Abstract
Bone defects caused by trauma, tumor, congenital abnormality and osteoarthritis, etc. have been substantially impacted the lives and health of human. Artificial bone implants, like bioceramic-based scaffolds, provide significant benefits over biological counterparts and are critical for bone repair and regeneration. However, it is highly probable that bacterial infections occur in the surgical procedures or on bioceramic-based scaffolds. Therefore, it is of great significance to obtain bioceramic-based scaffolds with integrative antibacterial and osteogenic functions for treating bone implant-associated infection and promoting bone repair. To fight against infection problems, bioceramic-based scaffolds with various antibacterial strategies are developed for bone repair and regeneration and also have made great progresses. This review summarizes recent progresses in bioceramic-based scaffolds with antibacterial function, which include drug-induced, ion-mediated, physical-activated and their combined antibacterial strategies according to specific antibacterial mechanism. Finally, the challenges and opportunities of antibacterial bioceramic-based scaffolds are discussed.
Keywords: Bioceramic-based scaffolds, Implant-associated infection, Antibacterial activity, Bone repair, Bone tissue engineering
Graphical abstract
Highlights
-
•
Bioceramic-based scaffolds with antibacterial function (BSAF) are reviewed.
-
•
BSAF have a great potential in treating bone infection and promoting bone repair.
-
•
Antibacterial strategies of BSAF include drug, ion, physical and combined ways.
-
•
The combined strategy may be the optimal approach in fighting bone infection.
-
•
Limitations, challenges and perspectives of BSAF are discussed.
1. Introduction
Bone defects caused by trauma, tumor, congenital abnormality and osteoarthritis have significantly obstructed patients’ quality of life. Although autologous, allogeneic and xenogeneic bone grafts have been generally accepted to be applied for bone repair, biological implants still possibly suffer from problems with lacking bone sources, carrying pathogens and/or inducing immune rejection [1]. Owing to outstanding biocompatibility, osteoconductivity and osteoinductivity, a variety of bioceramics have been widely applied in bone defects repair and bone tissue regeneration, such as HA (Ca10(PO4)6OH2), β-TCP (Ca3(PO4)2), akermanite (Ca2MgSi2O7), and 45S5 (24.5Na2O-24.5CaO–45SiO2–6P2O5), etc [[2], [3], [4], [5], [6]]. Generally, three dimensional (3D) porous scaffolds play a key role in bone defect repair and bone tissue engineering. They can provide mechanical support to resist external stress, maintain the original shape and integrity of tissues, connect with the surrounding tissues and guide the tissue to grow [[7], [8], [9]]. The porous structure facilitates cell migration and growth as well as the transportation of nutrients and metabolites, stimulating bone integration and revascularization [8,[10], [11], [12]]. On the other hand, some scaffolds can also release bioactive ions, thereby promoting physiological behavior of cells and serving the purpose of treatment [7,10,12]. Hence, 3D porous scaffolds are one of the key elements in bone tissue engineering. In response to the characteristics and technical requirements of bioceramics, a series of technologies have been applied for fabricating bioceramic-based scaffolds, such as templating method, freeze drying, foaming method, electrospinning and 3D printing [7,10,12,13].
Despite their superior osteoinductivity, osteoconductivity, biodegradability and biocompatibility, bioceramic-based scaffolds are still confronted with some serious problems in clinical applications, especially bone implant-associated infection. Additionally, clinical studies have also demonstrated that it is extremely easy to generate pathogenic bacterial infections during orthopedic surgery or caused by infected implants, resulting in the second surgical trauma of patients [14,15]. Without the integration with antibacterial strategies, bioceramic-based scaffolds can hardly possess antibacterial properties, and thus must rely on extraneous antibacterial strategies to fight against bone implant-associated infection. Antibiotics are by far the most effective antibacterial strategy commonly used to treat bone implant-associated infection, but systemic administration is often employed to achieve the antibacterial effects in clinic. The primary challenges in treating orthopedic infections are the bacterial colonization and biofilm formation on implants, which makes it difficult for drugs and body immunity to work; and bacteria spreading along implants invade the surrounding tissues, causing bone regeneration to be delayed. Also, drug resistance induced by the overuse of antibiotics may lead to more serious infections [[14], [15], [16], [17]]. Because systemic administration may result in serious side effects and bacterial drug resistance, it is vital to employing a local antibacterial strategy by integrating a potentially antibacterial function into the bioceramic-based scaffolds for eliminating the risk of postoperative infection [16,17]. For example, MBG with porous characteristics as a carrier achieved the sustained antibacterial drug release from the grafts [18]. The antibacterial substance was modified on the surface of the scaffolds by simple immersion to form an antibacterial coating [19]. Moreover, to achieve antibacterial aims, tricalcium phosphate scaffolds doped with metal ions of silver or zinc were prepared for long-term release of antibacterial ions [20,21]. Besides, forsterite scaffolds with photothermal antibacterial properties were fabricated by combining 3D printing and polymer-derived ceramics method [22]. Therefore, the doping and release of ions, the loading and release of drugs, the physical effects induced by light, heat and sound, as well as the synergistic effects of these strategies can all achieve an antibacterial goal [[18], [19], [20], [21], [22], [23], [24], [25]]. Additionally, the efficient utilization of existing antibacterial strategies and development of novel antibacterial agents is particularly vital to fighting resistant bacteria as well [26].
Through the implementation of these strategies, bioceramic-based scaffolds can realize the inhibition and killing effect on bacteria. However, the key to scaffold design is to introduce antibacterial functions without reducing the biocompatibility and bone formation ability. Hence, the releasing concentration of ions and drugs, the effects of physical strategy and combined strategy, as well as the long-term consequences of these antibacterial strategies should be considered. For example, the authors of ref. [27] demonstrated that the long-term drug release ability and bioactivity of the scaffolds could be improved through appropriate design of the scaffolds and the reasonable loading mode of drugs. The combination of biocompatibility, osteogenic ability and antibacterial property of the scaffolds can be achieved by the proper scaffold design, selection of suitable physical strategies and optimization of process parameters [22,28]. Due to the synergistic effect of a well-combined strategy, bioceramic-based scaffolds can possess superior angiogenesis, osteogenesis, and antibacterial activity [29,30]. Thus, appropriate scaffold design strategies and antibacterial agent selections can endow bioceramic-based scaffolds with dual functions of fighting bone implant-associated infection and promoting bone repair.
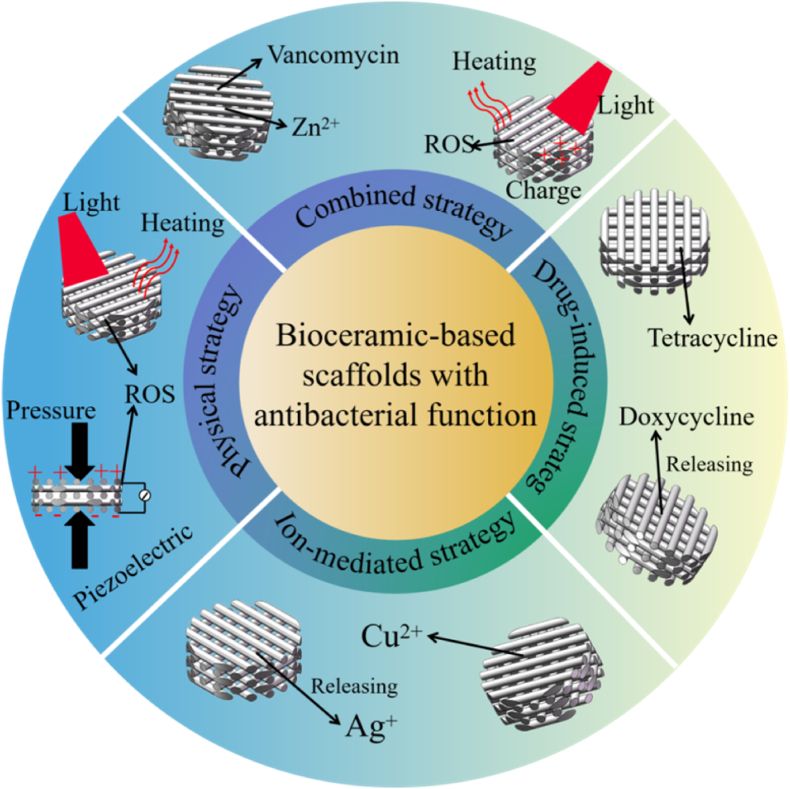
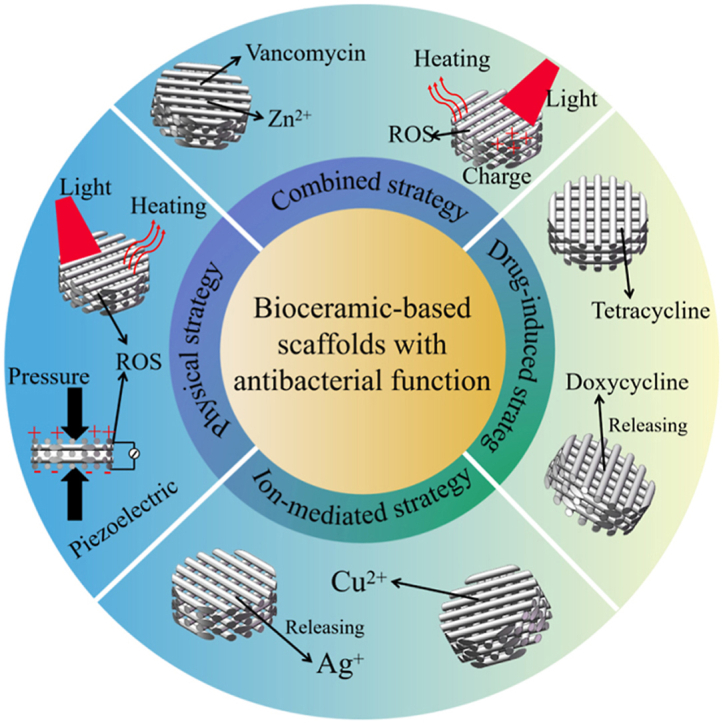
Interestingly, the bioceramic-based scaffolds themselves or after appropriate functionalization endow them with antibacterial function based on different mechanisms to fight bone implant-associated infection and promote bone repair. Therefore, this review summarizes the developments and achievements of bioceramic-based scaffolds with different antibacterial strategies for treating bone implant-associated infection and bone defects, including bioceramic-based scaffolds with drug-induced, ion-mediated, physical-activated and combined antibacterial strategies (Fig. 1). In detail, the preparation, antibacterial mechanism, antibacterial property and osteogenesis effect of each type of bioceramic-based scaffolds are introduced. Finally, a summary of current antibacterial strategies for bioceramic-based scaffolds is provided, as well as future outlooks. The functionalization of bioceramic-based scaffolds is extremely significant for bone repair and regeneration, among which antibacterial property is undoubtedly indispensable to solve the problem of bone implant-associated infection. It is expected that such bioceramic-based scaffolds with antibacterial and osteogenetic functions will be widely used in clinical applications.
Fig. 1.
Antibacterial strategies of bioceramic-based scaffolds based on various antibacterial mechanisms [[31], [32], [33], [34], [35], [36], [37], [38]].
2. Bioceramic-based scaffolds with drug-induced antibacterial function
Currently, a most commonly used way to fight against bone implant-associated infection is drug therapy [[14], [15], [16], [17]]. However, owing to general toxicity, pathogenic bacteria resistance, visceral complications and other serious side effects, systemic administration of antibacterial drugs or untargeted drug delivery is not the optimal strategy. To achieve local and targeted therapeutic effects in a real sense, antibiotics or drugs can be introduced into bionic bone scaffolds, with the bioceramic materials and the scaffold structural characteristics being possible for controlling their release [16,17]. For the combination of drug and scaffold, a lot of issues, such as the physical and chemical properties, the encapsulation and delivery efficiencies of drugs, and the preparation, material composition and scaffold structure, are necessary to be considered [[16], [17], [18]]. This efficient and less harmful antibacterial strategy focuses on successfully matching antibacterial delivery system to the 3D structure of the scaffolds, whereas the loading and releasing of drugs are the keys to achieving the antibacterial effects of bioceramic-based scaffolds. Some typical scaffolds with antibacterial function induced by drugs are summarized in Table 1.
Table 1.
Some typical bioceramic-based scaffolds loaded with different drugs for bone implant-associated infection.
