the indicated time points. The acellular area was measured by ImageJ (n = 5). C: Representative Western blots of nonphosphorylated β-catenin (NP-β-catenin) and EGFR in HCEC with indicated treatment. HCEC were treated with 20% LCM or WCM, with or without 12 µmol/mL kallistatin for 48 h. Protein levels of NP-β-catenin (D) and EGFR (E) in panel C were quantified by densitometry (n = 3). Values are mean ± SEM. *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001.
Discussion
Increasing evidence suggests that various corneal components (epithelium, stroma, nerves, and endothelium) are affected by diabetes (39,40). Delayed corneal epithelial wound healing may lead to sight-threatening complications, including ocular surface irregularities, microbial keratitis, and corneal scarring (4). However, the molecular mechanism for the diabetes-induced corneal wound-healing deficiency remains elusive.
Numerous studies have confirmed that the Wnt/β-catenin signaling pathway is essential for cell proliferation, differentiation, and migration (9,41–43). Using Wnt activators and inhibitors, the current study provided evidence supporting that canonical Wnt signaling plays an important role in corneal wound healing and that diabetes suppressed Wnt signaling in the cornea, which represents a mechanism for the impaired cornea wound healing in diabetes. Toward the mechanism for the dysregulation of Wnt signaling in the diabetic cornea, we found kallistatin, an endogenous inhibitor of Wnt signaling, is upregulated in the diabetic cornea. High levels of kallistatin alone are sufficient to suppress Wnt signaling and delay corneal wound healing, suggesting that diabetes-induced increases of kallistatin suppress cornea epithelial wound healing in diabetes through Wnt/β-catenin signaling. Our results also suggest that the Wnt signaling pathway promotes wound healing at least in part through upregulation of EGFR expression in corneal epithelial cells. These results provided in vivo and in vitro evidence indicating that elevated levels of kallistatin in the diabetic cornea contribute to cornea wound healing delay by suppressing Wnt signaling activation. These findings provided new insights into the regulation of cornea wound healing, which has the potential to contribute to the development of new therapeutic strategies for impaired corneal wound healing in diabetes.
β-Catenin is an essential effector of the canonical Wnt pathway (11). In the current study, we identified increased nonphosphorylated β-catenin levels in the wounded cornea. To verify Wnt signaling activation during wound healing, we also measured β-catenin activity using Axin2-lacZ mice, a commonly accepted Wnt reporter model. The results confirmed the increased Wnt pathway activation during the epithelial wound healing process. Limbal stem cells (LSCs) located at the corneoscleral junction play an essential role in corneal epithelium renewal and corneal wound healing (44–46). As shown by X-gal staining, Wnt signaling is activated in the limbus area and peripheral cornea during wound healing, suggesting that Wnt signaling activation in LSCs may contribute to the corneal epithelial repair. Furthermore, accumulating evidence has shown that Wnt signaling may actively participate in stem cell self-renewal and differentiation (47–49). Whether targeting the Wnt pathway in LSCs directly has therapeutic potential for cornea wound healing requires further investigation.
The mechanism by which Wnt/β-catenin signaling regulates corneal wound repair is not entirely understood. The canonical Wnt signal activation causes an accumulation of nonphosphorylated β-catenin in the cytoplasm and its eventual translocation into the nucleus to act as a transcription factor (11). β-Catenin activates expression of a number of growth factors and their receptors (10,50) that regulate cell growth, proliferation, migration, differentiation, and adhesion during wound healing (38). EGFR is one of the target genes regulated by Wnt/β-catenin signaling in multiple tissues (51–53). Wnt ligands have been shown to activate EGFR signaling, which increases the proliferation and invasion of fibroblast and glioma cells (54,55). Consistently, we found that EGFR is upregulated in corneal cells by Wnt signaling, which represents a mechanism for Wnt signaling to promote corneal epithelial cell proliferation and wound healing. Nakamura et al. (56) reported that EGFR inhibition affects epithelial cell proliferation and stratification during corneal epithelial wound healing.
