Abstract
The median bioavailabilities of aciclovir after administration of aciclovir and its prodrug valaciclovir were 21.5 and 70.1%, respectively, in 12 patients with malignant hematological diseases with leukopenia after chemotherapy. The interindividual variations of the bioavailability were 48.5 and 21.0% after administration of aciclovir and valaciclovir, respectively. Neither the bioavailability nor the interindividual variation of area under the concentration-time curve of oral aciclovir or valaciclovir differed from that reported in healthy volunteers.
Aciclovir is a purine nucleoside analogue with activity against human herpesviruses. The efficacy of oral aciclovir is limited as a result of its low bioavailability. Valaciclovir, the l-valyl ester of aciclovir, has been developed as a prodrug to improve bioavailability. Aciclovir is used in prophylaxis against herpesvirus infections in patients with leukopenia after chemotherapy for malignant diseases. Chemotherapy often causes damage of the intestinal mucosa, which could influence the absorption of drugs, jeopardizing its efficacy (10). A drug with more reliable absorption is of particular value in this clinical setting. The aim of the present study was to compare the bioavailabilities of aciclovir after administration of aciclovir and valaciclovir to patients with leukopenia following intensive chemotherapy for acute leukemia or lymphoma.
Twelve patients (median age, 62 years; range, 29 to 80 years; eight females) were included in the study. Eight patients had acute myelocytic leukemia, two had acute lymphoblastic leukemia, and two had high-grade non-Hodgkin's lymphoma. The patients with non-Hodgkin's lymphoma received high-dose chemotherapy followed by autologous stem cell support. The patients with acute myelocytic leukemia received treatment with cytosine arabinoside (Ara-C) and antracyclines or mitoxantrone with or without etoposide or thioguanine. The patients with acute lymphoblastic leukemia received an induction regimen consisting of Ara-C, cyclophosphamide, daunorubicin, and vincristine. The study was performed when the patients' polymorphonuclear cell counts were <0.5 × 109/liter, at a median time of 9 days (range, 5 to 13) after start of the cytostatic course. During the study, two patients had clinical signs of oral mucositis, six had fever, requiring antibiotic treatment, and one had diarrhea. All patients had normal renal and hepatic functions.
The study was approved by the Local Ethics Committee and the Swedish Medical Products Agency, and informed consent was obtained before the patients were included in the study.
The patients were treated with 400 mg of aciclovir administered as a 1-h infusion, 400 mg of aciclovir orally, and 500 mg of valaciclovir (corresponding to 349 mg of aciclovir) orally, respectively, on 3 consecutive days in a balanced randomized design. In addition, 400 mg of aciclovir was administered orally each night after blood sampling was completed. An infusion pump was used for intravenous administration of aciclovir (Zovirax; Glaxo Wellcome AB, Mölndal, Sweden). Oral aciclovir and valaciclovir (Zovirax and Valtrex; Glaxo Wellcome AB) were administered with 100 ml of water. There were no restrictions on intake of food and beverages during the study. Infusions were started and oral drugs were administered between 8 and 9 a.m.
Blood samples, 1 ml each, were drawn from a central venous access immediately prior to and at appropriate times after administration of oral valaciclovir (10, 20, 30, and 45 min; 1, 2, 3, 4, 5, and 7 h), intravenous aciclovir (5, 10, 15, 20, and 30 min; 1, 2, 3, 5, and 7 h), or oral aciclovir (15, 30, and 45 min; 1, 1.5, 2, 3, 4, 6, 8, and 10 h). The samples were collected into glass test tubes. The serum fractions were separated by centrifugation, transferred into microcentrifuge tubes, and stored at −70°C until analysis.
Aciclovir in serum was quantified by reversed-phase liquid chromatography (12). Pharmacokinetic analyses were performed by compartment analysis using the JANA stripping program (5) and the PC-NONLIN program (version 2.0; Statistical Consultants Inc., 1986). The reciprocal of measured serum concentrations were used as weights in the iterative procedures. The optimal pharmacokinetic models were established by visual inspection of the fitted serum-concentration time curves and from the weighted squared residuals by using the F-ratio test (3).
Calculations of median values and their approximate 95% confidence intervals (95% CI) were based on the Wilcoxon sign rank test as outlined by Tukey. The Mann-Whitney U test was used for the comparison of values from two independent populations. Associations were established by the Spearman rank correlation test. Miller's jackknife test was used to compare the dispersion of data from two populations. P values of <0.05 were considered statistically significant.