| Drug categories | Scaffolds (e.g.) | Drugs loaded in scaffolds | Antibacterial mechanisms | Bacterial species | Ref. |
|---|---|---|---|---|---|
| Tetracyclines | PCL/MBG | Doxycycline | Inhibiting synthesis of protein | S. aureus, E. coil, S. epidermidis, P. aeruginosa, | [32,39] |
| Gelatin/HA | Tetracycline | S. aureus | [40] | ||
| β-lactams | CS/calcium phosphate cements; | Penicillin | Inhibiting synthesis of cell walls | S. aureus | [41] |
| Agarose/nano hydroxycarbonateapatite | Cephalexin | [42] | |||
| Aminoglycosides | TiO2 scaffold; Poly (glycidyl methacrylate)/hydroxyapatite |
Gentamicin | Inhibiting synthesis of protein | S. aureus, S. epidermidis, E. coli | [25,43,44] |
| Quinolones | Polyurethane/silica/nano-hydroxyapatite | Levofloxacin | Inhibiting synthesis or function of nucleic acid | S. aureus, E. coli | [45] |
| Monticellite | Ciprofloxacin | [46] | |||
| Glycopeptides | Baghdadite (Ca3ZrSi2O9) | Vancomycin | Inhibiting synthesis of cell wall | S. aureus | [27,33,[47], [48], [49], [50], [51], [52]] |
| Other drugs | Alginate/HA | Chlorhexidine | Breaking osmotic barrier of cell membranes | S. aureus, E. coli, | [53,54] |
| Alginate/calcium phosphate | Berberine | Reducing the number of bacteria fimbria | S. aureus, E. coli, | [55] | |
| HA/calcium sulphate | Rifampicin | Inhibiting synthesis or function of nucleic acid | S. aureus | [56] |
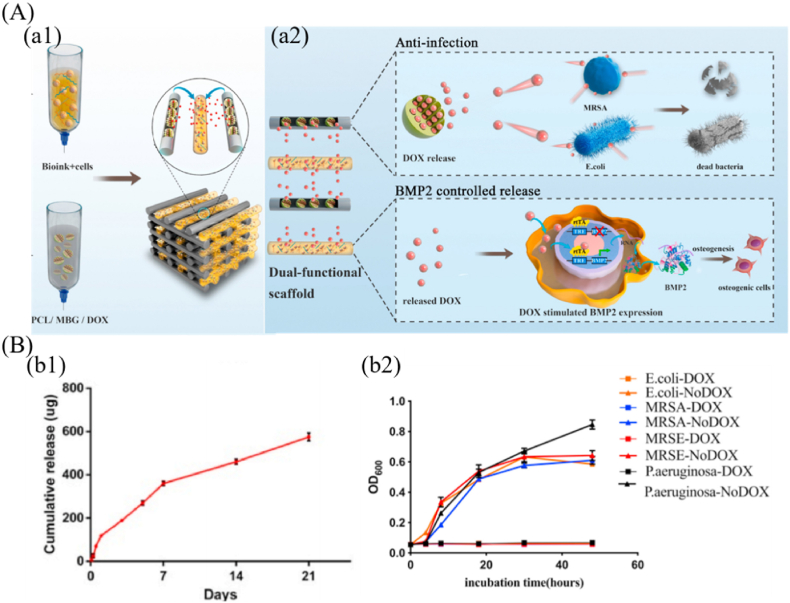
Tetracycline antibiotics, which have broad-spectrum antibacterial property, low toxicity and high efficiency, have been widely used to functionalize bioceramic scaffolds for applications in orthopedics. For instance, doxycycline-loaded Mg–Ca–TiO2 scaffolds with excellent drug release capacity, cytocompatibility, bioactivity and antibacterial activity, were prepared by using a space holder method [39]. The doxycycline release rate from the scaffolds could be adjusted by altering the loaded doxycycline concentrations and the pore characteristics of Mg–Ca–TiO2 scaffolds. The existence of an inhibition zone around the scaffold confirmed their inhibition abilities against S. aureus and E. coli bacteria. It demonstrated that appropriate structural design endowed the scaffolds with better drug loading capacity. Furthermore, biopolymer/bioceramic composite scaffolds can be used to regulate drug release as well [32,40]. For example, the authors of the study [32] fabricated a cell-laden bioink and doxycycline-loaded PCL/MBG scaffold by 3D printing with a double nozzle (Fig. 2). Doxycycline was loaded into the mesoporous channels of MBG and then mixed with molten PCL, which allowed sustained release of the antibiotic from the scaffolds. Doxycycline release profile revealed a burst release of ∼150 μg within 1 day, followed by a slow cumulative release to reach ∼400 μg at day 7 and ∼600 μg at day 21, respectively (Fig. 2 B (b1)). The preliminary results demonstrated that doxycycline significantly stimulated BMP-2 expression at a dose of 1000 ng/mL. In vitro and in vivo results also confirmed that the cell-laden scaffolds with doxycycline loading significantly inhibited bacterial adhesion and enhanced broad-spectrum antibacterial activity (Fig. 2 B (b2)), and promoted osteoblast differentiation. Therefore, such bioprinted scaffolds, with the ability to induce bone regeneration and inhibit bacterial infection, have shown a high promise for repairing infectious bone defects. Moreover, β-lactams antibiotics are widely used and effective drugs for the treatment of bone implant-associated infection owing to their high safety window with relatively few side effects. For example, 3D porous agarose/nano hydroxycarbonateapatite scaffolds containing VEGF and cephalexin, were fabricated by the combination of templating and freeze-drying methods [42]. The simultaneous release of both molecules promoted angiogenesis in chicken embryos, and also produced a local cephalexin concentration capable of suppressing S. aureus growth. Therefore, a better controlled-release effect of antibacterial drugs can be achieved by the combination of scaffold preparation methods and sustained release strategies of antibiotics, which makes bioceramic-based scaffolds possess dual functions of treating bone implant-associated infection and promoting bone repair.
Fig. 2.
Doxycycline (DOX)-loaded bioceramic-based scaffolds with antibacterial property. (A) Schematic diagram of 3D-bioprinted scaffold for promoting bone repair and inhibiting bone implant-associated infection. (a1) fabrication of 3D bioprinting scaffolds and (a2) mechanisms of antibacterial property and BMP2 controlled release ability of scaffolds. (B) Doxycycline release and in vitro antibacterial effects. (b1) doxycycline release curve within 21 days and (b2) broad-spectrum antibacterial effects of scaffolds with doxycycline (reprinted with permission from ref. [32]).
Quinolones drugs represented by levofloxacin and ciprofloxacin are also widely used in the treatment of bone implant-associated infection due to their ability to penetrate into trabecular and cortical bone and thus minimize the risk of resistance selection [45,46]. For example, a novel polyurethane/mesoporous silica/nano-hydroxyapatite scaffold containing levofloxacin was developed by a foaming method [45]. The sustained release behavior of levofloxacin was significantly increased by encapsulating it in mesoporous silica nanoparticles. In vitro results demonstrated that this scaffold could improve antibacterial activity by inhibiting bacterial adhesion and colonies, and possess favorable biocompatibility and osteoinduction by promoting proliferation and differentiation of BMSCs. Furthermore, the authors of another work [46] prepared a new multifunctional monticellite-ciprofloxacin scaffold by a space holder method with NaCl. Interconnected pores with a size range of 300–420 μm are vital to loading ciprofloxacin as well as allowing bone growth and vascularization. Antibacterial tests indicated that the antibacterial effects of scaffolds were strongly related to the ciprofloxacin concentration, with the scaffold containing 6% ciprofloxacin exhibiting the highest bacterial inhibition. Furthermore, the monticellite scaffold loaded with 3% ciprofloxacin resulted in greater cell attachment, cell proliferation and cell viability than for the scaffolds containing 6% ciprofloxacin. The balance of compressive strength, bioactivity, antibacterial performance and biocompatibility can be acquired by regulating the proper loading ratio of ciprofloxacin in scaffold to fight against bone implant-associated infection. Obviously, loading quinolones on bioceramic-based scaffolds by appropriate means could achieve better drug sustained release, antibacterial activity and osteogenesis ability.
Possessing an obvious effect on gram-negative bacteria, aminoglycoside antibiotics represented by gentamicin have also been widely utilized to treat bone implant-associated infection. It was an excellent strategy that the polymers loaded with antibacterial drugs were coated on bioceramic scaffolds [25,43]. For example, TiO2 scaffolds fabricated by a polymer sponge replication method were coated with gentamicin-loaded PLGA microparticles, and the pattern of ‘burst release with following sustained release’ was sought to avoid perioperative bone implant-associated infection [43]. In vitro tests verified the antibacterial activity of the released gentamicin from the scaffold against Staphylococcus spp. as well as the cytocompatibility of scaffolds with osteoblast-like cells. Most release principles of antibiotics loaded on scaffolds are based on their concentration gradient diffusion; however, the fatal problem with this mode is that the drugs show a rapidly declining tendency and eventually limit the antibacterial effects. Therefore, if the scaffolds have smart drug release behavior according to the environmental changes, the utilization efficiency and release time of drugs will be greatly improved. Given this, the researchers of ref. [44] designed a novel self-adaptive antibacterial porous poly (glycidyl methacrylate)/hydroxyapatite/gentamicin implant with long-term responses for therapy of infected bone defects, in which the release of gentamicin could be triggered by the acidic environment created by the metabolism of bacteria. The cumulative release ratio reached 35% and virtually 0% at day 7 under pH = 5.0 and 7.4 respectively, demonstrating that gentamicin was gradually released from the implant under weakly acidic conditions, and hardly released under neutral conditions. The highly effective in vivo antibacterial therapy with the implant was evaluated in one infected bone defect rabbit model. Because of the high drug loading capacity, responsiveness to acidic environments, and chemical stability of scaffolds in neutral conditions, a long-term antibacterial effect was readily acquired. This design strategy of sustainable self-adaptive antibacterial implants provides a promising concept for the prevention and therapy of bone implant-associated infections.
Glycopeptides drugs, such as vancomycin, are narrow-spectrum antibiotics that are exclusively effective against gram-positive bacteria, particularly sensitive to resistant S. aureus. Vancomycin, as an antibacterial agent loaded in scaffolds, has been widely used in bone implant-associated infections [47,48]. For instance, the authors of study [47] prepared vancomycin-loaded baghdadite (Ca3ZrSi2O9) scaffolds with a pore size of 300–400 μm and a total porosity of 80–82% by a space holder method. Such porous structure made the drug release behavior of the scaffolds follow a burst release during the first 12 h and a sustained release afterwards, as well as exhibited a concentration-dependent tendency, i.e., the higher the concentration, the faster the release. Combined antibacterial evaluation with the preliminary biocompatibility, the vancomycin-loaded (3 wt%) scaffold was a highly prospective candidate for preventing post-surgery infections, as well as for bone tissue engineering. On the other hand, more studies are being focused on bioceramic-based composite scaffolds that combine bioceramics and polymers as drug carriers [27,33,[49], [50], [51], [52]]. For example, Gelatin/biphasic calcium phosphate/45S5 glass composite scaffolds with vancomycin and BMP-2 loading could be applied for treating bone implant-associated infection and promoting bone regeneration [33]. Another kind of vancomycin-loaded PLGA/MBG composite scaffolds was manufactured by a freeze-drying method [52]. In this work, the vancomycin encapsulated in the mesoporous channels of MBG was rapidly released from the PLGA/MBG scaffolds within the first 3 days, followed by a slower and more moderate release profile. In vitro results revealed that loading vancomycin onto the PLGA/MBG scaffolds could inhibit biofilm formation, enhance antibacterial effect and ultimately improve cytocompatibility and osteoblastic differentiation compared with pure PLGA scaffolds. Undoubtedly, such novel inorganic-organic composite scaffolds are considered as potential materials for the treatment of infected bone defects.
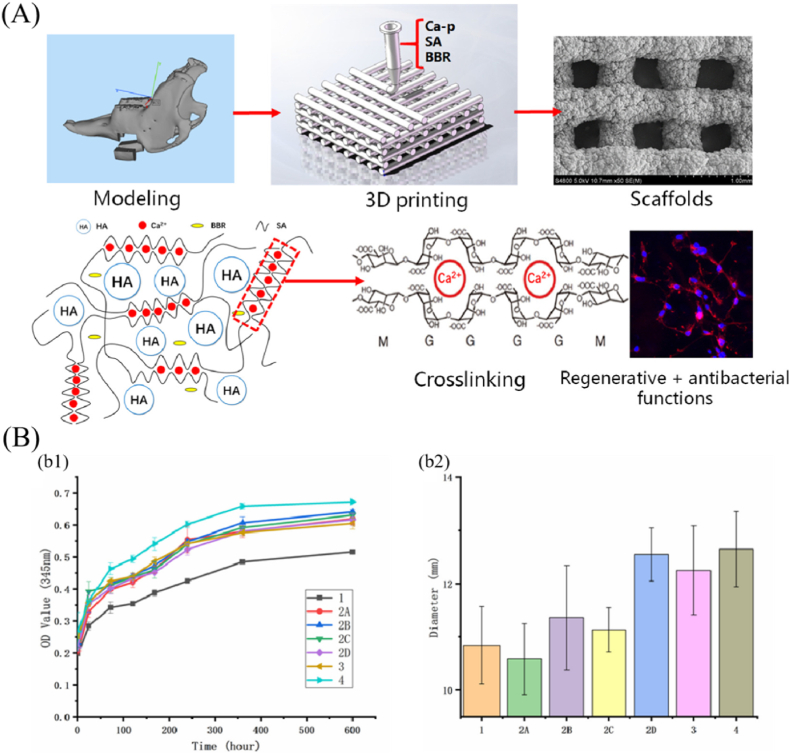
In addition, many other antibacterial drugs, such as chlorhexidine, berberine and rifampicin, have also been used to load onto bioceramic-based scaffolds for bone implant-associated infection. As a common antibacterial agent, chlorhexidine has a broad-spectrum bactericidal effect and low drug resistance. Loading chlorhexidine into bioceramic-based composite scaffolds gave the scaffolds strong antibacterial properties, indicating the potential for biomedical applications, particularly controlled drug delivery in dentistry [53,54]. Berberine, an alkaloid component extracted from botanicals, has been long used as a heat-clearing, detoxifying and antibacterial drug. For instance, the researchers of ref. [55] loaded berberine into an alginate/calcium phosphate composite scaffold prepared by 3D printing for fighting bacterial infection during bone repair (Fig. 3). As shown in Fig. 3 (A), the preparation procedures involved combining calcium phosphate powders, berberine and sodium alginate to modulate the printing inks, and then fabricating the porous scaffolds by direct extrusion 3D printing and cross-linked method in situ. According to the release profile, berberine was rapidly released from the scaffold in the early stages, followed by a gradual transition to stable release (Fig. 3 B (b1)). In vitro biological tests showed that berberine-loaded scaffolds possessed outstanding antibacterial property (Fig. 3 B (b2)) and promoted the adhesion and proliferation of MC3T3 cells. Therefore, 3D printed calcium phosphate scaffolds possess the controlled-release capacity of berberine, excellent antibacterial property, and is a promising biomaterial for jaw repair. Rifampicin, a first line antituberculosis drug, has been shown to have a potent bactericidal effect on S. aureus by eradicating both adherent and stationery-phase staphylococci. For example, the researchers of ref. [56] designed a nanohydroxyapatite-based scaffold adopted as the drug carrier to treat bone implant-associated infection by local and sustained delivery of rifampicin. Based on in vivo and in vitro antibacterial and osteogenetic experiments, this work demonstrated that the nanohydroxyapatite-based bioceramic scaffolds, as a carrier of rifampicin, could eliminate bacterial infection while simultaneously promoting bone repair and regeneration.
Fig. 3.
3D printed scaffolds loaded with berberine for bone implant-associated infection and bone repair. (A) Schematic diagram of the scaffold fabrication process; (B) Berberine release profile and statistical analysis of antibacterial results. (b1) release curves of berberine from scaffolds, (b2) the effects different scaffolds on statistical diameter of the bacteriostatic zones (reprinted with permission from ref. [55]).
Consequently, a variety of studies proposed a local delivery strategy for antibacterial drugs based on the loading of antibiotics into bioceramic-based scaffolds, which could inhibit the adhesion and massive proliferation of bacteria, as well as improve the osteogenesis property. Although antibiotics remain the most effective antibacterial strategy by far, the problem of bacterial drug resistance is already imminent. With the exception of the intrinsic resistance, drug resistance is primarily acquired from the usage of drugs, particularly drug abuse. Therefore, avoiding drug abuse and increasing utilization efficiency may contribute to reducing the incidence of drug resistance while simultaneously increasing antibacterial capacity. The discussion in this section demonstrated how the controlled release property and utilization efficiency of drugs could be improved by the design and manufacture of bioceramic-based scaffolds and the ingenious loading of drugs on the scaffolds. In addition, they could be improved by developing smart responsive scaffold materials that could respond to the infected microenvironment and release minute amounts of drugs directly into the infected area to kill bacteria. The development and application of novel antibacterial agents may also be critical for reducing drug resistance and increasing antibacterial effect.
3. Bioceramic-based scaffolds with ion-mediated antibacterial functions
While modifications to bioceramic-based scaffolds are required to increase their capacity for infection treatment, the degradation properties of bioceramics doped with antibacterial ions enable them to function adequately against bacteria on their own; the key is the doping of functional ions and the control of degradation behavior. For example, the degradation behaviors of most bioceramic-based scaffolds may induce the release of ions into the surrounding microenvironment, which could result in the increase of pH and is detrimental to the growth of acidophilic bacteria [23]. Although the ions released from the scaffolds can raise the pH and theoretically inhibit bacterial growth by regulating the microenvironment, bacteria cannot be effectively killed, especially in the case of bacterial biofilm formation. So far, few bioceramic scaffolds are actually demonstrated to have significant antibacterial activities. Therefore, it is necessary to introduce functional elements into the scaffolds for killing bacteria, such as Ag+, Zn2+, Cu2+ and La3+, etc. (Table 2). These elements can be doped, incorporated, or coated into bioceramic-based scaffolds in the form of oxide, ions, or micro-nano particles.