In this study, we found that wound-induced Wnt signaling activation was significantly suppressed in diabetic mouse corneas. The causes of suppressed Wnt signaling in the diabetic cornea were not yet known. We previously found that serum kallistatin levels are increased in the circulation of patients with diabetes, which may contribute to impaired skin wound healing through blocking LRP6, an essential coreceptor in the Wnt/β-catenin pathway (16,25). Here, we found for the first time that levels of kallistatin were increased in the corneas from human donors with diabetes compared with subjects without diabetes. To define the role of the diabetes-induced increases of kallistatin levels in corneal wound healing deficiency, we used kallistatin transgenic mice and showed that elevated levels of kallistatin alone in nondiabetic KS-TG mice resulted in delayed corneal wound healing, recapitulating phenotypes of the diabetic cornea. We also found that local application of kallistatin alone delayed mouse corneal wound closure. Also, in vitro study revealed that kallistatin significantly suppressed HCEC proliferation and migration induced by the Wnt ligand in a concentration-dependent manner. These in vivo and in vitro results confirmed that kallistatin directly acted on the Wnt signal pathway in cornea epithelial cells and delayed corneal wound healing. Subconjunctival injection of kallistatin protein alone is sufficient to delay corneal wound healing. Consistently, activation of Wnt signaling at the β-catenin level, downstream of the kallistatin binding site, offset the inhibitory effect of kallistatin on HCEC proliferation. Taken together, these observations suggest for the first time that the increased levels of kallistatin in the diabetic cornea are responsible, at least in part, for the Wnt signaling inhibition in the cornea, leading to delayed corneal wound healing in diabetes. Therefore, these findings suggest that diabetes-induced upregulation of kallistatin levels contributes to DK and represents a potential drug target.
Previously, we found that VLDLR inhibits Wnt signaling by dimerizing with LRP6 through its extracellular domain (VLN) (20,32). Further, we showed that VLN is naturally shed into the extracellular space to function as a soluble Wnt inhibitor (20). However, the function of VLDLR in the cornea has not been carefully studied. The current study demonstrated that Wnt signaling is overactivated in the cornea of Vldlr−/− mice, suggesting that VLDLR also regulates Wnt signaling in the cornea. Inconsistent, corneal wound healing is enhanced in Vldlr−/− mice. Since the full-length VLDLR also has other functions, such as lipid transport and metabolism, Vldlr−/− mice manifested a disturbed lipid profile. To exclude possible impacts of the systemic lipid disturbance, we overexpressed VLN locally using adenovirus vector subconjunctival injection. The local expression of VLN alone impaired corneal wound healing, further supporting that the impact of VLDLR on wound healing is through regulation of Wnt signaling in the cornea. To verify the impacts of Wnt inhibition on wound healing, we also injected a monoclonal antibody Mab2F1 that specifically binds to LRP6, blocking Wnt signaling activation (13). Consistent with VLN, this specific Wnt-blocking antibody also delayed corneal wound healing.
To further define the cell target of Wnt regulation, we used cultured primary HCECs. Wnt activation by the Wnt ligand indeed promoted migration and proliferation of corneal epithelial cells. Consistent with the in vivo observation, kallistatin blocked Wnt signaling in these cells, subsequently downregulating EGFR expression and inhibiting proliferation and migration of cornea epithelial cells. Therefore, it is plausible to suggest that upregulation of EGFR in epithelial cells may represent a mechanism for the function of Wnt signaling in promoting wound healing.
Lithium activates Wnt singling at the GSK3β level (57). Our results demonstrated that activation of Wnt signaling downstream of LRP6 by lithium offsets the inhibitory effect of kallistatin, which blocks LRP6, on corneal wound healing in KS-Tg mice. Furthermore, in vitro study showed that activation of Wnt signaling alone by the Wnt ligand promoted HCEC proliferation and migration, suggesting that Wnt signaling directly targets corneal epithelial cells and promotes epithelial wound repair. Our findings suggested that early local modulation of kallistatin/Wnt/β-catenin activities may benefit the management of diabetic corneal complications.
Wnt signaling regulation is a complex process. It has been well established that Wnt signaling is overactivated in the retina and kidney of diabetic animal models, contributing to diabetic retinopathy and nephropathy (11,58). In contrast, the Wnt pathway is suppressed in the skin under diabetes, which plays a role in impaired skin wound healing in diabetes (16). The current study demonstrated that Wnt signaling is downregulated in the diabetic cornea, a change similar to that in the diabetic skin. It remains to be studied how diabetes confers the differential regulation of Wnt signaling in different tissues.
In conclusion, the current study identified that diabetes-induced kallistatin overexpression may be responsible, at least in part, for the deficient cornea wound healing through suppression of Wnt signaling in diabetic cornea. Further studies are warranted to determine whether inhibition of kallistatin or activation of Wnt signaling in the cornea may have therapeutic potential in DK.