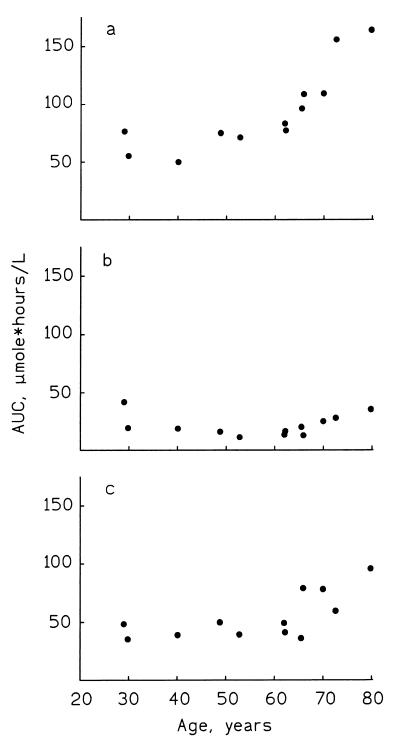
A two-compartment model was used to describe the serum-concentration time course after intravenous administration. The area under the serum-concentration time curve (AUC) increased with increasing age of the patients (P < 0.0001) (Fig. 1a). Multiple regression with stepwise variable selection showed that only age affected AUC among the independent variables tested, including age, body weight, body mass index, body surface area, and serum creatinine. After oral administration of aciclovir, the serum-concentration time course was described by the one-compartment model with either a zero-order (five patients) or first-order (seven patients) absorption phase. The pharmacokinetic data are summarized in Table 1. The AUC values were not affected by the age of the patients (Fig. 1b). The median bioavailability was 21.5% (95% CI, 17.9 to 33.2%).
FIG. 1.
AUCs of aciclovir in relationship to patient age. (a) Aciclovir (400 mg) given as a 1-h infusion (rs = 0.91, P < 0.0001); (b) oral administration of 400 mg of aciclovir (rs = 0.18, P = 0.59); (c) oral administration of 500 mg of valaciclovir (rs = 0.68, P = 0.015).
TABLE 1.
Pharmacokinetics of aciclovir
| Parameter | Median value (95% CI)
|
||
|---|---|---|---|
| Intravenous aciclovir (400 mg) | Oral aciclovir (400 mg) | Oral valaciclovir (500 mg) | |
| AUC (μmol · h/liter) | 89.7 (73.0–117.5) | 19.7 (15.6–27.5) | 49.7 (41.1–67.3) |
| Cmax (μmol/liter) | 41.6 (33.6–52.3) | 2.5 (2.1–3.1) | 12.0 (8.5–16.5) |
| Tmax (h) | 1.0 (end of infusion) | 2.4 (1.4–4.3) | 2.5 (1.5–3.4) |
| t1/2 (h)a | 2.3 (2.0–2.7) | 3.7 (3.1–5.0) | 2.1 (1.7–2.7) |
| Cmax/AUC | 0.14 (0.11–0.16) | 0.23 (0.20–0.26) | |
| Bioavailability (%) | 21.5 (17.9–33.2) | 70.1 (58.5–78.4) | |
t1/2, half-life.
After oral administration of valaciclovir, the serum concentration time course of aciclovir was described by the one-compartment model with a zero-order absorption phase. The AUC values increased with increasing age of the patients (P = 0.015) (Fig. 1c). The median bioavailability of aciclovir after oral administration of valaciclovir was 70.1% (95% CI, 58.5 to 78.4%). The AUC values and bioavailability of aciclovir after oral administration of aciclovir and valaciclovir did not differ between patients with clinical signs of oral mucositis and other patients in this study.
The median bioavailability of aciclovir was three times (95% CI, 2.4 to 4) higher after administration of valaciclovir than after administration of the intact drug. The interindividual variations in bioavailability of aciclovir were 48.5 and 21.0% (coefficient of variation) after administration of aciclovir and valaciclovir, respectively (P = 0.05). There were no differences in time to maximum concentration of drug in serum (Tmax) for aciclovir after administration as intact drug and valaciclovir. The maximum concentration of drug in serum (Cmax) and the ratio of Cmax to AUC were higher after administration of the prodrug (P = 0.0005 and 0.0005, respectively).
The findings in the present study indicate that the pharmacokinetics of aciclovir after oral administration of valaciclovir are age dependent, which is in agreement with the findings of a previous study (14). Aciclovir is eliminated from the body primarily by urinary excretion, and the observed increase of AUC with increasing age of the patients is due most likely to a decrease in renal function with increasing age. A relationship between aciclovir clearance and renal function expressed by creatinine clearance has been reported previously (2).
Administration of oral aciclovir as a prodrug facilitates dosing, since the bioavailability is threefold higher with a considerably lower interindividual variability than after administration of aciclovir. Additional advantages of valaciclovir include higher Cmax values and a higher absorption rate, expressed by the ratio of Cmax to AUC (6). In fact, oral valaciclovir has been used as a substitute for intravenous aciclovir therapy (S. Eksborg, M. Kalin, N. Pal, and S. Söderhäll, Abstr. 8th Int. Congr. Infect. Dis., Boston, Mass., abstr. 47.025, 1998).
Cytotoxic drugs have effects on the dividing cells of the gastrointestinal mucosa, and a decreased absorption of ciprofloxacin has been reported in patients during the neutropenic period following chemotherapy for hematological malignancies (10). However, changes in permeability do not seem to correlate with clinical signs of oral mucositis (9). In this study, neither the bioavailabilities nor the interindividual variations in AUC of oral aciclovir and valaciclovir differed from that reported in healthy volunteers (1, 4, 11, 13, 15).