Table 2.
Ions released from bioceramic-based scaffolds for treating bone implant-associated infection.
| Categories | Scaffolds (e.g.) | Released ions | Antibacterial mechanisms | Bacteria used for antibacterial assays | Ref. |
|---|---|---|---|---|---|
| Alkali and alkaline earth ions | CaO–P2O5–SiO2–RbO MBG scaffold; Forsterite scaffold |
Ca2+, Na+, Mg2+, Rb+ | Inducing the increase of solution pH and osmotic pressure | S. aureus, E. coli, S. epidermidis, P. aeruginosa | [30,[57], [58], [59], [60], [61], [62]] |
| Heavy metal ions | Ag@rGO modified β-TCP scaffold | Ag+ | Inducing ROS; Breaking bacterial cell membranes | S. aureus, E.coli, Bacillus subtills | [19,35,[63], [64], [65], [66], [67], [68], [69], [70], [71], [72], [73], [74]] |
| PCL/SiO2–CaO–P2O5–ZnO MBG scaffold | Zn2+ | Destabilizing membrane and enhancing permeability; Deactivating nucleic acids and enzymes; Killing bacterial by inducing ROS | E. coli, S. aureus | [[75], [76], [77], [78], [79], [80], [81]] | |
| SiO2–CaO–P2O5–CuO MBG scaffold | Cu2+ | Disrupting bacterial cell wall; Inhibiting DNA replication; Inducing ROS to inhibit bacterial growth | E. coli, S. aureus | [[82], [83], [84], [85]] | |
| Other ions | Na2O–CaO–P2O5–La2O3 BG scaffold | La3+ | Causing leakage of cell contents; Inactivating genetic materials, enzymes and proteins; Bacterial apoptosis | S. aureus, E. coli | [86] |
| ZrO2 modified Chitosan/poly (ethylene glycol)/nano-hydroxyapatite scaffold | Zr4+ | Interacting with sulphur-containing proteins and DNA; Attacking respiratory chain; Inhibiting cell division and causing death | E. coli, B. ereus, L. fusiformis | [87] | |
| KI-loaded bilayer scaffold | I− | Damaging cell membrane by reacting with respiratory chain-associated enzymes and membrane proteins | S. aureus, E. coli | [88] |
Bioglass (BG) with inherent antibacterial properties, as one type of perfect bioceramics, not only has exceptional osteoinductive potential, but also possesses distinct antibacterial behaviors [14,57]. For example, the authors of study [60] confirmed the outstanding antibacterial properties of the SiO2–CaO–Na2O–P2O5 glass scaffolds according to systematic in vitro evaluations. New bioglass scaffolds composed of SiO2–P2O5–CaO–Na2O–SrO–F or SiO2–Na2O–Al2O3–CaO–B2O3 were also demonstrated to have good antibacterial properties [58,59]. In addition, due to its excellent antibacterial property, high osteogenetic activity and mechanical strength, forsterite (Mg2SiO4) scaffold has become one of the ideal materials in bone repair [61,62]. The possible antibacterial mechanism was that alkaline ions released from these scaffolds increased the solution pH and osmotic pressure, resulting in cell membrane depolarization and bacterial mortality [[58], [59], [60], [61], [62]]. Although certain bioceramics have inherent antibacterial capabilities, future research is hampered by their limited antibacterial properties. Given this, particular antibacterial metal agents, such as elemental substances, oxides, and ions, must be introduced into the scaffolds [69,89]. The requirements lie in the fact that these excellent dopants are biocompatible for normal cells, lethal to drug-resistant bacteria and do not interfere with the effective osteogenesis of scaffolds [90].
Silver (Ag) is the most well-known antibacterial agent used in different bioceramic-based scaffolds. Nevertheless, its antibacterial actions should consider to avoid adverse effect on the osteogenesis, biocompatibility, or bioactivity [91,92]. With its significant antibacterial ability, Ag could be introduced into different biocereamic-based scaffolds using various procedures such as coating, doping, and mixing, in diverse forms including ions, particles, and oxides [19,35,[63], [64], [65], [66], [67], [68], [69], [70], [71], [72], [73], [74]]. Among these, the sponge replication method was applied for preparing Ag-containing antibacterial glass-ceramic scaffolds with good HA mineralization, osteogenesis, and antibacterial property [72]. In addition, the reduction synthesis method of silver nanoparticles can be used to construct antibacterial coatings on bioceramic-based scaffolds as well. The researchers of another work [19], for example, modified the 3D printed β-TCP scaffolds with Ag@rGO coating to treat bone implant-associated infection and promote bone repair. Silver-modified scaffolds had strong antibacterial effect at a low concentration (3.5 mg/mL) without reducing the cell viability. The in vitro antibacterial results demonstrated that the scaffolds with Ag@rGO nanocomposites presented excellent antibacterial activity. Furthermore, the scaffolds coated with Ag@rGO nanocomposites significantly accelerated the osteogenic differentiation of rabbit bone marrow stromal cells by improving their ALP activity and bone–related gene expression (OCN, Runx-2, OPN and BSP). It reveals that bifunctional scaffolds with antibacterial activity and osteogenesis ability are favorable to the restoration of large-bone defects while treating infections.
Znic (Zn), which has good osteogenesis, vascularization, and antibacterial property, is primarily derived from metal zinc, zinc salt compounds and zinc oxide, where controlled release of zinc ions is important for bioceramic-based scaffolds [[75], [76], [77], [78], [79]]. For example, the PCL/SiO2–CaO–P2O5–ZnO MBG scaffold was prepared by using the sol-gel method and 3D printing, and the mesoporous structure of MBG induced its good degradability, bioactivity and antibacterial properties [77]. The released Zn2+ amount was higher than the reported average Zn2+ ion concentration in human plasma (0.95–1.30 ppm), but lower than the in vitro toxic levels (5.9–6.1 ppm), showing antibacterial activity against S. aureus. It was demonstrated that Zn2+ released from the scaffolds could penetrate the cell walls and caused the cell contents to flow out, deactivate nucleic acids and enzymes, and activate ROS to kill bacteria [80,81]. Obviously, the scaffolds would be a viable choice for bone regeneration applications due to its bioactivity and antibacterial property. Furthermore, the researchers of another work [78] fabricated one kind of yttrium oxide-stabilized zirconium oxide (3Y–ZrO2) scaffolds using 3D printing and coated them with nanoscale zinc oxide. It was proven by in vitro and in vivo evaluations that the as-prepared hip prosthesis could exactly match the corresponding parts, as well as exhibit high biocompatibility and outstanding antibacterial activity. Therefore, the introduction of Zn into bioceramic-based scaffolds endowed them with dual antibacterial and osteogenesis functions, showing a great clinical potential.
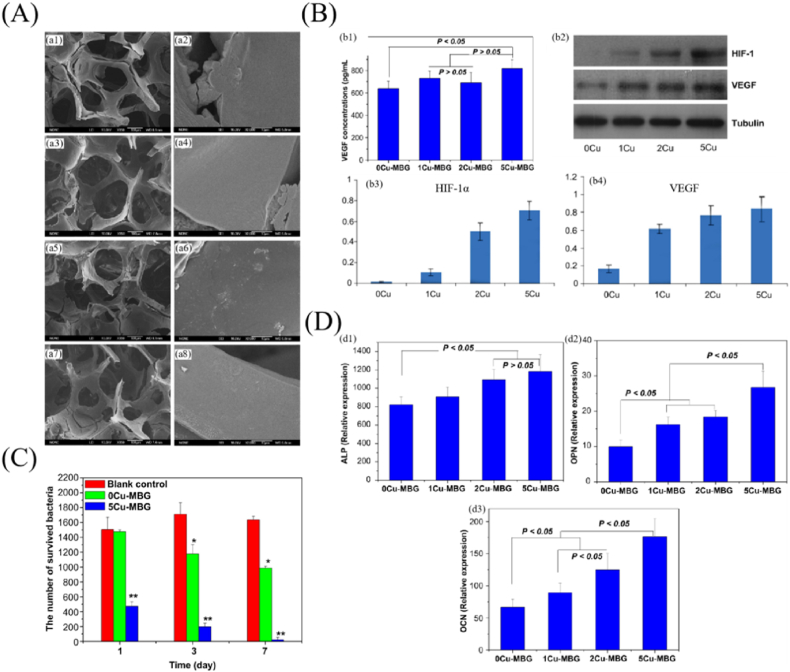
Copper (Cu) is a commonly utilized therapeutic agent with outstanding angiogenic and antibacterial activities, particularly against gram-positive and gram-negative bacteria. Nevertheless, excessive concentrations can cause cytotoxicity and even the apoptosis of normal cells [[82], [83], [84], [85],93]. Therefore, it is very critical to control the sustained release of Cu2+ by ingenious designs and effective methods. To achieve such goal, the authors of ref. [82] prepared an active antibacterial coating on the alginate/BG scaffolds by cross-linking copper ions with alginate, which provided the scaffolds with outstanding antibacterial property and biocompatibility. Another copper source was copper oxide, which was introduced into scaffolds as antibacterial dopants for bioceramic scaffolds. For example, the authors of ref. [93] designed one type of copper-containing MBG scaffolds with multifunctional ibuprofen delivery capacity, bioactivity, angiogenesis, osteogenesis and antibacterial activity, and the mesoporous structure significantly improved these performances (Fig. 4). To be specific, SiO2–CaO–P2O5–CuO scaffolds and their ionic extracts could stimulate HIF-1α and VEGF expression in human bone marrow stromal cells (Fig. 4 (B)) and significantly promote the osteogenic differentiation of hBMSCs by improving bone-related gene expression of ALP, OPN and OCN (Fig. 4 (D)). On the other hand, the Cu-containing MBG scaffolds significantly inhibited bacterial viability owing to a sustained release of Cu2+ from the scaffolds (Fig. 4 (C)). The antibacterial mechanism might be that the Cu2+ released from the scaffolds could disrupt bacterial cell walls, thereby inhibiting bacterial DNA replication and inducing ROS to kill bacteria [[82], [83], [84], [85],93]. Thus, it was possible to achieve the ideal angiogenesis, osteogenesis, and antibacterial effects by properly incorporating Cu into MBG scaffolds and managing the release concentration of Cu2+. As a consequence, appropriate strategies for controlling the degradation performance of bioceramic-based scaffolds is critical for establishing controlled release of Cu2+ and achieving biological and antibacterial properties.
Fig. 4.
SiO2–CaO–P2O5–CuO MBG scaffolds with angiogenesis, osteogenesis and antibacterial activity. (A) SEM images of the MBG scaffolds. (a1, a2) 0Cu-MBG, (a3, a4) 1Cu-MBG, (a5, a6) 2Cu-MBG, and (a7, a8) 5Cu-MBG. (B) Angiogenesis capacity of the scaffolds. (b1) VEGF secretion by ELISA, (b2) HIF-1a, VEGF and Tubulin expression by western blotting, (b3) HIF-1a expression and (b4) VEGF expression for hBMSCs. (C) Antibacterial evaluation of the scaffolds. (D) Osteogenesis assay of the ionic extracts from Cu-MBG particles. (d1) ALP, (d2) OPN and (d3) OCN for hBMSCs (reprinted with permission from ref. [93]).
Interestingly, there are additional critical elements that may be employed to treat bone implant-associated infection and promote bone repair. For example, the researchers of work [86] investigated the biological activity and antibacterial mechanism of lanthanum (La)-doped phosphate-based BG scaffolds. It demonstrated that the La3+ could successfully replace Ca2+ to penetrate into the bacterial cells, resulting in the leakage of cell contents, the inactivation of genetic materials, enzymes and proteins, and finally the apoptosis of bacteria. In addition, zirconia nanoparticles could be selected as a reinforcement and antibacterial agent of nanoscale HA to construct a composite scaffold with significant antibacterial activity [87]. The findings indicated that the zirconia nanoparticles may interact with phosphorus-containing compounds in bacterial cell membranes, such as sulphur-containing proteins and DNA, before entering the cell and attacking the respiratory chain, ultimately killing the bacteria. Additionally, another study reported a multifunctional bilayer scaffold with antibacterial activity for wounds that are infected or chronic [88]. The potent antibacterial activity of nanofibrous sheet may be attributed to the role of iodine in destroying the cell membranes through reactions with respiratory chain-associated enzymes and membrane proteins.
As demonstrated in the preceding investigations, the ion-mediated antibacterial strategy can provide a long-term release of ions and an optimum antibacterial effect by carefully selecting and optimizing materials and processes. Through numerous examples in this section, it was discovered that there was an optimal concentration range of ion release for ion-mediated antibacterial scaffolds, with the biocompatibility being adversely affected beyond a certain point. However, the release behavior of bioceramic-based scaffolds can be a sudden tendency, especially for bioactive materials, so the concentration range of ions released should be strictly managed in considering ion-mediated antibacterial methods. Because the majority of antibacterial ions used in ion-mediated strategies are heavy metal elements, the biocompatibility of the ions requires additional considerations and attentions. On the other hand, antibacterial ions commonly possess additional functions such as osteogenesis, angiogenesis, and immunoregulation, necessitating an analysis of the type and amount of ions used in relation to the specific application. Therefore, considering the use of an ion-mediated antibacterial strategy, it is necessary to take into account all of the influencing impact factors in order to achieve the optimal effect.
4. Bioceramic-based scaffolds with physical antibacterial functions
Endowing bioceramic-based scaffolds with physical antibacterial functions is another important antibacterial strategy, which is based on their physical properties, such as surface charge and topological structure, or the change of scaffolds’ surrounding microenvironment induced by external stimulation (light, magnetic field and ultrasound) to kill bacteria [22,36,38,[94], [95], [96]]. Nanomaterials and nanostructures have unique physical and chemical properties, which may play an important role in physical-activated antibacterial strategies, especially in fighting drug-resistant bacteria [[97], [98], [99]]. Table 3 shows the typical categories of physical antibacterial scaffolds for bone implant-associated infection, and the following specific examples are provided for expounding this antibacterial strategy.
Table 3.