Article Information
Funding. This study was supported by National Institutes of Health National Eye Institute grants (EY019309, EY012231, EY028949, EY032930, EY032931) and Fujian Provincial Natural Science Foundation (grant 2018J01310). The authors like to thank the technical support from the Diabetic Animal Core and Histology and Image Core of Diabetes Center of Biomedical Research Excellence (CoBRE) by National Institute of General Medical Sciences (GM122744), and the Vision Core supported by National Eye Institute P30 (EY021725).
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. W.L. and L.H. performed experiments, acquired and analyzed data, and wrote the manuscript. X.M., L.D., and R.C. conducted experiments and acquired data. M.D. generated the kallistatin protein and Mab2F1 antibody used in this study. D.K. and J.-x.M. designed the research, analyzed data, and wrote and edited the manuscript. J.-x.M. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Prior Presentation. Parts of this study were presented in abstract form at the 2020 Trans-Agency Scientific Meeting on Developing Medical Countermeasures to Treat the Acute and Chronic Effects of Ocular Chemical Toxicity, Bethesda, MD, 25–26 February 2020.
Footnotes
W.L. and L.H. contributed equally to this work.
This article contains supplementary material online at https://doi.org/10.2337/figshare.17739422.
References
- 1. American Diabetes Association . Diagnosis and classification of diabetes mellitus. Diabetes Care 2014;37(Suppl. 1):S81–S90 [DOI] [PubMed] [Google Scholar]
- 2. Misra SL, Patel DV, McGhee CN, et al. Peripheral neuropathy and tear film dysfunction in type 1 diabetes mellitus. J Diabetes Res 2014;2014:848659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Misra SL, Goh YW, Patel DV, Riley AF, McGhee CN. Corneal microstructural changes in nerve fiber, endothelial and epithelial density after cataract surgery in patients with diabetes mellitus. Cornea 2015;34:177–181 [DOI] [PubMed] [Google Scholar]
- 4. Bu Y, Shih KC, Kwok SS, et al. Experimental modeling of cornea wound healing in diabetes: clinical applications and beyond. BMJ Open Diabetes Res Care 2019;7:e000779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Shih KC, Lam KS, Tong L. A systematic review on the impact of diabetes mellitus on the ocular surface. Nutr Diabetes 2017;7:e251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mirza S, Hossain M, Mathews C, et al. Type 2-diabetes is associated with elevated levels of TNF-alpha, IL-6 and adiponectin and low levels of leptin in a population of Mexican Americans: a cross-sectional study. Cytokine 2012;57:136–142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ni S, Yu J, Bao F, Li J, Elsheikh A, Wang Q. Effect of glucose on the stress-strain behavior of ex-vivo rabbit cornea. Exp Eye Res 2011;92:353–360 [DOI] [PubMed] [Google Scholar]
- 8. Barsegian A, Lee J, Salifu MO, McFarlane SI. Corneal neuropathy: an underrated manifestation of diabetes mellitus. J Clin Endocrinol Diabetes 2018;2:JCED-111 [Google Scholar]
- 9. Mohammed MK, Shao C, Wang J, et al. Wnt/β-catenin signaling plays an ever-expanding role in stem cell self-renewal, tumorigenesis and cancer chemoresistance. Genes Dis 2016;3:11–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. MacDonald BT, Tamai K, He X. Wnt/beta-catenin signaling: components, mechanisms, and diseases. Dev Cell 2009;17:9–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chen Q, Ma JX. Canonical Wnt signaling in diabetic retinopathy. Vision Res 2017;139:47–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Liu Q, Li J, Cheng R, et al. Nitrosative stress plays an important role in Wnt pathway activation in diabetic retinopathy. Antioxid Redox Signal 2013;18:1141–1153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lee K, Hu Y, Ding L, et al. Therapeutic potential of a monoclonal antibody blocking the Wnt pathway in diabetic retinopathy. Diabetes 2012;61:2948–2957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zerlin M, Julius MA, Kitajewski J. Wnt/frizzled signaling in angiogenesis. Angiogenesis 2008;11:63–69 [DOI] [PubMed] [Google Scholar]
- 15. Wang Z, Liu CH, Huang S, Chen J. Wnt signaling in vascular eye diseases. Prog Retin Eye Res 2019;70:110–133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. McBride JD, Jenkins AJ, Liu X, et al. Elevated circulation levels of an antiangiogenic SERPIN in patients with diabetic microvascular complications impair wound healing through suppression of Wnt signaling. J Invest Dermatol 2014;134:1725–1734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Yang S, Zhang Y, Zhang Z, et al. Insulin promotes corneal nerve repair and wound healing in type 1 diabetic mice by enhancing Wnt/β-catenin signaling. Am J Pathol 2020;190:2237–2250 [DOI] [PubMed] [Google Scholar]