Acknowledgments
The study was supported by a grant from Glaxo Wellcome AB.
We thank Eva Johansson, Ingela Lundström, Mia Gustavsson, and Annika Lindberg for skillful technical assistance and J.-O. Svensson for performing the aciclovir analysis.
REFERENCES
- 1.Al-Yamani M, Al-Khamis J K I, El-Sayed Y M, Bawazir S A, Al-Rashood K A, Gouda M W. Comparative bioavailability of two tablet formulations of aciclovir in healthy volunteers. Int J Clin Pharmacol Ther. 1998;36:222–226. [PubMed] [Google Scholar]
- 2.Blum M R, Liao S H T, de Miranda P. Overview of aciclovir pharmacokinetic disposition in adults and children. Am J Med. 1982;73(Suppl. 1A):186–192. doi: 10.1016/0002-9343(82)90088-2. [DOI] [PubMed] [Google Scholar]
- 3.Boxenbaum H G, Riegelman S, Elashoff R M. Statistical estimations in pharmacokinetics. J Pharm Biopharm. 1974;2:123–148. doi: 10.1007/BF01061504. [DOI] [PubMed] [Google Scholar]
- 4.De Miranda P, Blum M R. Pharmacokinetics of aciclovir after intravenous and oral administration. J Antimicrob Chemother. 1983;12(Suppl. B):29–37. doi: 10.1093/jac/12.suppl_b.29. [DOI] [PubMed] [Google Scholar]
- 5.Dunne A. JANA: a new iterative polyexponential curve stripping program. Computer Methods Programs Biomed. 1985;20:269–275. doi: 10.1016/0169-2607(85)90085-9. [DOI] [PubMed] [Google Scholar]
- 6.Endrenyi L, Fritsch S, Yan W. Cmax/AUC is a clearer measure than Cmax for absorption rates in investigations of bioequivalence. Int J Clin Pharmacol Ther Toxicol. 1991;29:394–399. [PubMed] [Google Scholar]
- 7.Gubbins P O, Bertch K E. Drug absorption in gastrointestinal disease and surgery: clinical, pharmacokinetic and therapeutic implications. Clin Pharmacokinet. 1991;21:431–447. doi: 10.2165/00003088-199121060-00004. [DOI] [PubMed] [Google Scholar]
- 8.Jacobson M A, Gallant J, Wang L H, Coakley D, Weller S, Gary D, Squires L, Smiley M L, Blum M R, Feinberg J. Phase I trial of valaciclovir, the l-valyl ester of aciclovir in patients with advanced human immunodeficiency virus disease. Antimicrob Agents Chemother. 1994;38:1534–1540. doi: 10.1128/aac.38.7.1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Johansson J E, Ekman T. Gastro-intestinal toxicity related to bone marrow transplantation: disruption of the intestinal barrier precedes clinical findings. Bone Marrow Transplant. 1997;19:921–925. doi: 10.1038/sj.bmt.1700765. [DOI] [PubMed] [Google Scholar]
- 10.Johnson E J, MacGowan A P, Potter M N, Stockley R J, White L O, Slade R R, Reevers D S. Reduced absorption of oral ciprofloxacin after chemotherapy for haematological malignancy. J Antimicrob Chemother. 1990;25:837–842. doi: 10.1093/jac/25.5.837. [DOI] [PubMed] [Google Scholar]
- 11.Soul-Lawton J, Seaber E, On N, Wootton R, Rolan P, Posner J. Absolute bioavailability and metabolic disposition of valaciclovir, the l-valyl ester of aciclovir, following oral administration to humans. Antimicrob Agents Chemother. 1995;39:2759–2764. doi: 10.1128/aac.39.12.2759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Svensson J-O, Barkholt L, Säwe J. Determination of aciclovir and its metabolite 9-carboxymethoxymethylguanine in serum and urine using solid-phase extraction and high-performance liquid chromatography. J Chromatogr B. 1997;690:363–366. doi: 10.1016/s0378-4347(96)00424-0. [DOI] [PubMed] [Google Scholar]
- 13.Vergin H, Kikuta C, Mascher H, Metz R. Pharmacokinetics and bioavailability of different formulations of aciclovir. Arzneim-Forsch. 1995;45:508–515. [PubMed] [Google Scholar]
- 14.Wang L H, Schultz M, Weller S, Smiley M L, Blum M R. Pharmacokinetics and safety of multiple-dose valaciclovir in geriatric volunteers with and without concomitant diuretic therapy. Antimicrob Agents Chemother. 1996;40:80–85. doi: 10.1128/aac.40.1.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Weller S, Blum M R, Doucette M, Burnette T, Cederberg D M, de Miranda P, Smiley M L. Pharmacokinetics of the aciclovir pro-drug valaciclovir after escalating single- and multiple-dose administration to normal volunteers. Clin Pharmacol Ther. 1993;54:595–605. doi: 10.1038/clpt.1993.196. [DOI] [PubMed] [Google Scholar]