Some typical bioceramic-based scaffolds with physical antibacterial functions for bone implant-associated infection.
| Categories | Scaffolds (e.g.) | Antibacterial mechanisms of scaffolds | Bacteria used for antibacterial assays | Ref. |
|---|---|---|---|---|
| Surface charge | Chitosan/zoledronic acid/nano hydroxyapatite scaffold; chitosan/zein/silica scaffold | Positively charged surface disrupting the negatively charged membrane of bacteria; Covering bacterial cell wall to block transport; Penetrating bacterial cell wall to prevent DNA replication | E. coli, S. aureus | [[100], [101], [102]] |
| Pressure (surface charge) | Potassium-sodium niobate scaffold; (Ba,Ca) (Ti,Zr)O3 scaffold | Piezoelectric effects inducing surface charge; Surface charge generating micro-electric field and ROS around the material to kill bacteria | E. coli, S. aureus | [38,94] |
| Photothermal effect | Free carbon-containing forsterite scaffold; Forsterite-hydroxyapatite scaffold | Photothermic effect generating ROS and increasing temperature to kill bacteria | S. aureus, E. coli, MRSA | [22,99,103] |
| Magnetothermal effect | Mg2SiO4–CoFe2O4 scaffold | Magnetothermal effect generating thermal energy and increasing temperature to kill bacteria | S. aureus, E. coli | [95,104] |
| Sonodynamic effect | Palacos (bone cement) scaffold | Attaching to certain cellular components and inducing damage under ultrasound irradiation; Generating ROS inducing oxidative damage to the cell wall | MRSA, S. aureus, E. Coli, P. aeruginosa |
[[105], [106], [107]] |
| Photocatalysis | GDY-modified TiO2 nanofiber scaffold; TiO2 scaffold | Generating ROS to kill bacteria | MRSA, S. aureus, E. coli | [36,108] |
Derived from the exoskeleton of crustaceans, CS is one kind of natural polymers that possesses excellent biocompatibility, biodegradability and antibacterial property, and has a wide range of applications in bone tissue engineering [109]. CS is a positively charged alkaline polysaccharide that disrupts the negatively charged outer membrane of microbes to exert antibacterial activity, which is one of the characteristics required in bone tissue engineering [100]. Owing to the fact that CS materials have poor mechanical property and osteogenic activity, it is necessary to develop CS/bioceramic composite scaffolds with dual osteogenic and antibacterial properties for bone repair. For example, chitosan/zoledronic acid/nano hydroxyapatite scaffolds were fabricated by freeze drying, which exhibited excellent biocompatibility, osteoinductivity, and antibacterial activity against clinically pathogenic S. aureus and E. coli (nearly 100% inhibition) [102]. Additionally, the zoledronic acid-loaded scaffolds showed excellent in vitro tumor inhibition efficiency against giant cell tumors of bone. Thus, the multifunctional composite scaffolds may provide significant benefits in repairing tumor-induced bone defects. Furthermore, the antibacterial activity of scaffolds was derived from their interaction with the bacterial cell wall, penetrating the cell wall to inhibit DNA replication and covering the cell wall of bacteria to hinder the transport of nutrients [102]. The antibacterial property of CS was insufficient to address the complicated requirements of bone implant-associated infection; however, it may be coupled with other antibacterial materials, such as ions and drugs, to enhance antibacterial capabilities, as detailed in the combined strategies section [[110], [111], [112], [113]].
Under the piezoelectric effect, the surface of piezoelectric ceramics will generate positive charge, which can also induce antibacterial activity [38,94]. For example, the recent study reported the antibacterial effect of potassium-sodium niobate ceramics by manipulating their piezoelectric properties [38]. The results showed that the piezoceramics were capable of decreasing the colonies of bacteria S. aureus, promoting the proliferation, adhesion and spreading of rat bone marrow mesenchymal stem cells. Results also revealed that the antibacterial ratio, the bacterial membrane shrinkage and the ROS production highly correlated with the number of positive charges on the surface of the piezoceramic materials, indicating that the possible antibacterial mechanism is derived from ROS induced by surface charge. Furthermore, the authors of another study [94] developed a lead-free piezoelectric (Ba,Ca) (Ti,Zr)O3 scaffold with enhanced antibacterial property for bone tissue engineering. The charged surfaces of scaffolds revealed good antibacterial responses, which were attributed to the micro-electric field around the materials formed by surface charge, and ROS generated by the decomposition of the surrounding solution. Therefore, it might be a new insight to apply the intrinsic electrical properties of biomaterials to solve the infective problems of bone implants.
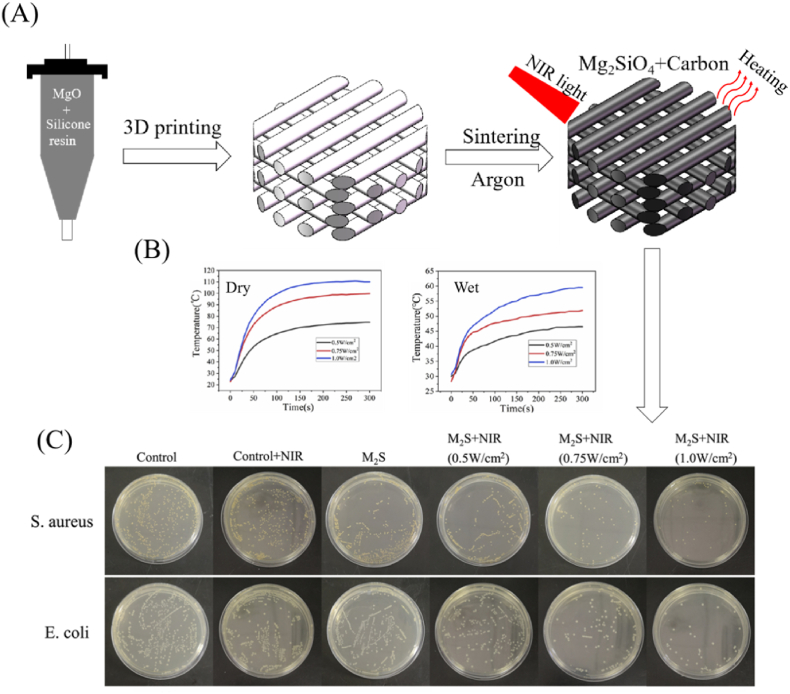
Physical thermal effect refers to the ability of certain materials and structures with specific physical properties, such as photothermal effect and sonodynamic effect, to absorb energy sources via light, sound, and other action pathways, causing lattice vibration, generating thermal energy, and increasing temperature. Physical thermal therapy is a safe and successful technique for treating bone implant-associated infection that primarily relies on the physical thermal effect to directly trigger heat generation and kill bacteria selectively [22,114]. The materials with physical thermal effect, such as carbon-based nanocomposites (graphene derivatives and carbon nanotubes) and metallic compound nanocomposites (copper sulfide and molybdenum sulfide), can be introduced into scaffolds in the form of a coating or matrix materials [114]. For example, the researchers of ref. [22] combined 3D printing with a polymer-derived-ceramics strategy to produce a porous forsterite scaffold with photothermal antibacterial activity (Fig. 5). The forsterite scaffolds were sintered at a high temperature in an argon atmosphere, producing free carbon with a strong photothermal effect. Obviously, the photothermal temperature of forsterite scaffolds could be controlled through NIR laser power density (Fig. 5 (B)). In vitro antibacterial experiments demonstrated that the scaffolds with free carbon exhibited excellent photothermal effect and were capable of inhibiting the growth of pathogenic bacteria (E. coli and S. aureus) under NIR irradiation (Fig. 5 (C)). Hence, forsterite scaffolds fabricated by the combining of 3D printing and polymer-derived-ceramics strategy would be an attractive choice for bone tissue engineering. In another example, multifunctional magnetic Mg2SiO4–CoFe2O4 scaffolds with magnetothermal effect were prepared by using the polymer sponge templating method, which can be similarly applied for treating bone implant-associated infection [95]. Such physical magntothermal antibacterial action, which is based on the susceptibility of various cells to heat, may effectively inhibit the proliferation of drug-resistant bacteria while preserving normal cell growth and differentiation [104].
Fig. 5.
Forsterite scaffolds with photothermal-induced antibacterial activity by 3D printing and polymer-derived ceramics strategy. (A) Schematic diagram for fabrication of forsterite scaffolds, (B) Photothermal properties of forsterite scaffolds under dry and wet conditions, and (C) In vitro evaluation of photothermal-induced antibacterial activity of forsterite scaffolds (reprinted with permission from ref. [22]).
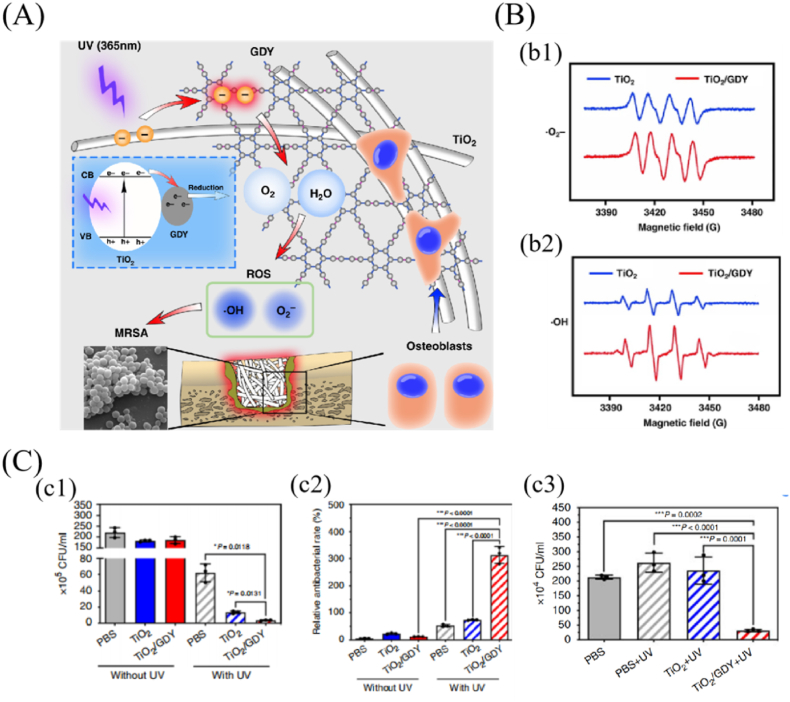
Photocatalytic reaction has the characteristics of high efficiency and low energy consumption, making it an attractive way for treating bone implant-associated infection. The principle is that photocatalytic agents form hole-electron pairs in the presence of light and then redox react with substrate molecules (such as O2 and H2O) at the interface to generate a sequence of ROS capable of killing a wide range of bacteria [36,96]. Although TiO2 has the photocatalytic activity to produce ROS, the recombination of generated electrons and holes limits its antibacterial ability. Therefore, a GDY-modified TiO2 nanofiber scaffold with enhanced photocatalytic antibacterial activity was designed for preventing implant infection (Fig. 6) [36]. Under UV irradiation (365 nm), the electron spin resonance spectra revealed that the signals of ROS (·OH and ·O2−) formed by GDY/TiO2 were higher than those by TiO2 (Fig. 6 (B)). Due to the capability of electron separation of GDY, free electrons from the photocatalytic-activated TiO2 were transported to the GDY surface and had a longer lifespan, considerably increasing the ROS production ability of TiO2 nanofibers. In vitro and in vivo antibacterial results also revealed that GDY-modified TiO2 nanofiber scaffold showed better antibacterial effects (MRSA) under UV irradiation than TiO2 without GDY (Fig. 6 (C)). Additionally, both in vitro and in vivo tests demonstrated that GDY-modified TiO2 nanofiber scaffold significantly promoted osteogenic differentiation than pure TiO2. Therefore, GDY-modified TiO2 nanofiber scaffold facilitated the process of bone tissue regeneration in drug-resistant bacteria-induced infection of bone implants. Whereas, photocatalytic agent, like biocompatible TiO2, needs UV light to produce antibacterial ROS, which directly limits their application on scaffolds implanted in the body. In comparison to UV light, NIR light could penetrate into the deeper tissues and may have more potential applications for photodynamic therapy of implants. A recent study reported that the photocatalytic ability of TiO2 was greatly improved by the dope of F, Yb and Ho, which could generate sufficient ROS to effectively remove the S. aureus biofilms of implants under 1060 nm laser irradiation for 15 min [108]. Thus, endowing the scaffolds with antibacterial functions that are triggered by catalytic activity, is a promising choice to treat bone implant-associated infection, even those caused by drug-resistant bacteria.
Fig. 6.
Graphdiyne-modified TiO2 nanofiber scaffold with osteogenesis and enhanced photocatalytic antibacterial activity irradiated by UV light. (A) Schematic diagram of the dual function of GDY/TiO2 in orthopedic implant infection. (B) Electron spin resonance for (b1) •O2− and (b2) •OH generation after TiO2 and GDY/TiO2 induced by UV. (C) In vitro and in vivo antibacterial evaluation of the GDY/TiO2 nanofiber scaffold. (c1) quantitative analysis of in vitro bacterial colonies, (c2) statistical analysis of the live/dead staining of in vitro antibacterial tests, (c3) quantitative analysis of the bacterial colonies of the infected femurs treated with TiO2 and GDY/TiO2 scaffolds (reprinted with permission from ref. [36]).
Encouragingly, physical-activated antibacterial strategies are the most probable approach to fighting drug-resistant bacteria based on the microenvironment changes triggered by physical effects to kill bacteria. Numerous examples also have demonstrated its ability to kill drug-resistant bacteria, which may be induced by the changes of microenvironment (such as heat, charge and ROS etc.) resulted from stimulating the bioceramic-based scaffolds or the sensitive materials on them by physical signals (ultrasonic, optical, magnetic etc.). It is difficult for bacteria to fundamentally adapt to these changes of the microenvironment in a short period of time, thus bacteria are directly or indirectly killed. Additionally, cells and bacteria tolerate differently to the changes of microenvironment, making it possible to merely kill bacteria without damaging the cells. More importantly, as shown in the numerous examples, the physical-activated antibacterial strategy allows the quantitative control of parameters, which contribute to the formation of a favorable antibacterial microenvironment. Therefore, physical-activated antibacterial strategies are expected to truly address the problem of bacterial resistance, leading to the goal of improving antibacterial effect and bone repair.
5. Bioceramic-based scaffolds with combined antibacterial functions
Each type of antibacterial strategies show great potential for treating infected bone defects, however, most cases indicated that relying on a single antibacterial strategy makes it difficult to meet the intricate requirements. A combination of antibacterial strategies on one scaffold, such as drug and ion therapy, drug and physical therapy, or physical and ion therapy, may be favorable to enhance the antibacterial effectiveness. It is critical to emphasize that, although the combined strategy entails additional demands on scaffold design and material selection, it is the most effective way to maximize antibacterial properties by integrating diverse antibacterial agents with scaffolds. Some typical combined antibacterial strategies of bioceramic-based scaffolds are listed in Table 4.
Table 4.