- 18. Chen Y, Hu Y, Lu K, Flannery JG, Ma JX. Very low density lipoprotein receptor, a negative regulator of the wnt signaling pathway and choroidal neovascularization. J Biol Chem 2007;282:34420–34428 [DOI] [PubMed] [Google Scholar]
- 19. Wang Z, Cheng R, Lee K, et al. Nanoparticle-mediated expression of a Wnt pathway inhibitor ameliorates ocular neovascularization. Arterioscler Thromb Vasc Biol 2015;35:855–864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lee K, Shin Y, Cheng R, et al. Receptor heterodimerization as a novel mechanism for the regulation of Wnt/β-catenin signaling. J Cell Sci 2014;127:4857–4869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Zhou GX, Chao L, Chao J. Kallistatin: a novel human tissue kallikrein inhibitor. Purification, characterization, and reactive center sequence. J Biol Chem 1992;267:25873–25880 [PubMed] [Google Scholar]
- 22. Lin WC, Chen CW, Huang YW, et al. Kallistatin protects against sepsis-related acute lung injury via inhibiting inflammation and apoptosis. Sci Rep 2015;5:12463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Liu X, Zhang B, McBride JD, et al. Antiangiogenic and antineuroinflammatory effects of kallistatin through interactions with the canonical Wnt pathway. Diabetes 2013;62:4228–4238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ma JX, King LP, Yang Z, Crouch RK, Chao L, Chao J. Kallistatin in human ocular tissues: reduced levels in vitreous fluids from patients with diabetic retinopathy. Curr Eye Res 1996;15:1117–1123 [DOI] [PubMed] [Google Scholar]
- 25. Jenkins AJ, McBride JD, Januszewski AS, et al. Increased serum kallistatin levels in type 1 diabetes patients with vascular complications. J Angiogenes Res 2010;2:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Qiu F, Meng T, Chen Q, et al. Fenofibrate-loaded biodegradable nanoparticles for the treatment of experimental diabetic retinopathy and neovascular age-related macular degeneration. Mol Pharm 2019;16:1958–1970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Zhou KK, Benyajati S, Le Y, Cheng R, Zhang W, Ma JX. Interruption of Wnt signaling in Müller cells ameliorates ischemia-induced retinal neovascularization. PLoS One 2014;9:e108454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kalha S, Kuony A, Michon F. Corneal epithelial abrasion with ocular burr as a model for cornea wound healing. J Vis Exp 2018:e58071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Al Alam D, Green M, Tabatabai Irani R, et al. Contrasting expression of canonical Wnt signaling reporters TOPGAL, BATGAL and Axin2(LacZ) during murine lung development and repair. PLoS One 2011;6:e23139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. de Roo JJD, Breukel C, Chhatta AR, et al. Axin2-mTurquoise2: a novel reporter mouse model for the detection of canonical Wnt signalling. Genesis 2017;55:10. [DOI] [PubMed] [Google Scholar]
- 31. Cormier N, Yeo A, Fiorentino E, Paxson J. Optimization of the wound scratch assay to detect changes in murine mesenchymal stromal cell migration after damage by soluble cigarette smoke extract. J Vis Exp 2015:e53414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Chen Q, Takahashi Y, Oka K, Ma JX. Functional differences of very-low-density lipoprotein receptor splice variants in regulating Wnt signaling. Mol Cell Biol 2016;36:2645–2654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Zhou T, Hu Y, Chen Y, et al. The pathogenic role of the canonical Wnt pathway in age-related macular degeneration. Invest Ophthalmol Vis Sci 2010;51:4371–4379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. McCubrey JA, Steelman LS, Bertrand FE, et al. Multifaceted roles of GSK-3 and Wnt/β-catenin in hematopoiesis and leukemogenesis: opportunities for therapeutic intervention. Leukemia 2014;28:15–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hedgepeth CM, Conrad LJ, Zhang J, Huang HC, Lee VM, Klein PS. Activation of the Wnt signaling pathway: a molecular mechanism for lithium action. Dev Biol 1997;185:82–91 [DOI] [PubMed] [Google Scholar]