Some typical bioceramic-based scaffolds with combined antibacterial strategies for bone implant-associated infection.
| Antibacterial strategies | Scaffolds (e.g.) | Specific objects | Bacteria used in antibacterial assays | Ref. |
|---|---|---|---|---|
| Combined drug-induced and drug-induced | PHBHHx/80SiO2–15CaO–5P2O5 MBG | Levofloxacin, vancomycin and rifampicin; Isoniazid and rifampicin | E. coli, S. aureus | [37,115,116] |
| Combined drug-induced and ion mediated | Enoxacin-loaded CaO–P2O5–SiO2–RbO MBG scaffold | Enoxacin and Rb2+; Vancomycin and Zn2+ | E. coli, S. aureus | [30,47] |
| Combined ion mediated and ion mediated | poly (octanediol citrate)/SiO2–CaO–ZnO-Ga2O3 scaffold | Zn2+ and Ga3+ | E. coli, S. aureus | [117] |
| Combined drug-induced and charge effect | Vancomycin-loaded CS/Si-doped hydroxyapatite scaffold | CS and chlorhexidine; CS and Penicillin; CS and vancomycin | E. coli, S. aureus | [41,113,118,119] |
| Combined charge effect and ion-mediated | Chitosan/polyethylene oxide/ZnO scaffold | CS and Zn2+; CS and Ag+; CS and Se-HA |
E. coli, S. aureus | [110,112,120] |
| Combined photothermal effect and ion-mediated | Forsterite scaffolds | Forsterite and free carbon | E. coli, S. aureus | [22] |
| Combined photothermal effect and charge effect | HA scaffold | BPs and ZnL2 | E. coli, S. aureus | [121] |
| Combined charge effect and physical therapy | Polyethylenimine/MXene@CeO2 scaffold | Cationic polyethylenimine and MXene (Ti3C2Tx) | MRSA, E. coli, S. aureus | [122] |
Some studies have developed a multi-drug method to improme the antibacterial efficacy [37,115,116]. For example, the authors of ref. [115] developed a 3D composite scaffold using fast prototyping and coating technology with effective multidrug sequential release function against bacteria biofilm. In this study, levofloxacin, vancomycin and rifampicin were loaded into the mesoporous nanocomposite bioceramics (hydroxyapatite embedded into amorphous MBG), polyvinyl alcohol biopolymer and the external coating of gelatinglutaraldehyde, respectively. The scaffolds containing levofloxacin, vancomycin and rifampicin demonstrated a sequential release manner that was an early and fast release of rifampicin, followed by a sustained and prolonged release of vancomycin and levofloxacin. Encouraging, the antibacterial results showed that such combined strategy was effective in destroying gram-positive and gram-negative bacteria biofilms and inhibiting their proliferation. Additionally, the researchers of another study [37] designed a hierarchical scaffold for localized isoniazid and rifampicin drug delivery and osteoarticular tuberculosis therapy. In the detailed experiments, isoniazid and rifampicin drugs were preloaded into chemically modified MBG and subsequently combined with PHBHHx by 3D printing. In vitro and in vivo results revealed that the scaffolds exhibited prolonged drug release, with the drug concentrations on the periphery tissues of defects remaining above isoniazid and rifampicin minimal inhibitory concentration even up to 12 weeks after surgery. These findings showed that such hierarchical scaffolds had potential uses in bone regeneration and local antibacterial treatment following osteoarticular tuberculosis debridement surgery. Consequently, the antibacterial effects were indeed enhanced by the combination of more drugs in the scaffolds and a sequential release profile.
Similarly, the drugs and ions loaded in bioceramic-based scaffolds can complement each other to treat bone implant-associated infection. For example, the authors of ref. [30] used the templating approach to design enoxacin-loaded and rubidium-containing MBG scaffolds. In vitro cell experiments also revealed that rubidium-containing MBG scaffolds promoted attachment, spreading morphology, proliferation, ALP activity, as well as bone related protein expression of hBMSCs. Obviously, the rubidium-containing MBG scaffolds were capable of loading and releasing Rb ions and enoxacin to continuously damage the bacterial cell membranes, with the synergistic effects significantly improving the antibacterial properties of the scaffolds. Due to the dual antibacterial activity and osteogenesis, such scaffolds had a very attractive prospect in treating bone implant-associated infection and promoting bone repair. Additionally, the simultaneous utilization of multiple ions has a synergistic impact on antibacterial function, allowing them to be further enhanced. For example, the authors of study [117] investigated the antibacterial properties of gallium (Ga) and zinc (Zn)-containing BG scaffolds. The Ga3+ and Zn2+ release ratios increased with the incubation time, reaching a maximum of 1.5 and 0.05 ppm, respectively. Additionally, in vitro antibacterial results revealed that the Zn2+ and Ga3+ released from the scaffolds could act synergistically to inhibit the formation of bacterial biofilms and exert more powerful antibacterial function.
CS, as a natural polymer, possesses good antibacterial property. It has also been demonstrated that the comprehensive antibacterial activity can be enhanced when it coupled with other drugs [41,113,118,119]. The authors of ref. [41] constructed an injectable and penicillin-loaded CS/calcium phosphate cement scaffold, which exhibited an initial burst penicillin release and following a steady decline. Such release behavior made the CS/calcium phosphate cements scaffolds achieve a minimal inhibitory concentration for S. aureus (0.03 μg/mL). Furthermore, such injectable scaffolds did not cause toxicity, and showed high viability of human umbilical cord mesenchymal stem cells. Thus, the combined strategy of CS and penicillin enhanced antibacterial performance and bone regeneration for the scaffold. Interestingly, the combination of various physical strategies with drugs is also an effective way to enhance antibacterial performance for bioceramic-based scaffolds. For example, the recent study reported a Mg2SiO4–CoFe2O4 composite scaffold with magnetothermal effect, which makes it possible to increase temperature of the scaffolds for killing bacteria under an alternating magnetic field [95]. Furthermore, the scaffolds also showed excellent controlled release of rifampicin for enhancing antibacterial performance.
Nowadays, developing synergistic physical-activated and ion-mediated strategies to enhance antibacterial activity raised an increasing attention [110,112,120]. For example, the researchers of ref. [110] prepared an Ag-containing CS/HA composite scaffold with antibacterial property for bone tissue engineering by a freeze-drying method. Here, the positively charged CS and released Ag+ contributed to the synergistic antibacterial effect for the scaffolds. Another study developed a 3D printed stimuli-responsive CS/polyethylene oxide/ZnO hydrogel scaffold with antibacterial activity [112]. The scaffold with CS was pH-responsive due to the acidic microenvironment of the bacterial infection zone, thereby accelerating the release of ZnO. Antibacterial experiments in vitro under different concentrations of nZnO indicated that nZnO at concentrations higher than 2 mg/mL resulted in more than 99.99% inhibition of E. coli growth. On the other side, nZnO could be photoactivated under UV light to induce an increased ROS production, thereby leading to a higher antibacterial effect. Thus, the synergistic actions of physical photoactivation of nZnO and ion (Zn2+) strategies improved the comprehensive antibacterial ability.
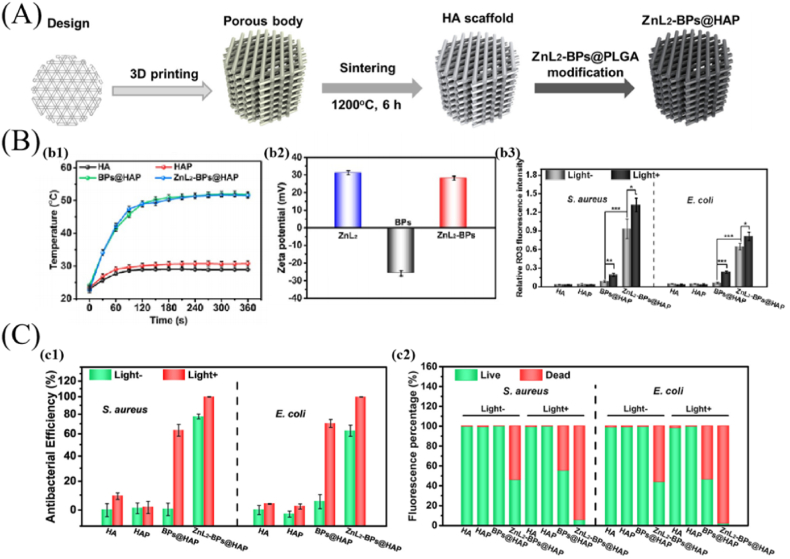
Nanomaterials, such as rGO, BPs and CuS, exhibit favorable photothermal effect due to their unique physical and chemical features. The photothermal effect can be applied for antibacterial purposes, but a single material's photothermal efficiency is often insufficient. Hence, a combination of two or more photothermal materials is expected to overcome the hurdles [31,121]. For example, the latest study reported a bone implant with antibacterial action by integrating ZnL2 with thermosensitivity and BPs with photothermal property on HA scaffold (Fig. 7) [121]. In detailed experiments, ZnL2-BPs were synthesized by ligating BPs with ZnL2 and then integrating them onto the surface of 3D-printed HA scaffolds to form ZnL2-BPs@HA scaffolds (Fig. 7 (A)). Antibacterial tests in vitro and in vivo revealed that the ZnL2-BPs@HA scaffolds exhibited superior antibacterial capability when exposed to light radiation compared to other groups (Fig. 7 (C) representing in vitro results). The results demonstrated that the synergistic effects of positively charged ZnL2-BPs and hyperthermia, as well as the continuous production of ROS over time, may induce irreparable damage to intracellular biomolecules and ultimately end in bacterial mortality (Fig. 7 (B)). Therefore, the combination of multiple materials on scaffolds can enhance the photothermal property and thus improve the antibacterial effect. Apart from their unique physical and chemical properties, nanoparticles also have physically destructive effect on bacterial membranes that can be used for fighting bacteria. For example, the authors of study [122] designed a multifunctional scaffold with bioactivity and antibacterial property based on MXene@CeO2 nanocomposites for infection-impaired skin multimodal therapy. The probable antibacterial mechanism was that the cationic polyethylenimine attracted the negatively charged bacterial cell membrane, disrupted the transmembrane potential and induced cell death. More importantly, the 2D nanosheets as “nano-knife” might physically injure the cell membranes of bacteria, destroy bacterial membrane integrity, and result in a synergistic inhibition of bacterial growth by directly physically interacting with bacteria membrane surfaces. This study was rarely related to bone repair, but it provided a novel idea for treating bone implant-associated infection.
Fig. 7.
ZnL2-BPs@HA scaffolds with photothermal effect for treating bone implant-associated infection. (A) Schematic illustration of the preparation process for ZnL2-BPs@HA scaffold. (B) Photothermal effect, surface charge of nanomaterials and ROS production by the scaffolds. (b1) photothermal heating curves of the scaffolds immersed in PBS upon NIR irradiation, (b2) zeta potentials of ZnL2, BPs and ZnL2-BPs, (b3) quantitative analysis of ROS production of different groups. (C) In vitro antibacterial effects of scaffolds after different treatments. (c1) antibacterial efficiency of the scaffolds with/without NIR irradiation, (c2) quantitative analysis of live/dead staining (reprinted with permission from ref. [121]).
Despite the superior antibacterial property, each antibacterial strategy has its own limitations, such as biocompatibility restrictions for ions, bacterial resistance induced by antibiotic drugs, and stimulus signals input and materials degradation issues for physical strategy. Obviously, the antibacterial property could be greatly improved by the synergistic effect of the combined antibacterial strategy, with the superiority of the combined strategy over the single one being demonstrated by numerous examples. The combined antibacterial strategy consisting of two or more antibacterial approaches with diverse mechanisms not only tends to hardly interfere with the mutual antibacterial effect, but perhaps reinforces it. The combined strategy possesses good antibacterial property for drug-resistant bacteria, which may be attributed to the synergistic effect as well. In addition, the single antibacterial strategy may have limited antibacterial activity against specific bacteria, while the combined strategy may confer a broad-spectrum antibacterial property on bioceramic-based scaffolds. Therefore, the combined strategy can be prioritized in the design of antibacterial bioceramic-based scaffolds, resulting in enhanced antibacterial properties for bioceramic-based scaffolds that can withstand bone implant-associated infection.
6. Summary and outlooks
Bioceramic-based scaffolds with high bioactivity, degradability, osteoinduction and osteoconduction are very useful for bone repair. They can provide mechanical support and facilitate cell migration and nutrient transportation, while the released active substances from them stimulate cell proliferation and differentiation, thereby promoting osteogenesis and angiogenesis, and ultimately bone repair. However, the prevention and treatment of bacterial infection is a major challenge in bone repair. Currently, introducing antibacterial function into bioceramic-based scaffolds is an effective strategy to fight bone implant-associated infection, and a variety of antibacterial bioceramic-based scaffolds have been developed based on different antibacterial mechanisms, including bioceramic-based scaffolds with drug-induced antibacterial functions, bioceramic-based scaffolds with ion-mediated antibacterial functions, bioceramic-based scaffolds with physical antibacterial functions, and bioceramic-based scaffolds with combined antibacterial functions. Although the drug-induced antibacterial strategy is still the most effective and widely used in clinical practice, it is imperative to develop replaceable antibacterial strategies due to the problem of drug resistance to bacteria. The ion-mediated antibacterial strategy can achieve favorable antibacterial effects owing to the capability of sustained release of antibacterial ions. Since the ions utilized in this strategy are often heavy metals, it faces biological safety risks and has great obstacles in the clinical applications. Therefore, it is critical to strike a balance between antibacterial capability and biocompatibility. Physical-activated antibacterial strategy is more promising, and the most likely to solve the problem of drug-resistant bacteria. However, the depth and breadth of researches are far from enough, especially in terms of antibacterial mechanisms. The combination of the aforementioned various strategies, particularly their synergy of multiple strategies, may effectively overcome the drawbacks of a single approach and enhance the comprehensive antibacterial effect. Hence, the combined antibacterial strategy is a comparatively ideal pathway. Due to the fact that multiple strategies are involved, it is a challenge to complete the design and preparation of the scaffolds without impairing their osteogenic performance.
Many antibacterial strategies of bioceramic-based scaffolds for treating bone implant-associated infection have been developed, nevertheless, the issue of bacterial resistance requires more attentions. Effective antibacterial strategies and the development of innovative antibacterial agents are critical in the battle against drug-resistant bacteria. Research on adaptive antibacterial scaffolds is a promising direction, which stimulates the scaffolds in vivo by specific microenvironment triggered by bacteria to produce timely antibacterial effect. And the antibacterial actions can be stimulated by acid, enzyme, temperature and touching responses in the infected sites, which enhances the availability of the antibacterial agents, and effectively avoids the emergence of drug-resistant bacteria. Developing antibiotic-free strategies that do not generate bacterial resistance to achieve a better antibacterial effect may be a more appropriate pathway. Antibacterial scaffolds are being developed in general with the goals of being efficient, long-term, non-toxic, antibiotic-free and broad-spectrum antibacterial, and ultimately solving the issue of bone implant-associated infection. It is hoped that the aims may be achieved gradually via advances in new antibacterial materials, such as biomaterials with physical-activated antibacterial function. Bacteria can be inhibited by constructing structures with specific physical and chemical properties on the surface of bioceramic-based scaffolds, or by stimulating the production of bactericidal substances via an external field. Researches on the antibacterial mechanisms of biomaterials and loading principals on the scaffolds may bring about a significant breakthrough in the treatment of tissue infection. Nonetheless, the biosafety and degradability of biomaterials, as well as their loading techniques on the scaffolds, should be highly concerned to meet the requirements in practical applications. Undoubtedly, it will be possible to endow bioceramic-based scaffolds with excellent antibacterial property to treat bone implant-associated infection, and ultimately promote bone repair.
Declaration of competing interest
The authors declare no competing financial interest.
CRediT authorship contribution statement
Chaoqian Zhao: Writing – original draft, Resources, Validation. Weiye Liu: Resources, Writing – original draft. Min Zhu: Conceptualization, Funding acquisition, Writing – review & editing. Chengtie Wu: Funding acquisition, Writing – review & editing. Yufang Zhu: Conceptualization, Supervision, Writing – review & editing, Funding acquisition.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (No. 51872185, 52072246, 32130062), Science and Technology Commission of Shanghai Municipality (No. 20442420300), and China Postdoctoral Science Foundation (No. 2021M703332).
Footnotes
Peer review under responsibility of KeAi Communications Co., Ltd.
Contributor Information
Min Zhu, Email: mzhu@usst.edu.cn.
Chengtie Wu, Email: chengtiewu@mail.sic.ac.cn.
Yufang Zhu, Email: zjf2412@163.com.
Abbreviations
- ALP
Alkaline phosphatase
- B. cereus
Bacillus cereus
- BPs
Black phosphorus nanosheets
- BMP-2
Bone morphogenetic protein-2
- (h)BMSCs
(Human) Bone mesenchymal stem cells
- BSP
Bone sialoprotein
- β-TCP
β-calcium phosphate
- CS
Chitosan
- E. coil
Escherichia coli
- HA
Hydroxyapatite
- GDY
Graphdiyne
- L. fusiformis
Lysinibacillus fusiformis
- MBG
Mesoporous bioglass
- MRSA
Methicillin-resistant staphylococcus aureus
- NIR
Near-infrared
- OCN
Osteocalcin
- OPN
Osteopontin
- PCL
Polycaprolactone
- P. aeruginosa
Pseudomonas aeruginosa
- PHBHHx
Poly(3-hydroxybuty rate-co-3-hydroxyhexanoate)
- PLGA
Poly(lactic-co-glycolic) acid
- ROS
Reactive oxide species
- rGO
Reduced graphene oxide
- Runx-2
Runt-related transcription factor 2
- S. aureus
Staphylococcus aureus
- S. epidermidis
Staphylococcus epidermidis
- VEGF
Vascular endothelial growth factor
- ZnL2
Zinc sulfonate ligand
References
- 1.Mebarki M., Coquelin L., Layrolle P., Battaglia S., Tossou M., Hernigou P., et al. Enhanced human bone marrow mesenchymal stromal cell adhesion on scaffolds promotes cell survival and bone formation. Acta Biomater. 2017;59:94–107. doi: 10.1016/j.actbio.2017.06.018. [DOI] [PubMed] [Google Scholar]
- 2.Shekhawat D., Singh A., Banerjee M.K., Singh T., Patnaik A. Bioceramic composites for orthopaedic applications: a comprehensive review of mechanical, biological, and microstructural properties. Ceram. Int. 2021;47:3013–3030. doi: 10.1016/j.ceramint.2020.09.214. [DOI] [Google Scholar]
- 3.Feng C., Zhang K., He R., Ding G., Xia M., Jin X., Xie C. S Additive manufacturing of hydroxyapatite bioceramic scaffolds: dispersion, digital light processing, sintering, mechanical properties, and biocompatibility. J. Adv. Ceram. 2020;9:360–373. doi: 10.1007/s40145-020-0375-8. [DOI] [Google Scholar]
- 4.Jodati H., Yilmaz B., Evis Z. Calcium zirconium silicate (baghdadite) ceramic as a biomaterial. Ceram. Int. 2020;46:21902–21909. doi: 10.1016/j.ceramint.2020.06.105. [DOI] [Google Scholar]
- 5.Kargozar S., Baino F., Hamzehlou S., Hill R.G., Mozafari M. Bioactive glasses: sprouting angiogenesis in tissue engineering. Trends Biotechnol. 2018;36:430–444. doi: 10.1016/j.tibtech.2017.12.003. [DOI] [PubMed] [Google Scholar]
- 6.Yao Y., Qin W., Xing B., Sha N., Jiao T., Zhao Z. High performance hydroxyapatite ceramics and a triply periodic minimum surface structure fabricated by digital light processing 3D printing. J. Adv. Ceram. 2021;10:39–48. doi: 10.1007/s40145-020-0415-4. [DOI] [Google Scholar]
- 7.Zhang L., Yang G.J., Johnson B.N., Jia X.F. Three-dimensional (3D) printed scaffold and material selection for bone repair. Acta Biomater. 2019;84:16–33. doi: 10.1016/j.actbio.2018.11.039. [DOI] [PubMed] [Google Scholar]
- 8.Jodati H., Yilmaz B., Evis Z. A review of bioceramic porous scaffolds for hard tissue applications: effects of structural features. Ceram. Int. 2020;46:15725–15739. doi: 10.1016/j.ceramint.2020.03.192. [DOI] [Google Scholar]
- 9.O'Brien F.J. Biomaterials & scaffolds for tissue engineering, Mater. Today Off. 2011;14:88–95. doi: 10.1016/S1369-7021(11)70058-X. [DOI] [Google Scholar]
- 10.Nikolova M.P., Chavali M.S. Recent advances in biomaterials for 3D scaffolds: a review. Bioact. Mater. 2019;4:271–292. doi: 10.1016/j.bioactmat.2019.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Abbasi N., Hamlet S., Love R.M., Nguyen N.T. Porous scaffolds for bone regeneration. J. Sci. 2020;5:1–9. doi: 10.1016/j.jsamd.2020.01.007. [DOI] [Google Scholar]
- 12.Roseti L., Parisi V., Petretta M., Cavallo C., Desando G., Bartolotti I., et al. Scaffolds for bone tissue engineering: state of the art and new perspectives. Mater. Sci. Eng. C-Mater. Biol. Appl. 2017;78:1246–1262. doi: 10.1016/j.msec.2017.05.017. [DOI] [PubMed] [Google Scholar]
- 13.Chen Z.W., Li Z.Y., Li J.J., Liu C.B., Lao C.S., Fu Y.L., et al. 3D printing of ceramics: a review. J. Eur. Ceram. Soc. 2019;39:661–687. doi: 10.1016/j.jeurceramsoc.2018.11.013. [DOI] [Google Scholar]
- 14.Fernandes J.S., Gentile P., Pires R.A., Reis R.L., Hatton P.V. Multifunctional bioactive glass and glass-ceramic biomaterials with antibacterial properties for repair and regeneration of bone tissue. Acta Biomater. 2017;59:2–11. doi: 10.1016/j.actbio.2017.06.046. [DOI] [PubMed] [Google Scholar]
- 15.Filipovic U., Dahmane R.G., Ghannouchi S., Zore A., Bohinc K. Bacterial adhesion on orthopedic implants. Adv. Colloid Interface Sci. 2020;283 doi: 10.1016/j.cis.2020.102228. [DOI] [PubMed] [Google Scholar]
- 16.Unnithan A.R., Arathyram R.S., Kim C.S. In: Nanotechnology Applications for Tissue Engineering. Thomas S., Grohens Y., Ninan N., editors. William Andrew Publishing; Oxford: 2015. Chapter 7 - scaffolds with antibacterial properties; pp. 103–123. [Google Scholar]
- 17.Vallet-Regi M., Lozano D., Gonzalez B., Izquierdo-Barba I. Biomaterials against bone infection. Adv. Healthc. Mater. 2020;9 doi: 10.1002/adhm.202000310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kargozar S., Montazerian M., Harnzehlou S., Kim H.W., Baino F. Mesoporous bioactive glasses: promising platforms for antibacterial strategies. Acta Biomater. 2018;81:1–19. doi: 10.1016/j.actbio.2018.09.052. [DOI] [PubMed] [Google Scholar]
- 19.Zhang Y.L., Zhai D., Xu M.C., Yao Q.Q., Zhu H.Y., Chang J., et al. 3D-printed bioceramic scaffolds with antibacterial and osteogenic activity. Biofabrication. 2017;9 doi: 10.1088/1758-5090/aa6ed6. [DOI] [PubMed] [Google Scholar]
- 20.Yuan J.J., Wang B.X., Han C., Huang X.Y., Xiao H.J., Lu X., et al. Nanosized-Ag-doped porous beta-tricalcium phosphate for biological applications. Mater. Sci. Eng. C-Mater. Biol. Appl. 2020;114 doi: 10.1016/j.msec.2020.111037. [DOI] [PubMed] [Google Scholar]
- 21.Ke D.X., Tarafder S., Vahabzadeh S., Bose S. Effects of MgO, ZnO, SrO, and SiO2 in tricalcium phosphate scaffolds on in vitro gene expression and in vivo osteogenesis. Mater. Sci. Eng. C-Mater. Biol. Appl. 2019;96:10–19. doi: 10.1016/j.msec.2018.10.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhu T., Zhu M., Zhu Y. Fabrication of forsterite scaffolds with photothermal-induced antibacterial activity by 3D printing and polymer-derived ceramics strategy. Ceram. Int. 2020;46:13607–13614. doi: 10.1016/j.ceramint.2020.02.146. [DOI] [Google Scholar]
- 23.Choudhary R., Chatterjee A., Venkatraman S.K., Koppala S., Abraham J., Swamiappan S. Antibacterial forsterite (Mg2SiO4) scaffold: a promising bioceramic for load bearing applications. Bioact. Mater. 2018;3:218–224. doi: 10.1016/j.bioactmat.2018.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stevanovic M., Filipovic N., Djurdjevic J., Lukic M., Milenkovic M., Boccaccini A. 45S5Bioglass (R)-based scaffolds coated with selenium nanoparticles or with poly(lactide-co-glycolide)/selenium particles: processing, evaluation and antibacterial activity. Colloids Surf. B Biointerfaces. 2015;132:208–215. doi: 10.1016/j.colsurfb.2015.05.024. [DOI] [PubMed] [Google Scholar]
- 25.Channasanon S., Udomkusonsri P., Chantaweroad S., Tesavibul P., Tanodekaew S. Gentamicin released from porous scaffolds fabricated by stereolithography. J. Healthc. Eng. 2017;2017 doi: 10.1155/2017/9547896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yu Q., Wu Z.Q., Chen H. Dual-function antibacterial surfaces for biomedical applications. Acta Biomater. 2015;16:1–13. doi: 10.1016/j.actbio.2015.01.018. [DOI] [PubMed] [Google Scholar]
- 27.Olalde B., Garmendia N., Saez-Martinez V., Argarate N., Nooeaid P., Morin F., et al. Multifunctional bioactive glass scaffolds coated with layers of poly(D,L-lactide-co-glycolide) and poly(n-isopropylacrylamide-co-acrylic acid) microgels loaded with vancomycin. Mater. Sci. Eng. C-Mater. Biol. Appl. 2013;33:3760–3767. doi: 10.1016/j.msec.2013.05.002. [DOI] [PubMed] [Google Scholar]
- 28.Bigham A., Foroughi F., Motamedi M., Rafienia M. Multifunctional nanoporous magnetic zinc silicate-ZnFe(2)0(4) core-shell composite for bone tissue engineering applications. Ceram. Int. 2018;44:11798–11806. doi: 10.1016/j.ceramint.2018.03.264. [DOI] [Google Scholar]
- 29.Diaz-Rodriguez P., Sanchez M., Landin M. Drug-loaded biomimetic ceramics for tissue engineering. Pharmaceutics. 2018;10 doi: 10.3390/pharmaceutics10040272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.He X., Liu Y., Tan Y.N., Grover L.M., Song J., Duan S.L., et al. Rubidium-containing mesoporous bioactive glass scaffolds support angiogenesis, osteogenesis and antibacterial activity. Mater. Sci. Eng. C-Mater. Biol. Appl. 2019;105 doi: 10.1016/j.msec.2019.110155. [DOI] [PubMed] [Google Scholar]
- 31.Zhang Z.J., Wang Y.K., Teng W.S.Y., Zhou X.Z., Ye Y.X., Zhou H., et al. An orthobiologics-free strategy for synergistic photocatalytic antibacterial and osseointegration. Biomaterials. 2021;274 doi: 10.1016/j.biomaterials.2021.120853. [DOI] [PubMed] [Google Scholar]
- 32.Wang M.Q., Li H.J., Yang Y.Q., Yuan K., Zhou F., Liu H.B., et al. A 3D-bioprinted scaffold with doxycycline-controlled BMP2-expressing cells for inducing bone regeneration and inhibiting bacterial infection. Bioact. Mater. 2021;6:1318–1329. doi: 10.1016/j.bioactmat.2020.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rajabi A., Esmaeili A. Preparation of three-phase nanocomposite antimicrobial scaffold BCP/Gelatin/45S5 glass with drug vancomycin and BMP-2 loading for bone regeneration. Colloids Surf. A Physicochem. Eng. Asp. 2020;606 doi: 10.1016/j.colsurfa.2020.125508. [DOI] [Google Scholar]
- 34.Wang J., Wang C., Jin K., Yang X., Gao L., Yao C., et al. Simultaneous enhancement of vascularization and contact-active antibacterial activity in diopside-based ceramic orbital implants. Mater. Sci. Eng. C-Mater. Biol. Appl. 2019;105:10. doi: 10.1016/j.msec.2019.110036. [DOI] [PubMed] [Google Scholar]
- 35.Shuai C.J., Xu Y., Feng P., Wang G.Y., Xiong S.X., Peng S.P. Antibacterial polymer scaffold based on mesoporous bioactive glass loaded with in situ grown silver. Chem. Eng. J. 2019;374:304–315. doi: 10.1016/j.cej.2019.03.273. [DOI] [Google Scholar]
- 36.Wang R., Shi M.S., Xu F.Y., Qiu Y., Zhang P., Shen K.L., et al. Graphdiyne-modified TiO2 nanofibers with osteoinductive and enhanced photocatalytic antibacterial activities to prevent implant infection. Nat. Commun. 2020;11 doi: 10.1038/s41467-020-18267-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhu M., Li K., Zhu Y.F., Zhang J.H., Ye X.J. 3D-printed hierarchical scaffold for localized isoniazid/rifampin drug delivery and osteoarticular tuberculosis therapy. Acta Biomater. 2015;16:145–155. doi: 10.1016/j.actbio.2015.01.034. [DOI] [PubMed] [Google Scholar]
- 38.Yao T.T., Chen J.Q., Wang Z.G., Zhai J.X., Li Y.F., Xing J., et al. The antibacterial effect of potassium-sodium niobate ceramics based on controlling piezoelectric properties. Colloids Surf. B Biointerfaces. 2019;175:463–468. doi: 10.1016/j.colsurfb.2018.12.022. [DOI] [PubMed] [Google Scholar]
- 39.Bakhsheshi-Rad H.R., Hamzah E., Staiger M.P., Dias G.J., Hadisi Z., Saheban M., et al. Drug release, cytocompatibility, bioactivity, and antibacterial activity of doxycycline loaded Mg-Ca-TiO2 composite scaffold. Mater. Des. 2018;139:212–221. doi: 10.1016/j.matdes.2017.10.072. [DOI] [Google Scholar]