- 36. Wang Z, Liu CH, Sun Y, et al. Pharmacologic activation of Wnt signaling by lithium normalizes retinal vasculature in a murine model of familial exudative vitreoretinopathy. Am J Pathol 2016;186:2588–2600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Słoniecka M, Le Roux S, Zhou Q, Danielson P. Substance P enhances keratocyte migration and neutrophil recruitment through interleukin-8. Mol Pharmacol 2016;89:215–225 [DOI] [PubMed] [Google Scholar]
- 38. Ljubimov AV, Saghizadeh M. Progress in corneal wound healing. Prog Retin Eye Res 2015;49:17–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Del Buey MA, Casas P, Caramello C, et al. An update on corneal biomechanics and architecture in diabetes. J Ophthalmol 2019;2019:7645352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Zhao H, He Y, Ren YR, Chen BH. Corneal alteration and pathogenesis in diabetes mellitus. Int J Ophthalmol 2019;12:1939–1950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ring A, Kim YM, Kahn M. Wnt/catenin signaling in adult stem cell physiology and disease. Stem Cell Rev Rep 2014;10:512–525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Tang D, He Y, Li W, Li H. Wnt/β-catenin interacts with the FGF pathway to promote proliferation and regenerative cell proliferation in the zebrafish lateral line neuromast. Exp Mol Med 2019;51:1–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Vlad-Fiegen A, Langerak A, Eberth S, Müller O. The Wnt pathway destabilizes adherens junctions and promotes cell migration via β-catenin and its target gene cyclin D1. FEBS Open Bio 2012;2:26–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Yoon JJ, Ismail S, Sherwin T. Limbal stem cells: central concepts of corneal epithelial homeostasis. World J Stem Cells 2014;6:391–403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Ksander BR, Kolovou PE, Wilson BJ, et al. ABCB5 is a limbal stem cell gene required for corneal development and repair. Nature 2014;511:353–357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Saghizadeh M, Kramerov AA, Svendsen CN, Ljubimov AV. Concise review: stem cells for corneal wound healing. Stem Cells 2017;35:2105–2114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Nusse R. Wnt signaling and stem cell control. Cell Res 2008;18:523–527 [DOI] [PubMed] [Google Scholar]
- 48. Lento W, Congdon K, Voermans C, Kritzik M, Reya T. Wnt signaling in normal and malignant hematopoiesis. Cold Spring Harb Perspect Biol 2013;5:a008011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Pei Y, Brun SN, Markant SL, et al. WNT signaling increases proliferation and impairs differentiation of stem cells in the developing cerebellum. Development 2012;139:1724–1733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Kim E, Lisby A, Ma C, et al. Promotion of growth factor signaling as a critical function of β-catenin during HCC progression. Nat Commun 2019;10:1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Tan X, Apte U, Micsenyi A, et al. Epidermal growth factor receptor: a novel target of the Wnt/beta-catenin pathway in liver. Gastroenterology 2005;129:285–302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Schlange T, Matsuda Y, Lienhard S, Huber A, Hynes NE. Autocrine WNT signaling contributes to breast cancer cell proliferation via the canonical WNT pathway and EGFR transactivation. Breast Cancer Res 2007;9:R63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Guturi KK, Mandal T, Chatterjee A, et al. Mechanism of β-catenin-mediated transcriptional regulation of epidermal growth factor receptor expression in glycogen synthase kinase 3 β-inactivated prostate cancer cells. J Biol Chem 2012;287:18287–18296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Kim SE, Choi KY. EGF receptor is involved in WNT3a-mediated proliferation and motility of NIH3T3 cells via ERK pathway activation. Cell Signal 2007;19:1554–1564 [DOI] [PubMed] [Google Scholar]
- 55. Paul I, Bhattacharya S, Chatterjee A, Ghosh MK. Current understanding on EGFR and Wnt/β-catenin signaling in glioma and their possible crosstalk. Genes Cancer 2013;4:427–446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Nakamura Y, Sotozono C, Kinoshita S. The epidermal growth factor receptor (EGFR): role in corneal wound healing and homeostasis. Exp Eye Res 2001;72:511–517 [DOI] [PubMed] [Google Scholar]
- 57. Rao AS, Kremenevskaja N, Resch J, Brabant G. Lithium stimulates proliferation in cultured thyrocytes by activating Wnt/beta-catenin signalling. Eur J Endocrinol 2005;153:929–938 [DOI] [PubMed] [Google Scholar]
- 58. Cheng R, Ding L, He X, Takahashi Y, Ma JX. Interaction of PPARα with the canonic Wnt pathway in the regulation of renal fibrosis. Diabetes 2016;65:3730–3743 [DOI] [PMC free article] [PubMed] [Google Scholar]