- 40.Kim H.W., Knowles J.C., Kim H.E. Porous scaffolds of gelatin-hydroxyapatite nanocomposites obtained by biomimetic approach: characterization and antibiotic drug release. J. Biomed. Mater. Res. Part B. 2005;74B:686–698. doi: 10.1002/jbm.b.30236. [DOI] [PubMed] [Google Scholar]
- 41.Wu S.Z., Lei L., Bao C.Y., Liu J., Weir M.D., Ren K., et al. An injectable and antibacterial calcium phosphate scaffold inhibiting Staphylococcus aureus and supporting stem cells for bone regeneration. Mater. Sci. Eng. C-Mater. Biol. Appl. 2021;120 doi: 10.1016/j.msec.2020.111688. [DOI] [PubMed] [Google Scholar]
- 42.Paris J.L., Lafuente-Gomez N., Cabanas M.V., Roman J., Pena J., Vallet-Regi M. Fabrication of a nanoparticle-containing 3D porous bone scaffold with proangiogenic and antibacterial properties. Acta Biomater. 2019;86:441–449. doi: 10.1016/j.actbio.2019.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rumian L., Tiainen H., Cibor U., Krok-Borkowicz M., Brzychczy-Wloch M., Haugen H.J., et al. Ceramic scaffolds enriched with gentamicin loaded poly(lactide-co-glycolide) microparticles for prevention and treatment of bone tissue infections. Mater. Sci. Eng. C-Mater. Biol. Appl. 2016;69:856–864. doi: 10.1016/j.msec.2016.07.065. [DOI] [PubMed] [Google Scholar]
- 44.Jin X., Xiong Y.H., Zhang X.Y., Wang R.X., Xing Y.G., Duan S., et al. Self-adaptive antibacterial porous implants with sustainable responses for infected bone defect therapy. Adv. Funct. Mater. 2019;29 doi: 10.1002/adfm.201807915. [DOI] [Google Scholar]
- 45.Kuang Z.P., Dai G.M., Wan R.J., Zhang D.L., Zhao C., Chen C., et al. Osteogenic and antibacterial dual functions of a novel levofloxacin loaded mesoporous silica microspheres/nano-hydroxyapatite/polyurethane composite scaffold. Genes Dis. 2021;8:193–202. doi: 10.1016/j.gendis.2019.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bakhsheshi-Rad H.R., Chen X.B., Ismail A.F., Aziz M., Hamzah E., Najafinezhad A. A new multifunctional monticellite-ciprofloxacin scaffold: preparation, bioactivity, biocompatibility, and antibacterial properties. Mater. Chem. Phys. 2019;222:118–131. doi: 10.1016/j.matchemphys.2018.09.054. [DOI] [Google Scholar]
- 47.Bakhsheshi-Rad H.R., Hamzah E., Ismail A.F., Aziz M., Hadisi Z., Kashefian M., et al. Novel nanostructured baghdadite-vancomycin scaffolds: in-vitro drug release, antibacterial activity and biocompatibility. Mater. Lett. 2017;209:369–372. doi: 10.1016/j.matlet.2017.08.027. [DOI] [Google Scholar]
- 48.Manchon A., Alkhraisat M.H., Rueda-Rodriguez C., Pintado C., Prados-Frutos J.C., Torres J., et al. Silicon bioceramic loaded with vancomycin stimulates bone tissue regeneration. J. Biomed. Mater. Res. Part B. 2018;106:2307–2315. doi: 10.1002/jbm.b.34040. [DOI] [PubMed] [Google Scholar]
- 49.Lian X.J., Wang Xu Mei, Cui Fu Zhai. In vitro antibacterial properties of vancomycin-loaded nano-hydroxyapatite/collagen/calcium sulfate hemihydrates (VCM/NHAC/CSH) bone substitute. Mater. Sci. Forum. 2013:745–746. doi: 10.4028/www.scientific.net/MSF.745-746.6. 6–12. [DOI] [Google Scholar]
- 50.Seidenstuecker M., Ruehe J., Suedkamp N.P., Serr A., Wittmer A., Bohner M., et al. Composite material consisting of microporous beta-TCP ceramic and alginate for delayed release of antibiotics. Acta Biomater. 2017;51:433–446. doi: 10.1016/j.actbio.2017.01.045. [DOI] [PubMed] [Google Scholar]
- 51.Hess U., Mikolajczyk G., Treccani L., Streckbein P., Heiss C., Odenbach S., et al. Multi-loaded ceramic beads/matrix scaffolds obtained by combining ionotropic and freeze gelation for sustained and tuneable vancomycin release. Mater. Sci. Eng. C-Mater. Biol. Appl. 2016;67:542–553. doi: 10.1016/j.msec.2016.05.042. [DOI] [PubMed] [Google Scholar]
- 52.Cheng T., Qu H.Y., Zhang G.Y., Zhang X.L. Osteogenic and antibacterial properties of vancomycin-laden mesoporous bioglass/PLGA composite scaffolds for bone regeneration in infected bone defects. Artif. Cell. Nanomed. Biotechnol. 2018;46:1935–1947. doi: 10.1080/21691401.2017.1396997. [DOI] [PubMed] [Google Scholar]
- 53.Sukhodub L.F., Sukhodub L.B., Litsis O., Prylutskyy Y. Synthesis and characterization of hydroxyapatite-alginate nanostructured composites for the controlled drug release. Mater. Chem. Phys. 2018;217:228–234. doi: 10.1016/j.matchemphys.2018.06.071. [DOI] [Google Scholar]
- 54.Vidal E., Guillem-Marti J., Ginebra M.P., Combes C., Ruperez E., Rodriguez D. Multifunctional homogeneous calcium phosphate coatings: toward antibacterial and cell adhesive titanium scaffolds. Surf. Coating. Technol. 2021;405 doi: 10.1016/j.surfcoat.2020.126557. [DOI] [Google Scholar]
- 55.Sun H., Hu C., Zhou C.C., Wu L.N., Sun J.X., Zhou X.D., et al. 3D printing of calcium phosphate scaffolds with controlled release o antibacterial functions for jaw bone repair. Mater. Des. 2020;189 doi: 10.1016/j.matdes.2020.108540. [DOI] [Google Scholar]
- 56.Qayoom I., Teotia A.K., Panjla A., Verma S., Kumar A. Local and sustained delivery of rifampicin from a bioactive ceramic carrier treats bone infection in rat tibia. ACS Infect. Dis. 2020;6:2938–2949. doi: 10.1021/acsinfecdis.0c00369. [DOI] [PubMed] [Google Scholar]
- 57.Bauer J., Silva A.S.E., Carvalho E.M., Ferreira P.V.C., Carvalho C.N., Manso A.P., et al. Dentin pretreatment with 45S5 and niobophosphate bioactive glass: effects on pH, antibacterial, mechanical properties of the interface and microtensile bond strength. J. Mech. Behav. Biomed. Mater. 2019;90:374–380. doi: 10.1016/j.jmbbm.2018.10.029. [DOI] [PubMed] [Google Scholar]
- 58.Dai L.L., Mei M.L., Chu C.H., Lo E.C.M. Antibacterial effect of a new bioactive glass on cariogenic bacteria. Arch. Oral Biol. 2020;117 doi: 10.1016/j.archoralbio.2020.104833. [DOI] [PubMed] [Google Scholar]
- 59.Esteban-Tejeda L., Zheng K., Prado C., Cabal B., Torrecillas R., Boccaccini A.R., et al. Bone tissue scaffolds based on antimicrobial SiO2-Na2O-Al2O3-CaO-B2O3 glass. J. Non-Cryst. Solids. 2016;432:73–80. doi: 10.1016/j.jnoncrysol.2015.05.040. [DOI] [Google Scholar]
- 60.Mubina M.S.K., Shailajha S., Sankaranarayanan R., Saranya L. In vitro bioactivity, mechanical behavior and antibacterial properties of mesoporous SiO2-CaO-Na2O-P2O5 nano bioactive glass ceramics. J. Mech. Behav. Biomed. Mater. 2019;100 doi: 10.1016/j.jmbbm.2019.103379. [DOI] [PubMed] [Google Scholar]
- 61.Kheradmandfard M., Kashani-Bozorg S.F., Noori-Alfesharaki A.H., Kharazi A.Z., Kheradmandfard M., Abutalebi N. Ultra-fast, highly efficient and green synthesis of bioactive forsterite nanopowder via microwave irradiation. Mater. Sci. Eng. C-Mater. Biol. Appl. 2018;92:236–244. doi: 10.1016/j.msec.2018.06.026. [DOI] [PubMed] [Google Scholar]
- 62.Saidi R., Fathi M., Salimijazi H. Synthesis and characterization of bioactive glass coated forsterite scaffold for tissue engineering applications, J. Alloy. Compd. 2017;727:956–962. doi: 10.1016/j.jallcom.2017.08.186. [DOI] [Google Scholar]
- 63.Newby P.J., El-Gendy R., Kirkham J., Yang X.B., Thompson I.D., Boccaccini A.R. Ag-doped 45S5 Bioglass (R)-based bone scaffolds by molten salt ion exchange: processing and characterisation. J. Mater. Sci. Mater. Med. 2011;22:557–569. doi: 10.1007/s10856-011-4240-8. [DOI] [PubMed] [Google Scholar]
- 64.Chatzistavrou X., Fenno J.C., Faulk D., Badylak S., Kasuga T., Boccaccini A.R., et al. Fabrication and characterization of bioactive and antibacterial composites for dental applications. Acta Biomater. 2014;10:3723–3732. doi: 10.1016/j.actbio.2014.04.030. [DOI] [PubMed] [Google Scholar]
- 65.Yazdimamaghani M., Vashaee D., Assefa S., Walker K.J., Madihally S.V., Kohler G.A., et al. Hybrid macroporous gelatin/bioactive-glass/nanosilver scaffolds with controlled degradation behavior and antimicrobial activity for bone tissue engineering. J. Biomed. Nanotechnol. 2014;10:911–931. doi: 10.1166/jbn.2014.1783. [DOI] [PubMed] [Google Scholar]
- 66.Hoover S., Tarafder S., Bandyopadhyay A., Bose S. Silver doped resorbable tricalcium phosphate scaffolds for bone graft applications. Mater. Sci. Eng. C-Mater. Biol. Appl. 2017;79:763–769. doi: 10.1016/j.msec.2017.04.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wang S., Li R.Y., Qing Y.A., Wei Y.Z., Wang Q.F., Zhang T.R., et al. Antibacterial activity of Ag-incorporated zincosilicate zeolite scaffolds fabricated by additive manufacturing. Inorg. Chem. Commun. 2019;105:31–35. doi: 10.1016/j.inoche.2019.04.026. [DOI] [Google Scholar]
- 68.Makvandi P., Ali G.W., Della Sala F., Abdel-Fattah W.I., Borzacchiello A. Hyaluronic acid/corn silk extract based injectable nanocomposite: a biomimetic antibacterial scaffold for bone tissue regeneration. Mater. Sci. Eng. C-Mater. Biol. Appl. 2020;107 doi: 10.1016/j.msec.2019.110195. [DOI] [PubMed] [Google Scholar]
- 69.Calabrese G., Petralia S., Franco D., Nocito G., Fabbi C., Forte L., et al. A new Ag-nanostructured hydroxyapatite porous scaffold: antibacterial effect and cytotoxicity study. Mater. Sci. Eng. C-Mater. Biol. Appl. 2021;118 doi: 10.1016/j.msec.2020.111394. [DOI] [PubMed] [Google Scholar]
- 70.Marsh A.C., Mellott N.P., Crimp M., Wren A., Hammer N., Chatzistavrou X. Ag-doped bioactive glass-ceramic 3D scaffolds: microstructural, antibacterial, and biological properties. J. Eur. Ceram. Soc. 2021;41:3717–3730. doi: 10.1016/j.jeurceramsoc.2021.01.011. [DOI] [Google Scholar]
- 71.Bellantone M., Williams H.D., Hench L.L. Broad-spectrum bactericidal activity of Ag2O-doped bioactive glass. Antimicrob. Agents Chemother. 2002;46:1940–1945. doi: 10.1128/AAC.46.6.1940-1945.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Miola M., Verne E., Vitale-Brovarone C., Baino F. Antibacterial bioglass-derived scaffolds: innovative synthesis approach and characterization. Int. J. Appl. Glass Sci. 2016;7:238–247. doi: 10.1111/ijag.12209. [DOI] [Google Scholar]
- 73.Srinivasan S., Kumar P.T.S., Nair S.V., Nair S.V., Chennazhi K.P., Jayakumar R. Antibacterial and bioactive alpha- and beta-chitin hydrogel/nanobioactive glass ceramic/nano silver composite scaffolds for periodontal regeneration. J. Biomed. Nanotechnol. 2013;9:1803–1816. doi: 10.1166/jbn.2013.1658. [DOI] [PubMed] [Google Scholar]
- 74.Saini R.K., Bagri L.P., Bajpai A.K. Nano-silver hydroxyapatite based antibacterial 3D scaffolds of gelatin/alginate/poly (vinyl alcohol) for bone tissue engineering applications. Colloids Surf. B Biointerfaces. 2019;177:211–218. doi: 10.1016/j.colsurfb.2019.01.064. [DOI] [PubMed] [Google Scholar]
- 75.Alavi M., Nokhodchi A. An overview on antimicrobial and wound healing properties of ZnO nanobiofilms, hydrogels, and bionanocomposites based on cellulose, chitosan, and alginate polymers. Carbohydr. Polym. 2020;227 doi: 10.1016/j.carbpol.2019.115349. [DOI] [PubMed] [Google Scholar]
- 76.Hoppe A., Guldal N.S., Boccaccini A.R. A review of the biological response to ionic dissolution products from bioactive glasses and glass-ceramics. Biomaterials. 2011;32:2757–2774. doi: 10.1016/j.biomaterials.2011.01.004. [DOI] [PubMed] [Google Scholar]
- 77.Sanchez-Salcedo S., Shruti S., Salinas A.J., Malavasi G., Menabue L., Vallet-Regi M. In vitro antibacterial capacity and cytocompatibility of SiO2-CaO-P2O5 meso-macroporous glass scaffolds enriched with ZnO. J. Math. Chem. B. 2014;2:4836–4847. doi: 10.1039/c4tb00403e. [DOI] [PubMed] [Google Scholar]
- 78.Li C., Ai F.R., Miao X.X., Liao H., Li F.S., Liu M.Z., et al. The return of ceramic implants": rose stem inspired dual layered modification of ceramic scaffolds with improved mechanical and anti-infective properties. Mater. Sci. Eng. C-Mater. Biol. Appl. 2018;93:873–879. doi: 10.1016/j.msec.2018.08.044. [DOI] [PubMed] [Google Scholar]
- 79.Felice B., Sanchez M.A., Socci M.C., Sappia L.D., Gomez M.I., Cruz M.K., et al. Controlled degradability of PCL-ZnO nanofibrous scaffolds for bone tissue engineering and their antibacterial activity. Mater. Sci. Eng. C-Mater. Biol. Appl. 2018;93:724–738. doi: 10.1016/j.msec.2018.08.009. [DOI] [PubMed] [Google Scholar]
- 80.Applerot G., Lipovsky A., Dror R., Perkas N., Nitzan Y., Lubart R., et al. Enhanced antibacterial activity of nanocrystalline ZnO due to increased ROS-mediated cell injury. Adv. Funct. Mater. 2009;19:842–852. doi: 10.1002/adfm.200801081. [DOI] [Google Scholar]
- 81.Saxena V., Hasan A., Pandey L.M. Effect of Zn/ZnO integration with hydroxyapatite: a review. Mater. Technol. 2018;33:79–92. doi: 10.1080/10667857.2017.1377972. [DOI] [Google Scholar]
- 82.Erol M.M., Mournio V., Newby P., Chatzistavrou X., Roether J.A., Hupa L., et al. Copper-releasing, boron-containing bioactive glass-based scaffolds coated with alginate for bone tissue engineering. Acta Biomater. 2012;8:792–801. doi: 10.1016/j.actbio.2011.10.013. [DOI] [PubMed] [Google Scholar]
- 83.Azeena S., Subhapradha N., Selvamurugan N., Narayan S., Srinivasan N., Murugesan R., et al. Antibacterial activity of agricultural waste derived wollastonite doped with copper for bone tissue engineering. Mater. Sci. Eng. C-Mater. Biol. Appl. 2017;71:1156–1165. doi: 10.1016/j.msec.2016.11.118. [DOI] [PubMed] [Google Scholar]
- 84.Baino F., Potestio I., Vitale-Brovarone C. Production and physicochemical characterization of Cu-doped silicate bioceramic scaffolds. Materials. 2018;11 doi: 10.3390/ma11091524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kaya S., Cresswell M., Boccaccini A.R. Mesoporous silica-based bioactive glasses for antibiotic-free antibacterial applications. Mater. Sci. Eng. C-Mater. Biol. Appl. 2018;83:99–107. doi: 10.1016/j.msec.2017.11.003. [DOI] [PubMed] [Google Scholar]
- 86.Youness R.A., Taha M.A., Ibrahim M., El-Kheshen A. FTIR spectral characterization, mechanical properties and antimicrobial properties of La-doped phosphate-based bioactive glasses. Silicon. 2018;10:1151–1159. doi: 10.1007/s12633-017-9587-0. [DOI] [Google Scholar]
- 87.Bhowmick A., Pramanik N., Jana P., Mitra T., Gnanamani A., Das M., et al. Development of bone-like zirconium oxide nanoceramic modified chitosan based porous nanocomposites for biomedical application. Int. J. Biol. Macromol. 2017;95:348–356. doi: 10.1016/j.ijbiomac.2016.11.052. [DOI] [PubMed] [Google Scholar]
- 88.Andrabi S.M., Singh P., Majumder S., Kumar A. A compositionally synergistic approach for the development of a multifunctional bilayer scaffold with antibacterial property for infected and chronic wounds. Chem. Eng. J. 2021;423:130219. doi: 10.1016/j.cej.2021.130219. [DOI] [Google Scholar]
- 89.Vitale-Brovarone C., Miola M., Balagna C., Verne E. 3D-glass-ceramic scaffolds with antibacterial properties for bone grafting. Chem. Eng. J. 2008;137:129–136. doi: 10.1016/j.cej.2007.07.083. [DOI] [Google Scholar]
- 90.Xu Q., Chang M.L., Zhang Y., Wang E.D., Xing M., Gao L., et al. PDA/Cu bioactive hydrogel with "hot ions effect" for inhibition of drug-resistant bacteria and enhancement of infectious skin wound healing. ACS Appl. Mater. Interfaces. 2020;12:31255–31269. doi: 10.1021/acsami.0c08890. [DOI] [PubMed] [Google Scholar]
- 91.Wen X.Z., Wang Q., Dai T.T., Shao J., Wu X.H., Jiang Z.Y., et al. Identification of possible reductants in the aqueous leaf extract of mangrove plant Rhizophora apiculata for the fabrication and cytotoxicity of silver nanoparticles against human osteosarcoma MG-63 cells. Mater. Sci. Eng. C-Mater. Biol. Appl. 2020;116 doi: 10.1016/j.msec.2020.111252. [DOI] [PubMed] [Google Scholar]
- 92.Hu C., Wu L.N., Zhou C.C., Sun H., Gao P., Xu X.J., et al. Berberine/Ag nanoparticle embedded biomimetic calcium phosphate scaffolds for enhancing antibacterial function. Nanotechnol. Rev. 2020;9:568–579. doi: 10.1515/ntrev-2020-0046. [DOI] [Google Scholar]
- 93.Wu C.T., Zhou Y.H., Xu M.C., Han P.P., Chen L., Chang J., et al. Copper-containing mesoporous bioactive glass scaffolds with multifunctional properties of angiogenesis capacity, osteostimulation and antibacterial activity. Biomaterials. 2013;34:422–433. doi: 10.1016/j.biomaterials.2012.09.066. [DOI] [PubMed] [Google Scholar]
- 94.Sugimoto H., Biggemann J., Fey T., Singh P., Khare D., Dubey A.K., et al. Lead-free piezoelectric (Ba,Ca)(Ti,Zr)O3 scaffolds for enhanced antibacterial property. Mater. Lett. 2021;297:129969. doi: 10.1016/j.matlet.2021.129969. [DOI] [Google Scholar]
- 95.Bigham A., Aghajanian A.H., Behzadzadeh S., Sokhani Z., Shojaei S., Kaviani Y., et al. Nanostructured magnetic Mg2SiO4-CoFe2O4 composite scaffold with multiple capabilities for bone tissue regeneration. Mater. Sci. Eng. C-Mater. Biol. Appl. 2019;99:83–95. doi: 10.1016/j.msec.2019.01.096. [DOI] [PubMed] [Google Scholar]
- 96.Wiedmer D., Cui C., Weber F., Petersen F.C., Tiainen H. Antibacterial surface coating for bone scaffolds based on the dark catalytic effect of titanium dioxide. ACS Appl. Mater. Interfaces. 2018;10:35784–35793. doi: 10.1021/acsami.8b12623. [DOI] [PubMed] [Google Scholar]
- 97.Wang Y., Yang Y.N., Shi Y.R., Song H., Yu C.Z. Antibiotic-free antibacterial strategies enabled by nanomaterials: progress and perspectives. Adv. Mater. 2020;32 doi: 10.1002/adma.201904106. [DOI] [PubMed] [Google Scholar]
- 98.Niu W., Chen M., Guo Y., Wang M., Luo M., Cheng W., et al. A multifunctional bioactive glass-ceramic nanodrug for post-surgical infection/cancer therapy-tissue regeneration. ACS Nano. 2021;15:14323–14337. doi: 10.1021/acsnano.1c03214. [DOI] [PubMed] [Google Scholar]
- 99.Hu D.F., Deng Y.Y., Jia F., Jin Q., Ji J. Surface charge switchable supramolecular nanocarriers for nitric oxide synergistic photodynamic eradication of biofilms. ACS Nano. 2020;14:347–359. doi: 10.1021/acsnano.9b05493. [DOI] [PubMed] [Google Scholar]
- 100.LogithKumar R., KeshavNarayan A., Dhivya S., Chawla A., Saravanan S., Selvamurugan N. A review of chitosan and its derivatives in bone tissue engineering. Carbohydr. Polym. 2016;151:172–188. doi: 10.1016/j.carbpol.2016.05.049. [DOI] [PubMed] [Google Scholar]
- 101.Zhou P.Y., Xia Y., Cheng X.S., Wang P.F., Xie Y., Xu S.G. Enhanced bone tissue regeneration by antibacterial and osteoinductive silica-HACC-zein composite scaffolds loaded with rhBMP-2. Biomaterials. 2014;35:10033–10045. doi: 10.1016/j.biomaterials.2014.09.009. [DOI] [PubMed] [Google Scholar]
- 102.Lu Y., Li M., Li L.H., Wei S.Z., Hu X.M., Wang X.L., et al. High-activity chitosan/nano hydroxyapatite/zoledronic acid scaffolds for simultaneous tumor inhibition, bone repair and infection eradication. Mater. Sci. Eng. C-Mater. Biol. Appl. 2018;82:225–233. doi: 10.1016/j.msec.2017.08.043. [DOI] [PubMed] [Google Scholar]
- 103.Liu W.Y., Zuo R.T., Zhu T.L., Zhu M., Zhao S.C., Zhu Y.F. Forsterite-hydroxyapatite composite scaffolds with photothermal antibacterial activity for bone repair. J. Adv. Ceram. 2021;10:1095–1106. doi: 10.1007/s40145-021-0494-x. [DOI] [Google Scholar]
- 104.Wang J.X., Wang L.T., Pan J., Zhao J.H., Tang J., Jiang D.J., et al. Magneto-based synergetic therapy for implant-associated infections via biofilm disruption and innate immunity regulation. Adv. Sci. 2021;8 doi: 10.1002/advs.202004010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Fan L.H., Muhammad A.I., Ismail B.B., Liu D.H. Sonodynamic antimicrobial chemotherapy: an emerging alternative strategy for microbial inactivation. Ultrason. Sonochem. 2021;75 doi: 10.1016/j.ultsonch.2021.105591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ensing G.T., Roeder B.L., Nelson J.L., van Horn J.R., van der Mei H.C., Busscher H.J., et al. Effect of pulsed ultrasound in combination with gentamicin on bacterial viability in biofilms on bone cements in vivo. J. Appl. Microbiol. 2005;99:443–448. doi: 10.1111/j.1365-2672.2005.02643.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Guan W., Tan L., Liu X.M., Cui Z.D., Zheng Y.F., Yeung K.W.K., et al. Ultrasonic interfacial engineering of red phosphorous-metal for eradicating MRSA infection effectively. Adv. Mater. 2021;33 doi: 10.1002/adma.202006047. [DOI] [PubMed] [Google Scholar]
- 108.Zhang G.N., Yang Y.Q., Shi J., Yao X.H., Chen W.Y., Wei X.C., et al. Near-infrared light II - assisted rapid biofilm elimination platform for bone implants at mild temperature. Biomaterials. 2021;269 doi: 10.1016/j.biomaterials.2020.120634. [DOI] [PubMed] [Google Scholar]
- 109.Zhao C.Q., Liu W.G., Xu Z.Y., Li J.G., Huang T.T., Lu Y.J., et al. Chitosan ducts fabricated by extrusion-based 3D printing for soft-tissue engineering. Carbohydr. Polym. 2020;236:7. doi: 10.1016/j.carbpol.2020.116058. [DOI] [PubMed] [Google Scholar]
- 110.Qiao Y.J., Zhai Z.J., Chen L.M., Liu H. Cytocompatible 3D chitosan/hydroxyapatite composites endowed with antibacterial properties: toward a self-sterilized bone tissue engineering scaffold. Sci. Bull. 2015;60:1193–1202. doi: 10.1007/s11434-015-0838-4. [DOI] [Google Scholar]
- 111.Gritsch L., Maqbool M., Mourino V., Ciraldo F.E., Cresswell M., Jackson P.R., et al. Chitosan/hydroxyapatite composite bone tissue engineering scaffolds with dual and decoupled therapeutic ion delivery: copper and strontium. J. Math. Chem. B. 2019;7:6109–6124. doi: 10.1039/c9tb00897g. [DOI] [PubMed] [Google Scholar]
- 112.Ramesh S., Kovelakuntla V., Meyer A.S., Rivero I.V. Three-dimensional printing of stimuli-responsive hydrogel with antibacterial activity. Bioprinting. 2020 doi: 10.1016/j.bprint.2020.e00106. [DOI] [Google Scholar]
- 113.El-Sayed S.A.M., Mabrouk M., Khallaf M.E., Abd El-Hady B.M., El-Meliegy E., Shehata M.R. Antibacterial, drug delivery, and osteoinduction abilities of bioglass/chitosan scaffolds for dental applications. J. Drug Deliv. Sci. Technol. 2020;57 doi: 10.1016/j.jddst.2020.101757. [DOI] [Google Scholar]
- 114.Xu J.W., Yao K., Xu Z.K. Nanomaterials with a photothermal effect for antibacterial activities: an overview. Nanoscale. 2019;11:8680–8691. doi: 10.1039/c9nr01833f. [DOI] [PubMed] [Google Scholar]
- 115.Garcia-Alvarez R., Izquierdo-Barba I., Vallet-Regi M. 3D scaffold with effective multidrug sequential release against bacteria biofilm. Acta Biomater. 2017;49:113–126. doi: 10.1016/j.actbio.2016.11.028. [DOI] [PubMed] [Google Scholar]
- 116.Camara-Torres M., Duarte S., Sinha R., Egizabal A., Alvarez N., Bastianini M., et al. 3D additive manufactured composite scaffolds with antibiotic-loaded lamellar fillers for bone infection prevention and tissue regeneration. Bioact. Mater. 2021;6:1073–1082. doi: 10.1016/j.bioactmat.2020.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Zeimaran E., Pourshahrestani S., Djordjevic I., Pingguan-Murphy B., Kadri N.A., Wren A.W., et al. Antibacterial properties of poly (octanediol citrate)/gallium-containing bioglass composite scaffolds. J. Mater. Sci. Mater. Med. 2016;27 doi: 10.1007/s10856-015-5620-2. [DOI] [PubMed] [Google Scholar]
- 118.Thanyaphoo S., Kaewsrichan J. Synthesis and evaluation of novel glass ceramics as drug delivery systems in osteomyelitis. J. Pharmacol. Sci. 2012;101:2870–2882. doi: 10.1002/jps.23230. [DOI] [PubMed] [Google Scholar]
- 119.Iviglia G., Cassinelli C., Bollati D., Baino F., Torre E., Morra M., et al. Engineered porous scaffolds for periprosthetic infection prevention. Mater. Sci. Eng. C-Mater. Biol. Appl. 2016;68:701–715. doi: 10.1016/j.msec.2016.06.050. [DOI] [PubMed] [Google Scholar]
- 120.Li K., Cai K.Y., Ran Q.C., Jiang D.M. Biomimetic triphase composite scaffolds with antibacterial and antitumor potentials for bone repair. Mater. Lett. 2019;256 doi: 10.1016/j.matlet.2019.126590. [DOI] [Google Scholar]
- 121.Wu Y., Liao Q., Wu L., Luo Y., Zhang W., Guan M., et al. ZnL2-BPs integrated bone scaffold under sequential photothermal mediation: a win-win strategy delivering antibacterial therapy and fostering osteogenesis thereafter. ACS Nano. 2021 doi: 10.1021/acsnano.1c06062. [DOI] [PubMed] [Google Scholar]
- 122.Zheng H., Wang S., Cheng F., He X., Liu Z., Wang W., et al. Bioactive anti-inflammatory, antibacterial, conductive multifunctional scaffold based on MXene@CeO2 nanocomposites for infection-impaired skin multimodal therapy. Chem. Eng. J. 2021;424:130148. doi: 10.1016/j.cej.2021.130148. [DOI] [Google Scholar]